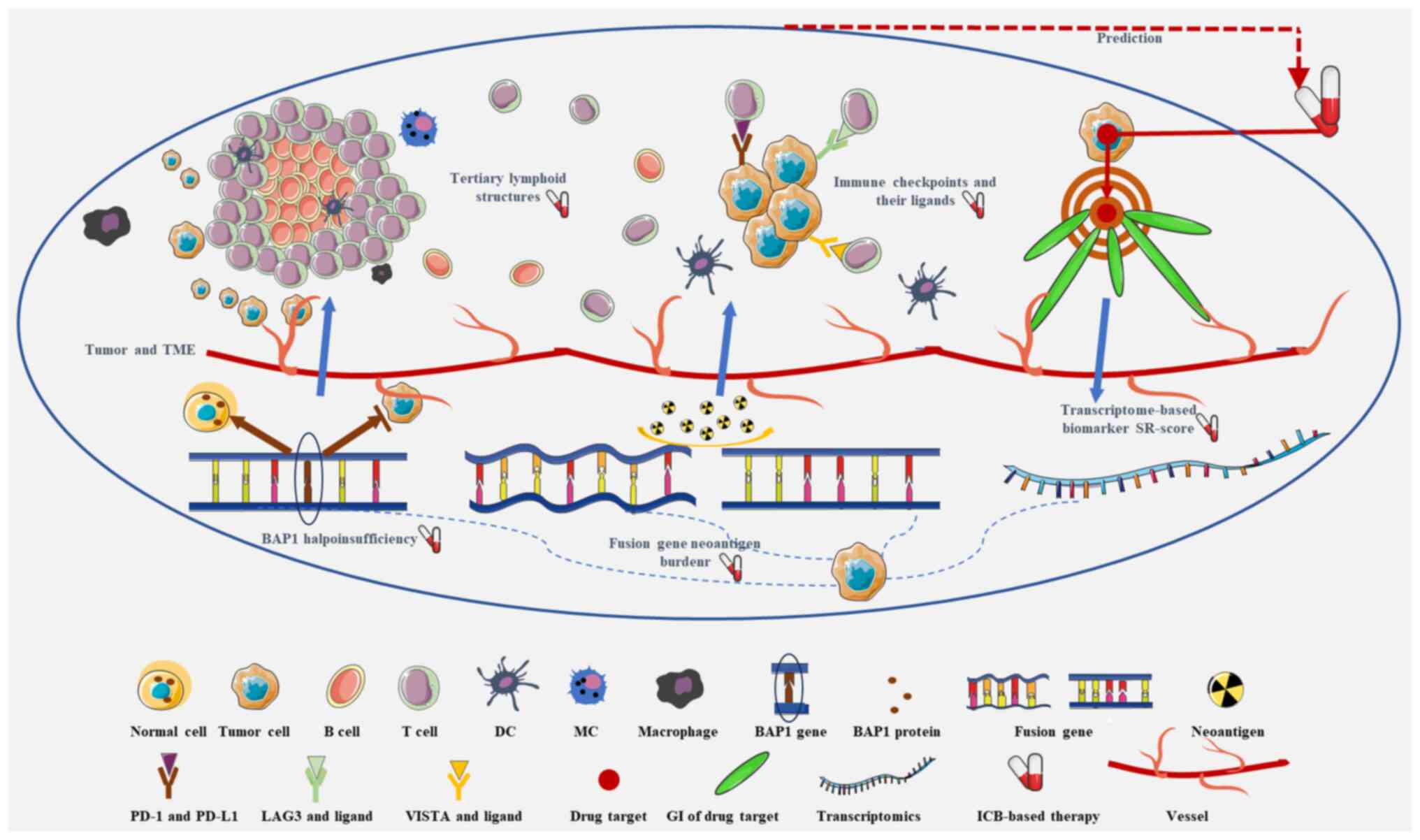
|
1
|
Kusamura S, Kepenekian V, Villeneuve L,
Lurvink RJ, Govaerts K, De Hingh IHJT, Moran BJ, Van der Speeten K,
Deraco M and Glehen O; PSOGI: Peritoneal mesothelioma:
PSOGI/EURACAN clinical practice guidelines for diagnosis, treatment
and follow-up. Eur J Surg Oncol. 47:36–59. 2021. View Article : Google Scholar
|
|
2
|
Greenbaum A and Alexander HR: Peritoneal
mesothelioma. Transl Lung Cancer Res. 9 (Suppl 1):S120–S132. 2020.
View Article : Google Scholar
|
|
3
|
Rui R, Zhou L and He S: Cancer
immunotherapies: Advances and bottlenecks. Front Immunol.
14:12124762023. View Article : Google Scholar
|
|
4
|
Scherpereel A, Mazieres J, Greillier L,
Lantuejoul S, Dô P, Bylicki O, Monnet I, Corre R, Audigier-Valette
C, Locatelli-Sanchez M, et al: Nivolumab or nivolumab plus
ipilimumab in patients with relapsed malignant pleural mesothelioma
(IFCT-1501 MAPS2): A multicentre, open-label, randomised,
non-comparative, phase 2 trial. Lancet Oncol. 20:239–253. 2019.
View Article : Google Scholar
|
|
5
|
Disselhorst MJ, Quispel-Janssen J,
Lalezari F, Monkhorst K, de Vries JF, van der Noort V, Harms E,
Burgers S and Baas P: Ipilimumab and nivolumab in the treatment of
recurrent malignant pleural mesothelioma (INITIATE): Results of a
prospective, single-arm, phase 2 trial. Lancet Respir Med.
