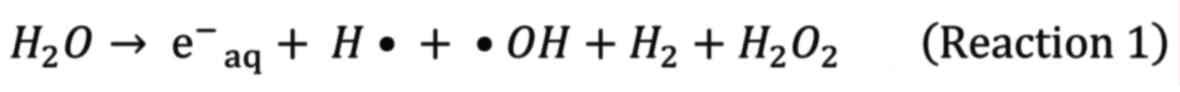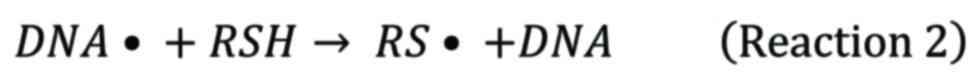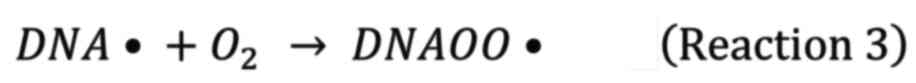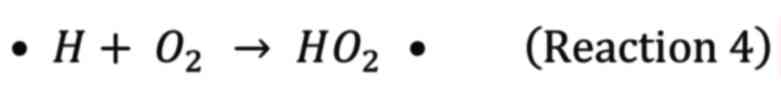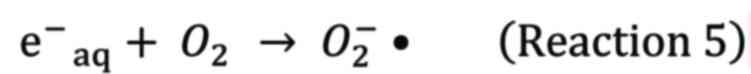Introduction
In recent years, due to the large number of cancer
cases and cancer-associated deaths, and the burden of rising
treatment costs, there has been an urgent need to improve the
therapeutic efficacy of malignant tumor treatment techniques.
Radiotherapy (RT) is one of the most widely used cancer treatments,
playing an irreplaceable role for curable malignancies. Despite
killing tumor cells, RT inevitably damages the healthy tissue
surrounding the tumor, significantly affecting the prognosis and
quality of life of treated patients. Therefore, a critical
principle underlying clinical RT dosimetry is maximizing the dose
to the target region while minimizing the dose to the adjacent
healthy tissue. In the past 30 years, advanced RT techniques, such
as intensity-modulated radiation therapy and image-guided radiation
therapy, have been used to precisely adjust the dose distribution
and improve the efficacy the tumor treatment (1). The use of particles such as protons
and heavy ions, which have unique radiophysical and biological
properties, as radiation sources, has also led to improvements in
RT techniques (2). Despite the
advances made in modern RT techniques, owing to the diversity of
tumor types, the radioresistance of tumors and the complexity of RT
techniques, the development of new high-precision, high-dose,
high-efficacy and low-toxicity RT techniques remains a constant
endeavor.
FLASH-RT may be a seminal technique due to its low
toxicity to healthy tissue and consequent promising clinical RT
applications, and has interested researchers worldwide. The term
FLASH was first coined by Favaudon et al (3) in 2014. The study demonstrated that
FLASH-RT could maintain toxicity to the tumor while sparing healthy
tissues surrounding the tumor compared with conventional RT
(CONV-RT). The mean dose rates of FLASH-RT (typically >40
Gy/sec) are generally several orders of magnitude higher than
CONV-RT (typically ≤0.01 Gy/sec). Currently, FLASH-RT has provided
exciting results as a new clinical treatment (4). Although FLASH-RT is expected to be a
breakthrough in tumor RT, the exact conditions and underlying
biological mechanisms behind the emergence of the FLASH effect
remain unclear. The present review discusses the factors that may
influence the emergence of the FLASH effect and highlights the
proposed hypotheses accounting for the mechanism of the FLASH
effect. At the same time, a comparison of in vivo and in
vitro research shows discrepancies in the biological effect of
FLASH irradiation. Therefore, the present review retrospectively
assesses the in vivo and in vitro research, and
considers the contributions of oxygen concentration and immune
response to this discrepancy. Meanwhile, current issues and the
future direction of FLASH-RT are discussed to provide a reference
for its clinical application.
Possible mechanisms responsible for the
FLASH effect
Although multiple studies (5,6) have
observed the FLASH effect, the underlying biological mechanism
responsible for the FLASH effect remains elusive. Some researchers
have proposed hypotheses to explain it, such as oxygen depletion
and immune regulation. However, these hypotheses have been
challenged with the deepening of the FLASH research.
Oxygen depletion hypothesis
mechanism
Dewey and Boag (7)
originally proposed the oxygen depletion hypothesis based on the
oxygen fixation model and the oxygen effect. It is well known that
radiation induces ionization of DNA and H2O, resulting
in direct and indirect damage to DNA, which may
lead to cell death. The radiolysis of H2O
created radicals such as hydrated electron
(e−aq), •OH, and H• (8).
Following irradiation, DNA radicals (DNA•) caused
by
hydroxyl radicals (•OH) reacting with DNA are
innocuous in anoxic conditions owing to the reaction with reducing
species
such as thiols.
Nevertheless, in the presence of oxygen, the DNA
radicals are fixed by oxygen to form irreversible deleterious
species.
Reaction 2 and reaction 3 are competitive reactions. As a
result, the radiological sensitivity enhances in the presence of
oxygen owing to the fixation of DNA damage. This is a simplified
illustration of the oxygen fixation model and oxygen effect. FLASH
irradiation delivers the total dose over an extremely short
duration with an ultra-high instantaneous dose
rate causing a high initial concentration of
radicals. Among these radicals, e−aq and H•
consume bulk oxygen, as a result, forming superoxide anions
(O2−•) and hydroperoxyl (HO2•),
respectively, leading to local oxygen depletion.
Therefore, in the absence of oxygen, the enhancement
of radioresistance improves the cell survival rate in normal
tissues. However, owing to low oxygen levels in tumors (hypoxic),
FLASH irradiation induces small change in radiosensitivity of
tumor. The physical and chemical events of the oxygen depletion
hypothesis, incidentally, occur at millisecond timescale (9–13).
This hypothesis seems convincing, as it is difficult
for reoxidation to occur during the duration of flash light
exposure, typically a millisecond timescale. Therefore, FLASH
irradiation induces less DNA insult in hypoxia resulting in an
increased survival rate. However, a critical question is whether
FLASH irradiation can deplete oxygen or not. Two studies have been
conducted to answer this question via constructed computational
model and direct measurement, respectively (14,15).
The direct measurements of oxygen level showed a small oxygen
change in the range of 1–3 mm Hg at a 20-Gy dose, with a dose rate
of 270 Gy/sec, suggesting that FLASH irradiation cannot deplete
oxygen in vivo. Normal tissues in vivo are in a
physoxic condition with a partial O2 pressure ranging
from 20–50 mmHg (15,16). Consequently, the oxygen depletion
hypothesis may not entirely account for the FLASH effect. Given the
potential for achieving local oxygen depletion if cells are at low
oxygen levels, the consideration that the FLASH effect occurs in
hypoxic cells, such as stem cells, as proposed by Pratx and Kapp
(9,17), is plausible. The computational model
showed that it is possible to achieve local oxygen depletion at a
large dose (e.g., 10 Gy) with ultra-high mean dose rates (e.g., 100
Gy/sec) in vivo (14).
Furthermore, the FLASH effect was observed at the range of 1.6–4.4%
oxygen concentration (12.24–33.66 mmHg) via in vitro
experiments (18).
Although there exists a discrepancy between the
computational model and direct measurement, the underlying
consensus is that FLASH irradiation is insufficient to deplete
oxygen at normoxic conditions, suggesting that the FLASH effect may
not be represented in vitro (normoxia, ~139 mmHg) (19). This deduction is consistent with the
in vitro experimental results that there is no significant
difference in cell survival rate between FLASH and CONV
irradiation. Consequently, we consider that the oxygen depletion
hypothesis may be only partly responsible for the FLASH effect.
