Introduction
Myeloid sarcoma (MS) of the breast is an extremely
rare disease, occurring as a manifestation in only 0.12% of
patients with acute myeloid leukemia (AML) (1). It is an extramedullary tumor
comprising immature cells derived from the myeloid lineage. While
MSs can affect various organs, including the breast, skin, lymph
nodes, intestines, bones and central nervous system, the isolated
involvement of the breast is extremely rare, with only a few
reported cases in the literature (2,3). The
clinical manifestations of breast MS are often nonspecific, which
makes the initial clinical assessment challenging, particularly in
patients who have exhibited no prior indication of myeloid lesions
or involvement of breast tissue. Although breast imaging plays a
crucial role in the initial evaluation of this disease, the imaging
features of MS may be similar to those of breast cancer or
lymphoma, rendering it challenging to distinguish between these
conditions (4). Currently, the
diagnosis of breast MS can only be confirmed using
immunohistochemical examinations and other histological tests.
Therefore, it is necessary for suspected malignant breast masses to
be confirmed through needle puncture pathology. In the context of
MS, a variety of chemotherapeutic regimens, including idarubicin,
high-dose cytarabine, cyclophosphamide and cisplatin, have been
used to induce remission (5).
Idarubicin is a DNA topoisomerase inhibitor that disrupts protein
synthesis and hampers DNA repair, thereby promoting cell death.
Cytarabine acts by inhibiting DNA polymerase during the DNA
synthesis phase, which interrupts DNA replication and reduces tumor
cell growth. Cyclophosphamide is metabolized within tumor cells to
form the potent phosphamide mustard inside, which alkylates DNA and
creates cross-links between DNA strands that inhibit the growth and
reproduction of tumor cells. Cisplatin is a cytotoxic agent that is
effective throughout the cell cycle, and is capable of killing
tumor cells at various stages of their growth (6). In addition, radiation therapy may be
used to reduce the risk of local recurrence (7).
The present case report describes a case of MS
originating from a single breast, its clinical and pathological
characteristics and therapeutic management.
Case report
A 58-year-old woman was admitted to The Affiliated
Hospital of Inner Mongolia Medical University (Hohhot, China) in
April 2022 after the discovery of a lump in her right breast within
the previous 2 months. The patient was generally healthy with no
notable medical history. Physical examination revealed symmetrical
breasts with no nipple retraction, discharge or ‘orange peel’
appearance. A hard lump measuring ~25×20×15 mm was detected at the
10 o'clock position in the right breast, ~2 cm away from the
nipple. The lump had an irregular surface and unclear boundaries.
However, it was movable and not adherent to the chest wall; in
addition, no tenderness was observed. No masses were detected in
the left breast, and no enlargement of the lymph nodes was observed
in either armpit.
Routine blood examination was within normal limits,
revealing a white blood cell count of 6.0×109 cells/l,
with neutrophil and lymphocyte percentages of 65.1 and 28.2%,
respectively, a hemoglobin level of 128 g/l and platelet count of
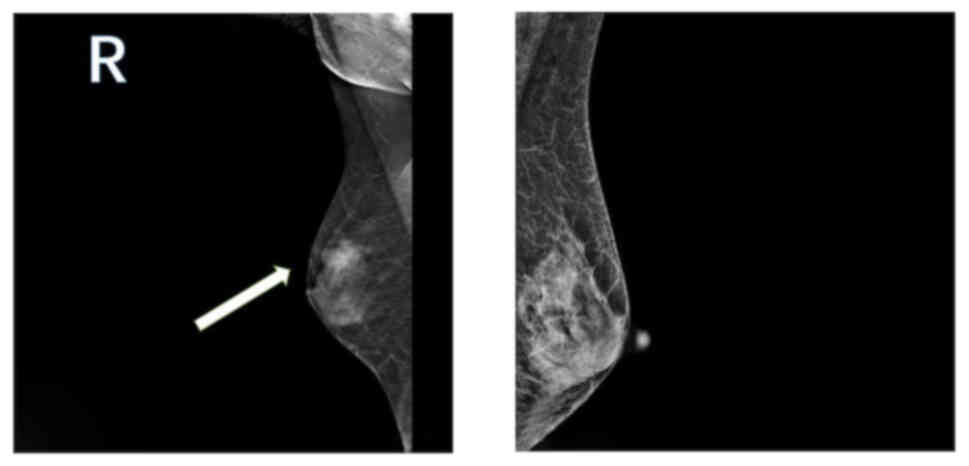
247×109 cells/l. Mammography revealed the presence of
uneven dense glandular tissue in both breasts. A nodular
high-density shadow with unclear boundaries, measuring ~21.6×14.7
mm, was evident in the upper outer quadrant of the right breast
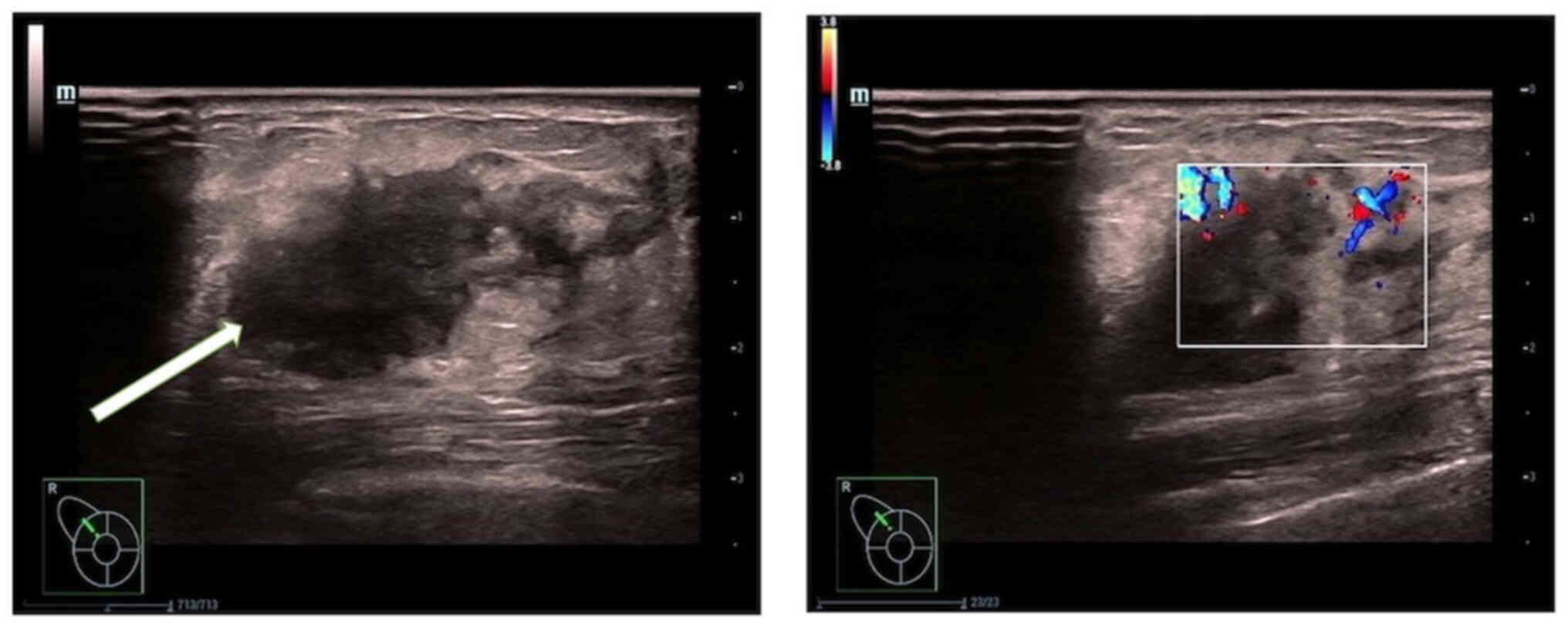
(Fig. 1). Breast ultrasonography
(Fig. 2) showed a hypoechoic nodule
measuring ~22.1×14.4 mm at the 11 o'clock position in the right
breast, approximately one finger breadth away from the nipple. The
nodule was irregularly shaped with unclear boundaries and
surrounding spicules. Rich linear blood-flow signals were observed
peripherally.