7:260–270. 2019. View Article : Google Scholar
|
|
6
|
Baas P, Scherpereel A, Nowak AK, Fujimoto
N, Peters S, Tsao AS, Mansfield AS, Popat S, Jahan T, Antonia S, et
al: First-line nivolumab plus ipilimumab in unresectable malignant
pleural mesothelioma (CheckMate 743): A multicentre, randomised,
open-label, phase 3 trial. Lancet. 397:375–386. 2021. View Article : Google Scholar
|
|
7
|
Raghav K, Liu S, Overman M, Morani A,
Willette A, Fournier K and Varadhachary G: Clinical efficacy of
immune checkpoint inhibitors in patients with advanced malignant
peritoneal mesothelioma. JAMA Netw Open. 4:e21199342021. View Article : Google Scholar
|
|
8
|
Raghav K, Liu S, Overman MJ, Willett AF,
Knafl M, Fu SC, Malpica A, Prasad S, Royal RE, Scally CP, et al:
Efficacy, safety, and biomarker analysis of combined PD-L1
(Atezolizumab) and VEGF (Bevacizumab) blockade in advanced
malignant peritoneal mesothelioma. Cancer Discov. 11:2738–2747.
2021. View Article : Google Scholar
|
|
9
|
Herbst RS, Soria JC, Kowanetz M, Fine GD,
Hamid O, Gordon MS, Sosman JA, McDermott DF, Powderly JD, Gettinger
SN, et al: Predictive correlates of response to the anti-PD-L1
antibody MPDL3280A in cancer patients. Nature. 515:563–567. 2014.
View Article : Google Scholar
|
|
10
|
Marabelle A, Fakih M, Lopez J, Shah M,
Shapira-Frommer R, Nakagawa K, Chung HC, Kindler HL, Lopez-Martin
JA, Miller WH Jr, et al: Association of tumour mutational burden
with outcomes in patients with advanced solid tumours treated with
pembrolizumab: Prospective biomarker analysis of the multicohort,
open-label, phase 2 KEYNOTE-158 study. Lancet Oncol. 21:1353–1365.
2020. View Article : Google Scholar
|
|
11
|
Sepulveda AR, Hamilton SR, Allegra CJ,
Grody W, Cushman-Vokoun AM, Funkhouser WK, Kopetz SE, Lieu C,
Lindor NM, Minsky BD, et al: Molecular biomarkers for the
evaluation of colorectal cancer: Guideline From the American
Society for Clinical Pathology, College of American Pathologists,
Association for Molecular Pathology, and American Society of
Clinical Oncology. J Clin Oncol. 35:1453–1486. 2017. View Article : Google Scholar
|
|
12
|
Lu S, Stein JE, Rimm DL, Wang DW, Bell JM,
Johnson DB, Sosman JA, Schalper KA, Anders RA, Wang H, et al:
Comparison of biomarker modalities for predicting response to
PD-1/PD-L1 checkpoint blockade: A systematic review and
meta-analysis. JAMA Oncol. 5:1195–1204. 2019. View Article : Google Scholar
|
|
13
|
André T, Shiu KK, Kim TW, Jensen BV,
Jensen LH, Punt C, Smith D, Garcia-Carbonero R, Benavides M, Gibbs
P, et al: Pembrolizumab in microsatellite-instability-high advanced
colorectal cancer. N Engl J Med. 383:2207–2218. 2020. View Article : Google Scholar
|
|
14
|
Bonneville R, Krook MA, Kautto EA, Miya J,
Wing MR, Chen HZ, Reeser JW, Yu L and Roychowdhury S: Landscape of
microsatellite instability across 39 cancer types. JCO Precis
Oncol. 2017.PO.17.00073. 2017. View Article : Google Scholar
|
|
15
|
Cedrés S, Ponce-Aix S, Iranzo P, Callejo
A, Pardo N, Navarro A, Martinez-Marti A, Gómez-Abecia S, Zucchiatti
AC, Sansano I, et al: Analysis of mismatch repair (MMR) proteins
expression in a series of malignant pleural mesothelioma (MPM)
patients. Clin Transl Oncol. 22:1390–1398. 2020. View Article : Google Scholar
|
|
16
|
Zhang Z, Liu S, Zhang B, Qiao L and Zhang
Y and Zhang Y: T cell dysfunction and exhaustion in cancer. Front
Cell Dev Biol. 8:172020. View Article : Google Scholar
|
|
17
|
Ott PA, Bang YJ, Piha-Paul SA, Razak ARA,
Bennouna J, Soria JC, Rugo HS, Cohen RB, O'Neil BH, Mehnert JM, et
al: T-Cell-Inflamed gene-expression profile, programmed death
ligand 1 expression, and tumor mutational burden predict efficacy
in patients treated with pembrolizumab across 20 cancers:
KEYNOTE-028. J Clin Oncol. 37:318–327. 2019. View Article : Google Scholar
|
|
18
|
Hiltbrunner S, Mannarino L, Kirschner MB,
Opitz I, Rigutto A, Laure A, Lia M, Nozza P, Maconi A, Marchini S,
et al: Tumor immune microenvironment and genetic alterations in
mesothelioma. Front Oncol. 11:6600392021. View Article : Google Scholar
|
|
19
|
Bentham R, Litchfield K, Watkins TBK, Lim
EL, Rosenthal R, Martínez-Ruiz C, Hiley CT, Bakir MA, Salgado R,
Moore DA, et al: Using DNA sequencing data to quantify T cell
fraction and therapy response. Nature. 597:555–560. 2021.
View Article : Google Scholar
|
|
20
|
Petitprez F, de Reyniès A, Keung EZ, Chen
TW, Sun CM, Calderaro J, Jeng YM, Hsiao LP, Lacroix L, Bougoüin A,
et al: B cells are associated with survival and immunotherapy
response in sarcoma. Nature. 577:556–560. 2020. View Article : Google Scholar
|
|
21
|
Khanal S, Wieland A and Gunderson AJ:
Mechanisms of tertiary lymphoid structure formation: Cooperation
between inflammation and antigenicity. Front Immunol.
