Introduction
Endometrial cancer (EC) is the most common
gynecological cancer in the United States, with a troubling rise in
related fatalities (1). This trend
is also evident in developing countries, where both incidence and
mortality rates are increasing (2,3).
According to global cancer statistics published by CA-Cancer
Journal for Clinicians in 2021, China reported 80,000 new cases of
EC in 2020 (4). Despite generally
favorable overall prognosis, mortality rates for EC are on the
rise. By 2035, EC is projected to become the sixth leading cause of
cancer-related deaths among women (5). Therefore, advancing early diagnostic
and prognostic evaluation techniques for EC is crucial, as these
improvements are key to enhancing survival rates for those affected
by the disease.
In recent years, the classification and treatment of
Patients with EC have become increasingly precise, representing a
major shift from tissue-based to gene-based approaches (6). Following the introduction of molecular
subtypes of EC by The Cancer Genome Atlas in 2013 (7), over a decade of research has confirmed
the predictive efficacy of these subtypes. These four molecular
classifications were incorporated into the guidelines by the
ESMO-ESGO-ESTRO consensus conference in 2016 and were officially
included in the FIGO staging criteria in 2023, promoting molecular
subtyping for all patients with EC. However, the present
classification standards have notable limitations: i) Existing
predictive factors are inadequate for fully assessing the risk of
recurrence, especially in the early stages (8); and ii) routine molecular profiling is
costly and numerous patients achieve favorable outcomes with
hysterectomy alone, suggesting that a more cost-effective approach
may be preferable.
The critical role of immunohistochemistry (IHC) in
risk stratification for patients with EC is well-documented,
demonstrating its practical application and high reproducibility
(9). Despite advances in algorithm
development, a need remains for a cost-effective and highly useful
predictive model to assess recurrence risk. In the present study,
basic clinical information and preoperative routine pathological
IHC results were used, including estrogen receptor (ER),
progesterone receptor (PR), P53, Ki67, lymph node metastasis (LNM),
lymph-vascular space invasion (LVSI) and other indicators, to
construct a predictive model. Survival outcomes associated with
various histological behaviors in previous patients were analyzed,
aiming to provide a more specific and sensitive model for patients
with EC.
Materials and methods
Patient population
A retrospective study was conducted of patients
diagnosed with EC at the Department of Obstetrics and Gynecology,
Peking University People's Hospital (PKUPH), from January 2006 to
December 2020. The inclusion criteria were: i) Age over 18 years;
ii) histologically confirmed diagnosis of EC; iii) undergoing total
hysterectomy with either systematic lymphadenectomy or sentinel
lymph node dissection (10); iv)
complete clinical information and postoperative pathological data.
The exclusion criteria were: i) Presence of additional malignant
tumors; ii) lack of medical records; iii) preoperative treatment
history; iv) other serious illnesses (such as stroke and heart
disease); and v) death from other causes during follow-up. Based on
these criteria, a total of 834 cases were selected for subsequent
analysis. The present study was approved (approval no. 2022PHB379)
by the Ethics Committee Board of Peking University People's
Hospital (Beijing, China), in accordance with the principles
outlined in the Declaration of Helsinki (2013). Informed consent
was obtained from all subjects.
IHC
All patients underwent IHC examination, with
approval from the Institutional Review Board of PKUPH for tissue
excision. Pathological surgical specimens were fixed in 4%
paraformaldehyde at 25°C for 48 h. After dehydration in a gradient
of ethanol and clarification in xylene, the tissue samples were
infiltrated with paraffin and embedded. The embedded tissue blocks
were then sectioned into 5 µm slices using a microtome. The
sections were incubated in a 60–65°C oven for 1–1.5 h, then
deparaffinized in a xylene and ethanol gradient. Antigen retrieval
was performed by incubating the sections in sodium citrate buffer
at 95°C for 10 min, followed by the addition of an endogenous
peroxidase blocker (cat. no. BF06060; Biodragon) to the tissue. The
sections were then washed with PBST (including 0.1% Tween-20) for 3
min × 3 times. The primary antibody was applied to the tissue and
incubated overnight at 4°C, followed by the addition of the
secondary antibody and incubation at room temperature for 30 min.
Detailed information about the antibodies has been added to the
supplementary materials (Table
SI). The sections' color was developed with
3,3′-diaminobenzidine (DAB), and the nuclei were stained with
hematoxylin at 25°C for 15 min. Two pathologists independently
assessed each sample in a blinded manner, without prior knowledge
of the patients' details. IHC staining for estrogen receptor (ER),
PR and P53 included both the percentage of positive nuclear
staining, from 0–100%, and staining intensity, which was graded on
a scale from 0 to 3. On this scale, 0 indicated negative, 1
indicated weak staining (+), 2 indicated moderate staining (++),
and 3 indicated strong staining (+++). Ki67 was evaluated based
solely on the percentage of positive nuclear staining. The
representative IHC images are attached (Fig. S1, Fig.
S2, Fig. S3, Fig. S4). In summary, the expression
patterns of these four IHC markers in the patients were derived
from the pathology reports and were reviewed and confirmed by two
experienced pathologists.
Construction of prognostic model
To develop a model with high accuracy and stability,
10 machine learning algorithms were integrated and 92 algorithm
combinations. The algorithms included RSF, elastic net, least
absolute shrinkage and selection operator (Lasso), Ridge, StepCox,
CoxBoost, partial least squares regression for Cox, supervised
principal components, generalized boosted regression modeling, and
survival support vector machine. One algorithm to filter the
variables was utilized and another to build the prognostic
signature. Out of 100 possible combinations of machine learning
algorithm pairs, eight were excluded because the final prognostic
signature included fewer than five genes. Leave-One-Out
Cross-Validation (LOOCV) is well-known for providing an unbiased
estimate and allowing comprehensive testing on each data point,
ensuring the accuracy of the predictive model. The principle of
this algorithm is as follows: One observation is selected as the
test data, while all remaining observations are used as the
training data. The model is then trained, and this process is
repeated for each observation in the dataset. The test error is
estimated by averaging the errors across all iterations.
The procedure for generating the signature was as
follows: i) The collected patient demographic and pathological
staining data were organized into numerical variables [age at
diagnosis, BMI, ER percentage, PR percentage, P53 percentage, Ki67
percentage, overall survival (OS) time] and categorical variables
[menopause status (premenopausal, postmenopausal), diabetes
mellitus (without, with), hypertension (without, with), number of
ER+ (0, 1, 2, 3), number of PR+ (0, 1, 2, 3),
number of P53+ (0, 1, 2, 3), survival status (alive, deceased),
ascites' cytology (negative, positive), histology [endometrioid
endometrial adenocarcinoma (EEA), other types], LNM (negative,
positive), lymph-vascular space invasion (negative, positive),
myometrial invasion (<50%, ≥50%), cervical invasion (negative,
positive), FIGO stage (I, II, III, IV) and grade (G1, G2, G3)]; ii)
identifying those factors highly associated with prognosis through
univariate Cox regression; iii) as previously mentioned, the
combination of the 92 algorithms were utilized to construct
predictive models for patients with EC; and iv) the Harrell
concordance index (C-index) was computed, with the model exhibiting
the highest average C-index being selected as the final model.