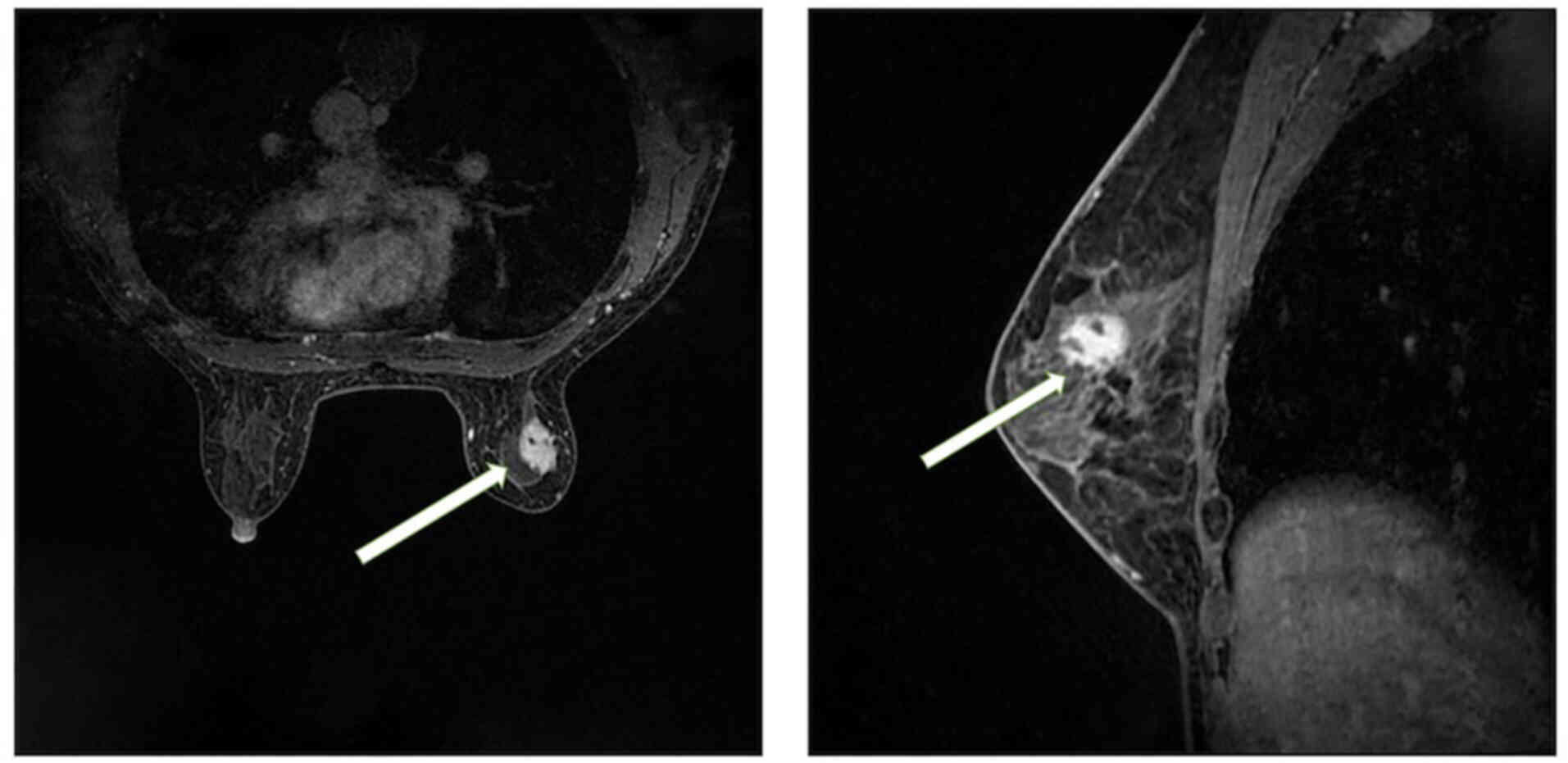
Breast magnetic resonance imaging (MRI; Fig. 3) showed a slightly elongated T2
signal in the upper outer quadrant of the right breast, which
exhibited high signal intensity on diffusion-weighted imaging. The
lesion measured ~18.5×14 mm and was observed to have irregular
edges, lobulation and spicules. Doubly
deprotonated-diethylenetriamine penta-acetic acid-enhanced MRI
scanning revealed markedly uneven enhancement of the lesion
accompanied by a dynamic curve with a plateau-shaped profile.