14:12676542023. View Article : Google Scholar
|
|
22
|
Trüb M and Zippelius A: Tertiary lymphoid
structures as a predictive biomarker of response to cancer
immunotherapies. Front Immunol. 12:6745652021. View Article : Google Scholar
|
|
23
|
Jacquelot N, Tellier J, Nutt SL and Belz
GT: Tertiary lymphoid structures and B lymphocytes in cancer
prognosis and response to immunotherapies. Oncoimmunology.
10:19005082021. View Article : Google Scholar
|
|
24
|
Schumacher TN and Thommen DS: Tertiary
lymphoid structures in cancer. Science. 375:eabf94192022.
View Article : Google Scholar
|
|
25
|
Schweiger T, Berghoff AS, Glogner C,
Glueck O, Rajky O, Traxler D, Birner P, Preusser M, Klepetko W and
Hoetzenecker K: Tumor-infiltrating lymphocyte subsets and tertiary
lymphoid structures in pulmonary metastases from colorectal cancer.
Clin Exp Metastasis. 33:727–739. 2016. View Article : Google Scholar
|
|
26
|
Siliņa K, Soltermann A, Attar FM, Casanova
R, Uckeley ZM, Thut H, Wandres M, Isajevs S, Cheng P,
Curioni-Fontecedro A, et al: Germinal centers determine the
prognostic relevance of tertiary lymphoid structures and are
impaired by corticosteroids in lung squamous cell carcinoma. Cancer
Res. 78:1308–1320. 2018. View Article : Google Scholar
|
|
27
|
Calderaro J, Petitprez F, Becht E, Laurent
A, Hirsch TZ, Rousseau B, Luciani A, Amaddeo G, Derman J, Charpy C,
et al: Intra-tumoral tertiary lymphoid structures are associated
with a low risk of early recurrence of hepatocellular carcinoma. J
Hepatol. 70:58–65. 2019. View Article : Google Scholar
|
|
28
|
Sofopoulos M, Fortis SP, Vaxevanis CK,
Sotiriadou NN, Arnogiannaki N, Ardavanis A, Vlachodimitropoulos D,
Perez SA and Baxevanis CN: The prognostic significance of
peritumoral tertiary lymphoid structures in breast cancer. Cancer
Immunol Immunother. 68:1733–1745. 2019. View Article : Google Scholar
|
|
29
|
He W, Zhang D, Liu H, Chen T, Xie J, Peng
L, Zheng X, Xu B, Li Q and Jiang J: The high level of tertiary
lymphoid structure is correlated with superior survival in patients
with advanced gastric cancer. Front Oncol. 10:9802020. View Article : Google Scholar
|
|
30
|
Lin Q, Tao P, Wang J, Ma L, Jiang Q, Li J,
Zhang G, Liu J, Zhang Y, Hou Y, et al: Tumor-associated tertiary
lymphoid structure predicts postoperative outcomes in patients with
primary gastrointestinal stromal tumors. Oncoimmunology.
9:17473392020. View Article : Google Scholar
|
|
31
|
Magara T, Nakamura M, Nojiri Y, Yoshimitsu
M, Kano S, Matsubara A, Kato H and Morita A: Tertiary lymphoid
structures correlate with better prognosis in cutaneous
angiosarcoma. J Dermatol Sci. 103:57–59. 2021. View Article : Google Scholar
|
|
32
|
Baker PM, Clement PB and Young RH:
Malignant peritoneal mesothelioma in women: A study of 75 cases
with emphasis on their morphologic spectrum and differential
diagnosis. Am J Clin Pathol. 123:724–737. 2005. View Article : Google Scholar
|
|
33
|
Benzerdjeb N, Dartigues P, Kepenekian V,
Valmary-Degano S, Mery E, Avérous G, Chevallier A, Laverriere MH,
Villa I, Harou O, et al: Tertiary lymphoid structures in
epithelioid malignant peritoneal mesothelioma are associated with
neoadjuvant chemotherapy, but not with prognosis. Virchows Arch.
479:765–772. 2021. View Article : Google Scholar
|
|
34
|
Gao J, Navai N, Alhalabi O, Siefker-Radtke
A, Campbell MT, Tidwell RS, Guo CC, Kamat AM, Matin SF, Araujo JC,
et al: Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with
cisplatin-ineligible operable high-risk urothelial carcinoma. Nat
Med. 26:1845–1851. 2020. View Article : Google Scholar
|
|
35
|
Helmink BA, Reddy SM, Gao J, Zhang S,
Basar R, Thakur R, Yizhak K, Sade-Feldman M, Blando J, Han G, et
al: B cells and tertiary lymphoid structures promote immunotherapy
response. Nature. 577:549–555. 2020. View Article : Google Scholar
|
|
36
|
Cabrita R, Lauss M, Sanna A, Donia M,
Skaarup Larsen M, Mitra S, Johansson I, Phung B, Harbst K,
Vallon-Christersson J, et al: Tertiary lymphoid structures improve
immunotherapy and survival in melanoma. Nature. 577:561–565. 2020.
View Article : Google Scholar
|
|
37
|
Lee HS, Jang HJ, Ramineni M, Wang DY,
Ramos D, Choi JM, Splawn T, Espinoza M, Almarez M, Hosey L, et al:
A phase II window of opportunity study of neoadjuvant PD-L1 versus
PD-L1 plus CTLA-4 blockade for patients with malignant pleural
mesothelioma. Clin Cancer Res. 29:548–559. 2023. View Article : Google Scholar
|
|
38
|
Chapel DB, Stewart R, Furtado LV, Husain
AN, Krausz T and Deftereos G: Tumor PD-L1 expression in malignant
pleural and peritoneal mesothelioma by Dako PD-L1 22C3 pharmDx and
Dako PD-L1 28-8 pharmDx assays. Hum Pathol. 87:11–17. 2019.
