Introduction
Digestive tract cancers, including esophageal,
gastric and colorectal cancer, are a type of prevalent malignant
tumor, characterized by high mortality rates worldwide (1). As reported in the Global Cancer
Statistics for 2020, there were 19.3 million new cases of cancer
and 10 million deaths linked to the disease. Within these
statistics, colorectal cancer has the third-highest incidence rate
at 10%, while it is the second leading cause of cancer-related
deaths at 9.4%. Gastric cancer, on the other hand, ranks fifth in
incidence at 5.6% and fourth in mortality rate at 7.7% (2). According to the World Health
Organization classification of tumors, tumors of the digestive
system encompass several types of cancer, including esophageal,
gastric, small intestinal, hepatocellular, gallbladder,
cholangiocarcinoma, pancreatic and colorectal cancer (3). Gastrointestinal cancer exhibits one of
the highest incidence and mortality rates among all types of cancer
(4), and is a leading cause of
cancer-related mortality (5). In
addition, gastric cancer (GC) is a particularly aggressive type of
malignancy, which originates from gastric mucosal epithelial cells
(6). As one of the most prevalent
malignant tumors of the digestive tract, GC is also characterized
by a very high invasive capacity (7). Therefore, its incidence and mortality
rates are very high worldwide, accounting for ~10% of all
cancer-related deaths, thus posing a serious threat to global human
health (8–12). Despite the decline in GC incidence
and progress in the development of novel treatment approaches, the
mortality rate among patients with GC remains high (13,14).
Therefore, further methods for predicting prognosis and identifying
novel biomarkers to improve patient outcomes are urgently
needed.
D-dimer, a fibrin degradation product, serves as a
conventional marker of clotting, with enhanced levels indicating a
hypercoagulable state (15,16). Emerging evidence has suggested that
tumor cells can release procoagulants or fibrinolytic substances,
thus attracting platelets and promoting tumor progression via the
excessive activation of coagulation (17–19).
Previous studies demonstrated that heightened D-dimer levels were
associated with a poor prognosis in several types of cancer
including lung, colorectal, pancreatic cancer and gastric cancer,
and more particularly in GC (20–22).
Due to the visibility and convenience of measuring D-dimers, Guan
et al (23) highlighted the
significance of D-dimer monitoring in patients with cancer,
including those with GC, as a reliable predictor of
thromboembolism. Other studies further supported the association
between high D-dimer levels and unfavorable outcomes in patients
with GC (24–27). However, a propensity matching
analysis performed by Liang et al (28) indicated that preoperative D-dimer
elevation was not an independent prognostic factor for GC.
Therefore, the present study aimed to investigate the association
between preoperative D-dimer levels and long-term postoperative
survival in patients with GC. To enhance the reliability of the
findings, previous research was expanded upon (24–27) by
incorporating additional covariates associated with prognosis and
survival, such as smoking and drinking history, hypertension,
diabetes, cardio-cerebral-renal diseases and immune-related markers
specific to GC. Furthermore, non-linear associations, beyond the
traditional linear analysis, were assessed. To evaluate the
potential effect of the baseline characteristics of patients with
GC on D-dimer prognostic assessments, patients who had undergone
radical cancer surgery were randomly selected and their
postoperative outcomes were assessed using a follow-up system. To
minimize bias, the data were subjected to univariate and
multivariate Cox regression analysis. A fitting curve was also
conducted, and inflection point analysis was performed to determine
the optimal cut off value.
Materials and methods
Study design
The target independent variable was preoperative
D-dimer level obtained at baseline. The dependent variable was
5-year overall survival (OS) period (1=death; 0=survive).
Study population
The data from randomly selected patients with GC
treated between January 24 and February 15, 2017 in Shanxi Province
Cancer Hospital were collected. Most patients underwent surgery
within 1 month of admission. To select patients who met the
inclusion criteria, the authors had free access to their data.
However, to ensure the privacy of the participants, following the
establishment of the database, their names were immediately
deleted. Therefore, no one, not even the authors, could ever
identify each patient in the database. The inclusion criteria were
