Urological cancers encompass malignancies that arise
in the organs associated with the urinary system, predominantly
comprising renal cell carcinoma (RCC), prostate cancer (PCa) and
bladder cancer (BCa). According to global cancer statistics for
2020, there were ~2.4 million novel instances of urinary system
neoplasms, constituting 12.5% of all malignancies worldwide
(1). The risk factors associated
with PCa primarily include familial predisposition, racial
background, advanced age, obesity and several environmental and
genetic influences. Adenocarcinomas can be categorized into
androgen-sensitive and androgen-insensitive subtypes. Treatment
modalities for PCa include active surveillance, chemotherapy,
radiotherapy, hormone therapy, surgical intervention and
cryotherapy (2). Sex, obesity,
hypertension, smoking and chronic kidney disease are risk factors
for RCC. Whilst it is possible to treat RCC surgically, local
recurrence occurs in 2–5% of patients and in 20–30% of patients
with distant metastases. Postoperative adjuvant therapy includes
hormone therapy, radiotherapy, immunotherapy, vaccines and targeted
drugs. These therapies, however, have not yielded any evidence of
improved patient survival (3). Risk
factors for BCa include smoking, parasitic infections, chronic
inflammation, sex, age, occupational exposure and genetic factors.
Depending on whether a tumor has invaded the bladder muscular
layer, it can be classified as non-muscle-invasive BCa or
muscle-invasive BCa. The treatment of BCa includes surgical
resection, immunotherapy, chemotherapy, radiotherapy and
antibody-drug conjugates (4). An
increasing amount of data indicate that investigating novel
therapeutic approaches for advanced urologic malignancies requires
a thorough understanding of the molecular pathways underlying
urologic neoplasia (5,6).
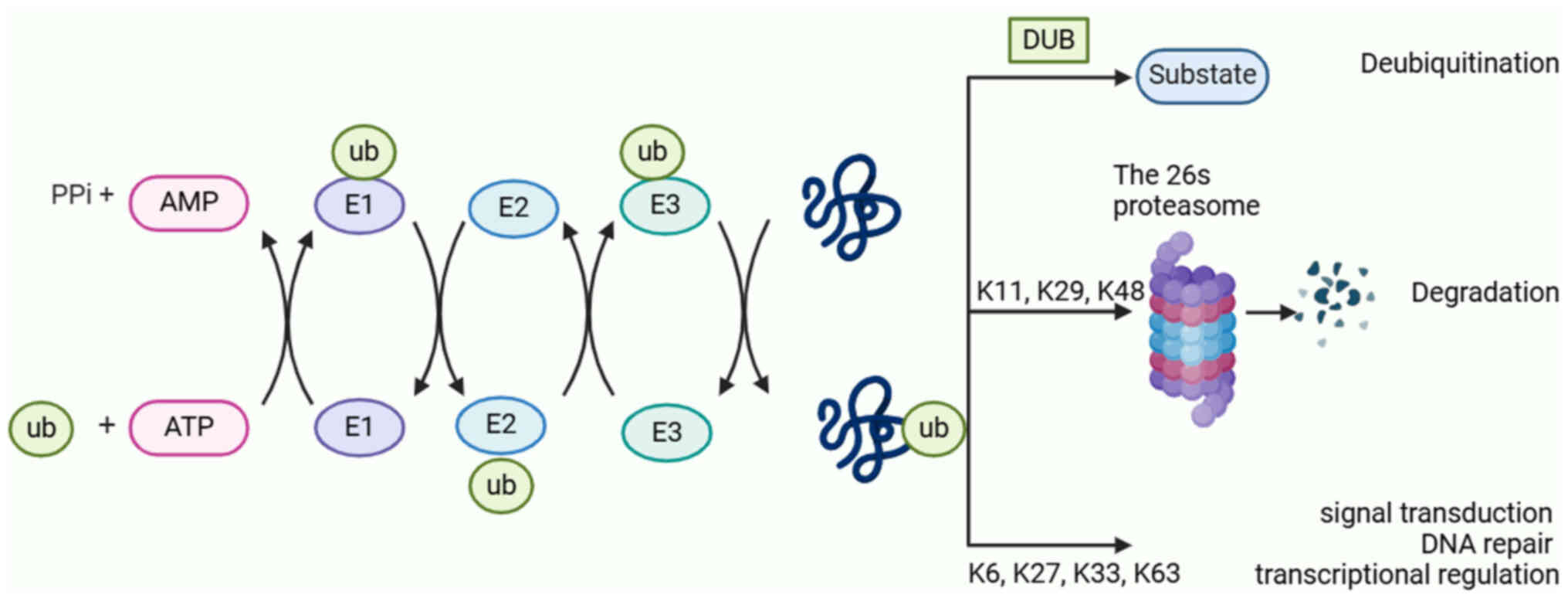
An important post-translational modification is
ubiquitination. The complex signaling network created by intricate
interactions between the ubiquitin-proteasome system (UPS) and its
substrates are necessary for regulating a number of bodily
physiological processes, including signal transmission, cellular
metabolism, immunological stress responses and cell cycle
progression (7). In eukaryotes, the
UPS is responsible for >80% of protein turnover. The main
components of the ubiquitination process are deubiquitylating
enzymes (DUBs), ubiquitin (Ub), ubiquitin-activating enzyme E1,
ubiquitin-conjugating enzyme E2, ubiquitin ligase enzyme E3 and 26S
proteasome (8). First, to create an
E1-Ub complex, unbound Ub molecules are activated by E1 in an
ATP-dependent manner and connected by thioester linkages. Next, the
activated Ub is transferred to the E2 active site cysteine residue.
Finally, the interaction between the E2-Ub complex and E3
facilitates the transfer of activated Ub to the lysine residue of
the substrate protein (9). A total
of seven lysine residues (K6, K11, K27, K29, K33, K48 and K63) are
notable features of ubiquitin. These residues are ubiquitinated to
produce different polyubiquitin chains (10). Target substrates are typically
delivered to the proteasome for destruction by polymerized
ubiquitinated chains of K11, K29 and K48. Despite this, K63, K6,
K27, K33 and other polymerized ubiquitinated chains, are involved
in many critical cellular processes, including transcriptional
control, DNA repair and signal transduction, whilst protecting
their target substrates from damage (Fig. 1) (11).
Abnormal regulation of DUBs has been extensively
studied and reported to be closely associated with the occurrence
and progression of human tumors, including malignant tumors of the
urinary system (12). It has been
reported that a large number of DUBs can act as both oncogenic
factors and tumor suppressors (12). Therefore, an exhaustive overview of
these DUBs may lead to innovative approaches for the treatment of
urinary system tumors. The present review provides an extensive
synopsis of the most recent developments in DUBs within urological
malignancies, encompassing their classification, structure and
function. Furthermore, it highlights the crucial regulatory
mechanisms through which DUBs modulate signaling pathways relevant
to urological tumors (Table I).
PI3K/AKT pathway. A key element of the PI3K/AKT
pathway is transmission of signals within cells, and its aberrant
activation can lead to rapid growth, reproduction and abnormal
regulation of angiogenesis in cancer cells (13). PI3K is an isodimer composed of the
regulatory subgroup p85 and catalytic subgroup p110, so it can be
categorized into PI3KI, PI3KII and PI3KIII types based on their
structure. PI3K is not primarily in a specific organelle, but acts
as an intracellular phosphatidylinositol kinase on the plasma
membrane of the cell (14).
Transmembrane tyrosine kinase growth factor receptors can activate
PI3K, such as EGFR and insulin-like growth factor receptor 1.
Phosphorylation of phosphatidylinositol 4,5-diphosphate (PIP2)
activated by PI3K is then converted to phosphatidylinositol
3,4,5-triphosphate (PIP3) (15).
Subsequently, 3-phosphoinositide-dependent kinase 1 (PDK1) and AKT
are recruited to the plasma membrane, where PDK1 phosphorylates
threonine at position 308 of AKT, resulting in AKT activation
(16). Once activated, AKT
phosphorylates its downstream targets, including mTOR. Therefore,
it is a major factor in the development and metastasis of cancer
(17,18).
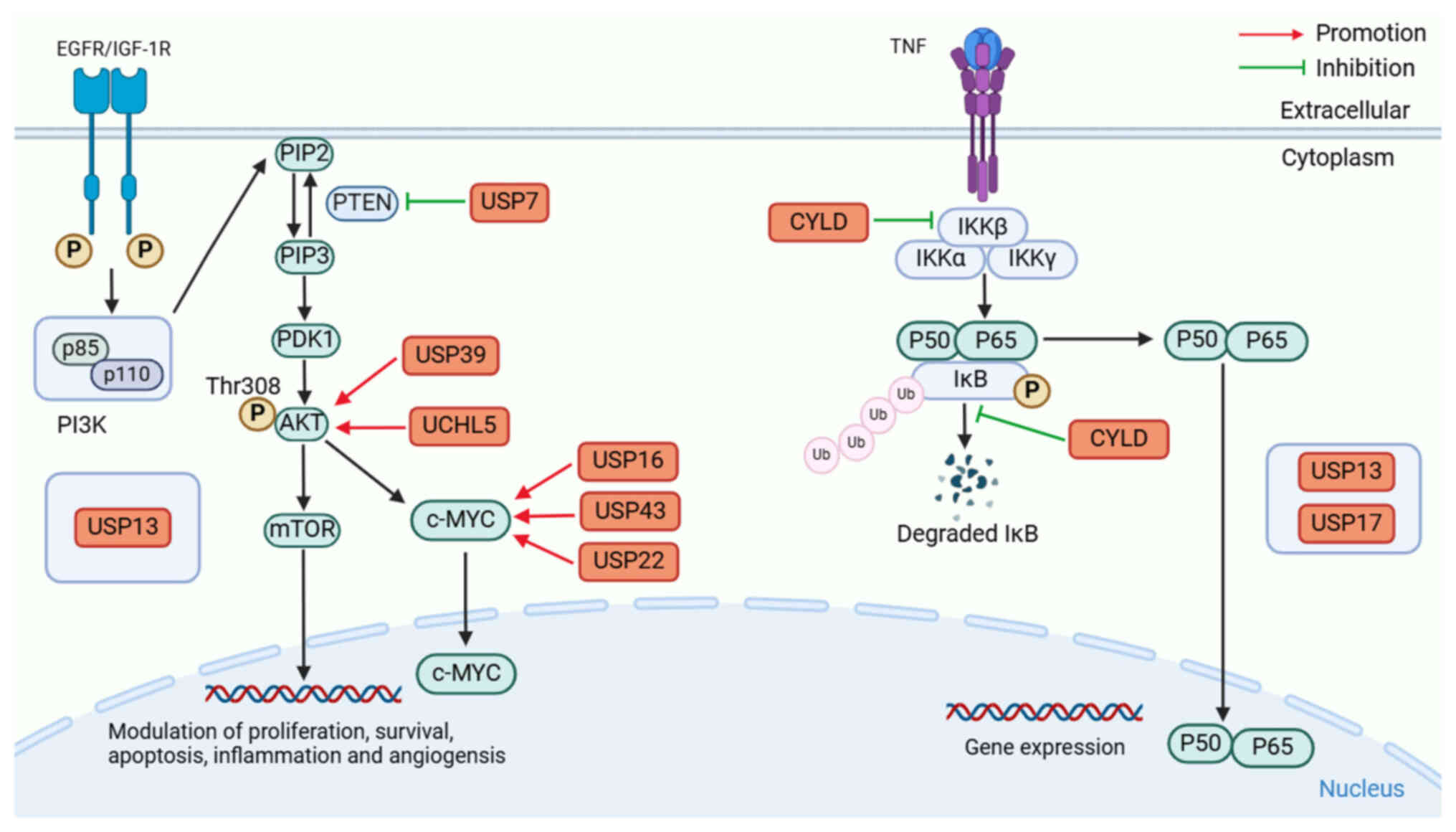
Of note, phosphatase and tensin homologue deleted on
chromosome 10 (PTEN) could inhibit the activation of the PI3K-PKB
signaling pathway by dephosphorylating PIP3 and converting it to
PIP2. When PTEN is mutated or absent, PIP3 accumulates in
cells and uncontrollably activates its downstream signaling
(19,20). Both deregulated ubiquitination and
deubiquitination can lead to detrimental impacts on PTEN levels and
subcellular partitioning, promoting tumorigenesis (21). Furthermore, a diverse range of DUBs,
including ubiquitin-specific protease (USP)7 and USP13, have been
reported to mediate the deubiquitination of PTEN, thereby
disrupting the function of PTEN to promote the PI3K/AKT signaling
pathway (21), and the
dysregulation of PTEN deubiquitination could lead to the
tumorigenesis of PCa (Fig. 2).
USP7, also known as herpes virus-associated
protease, is a DUB enzyme belonging to the USPs family. It is a
cysteine peptidase that comprises five distinct domains (22). USP7 is mostly found in the nucleus
and is essential for controlling the stability of several proteins
involved in several cellular activities, including immunological
response, infection by viruses, DNA damage reaction,
transcriptional regulation and epigenetic control of gene
overexpression (23).
Mechanistically, USP7 may interact with K13 and K289 to
deubiquitinate PTEN in vivo and in vitro, reducing
its single-stranded structure and leading to nuclear rejection and
PTEN inactivation, thereby promoting cancer occurrence and
metastasis. Song et al (24)
reported that USP7-mediated deubiquitination of PTEN could lead to
a reduction in its inhibitory effect on PCa cell proliferation.
