Introduction
Glioma is a highly aggressive and malignant brain
tumor that originates from glial cells (1) and provides structural and functional
support to neurons. Glioblastoma multiforme (GBM) is the most
common and fatal type of primary brain tumor (2), accounting for ~50% of all gliomas
(3). Common signs include cognitive
decline and changes in personality or behavior. As the tumor
expands, it puts increased pressure on the surrounding brain
tissue, leading to neurological deficits (4). Despite extensive research and
advancements in medical science, the prognosis for patients
diagnosed with glioma remains poor (5).
The aberrant reactivation of epithelial-mesenchymal
transition (EMT) is associated with the malignant characteristics
of tumor cells during cancer progression and metastasis (6). Studies have indicated that centromere
protein F (CENPF) serves a pivotal role in tumor metastasis, as it
can promote EMT progression in hepatocellular carcinoma and
pancreatic cancer (7,8). Through in vitro experiments,
Huang et al (9) reported
that the CENPF/CDK1 signaling pathway facilitated the progression
of adrenocortical carcinoma by regulating the G2/M phase of the
cell cycle. Furthermore, Han et al (10) reported that in vitro CENPF
modulates the proliferation, apoptosis and cell cycle of thyroid
carcinoma cells, impacting tumor growth in mice.
Significant constituents of the kinesin family
include kinesin superfamily protein 20A (KIF20A) and kinesin
superfamily protein 4A (KIF4A). A previous study reported that the
upregulation of KIF20A promotes tumor proliferation and invasion in
renal clear cell carcinoma, with associations with adverse clinical
outcomes (11). Huang et al
(12) identified KIF20A as a
prognostic marker in patients with estrogen receptor-positive
breast cancer receiving tamoxifen adjuvant endocrine therapy. KIF4A
enhances cell proliferation and migration via Hippo signaling,
predicting poor prognosis in esophageal squamous cell carcinoma
(13). Jin and Ye (14) reported that KIF4A promotes ovarian
cancer cell proliferation and inhibits apoptosis by upregulating
BUB1 mitotic checkpoint serine/threonine kinase (BUB1) expression.
Moreover, marker of proliferation Ki-67 (MKI67) is already a widely
utilized proliferation marke (15).
Hu et al (16) reported its
upregulation in uterine leiomyosarcoma, suggesting its potential as
a diagnostic biomarker. Meng et al (17) also reported that KIF20A stimulates
the expression of MKI67, promoting the growth and metastasis of
bladder cancer.
Characterized by rapid growth and invasion into
surrounding brain tissue (18),
glioma is a devastating brain tumor (19). With limited treatment options and
high recurrence rates, glioma poses significant challenges to
patients, caregivers and health care professionals alike. Ongoing
research and advancements in the field of neuro-oncology will offer
hope for improved management and outcomes in the battle against
this formidable malignancy.
Materials and methods
Data origination
The TCGA-glioma data were downloaded from the Cancer
Genome Atlas database (TCGA; http://www.cancer.gov/ccg/research/genome-sequencing/tcga).
GSE111260 (20) and GSE16011
(21) series profiles from the Gene
Expression Omnibus database (GEO; http://www.ncbi.nlm.nih.gov/geo/). From TCGA, gene
expression data from 1,097 glioma samples and 5 adjacent normal
samples was obtained. GSE111260 comprises 67 glioma samples and 3
control samples, whereas GSE16011 includes 276 glioma samples of
all histologies and 8 control samples. In each dataset,
differentially expressed genes (DEGs) were subsequently screened
using the R ‘limma’ package (version 3.44.1; The R Foundation) with
the following screening requirements: False discovery rate (FDR)
<0.05 and |log2-fold change (FC)|>1, where
log2FC <-1 indicates downregulation and
log2FC >1 indicates upregulation. The ‘ggplot2’
package (version 3.3.5) in R (version 4.0.2; The R Foundation) was
used to design the volcano maps (22), and the overlapping DEGs were checked
and displayed using Venn diagrams (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Functional analyses and
protein-protein interaction (PPI) network construction
Gene Ontology (GO) and Kyoto Encyclopedia of Genes
and Genomes (KEGG) enrichment analyses were performed using the R
software packages clusterProfiler (version 4.12.6), enrichplot
(version 1.24.4) and ggplot2 (The R Foundation; FDR <0.05).
Biological process, molecular function and cellular component are
the three separate branches of GO. The PPI network was then created
using the CytoHubba plug-in (version 0.1; http://apps.cytoscape.org/apps/cytohubba) and the
Search Tool for the Retrieval of Interacting Genes (version 12.0;
http://string-db.org/) in the Cytoscape program
(version 3.10.2; http://cytoscape.org/). Subnetworks of the overlapping
DEGs were generated using the Molecular Complex Detection (MCODE;
version 2.0.3; http://apps.cytoscape.org/apps/mcode) 1 and 2
algorithms.
Development and validation of the
prognostic signature model
In the univariate Cox proportional hazard regression
analysis, the R package c060 (version 0.2–4; The R Foundation) was
used, and the stability selection approach was used to further
restrict the scope. Using the R package glmnet (version 2.0–16; The
R Foundation) and the genes chosen in earlier rounds, a least
absolute shrinkage and selection operator (LASSO) Cox model was
used to construct a prognostic model. To establish the ideal LASSO
penalty parameter value, a 10-fold cross-validation was performed.
Patients with gliomas in the TCGA cohort were separated into high-
and low-risk groups according to the median risk score. The
survival status of patients in the two groups is presented in
scatter plots. A heatmap was created using ‘pheatmap’ software
(version 1.0.12; http://rdrr.io/cran/pheatmap/) to display the
differential expression of hallmark genes between groups. The best
risk score cut-off was assessed and a Kaplan-Meier overall survival
(OS) curve was produced. Finally, for thorough analyses using
receiver operating characteristic curve analysis, the area under
the curve (AUC) values of the 1st, 3rd and 5th years were
computed.
Gene Set Cancer Analysis (GSCA)
The present study assessed the changes in the
expression of 16 prognostic signature genes, namely, single
nucleotide variants (SNVs) and copy number variations (CNVs), in
lower-grade gliomas (LGGs) and GBMs using GSCA (23). The top 10 mutated genes were chosen
for further study, and their waterfall plots displayed detailed
information.
Development of a prognostic
nomogram
The prognostic importance of the top 10 mutated
genes, grade, age and sex were evaluated for their prognostic
importance using univariate and multivariate regression analysis.
Subsequently, using important factors (CENPF, KIF20A, KIF4A, MKI67
and age), a predictive nomogram was created. The performance of the
nomogram in predicting the 1-, 3- and 5-year OS times of patients
with glioma was assessed using a calibration chart.
Tumor Immune Estimation Resource
(TIMER)
The TIMER (https://cistrome.shinyapps.io/timer/) (24) provides a systematic study of the
prevalence of immune infiltrates in a range of cancers. The TIMER
scores were used to evaluate the relationships between immune cells
(CD4+ T cells, B cells, CD8+ T cells,
macrophages, neutrophils and myeloid dendritic cells) and the
expression of prognostic hub genes (25). These investigations led to the
identification of crucial genes.
The Human Protein Atlas (HPA)
The HPA (26) uses a
combination of omics technologies to demonstrate every human
protein. The present study detected the protein level of CENPF
using the HPA and the mRNA level using Gene Expression Profiling
Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/) in the glioma and normal
groups. In addition, the effects of CENPF expression on primary and
recurrent glioma, OS and disease-specific survival (DSS) status in
patients with glioma were assessed. A log-rank test was used to
assess the effect of gene expression on survival.
Cell culture and transfection
Human U87 MG cells (U87; glioblastoma of unknown
origin) were purchased from the American Type Culture Collection
(ATCC; cat. no. HTB-14). Human U251 MG cells (U251) were purchased
from Shanghai Anwei Biotechnology Co., Ltd. (cat. no.
AW-CELLS-H0379). The cells used in the present study were subjected
to short tandem repeat analysis. In RPMI-1640 media (cat. no.
PM150110; Procell Life Science & Technology Co., Ltd.)
supplemented with 10% FBS (cat. no. 164210; Procell Life Science
& Technology Co., Ltd.), the human glioma cell lines U87 and
U251 were grown and incubated at 37°C with 5% CO2.
Small interfering (si)RNAs targeting CENPF
(si-CENPF-1, 2 and 3) were generated and synthesized by Hanbio
Biotechnology Co., Ltd. The siRNA sequences used were as follows:
si-CENPF-1, 5′-GCGCAGAAUCAAGAGCUAA-3′; si-CENPF-2,
5′-CCCAAGAGAAUGGGACUCUUA-3′; si-CENPF-3,
5′-GCGAGUCAGAUCAAGGAGAAU-3′; and si-negative control (si-NC),
5′-UUCUCCGAACGUGUCACGUTT-3′. Lipofectamine™ 2000 (Invitrogen™;
Thermo Fisher Scientific, Inc.) was used to transfect these
nucleotides into U87 and U251 cells following the manufacturer's
instructions. U87 and U251 cells were divided into three groups:
si-NC (negative control), si-CENPF-1, si-CENPF-2, and si-CENPF-3.
According to the grouping, each well was transfected with 1 µg of
the corresponding siRNA and incubated for 5 h at 37°C for
transfection. Subsequently, U87 and U251 cells were incubated for
an additional 48 h at 37°C before further experimentation.
Reverse transcription-quantitative
(q)PCR
Using TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), total RNA was extracted from the U87 or
U251 cells as directed by the manufacturer. The GoScript™ Reverse
Transcription (RT-PCR) Kit (cat. no. A2790; Promega Coporation) was
utilized to transcribe 2 µg total RNA into complementary DNA
(cDNA). The reaction parameters were as follows: Iincubation at
37°C for 10 min, followed by 42°C for 45 min and then 70°C for 5
min, after which the mixture was cooled on ice for 5 min.
Subsequently, the following components were added: 4 µl of
GoScript™ 5X reaction buffer, 1.7 µl MgCl2 (final
concentration of 2 mM), 1 µl 0.5 mM dNTPs, 0.3 µl ribonuclease
inhibitor (20 units), 1 µl reverse transcriptase and distilled
deionized water (ddH2O) to achieve a final volume of 15
µl. After thorough mixing, the samples were incubated at 42°C for
60 min, followed by inactivation at 70°C for 15 min. cDNA was
subjected to qPCR using ChamQ Universal SYBR qPCR Master Mix (cat.
no. Q711-02; Vazyme Biotech Co., Ltd.). The thermocycling
conditions were as follows: An initial denaturation at 95°C for 2
min, followed by 40 cycles of denaturation at 95°C for 30 sec,
annealing at 95°C for 10 sec and a final extension at 60°C for 30
sec. GAPDH was used as the internal control. The 2−ΔΔCq
approach was used to assess the relative fold changes in expression
(27). The sequences of the primers
used included: CENPF forward, 5′-AAAGAAACAGACGGAACAACTG-3′ and
reverse, 5′-CCAAGCAAAGACCGAGAACT-3′; and GAPDH forward,
5′-TGAAGGTCGGAGTCAACGGATTTGG-3′ and reverse,
5′-GGAGGCCATGTGGGCCATGAG-3′.