Other mechanisms must exist to explain the FLASH effect. In fact,
some studies have pointed out that the interactions among
radiochemical radicals are pivotal for explaining the FLASH effect
(20–22).
Mechanism of free radical
interaction
As early as 1969, Berry et al (20) proposed that FLASH irradiation
induced a high local initial free radical concentration resulting
in radical-radical interaction. As a result, the number of free
radicals reduces and subsequently induces less damage to the
biomolecule. In addition, as mentioned by Koch (21), the deposited energy followed by
electron track is nonhomogeneous and may cause a local high radical
density. In high radical density areas, radical-radical interaction
can occur. Based on this assertion, the damage to DNA was simulated
in a simulation box full of H2O and O2
following FLASH irradiation. The results demonstrated that the
levels of innoxious non-ROS are higher at FLASH irradiation than at
CONV irradiation. In addition, the population of ROS at the initial
time of FLASH irradiation is high and rapidly decreases, and is
ultimately lower than that at CONV irradiation with time (22). This simulation is consistent with
the aforementioned assertion suggesting that the interaction among
high-density radicals reduces the free radicals that may damage
biomolecules. Moreover, an in vivo study also observed lower
levels of ROS at FLASH irradiation than at CONV irradiation
(23). Therefore, the interaction
of radicals resulting in the reduction of deleterious radicals may
be responsible for the FLASH effect.
Immune regulation mechanism
Some studies have suggested that the FLASH effect
may relate to immune regulation. Several experiments have shown
that FLASH irradiation activates different inflammatory response
pathways and induces less activation of gliocytes compared with
CONV radiation in the brain (23–25).
However, these studies only showed an association between immune
regulation and the FLASH effect, not causation, and the tangible
mechanism of FLASH radiation resulting in a reduction of
inflammation and ultimately causing the FLASH effect remains
elusive. Meanwhile, Jin et al (26) proposed that FLASH-RT could control
normal tissue toxicity and tumors by reducing the killing of
circulating immune cells. This relative protection of the immune
system allows the body to mitigate the toxicity of radiation to
normal cells and achieve tumor control. Nevertheless, this study is
only a theoretical simulation and needs to be experimentally
verified.
DNA integrity hypothesis
Shi et al (6)
suggested that the FLASH effect may be related to the integrity of
DNA. The deposition of a CONV-RT dose takes hundreds of seconds,
which means that some DNA molecules break due to the energy levels
before the dose transfer is completed, resulting in partial DNA
damage and damage to the integrity of the DNA. This means that
during FLASH radiation, DNA breaks and instability rarely occurs
until dose delivery is complete. On the other hand, genomic
instability has been considered as a marker of cancer for more than
a decade (27). Therefore, in tumor
cells, even in FLAS-RT, due to the inherent instability of the
genome, a large amount of tumor cell DNA damage will be caused, so
as to achieve the same tumor killing effect as CONV-RT.
In conclusion, the proposed hypotheses tried to
explain the FLASH effect from different perspectives. However, none
can fully explain the mechanism of the FLASH effect. It is possible
that the FLASH effect depends on the combination of all mechanisms.
For demonstrating these hypotheses, practical validation
experiments are indispensable.
Differences between in vitro and
in vivo experiments
The previous fundamental studies of FLASH-RT are
chiefly in vitro studies, in which the observed phenomena
are quite different from those in in vivo studies (Tables SI and SII). Through examination of these
studies, it can be observed that there is no difference in cell
survival between FLASH and CONV irradiation, suggesting that the
FLASH effect cannot occur at the cell level. However, nearly all
in vivo studies observed the FLASH effect in a variety of
animal models (5,6,18,28).
What caused this differential phenomenon and why the FLASH effect
emerged in a number of in vivo studies is worth considering.
Therefore, the in vitro and in vivo studies were we
retrospectively assessed to decipher the potential reason
responsible for this.
In vitro research
Several reviews have meticulously depicted the in
vitro results of FLASH irradiation (29–32).
Given that, the present review aims to systematically list in
vitro studies with experimental conditions, assay endpoint and
the occurrence of the FLASH effect to dissect the regular patterns
that produce the FLASH effect. Table
SI shows that the assay endpoint of irradiation-induced
biological effects is frequently characterized by cell colony
formation, DNA double-strand breaks (DSBs) and cell arrest.
Although there are differences in physical irradiation parameters,
the biological effects are generally consistent in these
studies.
A cell colony assay is considered a gold standard to
validate the radiobiological effect of irradiation in in
vitro studies. There was no difference in cell colony formation
rate between FLASH and CONV irradiation in most studies (33–41).
However, a few studies reported that the cell survival rate at
FLASH irradiation was higher than at CONV irradiation (7,18,20,28).
Notably, one of these studies showed the significance of cell
survival rate between FLASH and CONV irradiation only in specific
O2 conditions (1% O2 in N2)
(7). In addition, physical
irradiation parameters and cell lines were similar between the
study by Fouillade et al (28) and that by Beddok et al
(38); however, they attained
discrepant results, suggesting that the emergence of the FLASH
effect was volatile in vitro.
Furthermore, the volatility of DSBs in some studies
also hinted that the FLASH effect was not ubiquitous in
vitro (28,37,39,42,43).
Notably, with one exception (43),
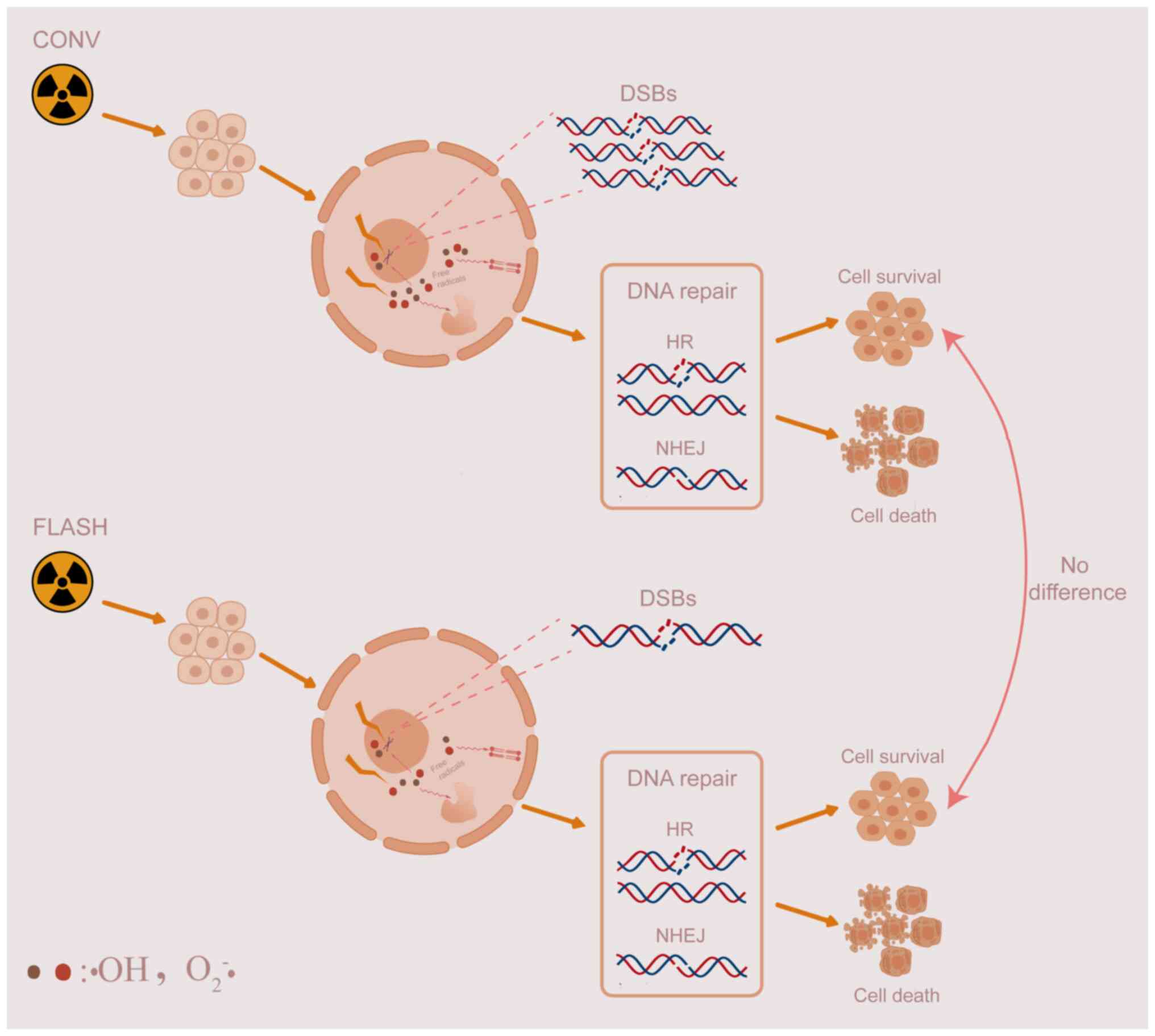
other studies indicated that the number of DSBs was significantly
different between FLASH and CONV irradiation in the normal cell
line, whereas the number of DBSs produced by the two irradiations
was not significantly different in tumour cell lines (Fig. 1).