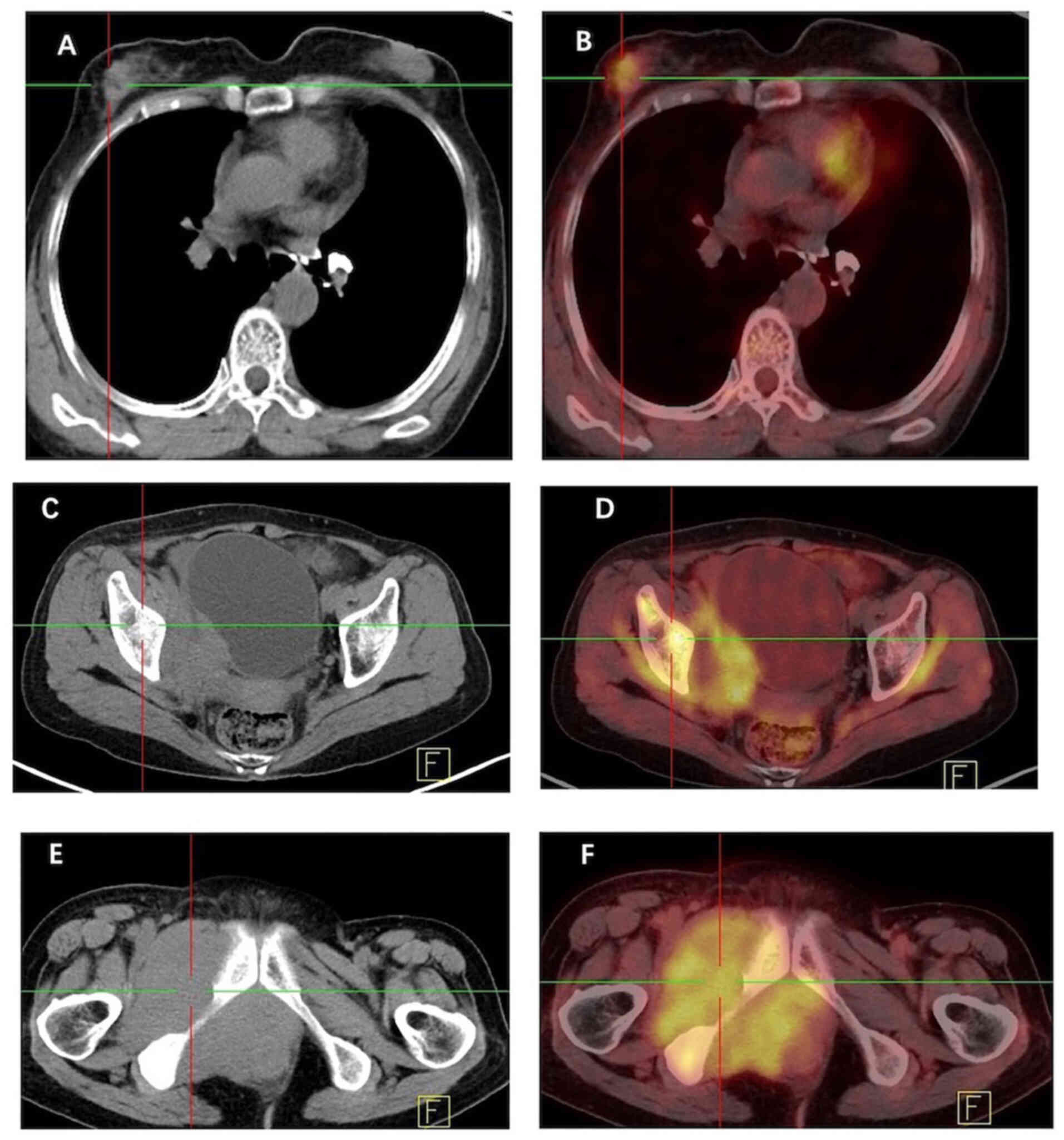
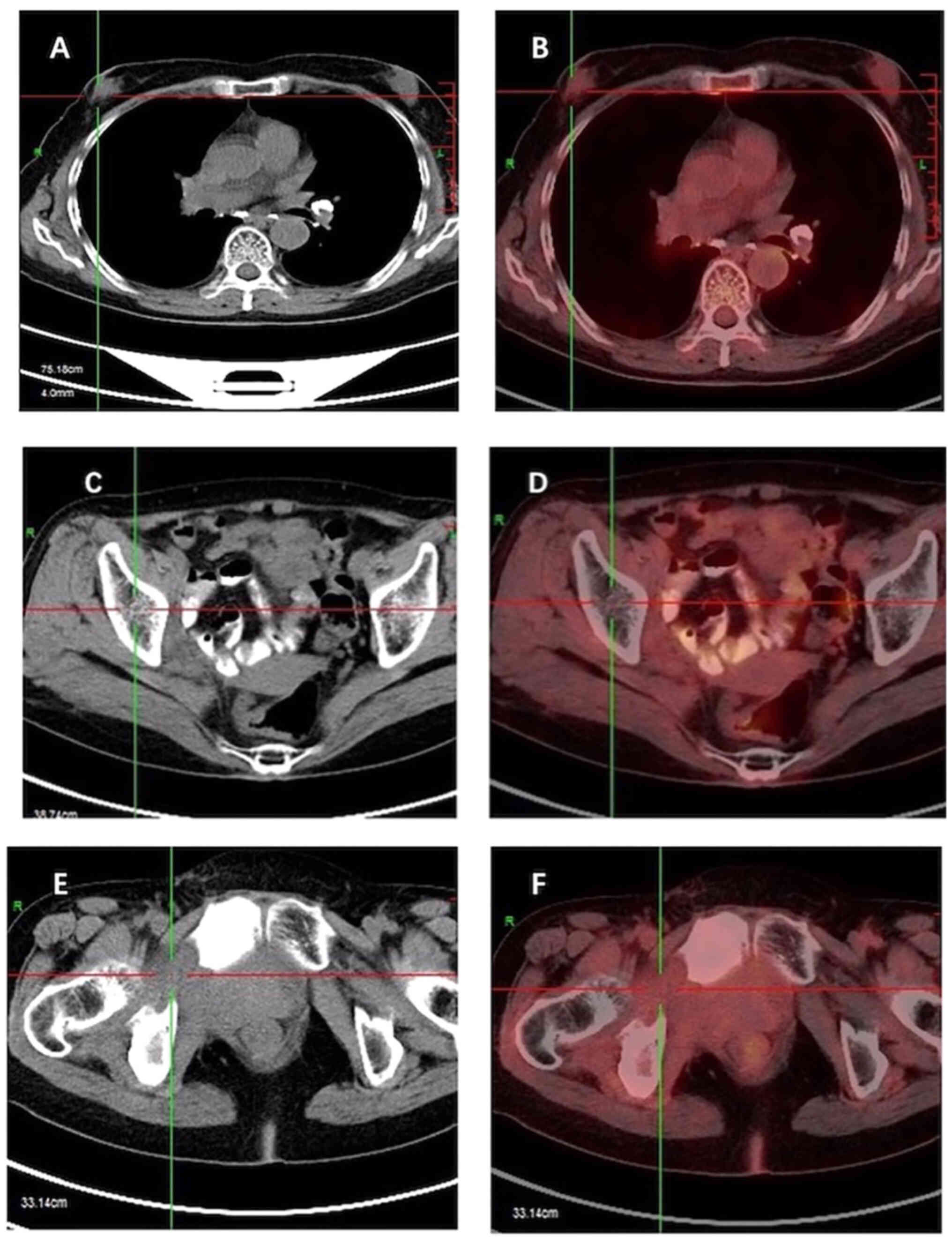
Furthermore, [18F] fluorodeoxyglucose (FDG) positron
emission tomography-computed tomography (PET-CT; Fig. 4) indicated heightened metabolism
within a nodular soft tissue shadow in the upper outer quadrant of
the right breast, suggesting the presence of a malignant lesion.
Additionally, bone destruction was observed in the right ilium,
pubic bone and ischium, accompanied by masses in the surrounding
soft tissue, indicating the presence of metastatic tumors in these
locations.
