Introduction
Breast cancer (BC) is one of the most common
malignant tumours worldwide (1);
however, 70–80% of early-stage non-metastatic BC cases are curable
(2). BC's Molecular typing is based
on the expression of oestrogen receptor (ER), progesterone receptor
(PR) and human epidermal growth factor receptor 2 (HER2). BC can be
further subdivided into luminal A- and B-type BC, HER2-positive BC
and triple-negative BC (TNBC). Among these subtypes, TNBC lacks the
expression of ER, PR and HER2, and accounts for 15–20% of all BC
cases. Furthermore, TNBC is characterized by a high rate of
systemic metastasis, insensitivity to conventional treatments and
susceptibility to drug resistance, thus leading to a poor
prognosis. The poor response of TNBC to treatment remains a major
problem in the field of BC research (3–5).
Locally advanced or metastatic TNBC, which is the
most invasive and immunogenic subtype, has the poorest prognosis
among all BC types (6). For
decades, various treatment options, including chemotherapy,
radiotherapy (RT) and surgery, have been available for TNBC; in
particular, chemotherapy has been the primary first-line treatment
(7,8). Surgical treatment of TNBC is confined
to local therapy and consists of surgical removal of the breast
tumour mass by mastectomy or breast-conserving surgery, followed by
radiation therapy [breast-conserving therapy (BCT)]. According to
previous studies, surgical treatment of TNBC and BC did not differ
in local control rates, and these studies have demonstrated a
higher rate of local recurrence in patients with TNBC subtypes
following BCT (9–11). Although the current guidelines for
adjuvant RT for TNBC are not much different from those for other BC
subtypes, the recommended RT regimen for TNBC depends on the extent
of surgery and lymph node status; however, no significant
prolongation of survival and a high recurrence rate are observed
after treatment (12). Although
chemotherapy is effective, the treatment of TNBC still faces a
series of challenges. Chemotherapy is successful in early-stage
TNBCs, but it is relatively ineffective in advanced-stage patients,
as reflected in the fact that metastatic TNBC has a 5-year survival
rate of only 12% (13) due to its
molecular heterogeneity, poor cell differentiation, high degree of
malignancy, lack of molecular targets, rapid metastasis and
resistance to chemotherapy drugs (14,15).
Although BC is not traditionally considered a
particularly immunogenic tumour, previous studies have shown that
immune checkpoint inhibitors (ICIs) or immunotherapies exert
promising effects on a wide range of malignancies that are
refractory to solid tumours (including advanced non-small cell lung
cancer, metastatic melanoma and metastatic bladder cancer)
(16–18). While TNBC has a higher degree of
stromal and intratumoural tumour-infiltrating lymphocytes that
recognize and attack tumour cells, non-TNBC has a lower mutational
load than other solid tumours, which is correlated with the number
of somatic mutations present in the tumour, high mutational loads
in TNBC and higher programmed death ligand 1 (PD-L1) expression on
the cell surface of TNBC compared with other BC subtypes. These
correlations suggest that some aggressive TNBCs may be immunogenic
(3,19,20).
Thus, the use of ICIs, including programmed cell death protein 1
(PD-1), PD-L1 and cytotoxic T-lymphocyte associated protein 4
(CLTA-4), have promising potential for the treatment of BC. In
TNBC, tumour immune infiltration, neoantigen levels, mutational
load, high genomic instability and high levels of immune markers
(such as PD-1 and PD-L1) are closely associated with tumour
response, recurrence and prognosis. Immunotherapy can improve the
prognosis of TNBC by remodelling the tumour microenvironment and
stimulating antitumour immune responses (21,22).
Programmed cell death proteins that are expressed on T cells bind
to the ligand PD-L1, which is expressed on tumour cells, thereby
mediating tumour immune escape by inhibiting antigen-specific
T-cell immune responses. Interfering with PD-1/PD-L1 interactions
via anti-PD-1 monoclonal antibodies (mAbs) or anti-PD-L1 mAbs
activates antitumour immune responses. In addition, PD-L1 is
expressed in other tumour-infiltrating immune cells, mainly
antigen-presenting cells such as dendritic cells and macrophages,
and PD-L1 expression on these immune cells plays an indispensable
role in the efficacy of PD-1/PD-L1 blockade therapy (23–25).
However, the results of several trials such as IMPASSION130 and
IMPASSION131 that examined the use of ICI immunotherapy or
combination chemotherapy to treat TNBC appear to be
inconsistent.
To further understand the clinical efficacy of
immunosuppressants in patients with locally advanced or metastatic
TNBC, the current meta-analysis examined randomized controlled
trials (RCTs) on patients with TNBC treated with ICIs, thus
providing a comprehensive assessment of the efficacy and safety
graded of PD-1/PD-L1 immunosuppressant treatment for locally
advanced or metastatic TNBC according to the National Cancer
Institute Common Terminology Criteria for Adverse Events (version
4.0) (26).
Materials and methods
Data sources and literature
search
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), Cochrane Library
(https://www.cochranelibrary.com/),
Embase (https://www.embase.com/) and MEDLINE
(https://www.nlm.nih.gov/medline)
databases were searched from December 2010 to July 19, 2023, to
identify trials that examined the efficacy and safety of
immunotherapy with ICIs for treating unresectable locally advanced
or metastatic TNBC. The following keywords were used in the
literature search: ‘Triple-negative breast cancer’, ‘programmed
cell death ligand 1 inhibitor’ and ‘immunotherapy’. The full search
strategy was as follows: ‘Triple-Negative Breast Cancer’ OR
‘Triple-Negative Breast Cancers’ OR ‘Triple-Negative Breast
Neoplasm’ OR ‘Triple Negative Breast Neoplasm’ OR ‘Triple-Negative
Breast Neoplasms’ AND ‘Immune Checkpoint Inhibitors’ OR ‘PD-1
inhibitor’ OR ‘PD-L1 inhibitor’ OR ‘PD-1/PD-L1 inhibitor’ OR
‘CTLA-4 inhibitor’ OR ‘Immune Checkpoint Blockade’ OR ‘PD-1
Blockade’ OR ‘PD-L1 blockade’ OR ‘CLTA-4 blockade’ OR ‘pd-1/pd-l1
blockade’. When duplicate studies were identified, the most recent
article or the higher-quality article was selected. Furthermore,
the reference lists of the retrieved studies and recently published
reviews were thoroughly searched to identify additional relevant
studies. The objective of the present meta-analysis was to examine
the study population, treatments/exposure factors, comparative
measures, outcome indicators and environmental criteria. The
current study was conducted in accordance with the Preferred
Reporting Items for Systematic reviews and Meta-Analyses 2020
checklist (27).
Screening criteria
The primary objective of the present meta-analysis
was to compare the safety and efficacy of immunotherapy involving
ICIs (PD-1/PD-L1 and CLTA-4 inhibitors) with the safety and
efficacy of other treatments for locally advanced or metastatic
TNBC. The inclusion criteria were as follows: i) RCTs or other
types of clinical trials; ii) patients with unresectable locally
advanced or metastatic TNBC only; and iii) patients in the
experimental group of the RCT received immunotherapy based on ICIs,
whereas the control group received other treatments. The exclusion
criteria were as follows: i) Patients without TNBC; ii) animal
experiments; iii) reviews, study reports, case reports, guidelines,
letters, conference abstracts and meta-analyses; iv) incomplete
studies; v) preclinical or phase 1 studies; and vi) early TNBC and
adjuvant therapies.
Two authors (YC and LS) adopted a screening strategy
for the retrieved literature and independently reviewed all the
titles and abstracts of the studies to determine whether they met
the inclusion criteria. Studies that did not meet the inclusion
criteria were promptly excluded. In cases of doubt, the full text
of the studies was screened. Disagreements regarding the inclusion
of a study were resolved by discussion with a third author (WY); if
a consensus could not be reached, the study was excluded.