as follows: i) Patients hospitalized in 2017; ii) who underwent
radical treatment and R0 resection; iii) who were diagnosed with GC
via postoperative pathological examination; iv) who had stage I–III
disease, in accordance with the TNM staging system established by
the American Joint Committee on Cancer (AJCC 7th ed., 2010)
(29); and v) who were aged ≥18
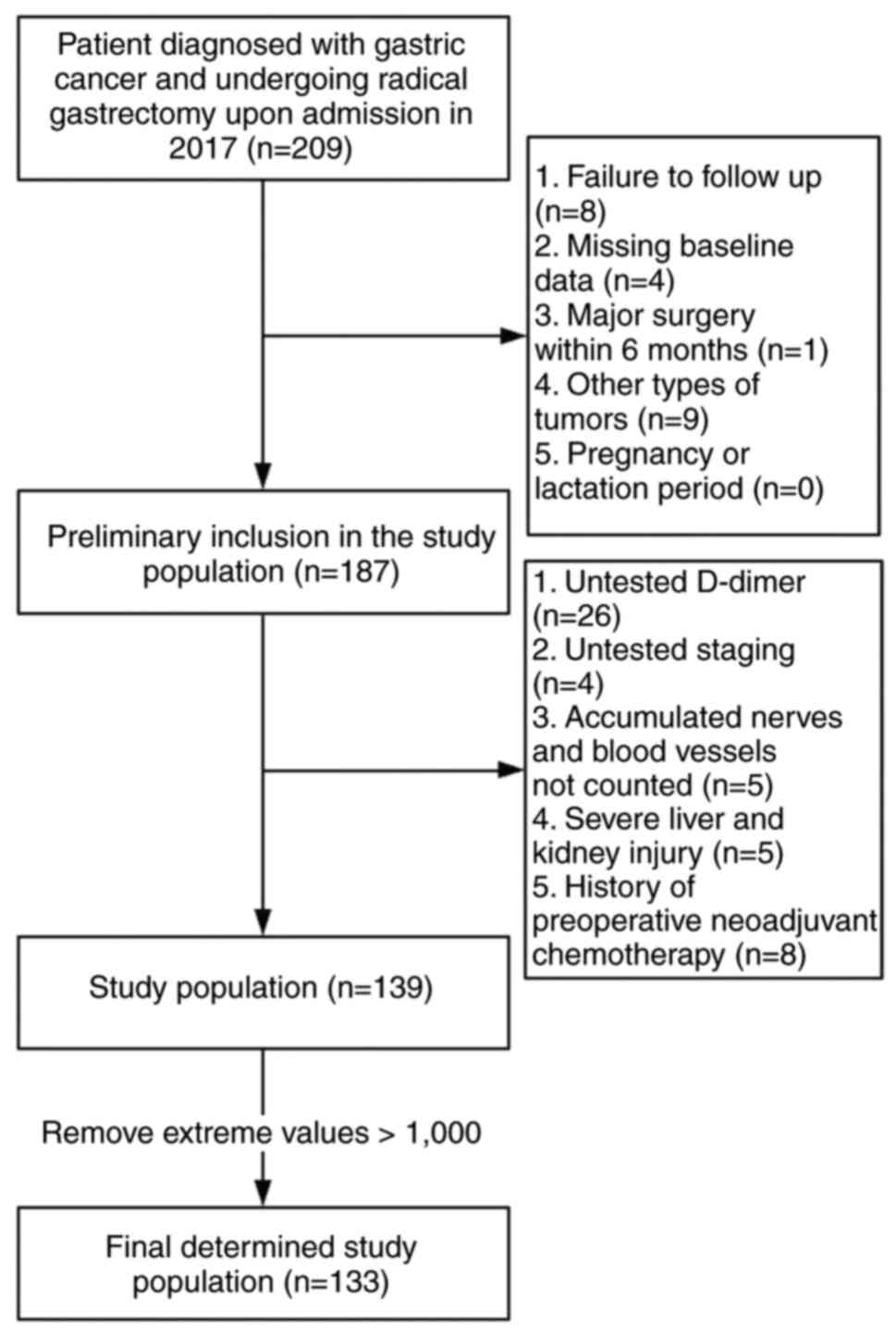
years old. The exclusion criteria were the following: i) Patients
who underwent major surgery within 6 months of the diagnosis; ii)
who failed to undergo follow-up; iii) with first preoperative data
in an external hospital; iv) with missing D-dimer levels and
baseline data; v) with unspecified staging; vi) unspecified
accumulation of nerves and vessels; vii) suffering from other types
of cancer; viii) with severe liver and kidney injury; ix) with a
history of preoperative neoadjuvant chemotherapy and x) pregnant or
breastfeeding patients (Fig.
1).
Variables
The preoperative D-dimer levels were considered as
the baseline levels. The first D-dimer levels (ng/ml) were measured
and recorded after hospitalization. Based on the published
guidelines and previous studies, the final outcome variables, such
as 5-year OS rate, were assessed. Survival time was recorded in
months. The selection criteria for confounding factors (covariates)
were as follows: i) Demographic data; ii) variables reported in the
previous literature that could affect preoperative D-dimer levels
or 5-year OS rate; and iii) those based on clinical experience (age
and smoking history, drinking history, hypertension history and
diabetes history). Therefore, the following variables were used to
construct the fully-adjusted model: i) Continuous variables,
including age, and carbohydrate antigen (CA) 50, CA199, CA724,
tissue polypeptide antigen and tumor-specific growth factor levels;
and ii) categorical variables, including severe cardiovascular and
cerebrovascular diseases, tumor site, Lauren classification,
Tumor-Node-Metastasis (TNM) classification, staging invasion of
vasculature and nerves, and postoperative neoadjuvant chemotherapy,
in accordance with the TNM staging system established by the
American Joint Committee on Cancer (AJCC 7th ed., 2010) (29) (Table
I).
 | Table I.Baseline data of variables. |
Table I.
Baseline data of variables.
| Variables | Total (n=133) | D-dimer_low
(n=66) | D-dimer_high
(n=67) | P-value |
|---|
| Mean age ± SD,
years | 60.3±9.2 | 57.4±9.1 | 63.1±8.5 | <0.001 |
| Sex |
|
|
| 0.037 |
|
Female | 28 (21.1) | 9 (13.6) | 19 (28.4) |
|
| Male | 105 (78.9) | 57 (86.4) | 48 (71.6) |
|
| Smoking status |
|
|
| 0.099 |
|
Non-smoker | 76 (57.1) | 33 (50.0) | 43 (64.2) |
|
|
Smoker | 57 (42.9) | 33 (50.0) | 24 (35.8) |
|
| Hypertension |
|
|
| 0.446 |
|
Presence | 101 (75.9) | 52 (78.8) | 49 (73.1) |
|
|
Absence | 32 (24.1) | 14 (21.2) | 18 (26.9) |
|
| Diseases |
|
|
| 0.037 |
|
Presence | 105 (78.9) | 57 (86.4) | 48 (71.6) |
|
|
Absence | 28 (21.1) | 9 (13.6) | 19 (28.4) |
|
| Site |
|
|
| 0.778 |
|
Proximal | 75 (56.4) | 36 (54.5) | 39 (58.2) |
|
| Gastric
body | 25 (18.8) | 14 (21.2) | 11 (16.4) |
|
|
Distal | 33 (24.8) | 16 (24.2) | 17 (25.4) |
|
| Lauren
classification |
|
|
| 0.336 |
|
Intestinal | 16 (28.6) | 9 (33.3) | 7 (24.1) |
|
|
Diffuse | 20 (35.7) | 11 (40.7) | 9 (31.0) |
|
| Mixed
type | 20 (35.7) | 7 (25.9) | 13 (44.8) |
|
| T stage |
|
|
| 0.037 |
| T1 | 15 (11.3) | 9 (13.6) | 6 (9.0) |
|
| T2 | 12 (9.0) | 10 (15.2) | 2 (3.0) |
|
| T3 | 40 (30.1) | 15 (22.7) | 25 (37.3) |
|
| T4 | 66 (49.6) | 32 (48.5) | 34 (50.7) |
|
| N stage |
|
|
| 0.012 |
| N0 | 38 (28.6) | 22 (33.3) | 16 (23.9) |
|
| N1 | 28 (21.1) | 17 (25.8) | 11 (16.4) |
|
| N2 | 25 (18.8) | 15 (22.7) | 10 (14.9) |
|
| N3 | 42 (31.6) | 12 (18.2) | 30 (44.8) |
|
| TNM stage |
|
|
| 0.07 |
| I | 14 (10.5) | 11 (16.7) | 3 (4.5) |
|
| II | 44 (33.1) | 21 (31.8) | 23 (34.3) |
|
|
III | 75 (56.4) | 34 (51.5) | 41 (61.2) |
|
| Nerve
involvement |
|
|
| 0.186 |
|
Negative | 75 (56.4) | 41 (62.1) | 34 (50.7) |
|
|
Positive | 58 (43.6) | 25 (37.9) | 33 (49.3) |
|
| Vessel
involvement |
|
|
| 0.323 |
|
Negative | 79 (59.4) | 42 (63.6) | 37 (55.2) |
|
|
Positive | 54 (40.6) | 24 (36.4) | 30 (44.8) |
|
| Postoperative
chemotherapy |
|
|
| 0.068 |
|
Negative | 65 (48.9) | 27 (40.9) | 38 (56.7) |
|
|
Positive | 68 (51.1) | 39 (59.1) | 29 (43.3) |
|
| Antithrombin-III,
% | 106.7±14.5 | 107.0±14.1 | 106.4±14.9 | 0.806 |
| HER-2 |
|
|
| 0.265 |
|
Negative | 51 (86.4) | 20 (80.0) | 31 (91.2) |
|
|
Positive | 8 (13.6) | 5 (20.0) | 3 (8.8) |
|
| TSGF (pg/ml) | 60.0±7.5 | 57.4±5.6 | 62.1±8.1 | 0.002 |
| VEGF (pg/ml) | 468.4±333.3 | 402.2±281.6 | 532.1±370.6 | 0.166 |