Statistical analysis
The χ2 test was applied to compare
categorical variables and the Wilcoxon rank-sum test or the
unpaired t-test were used to assess continuous variables. Fisher's
exact test was employed for the analysis of sample data with
theoretical frequencies <5. The correlation between two
continuous variables was evaluated using the Pearson correlation
coefficient. The optimal cut-off value was determined with the
survminer package. C-indices were compared using the Compare C
package. Cox regression and Kaplan-Meier analyses followed by the
log-rank test were conducted with the survival package. ROC
analysis was performed with the pROC package, and the area under
the curve (AUC) for survival variables was assessed using the time
ROC package. All data analyses were conducted with R version 4.3.2
(http://www.R-project.org; The R
Foundation) and EmpowerStats (http://www.empowerstats.com; X&Y Solutions, Inc.).
A two-tailed significance level of P<0.05 was considered to
indicate a statistically significant difference.
Results
Clinical and pathological feature
In the present risk model, a total of 834 patients
with EC were randomly assigned to two groups: The training cohort
(n=566) and the validation cohort (n=278), in a 2:1 ratio. The
clinical baseline features and clinicopathological characteristics
of patients are presented in Tables
I and II. Based on the
P-values obtained from the unpaired t-test and Fisher's exact test,
the differences between the two groups were found to be
statistically non-significant. Both cohorts predominantly consist
of middle-aged and overweight patients, with mean ages of 56.49 and
55.85 years, and mean body mass indexes of 26.35 and 26.18 in the
training and validation cohorts, respectively. In the training
cohort, 364 patients (65.47%) are postmenopausal, while 192
patients (34.53%) are not. By contrast, the validation cohort
includes 101 patients with premenopausal (36.33%) and 177 patients
with non-premenopausal (63.67%). Most patients are staged as FIGO
Stage I, comprising 77.34% (430/556) of the training cohort and
85.25% (237/278) of the validation cohort.
 | Table I.Baseline Information and Clinical
Features of EC Patients - Continuous Variables |
Table I.
Baseline Information and Clinical
Features of EC Patients - Continuous Variables
|
| Training cohort | Validation
cohort |
|
|---|
|
|
|
|
|
|---|
| Variables | Mean ± SD | Mean ± SD | P-value |
|---|
| Age at diagnosis | 56.49±9.33 | 55.85±9.59 | 0.457 |
| Body mass index
(kg/m2) | 26.35±4.59 | 26.18±4.12 | 0.780 |
| ER percentage | 0.80±0.29 | 0.68±0.34 | 0.183 |
| PR percentage | 0.76±0.34 | 0.70±0.36 | 0.716 |
| P53 percentage | 0.37±0.45 | 0.35±0.45 | 0.838 |
| Ki67
percentage | 0.35±0.24 | 0.38±0.21 | 0.394 |
| Overall survival
time (days) |
2106.24±1350.77 |
2185.26±1413.85 | 0.629 |
| Myometrial
infiltration |
|
| 0.482 |
|
<50% | 426 (76.62) | 220 (79.14) |
|
|
≥50% | 130 (23.38) | 58 (20.86) |
|
| Cervical
invasion |
|
| 0.713 |
|
Negative | 490 (88.13) | 256 (92.09) |
|
|
Positive | 66 (11.87) | 22 (7.91) |
|
| FIGO stage |
|
| 0.859 |
| I | 430 (77.34) | 237 (85.25) |
|
| II | 30 (5.40) | 12 (4.32) |
|
|
III | 82 (14.75) | 22 (7.91) |
|
| IV | 14 (2.52) | 7 (2.52) |
|
| Grade |
|
| 0.299 |
| G1 | 205 (36.87) | 106 (38.13) |
|
| G2 | 239 (42.99) | 126 (45.32) |
|
| G3 | 112 (20.14) | 46 (16.55) |
|
 | Table II.Baseline Information and Clinical
Features of EC Patients - Categorical Variables |
Table II.
Baseline Information and Clinical
Features of EC Patients - Categorical Variables
| Variables | N (%) | N (%) | P-value |
|---|
| Menopause
status |
|
| 0.886 |
|
Premenopausal | 192 (34.53) | 101 (36.33) |
|
|
Postmenopausal | 364 (65.47) | 177 (63.67) |
|
| Diabetes
mellitus |
|
| 0.791 |
|
Without | 423 (76.08) | 213 (76.62) |
|
|
With | 133 (23.92) | 65 (23.38) |
|
| Hypertension |
|
|
|
|
Without | 319 (57.37) | 170 (61.15) | 0.224 |
|
With | 237 (42.63) | 108 (38.85) |
|
| Number of ER + |
|
| 0.395 |
| 0 | 29 (5.06) | 22 (8.44) |
|
| 1 | 352 (63.29) | 200 (72.15) |
|
| 2 | 112 (20.25) | 28 (9.70) |
|
| 3 | 63 (11.39) | 28 (9.70) |
|
| Number of PR + |
|
| 0.718 |
| 0 | 39 (7.17) | 30 (12.66) |
|
| 1 | 385 (69.20) | 164 (69.20) |
|
| 2 | 66 (11.81) | 9 (3.80) |
|
| 3 | 66 (11.81) | 34 (14.35) |
|
| Number of P53 |
|
| 0.201 |
| 0 | 217 (39.24) | 123 (44.30) |
|
| 1 | 317 (56.97) | 143 (51.48) |
|
| 2 | 15 (2.53) | 5 (1.69) |
|
| 3 | 7 (1.27) | 7 (2.53) |
|
| Survival
status |
|
| 0.528 |
|
Alive | 498 (89.57) | 253 (91.01) |
|
|
Death | 58 (10.43) | 25 (8.99) |
|
| Ascites
cytology |
|
| 0.872 |
|
Negative | 500 (92.25) | 259 (95.22) |
|
|
Positive | 42 (7.75) | 13 (4.78) |
|
| Histology |
|
| 0.946 |
|
Endometrioid endometrial
adenocarcinoma | 508 (91.37) | 252 (90.65) |
|
| Other
types | 48 (8.63) | 26 (9.35) |
|
| Lymph node
metastasis |
|
| 0.163 |
|
Negative | 502 (90.29) | 260 (93.53) |
|
|
Positive | 54 (9.71) | 18 (6.47) |
|
| Lymph-vascular
space invasion |
|
| 0.844 |
|
Negative | 460 (82.73) | 229 (82.37) |
|
|
Positive | 96 (17.27) | 49 (17.63) |
|
Establishment of machine-learning
model for pathology prediction
The present study analyzed 19 characteristic factors
of patients with EC. Except for menopausal status, diabetes and
hypertension, univariate Cox analysis revealed that the impact of
the remaining factors on OS was statistically significant (Table III). Additionally, ROC curves were
plotted for models incorporating four factors, three factors, and
two factors, respectively (Figs.