Hematoxylin and eosin staining of the right breast
mass biopsy revealed numerous compressed cells with distinct
morphological features (Fig. 5A and
B) indicating the high likelihood of B-lymphoblastic
lymphoma/leukemia. However, following needle biopsy, it was decided
that an excisional biopsy was necessary as the small size of the
needle aspiration biopsy sample and the challenges in accurately
determining the nature of the tumor rendered the results
inconclusive. Consequently, the patient underwent surgery to excise
the right breast mass and an intraoperative frozen section
examination was performed. During surgery, the tumor was found
within the breast tissue. It exhibited an incomplete capsule and
the cut surface had an appearance resembling that of fish flesh.
The frozen section examination suggested the presence of breast
lymphoma; therefore, further evaluations using paraffin sections
and immunohistochemistry were recommended. The final pathology
report indicated the widespread infiltration of immature tumor
cells, with only a few remaining small ducts and acini. Scattered
eosinophils were also reported.
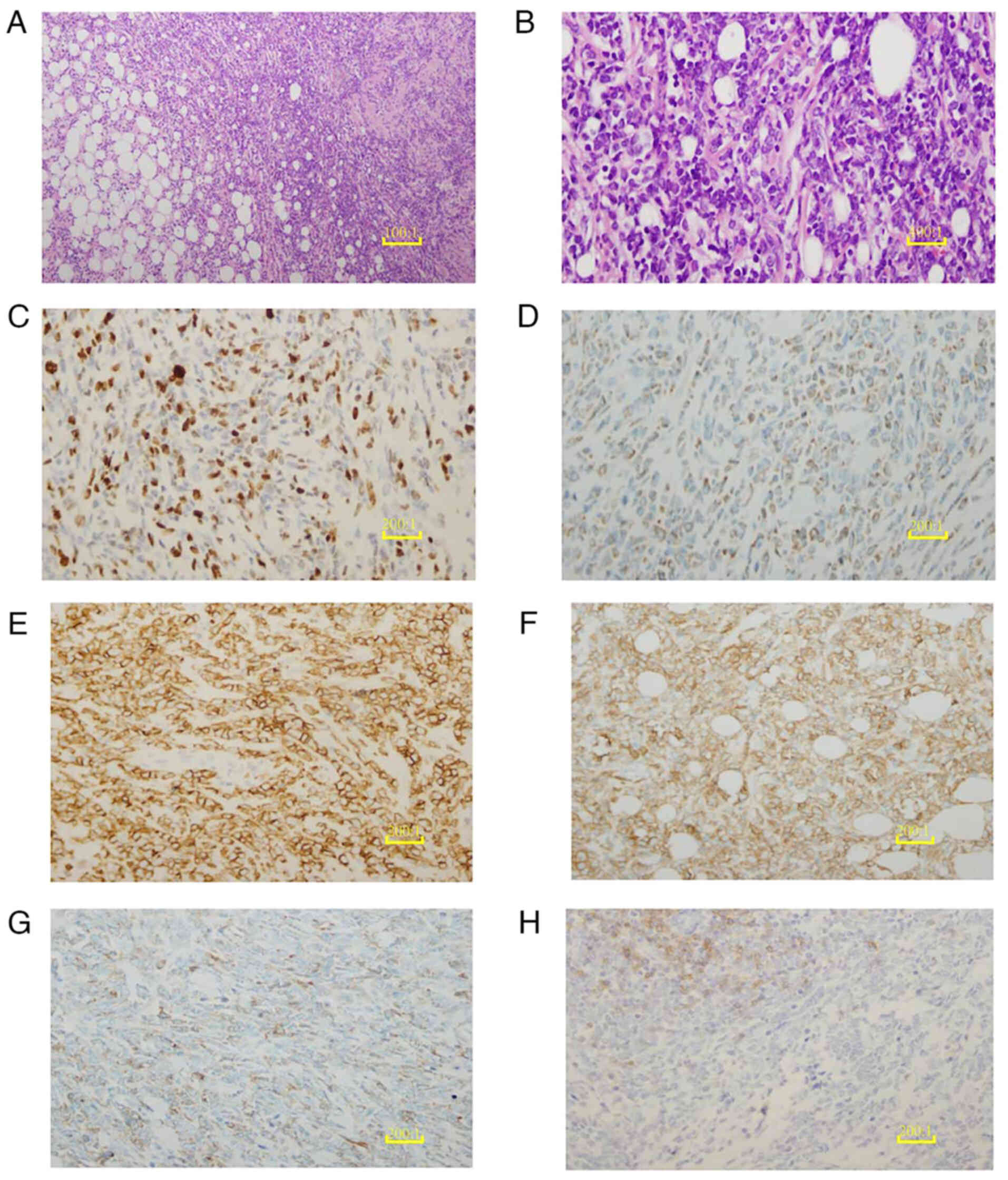 | Figure 5.Histopathological and
immunohistochemical analysis of the breast mass. (A) H&E
staining of the breast mass biopsy tissue reveals diffuse,
sheet-like growth with disrupted ductal structures (scale bar, 10
µm). (B) The H&E-stained breast mass biopsy tissue is
extensively infiltrated by medium-sized malignant cells with round
follicular nuclei containing finely dispersed chromatin and small
nucleoli (scale bar, 2.5 µm). (C) Ki-67 shows a proliferation index
of 50%, serving as a marker for cancer cells. (D) Myeloid
differentiation of the tumor cells is illustrated with a
myeloperoxidase immunohistochemical stain. (E) Positive expression
of CD43 is revealed by immunohistochemical staining, supporting the
diagnosis of myeloid sarcoma. (F) Tumor cells surrounding the
breast ductal epithelium are positive for CD117 immunohistochemical
staining, indicating their myeloid origin. (G) The hematopoietic
progenitor origin of the tumor cells is indicated by weakly
positive CD34 immunohistochemical staining. (H) Weak CD19
immunohistochemical staining indicates the reduced or abnormal
expression of B-cell markers in the tumor cells. (C-H), scale bar,
5 µm. H&E, hematoxylin and eosin; CD, cluster of
differentiation. |
Immunohistochemical analyses were conducted on 4-mµm
sections of whole tumor tissues containing in situ and/or
invasive regions, using autostainers such as the Ultra System from
Roche Diagnostics or the Autostainer Link 48 from DakoCytomation
following the manufacturer's instructions. Immunohistochemical
analyses revealed the expression of paired box 5, cluster of
differentiation (CD)43, CD34, CD99, myeloperoxidase (MPO) and CD117
in the tumor cells. In addition, ~50% of the tumor cells tested
positive for Ki-67, while no expression of cytokeratin, CD20, CD3,
CD5 and CD4 was detected. These findings were consistent with the
diagnosis of MS, as shown in Fig.