Data extraction
The following data were extracted from the included
studies: Name of the first author, year of publication, duration of
the trial, authors' country, name of the RCT, phase of the trial,
number of patients, patients' age group, patients' ethnicity,
clinicopathological characteristics of the population enrolled,
treatment regimen, follow-up time, and primary and secondary
outcomes of the trial (Tables I and
SI). It was also noted whether
patients were included in the intent-to-treat (ITT) population,
which included all the patients who had undergone randomization,
and PD-L1 status subgroups were also extracted. To ensure the
validity and accuracy of the extracted data, the aforementioned two
authors independently extracted the data from the studies that met
the inclusion criteria, and the data were then cross-validated.
Disagreements between the two authors were resolved by consulting
with the aforementioned third author.
 | Table I.Basic characteristics of the studies
included in the present meta-analysis. |
Table I.
Basic characteristics of the studies
included in the present meta-analysis.
|
|
|
|
|
| Treatment plan | Patients with
triple-negative breast cancer (n) |
|
|---|
| First author/s,
year | Trial name | Trial phase | Primary
outcomes | Secondary
outcomes |
|
|
|
|---|
| Experimental | Control | Experimental | Control | (Refs.) |
|---|
| Schmid et
al, 2018 | IMpassion130 | III | PFS, OS | OR, CR, PR, DOR,
AEs | Atezolizumab plus
nab-paclitaxel | Placebo plus
nab-paclitaxel | 451 | 451 | (29) |
| Brufsky et
al, 2021 | COLET | II | PFS, OR, PR,
CR | AEs, DOR, OS | Cobimetinib plus
atezolizumab plus paclitaxel | Cobimetinib plus
paclitaxel | 32 | 47 | (35) |
| Cortes et
al, 2022 | KEYNOTE-355 | III | PFS | OR, DOR, DCR | Pembrolizumab plus
chemotherapy | Placebo plus
chemotherapy | 566 | 281 | (32) |
| Bachelot et
al, 2021 | SAFIR02-BREAST
IMMUNO | II | PFS | OS, ORR, AEs | Durvalumab | Maintenance
chemotherapy | 47 | 35 | (31) |
| Hattori et
al, 2023 | KEYNOTE-355 | III | PFS, OS | AEs, ORR, DOR,
DCR | Pembrolizumab plus
chemotherapy | Placebo plus
chemotherapy | 61 | 26 | (33) |
| Miles et al,
2021 | IMpassion131 | III | PFS | OS, ORR, AEs | Atezolizumab plus
paclitaxel | Placebo plus
paclitaxel | 431 | 220 | (36) |
| Røssevold et
al, 2022 | ALICE | IIb | PFS | ORR, DOR, DRR, CBR,
OS | Atezolizumab plus
chemotherapy | Placebo plus
chemotherapy | 40 | 28 | (30) |
| Winer et al,
2021 | KEYNOTE-119 | III | OS | PFS, ORR, DOR, DCR,
AEs | Pembrolizumab | Chemotherapy | 312 | 310 | (34) |
Literature quality assessment
The quality of each RCT was assessed using the
Cochrane Collaboration tool in the Review Manager version 5.4
software (The Cochrane Collaboration). The quality of the
literature was assessed across the following domains: i)
Randomization sequence; ii) allocation concealment; iii) blinding
of participants and investigators; iv) blinding of outcome
assessors; v) completeness of outcome data; vi) selective reporting
of outcomes; and vii) other risks of bias. Each domain was rated as
‘low risk’, ‘high risk’ or ‘unclear risk’. Two authors
independently assessed the quality of the literature, and
disagreements were resolved via discussion with a third author to
reach a consensus.
Statistical analysis
Statistical analyses were performed using Review
Manager version 5.4, and hazard ratio (HR) and standard error were
calculated and recorded by selecting the generalized inverse
variance from the collected HR data for progression-free survival
(PFS) and overall survival (OS). By contrast, dichotomous data
types were selected to calculate the objective response rate (ORR)
and adverse events. The heterogeneity of the studies was estimated
by using the Cochrane Q test and the I2 statistic, where
P<0.1 or I2>50% were considered to indicate
significant heterogeneity among the included studies. A random
effects model was used regardless of I2>50% or ≤50%.
Sensitivity analyses were not performed because <10 articles
were included, and therefore it would have been difficult to detect
the cause of asymmetry with such a small, number of studies
included in the present meta-analysis (28).
Results
Research options
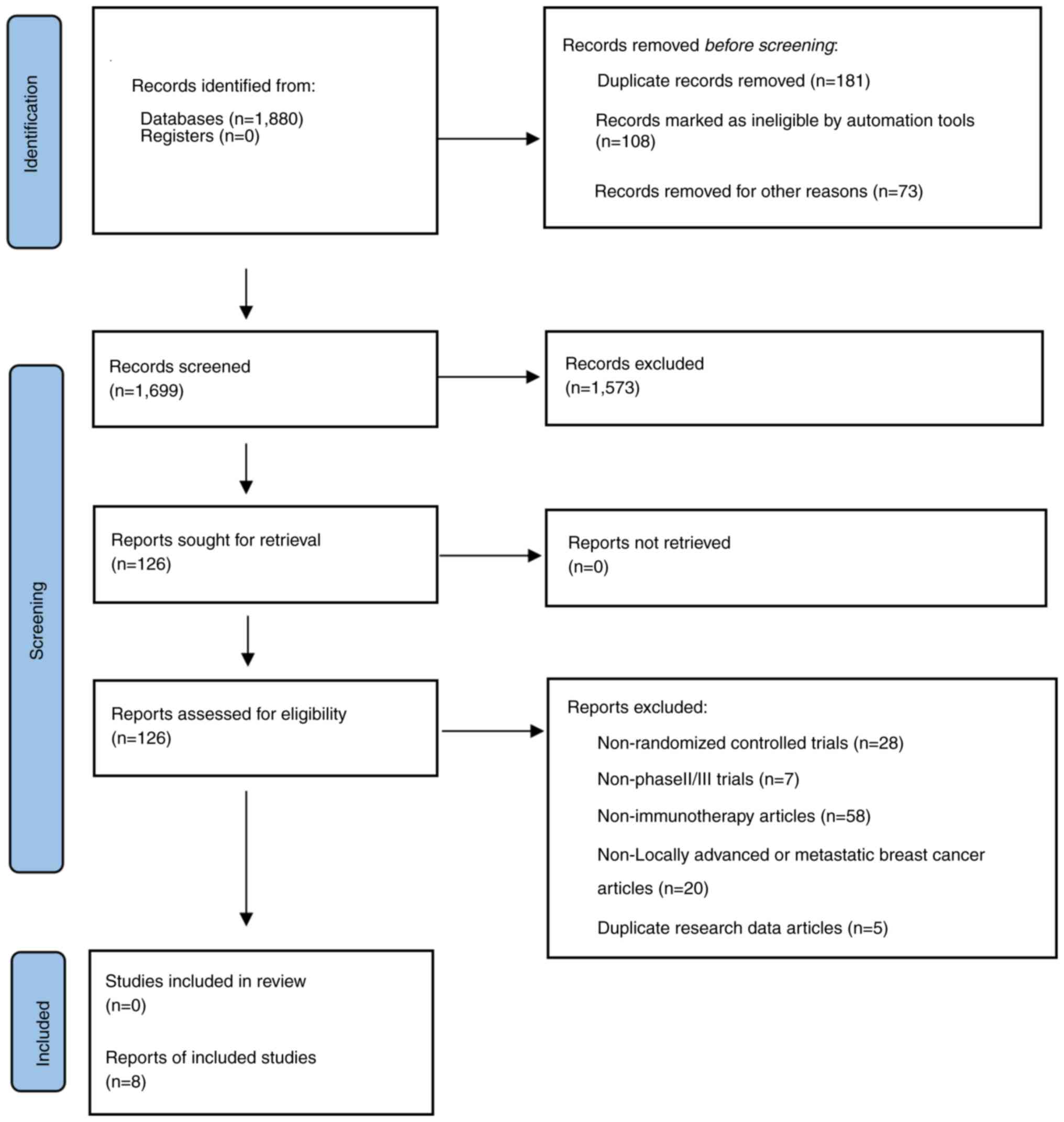
The study selection process is shown in Fig. 1. A total of 1,880 articles related
to TNBC and PD-1/PD-L1 inhibitors were retrieved from the PubMed,
Cochrane Library, Embase and MEDLINE databases. No additional
articles were retrieved from other sources. A total of 181 articles
were excluded due to being duplicates; 108 of these articles were
identified via duplicate searches performed by the literature
manager, and the authors deleted the remaining 73 articles by
comparing the years, journals, titles and abstracts between the
articles. A total of 1,573 articles were excluded after screening
the titles and abstracts. The full texts of the remaining 126
articles were screened. A total of 118 articles were excluded for
the following reasons: 28 were not RCTs; 7 were preclinical or
phase I clinical trials; 58 involved adjuvant therapy but not
immunotherapy for TNBC; 20 were trials of early-stage TNBC or other
stages rather than locally advanced or metastatic breast cancer;
and 5 reported the same data as other included studies.