| sIL-2R (U/ml) | 334.8±80.8 | 331.9±87.4 | 337.2±75.7 | 0.759 |
| AFP (ug/l) | 4.6±13.7 | 6.7±19.6 | 2.6±2.2 | 0.123 |
| CEA (ug/l) | 5.2±12.7 | 3.7±10.5 | 6.7±14.5 | 0.186 |
| CA242, U/ml) | 25.8±58.8 | 15.9±35.7 | 35.6±74.2 | 0.063 |
| CA724 (U/ml) | 10.0±24.7 | 5.6±11.2 | 14.4±32.7 | 0.046 |
| CA50 (U/ml) | 2.6±6.5 | 1.2±1.7 | 3.9±8.8 | 0.043 |
| CA199 (U/ml) | 42.7±72.9 | 29.9±46.4 | 55.8±90.9 | 0.048 |
| TPS (U/l) | 107.6±84.7 | 112.3±92.9 | 103.1±76.7 | 0.59 |
| TPA (ng/ml) | 1.0±3.7 | 0.6±1.2 | 1.3±5.1 | 0.357 |
| 5-year OS |
|
|
| 0.011 |
|
Survival | 72 (54.1) | 43 (65.2) | 29 (43.3) |
|
|
Death | 61 (45.9) | 23 (34.8) | 38 (56.7) |
|
Follow-up procedure
Follow-up was conducted according to Shanxi Province
Cancer Hospital's follow-up system. The follow-up period was 5
years, and the follow-up frequency is once a month. The cutoff date
for follow-up was December 2022.
Statistical analysis
Mean value of the continuous variable D-dimer was
used to generate a binary categorical variable (D-dimer_high and
D-dimer_low groups. The remaining categorical variables are
expressed as frequency or percentage. The differences between the
D-dimer_high and D-dimer_low groups were compared using the
χ2 test for categorical variables, and the unpaired
t-test and Mann-Whitney U test for continuous variables with normal
and skewed distribution, respectively. Sensitivity analysis was
performed to ensure the robustness of the data analysis. The
continuous (D-dimer level) and binary (D-dimer_high and
D-dimer_low) variables were assessed to verify the consistent
significance of their effect values and identify potential
non-linearity. In the multivariate analysis of the continuous
variables, D-dimer levels were multiplied by 0.1 to enhance their
significance (Table II). Therefore, univariate and
multivariate Cox proportional hazard models were employed. A total
of three models were constructed. Model 1, which was adjusted for
age and diseases, model 2, which was adjusted for the variables of
model 1 plus tumor-related description features, and model 3, which
was adjusted for the variables of model 2 plus other influential
covariates (Table III). To address the non-linearity of the
preoperative D-dimer levels and 5-year OS rates, a generalized
additive model and smooth curve fitting (penalized spline method)
were performed (Fig. 2). When
non-linearity was obtained, the inflection point was first
calculated using a recursive algorithm, and a two-piecewise Cox
proportional hazard model on both sides of the inflection point was
then constructed (Table IV). Furthermore, to more
intuitively display the curve association of its existence,
Kaplan-Meier (K-M) survival curves at the 2nd and 5th percentiles
were plotted, the cutting standards were a mean of 2 and 5 equal
parts, respectively (Figs. 3 and
4). All analyses were performed
using R Statistical Software (http://www.R-project.org, The R Foundation) and Free
Statistics analysis platform (FreeClinical Medical Technology Co.,
Ltd.). A two-tailed test was performed and P<0.05 was considered
to indicate a statistically significant difference. Due to missing
values in some covariates, to analyze the data more accurately
without adding new ones or reducing sample size, the dummy variable
imputation method was applied.
Results
Baseline characteristics of the
selected participants
A total of 133 participants were included in the
final data analysis (Fig. 1). The
baseline characteristics of participants allocated to the
D-dimer_high and D-dimer_low groups are listed in Table I. The mean age of the selected
participants was 60.3±9.2 years (range, 34–85 years old, with 78.9%
being male. To ensure consistency in the high group and result
stability, due to the limited sample size, extreme values of
>1,000 ng/ml were excluded.
Univariate analysis
The results of the univariate analysis are shown in
Table II. The preliminary results
revealed that the effect value of D-dimer levels as a binary
variable was significantly greater compared with that obtained from
D-dimer levels as a continuous variable. The aforementioned
preliminary findings suggested that there was a curved association
between these variables. In the current analysis, as many and
appropriate covariates as possible were included in the multi-model
and multi-factor analysis (Tables I
and II). Covariates included in
Cox multivariate analysis and curve fitting were determined as age,
presence of disease, site, Lauren classification, T stage, N stage,
stating, nerve involvement, vessel involvement, postoperative
chemotherapy, TPA, CA50, CA199, CA724 and TSGF.
 | Table II.Cox single factor regression
analysis. |
Table II.