Formerly known as isopeptidase T, USP13 is a member
of the USPs family of enzymes and is categorized as a
deubiquitinating enzyme. Its roles include controlling the course
of the cell period, repairing damage to DNA systems, myoblast
development, endoplasmic reticulum quality control mechanisms and
autophagy. Many malignant tumors, including ovarian cancer (OC),
cervical carcinoma (CC) and hepatocellular carcinoma (HCC), have
elevated USP13 expression. Enhanced USP13 activity promotes tumor
cell proliferation by upregulating a range of carcinogenic factors
such as myeloid cell leukemia-1 and c-MYC. However, in certain
malignancies such as breast cancer (BC) and colorectal cancer
(CRC), the overexpression of USP13 exhibits a tumor-suppressive
effect through the upregulation of PTEN (25,26).
Recently, Cui et al (27)
reported that USP13 was involved in PCa by upregulating the
PI3K/AKT pathway. Although USP13 can promote PI3K-AKT-induced
invasion and metastasis of PCa (27), the underlying mechanism requires
further exploration.
The DUBs family of enzymes includes USP39, which has
a central zinc finger ubiquitin-binding domain and a ubiquitin
C-terminal hydrolase domain (28).
It is involved in the regulation of several biological processes
such as cell proliferation, cell cycle progression, apoptosis and
pre-messenger RNA splicing (29).
Upregulation of USP39 has also been implicated in the pathogenesis
of HCC, medullary thyroid carcinoma, oral squamous cell carcinoma
(OSCC) and CRC (30–33). Xu et al (34) first determined that the knockdown of
USP39 significantly impedes the phosphorylation of AKT at
Ser473, therefore blocking the activity of the AKT signaling
pathway in RCC. However, more investigation is needed to clarify
the precise processes behind the function of USP39 in regulating
AKT signaling.
UCHL5, also known as ubiquitin carboxy-terminal
hydrolase 37, is an important factor regulating deubiquitination
and has been reported to be abnormally high expressed in numerous
cancers, including CSCC, esophageal squamous cell carcinoma (ESCC),
epithelial OC, non-small cell lung cancer (NSCLC) and pancreatic
carcinoma (PC) (35–39). Recently, Cao et al (40) reported that overexpression of UCHL5
could activate the AKT/mTOR pathway and further activate the
downstream target c-MYC, thus promoting the proliferation and
migration of BCa. It remains unknown, therefore, how UCHL5 controls
the AKT signaling pathway.
The protein complex known as nuclear factor κB
(NF-κB) is an essential nuclear transcription factor in cells and
is associated with cancer, inflammatory and autoimmune diseases,
viral infections and abnormal development of the immune system
(41). It represents an important
family of structurally similar transcription factors (Rel
proteins), consisting of five members: NF-κB1 (p50), NF-κB2 (p52),
RelA (p65), RelB and c-Rel (42).
The signal transduction pathway of NF-κB activation primarily
comprises two distinct modes: Canonical and non-canonical (43). The NF-κB protein typically forms
homologous or heterodimers with p65 and p50 or is rendered inactive
in the cytoplasm by binding to the suppressor IκB, thus causing a
trimeric complex to form. Upon binding of the upstream signaling
factor tumor necrosis factor (TNF) to the cell membrane surface
receptor, conformational changes occur within the receptor,
transmitting the signal to IKK kinase (IκB kinase), causing the IKK
complex made up of IKK-α, IKK-β and IKK-γ to become activated. This
IKK complex then phosphorylates the IκB protein, causing it to
separate from the trimeric complex. Subsequently, nuclear
localization sequences (NLS) are exposed on the NF-κB dimer,
allowing for rapid translocation from the cytoplasm into the
nucleus for the activation of target genes (44).
TNF receptor-associated factor (TRAF)2, a
bifunctional protein that acts as an adaptor and ubiquitin E3
ligase, is one of the major mediators of the TNF receptor. It
possesses a carboxy-terminal TRAF domain that can be further
divided into two subdomains known as TRAF-N and TRAF-C domains
(45). TRAF2 protein induces the
activation of IκB kinase (IKKα and IKKi/ε), leading to the
phosphorylation of IκBα. Additionally, it promotes nuclear
translocation and phosphorylation of p65/RelA to promote the
downstream signaling cascade of the NF-κB pathway (46).
As a negative regulator of the NF-κB pathway, the
CYLD protein belongs to the USPs and consists of three glycine-rich
cytoskeletal associated protein domains, two proline-rich motifs, a
phosphorylation region and the USP catalytic domain (51). Emerging evidence suggests that CYLD
is associated with the pathogenesis of several diseases, including
cancer, infection, pulmonary fibrosis, neurodegeneration and
cardiovascular dysfunction (52).
The fundamental process involves CYLD mediating TRAF2 or BCL-3
deubiquitination, which disrupts NF-κB signaling (53). Sim et al (54) reported that CYLD can inhibit IKKβ,
stabilize I-κBα and retain the NF-κB heterodimer in the cytoplasm,
leading to the blocking of p65/p50 nuclear translocation, hence
preventing RCC cells from proliferating. Notably, Yuan et al
(55) reported that the CYLD
protein functions by inhibiting the ubiquitination of IκB and
retaining the NF-κB heterodimer p65/p50 in the cytoplasm, thereby
suppressing the proliferation, migration and invasion of BCa
cells.
USP13 may function as a putative oncogenic protein
in PCa by activating the PI3K/AKT signaling pathway (27). Notably, Man et al (56,57)
reported that USP13 deubiquitinates and stabilizes PTEN protein,
and PTEN protein suppresses NF-κB activation by inhibiting the
PI3K/AKT pathway, thereby preventing the nuclear translocation and
DNA-binding ability of NF-κB subunits. In conclusion, low USP13
expression can promote the occurrence and progression of BCa.
Nevertheless, the chemical control technique of USP13 over the
NF-κB signaling pathway remains poorly understood, and further
research is needed.
USP17, known as DUB3, comprises a catalytic USP
domain, along with two hyaluronic acid (and RNA) binding motifs
(58), which is controlled by
interleukin (IL)-4 and IL-6 cytokines. The formation of T helper
cell 17 cells, inflammation, cell motility and carcinogenesis are
all associated with the abnormal expression of USP17 (59). Research has demonstrated an
association between the expression and function of USP17 and
several cancer types, including OSCC, NSCLC, BC, CRC, CSCC and
osteosarcoma (OS) (58). USP13 and
USP17, as deubiquitinating enzymes, are not localized in a specific
organelle, but widely distributed in the cytoplasm and nucleus.
Under different conditions, the localization of USP13 and USP17 in
the cell may change (60). Baohai
et al (61) reported that
the inhibition of USP17 hinders NF-κB signaling through the
facilitation of reactive oxygen species generation to inhibit the
progression of PCa. However, more research is needed to elucidate
how USP17 controls these genes or how it regulates the NF-κB
signaling pathway.
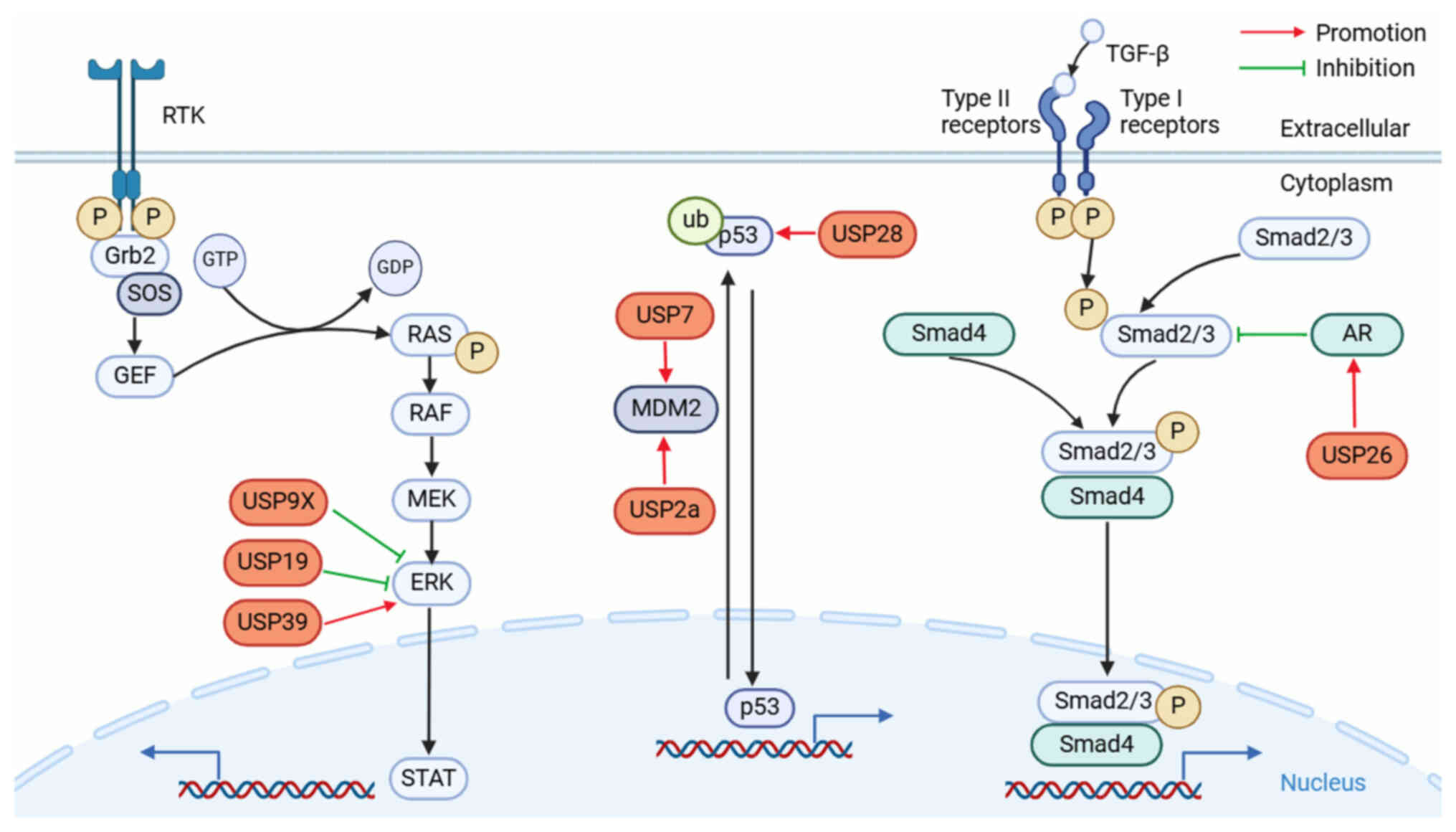
The MAPK signaling pathway, a crucial component of
the eukaryotic signal transduction network, functions as a major
signaling cascade that regulates the processes of apoptosis
(programmed cell death), cell division, proliferation, and the
cellular reaction of irritability in both healthy and pathological
settings. MAPK is a family of serine-threonine kinases that has
undergone evolutionary conservation. It is composed of four
separate subfamilies: ERK, p38, JNK and BMK1 (sometimes referred to
as ERK5), which correspond to four conventional MAPK pathways
(62). The most notable signaling
cycle among all MAPK signaling pathways is the RAS/RAF/MEK/ERK
pathway (63). Binding of
growth-promoting elements to their receptors triggers activation of
receptor tyrosine kinase (RTK). This, in turn, leads to the
recruitment of the growth factor receptor bound protein 2 and
subsequently activates guanine nucleotide exchange factors. The
signal is then transmitted to the RAS, which directly interacts
with the RAF to form a transient membrane-anchored signal (64). Activated RAF triggers the MEK by
making a serine site in its catalytic loop phosphorylated and ERK
is subsequently activated by MEK. This then phosphorylates several
substrates connected to the cytoplasm and the membrane of the cell
(65). Cancer mutations with the
highest prevalence are driver mutations in RAS, primarily KRAS,
which occurs in ~30% of all types of cancer and affects ~10% of all
patients with cancer (66,67). Targeting this route has been
reported in multiple studies to have a major impact on the growth
and progression of urinary system cancers by involving multiple
DUBs (34,68,69)
(Fig. 3).
On chromosome Xp11.4, USP9X is a highly conserved
DUB that is a member of the USP group (70). The expression level of USP9X is
widely associated with cell cycle regulation, signaling pathway
conduction, ribosomal stalling and tumorigenesis. Prior research
has demonstrated the marked impact USP9X serves in many
malignancies, including laryngeal carcinoma, BC, glioblastoma and
lung cancer (71–73). Zhang et al (68) reported that USP9X may act as a tumor
suppressor in PCa. Compared with healthy tissues, USP9X expression
was downregulated in PCa tissues, and ERK was notably increased.
Furthermore, USP9X depletion upregulated ERK signaling,
resulting in an increase in matrix metallopeptidase 9 protein
levels and dynamin-related protein 1 phosphorylation, thereby
further promoting cancer cell invasion. These results indicate that
USP9X is an efficient tumor suppressor; nevertheless, more
investigation is needed to identify USP9X substrates.
USP19 is a DUB that is essential for lipogenesis,
cellular metabolism and immunological responses (74,75).
Furthermore, prior research has indicated that USP19 is implicated
in a number of malignancies, such as stomach cancer (GC), CRC and
HCC (74,76,77).
Hu et al (69) analyzed The
Cancer Genome Atlas and Gene Expression Omnibus databases and
reported that USP19 expression was downregulated in RCC and that
USP19 knockdown led to tumor progression and poor prognosis.
Notably, the study reported that USP19 knockdown enhanced ERK
phosphorylation, and USP19 overexpression markedly reduced ERK
levels, which in turn promoted clear cell RCC proliferation both
in vitro and in vivo. These findings suggest that USP19
expression is linked to ERK pathway activity and may have
tumor-suppressive properties. Nevertheless, more research is
required to elucidate the underlying mechanisms.
