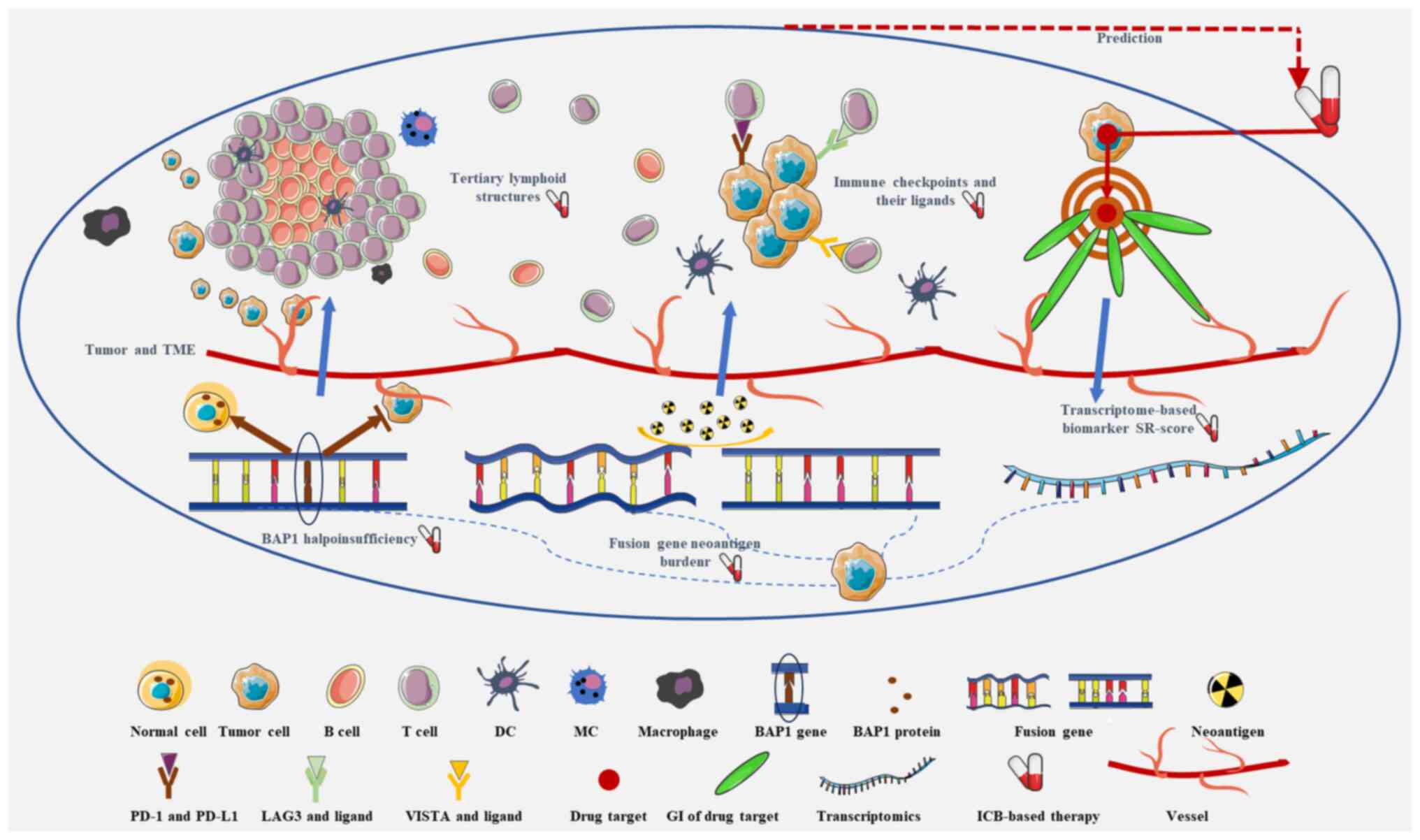
Malignant peritoneal mesothelioma (MPeM) is a rare
and highly lethal type of cancer. Approximately 500 to 700 new
cases are diagnosed annually in the United States and median
overall survival (OS) is 6 months to 1 year. Platinum + pemetrexed
with or without bevacizumab is the standard first-line therapy for
patients with advanced disease; however, its efficacy is limited
(1,2).
Immune checkpoint blockade (ICB)-based therapy has
revolutionized the treatment of several types of solid tumors.
Currently, approved ICB treatments worldwide include
anti-programmed death-1 (PD-1)/anti-programmed death-ligand 1
(PD-L1), anti-cytotoxic T lymphocyte-associated protein 4 (CTLA-4)
and anti-lymphocyte activation gene-3 (LAG-3) treatment (3). Given the promising results reported
for malignant pleural mesothelioma (MPM), certain small studies
have focused on the efficacy of ICB-based therapy for MPeM
(4–6). In a real-world study, 29 patients with
MPeM were treated with nivolumab + ipilimumab or a single immune
checkpoint inhibitor as the second-line treatment (7). The objective response rate (ORR) was
19.2% (5/26; 95% CI, 6.6–39.4). In a phase II single-center study
(8), 20 patients with MPeM were
treated with atezolizumab + bevacizumab as the second-line
treatment and the ORR was 40% (8/20; 95% CI 19.1–64.0). Both
studies demonstrated the encouraging clinical activity of ICB
treatment for MPeM.
However, it is evident that only a subset of
patients with MPeM may benefit from ICB-based therapy. Identifying
patients who may benefit from ICB-based therapy is a subject of
research. To date, tumor mutation burden (TMB), expression of PD-L1
and microsatellite instability (MSI) are the three biomarkers
validated for predicting the response to ICB; however, there is no
widely accepted method for prediction based on biomarkers, and
their applications vary by disease site (9–11).
Certain patients with TMB-low and PD-L1 negative tumors also
exhibit marked treatment responses; meanwhile, other patients with
MSI-high tumors show primary or secondary resistance to ICB therapy
(12,13). Other biomarkers, including the tumor
microenvironment (TME) and the components in the TME, host immune
response patterns based on transcriptomic and proteomic analysis,
as well as specific mutations (such as gene fusions) or clonal
mutations, are currently being explored. Malignant mesothelioma has
been traditionally assumed to be a TMB-low tumor with extremely low
MSI prevalence (14,15). Therefore, there is an urgent need to
develop appropriate biomarkers for predicting the efficacy of
ICB-based therapy (including, but not limited to,
anti-PD-1/anti-PD-L1 treatment) for patients with MPeM. The present
review focuses on five types of promising biomarkers currently
available, highlighting evidence that supports the predictive role
of these biomarkers for ICB-based therapy for MPeM (Fig. 1 and Table I). Additionally, the limitations of
each type of biomarker are discussed and possible methods for
addressing these problems are mentioned.
It is well established that the TME is a complex
system. It includes multiple cellular and non-cellular components
and serves an important role in several stages of tumor development
and progression, including metastasis, and evasion of immune
monitoring and treatment. Initially, the majority of tumor
immunology research focused on how the function and relative
abundance of T cells and macrophages within the TME mediated
effector responses (16–19). Recently, it was reported that other
tumor-infiltrating immune subsets, such as B cells and mast cells,
were essential for effector responses (20,21).
TLS are organized aggregates of immune cells,
characterized by an inner zone of CD20+ B cells surrounded by CD3+
T cells. In addition to B and T cell populations, TLS are also
populated by dendritic cells (DC), macrophages and other immune
cell types (22–24). Several studies have reported that
TLS were detectable in certain types of tumors, such as cutaneous
angiosarcoma, colorectal cancer, hepatocellular carcinoma, gastric
cancer, gastrointestinal stromal tumor, breast cancer and lung
squamous cell carcinoma (25–31).
However, studies focusing on TLS in MPeMs are limited. In a study
published in 2005, it was reported that there was a large degree of
infiltration of lymphocytes and plasma cells, including lymphoid
aggregates and follicles within the omental fat or omental fibrous
tissue surrounding the areas of an invading tumor in 13/75 female
patients with MPeM (32). This
morphological change is akin to a TLS. Recently, Benzerdjeb et
al (33) reported there were
numerous lymphoid aggregates with or without germinal centers in
52/138 cases with epithelioid MPeM. Another study demonstrated that
TLS were present in MPeMs (32).
Additionally, the study reported that neoadjuvant chemotherapy
could induce the formation of TLS; however, the study did not
report on the relationship between the presence of TLS and the
response to ICB therapy.