Cox single factor regression
analysis.
| Variables | HR (95% CI) | P-value |
|---|
| D-dimer*0.1 (cont.
var.) | 1.02
(1.00–1.03) | 0.017 |
| D-dimer_cut: High
vs. Low | 2.07
(1.23–3.48) | 0.006 |
| Age | 1.04
(1.01–1.07) | 0.017 |
| Sex: Male vs.
female | 1.12
(0.59–2.10) | 0.734 |
| Smoking status:
Smoker vs. non-smoker | 0.65
(0.38–1.10) | 0.108 |
| Hypertension:
Presence vs. absence | 1.32
(0.76–2.29) | 0.326 |
| Diseases: Presence
vs. absence | 1.50
(0.85–2.62) | 0.159 |
| Site |
| 0.992 |
|
Proximal | Reference |
|
| Gastric
body | 0.96
(0.49–1.88) | 0.898 |
|
Distal | 0.99
(0.54–1.81) | 0.972 |
| Lauren
classification |
| 0.147 |
|
Intestinal | Reference |
|
|
Diffuse | 0.39
(0.15–1.02) | 0.054 |
| Mixed
type | 0.61
(0.26–1.44) | 0.26 |
| T stage |
| 0.008 |
| T1 | Reference |
|
| T2 | 1.36
(0.27–6.75) | 0.705 |
| T3 | 2.18
(0.63–7.47) | 0.217 |
| T4 | 3.96
(1.22–12.84) | 0.022 |
| N stage |
| <0.001 |
| N0 | Reference |
|
| N1 | 1.43
(0.54–3.80) | 0.478 |
| N2 | 3.29
(1.38–7.85) | 0.007 |
| N3 | 5.81
(2.66–12.69) | <0.001 |
| TNM stage |
| <0.001 |
| I | Reference |
|
| II | 2.57
(0.59–11.25) | 0.210 |
|
III | 5.55
(1.35–22.93) | 0.018 |
| Nerve: Invasion vs.
not | 2.65
(1.59–4.42) | <0.001 |
| Vessel: Invasion
vs. not | 2.78
(1.66–4.64) | <0.001 |
| Postoperative
chemotherapy: | 0.72
(0.43–1.19) | 0.200 |
| Treatment vs.
not |
|
|
| Antithrombin
III | 0.99
(0.97–1.01) | 0.390 |
| HER2: 1 vs. 0 | 0.97
(0.37–2.51) | 0.943 |
| TSGF | 1.02
(0.98–1.06) | 0.285 |
| VEGF | 1.00
(0.99–1.00) | 0.566 |
| sIL2R | 0.99
(0.99–1.00) | 0.079 |
| AFP | 0.99
(0.97–1.02) | 0.737 |
| CEA | 1.01
(0.99–1.02) | 0.205 |
| CA242 | 1.00
(0.99–1.01) | 0.156 |
| CA72.4 | 1.01
(1.00–1.02) | 0.072 |
| CA50 | 1.03
(1.00–1.05) | 0.058 |
| CA199 | 1.00
(0.99–1.01) | 0.087 |
| TPS | 1.00
(0.99–1.00) | 0.954 |
| TPA | 1.10
(1.03–1.17) | 0.003 |
Multi-model and multi-factor
analysis
In the present study, three models were constructed
to analyze the independent effects of preoperative D-dimer levels
on the 5-year OS rate using a multivariate Cox proportional hazard
model. The effect sizes [hazard ratio (HR) and 95% confidence
interval (CI)] of D-dimer levels as a binary and continuous
variable in each model are listed in Table III. In model 1, when D-dimer
levels served as a continuous variable, the effect size indicated
that a one-unit change in preoperative D-dimer levels was
associated with the risk of death. Conversely, when D-dimer levels
were used as a binary variable, the effect size suggested that
there was an increased risk of mortality in the D-dimer_High group
compared with the D-dimer_Low group. For example, the effect size
of the 5-year OS rate in model 1 (D-dimer levels, continuous
variable) showed that a one-unit change in preoperative D-dimer
levels was associated with a change in the risk of death (HR=1.01;
95% CI, 1.00–1.03), however this was not significant. Similarly,
the effect size of the 5-year OS rate in model 1 (D-dimer levels,
binary variable) revealed an association between the mortality risk
and D-dimer levels (low and high D-dimer groups; HR=1.74; 95 CI,
1.00–3.01). In model 2, when D-dimer levels served as a continuous
variable, a one-unit increase in preoperative D-dimer levels was
associated with an increased mortality risk (HR=1.01; 95% CI,
1.00–1.03), however this was not significant. On the other hand,
when D-dimer levels served as binary variable in the same model, a
greater mortality risk was obtained in the D-dimer_high group
compared with the D-dimer_low group (HR=1.67; 95% CI, 0.93–2.98),
however this was not significant. In the fully adjusted model,
which was adjusted for as many covariates as possible within an
appropriate range (model 3), there was no statistical significance
in the linear associations among the different covariates.
 | Table III.Cox multivariate regression
analysis. |
Table III.
Cox multivariate regression
analysis.