5. As the pathological examination indicated MS, the patient
underwent a bone marrow biopsy and peripheral blood examination to
confirm the diagnosis and assess the extent of the disease. These
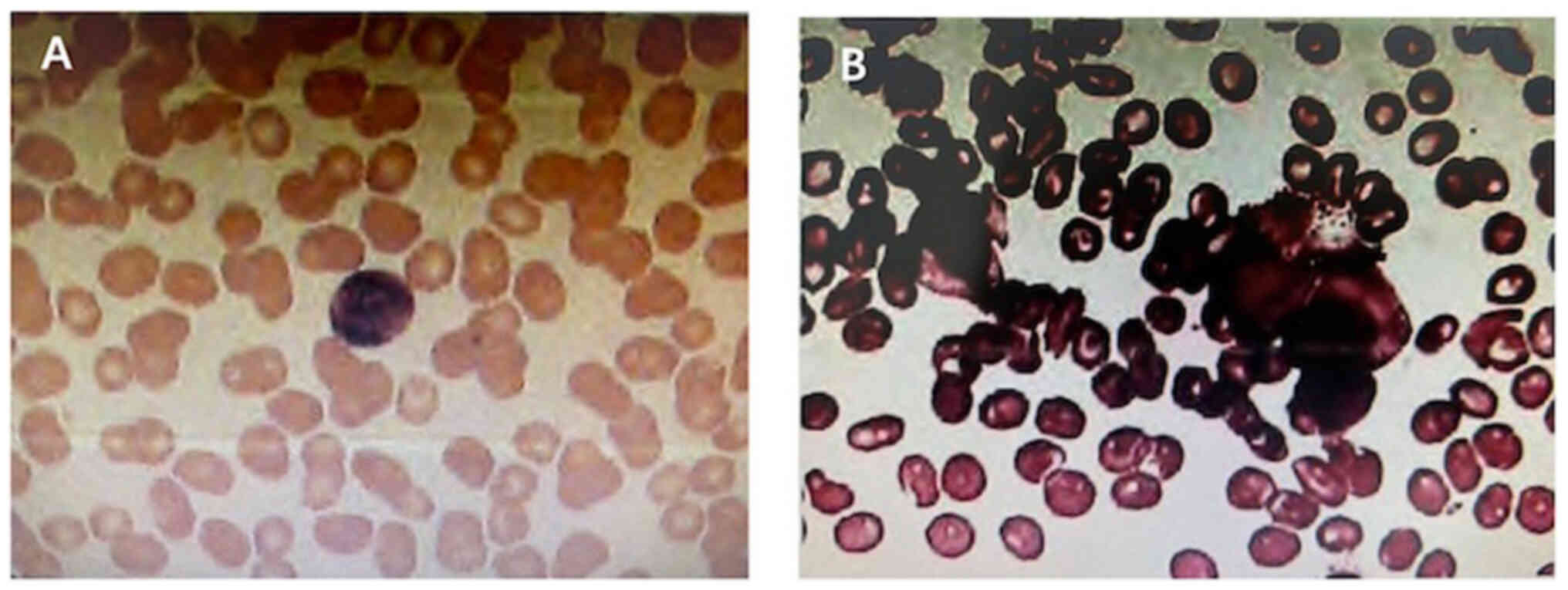
did not identify any blast cells (Fig.
6). As the results from these procedures revealed no
abnormalities, the possibility that the tumor had metastasized from
the bone marrow to the breast was excluded. Thus, a diagnosis of
primary MS of the breast with concurrent malignant bone metastasis
was made.
Although there is no specific treatment for MS, the
current systemic chemotherapy regimens for AML are considered to be
suitable first-line treatment approaches for MS. These mainly
comprise cytarabine combined with anthracyclines or
homoharringtonine, sometimes used in combination with etoposide
(8). Considering that the cells
observed in the pathological examination were predominantly
primitive marrow cells and included some cells indicative of B-cell
lymphoblastic lymphoma, a decision was made to use the ESHAP
chemotherapy regimen, based on National Comprehensive Cancer
Network guidelines (9). The ESHAP
regimen is a second-line treatment option that includes etoposide,
methylprednisolone, cytarabine and cisplatin. These drugs work
synergistically to collectively target Ewing's sarcoma and B-cell
lymphoblastic lymphoma without cross-resistance. Following surgery,
the patient was transferred to the oncology department at the
hospital to undergo six cycles of ESHAP regimen chemotherapy and 28
cycles of radiation therapy, each at a dose of 56 cGy. A follow-up
contrast-enhanced [18F] FDG PET-CT scan at 12 months did
not show any tumor residue or recurrence (Fig. 7). At the 2-year follow-up, the
patient was thriving and disease-free.
Discussion
In the current case, a tumor infiltrating the breast
parenchyma and comprising medium-sized immature cells with rounded
nuclei surrounded by a thin rim of eosinophilic cytoplasm was
identified and assessed. The tumor cells exhibited positive
immunostaining for MPO, CD43 and CD117, suggesting their myeloid
nature. Follow-up bone marrow biopsy and peripheral blood
examination did not identify any blast cells, leading to the
conclusion that this tumor was a primary MS of the breast. MS,
which is composed of malignant immature cells, is a rare
extramedullary tumor mainly associated with AML. According to the
World Health Organization classification, MS is a major subgroup of
myeloid neoplasms and acute leukemia (10). Typically, it is detected in patients
who are diagnosed with myeloid leukemia; it may manifest
concurrently with AML or as an initial presentation of relapse in
patients with previously treated AML (11).
MS is rarely an isolated finding preceding myeloid
leukemia of the blood or bone marrow, and MS of the breast is even
rarer (12). Although MS can
develop in individuals aged between 5 months and 89 years, these
tumors predominantly affect younger individuals and children, with
no distinct difference in the incidence rates between males and
females (13). According to a study
conducted by Viadana et al (14), among 503 patients with leukemia who
underwent biopsies, only four of 235 patients with AML exhibited
breast involvement. In addition, Naamo et al (15) reported the case of a 27-year-old
female who presented with a palpable right breast lump, the biopsy
of which showed breast tissue with diffuse infiltration of blasts
compatible with MS. Furthermore, a study conducted by Amiraian
et al (16) identified MS in
both breasts of a 63-year-old woman with relapsed AML. To identify
further cases, comprehensive searches in the PubMed, Embase and
Cochrane Library databases for the years 2013–2023 were conducted.
These yielded the reports of 14 patients with MS of the breast,
excluding the patient presented in the present case report. The
detailed clinical characteristics of these patients are presented
in Table I (15–27).
It may be observed that, while the clinical and pathological
characteristics of these cases of breast MS are quite similar, the
treatment plans varied considerably.
 | Table I.Clinical characteristics of patients
with breast myeloid sarcoma from 2014 to 2022. |
Table I.