TLS are an important component of the TME and serve
a critical role in regulating tumor-specific immune responses
(24,32). A growing body of evidence has
suggested that TLS can serve as a predictive biomarker for the
response to ICB treatment in certain types of solid tumors. The
presence of TLS in pre-treatment biopsies of melanoma, renal cell
carcinoma, soft tissue sarcoma and urothelial carcinoma has been
reported to be associated with the response to anti-PD-1 or
anti-PD-1 + anti-CTLA-4 treatment (20,24,34–36).
These studies highlighted that there was a higher density of TLS in
pre-treatment tumor tissues of responder patients as compared to
non-responder patients. Furthermore, in a randomized phase II
study, patients with MPM were treated using a single cycle of
durvalumab + tremelimumab or durvalumab alone in a neoadjuvant
setting (37). There was a marked
increase in TLS density following ICB combination therapy, a
greater increase in TLS formation in tumors that had partial
remission (PR), and a higher pre-treatment TLS density associated
with PR. The study also reported that pre-treatment TLS was more
closely associated with the efficacy of ICB treatment, and TLS
formation could be induced following ICB treatment just as with
chemotherapy (33,37). This evidence suggests that
pre-treatment TLS may fully represent the initial tumor status and
have a greater impact on the response to ICB therapy. Thus, the
presence of TLS pre-treatment is more accurate in predicting the
response to ICB treatment, as it implicates that the tumor and the
host under the influence of the tumor may already be generating an
antitumor immune response, which is potentially enhanced by ICB
therapy and chemotherapy.
The findings from the limited body of studies
indicate that TLS are present in MPeM and the presence of TLS
pre-treatment has potential as a biomarker for predicting the
efficacy of ICB therapy. Notably, TLS is a readily testable and
acquirable indicator using immunohistochemistry in the clinic
(23). However, there are no
studies on the predictive role of TLS for ICB treatment in MPeM, to
the best of our knowledge. Additionally, the underlying mechanism
of TLS formation is not clear, and this may be critical for
understanding the predictive value of TLS status in determining the
efficacy of ICB therapy. Therefore, additional studies are required
to verify the relationship between the TLS and ICB treatment in
MPeM.
PD-L1 expression is the most commonly used biomarker
for predicting the benefits of anti-PD-1/anti-PD-L1 treatment, and
it has been reported that PD-L1 is expressed in mesotheliomas
(38–40). A study by Chapel et al
(38) used Dako PD-L1 IHC 22C3
pharmDx and Dako PD-L1 IHC 28–8 pharmDx (Agilent Technologies,
Inc.) to detect PD-L1 expression in mesothelioma, with a 22 and 27%
positive tumor proportion score (TPS; cutoff ≥1%), respectively.
The proportion of cases with positive PD-L1 expression was notably
higher among MPeMs compared with that in MPMs (22C3 assay: MPM, 18%
and MPeM 54%; 28-8 assay: MPM, 24% and MPeM, 54%). In another
study, the positive combined positive score (CPS) and positive TPS
(both cutoff ≥1%; 22C3 assay) were 76 and 43% among MPeMs,
respectively (39). More aggressive
biphasic/sarcomatoid MPeMs had a higher CPS and TPS. However, a
study by Pezzuto et al (40)
reported that there were only 2% positive TPS (cutoff ≥1%; 22C3
assay) cases among 43 patients with MPeM. It was hypothesized that
this discrepancy was attributed to the small sample size of
non-epithelioid cases, which are more commonly positive for
PD-L1.
The role of PD-L1 in predicting the response to
ICB-based therapy for MPeM is also contested. In a phase II trial,
56 patients with MPM and eight patients with MPeM were treated with
pembrolizumab alone (41). The ORR
was 20 and 12.5%, respectively. Notably, PD-L1 expression was not
associated with ORR. Moreover, Raghav et al (8) reported 20 patients with MPeM treated
with atezolizumab + bevacizumab. The ORR was 40% and responses were
reported in both PD-L1-positive and -negative cases. Given these
contradictory results, there is no consensus on identifying
patients with MPeM who would benefit from anti-PD-1/anti-PD-L1
treatment based solely on PD-L1 expression. Therefore, there is no
solid evidence to support PD-L1 expression as a biomarker for
predicting the efficacy of anti-PD-1/anti-PD-L1 treatment for MPeM.
It is necessary to determine PD-L1 expression status and the
association between PD-L1 expression and the efficacy of
anti-PD-1/anti-PD-L1 treatment in patients with MPeM. Additionally,
there is a need to optimize other histological and molecular
characteristics as biomarkers to guide ICB-based therapy for
MPeM.
VISTA is a negative checkpoint regulator, which is
expressed in most hematopoietic cells. In addition, VISTA is
expressed in numerous types of tumor cells (42–45).
Several studies have reported notable upregulated expression of
VISTA in immunohistochemical analysis of malignant mesothelioma
tissues and in the normal mesothelium, as well as higher VISTA
expression levels in the epithelioid subtype (46–50).
VISTA serves a role in regulating the steady state
of both lymphoid and myeloid cells involved in the immune system
(53–56). Liu et al (57) reported that VISTA/PD-1 double
deficient knockout (KO) mice exhibited notably higher levels of
chronic inflammation and activation of T cells than the single KO
mice. This suggests that VISTA had a nonredundant role, distinct
from the PD-1/PD-L1 pathway. Moreover, an ex vivo study
supported the aforementioned result, in which pro-inflammatory
factors were upregulated and anti-inflammatory factors were
downregulated when T cells were co-cultured with MCF7 cells with
VISTA and CTLA-4 expression knocked down. Combined knockdown of
VISTA and CTLA-4 inhibited MCF7 breast cancer cell development
(58). In a study of liver cancer
using a mouse model, symptoms and tumor growth were reduced using
anti-VISTA monoclonal antibodies (mAbs), resulting in a reduction
in mouse mortality (59). In the
study of CT26 colon cancer cells and less immunogenic B16BL6
melanoma mice models, the combinatorial treatment of VISTA and
PD-L1 mAbs led to suppressed tumor growth and tumor regression and
conferred a survival advantage compared with VISTA mAb alone or
PD-L1 mAb alone, respectively (57). Treatment with the anti-VISTA
antibody KVA12123 mediated strong antitumor activity and showed
enhanced efficacy in combination with anti-PD-1 treatment in
several tumor models, including bladder cancer, colon cancer,
lymphoma and melanoma (60). These
results suggest that anti-VISTA treatment with anti-PD-1/PD-L1 or
anti-CTLA-4 treatment may contribute to the acquisition of an
immuno-active TME, enhancing antitumor responses and improving
efficacy.
Given the high frequency of upregulated VISTA
expression in MPeM and the marked benefits from VISTA blockade
treatment in preclinical models, VISTA blockade, particularly
combined with anti-PD-1/PD-L1 or anti-CTLA-4 treatment, is a
promising approach for management of MPeM. The small molecule
immune checkpoint inhibitor CA-170, which targets both PD-L1 and
VISTA, has been reported to exhibit antitumor efficacy
preclinically (61). There is
currently an ongoing phase I study in which patients with tumors
with high levels of VISTA expression, including malignant
mesothelioma, are being treated with CA-170 (62). Therefore VISTA, particularly when
combined with PD-L1, may serve as a suitable biomarker and
therapeutic target to improve the outcomes in patients receiving
anti-PD-1/PD-L1 or anti-CTLA-4 treatment.
In addition to PD-L1 and VISTA, LAG-3 may also serve
as a potential biomarker for guiding ICB-based therapy for MPeM.
LAG-3 is expressed on the membranes of immune cells, including T
cells, and it negatively regulates T-cell proliferation and
effector T-cell function (63).
Upregulated LAG-3 mRNA expression was detected in malignant
mesothelioma under specific settings. In a comprehensive analysis
of 19 cases of treatment-naive MPeM in 18 patients, high levels of
LAG-3 mRNA expression were detected in MPeM with
BRCA1-associated protein-1 (BAP1) haploinsufficiency
(64). In another analysis of MPM
datasets from The Cancer Genome Atlas (TCGA; n=86) and Memorial
Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer
Targets (n=61), the mRNA expression levels of LAG-3 and VISTA were
markedly higher in patients with tumors with BAP1 mutations [not
accompanied with neurofibromatosis type 2 (NF2) and
cyclin-dependent kinase inhibitor 2A/B (CDKN2A/B) mutations] than
tumors with NF2 or CDKN2A/B mutation alone, or BAP1 accompanied
with NF2 and CDKN2A/B mutations (65).