Consequently, we could speculate that FLASH
irradiation, relative to CONV irradiation, induces less molecule
damage in normal cells, but has equivalent toxicity in tumor cells.
This phenomenon is analogous to what was observed in animal models
(Table SII), suggesting that the
FLASH effect seems only occur at the molecular level in normal
cells rather than in tumor cells in vitro. One study showed
no difference in DSBs between FLASH and CONV irradiation in a
normal cell line (43). It is
plausible that different irradiation sources may account for this
result. FLASH proton irradiation with high linear energy transfer
(LET) and relative biological effectiveness may induce a biological
effect equivalent to low LET CONV X-ray irradiation.
As aforementioned, a regular pattern can be observed
in which the FLASH effect barely occurs at the cellular level and
only occurs in normal cells rather than tumor cells at the
molecular level in vitro. According to the aforementioned
hypotheses, the disappearance of the FLASH effect at cellular level
can be attributed to the oxygen concentration being too high to
deplete oxygen in vitro. However, there are still some
uncertainties remaining: i) the reason for the sparing effect at
the molecular level in normal cells; ii) the reason that FLASH
irradiation cannot magnify the FLASH effect from molecular level to
cellular level; and iii) what is responsible for the discrepant
results between normal and tumor cells at the molecular level. The
present review attempts to answer these questions based on known
experimental results and hypotheses. For point i), we consider that
the interaction among radicals plays a vital role, since the oxygen
depletion hypothesis is not eligible in vitro. The reduction
of deleterious radicals inducing lower DSBs level is
persuasive.
For point ii), there are no experimental results or
proposed hypotheses for reference. Therefore, an explanation must
be proposed via the theoretical mechanism. DNA damage is generally
considered the central cause of cell death after irradiation,
especially after clinical RT doses. Undeniably, irradiation-induced
cell death is comprised of other pathways, such as
membrane-dependent signaling pathways and bystander responses,
which are associated with oxidative damage to all biomolecules
(nucleic acids, proteins and lipids) (44–47).
In addition, DNA damage induced by irradiation triggers the DNA
repair pathways consisting of non-homologous end joining and
homologous recombination to repair DNA lesions. Incorrect and
unrepaired DNA lesions cause chromosomal aberrations related to
cell death (48). In this respect,
a previous study indicated no sparing effect in chromosomal
aberrations after FLASH and CONV irradiation (49). Consequently, we consider that the
process of DNA repair and oxidative stress responses may diminish
the sparing effect from the molecular level to a cellular effect
(Fig. 1).
For point iii), given that normal cells and tumor
cells are in the same context with regard to culture conditions
in vitro, the oxygen depletion hypothesis does not seem
suitable to explain the discrepant results in DSBs between normal
and tumor cells at the molecular level. However, oxidative stress
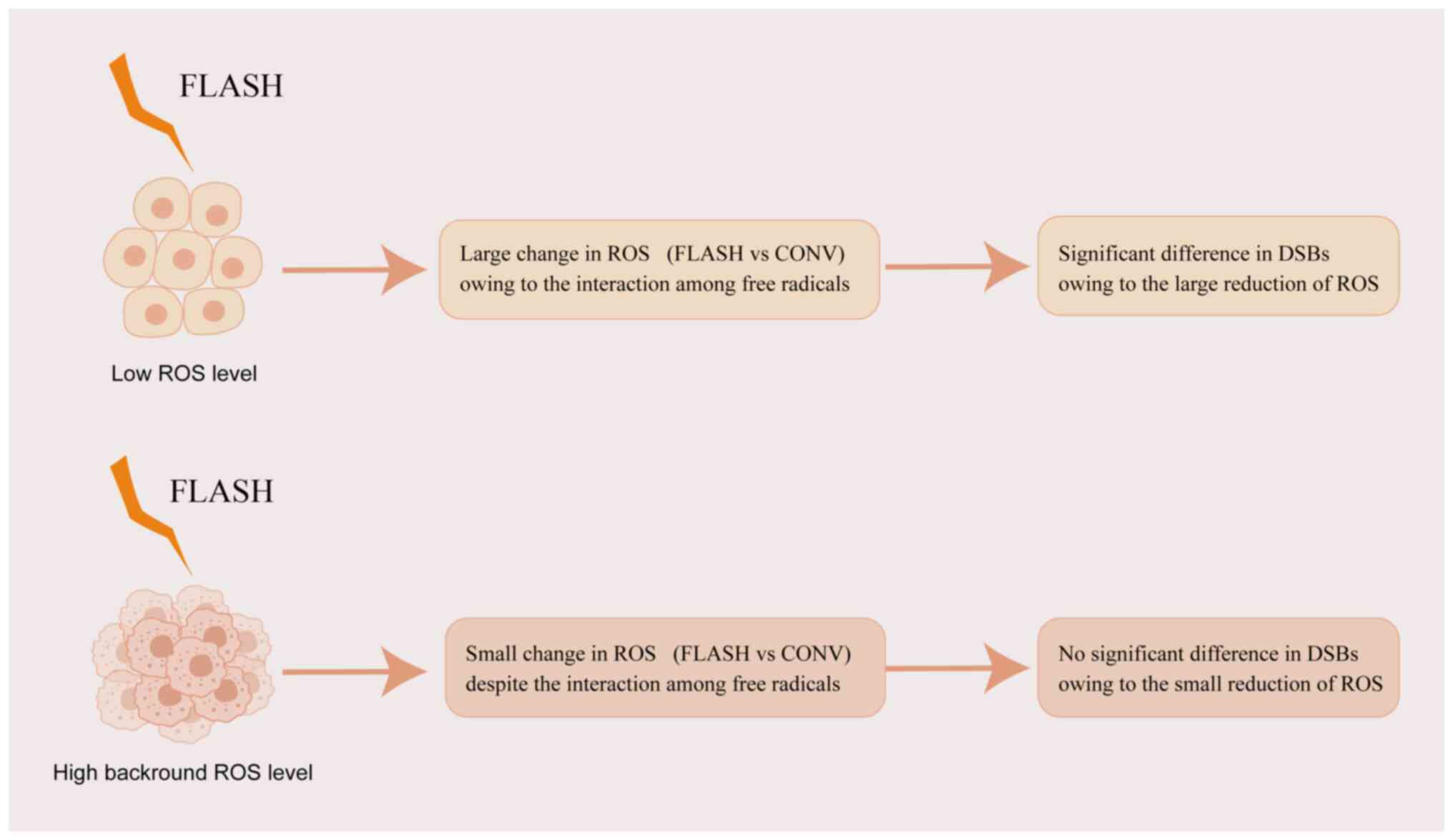
caused by radiation may be associated with the discrepancy. Tumor
cells, relative to normal cells, display higher background levels
of ROS and have robust Fenton-type reactions, which could magnify
the production of ROS (50,51). In addition, according to the
aforementioned hypotheses, FLASH irradiation induces a lower level
of ROS owing to the interaction among free radicals compared with
CONV irradiation. Therefore, the reduction of ROS induced by FLASH
irradiation may be a small change in tumor cells due to the high
background level of ROS. By contrast, the reduction of ROS induced
by FLASH irradiation is a large change in normal cells.