Research characteristics and quality
assessment
In total, eight studies (29–36)
were ultimately included in the present meta-analysis. All of them
used immunotherapy with immune checkpoint inhibition to treat
locally advanced or metastatic TNBC. A total of 3,338 patients were
included in the above studies, with 1,940 patients in the
experimental group and 1,398 patients in the control group. Six of
the RCTs examined the use of immunosuppressants in combination with
other treatments versus other treatments alone, while two RCTs
compared the use of immunosuppressant monotherapy and chemotherapy.
Regarding the blinding method used across the study trials, three
studies were open-label trials, three were double-blind
(participants and investigators) and the other two were
quadruple-blind (participants, investigators, care providers and
outcome assessors). Among the eight included studies, seven
reported OS outcomes for all patients with TNBC. The subjects were
divided into PD-L1-negative [PD-L1(−)] and PD-L1-positive
[PD-L1(+)] subgroups according to PD-L1 expression. Six studies
reported OS for PD-L1(+) patients; all the studies reported PFS for
patients with TNBC and four studies reported PFS for PD-L1(+)
patients. All the studies defined the PD-L1(+) population as
patients in whom the number of PD-L1-stained tumour-infiltrating
immune cells in the total tumour area in the metastatic lesion
samples was >1. By contrast, the PD-L1(−) population was defined
as patients in whom the number of PD-L1-stained tumour-infiltrating
immune cells was ≤1% of the total tumour area in metastatic lesion
samples in the ITT population.
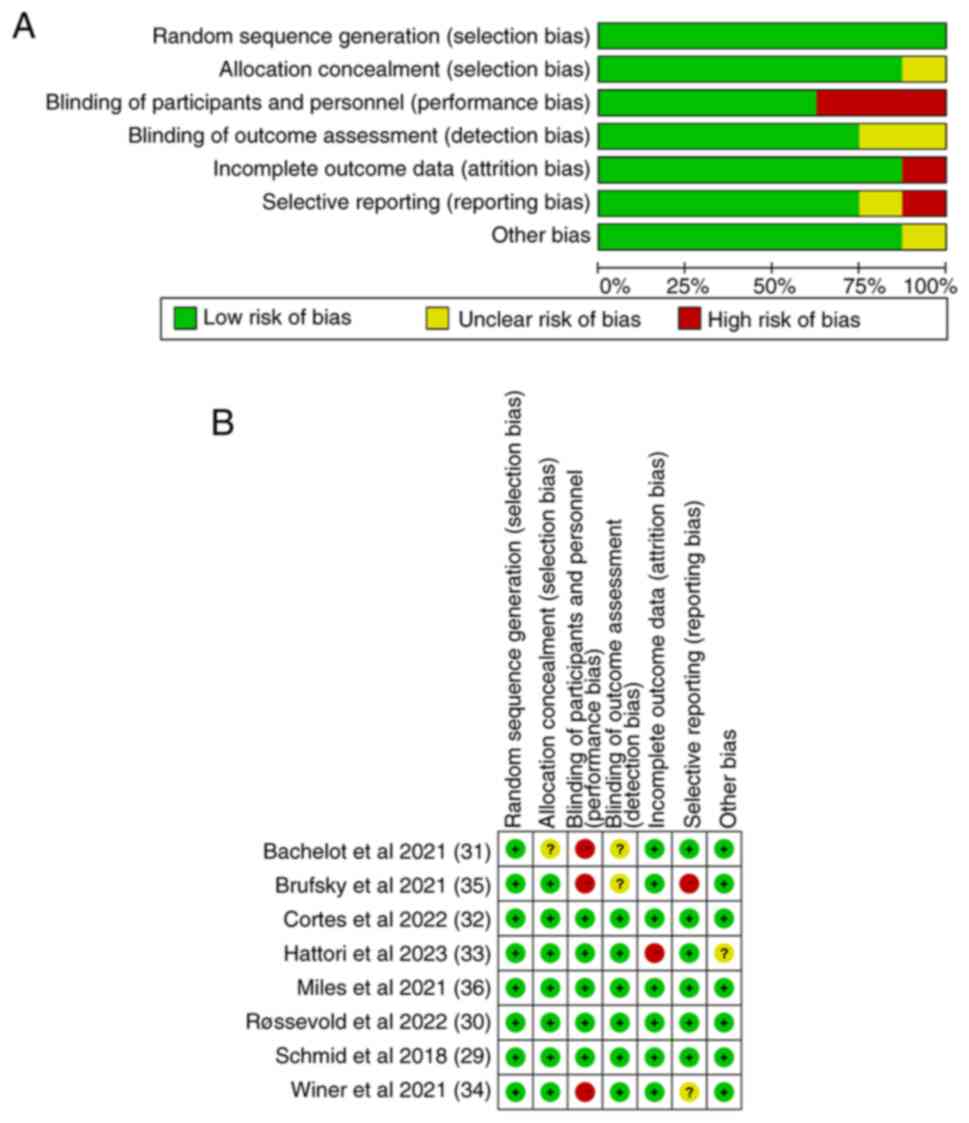
The results of the Cochrane risk of bias assessment
are shown in Fig. 2. All eight
studies had a relatively high standard of inclusion, and most of
them had a low risk of bias. In the risk of bias assessment chart,
green indicates a low risk of bias, while red indicates a high risk
of bias and yellow indicates an unclear risk of bias.
