Introduction
Liver cancer has become one of the leading
malignancies worldwide, with a continuous increase in annual
incidence from 1990 to 2015 (1). It
has been estimated that incident cases of liver cancer will exceed
1 million by 2025 (2).
Hepatocellular carcinoma represents the most common form of liver
cancer and comprises 75–85% of total liver cancer cases (3,4).
Hepatic disorders due to unhealthy lifestyles and hepatitis B virus
infection are becoming the major etiologies of liver cancer
(5,6). For instance, there is an increased
risk of liver cancer development in patients with non-alcoholic
steatohepatitis (7). Surgical
resection together with adjuvant chemotherapy remains as the
mainstay of liver cancer treatment; however, postoperative
recurrence and metastasis can seriously undermine the prognosis of
patients with liver cancer (8).
According to the World Health Organization, the recurrence rate of
liver cancer at 2 years post-surgery in 2018 was as high as 61.6%
(9). Currently, there is a lack of
therapeutic strategies for the prevention and treatment of liver
cancer metastasis (10).
Neo-angiogenesis is a key feature in the clinical
progression of liver cancer. The hypervascular nature of hepatic
tumors implies the significance of neo-vascularization in the
pathophysiological progression of these tumors (11). Several angiogenic pathways have been
found to be dysregulated in liver cancer. Tumor cells can secrete
pro-angiogenic factors, including vascular endothelial growth
factor A (VEGFA), angiopoietin 2 (Ang2), fibroblast growth factor 2
(FGF2) and platelet-derived growth factor A (PDGFA), which bind to
receptors expressed on endothelial cells to promote angiogenesis
(12). Hypoxia has been recognized
as an important factor that upregulates expression of VEGFA, FGF2
and PDGFA in tumor cells (13).
Moreover, overexpression of extracellular matrix remodelers such as
matrix metalloproteinases (MMP)2 and 9 can contribute to
neo-angiogenesis, local tissue invasion and metastasis (14,15).
Furthermore, production of inflammatory cytokines such as tumor
necrosis factor (TNF)-α and interleukin (IL)-1β is also implicated
in the angiogenesis and invasion of liver cancer (16–18).
As hyper-vascularization facilitates tumor growth, tissue invasion
and metastasis, anti-angiogenic agents have been proposed to
normalize the tumor vasculature and improve the efficacy of other
treatments such as chemotherapy and radiation (19).
Plasmodium is a single-cell protozoan
responsible for malaria which inhabits hepatocytes to enter a
dormant state, and the subsequent reproduction of merozoites leads
to hepatocyte rupture (20).
Accumulating evidence suggests that Plasmodium infection
suppresses tumor growth and metastasis in a murine Lewis lung
cancer model (21,22). There is also evidence that malaria
incidence and cancer mortality are inversely associated (23). Furthermore, Plasmodium
infection could curtail recurrence and metastasis of liver cancer
by suppressing epithelial-mesenchymal transition (24). Although Plasmodium infection
has been reported to inhibit angiogenesis by modulating the
infiltration of tumor-associated macrophages in liver cancer
(25), the effect of
Plasmodium infection on tumor cell-derived angiogenic
signaling remains unclear.
The present study established a murine model of
implanted HepG2 cells and assessed the impact of Plasmodium
infection on vascularization and tumorigenesis. HepG2 cells were
injected into the left liver lobe of nude mice as a model of in
situ hepatic tumorigenesis. Plasmodium yoelii
parasitized erythrocytes were administered in the animal model of
liver cancer to introduce Plasmodium infection. The tumor
growth and microvascular density were determined in the presence or
absence of Plasmodium infection. The expression levels of
hypoxia-inducible factor 1α (HIF-1α) and angiogenesis-related
factors were evaluated using western blotting and reverse
transcription-quantitative PCR analysis.
Materials and methods
Animal model of liver cancer and
Plasmodium infection
BALB/c nude mice (male; 4–5 weeks old; weight, 20–25
g; n=40 mice; n=15 for Plasmodium propagation; n=25 for
in situ liver cancer model) were purchased from Shanghai
Laboratory Animal Center Co., Ltd. and raised at a standard
specific pathogen-free facility with a 12 h-light/dark cycle. All
animal experiments described in the present study were performed in
collaboration with Yunnan Bestai Biotechnology Co., Ltd., a
qualified animal research facility. The animals were housed under
controlled conditions with a temperature of 22±2°C, relative
humidity of 50–60% and a 12-h light/dark cycle. They had free
access to standard laboratory food and water ad libitum. The
animal protocols in the present study were approved by the
Experimental Animal Ethics Committee of Yunnan Bestai Biotechnology
Co (Kunming, China; approval no. BST-MICE-20221229-01).
HepG2 cells were purchased from The Cell Bank of
Type Culture Collection of The Chinese Academy of Sciences, and
cultured in DMEM high glucose medium containing 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.), 100 U/ml penicillin and 100 µg/ml
streptomycin (Beyotime Institute of Biotechnology) in a humidified
incubator containing 5% CO2 at 37°C. This cell line was
authenticated by the supplier through STR profiling.
To stably express HIF-1α, HepG2 cells were infected
with a lentivirus carrying the cDNA of HIF-1α (synthesized by
GenScript Biotech Corporation). The empty lentiviral vector was
used as a negative control. Lentivirus was prepared in 293T cells
in a 10 cm dish at 70–80% confluence. 293T cells were transfected
with 12 µg pLX304-HIF-1α expression plasmid and 12 µg
MISSION® Lentiviral Packaging Mix (cat. no. SHP001;
Sigma-Aldrich; Merck KGaA) using Lipofectamine™ 3000 transfection
reagent (Invitrogen™; Thermo Fisher Scientific, Inc.) for 48 h at
37°C. The lentivirus-containing supernatant was collected 48 h
post-transfection, filtered through a 0.45 µm filter to remove
cells and debris, and used to transduce HepG2 cells at an
multiplicity of infection of 10 in the presence of 8 µg/ml
polybrene. After 48 h, transduced HepG2 cells overexpressing HIF-1α
were selected with 10 µg/ml blasticidin for 2 weeks to eliminate
uninfected cells, and HIF-1α overexpression was confirmed by
western blotting, as described below.
The Plasmodium yoelii (Py) nonlethal strain
(Py17XNL) was purchased from the Malaria Research and Reference
Reagent Resource Center, and propagated in 8-week old BALB/c mice
(male; weight, 30–35 g; housed as aforementioned) at an initial
injection dose of 5×105 infected red blood cells
(25). To isolate parasitized
erythrocytes, blood was collected from the donor mice with >20%
parasitemia via cardiac puncture under anesthesia with 240 mg/kg
Avertin (1.25% Tribromoethanol) through intraperitoneal injection.
Animals were euthanized after blood collection via carbon dioxide
asphyxiation, followed by cervical dislocation. The blood was
immediately transferred to heparinized tubes to prevent
coagulation. Erythrocytes were separated from whole blood by
centrifugation at 800 × g for 10 min at 4°C. The erythrocyte pellet
was washed twice with sterile PBS, resuspended in sterile saline,
and counted using a hemocytometer. The blood parasitemia level in
the donor mice (% red blood cells infected by the malaria parasite)
was determined by examining thin blood smears with a light
microscope (Olympus BX53; Olympus Corporation) at 1,000×
magnification under oil immersion. Blood samples with parasitemia
level >20% were used to prepare a desirable amount of
parasitized erythrocytes for further infection. Based on the
parasitemia level determined by the blood smear examination, the
appropriate volume of erythrocyte suspension containing
5×105 parasitized erythrocytes was calculated and
diluted to a final volume of 100–200 µl with sterile saline for
intraperitoneal injection into the recipient mice.