Western blotting (WB)
RIPA buffer containing 1 mM PMSF was used to lyse
the total protein of the cells (Beyotime Institute of
Biotechnology). A Pierce™ BCA protein assay kit (Thermo Fisher
Scientific, Inc.) was used to assess the protein concentration.
Protein samples (30 µg) were separated using 10% SDS-PAGE and
transferred to a polyvinylidene difluoride membrane. Following an
overnight incubation at 4°C with diluted primary antibodies, the
membrane was blocked indoors for 1 h in 5% nonfat milk at room
temperature. The primary antibodies used for WB were as follows:
anti-CENPF (1:1,000; cat. no. Ab5; Abcam), anti-p21 (1:1,000; cat.
no. ab109520; Abcam), anti-CDK1 (1:1,000; cat. no. ab265590;
Abcam), anti-vimentin (1:1,000; cat. no. ab92547; Abcam),
anti-E-cadherin (1:10,000; cat. no. ab40772; Abcam) and anti-GAPDH
(1:8,000; cat. no. ab128915; Abcam) antibodies. Horseradish
peroxidase-labelled secondary antibodies (1:5,000; cat. no.
ab205718; Abcam) were then applied to the membrane for 1 h indoors
at room temperature. The ChemiDoc™ Touch Imaging System (Bio-Rad
Laboratories, Inc.) was used to capture the signal after it had
been visualized using ECL reagent (cat. no. KGC4902; Nanjing KeyGen
Biotech Co., Ltd.).
Cell proliferation and colony
formation assays
In 96-well plates with 100 µl culture media, 1,000
U87 or U251 cells per well were cultured. Cell Counting Kit-8
(CCK-8) reagent (10 µl; Beyotime Institute of Biotechnology) was
added to each well and incubated for 2 h. The colorimetric
absorbance at 450 nm was measured using an enzyme marker (Molecular
Devices, LLC).
A total of 1.5×103 treated U87 or U251
cells were plated three times onto 6-well plates for the colony
formation assay. The inoculated cells were cultured for another 14
days at 37°C with medium renewal every 3 days. Subsequently, the
U87 and U251 cells were washed with PBS and fixed at room
temperature with 1 ml of 4% paraformaldehyde (cat. no. P0099;
Beyotime Institute of Biotechnology) to a final concentration of 2%
for 15 min. The U87 or U251 cells were then washed again with PBS.
The formed colonies were subsequently stained with 0.5% crystal
violet (cat. no. C0121; Beyotime Institute of Biotechnology) for 5
min at room temperature. The number of cell colonies was quantified
using ImageJ software (version 3.0; National Institutes of Health),
and the colony formation rate was calculated. Images were captured
under a light microscope (Olympus, Tokyo, Japan).
Transwell migration and invasion
assays
Migration and invasion assays were performed using
well plates with an 8-µm pore size filter insert (Corning, Inc.)
with or without diluted Matrigel (precoated for 1 h at 37°C; Becton
Dickinson and Company). The upper compartment was filled with U87
or U251 cells (5×104/well) in medium without serum, and
the lower chamber contained RPMI-1640 medium supplemented with 10%
FBS. The cells were incubated at 37°C for 48 h before being
immobilized and stained with DAPI (Beyotime Institute of
Biotechnology) for 10 min at room temperature. Cells in the lower
chamber were subsequently counted in five arbitrary regions using a
light microscope.
Cell cycle assay
U87 or U251 cells were harvested using 0.05% trypsin
(MilliporeSigma) for digestion and washed with pre-cooled PBS. The
treated U87 or U251 cells (1×106) were collected and
fixed with 75% ethanol at −20°C for 3 h. The cells were washed
twice with PBS after the ethanol was removed and then resuspended
in 1 ml DNA staining solution and 10 µl permeabilization solution
(cat. no. CCS012; Multi Sciences Biotech Co., Ltd.) in the dark for
30 min at room temperature. A CytoFLEX S flow cytometer (Beckman
Coulter, Inc.) was used for analysis using the FACS LSR II system
(BD Biosciences).
Establishment of animal models
The Ethical Committee of the Second Affiliated
Hospital of Anhui Medical University (Hefei, China) approved the
animal experiments in the present study (approval no.
LLSC20230730). The experiments were performed in accordance with
the Guide for the Care and Use of Laboratory Animals (GB/T
35892-2018; Standardization Administration of the People's Republic
of China) (28). U251 cells
transfected with si-NC or si-CENPF were harvested at a
concentration of 1×107 cells/ml. A total of 12 male
BALB/c nude mice (4–5 weeks old; 15–22 g) were purchased from
Beijing Vital River Laboratory Animal Technology Co., Ltd. Mice
were housed under controlled environmental conditions (temperature,
22±2°C; humidity, 55±10%; 12-h light/dark cycle) and had free
access to standard laboratory food and water. Prior to the start of
the experiment, the animals were acclimatized to the laboratory
conditions for 6 days to minimize physiological changes related to
stress. The mice were randomly divided into two groups (n=6 in each
group): the NC group and the si-CENPF (injected with CENPF
knockdown) group. The transfected U251 cells (1×106)
were first injected subcutaneously into the posterior flanks of the
mice. A digital caliper was used once a week to measure the tumor
diameters. After 28 days, the mice were euthanized by cervical
dislocation following 4% isoflurane anesthesia. The tumor
xenografts were excised, imaged and weighed (g). The following
humane endpoints were established: Tumor diameter, >2.0 cm;
weight loss, >20%; and overall poor condition. In the present
study, no mouse reached the humane endpoint.
Statistical analysis
The results from ≥3 separate tests are presented as
the mean ± standard deviation. For statistical analysis, IBM SPSS
Statistics for Windows, version 17.0 statistical software (IBM
Corp.) was used. Significant differences between groups were
evaluated using the unpaired Student's t test. One-way ANOVA
followed by Tukey's post hoc test was used for multiple
comparisons. The Wilcoxon rank-sum test was employed to analyze the
mRNA levels of CENPF between glioma and normal tissue samples.
Spearman's correlation analysis was used to assess the correlation
between CENPF, the G2M checkpoint and EMT markers in glioma.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Identification of DEGs from the TCGA
glioma, GSE111260 and GSE16011 datasets
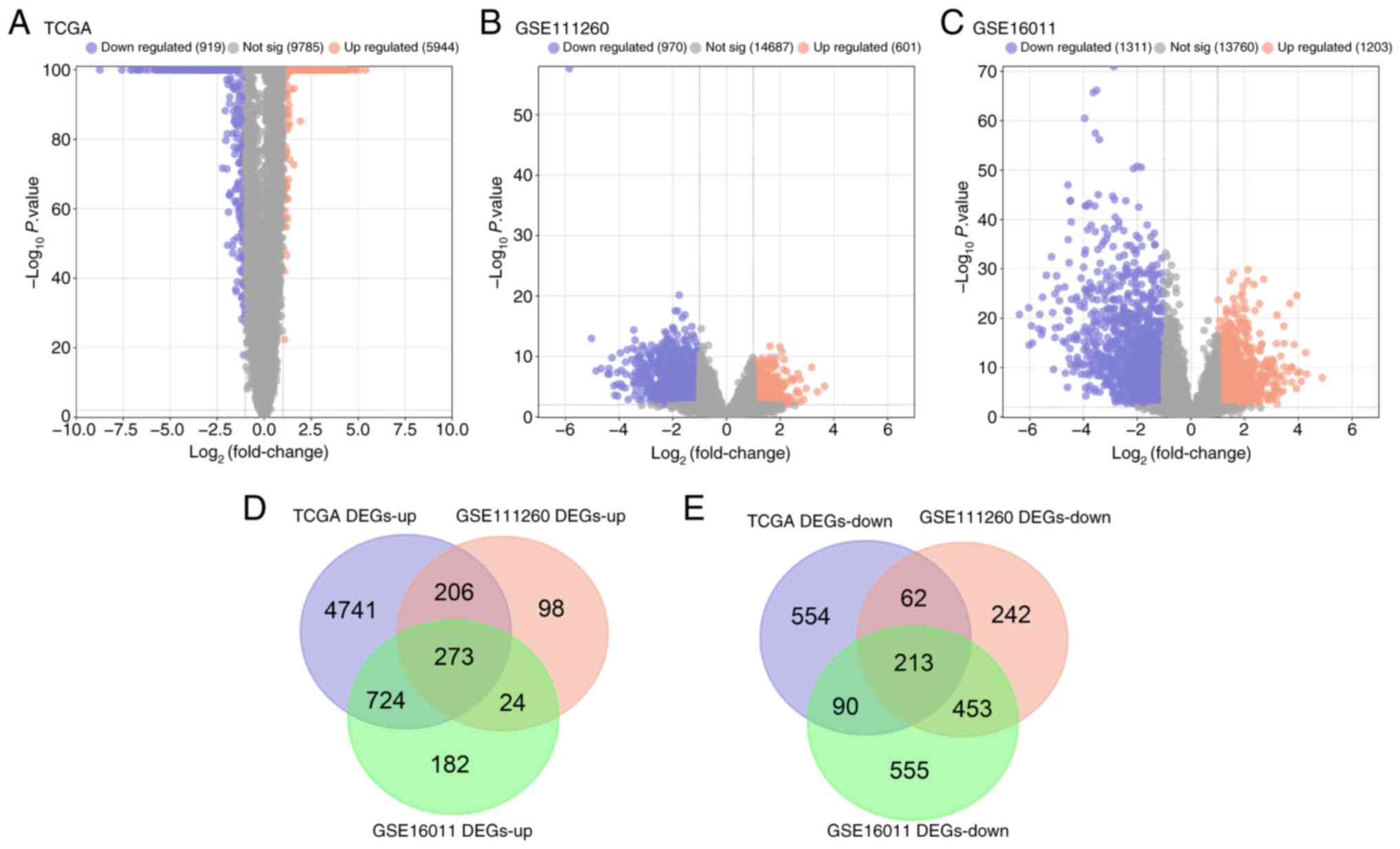
After a series of bioinformatics analyses, 5,944 up-
and 919 downregulated DEGs were identified from TCGA glioma
samples, 601 up- and 970 downregulated DEGs from GSE111260 and
1,203 up- and 1,311 downregulated DEGs from GSE16011 (Fig. 1A-C). Subsequently, using a Venn
diagram, 273 overlapping up- and 213 overlapping downregulated DEGs
were identified (Fig. 1D and E) and
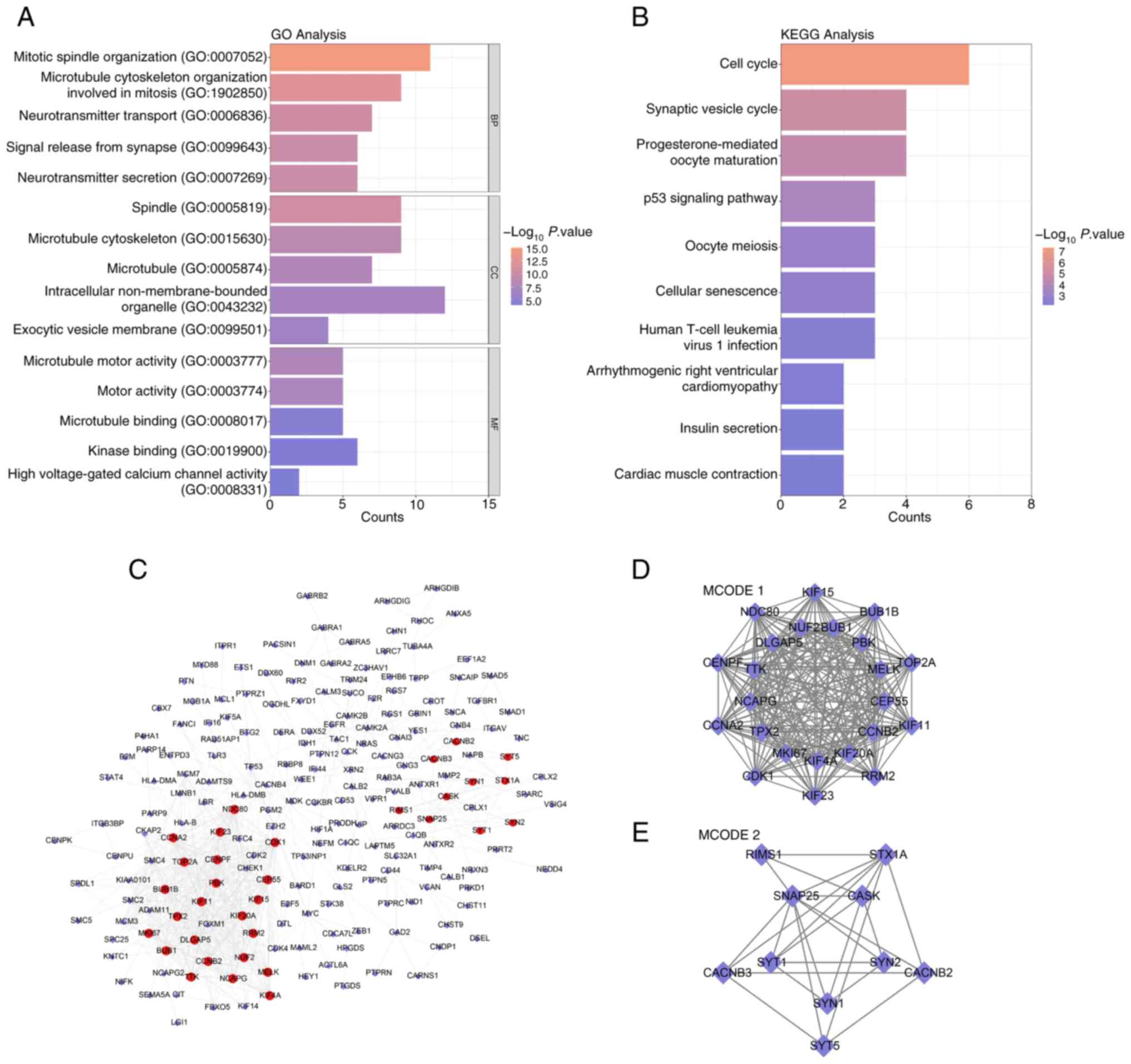
analyzed using functional analyses. According to the GO terms, the
overlapping DEGs were enriched in the terms ‘mitotic spindle
organization’ and ‘neurotransmitter transport’ (Fig. 2A). The enriched KEGG pathways were
related to pathways such as oocyte meiosis, the synaptic vesicle
cycle and the cell cycle (Fig. 2B).