Preclinical experiments have indicated that LAG-3
and PD-1 may have a synergistic effect in inhibiting T-cell
activation and promoting tumor immune evasion. Simultaneous
blockade of both receptors has a more potent immune response
compared with blocking either receptor alone (66,67).
In the AB1-HA BALB/cJ mesothelioma mice model, a delay in tumor
growth and a notable improvement in survival were observed for
anti-PD-L1 and anti-PD-L1 + anti-LAG-3 treatment when compared with
the PBS control group. The combination of PD-L1 and LAG-3 blockade
differed more from the PBS control than the anti-PD-L1 monotherapy,
suggesting that anti-LAG-3 treatment had an additional effect
(68). Notably, anti-LAG-3-based
treatment has been used in the clinic. The US Food and Drug
Administration approved the combination of relatlimab (anti-LAG-3
mAb) and nivolumab (anti-PD-1 mAb) for the treatment of
unresectable or metastatic melanoma in 2022 (69). The approval was based on a phase
II/III randomized trial, in which treatment with relatlimab +
nivolumab was associated with a 47.7% 12-month progression-free
survival (PFS) in patients with melanoma, compared with 36% in
those who underwent nivolumab monotherapy. In a prespecified
exploratory analysis, median PFS estimates were markedly longer for
patients with a LAG-3 expression of ≥1% than those with a LAG-3
expression of <1% (63).
Similar to VISTA, LAG-3 blockade, particularly when
combined with anti-PD-1/PD-L1 treatment, is promising for MPeM.
Therefore LAG-3, particularly when combined with PD-L1, may serve
as a biomarker to guide the blockade of VISTA + PD-1 treatment.
Additionally, it is common that drugs approved for common tumors
are also used to treat rarer tumors clinically (70–72).
The potential use of relatlimab provides a potential opportunity
for the treatment of MPeM.
Neoantigens are tumor-specific antigens that can
stimulate an antitumor immune response (73). An increasing number of studies have
reported that high clonal neoantigen burden (present in all tumor
cells) and low neoantigen intratumor heterogeneity are associated
with the efficacy of ICB treatment (73–76).
Previously, it was hypothesized that single-nucleotide variants
(SNVs) and insertions or deletions (indels) were the most common
source of tumor neoantigen acquisition. At present, gene fusions
are also suggested to be a notable means of acquisition of tumor
neoantigens with higher immunogenic potential (77–79).
Analysis of whole genomes from 2,528 tumors revealed that gene
fusions from genomic rearrangement notably contributed to
neoantigen formation in both quantity and quality (77). By comparing TCGA fusion candidate
neoantigens with TCGA SNVs and indel candidate neoantigens, it was
reported that gene fusions generated six-fold more candidate
neoantigens and 11-fold more specific candidate neoantigens
(78).
Chromosomal rearrangements are the source of gene
fusions and they are common in malignant mesotheliomas (80–83).
Yoshikawa et al (84)
reported multiple noncontiguous minute deletions on chromosome 3p21
in malignant mesotheliomas, where the BAP1 gene is located.
This genetic change was attributed to chromothripsis, which is a
mutational phenomenon in which the rapid accrual of hundreds of
rearrangements occurs (80). Oey
et al (81) reported that
8/9 tested mesotheliomas had complex rearranged genomes with
evidence of chromothripsis and chromoplexy, the latter representing
a series of linked translocations or weaving of chromosomal
fragments (80). In the study by
Bueno et al (82), gene
fusions from chromosomal rearrangements and splice alterations were
frequent mechanisms for the inactivation of the driver genes of
malignant mesothelioma, such as NF2, BAP1 and SET domain
containing 2. Neoantigen prediction analysis revealed that
neoantigens formed from gene fusions. Mansfield et al
(83) reported that there were
1,535 chromosomal rearrangements by Mate-pair sequencing in 22
treatment-naive cases of malignant mesothelioma specimens and
several of these abnormalities were obtained due to chromothripsis
and chromoplexy. The study also demonstrated that gene fusions from
chromosomal rearrangements could result in neoantigen formation.
Together, these results demonstrated that chromosomal
rearrangements were common in malignant mesotheliomas, in
accordance with the complex structural and numerical abnormalities
of the karyotype reported in other studies (85,86).
Malignant mesotheliomas may exhibit marked neoantigen formation
from rearrangement-related gene fusions. However, chromosomal
rearrangements and rearrangement-related gene fusions were notably
underestimated due to the limitations of previous detection
techniques and approaches, such as standard next-generation
sequencing technology (80,82).
Fusion gene neoantigens tend to have higher
immunogenic potential and trigger more effective adaptive immune
responses. Analysis of whole genomes from 2,528 tumors observed the
derived neoantigens from genomic rearrangements, especially the
clonal neoantigens, were extensively immuno-edited, which suggested
their immunogenic potential (77).
A comprehensive analysis of tumor neoantigens reported that
candidate neoantigens with the highest immunogenic potential were
produced by fusion genes in 32.2% of cases in a TCGA dataset
(78). By analyzing 522 cases of
head and neck squamous cell carcinoma with a low TMB, it was
reported that fusion-associated neoantigens were able to stimulate
a more potent T cell response compared with missense
mutation-associated neoantigens (79). Fusion gene neoantigens with the
immunogenic potential to elicit an adaptive immune response were
also observed in mesothelioma. Mansfield et al (83) estimated that 1,535 chromosomal
rearrangements in malignant mesothelioma specimens resulted in the
expression of 179 novel peptides. A number of the 179 candidate
neoantigens could bind to patient-specific human leukocyte antigen
molecules and improve the expansion of tumor-infiltrating T cells
in the TME (83). These results
revealed that neoantigens from chromosomal rearrangement-related
gene fusions in malignant mesotheliomas had the potential to elicit
more effective anti-neoplasm immune responses.
Conventionally, TMB is used to estimate the
neoantigen burden and has been shown to predict the efficacy of
anti-PD-1/anti-PD-L1 treatment for several tumors (87–90).
However, malignant mesotheliomas, including MPeM, are commonly
viewed as tumors with a low TMB. This is attributed to the
disadvantages of conventional detection techniques and approaches
(84). Notably, Kosari et al
(91) used the burden of tumor
junction from chromosomal rearrangements as a surrogate biomarker
for determining the fusion gene neoantigen burden. It was reported
that tumor junction burden was associated with improved survival
outcomes in patients with mesothelioma treated with ICB in the
presence of antigen processing and presentation gene set
expression. Moreover, it was revealed that antigen processing and
presentation were essential for the role of tumor junction burden.
Another study also emphasized that a high burden of neopeptides
could successfully predict the response to PD-1 inhibitors in
patients with advanced MPM or melanoma when accompanied by
upregulated expression of major histocompatibility complex proteins
specific for the neopeptides (92).
These studies suggest that fusion gene neoantigen burden could be
used instead of TMB and that it serves a predictive role in ICB
treatment in mesothelioma when antigen processing and presentation
are taken into account.
Together, the available studies suggest that
malignant mesotheliomas exhibit a higher degree of neoantigen
formation, and this can be attributed to chromosomal
rearrangement-related gene fusions. When neoantigens are processed
and presented appropriately, they have a notable immunogenic
potential to affect effector cells in the TME, and the burden of
these neoantigens has potential as a biomarker for predicting the
response to ICB treatment for patients with MPeM. However,
prospective studies are required to validate these findings.
Therefore, BAP1 haploinsufficiency alone may not be
adequate for predicting the response to ICB-based therapy. In a
retrospective analysis of the JAVELIN Renal 101 and
checkmate-009/010/025 trials, the BAP1 score was developed using
the top 20 BAP1 mutation-associated differentially expressed genes.
The BAP1-score was a significant predictor of the immune
microenvironment and the clinical benefits of ICB-based therapies
in patients with advanced clear cell renal cell carcinoma (98). Therefore, BAP1
haploinsufficiency-based biomarkers may be superior to BAP1
haploinsufficiency alone. The value of BAP1 haploinsufficiency or
BAP1 haploinsufficiency-based biomarkers both warrant further
investigation.