Clinical characteristics of patients
with breast myeloid sarcoma from 2014 to 2022.
| First author/s,
year | Patient | Age, years | First symptom | Tumor location | Tumor size | AML link | CD43, CD34 | CD117, MPO | Treatment | Survival | (Refs.) |
|---|
| Naamo, 2022 | P1 | 27 | Painless breast
mass | Right breast
upper | 65×34×64 mm | No | (+), (+) | (+), (+) | Chemotherapy with
Cytosar, Cerubidine and Mylotarg | Alive, 12
months | (15) |
| Amiraian, 2022 | P2 | 63 | Rapidly enlarging
breast masses | Multiple lumps in
both breasts | - | Yes | (++), (++) | -, (+) | Salvage
chemotherapy, allogeneic bone marrow transplant | Deceased, 2 years 9
months | (16) |
| Ding, 2022 | P3 | 34 | Painless breast
mass | Right breast | 31×1.5 mm | Yes | -, - | -, (+) | Chemotherapy after
surgery and radiotherapy | Alive, 7 years | (17) |
| Huang, 2021 | P4 | 34 | Rapidly enlarging
breast masses | Multiple lumps in
both breasts | - | Yes | (+++), (+++) | (+), (+) | Chemotherapy | Deceased, 1
year | (18) |
| Varol, 2021 | P5 | 31 | Painless breast
mass | Multiple lumps in
both breasts | - | Yes | (+), (+) | (+), (+) | Systemic
chemotherapy | Alive, 4 years | (19) |
| Kim and Kim,
2019 | P6 | 24 | Painless breast
mass | Upper right
breast | 43 mm | Yes | -, - | -, (+) | - | Alive, 1 year 6
months | (20) |
| Wu, 2019 | P7 | 42 | Painless breast
mass | Upper right
breast | 20×18 mm | No | -, - | -, (+) | 2 cycles of IA
regimen chemotherapy after surgery | Alive, 1 year | (21) |
| Gomaa, 2018 | P8 | 29 | Painless breast
mass | Upper left
breast | 15×12×4 mm | No | -, (+) | (+), (−) | Radiotherapy and
chemotherapy | Alive, 6
months | (22) |
| Uhida, 2016 | P9 | 28 | Painless breast
mass | Right breast | - | No | -, - | -, - | Induction
chemotherapy and stem cell transplantation | Deceased, 1
year | (23) |
| Stewart, 2015 | P10 | 46 | Enlarging, tender
breast mass | Upper right
breast | 44 mm | Yes | -, (+) | (+), (+) | - | N/A | (24) |
| Nalwa, 2015 | P11 | 33 | Painless breast
mass | Left breast | - | No | -, (+) | (+),- | High dose
cytarabine (HDAC) regimen | Alive, 1 year | (25) |
| Gunduz, 2014 | P12 | 33 | Painless breast
mass | Upper right
breast | 33 mm | Yes | -, - | -, - | Ara-C, etoposide
and idarubicin chemotherapy | Deceased, 1 year 6
months | (26) |
| Fu and Luo,
2014 | P13 | 59 | Painless breast
mass | Upper left
breast | 18×12 mm | No | -, - | -, (+) | Chemotherapy with
the MA regimen and consolidation chemotherapy | Alive, 4 years | (27) |
| Fu and Luo,
2014 | P14 | 39 | Painless breast
mass | Lower left
breast | 20×15 mm | No | -, - | -, (+) | Succumbed to fungal
pneumonia without systemic chemotherapy | Deceased, 4
months | (27) |
MS of the breast lacks specific clinical features
and typically appears as palpable nodules in one or both breasts,
which may or may not be painful. These nodules can be mistaken for
primary breast cancer (11). In
this scenario, mammography typically identifies large, irregular,
non-calcified masses with poorly defined borders (11), while ultrasonography commonly
reveals hypoechoic lesions that are either homogeneous or
heterogeneous and hypervascularized on color Doppler scans
(12,20). Additionally, CT and MRI are often
used for tumor localization, as these techniques are helpful in
distinguishing MS from other masses. Specifically, MRI can be an
effective diagnostic tool, which reveals MS of the breast as
hypointense lesions on T1-weighted images and hyperintense lesions
on T2-weighted images, with inhomogeneous enhancement (18). Furthermore, [18F]
FDG-PET-CT imaging has emerged as a valuable tool for studying and
monitoring extramedullary acute myelocytic leukemia (28). However, given that typical imaging
characteristics of MS of the breast are lacking, it can lead to a
misdiagnosis of primary breast malignancy, lymphoma, other neoplasm
or inflammation (12,18). Although these findings are
non-specific, they are suspicious and necessitate a biopsy.
Accordingly, it is imperative to pay close attention to the
characteristic clinical, radiographic and pathological findings
when diagnosing isolated cases of breast MS.
Pathologically, MS typically exhibits either a
diffuse growth pattern or a single-cell infiltrating growth
pattern. Based on the proportion of immature granulocytes at
different stages of differentiation, MS can be categorized into
three pathological types, namely blast cell, partially
differentiated and differentiated (29). The blast cell type predominantly
comprises myeloblasts with only a few differentiated promyelocytes,
while the partially differentiated subtype is characterized by both
myeloblasts and promyelocytes, and the differentiated subtype is
predominantly composed of promyelocytes and granulocytes in later
stages of maturity. Notably, eosinophilic granulocytes are
prominent in the differentiated subtype. Nevertheless, the accurate
diagnosis of MS using routine histological slices is challenging.
This renders immunohistochemical assessment necessary to prevent
the misdiagnosis of diffuse large B-cell lymphoma (30). In addition to these diagnostic
complexities, specific genetic alterations such as t (8;21) (q22;
q22.1), inv (16) (p13.1q22) or t
(16;16) (p13.1; q22), as well as nucleophosmin 1 mutations, have
been associated with MS (31).
Immunohistochemistry is crucial in the diagnosis of
MS. Among the various markers, MPO is the most effective for
distinguishing MS and is expressed in as many as 93% of myeloid
tumors. However, its expression levels vary depending on the degree
of differentiation (31).
Nevertheless, a panel including MPO, CD43 and CD20 as markers has
been shown to effectively differentiate >96% of MS cases
(32). Notably, CD43 exhibits high
sensitivity but poor specificity, as it is expressed in almost all
cases of MS. Therefore, if tumor cells of unknown origin express
CD43 but are negative for CD3, MS should be considered. By
contrast, CD117 is mainly expressed in immature myeloid tumors and
is absent in lymphomas, rendering it a sensitive indicator of
myeloid tumors (33). Although CD20
is a characteristic differentiation antigen of B cells, most
studies suggest that MS is CD20-negative, while other studies have
reported a CD20 expression rate of 13% (34,35).
Therefore, it is crucial to select the appropriate antibody
combination for use in the immunohistochemical examination of
MS.
The treatment approaches for primary MS of the
breast include surgical resection, local radiotherapy and systemic
chemotherapy. However, it has been noted that surgical resection
and local radiotherapy alone are not effective in delaying the
transformation of MS into AML or improving its prognosis (36). Therefore, primary MS is considered a
systemic disease and requires systemic treatment. The
administration of systemic chemotherapy is recommended for all
solitary MS lesions in patients who have undergone surgical
resection. A variety of chemotherapy regimens that induce AML
remission have been used in the context of MS, including idarubicin
and cytarabine; fludarabine, high dose cytarabine, idarubicin and
granulocyte-colony stimulating factor (G-CSF); cyclophosphamide,
cytarabine, topotecan and G-CSF; and daunorubicin and cytarabine
(5).