In addition, the PPI network of the overlapping genes revealed the
interactions between genes (Fig.
2C). The genes in the subnetworks (Fig. 2D and E) generated by MCODE 1 (23
nodes) and 2 (10 nodes) were chosen for further study.
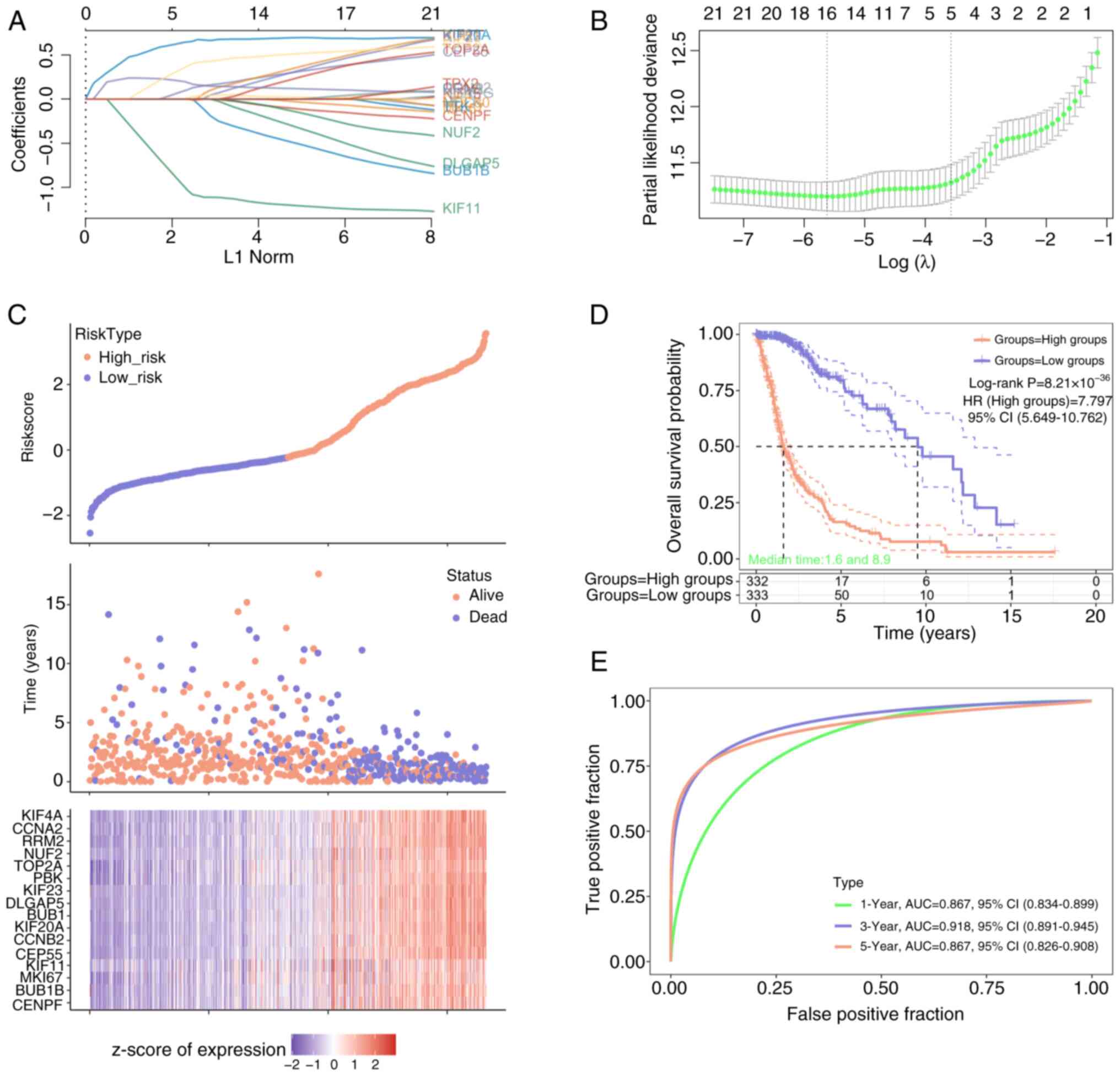
Screening of 16 prognostic signature
genes
On the basis of the 33 genes identified by the MCODE
algorithm, LASSO-Cox analysis was used to select the optimal
threshold parameter (λ=16) for the risk score model (Fig. 3A and B). Patients with glioma were
subsequently classified into high (n=332) and low (n=333) risk
score groups (Fig. 3C), with the
number of deaths increasing from low to high risk scores. Moreover,
16 prognostic signature genes were obtained in which the expression
increased from low to high risk scores. The OS probability was
markedly greater in the low-risk group compared with that in the
high-risk group (Fig. 3D), and the
model had the best performance in year 3 comprare with that in year
1 and 5 (Fig. 3E).
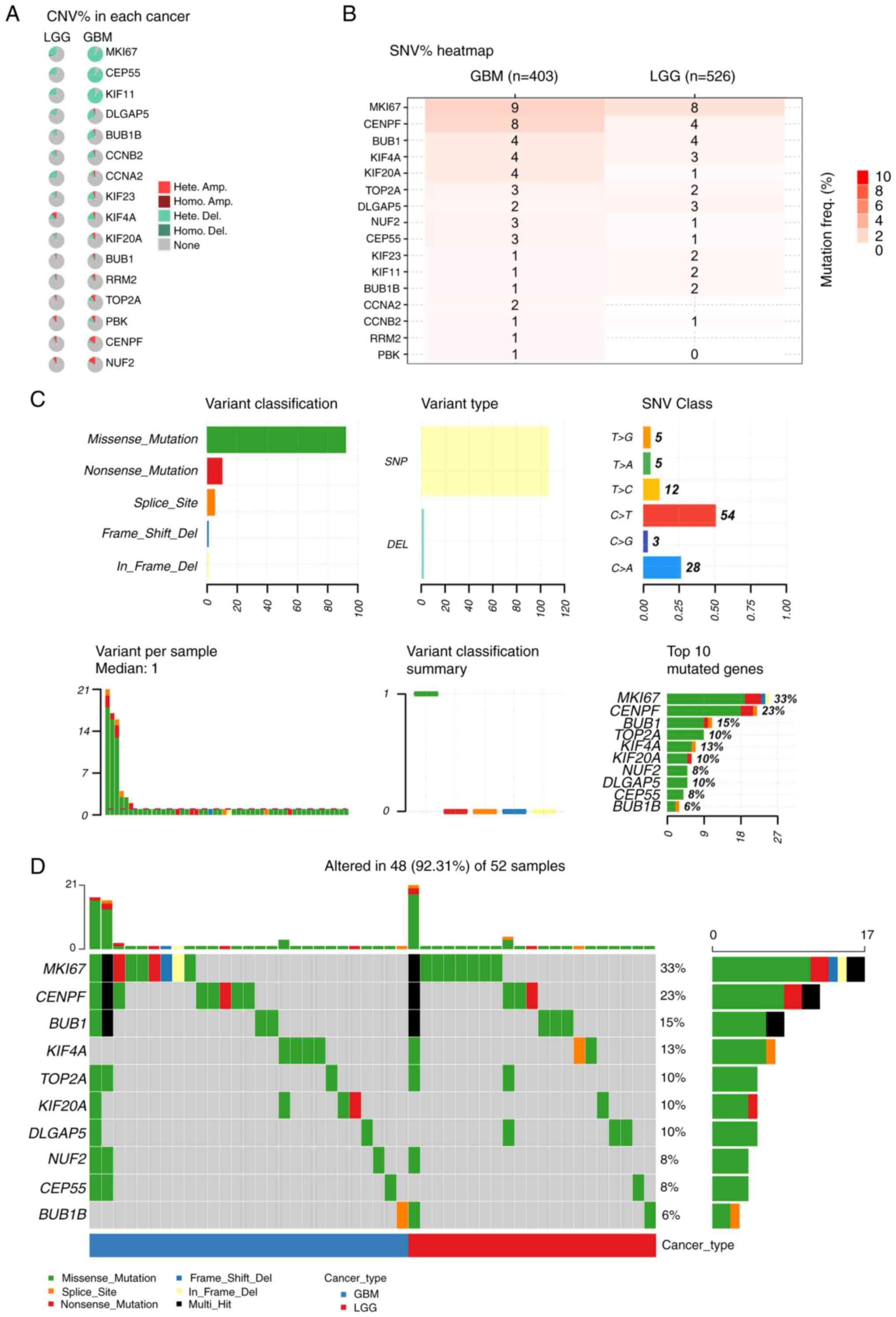
Top 10 mutated genes identified by
GSCA
To identify the most frequently mutated genes, the
online database GSCA was first searched for genes with CNV and SNV
in LGG and GBM, respectively. MKI67, centrosomal protein 55
(CEP55), kinesin family member 11 (KIF11) and DLG-associated
protein 5 (DLGAP5) were the top mutated genes according to CNV %
analysis, especially in GBM (Fig.