USP39 is a member of the USP family of DUBs, with a
central zinc finger ubiquitin-binding domain and a ubiquitin
C-terminal hydrolase domain (78).
USP39 is involved in multiple cellular processes, including
spliceosome formation, spindle stabilization and the cell cycle
(79). Many studies have reported
that USP39 is overexpressed in HCC, BC, colon cancer (CC) and RCC
(78,80,81).
Xu et al (34) reported that
USP39 promotes the MAPK signaling and exerts an oncogenic effect in
RCC. Small interfering RNA was used to reduce USP39 expression in
RCC cells, and the study reported that this markedly reduced the
ability of the cells to proliferate and spread. Mechanistically,
the deletion of USP39 led to a notable reduction in the
expression levels of apoptotic proteins and G2/M phase-associated
proteins. Furthermore, phosphorylation of ERK at the Thr202/Tyr204
region was dramatically suppressed by downregulating USP39, which
prevented the MAPK pathway from being activated. The aforementioned
findings suggest that USP39 is a potential target for RCC
therapy.
The TP53 gene, commonly referred to as p53, encodes
the tumor protein, which is a known tumor suppressor in humans and
has been identified as the most strongly associated with human
tumorigenesis to date. It is also associated with ~50% of all human
tumors (82). Human p53 is a 4×393
amino acid homotetramer consisting of an intrinsically disordered
N-terminal trans-activation domain (TAD), proproline (Pro)-rich
region, structured DNA-binding domain, tetrameration domain linked
by an elastic linker, and intrinsically disordered C-terminal
regulatory domain (83). Cell cycle
arrest, DNA repair, metabolic changes, antioxidant and
anti-angiogenic effects, autophagy, senescence and apoptosis are
just a few of the reactions that p53 orchestrates. It can activate
multiple transcriptional targets in response to cellular stress or
DNA damage (84).
Mouse double minute 2 homolog (MDM2) is an E3 ligase
composed of a p53-binding domain at the N-terminus, a central
region containing a NLS, a nuclear export signal and a RING finger
domain at the C-terminus (85).
MDM2 negatively regulates p53 by binding to its transcriptional
activation domain to prevent the interaction of transcription
elements, mediating p53 covalent binding to ubiquitin proteins to
be degraded by proteolytic enzymes, and expulsion from the nucleus
(86,87). A comprehensive overview of several
DUBs that control p53 signaling may offer novel insights into the
occurrence and progression of tumors of the urinary system
(Fig. 3).
The enzyme known as USP2a deubiquitinates cysteine
proteases. USP2a regulates the function of multiple important cell
growth regulators and signal transduction factors. USP2a serves a
role in carcinogenesis, stimulation of NF-κB and interferon
modulation (88,89). USP2a is upregulated in several
cancers, including BC, HCC and OC (90–92).
Kim et al (93) assessed the
function of USP2a in BCa cells and reported that USP2a stabilizes
and deubiquitinates MDM2, which is a specific target of USP2a. The
study also reported that silencing USP2a notably decreased MDM2
protein levels and cell proliferation capacity, implying that USP2a
is an MDM2-associated positive regulator. In addition, it was also
reported that during the development of PCa, USP2a was
overexpressed, leading to the inhibition of p53 by stabilizing
MDM2. Therefore, the inhibition of USP2a activity may provide a
novel strategy for cancer treatment (94).
USP7 may activate the PI3K/AKT signaling pathway.
USP7 participates in the regulation of multiple cellular pathways
and its expression is often dysregulated in human malignancies
(22). Studies have reported that
USP7 expression is upregulated in PCa and strongly associated with
tumor progression (95,96). Mechanistically, USP7 stabilizes MDM2
through its specific deubiquitinase activity, which increases the
intracellular level of MDM2 and downregulates p53, leading to the
promotion of tumor development. Consequently, the creation of USP7
inhibitors is a successful patient therapy strategy (97).
A member of the USPs family, USP28 possesses both a
USP domain and a C-terminal extension domain, is located on
chromosome 11q23 (98). In
addition, studies have reported an associated between USP28 and
certain malignancies, such as PC, CRC and GC (99–101).
USP28 was reported to be overexpressed in BCa and associated with
tumor invasion and growth. Immunohistochemistry and reverse
transcription-quantitative PCR revealed that USP28 and p53 were
overexpressed in BCa cells, and there was a marked association
between them (102).
Mechanistically, USP28 deubiquitinates and stabilizes p53 in BCa
cells. However, owing to the limited association studies on p53 and
USP28, more research is needed to determine the foundational
processes. Taken together, USP28 is expected to be a prognostic
marker for BCa (102,103).
The TGF-β superfamily is the largest group of
secreted growth factors, comprising several structurally and
functionally related subfamilies, including TGF-βs, bone
morphogenetic proteins, growth differentiation factors, mullerian
inhibitor substance, activins and inhibins (104). It regulates several biological
processes, including cell proliferation, differentiation, cell-cell
interaction, immune regulation, extracellular matrix synthesis and
inflammatory responses (105). All
TGF-β and TGF-β-related family members bind to Type II receptors
and recruit Type I receptors, which phosphorylate and activate Type
I receptors (106). Type I
receptors in turn phosphorylate and activate the downstream SMA and
MAD-related protein (Smad)2 and Smad3. The C-terminally
phosphorylated Smad2 and Smad3 recruit Smad4 to assemble into
complexes and go into the nucleus to control TGF-β target gene
transcription (107). In the
nucleus, SMAD complexes activate specific genes through
collaborative interactions with other DNA-binding and coactivator
(or co-inhibitor) proteins (108).
TGF-β serves a dual role in tumorigenesis. During
the early stages of tumor formation, TGF-β exerts its inhibitory
effects primarily by inducing cell cycle arrest and activating the
apoptotic pathways in cancer cells. However, as the tumor
progresses, the suppressive effect of TGF-β on tumor cell
proliferation diminishes or even disappears, leading to increased
TGF-β secretion. Consequently, at this stage, TGF-β can act as a
growth-promoting factor for tumor cells (Fig. 3) (109).
On the X chromosome, USP26 is expressed only in the
testes of mice and humans and is believed to be a retrogene derived
from autosomal USP39 (110).
According to certain research, many cancers, including anaplastic
thyroid carcinoma, CSCC and ESCC, express USP26 aberrantly
(111–113). Analysis of USP26 expression in
humanity extragonadal and testicular tissues revealed the varied
roles of USP26 in cell differentiation and carcinogenesis (114). Mechanistically, Dirac and Bernards
(115) reported that when USP26
was overexpressed, AR polyubiquitination was strongly inhibited,
resulting in enhanced AR signaling. Notably, Cai et al
(116) reported that androgen
receptors (ARs) could block the TGF-β signaling pathway by directly
acting on the substrate Smad3 via the TGF-β type I receptor. In
conclusion, overexpression of USP26 can prevent PCa cells from
proliferating and spreading.
MYC is a prominent transcriptional regulator
encompassing L-MYC, N-MYC and c-MYC, that are located on
chromosomes 8, 2 and 1, respectively. The aberrant expression or
activity of any members within this family has been demonstrated to
play a role in tumor development. N-MYC and L-MYC exhibit
tissue-specific expression patterns, primarily in the lung and
nervous system (117). The
N-terminal TAD, the MYC box domains (MB0-IV), the carboxy-terminus
basic-helix-loop-helix-leucine zipper (bHLHZ), a PEST domain (rich
in proline, glutamic acid, serine and threonine), and a NLS are the
primary domains of MYC (118–122). The c-MYC oncogene signaling
pathway regulates several biological processes including apoptotic
cell death, proliferation, survival and differentiation (123). A number of upstream signaling
routes, including the traditional PI3K/AKT/mTOR pathway can
phosphorylate MYC protein, thereby enhancing its DNA-binding
ability (124). Subsequently,
phosphorylated MYC forms a dimer with its natural ligand Max and
initiates downstream DNA transcription to facilitate cellular
growth and proliferation (125).
Evidently, DUBs are engaged in the formation and development of
urinary system neoplasms targeting the MYC signaling pathway, such
as USP16 and USP43 (Fig. 2).
USP16 protein is a histone H2A-specific
deubiquitination enzyme, and human chromosome 21 is home to its
coding gene (126). USP16 is
involved in the regulation of gene expression, cell-cycle
progression and several other cellular functions (127). USP16 is abundantly expressed in
many types of tumors originating from diverse tissues, such as
gallbladder cancer, lung tumorigenesis, atherosclerosis and
coronary artery diseases (128–130). Ge et al (131) reported that USP16 knockdown
markedly inhibited PCa cell growth in vitro and in
vivo. Mechanistically, the deubiquitination of USP16 stabilizes
c-MYC to promote the initiation and progression of PCa. However,
the specific mechanisms involved are not yet fully understood.
USP43 is a USP family member. The cDNA sequence of
USP43 is 3,369 base pairs long and has an open reading frame that
codes for a protein with 1,123 amino acids (132). Prior research reported that
overexpression of USP43 could facilitate the growth of a number of
malignancies, including CRC, BC, lung squamous cell carcinoma, PC
and OS (133–137). USP43 deubiquitinates c-MYC at K148
and K289, primarily through deubiquitinase activity, which promotes
BCa metastasis. The disruption of F-box and WD repeat domain
containing 7 (FBXW7) accessibility and increased probability of
interaction with c-MYC are caused by the overexpression of USP43
protein in BCa, thereby impeding c-MYC degradation (138). These findings imply that USP43
contributes to the growth of tumors in BCa and may serve as a
marker for the eventual course of the disease.
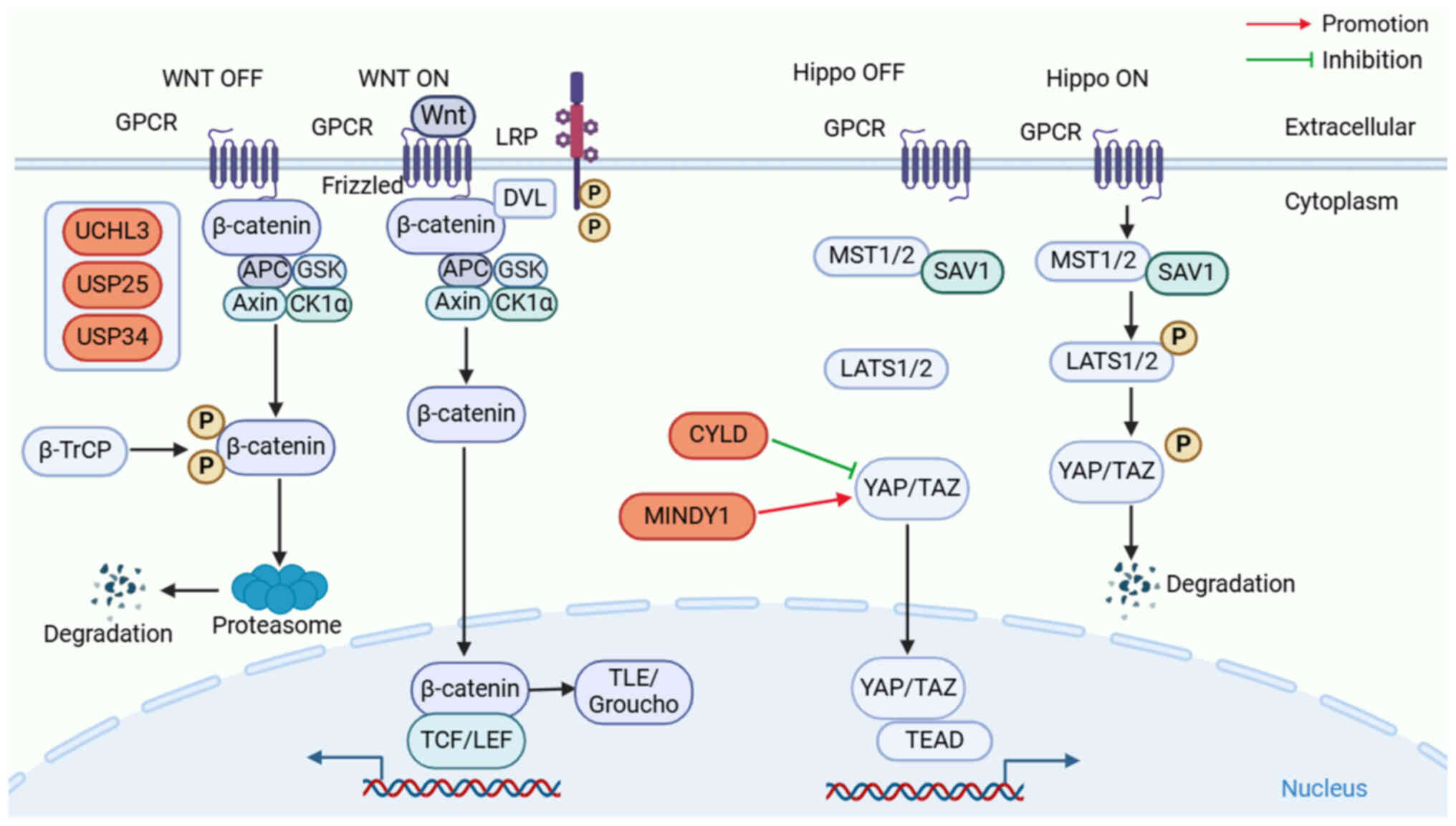
Wnt was initially derived from mouse breast cancer
integrase-1 and Drosophila wingless. Considering the substantial
functional protein similarities between these two genes,
researchers opted to merge them, resulting in the designation of
the Wnt gene (139). Wnt signaling
is highly evolutionary conserved and serves a key role in
organogenesis, tissue homeostasis, tissue regeneration and tumor
formation (140). When Wnt ligands
are not present, the destruction complex (DC) consisting of
Axin/adenomatous polyposis coli (APC)/glycogen synthase kinase-3β
(GSK3β)/casein kinase 1α (CK1α), then subsequently ubiquitinated by
β-transducin repeat-containing protein (β-TrCP), which maintains
the concentration of β-catenin in cells at a low level (141). When secreted Wnt ligands, such as
Wnt3a and Wnt1, bind to Frizzled receptors and low-density
lipoprotein receptor-related protein (LRP) co-receptors, LRP
receptors are phosphorylated by CK1α and GSK3β, and disheveled
protein is recruited to the plasma membrane and activated, leading
to inactivation of the destruction complex DC. This stops β-catenin
from constitutively degrading and from building up in the nucleus
(142). In the nucleus, β-catenin
activates Wnt-responsive gene transcription by dislocating the
transducing-like enhancer protein (Groucho) complex and recruiting
histone-modified coactivators, such as the CREB-binding
protein/p300, brahma-related gene 1, B-cell lymphoma 9 and pygopus
to form active complexes with lymphoid enhancer-binding factor and
T-cell factor proteins, stimulating the transcriptional activity of
Wnt target genes and eliciting cellular reactions (143). The present section focusses on
elucidating the role of DUBs in regulating the Wnt/β-catenin
pathway and exploring how dysregulation of this intricate network
can contribute to the pathogenesis of tumors of the urinary system
(Fig. 4).