Consequently, the small change of ROS is insufficient to cause the
significant difference in DSBs in tumor cells, which is different
from normal cells (Fig. 2).
Although the present review observes and illustrates
the regular pattern of FLASH effects produced in vitro and
proposes mechanistic insights to explain this pattern, it is
essential to point out that this pattern is not rigorous owing to
the limited experimental results. Further studies are indispensable
to validate the accuracy of this pattern.
In vivo research
Unlike the ambiguous results in vitro, the
FLASH effect ubiquitously occurs in animal models after FLASH
irradiation. Currently, in various animal models (e.g., mice,
zebrafish, pigs and cats), the FLASH effect has been reported to
occur in the central nervous system, lung, intestines and skin
(3,6,23,52,53).
The study of FLASH irradiation in vivo
commenced in the study by Favaudon et al (3). The study observed lung fibrosis
formation and lung tumor survival after FLASH and CONV electron
irradiation. The results indicated that FLASH irradiation induced
less lung fibrosis than CONV irradiation. By contrast, FLASH and
CONV irradiation possessed comparable tumour killing capacity,
suggesting that FLASH irradiation diminishes the insult to normal
tissues, and reduces the incidence and severity of post-irradiation
complications (3). A further study
indicated that FLASH irradiation protected lung progenitor cells
from excessive damage, and induced less cell senescence and DNA
damage in the lung. However, the FLASH effect disappeared in
Terc−/− mice, indicating that Terc may be an
important gene target for FLASH irradiation to alleviate lung
injury (28). Another study also
indicated that FLASH irradiation could attenuate the
radiation-induced insult to intrathoracic tissues (54). This study demonstrated that use of
high energy X-rays as a FLASH irradiation source for sparing normal
tissue is technically feasible.
Montay-Gruel et al (23–25,55,56)
from Lausanne University Hospital, Switzerland, focused on the
phenotypic and molecular characteristics of the brain exposed to
FLASH irradiation, and united other institutions to validate the
FLASH effect on the brain. Severe brain damage, cognitive deficits
and neurogenic decompensation at the mean dose rate of ≤30 Gy/sec
were observed in normal mouse brains at a 10-Gy dose. However, at
higher dose rates, especially those >100 Gy/sec, the cognitive
function was spared, suggesting that the sparing effect was more
pronounced at higher dose rates. Subsequently, pulsed X-rays were
verified as a FLASH irradiation source that could help retain
cognitive memory capacity (24).
The study indicated that the sparing of cognitive function is
associated with the reduction in ROS and that following the relief
of oxidative stress, it limits microglial activation and attenuates
neuroinflammatory responses (23).
Moreover, the difference in astrogliosis and neuroinflammatory
responses between FLASH and CONV irradiation may contribute to the
sparing effect in the brain. FLASH irradiation did not trigger TLR4
expression and astrogliosis compared with CONV irradiation,
although both irradiations induced the activation of the complement
cascade (25). Normal mice were
used as the subjects in the aforementioned studies, which cannot
indicate that FLASH irradiation possesses the same antitumour
effect as CONV irradiation; therefore, Montay-Gruel et al
(56) established an orthotopic
murine glioblastoma model and simulated clinical fractioned RT to
investigate the feasibility of FLASH-RT. The results showed that
fractioned FLASH-RT could delay tumor growth similar to fractioned
CONV-RT, and the FLASH effect was more pronounced after the
delivery of hypo-fractionated regimens (56). Other studies indicated that the
reduction of ROS, the neuroinflammatory response, the retention of
microvascular integrity and dendritic spine density, the reduction
of toxicity to the endocrine system and the decrease of apoptosis
in neurogenic regions may be related to the sparing of cognition
after FLASH irradiation to a substantial degree (57–59).
In addition to the brain and thorax, other
anatomical sites of mice, such as the abdomen (54,60–62),
skin (63,64) and even the whole body (65), were probed after FLASH irradiation.
A series of sparing phenomena, such as the retention of
gastrointestinal function, the low mortality of stem cells, the
attenuation of the gastrointestinal syndrome, the proliferation of
intestinal crypt cells and the high survival rates of mice, were
observed after abdominal FLASH irradiation. Moreover, mice with
pancreatic flank tumors subjected to abdominal FLASH and CONV
irradiation showed iso-efficient tumor delay, suggesting that the
FLASH effect can occur in the abdomen (62). Compared with CONV irradiation,
electron and proton FLASH irradiation induced lower toxicity to the
skin (63,64). Whole-body FLASH irradiation induced
less toxicity to hematopoietic stem cells in immunocompromised
mice, suggesting that FLASH-RT may be well applied to the treatment
of acute lymphoid leukemia (65).
For translation to clinical applications, large
mammals such as mini-pig, cat and canine patients subjected to
FLASH irradiation were used to evaluate the safety and feasibility
of FLASH-RT (52,66). The overall results indicated that
FLASH irradiation induced lower toxicity to the normal tissues,
with slight side effects, and inhibited tumor growth, suggesting
that the FLASH effect can also occur in higher mammals (52).
Although most studies revealed the FLASH effect was
ubiquitous in vivo, some studies failed to observe the FLASH
effect at all (40,53,54,67).
For example, in one study, at a dose rate of 35 Gy/sec, the
electron FLASH irradiation induced more mucosal damage in the
gastrointestinal tract compared with CONV irradiation, suggesting
the reproducibility of the FLASH effect may be more stringent for
the dose rate range (40).
Furthermore, the FLASH effect may be concealed by the high dose of
FLASH and CONV irradiation, and the radioresistance of different
tissues (54).
The present review summarizes the in vivo
research data consisting of assay conditions and physical
irradiation parameters in Table
SII. In contrast to the ambiguous results in vitro, the
FLASH effect could be produced by different adapted irradiation
parameters in the majority of in vivo research. It is worth
considering what accounts for this discrepant phenomenon between
in vitro and in vivo studies. As aforementioned, the
oxygen level is much lower in vivo in comparison with that
in vitro, which may be sufficient to achieve oxygen
depletion, resulting in the FLASH effect. In addition, in
vivo studies, relative to in vitro studies, are
implemented in the systematic organism encompassing complex
interactions of all tissues. Therefore, as researchers have found,
immune responses may also be responsible for the discrepancy.
Factors influencing the FLASH effect
We attempt to explore the occurrence patterns of
the FLASH effect through currently published studies. Physical
irradiation parameters, such as the mean dose rate, instantaneous
dose rate, pulse width, total exposure time, pulse repetition
frequency, total dose and fractioned dose, are noted to be
different between FLASH and CONV irradiation, which is presumably
responsible for the occurrence of the FLASH effect. In addition,
the levels of oxygen may also be a key factor contributing to
it.