4A). KIF20A, KIF4A, BUB1, CENPF and MKI67 demonstrated a
notable mutation frequency in both LGG and GBM, according to SNV %
analysis (Fig. 4B). Furthermore,
missense mutations were demonstrated to account for the vast
majority of gene mutations in glioma, with most mutations occurring
in SNPs (Fig. 4C). In glioma, point
mutations frequently convert base C to base T and base C to base A
(Fig. 4C). Additionally, the top 10
genes with the greatest mutation rate were identified (Fig. 4D), and different mutations in these
genes were revealed in 48/52 glioma samples, accounting for 92.31%
of the total. The distribution and locations of missense mutations
in the top 10 mutated genes in GBM and LGG were also identified
(Fig. S1).
Prognostic hub genes screened via the
nomogram
Combining the mutated genes and clinical factors,
univariate and multivariate Cox analyses were performed to screen
individual variables, including CENPF, KIF20A, KIF4A, MKI67 and age
(Fig. 5A and B). Subsequently, a
prognostic nomogram was constructed using the aforementioned
variables (Fig. 5C) and its
predictive ability was the greatest at year 1 (Fig. 5D). These findings indicate that
CENPF, KIF20A, KIF4A and MKI67 could be promising biomarkers for
glioma prognosis.
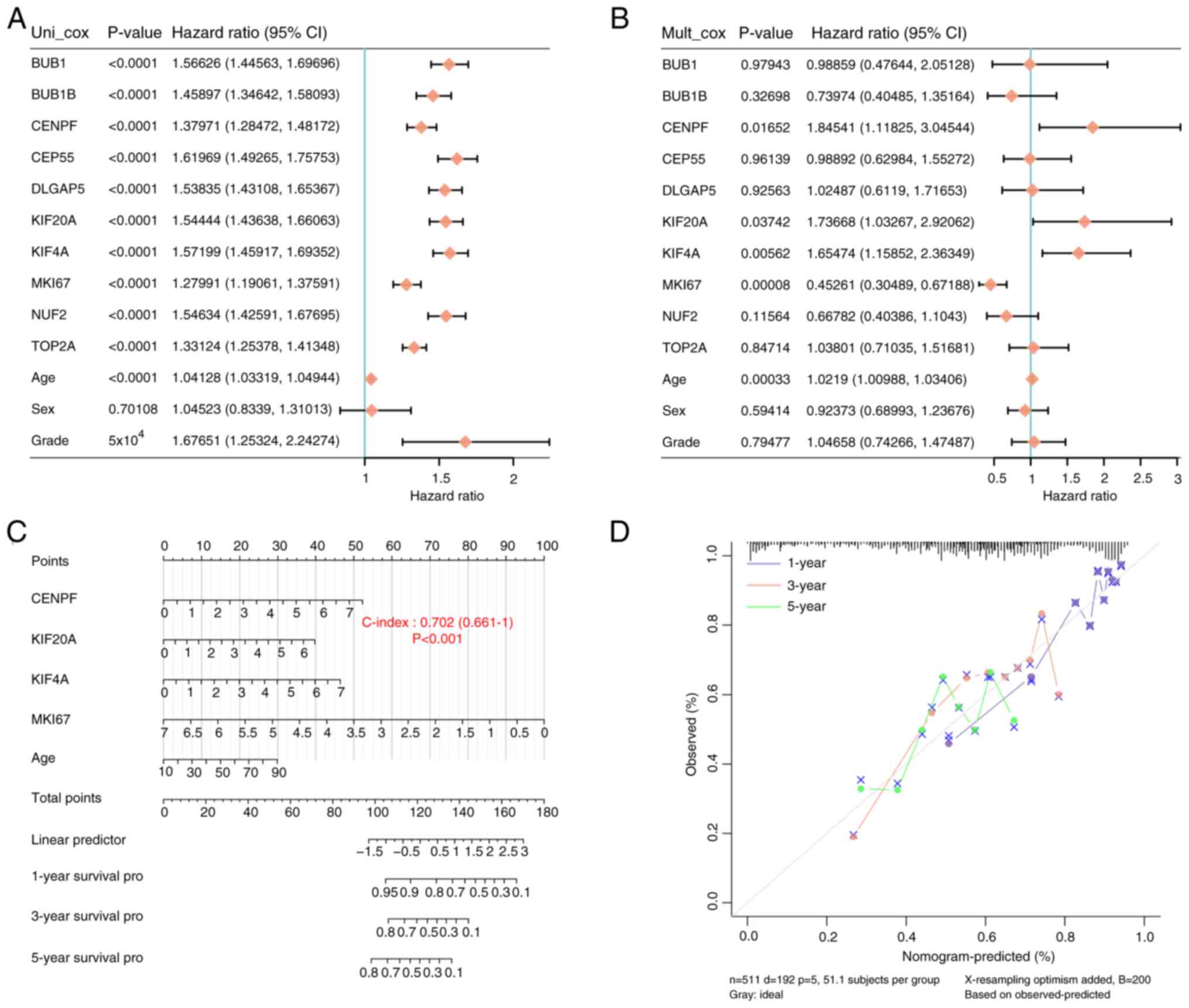 | Figure 5.A total of four prognostic hub genes
were screened in a nomogram. (A) Univariate and (B) multivariate
Cox regression analysis was performed to identify significant
variables in glioma. (C) A prognostic nomogram was constructed,
integrating the significant variables (CENPF, KIF20A, KIF4A, MKI67
and age). (D) Predictive ability of the prognostic nomogram was
assessed by a 45° diagonal line to predict the 1-, 3- and 5-year
survival status. CENPF, centromere protein F; KIF20A, kinesin
superfamily protein 20A; KIF4A, kinesin superfamily protein 4A;
MKI67, marker of proliferation Ki-67; CI, confidence interval. |
Immunoassay of prognostic hub genes
and immune cells
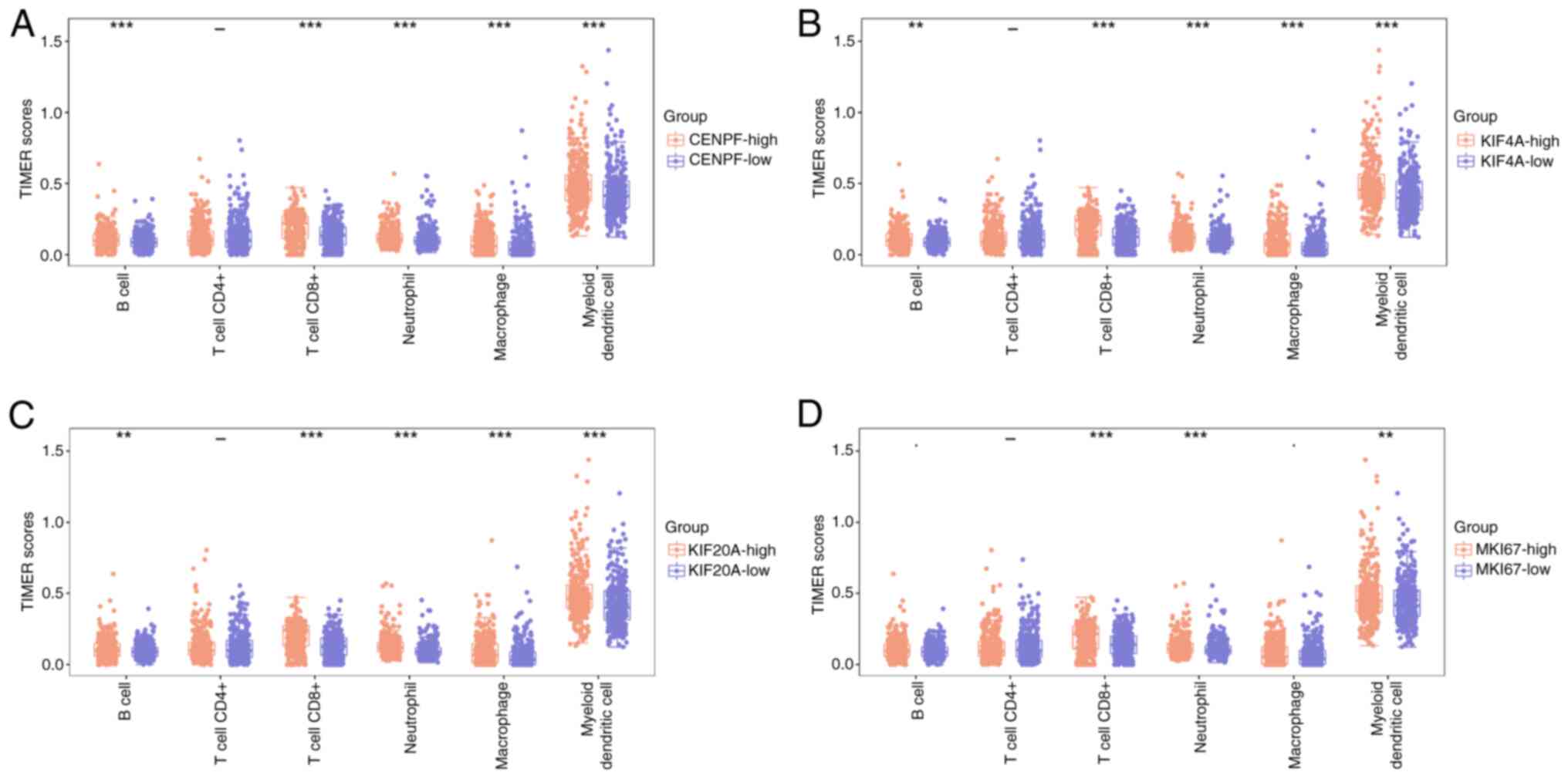
The TIMER scores of immune cells in the high- and
low-expression groups were assessed for CENPF, KIF20A, KIF4A and
MKI67. The results revealed that all immune cells had high TIMER
scores in the high-score groups, and myeloid dendritic cells had
the highest scores in each boxplot (Fig. 6). Notably, the difference in TIMER
scores between the high- and low-CENPF expression groups was more
pronounced compared with those in the KIF20A, KIF4A and MKI67 gene
groups. The aforementioned results indicate that CENPF is a key
gene.