UCHL3, a cysteine protease, belongs to the UCH
family and is a cysteine protease. The UCHL3 gene is located at
13q22.2 and consists of two main structures: A six-stranded
antiparallel β-sheet and eight α-helices (144,145). Overexpression of UCHL3 has been
reported in several cancers, including HCC, CSCC, CRC and BCa
(146–148). CTNNB1 is an important downstream
transcriptional coactivator of the Wnt signaling pathway. In most
malignant tumors, accumulation of CTNNB1 promotes Wnt/β-catenin
signaling, thereby promoting tumor progression (149). Liu et al (150) reported that overexpression of
UCHL3 was closely associated with proliferation, invasion and
migration in BCa, and coimmunoprecipitation demonstrated that UCHL3
deubiquitinated and stabilized CTNNB1, which triggered the
activation of the Wnt signaling pathway. These findings imply that
UCHL3 contributes to the development of BCa in a pro-carcinogenic
manner.
Full-length human USP25 contains 1,055 amino acids
and is part of the USPs family. Structurally, USP25 has a classical
structure similar to most USPs, including three substructural
domains: Finger, palm and thumb (151). USP25 is involved in several
cellular processes, including immune responses, inflammatory
responses and metabolic regulation (152,153). In addition, there have been
reports linking USP25 to malignant tumors, Alzheimer's disease and
cardiomegaly (154,155). Tankyase (TNKS) is an important
regulator of Wnt signaling. The TNKS anchor protein repeat
sequences collaborate with the C-terminal peptide of USP25 to
enhance the stability of TNKS and reduce the stability of AXIN1,
thus promoting the Wnt/β-catenin pathway. Furthermore, Cheng et
al (156) reported that
selective inhibition of TNKS-USP25 intermolecular interactions was
effective in inhibiting prostate tumor development, suggesting that
the therapeutic exploitation of this inhibitor may provide
opportunities for patients with Wnt pathway-dependent PCa.
In chromosome 2p15, there is a gene for USP34, a
deubiquitinase belonging to the most extensive family of
deubiquitinating enzymes (157). A
number of diseases, such as cancer, gliomas and bone disease, are
linked to USP dysregulation (158,159). USP34 serves a critical role in the
Wnt signaling pathway, and it has been reported that the function
of USP34 affects Axin degradation and β-catenin-mediated
transcription (160). NanoRNAs, or
small nucleolar RNAs, serve a role in the production of proteins
and are associated with a number of illnesses, including cancer.
Recently, a study reported that SNORA70B and its hose gene
USP34 might directly regulate Wnt pathway to promote
tumorigenesis in RCC. However, relevant proteins remain to be
investigated (161).
CYLD may promote the NF-κB pathway by activating
NF-κB/p65 and acting as a potential tumor suppressor in BCa
(55). Notably, Gu et al
(167) reported that the CYLD
protein upregulates the levels of downstream ferroptosis-related
proteins acyl-CoA synthetase long chain family member 4 and
transferrin receptor whilst inhibiting the ubiquitination of YAP,
inhibiting PCa progression by promoting ferroptosis. These findings
may contribute to a more profound understanding of the molecular
mechanisms underlying PCa, thereby facilitating enhanced
therapeutic strategies for ferroptosis.
MINDY1, also known as FAM63A, belongs to a crucial
member of DUBs. It contains a ub binding domain (UBD) that can bind
K48-linked polyUb chains and an unknown functional domain (DUF544)
(168). Previous studies have
reported that MINDY1 is abnormally highly expressed in BC and HCC
(169,170). The novel key regulator MINDY1
serves a crucial role in maintaining stem cell self-renewal by
enhancing the stability of core self-renewal proteins (171). According to Luo et al
(172), MINDY1 may encourage the
growth of BCa cells both in vivo and in vitro. It has
been reported that MINDY1 binds to YAP and increases its stability
by eliminating the K48-linked ubiquitin ring from YAP, indicating
that it may be a potential target for the intervention of BCa.
The AR signaling pathway serves an important role
in all phases of prostate carcinogenesis (173). USP10 is a multifunctional
deubiquitinating enzyme located on chromosome 16q24.1, which serves
an important role in several signaling pathways, including the p53,
mTOR and AR signaling pathway (174–176). In the regulation of gene
transcription, H2A is a core histone. It was reported that USP10
mediates the activation of the AR signaling pathway by
deubiquitinating and stabilizing H2A. It is also able to
deubiquitinate H2A.Z (H2A mutant), which is able to bind to
enhancers and promoters that bind to PSA and kallikrein-like 2
genes, and thus is involved in the regulation of the AR pathway
(177).
USP22 is widely expressed in mammals and is found
on the long limb of chromosome 17 in the human genome. It comprises
13 exons and has a cDNA with 1,578 base pairs. USP22 inhibits
protein breakdown by deubiquitinating substrate proteins, which is
how it regulates gene transcription, injury to DNA repair and
immune escape (183,184). It was reported that USP22
expression is markedly elevated in malignant tumors, such as HCC,
NSCLC and CC, indicating that it may be a significant
pro-carcinogenic factor (185–187). In BCa, high expression of USP22
was detected, which was inhibited by transfection of 15/21
asymmetric interfering RNA, which specifically targets USP22,
leading to EJ cell cycle arrest and inhibition of cell
proliferation (188). Similarly,
USP22 is a crucial biological target and AR regulator in PCa. USP22
not only controls PCa advances as AR accumulation and signaling but
also stabilizes MYC expression in cancer cells but also accelerates
its growth. In conclusion, USP22 may offer a new viewpoint on the
care of patients with urological tumors (189,190).
Urologic cancers are one of the common cancers with
a marked increase of incidence in recent years. Traditional
treatment options mainly include surgical resection, chemotherapy,
radiotherapy, immunotherapy and hormonal therapy. Early surgical
resection is an effective therapeutic maneuver, but the majority of
patients still die of recurrence and metastasis; therefore, it is
important to discover new therapeutic strategies (191). DUBs can regulate the levels of
proteins that are not responsive or directly inhibited by
conventional targeted therapies, including targets that are not
patentable. Examples of these potential targets include
transcription factors, drug-resistant enzymes and proteins involved
in protein interactions that lack distinctive features and pose
challenges for intervention with small molecules (192). Given the important role of DUBs in
cancer development, the present review summarizes the inhibitors
targeting DUBs.
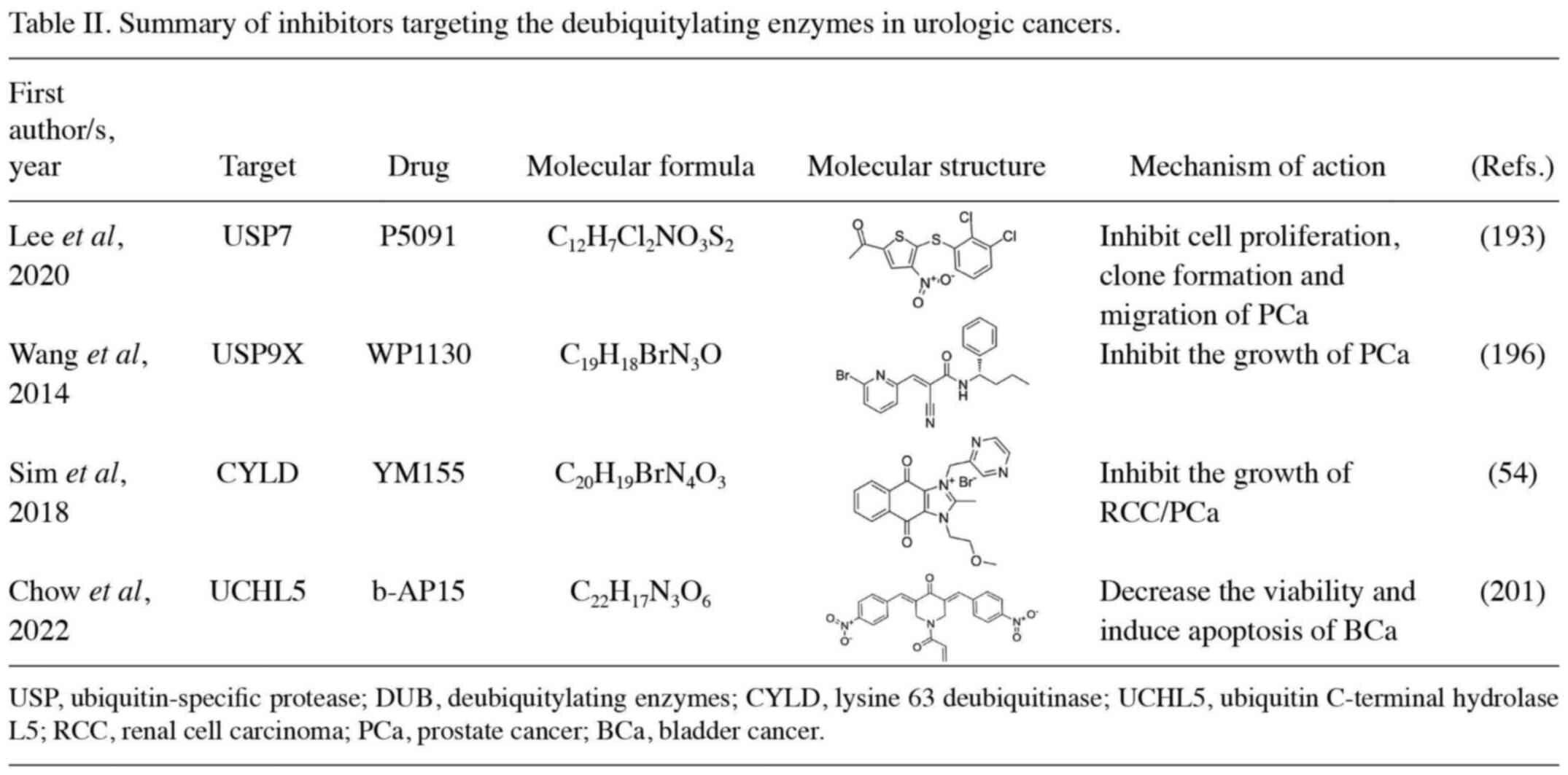
USP7 is currently one of the most extensively
studied DUBs, and several inhibitors have been developed based on
its pivotal role in the p53 pathway (22). P5091, an inhibitor of USP7, can
prevent PCa cells from migrating, invading and creating spheres,
especially in combination with EZH2 inhibitors. The decrease in PCa
cells count induced by P5091 treatment is attributed, at least
partially, to apoptosis mediated by caspases (193). P22077 has been reported to induce
apoptosis in HCC and PC, which is expected to be a promising target
for the treatment of PCa (194,195). DUB inhibitors P22077 and P50429
covalently modify USP7, leading to cysteine catalysis and inducing
conformational changes in enzymes associated with active site
rearrangement, thereby resulting in the inhibition of enzyme
activity (196). In recent years,
researchers have also developed a new generation of allosteric
inhibitors, such as XL188 and FT671, which can co-crystallize with
the catalytic core of USP7 (197,198). ETS-related gene (ERG) is a
proto-oncogene and a member of the E-twentysix transcription factor
family, which is overexpressed in 40% of patients with PCa. WP1130
is an inhibitor of USP9X and has been reported to degrade ERG,
resulting in the blockage of PCa-related gene expression and
inhibition of tumor progression (196). Prior research has demonstrated the
effectiveness of YM155, an inhibitor of survivin in small
molecules, in reducing the viability of RCC cells generated from
patients and immortalized cells. By activating the deubiquitinizing
enzyme CYLD, it results in IKKβ inhibition, IκBα stabilization, as
well as the cytosolic persistence of NF-κB heterodimers. As a
result, transcription of the NF-κB target gene survivin is reduced
(54). Xu et al (199) reported that YM155 inhibited
survivin in vitro and in vivo human
hormone-refractory PCa cells and demonstrated potent antitumor
activity. Moderate single-agent activity was also reported in a
phase I study in a large number of previously treated patients.
Furthermore, a phase II study of YM155 in combination with
docetaxel for castration-resistant PCa reported that YM155 showed
good activity (study ID no. NCT00514267) (200). b-AP15, an inhibitor of UCHL5, can
enhance the accumulation of polyubiquitinated proteins and
subsequent endoplasmic reticulum stress, thereby decreasing BCa
cell viability and inducing apoptosis. In addition, Chow et
al (201) reported that a
combination of b-AP15 and cisplatin showed better therapeutic
efficacy than monotherapy (Table
II).