View Article : Google Scholar
|
|
39
|
Gazivoda VP, Kangas-Dick AW, Greenbaum AA,
Roshal J, Chen C, Moore DF, Langan RC, Kennedy TJ, Minerowicz C and
Alexander HR: Expression of PD-L1 in patients with malignant
peritoneal mesothelioma: A pilot study. J Surg Res. 277:131–137.
2022. View Article : Google Scholar
|
|
40
|
Pezzuto F, Vimercati L, Fortarezza F,
Marzullo A, Pennella A, Cavone D, Punzi A, Caporusso C, d'Amati A,
Lettini T and Serio G: Evaluation of prognostic histological
parameters proposed for pleural mesothelioma in diffuse malignant
peritoneal mesothelioma. A short report. Diagn Pathol. 16:642021.
View Article : Google Scholar
|
|
41
|
Desai A, Karrison T, Rose B, Tan Y, Hill
B, Pemberton E, Straus C, Seiwert T and Kindler HL: OA08.03 phase
II Trial of pembrolizumab (NCT02399371) in previously-treated
malignant mesothelioma (MM): Final analysis. J Thorac Oncol.
13:S3392018. View Article : Google Scholar
|
|
42
|
Zong L, Mo S, Yu S, Zhou Y, Zhang M, Chen
J and Xiang Y: Expression of the immune checkpoint VISTA in breast
cancer. Cancer Immunol Immunother. 69:1437–1446. 2020. View Article : Google Scholar
|
|
43
|
Zong L, Zhou Y, Zhang M, Chen J and Xiang
Y: VISTA expression is associated with a favorable prognosis in
patients with high-grade serous ovarian cancer. Cancer Immunol
Immunother. 69:33–42. 2020. View Article : Google Scholar
|
|
44
|
Xie S, Huang J, Qiao Q, Zang W, Hong S,
Tan H, Dong C, Yang Z and Ni L: Expression of the inhibitory B7
family molecule VISTA in human colorectal carcinoma tumors. Cancer
Immunol Immunother. 67:1685–1694. 2018. View Article : Google Scholar
|
|
45
|
Noelle RJ, Lines JL, Lewis LD, Martell RE,
Guillaudeux T, Lee SW, Mahoney KM, Vesely MD, Boyd-Kirkup J,
Nambiar DK and Scott AM: Clinical and research updates on the VISTA
immune checkpoint: Immuno-oncology themes and highlights. Front
Oncol. 13:12250812023. View Article : Google Scholar
|
|
46
|
Hmeljak J, Sanchez-Vega F, Hoadley KA,
Shih J, Stewart C, Heiman D, Tarpey P, Danilova L, Drill E, Gibb
EA, et al: Integrative molecular characterization of malignant
pleural mesothelioma. Cancer Discov. 8:1548–1565. 2018. View Article : Google Scholar
|
|
47
|
Alcala N, Mangiante L, Le-Stang N,
Gustafson CE, Boyault S, Damiola F, Alcala K, Brevet M,
Thivolet-Bejui F, Blanc-Fournier C, et al: Redefining malignant
pleural mesothelioma types as a continuum uncovers immune-vascular
interactions. EBioMedicine. 48:191–202. 2019. View Article : Google Scholar
|
|
48
|
Muller S, Victoria Lai W, Adusumilli PS,
Desmeules P, Frosina D, Jungbluth A, Ni A, Eguchi T, Travis WD,
Ladanyi M, et al: V-domain Ig-containing suppressor of T-cell
activation (VISTA), a potentially targetable immune checkpoint
molecule, is highly expressed in epithelioid malignant pleural
mesothelioma. Mod Pathol. 33:303–311. 2020. View Article : Google Scholar
|
|
49
|
Chung YS, Kim M, Cha YJ, Kim KA and Shim
HS: Expression of V-set immunoregulatory receptor in malignant
mesothelioma. Mod Pathol. 33:263–270. 2020. View Article : Google Scholar
|
|
50
|
Offin M, Yang SR, Egger J, Jayakumaran G,
Spencer RS, Lopardo J, Nash GM, Cercek A, Travis WD, Kris MG, et
al: Molecular characterization of peritoneal mesotheliomas. J
Thorac Oncol. 17:455–460. 2022. View Article : Google Scholar
|
|
51
|
Gao J, Ward JF, Pettaway CA, Shi LZ,
Subudhi SK, Vence LM, Zhao H, Chen J, Chen H, Efstathiou E, et al:
VISTA is an inhibitory immune checkpoint that is increased after
ipilimumab therapy in patients with prostate cancer. Nat Med.
23:551–555. 2017. View Article : Google Scholar
|
|
52
|
Kakavand H, Jackett LA, Menzies AM, Gide
TN, Carlino MS, Saw RPM, Thompson JF, Wilmott JS, Long GV and
Scolyer RA: Negative immune checkpoint regulation by VISTA: A
mechanism of acquired resistance to anti-PD-1 therapy in metastatic
melanoma patients. Mod Pathol. 30:1666–1676. 2017. View Article : Google Scholar
|
|
53
|
ElTanbouly MA, Zhao Y, Nowak E, Li J,
Schaafsma E, Le Mercier I, Ceeraz S, Lines JL, Peng C, Carriere C,
et al: VISTA is a checkpoint regulator for naïve T cell quiescence
and peripheral tolerance. Science. 367:eaay05242020. View Article : Google Scholar
|
|
54
|
Shekari N, Shanehbandi D, Kazemi T,
Zarredar H, Baradaran B and Jalali SA: VISTA and its ligands: The
next generation of promising therapeutic targets in immunotherapy.