S5 and S6), demonstrating that
the model including four IHC factors had the best predictive
performance (AUC=0.951). A machine learning-based pathology-related
model incorporating these 16 selected factors was developed.
 | Table III.The univariate COX analysis of OS and
RFS. |
Table III.
The univariate COX analysis of OS and
RFS.
| Variables | OS | RFS |
|---|
| Age at
diagnosis | 1.07 (1.02,1.11)
0.0021 | 1.05 (1.02,1.09)
0.0047 |
| Body mass index
(kg/m2) | 1.83 (1.05,3.60)
0.0458 | 2.35 (1.05,5.27)
0.0371 |
| ER percentage | 0.17 (0.06,0.48)
0.0009 | 0.20 (0.08,0.49)
0.0005 |
| PR percentage | 0.10 (0.04,0.28)
<0.0001 | 0.11 (0.05,0.27)
<0.0001 |
| P53 percentage | 6.18 (2.37,16.10)
0.0002 | 5.63 (2.56,12.40)
<0.0001 |
| Ki67
percentage | 4.69 (2.02,10.88)
0.0003 | 2.92 (1.59,5.36)
0.0005 |
| Menopause
status |
|
|
|
Premenopausal | 1.0 | 1.0 |
|
Postmenopausal | 2.29 (0.85,6.13)
0.0995 | 2.99 (1.22,7.37)
0.0171 |
| Diabetes
mellitus |
|
|
|
Without | 1.0 | 1.0 |
|
With | 0.49 (0.15,1.65)
0.2501 | 0.67 (0.27,1.67)
0.3922 |
| Hypertension |
|
|
|
Without | 1.0 | 1.0 |
|
With | 1.26 (0.56,2.80)
0.5787 | 0.97 (0.48,1.95)
0.9212 |
| Number of
ER+ |
|
|
| 0 | 1.0 | 1.0 |
| 1 | 0.20 (0.08,0.52)
0.0009 | 0.15 (0.06,0.35)
<0.0001 |
| 2 | 0.05 (0.01,0.38)
0.0042 | 0.06 (0.01,0.31)
0.0007 |
| 3 | 0.15 (0.03,0.72)
0.0183 | 0.14 (0.03,0.55)
0.0050 |
| Number of
PR+ |
|
|
| 0 | 1.0 | 1.0 |
| 1 | 0.15 (0.06,0.34)
<0.0001 | 0.14 (0.07,0.31)
<0.0001 |
| 2 | 0.08 (0.01,0.64)
0.0171 | 0.07 (0.01,0.57)
0.0131 |
| 3 | 0.06 (0.01,0.49)
0.0082 | 0.08 (0.02,0.38)
0.0014 |
| Number of P53 |
|
|
| 0 | 1.0 | 1.0 |
| 1 | 8.81 (2.05, 37.84)
0.0034 | 5.26 (1.96, 14.10)
0.0010 |
| 2 | 19.76 (2.78,
140.50) 0.0029 | 9.15 (1.53, 54.59)
0.0151 |
| 3 | 9.40 (0.85, 103.80)
0.0673 | 10.46 (1.72, 63.59)
0.0108 |
| Ascites'
cytology |
|
|
|
Negative | 1.0 | 1.0 |
|
Positive | 10.08 (4.08, 24.90)
<0.0001 | 11.65 (4.72, 28.74)
<0.0001 |
| Histology |
|
|
|
EEA | 1.0 | 1.0 |
| Other
types | 11.45 (5.08, 25.84)
<0.0001 | 12.03 (5.73, 25.26)
<0.0001 |
| Lymph node
metastasis |
|
|
|
Negative | 1.0 | 1.0 |
|
Positive | 21.02 (8.96, 49.30)
<0.0001 | 14.44 (6.78, 30.76)