Expression and survival analysis of
CENPF in glioma
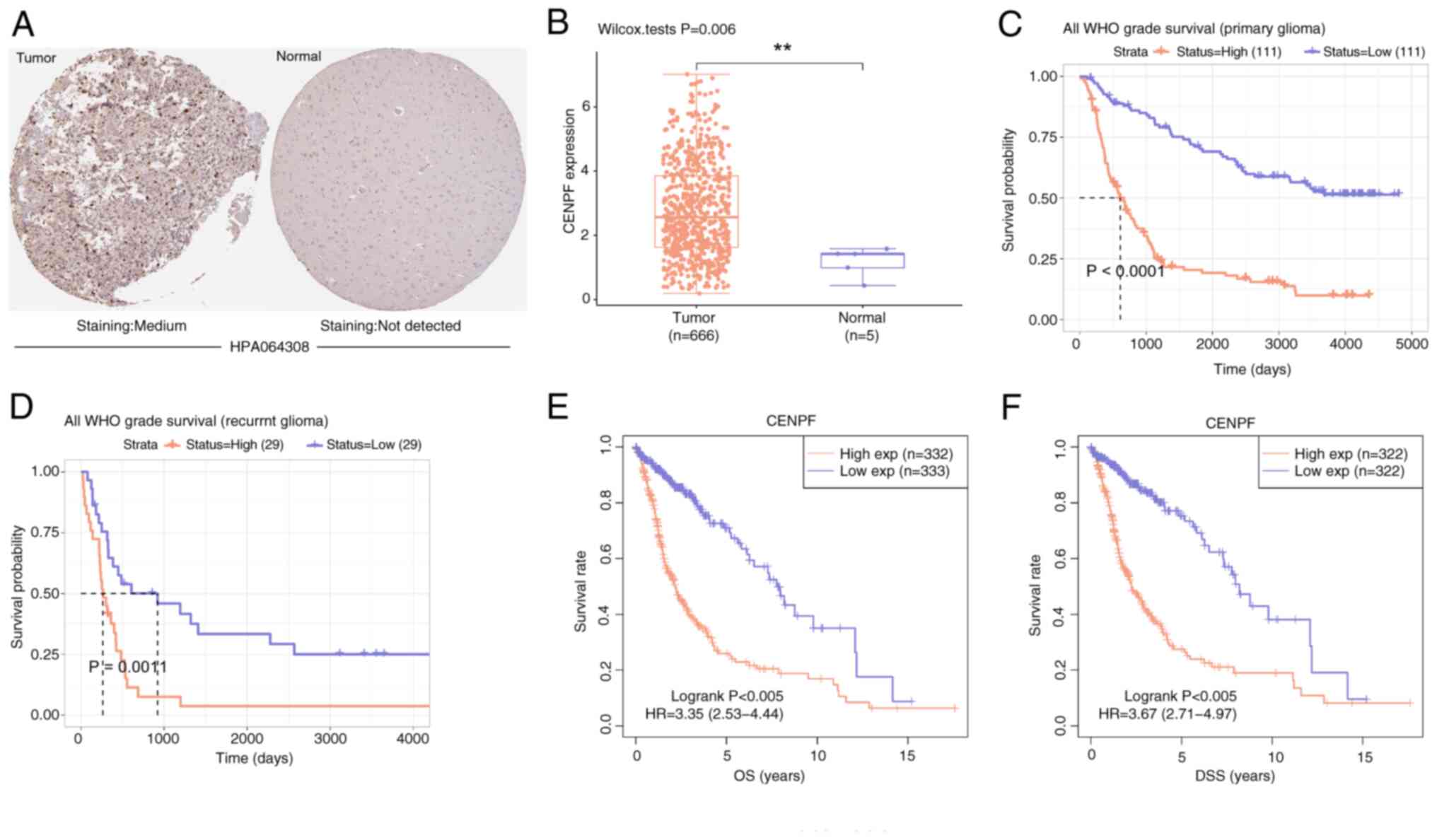
By using the HPA and GEPIA databases, it was
demonstrated that the protein and mRNA levels of CENPF were both
markedly higher in the glioma group than in the normal group
(Fig. 7A and B). Moreover, low
CENPF expression was significantly associated with an improved
survival probability compared with high CENPF expression,
particularly in primary glioma (Fig. 7C
and D). Similarly, low CENPF expression was significantly
associated with improved OS and DSS probabilities compared with
high CENPF expression(Fig. 7E and
F). These findings indicate that CENPF may be an oncogene in
glioma.
CENPF knockdown inhibits glioma
proliferation and metastasis and induces G2 arrest in vitro
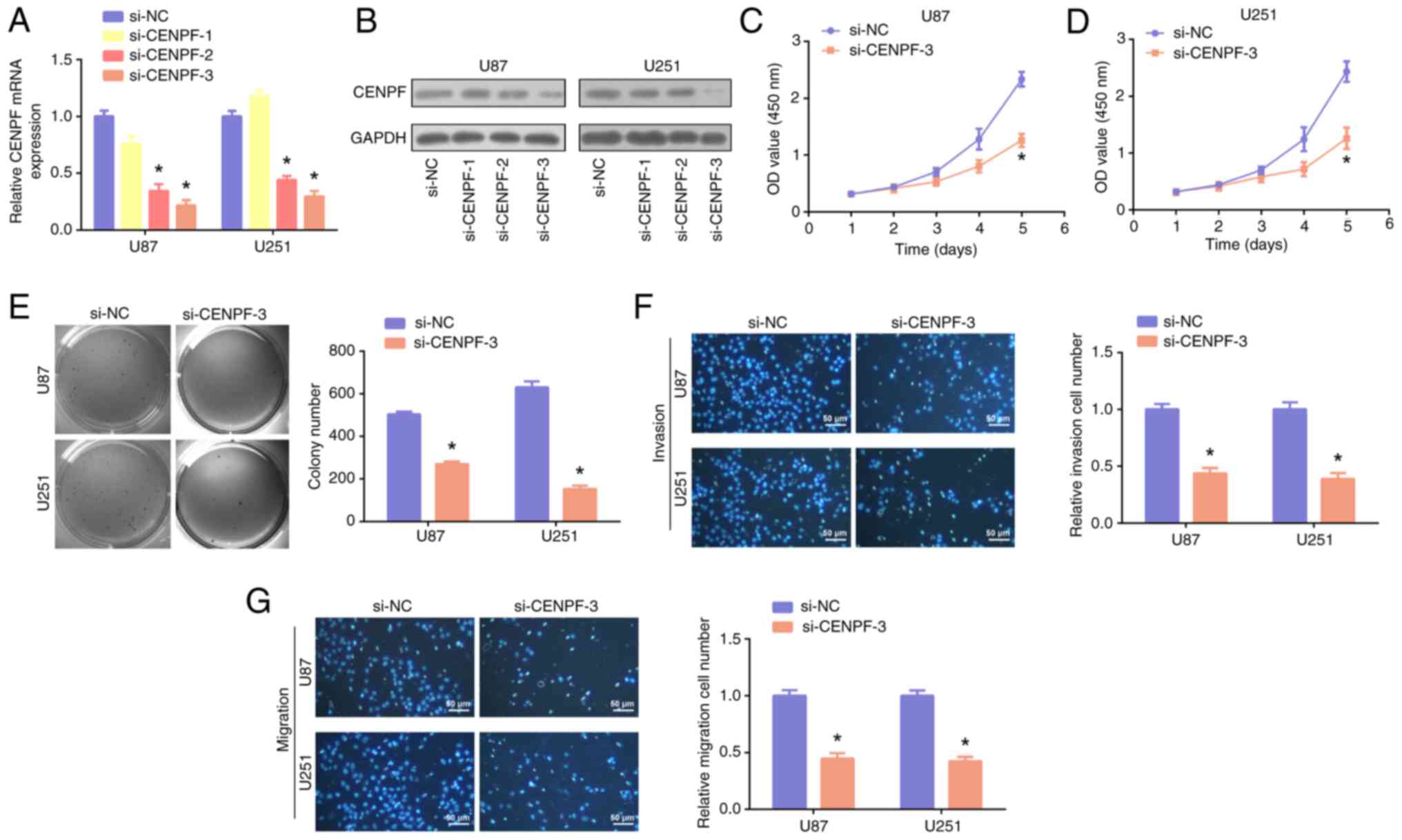
CENPF was subsequently knocked down in U87 and U251
cells, and the results of PCR and WB revealed that si-CENPF-3 had
the greatest knockdown efficiency (Fig.
8A and B). By performing CCK-8 (Fig. 8C and D), colony formation (Fig. 8E) and Transwell (Fig. 8F and G) assays, it was demonstrated
that CENPF knockdown significantly inhibited the proliferation,
invasion and migration of glioma cells, in comparison with
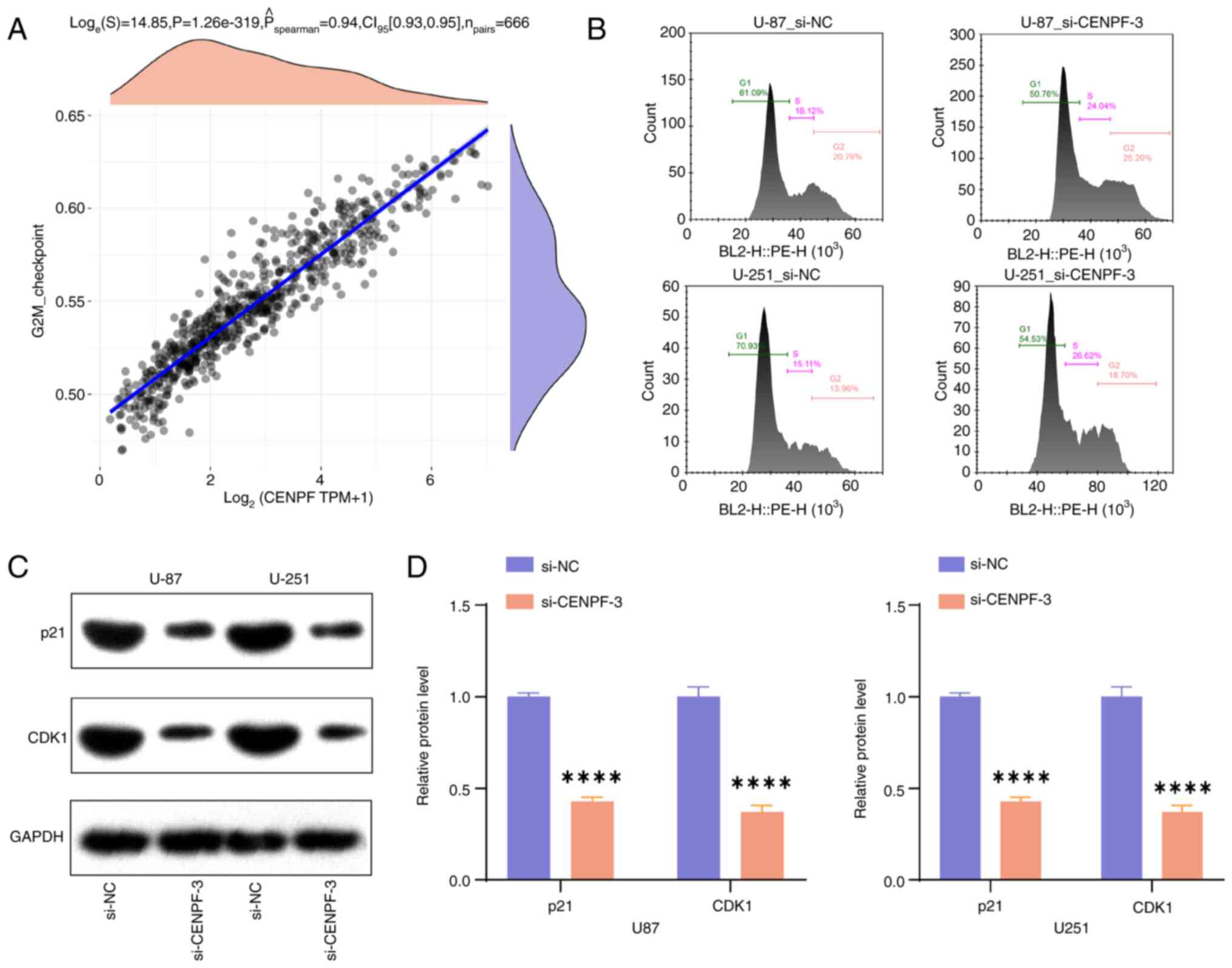
controls. Moreover, it was demonstrated that CENPF was positively
correlated with the G2M checkpoint (Fig. 9A). A cell cycle assay revealed that
the glioma cells in the G2 phase markedly increased in the
si-CENPF-3 group (Fig. 9B), and
si-CENPF-3 was significantly associated with reduced p21 and CDK1
levels, in comparison with controls (Fig. 9C and D). Therefore, the results
indicate that CENPF knockdown could induce G2 arrest in glioma.