Cancer Cell Int. 23:2652023. View Article : Google Scholar
|
|
55
|
ElTanbouly MA, Schaafsma E, Noelle RJ and
Lines JL: VISTA: Coming of age as a multi-lineage immune
checkpoint. Clin Exp Immunol. 200:120–130. 2020. View Article : Google Scholar
|
|
56
|
ElTanbouly MA, Croteau W, Noelle RJ and
Lines JL: VISTA: A novel immunotherapy target for normalizing
innate and adaptive immunity. Semin Immunol. 42:1013082019.
View Article : Google Scholar
|
|
57
|
Liu J, Yuan Y, Chen W, Putra J,
Suriawinata AA, Schenk AD, Miller HE, Guleria I, Barth RJ, Huang
YH, et al: Immune-checkpoint proteins VISTA and PD-1 nonredundantly
regulate murine T-cell responses. Proc Natl Acad Sci USA.
112:6682–6687. 2015. View Article : Google Scholar
|
|
58
|
Hosseinkhani N, Hemmat N, Baghbani E,
Baghbanzadeh A, Kazemi T, Mokhtarzadeh A, Jafarlou M, Amin
Doustvandi M and Baradaran B: Dual silencing of tumor-intrinsic
VISTA and CTLA-4 stimulates T-cell mediated immune responses and
inhibits MCF7 breast cancer development. Gene. 896:1480432024.
View Article : Google Scholar
|
|
59
|
Lei CJ, Wang B, Long ZX, Ren H, Pan QY and
Li Y: Investigation of PD-1H in DEN-induced mouse liver cancer
model. Eur Rev Med Pharmacol Sci. 22:5194–5199. 2018.
|
|
60
|
Iadonato S, Ovechkina Y, Lustig K, Cross
J, Eyde N, Frazier E, Kabi N, Katz C, Lance R, Peckham D, et al: A
highly potent anti-VISTA antibody KVA12123-a new immune checkpoint
inhibitor and a promising therapy against poorly immunogenic
tumors. Front Immunol. 14:13116582023. View Article : Google Scholar
|
|
61
|
Sasikumar PG, Sudarshan NS, Adurthi S,
Ramachandra RK, Samiulla DS, Lakshminarasimhan A, Ramanathan A,
Chandrasekhar T, Dhudashiya AA, Talapati SR, et al: PD-1 derived
CA-170 is an oral immune checkpoint inhibitor that exhibits
preclinical anti-tumor efficacy. Commun Biol. 4:6992021. View Article : Google Scholar
|
|
62
|
Curis Inc: A Study of CA-170 (Oral PD-L1,
PD-L2 and VISTA Checkpoint Antagonist) in Patients With Advanced
Tumors and Lymphomas. 2016. https://ClinicalTrials.gov/show/NCT02812875
|
|
63
|
Tawbi HA, Schadendorf D, Lipson EJ,
Ascierto PA, Matamala L, Castillo Gutiérrez E, Rutkowski P, Gogas
HJ, Lao CD, De Menezes JJ, et al: Relatlimab and nivolumab versus
nivolumab in untreated advanced melanoma. N Engl J Med. 386:24–34.
2022. View Article : Google Scholar
|
|
64
|
Shrestha R, Nabavi N, Lin YY, Mo F,
Anderson S, Volik S, Adomat HH, Lin D, Xue H, Dong X, et al: BAP1
haploinsufficiency predicts a distinct immunogenic class of
malignant peritoneal mesothelioma. Genome Med. 11:82019. View Article : Google Scholar
|
|
65
|
Osmanbeyoglu HU, Palmer D, Sagan A,
Sementino E, Becich MJ and Testa JR: Isolated BAP1 genomic
alteration in malignant pleural mesothelioma predicts distinct
immunogenicity with implications for immunotherapeutic response.
Cancers (Basel). 14:56262022. View Article : Google Scholar
|
|
66
|
Zelba H, Bedke J, Hennenlotter J, Mostböck
S, Zettl M, Zichner T, Chandran A, Stenzl A, Rammensee HG and
Gouttefangeas C: PD-1 and LAG-3 dominate checkpoint
receptor-mediated T-cell inhibition in renal cell carcinoma. Cancer
Immunol Res. 7:1891–1899. 2019. View Article : Google Scholar
|
|
67
|
Woo SR, Turnis ME, Goldberg MV, Bankoti J,
Selby M, Nirschl CJ, Bettini ML, Gravano DM, Vogel P, Liu CL, et
al: Immune inhibitory molecules LAG-3 and PD-1 synergistically
regulate T-cell function to promote tumoral immune escape. Cancer
Res. 72:917–927. 2012. View Article : Google Scholar
|
|
68
|
Marcq E, Van Audenaerde JRM, De Waele J,
Merlin C, Pauwels P, van Meerbeeck JP, Fisher SA and Smits ELJ: The
Search for an Interesting Partner to Combine with PD-L1 Blockade in
Mesothelioma: Focus on TIM-3 and LAG-3. Cancers (Basel).
13:2822021. View Article : Google Scholar
|
|
69
|
Aggarwal V, Workman CJ and Vignali DAA:
LAG-3 as the third checkpoint inhibitor. Nat Immunol. 24:1415–1422.