| A, D-dimer levels
as binary variables |
|---|
|
|---|
| Model | Variable | Total patients,
n | Events, n (%) | Follow-up time,
days | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI) | Adjusted
P-value |
|---|
| Model 1 | D-dimer_low | 66 | 23 (34.8) | 3,166 | 1 (Reference) | | 1 (Reference) |
|
|
| D-dimer_high | 67 | 38 (56.7) | 2,405 | 2.07
(1.23–3.48) | 0.006 | 1.74
(1.00–3.01) | 0.048 |
| Model 2 | D-dimer_low | 66 | 23 (34.8) | 3,166 | 1 (Reference) | | 1 (Reference) |
|
|
| D-dimer_high | 67 | 38 (56.7) | 2,405 | 2.07
(1.23–3.48) | 0.006 | 1.67
(0.93–2.98) | 0.083 |
| Model 3 | D-dimer_low | 66 | 23 (34.8) | 3,166 | 1 (Reference) | | 1 (Reference) |
|
|
| D-dimer_high | 67 | 38 (56.7) | 2,405 | 2.07
(1.23–3.48) | 0.006 | 1.55
(0.81–3.00) | 0.188 |
|
| B, D-dimer
levels as continuous variables |
|
| Model |
Variable | Total patients,
n | Events, n
(%) | Follow-up time,
days | Crude HR (95%
CI) | Crude
P-value | Adjusted HR (95%
CI) | Adjusted
P-value |
|
| Model 1 | D-dimer*0.1 | 133 | 61 (45.9) | 5,571 | 1.02
(1.00–1.03) | 0.017 | 1.01
(1.00–1.03) | 0.082 |
| Model 2 | D-dimer*0.1 | 133 | 61 (45.9) | 5,571 | 1.02
(1.00–1.03) | 0.017 | 1.01
(1.00–1.03) | 0.179 |
| Model 3 | D-dimer*0.1 | 133 | 61 (45.9) | 5,571 | 1.02
(1.00–1.03) | 0.017 | 1.01
(0.99–1.03) | 0.485 |
Non-linearity of preoperative D-dimer
levels and 5-year OS rate
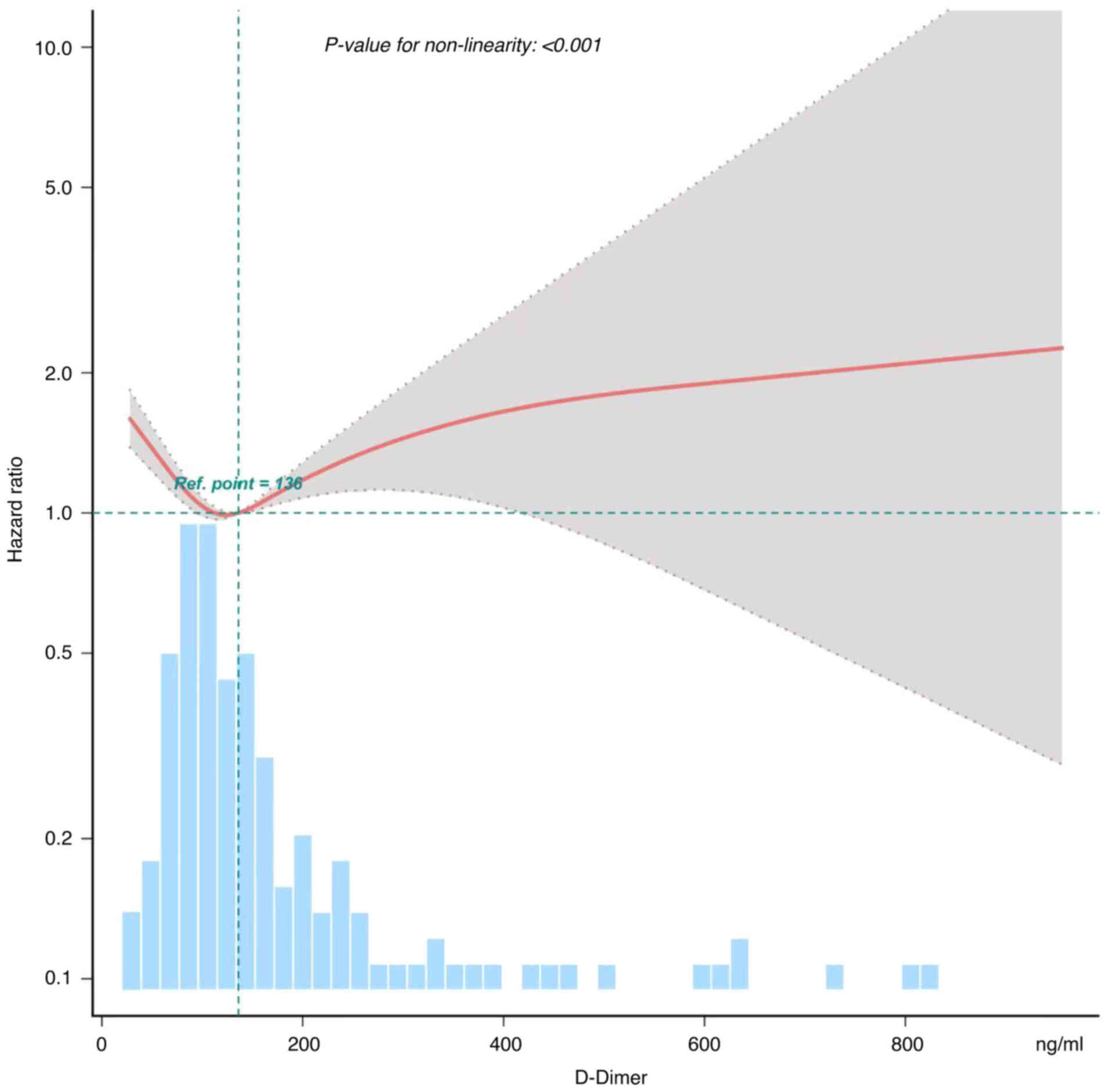
In the present study, the non-linear association
between preoperative D-dimer levels and 5-year OS rate was also
assessed (Fig. 2). The smooth curve
and the results from the Generalized Additive Model revealed a
non-linear association between preoperative D-dimer levels and
5-year OS rate, even after adjusting for different variables, such
as sex, diseases, tumor site, T stage, N stage, TNM staging, Lauren
classification, nerve involvement, vessel involvement,
postoperative chemotherapy and various tumor markers. Both a Cox
proportional hazard model and a two-piecewise Cox proportional
hazard model were utilized to analyze the association. The best
model was selected based on the P-value of the log likelihood-ratio
test. As P-value of <0.05 was obtained, the two-piecewise Cox
proportional hazard model was selected. This model could more
accurately capture the association. From Fig. 2, it can be observed that there is a
U-shaped curve association between preoperative D-dimer levels and
prognosis of gastric cancer. Both excessively high and low levels
of D-dimer suggest lower 5-year survival rates in patients, further
indicating a poor prognosis for gastric cancer. Through this model
and a recursive algorithm, an inflection point of 110.449 was
calculated. The effect size on the left and right sides of the
inflection point was 0.451 and 1.0036, respectively (Table IV).
 | Table IV.Table data format for inflection
point analysis in Fig. 2. |
Table IV.