Antitumour efficacy results
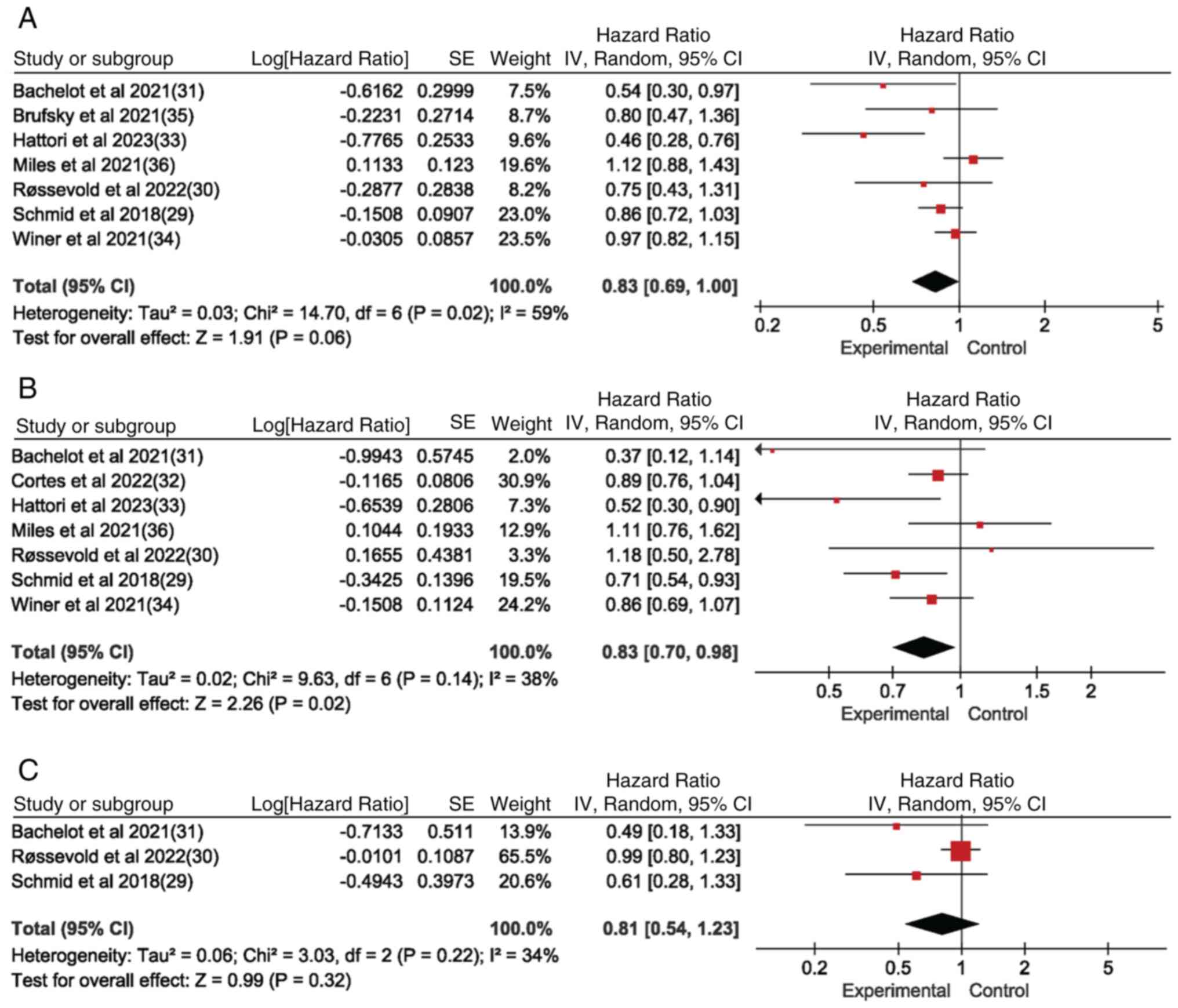
OS in the ITT population, and in the
PD-L1-positive and PD-L1-negative populations
Seven of the eight studies reported OS for the ITT
population, while seven studies reported OS for the PD-L1(+)
population and three studies reported OS for the PD-L1(−)
population. The OS of the experimental group was significantly
superior to that of the control group in the PD-L1(+) population
[hazard ratio (HR)=0.83; 95% confidence interval (CI)=0.70-0.98;
P<0.05; I2=38%] (Fig.
3B) and in the ITT population (Fig.
3A) (HR=0.83; 95% CI=0.69-1.00; P<0.05; I2=59%).
By contrast, there was no significant difference in the OS of the
PD-L1(−) population (Fig. 3C).
Forest plot results in Fig. 3
showed that PD-1/PD-L1 inhibitor-based immunotherapy improved OS in
the ITT population as well as in the PD-L1(+) population; however,
there was no effect on OS in the PD-L1(−) subgroup-based
population, which indicates that PD-1/PD-L1 inhibitor-based
immunotherapy has an impact on OS in metastatic and advanced
locally unresectable TNBC, and correlates with PD-L1 subtype
subgroups, since the PD-L1(+) group in the ITT population is
affected.
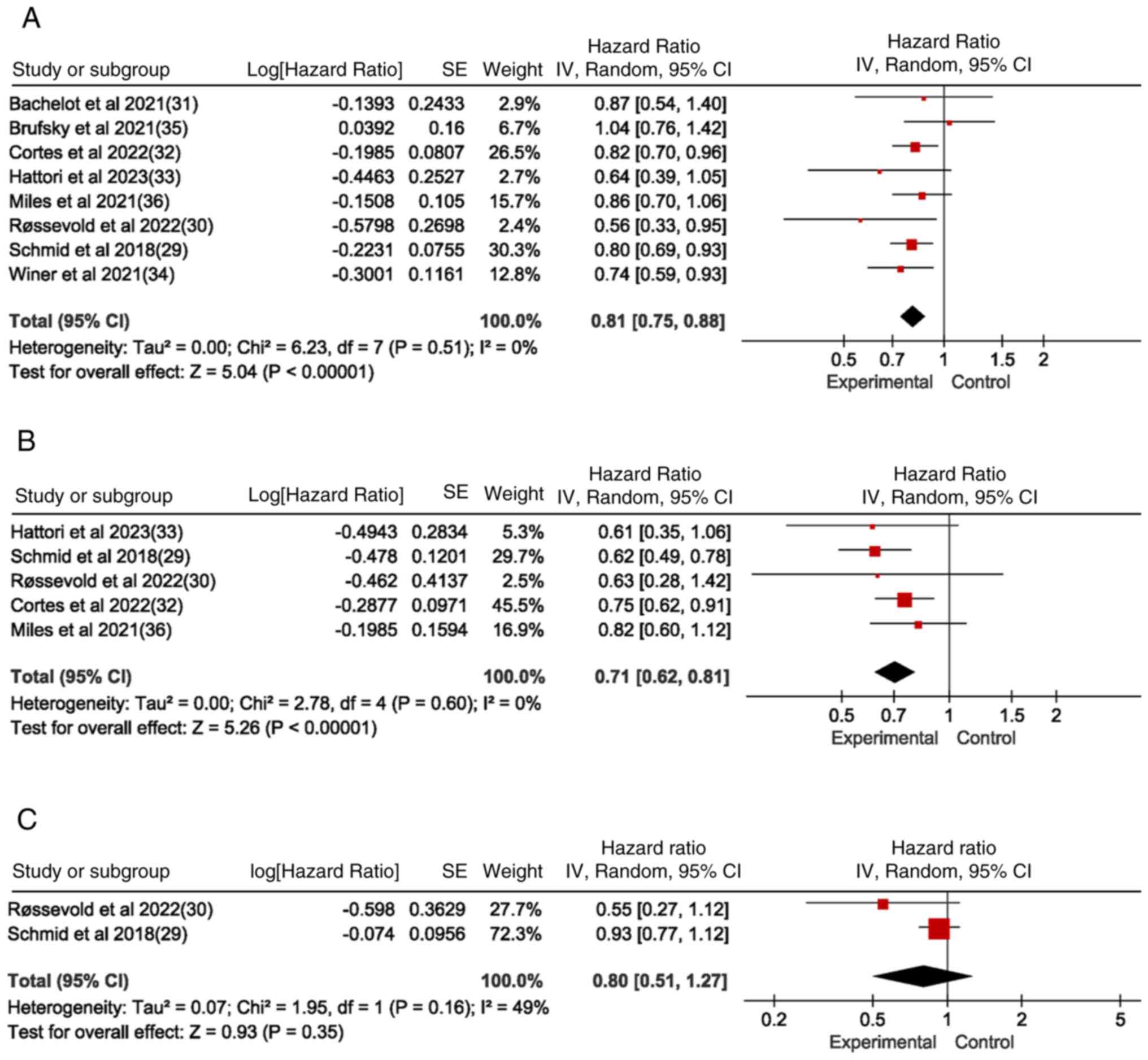
PFS in the ITT population, and in the
PD-LI-positive and -negative population
Eight studies reported PFS for the ITT population,
five of which (29,30,32,33,36)
reported PFS for the PD-L1(+) population. Of these, two (29,30)
reported PFS for the PD-L1(−) population, while the others did not
report PFS for the PD-L1 subgroup. The PFS in the experimental
group was significantly higher compared with that in the control
group for both the ITT population (Fig.
4A) and the PD-L1(+) population (Fig. 4B) (ITT: HR=0.81, 95% CI=0.75-0.88,
P<0.05, I2=0%; PD-L1-positive: HR=0.71, 95%
CI=0.62-0.81, P<0.05, I2=0%). However, the PFS of the
experimental and control groups was not statistically significant
in the PD-L1(−) population (Fig.
4C). Forest plot results in Fig.
4 illustrate that PD-1/PD-L1 inhibitor-based immunotherapy
significantly prolongs PFS in the ITT population as well as in
PD-L1(+) population, confirming the strong association between
PD-1/PD-L1 inhibitor therapy for TNBC and PD-L1 subtype status.