CENPF suppresses the progression of
glioma by regulating the EMT pathway
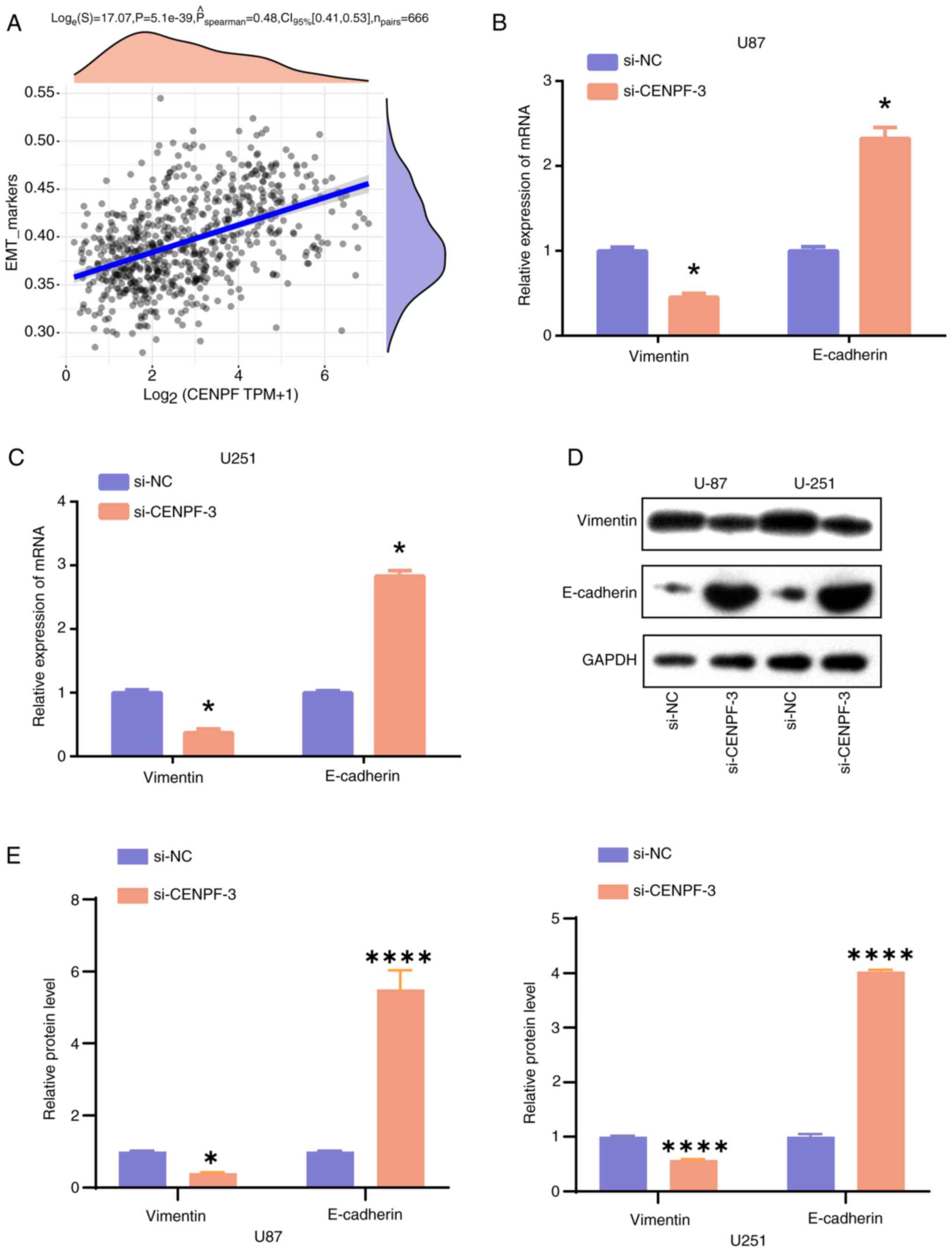
Spearman's correlation analysis demonstrated that
CENPF was positively correlated with EMT markers (Fig. 10A), indicating that the EMT pathway
may be involved in the mechanism of CENPF in glioma. Furthermore,
the PCR and WB assays revealed significantly decreased Vimentin and
elevated E-cadherin in both U87 and U251 cells of the si-CENPF
group compared with those of the si-NC group (Fig. 10B-E). Therefore, the findings
indicate that CENPF suppressed the progression of glioma by
regulating the EMT pathway.
CENPF knockdown blocks the
tumorigenesis of glioma in vivo
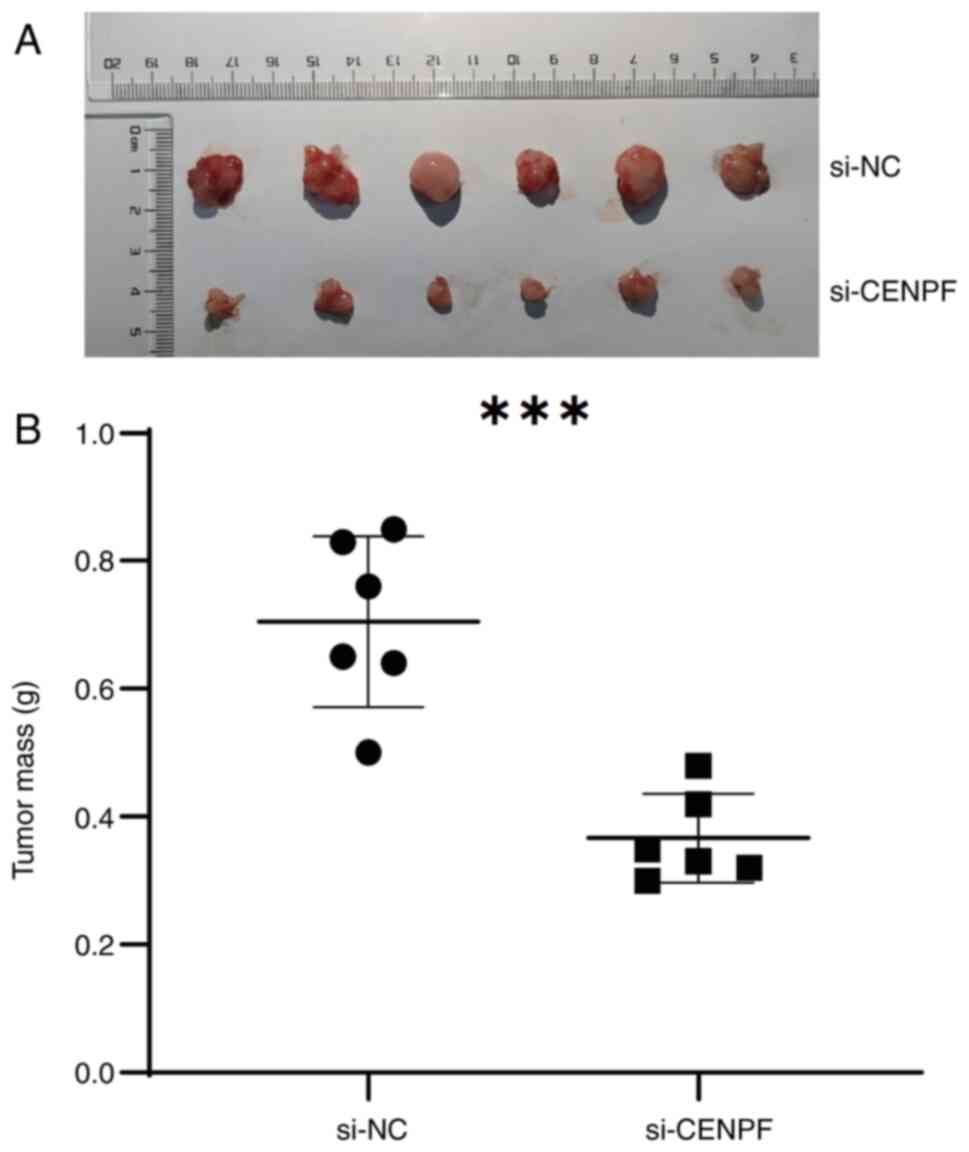
In the constructed animal models, tumor tissues were
collected and measured. The sizes of the tumors in the si-CENPF
group were notably smaller than those in the si-NC group (Fig. 11A). In addition, the tumor mass in
the si-CENPF group was significantly lower than that in the si-NC
group (Fig. 11B). These findings
confirm that CENPF knockdown suppressed the tumorigenesis of glioma
in mice.
Discussion
Currently, the diagnosis of glioma typically
involves imaging techniques such as MRI or CT (29), followed by a biopsy to confirm the
presence of malignant glial cells. Standard treatment for glioma
(30) usually involves a
combination of surgical resection, radiation therapy and
chemotherapy (31). However, this
type of brain tumor tends to develop quickly and infiltrate
surrounding tissues, essentially prohibiting thorough surgical
removal. The rapid and invasive growth of glioma is attributed to
its highly proliferative glial cells (32), which are characterized by an
increased capacity for angiogenesis and resistance to apoptosis
(33). Therefore, early detection,
personalized treatment strategies and innovative therapeutic
interventions hold the key to enhancing the overall survival and
quality of life of patients with glioma.
In the present study, glioma DEGs were identified
using data from the TCGA, GSE111260 and GSE16011 datasets. The
overlapping DEGs obtained from these datasets were found to be
significantly enriched in spindle, Microtubule motor activity, Cell
cycle and Synaptic vesicle cycle. Through further application of
the MCODE 1 and 2 algorithms, 33 genes associated with prognosis
were identified, from which 16 prognostic signature genes were
selected in the risk score model for gene mutation analysis.
Subsequently, the top 10 mutated genes was used to construct a
prognostic nomogram. Finally, four key prognostic hub genes were
identified, namely, CENPF, KIF20A, KIF4A and MKI67. These genes
exhibited the potential to serve as valuable prognostic biomarkers
in glioma.