A total of 2×106 HepG2 cells/animal were
injected into the left liver lobe of nude mice as an in situ
tumor growth model of liver cancer (26–28),
after anesthetization with 240 mg/kg Avertin (1.25%
Tribromoethanol) through intraperitoneal injection. A total of 7
days after tumor cell injection, the mice were intraperitoneally
injected with 5×105 parasitized erythrocytes or
uninfected erythrocytes (control) from the donor mice. On day 17
post-parasite injection, the mice were sacrificed for tissue
collection. For euthanasia, a chamber was connected to a carbon
dioxide cylinder with a flow rate to displace 40% of the cage
volume/minute. Mice were placed into the euthanizing chamber for 10
min until no movement was observed. Animal death was further
assured by subsequent cervical dislocation. All the animals
(including the donor mice) were euthanized by the same method. A
total of 15 donor BALB/c mice were used for Py propagation and
erythrocyte collection. For tumor formation experiments, a total of
25 BALB/c nude mice were used, in which 5 mice were used for the
initial in situ tumor model validation experiment. The
remaining 20 mice were divided into 4 groups with 5 animals/group
as follows: i) Sham (normal liver group); ii) HepG2 injection; iii)
HepG2 + Py; and iv) HepG2 (HIF-1α) + Py.
Animal health and behavior were monitored daily
throughout the experiment, with increased frequency to twice daily
following tumor cell injection and Plasmodium infection.
Trained personnel handled the animals to reduce stress and
discomfort, and adequate anesthesia was provided for all surgical
procedures. Welfare considerations included providing environmental
enrichment, maintaining appropriate housing conditions, and
minimizing handling stress. Animal health status was regularly
monitored, and humane endpoints were established and implemented
promptly, including euthanasia if animals showed signs of severe
illness, significant weight loss (>20% initial body weight), a
tumor size >2 cm, or any conditions causing obvious pain or
distress that could not be alleviated by analgesics.
To accurately measure tumor weight, the present
study used a meticulous isolation process. After harvesting the
livers, visible tumor nodules were carefully excised using sterile
surgical instruments. Fine dissection was then performed under a
dissecting microscope to remove any remaining normal liver tissue
from the tumor mass. The isolated tumor tissue was gently washed in
cold PBS to remove blood and debris, then carefully blotted on
sterile filter paper to remove excess fluid. Immediately after this
preparation, the cleaned and dried tumor tissue was weighed using a
high-precision analytical balance (ME204E; Mettler Toledo). This
process was repeated for all tumor nodules in each liver, with the
total weight of tumor tissue from each liver recorded for
subsequent comparisons between groups.
Immunohistochemistry (IHC) of
CD31
Immunohistochemical staining of CD31 was performed
using 5-µm sections of formalin-fixed paraffin-embedded tumor
tissues using the VENTANA BenchMark Special Stain system (Roche
Diagnostics). Tissues were fixed in 10% neutral buffered formalin
at 4°C for 24 h before paraffin embedding. After deparaffinization
in xylene (3 changes, 5 min each) at 60°C and hydration through a
descending ethanol series (100, 95, 80 and 70%; 5 min each),
followed by washing in distilled water for 5 min, antigen retrieval
was performed using a citrate unmasking solution (10X;
SignalStain® Citrate Unmasking Solution; cat. no. 14746;
Cell Signaling Technology, Inc.) for 10 min at a sub-boiling
temperature (95–98°C). After cooling, the sections were washed in
distilled H2O and incubated in 3% hydrogen peroxide for
10 min at 37°C. The sections were then washed three times in TBST
buffer (Tris-buffered saline with 0.1% Tween-20; 5 min each time),
and blocked for 1 h at room temperature in TBST with 5% normal goat
serum (Cell Signaling Technologies, Inc.). Anti-CD31 antibodies
(1:100; cat. no. ab124432; Abcam) was applied to stain the sections
overnight at 4°C. After washing, the sections were incubated with 3
drops of SignalStain® Boost IHC Detection Reagent
(horseradish peroxidase, rabbit; cat. no. 8114; Cell Signaling
Technology, Inc.) for 30 min at room temperature. Signal
development was performed using 200 µl SignalStain® DAB
Substrate Kit (cat. no. 8059; Cell Signaling Technology, Inc.) for
5 min. After washing and dehydration, the sections were mounted
with coverslips using mounting medium (cat. no. 14177; Cell
Signaling Technology, Inc.). Images were captured under a Leica
DMI6000 microscope (Leica Microsystems GmbH) at ×200
magnification.
Giemsa staining
Liver tumor tissues were fixed in 4%
paraformaldehyde overnight at 4°C and then cut into 50-µm sections.
Sections were treated with 3% hydrogen peroxide and 0.25% Triton
X-100 in 1X TBS for 30 min. Tissue sections were then stained with
the Giemsa Stain Kit (cat. no. ab150670; Abcam) at room temperature
(22–25°C) for 15 min, based on the supplier's instructions. Nuclei
were counterstained with hematoxylin solution at room temperature
(22–25°C) for 15 min. Images were captured under a Leica DMI6000
microscope (Leica Microsystems GmbH).
Terminal deoxynucleotidyl transferase
dUTP nick end labeling (TUNEL)
Cell death events in tumor tissues were assessed
using the Biotin TUNEL Staining Kit (cat. no. T2191, Beijing
Solarbio Science & Technology Co., Ltd.). Tissue samples were
fixed in 10% neutral buffered formalin at 4°C for 24 h. The fixed
tissues were then dehydrated and paraffin-embedded following
standard histological procedures. Paraffin-embedded tumor tissues
were processed into 5-µm sections. After deparaffinization in
xylene (3 changes, 5 min) at 60°C and hydration through a
descending ethanol series (100, 95, 80 and 70%; 5 min), followed by
washing in distilled water for 5 min, tissue sections were
incubated with 20 µg/ml Protease K at 37°C for 15 min, followed by
treatment with 3% hydrogen peroxide at room temperature for 10 min.
The sections were then labeled with a working solution containing
TdT enzyme, Biotin-dUTP and a Biotin labeling solution at 37°C for
1 h. After washing with TBST buffer (Tris-buffered saline with 0.1%
Tween-20; 5 min each time), the sections were incubated with a
Streptavidin-horseradish peroxidase solution at room temperature
for 30 min. The staining signal was developed using 0.2 ml DAB
reagent at room temperature for 2–5 min. After dehydration with 95%
ethanol, the sections were treated with xylene for 5 min before
observation. The nuclei were then counterstained with hematoxylin
(0.1% w/v) for 5 min at room temperature. After rinsing in running
tap water for 5 min, sections were dehydrated through an ascending
ethanol series, cleared in xylene and mounted using DPX mounting
medium (Sigma-Aldrich). Stained sections were observed using an
Olympus BX53 light microscope. A total of five random fields of
view were examined for each section at 400× magnification.
Reverse transcription
(RT)-quantitative PCR (qPCR) analysis
RNA samples were extracted from tissues using Trizol
reagent (Beyotime Institution of Biotechnology). A total of 1 µg
RNA sample was reverse transcribed into complementary DNA using the
PrimeScript™ RT Reagent Kit (cat. no. RR037A; Takara Bio, Inc.).
The reverse transcription reaction was performed under the
following conditions: 37°C for 15 min (reverse transcription),
followed by 85°C for 5 sec (inactivation of reverse transcriptase)
and then cooled to 4°C.. qPCR was performed using the 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc.) using the SYBR premix master mix (cat. no. SR1110; Beijing
Solarbio Science & Technology Co., Ltd.). The PCR cycling
conditions were as follows: Initial denaturation at 95°C for 30
sec, followed by 40 cycles of denaturation at 95°C for 5 sec and
annealing/extension at 60°C for 30 sec. Relative gene expression
was determined by the 2−ΔΔCq method (29), using β-actin as the reference gene.
