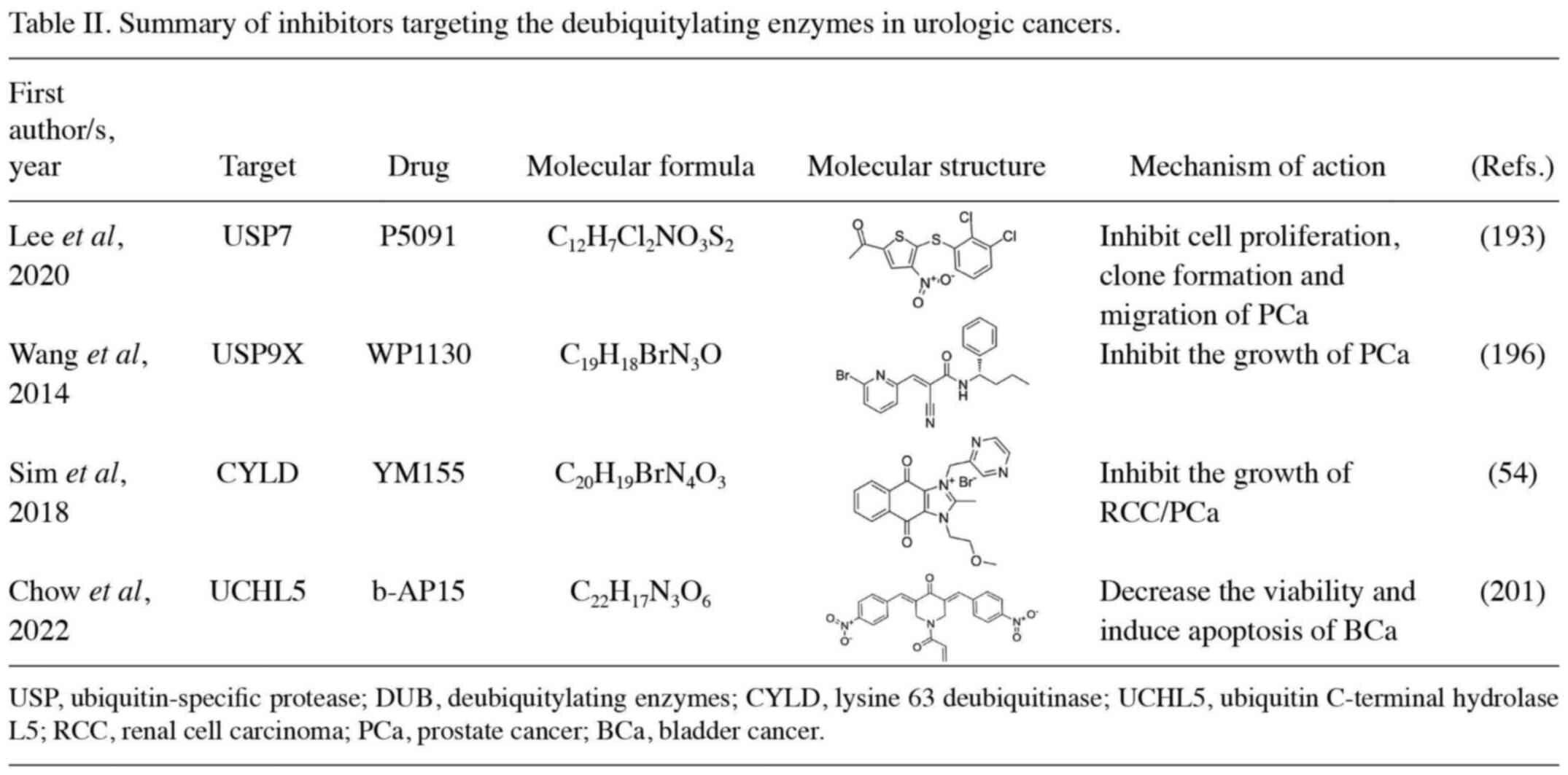
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global Cancer Statistics 2020:
GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36
Cancers in 185 Countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nguyen-Nielsen M and Borre M: Diagnostic
and Therapeutic Strategies for Prostate Cancer. Semin Nucl Med.
46:484–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bahadoram S, Davoodi M, Hassanzadeh S,
Bahadoram M, Barahman M and Mafakher L: Renal cell carcinoma: An
overview of the epidemiology, diagnosis, and treatment. G Ital
Nefrol. 39:2022–vol3. 2022.PubMed/NCBI
|
|
4
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder Cancer: A Review. JAMA. 324:1980–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gao H, Yin J, Ji C, Yu X, Xue J, Guan X,
Zhang S, Liu X and Xing F: Targeting ubiquitin specific proteases
(USPs) in cancer immunotherapy: From basic research to preclinical
application. J Exp Clin Cancer Res. 42:2252023. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng LL, Wang LT, Pang YW, Sun LP and Shi
L: Recent advances in the development of deubiquitinases inhibitors
as antitumor agents. Eur J Med Chem. 266:1161612024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Popovic D, Vucic D and Dikic I:
Ubiquitination in disease pathogenesis and treatment. Nat Med.
20:1242–1253. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dagar G, Kumar R, Yadav KK, Singh M and
Pandita TK: Ubiquitination and deubiquitination: Implications on
cancer therapy. Biochim Biophys Acta Gene Regul Mech.
1866:1949792023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han S, Wang R, Zhang Y, Li X, Gan Y, Gao
F, Rong P, Wang W and Li W: The role of ubiquitination and
deubiquitination in tumor invasion and metastasis. Int J Biol Sci.
18:2292–2303. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ye Z, Yang J, Jiang H and Zhan X: The
roles of protein ubiquitination in tumorigenesis and targeted drug
discovery in lung cancer. Front Endocrinol (Lausanne).
14:12201082023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou Z, Zheng K, Zhou S, Yang Y, Chen J
and Jin X: E3 ubiquitin ligases in nasopharyngeal carcinoma and
implications for therapies. J Mol Med (Berl). 101:1543–1565. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Dewson G, Eichhorn PJA and Komander D:
Deubiquitinases in cancer. Nat Rev Cancer. 23:842–862. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coutte L, Dreyer C, Sablin MP, Faivre S
and Raymond E: PI3K-AKT-mTOR pathway and cancer. Bull Cancer.
99:173–180. 2012.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cantley LC: The phosphoinositide 3-kinase
pathway. Science. 296:1655–1657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vanhaesebroeck B, Whitehead MA and Piñeiro
R: Molecules in medicine mini-review: Isoforms of PI3K in biology
and disease. J Mol Med (Berl). 94:5–11. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gagliardi PA, Puliafito A and Primo L:
PDK1: At the crossroad of cancer signaling pathways. Semin Cancer
Biol. 48:27–35. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tan AC: Targeting the PI3K/Akt/mTOR
pathway in non-small cell lung cancer (NSCLC). Thorac Cancer.