<0.0001 |
| Lymph-vascular
space invasion |
|
|
|
Negative | 1.0 | 1.0 |
|
Positive | 8.66 (3.84, 19.53)
<0.0001 | 3.93 (1.90, 8.15)
0.0002 |
| Myometrial
infiltration |
|
|
|
<50% | 1.0 | 1.0 |
|
≥50% | 15.33 (4.57, 51.42)
<0.0001 | 7.08 (3.22, 15.55)
<0.0001 |
| FIGO stage |
|
|
| I | 1.0 | 1.0 |
| II | 7.96 (1.46, 43.47)
0.0167 | 5.61 (1.42, 22.07)
0.0137 |
|
III | 17.00 (5.23, 55.22)
<0.0001 | 10.14 (4.13, 24.90)
<0.0001 |
| IV | 149.67 (43.33,
517.00) <0.0001 | 71.00 (20.46,
246.32) <0.0001 |
| Grade |
|
|
| G1 | 1.0 | 1.0 |
| G2 | 0.47 (0.08, 2.79)
0.4025 | 2.32 (0.46, 11.64)
0.3079 |
| G3 | 10.22 (3.02, 34.54)
0.0002 | 25.46 (5.90,
109.86) <0.0001 |
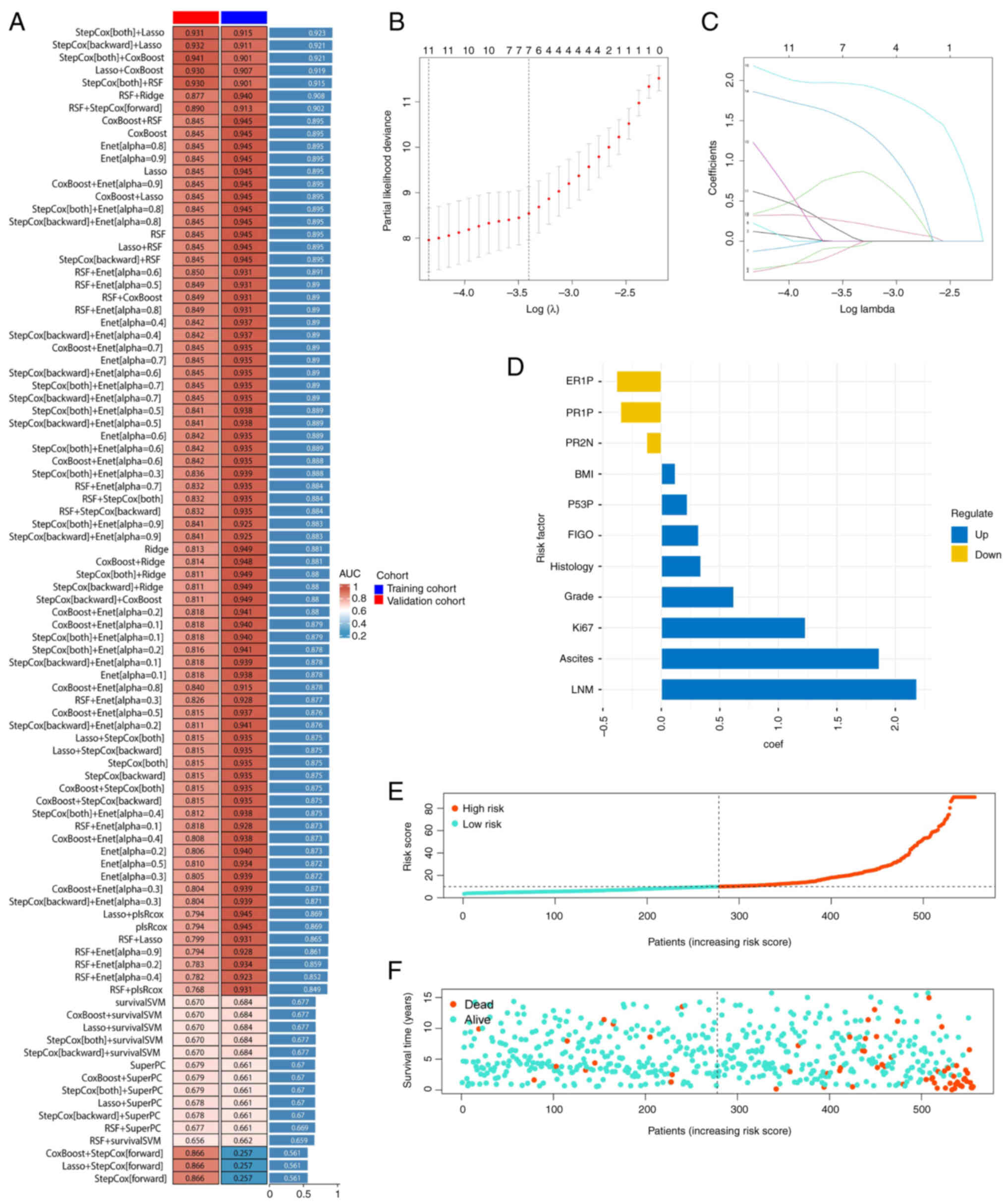
In the EC dataset, 92 prediction models were applied
using the LOOCV framework and the C-index for each model was
calculated (Fig. 1A). The Lasso and
stepwise Cox models were selected, which revealed the highest
average C-index of 0.923. In Lasso regression, the optimal λ value
was identified by minimizing the partial likelihood deviance using
the LOOCV framework (Fig. 1B).
Through stepwise Cox proportional hazards regression, a final set
of 11 factors were determined from the original 16 factors
(Fig. 1C). A risk score was
calculated for each patient using the regression coefficients
(Fig. 1D). The median risk score
was used in each cohort as the threshold to stratify patients
(Fig. 1E). As risk scores
increased, survival time decreased, and the mortality rate
increased (Fig. 1F). By combining
multiple machine learning algorithms, the accuracy of the present
study's predictive model has been significantly improved.
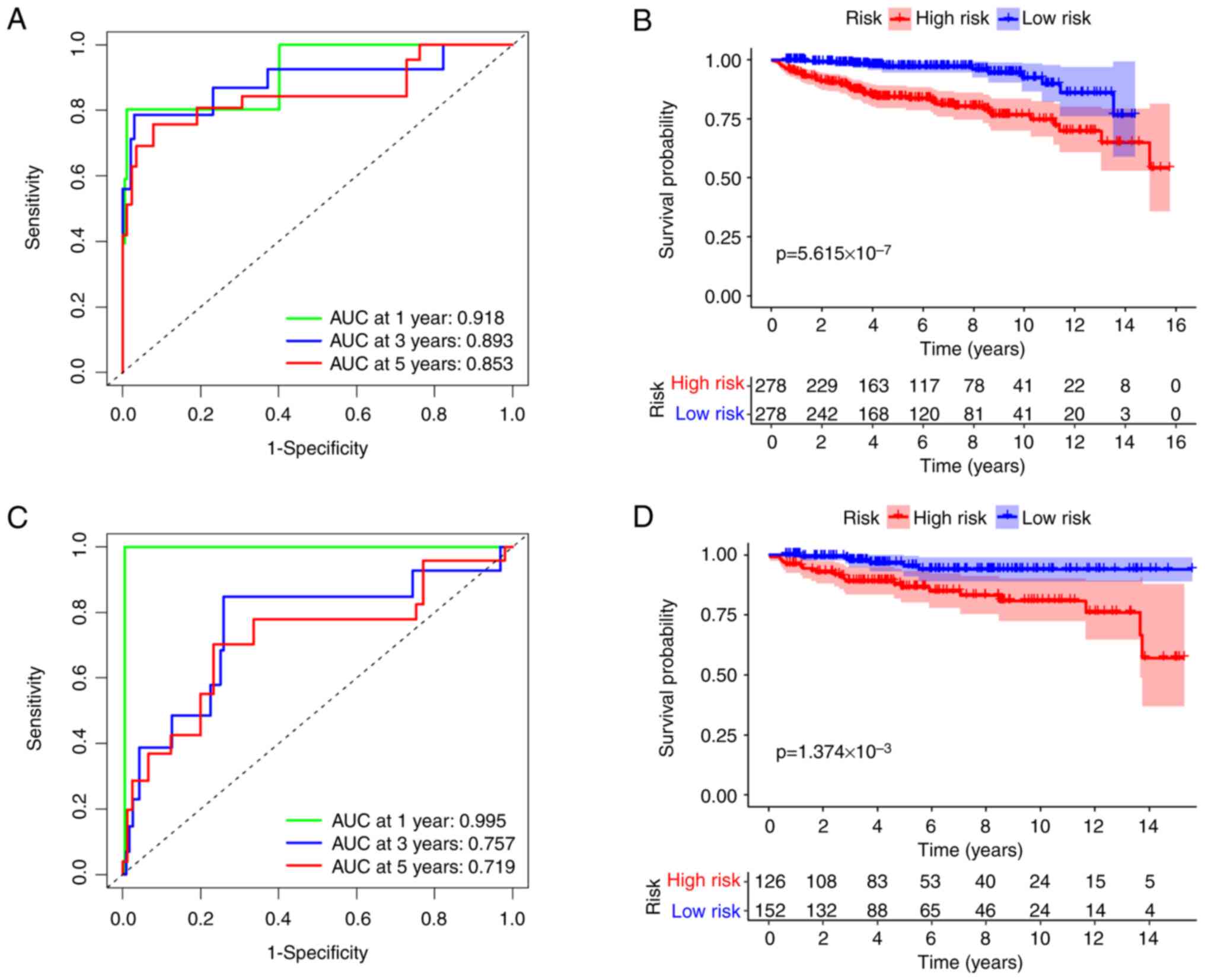
Evaluation of the pathological
prediction model in OS
Kaplan-Meier plots and ROC curves were used to
evaluate the relationship between risk scores and prognosis in
patients with EC. The model demonstrated superior accuracy
according to ROC analysis. In the training cohort, the AUC for
predicting OS at 1, 3 and 5 years was 0.918, 0.893 and 0.853,
respectively (Fig. 2A). In the
validation cohort, the AUCs were 0.995, 0.757 and 0.719,
respectively (Fig. 2C).