The mean dose rate is a critical physical parameter
to distinguish between CONV and FLASH irradiation. Therefore, the
mean dose rate is a key factor influencing the occurrence of the
FLASH effect. For example, in a previous study, the proportion of
normal human lung fibroblast senescent cells decreased with
increasing irradiation dose rates (39). A gradient of mean dose rates of
irradiation showed that 30 Gy/sec was the threshold for displaying
the sparing effect (55). However,
the minimum mean dose rate representing the FLASH effect is
uncertain, although most studies define FLASH irradiation with a
mean dose rate of ≥40 Gy/sec, as initially stated by Favaudon et
al (3). We consider that the
scope of the mean dose rate could potentially be extended. There
are some pieces of evidence to support this consideration. In one
study, at a dose rate of 37 Gy/sec, the sparing effect of cognitive
function was observed (24).
Another study simulated veritable biological responses after FLASH
irradiation in animal models and deduced that the minimum dose rate
to display the FLASH effect was 57 Gy/sec, which is close to the
mean dose rate applied in various preclinical experiments (68). As alluded to here, the occurrence of
the FLASH effect demands an adapted mean dose rate. However, a
solid minimum mean dose rate is not currently attainable owing to
the limited data available.
Researchers are realizing that it is not rigorous
to define FLASH irradiation by the mean dose rate alone for further
research. Other physical irradiation parameters, such as the total
exposure time and the instantaneous dose rate, may be equally
important as the mean dose rate. One review has elegantly indicated
that the FLASH effect is associated with the combination of
relevant parameters, such as the number of pulses, instantaneous
dose rate and total exposure time (<200 msec) (69). In addition, the fractionated dose
may be an important factor influencing the FLASH effect. In
traditional clinical RT regimens, a fractionated dose (<10 Gy)
is usually used. However, most in vivo studies performed
FLASH irradiation at single doses of ≥10 Gy. Given this, a study
simulated clinical RT to explore whether the FLASH effect occurs in
the fractioned FLASH-RT regimen or not (56). Results showed that the benefits of
FLASH-RT were more pronounced in the hypo-fractionated RT regimens
(7–10 Gy), while the FLASH effect was not observed when the single
dose was too large (14 Gy).
In addition to the physical parameters of FLASH
irradiation, the level of oxygen also affects the occurrence of the
FLASH effect. As aforementioned, the sparing effect is ubiquitous
in vivo rather than in vitro. The current review has
shown that the level of oxygen plays an important role in this
discrepancy. Another review also has discussed the importance of
oxygen, which may be responsible for this discrepant phenomenon
(70). Therefore, we consider the
level of O2 may be directly associated with the FLASH
effect.
Challenges and prospects
The investigation into FLASH irradiation is a
treasure trove that has yet to be mined fully. Despite the fact
that the FLASH effect has been demonstrated in a number of studies,
it is still a very young research field, and there are many
challenges to overcome from the pre-clinical research to clinical
practice. Several issues still need to be addressed to bring FLASH
radiation to maturity. For example: i) The reproducibility
conditions for the FLASH effect are not entirely clear. As
aforementioned, certain studies have failed to observe this
phenomenon (40,43,53,65).
We consider that the key parameters that would reproduce the FLASH
effect in these studies are not being used at the required
standards. Therefore, the FLASH effect cannot be observed. To best
investigate the mechanism of the FLASH effect, the key factors that
may influence the occurrence of the FLASH effect must be figured
out. ii) There are still technical challenges to achieving FLASH
dose rates with clinically modified equipment. The majority of
studies use clinical linear electron accelerators retrofitted to
emit FLASH electron beams, owing to the lack of difficulty in
developing such retrofits. However, the electron beam can only
treat superficial tumors due to its limited dose distribution at
depth (71). Future treatment of
deep tumors will require FLASH X-rays or proton beams; however,
there are technical challenges to be resolved to prepare clinical
equipment capable of delivering FLASH X-rays. Currently, the
apparatus producing FLASH X-rays are only available at large
facilities, such as the European Synchrotron Radiation Facility in
Europe (24) and the Platform for
Advanced RT Research in China (54). When treating tumors with a proton
beam, the beam must be scattered or scanned to cover the target
volume. However, the scanning may reduce the dose rate and
ultimately cannot trigger the FLASH effect (72). Therefore, beam flow systems,
scanning speeds and monitoring of ionization chambers available for
FLASH proton RT systems also need to be modified (73). iii) FLASH dosimetry and dose
monitoring systems need to be improved. Given that FLASH
irradiation delivers a dose in an instant period, there is a
clinical need for monitoring systems that can achieve real-time
monitoring of FLASH irradiation dose. It is vital to develop
methods and dose monitoring systems for accurately measuring the
delivered dose of FLASH irradiation. Several studies have made
progress in this area (74–77). McManus et al (74) demonstrated that conventional alanine
dosimeters, film dosimeters and pyroelectric dosimeters are all
suitable for absolute dose measurements of FLASH irradiation. Jorge
et al (75) and Petersson
et al (76) developed an
empirical ionization chamber model for FLASH radiation dosimetry.
However, the dose monitoring is inaccurate, when the dose rate is
too high in each pulse. Therefore, further development of
appropriate ionization chambers and empirical models is needed. In
addition, Oraiqat et al (77) established an image-guided approach
for the real-time measurement of deep tissue doses during FLASH-RT.
Although these studies have built the foundation for a clinical
shift to FLASH-RT, the accuracy of related technologies remains to
be verified. iv) The safety of FLASH-RT requires further
validation. Although the first FLASH-RT patient was treated well
(4), the treatment of superficial
tumors alone is not sufficient for clinical RT. One clinical study
alone is not representative, and the feasibility and safety of
FLASH-RT need to be verified in the future to ensure as much safety
as possible. v) The mechanism of the FLASH effect still needs to be
experimentally investigated. The role of the oxygen depletion
hypothesis and other hypotheses in the FLASH effect, and the
relationship between these hypotheses, still require further
study.
To conclude, researchers in previous studies tended
to primarily devise irradiation parameters based on average dose
rate during experimental design, ignoring significant physical
parameters, such as instantaneous dose rate, pulse width and
radiation dose fractionation. The optimization of radiation
parameters is crucial for the future application of FLASH
technology. Therefore, researchers must delve deeper into the
rationality of physical irradiation parameters and endeavor to
devise experiments that encompass a variety of these parameters.
This will facilitate a clearer understanding of the conditions
under which the FLASH effect manifests. At the same time,
researchers must not overlook the long-term consequences of FLASH
irradiation in future investigations, as they are intricately
linked to the prognosis of patients in potential clinical
applications. The FLASH-RT could be a revolutionary advancement in
clinical RT in the future. Some radiation-resistant tumors that
require larger doses for treatment would be well treated via
FLASH-RT, which could provide a high dose threshold to overcome
excessive toxicity to healthy tissues surrounding tumors.
Furthermore, preclinical studies have shown that all irradiation
sources, such as electron beams, X-rays and proton beams, can
achieve the FLASH effect, suggesting the universality of FLASH-RT
in the future. In terms of laboratory and preclinical research, it
is advisable to embark on studies focusing on the integration of
FLASH-RT in tumor RT with immunotherapy, with the aim of thoroughly
elucidating the underlying mechanisms of FLASH-RT and assessing the
potential value of this combined therapeutic approach. In summary,
this comprehensive review, embracing pivotal research and pertinent
overviews associated with FLASH-RT, serves as a valuable resource
for a profound exploration of the mechanisms underlying the diverse
applications of this technology.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
SZ and MHL conceived the study, searched the
literature and wrote the original manuscript. MHL, GFD, and CZW
participated in the writing and subsequent revision of the article.
All authors have read and approved the final manuscript. Data
authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yi J, Huang X, Gao L, Luo J, Zhang S, Wang
K, Qu Y, Xiao J and Xu G: Intensity-modulated radiotherapy with
simultaneous integrated boost for locoregionally advanced
nasopharyngeal carcinoma. Radiat Oncol. 9:562014. View Article : Google Scholar
|
|
2
|
Luhr A, von Neubeck C, Pawelke J, Seidlitz
A, Peitzsch C, Bentzen SM, Bortfeld T, Debus J, Deutsch E,
Langendijk JA, et al: ‘Radiobiology of Proton Therapy’: Results of
an International expert workshop. Radiother Oncol. 128:56–67. 2018.