2023. View Article : Google Scholar
|
|
70
|
Feng L, Gao X, Jiao Z, Wang Z and Min F:
BTK inhibitor combined with anti-PD-1 monoclonal antibody for the
treatment of CD20-negative primary central nervous system lymphoma:
A case report. Oncol Lett. 25:482022. View Article : Google Scholar
|
|
71
|
Maccaroni E, Lunerti V, Agostinelli V,
Giampieri R, Zepponi L, Pagliacci A and Berardi R: New insights
into hormonal therapies in uterine sarcomas. Cancers (Basel).
14:9212022. View Article : Google Scholar
|
|
72
|
Pan C, Yu T, Han L, Hao D, Yang M, Li L,
Chu L and Ni Q: Surufatinib combined camrelizumab as a valuable
third-line rescue therapy for a patient with extensive-stage for
small-cell lung cancer: A case report and literature review.
Anticancer Drugs. 35:271–276. 2024. View Article : Google Scholar
|
|
73
|
Peri A, Salomon N, Wolf Y, Kreiter S,
Diken M and Samuels Y: The landscape of T cell antigens for cancer
immunotherapy. Nat Cancer. 4:937–954. 2023. View Article : Google Scholar
|
|
74
|
McGranahan N, Furness AJ, Rosenthal R,
Ramskov S, Lyngaa R, Saini SK, Jamal-Hanjani M, Wilson GA, Birkbak
NJ, Hiley CT, et al: Clonal neoantigens elicit T cell
immunoreactivity and sensitivity to immune checkpoint blockade.
Science. 351:1463–1469. 2016. View Article : Google Scholar
|
|
75
|
Anzar I, Malone B, Samarakoon P, Vardaxis
I, Simovski B, Fontenelle H, Meza-Zepeda LA, Stratford R, Keung EZ,
Burgess M, et al: The interplay between neoantigens and immune
cells in sarcomas treated with checkpoint inhibition. Front
Immunol. 14:12264452023. View Article : Google Scholar
|
|
76
|
Nguyen KB, Roerden M, Copeland CJ,
Backlund CM, Klop-Packel NG, Remba T, Kim B, Singh NK, Birnbaum ME,
Irvine DJ and Spranger S: Decoupled neoantigen cross-presentation
by dendritic cells limits anti-tumor immunity against tumors with
heterogeneous neoantigen expression. Elife. 12:e852632023.
View Article : Google Scholar
|
|
77
|
Shi Y, Jing B and Xi R: Comprehensive
analysis of neoantigens derived from structural variation across
whole genomes from 2528 tumors. Genome Biol. 24:1692023. View Article : Google Scholar
|
|
78
|
Wei Z, Zhou C, Zhang Z, Guan M, Zhang C,
Liu Z and Liu Q: The landscape of tumor fusion neoantigens: A
pan-cancer analysis. iScience. 21:249–260. 2019. View Article : Google Scholar
|
|
79
|
Yang W, Lee KW, Srivastava RM, Kuo F,
Krishna C, Chowell D, Makarov V, Hoen D, Dalin MG, Wexler L, et al:
Immunogenic neoantigens derived from gene fusions stimulate T cell
responses. Nat Med. 25:767–775. 2019. View Article : Google Scholar
|
|
80
|
Cortés-Ciriano I, Lee JJ, Xi R, Jain D,
Jung YL, Yang L, Gordenin D, Klimczak LJ, Zhang CZ, Pellman DS, et
al: Comprehensive analysis of chromothripsis in 2,658 human cancers
using whole-genome sequencing. Nat Genet. 52:331–341. 2020.
View Article : Google Scholar
|
|
81
|
Oey H, Daniels M, Relan V, Chee TM,
Davidson MR, Yang IA, Ellis JJ, Fong KM, Krause L and Bowman RV:
Whole-genome sequencing of human malignant mesothelioma tumours and
cell lines. Carcinogenesis. 40:724–734. 2019. View Article : Google Scholar
|
|
82
|
Bueno R, Stawiski EW, Goldstein LD,
Durinck S, De Rienzo A, Modrusan Z, Gnad F, Nguyen TT, Jaiswal BS,
Chirieac LR, et al: Comprehensive genomic analysis of malignant
pleural mesothelioma identifies recurrent mutations, gene fusions
and splicing alterations. Nat Genet. 48:407–416. 2016. View Article : Google Scholar
|
|
83
|
Mansfield AS, Peikert T, Smadbeck JB,
Udell JBM, Garcia-Rivera E, Elsbernd L, Erskine CL, Van Keulen VP,
Kosari F, Murphy SJ, et al: Neoantigenic potential of complex
chromosomal rearrangements in mesothelioma. J Thorac Oncol.
14:276–287. 2019. View Article : Google Scholar
|
|
84
|
Yoshikawa Y, Emi M, Hashimoto-Tamaoki T,
Ohmuraya M, Sato A, Tsujimura T, Hasegawa S, Nakano T, Nasu M,
Pastorino S, et al: High-density array-CGH with targeted NGS unmask
multiple noncontiguous minute deletions on chromosome 3p21 in
mesothelioma. Proc Natl Acad Sci USA. 113:13432–13437. 2016.
View Article : Google Scholar
|
|
85
|
Relan V, Morrison L, Parsonson K, Clarke
BE, Duhig EE, Windsor MN, Matar KS, Naidoo R, Passmore L, McCaul E,
et al: Phenotypes and karyotypes of human malignant mesothelioma
cell lines. PLoS One. 8:e581322013. View Article : Google Scholar
|
|
86
|
Panagopoulos I, Andersen K, Brunetti M,
Gorunova L, Davidson B, Lund-Iversen M, Micci F and Heim S: Genetic
pathways in peritoneal mesothelioma tumorigenesis. Cancer Genomics
Proteomics. 20:363–374. 2023. View Article : Google Scholar
|
|
87
|
Verdegaal EM, de Miranda NF, Visser M,
Harryvan T, van Buuren MM, Andersen RS, Hadrup SR, van der Minne
CE, Schotte R, Spits H, et al: Neoantigen landscape dynamics during
human melanoma-T cell interactions. Nature. 536:91–95. 2016.