11:511–518. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yu Z, Li H, Zhu J, Wang H and Jin X: The
roles of E3 ligases in Hepatocellular carcinoma. Am J Cancer Res.
12:1179–1214. 2022.PubMed/NCBI
|
|
19
|
Ngeow J and Eng C: PTEN in Hereditary and
Sporadic Cancer. Cold Spring Harb Perspect Med. 10:a0360872020.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Noorolyai S, Shajari N, Baghbani E,
Sadreddini S and Baradaran B: The relation between PI3K/AKT
signalling pathway and cancer. Gene. 698:120–128. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Christine A, Park MK, Song SJ and Song MS:
The equilibrium of tumor suppression: DUBs as active regulators of
PTEN. Exp Mol Med. 54:1814–1821. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Saha G, Roy S, Basu M and Ghosh MK: USP7-a
crucial regulator of cancer hallmarks. Biochim Biophys Acta Rev
Cancer. 1878:1889032023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pozhidaeva A and Bezsonova I: USP7:
Structure, substrate specificity, and inhibition. DNA Repair
(Amst). 76:30–39. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Song MS, Salmena L, Carracedo A, Egia A,
Lo-Coco F, Teruya-Feldstein J and Pandolfi PP: The
deubiquitinylation and localization of PTEN are regulated by a
HAUSP-PML network. Nature. 455:813–817. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wang Q, Sun Z, Xia W, Sun L, Du Y, Zhang Y
and Jia Z: Role of USP13 in physiology and diseases. Front Mol
Biosci. 9:9771222022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin J, He J, Li X, Ni X and Jin X: The
role of ubiquitination and deubiquitination in PI3K/AKT/mTOR
pathway: A potential target for cancer therapy. Gene.
889:1478072023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cui X, Yu H, Yao J, Li J, Li Z and Jiang
Z: ncRNA-mediated overexpression of ubiquitin-specific proteinase
13 contributes to the progression of prostate cancer via modulating
AR signaling, DNA damage repair and immune infiltration. BMC
Cancer. 22:13502022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhao Y, Zhang B, Lei Y, Sun J, Zhang Y,
Yang S and Zhang X: Knockdown of USP39 induces cell cycle arrest
and apoptosis in melanoma. Tumour Biol. 37:13167–13176. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan J, Li X, Zhang G, Cheng W, Wang W,
Lei Y, Ma Q and Song G: USP39 mediates p21-dependent proliferation
and neoplasia of colon cancer cells by regulating the
p53/p21/CDC2/cyclin B1 axis. Mol Carcinog. 60:265–278. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yuan X, Sun X, Shi X, Jiang C, Yu D, Zhang
W, Guan W, Zhou J, Wu Y, Qiu Y and Ding Y: USP39 promotes the
growth of human hepatocellular carcinoma in vitro and in vivo.
Oncol Rep. 34:823–832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
An Y, Yang S, Guo K, Ma B and Wang Y:
Reduced USP39 expression inhibits malignant proliferation of
medullary thyroid carcinoma in vitro. World J Surg Oncol.
13:2552015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li KY, Zhang J, Jiang LC, Zhang B, Xia CP,
Xu K, Chen HY, Yang QZ, Liu SW and Zhu H: Knockdown of USP39 by
lentivirus-mediated RNA interference suppresses the growth of oral
squamous cell carcinoma. Cancer Biomark. 16:137–144. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xing Z, Sun F, He W, Wang Z, Song X and
Zhang F: Downregulation of ubiquitin-specific peptidase 39
suppresses the proliferation and induces the apoptosis of human
colorectal cancer cells. Oncol Lett. 15:5443–5450. 2018.PubMed/NCBI
|
|
34
|
Xu Y, Zhu MR, Zhang JY, Si GM and Lv JJ:
Knockdown of ubiquitin-specific peptidase 39 inhibits the malignant
progression of human renal cell carcinoma. Mol Med Rep.
17:4729–4735. 2018.PubMed/NCBI
|
|
35
|
Rolén U, Kobzeva V, Gasparjan N, Ovaa H,
Winberg G, Kisseljov F and Masucci MG: Activity profiling of
deubiquitinating enzymes in cervical carcinoma biopsies and cell
lines. Mol Carcinog. 45:260–269. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen Y, Fu D, Xi J, Ji Z, Liu T, Ma Y,
Zhao Y, Dong L, Wang Q and Shen X: Expression and clinical
significance of UCH37 in human esophageal squamous cell carcinoma.
Dig Dis Sci. 57:2310–2317. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang L, Chen YJ, Xu K, Wang YY, Shen XZ
and Tu RQ: High expression of UCH37 is significantly associated
with poor prognosis in human epithelial ovarian cancer. Tumour
Biol. 35:11427–11433. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chen Z, Niu X, Li Z, Yu Y, Ye X, Lu S and
Chen Z: Effect of ubiquitin carboxy-terminal hydrolase 37 on
apoptotic in A549 cells. Cell Biochem Funct. 29:142–148. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Cutts AJ, Soond SM, Powell S and Chantry
A: Early phase TGFβ receptor signalling dynamics stabilised by the
deubiquitinase UCH37 promotes cell migratory responses. Int J
Biochem Cell Biol. 43:604–612. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Cao Y, Yan X, Bai X, Tang F, Si P, Bai C,
Tuoheti K, Guo L, Yisha Z and Liu T and Liu T: UCHL5 Promotes
Proliferation and Migration of Bladder Cancer Cells by Activating
c-Myc via AKT/mTOR Signaling. Cancers (Basel). 14:55382022.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Oeckinghaus A, Hayden MS and Ghosh S:
Crosstalk in NF-κB signaling pathways. Nat Immunol. 12:695–708.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Lawrence T: The nuclear factor NF-kappaB
pathway in inflammation. Cold Spring Harb Perspect Biol.
1:a0016512009. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hayden MS and Ghosh S: Shared principles
in NF-kappaB signaling. Cell. 132:344–362. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Au PY and Yeh WC: Physiological roles and
mechanisms of signaling by TRAF2 and TRAF5. Adv Exp Med Biol.
597:32–47. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Zhang W, Zhang X, Wu XL, He LS, Zeng XF,
Crammer AC and Lipsky PE: Competition between TRAF2 and TRAF6
regulates NF-kappaB activation in human B lymphocytes. Chin Med Sci
J. 25:1–12. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Liu H, Zeng L, Yang Y, Guo C and Wang H:
Bcl-3: A Double-Edged Sword in Immune Cells and Inflammation. Front
Immunol. 13:8476992022. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Franzoso G, Bours V, Azarenko V, Park S,
Tomita-Yamaguchi M, Kanno T, Brown K and Siebenlist U: The
oncoprotein Bcl-3 can facilitate NF-kappa B-mediated
transactivation by removing inhibiting p50 homodimers from select
kappa B sites. EMBO J. 12:3893–3901. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Fujita T, Nolan GP, Liou HC, Scott ML and
Baltimore D: The candidate proto-oncogene bcl-3 encodes a
transcriptional coactivator that activates through NF-kappa B p50
homodimers. Genes Dev. 7:1354–1363. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tang W, Wang H, Claudio E, Tassi I, Ha HL,
Saret S and Siebenlist U: The oncoprotein and transcriptional
regulator Bcl-3 governs plasticity and pathogenicity of autoimmune
T cells. Immunity. 41:555–566. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Marín-Rubio JL, Raote I, Inns J,
Dobson-Stone C and Rajan N: CYLD in health and disease. Dis Model
Mech. 16:dmm0500932023. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Mathis BJ, Lai Y, Qu C, Janicki JS and Cui
T: CYLD-mediated signaling and diseases. Curr Drug Targets.