Safety analysis
Adverse event rates were used to assess the safety
of ICI-based immunotherapy or immunotherapy in combination with
other drugs. Specifically, the rates of serious adverse events
(SAEs) and immune-related adverse events (irAEs) in the included
studies were examined to determine the safety of the therapies.
There was no significant difference in the rate of adverse events
between the immunotherapy alone group and the immunotherapy
combined with chemotherapy group. Therefore, the main adverse
events examined were SAEs (grade ≥3). Table SII shows that immunosuppressant
treatment combined with other therapies for TNBC was associated
with the following adverse events: Alopecia [relative risk
(RR)=0.89; 95% CI=0.70-1.14; P=0.36], anaemia (RR=0.96; 95%
CI=0.75-1.22; P=0.72), cough (RR=1.29; 95% CI=1.06-1.57; P=0.01),
diarrhoea (RR=0.83; 95% CI=0.59-1.19; P=0.31), loss of appetite
(RR=0.64; 95% CI=0.29-1.39; P=0.26), fatigue (RR=1.02; 95%
CI=0.92-1.13; P=0.74), hypothyroidism (RR=4.52; 95% CI=2.95-6.94;
P<0.00001), nausea (RR=0.93; 95% CI=0.76-1.14; P=0.49),
neutropenia (RR=0.97; 95% CI=0.68-1.38; P=0.86), fever (RR=1.42;
95% CI=1.15-1.83; P=0.002), rash (RR=1.10; 95% CI=0.90-1.33;
P=0.36), pruritus (RR=0.80; 95% CI=0.26-2.48; P=0.70) and weakness
(RR=1.00; 95% CI=0.81-1.23; P=0.99). Among them, only
hypothyroidism, fever and cough showed significant associations
with the ICI + chemotherapy combination treatment (P<0.05). Six
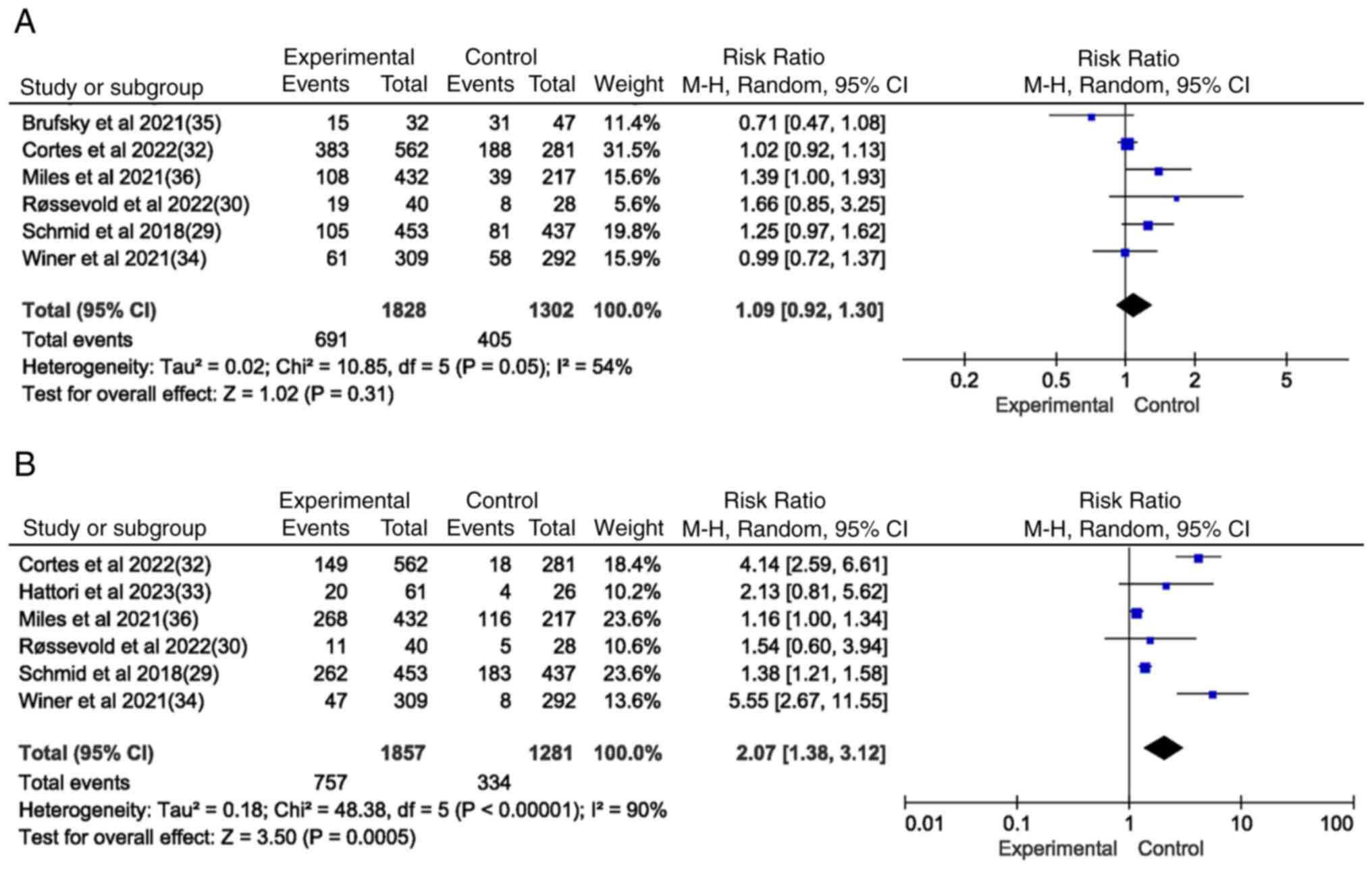
studies documented SAEs (Fig. 5A);
immunotherapy based on ICIs in combination with other therapies was
associated with 691 SAEs among 1,828 patients compared with 405
SAEs among the 1,302 control patients receiving other therapies.
Furthermore, there was no significant difference in the rate of
SAEs associated with immunotherapy with ICIs alone or combined with
other therapies and the rate of SAEs associated with other
therapies (P=0.31; P>0.05).
In six of the included studies, there was a
significant difference in irAEs between the experimental group and
the control group (P=0.0005) (Fig.