View Article : Google Scholar
|
|
88
|
Samstein RM, Lee CH, Shoushtari AN,
Hellmann MD, Shen R, Janjigian YY, Barron DA, Zehir A, Jordan EJ,
Omuro A, et al: Tumor mutational load predicts survival after
immunotherapy across multiple cancer types. Nat Genet. 51:202–206.
2019. View Article : Google Scholar
|
|
89
|
Ren Y, Cherukuri Y, Wickland DP, Sarangi
V, Tian S, Carter JM, Mansfield AS, Block MS, Sherman ME, Knutson
KL, et al: HLA class-I and class-II restricted neoantigen loads
predict overall survival in breast cancer. Oncoimmunology.
9:17449472020. View Article : Google Scholar
|
|
90
|
Zou XL, Li XB, Ke H, Zhang GY, Tang Q,
Yuan J, Zhou CJ, Zhang JL, Zhang R and Chen WY: Prognostic value of
neoantigen load in immune checkpoint inhibitor therapy for cancer.
Front Immunol. 12:6890762021. View Article : Google Scholar
|
|
91
|
Kosari F, Disselhorst M, Yin J, Peikert T,
Udell J, Johnson S, Smadbeck J, Murphy S, McCune A, Karagouga G, et
al: Tumor junction burden and antigen presentation as predictors of
survival in mesothelioma treated with immune checkpoint inhibitors.
J Thorac Oncol. 17:446–454. 2022. View Article : Google Scholar
|
|
92
|
Lee HS, Jang HJ, Choi JM, Zhang J, de
Rosen VL, Wheeler TM, Lee JS, Tu T, Jindra PT, Kerman RH, et al:
Comprehensive immunoproteogenomic analyses of malignant pleural
mesothelioma. JCI Insight. 3:e985752018. View Article : Google Scholar
|
|
93
|
Tandon RT, Jimenez-Cortez Y, Taub R and
Borczuk AC: Immunohistochemistry in peritoneal mesothelioma: A
single-center experience of 244 cases. Arch Pathol Lab Med.
142:236–242. 2018. View Article : Google Scholar
|
|
94
|
Leblay N, Leprêtre F, Le Stang N,
Gautier-Stein A, Villeneuve L, Isaac S, Maillet D, Galateau-Sallé
F, Villenet C, Sebda S, et al: BAP1 is altered by copy number loss,
mutation, and/or loss of protein expression in more than 70% of
malignant peritoneal mesotheliomas. J Thorac Oncol. 12:724–733.
2017. View Article : Google Scholar
|
|
95
|
Gezgin G, Dogrusöz M, van Essen TH, Kroes
WGM, Luyten GPM, van der Velden PA, Walter V, Verdijk RM, van Hall
T, van der Burg SH and Jager MJ: Genetic evolution of uveal
melanoma guides the development of an inflammatory
microenvironment. Cancer Immunol Immunother. 66:903–912. 2017.
View Article : Google Scholar
|
|
96
|
Lai J, Zhou Z, Tang XJ, Gao ZB, Zhou J and
Chen SQ: A tumor-specific neo-antigen caused by a frameshift
mutation in BAP1 is a potential personalized biomarker in malignant
peritoneal mesothelioma. Int J Mol Sci. 17:7392016. View Article : Google Scholar
|
|
97
|
Rizzolo A, Ah-Lan KC, Nu TNT and Alcindor
T: Response to Ipilimumab and nivolumab in a patient with malignant
peritoneal mesothelioma. Clin Colorectal Cancer. 21:371–374. 2022.
View Article : Google Scholar
|
|
98
|
Liu K, Huang Y, Xu Y, Wang G, Cai S, Zhang
X and Shi T: BAP1-related signature predicts benefits from
immunotherapy over VEGFR/mTOR inhibitors in ccRCC: A retrospective
analysis of JAVELIN Renal 101 and checkmate-009/010/025 trials.
Cancer Immunol Immunother. 72:2557–2572. 2023. View Article : Google Scholar
|
|
99
|
Topalian SL, Taube JM, Anders RA and
Pardoll DM: Mechanism-driven biomarkers to guide immune checkpoint
blockade in cancer therapy. Nat Rev Cancer. 16:275–287. 2016.
View Article : Google Scholar
|
|
100
|
Ayers M, Lunceford J, Nebozhyn M, Murphy
E, Loboda A, Kaufman DR, Albright A, Cheng JD, Kang SP, Shankaran
V, et al: IFN-γ-related mRNA profile predicts clinical response to
PD-1 blockade. J Clin Invest. 127:2930–2940. 2017. View Article : Google Scholar
|
|
101
|
Jiang P, Gu S, Pan D, Fu J, Sahu A, Hu X,
Li Z, Traugh N, Bu X, Li B, et al: Signatures of T cell dysfunction
and exclusion predict cancer immunotherapy response. Nat Med.