In the present case, an integrated treatment
approach for breast MS was used. This combined lumpectomy with
systemic chemotherapy, which mirrors protocols typically used in
AML, and was followed up with local radiotherapy aimed at achieving
a cure. At 24 months post-treatment, the patient remained in good
health without any signs of disease relapse. This case shares
similarities with patients described in previous literature, such
as the patient undergoing complete excision of the local tumor and
receiving systemic chemotherapy predominantly consisting of
cytarabine and doxorubicin. However, a notable difference is that
the current case also underwent 28 cycles of radiotherapy following
the completion of chemotherapy. Although the treatment methods for
breast MS have not yet been standardized, the majority of studies
have concluded that all patients should undergo either mastectomy
or tumor resection surgery, along with standard systemic
chemotherapy (5,37). The case described in the present
study underwent tumor resection surgery and systemic chemotherapy,
and one year later, no local recurrence of the breast was
detected.
It has been suggested that anti-leukemia
chemotherapy administered shortly after surgery aids in controlling
the development of MS and improving its prognosis. For MS, the
preferred treatment regimen uses anthracyclines in combination with
cytarabine (38). Allogeneic
hematopoietic stem cell transplantation has also been indicated to
be an effective alternative treatment (39). In addition, molecular developments
have facilitated the development of highly targeted therapies for
patients with MS, including those associated with breakpoint
cluster region-Abelson 1, Fms-like tyrosine kinase 3-internal
tandem duplication and FIP1-like 1-platelet derived growth factor
receptor a gene mutations, thereby yielding promising results
(40).
In conclusion, MS of the breast is a rare malignant
neoplasm of myeloid origin that is often misdiagnosed. It
originates from myeloid cells and requires intensive systemic
chemotherapy, allogeneic hematopoietic stem cell transplantation,
surgical resection and/or radiotherapy for effective treatment.
However, the lack of a standard treatment approach for breast MS
poses a considerable challenge. Once MS has been diagnosed, the
prompt initiation of induction chemotherapy is recommended. The
study of additional cases is essential to enhance clinical practice
and improve the outcomes of patients with MS. Future prospective
multicenter studies are necessary to gain an improved understanding
of MS and guide its diagnostic and treatment approaches.
Acknowledgements
Not applicable.
Funding
This study was supported by the Natural Science Foundation of
Inner Mongolia Autonomous Region (grant no. 2023QN08047), the Youth
Exploration Project of Affiliated Hospital of Inner Mongolia
Medical University (grant no. 2022NYFYTS022) and the Science and
Technology Program of Inner Mongolia Autonomous Region (grant no.
2023YFSH0039).
Availability of data and materials
The data generated in the present study maybe
requested from the corresponding author.
Authors' contributions
ZZ, YC, RZ and ML contributed to study conception
and design, and performed material preparation, data collection and
analysis. The first draft of the manuscript was written by ZZ, and
all authors commented on previous versions of the manuscript. ZZ
and YC confirm the authenticity of all the raw data. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
This study was performed in line with the principles
of the Declaration of Helsinki. Approval was granted by the Ethics
Inner Mongolia Medical University in accordance with the
regulations of the ethics committee for the publication of case
reports [ethics approval no. WZ (2023072)]. Written informed
consent was obtained from the participant for inclusion in the
study.
Patient consent for publication
The patient provided written informed consent for
publication of her data and images in this case report.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MRI
|
magnetic resonance imaging
|
|
FDG
|
fluorodeoxyglucose
|
|
PET-CT
|
positron emission tomography-computed
tomography
|
|
CD
|
cluster of differentiation
|
|
MPO
|
myeloperoxidase
|
|
MS
|
myeloid sarcoma
|
|
AML
|
acute myeloid leukemia
|
|
G-CSF
|
granulocyte-colony stimulating
factor
|
|
FLAG
|
fludarabine + high dose cytarabine +
G-CSF
|
|
H&E
|
hematoxylin and eosin
|
References
|
1
|
Goyal G, Bartley AC, Patnaik MM, Litzow
MR, Al-Kali A and Go RS: Clinical features and outcomes of
extramedullary myeloid sarcoma in the United States: Analysis using
a national data set. Blood Cancer J. 7:e5922017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fitoz S, Atasoy C, Yavuz K, Gozdasoglu S,
Erden I and Akyar S: Granulocytic sarcoma. Cranial and breast
involvement. Clin Imaging. 26:166–169. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ngu IW, Sinclair EC, Greenaway S and
Greenberg ML: Unusual presentation of granulocytic sarcoma in the
breast: A case report and review of the literature. Diagn
Cytopathol. 24:53–57. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Cunningham I: A clinical review of breast
involvement in acute leukemia. Leuk Lymphoma. 47:2517–2526. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yamauchi K and Yasuda M: Comparison in
treatments of nonleukemic granulocytic sarcoma: Report of two cases
and a review of 72 cases in the literature. Cancer. 94:1739–1746.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kewalramani T, Zelenetz AD, Nimer SD,
Portlock C, Straus D, Noy A, O'Connor O, Filippa DA,
Teruya-Feldstein J, Gencarelli A, et al: Rituximab and ICE as
second-line therapy before autologous stem cell transplantation for
relapsed or primary refractory diffuse large B-cell lymphoma.
Blood. 103:3684–3688. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miyazaki C, Shiozawa M, Koike R, Ogihara
K, Sasaki Y, Shiba S, Nishida S, Sakuragi M, Mizunuma H, Fujita T,
et al: Neoadjuvant chemotherapy for primary sarcoma of the breast:
A case report. J Med Case Rep. 13:2892019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu L, Li X, Su J, Chang C, He Q, Zhang X,
Xu L, Song L and Pu Q: Effect of low-dose cytarabine,
homoharringtonine and granulocyte colony-stimulating factor priming
regimen on patients with advanced myelodysplastic syndrome or acute
myeloid leukemia transformed from myelodysplastic syndrome. Leuk
Lymphoma. 50:1461–1467. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
National Comprehensive Cancer Network, .
NCCN clinical practice guidelines in oncology: Antiemesis. Version
3.2024. http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf
|
|
10
|
Grantham JT, Howell DM, Bacaj PJ, Coad JE
and Vos JA: Myeloid sarcoma of the bladder in the setting of
refractory anemia with excess blasts-2 (RAEB-2). W V Med J.