Furthermore, the OS rate for the high-risk group was significantly
lower compared with the low-risk group, with
P=5.615×10−7 (Fig. 2B)
for the training cohort and P=1.374×10−3 (Fig. 2D) for the validation cohort. The
present study's model clearly assessed patient risk severity
effectively and demonstrated strong predictive capability for
recent events.
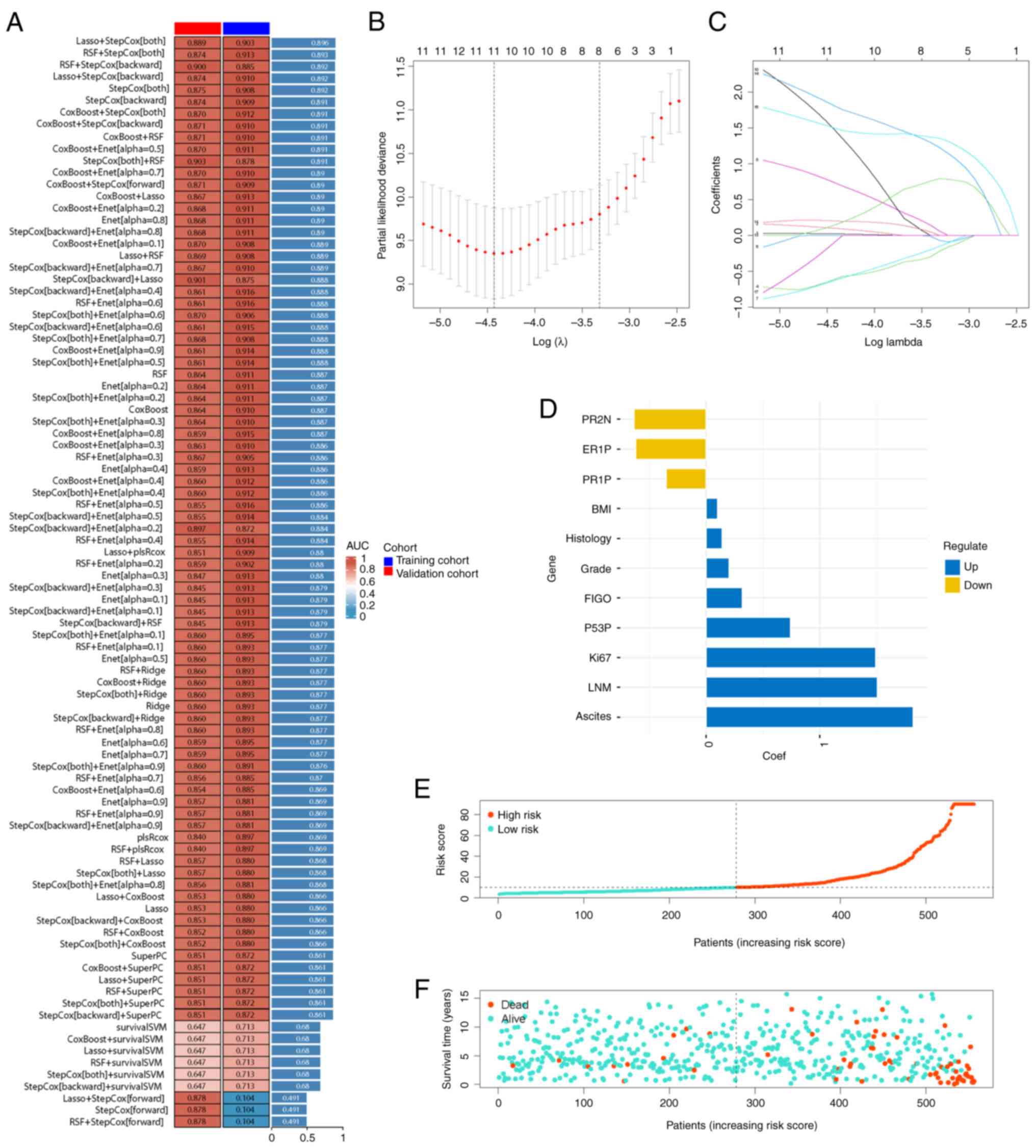
Application of this model in
recurrence-free survival (RFS)
A similar approach was used to develop a prognostic
model for RFS in patients with EC. The integration of Lasso and
stepwise Cox methods demonstrated superior statistical power,
achieving a C-index of 0.896 for the training group (Fig. 3A). After filtering out 11 factors
(Fig. 3B and C), patients were
categorized into high-risk and low-risk groups using the new risk
calculation formula (Fig. 3D and
E). Patients in the high-risk group exhibited a shorter time to
recurrence, as demonstrated by a denser concentration of red dots
in the lower right corner (Fig.
3F).
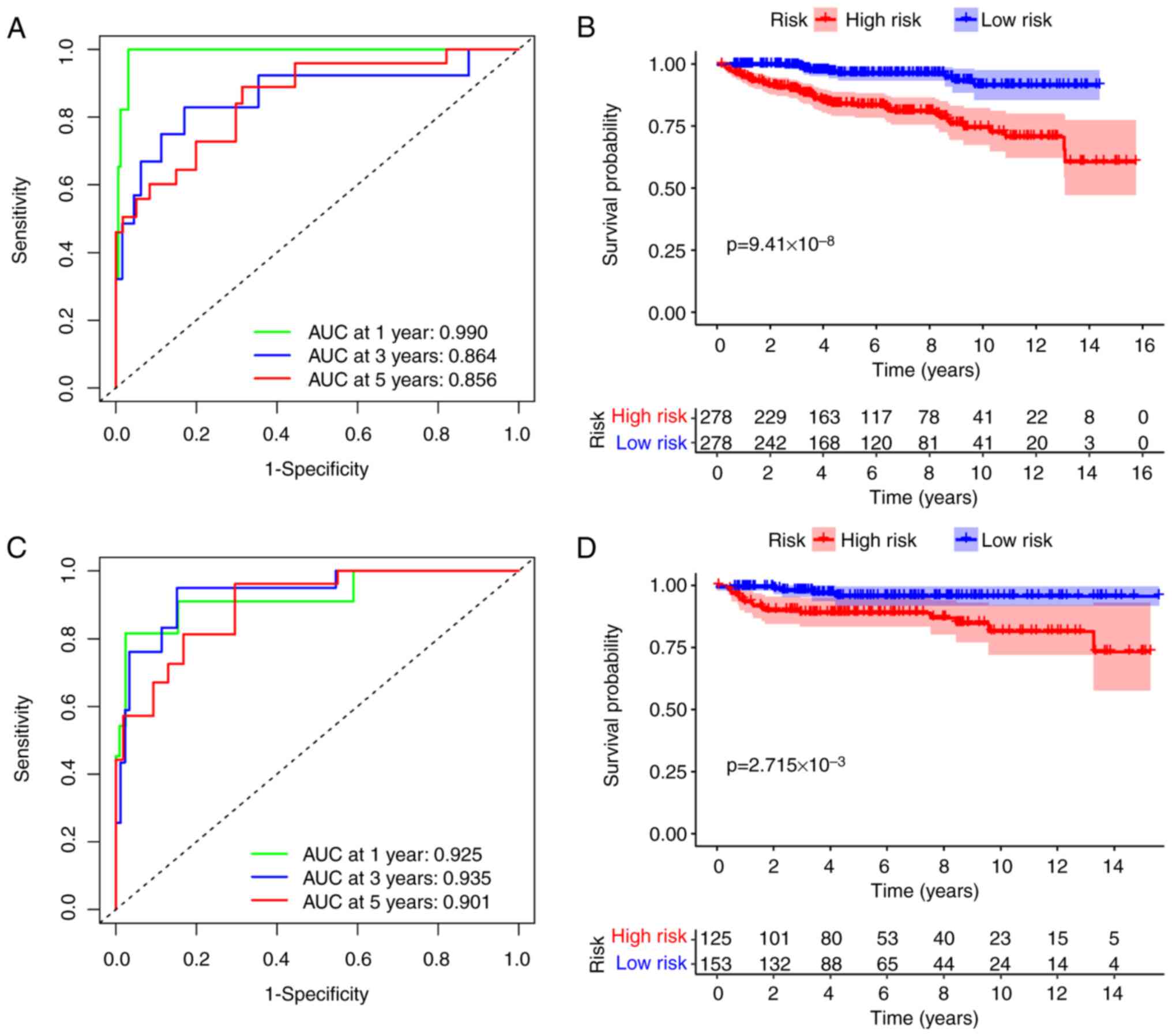
The present study's model demonstrated exceptional
predictive capability, with AUC values exceeding 0.85 for both
cohorts over a 5-year period. Notably, the training and validation
cohorts achieved an AUC value of 0.99 and 0.925 at 1 year,
respectively (Fig. 4A and C,
respectively). In the training cohort, patients in the low-risk
group had a significantly improved RFS compared with those in the
high-risk group, with a P=9.41×10−8 (Fig. 4B). The validation cohort revealed
similar outcomes, with a P=2.715×10−3 (Fig. 4D). These results indicated that the
present study's model provides outstanding predictive performance
for both OS and RFS.