For human genes, the following primers were used: MMP-2, (forward)
5′-CCCTTTGACGGTAAGGACGGACTC-3′ and (reverse)
5′-GCCCTGGAAGCGGAATGGAA-3′; MMP-9, (forward)
5′-CTATGGTCCTCGCCCTGAACCTG-3′ and (reverse)
5′-AAGGCACAGTAGTGGCCGTAGAAGG-3′; IL-1β, (forward)
5′-CCGACCACCACTACAGCAAG-3′ and (reverse)
5′-ATGGACCAGACATCACCAAGC-3′; IL-6, (forward)
5′-ATGTGTGAAAGCAGCAAAGAGGCAC-3′ and (reverse)
5′-GTGCCTCTTTGCTGCTTTCACACAT-3′; TNF-α, (forward)
5′-CCCGAGTGACAAGCCTGTAGCC-3′ and (reverse)
5′-CCCTTGAAGAGGACCTGGGAGTAGAT-3′; and β-actin, (forward)
5′-CATGTACGTTGCTATCCAGGC-3′ and (reverse)
5′-CTCCTTAATGTCACGCACGAT-3′. For mouse genes, the following primers
were used: MMP-2, (forward) 5′-TCAACGGTCGGGAATACAGCA-3′ and
(reverse) 5′-CCACCCACAGTGGACATAGCG-3′; MMP-9, (forward)
5′-CGGCAACGGAGAAGGCAAAC-3′ and (reverse)
5′-CGTCTATGTCGTCTTTATTCAGAGGGA-3′; IL-1β, (forward)
5′-CTCGTGCTGTCGGACCCAT-3′ and (reverse)
5′-CAGGCTTGTGCTCTGCTTGTGA-3′; IL-6, (forward)
5′-TGCCTTCTTGGGACTGATG-3′ and (reverse)
5′-TCTGGCTTTGTCTTTCTTGTTA-3′; TNF-α, (forward)
5′-ACTCCAGGCGGTGCCTATGTC-3′ and (reverse)
5′-GCTCCTCCACTTGGTGGTTTGT-3′; and β-actin, (forward)
5′-GTGACGTTGACATCCGTAAAGA-3′ and (reverse)
5′-GCCGGACTCATCGTACTCC-3′.
Western blotting
Protein sample extraction was performed using a RIPA
buffer (cat. no. P0013B; Beyotime Institute of Biotechnology),
supplemented with a protease inhibitor cocktail (cat. no. 78430,
Thermo Fisher Scientific, Inc.) on ice for 15 min. Sample
concentration was determined using a BCA assay kit (cat. no. P0012;
Beyotime Institute of Biotechnology). After denaturation, 20 µg of
total protein per lane was separated by 12% SDS-PAGE and then
transferred onto a PVDF membrane. The membrane was probed with the
following primary antibodies: anti-β-actin (1:2,000; cat. no.
ab8227; Abcam), anti-HIF-1α (1:1,000; cat. no. ab51608; Abcam),
anti-VEGFA (1:1,000; cat. no. ab46154; Abcam), and anti-Ang2
(1:1,000; cat. no. ab155106; Abcam) at 4°C overnight. After washing
using TBST with 5% Tween-20 buffer, horseradish
peroxidase-conjugated secondary antibodies (1:2,000; ab205718;
Abcam) were applied at room temperature for 1 h. Signals of protein
bands were developed using the BeyoECL Plus Enhanced
Chemiluminescence Western Blotting Substrate Kit (cat. no. P0018M;
Beyotime Institute of Biotechnology). The western blot results were
semi-quantified by densitometric analysis using ImageJ software
(version 1.53c; National Institutes of Health).
Statistical analysis
Data are presented as mean ± standard deviation.
Data analysis was performed using GraphPad Prism software (version
9.3.1; Dotmatics). Comparisons between two groups were assessed
using an unpaired Student's t-test, and one-way ANOVA was used for
multiple comparisons, followed by Tukey's post hoc test for
pairwise comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Plasmodium infection suppresses the
tumorigenesis of HepG2 cells in nude mice
To assess the effect of Plasmodium on the
tumorigenesis of liver cancer, HepG2 cells were injected into the
left liver lobe of nude mice as a preliminary experiment to
demonstrate in situ tumor formation. Liver tissues were
harvested 7 and 21 days after HepG2 cell injection, with liver
tissues from the non-injected normal group serving as controls.
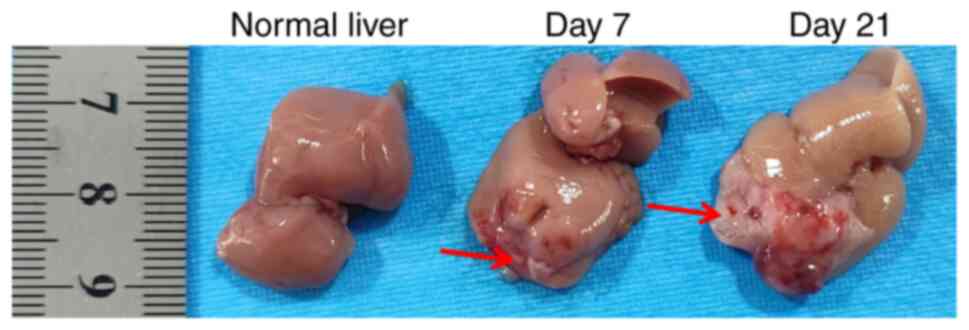
Tumor formation in liver tissues was observed 7 days
post-injection, with increased in situ tumor growth evident
21 days post-injection (Fig. 1). A
total of 7 days after tumor cell injection, the mice were
intraperitoneally injected with Py parasitized erythrocytes (HepG2
+ Py group) or uninfected erythrocytes (as the control: HepG2
group). On day 17 post-parasite injection, the mice were euthanized
and xenograft tumors were collected for histological analysis.
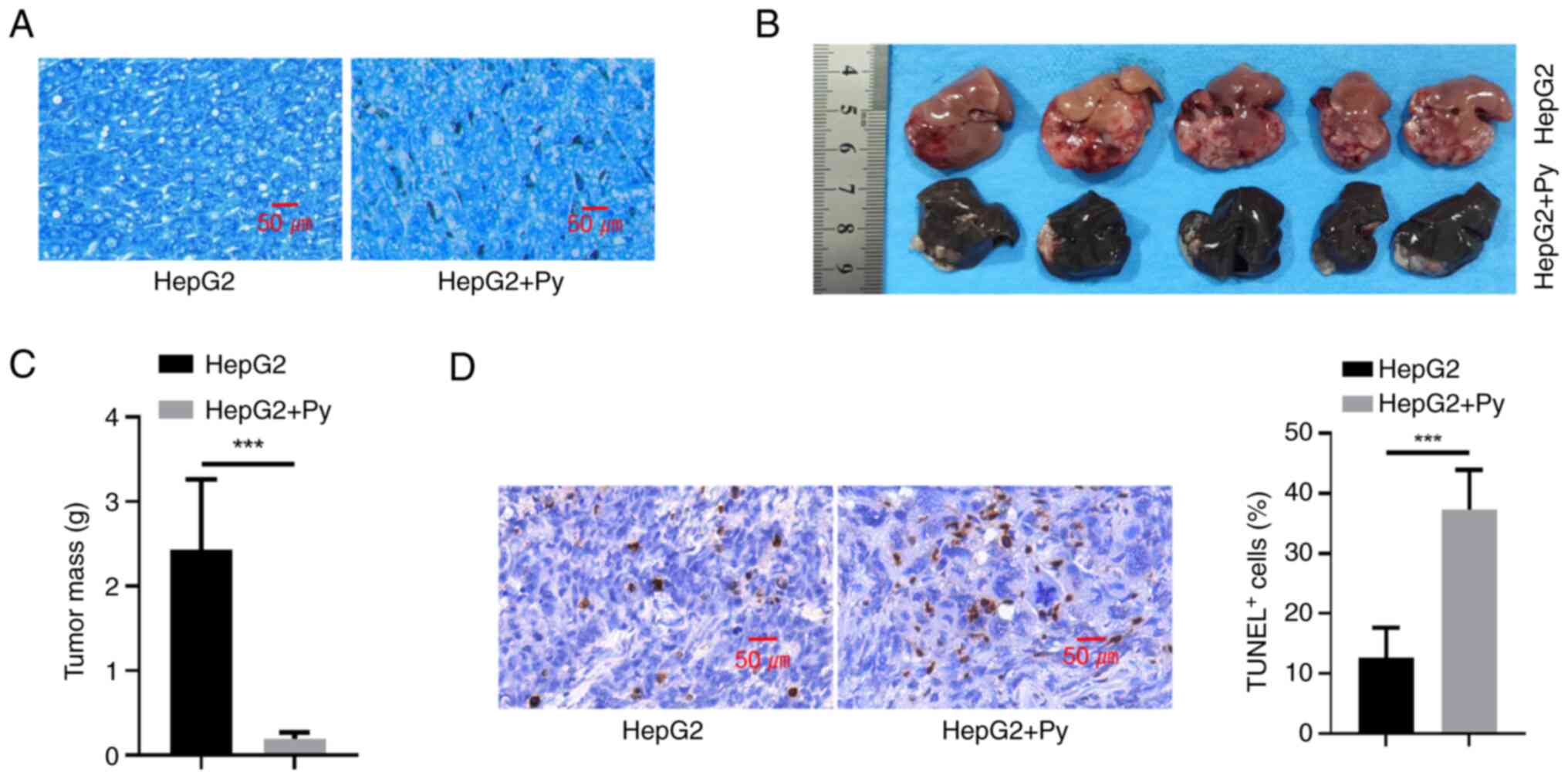
Giemsa staining revealed that in the HepG2 + Py group, infection of
Py was associated with the deposition of hemozoin (the byproduct of
hemoglobin digestion by Plasmodium) (20) in tumor tissues (Fig. 2A). Plasmodium infection also
significantly suppressed tumor formation of HepG2 cells in nude
mice compared with the uninfected control group (Fig. 2B and C). Furthermore, detection of
apoptotic events by TUNEL staining revealed that Plasmodium
infection was associated with a significant increase in the cell
death of tumor tissues, compared with the uninfected control group
(Fig. 2D).