View Article : Google Scholar
|
|
3
|
Favaudon V, Caplier L, Monceau V,
Pouzoulet F, Sayarath M, Fouillade C, Poupon MF, Brito I, Hupé P,
Bourhis J, et al: Ultrahigh dose-rate FLASH irradiation increases
the differential response between normal and tumor tissue in mice.
Sci Transl Med. 6:245ra932014. View Article : Google Scholar
|
|
4
|
Bourhis J, Sozzi WJ, Jorge PG, Gaide O,
Bailat C, Duclos F, Patin D, Ozsahin M, Bochud F, Germond JF, et
al: Treatment of a first patient with FLASH-radiotherapy. Radiother
Oncol. 139:18–22. 2019. View Article : Google Scholar
|
|
5
|
Dai Y, Liang R, Wang J, Zhang J, Wu D,
Zhao R, Liu Z and Chen F: Fractionated FLASH radiation in
xenografted lung tumors induced FLASH effect at a split dose of 2
Gy. Int J Radiat Biol. 99:1542–1549. 2023. View Article : Google Scholar
|
|
6
|
Shi X, Yang Y, Zhang W, Wang J, Xiao D,
Ren H, Wang T, Gao F, Liu Z, Zhou K, et al: FLASH X-ray spares
intestinal crypts from pyroptosis initiated by cGAS-STING
activation upon radioimmunotherapy. Proc Natl Acad Sci USA.
119:e22085061192022. View Article : Google Scholar
|
|
7
|
Dewey DL and Boag JW: Modification of the
oxygen effect when bacteria are given large pulses of radiation.
Nature. 183:1450–1451. 1959. View Article : Google Scholar
|
|
8
|
Land EJ: Pulse radiolysis and flash
photolysis: Some applications in biology and medicine. Biochimie.
62:207–221. 1980. View Article : Google Scholar
|
|
9
|
Pratx G and Kapp DS: A computational model
of radiolytic oxygen depletion during FLASH irradiation and its
effect on the oxygen enhancement ratio. Phys Med Biol.
64:1850052019. View Article : Google Scholar
|
|
10
|
Becker D and Sevilla MD: 3 - The Chemical
Consequences of Radiation Damage to DNA: Advances in Radiation
Biology. Elsevier. 1993.121–80
|
|
11
|
Alper T and Howard-Flanders P: Role of
oxygen in modifying the radiosensitivity of E. coli B. Nature.
178:978–979. 1956. View Article : Google Scholar
|
|
12
|
Van den Heuvel F, Vella A, Fiorini F,
Brooke M, Hill MA and Maughan T: Incorporating oxygenation levels
in analytical DNA-damage models-quantifying the oxygen fixation
mechanism. Phys Med Biol. 66:1450052021. View Article : Google Scholar
|
|
13
|
Wilson JD, Hammond EM, Higgins GS and
Petersson K: Ultra-High dose rate (FLASH) radiotherapy: Silver
bullet or fool's gold? Front Oncol. 9:15632020. View Article : Google Scholar
|
|
14
|
Petersson K, Adrian G, Butterworth K and
McMahon SJ: A quantitative analysis of the role of oxygen tension
in FLASH radiation therapy. Int J Radiat Oncol Biol Phys.
107:539–547. 2020. View Article : Google Scholar
|
|
15
|
Cao X, Zhang R, Esipova TV, Allu SR,
Ashraf R, Rahman M, Gunn JR, Bruza P, Gladstone DJ, Williams BB, et
al: Quantification of oxygen depletion during FLASH irradiation in
vitro and in vivo. Int J Radiat Oncol Biol Phys. 111:240–248. 2021.
View Article : Google Scholar
|
|
16
|
McKeown SR: Defining normoxia, physoxia
and hypoxia in tumours-implications for treatment response. Br J
Radiol. 87:201306762014. View Article : Google Scholar
|
|
17
|
Pratx G and Kapp DS: Ultra-High-Dose-Rate
FLASH irradiation may spare hypoxic stem cell niches in normal
tissues. Int J Radiat Oncol Biol Phys. 105:190–192. 2019.
View Article : Google Scholar
|
|
18
|
Adrian G, Konradsson E, Lempart M, Bäck S,
Ceberg C and Petersson K: The FLASH effect depends on oxygen
concentration. Br J Radiol. 93:201907022020. View Article : Google Scholar
|
|
19
|
Keeley TP and Mann GE: Defining
physiological normoxia for improved translation of cell physiology
to animal models and humans. Physiol Rev. 99:161–234. 2019.
View Article : Google Scholar
|
|
20
|
Berry RJ, Hall EJ, Forster DW, Storr TH
and Goodman MJ: Survival of mammalian cells exposed to X rays at
ultra-high dose-rates. Br J Radiol. 42:102–107. 1969. View Article : Google Scholar
|
|
21
|
Koch CJ: Re: Differential impact of FLASH
versus conventional dose rate irradiation: Spitz et al. Radiother
Oncol. 139:62–63. 2019. View Article : Google Scholar
|
|
22
|
Abolfath R, Grosshans D and Mohan R:
Oxygen depletion in FLASH ultra-high-dose-rate radiotherapy: A
molecular dynamics simulation. Med Phys. 47:6551–6561. 2020.
View Article : Google Scholar
|
|
23
|
Montay-Gruel P, Acharya MM, Petersson K,
Alikhani L, Yakkala C, Allen BD, Ollivier J, Petit B, Jorge PG,
Syage AR, et al: Long-term neurocognitive benefits of FLASH
radiotherapy driven by reduced reactive oxygen species. Proc Natl
Acad Sci USA. 116:10943–10951. 2019. View Article : Google Scholar
|
|
24
|
Montay-Gruel P, Bouchet A, Jaccard M,
Patin D, Serduc R, Aim W, Petersson K, Petit B, Bailat C, Bourhis
J, et al: X-rays can trigger the FLASH effect: Ultra-high dose-rate
synchrotron light source prevents normal brain injury after whole
brain irradiation in mice. Radiother Oncol. 129:582–588. 2018.
View Article : Google Scholar
|
|
25
|
Montay-Gruel P, Markarian M, Allen BD,
Baddour JD, Giedzinski E, Jorge PG, Petit B, Bailat C, Vozenin MC,
Limoli C and Acharya MM: Ultra-High-Dose-Rate FLASH irradiation
limits reactive gliosis in the brain. Radiat Res. 194:636–645.
2020. View Article : Google Scholar
|
|
26
|
Jin JY, Gu A, Wang W, Oleinick NL, Machtay
M and Spring Kong FM: Ultra-high dose rate effect on circulating
immune cells: A potential mechanism for FLASH effect? Radiother
Oncol. 149:55–62. 2020. View Article : Google Scholar
|
|
27
|
Hanahan D: Hallmarks of Cancer: New
Dimensions. Cancer Discov. 12:31–46. 2022. View Article : Google Scholar
|
|
28
|
Fouillade C, Curras-Alonso S, Giuranno L,
Quelennec E, Heinrich S, Bonnet-Boissinot S, Beddok A, Leboucher S,
Karakurt HU, Bohec M, et al: FLASH irradiation spares lung
progenitor cells and limits the incidence of radio-induced
senescence. Clin Cancer Res. 26:1497–1506. 2020. View Article : Google Scholar
|
|
29
|
Marcu LG, Bezak E, Peukert DD and Wilson
P: Translational Research in FLASH radiotherapy-from
radiobiological mechanisms to in vivo results. Biomedicines.