16:284–294. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Massoumi R: CYLD: A deubiquitination
enzyme with multiple roles in cancer. Future Oncol. 7:285–297.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sim MY, Yuen JSP and Go ML: Anti-survivin
effect of the small molecule inhibitor YM155 in RCC cells is
mediated by time-dependent inhibition of the NF-κB pathway. Sci
Rep. 8:102892018. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yuan H, Wei S, Ren Z, Li F, Liu B, Liu R
and Zhang X: KLHL21/CYLD signaling confers aggressiveness in
bladder cancer through inactivating NF-κB signaling. Int
Immunopharmacol. 114:1092022023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Man X, Piao C, Lin X, Kong C, Cui X and
Jiang Y: USP13 functions as a tumor suppressor by blocking the
NF-kB-mediated PTEN downregulation in human bladder cancer. J Exp
Clin Cancer Res. 38:2592019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Man X, Piao C, Lin X, Kong C, Cui X and
Jiang Y: Correction to: USP13 functions as a tumor suppressor by
blocking the NF-kB-mediated PTEN downregulation in human bladder
cancer. J Exp Clin Cancer Res. 40:3862021. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Yang GF, Zhang X, Su YG, Zhao R and Wang
YY: The role of the deubiquitinating enzyme DUB3/USP17 in cancer: A
narrative review. Cancer Cell Int. 21:4552021. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Han L, Yang J, Wang X, Wu Q, Yin S, Li Z,
Zhang J, Xing Y, Chen Z, Tsun A, et al: The E3 deubiquitinase USP17
is a positive regulator of retinoic acid-related orphan nuclear
receptor γt (RORγt) in Th17 cells. J Biol Chem. 289:25546–25555.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Haq S and Ramakrishna S: Deubiquitylation
of deubiquitylases. Open Biol. 7:1700162017. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Baohai X, Shi F and Yongqi F: Inhibition
of ubiquitin specific protease 17 restrains prostate cancer
proliferation by regulation of epithelial-to-mesenchymal transition
(EMT) via ROS production. Biomed Pharmacother. 118:1089462019.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Fang JY and Richardson BC: The MAPK
signalling pathways and colorectal cancer. Lancet Oncol. 6:322–327.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Guo YJ, Pan WW, Liu SB, Shen ZF, Xu Y and
Hu LL: ERK/MAPK signalling pathway and tumorigenesis. Exp Ther Med.
19:1997–2007. 2020.PubMed/NCBI
|
|
64
|
Santarpia L, Lippman SM and El-Naggar AK:
Targeting the MAPK-RAS-RAF signaling pathway in cancer therapy.
Expert Opin Ther Targets. 16:103–119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Wu PK, Becker A and Park JI: Growth
Inhibitory Signaling of the Raf/MEK/ERK Pathway. Int J Mol Sci.
21:54362020. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Maik-Rachline G, Hacohen-Lev-Ran A and
Seger R: Nuclear ERK: Mechanism of Translocation, Substrates, and
Role in Cancer. Int J Mol Sci. 20:11942019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Sanchez-Vega F, Mina M, Armenia J, Chatila
WK, Luna A, La KC, Dimitriadoy S, Liu DL, Kantheti HS, Saghafinia
S, et al: Oncogenic signaling pathways in the cancer genome atlas.
Cell. 173:321–337.e10. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Zhang J, Wang J, Luan T, Zuo Y, Chen J,
Zhang H, Ye Z, Wang H and Hai B: Deubiquitinase USP9X regulates the
invasion of prostate cancer cells by regulating the ERK pathway and
mitochondrial dynamics. Oncol Rep. 41:3292–3304. 2019.PubMed/NCBI
|
|
69
|
Hu W, Su Y, Fei X, Wang X, Zhang G, Su C,
Du T, Yang T, Wang G, Tang Z and Zhang J: Ubiquitin specific
peptidase 19 is a prognostic biomarker and affect the proliferation
and migration of clear cell renal cell carcinoma. Oncol Rep.
43:1964–1974. 2020.PubMed/NCBI
|
|
70
|
Meng Y, Hong C, Yang S, Qin Z, Yang L and
Huang Y: Roles of USP9X in cellular functions and tumorigenesis
(Review). Oncol Lett. 26:5062023. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Wan YF, Zhang CY, Cheng XW, Liu LS, Zhou
T, Gao JK, Zhu HQ and Liu YH: USP9X expression is functionally
related to laryngeal cancer. J Cancer. 14:591–599. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Jaiswal A, Murakami K, Elia A, Shibahara
Y, Done SJ, Wood SA, Donato NJ, Ohashi PS and Reedijk M:
Therapeutic inhibition of USP9x-mediated Notch signaling in
triple-negative breast cancer. Proc Natl Acad Sci USA.
118:e21015921182021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Jie X, Fong WP, Zhou R, Zhao Y, Zhao Y,
Meng R, Zhang S, Dong X, Zhang T, Yang K, et al: USP9X-mediated
KDM4C deubiquitination promotes lung cancer radioresistance by
epigenetically inducing TGF-β2 transcription. Cell Death Differ.
28:2095–2111. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Zhu Y, Gu L, Lin X, Zhou X, Lu B, Liu C,
Lei C, Zhou F, Zhao Q, Prochownik EV and Li Y: USP19 exacerbates
lipogenesis and colorectal carcinogenesis by stabilizing ME1. Cell
Rep. 37:1101742021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee JG, Kim W, Gygi S and Ye Y:
Characterization of the deubiquitinating activity of USP19 and its
role in endoplasmic reticulum-associated degradation. J Biol Chem.
289:3510–3517. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Dong Z, Guo S, Wang Y, Zhang J, Luo H,
Zheng G, Yang D, Zhang T, Yan L, Song L, et al: USP19 Enhances
MMP2/MMP9-Mediated tumorigenesis in gastric cancer. Onco Targets
Ther. 13:8495–8510. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Tyagi A, Karapurkar JK, Colaco JC,
Sarodaya N, Antao AM, Kaushal K, Haq S, Chandrasekaran AP, Das S,
Singh V, et al: USP19 Negatively Regulates p53 and promotes
cervical cancer progression. Mol Biotechnol. 66:2032–2045. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Li X, Yuan J, Song C, Lei Y, Xu J, Zhang
G, Wang W and Song G: Deubiquitinase USP39 and E3 ligase TRIM26
balance the level of ZEB1 ubiquitination and thereby determine the
progression of hepatocellular carcinoma. Cell Death Diffe.