Plasmodium infection attenuates
angiogenesis in tumor and para-cancerous hepatic tissues
Vascularization in tumor tissues of each
experimental group was assessed. Analysis of microvascular density
(MVD) by IHC staining of CD31 revealed that, compared with in
normal hepatic tissues without tumor cell injection, there was a
marked increase in MVD in tumor tissue formed by HepG2 cells.
However, Plasmodium infection notably reduced the MVD in
tumor tissues (Fig. 3A). Western
blot analysis of CD31 expression levels in tumor tissues
demonstrated consistent results with the IHC staining (Fig. 3B). Furthermore, the results revealed
that CD31 expression level in hepatic tissues was significantly
increased after HepG2 cell injection compared with the sham group
without tumor cell injection, whilst Plasmodium infection
significantly suppressed its upregulation (Fig. 3B). Subsequently, the protein levels
of HIF-1α, VEGFA and Ang2, which are implicated in angiogenesis,
were assessed. The findings demonstrated that the protein levels of
HIF-1α, VEGFA and Ang2 were significantly increased in both tumor
and para-cancerous hepatic tissues when compared with hepatic
tissues in the sham group. Infection of Plasmodium
significantly reduced their expression in both tumor and
para-cancerous tissues (Fig. 3C).
Moreover, RT-qPCR analysis of MMP-2, MMP-9 and inflammatory
cytokines (IL-1β, IL-6, TNF-α) also revealed that these genes were
significantly upregulated in both tumor and para-cancerous tissues
from the mice injected with HepG2 cells when compared with the sham
group, whilst Plasmodium infection significantly reduced
their expression in comparison with the group injected with HepG2
cells alone (Fig. 3D). These
results indicate that Plasmodium suppresses
neo-vascularization during tumorigenesis of liver cancer.
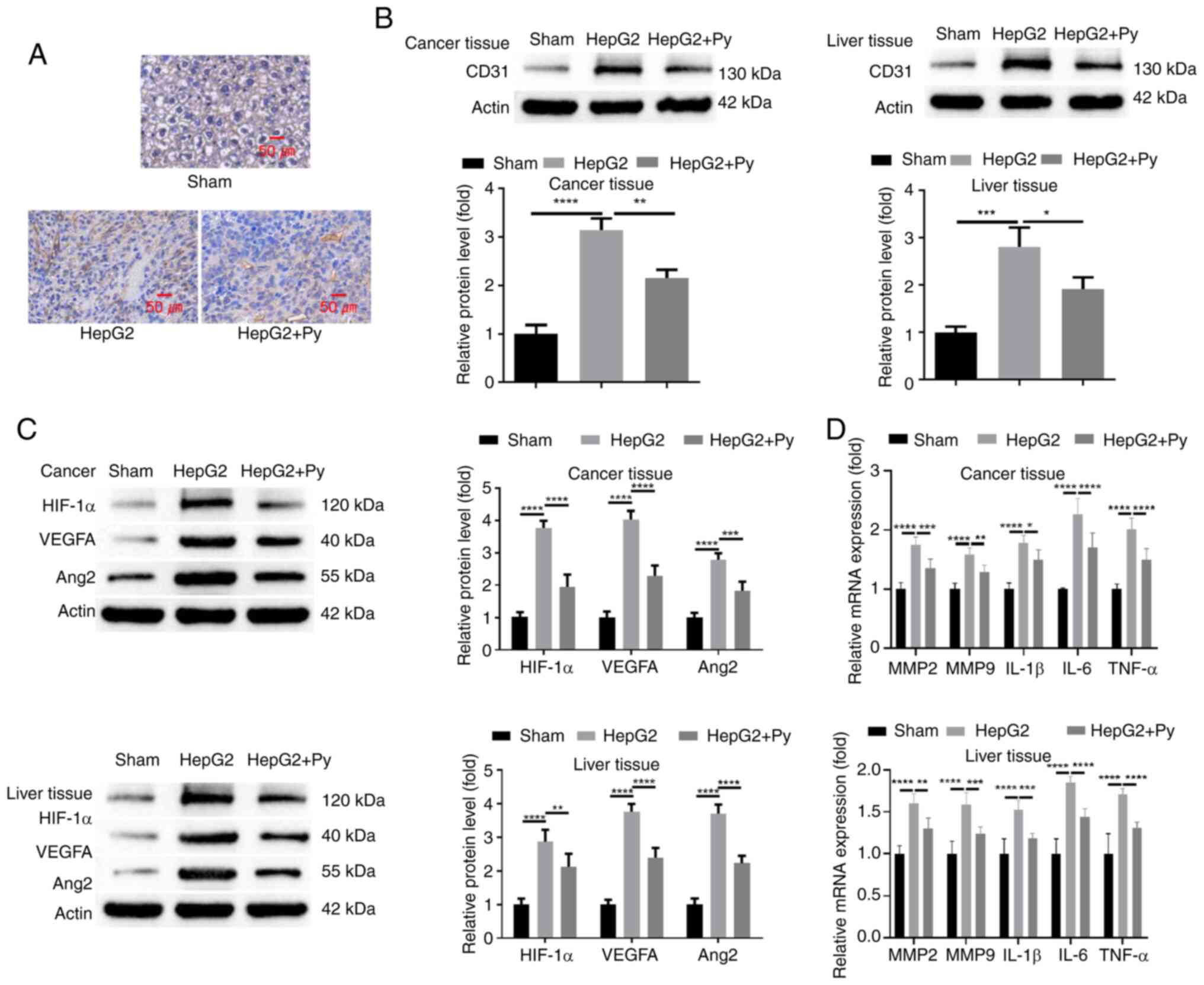 | Figure 3.Plasmodium infection
attenuates angiogenesis in hepatic tumor tissues and para-cancerous
tissues. (A) Analysis of microvascular density by
immunohistochemical staining of CD31 in normal hepatic tissues
(sham group) and tumor tissues with or without Py infection (HepG2
+ Py and HepG2 groups, respectively). Western blot analysis of (B)
CD31 and (C) HIF-1α, VEGFA and Ang2 protein levels in normal
hepatic tissues (sham group), and tumor and para-cancerous hepatic
tissues with or without Py infection. (D) Reverse
transcription-quantitative PCR analysis of MMP-2, MMP-9 and
inflammatory cytokines (IL-1β, IL-6 TNF-α) in normal hepatic
tissues (sham group), and tumor and para-cancerous hepatic tissues
with or without Py infection. Tissues for analysis in the sham
group were normal hepatic tissues without HepG2 cell injection. n=5
in each group. *P<0.05; **P<0.01; ***P<0.001;
****P<0.0001. Py, Plasmodium yoelii; HIF-1α,
hypoxia-inducible factor 1α; VEGFA, vascular endothelial growth
factor A; Ang2, angiopoietin 2; MMP, matrix metalloproteinase; IL,
interleukin; TNF, tumor necrosis factor. |
Plasmodium infection hinders the
tumorigenesis of HepG2 cells by downregulating HIF-1α
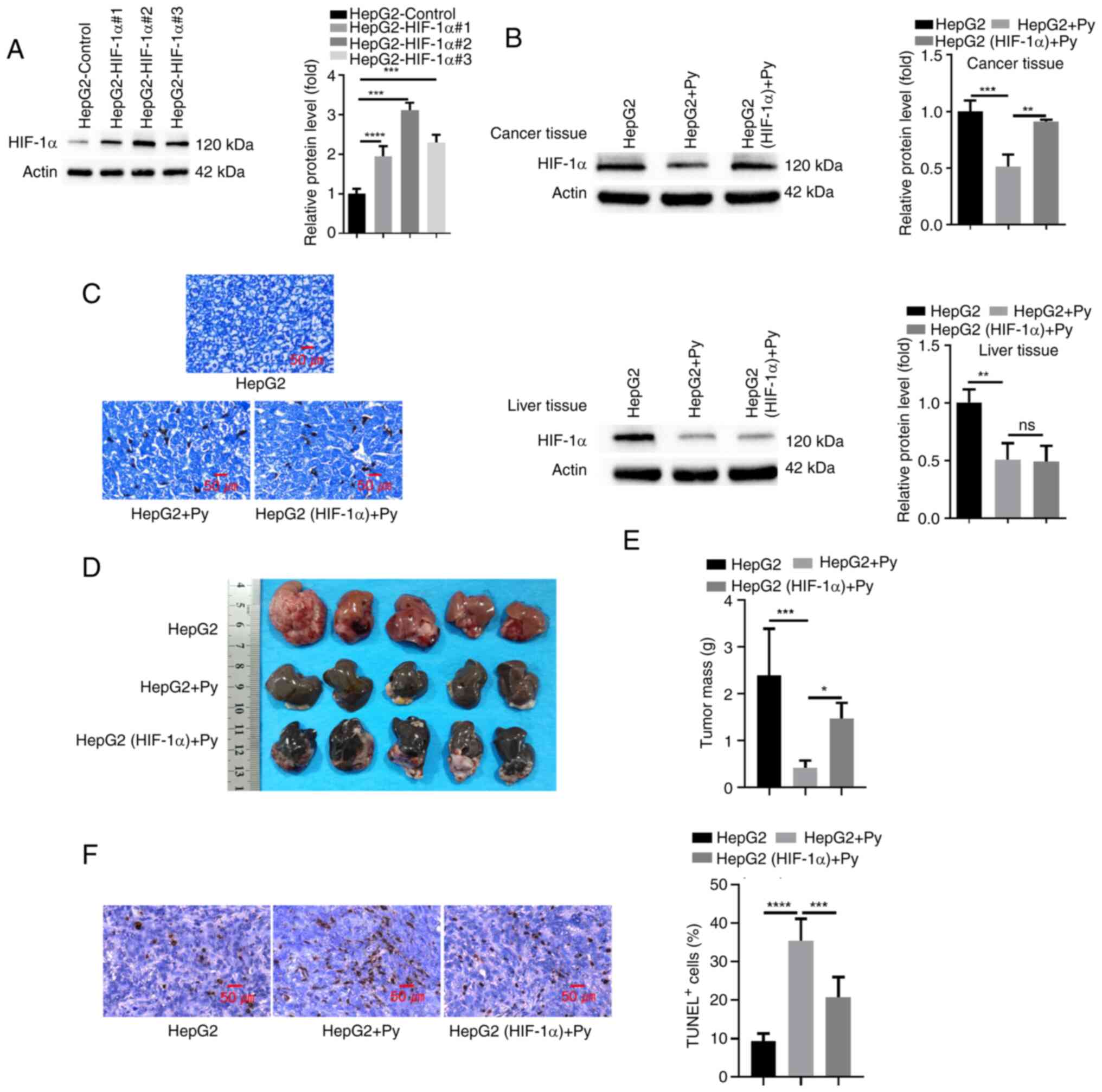
To assess the role of HIF-1α in mediating the effect
of Plasmodium infection on tumorigenesis, HepG2 cells or