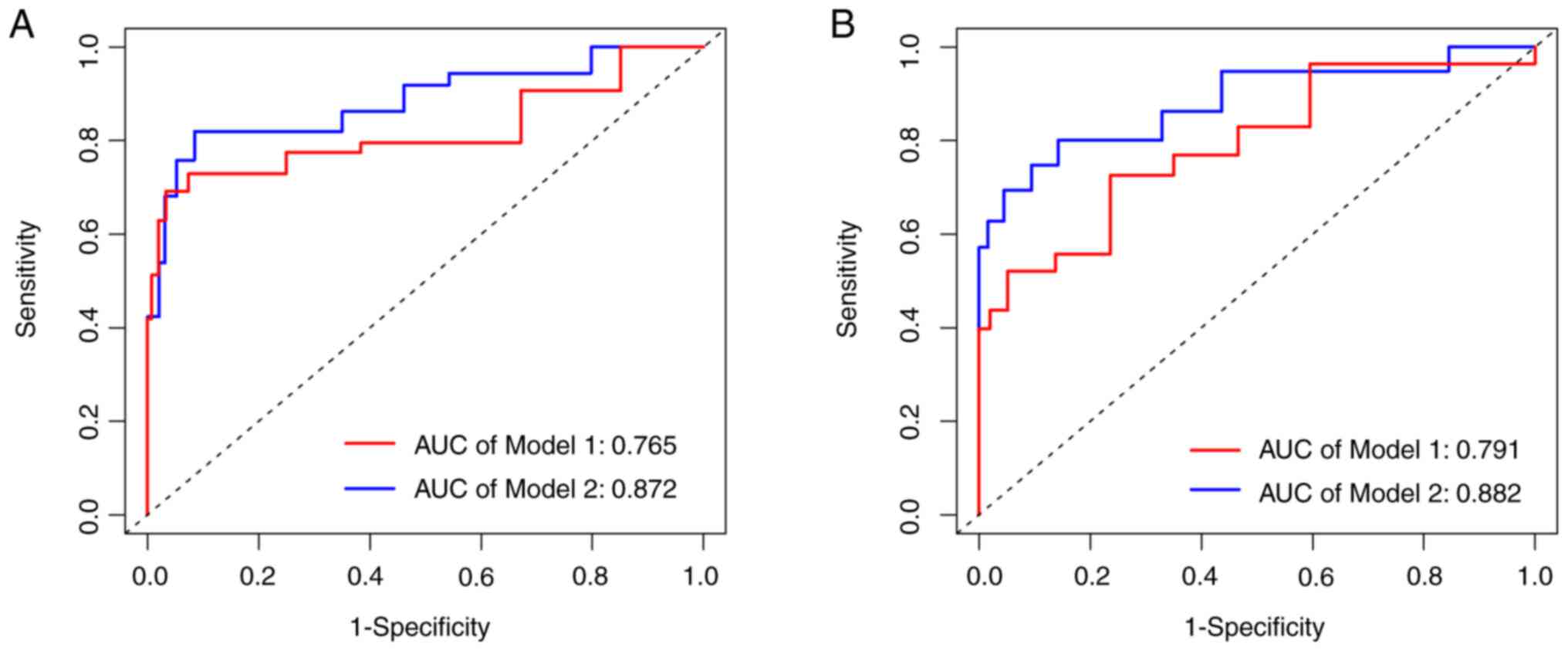
Advantages of introducing IHC
markers
To evaluate the enhanced predictive efficacy of
incorporating IHC markers for OS and RFS in patients with EC, the
IHC-related indicators were removed and the impact on curve values
for both scenarios was assessed, pre- and post-exclusion. It was
found that including these four factors, actually improved
diagnostic accuracy, with one AUC value increasing from 0.765 to
0.872 (Fig. 5A) and another from
0.791 to 0.882 (Fig. 5B).
In summary, the model of the present study
demonstrated robust predictive performance, demonstrating high
accuracy and reliability in forecasting both OS and RFS across
patients with EC. This predictive capability highlights its
potential utility in clinical decision-making and personalized
treatment planning.
Discussion
EC ranks as the second most common gynecologic
malignancy, with increasing incidence and mortality rates (4). In China, EC exhibits similar trends,
with five-year survival rates varying based on FIGO staging. For
patients diagnosed at an early stage (FIGO stage I), the five-year
survival rate is ~90%. By contrast, for those with advanced-stage
disease (FIGO stage IV), the survival rate significantly declines
to ~15% (11). Research has
identified numerous indicators that are strongly associated with
poor prognosis in patients with EC (12,13).
However, there is currently no comprehensive scoring system that
assigns weights to these indicators and calculates a risk score for
each patient. Such a system would enable stratification of OS and
RFS risk levels. Therefore, there is an urgent need to develop an
effective method to optimize treatment selection and improve
patient survival outcomes.