28:2315–2332. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhu X, Ma J, Lu M, Liu Z, Sun Y and Chen
H: The Deubiquitinase USP39 promotes esophageal squamous cell
carcinoma malignancy as a splicing factor. Genes (Basel).
13:8192022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Zhang Z, Liu W, Bao X, Sun T, Wang J, Li M
and Liu C: USP39 facilitates breast cancer cell proliferation
through stabilization of FOXM1. Am J Cancer Res. 12:3644–3661.
2022.PubMed/NCBI
|
|
81
|
Yuan J, Li X, Zhang Y, Zhang G, Cheng W,
Wang W, Lei Y and Song G: USP39 attenuates the antitumor activity
of cisplatin on colon cancer cells dependent on p53. Cell Biol
Toxicol. 39:1995–2010. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Huang J: Current developments of targeting
the p53 signaling pathway for cancer treatment. Pharmacol Ther.
220:1077202021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Joerger AC and Fersht AR: The p53 Pathway:
Origins, inactivation in cancer, and emerging therapeutic
approaches. Annu Rev Biochem. 85:375–404. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lahalle A, Lacroix M, De Blasio C, Cissé
MY, Linares LK and Le Cam L: The p53 pathway and metabolism: The
tree that hides the forest. Cancers (Basel). 13:1332021. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Zhao Y, Yu H and Hu W: The regulation of
MDM2 oncogene and its impact on human cancers. Acta Biochim Biophys
Sin (Shanghai). 46:180–189. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Kwon SK, Saindane M and Baek KH: p53
stability is regulated by diverse deubiquitinating enzymes. Biochim
Biophys Acta Rev Cancer. 1868:404–411. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Klein AM, de Queiroz RM, Venkatesh D and
Prives C: The roles and regulation of MDM2 and MDMX: It is not just
about p53. Genes Dev. 35:575–601. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ren Y, Zhao P, Liu J, Yuan Y, Cheng Q, Zuo
Y, Qian L, Liu C, Guo T, Zhang L, et al: Deubiquitinase USP2a
sustains interferons antiviral activity by restricting
ubiquitination of activated STAT1 in the Nucleus. PLoS Pathog.
12:e10057642016. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li Y, He X, Wang S, Shu HB and Liu Y:
USP2a positively regulates TCR-induced NF-κB activation by bridging
MALT1-TRAF6. Protein Cell. 4:62–70. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Allende-Vega N, Sparks A, Lane DP and
Saville MK: MdmX is a substrate for the deubiquitinating enzyme
USP2a. Oncogene. 29:432–441. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Xiong B, Huang J, Liu Y, Zou M, Zhao Z,
Gong J, Wu X and Qiu C: Ubiquitin-specific protease 2a promotes
hepatocellular carcinoma progression via deubiquitination and
stabilization of RAB1A. Cell Oncol (Dordr). 44:329–343. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Selvendiran K, Ahmed S, Dayton A, Ravi Y,
Kuppusamy ML, Bratasz A, Rivera BK, Kálai T, Hideg K and Kuppusamy
P: HO-3867, a synthetic compound, inhibits the migration and
invasion of ovarian carcinoma cells through downregulation of fatty
acid synthase and focal adhesion kinase. Mol Cancer Res.
8:1188–1197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Kim J, Keay SK, You S, Loda M and Freeman
MR: A synthetic form of frizzled 8-associated antiproliferative
factor enhances p53 stability through USP2a and MDM2. PLoS One.
7:e503922012. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Stevenson LF, Sparks A, Allende-Vega N,
Xirodimas DP, Lane DP and Saville MK: The deubiquitinating enzyme
USP2a regulates the p53 pathway by targeting Mdm2. EMBO J.
26:976–986. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Ballar Kirmizibayrak P,
Erbaykent-Tepedelen B, Gozen O and Erzurumlu Y: Divergent
modulation of proteostasis in prostate cancer. Adv Exp Med Biol.
1233:117–151. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Park SH, Fong KW, Kim J, Wang F, Lu X, Lee
Y, Brea LT, Wadosky K, Guo C, Abdulkadir SA, et al:
Posttranslational regulation of FOXA1 by Polycomb and BUB3/USP7
deubiquitin complex in prostate cancer. Sci Adv. 7:eabe22612021.
View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Sun T, Lee GS, Oh WK, Pomerantz M, Yang M,
Xie W, Freedman ML and Kantoff PW: Single-nucleotide polymorphisms
in p53 pathway and aggressiveness of prostate cancer in a Caucasian
population. Clin Cancer Res. 16:5244–5251. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ren X, Jiang M, Ding P, Zhang X, Zhou X,
Shen J, Liu D, Yan X and Ma Z: Ubiquitin-specific protease 28: The
decipherment of its dual roles in cancer development. Exp Hematol
Oncol. 12:272023. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Chen L, Xu Z, Li Q, Zheng C, Du Y, Yuan R
and Peng X: USP28 facilitates pancreatic cancer progression through
activation of Wnt/β-catenin pathway via stabilising FOXM1. Cell
Death Dis. 12:8872021. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Zhao LJ, Zhang T, Feng XJ, Chang J, Suo
FZ, Ma JL, Liu YJ, Liu Y, Zheng YC and Liu HM: USP28 contributes to
the proliferation and metastasis of gastric cancer. J Cell Biochem.
120:7657–7666. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Wang H, Meng Q, Ding Y, Xiong M, Zhu M,
Yang Y, Su H, Gu L, Xu Y, Shi L, et al: USP28 and USP25 are
downregulated by Vismodegib in vitro and in colorectal cancer cell
lines. FEBS J. 288:1325–1342. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Devrim T, Ataç F, Devrim AK and Balcı M:
The concomitant use of USP28 and p53 to predict the progression of
urothelial carcinoma of the bladder. Pathol Res Pract.
216:1527742020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Fong CS, Mazo G, Das T, Goodman J, Kim M,
O'Rourke BP, Izquierdo D and Tsou MF: 53BP1 and USP28 mediate
p53-dependent cell cycle arrest in response to centrosome loss and
prolonged mitosis. Elife. 5:e162702016. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Tzavlaki K and Moustakas A: TGF-β
Signaling. Biomolecules. 10:4872020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Derynck R and Budi EH: Specificity,
versatility, and control of TGF-β family signaling. Sci Signal.
12:eaav51832019. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Hata A and Chen YG: TGF-β Signaling from
Receptors to Smads. Cold Spring Harb Perspect Biol. 8:a0220612016.
View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Peng D, Fu M, Wang M, Wei Y and Wei X:
Targeting TGF-β signal transduction for fibrosis and cancer
therapy. Mol Cancer. 21:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhang Y, Alexander PB and Wang XF: TGF-β
family signaling in the control of cell proliferation and survival.