In summary, the AR and p53 pathways are considered
as the primary signaling pathways in PCa, with USP7 emerging as a
promising therapeutic target due to its extensive drug development.
Currently, there is a lack of reported information regarding the
key signaling pathways involved in DUBs in RCC and BCa.
Nevertheless, CYLD and UCHL5 are likely to be pivotal DUBs in RCC
and BCa respectively, given their ongoing development of relevant
inhibitors. Facilitating the advancement of DUB inhibitors and
commencing clinical trials necessitates multifaceted endeavors and
collaboration. By enhancing fundamental research, devising
efficient screening and validation technologies, fostering
interdisciplinary cooperation, promoting clinical trials and
reinforcing international cooperation and exchanges, it is
anticipated that the development process of DUBs inhibitors will be
expedited along with their early implementation in clinical
therapy.
AR, a member of the steroid hormone receptor
family, is expressed in PCa and BCa (202). When androgens bind to AR, the AR
is released and translocated into the nucleus, thus promoting gene
transcription and accelerating tumor progression. Therefore,
inhibitors targeting the AR signaling pathway are important
therapeutic approaches for such patients (203). Bicalutamide is an androgen
receptor blocker that blocks testosterone production, thereby
decreasing hormone levels and inhibiting the growth and
proliferation of PCa cells (204).
Apalutamide is a second-generation nonsteroidal AR inhibitor
currently used for the treatment of PCa. Mechanistically, it can
successfully stop androgens from binding to the receptor and from
transferring AR into the nucleus of tumor cells, and serve a role
in inhibiting the growth of tumor cells (205). Enzalutamide is an AR antagonist
that exhibits substantial improvement in metastatic
hormone-sensitive PCa, much like apalutamide does (206). Abiraterone is an androgen
synthesis inhibitor that blocks CYP17-mediated androgen production,
thereby inhibiting the growth of PCa cells (207).
The primary mechanism of action for immune
checkpoint inhibitors (ICIs) is the blockade of inhibitory
signaling pathways within the immune system, thereby reinvigorating
the recognition and cytotoxicity capabilities of immune cells, such
as T cells, towards tumor cells. In urinary system cancer, key
targets for this drug class encompass programmed death protein 1
(PD-1) and its ligand (PD-L1), as well as cytotoxic T
lymphocyte-associated antigen 4. The US Food and Drug
Administration has thus far granted approval for three PD-L1
inhibitors (atezolizumab, avelumab and durvalumab) in the treatment
of urothelial cancer (208). PD-1
drugs have been approved for the treatment of patients with
advanced BCa and RCC, notably improving patient survival and
quality of life (209). A recent
study by Kuang et al (210)
reported that Thr288, Arg292 and Asp293 of USP2 bind to PD-L1
through the resolution of K48 link polyubiquitination at the PD-L1
lysine 270 site. USP2 depletion led to endoplasmic
reticulum-related degradation of PD-L1, which weakened PD-L1/PD-1
interactions and sensitivities T cells to cancer cells.
Tyrosine kinase inhibitors (TKIs) block the signal
transduction pathway of tumor cells by binding to the kinase domain
of their target RTK, thereby inhibiting the proliferation, invasion
and metastasis of tumor cells. The combination of TKIs and ICIs is
considered a primary treatment option for patients with advanced
RCC (211). The latest research
findings indicate that cabozantinib has an impact on the tumor
microenvironment and reinstates T cells activity, thereby
suggesting that its combination therapy with ICIs could potentially
synergistically target the growth of both primary and metastatic
PCa (212). McCann et al
(213) reported that the deletion
of USP17 in EGFR WT NSCLC cells, when combined with EGFR TKI
treatment, resulted in apoptosis induction. This suggests that
targeting USP17 could enhance therapeutic efficacy and broaden the
patient population responsive to these drugs. However, there are no
reports of DUBs and TKIs in urinary system tumors, to the best of
our knowledge.
Rapamycin is a first-generation mTOR inhibitor that
selectively inhibit the activity of mTOR by binding to
FK506-binding protein-12 and forming ternary complexes with mTOR
(214). Everolimus and
temsirolimus are used for advanced and metastatic kidney cancer
(215,216) and the efficacy of everolimus in
the treatment of kidney cancer has been demonstrated by the
RECORD-1 study (217). The
application of mTOR inhibitors in urinary system tumors is
primarily focused on the treatment of RCC, and has demonstrated
marked efficacy. Notably, Roldán-Romero et al (218) reported that the involvement of
USP9X in the regulation of p62-mediated autophagy through
ubiquitination potentially led to chromophobe renal cell carcinoma
cells sensitization to temsirolimus due to the ablation of USP9X.
This also suggests the administration of mTOR inhibitors presents a
potential therapeutic avenue for tumors harboring USP9X mutations.
Simultaneously, its research and utilization in other urologic
cancers are progressively expanding.
Men are more likely to acquire cancers of the
urinary system, such as RCC, PCa and BCa, than women. Conventional
surgery, chemotherapy and radiotherapy can only enhance the quality
of life of patients but cannot significantly improve their survival
time. The occurrence of these tumors is associated with several
factors, among which gene mutations and abnormal gene expression
serve a crucial role. Deubiquitinating enzymes regulate protein
ubiquitination levels and participate in cell cycle regulation,
signal transduction pathways and gene transcription processes,
thereby influencing tumor initiation and progression. Previous
studies have indicated that certain deubiquitinating enzymes
exhibit aberrant expression patterns in urological tumors. Such
dysregulation may lead to uncontrolled cell proliferation and
impaired apoptosis mechanisms that promote tumor formation and
advancement. Furthermore, deubiquitinating enzymes may interact
with other genes or signaling pathways related to tumorigenesis.
The present review focused on the regulation of deubiquitinating
enzymes in several signaling pathways, including the PI3K/AKT,
NF-κB, RAS/RAF/MEK/ERK, p53, TGF-β, MYC, Wnt/β-catenin and Hippo
pathways.
USP7, USP9X and UCHL5 were explored as possible
therapeutic focal points in the context of specific treatment for
urologic tumors. Nonetheless, the development of DUB inhibitors is
in the preliminary phase, with numerous unresolved queries. As an
illustration, the specific substrates and subsequent effectors of
certain DUBs in several pathways remain unidentified, encompassing
the RAS/RAF/MEK/ERK pathway, the Wnt/β-catenin pathway and others.
Despite these advances, much remains unknown regarding the
mechanism of deubiquitination in urologic tumors. In summary, the
mechanism of deubiquitinating enzymes in urologic tumors remains to
be further studied; however, they have shown promise as potential
targets for cancer treatment and prognosis evaluation. In order to
provide new ideas and approaches for the clinical treatment of
urinary system malignancies, future research should concentrate on
elucidating the specifics of the mechanism of action and creating
tailored therapy techniques.
Not applicable.
The present work was supported by the TCM Science and Technology
Project of Zhejiang Province (grant no. 2024ZL152), the National
Natural Science Foundation of China (grant no. 32270821), the
Natural Science Foundation of Zhejiang (grant no. LY24C050001) and
the K.C. Wong Magna Fund in Ningbo University.
Not applicable.
LW conceptualized the study, wrote original draft,
wrote the review and edited the manuscript. JW and LC
conceptualized the study, wrote the review and edited the
manuscript. XJ and JC revised the manuscript. All authors have read
and approved the final manuscript. Data authentication is not
applicable.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36
Cancers in 185 Countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen-Nielsen M and Borre M: Diagnostic
and Therapeutic Strategies for Prostate Cancer. Semin Nucl Med.
46:484–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bahadoram S, Davoodi M, Hassanzadeh S,
Bahadoram M, Barahman M and Mafakher L: Renal cell carcinoma: An
overview of the epidemiology, diagnosis, and treatment. G Ital
Nefrol. 39:2022–vol3. 2022.PubMed/NCBI
|
|
4
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder Cancer: A Review. JAMA. 324:1980–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao H, Yin J, Ji C, Yu X, Xue J, Guan X,
Zhang S, Liu X and Xing F: Targeting ubiquitin specific proteases
(USPs) in cancer immunotherapy: From basic research to preclinical
application. J Exp Clin Cancer Res. 42:2252023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng LL, Wang LT, Pang YW, Sun LP and Shi
L: Recent advances in the development of deubiquitinases inhibitors
as antitumor agents. Eur J Med Chem. 266:1161612024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Popovic D, Vucic D and Dikic I:
Ubiquitination in disease pathogenesis and treatment. Nat Med.
20:1242–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dagar G, Kumar R, Yadav KK, Singh M and
Pandita TK: Ubiquitination and deubiquitination: Implications on
cancer therapy. Biochim Biophys Acta Gene Regul Mech.
1866:1949792023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han S, Wang R, Zhang Y, Li X, Gan Y, Gao
F, Rong P, Wang W and Li W: The role of ubiquitination and
deubiquitination in tumor invasion and metastasis. Int J Biol Sci.
18:2292–2303. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye Z, Yang J, Jiang H and Zhan X: The
roles of protein ubiquitination in tumorigenesis and targeted drug
discovery in lung cancer. Front Endocrinol (Lausanne).
14:12201082023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Z, Zheng K, Zhou S, Yang Y, Chen J
and Jin X: E3 ubiquitin ligases in nasopharyngeal carcinoma and
implications for therapies. J Mol Med (Berl). 101:1543–1565. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dewson G, Eichhorn PJA and Komander D:
Deubiquitinases in cancer. Nat Rev Cancer. 23:842–862. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coutte L, Dreyer C, Sablin MP, Faivre S
and Raymond E: PI3K-AKT-mTOR pathway and cancer. Bull Cancer.
99:173–180. 2012.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vanhaesebroeck B, Whitehead MA and Piñeiro
R: Molecules in medicine mini-review: Isoforms of PI3K in biology
and disease. J Mol Med (Berl). 94:5–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gagliardi PA, Puliafito A and Primo L:
PDK1: At the crossroad of cancer signaling pathways. Semin Cancer
Biol. 48:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan AC: Targeting the PI3K/Akt/mTOR
pathway in non-small cell lung cancer (NSCLC). Thorac Cancer.
11:511–518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Z, Li H, Zhu J, Wang H and Jin X: The
roles of E3 ligases in Hepatocellular carcinoma. Am J Cancer Res.
12:1179–1214. 2022.PubMed/NCBI
|
|
19
|
Ngeow J and Eng C: PTEN in Hereditary and
Sporadic Cancer. Cold Spring Harb Perspect Med. 10:a0360872020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christine A, Park MK, Song SJ and Song MS:
The equilibrium of tumor suppression: DUBs as active regulators of
PTEN. Exp Mol Med. 54:1814–1821. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saha G, Roy S, Basu M and Ghosh MK: USP7-a
crucial regulator of cancer hallmarks. Biochim Biophys Acta Rev
Cancer. 1878:1889032023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pozhidaeva A and Bezsonova I: USP7:
Structure, substrate specificity, and inhibition. DNA Repair
(Amst). 76:30–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song MS, Salmena L, Carracedo A, Egia A,
Lo-Coco F, Teruya-Feldstein J and Pandolfi PP: The
deubiquitinylation and localization of PTEN are regulated by a
HAUSP-PML network. Nature. 455:813–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Sun Z, Xia W, Sun L, Du Y, Zhang Y
and Jia Z: Role of USP13 in physiology and diseases. Front Mol
Biosci. 9:9771222022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin J, He J, Li X, Ni X and Jin X: The
role of ubiquitination and deubiquitination in PI3K/AKT/mTOR
pathway: A potential target for cancer therapy. Gene.
889:1478072023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui X, Yu H, Yao J, Li J, Li Z and Jiang
Z: ncRNA-mediated overexpression of ubiquitin-specific proteinase
13 contributes to the progression of prostate cancer via modulating
AR signaling, DNA damage repair and immune infiltration. BMC
Cancer. 22:13502022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Zhang B, Lei Y, Sun J, Zhang Y,
Yang S and Zhang X: Knockdown of USP39 induces cell cycle arrest
and apoptosis in melanoma. Tumour Biol. 37:13167–13176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan J, Li X, Zhang G, Cheng W, Wang W,
Lei Y, Ma Q and Song G: USP39 mediates p21-dependent proliferation
and neoplasia of colon cancer cells by regulating the
p53/p21/CDC2/cyclin B1 axis. Mol Carcinog. 60:265–278. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan X, Sun X, Shi X, Jiang C, Yu D, Zhang
W, Guan W, Zhou J, Wu Y, Qiu Y and Ding Y: USP39 promotes the
growth of human hepatocellular carcinoma in vitro and in vivo.
Oncol Rep. 34:823–832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An Y, Yang S, Guo K, Ma B and Wang Y:
Reduced USP39 expression inhibits malignant proliferation of
medullary thyroid carcinoma in vitro. World J Surg Oncol.
13:2552015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li KY, Zhang J, Jiang LC, Zhang B, Xia CP,
Xu K, Chen HY, Yang QZ, Liu SW and Zhu H: Knockdown of USP39 by
lentivirus-mediated RNA interference suppresses the growth of oral
squamous cell carcinoma. Cancer Biomark. 16:137–144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xing Z, Sun F, He W, Wang Z, Song X and
Zhang F: Downregulation of ubiquitin-specific peptidase 39
suppresses the proliferation and induces the apoptosis of human
colorectal cancer cells. Oncol Lett. 15:5443–5450. 2018.PubMed/NCBI
|
|
34
|
Xu Y, Zhu MR, Zhang JY, Si GM and Lv JJ:
Knockdown of ubiquitin-specific peptidase 39 inhibits the malignant
progression of human renal cell carcinoma. Mol Med Rep.