Members of the kinesin superfamily of motor proteins
include KIF4A and KIF20A. KIF4A functions as a motor protein that
is based on microtubules and is related to the organization and
dynamics of the mitotic spindle (34), which are essential for proper cell
division and genomic stability (35). Hou et al (36) demonstrated that KIF4A enhances cell
proliferation and promotes colorectal cancer development by
promoting cell cycle progression both in vitro and in
vivo. Additionally, Hou et al (37) reported that KIF4A overexpression
enhances the proliferation and migration of hepatocellular
carcinoma cells, whereas KIF4A knockdown reduces cell proliferation
and migration, suggesting a potential role for KIF4A in mediating
tumorigenesis and progression. Jin and Ye (14) reported that KIF4A regulates the
expression of BUB1, inhibiting apoptosis and promoting ovarian
cancer progression. Zhang et al (38) reported that Rac1/Cdc42
transcriptional suppression by KIF4A, which causes cytoskeletal
reorganization in glioma cells, promotes the formation of gliomas.
KIF20A is involved primarily in regulating microtubule dynamics
during cell division (39) and is
essential for proper cytokinesis and cell cycle progression
(40). Previous research linked the
overexpression of KIF20A with several cancers. KIF20A was first
discovered to be overexpressed in pancreatic ductal adenocarcinoma
(PDAC), and its knockdown in PDAC cell lines severely inhibited
cell growth (41). Further studies
in human liver cancer cell lines have also reported elevated levels
of KIF20A, whereas it is undetectable in normal human liver cells
(42). Yan et al (43) demonstrated that KIF20A RNAi
inhibited the viability of gastric cancer (GC) SGC7901 cells.
Peptides derived from KIF20A used alone as immunotherapy vaccines
or in combination with other peptides/chemotherapy drugs have
achieved notably higher OS rates in GC treatment (44,45).
Copello and Burnstein (46)
reported that KIF20A promotes progression to castration-resistant
prostate cancer by activating the androgen receptor via autocrine
mechanisms. Research has reported that elevated KIF20A levels are
associated with poor prognosis in patients with GBM (47).
In addition, MKI67 is a nuclear protein associated
with cell proliferation. Under normal conditions, MKi67 shows
cortical nucleolar localization during interphase and is recruited
to condensed chromosomes during mitosis (48). The MKi67 gene is located on
chromosome 10q25-ter and primarily encodes two MKi67 isoforms (345
and 395 kDa) (49,50). The expression of the MKi67 protein
can be assessed in the nuclei of cells in the G1, S, G2 and mitotic
phases but not in the quiescent G0 phase (51). The expression of MKI67 is widely
used as a biomarker for assessing cell proliferation in several
types of cancers (52), including
glioma (53). High expression of
Ki67 in cancer cells can be considered a prognostic predictor for
cancer (54). Substantial evidence
supports the role of MKi67 in cancer diagnosis (55). In a study involving patients with
liver hepatocellular carcinoma (LIHC) who underwent surgery, high
MKi67 expression in cancer tissues was reported to predict
increased tumor grade and early cancer recurrence (56). Moreover, MKi67 staining has been
widely used to predict postoperative survival rates and even
survival rates after liver transplantation in patients with LIHC
(57).
CENPF is a human gene that encodes the centromere
protein F, a crucial component of the kinetochore complex. It has
been extensively studied in several fields, including cell biology,
cancer research and genomics, and is associated with the prognosis
of patients with non-small cell lung cancer and prostate cancer
(58,59). Additionally, overexpression of CENPF
is associated with poor prognosis and bone metastasis in patients
with breast cancer (60). Moreover,
in hepatocellular carcinoma, high CENPF levels are associated with
poor prognosis and aggressive tumor behavior (61). Moreover, one study analyzed genomic
data from patients with glioma and identified CENPF as one of the
notably amplified genes in tumor samples (62). However, to fully understand the
molecular processes of CENPF in glioma, further study is
necessary.
Through immunoassays targeting hub genes, CENPF
emerged as a key gene in the present study, and its significant
association with glioma prognosis was revealed. High CENPF
expression was associated with the poor prognosis of patients with
glioma. In in vitro and in vivo assays, CENPF was
observed to be upregulated in glioma, and its knockdown inhibited
glioma progression and metastasis, as demonstrated by data from
public databases. Collectively, these findings establish CENPF as
an oncogene in glioma. Furthermore, given the close relationship of
CENPF with the cell cycle, flow cytometry was used to assess its
impact on cell cycle regulation in glioma. The results demonstrated
that CENPF knockdown led to G2 arrest in the cell cycle.
EMT is a complex biological process (63). In glioma, EMT promotes cell invasion
into surrounding brain tissue and facilitates their ability to
migrate to distant sites, contributing to tumor spread and
metastasis (64). As the EMT
pathway may be involved in the mechanism of CENPF in glioma, the
present study assessed the levels of EMT markers in glioma cells
with CENPF knockdown. The results revealed decreased vimentin and
elevated E-cadherin levels, suggesting that CENPF promotes glioma
development by regulating the EMT pathway.
The present study has several limitations. Whilst
in vitro and in vivo experiments were performed to
assess the role of the CENPF gene in glioma, additional experiments
are needed to confirm its mechanisms. The present study also used
multiple public databases and mouse models to analyze the role of
CENPF in glioma but lacked validation in clinical samples. In
future studies, more clinical data should be collected, including
clinical samples, clinical characteristics and survival data to
perform further validation of the expression level and prognostic
value of CENPF in glioma clinical samples.
In conclusion, the present study assessed the
molecular landscape and potential prognostic biomarkers in glioma,
a highly aggressive and lethal brain tumor. Through joint analysis
of the TCGA, GSE111260 and GSE16011 datasets, 486 genes associated
with glioma were identified. Comprehensive bioinformatics analyses,
including PPI networks, risk score models, gene mutation analyses
and diagnostic models, revealed potential prognostic biomarkers for
glioma (CENPF, KIF20A, KIF4A and MKI67). CENPF was significantly
upregulated in glioma and was associated with poor patient
prognosis. In vitro functional experiments demonstrated that
CENPF promotes the proliferation and metastasis of glioma cells,
promoting glioma progression through the regulation of the EMT
pathway. In vivo experiments indicated that downregulation
of CENPF expression inhibits tumor progression in glioma. Overall,
the present study contributes to the understanding of glioma
biology and provides a basis for further investigations and the
development of personalized approaches for glioma diagnosis,
treatment and prognosis.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JL, LL, GZ, ZY, DZ and BZ contributed to the study
conception and design. Material preparation, data collection and
analysis were performed by JL, LL, GZ and ZY. The first draft of
the manuscript was written by DZ and BZ, and all authors commented
on previous versions of the manuscript. JL and BZ confirm the
authenticity of all the raw data. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments in the present study were
approved by The Ethical Committee of the Second Affiliated Hospital
of Anhui Medical University (Hefei, China; approval no.
LLSC20230730}.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Weller M, Wick W, Aldape K, Brada M,
Berger M, Pfister SM, Nishikawa R, Rosenthal M, Wen PY, Stupp R and
Reifenberger G: Glioma. Nat Rev Dis Primers. 1:150172015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Spanish DF: Types of brain tumors. Health.
2021.
|
|
3
|
Alifieris C and Trafalis DT: Glioblastoma
multiforme: Pathogenesis and treatment. Pharmacol Ther. 152:63–82.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Peeters MCM, Dirven L, Koekkoek JAF,
Gortmaker EG, Fritz L, Vos MJ and Taphoorn MJB: Prediagnostic
symptoms and signs of adult glioma: The patients' view. J
Neurooncol. 146:293–301. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Roda E and Bottone MG: Editorial: Brain
cancers: New perspectives and therapies. Front Neurosci.
16:8574082022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang Y, Hong W and Wei X: The molecular
mechanisms and therapeutic strategies of EMT in tumor progression
and metastasis. J Hematol Oncol. 15:1292022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chen H, Wu F, Xu H, Wei G, Ding M, Xu F,
Deivasigamani A, Zhou G, Hui KM and Xia H: Centromere protein F
promotes progression of hepatocellular carcinoma through ERK and
cell cycle-associated pathways. Cancer Gene Ther. 29:1033–1042.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chen H, Wang X, Wu F, Mo X, Hu C, Wang M,
Xu H, Yao C, Xia H and Lan L: Centromere protein F is identified as
a novel therapeutic target by genomics profile and contributing to
the progression of pancreatic cancer. Genomics. 113:1087–1095.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang YG, Li D, Wang L, Su XM and Tang XB:
CENPF/CDK1 signaling pathway enhances the progression of
adrenocortical carcinoma by regulating the G2/M-phase cell cycle. J
Transl Med. 20:782022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Han Y, Xu S, Cheng K, Diao C, Liu S, Zou W
and Bi Y: CENPF promotes papillary thyroid cancer progression by
mediating cell proliferation and apoptosis. Exp Ther Med.
21:4012021. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ren X, Chen X, Ji Y, Li L, Li Y, Qin C and
Fang K: Upregulation of KIF20A promotes tumor proliferation and
invasion in renal clear cell carcinoma and is associated with
adverse clinical outcome. Aging (Albany NY). 12:25878–25894. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang X, Li S, Gao W, Shi J, Cheng M, Mi
Y, Liu Y, Sang M, Li Z and Geng C: KIF20A is a prognostic marker
for female patients with estrogen receptor-positive breast cancer
and receiving tamoxifen as adjuvant endocrine therapy. Int J Gen
Med. 16:3623–3635. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun X, Chen P, Chen X, Yang W, Chen X,
Zhou W, Huang D and Cheng Y: KIF4A enhanced cell proliferation and
migration via Hippo signaling and predicted a poor prognosis in
esophageal squamous cell carcinoma. Thorac Cancer. 12:512–524.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jin W and Ye L: KIF4A knockdown suppresses
ovarian cancer cell proliferation and induces apoptosis by
downregulating BUB1 expression. Mol Med Rep. 24:5162021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Andrés-Sánchez N, Fisher D and Krasinska
L: Physiological functions and roles in cancer of the proliferation
marker Ki-67. J Cell Sci. 135:jcs258932022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hu X, Zhang H, Zheng X, Lin Z, Feng G,
Chen Y, Pan Q and Ni F: STMN1 and MKI67 are upregulated in uterine
leiomyosarcoma and are potential biomarkers for its diagnosis. Med
Sci Monit. 26:e9237492020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Meng X, Li W, Yuan H, Dong W, Xiao W and
Zhang X: KDELR2-KIF20A axis facilitates bladder cancer growth and
metastasis by enhancing Golgi-mediated secretion. Biol Proced
Online. 24:122022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Comba A, Faisal SM, Varela ML, Hollon T,
Al-Holou WN, Umemura Y, Nunez FJ, Motsch S, Castro MG and
Lowenstein PR: Uncovering spatiotemporal heterogeneity of
high-grade gliomas: From disease biology to therapeutic
implications. Front Oncol. 11:7037642021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pace A, Lombardi G, Villani V, Benincasa
D, Abbruzzese C, Cestonaro I, Corrà M, Cerretti G, Caccese M,
Silvani A, et al: Repurposing Chlorpromazine as add-on in the
adjuvant phase of first-line glioblastoma therapeutic protocol in
patients carrying hypo-/un-methylated MGMT gene promoter: RACTAC, a
phase II multicenter single arm clinical trial. medRxiv.