Cold Spring Harb Perspect Biol. 9:a0221452017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Haque S and Morris JC: Transforming growth
factor-β: A therapeutic target for cancer. Hum Vaccin Immunother.
13:1741–1750. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Sakai K, Ito C, Wakabayashi M, Kanzaki S,
Ito T, Takada S, Toshimori K, Sekita Y and Kimura T: Usp26 mutation
in mice leads to defective spermatogenesis depending on genetic
background. Sci Rep. 9:137572019. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Tang J, Luo Y and Xiao L: USP26 promotes
anaplastic thyroid cancer progression by stabilizing TAZ. Cell
Death Dis. 13:3262022. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Ye Y, Li M, Pan Q, Fang X, Yang H, Dong B,
Yang J, Zheng Y, Zhang R and Liao Z: Machine learning-based
classification of deubiquitinase USP26 and its cell proliferation
inhibition through stabilizing KLF6 in cervical cancer. Comput Biol
Med. 168:1077452024. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Li G, Qi HW, Dong HG, Bai P, Sun M and Liu
HY: Targeting deubiquitinating enzyme USP26 by microRNA-203
regulates Snail1's pro-metastatic functions in esophageal cancer.
Cancer Cell Int. 20:3552020. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Wosnitzer MS, Mielnik A, Dabaja A,
Robinson B, Schlegel PN and Paduch DA: Ubiquitin Specific Protease
26 (USP26) expression analysis in human testicular and extragonadal
tissues indicates diverse action of USP26 in cell differentiation
and tumorigenesis. PLoS One. 9:e986382014. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Dirac AM and Bernards R: The
deubiquitinating enzyme USP26 is a regulator of androgen receptor
signaling. Mol Cancer Res. 8:844–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Cai Q, Chen Y, Zhang D, Pan J, Xie Z, Ma
S, Liu C, Zuo J, Zhou X, Quan C, et al: Loss of epithelial AR
increase castration resistant stem-like prostate cancer cells and
promotes cancer metastasis via TGF-β1/EMT pathway. Transl Androl
Urol. 9:1013–1027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Allen-Petersen BL and Sears RC: Mission
Possible: Advances in MYC Therapeutic Targeting in Cancer.
BioDrugs. 33:539–553. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Blackwood EM and Eisenman RN: Max: A
helix-loop-helix zipper protein that forms a sequence-specific
DNA-binding complex with Myc. Science. 251:1211–1217. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Blackwood EM, Lüscher B, Kretzner L and
Eisenman RN: The Myc:Max protein complex and cell growth
regulation. Cold Spring Harb Symp Quant Biol. 56:109–117. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Helander S, Montecchio M, Pilstål R, Su Y,
Kuruvilla J, Elvén M, Ziauddin JME, Anandapadamanaban M, Cristobal
S, Lundström P, et al: Pre-anchoring of Pin1 to unphosphorylated
c-Myc in a fuzzy complex regulates c-Myc activity. Structure.
23:2267–2279. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Kato GJ, Barrett J, Villa-Garcia M and
Dang CV: An amino-terminal c-myc domain required for neoplastic
transformation activates transcription. Mol Cell Biol.
10:5914–5920. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Prendergast GC and Ziff EB:
Methylation-sensitive sequence-specific DNA binding by the c-Myc
basic region. Science. 251:186–189. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Ashrafizadeh M, Zarabi A, Hushmandi K,
Moghadam ER, Hashemi F, Daneshi S, Hashemi F, Tavakol S,
Mohammadinejad R, Najafi M, et al: C-Myc signaling pathway in
treatment and prevention of brain tumors. Curr Cancer Drug Targets.
21:2–20. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Park JH, Pyun WY and Park HW: Cancer
Metabolism: Phenotype, signaling and therapeutic targets. Cells.
9:23082020. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Chen H, Liu H and Qing G: Targeting
oncogenic Myc as a strategy for cancer treatment. Signal Transduct
Target Ther. 3:52018. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Yang S, Wang J, Guo S, Huang D, Lorigados
IB, Nie X, Lou D, Li Y, Liu M, Kang Y, et al: Transcriptional
activation of USP16 gene expression by NFκB signaling. Mol Brain.
12:1202019. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zheng J, Chen C, Guo C, Caba C, Tong Y and
Wang H: The pleiotropic ubiquitin-specific peptidase 16 and its
many substrates. Cells. 12:8862023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Liu S, Li H, Zhu Y, Ma X, Shao Z, Yang Z,
Cai C, Wu Z, Li M, Gong W and Wu X: LncRNA MNX1-AS1 sustains
inactivation of Hippo pathway through a positive feedback loop with
USP16/IGF2BP3 axis in gallbladder cancer. Cancer Lett.
547:2158622022. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Xu G, Yang Z, Ding Y, Liu Y, Zhang L, Wang
B, Tang M, Jing T, Jiao K, Xu X, et al: The deubiquitinase USP16
functions as an oncogenic factor in K-RAS-driven lung
tumorigenesis. Oncogene. 40:5482–5494. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Li Y, Rao Y, Zhu H, Jiang B and Zhu M:
USP16 regulates the stability and function of LDL receptor by
Deubiquitination. Int Heart J. 61:1034–1040. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Ge J, Yu W, Li J, Ma H, Wang P, Zhou Y,
Wang Y, Zhang J and Shi G: USP16 regulates castration-resistant
prostate cancer cell proliferation by deubiquitinating and
stablizing c-Myc. J Exp Clin Cancer Res. 40:592021. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
He L, Liu X, Yang J, Li W, Liu S, Liu X,
Yang Z, Ren J, Wang Y, Shan L, et al: Imbalance of the reciprocally
inhibitory loop between the ubiquitin-specific protease USP43 and
EGFR/PI3K/AKT drives breast carcinogenesis. Cell Res. 28:934–951.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Ye DX, Wang SS, Huang Y, Wang XJ and Chi
P: USP43 directly regulates ZEB1 protein, mediating proliferation
and metastasis of colorectal cancer. J Cancer. 12:404–416. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Xue Y, Li M, Hu J, Song Y, Guo W, Miao C,
Ge D, Hou Y, Wang X, Huang X, et al: Ca(v)2.2-NFAT2-USP43 axis
promotes invadopodia formation and breast cancer metastasis through
cortactin stabilization. Cell Death Dis. 13:8122022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Sun Q, Zhang H, Zong L, Julaiti A, Jing X
and Zhang L: Prognostic value and oncogenic effects of
ubiquitin-specific protease 43 in lung squamous cell carcinoma.