Table data format for inflection
point analysis in Fig. 2.
| Item | BK.HR | P-value |
|---|
| E_BK1 | 110.449
(106.956–113.942) |
|
| Slope 1 | 0.451
(0.429–0.474) | <0.001 |
| Slope 2 | 1.004
(0.997–1.011) | 0.3128 |
| Likelihood ratio
test | - | 0.002 |
| Non-linear
test*1 | - | 0.006 |
| Non-linear
test*2 | - | <0.001 |
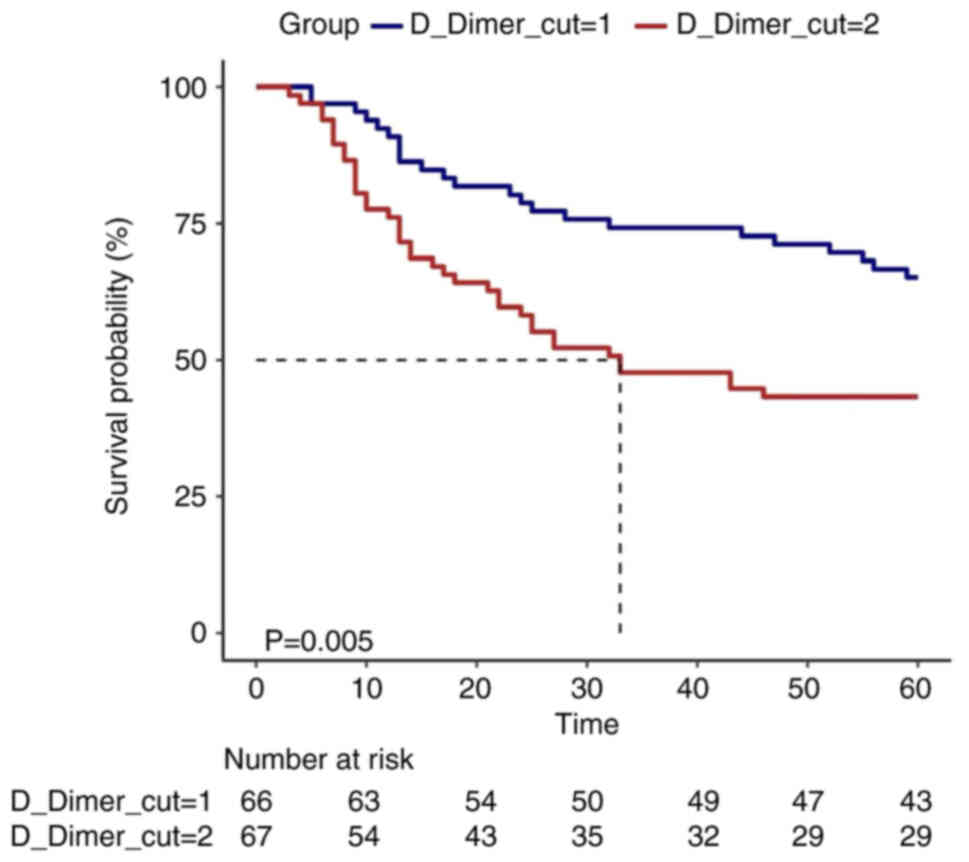
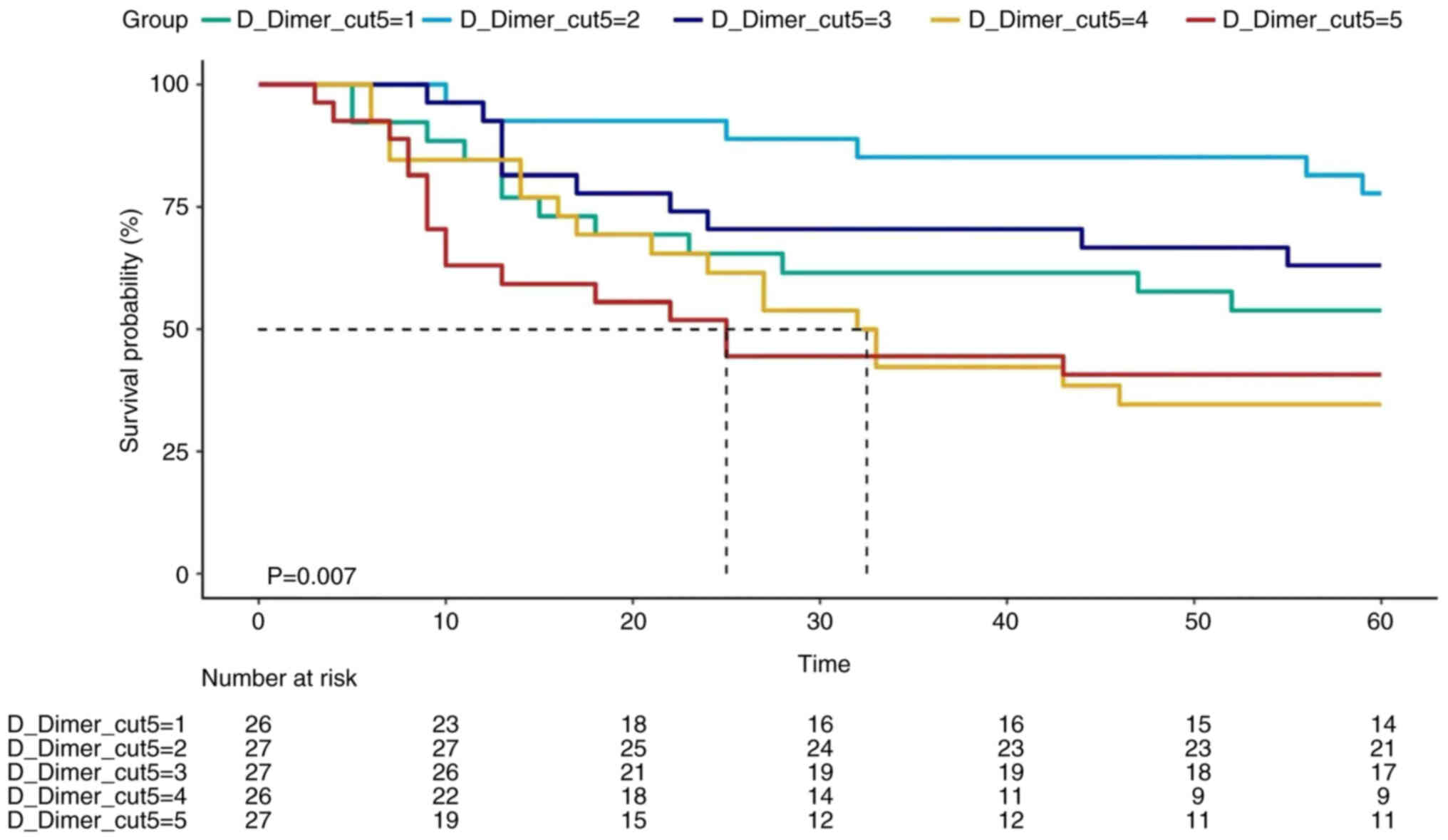
K-M survival curves
To represent the association between D-dimer levels
and survival, K-M survival curves were plotted by dividing
distribution at two and five equal parts. K-M survival curve
indicated that the survival time was longer in the D-dimer_high
group compared with that in the D-dimer_low group. This finding was
consistent with that reported in a previous study (27). However, D-dimer_lowest group ranked
third in D-dimer survival, thus clearly supporting the presence of
a curved association (Figs. 3 and
4).