2023.2023.02. 21.23286088. 2023.
|
|
20
|
Jeanmougin M, Håvik AB, Cekaite L, Brandal
P, Sveen A, Meling TR, Ågesen TH, Scheie D, Heim S, Lothe RA and
Lind GE: Improved prognostication of glioblastoma beyond molecular
subtyping by transcriptional profiling of the tumor
microenvironment. Mol Oncol. 14:1016–1027. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gravendeel LA, Kouwenhoven MC, Gevaert O,
de Rooi JJ, Stubbs AP, Duijm JE, Daemen A, Bleeker FE, Bralten LB,
Kloosterhof NK, et al: Intrinsic Gene Expression Profiles of
Gliomas Are a Better Predictor of Survival than Histology. Cancer
Res. 69:9065–9072. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Berker Y, Muti IH and Cheng LL:
Visualizing metabolomics data with R. NMR Biomed. 36:e48652023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu CJ, Hu FF, Xie GY, Miao YR, Li XW,
Zeng Y and Guo AY: GSCA: An integrated platform for gene set cancer
analysis at genomic, pharmacogenomic and immunogenomic levels.
Brief Bioinform. 24:bbac5582023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li T, Fan J, Wang B, Traugh N, Chen Q, Liu
JS, Li B and Liu XS: TIMER: A web server for comprehensive analysis
of tumor-infiltrating immune cells. Cancer Res. 77:e108–e110. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ghouzlani A, Kandoussi S, Tall M, Reddy
KP, Rafii S and Badou A: Immune checkpoint inhibitors in human
glioma microenvironment. Front Immunol. 12:6794252021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Thul PJ and Lindskog C: The human protein
atlas: A spatial map of the human proteome. Protein Sci.
27:233–244. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Clark JA and Sun D: Guidelines for the
ethical review of laboratory animal welfare People's Republic of
China National Standard GB/T 35892-2018 [Issued 6 February 2018
Effective from 1 September 2018]. Animal Model Exp Med. 3:103–113.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kan LK, Drummond K, Hunn M, Williams D,
O'Brien TJ and Monif M: Potential biomarkers and challenges in
glioma diagnosis, therapy and prognosis. BMJ Neurol Open.
2:e0000692020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yang K, Wu Z, Zhang H, Zhang N, Wu W, Wang
Z, Dai Z, Zhang X, Zhang L, Peng Y, et al: Glioma targeted therapy:
Insight into future of molecular approaches. Mol Cancer. 21:1–32.
2022. View Article : Google Scholar
|
|
31
|
Bush NAO, Chang SM and Berger MS: Current
and future strategies for treatment of glioma. Neurosurg Rev.
40:1–14. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhuang Q, Yang H and Mao Y: The
oncogenesis of glial cells in diffuse gliomas and clinical
opportunities. Neurosci Bull. 39:393–408. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Escamilla-Ramírez A, Castillo-Rodríguez
RA, Zavala-Vega S, Jimenez-Farfan D, Anaya-Rubio I, Briseño E,
Palencia G, Guevara P, Cruz-Salgado A, Sotelo J and Trejo-Solís C:
Autophagy as a potential therapy for malignant glioma.
Pharmaceuticals (Basel). 13:1562020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Vicente JJ and Wordeman L: The
quantification and regulation of microtubule dynamics in the
mitotic spindle. Curr Opin Cell Biol. 60:36–43. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang J, An L, Zhao R, Shi R, Zhou X, Wei
S, Zhang Q, Zhang T, Feng D, Yu Z and Wang H: KIF4A promotes
genomic stability and progression of endometrial cancer through
regulation of TPX2 protein degradation. Mol Carcinog. 62:303–318.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hou PF, Jiang T, Chen F, Shi PC, Li HQ,
Bai J and Song J: KIF4A facilitates cell proliferation via
induction of p21-mediated cell cycle progression and promotes
metastasis in colorectal cancer. Cell Death Dis. 9:4772018.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hou G, Dong C, Dong Z, Liu G, Xu H, Chen
L, Liu L, Wang H and Zhou W: Upregulate kif4a enhances
proliferation, invasion of hepatocellular carcinoma and indicates
poor prognosis across human cancer types. Sci Rep. 7:41482017.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang H, Meng S, Chu K, Chu S, Fan YC, Bai
J and Yu ZQ: KIF4A drives gliomas growth by transcriptional
repression of Rac1/Cdc42 to induce cytoskeletal remodeling in
glioma cells. J Cancer. 13:3640–3651. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wu WD, Yu KW, Zhong N, Xiao Y and She ZY:
Roles and mechanisms of Kinesin-6 KIF20A in spindle organization
during cell division. Eur J Cell Biol. 98:74–80. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Qiu R, Wu J, Gudenas B, Northcott PA,
Wechsler-Reya RJ and Lu Q: Depletion of kinesin motor KIF20A to
target cell fate control suppresses medulloblastoma tumour growth.
Commun Biol. 4:5522021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Taniuchi K, Nakagawa H, Nakamura T, Eguchi
H, Ohigashi H, Ishikawa O, Katagiri T and Nakamura Y:
Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with
membrane trafficking of discs large homologue 5, can attenuate
growth of pancreatic cancer cell. Cancer Res. 65:105–112. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gasnereau I, Boissan M, Margall-Ducos G,
Couchy G, Wendum D, Bourgain-Guglielmetti F, Desdouets C, Lacombe
ML, Zucman-Rossi J and Sobczak-Thépot J: KIF20A mRNA and its
product MKlp2 are increased during hepatocyte proliferation and
hepatocarcinogenesis. Am J Pathol. 180:131–140. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yan GR, Zou FY, Dang BL, Zhang Y, Yu G,
Liu X and He QY: Genistein-induced mitotic arrest of gastric cancer
cells by downregulating KIF20A, a proteomics study. Proteomics.
12:2391–2399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Marin-Acevedo JA, Dholaria B, Soyano AE,
Knutson KL, Chumsri S and Lou Y: Next generation of immune
checkpoint therapy in cancer: New developments and challenges. J
Hematol Oncol. 11:392018. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Fujiwara Y, Okada K, Omori T, Sugimura K,
Miyata H, Ohue M, Kobayashi S, Takahashi H, Nakano H, Mochizuki C,
et al: Multiple therapeutic peptide vaccines for patients with
advanced gastric cancer. Int J Oncol. 50:1655–1662. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Copello VA and Burnstein KL: The kinesin
KIF20A promotes progression to castration-resistant prostate cancer
through autocrine activation of the androgen receptor. Oncogene.
41:2824–2832. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Han L and Wang J: Bioinformatics Analysis
of KIF20A, a Potential Therapeutic Target for Glioblastoma.
Accepted at. January 5–2021. View Article : Google Scholar
|
|
48
|
Verheijen R, Kuijpers HJ, Schlingemann RO,
Boehmer AL, van Driel R, Brakenhoff GJ and Ramaekers FC: Ki-67
detects a nuclear matrix-associated proliferation-related antigen.
I. Intracellular localization during interphase. J Cell Sci.
92:123–130. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Schlüter C, Duchrow M, Wohlenberg C,
Becker MH, Key G, Flad HD and Gerdes J: The cell
proliferation-associated antigen of antibody Ki-67: A very large,
ubiquitous nuclear protein with numerous repeated elements,
representing a new kind of cell cycle-maintaining proteins. J Cell
Biol. 123:513–522. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Duchrow M, Schlüter C, Wohlenberg C, Flad
HD and Gerdes J: Molecular characterization of the gene locus of
the human cell proliferation-associated nuclear protein defined by
monoclonal antibody Ki-67. Cell Prolif. 29:1–12. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Gerdes J, Lemke H, Baisch H, Wacker HH,
Schwab U and Stein H: Cell cycle analysis of a cell
proliferation-associated human nuclear antigen defined by the
monoclonal antibody Ki-67. J Immunol. 133:1710–1715. 1984.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Xiong DD, Zeng CM, Jiang L, Luo DZ and
Chen G: Ki-67/MKI67 as a predictive biomarker for clinical outcome
in gastric cancer patients: An updated meta-analysis and systematic
review involving 53 studies and 7078 Patients. J Cancer.
10:5339–5354. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Zeng S, Li W, Ouyang H, Xie Y, Feng X and
Huang L: A novel prognostic pyroptosis-related gene signature
correlates to oxidative stress and immune-related features in
gliomas. Oxid Med Cell Longev. 31:42561162023.PubMed/NCBI
|
|
54
|
Visapää H, Bui M, Huang Y, Seligson D,
Tsai H, Pantuck A, Figlin R, Rao JY, Belldegrun A, Horvath S and
Palotie A: Correlation of Ki-67 and gelsolin expression to clinical
outcome in renal clear cell carcinoma. Urology. 61:845–850. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zini L, Porpiglia F and Fassnacht M:
Contemporary management of adrenocortical carcinoma. Eur Urol.
60:1055–1065. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Nakanishi K, Sakamoto M, Yamasaki S, Todo
S and Hirohashi S: Akt phosphorylation is a risk factor for early
disease recurrence and poor prognosis in hepatocellular carcinoma.
Cancer. 103:307–312. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Guzman G, Alagiozian-Angelova V,
Layden-Almer JE, Layden TJ, Testa G, Benedetti E, Kajdacsy-Balla A
and Cotler SJ: p53, Ki-67, and serum alpha feto-protein as
predictors of hepatocellular carcinoma recurrence in liver
transplant patients. Mod Pathol. 18:1498–1503. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li R, Wang X, Zhao X, Zhang X, Chen H, Ma
Y and Liu Y: Centromere protein F and Forkhead box M1 correlation
with prognosis of non-small cell lung cancer. Oncol Lett.
19:1368–1374. 2020.PubMed/NCBI
|
|
59
|
Aytes A, Mitrofanova A, Lefebvre C,
Alvarez MJ, Castillo-Martin M, Zheng T, Eastham JA, Gopalan A,
Pienta KJ, Shen MM, et al: Cross-species regulatory network
analysis identifies a synergistic interaction between FOXM1 and
CENPF that drives prostate cancer malignancy. Cancer Cell.
25:638–651. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Sun J, Huang J, Lan J, Zhou K, Gao Y, Song
Z, Deng Y, Liu L, Dong Y and Liu X: Overexpression of CENPF
correlates with poor prognosis and tumor bone metastasis in breast
cancer. Cancer Cell Int. 19:2642019. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Dai Y, Liu L, Zeng T, Zhu YH, Li J, Chen
L, Li Y, Yuan YF, Ma S and Guan XY: Characterization of the
oncogenic function of centromere protein F in hepatocellular
carcinoma. Biochem Biophys Res Commun. 436:711–718. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Zhang M, Zhang Q, Bai J, Zhao Z and Zhang
J: Transcriptome analysis revealed CENPF associated with glioma
prognosis. Math Biosci Eng. 18:2077–2096. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Brabletz S, Schuhwerk H, Brabletz T and
Stemmler MP: Dynamic EMT: A multi-tool for tumor progression. EMBO
J. 40:e1086472021. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Zhang G, Feng W and Wu J: Down-regulation
of SEPT9 inhibits glioma progression through suppressing
TGF-β-induced epithelial-mesenchymal transition (EMT). Biomed
Pharmacother. 125:1097682020. View Article : Google Scholar : PubMed/NCBI
|