Tohoku J Exp Med. 257:135–145. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Zhao Z, Lin Z, Guo X, Al-Danakh A, He H,
Qin H, Ma C, Zhang N and Tan G: Ubiquitin-specific protease 43
impacts pancreatic ductal adenocarcinoma prognosis by altering its
proliferation and infiltration of surrounding immune cells. J
Immunol Res. 2023:43113882023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Lavaud M, Mullard M, Tesfaye R, Amiaud J,
Legrand M, Danieau G, Brion R, Morice S, Regnier L, Dupuy M, et al:
Overexpression of the Ubiquitin Specific Proteases USP43, USP41,
USP27x and USP6 in Osteosarcoma Cell Lines: Inhibition of
Osteosarcoma Tumor Growth and Lung Metastasis Development by the
USP Antagonist PR619. Cells. 10:22682021. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Li M, Yu J, Ju L, Wang Y, Jin W, Zhang R,
Xiang W, Ji M, Du W, Wang G, et al: USP43 stabilizes c-Myc to
promote glycolysis and metastasis in bladder cancer. Cell Death
Dis. 15:442024. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Nusse R and Varmus HE: Many tumors induced
by the mouse mammary tumor virus contain a provirus integrated in
the same region of the host genome. Cell. 31:99–109. 1982.
View Article : Google Scholar : PubMed/NCBI
|
|
140
|
Zou G and Park JI: Wnt signaling in liver
regeneration, disease, and cancer. Clin Mol Hepatol. 29:33–50.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Steinhart Z and Angers S: Wnt signaling in
development and tissue homeostasis. Development. 145:dev1465892018.
View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Hafez N, Modather El-Awadly Z and Arafa
RK: UCH-L3 structure and function: Insights about a promising drug
target. Eur J Med Chem. 227:1139702022. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Zhu T, Xu L, Peng J, Chen M and Xu H:
Molecular characteristics and immune function of ubiquitin
C-terminal hydrolase-L3 in Macrobrachium nipponense. Fish Shellfish
Immunol. 121:295–304. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Ma Q, Lu Q, Lei X, Zhao J, Sun W, Wang J,
Zhu Q and Huang D: UCHL3 promotes hepatocellular carcinoma cell
migration by de-ubiquitinating and stabilizing Vimentin. Front
Oncol. 13:10884752023. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Zhang Y, Liu JB, Liu J, Liu M, Liu HL and
Zhang J: UCHL3 promotes cervical cancer development and metastasis
by stabilizing NRF2 via deubiquitination. Biochem Biophys Res
Commun. 641:132–138. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
148
|
Li J, Zheng Y, Li X, Dong X, Chen W, Guan
Z and Zhang C: UCHL3 promotes proliferation of colorectal cancer
cells by regulating SOX12 via AKT/mTOR signaling pathway. Am J
Transl Res. 12:6445–6454. 2020.PubMed/NCBI
|
|
149
|
Moroney MR, Woodruff E, Qamar L, Bradford
AP, Wolsky R, Bitler BG and Corr BR: Inhibiting Wnt/beta-catenin in
CTNNB1-mutated endometrial cancer. Mol Carcinog. 60:511–523. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Liu T, Fan MQ, Xie XX, Shu QP, Du XH, Qi
LZ, Zhang XD, Zhang MH, Shan G, Du RL and Li SZ: Activation of
CTNNB1 by deubiquitinase UCHL3-mediated stabilization facilitates
bladder cancer progression. J Transl Med. 21:6562023. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Zhou L, Qin B, Yassine DM, Luo M, Liu X,
Wang F and Wang Y: Structure and function of the highly homologous
deubiquitinases ubiquitin specific peptidase 25 and 28: Insights
into their pathophysiological and therapeutic roles. Biochem
Pharmacol. 213:1156242023. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Zhong B, Liu X, Wang X, Liu X, Li H,
Darnay BG, Lin X, Sun SC and Dong C: Ubiquitin-specific protease 25
regulates TLR4-dependent innate immune responses through
deubiquitination of the adaptor protein TRAF3. Sci Signal.
6:ra352013. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Liu B, Miao X, Shen J, Lou L, Chen K, Mei
F, Chen M, Su X, Du X, Zhu Z, et al: USP25 ameliorates diabetic
nephropathy by inhibiting TRAF6-mediated inflammatory responses.
Int Immunopharmacol. 124((Pt A)): 1108772023. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Ye B, Zhou H, Chen Y, Luo W, Lin W, Zhao
Y, Han J, Han X, Huang W, Wu G, et al: USP25 Ameliorates
Pathological Cardiac Hypertrophy by Stabilizing SERCA2a in
Cardiomyocytes. Circ Res. 132:465–480. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
155
|
Zheng Q, Song B, Li G, Cai F, Wu M, Zhao
Y, Jiang L, Guo T, Shen M, Hou H, et al: USP25 inhibition
ameliorates Alzheimer's pathology through the regulation of APP
processing and Aβ generation. J Clin Invest. 132:e1521702022.
View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Cheng H, Li X, Wang C, Chen Y, Li S, Tan
J, Tan B and He Y: Inhibition of tankyrase by a novel small
molecule significantly attenuates prostate cancer cell
proliferation. Cancer Lett. 443:80–90. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
157
|
Gu Z, Lin C, Hu J, Xia J, Wei S and Gao D:
USP34 Regulated Human Pancreatic Cancer Cell Survival via AKT and
PKC Pathways. Biol Pharm Bull. 42:573–579. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Guo YC, Wang MY, Zhang SW, Wu YS, Zhou CC,
Zheng RX, Shao B, Wang Y, Xie L, Liu WQ, et al: Ubiquitin-specific
protease USP34 controls osteogenic differentiation and bone
formation by regulating BMP2 signaling. EMBO J. 37:e993982018.
View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Zhu Q, Liang P, Meng H, Li F, Miao W, Chu
C, Wang W, Li D, Chen C, Shi Y, et al: Stabilization of Pin1 by
USP34 promotes Ubc9 isomerization and protein sumoylation in glioma
stem cells. Nat Commun. 15:402024. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Lui TT, Lacroix C, Ahmed SM, Goldenberg
SJ, Leach CA, Daulat AM and Angers S: The ubiquitin-specific
protease USP34 regulates axin stability and Wnt/β-catenin
signaling. Mol Cell Biol. 31:2053–2065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Zhao Y, Yan Y, Ma R, Lv X, Zhang L, Wang
J, Zhu W, Zhao L, Jiang L, Zhao L, et al: Expression signature of
six-snoRNA serves as novel non-invasive biomarker for diagnosis and
prognosis prediction of renal clear cell carcinoma. J Cell Mol Med.
24:2215–2228. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Ma S, Meng Z, Chen R and Guan KL: The
Hippo Pathway: Biology and Pathophysiology. Annu Rev Biochem.
88:577–604. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Lee U, Cho EY and Jho EH: Regulation of
Hippo signaling by metabolic pathways in cancer. Biochim Biophys
Acta Mol Cell Res. 1869:1192012022. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wu Z and Guan KL: Hippo Signaling in
Embryogenesis and Development. Trends Biochem Sci. 46:51–63. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
165
|
Cao Z, An L, Han Y, Jiao S and Zhou Z: The
Hippo signaling pathway in gastric cancer. Acta Biochim Biophys Sin
(Shanghai). 55:893–903. 2023.PubMed/NCBI
|
|
166
|
Zhu N, Yang R, Wang X, Yuan L, Li X, Wei F
and Zhang L: The Hippo signaling pathway: From multiple signals to
the hallmarks of cancers. Acta Biochim Biophys Sin (Shanghai).