It has been reported that certain tumor
transcriptomic changes can be used as biomarkers to guide tumor
therapy (99–102). However, the predictive roles of
these biomarkers are limited to a highly specific clinical context
and treatments. Lee et al (103) developed a uniform systematic
approach, SynthEtic LEthality and rescue-mediated precision
onCology via the Transcriptome (SELECT), which does not train any
model parameters by looking at the test data. Using SELECT, the
best drug was selected for a given patient based on the tumor
pre-treatment transcriptome data. Unlike commonly matching drugs
based on the transcriptome-based expression of their targets,
SELECT focused on identifying and utilizing the genetic interaction
(GI) of drug targets as the biologically testable biomarkers for
the prediction of a therapeutic response. The study analyzed data
obtained from TCGA and found notable pan-cancer synthetic rescue
(SR) interaction partners of PD-1/PD-L1 and CTLA-4. SR interaction
is described as the inactivation of one gene reducing cell
viability, but the alteration of another gene's activity-rescuing
viability. The study defined SR scores, where tumors with higher SR
scores were predicted to respond better to ICB treatment
(anti-PD-1/anti-PD-L1 and anti-CTLA-4 treatment). Higher SR scores
were associated with better responses to ICB treatment based on an
analysis of 21 immune checkpoint therapy datasets. Subsequently,
Nair et al (104) used the
same parameters and procedure described by Lee et al
(103) to predict the response to
anti-PD-1 treatment in an National Cancer Institute mesothelioma
patient cohort, which included an equal proportion of patients with
pleural and peritoneal mesothelioma. SR scores were able to
accurately predict the response to anti-PD-1 treatment in these
patients with mesothelioma.
ENLIGHT was designed and developed on the basis of
SELECT, which is a transcriptomics-based computational approach
that identifes clinically relevant genetic interactions and uses
them to predict responses to a variety of therapies in multiple
cancer types. ENLIGHT-matching scores (EMS) could be used to
predict the efficacy of immunotherapies (including ICBs and mAbs)
more accurately than targeted small molecules. EMS was comparable
with other ICB-specific biomarkers, including the proliferation
signature, cytolytic index and interferon (IFN)-γ signature. The
combination of EMS and IFN-γ was more accurate than either of them
alone or the other combinations in the ICB cohorts (105). Similar to SELECT, ENLIGHT did not
require training on previous treatment response data, which is
useful for rare tumors, such as MPeM (105).
Therefore, transcriptome-based biomarkers are
promising, as they seem to provide more comprehensive and valuable
information. Nevertheless, the veracity of the SR-score, EMS and
ENLIGHT-DeepPT require further testing in carefully controlled
prospective clinical trials.
ICB-based therapy has rapidly emerged as a principal
therapeutic modality for numerous solid tumors owing to its
efficacy. Notably, the efficacy of ICB-based therapy depends on
multiple factors, from tumor-specific neoantigen formation to
neoantigen processing and presentation, then to tumor cell
interactions with effector cells and the TME, among other factors.
Regarding ICB-based therapy, the goal of anti-PD-1/anti-PD-L1
therapy is to reactivate cytotoxic T cells within the TME against
tumor cells. Therefore, it is inevitable that the majority of
tumors show primary or secondary resistance. Thus, it is critical
to stratify patients with MPeM and develop novel therapeutic
strategies targeting the other factors underlying the pathogenesis
of MPeM.
At present, there have been several exploratory
studies regarding MPeM treatment, primarily targeting the
components beyond PD-1/PD-L1 within the TME, or targeting abnormal
molecular and signaling pathway alterations within tumor cells. The
former includes targeting mast cells to restore/promote T cell
infiltration within the TME, chemokines and cytokines for the
modulation of TLS neogenesis, and vascular endothelial growth
factor/vascular endothelial growth factor receptor to inhibit
neovascularization, among other approaches (47,107–109). The latter includes targeting the
PI3K/mTOR pathway, Hippo-Yes-associated protein pathway,
BAP1-/BRCA1-deficiency, anaplastic lymphoma kinase rearrangements,
focal adhesion kinase phosphorylation, fibroblast growth factor
receptor inhibition and histone deacetylase inhibitor inhibition,
among other targets (110–116). Although these therapeutic
approaches have shown antitumor efficacy to a certain degree,
further studies are still required. Moreover, given the complicated
alterations that can occur within tumor cells themselves and the
TME, combination treatment seems to be the most promising approach
(8).
The aim of the present review was to highlight
predictive biomarkers to guide ICB-based therapy (Fig. 1). The aforementioned biomarkers only
reflect a small portion of the tumor-associated alterations that
likely occur in MPeM. Therefore, combining multiple parameters to
develop predictive biomarker profiles is a more common-sense
approach. This principle is also applicable to other therapeutic
strategies, including combination treatment. However, this requires
not only a deep understanding of the alterations of tumors
themselves and the TME, but also the development of appropriate
tools to analyze these alterations. In this regard, artificial
intelligence-based approaches may be a valuable tool (117,118).
In conclusion, a wider range of appropriate
biomarkers in MPeM pathogenesis are also required to guide
ICB-based therapy and other novel therapeutic strategies.
Not applicable.
Funding: No funding was received.
Not applicable.
CW performed the review. CW, YZ and WL contributed
to the writing and editing of the manuscript. All authors have read
and approved the final manuscript and have full access to all the
data in the study and take responsibility for the integrity and
security of the data. Data authentication is not applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Kusamura S, Kepenekian V, Villeneuve L,
Lurvink RJ, Govaerts K, De Hingh IHJT, Moran BJ, Van der Speeten K,
Deraco M and Glehen O; PSOGI: Peritoneal mesothelioma:
PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment
and follow-up. Eur J Surg Oncol. 47:36–59. 2021. View Article : Google Scholar
|
|
2
|
Greenbaum A and Alexander HR: Peritoneal
mesothelioma. Transl Lung Cancer Res. 9 (Suppl 1):S120–S132. 2020.
View Article : Google Scholar
|
|
3
|
Rui R, Zhou L and He S: Cancer
immunotherapies: Advances and bottlenecks. Front Immunol.
14:12124762023. View Article : Google Scholar
|
|
4
|
Scherpereel A, Mazieres J, Greillier L,
Lantuejoul S, Dô P, Bylicki O, Monnet I, Corre R, Audigier-Valette
C, Locatelli-Sanchez M, et al: Nivolumab or nivolumab plus
ipilimumab in patients with relapsed malignant pleural mesothelioma
(IFCT-1501 MAPS2): A multicentre, open-label, randomised,
non-comparative, phase 2 trial. Lancet Oncol. 20:239–253. 2019.
View Article : Google Scholar
|
|
5
|
Disselhorst MJ, Quispel-Janssen J,
Lalezari F, Monkhorst K, de Vries JF, van der Noort V, Harms E,
Burgers S and Baas P: Ipilimumab and nivolumab in the treatment of
recurrent malignant pleural mesothelioma (INITIATE): Results of a
prospective, single-arm, phase 2 trial. Lancet Respir Med.
7:260–270. 2019. View Article : Google Scholar
|
|
6
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar
|
|
7
|
Raghav K, Liu S, Overman M, Morani A,
Willette A, Fournier K and Varadhachary G: Clinical efficacy of
immune checkpoint inhibitors in patients with advanced malignant
peritoneal mesothelioma. JAMA Netw Open. 4:e21199342021. View Article : Google Scholar
|
|
8
|
Raghav K, Liu S, Overman MJ, Willett AF,
Knafl M, Fu SC, Malpica A, Prasad S, Royal RE, Scally CP, et al:
Efficacy, safety, and biomarker analysis of combined PD-L1
(Atezolizumab) and VEGF (Bevacizumab) blockade in advanced
malignant peritoneal mesothelioma. Cancer Discov. 11:2738–2747.