cells transduced with a lentivirus expressing HIF-1α were injected
into the left liver lobe of nude mice in the presence or absence of
Plasmodium infection. A total of three different clones of
HepG2 cells transduced with HIF-1α expression lentivirus were
initially generated, and clone 2, which demonstrated the highest
HIF-1α expression, was used for subsequent liver injection
(Fig. 4A). Western blot analysis of
HIF-1α expression in the tumor tissues demonstrated that
Plasmodium infection significantly reduced HIF-1α expression
when compared with the uninfected group, and HIF-1α expression
level was significantly restored in tumor tissues with
lentivirus-mediated HIF-1α overexpression (Fig. 4B). However, HIF-1α expression
remained at a low level in para-cancerous hepatic tissues upon
Plasmodium infection, regardless of the overexpression of
HIF-1α in tumor tissues. Giemsa staining revealed that infection of
Py led to an equal level of hemozoin deposition in tumors with or
without HIF-1α overexpression (Fig.
4C), indicating that HIF-1α expression level did not affect
Plasmodium infection. However, restoration of HIF-1α
expression in HepG2 cells significantly promoted tumor growth under
the condition of Plasmodium infection compared with the
group without ectopic HIF-1α overexpression (Fig. 4D and E). Furthermore, TUNEL staining
in tumor sections revealed that Plasmodium infection
significantly induced cell death in tumor tissues compared with the
group without infection, and restoration of HIF-1α expression
partially rescued this effect when compared with the group without
ectopic HIF-1α overexpression (Fig.
4F). Together, the findings imply that suppression of HIF-1α
expression contributes to the anti-tumorigenic effect of Py
infection.
Plasmodium infection curbs
angiogenesis in hepatic tumor development through targeting
HIF-1α
The present study then evaluated whether HIF-1α
expression regulates vascularization during hepatic tumor
development upon Py infection. Analysis of MVD by anti-CD31 IHC
staining revealed that Plasmodium infection markedly reduced
the MVD in tumor tissues compared with the group without infection,
whilst in the group with HIF-1α overexpression, the level of MVD
was largely restored when compared with the group without ectopic
HIF-1α overexpression (Fig. 5A).
Western blot analysis of CD31 expression levels in tumor tissues of
the different groups demonstrated consistent results with the IHC
staining (Fig. 5B). Furthermore,
the protein levels of angiogenic factors (VEGFA and Ang2), which
were significantly suppressed upon infection with Plasmodium
compared with the group without infection, were significantly
increased after HIF-1α overexpression in the HepG2 cells (Fig. 5C). RT-qPCR analysis of MMP-2, MMP-9
and inflammatory cytokines (IL-1β, IL-6 TNF-α) also revealed that
HIF-1α overexpression significantly reduced the suppressive effect
of Plasmodium infection on their expression when compared
with the group without ectopic HIF-1α overexpression (Fig. 5D). Together, these findings indicate
that Plasmodium suppresses neo-vascularization during
tumorigenesis of liver cancer through targeting HIF-1α.