55:904–913. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
167
|
Gu Y, Wu S, Fan J, Meng Z, Gao G, Liu T,
Wang Q, Xia H, Wang X and Wu K: CYLD regulates cell ferroptosis
through Hippo/YAP signaling in prostate cancer progression. Cell
Death Dis. 15:792024. View Article : Google Scholar : PubMed/NCBI
|
|
168
|
Abdul Rehman SA, Kristariyanto YA, Choi
SY, Nkosi PJ, Weidlich S, Labib K, Hofmann K and Kulathu Y: MINDY-1
is a member of an evolutionarily conserved and structurally
distinct new family of deubiquitinating enzymes. Mol Cell.
63:146–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
169
|
Tang J, Luo Y, Long G and Zhou L: MINDY1
promotes breast cancer cell proliferation by stabilizing estrogen
receptor α. Cell Death Dis. 12:9372021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Xia BL, Liu KW, Huang HX, Shen MM, Wang B
and Gao J: Deubiquitinating enzyme MINDY1 is an independent risk
factor for the maintenance of stemness and poor prognosis in liver
cancer cells. Zhonghua Gan Zang Bing Za Zhi. 31:518–523. 2023.(In
Chinese). PubMed/NCBI
|
|
171
|
James C, Zhao TY, Rahim A, Saxena P,
Muthalif NA, Uemura T, Tsuneyoshi N, Ong S, Igarashi K, Lim CY, et
al: MINDY1 Is a Downstream Target of the Polyamines and Promotes
Embryonic Stem Cell Self-Renewal. Stem Cells. 36:1170–1178. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Luo Y, Zhou J, Tang J, Zhou F, He Z and
Liu T and Liu T: MINDY1 promotes bladder cancer progression by
stabilizing YAP. Cancer Cell Int. 21:3952021. View Article : Google Scholar : PubMed/NCBI
|
|
173
|
Dai C, Heemers H and Sharifi N: Androgen
signaling in prostate cancer. Cold Spring Harb Perspect Med.
7:a0304522017. View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Deng CC, Zhu DH, Chen YJ, Huang TY, Peng
Y, Liu SY, Lu P, Xue YH, Xu YP, Yang B and Rong Z: TRAF4 Promotes
Fibroblast Proliferation in Keloids by Destabilizing p53 via
Interacting with the Deubiquitinase USP10. J Invest Dermatol.
139:1925–1935.e5. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Zeng Z, Wu HX, Zhan N, Huang YB, Wang ZS,
Yang GF, Wang P and Fu GH: Prognostic significance of USP10 as a
tumor-associated marker in gastric carcinoma. Tumour Biol.
35:3845–3853. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
176
|
Lu C, Ning Z, Wang A, Chen D, Liu X, Xia
T, Tekcham DS, Wang W, Li T, Liu X, et al: USP10 suppresses tumor
progression by inhibiting mTOR activation in hepatocellular
carcinoma. Cancer Lett. 436:139–148. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Ye Z, Chen J, Huang P, Xuan Z and Zheng S:
Ubiquitin-specific peptidase 10, a deubiquitinating enzyme:
Assessing its role in tumor prognosis and immune response. Front
Oncol. 12:9901952022. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Kong L and Jin X: Dysregulation of
deubiquitination in breast cancer. Gene. 902:1481752024. View Article : Google Scholar : PubMed/NCBI
|
|
179
|
An T, Lu Y, Yan X and Hou J: Insights Into
the Properties, Biological Functions, and Regulation of USP21.
Front Pharmacol. 13:9440892022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Chen Y, Wang L, Jin J, Luan Y, Chen C, Li
Y, Chu H, Wang X, Liao G, Yu Y, et al: p38 inhibition provides
anti-DNA virus immunity by regulation of USP21 phosphorylation and
STING activation. J Exp Med. 214:991–1010. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
181
|
Liu J, Kruswick A, Dang H, Tran AD, Kwon
SM, Wang XW and Oberdoerffer P: Ubiquitin-specific protease 21
stabilizes BRCA2 to control DNA repair and tumor growth. Nat
Commun. 8:1372017. View Article : Google Scholar : PubMed/NCBI
|
|
182
|
Chen Y, Zhou B and Chen D: USP21 promotes
cell proliferation and metastasis through suppressing EZH2
ubiquitination in bladder carcinoma. Onco Targets Ther. 10:681–689.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Lin Z, Yang H, Kong Q, Li J, Lee SM, Gao
B, Dong H, Wei J, Song J, Zhang DD and Fang D: USP22 antagonizes
p53 transcriptional activation by deubiquitinating Sirt1 to
suppress cell apoptosis and is required for mouse embryonic
development. Mol Cell. 46:484–494. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Feng T, Ling S, Xu C, Ying L, Su D and Xu
X: Ubiquitin-specific peptidase 22 in cancer. Cancer Lett.
514:30–37. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Zeng K, Xie W, Wang C, Wang S, Liu W, Su
Y, Lin L, Zou R, Sun G, Zhou B, et al: USP22 upregulates
ZEB1-mediated VEGFA transcription in hepatocellular carcinoma. Cell
Death Dis. 14:1942023. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Xu G, Cai J, Wang L, Jiang L, Huang J, Hu
R and Ding F: MicroRNA-30e-5p suppresses non-small cell lung cancer
tumorigenesis by regulating USP22-mediated Sirt1/JAK/STAT3
signaling. Exp Cell Res. 362:268–278. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
187
|
Yuan X, Wang H, Xu A, Zhu X, Zhan Y and
Wang W: Ubiquitin-specific peptidase 22 promotes proliferation and
metastasis in human colon cancer. Oncol Lett. 18:5567–5576.
2019.PubMed/NCBI
|
|
188
|
Lv L, Xiao XY, Gu ZH, Zeng FQ, Huang LQ
and Jiang GS: Silencing USP22 by asymmetric structure of
interfering RNA inhibits proliferation and induces cell cycle
arrest in bladder cancer cells. Mol Cell Biochem. 346:11–21. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Guo J, Zhao J, Fu W, Xu Q and Huang D:
Immune Evasion and Drug Resistance Mediated by USP22 in Cancer:
Novel Targets and Mechanisms. Front Immunol. 13:9183142022.