9:1812021. View Article : Google Scholar
|
|
30
|
Esplen N, Mendonca MS and Bazalova-Carter
M: Physics and biology of ultrahigh dose-rate (FLASH) radiotherapy:
A topical review. Phys Med Biol. 65:23TR032020. View Article : Google Scholar
|
|
31
|
Borghini A, Vecoli C, Labate L, Panetta D,
Andreassi MG and Gizzi LA: FLASH ultra-high dose rates in
radiotherapy: Preclinical and radiobiological evidence. Int J
Radiat Biol. 98:127–135. 2022. View Article : Google Scholar
|
|
32
|
Omyan G, Musa AE, Shabeeb D, Akbardoost N
and Gholami S: Efficacy and toxicity of FLASH radiotherapy: A
systematic review. J Cancer Res Ther. 16:1203–1209. 2020.
View Article : Google Scholar
|
|
33
|
Tillman C, Grafstrom G, Jonsson AC,
Jönsson BA, Mercer I, Mattsson S, Strand SE and Svanberg S:
Survival of mammalian cells exposed to ultrahigh dose rates from a
laser-produced plasma x-ray source. Radiology. 213:860–865. 1999.
View Article : Google Scholar
|
|
34
|
Shinohara K, Nakano H, Miyazaki N, Tago M
and Kodama R: Effects of single-pulse (<=1 ps) X-rays from
laser-produced plasmas on mammalian cells. J Radiat Res.
45:509–514. 2004. View Article : Google Scholar
|
|
35
|
Auer S, Hable V, Greubel C, Drexler GA,
Schmid TE, Belka C, Dollinger G and Friedl AA: Survival of tumor
cells after proton irradiation with ultra-high dose rates. Radiat
Oncol. 6:1392011. View Article : Google Scholar
|
|
36
|
Doria D, Kakolee KF, Kar S, Litt SK,
Fiorini F, Ahmed H, Green S, Jeynes JCG, Kavanagh J, Kirby D, et
al: Biological effectiveness on live cells of laser driven protons
at dose rates exceeding 109Gy/s. AIP Advances. 2:0112092012.
View Article : Google Scholar
|
|
37
|
Laschinsky L, Baumann M, Beyreuther E,
Enghardt W, Kaluza M, Karsch L, Lessmann E, Naumburger D, Nicolai
M, Richter C, et al: Radiobiological effectiveness of laser
accelerated electrons in comparison to electron beams from a
conventional linear accelerator. J Radiat Res. 53:395–403. 2012.
View Article : Google Scholar
|
|
38
|
Beddok A, Fouillade C and Quelennec Enad
Favaudon V: OC-0030:: In vitro study of FLASH vs. conventional
dose-rate irradiation: Cell viability and DNA damage repair.
Radiotherapy and Oncology. 123:S9–S10. 2017. View Article : Google Scholar
|
|
39
|
Buonanno M, Grilj V and Brenner DJ:
Biological effects in normal cells exposed to FLASH dose rate
protons. Radiother Oncol. 139:51–55. 2019. View Article : Google Scholar
|
|
40
|
Venkatesulu BP, Sharma A, Pollard-Larkin
JM, Sadagopan R, Symons J, Neri S, Singh PK, Tailor R, Lin SH and
Krishnan S: Ultra high dose rate (35 Gy/sec) radiation does not
spare the normal tissue in cardiac and splenic models of
lymphopenia and gastrointestinal syndrome. Sci Rep. 9:171802019.
View Article : Google Scholar
|
|
41
|
Kiefer J and Ebert M: The effect of
ultra-high dose-rate beta-ray irradiation in aerobic and hypoxic
conditions on the survival of diploid yeast. Biophysik. 6:271–274.
1970. View Article : Google Scholar
|
|
42
|
Zlobinskaya O, Dollinger G, Michalski D,
Hable V, Greubel C, Du G, Multhoff G, Röper B, Molls M and Schmid
TE: Induction and repair of DNA double-strand breaks assessed by
gamma-H2AX foci after irradiation with pulsed or continuous proton
beams. Radiat Environ Biophys. 51:23–32. 2012. View Article : Google Scholar
|
|
43
|
Hanton F, Chaudhary P, Doria D, Gwynne D,
Maiorino C, Scullion C, Ahmed H, Marshall T, Naughton K, Romagnani
L, et al: DNA DSB repair dynamics following irradiation with
laser-driven protons at ultra-high dose rates. Sci Rep. 9:44712019.
View Article : Google Scholar
|
|
44
|
Prise KM, Schettino G, Folkard M and Held
KD: New insights on cell death from radiation exposure. Lancet
Oncol. 6:520–528. 2005. View Article : Google Scholar
|
|
45
|
Desouky O, Ding N and Zhou G: Targeted and
non-targeted effects of ionizing radiation. J Radiat Res Appl Sci.
8:247–254. 2015.
|
|
46
|
Kim W, Lee S, Seo D, Kim D, Kim K, Kim E,
Kang J, Seong KM, Youn H and Youn B: Cellular stress responses in
radiotherapy. Cells. 8:11052019. View Article : Google Scholar
|
|
47
|
Jeggo PA and Löbrich M: DNA double-strand
breaks: Their cellular and clinical impact? Oncogene. 26:7717–7719.
2007. View Article : Google Scholar
|
|
48
|
Nikjoo H, Emfietzoglou D, Liamsuwan T,
Taleei R, Liljequist D and Uehara S: Radiation track, DNA damage
and response-a review. Rep Prog Phys. 79:1166012016. View Article : Google Scholar
|
|
49
|
Purrott RJ and Reeder EJ: Chromosome
aberration yields induced in human lymphocytes by 15 MeV electrons
given at a conventional dose-rate and in microsecond pulses. Int J
Radiat Biol Relat Stud Phys Chem Med. 31:251–256. 1977. View Article : Google Scholar
|
|
50
|
Spitz DR, Buettner GR, Petronek MS,
St-Aubin JJ, Flynn RT, Waldron TJ and Limoli CL: An integrated
physico-chemical approach for explaining the differential impact of
FLASH versus conventional dose rate irradiation on cancer and
normal tissue responses. Radiother Oncol. 139:23–27. 2019.
View Article : Google Scholar
|
|
51
|
Benfeitas R, Uhlen M, Nielsen J and
Mardinoglu A: New challenges to study heterogeneity in cancer redox
metabolism. Front Cell Dev Biol. 5:652017. View Article : Google Scholar
|
|
52
|
Vozenin MC, De Fornel P, Petersson K,
Favaudon V, Jaccard M, Germond JF, Petit B, Burki M, Ferrand G,
Patin D, et al: The advantage of FLASH radiotherapy confirmed in
mini-pig and cat-cancer patients. Clin Cancer Res. 25:35–42. 2019.
View Article : Google Scholar
|
|
53
|
Beyreuther E, Brand M, Hans S, Hideghéty
K, Karsch L, Leßmann E, Schürer M, Szabó ER and Pawelke J:
Feasibility of proton FLASH effect tested by zebrafish embryo
irradiation. Radiother Oncol. 139:46–50. 2019. View Article : Google Scholar
|
|
54
|
Gao F, Yang Y, Zhu H, Wang J, Xiao D, Zhou
Z, Dai T, Zhang Y, Feng G, Li J, et al: First demonstration of the
FLASH effect with ultrahigh dose rate high-energy X-rays. Radiother
Oncol. 166:44–50. 2022. View Article : Google Scholar
|
|
55
|
Montay-Gruel P, Petersson K, Jaccard M,
Boivin G, Germond JF, Petit B, Doenlen R, Favaudon V, Bochud F,
Bailat C, et al: Irradiation in a flash: Unique sparing of memory
in mice after whole brain irradiation with dose rates above
100Gy/s. Radiother Oncol. 124:365–369. 2017. View Article : Google Scholar
|
|
56
|
Montay-Gruel P, Acharya MM, Goncalves
Jorge P, Petit B, Petridis IG, Fuchs P, Leavitt R, Petersson K,
Gondré M, Ollivier J, et al: Hypofractionated FLASH-RT as an
effective treatment against glioblastoma that reduces
neurocognitive side effects in mice. Clin Cancer Res. 27:775–784.