2021. View Article : Google Scholar
|
|
9
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar
|
|
10
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020. View Article : Google Scholar
|
|
11
|
Sepulveda AR, Hamilton SR, Allegra CJ,
Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C,
Lindor NM, Minsky BD, et al: Molecular biomarkers for the
evaluation of colorectal cancer: Guideline From the American
Society for Clinical Pathology, College of American Pathologists,
Association for Molecular Pathology, and American Society of
Clinical Oncology. J Clin Oncol. 35:1453–1486. 2017. View Article : Google Scholar
|
|
12
|
Lu S, Stein JE, Rimm DL, Wang DW, Bell JM,
Johnson DB, Sosman JA, Schalper KA, Anders RA, Wang H, et al:
Comparison of biomarker modalities for predicting response to
PD-1/PD-L1 checkpoint blockade: A systematic review and
meta-analysis. JAMA Oncol. 5:1195–1204. 2019. View Article : Google Scholar
|
|
13
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar
|
|
14
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017.PO.17.00073. 2017. View Article : Google Scholar
|
|
15
|
Cedrés S, Ponce-Aix S, Iranzo P, Callejo
A, Pardo N, Navarro A, Martinez-Marti A, Gómez-Abecia S, Zucchiatti
AC, Sansano I, et al: Analysis of mismatch repair (MMR) proteins
expression in a series of malignant pleural mesothelioma (MPM)
patients. Clin Transl Oncol. 22:1390–1398. 2020. View Article : Google Scholar
|
|
16
|
Zhang Z, Liu S, Zhang B, Qiao L and Zhang
Y and Zhang Y: T cell dysfunction and exhaustion in cancer. Front
Cell Dev Biol. 8:172020. View Article : Google Scholar
|
|
17
|
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA,
Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, et
al: T-Cell-Inflamed gene-expression profile, programmed death
ligand 1 expression, and tumor mutational burden predict efficacy
in patients treated with pembrolizumab across 20 cancers:
KEYNOTE-028. J Clin Oncol. 37:318–327. 2019. View Article : Google Scholar
|
|
18
|
Hiltbrunner S, Mannarino L, Kirschner MB,
Opitz I, Rigutto A, Laure A, Lia M, Nozza P, Maconi A, Marchini S,
et al: Tumor immune microenvironment and genetic alterations in
mesothelioma. Front Oncol. 11:6600392021. View Article : Google Scholar
|
|
19
|
Bentham R, Litchfield K, Watkins TBK, Lim
EL, Rosenthal R, Martínez-Ruiz C, Hiley CT, Bakir MA, Salgado R,
Moore DA, et al: Using DNA sequencing data to quantify T cell
fraction and therapy response. Nature. 597:555–560. 2021.
View Article : Google Scholar
|
|
20
|
Petitprez F, de Reyniès A, Keung EZ, Chen
TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A,
et al: B cells are associated with survival and immunotherapy
response in sarcoma. Nature. 577:556–560. 2020. View Article : Google Scholar
|
|
21
|
Khanal S, Wieland A and Gunderson AJ:
Mechanisms of tertiary lymphoid structure formation: Cooperation
between inflammation and antigenicity. Front Immunol.
14:12676542023. View Article : Google Scholar
|
|
22
|
Trüb M and Zippelius A: Tertiary lymphoid
structures as a predictive biomarker of response to cancer
immunotherapies. Front Immunol. 12:6745652021. View Article : Google Scholar
|
|
23
|
Jacquelot N, Tellier J, Nutt SL and Belz
GT: Tertiary lymphoid structures and B lymphocytes in cancer
prognosis and response to immunotherapies. Oncoimmunology.
10:19005082021. View Article : Google Scholar
|
|
24
|
Schumacher TN and Thommen DS: Tertiary
lymphoid structures in cancer. Science. 375:eabf94192022.
View Article : Google Scholar
|
|
25
|
Schweiger T, Berghoff AS, Glogner C,
Glueck O, Rajky O, Traxler D, Birner P, Preusser M, Klepetko W and
Hoetzenecker K: Tumor-infiltrating lymphocyte subsets and tertiary
lymphoid structures in pulmonary metastases from colorectal cancer.
Clin Exp Metastasis. 33:727–739. 2016. View Article : Google Scholar
|
|
26
|
Siliņa K, Soltermann A, Attar FM, Casanova
R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P,
Curioni-Fontecedro A, et al: Germinal centers determine the
prognostic relevance of tertiary lymphoid structures and are
impaired by corticosteroids in lung squamous cell carcinoma. Cancer
Res. 78:1308–1320. 2018. View Article : Google Scholar
|
|
27
|
Calderaro J, Petitprez F, Becht E, Laurent
A, Hirsch TZ, Rousseau B, Luciani A, Amaddeo G, Derman J, Charpy C,
et al: Intra-tumoral tertiary lymphoid structures are associated
with a low risk of early recurrence of hepatocellular carcinoma. J
Hepatol. 70:58–65. 2019. View Article : Google Scholar
|
|
28
|
Sofopoulos M, Fortis SP, Vaxevanis CK,
Sotiriadou NN, Arnogiannaki N, Ardavanis A, Vlachodimitropoulos D,
Perez SA and Baxevanis CN: The prognostic significance of
peritumoral tertiary lymphoid structures in breast cancer. Cancer
Immunol Immunother. 68:1733–1745. 2019. View Article : Google Scholar
|
|
29
|
He W, Zhang D, Liu H, Chen T, Xie J, Peng
L, Zheng X, Xu B, Li Q and Jiang J: The high level of tertiary
lymphoid structure is correlated with superior survival in patients
with advanced gastric cancer. Front Oncol. 10:9802020. View Article : Google Scholar
|
|
30
|
Lin Q, Tao P, Wang J, Ma L, Jiang Q, Li J,
Zhang G, Liu J, Zhang Y, Hou Y, et al: Tumor-associated tertiary
lymphoid structure predicts postoperative outcomes in patients with
primary gastrointestinal stromal tumors. Oncoimmunology.
9:17473392020. View Article : Google Scholar
|
|
31
|
Magara T, Nakamura M, Nojiri Y, Yoshimitsu
M, Kano S, Matsubara A, Kato H and Morita A: Tertiary lymphoid
structures correlate with better prognosis in cutaneous
angiosarcoma. J Dermatol Sci. 103:57–59. 2021. View Article : Google Scholar
|
|
32
|
Baker PM, Clement PB and Young RH:
Malignant peritoneal mesothelioma in women: A study of 75 cases
with emphasis on their morphologic spectrum and differential
diagnosis. Am J Clin Pathol. 123:724–737. 2005. View Article : Google Scholar
|
|
33
|
Benzerdjeb N, Dartigues P, Kepenekian V,
Valmary-Degano S, Mery E, Avérous G, Chevallier A, Laverriere MH,
Villa I, Harou O, et al: Tertiary lymphoid structures in
epithelioid malignant peritoneal mesothelioma are associated with
neoadjuvant chemotherapy, but not with prognosis. Virchows Arch.
479:765–772. 2021. View Article : Google Scholar
|
|
34
|
Gao J, Navai N, Alhalabi O, Siefker-Radtke
A, Campbell MT, Tidwell RS, Guo CC, Kamat AM, Matin SF, Araujo JC,
et al: Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with
cisplatin-ineligible operable high-risk urothelial carcinoma. Nat
Med. 26:1845–1851. 2020. View Article : Google Scholar
|
|
35
|
Helmink BA, Reddy SM, Gao J, Zhang S,
Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et
al: B cells and tertiary lymphoid structures promote immunotherapy
response. Nature. 577:549–555. 2020. View Article : Google Scholar
|
|
36
|
Cabrita R, Lauss M, Sanna A, Donia M,
Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K,
Vallon-Christersson J, et al: Tertiary lymphoid structures improve
immunotherapy and survival in melanoma. Nature. 577:561–565. 2020.
View Article : Google Scholar
|
|
37
|
Lee HS, Jang HJ, Ramineni M, Wang DY,
Ramos D, Choi JM, Splawn T, Espinoza M, Almarez M, Hosey L, et al:
A phase II window of opportunity study of neoadjuvant PD-L1 versus
PD-L1 plus CTLA-4 blockade for patients with malignant pleural
mesothelioma. Clin Cancer Res. 29:548–559. 2023. View Article : Google Scholar
|
|
38
|
Chapel DB, Stewart R, Furtado LV, Husain
AN, Krausz T and Deftereos G: Tumor PD-L1 expression in malignant
pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and
Dako PD-L1 28-8 pharmDx assays. Hum Pathol. 87:11–17. 2019.
View Article : Google Scholar
|
|
39
|
Gazivoda VP, Kangas-Dick AW, Greenbaum AA,
Roshal J, Chen C, Moore DF, Langan RC, Kennedy TJ, Minerowicz C and
Alexander HR: Expression of PD-L1 in patients with malignant
peritoneal mesothelioma: A pilot study. J Surg Res. 277:131–137.
2022. View Article : Google Scholar
|
|
40
|
Pezzuto F, Vimercati L, Fortarezza F,
Marzullo A, Pennella A, Cavone D, Punzi A, Caporusso C, d'Amati A,
Lettini T and Serio G: Evaluation of prognostic histological
parameters proposed for pleural mesothelioma in diffuse malignant
peritoneal mesothelioma. A short report. Diagn Pathol. 16:642021.
View Article : Google Scholar
|
|
41
|
Desai A, Karrison T, Rose B, Tan Y, Hill
B, Pemberton E, Straus C, Seiwert T and Kindler HL: OA08.03 phase
II Trial of pembrolizumab (NCT02399371) in previously-treated
malignant mesothelioma (MM): Final analysis. J Thorac Oncol.