View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Nag N and Dutta S: Deubiquitination in
prostate cancer progression: Role of USP22. J Cancer Metastasis
Treat. 6:162020.PubMed/NCBI
|
|
191
|
Li C, Zeng X, Qiu S, Gu Y and Zhang Y:
Nanomedicine for urologic cancers: Diagnosis and management. Semin
Cancer Biol. 86:463–475. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Schauer NJ, Magin RS, Liu X, Doherty LM
and Buhrlage SJ: Advances in Discovering Deubiquitinating Enzyme
(DUB) Inhibitors. J Med Chem. 63:2731–2750. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Lee JE, Park CM and Kim JH: USP7
deubiquitinates and stabilizes EZH2 in prostate cancer cells. Genet
Mol Biol. 43:e201903382020. View Article : Google Scholar : PubMed/NCBI
|
|
194
|
Zhang W, Zhang J, Xu C, Zhang S, Bian S,
Jiang F, Ni W, Qu L, Lu C, Ni R, et al: Ubiquitin-specific protease
7 is a drug-able target that promotes hepatocellular carcinoma and
chemoresistance. Cancer Cell Int. 20:282020. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Chen H, Zhu X, Sun R, Ma P, Zhang E, Wang
Z, Fan Y, Zhou G and Mao R: Ubiquitin-specific protease 7 is a
druggable target that is essential for pancreatic cancer growth and
chemoresistance. Invest New Drugs. 38:1707–1716. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Wang S, Kollipara RK, Srivastava N, Li R,
Ravindranathan P, Hernandez E, Freeman E, Humphries CG, Kapur P,
Lotan Y, et al: Ablation of the oncogenic transcription factor ERG
by deubiquitinase inhibition in prostate cancer. Proc Natl Acad Sci
USA. 111:4251–4256. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Stolte B, Iniguez AB, Dharia NV, Robichaud
AL, Conway AS, Morgan AM, Alexe G, Schauer NJ, Liu X, Bird GH, et
al: Genome-scale CRISPR-Cas9 screen identifies druggable
dependencies in TP53 wild-type Ewing sarcoma. J Exp Med.
215:2137–2155. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Turnbull AP, Ioannidis S, Krajewski WW,
Pinto-Fernandez A, Heride C, Martin ACL, Tonkin LM, Townsend EC,
Buker SM, Lancia DR, et al: Molecular basis of USP7 inhibition by
selective small-molecule inhibitors. Nature. 550:481–486. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Xu S, Adisetiyo H, Tamura S, Grande F,
Garofalo A, Roy-Burman P and Neamati N: Dual inhibition of survivin
and MAOA synergistically impairs growth of PTEN-negative prostate
cancer. Br J Cancer. 113:242–251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Tolcher AW, Quinn DI, Ferrari A, Ahmann F,
Giaccone G, Drake T, Keating A and de Bono JS: A phase II study of
YM155, a novel small-molecule suppressor of survivin, in
castration-resistant taxane-pretreated prostate cancer. Ann Oncol.
23:968–973. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Chow PM, Dong JR, Chang YW, Kuo KL, Lin
WC, Liu SH and Huang KH: The UCHL5 inhibitor b-AP15 overcomes
cisplatin resistance via suppression of cancer stemness in
urothelial carcinoma. Mol Ther Oncolytics. 26:387–398. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Jamroze A, Chatta G and Tang DG: Androgen
receptor (AR) heterogeneity in prostate cancer and therapy
resistance. Cancer Lett. 518:1–9. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
He Y, Xu W, Xiao YT, Huang H, Gu D and Ren
S: Targeting signaling pathways in prostate cancer: mechanisms and
clinical trials. Signal Transduct Target Ther. 7:1982022.
View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Schellhammer PF and Davis JW: An
evaluation of bicalutamide in the treatment of prostate cancer.
Clin Prostate Cancer. 2:213–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Chi KN, Agarwal N, Bjartell A, Chung BH,
Pereira de Santana Gomes AJ, Given R, Juárez Soto Á, Merseburger
AS, Özgüroğlu M, Uemura H, et al: Apalutamide for metastatic,
castration-sensitive prostate cancer. N Engl J Med. 381:13–24.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Armstrong AJ, Szmulewitz RZ, Petrylak DP,
Holzbeierlein J, Villers A, Azad A, Alcaraz A, Alekseev B, Iguchi
T, Shore ND, et al: ARCHES: A Randomized, phase III study of
androgen deprivation therapy with enzalutamide or placebo in men
with metastatic hormone-sensitive prostate cancer. J Clin Oncol.
37:2974–2986. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Fizazi K, Tran N, Fein L, Matsubara N,
Rodriguez-Antolin A, Alekseev BY, Özgüroğlu M, Ye D, Feyerabend S,
Protheroe A, et al: Abiraterone plus prednisone in metastatic,
castration-sensitive prostate cancer. N Engl J Med. 377:352–360.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Isaacsson Velho P and Antonarakis ES:
PD-1/PD-L1 pathway inhibitors in advanced prostate cancer. Expert
Rev Clin Pharmacol. 11:475–486. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
209
|
Wu X, Gu Z, Chen Y, Chen B, Chen W, Weng L
and Liu X: Application of PD-1 Blockade in Cancer Immunotherapy.
Comput Struct Biotechnol J. 17:661–674. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Kuang Z, Liu X, Zhang N, Dong J, Sun C,
Yin M, Wang Y, Liu L, Xiao D, Zhou X, et al: USP2 promotes tumor
immune evasion via deubiquitination and stabilization of PD-L1.
Cell Death Differ. 30:2249–2264. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Sweeney PL, Suri Y, Basu A, Koshkin VS and
Desai A: Mechanisms of tyrosine kinase inhibitor resistance in
renal cell carcinoma. Cancer Drug Resist. 6:858–873. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Ebrahimi N, Fardi E, Ghaderi H, Palizdar
S, Khorram R, Vafadar R, Ghanaatian M, Rezaei-Tazangi F, Baziyar P,
Ahmadi A, et al: Receptor tyrosine kinase inhibitors in cancer.
Cell Mol Life Sci. 80:1042023. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
McCann AP, Smyth P, Cogo F, McDaid WJ,
Jiang L, Lin J, Evergren E, Burden RE, Van Schaeybroeck S, Scott CJ
and Burrows JF: USP17 is required for trafficking and oncogenic
signaling of mutant EGFR in NSCLC cells. Cell Commun Signal.
16:772018. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Popova NV and Jücker M: The Role of mTOR
signaling as a therapeutic target in cancer. Int J Mol Sci.
22:17432021. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
Hasskarl J: Everolimus. Recent Results
Cancer Res. 211:101–123. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
216
|
Stock C, Zaccagnini M, Schulze M, Teber D
and Rassweiler JJ: Temsirolimus. Recent Results Cancer Res.
184:189–197. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Porta C, Calvo E, Climent MA, Vaishampayan
U, Osanto S, Ravaud A, Bracarda S, Hutson TE, Escudier B, Grünwald
V, et al: Efficacy and safety of everolimus in elderly patients
with metastatic renal cell carcinoma: an exploratory analysis of
the outcomes of elderly patients in the RECORD-1 Trial. Eur Urol.
61:826–833. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Roldán-Romero JM, Valdivia C, Santos M,
Lanillos J, Maroto P, Anguera G, Calsina B, Martinez-Montes A,
Monteagudo M, Mellid S, et al: Deubiquitinase USP9X loss sensitizes
renal cancer cells to mTOR inhibition. Int J Cancer. 153:1300–1312.
2023. View Article : Google Scholar : PubMed/NCBI
|