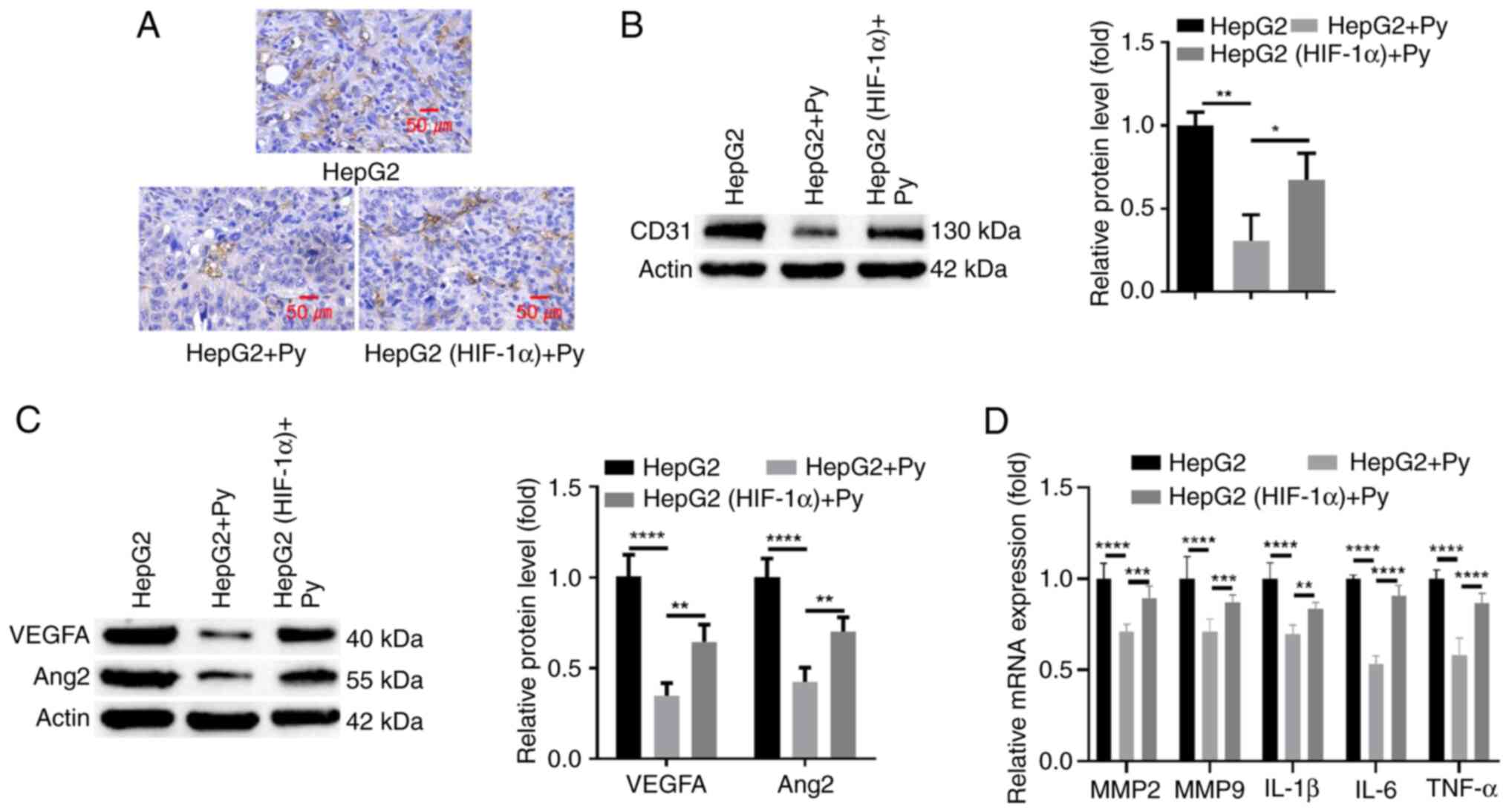 | Figure 5.Plasmodium infection curbs
angiogenesis during hepatic tumor development through targeting
HIF-1α. HepG2 cells or cells transduced with a lentivirus
expressing HIF-1α were injected into the left liver lobe of nude
mice in the presence or absence of Plasmodium infection. (A)
Analysis of microvascular density using immunohistochemical
staining of CD31 in tumor tissue sections. Western blot analysis of
(B) CD31 and (C) HIF-1α, VEGFA and Ang2 protein levels in tumor
tissues. (D) Reverse transcription-quantitative PCR analysis of
MMP-2, MMP-9 and inflammatory cytokines (IL-1β, IL-6 TNF-α) in
tumor tissues. n=5 in each group. *P<0.05; **P<0.01;
***P<0.001; ****P<0.0001. Py, Plasmodium yoelii;
HIF-1α, hypoxia-inducible factor 1α; VEGFA, vascular endothelial
growth factor A; Ang2, angiopoietin 2; MMP, matrix
metalloproteinase; IL, interleukin; TNF, tumor necrosis factor. |
Discussion
The present study demonstrated that
Plasmodium infection could suppress tumorigenesis and
vascularization in a mouse model of implanted HepG2 cells.
Plasmodium infection suppressed the expression of
pro-angiogenic factors (VEGFA and ANG2), MMP2, MMP9 and
inflammatory cytokines (TNF-α, IL-6 and IL-1β), which are
implicated in the angiogenesis and invasion of hepatic tumor cells
(30–33). Moreover, the findings indicate that
downregulation of HIF-1α is associated with the anti-angiogenic
effect of Plasmodium infection. The results of the present
study also demonstrate that Plasmodium infection may be
combined with anti-angiogenic agents to limit vascularization and
progression of liver cancer.
After delivery to the blood by mosquitoes,
Plasmodium parasites can inhabit hepatocytes in a dormant
state or mature and reproduce in hepatocytes, which significantly
alters the physiological features of liver tissues (34,35).
The Plasmodium berghei NK65 strain has been reported to
induce oxidative stress in mouse liver tissues and Plasmodium
chabaudi infection can trigger liver inflammation, and increase
production of serum liver enzymes (36,37).
In addition, Plasmodium falciparum has been reported to
promote anaerobic glycolysis in hepatocytes to produce lactic acid,
which may be linked with the development of hypoglycemia and lactic
acidosis (38).
Global epidemiological analysis has revealed that
the worldwide malaria incidence is inversely associated with
mortality in patients with solid cancer, including lung, breast,
stomach and colon cancer (23). It
has been well-documented that Plasmodium infection could
exert an anticancer effect, especially in liver cancer (39). Plasmodium parasites could
impinge on several regulatory pathways involved in cell survival,
proliferation, autophagy and p53 signaling in liver cells (40), which may underlie the
anti-tumorigenic effect. Furthermore, Plasmodium has been
recognized as an immunomodulatory agent that boosts anticancer
immunity (41). Plasmodium
infection suppresses the release of cytokines and chemokines
responsible for the recruitment of regulatory T cells and
tumor-associated macrophages in tumor tissues, thus ameliorating
the immunosuppressive tumor microenvironment (21,25).
In addition, Plasmodium parasites have been reported to
serve as a cancer vaccine to induce tumor antigen-specific T cell
responses in a murine liver cancer model (42).
The results of the present study also support that
Plasmodium infection could suppress tumorigenesis in a mouse
model of implanted HepG2 cells. As a highly vascularized
malignancy, neo-angiogenesis is critical for tumor growth and
progression of liver cancer (31).
The present study demonstrated that Plasmodium infection
significantly suppressed angiogenesis in hepatic tumor tissues
derived from HepG2 cells in nude mice, which may be a key factor
limiting tumorigenesis of liver cancer cells upon Plasmodium
infection. There are several lines of evidence supporting the
anti-angiogenic effect of Plasmodium infection in liver
cancer. Plasmodium infection could curb tumor angiogenesis
by reducing infiltration of tumor-associated macrophages in a mouse
model of implanted liver cancer cells (25). Furthermore, Plasmodium
infection could modulate expression of microRNAs and long
non-coding RNA (F66), which target the VEGF/VEGFR2 signaling
pathway to limit angiogenesis (22,43).
The present study revealed that Plasmodium
infection reduced the expression levels of HIF-1α in hepatic
tumors, and HIF-1α overexpression restored angiogenesis and tumor
growth under the condition of Plasmodium infection. Hypoxia
is a key hallmark of the tumor microenvironment, which leads to the
activation of hypoxia-induced gene expression and responses by
stabilizing HIF-1α (44).
Activation of HIF-1α signaling has a significant impact on cancer
cell metabolism, but also impinges on vasculature formation
(45). In both physiological and
pathophysiological conditions, HIF-1α serves as a master regulator
of angiogenesis by upregulating pro-angiogenic factors such as
VEGFs (46). The extracellular
matrix remodeling factors (such as MMP-2 and MMP-9) are also
transcriptional targets of HIF-1α (47,48).
Moreover, high HIF-1α expression is associated with a poor
prognosis in patients with liver cancer (49), and activation of the hypoxic
signaling pathway is closely associated with poor prognosis in
patients with liver cancer (50,51).