In the present study, a predictive model was
developed and validated to estimate the prognosis of patients with
EC in terms of OS and RFS. These findings revealed that the model
incorporating IHC indices exhibits superior predictive value
compared with clinical models. Information on four IHC-related
markers was included: ER, PR, Ki67 and P53. The emphasis on IHC
results is well-supported, as numerous studies have revealed that
these factors are strongly correlated with disease malignancy
(14,15). Furthermore, some of these indices
can indicate the molecular subtype of the disease, which is
particularly beneficial for patients who cannot undergo genetic
testing. This provides significant clinical advantages. When the
present predictive model identifies a patient as belonging to the
high-risk group, it guides clinicians to promptly administer an
appropriate and comprehensive chemotherapy regimen following
staging surgery, with the goal of improving the patient's long-term
survival rate.
It has been indicated that pre-operative IHC
biomarkers effectively evaluate patient prognosis, guiding
subsequent surgical and adjuvant treatment plans. A previous study
assessed the accuracy of P53 IHC in predicting TP53 mutations
identified by next-generation sequencing in EC biopsy samples,
finding a concordance rate of ≥95% (16). Moreover, IHC for P53, either alone
or in combination with TP53 sequencing, is particularly useful for
identifying specific high-risk tumor genotypes/phenotypes, which
significantly improves patient outcomes (17).
A large retrospective study investigated the impact
of ER expression on oncologic outcomes within a new risk
classification for EC. The aforementioned study, which included 891
patients with EC, found that the ER 01+ phenotype was significantly
associated with more advanced stages, higher rates of metastasis,
and poorer prognoses (18). Current
research confirms that incorporating the absence of ER and PR into
clinical risk stratification helps identify high-risk patients with
stage I–II EEA (19). Additionally,
the absence of PR expression is an important independent predictor
of tumor recurrence in these patients (20). Multivariate regression analysis has
established that a Ki67 index of ≥33% is a significant independent
predictor of recurrence. Patients with high Ki67 levels had notably
poorer RFS and OS compared with those with lower Ki67 levels
(P<0.001 and P=0.029, respectively) (21). The combined prognostic value of ER,
PR and P53 with Ki67 surpassed the predictive accuracy of each
individual marker. However, to date, no studies have combined
oncological behavior with IHC expression to jointly predict OS and
RFS in patients with EC. Additionally, research utilizing advanced
technologies such as machine learning to enhance predictive
accuracy in this context remains lacking.
Furthermore, the present study's model can assist
patients with EC who have ambiguous FIGO staging by stratifying
them based on their risk scores. This stratification allows us to
refine the surgical plan and ensure a more comprehensive resection.
Predictive models are already widely used in the preoperative
diagnosis of EC. LNM is a significant risk factor for poor
long-term prognosis, with LVSI (22) and a high metabolic syndrome score
(23) serving as indicators for its
occurrence. For instance, Yang et al (24) developed a nomogram to predict the
probability of lymph node positivity in patients with stage IIIC
EC. This nomogram demonstrated higher efficacy compared with FIGO
staging. Moreover, numerous emerging indicators have been revealed
to be associated with patient prognosis, including L1CAM (25), EPPK1 (26), FOXM1 (27) and TNFRSF4 (28). In the future, the authors plan to
incorporate these indicators to further refine and enhance the
predictive model. Compared with previous models developed at Peking
University People's Hospital, the model in the present study
demonstrated significant improvements. Notably, the incorporation
of IHC indicators has substantially enhanced the predictive
efficacy of this model.
With advancements in algorithms, machine learning
has become widely used in model construction. Several studies have
evaluated the impact of different algorithms on improving model
performance. A recent study found that Random Forest is optimal for
assessing OS and RFS in high-grade EC (29). Additionally, a model incorporating
the latest algorithms can preoperatively predict the histology,
stage and grade of EC, thereby assisting doctors in achieving more
accurate diagnoses and predictive outcomes (30). By evaluating 92 algorithm
combinations, a scoring criterion was established to calculate
individual risk scores for each patient. This scoring system
allowed to stratify patients into low-risk and high-risk groups.
The OS and RFS rates at 1, 3 and 5 years for each group were
calculated. In both the training and validation cohorts, the AUC
values demonstrated favorable performance across the three time
points. Notably, including four indicators significantly enhanced
the AUC values for both OS and RFS, strongly supporting the
validity of the hypothesis. For example, patients with stage IA EC
typically do not receive chemotherapy after comprehensive staging
surgery. However, their risk of recurrence remains relatively high
after 5 years. In such cases, the model of the present study could
be used to evaluate the patient by collecting their clinical data
and pathological information. If the model indicates that the
patient is ‘high risk’, consideration could be given to
administering a PC regimen (paclitaxel + platinum-based
chemotherapy) in hopes of achieving improved long-term survival
outcomes. Overall, a robust predictive model that greatly supports
the development of precise treatment strategies for patients with
EC with EC has been developed.
The model can be easily replicated by using patient
demographics and IHC outcomes, which facilitates clinical
application and adoption. However, several limitations must be
acknowledged. First, the data were derived from a single
institution, which necessitates further external validation to
confirm the reliability of the model. Furthermore, AI models were
not applied in the process of obtaining pathology reports. Although
the reports were jointly reviewed by two experienced pathologists,
heterogeneity still exists. Additionally, the authors are planning
a prospective study to determine whether this model improves
clinical outcomes in patients with risk stratification. Due to
limitations in the present study duration, results from the present
study are not yet available for publication. Finally, the authors
have not developed a publicly accessible platform, such as a
website, for physicians to use in prognosticating patient outcomes
with EC. The absence of such a tool may have hindered the broader
dissemination and practical application of our predictive model in
clinical settings. Nonetheless, to the best of the authors'
knowledge, this is the first study to incorporate these four IHC
results as indicators and to use the largest sample size. Further
multi-center validations and subsequent prospective studies are
necessary to assess the effectiveness of this model in real-world
scenarios.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Key Technology
Research and Development Program of China (grant nos.
2022YFC2704400 and 2022YFC2704401), the Research and Development
Fund of Peking University People's Hospital (grant no.
RDJP2023-19), the National Natural Science Foundation of China
(grant nos. 82103419, 82230050 and 81874108) and the Natural
Science Foundation of Beijing Municipality (grant no. 7234394).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RQW, JYW, XCL and JLW contributed to the study
conception and design. AXZ, YMW and XCL performed material
preparation, data collection and analysis. RQW and JYW wrote the
first draft of the manuscript. AXZ, JYW, XCL and JLW provided
comments on previous versions of the manuscript. XCL and JLW
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved (approval no.