5B), suggesting that immunotherapy based on ICIs alone or in
combination with other treatments is more likely to cause irAEs in
patients with TNBC. Furthermore, this finding suggests that the
occurrence of irAEs is predominantly associated with abnormalities
in thyroid function (including hyperthyroidism and hypothyroidism)
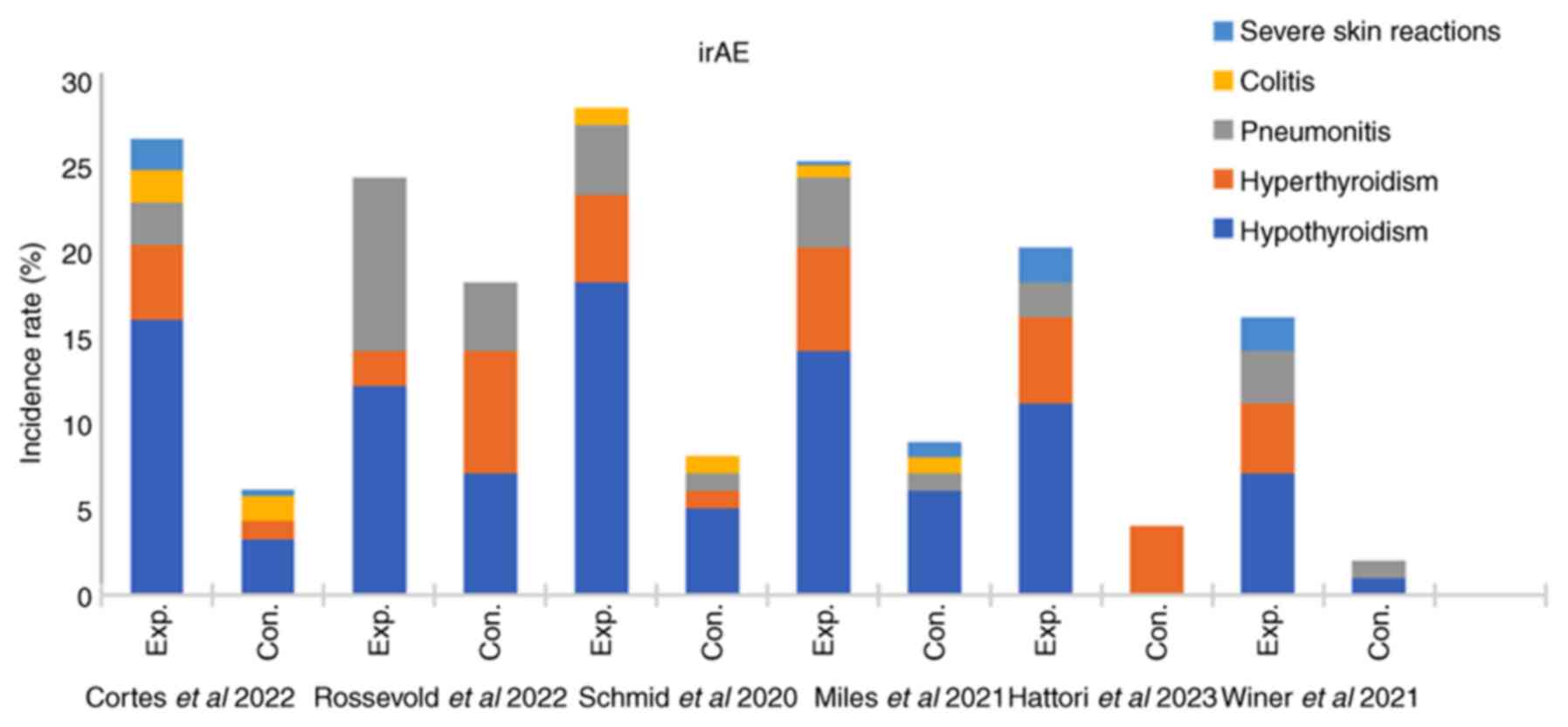
(Fig. 6).
Discussion
TNBC is characterized by high immunogenicity, high
invasiveness and poor prognosis (37). In recent years, immunotherapy has
emerged as an effective treatment for cancer (38–40).
The present meta-analysis aimed to assess the efficacy and safety
of PD-L1/PD-1 inhibitors for patients with TNBC by evaluating
whether the ICIs were combined with chemotherapy, and to determine
the clinical significance of PD-L1 status with respect to the use
of ICIs to treat patients with TNBC (34). The results revealed that
immunotherapy based on ICIs alone or combined with other therapies
for the treatment of locally advanced or metastatic TNBC affected
the PFS of the ITT population (P<0.05) but did not significantly
impact the OS of patients with TNBC. Subgroup analyses stratified
based on PD-L1 receptor status revealed that ICI-based
immunotherapy alone or in combination with other therapies
significantly prolonged PFS and OS in the PD-L1(+) subgroup;
however, no statistically significant differences were observed in
the PD-L1(−) subgroup.
Analysis of several RCTs revealed that ICIs alone or
combined with chemotherapy prolonged patients' OS and PFS. The
phase III RCT KEYNOTE119 (34),
which compared pembrolizumab immunotherapy and chemotherapy for the
treatment of metastatic TNBC, revealed that PD-1/PD-L1 inhibitor
monotherapy did not significantly improve the ORR or OS of patients
with metastatic TNBC who had received other therapies, while
pembrolizumab treatment was effective among individuals with
increased PD-L1 levels in the tumour microenvironment, thus
suggesting that the clinical benefits produced by pembrolizumab
monotherapy for metastatic TNBC may be associated with the
expression of PD-L1 in the tumour microenvironment; thus, the use
of immune checkpoint inhibition alone in the treatment of locally
advanced or metastatic TNBC is ineffective and prone to drug
resistance. On the other hand, the randomized, double-blind,
controlled phase III IMpassion130 clinical trial (29) evaluated the efficacy and safety of
atezolizumab in combination with nab-paclitaxel versus placebo in
combination with nab-paclitaxel for the treatment of locally
advanced or metastatic TNBC. The results revealed that there was no
significant difference in OS for ICI + nab-paclitaxel versus
placebo + paclitaxel, although ICI + nab-paclitaxel was superior to
placebo + paclitaxel in the ITT population. There was a significant
difference in the PD-L1(+) population, suggesting that ICI
atezolizumab + nab-paclitaxel has a clinically meaningful effect on
OS and PFS in the PD-L1(+) population. In the KEYNOTE-355 trial
(27), the use of pembrolizumab
plus chemotherapy for TNBC led to improvements in PFS and OS
compared with placebo plus chemotherapy. By contrast, the Impassion
131 trial (36), a randomized,
placebo-controlled, double-blind trial that evaluated the efficacy
of first-line paclitaxel alone or in combination with atezolizumab
for treating unresectable locally advanced or metastatic TNBC,
revealed that atezolizumab in combination with paclitaxel exhibited
significant efficacy for treating metastatic TNBC.
A safety analysis was conducted in the present
study, which focused on adverse events caused by PD-1/PD-L1
inhibitors, SAEs (grade ≥3 adverse events) and immune-related
adverse events. The safety analysis of immunosuppressant treatment
for patients with TNBC revealed that there was no statistically
significant difference in the incidence of SAEs, but there was a
statistically significant difference in the incidence of irAEs
between the experimental group and the control group. Specifically,
the incidence rates of hypothyroidism, hyperthyroidism,
pneumonitis, hepatitis and adrenal insufficiency significantly
increased after the addition of ICIs (41). Adverse events may affect the
patient's choice of treatment; therefore, it is important to inform
patients of such events when choosing PD-1/PD-L1 therapy for
patients with TNBC, and clinicians must remain vigilant in
recognizing and intervening in the prevention of serious
complications such as hypothyroidism, pneumonia and neutropenia
(42,43).
Compared with chemotherapy alone, treatment with
PD-1/PD-L1 and CLTA-4 ICIs significantly increased the occurrence
of irAEs, including severe pneumonia, hypothyroidism and
hypoadrenocorticism, in patients with TNBC. However, the incidence
of irAEs was relatively low. Serious immune-related adverse events
included thyroid dysfunction (hypothyroidism and hyperthyroidism),
severe skin events, colitis and pneumonia (43). Although the aetiological mechanism
of TNBC that leads to serious immune-related adverse events is
unclear, the physiopathology of immune-associated thyroid
dysfunction has been described for thyroiditis with concomitant
thyroid destruction, which is mediated by T-cell toxicity, natural
killer cells and PD-1/PD-L1 expression in thyroid cells (44). Severe skin events are also due to
the blockade of PD-1/PD-L1 inhibitors, which may lead to the
activation of nonspecific T lymphocytes that target
antigen-carrying keratinocytes and other skin cells, resulting in
skin toxicities such as rashes, purpura and other skin disorders
(45). For pneumonia, it has been
suggested that alveolar macrophages become overactivated in
patients receiving PD-1 inhibitors; this hypothesis is supported by
the fact that interstitial macrophages and alveolar cells express
repulsive guidance molecule B on their surfaces, which may be a
ligand for PD-L2 (46).
Data for the current meta-analysis were collected up
to July 19, 2023, thus including more data than similar
meta-analyses. In the meta-analysis by Zhang et al (47), data for the analysis were collected
up to October 2021, and the results showed that immunotherapy based
on PD-1/PD-L1 inhibitors had no effect on OS in the ITT population
(HR=0.90; 95% CI=0.78-1.04; P=0.144; I2=24.0%) versus
the PD-L1(+) population, and was not associated with PD-L1 status.