17:4729–4735. 2018.PubMed/NCBI
|
|
35
|
Rolén U, Kobzeva V, Gasparjan N, Ovaa H,
Winberg G, Kisseljov F and Masucci MG: Activity profiling of
deubiquitinating enzymes in cervical carcinoma biopsies and cell
lines. Mol Carcinog. 45:260–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Fu D, Xi J, Ji Z, Liu T, Ma Y,
Zhao Y, Dong L, Wang Q and Shen X: Expression and clinical
significance of UCH37 in human esophageal squamous cell carcinoma.
Dig Dis Sci. 57:2310–2317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Chen YJ, Xu K, Wang YY, Shen XZ
and Tu RQ: High expression of UCH37 is significantly associated
with poor prognosis in human epithelial ovarian cancer. Tumour
Biol. 35:11427–11433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Z, Niu X, Li Z, Yu Y, Ye X, Lu S and
Chen Z: Effect of ubiquitin carboxy-terminal hydrolase 37 on
apoptotic in A549 cells. Cell Biochem Funct. 29:142–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cutts AJ, Soond SM, Powell S and Chantry
A: Early phase TGFβ receptor signalling dynamics stabilised by the
deubiquitinase UCH37 promotes cell migratory responses. Int J
Biochem Cell Biol. 43:604–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao Y, Yan X, Bai X, Tang F, Si P, Bai C,
Tuoheti K, Guo L, Yisha Z and Liu T and Liu T: UCHL5 Promotes
Proliferation and Migration of Bladder Cancer Cells by Activating
c-Myc via AKT/mTOR Signaling. Cancers (Basel). 14:55382022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Au PY and Yeh WC: Physiological roles and
mechanisms of signaling by TRAF2 and TRAF5. Adv Exp Med Biol.
597:32–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang W, Zhang X, Wu XL, He LS, Zeng XF,
Crammer AC and Lipsky PE: Competition between TRAF2 and TRAF6
regulates NF-kappaB activation in human B lymphocytes. Chin Med Sci
J. 25:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu H, Zeng L, Yang Y, Guo C and Wang H:
Bcl-3: A Double-Edged Sword in Immune Cells and Inflammation. Front
Immunol. 13:8476992022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Franzoso G, Bours V, Azarenko V, Park S,
Tomita-Yamaguchi M, Kanno T, Brown K and Siebenlist U: The
oncoprotein Bcl-3 can facilitate NF-kappa B-mediated
transactivation by removing inhibiting p50 homodimers from select
kappa B sites. EMBO J. 12:3893–3901. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fujita T, Nolan GP, Liou HC, Scott ML and
Baltimore D: The candidate proto-oncogene bcl-3 encodes a
transcriptional coactivator that activates through NF-kappa B p50
homodimers. Genes Dev. 7:1354–1363. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang W, Wang H, Claudio E, Tassi I, Ha HL,
Saret S and Siebenlist U: The oncoprotein and transcriptional
regulator Bcl-3 governs plasticity and pathogenicity of autoimmune
T cells. Immunity. 41:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Marín-Rubio JL, Raote I, Inns J,
Dobson-Stone C and Rajan N: CYLD in health and disease. Dis Model
Mech. 16:dmm0500932023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mathis BJ, Lai Y, Qu C, Janicki JS and Cui
T: CYLD-mediated signaling and diseases. Curr Drug Targets.
16:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Massoumi R: CYLD: A deubiquitination
enzyme with multiple roles in cancer. Future Oncol. 7:285–297.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sim MY, Yuen JSP and Go ML: Anti-survivin
effect of the small molecule inhibitor YM155 in RCC cells is
mediated by time-dependent inhibition of the NF-κB pathway. Sci
Rep. 8:102892018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yuan H, Wei S, Ren Z, Li F, Liu B, Liu R
and Zhang X: KLHL21/CYLD signaling confers aggressiveness in
bladder cancer through inactivating NF-κB signaling. Int
Immunopharmacol. 114:1092022023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Man X, Piao C, Lin X, Kong C, Cui X and
Jiang Y: USP13 functions as a tumor suppressor by blocking the
NF-kB-mediated PTEN downregulation in human bladder cancer. J Exp
Clin Cancer Res. 38:2592019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Man X, Piao C, Lin X, Kong C, Cui X and
Jiang Y: Correction to: USP13 functions as a tumor suppressor by
blocking the NF-kB-mediated PTEN downregulation in human bladder
cancer. J Exp Clin Cancer Res. 40:3862021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang GF, Zhang X, Su YG, Zhao R and Wang
YY: The role of the deubiquitinating enzyme DUB3/USP17 in cancer: A
narrative review. Cancer Cell Int. 21:4552021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Han L, Yang J, Wang X, Wu Q, Yin S, Li Z,
Zhang J, Xing Y, Chen Z, Tsun A, et al: The E3 deubiquitinase USP17
is a positive regulator of retinoic acid-related orphan nuclear
receptor γt (RORγt) in Th17 cells. J Biol Chem. 289:25546–25555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Haq S and Ramakrishna S: Deubiquitylation
of deubiquitylases. Open Biol. 7:1700162017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Baohai X, Shi F and Yongqi F: Inhibition
of ubiquitin specific protease 17 restrains prostate cancer
proliferation by regulation of epithelial-to-mesenchymal transition
(EMT) via ROS production. Biomed Pharmacother. 118:1089462019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
64
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu PK, Becker A and Park JI: Growth
Inhibitory Signaling of the Raf/MEK/ERK Pathway. Int J Mol Sci.
21:54362020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Maik-Rachline G, Hacohen-Lev-Ran A and
Seger R: Nuclear ERK: Mechanism of Translocation, Substrates, and
Role in Cancer. Int J Mol Sci. 20:11942019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sanchez-Vega F, Mina M, Armenia J, Chatila
WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia
S, et al: Oncogenic signaling pathways in the cancer genome atlas.
Cell. 173:321–337.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang J, Wang J, Luan T, Zuo Y, Chen J,
Zhang H, Ye Z, Wang H and Hai B: Deubiquitinase USP9X regulates the
invasion of prostate cancer cells by regulating the ERK pathway and
mitochondrial dynamics. Oncol Rep. 41:3292–3304. 2019.PubMed/NCBI
|
|
69
|
Hu W, Su Y, Fei X, Wang X, Zhang G, Su C,
Du T, Yang T, Wang G, Tang Z and Zhang J: Ubiquitin specific
peptidase 19 is a prognostic biomarker and affect the proliferation
and migration of clear cell renal cell carcinoma. Oncol Rep.
43:1964–1974. 2020.PubMed/NCBI
|
|
70
|
Meng Y, Hong C, Yang S, Qin Z, Yang L and
Huang Y: Roles of USP9X in cellular functions and tumorigenesis
(Review). Oncol Lett. 26:5062023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wan YF, Zhang CY, Cheng XW, Liu LS, Zhou
T, Gao JK, Zhu HQ and Liu YH: USP9X expression is functionally
related to laryngeal cancer. J Cancer. 14:591–599. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jaiswal A, Murakami K, Elia A, Shibahara
Y, Done SJ, Wood SA, Donato NJ, Ohashi PS and Reedijk M:
Therapeutic inhibition of USP9x-mediated Notch signaling in
triple-negative breast cancer. Proc Natl Acad Sci USA.
118:e21015921182021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jie X, Fong WP, Zhou R, Zhao Y, Zhao Y,
Meng R, Zhang S, Dong X, Zhang T, Yang K, et al: USP9X-mediated
KDM4C deubiquitination promotes lung cancer radioresistance by
epigenetically inducing TGF-β2 transcription. Cell Death Differ.
28:2095–2111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhu Y, Gu L, Lin X, Zhou X, Lu B, Liu C,
Lei C, Zhou F, Zhao Q, Prochownik EV and Li Y: USP19 exacerbates
lipogenesis and colorectal carcinogenesis by stabilizing ME1. Cell
Rep. 37:1101742021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee JG, Kim W, Gygi S and Ye Y:
Characterization of the deubiquitinating activity of USP19 and its
role in endoplasmic reticulum-associated degradation. J Biol Chem.
289:3510–3517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dong Z, Guo S, Wang Y, Zhang J, Luo H,
Zheng G, Yang D, Zhang T, Yan L, Song L, et al: USP19 Enhances
MMP2/MMP9-Mediated tumorigenesis in gastric cancer. Onco Targets
Ther. 13:8495–8510. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tyagi A, Karapurkar JK, Colaco JC,
Sarodaya N, Antao AM, Kaushal K, Haq S, Chandrasekaran AP, Das S,
Singh V, et al: USP19 Negatively Regulates p53 and promotes
cervical cancer progression. Mol Biotechnol. 66:2032–2045. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li X, Yuan J, Song C, Lei Y, Xu J, Zhang
G, Wang W and Song G: Deubiquitinase USP39 and E3 ligase TRIM26
balance the level of ZEB1 ubiquitination and thereby determine the
progression of hepatocellular carcinoma. Cell Death Diffe.
28:2315–2332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhu X, Ma J, Lu M, Liu Z, Sun Y and Chen
H: The Deubiquitinase USP39 promotes esophageal squamous cell
carcinoma malignancy as a splicing factor. Genes (Basel).
13:8192022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang Z, Liu W, Bao X, Sun T, Wang J, Li M
and Liu C: USP39 facilitates breast cancer cell proliferation
through stabilization of FOXM1. Am J Cancer Res. 12:3644–3661.
2022.PubMed/NCBI
|
|
81
|
Yuan J, Li X, Zhang Y, Zhang G, Cheng W,
Wang W, Lei Y and Song G: USP39 attenuates the antitumor activity
of cisplatin on colon cancer cells dependent on p53. Cell Biol
Toxicol. 39:1995–2010. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang J: Current developments of targeting
the p53 signaling pathway for cancer treatment. Pharmacol Ther.
220:1077202021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Joerger AC and Fersht AR: The p53 Pathway:
Origins, inactivation in cancer, and emerging therapeutic
approaches. Annu Rev Biochem. 85:375–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lahalle A, Lacroix M, De Blasio C, Cissé
MY, Linares LK and Le Cam L: The p53 pathway and metabolism: The
tree that hides the forest. Cancers (Basel). 13:1332021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhao Y, Yu H and Hu W: The regulation of
MDM2 oncogene and its impact on human cancers. Acta Biochim Biophys
Sin (Shanghai). 46:180–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kwon SK, Saindane M and Baek KH: p53
stability is regulated by diverse deubiquitinating enzymes. Biochim
Biophys Acta Rev Cancer. 1868:404–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Klein AM, de Queiroz RM, Venkatesh D and
Prives C: The roles and regulation of MDM2 and MDMX: It is not just
about p53. Genes Dev. 35:575–601. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ren Y, Zhao P, Liu J, Yuan Y, Cheng Q, Zuo
Y, Qian L, Liu C, Guo T, Zhang L, et al: Deubiquitinase USP2a
sustains interferons antiviral activity by restricting
ubiquitination of activated STAT1 in the Nucleus. PLoS Pathog.
12:e10057642016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li Y, He X, Wang S, Shu HB and Liu Y:
USP2a positively regulates TCR-induced NF-κB activation by bridging
MALT1-TRAF6. Protein Cell. 4:62–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Allende-Vega N, Sparks A, Lane DP and
Saville MK: MdmX is a substrate for the deubiquitinating enzyme
USP2a. Oncogene. 29:432–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xiong B, Huang J, Liu Y, Zou M, Zhao Z,
Gong J, Wu X and Qiu C: Ubiquitin-specific protease 2a promotes
hepatocellular carcinoma progression via deubiquitination and
stabilization of RAB1A. Cell Oncol (Dordr). 44:329–343. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Selvendiran K, Ahmed S, Dayton A, Ravi Y,
Kuppusamy ML, Bratasz A, Rivera BK, Kálai T, Hideg K and Kuppusamy
P: HO-3867, a synthetic compound, inhibits the migration and
invasion of ovarian carcinoma cells through downregulation of fatty
acid synthase and focal adhesion kinase. Mol Cancer Res.
8:1188–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim J, Keay SK, You S, Loda M and Freeman
MR: A synthetic form of frizzled 8-associated antiproliferative
factor enhances p53 stability through USP2a and MDM2. PLoS One.
7:e503922012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stevenson LF, Sparks A, Allende-Vega N,
Xirodimas DP, Lane DP and Saville MK: The deubiquitinating enzyme
USP2a regulates the p53 pathway by targeting Mdm2. EMBO J.
26:976–986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ballar Kirmizibayrak P,
Erbaykent-Tepedelen B, Gozen O and Erzurumlu Y: Divergent
modulation of proteostasis in prostate cancer. Adv Exp Med Biol.
1233:117–151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Park SH, Fong KW, Kim J, Wang F, Lu X, Lee
Y, Brea LT, Wadosky K, Guo C, Abdulkadir SA, et al:
Posttranslational regulation of FOXA1 by Polycomb and BUB3/USP7
deubiquitin complex in prostate cancer. Sci Adv. 7:eabe22612021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sun T, Lee GS, Oh WK, Pomerantz M, Yang M,
Xie W, Freedman ML and Kantoff PW: Single-nucleotide polymorphisms
in p53 pathway and aggressiveness of prostate cancer in a Caucasian
population. Clin Cancer Res. 16:5244–5251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ren X, Jiang M, Ding P, Zhang X, Zhou X,
Shen J, Liu D, Yan X and Ma Z: Ubiquitin-specific protease 28: The
decipherment of its dual roles in cancer development. Exp Hematol
Oncol. 12:272023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen L, Xu Z, Li Q, Zheng C, Du Y, Yuan R
and Peng X: USP28 facilitates pancreatic cancer progression through
activation of Wnt/β-catenin pathway via stabilising FOXM1. Cell
Death Dis. 12:8872021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhao LJ, Zhang T, Feng XJ, Chang J, Suo
FZ, Ma JL, Liu YJ, Liu Y, Zheng YC and Liu HM: USP28 contributes to
the proliferation and metastasis of gastric cancer. J Cell Biochem.