Therefore, the findings of the present study suggest that
Plasmodium infection could restrain angiogenesis in liver
cancer by curtailing HIF-1α expression. Suppressed angiogenesis may
impair nutrient and oxygen supply to developing tumor tissues and
hinder local invasion of cancerous cells. Future work is warranted
to further dissect the mechanisms by which Plasmodium
infection attenuates HIF-1α expression in liver cancer.
The current study presents intriguing findings that
Plasmodium infection can suppress tumor growth and
angiogenesis in a mouse model of liver cancer, potentially through
downregulation of HIF-1α. However, the use of an immunodeficient
mouse model, the lack of evidence for specific targeting of tumor
cells without affecting normal tissues, the incomplete
understanding of the underlying mechanisms, and the uncertain
translational potential to human malaria parasites limit the
current clinical applicability. Addressing these limitations
through further research using immunocompetent models of hepatic
cancer, comprehensive toxicity analyses, mechanistic studies, and
exploration of attenuated or inactivated parasite forms is crucial
to assess the feasibility and safety of potential
Plasmodium-based anticancer interventions. Furthermore,
although the gene expression of MMP9, MMP8, IL-1B and IL-6 were
analyzed in the present study, these factors were not evaluated at
the protein level, which could provide additional insights into
their functional roles in the observed effect.
In conclusion, the present study demonstrated the
anti-angiogenic and anti-tumorigenic effects of Plasmodium
infection in a mouse model of implanted HepG2 cells. Reduced HIF-1α
expression in hepatic tumor tissues may account for the
anti-angiogenic effect of Plasmodium infection. Furthermore,
Plasmodium parasites may be used jointly with other
anti-angiogenic agents to limit neo-vascularization in liver cancer
treatment.
Acknowledgements
Not applicable.
Funding
The present work was supported by the Yunnan Health Training
Project of High Level Talents (grant no. H-2019039).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YL conceived and designed the study. HC, ML, RW and
XC were responsible for data collection. ML, RW and XC performed
the analysis and interpretation of results. RW, XC and YL prepared
the draft manuscript. RW, XC and YL confirm the authenticity of all
the raw data. All authors read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
The animal protocols in the present study were
approved by the Experimental Animal Ethics Committee of Yunnan
Bestai Biotechnology Co (Kunming, China; approval no.
BST-MICE-20221229-01).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Oh JH and Jun DW: The latest global burden
of liver cancer: A past and present threat. Clin Mol Hepatol.
29:355–357. 2023. View Article : Google Scholar
|
|
2
|
Llovet JM, Kelley RK, Villanueva A, Singal
AG, Pikarsky E, Roayaie S, Lencioni R, Koike K, Zucman-Rossi J and
Finn RS: Hepatocellular carcinoma. Nat Rev Dis Primers. 7:62021.
View Article : Google Scholar
|
|
3
|
Gilles H, Garbutt T and Landrum J:
Hepatocellular Carcinoma. Crit Care Nurs Clin North Am. 34:289–301.
2022. View Article : Google Scholar
|
|
4
|
Ganesan P and Kulik LM: Hepatocellular
carcinoma: New developments. Clin Liver Dis. 27:85–102. 2023.
View Article : Google Scholar
|
|
5
|
Siegel AB and Zhu AX: Metabolic syndrome
and hepatocellular carcinoma: Two growing epidemics with a
potential link. Cancer. 115:5651–5661. 2009. View Article : Google Scholar
|
|
6
|
Jiang Y, Han Q, Zhao H and Zhang J: The
Mechanisms of HBV–Induced Hepatocellular Carcinoma. J Hepatocell
Carcinoma. 8:435–450. 2021. View Article : Google Scholar
|
|
7
|
Powell EE, Wong VW and Rinella M:
Non-alcoholic fatty liver disease. Lancet. 397:2212–2224. 2021.
View Article : Google Scholar
|
|
8
|
Wen T, Jin C, Facciorusso A, Donadon M,
Han HS, Mao Y, Dai C, Cheng S, Zhang B, Peng B, et al:
Multidisciplinary management of recurrent and metastatic
hepatocellular carcinoma after resection: An international expert
consensus. Hepatobiliary Surg Nutr. 7:353–371. 2018. View Article : Google Scholar
|
|
9
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar
|
|
10
|
Lee JC, Hung HC, Wang YC, Cheng CH, Wu TH,
Lee CF, Wu TJ, Chou HS, Chan KM and Lee WC: Risk score model for
microvascular invasion in hepatocellular carcinoma: The Role of
tumor burden and alpha-fetoprotein. Cancers (Basel). 13:44032021.
View Article : Google Scholar
|
|
11
|
Morse MA, Sun W, Kim R, He AR, Abada PB,
Mynderse M and Finn RS: The role of angiogenesis in hepatocellular
carcinoma. Clin Cancer Res. 25:912–920. 2019. View Article : Google Scholar
|
|
12
|
Moawad AW, Szklaruk J, Lall C, Blair KJ,
Kaseb AO, Kamath A, Rohren SA and Elsayes KM: Angiogenesis in
hepatocellular carcinoma; pathophysiology, targeted therapy, and
role of imaging. J Hepatocell Carcinoma. 7:77–89. 2020. View Article : Google Scholar
|
|
13
|
Pinto E, Pelizzaro F, Farinati F and Russo
FP: Angiogenesis and hepatocellular carcinoma: From molecular
mechanisms to systemic therapies. Medicina (Kaunas). 59:11152023.
View Article : Google Scholar
|
|
14
|
Deryugina EI and Quigley JP: Tumor
angiogenesis: MMP-mediated induction of intravasation- and
metastasis-sustaining neovasculature. Matrix Biol. 44–46. 94–112.
2015.
|
|
15
|
Geervliet E and Bansal R: Matrix
Metalloproteinases as potential biomarkers and therapeutic targets
in liver diseases. Cells. 9:12122020. View Article : Google Scholar
|
|
16
|
Hammam O, Mahmoud O, Zahran M, Sayed A,
Salama R, Hosny K and Farghly A: A possible role for TNF-α in
coordinating inflammation and angiogenesis in chronic liver disease
and hepatocellular carcinoma. Gastrointest Cancer Res. 6:107–114.
2013.
|
|
17
|
Pocino K, Stefanile A, Basile V, Napodano
C, D'Ambrosio F, Di Santo R, Callà CAM, Gulli F, Saporito R, Ciasca
G, et al: Cytokines and hepatocellular carcinoma: biomarkers of a
deadly embrace. J Pers Med. 13:52022. View Article : Google Scholar
|
|
18
|
Tak KH, Yu GI, Lee MY and Shin DH:
Association between polymorphisms of interleukin 1 family genes and
hepatocellular carcinoma. Med Sci Monit. 24:3488–3495. 2018.
View Article : Google Scholar
|
|
19
|
Zhu AX, Duda DG, Sahani DV and Jain RK:
HCC and angiogenesis: Possible targets and future directions. Nat
Rev Clin Oncol. 8:292–301. 2011. View Article : Google Scholar
|
|
20
|
Tavares J, Formaglio P, Thiberge S,
Mordelet E, Van Rooijen N, Medvinsky A, Ménard R and Amino R: Role
of host cell traversal by the malaria sporozoite during liver
infection. J Exp Med. 210:905–915. 2013. View Article : Google Scholar
|
|
21
|
Adah D, Yang Y, Liu Q, Gadidasu K, Tao Z,
Yu S, Dai L, Li X, Zhao S, Qin L, et al: Plasmodium infection
inhibits the expansion and activation of MDSCs and Tregs in the
tumor microenvironment in a murine Lewis lung cancer model. Cell
Commun Signal. 17:322019. View Article : Google Scholar
|
|
22
|
Yang Y, Liu Q, Lu J, Adah D, Yu S, Zhao S,
Yao Y, Qin L, Qin L and Chen X: Exosomes from Plasmodium-infected
hosts inhibit tumor angiogenesis in a murine Lewis lung cancer
model. Oncogenesis. 6:e3512017. View Article : Google Scholar
|
|
23
|
Qin L, Chen C, Chen L, Xue R, Ou-Yang M,
Zhou C, Zhao S, He Z, Xia Y, He J, et al: Worldwide malaria
incidence and cancer mortality are inversely associated. Infect
Agent Cancer. 12:142017. View Article : Google Scholar
|
|
24
|
Liang Y, Chen X, Tao Z, Ma M, Adah D, Li
X, Dai L, Ding W, Fanuel S, Zhao S, et al: Plasmodium infection
prevents recurrence and metastasis of hepatocellular carcinoma
possibly via inhibition of the epithelial-mesenchymal transition.