13:S3392018. View Article : Google Scholar
|
|
42
|
Zong L, Mo S, Yu S, Zhou Y, Zhang M, Chen
J and Xiang Y: Expression of the immune checkpoint VISTA in breast
cancer. Cancer Immunol Immunother. 69:1437–1446. 2020. View Article : Google Scholar
|
|
43
|
Zong L, Zhou Y, Zhang M, Chen J and Xiang
Y: VISTA expression is associated with a favorable prognosis in
patients with high-grade serous ovarian cancer. Cancer Immunol
Immunother. 69:33–42. 2020. View Article : Google Scholar
|
|
44
|
Xie S, Huang J, Qiao Q, Zang W, Hong S,
Tan H, Dong C, Yang Z and Ni L: Expression of the inhibitory B7
family molecule VISTA in human colorectal carcinoma tumors. Cancer
Immunol Immunother. 67:1685–1694. 2018. View Article : Google Scholar
|
|
45
|
Noelle RJ, Lines JL, Lewis LD, Martell RE,
Guillaudeux T, Lee SW, Mahoney KM, Vesely MD, Boyd-Kirkup J,
Nambiar DK and Scott AM: Clinical and research updates on the VISTA
immune checkpoint: Immuno-oncology themes and highlights. Front
Oncol. 13:12250812023. View Article : Google Scholar
|
|
46
|
Hmeljak J, Sanchez-Vega F, Hoadley KA,
Shih J, Stewart C, Heiman D, Tarpey P, Danilova L, Drill E, Gibb
EA, et al: Integrative molecular characterization of malignant
pleural mesothelioma. Cancer Discov. 8:1548–1565. 2018. View Article : Google Scholar
|
|
47
|
Alcala N, Mangiante L, Le-Stang N,
Gustafson CE, Boyault S, Damiola F, Alcala K, Brevet M,
Thivolet-Bejui F, Blanc-Fournier C, et al: Redefining malignant
pleural mesothelioma types as a continuum uncovers immune-vascular
interactions. EBioMedicine. 48:191–202. 2019. View Article : Google Scholar
|
|
48
|
Muller S, Victoria Lai W, Adusumilli PS,
Desmeules P, Frosina D, Jungbluth A, Ni A, Eguchi T, Travis WD,
Ladanyi M, et al: V-domain Ig-containing suppressor of T-cell
activation (VISTA), a potentially targetable immune checkpoint
molecule, is highly expressed in epithelioid malignant pleural
mesothelioma. Mod Pathol. 33:303–311. 2020. View Article : Google Scholar
|
|
49
|
Chung YS, Kim M, Cha YJ, Kim KA and Shim
HS: Expression of V-set immunoregulatory receptor in malignant
mesothelioma. Mod Pathol. 33:263–270. 2020. View Article : Google Scholar
|
|
50
|
Offin M, Yang SR, Egger J, Jayakumaran G,
Spencer RS, Lopardo J, Nash GM, Cercek A, Travis WD, Kris MG, et
al: Molecular characterization of peritoneal mesotheliomas. J
Thorac Oncol. 17:455–460. 2022. View Article : Google Scholar
|
|
51
|
Gao J, Ward JF, Pettaway CA, Shi LZ,
Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al:
VISTA is an inhibitory immune checkpoint that is increased after
ipilimumab therapy in patients with prostate cancer. Nat Med.
23:551–555. 2017. View Article : Google Scholar
|
|
52
|
Kakavand H, Jackett LA, Menzies AM, Gide
TN, Carlino MS, Saw RPM, Thompson JF, Wilmott JS, Long GV and
Scolyer RA: Negative immune checkpoint regulation by VISTA: A
mechanism of acquired resistance to anti-PD-1 therapy in metastatic
melanoma patients. Mod Pathol. 30:1666–1676. 2017. View Article : Google Scholar
|
|
53
|
ElTanbouly MA, Zhao Y, Nowak E, Li J,
Schaafsma E, Le Mercier I, Ceeraz S, Lines JL, Peng C, Carriere C,
et al: VISTA is a checkpoint regulator for naïve T cell quiescence
and peripheral tolerance. Science. 367:eaay05242020. View Article : Google Scholar
|
|
54
|
Shekari N, Shanehbandi D, Kazemi T,
Zarredar H, Baradaran B and Jalali SA: VISTA and its ligands: The
next generation of promising therapeutic targets in immunotherapy.
Cancer Cell Int. 23:2652023. View Article : Google Scholar
|
|
55
|
ElTanbouly MA, Schaafsma E, Noelle RJ and
Lines JL: VISTA: Coming of age as a multi-lineage immune
checkpoint. Clin Exp Immunol. 200:120–130. 2020. View Article : Google Scholar
|
|
56
|
ElTanbouly MA, Croteau W, Noelle RJ and
Lines JL: VISTA: A novel immunotherapy target for normalizing
innate and adaptive immunity. Semin Immunol. 42:1013082019.
View Article : Google Scholar
|
|
57
|
Liu J, Yuan Y, Chen W, Putra J,
Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang
YH, et al: Immune-checkpoint proteins VISTA and PD-1 nonredundantly
regulate murine T-cell responses. Proc Natl Acad Sci USA.
112:6682–6687. 2015. View Article : Google Scholar
|
|
58
|
Hosseinkhani N, Hemmat N, Baghbani E,
Baghbanzadeh A, Kazemi T, Mokhtarzadeh A, Jafarlou M, Amin
Doustvandi M and Baradaran B: Dual silencing of tumor-intrinsic
VISTA and CTLA-4 stimulates T-cell mediated immune responses and
inhibits MCF7 breast cancer development. Gene. 896:1480432024.
View Article : Google Scholar
|
|
59
|
Lei CJ, Wang B, Long ZX, Ren H, Pan QY and
Li Y: Investigation of PD-1H in DEN-induced mouse liver cancer
model. Eur Rev Med Pharmacol Sci. 22:5194–5199. 2018.
|
|
60
|
Iadonato S, Ovechkina Y, Lustig K, Cross
J, Eyde N, Frazier E, Kabi N, Katz C, Lance R, Peckham D, et al: A
highly potent anti-VISTA antibody KVA12123-a new immune checkpoint
inhibitor and a promising therapy against poorly immunogenic
tumors. Front Immunol. 14:13116582023. View Article : Google Scholar
|
|
61
|
Sasikumar PG, Sudarshan NS, Adurthi S,
Ramachandra RK, Samiulla DS, Lakshminarasimhan A, Ramanathan A,
Chandrasekhar T, Dhudashiya AA, Talapati SR, et al: PD-1 derived
CA-170 is an oral immune checkpoint inhibitor that exhibits
preclinical anti-tumor efficacy. Commun Biol. 4:6992021. View Article : Google Scholar
|
|
62
|
Curis Inc: A Study of CA-170 (Oral PD-L1,
PD-L2 and VISTA Checkpoint Antagonist) in Patients With Advanced
Tumors and Lymphomas. 2016. https://ClinicalTrials.gov/show/NCT02812875
|
|
63
|
Tawbi HA, Schadendorf D, Lipson EJ,
Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas
HJ, Lao CD, De Menezes JJ, et al: Relatlimab and nivolumab versus
nivolumab in untreated advanced melanoma. N Engl J Med. 386:24–34.
2022. View Article : Google Scholar
|
|
64
|
Shrestha R, Nabavi N, Lin YY, Mo F,
Anderson S, Volik S, Adomat HH, Lin D, Xue H, Dong X, et al: BAP1
haploinsufficiency predicts a distinct immunogenic class of
malignant peritoneal mesothelioma. Genome Med. 11:82019. View Article : Google Scholar
|
|
65
|
Osmanbeyoglu HU, Palmer D, Sagan A,
Sementino E, Becich MJ and Testa JR: Isolated BAP1 genomic
alteration in malignant pleural mesothelioma predicts distinct
immunogenicity with implications for immunotherapeutic response.
Cancers (Basel). 14:56262022. View Article : Google Scholar
|
|
66
|
Zelba H, Bedke J, Hennenlotter J, Mostböck
S, Zettl M, Zichner T, Chandran A, Stenzl A, Rammensee HG and
Gouttefangeas C: PD-1 and LAG-3 dominate checkpoint
receptor-mediated T-cell inhibition in renal cell carcinoma. Cancer
Immunol Res. 7:1891–1899. 2019. View Article : Google Scholar
|
|
67
|
Woo SR, Turnis ME, Goldberg MV, Bankoti J,
Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et
al: Immune inhibitory molecules LAG-3 and PD-1 synergistically
regulate T-cell function to promote tumoral immune escape. Cancer
Res. 72:917–927. 2012. View Article : Google Scholar
|
|
68
|
Marcq E, Van Audenaerde JRM, De Waele J,
Merlin C, Pauwels P, van Meerbeeck JP, Fisher SA and Smits ELJ: The
Search for an Interesting Partner to Combine with PD-L1 Blockade in
Mesothelioma: Focus on TIM-3 and LAG-3. Cancers (Basel).