120:7657–7666. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang H, Meng Q, Ding Y, Xiong M, Zhu M,
Yang Y, Su H, Gu L, Xu Y, Shi L, et al: USP28 and USP25 are
downregulated by Vismodegib in vitro and in colorectal cancer cell
lines. FEBS J. 288:1325–1342. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Devrim T, Ataç F, Devrim AK and Balcı M:
The concomitant use of USP28 and p53 to predict the progression of
urothelial carcinoma of the bladder. Pathol Res Pract.
216:1527742020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fong CS, Mazo G, Das T, Goodman J, Kim M,
O'Rourke BP, Izquierdo D and Tsou MF: 53BP1 and USP28 mediate
p53-dependent cell cycle arrest in response to centrosome loss and
prolonged mitosis. Elife. 5:e162702016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tzavlaki K and Moustakas A: TGF-β
Signaling. Biomolecules. 10:4872020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Derynck R and Budi EH: Specificity,
versatility, and control of TGF-β family signaling. Sci Signal.
12:eaav51832019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hata A and Chen YG: TGF-β Signaling from
Receptors to Smads. Cold Spring Harb Perspect Biol. 8:a0220612016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Peng D, Fu M, Wang M, Wei Y and Wei X:
Targeting TGF-β signal transduction for fibrosis and cancer
therapy. Mol Cancer. 21:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang Y, Alexander PB and Wang XF: TGF-β
family signaling in the control of cell proliferation and survival.
Cold Spring Harb Perspect Biol. 9:a0221452017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Haque S and Morris JC: Transforming growth
factor-β: A therapeutic target for cancer. Hum Vaccin Immunother.
13:1741–1750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sakai K, Ito C, Wakabayashi M, Kanzaki S,
Ito T, Takada S, Toshimori K, Sekita Y and Kimura T: Usp26 mutation
in mice leads to defective spermatogenesis depending on genetic
background. Sci Rep. 9:137572019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tang J, Luo Y and Xiao L: USP26 promotes
anaplastic thyroid cancer progression by stabilizing TAZ. Cell
Death Dis. 13:3262022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ye Y, Li M, Pan Q, Fang X, Yang H, Dong B,
Yang J, Zheng Y, Zhang R and Liao Z: Machine learning-based
classification of deubiquitinase USP26 and its cell proliferation
inhibition through stabilizing KLF6 in cervical cancer. Comput Biol
Med. 168:1077452024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Li G, Qi HW, Dong HG, Bai P, Sun M and Liu
HY: Targeting deubiquitinating enzyme USP26 by microRNA-203
regulates Snail1's pro-metastatic functions in esophageal cancer.
Cancer Cell Int. 20:3552020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wosnitzer MS, Mielnik A, Dabaja A,
Robinson B, Schlegel PN and Paduch DA: Ubiquitin Specific Protease
26 (USP26) expression analysis in human testicular and extragonadal
tissues indicates diverse action of USP26 in cell differentiation
and tumorigenesis. PLoS One. 9:e986382014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dirac AM and Bernards R: The
deubiquitinating enzyme USP26 is a regulator of androgen receptor
signaling. Mol Cancer Res. 8:844–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Cai Q, Chen Y, Zhang D, Pan J, Xie Z, Ma
S, Liu C, Zuo J, Zhou X, Quan C, et al: Loss of epithelial AR
increase castration resistant stem-like prostate cancer cells and
promotes cancer metastasis via TGF-β1/EMT pathway. Transl Androl
Urol. 9:1013–1027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Allen-Petersen BL and Sears RC: Mission
Possible: Advances in MYC Therapeutic Targeting in Cancer.
BioDrugs. 33:539–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Blackwood EM and Eisenman RN: Max: A
helix-loop-helix zipper protein that forms a sequence-specific
DNA-binding complex with Myc. Science. 251:1211–1217. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Blackwood EM, Lüscher B, Kretzner L and
Eisenman RN: The Myc:Max protein complex and cell growth
regulation. Cold Spring Harb Symp Quant Biol. 56:109–117. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Helander S, Montecchio M, Pilstål R, Su Y,
Kuruvilla J, Elvén M, Ziauddin JME, Anandapadamanaban M, Cristobal
S, Lundström P, et al: Pre-anchoring of Pin1 to unphosphorylated
c-Myc in a fuzzy complex regulates c-Myc activity. Structure.
23:2267–2279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kato GJ, Barrett J, Villa-Garcia M and
Dang CV: An amino-terminal c-myc domain required for neoplastic
transformation activates transcription. Mol Cell Biol.
10:5914–5920. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Prendergast GC and Ziff EB:
Methylation-sensitive sequence-specific DNA binding by the c-Myc
basic region. Science. 251:186–189. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ashrafizadeh M, Zarabi A, Hushmandi K,
Moghadam ER, Hashemi F, Daneshi S, Hashemi F, Tavakol S,
Mohammadinejad R, Najafi M, et al: C-Myc signaling pathway in
treatment and prevention of brain tumors. Curr Cancer Drug Targets.
21:2–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Park JH, Pyun WY and Park HW: Cancer
Metabolism: Phenotype, signaling and therapeutic targets. Cells.
9:23082020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chen H, Liu H and Qing G: Targeting
oncogenic Myc as a strategy for cancer treatment. Signal Transduct
Target Ther. 3:52018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yang S, Wang J, Guo S, Huang D, Lorigados
IB, Nie X, Lou D, Li Y, Liu M, Kang Y, et al: Transcriptional
activation of USP16 gene expression by NFκB signaling. Mol Brain.
12:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zheng J, Chen C, Guo C, Caba C, Tong Y and
Wang H: The pleiotropic ubiquitin-specific peptidase 16 and its
many substrates. Cells. 12:8862023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu S, Li H, Zhu Y, Ma X, Shao Z, Yang Z,
Cai C, Wu Z, Li M, Gong W and Wu X: LncRNA MNX1-AS1 sustains
inactivation of Hippo pathway through a positive feedback loop with
USP16/IGF2BP3 axis in gallbladder cancer. Cancer Lett.
547:2158622022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xu G, Yang Z, Ding Y, Liu Y, Zhang L, Wang
B, Tang M, Jing T, Jiao K, Xu X, et al: The deubiquitinase USP16
functions as an oncogenic factor in K-RAS-driven lung
tumorigenesis. Oncogene. 40:5482–5494. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li Y, Rao Y, Zhu H, Jiang B and Zhu M:
USP16 regulates the stability and function of LDL receptor by
Deubiquitination. Int Heart J. 61:1034–1040. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ge J, Yu W, Li J, Ma H, Wang P, Zhou Y,
Wang Y, Zhang J and Shi G: USP16 regulates castration-resistant
prostate cancer cell proliferation by deubiquitinating and
stablizing c-Myc. J Exp Clin Cancer Res. 40:592021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
He L, Liu X, Yang J, Li W, Liu S, Liu X,
Yang Z, Ren J, Wang Y, Shan L, et al: Imbalance of the reciprocally
inhibitory loop between the ubiquitin-specific protease USP43 and
EGFR/PI3K/AKT drives breast carcinogenesis. Cell Res. 28:934–951.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ye DX, Wang SS, Huang Y, Wang XJ and Chi
P: USP43 directly regulates ZEB1 protein, mediating proliferation
and metastasis of colorectal cancer. J Cancer. 12:404–416. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Xue Y, Li M, Hu J, Song Y, Guo W, Miao C,
Ge D, Hou Y, Wang X, Huang X, et al: Ca(v)2.2-NFAT2-USP43 axis
promotes invadopodia formation and breast cancer metastasis through
cortactin stabilization. Cell Death Dis. 13:8122022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Sun Q, Zhang H, Zong L, Julaiti A, Jing X
and Zhang L: Prognostic value and oncogenic effects of
ubiquitin-specific protease 43 in lung squamous cell carcinoma.
Tohoku J Exp Med. 257:135–145. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhao Z, Lin Z, Guo X, Al-Danakh A, He H,
Qin H, Ma C, Zhang N and Tan G: Ubiquitin-specific protease 43
impacts pancreatic ductal adenocarcinoma prognosis by altering its
proliferation and infiltration of surrounding immune cells. J
Immunol Res. 2023:43113882023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lavaud M, Mullard M, Tesfaye R, Amiaud J,
Legrand M, Danieau G, Brion R, Morice S, Regnier L, Dupuy M, et al:
Overexpression of the Ubiquitin Specific Proteases USP43, USP41,
USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of
Osteosarcoma Tumor Growth and Lung Metastasis Development by the
USP Antagonist PR619. Cells. 10:22682021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li M, Yu J, Ju L, Wang Y, Jin W, Zhang R,
Xiang W, Ji M, Du W, Wang G, et al: USP43 stabilizes c-Myc to
promote glycolysis and metastasis in bladder cancer. Cell Death
Dis. 15:442024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zou G and Park JI: Wnt signaling in liver
regeneration, disease, and cancer. Clin Mol Hepatol. 29:33–50.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Steinhart Z and Angers S: Wnt signaling in
development and tissue homeostasis. Development. 145:dev1465892018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hafez N, Modather El-Awadly Z and Arafa
RK: UCH-L3 structure and function: Insights about a promising drug
target. Eur J Med Chem. 227:1139702022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhu T, Xu L, Peng J, Chen M and Xu H:
Molecular characteristics and immune function of ubiquitin
C-terminal hydrolase-L3 in Macrobrachium nipponense. Fish Shellfish
Immunol. 121:295–304. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ma Q, Lu Q, Lei X, Zhao J, Sun W, Wang J,
Zhu Q and Huang D: UCHL3 promotes hepatocellular carcinoma cell
migration by de-ubiquitinating and stabilizing Vimentin. Front
Oncol. 13:10884752023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zhang Y, Liu JB, Liu J, Liu M, Liu HL and
Zhang J: UCHL3 promotes cervical cancer development and metastasis
by stabilizing NRF2 via deubiquitination. Biochem Biophys Res
Commun. 641:132–138. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li J, Zheng Y, Li X, Dong X, Chen W, Guan
Z and Zhang C: UCHL3 promotes proliferation of colorectal cancer
cells by regulating SOX12 via AKT/mTOR signaling pathway. Am J
Transl Res. 12:6445–6454. 2020.PubMed/NCBI
|
|
149
|
Moroney MR, Woodruff E, Qamar L, Bradford
AP, Wolsky R, Bitler BG and Corr BR: Inhibiting Wnt/beta-catenin in
CTNNB1-mutated endometrial cancer. Mol Carcinog. 60:511–523. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Liu T, Fan MQ, Xie XX, Shu QP, Du XH, Qi
LZ, Zhang XD, Zhang MH, Shan G, Du RL and Li SZ: Activation of
CTNNB1 by deubiquitinase UCHL3-mediated stabilization facilitates
bladder cancer progression. J Transl Med. 21:6562023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhou L, Qin B, Yassine DM, Luo M, Liu X,
Wang F and Wang Y: Structure and function of the highly homologous
deubiquitinases ubiquitin specific peptidase 25 and 28: Insights
into their pathophysiological and therapeutic roles. Biochem
Pharmacol. 213:1156242023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhong B, Liu X, Wang X, Liu X, Li H,
Darnay BG, Lin X, Sun SC and Dong C: Ubiquitin-specific protease 25
regulates TLR4-dependent innate immune responses through
deubiquitination of the adaptor protein TRAF3. Sci Signal.
6:ra352013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu B, Miao X, Shen J, Lou L, Chen K, Mei
F, Chen M, Su X, Du X, Zhu Z, et al: USP25 ameliorates diabetic
nephropathy by inhibiting TRAF6-mediated inflammatory responses.
Int Immunopharmacol. 124((Pt A)): 1108772023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ye B, Zhou H, Chen Y, Luo W, Lin W, Zhao
Y, Han J, Han X, Huang W, Wu G, et al: USP25 Ameliorates
Pathological Cardiac Hypertrophy by Stabilizing SERCA2a in
Cardiomyocytes. Circ Res. 132:465–480. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zheng Q, Song B, Li G, Cai F, Wu M, Zhao
Y, Jiang L, Guo T, Shen M, Hou H, et al: USP25 inhibition
ameliorates Alzheimer's pathology through the regulation of APP
processing and Aβ generation. J Clin Invest. 132:e1521702022.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Cheng H, Li X, Wang C, Chen Y, Li S, Tan
J, Tan B and He Y: Inhibition of tankyrase by a novel small
molecule significantly attenuates prostate cancer cell
proliferation. Cancer Lett. 443:80–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Gu Z, Lin C, Hu J, Xia J, Wei S and Gao D:
USP34 Regulated Human Pancreatic Cancer Cell Survival via AKT and
PKC Pathways. Biol Pharm Bull. 42:573–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Guo YC, Wang MY, Zhang SW, Wu YS, Zhou CC,
Zheng RX, Shao B, Wang Y, Xie L, Liu WQ, et al: Ubiquitin-specific
protease USP34 controls osteogenic differentiation and bone
formation by regulating BMP2 signaling. EMBO J. 37:e993982018.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhu Q, Liang P, Meng H, Li F, Miao W, Chu
C, Wang W, Li D, Chen C, Shi Y, et al: Stabilization of Pin1 by
USP34 promotes Ubc9 isomerization and protein sumoylation in glioma
stem cells. Nat Commun. 15:402024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Lui TT, Lacroix C, Ahmed SM, Goldenberg
SJ, Leach CA, Daulat AM and Angers S: The ubiquitin-specific
protease USP34 regulates axin stability and Wnt/β-catenin
signaling. Mol Cell Biol. 31:2053–2065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhao Y, Yan Y, Ma R, Lv X, Zhang L, Wang
J, Zhu W, Zhao L, Jiang L, Zhao L, et al: Expression signature of
six-snoRNA serves as novel non-invasive biomarker for diagnosis and
prognosis prediction of renal clear cell carcinoma. J Cell Mol Med.