Mol Med Rep. 23:4182021. View Article : Google Scholar
|
|
25
|
Wang B, Li Q, Wang J, Zhao S, Nashun B,
Qin L and Chen X: Plasmodium infection inhibits tumor angiogenesis
through effects on tumor-associated macrophages in a murine
implanted hepatoma model. Cell Commun Signal. 18:1572020.
View Article : Google Scholar
|
|
26
|
Blidisel A, Marcovici I, Coricovac D, Hut
F, Dehelean CA and Cretu OM: Experimental models of hepatocellular
carcinoma-a preclinical perspective. Cancers (Basel). 13:36512021.
View Article : Google Scholar
|
|
27
|
Li J, Wang X, Ren M, He S and Zhao Y:
Advances in experimental animal models of hepatocellular carcinoma.
Cancer Med. 12:15261–15276. 2023. View Article : Google Scholar
|
|
28
|
Liu S, Huang F, Ru G, Wang Y, Zhang B,
Chen X and Chu L: Mouse models of hepatocellular carcinoma:
Classification, advancement, and application. Front Oncol.
12:9028202022. View Article : Google Scholar
|
|
29
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar
|
|
30
|
Yang YM, Kim SY and Seki E: Inflammation
and liver cancer: Molecular mechanisms and therapeutic targets.
Semin Liver Dis. 39:26–42. 2019. View Article : Google Scholar
|
|
31
|
Yao C, Wu S, Kong J, Sun Y, Bai Y, Zhu R,
Li Z, Sun W and Zheng L: Angiogenesis in hepatocellular carcinoma:
Mechanisms and anti-angiogenic therapies. Cancer Biol Med.
20:25–43. 2023. View Article : Google Scholar
|
|
32
|
Sanz-Cameno P, Trapero-Marugán M, Chaparro
M, Jones EA and Moreno-Otero R: Angiogenesis: From chronic liver
inflammation to hepatocellular carcinoma. J Oncol. 2010:2721702010.
View Article : Google Scholar
|
|
33
|
Quintero-Fabián S, Arreola R,
Becerril-Villanueva E, Torres-Romero JC, Arana-Argáez V,
Lara-Riegos J, Ramírez-Camacho MA and Alvarez-Sánchez ME: Role of
Matrix Metalloproteinases in Angiogenesis and Cancer. Front Oncol.
9:13702019. View Article : Google Scholar
|
|
34
|
Kluck GEG, Wendt CHC, Imperio GED, Araujo
MFC, Atella TC, da Rocha I, Miranda KR and Atella GC: Plasmodium
infection induces dyslipidemia and a hepatic lipogenic state in the
host through the inhibition of the AMPK-ACC pathway. Sci Rep.
9:146952019. View Article : Google Scholar
|
|
35
|
Balasubramanian L, Zuzarte-Luís V, Syed T,
Mullick D, Deb S, Ranga-Prasad H, Meissner J, Almeida A,
Furstenhaupt T, Siddiqi K, et al: Association of plasmodium berghei
with the apical domain of hepatocytes is necessary for the
parasite's liver stage development. Front Cell Infect Microbiol.
9:4512020. View Article : Google Scholar
|
|
36
|
Scaccabarozzi D, Deroost K, Corbett Y,
Lays N, Corsetto P, Salè FO, Van den Steen PE and Taramelli D:
Differential induction of malaria liver pathology in mice infected
with Plasmodium chabaudi AS or Plasmodium berghei NK65. Malar J.
17:182018. View Article : Google Scholar
|
|
37
|
Deroost K, Lays N, Pham TT, Baci D, Van
den Eynde K, Komuta M, Prato M, Roskams T, Schwarzer E, Opdenakker
G and Van den Steen PE: Hemozoin induces hepatic inflammation in
mice and is differentially associated with liver pathology
depending on the Plasmodium strain. PLoS One. 9:e1135192014.
View Article : Google Scholar
|
|
38
|
Daily JP, Scanfeld D, Pochet N, Le Roch K,
Plouffe D, Kamal M, Sarr O, Mboup S, Ndir O, Wypij D, et al:
Distinct physiological states of Plasmodium falciparum in
malaria-infected patients. Nature. 450:1091–1095. 2017. View Article : Google Scholar
|
|
39
|
Ding H, Wu S, Jin Z, Zheng B, Hu Y, He K,
Lu S and Zhuo X: Anti-Tumor effect of parasitic protozoans.
Bioengineering (Basel). 9:3952022. View Article : Google Scholar
|
|
40
|
Kaushansky A, Ye AS, Austin LS,
Mikolajczak SA, Vaughan AM, Camargo N, Metzger PG, Douglass AN,
MacBeath G and Kappe SH: Suppression of host p53 is critical for
Plasmodium liver-stage infection. Cell Rep. 3:630–637. 2013.
View Article : Google Scholar
|
|
41
|
Chen X, Qin L, Hu W and Adah D: The
mechanisms of action of Plasmodium infection against cancer. Cell
Commun Signal. 19:742021. View Article : Google Scholar
|
|
42
|
Liu Q, Yang Y, Tan X, Tao Z, Adah D, Yu S,
Lu J, Zhao S, Qin L, Qin L and Chen X: Plasmodium parasite as an
effective hepatocellular carcinoma antigen glypican-3 delivery
vector. Oncotarget. 8:24785–24796. 2017. View Article : Google Scholar
|
|
43
|
Qin L, Zhong M, Adah D, Qin L, Chen X, Ma
C, Fu Q, Zhu X, Li Z, Wang N and Chen Y: A novel tumour suppressor
lncRNA F630028O10Rik inhibits lung cancer angiogenesis by
regulating miR-223-3p. J Cell Mol Med. 24:3549–3559. 2020.
View Article : Google Scholar
|
|
44
|
Jun JC, Rathore A, Younas H, Gilkes D and
Polotsky VY: Hypoxia-Inducible Factors and Cancer. Curr Sleep Med
Rep. 3:1–10. 2017. View Article : Google Scholar
|
|
45
|
Kierans SJ and Taylor CT: Regulation of
glycolysis by the hypoxia-inducible factor (HIF): Implications for
cellular physiology. J Physiol. 599:23–37. 2021. View Article : Google Scholar
|
|
46
|
Lv X, Li J, Zhang C, Hu T, Li S, He S, Yan
H, Tan Y, Lei M, Wen M and Zuo J: The role of hypoxia-inducible
factors in tumor angiogenesis and cell metabolism. Genes Dis.
4:19–24. 2016. View Article : Google Scholar
|
|
47
|
Manuelli V, Pecorari C, Filomeni G and
Zito E: Regulation of redox signaling in HIF-1-dependent tumor
angiogenesis. FEBS J. 289:5413–5425. 2022. View Article : Google Scholar
|
|
48
|
Zhao L, Liu Z, Yang F, Zhang Y, Xue Y,
Miao H, Liao X, Huang H and Li G: Intrabody against prolyl
hydroxylase 2 promotes angiogenesis by stabilizing
hypoxia-inducible factor-1α. Sci Rep. 9:118612019. View Article : Google Scholar
|
|
49
|
Zheng SS, Chen XH, Yin X and Zhang BH:
Prognostic significance of HIF-1α expression in hepatocellular
carcinoma: A meta-analysis. PLoS One. 8:e657532013. View Article : Google Scholar
|
|
50
|
Li Q, Jin L and Jin M: Novel
hypoxia-related gene signature for risk stratification and
prognosis in hepatocellular carcinoma. Front Genet. 12:6138902021.
View Article : Google Scholar
|
|
51
|
Deng F, Chen D, Wei X, Lu S, Luo X, He J,
Liu J, Meng T, Yang A and Chen H: Development and validation of a
prognostic classifier based on HIF-1 signaling for hepatocellular
carcinoma. Aging (Albany NY). 12:3431–3450. 2020. View Article : Google Scholar
|