13:2822021. View Article : Google Scholar
|
|
69
|
Aggarwal V, Workman CJ and Vignali DAA:
LAG-3 as the third checkpoint inhibitor. Nat Immunol. 24:1415–1422.
2023. View Article : Google Scholar
|
|
70
|
Feng L, Gao X, Jiao Z, Wang Z and Min F:
BTK inhibitor combined with anti-PD-1 monoclonal antibody for the
treatment of CD20-negative primary central nervous system lymphoma:
A case report. Oncol Lett. 25:482022. View Article : Google Scholar
|
|
71
|
Maccaroni E, Lunerti V, Agostinelli V,
Giampieri R, Zepponi L, Pagliacci A and Berardi R: New insights
into hormonal therapies in uterine sarcomas. Cancers (Basel).
14:9212022. View Article : Google Scholar
|
|
72
|
Pan C, Yu T, Han L, Hao D, Yang M, Li L,
Chu L and Ni Q: Surufatinib combined camrelizumab as a valuable
third-line rescue therapy for a patient with extensive-stage for
small-cell lung cancer: A case report and literature review.
Anticancer Drugs. 35:271–276. 2024. View Article : Google Scholar
|
|
73
|
Peri A, Salomon N, Wolf Y, Kreiter S,
Diken M and Samuels Y: The landscape of T cell antigens for cancer
immunotherapy. Nat Cancer. 4:937–954. 2023. View Article : Google Scholar
|
|
74
|
McGranahan N, Furness AJ, Rosenthal R,
Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak
NJ, Hiley CT, et al: Clonal neoantigens elicit T cell
immunoreactivity and sensitivity to immune checkpoint blockade.
Science. 351:1463–1469. 2016. View Article : Google Scholar
|
|
75
|
Anzar I, Malone B, Samarakoon P, Vardaxis
I, Simovski B, Fontenelle H, Meza-Zepeda LA, Stratford R, Keung EZ,
Burgess M, et al: The interplay between neoantigens and immune
cells in sarcomas treated with checkpoint inhibition. Front
Immunol. 14:12264452023. View Article : Google Scholar
|
|
76
|
Nguyen KB, Roerden M, Copeland CJ,
Backlund CM, Klop-Packel NG, Remba T, Kim B, Singh NK, Birnbaum ME,
Irvine DJ and Spranger S: Decoupled neoantigen cross-presentation
by dendritic cells limits anti-tumor immunity against tumors with
heterogeneous neoantigen expression. Elife. 12:e852632023.
View Article : Google Scholar
|
|
77
|
Shi Y, Jing B and Xi R: Comprehensive
analysis of neoantigens derived from structural variation across
whole genomes from 2528 tumors. Genome Biol. 24:1692023. View Article : Google Scholar
|
|
78
|
Wei Z, Zhou C, Zhang Z, Guan M, Zhang C,
Liu Z and Liu Q: The landscape of tumor fusion neoantigens: A
pan-cancer analysis. iScience. 21:249–260. 2019. View Article : Google Scholar
|
|
79
|
Yang W, Lee KW, Srivastava RM, Kuo F,
Krishna C, Chowell D, Makarov V, Hoen D, Dalin MG, Wexler L, et al:
Immunogenic neoantigens derived from gene fusions stimulate T cell
responses. Nat Med. 25:767–775. 2019. View Article : Google Scholar
|
|
80
|
Cortés-Ciriano I, Lee JJ, Xi R, Jain D,
Jung YL, Yang L, Gordenin D, Klimczak LJ, Zhang CZ, Pellman DS, et
al: Comprehensive analysis of chromothripsis in 2,658 human cancers
using whole-genome sequencing. Nat Genet. 52:331–341. 2020.
View Article : Google Scholar
|
|
81
|
Oey H, Daniels M, Relan V, Chee TM,
Davidson MR, Yang IA, Ellis JJ, Fong KM, Krause L and Bowman RV:
Whole-genome sequencing of human malignant mesothelioma tumours and
cell lines. Carcinogenesis. 40:724–734. 2019. View Article : Google Scholar
|
|
82
|
Bueno R, Stawiski EW, Goldstein LD,
Durinck S, De Rienzo A, Modrusan Z, Gnad F, Nguyen TT, Jaiswal BS,
Chirieac LR, et al: Comprehensive genomic analysis of malignant
pleural mesothelioma identifies recurrent mutations, gene fusions
and splicing alterations. Nat Genet. 48:407–416. 2016. View Article : Google Scholar
|
|
83
|
Mansfield AS, Peikert T, Smadbeck JB,
Udell JBM, Garcia-Rivera E, Elsbernd L, Erskine CL, Van Keulen VP,
Kosari F, Murphy SJ, et al: Neoantigenic potential of complex
chromosomal rearrangements in mesothelioma. J Thorac Oncol.
14:276–287. 2019. View Article : Google Scholar
|
|
84
|
Yoshikawa Y, Emi M, Hashimoto-Tamaoki T,
Ohmuraya M, Sato A, Tsujimura T, Hasegawa S, Nakano T, Nasu M,
Pastorino S, et al: High-density array-CGH with targeted NGS unmask
multiple noncontiguous minute deletions on chromosome 3p21 in
mesothelioma. Proc Natl Acad Sci USA. 113:13432–13437. 2016.
View Article : Google Scholar
|
|
85
|
Relan V, Morrison L, Parsonson K, Clarke
BE, Duhig EE, Windsor MN, Matar KS, Naidoo R, Passmore L, McCaul E,
et al: Phenotypes and karyotypes of human malignant mesothelioma
cell lines. PLoS One. 8:e581322013. View Article : Google Scholar
|
|
86
|
Panagopoulos I, Andersen K, Brunetti M,
Gorunova L, Davidson B, Lund-Iversen M, Micci F and Heim S: Genetic
pathways in peritoneal mesothelioma tumorigenesis. Cancer Genomics
Proteomics. 20:363–374. 2023. View Article : Google Scholar
|
|
87
|
Verdegaal EM, de Miranda NF, Visser M,
Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne
CE, Schotte R, Spits H, et al: Neoantigen landscape dynamics during
human melanoma-T cell interactions. Nature. 536:91–95. 2016.
View Article : Google Scholar
|
|
88
|
Samstein RM, Lee CH, Shoushtari AN,
Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ,
Omuro A, et al: Tumor mutational load predicts survival after
immunotherapy across multiple cancer types. Nat Genet. 51:202–206.
2019. View Article : Google Scholar
|
|
89
|
Ren Y, Cherukuri Y, Wickland DP, Sarangi
V, Tian S, Carter JM, Mansfield AS, Block MS, Sherman ME, Knutson
KL, et al: HLA class-I and class-II restricted neoantigen loads
predict overall survival in breast cancer. Oncoimmunology.
9:17449472020. View Article : Google Scholar
|
|
90
|
Zou XL, Li XB, Ke H, Zhang GY, Tang Q,
Yuan J, Zhou CJ, Zhang JL, Zhang R and Chen WY: Prognostic value of
neoantigen load in immune checkpoint inhibitor therapy for cancer.
Front Immunol. 12:6890762021. View Article : Google Scholar
|
|
91
|
Kosari F, Disselhorst M, Yin J, Peikert T,
Udell J, Johnson S, Smadbeck J, Murphy S, McCune A, Karagouga G, et
al: Tumor junction burden and antigen presentation as predictors of
survival in mesothelioma treated with immune checkpoint inhibitors.
J Thorac Oncol. 17:446–454. 2022. View Article : Google Scholar
|
|
92
|
Lee HS, Jang HJ, Choi JM, Zhang J, de
Rosen VL, Wheeler TM, Lee JS, Tu T, Jindra PT, Kerman RH, et al:
Comprehensive immunoproteogenomic analyses of malignant pleural
mesothelioma. JCI Insight. 3:e985752018. View Article : Google Scholar
|
|
93
|
Tandon RT, Jimenez-Cortez Y, Taub R and
Borczuk AC: Immunohistochemistry in peritoneal mesothelioma: A
single-center experience of 244 cases. Arch Pathol Lab Med.