2022PHB379) by the Ethics Committee Board of Peking University
People's Hospital, in accordance with the principles of the
Declaration of Helsinki. Informed consent was obtained from all
patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Makker V, MacKay H, Ray-Coquard I, Levine
DA, Westin SN, Aoki D and Oaknin A: Endometrial cancer. Nat Rev Dis
Primers. 7:882021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Medina HN, Penedo FJ, Joachim C,
Deloumeaux J, Koru-Sengul T, Macni J, Bhakkan B, Peruvien J,
Schlumbrecht MP and Pinheiro PS: Endometrial cancer risk and trends
among distinct African descent populations. Cancer. 129:2717–2726.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Piechocki M, Koziołek W, Sroka D, Matrejek
A, Miziołek P, Saiuk N, Sledzik M, Jaworska A, Bereza K, Pluta E
and Banas T: Trends in incidence and mortality of gynecological and
breast cancers in Poland (1980–2018). Clin Epidemiol. 14:95–114.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitric C and Bernardini MQ: Endometrial
cancer: Transitioning from histology to genomics. Curr Oncol.
29:741–757. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cancer Genome Atlas Research Network, .
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bruno V, Betti M, D'Ambrosio L, Massacci
A, Chiofalo B, Pietropolli A, Piaggio G, Ciliberto G, Nisticò P,
Pallocca M, et al: Machine learning endometrial cancer risk
prediction model: Integrating guidelines of European society for
medical oncology with the tumor immune framework. Int J Gynecol
Cancer. 33:1708–1714. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Perrone E, De Felice F, Capasso I,
Distefano E, Lorusso D, Nero C, Arciuolo D, Zannoni GF, Scambia G
and Fanfani F: The immunohistochemical molecular risk
classification in endometrial cancer: A pragmatic and
high-reproducibility method. Gynecol Oncol. 165:585–593. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rossi EC, Kowalski LD, Scalici J, Cantrell
L, Schuler K, Hanna RK, Method M, Ade M, Ivanova A and Boggess F: A
comparison of sentinel lymph node biopsy to lymphadenectomy for
endometrial cancer staging (FIRES trial): A multicentre,
prospective, cohort study. Lancet Oncol. 18:384–392. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Njoku K, Barr CE and Crosbie EJ: Current
and emerging prognostic biomarkers in endometrial cancer. Front
Oncol. 12:8909082022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Coll-de la Rubia E, Martinez-Garcia E,
Dittmar G, Gil-Moreno A, Cabrera S and Colas E: Prognostic
biomarkers in endometrial cancer: A systematic review and
meta-analysis. J Clin Med. 9:19002020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vrede SW, van Weelden WJ, Visser NCM,
Bulten J, van der Putten LJM, van de Vijver K, Santacana M, Colas
E, Gil-Moreno A, Moiola CP, et al: Immunohistochemical biomarkers
are prognostic relevant in addition to the ESMO-ESGO-ESTRO risk
classification in endometrial cancer. Gynecol Oncol. 161:787–794.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Talhouk A, McConechy MK, Leung S, Yang W,
Lum A, Senz J, Boyd N, Pike J, Anglesio M, Kwon JS, et al:
Confirmation of ProMisE: A simple, genomics-based clinical
classifier for endometrial cancer. Cancer. 123:802–813. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Singh N, Piskorz AM, Bosse T,
Jimenez-Linan M, Rous B, Brenton JD, Gilks CB and Köbel M: p53
immunohistochemistry is an accurate surrogate for TP53 mutational
analysis in endometrial carcinoma biopsies. J Pathol. 250:336–345.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Thiel KW, Devor EJ, Filiaci VL, Mutch D,
Moxley K, Secord AA, Tewari KS, McDonald ME, Mathews C, Cosgrove C,
et al: TP53 sequencing and p53 immunohistochemistry predict
outcomes when bevacizumab is added to frontline chemotherapy in
endometrial cancer: An NRG Oncology/Gynecologic oncology group
study. J Clin Oncol. 40:3289–3300. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Perrone E, Capasso I, De Felice F,
Giannarelli D, Dinoi G, Petrecca A, Palmieri L, Foresta A, Nero C,
Arciuolo D, et al: Back to the future: The impact of oestrogen
receptor profile in the era of molecular endometrial cancer
classification. Eur J Cancer. 186:98–112. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guan J, Xie L, Luo X, Yang B, Zhang H, Zhu
Q and Chen X: The prognostic significance of estrogen and
progesterone receptors in grade I and II endometrioid endometrial
adenocarcinoma: Hormone receptors in risk stratification. J Gynecol
Oncol. 30:e132019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huvila J, Talve L, Carpén O, Edqvist PH,
Pontén F, Grénman S and Auranen A: Progesterone receptor negativity
is an independent risk factor for relapse in patients with early
stage endometrioid endometrial adenocarcinoma. Gynecol Oncol.
130:463–469. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jia M, Pi J, Zou J, Feng M, Chen H, Lin C,
Yang S and Xiao X: The potential value of ki-67 in prognostic
classification in early low-risk endometrial cancer. Cancer
Control. 30:107327482312069292023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Z, Zhang S, Ma Y, Li W, Tian J and
Liu T: A nomogram prediction model for lymph node metastasis in
endometrial cancer patients. BMC Cancer. 21:7482021. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Feng X, Li XC, Yang X, Cheng Y, Dong YY,
Wang JY, Zhou JY and Wang JL: Metabolic syndrome score as an
indicator in a predictive nomogram for lymph node metastasis in
endometrial cancer. BMC Cancer. 23:6222023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang XL, Huang H, Kou LN, Lai H, Chen XP
and Wu DJ: Construction and validation of a prognostic model for
stage IIIC endometrial cancer patients after surgery. Eur J Surg
Oncol. 48:1173–1180. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van der Putten LJM, Visser NCM, van de
Vijver K, Santacana M, Bronsert P, Bulten J, Hirschfeld M, Colas E,
Gil-Moreno A, Garcia A, et al: Added value of estrogen receptor,
progesterone receptor, and L1 cell adhesion molecule expression to
histology-based endometrial carcinoma recurrence prediction models:
An ENITEC collaboration study. Int J Gynecol Cancer. 28:514–523.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu L, Yuan S, Yao S, Cao W and Wang L:
EPPK1 as a prognostic biomarker in type I endometrial cancer and
its correlation with immune infiltration. Int J Gen Med.
17:1677–1694. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen J, Yang P, Li S and Feng Y: Increased
FOXM1 expression was associated with the prognosis and the
recruitment of neutrophils in endometrial cancer. J Immunol Res.
2023:54375262023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ma H, Feng PH, Yu SN, Lu ZH, Yu Q and Chen
J: Identification and validation of TNFRSF4 as a high-profile
biomarker for prognosis and immunomodulation in endometrial
carcinoma. BMC Cancer. 22:5432022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Piedimonte S, Feigenberg T, Drysdale E,
Kwon J, Gotlieb WH, Cormier B, Plante M, Lau S, Helpman L, Renaud
MC, et al: Predicting recurrence and recurrence-free survival in
high-grade endometrial cancer using machine learning. J Surg Oncol.
126:1096–1103. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Feng Y, Wang Z, Xiao M, Li J, Su Y,
Delvoux B, Zhang Z, Dekker A, Xanthoulea S, Zhang Z, et al: An
applicable machine learning model based on preoperative
examinations predicts histology, stage, and grade for endometrial
cancer. Front Oncol. 12:9045972022. View Article : Google Scholar : PubMed/NCBI
|