2021. View Article : Google Scholar
|
|
57
|
Simmons DA, Lartey FM, Schuler E, Rafat M,
King G, Kim A, Ko R, Semaan S, Gonzalez S, Jenkins M, et al:
Reduced cognitive deficits after FLASH irradiation of whole mouse
brain are associated with less hippocampal dendritic spine loss and
neuroinflammation. Radiother Oncol. 139:4–10. 2019. View Article : Google Scholar
|
|
58
|
Alaghband Y, Cheeks SN, Allen BD,
Montay-Gruel P, Doan NL, Petit B, Jorge PG, Giedzinski E, Acharya
MM, Vozenin MC and Limoli CL: Neuroprotection of radiosensitive
juvenile mice by ultra-high dose rate FLASH irradiation. Cancers
(Basel). 12:16712020. View Article : Google Scholar
|
|
59
|
Allen BD, Acharya MM, Montay-Gruel P,
Jorge PG, Bailat C, Petit B, Vozenin MC and Limoli C: Maintenance
of tight junction integrity in the absence of vascular dilation in
the brain of mice exposed to ultra-high-dose-rate FLASH
irradiation. Radiat Res. 194:625–635. 2020. View Article : Google Scholar
|
|
60
|
Loo BW, Schuler E, Lartey FM, Rafat M,
King GJ, Trovati S, Koong AC and Maxim PG: Delivery of ultra-rapid
flash radiation therapy and demonstration of normal tissue sparing
after abdominal irradiation of mice: International Journal of
Radiation Oncology*Biology*Physics. 98:pE162017.
|
|
61
|
Levy K, Natarajan S, Wang J, Chow S,
Eggold JT, Loo PE, Manjappa R, Melemenidis S, Lartey FM, Schüler E,
et al: Abdominal FLASH irradiation reduces radiation-induced
gastrointestinal toxicity for the treatment of ovarian cancer in
mice. Sci Rep. 10:216002020. View Article : Google Scholar
|
|
62
|
Diffenderfer ES, Verginadis II, Kim MM,
Shoniyozov K, Velalopoulou A, Goia D, Putt M, Hagan S, Avery S, Teo
K, et al: Design, implementation, and in vivo validation of a novel
proton FLASH radiation therapy system. Int J Radiat Oncol Biol
Phys. 106:440–448. 2020. View Article : Google Scholar
|
|
63
|
Soto LA, Casey KM, Wang J, Blaney A,
Manjappa R, Breitkreutz D, Skinner L, Dutt S, Ko RB, Bush K, et al:
FLASH irradiation results in reduced severe skin toxicity compared
to conventional-dose-rate irradiation. Radiat Res. 194:618–624.
2020. View Article : Google Scholar
|
|
64
|
Cunningham S, McCauley S, Vairamani K,
Speth J, Girdhani S, Abel E, Sharma RA, Perentesis JP, Wells SI,
Mascia A and Sertorio M: FLASH proton pencil beam scanning
irradiation minimizes radiation-induced leg contracture and skin
toxicity in mice. Cancers (Basel). 13:10122021. View Article : Google Scholar
|
|
65
|
Chabi S, To THV, Leavitt R, Poglio S,
Jorge PG, Jaccard M, Petersson K, Petit B, Roméo PH, Pflumio F, et
al: Ultra-high-dose-rate FLASH and conventional-dose-rate
irradiation differentially affect human acute lymphoblastic
leukemia and normal hematopoiesis. Int J Radiat Oncol Biol Phys.
109:819–829. 2021. View Article : Google Scholar
|
|
66
|
Konradsson E, Arendt ML, Bastholm Jensen
K, Børresen B, Hansen AE, Bäck S, Kristensen AT, Munck Af
Rosenschöld P, Ceberg C and Petersson K: Establishment and initial
experience of clinical FLASH radiotherapy in canine cancer
patients. Front Oncol. 11:6580042021. View Article : Google Scholar
|
|
67
|
Smyth LML, Donoghue JF, Ventura JA,
Livingstone J, Bailey T, Day LRJ, Crosbie JC and Rogers PAW:
Comparative toxicity of synchrotron and conventional radiation
therapy based on total and partial body irradiation in a murine
model. Sci Rep. 8:120442018. View Article : Google Scholar
|
|
68
|
Zhou S, Zheng D, Fan Q, Yan Y, Wang S, Lei
Y, Besemer A, Zhou C and Enke C: Minimum dose rate estimation for
pulsed FLASH radiotherapy: A dimensional analysis. Med Phys.
47:3243–3249. 2020. View Article : Google Scholar
|
|
69
|
Bourhis J, Montay-Gruel P, Gonçalves Jorge
P, Bailat C, Petit B, Ollivier J, Jeanneret-Sozzi W, Ozsahin M,
Bochud F, Moeckli R, et al: Clinical translation of FLASH
radiotherapy: Why and how? Radiother Oncol. 139:11–17. 2019.
View Article : Google Scholar
|
|
70
|
Vozenin MC, Hendry JH and Limoli CL:
Biological benefits of ultra-high dose rate FLASH radiotherapy:
Sleeping beauty awoken. Clin Oncol (R Coll Radiol). 31:407–415.
2019. View Article : Google Scholar
|
|
71
|
Rahman M, Trigilio A, Frenciosini G,
Moeckli R, Zhang R and Böhlen TT: FLASH radiotherapy treatment
planning and models for electron beams. Radiother Oncol.
175:210–221. 2022. View Article : Google Scholar
|
|
72
|
van Marlen P, Dahele M, Folkerts M, Abel
E, Slotman BJ and Verbakel WFAR: Bringing FLASH to the Clinic:
Treatment planning considerations for ultrahigh dose-rate proton
beams. Int J Radiat Oncol Biol Phys. 106:621–629. 2020. View Article : Google Scholar
|
|
73
|
Jolly S, Owen H, Schippers M and Welsch C:
Technical challenges for FLASH proton therapy. Phys Med. 78:71–82.
2020. View Article : Google Scholar
|
|
74
|
McManus M, Romano F, Lee ND, Farabolini W,
Gilardi A, Royle G, Palmans H and Subiel A: The challenge of
ionisation chamber dosimetry in ultra-short pulsed high dose-rate
Very High Energy Electron beams. Sci Rep. 10:90892020. View Article : Google Scholar
|
|
75
|
Jorge PG, Jaccard M, Petersson K, Gondré
M, Durán MT, Desorgher L, Germond JF, Liger P, Vozenin MC, Bourhis
J, et al: Dosimetric and preparation procedures for irradiating
biological models with pulsed electron beam at ultra-high
dose-rate. Radiother Oncol. 139:34–39. 2019. View Article : Google Scholar
|
|
76
|
Petersson K, Jaccard M, Germond JF,
Buchillier T, Bochud F, Bourhis J, Vozenin MC and Bailat C: High
dose-per-pulse electron beam dosimetry-A model to correct for the
ion recombination in the Advanced Markus ionization chamber. Med
Phys. 44:1157–1167. 2017. View Article : Google Scholar
|
|
77
|
Oraiqat I, Zhang W, Litzenberg D, Lam K,
Ba Sunbul N, Moran J, Cuneo K, Carson P, Wang X and El Naqa I: An
ionizing radiation acoustic imaging (iRAI) technique for real-time
dosimetric measurements for FLASH radiotherapy. Med Phys.
47:5090–5101. 2020. View Article : Google Scholar
|