However, the PFS in the ITT population (HR=0.82; 95% CI=0.76-1.14;
P<0.001; I2=0%) and in the PD-L1(+) population
(HR=0.68; 95% CI=0.6-0.76; P<0.001) was significantly prolonged
in the ITT and PD-L1(+) populations. Wang (48) collected data through August 30, 2021
for treatments targeting early to mid- and late-stage TNBC, and the
analysis showed that, regarding the PFS and OS of all subjects
treated with or without PD-1/PD-L1 inhibitors, there was no
significant effect on PD-L1 subgroup status, while the results of
PD-L1 subgroup status showed that treatment of the PD-L1(+) TNBC
population with PD-1/PD-L1 inhibitors significantly improved their
PFS and OS (P<0.05); the safety analysis was the same as in the
present analysis, with a significant effect on SAEs and immune
adverse events. The study by Yu et al (49) was a meta-analysis focused on
immunotherapy for metastatic TNBC, excluding data on locally
advanced unresectable TNBC, and the results showed that there was a
significant difference in PFS between the ITT population and the
PD-L1(+) group, which was similar to the results of the present
meta-analysis, whereas the OS of the ITT population was not
significantly different, which is in disagreement with the present
analysis that may be due to the addition of OS data for the
intention-to-treat population with locally advanced unresectable
TNBC (33), and the OS of the
PD-L1(+) group was significantly different from that of the ITT
population, which is similar to the present results. The novelty of
the present meta-analysis is the significant effect of time and
trial size on PFS and OS in the ITT and PD-L1(+) populations
receiving PD-1/PD-L1-based immunotherapy, which differs from
similar previous analyses in that immunotherapy had a significant
effect on the OS of ITT patients with locally advanced unresectable
and metastatic TNBC receiving PD-1/PD-L1-based immunotherapy.
Therefore, it may be hypothesized that the incorporation of
supplementary clinical data pertaining to immunosuppressive therapy
in patients diagnosed with metastatic and locally advanced
unresectable TNBC has the potential to impact OS within the
targeted treatment population.
The present meta-analysis has several limitations.
First, only eight studies were included, and some of the clinical
trials did not report long-term survival outcomes, such as
KEYNOTE-522, which did not report patient OS. Second, some studies
did not stratify PFS or OS analyses based on PD-L1 subgroup status,
which may have affected the data results and potentially
contributed to the inability to determine the benefits of ICIs for
the treatment of locally advanced or metastatic TNBC. In addition,
the multiple dosing regimens and delivery modes of treatment
included in the present meta-analysis difficulted the determination
of the optimal treatment regimen. Therefore, it is necessary to
conduct numerous RCTs of relevant treatment regimens and to obtain
clinical treatment data in the future in order to refine the use of
other therapies combined with ICI immunotherapy for TNBC.
In conclusion, the present meta-analysis revealed
that treatment with ICIs in combination with other therapies
prolonged PFS in patients with locally advanced or metastatic TNBC
compared with other therapies, and that immunotherapies also
significantly prolonged OS and PFS in patients with TNBC in the
PD-L1(+) subgroup and in the ITT population (OS), but not in the
PD-L1(−) subgroup (OS and PFS). The main adverse reaction of any
grade arising from the use of ICIs was hypothyroidism.
Additionally, hyperthyroidism accounted for a relatively high
proportion of adverse events, but this adverse effect was generally
manageable.
In summary, the present study has illustrated that
PD-1/PD-L1 inhibitors are effective in the treatment of TNBC,
although a significant association with PD-L1 subgroup status was
observed. Targeting the adverse effects associated with
immunotherapy may affect patient selection and clinical
application, but may have a relatively significant efficacy in OS
and PFS in the ITT and PD-L1(+) population. Future studies should
focus on PD-L1 expression status, as this parameter may help to
optimize personalized treatment for patients.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Jiangxi Provincial
Natural Science Foundation (grant no. 20202BABL206079).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YC, LS, WY, HX and CL contributed to the conception
and development of the present study. YC edited the entire
manuscript and contributed to data visualization; WY proposed the
direction and purpose of the study, and guided and reviewed the
article. LS and YC reviewed and collected a large quantity of
literature and data, participated in literature screening, and
discussed the article's inclusion criteria. LS and YC confirm the
authenticity of all the raw data. CL guided the overall framework
of the article and provided comments on the article's structure. HX
and WY reviewed the final draft of the manuscript and provided
comments on the revisions. All the authors have read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nik-Zainal S, Davies H, Staaf J,
Ramakrishna M, Glodzik D, Zou X, Martincorena I, Alexandrov LB,
Martin S, Wedge DC, et al: Landscape of somatic mutations in 560
breast cancer whole-genome sequences. Nature. 534:47–54. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Harbeck N, Penault-Llorca F, Cortes J,
Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J and Cardoso F:
Breast cancer. Nat Rev Dis Primers. 5:662019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhou Y, Tian Q, Wang BY and Yang J, Zhao
SD and Yang J: The prognostic significance of TILs as a biomarker
in triple-negative breast cancer: What is the role of TILs in TME
of TNBC? Eur Rev Med Pharmacol Sci. 25:2885–2897. 2021.PubMed/NCBI
|
|
4
|
Luo C, Wang P, He S, Zhu J, Shi Y and Wang
J: Progress and prospect of immunotherapy for triple-negative
breast cancer. Front Oncol. 12:9190722022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen X, Feng L, Huang Y, Wu Y and Xie N:
Mechanisms and strategies to overcome PD-1/PD-L1 blockade
resistance in triple-negative breast cancer. Cancers (Basel).
15:1042022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
He R, Yuan X, Chen Z and Zheng Y: Combined
immunotherapy for metastatic triple-negative breast cancer based on
PD-1/PD-L1 immune checkpoint blocking. Int Immunopharmacol.
113:1094442022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
So JY, Ohm J, Lipkowitz S and Yang L:
Triple negative breast cancer (TNBC): Non-genetic tumor
heterogeneity and immune microenvironment: Emerging treatment
options. Pharmacol Ther. 237:1082532022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Obidiro O, Battogtokh G and Akala EO:
Triple negative breast cancer treatment options and limitations:
Future outlook. Pharmaceutics. 15:17962023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Voduc KD, Cheang MC, Tyldesley S, Gelmon
K, Nielsen TO and Kennecke H: Breast cancer subtypes and the risk
of local and regional relapse. J Clin Oncol. 28:1684–1691. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Solin LJ, Hwang WT and Vapiwala N: Outcome
after breast conservation treatment with radiation for women with
triple-negative early-stage invasive breast carcinoma. Clin Breast
Cancer. 9:96–100. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panoff JE, Hurley J, Takita C, Reis IM,
Zhao W, Sujoy V, Gomez CR, Jorda M, Koniaris L and Wright JL: Risk
of locoregional recurrence by receptor status in breast cancer
patients receiving modern systemic therapy and post-mastectomy
radiation. Breast Cancer Res Treat. 128:899–906. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pan XB, Qu S, Jiang YM and Zhu XD: Triple
negative breast cancer versus non-triple negative breast cancer
treated with breast conservation surgery followed by radiotherapy:
A systematic review and meta-analysis. Breast Care (Basel).
10:413–416. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chapdelaine AG and Sun G: Challenges and
opportunities in developing targeted therapies for triple negative
breast cancer. Biomolecules. 13:12072023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chang-Qing Y, Jie L, Shi-Qi Z, Kun Z,
Zi-Qian G, Ran X, Hui-Meng L, Ren-Bin Z, Gang Z, Da-Chuan Y and
Chen-Yan Z: Recent treatment progress of triple negative breast
cancer. Prog Biophys Mol Biol. 151:40–53. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Maqbool M, Bekele F and Fekadu G:
Treatment strategies against triple-negative breast cancer: An
updated review. Breast Cancer (Dove Med Press). 14:15–24.