142:236–242. 2018. View Article : Google Scholar
|
|
94
|
Leblay N, Leprêtre F, Le Stang N,
Gautier-Stein A, Villeneuve L, Isaac S, Maillet D, Galateau-Sallé
F, Villenet C, Sebda S, et al: BAP1 is altered by copy number loss,
mutation, and/or loss of protein expression in more than 70% of
malignant peritoneal mesotheliomas. J Thorac Oncol. 12:724–733.
2017. View Article : Google Scholar
|
|
95
|
Gezgin G, Dogrusöz M, van Essen TH, Kroes
WGM, Luyten GPM, van der Velden PA, Walter V, Verdijk RM, van Hall
T, van der Burg SH and Jager MJ: Genetic evolution of uveal
melanoma guides the development of an inflammatory
microenvironment. Cancer Immunol Immunother. 66:903–912. 2017.
View Article : Google Scholar
|
|
96
|
Lai J, Zhou Z, Tang XJ, Gao ZB, Zhou J and
Chen SQ: A tumor-specific neo-antigen caused by a frameshift
mutation in BAP1 is a potential personalized biomarker in malignant
peritoneal mesothelioma. Int J Mol Sci. 17:7392016. View Article : Google Scholar
|
|
97
|
Rizzolo A, Ah-Lan KC, Nu TNT and Alcindor
T: Response to Ipilimumab and nivolumab in a patient with malignant
peritoneal mesothelioma. Clin Colorectal Cancer. 21:371–374. 2022.
View Article : Google Scholar
|
|
98
|
Liu K, Huang Y, Xu Y, Wang G, Cai S, Zhang
X and Shi T: BAP1-related signature predicts benefits from
immunotherapy over VEGFR/mTOR inhibitors in ccRCC: A retrospective
analysis of JAVELIN Renal 101 and checkmate-009/010/025 trials.
Cancer Immunol Immunother. 72:2557–2572. 2023. View Article : Google Scholar
|
|
99
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar
|
|
100
|
Ayers M, Lunceford J, Nebozhyn M, Murphy
E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran
V, et al: IFN-γ-related mRNA profile predicts clinical response to
PD-1 blockade. J Clin Invest. 127:2930–2940. 2017. View Article : Google Scholar
|
|
101
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018. View Article : Google Scholar
|
|
102
|
Cui C, Xu C, Yang W, Chi Z, Sheng X, Si L,
Xie Y, Yu J, Wang S, Yu R, et al: Ratio of the interferon-γ
signature to the immunosuppression signature predicts anti-PD-1
therapy response in melanoma. NPJ Genom Med. 6:72021. View Article : Google Scholar
|
|
103
|
Lee JS, Nair NU, Dinstag G, Chapman L,
Chung Y, Wang K, Sinha S, Cha H, Kim D, Schperberg AV, et al:
Synthetic lethality-mediated precision oncology via the tumor
transcriptome. Cell. 184:2487–2502.e13. 2021. View Article : Google Scholar
|
|
104
|
Nair NU, Jiang Q, Wei JS, Misra VA, Morrow
B, Kesserwan C, Hermida LC, Lee JS, Mian I, Zhang J, et al: Genomic
and transcriptomic analyses identify a prognostic gene signature
and predict response to therapy in pleural and peritoneal
mesothelioma. Cell Rep Med. 4:1009382023. View Article : Google Scholar
|
|
105
|
Dinstag G, Shulman ED, Elis E, Ben-Zvi DS,
Tirosh O, Maimon E, Meilijson I, Elalouf E, Temkin B, Vitkovsky P,
et al: Clinically oriented prediction of patient response to
targeted and immunotherapies from the tumor transcriptome. Med.
4:15–30.e8. 2023. View Article : Google Scholar
|
|
106
|
Hoang DT, Dinstag G, Shulman ED, Hermida
LC, Ben-Zvi DS, Elis E, Caley K, Sammut SJ, Sinha S, Sinha N, et
al: A deep-learning framework to predict cancer treatment response
from histopathology images through imputed transcriptomics. Nat
Cancer. Jul 3–2024.(Epub ahead of print). View Article : Google Scholar
|
|
107
|
Panagi M, Mpekris F, Voutouri C,
Hadjigeorgiou AG, Symeonidou C, Porfyriou E, Michael C, Stylianou
A, Martin JD, Cabral H, et al: Stabilizing tumor-resident mast
cells restores T-Cell infiltration and sensitizes sarcomas to PD-L1
inhibition. Clin Cancer Res. 30:2582–2597. 2024. View Article : Google Scholar
|
|
108
|
Sautès-Fridman C, Lawand M, Giraldo NA,
Kaplon H, Germain C, Fridman WH and Dieu-Nosjean MC: Tertiary
lymphoid structures in cancers: Prognostic value, regulation, and
manipulation for therapeutic intervention. Front Immunol.
7:4072016. View Article : Google Scholar
|
|
109
|
Yang ZR, Su YD, Ma R, Wu HL and Li Y:
Efficacy and adverse events of apatinib salvage treatment for
refractory diffuse malignant peritoneal mesothelioma: A pilot
study. Front Oncol. 12:8118002022. View Article : Google Scholar
|
|
110
|
Zauderer MG, Alley EW, Bendell J,
Capelletto E, Bauer TM, Callies S, Szpurka AM, Kang S, Willard MD,
Wacheck V and Varghese AM: Phase 1 cohort expansion study of
LY3023414, a dual PI3K/mTOR inhibitor, in patients with advanced
mesothelioma. Invest New Drugs. 39:1081–1088. 2021. View Article : Google Scholar
|
|
111
|
Yang H, Hall SRR, Sun B, Zhao L, Gao Y,
Schmid RA, Tan ST, Peng RW and Yao F: NF2 and Canonical Hippo-YAP
pathway define distinct tumor subsets characterized by different
immune deficiency and treatment implications in human pleural
mesothelioma. Cancers (Basel). 13:15612021. View Article : Google Scholar
|
|
112
|
Fennell DA, King A, Mohammed S, Branson A,
Brookes C, Darlison L, Dawson AG, Gaba A, Hutka M, Morgan B, et al:
Rucaparib in patients with BAP1-deficient or BRCA1-deficient
mesothelioma (MiST1): An open-label, single-arm, phase 2a clinical
trial. Lancet Respir Med. 9:593–600. 2021. View Article : Google Scholar
|
|
113
|
Hung YP, Dong F, Watkins JC, Nardi V,
Bueno R, Dal Cin P, Godleski JJ, Crum CP and Chirieac LR:
Identification of ALK rearrangements in malignant peritoneal
mesothelioma. JAMA Oncol. 4:235–238. 2018. View Article : Google Scholar
|
|
114
|
Li Petri G, Pecoraro C, Randazzo O, Zoppi
S, Cascioferro SM, Parrino B, Carbone D, El Hassouni B, Cavazzoni
A, Zaffaroni N, et al: New Imidazo[2,1-b][1,3,4]Thiadiazole
derivatives inhibit FAK phosphorylation and potentiate the
antiproliferative effects of gemcitabine through modulation of the
human equilibrative nucleoside transporter-1 in peritoneal
mesothelioma. Anticancer Res. 40:4913–4919. 2020. View Article : Google Scholar
|
|
115
|
Quispel-Janssen JM, Badhai J, Schunselaar
L, Price S, Brammeld J, Iorio F, Kolluri K, Garnett M, Berns A,
Baas P, et al: Comprehensive pharmacogenomic profiling of malignant
pleural mesothelioma identifies a subgroup sensitive to FGFR
inhibition. Clin Cancer Res. 24:84–94. 2018. View Article : Google Scholar
|
|
116
|
Bensaid D, Blondy T, Deshayes S, Dehame V,
Bertrand P, Grégoire M, Errami M and Blanquart C: Assessment of new
HDAC inhibitors for immunotherapy of malignant pleural
mesothelioma. Clin Epigenetics. 10:792018. View Article : Google Scholar
|
|
117
|
McGale JP, Chen DL, Trebeschi S, Farwell
MD, Wu AM, Cutler CS, Schwartz LH and Dercle L: Artificial
intelligence in immunotherapy PET/SPECT imaging. Eur Radiol. Feb
15–2024.(Epub ahead of print). View Article : Google Scholar
|
|
118
|
Addala V, Newell F, Pearson JV, Redwood A,
Robinson BW, Creaney J and Waddell N: Computational immunogenomic
approaches to predict response to cancer immunotherapies. Nat Rev
Clin Oncol. 21:28–46. 2024. View Article : Google Scholar
|