Discussion
It is widely accepted that several factors can
affect the association between D-dimer levels and GC prognosis.
Therefore, the results have always been controversial. Based on the
risk of biases, in the present study, several measures were taken
to obtain more rigorous results. The preoperative D-dimer levels,
at initial admission, were considered as the baseline levels.
Therefore, to avoid the effect of different surgical methods and
types on D-dimer levels, these levels were not determined
postoperatively. Regarding the research methodology, multiple
factor and multiple model regression analyses were performed by
incorporating as many covariates as possible. Therefore, the
presence of similar results among different models was
verified.
The results of the current study revealed a positive
association between preoperative D-dimer levels and 5-year OS rate,
even after adjusting for other covariates. Therefore, it was
hypothesized that preoperative D-dimer levels could exploit the
strong invasive capacity of GC and its susceptibility to
metastasis, thus further suggesting that blood D-dimer levels could
predict tumor status. Notably, the results also showed that the
effect sizes on the left and right sides of the inflection point
were not consistent [left, 0.451 (0.429–0.474); right, 1.0036
(0.9967–1.0105)]. The aforementioned findings indicated a U-shaped
independent association between preoperative D-dimer levels and
5-year OS rate. Therefore, different effects were obtained in
different intervals. More particularly, preoperative D-dimer levels
of <100 ng/ml and >200 ng/ml were associated with worse and
better prognosis in GC, respectively. The aforementioned results
not only indicated that preoperative D-dimer levels were an
independent risk factor for GC prognosis within a specific
interval, but also supported that the malignant status of a tumor
could not simply directly associated with the coagulation status of
the blood, since low D-dimer levels were also associated with poor
tumor prognosis. In fact, this finding is also consistent with the
clinical work experience, since patients with GC commonly first
experience hypercoagulability, followed by hypocoagulability and
bleeding. This observation further suggested that in patients with
GC, preoperative D-dimer levels could not be necessarily lower
compared with those in healthy patients. According to existing
confirmed tumor physiology, patients with advanced gastric cancer
typically experience more severe tumor infiltration depth and
vascular invasion, which may form cancer emboli and further lead to
hypercoagulable blood (30–32). However, the low coagulation state
that occurs after high blood coagulation cannot be explained by
existing research. We speculate that this may be due to the
long-term hypercoagulability of blood in late-stage tumors, which
leads to a large consumption of platelets and procoagulant factors.
By contrast, anticoagulant factors will increase compensatorily,
causing the blood to gradually transition from hypercoagulability
to hypercoagulability. Therefore, in clinical practice, further
decisions should be made based on a comprehensive evaluation of the
actual staging of the tumor, and vigilance should be maintained in
the face of low coagulation after high coagulation in patients.
Once the risk of bleeding is detected, timely rescue measures
should be taken. Overall, the results of the current study
demonstrated that the malignancy of GC was not directly associated
with the coagulation status of the blood, but it could also be
associated with other factors involved. For example, changes in
procoagulant and anticoagulant factors caused by long-term
hypercoagulability in the blood. Therefore, the malignancy of
gastric cancer cannot be directly inferred based on blood
hypercoagulability, and low coagulation status may also predict
poor prognosis.
Kim and Song (27)
suggested that high D-dimer levels immediately after surgery were
significantly associated with advanced T and TNM stages (P=0.001
and P=0.006, respectively). Patients in the high D-dimer levels
group displayed significantly lower overall and disease-free
survival rate compared with those in the low D-dimer levels group.
The aforementioned association was evident in the D-dimer levels
before surgery, immediately after surgery, on postoperative day 1
and on postoperative day 30. The multivariate analysis, adjusted
only for TNM stage and cure rate, in a sample of 666 participants,
identified immediate postoperative D-dimer levels as an independent
prognostic factor for OS (HR, 2.52; P=0.010). The research results
by Kim and Song (27) were not
completely consistent with the results of the current study.
Therefore, in the present study, the multivariate analysis was only
adjusted for TNM staging (Table V)
and the results were consistent with those of the study by Kim and
Song (27). The results of the
current study were also consistent with other previous studies;
however, without a clear linear association.
 | Table V.Multi factor regression analysis
conducted by simulating the experiment by Kim and Song (27). |
Table V.
Multi factor regression analysis
conducted by simulating the experiment by Kim and Song (27).
| Variable | Total patients,
n | Events, n (%) | Follow-up time,
days | Crude HR (95%
CI) | Crude P-value | Adjusted HR (95%
CI) | Adjusted
P-value |
|---|
| D-dimer_low | 66 | 23 (34.8) | 3,166 | 1 (Reference) | - | 1.00
(Reference) | - |
| D-dimer_high | 67 | 38 (56.7) | 2,405 | 2.07
(1.23–3.48) | 0.006 | 1.91
(1.13–3.22) | 0.015 |
Liang et al (28) reported that patients with GC and
increased D-dimer levels (EG group) were more likely to have tumors
with a size of ≥5 cm (67.5 vs. 55.8%; P=0.006), an increased
average age (64.0±10.8 vs. 60.5±11.6 years; P<0.001) and
advanced T, N and TNM stages, compared with patients with normal
D-dimer levels (NG group). In addition, the 5-year OS of patients
with elevated D-dimer levels was significantly lower than that in
patients with normal levels (27.0 vs. 42.6%; P<0.001), thus
indicating that D-dimer levels were not an independent prognostic
factor for OS in the multivariate analysis (HR=1.13; 95% CI,
0.92–1.39; P=0.236). After matching, 163 patients in the EG and NG
groups with the same characteristics were selected. The 5-year OS
rate for patients in the EG group was 27.0% compared with 25.8%
recorded for patients in the NG group (P=0.809). This finding was
consistent with that obtained in the multi-model and multi-factor
analysis of the present study, where the D-dimer levels served as a
continuous variable. However, the abovementioned study mainly
focused on exploring linear associations and not non-linear ones.