111:34–36. 2015.PubMed/NCBI
|
|
11
|
Nicosia L, Latronico A, Farina M, Bozzini
AC, Baratella P, Galimberti VE, Fiori S, Montesano M and Cassano E:
Myeloid sarcoma of the breast: A pathology that should not be
forgotten. Ecancermedicalscience. 14:11602020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thachil J, Richards RM and Copeland G:
Granulocytic sarcoma-a rare presentation of a breast lump. Ann R
Coll Surg Engl. 89:W7–W9. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu T, Xu G, Xu X, Yang J and Ding L:
Myeloid sarcoma derived from the gastrointestinal tract: A case
report and review of the literature. Oncol Lett. 11:4155–4159.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Viadana E, Bross ID and Pickren JW: An
autopsy study of the metastatic patterns of human leukemias.
Oncology. 35:87–96. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Naamo S, Naamo S, Sarker S, Vasconez M and
Froicu M: Breast manifestation of extramedullary myeloid sarcoma: A
case report. Radiol Case Rep. 17:4660–4665. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Amiraian D, McDonough M and Geiger X:
Bilateral myeloid sarcoma of the breast: A case report with
radiological and pathological correlation. Cureus.
14:e247312022.PubMed/NCBI
|
|
17
|
Ding Y, Xi D, Chen Y and Gu W: Myeloid
sarcoma of the breast as a first manifestation of acute myeloid
leukemia: A case report. Asian J Surg. 45:1622–1623. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang C, Fei S, Yao J, Chen P, Luo J, Wang
Y, Li J and Wang W: Breast myeloid sarcoma presenting as a palpable
breast lump after allogeneic stem cell transplantation for acute
myelomonocytic leukemia: A rare case report. World J Surg Oncol.
19:2892021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Varol E, Kiraz U, Guler SA, Vural Ç,
Gülbaş Z and Utkan NZ: Breast recurrence of acute myeloid leukemia
after bone marrow transplantation: A case report about myeloid
sarcoma of the breast. Eur J Breast Health. 17:292–295. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kim SJ and Kim WG: Sonographic features of
a myeloid sarcoma of the breast as a relapse of acute myeloid
leukemia after stem-cell transplantation: A case report. Am J Case
Rep. 20:612–619. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wu HY, Liu L, Gu L and Luo YH: Clinical
characteristics and management of primary granulocytic sarcoma of
the breast: A case report. Medicine (Baltimore). 98:e166482019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Gomaa W and Ghanim A, Emam E, Bayoumi K
and Ghanim A: Primary myeloid sarcoma of the breast: A case report
and review of literature. J Microsc Ultrastruct. 6:212–214.
2018.PubMed/NCBI
|
|
23
|
Uchida E, Watanabe K, Oshikawa G,
Sakashita C, Kurosu T, Fukuda T, Arai A, Murakami N, Miura O and
Yamamoto M: Refractory primary myeloid sarcoma of the breast with
MLL-AF9 rearrangement. Rinsho Ketsueki. 57:47–51. 2016.PubMed/NCBI
|
|
24
|
Stewart RL, Dell CM and Samayoa L: Myeloid
sarcoma of the breast misdiagnosed as poorly differentiated mammary
carcinoma with lobular features. Breast J. 21:192–193. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Nalwa A, Nath D, Suri V, Jamaluddin MA and
Srivastava A: Myeloid sarcoma of the breast in an aleukemic
patient: A rare entity in an uncommon location. Malays J Pathol.
37:63–66. 2015.PubMed/NCBI
|
|
26
|
Gündüz E, Akay MO, Karagülle M and Ak IS:
isolated granulocytic sarcoma of the breast after allogeneic stem
cell transplantation: A rare involvement also detected by
18FDG-PET/CT. Turk J Haematol. 31:88–91. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fu J and Luo J: Granulocytic sarcoma of
the breast in acute myeloid leukemia: Two case reports. Oncol Lett.
7:145–147. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Avni B and Koren-Michowitz M: Myeloid
sarcoma: Current approach and therapeutic options. Ther Adv
Hematol. 2:309–316. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li JM, Liu WP, Zhang MH, Wei X, Gu JM, Han
AJ, Wu WQ and Chen XY: Clinicopathologic and immunophenotypic
analysis of myeloid sarcoma. Zhonghua Bing Li Xue Za Zhi.
35:606–611, (In Chinese). PubMed/NCBI
|
|
30
|
Pileri SA, Ascani S, Cox MC, Campidelli C,
Bacci F, Piccioli M, Piccaluga PP, Agostinelli C, Asioli S, Novero
D, et al: Myeloid sarcoma: Clinico-pathologic, phenotypic and
cytogenetic analysis of 92 adult patients. Leukemia. 21:340–350.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Estey EH: Acute myeloid leukemia: 2019
update on risk-stratification and management. Am J Hematol.
93:1267–1291. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Traweek ST, Arber DA, Rappaport H and
Brynes R: Extramedullary myeloid cell tumors. An
immunohistochemical and morphologic study of 28 cases. Am J Surg
Pathol. 17:1011–1019. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Audouin J, Comperat E, Le Tourneau A,
Camilleri-Broët S, Adida C, Molina T and Diebold J: Myeloid
sarcoma: Clinical and morphologic criteria useful for diagnosis.
Int J Surg Pathol. 11:271–282. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Mourad W, Kfoury H and Al Husseini H: The
value of CD34, myeloperoxidase and chloroacetate esterase (Leder)
stain in the diagnosis of granulocytic sarcoma. Ann Saudi Med.
21:287–291. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
He J, Zhu L, Ye X, Li L, Zhu J, Zhang J,
Xie W, Shi J, Zheng W, Wei G, et al: Clinical characteristics and
prognosis of nonleukemic myeloid sarcoma. Am J Med Sci.
347:434–438. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Florou D, Katsara M, Feehan J, Dardiotis E
and Apostolopoulos V: Anti-CD20 agents for multiple sclerosis:
Spotlight on ocrelizumab and ofatumumab. Brain Sci. 10:7582020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Huang XE, Li YJ and Zhou XD: Granulocytic
sarcoma of the breast: A case report. Oncol Lett. 10:2447–2449.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Widhalm G, Dietrich W, Müllauer L,
Streubel B, Rabitsch W, Kotter MR, Knosp E and Roessler K: Myeloid
sarcoma with multiple lesions of the central nervous system in a
patient without leukemia. Case report. J Neurosurg. 105:916–919.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tsimberidou AM, Kantarjian HM, Wen S,
Keating MJ, O'Brien S, Brandt M, Pierce S, Freireich EJ, Medeiros
LJ and Estey E: Myeloid sarcoma is associated with superior
event-free survival and overall survival compared with acute
myeloid leukemia. Cancer. 113:1370–1378. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Védy D, Muehlematter D, Rausch T, Stalder
M, Jotterand M and Spertini O: Acute myeloid leukemia with myeloid
sarcoma and eosinophilia: Prolonged remission and molecular
response to imatinib. J Clin Oncol. 28:e33–e35. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Spak DA, Plaxco JS, Santiago L, Dryden MJ
and Dogan BE: BI-RADS((R)) fifth edition: A summary of changes.
Diagn Interv Imaging. 98:179–190. 2017. View Article : Google Scholar : PubMed/NCBI
|