24:1550–1558. 2018. View Article : Google Scholar
|
|
102
|
Cui C, Xu C, Yang W, Chi Z, Sheng X, Si L,
Xie Y, Yu J, Wang S, Yu R, et al: Ratio of the interferon-γ
signature to the immunosuppression signature predicts anti-PD-1
therapy response in melanoma. NPJ Genom Med. 6:72021. View Article : Google Scholar
|
|
103
|
Lee JS, Nair NU, Dinstag G, Chapman L,
Chung Y, Wang K, Sinha S, Cha H, Kim D, Schperberg AV, et al:
Synthetic lethality-mediated precision oncology via the tumor
transcriptome. Cell. 184:2487–2502.e13. 2021. View Article : Google Scholar
|
|
104
|
Nair NU, Jiang Q, Wei JS, Misra VA, Morrow
B, Kesserwan C, Hermida LC, Lee JS, Mian I, Zhang J, et al: Genomic
and transcriptomic analyses identify a prognostic gene signature
and predict response to therapy in pleural and peritoneal
mesothelioma. Cell Rep Med. 4:1009382023. View Article : Google Scholar
|
|
105
|
Dinstag G, Shulman ED, Elis E, Ben-Zvi DS,
Tirosh O, Maimon E, Meilijson I, Elalouf E, Temkin B, Vitkovsky P,
et al: Clinically oriented prediction of patient response to
targeted and immunotherapies from the tumor transcriptome. Med.
4:15–30.e8. 2023. View Article : Google Scholar
|
|
106
|
Hoang DT, Dinstag G, Shulman ED, Hermida
LC, Ben-Zvi DS, Elis E, Caley K, Sammut SJ, Sinha S, Sinha N, et
al: A deep-learning framework to predict cancer treatment response
from histopathology images through imputed transcriptomics. Nat
Cancer. Jul 3–2024.(Epub ahead of print). View Article : Google Scholar
|
|
107
|
Panagi M, Mpekris F, Voutouri C,
Hadjigeorgiou AG, Symeonidou C, Porfyriou E, Michael C, Stylianou
A, Martin JD, Cabral H, et al: Stabilizing tumor-resident mast
cells restores T-Cell infiltration and sensitizes sarcomas to PD-L1
inhibition. Clin Cancer Res. 30:2582–2597. 2024. View Article : Google Scholar
|
|
108
|
Sautès-Fridman C, Lawand M, Giraldo NA,
Kaplon H, Germain C, Fridman WH and Dieu-Nosjean MC: Tertiary
lymphoid structures in cancers: Prognostic value, regulation, and
manipulation for therapeutic intervention. Front Immunol.
7:4072016. View Article : Google Scholar
|
|
109
|
Yang ZR, Su YD, Ma R, Wu HL and Li Y:
Efficacy and adverse events of apatinib salvage treatment for
refractory diffuse malignant peritoneal mesothelioma: A pilot
study. Front Oncol. 12:8118002022. View Article : Google Scholar
|
|
110
|
Zauderer MG, Alley EW, Bendell J,
Capelletto E, Bauer TM, Callies S, Szpurka AM, Kang S, Willard MD,
Wacheck V and Varghese AM: Phase 1 cohort expansion study of
LY3023414, a dual PI3K/mTOR inhibitor, in patients with advanced
mesothelioma. Invest New Drugs. 39:1081–1088. 2021. View Article : Google Scholar
|
|
111
|
Yang H, Hall SRR, Sun B, Zhao L, Gao Y,
Schmid RA, Tan ST, Peng RW and Yao F: NF2 and Canonical Hippo-YAP
pathway define distinct tumor subsets characterized by different
immune deficiency and treatment implications in human pleural
mesothelioma. Cancers (Basel). 13:15612021. View Article : Google Scholar
|
|
112
|
Fennell DA, King A, Mohammed S, Branson A,
Brookes C, Darlison L, Dawson AG, Gaba A, Hutka M, Morgan B, et al:
Rucaparib in patients with BAP1-deficient or BRCA1-deficient
mesothelioma (MiST1): An open-label, single-arm, phase 2a clinical
trial. Lancet Respir Med. 9:593–600. 2021. View Article : Google Scholar
|
|
113
|
Hung YP, Dong F, Watkins JC, Nardi V,
Bueno R, Dal Cin P, Godleski JJ, Crum CP and Chirieac LR:
Identification of ALK rearrangements in malignant peritoneal
mesothelioma. JAMA Oncol. 4:235–238. 2018. View Article : Google Scholar
|
|
114
|
Li Petri G, Pecoraro C, Randazzo O, Zoppi
S, Cascioferro SM, Parrino B, Carbone D, El Hassouni B, Cavazzoni
A, Zaffaroni N, et al: New Imidazo[2,1-b][1,3,4]Thiadiazole
derivatives inhibit FAK phosphorylation and potentiate the
antiproliferative effects of gemcitabine through modulation of the
human equilibrative nucleoside transporter-1 in peritoneal
mesothelioma. Anticancer Res. 40:4913–4919. 2020. View Article : Google Scholar
|
|
115
|
Quispel-Janssen JM, Badhai J, Schunselaar
L, Price S, Brammeld J, Iorio F, Kolluri K, Garnett M, Berns A,
Baas P, et al: Comprehensive pharmacogenomic profiling of malignant
pleural mesothelioma identifies a subgroup sensitive to FGFR
inhibition. Clin Cancer Res. 24:84–94. 2018. View Article : Google Scholar
|
|
116
|
Bensaid D, Blondy T, Deshayes S, Dehame V,
Bertrand P, Grégoire M, Errami M and Blanquart C: Assessment of new
HDAC inhibitors for immunotherapy of malignant pleural
mesothelioma. Clin Epigenetics. 10:792018. View Article : Google Scholar
|
|
117
|
McGale JP, Chen DL, Trebeschi S, Farwell
MD, Wu AM, Cutler CS, Schwartz LH and Dercle L: Artificial
intelligence in immunotherapy PET/SPECT imaging. Eur Radiol. Feb
15–2024.(Epub ahead of print). View Article : Google Scholar
|
|
118
|
Addala V, Newell F, Pearson JV, Redwood A,
Robinson BW, Creaney J and Waddell N: Computational immunogenomic
approaches to predict response to cancer immunotherapies. Nat Rev
Clin Oncol. 21:28–46. 2024. View Article : Google Scholar
|