2022.PubMed/NCBI
|
|
16
|
Sandler A, Gray R, Perry MC, Brahmer J,
Schiller JH, Dowlati A, Lilenbaum R and Johnson DH:
Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell
lung cancer. N Engl J Med. 355:2542–2550. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hodi FS, O'Day SJ, McDermott DF, Weber RW,
Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel
JC, et al: Improved survival with ipilimumab in patients with
metastatic melanoma. N Engl J Med. 363:711–723. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Powles T, Park SH, Voog E, Caserta C,
Valderrama BP, Gurney H, Kalofonos H, Radulović S, Demey W, Ullén
A, et al: Avelumab maintenance therapy for advanced or metastatic
urothelial carcinoma. N Engl J Med. 383:1218–1230. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Abad NM, Calabuig-Fariñas S, de Mena ML,
Torres-Martínez S, González CG, García JÁ, González-Cruz VI and
Herrero CC: Programmed death-ligand 1 (PD-L1) as immunotherapy
biomarker in breast cancer. Cancers (Basel). 14:3072022. View Article : Google Scholar
|
|
20
|
Qureshi S, Chan N, George M, Ganesan S,
Toppmeyer D and Omene C: Immune checkpoint inhibitors in triple
negative breast cancer: The search for the optimal biomarker.
Biomark Insights. 17:117727192210787742022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Y, Zhu X, Tang C, Guan X and Zhang W:
Progress and challenges of immunotherapy in triple-negative breast
cancer. Biochim Biophys Acta Rev Cancer. 1876:1885932021.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li Y, Zhang H, Merkher Y, Chen L, Liu N,
Leonov S and Chen Y: Recent advances in therapeutic strategies for
triple-negative breast cancer. J Hematol Oncol. 15:1212022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tang F and Zheng P: Tumor cells versus
host immune cells: Whose PD-L1 contributes to PD-1/PD-L1 blockade
mediated cancer immunotherapy? Cell Biosci. 8:342018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang H, Liang Y, Anders RA, Taube JM, Qiu
X, Mulgaonkar A, Liu X, Harrington SM, Guo J, Xin Y, et al: PD-L1
on host cells is essential for PD-L1 blockade-mediated tumor
regression. J Clin Invest. 128:580–588. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Khan M, Du K, Ai M, Wang B, Lin J, Ren A,
Chen C, Huang Z, Qiu W, Yuan Y and Tian Y: PD-L1 expression as
biomarker of efficacy of PD-1/PD-L1 checkpoint inhibitors in
metastatic triple negative breast cancer: A systematic review and
meta-analysis. Front Immunol. 14:10603082023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
National Institutes of Health, . National
Cancer Institute, U.S. Department of Health and Human Services.,
Common Terminology Criteria for Adverse Events (CTCAE) Version
4.0.
|
|
27
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sterne JA, Sutton AJ, Ioannidis JP, Terrin
N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH,
et al: Recommendations for examining and interpreting funnel plot
asymmetry in meta-analyses of randomised controlled trials. BMJ.
343:d40022011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Schmid P, Adams S, Rugo HS, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Hegg R, Im SA, Wright GS, et al:
Atezolizumab and nab-paclitaxel in advanced triple-negative breast
cancer. N Engl J Med. 379:2108–2121. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Røssevold AH, Andresen NK, Bjerre CA,
Gilje B, Jakobsen EH, Raj SX, Falk RS, Russnes HG, Jahr T,
Mathiesen RR, et al: Atezolizumab plus anthracycline-based
chemotherapy in metastatic triple-negative breast cancer: The
randomized, double-blind phase 2b ALICE trial. Nat Med.
28:2573–2583. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bachelot T, Filleron T, Bieche I, Arnedos
M, Campone M, Dalenc F, Coussy F, Sablin MP, Debled M,
Lefeuvre-Plesse C, et al: Durvalumab compared to maintenance
chemotherapy in metastatic breast cancer: The randomized phase II
SAFIR02-BREAST IMMUNO trial. Nat Med. 27:250–255. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Cortes J, Rugo HS, Cescon DW, Im SA, Yusof
MM, Gallardo C, Lipatov O, Barrios CH, Perez-Garcia J, Iwata H, et
al: Pembrolizumab plus chemotherapy in advanced triple-negative
breast cancer. N Engl J Med. 387:217–226. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hattori M, Masuda N, Takano T, Tsugawa K,
Inoue K, Matsumoto K, Ishikawa T, Itoh M, Yasojima H, Tanabe Y, et
al: Pembrolizumab plus chemotherapy in Japanese patients with
triple-negative breast cancer: Results from KEYNOTE-355. Cancer
Med. 12:10280–10293. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Winer EP, Lipatov O, Im SA, Goncalves A,
Muñoz-Couselo E, Lee KS, Schmid P, Tamura K, Testa L, Witzel I, et
al: Pembrolizumab versus investigator-choice chemotherapy for
metastatic triple-negative breast cancer (KEYNOTE-119): A
randomised, open-label, phase 3 trial. Lancet Oncol. 22:499–511.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brufsky A, Kim SB, Zvirbule Ž, Eniu A,
Mebis J, Sohn JH, Wongchenko M, Chohan S, Amin R, Yan Y, et al: A
phase II randomized trial of cobimetinib plus chemotherapy, with or
without atezolizumab, as first-line treatment for patients with
locally advanced or metastatic triple-negative breast cancer
(COLET): Primary analysis. Ann Oncol. 32:652–660. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Miles D, Gligorov J, André F, Cameron D,
Schneeweiss A, Barrios C, Xu B, Wardley A, Kaen D, Andrade L, et
al: Primary results from IMpassion131, a double-blind,
placebo-controlled, randomised phase III trial of first-line
paclitaxel with or without atezolizumab for unresectable locally
advanced/metastatic triple-negative breast cancer. Ann Oncol.
32:994–1004. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Schmid P, Rugo HS, Adams S, Schneeweiss A,
Barrios CH, Iwata H, Diéras V, Henschel V, Molinero L, Chui SY, et
al: Atezolizumab plus nab-paclitaxel as first-line treatment for
unresectable, locally advanced or metastatic triple-negative breast
cancer (IMpassion130): Updated efficacy results from a randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:44–59. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Robert C: A decade of immune-checkpoint
inhibitors in cancer therapy. Nat Commun. 11:38012020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ledford H, Else H and Warren M: Cancer
immunologists scoop medicine nobel prize. Nature. 562:20–21. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Le DT, Uram JN, Wang H, Bartlett BR,
Kemberling H, Eyring AD, Skora AD, Luber BS, Azad NS, Laheru D, et
al: PD-1 blockade in tumors with mismatch-repair deficiency. N Engl
J Med. 372:2509–2520. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Derakhshan F and Reis-Filho JS:
Pathogenesis of triple-negative breast cancer. Annu Rev Pathol.
17:181–204. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xiao BY, Lin GH, Zhao YX and Wang BC: The
efficacy and safety of PD-1/PD-L1 inhibitors in breast cancer: A
systematic review and meta-analysis. Transl Cancer Res.
9:3804–3818. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang Y, Wang J, Hu T, Wang H, Long M and
Liang B: Adverse events of PD-1 or PD-L1 inhibitors in
triple-negative breast cancer: A systematic review and
meta-analysis. Life (Basel). 12:19902022.PubMed/NCBI
|
|
44
|
Baraibar I, Melero I, Ponz-Sarvise M and
Castanon E: Safety and tolerability of immune checkpoint inhibitors
(PD-1 and PD-L1) in cancer. Drug Saf. 42:281–294. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Patel AB and Pacha O: Skin reactions to
immune checkpoint inhibitors. Adv Exp Med Biol. 995:175–184. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Nguyen LT and Ohashi PS: Clinical blockade
of PD1 and LAG3-potential mechanisms of action. Nat Rev Immunol.
15:45–56. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhang W, He Y, Tang Y, Dai W, Si Y, Mao F,
Xu J, Yu C and Sun X: A meta-analysis of application of PD-1/PD-L1
inhibitor-based immunotherapy in unresectable locally advanced
triple-negative breast cancer. Immunotherapy. 15:1073–1088. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Wang C: A meta-analysis of efficacy and
safety of PD-1/PD-L1 inhibitors in triple-negative breast cancer. J
Oncol. 2022:24072112022.PubMed/NCBI
|
|
49
|
Yu Y, Jin X, Zhu X, Xu Y, Si W and Zhao J:
PD-1/PD-L1 immune checkpoint inhibitors in metastatic
triple-negative breast cancer: A systematic review and
meta-analysis. Front Immunol. 14:12066892023. View Article : Google Scholar : PubMed/NCBI
|