24:2215–2228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Ma S, Meng Z, Chen R and Guan KL: The
Hippo Pathway: Biology and Pathophysiology. Annu Rev Biochem.
88:577–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Lee U, Cho EY and Jho EH: Regulation of
Hippo signaling by metabolic pathways in cancer. Biochim Biophys
Acta Mol Cell Res. 1869:1192012022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wu Z and Guan KL: Hippo Signaling in
Embryogenesis and Development. Trends Biochem Sci. 46:51–63. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Cao Z, An L, Han Y, Jiao S and Zhou Z: The
Hippo signaling pathway in gastric cancer. Acta Biochim Biophys Sin
(Shanghai). 55:893–903. 2023.PubMed/NCBI
|
|
166
|
Zhu N, Yang R, Wang X, Yuan L, Li X, Wei F
and Zhang L: The Hippo signaling pathway: From multiple signals to
the hallmarks of cancers. Acta Biochim Biophys Sin (Shanghai).
55:904–913. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Gu Y, Wu S, Fan J, Meng Z, Gao G, Liu T,
Wang Q, Xia H, Wang X and Wu K: CYLD regulates cell ferroptosis
through Hippo/YAP signaling in prostate cancer progression. Cell
Death Dis. 15:792024. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Abdul Rehman SA, Kristariyanto YA, Choi
SY, Nkosi PJ, Weidlich S, Labib K, Hofmann K and Kulathu Y: MINDY-1
is a member of an evolutionarily conserved and structurally
distinct new family of deubiquitinating enzymes. Mol Cell.
63:146–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Tang J, Luo Y, Long G and Zhou L: MINDY1
promotes breast cancer cell proliferation by stabilizing estrogen
receptor α. Cell Death Dis. 12:9372021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Xia BL, Liu KW, Huang HX, Shen MM, Wang B
and Gao J: Deubiquitinating enzyme MINDY1 is an independent risk
factor for the maintenance of stemness and poor prognosis in liver
cancer cells. Zhonghua Gan Zang Bing Za Zhi. 31:518–523. 2023.(In
Chinese). PubMed/NCBI
|
|
171
|
James C, Zhao TY, Rahim A, Saxena P,
Muthalif NA, Uemura T, Tsuneyoshi N, Ong S, Igarashi K, Lim CY, et
al: MINDY1 Is a Downstream Target of the Polyamines and Promotes
Embryonic Stem Cell Self-Renewal. Stem Cells. 36:1170–1178. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Luo Y, Zhou J, Tang J, Zhou F, He Z and
Liu T and Liu T: MINDY1 promotes bladder cancer progression by
stabilizing YAP. Cancer Cell Int. 21:3952021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Dai C, Heemers H and Sharifi N: Androgen
signaling in prostate cancer. Cold Spring Harb Perspect Med.
7:a0304522017. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Deng CC, Zhu DH, Chen YJ, Huang TY, Peng
Y, Liu SY, Lu P, Xue YH, Xu YP, Yang B and Rong Z: TRAF4 Promotes
Fibroblast Proliferation in Keloids by Destabilizing p53 via
Interacting with the Deubiquitinase USP10. J Invest Dermatol.
139:1925–1935.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS,
Yang GF, Wang P and Fu GH: Prognostic significance of USP10 as a
tumor-associated marker in gastric carcinoma. Tumour Biol.
35:3845–3853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Lu C, Ning Z, Wang A, Chen D, Liu X, Xia
T, Tekcham DS, Wang W, Li T, Liu X, et al: USP10 suppresses tumor
progression by inhibiting mTOR activation in hepatocellular
carcinoma. Cancer Lett. 436:139–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ye Z, Chen J, Huang P, Xuan Z and Zheng S:
Ubiquitin-specific peptidase 10, a deubiquitinating enzyme:
Assessing its role in tumor prognosis and immune response. Front
Oncol. 12:9901952022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Kong L and Jin X: Dysregulation of
deubiquitination in breast cancer. Gene. 902:1481752024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
An T, Lu Y, Yan X and Hou J: Insights Into
the Properties, Biological Functions, and Regulation of USP21.
Front Pharmacol. 13:9440892022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Chen Y, Wang L, Jin J, Luan Y, Chen C, Li
Y, Chu H, Wang X, Liao G, Yu Y, et al: p38 inhibition provides
anti-DNA virus immunity by regulation of USP21 phosphorylation and
STING activation. J Exp Med. 214:991–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Liu J, Kruswick A, Dang H, Tran AD, Kwon
SM, Wang XW and Oberdoerffer P: Ubiquitin-specific protease 21
stabilizes BRCA2 to control DNA repair and tumor growth. Nat
Commun. 8:1372017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Chen Y, Zhou B and Chen D: USP21 promotes
cell proliferation and metastasis through suppressing EZH2
ubiquitination in bladder carcinoma. Onco Targets Ther. 10:681–689.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao
B, Dong H, Wei J, Song J, Zhang DD and Fang D: USP22 antagonizes
p53 transcriptional activation by deubiquitinating Sirt1 to
suppress cell apoptosis and is required for mouse embryonic
development. Mol Cell. 46:484–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Feng T, Ling S, Xu C, Ying L, Su D and Xu
X: Ubiquitin-specific peptidase 22 in cancer. Cancer Lett.
514:30–37. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zeng K, Xie W, Wang C, Wang S, Liu W, Su
Y, Lin L, Zou R, Sun G, Zhou B, et al: USP22 upregulates
ZEB1-mediated VEGFA transcription in hepatocellular carcinoma. Cell
Death Dis. 14:1942023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Xu G, Cai J, Wang L, Jiang L, Huang J, Hu
R and Ding F: MicroRNA-30e-5p suppresses non-small cell lung cancer
tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3
signaling. Exp Cell Res. 362:268–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yuan X, Wang H, Xu A, Zhu X, Zhan Y and
Wang W: Ubiquitin-specific peptidase 22 promotes proliferation and
metastasis in human colon cancer. Oncol Lett. 18:5567–5576.
2019.PubMed/NCBI
|
|
188
|
Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ
and Jiang GS: Silencing USP22 by asymmetric structure of
interfering RNA inhibits proliferation and induces cell cycle
arrest in bladder cancer cells. Mol Cell Biochem. 346:11–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Guo J, Zhao J, Fu W, Xu Q and Huang D:
Immune Evasion and Drug Resistance Mediated by USP22 in Cancer:
Novel Targets and Mechanisms. Front Immunol. 13:9183142022.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Nag N and Dutta S: Deubiquitination in
prostate cancer progression: Role of USP22. J Cancer Metastasis
Treat. 6:162020.PubMed/NCBI
|
|
191
|
Li C, Zeng X, Qiu S, Gu Y and Zhang Y:
Nanomedicine for urologic cancers: Diagnosis and management. Semin
Cancer Biol. 86:463–475. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Schauer NJ, Magin RS, Liu X, Doherty LM
and Buhrlage SJ: Advances in Discovering Deubiquitinating Enzyme
(DUB) Inhibitors. J Med Chem. 63:2731–2750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Lee JE, Park CM and Kim JH: USP7
deubiquitinates and stabilizes EZH2 in prostate cancer cells. Genet
Mol Biol. 43:e201903382020. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhang W, Zhang J, Xu C, Zhang S, Bian S,
Jiang F, Ni W, Qu L, Lu C, Ni R, et al: Ubiquitin-specific protease
7 is a drug-able target that promotes hepatocellular carcinoma and
chemoresistance. Cancer Cell Int. 20:282020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Chen H, Zhu X, Sun R, Ma P, Zhang E, Wang
Z, Fan Y, Zhou G and Mao R: Ubiquitin-specific protease 7 is a
druggable target that is essential for pancreatic cancer growth and
chemoresistance. Invest New Drugs. 38:1707–1716. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Wang S, Kollipara RK, Srivastava N, Li R,
Ravindranathan P, Hernandez E, Freeman E, Humphries CG, Kapur P,
Lotan Y, et al: Ablation of the oncogenic transcription factor ERG
by deubiquitinase inhibition in prostate cancer. Proc Natl Acad Sci
USA. 111:4251–4256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Stolte B, Iniguez AB, Dharia NV, Robichaud
AL, Conway AS, Morgan AM, Alexe G, Schauer NJ, Liu X, Bird GH, et
al: Genome-scale CRISPR-Cas9 screen identifies druggable
dependencies in TP53 wild-type Ewing sarcoma. J Exp Med.
215:2137–2155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Turnbull AP, Ioannidis S, Krajewski WW,
Pinto-Fernandez A, Heride C, Martin ACL, Tonkin LM, Townsend EC,
Buker SM, Lancia DR, et al: Molecular basis of USP7 inhibition by
selective small-molecule inhibitors. Nature. 550:481–486. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Xu S, Adisetiyo H, Tamura S, Grande F,
Garofalo A, Roy-Burman P and Neamati N: Dual inhibition of survivin
and MAOA synergistically impairs growth of PTEN-negative prostate
cancer. Br J Cancer. 113:242–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Tolcher AW, Quinn DI, Ferrari A, Ahmann F,
Giaccone G, Drake T, Keating A and de Bono JS: A phase II study of
YM155, a novel small-molecule suppressor of survivin, in
castration-resistant taxane-pretreated prostate cancer. Ann Oncol.
23:968–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Chow PM, Dong JR, Chang YW, Kuo KL, Lin
WC, Liu SH and Huang KH: The UCHL5 inhibitor b-AP15 overcomes
cisplatin resistance via suppression of cancer stemness in
urothelial carcinoma. Mol Ther Oncolytics. 26:387–398. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Jamroze A, Chatta G and Tang DG: Androgen
receptor (AR) heterogeneity in prostate cancer and therapy
resistance. Cancer Lett. 518:1–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
He Y, Xu W, Xiao YT, Huang H, Gu D and Ren
S: Targeting signaling pathways in prostate cancer: mechanisms and
clinical trials. Signal Transduct Target Ther. 7:1982022.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Schellhammer PF and Davis JW: An
evaluation of bicalutamide in the treatment of prostate cancer.
Clin Prostate Cancer. 2:213–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Chi KN, Agarwal N, Bjartell A, Chung BH,
Pereira de Santana Gomes AJ, Given R, Juárez Soto Á, Merseburger
AS, Özgüroğlu M, Uemura H, et al: Apalutamide for metastatic,
castration-sensitive prostate cancer. N Engl J Med. 381:13–24.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Armstrong AJ, Szmulewitz RZ, Petrylak DP,
Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi
T, Shore ND, et al: ARCHES: A Randomized, phase III study of
androgen deprivation therapy with enzalutamide or placebo in men
with metastatic hormone-sensitive prostate cancer. J Clin Oncol.
37:2974–2986. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Isaacsson Velho P and Antonarakis ES:
PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert
Rev Clin Pharmacol. 11:475–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 Blockade in Cancer Immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kuang Z, Liu X, Zhang N, Dong J, Sun C,
Yin M, Wang Y, Liu L, Xiao D, Zhou X, et al: USP2 promotes tumor
immune evasion via deubiquitination and stabilization of PD-L1.
Cell Death Differ. 30:2249–2264. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sweeney PL, Suri Y, Basu A, Koshkin VS and
Desai A: Mechanisms of tyrosine kinase inhibitor resistance in
renal cell carcinoma. Cancer Drug Resist. 6:858–873. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Ebrahimi N, Fardi E, Ghaderi H, Palizdar
S, Khorram R, Vafadar R, Ghanaatian M, Rezaei-Tazangi F, Baziyar P,
Ahmadi A, et al: Receptor tyrosine kinase inhibitors in cancer.
Cell Mol Life Sci. 80:1042023. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
McCann AP, Smyth P, Cogo F, McDaid WJ,
Jiang L, Lin J, Evergren E, Burden RE, Van Schaeybroeck S, Scott CJ
and Burrows JF: USP17 is required for trafficking and oncogenic
signaling of mutant EGFR in NSCLC cells. Cell Commun Signal.
16:772018. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Popova NV and Jücker M: The Role of mTOR
signaling as a therapeutic target in cancer. Int J Mol Sci.
22:17432021. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Hasskarl J: Everolimus. Recent Results
Cancer Res. 211:101–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Stock C, Zaccagnini M, Schulze M, Teber D
and Rassweiler JJ: Temsirolimus. Recent Results Cancer Res.
184:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Porta C, Calvo E, Climent MA, Vaishampayan
U, Osanto S, Ravaud A, Bracarda S, Hutson TE, Escudier B, Grünwald
V, et al: Efficacy and safety of everolimus in elderly patients
with metastatic renal cell carcinoma: an exploratory analysis of
the outcomes of elderly patients in the RECORD-1 Trial. Eur Urol.
61:826–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Roldán-Romero JM, Valdivia C, Santos M,
Lanillos J, Maroto P, Anguera G, Calsina B, Martinez-Montes A,
Monteagudo M, Mellid S, et al: Deubiquitinase USP9X loss sensitizes
renal cancer cells to mTOR inhibition. Int J Cancer. 153:1300–1312.
2023. View Article : Google Scholar : PubMed/NCBI
|