Furthermore, the study by Liang et al (28) set the threshold for distinguishing
patients with high D-dimer levels from those with low D-dimer
levels to a relatively high value, thus resulting in the inability
to identify turning points in the curve fitting.
The current study has significant value clinically,
since it could provide valuable clinical insights in the following
two main areas: i) Firstly, the study demonstrated that there was a
different association between the preoperative D-dimer levels and
5-year OS in different intervals. Therefore, preoperative D-dimer
levels <200 ng/ml were associated with a worse prognosis, while
D-dimer levels 100–200 ng/ml were associated with a better
prognosis in GC. Secondly, the findings of the current study could
also provide novel insights for future studies on the development
of diagnostic or predictive models for assessing 5-year OS in
patients with GC.
However, the present study has some limitations.
Firstly, since it was necessary to record the effects of numerous
covariates on D-dimer levels, a relatively small sample size was
included in the study. Consequently, the sample continuity weakened
after the inflection point (the rising segment on the right side of
the U-shaped curve inflection point), thus resulting in
insignificant P-values in the right segment of the inflection point
analysis. In terms of statistics, due to the limited sample size,
multivariate Cox proportional hazards models may lack sufficient
statistical power. Furthermore, the exclusion of particular
patients from the study could limit the generalizability of the
study's findings to these individuals.
Despite its limitations, this study has also several
strengths. Firstly, it focused on preoperative (on admission)
D-dimer levels to avoid significant fluctuations in D-dimer values
caused by different surgical types and incision size/location.
Secondly, the non-linearity issue was also fully addressed and
further explored. Furthermore, since the present observational
study was susceptible to potential confounding factors, a strict
statistical adjustment was applied to minimize residual
confounders. To explore the linear and non-linear association of
the independent target variable, it was evaluated as both a
continuous and categorical variable. In addition, to enhance the
clarity of the results, these were presented in fitting curves,
inflection point analysis graphs and K-M survival curves. To the
best of our knowledge, the current study is the first to reveal
that there was a non-linear association between preoperative
D-dimer levels and 5-year OS rate in patients with GC after
surgery. Additionally, a different association between the two
variables was recorded in different intervals; preoperative D-dimer
levels <100 ng/ml and >200 ng/ml were associated with better
and worse prognosis in GC, respectively. Finally, although only 209
cases of patients with GC were included in the study, the samples
were collected from Chinese Academy of Medical Sciences Cancer
Hospital Shanxi Hospital (Shanxi, China), which attracts patients
from different regions of the Chinese Mainland, thus enhancing the
representativeness of the results. The random selection of cases in
2017 over a 3-month period ensured the randomness of the samples.
Additionally, the robust diagnosis, treatment and follow-up systems
of the Chinese Academy of Medical Sciences Cancer Hospital
contributed to the accuracy of the data. Overall, the results of
the present study could provide novel insights into the prognosis
of GC in Mainland China.
Acknowledgements
The authors would like to thank thank Dr Jie Liu
(Department of Vascular and Endovascular Surgery, Chinese People's
Liberation Army General Hospital, Beijing China) for the
statistical support, study design consultations, helpful reviewing
and comments regarding the manuscript.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YZ, JH, RY, SW, KZ and HL contributed to the study
conception and design. Material preparation, data collection and
analysis were performed by HL, YZ, RY and JH. The first draft of
the manuscript was written by YZ, and all authors commented on
previous versions of the manuscript. All authors read and approved
the final manuscript. HL and YZ confirm the authenticity of all the
raw data.
Ethics approval and consent to
participate
This is a retrospective observational study that has
been reviewed by the Ethics Committee of Shanxi Cancer Hospital
(ethical code: KY2023138; project approval number: IIT-2023-136L).
Due to the fact that this study was based on baseline data obtained
from patient case data and remaining biological specimen data, and
there were no adverse effects on patients, an exemption for
informed consent was obtained.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chen S, Zhou D, Yu J, Ruan R, Liu Y, Li Y,
Shen Q and Wang S: A novelly developed bipolar needle knife can be
an alternative device choice for endoscopic submucosal dissection
(with video). Front Med (Lausanne). 9:8886352022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li D, Wang R, Wu N and Yu Y: LncRNA HULC
as a potential predictor of prognosis and clinicopathological
features in patients with digestive system tumors: A meta-analysis.
Aging (Albany NY). 14:1797–811. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu T, Song C, Zhang Y, Siyin ST, Zhang Q,
Song M, Cao L and Shi H: Hepatitis B virus infection and the risk
of gastrointestinal cancers among Chinese population: A prospective
cohort study. Int J Cancer. 150:1018–1028. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Wang R, Lai Q, Tang L, Tao Y, Yao Y, Liu
Y, Lu Y, Shen C, Lu R, Fan C, et al: A novel 5T4-targeting
antibody-drug conjugate H6-DM4 exhibits potent therapeutic efficacy
in gastrointestinal tumor xenograft models. Am J Cancer Res.
8:610–623. 2018.PubMed/NCBI
|
|
6
|
Ji J, Wang Z, Sun W, Li Z, Cai H, Zhao E
and Gui H: Effects of cynaroside on cell proliferation, apoptosis,
migration and invasion though the MET/AKT/mTOR axis in gastric
cancer. Int J Mol Sci. 22:121252021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
You X, Wu J, Zhao X, Jiang X, Tao W, Chen
Z, Huang C, Zheng T and Shen X: Fibroblastic galectin-1-fostered
invasion and metastasis are mediated by TGF-β1-induced
epithelial-mesenchymal transition in gastric cancer. Aging (Albany
NY). 13:18464–18481. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bray F, Ferlay J, Soerjomataram I, Siegel
RL, Torre LA and Jemal A: Global cancer statistics 2018: GLOBOCAN
estimates of incidence and mortality worldwide for 36 cancers in
185 countries. CA Cancer J Clin. 68:394–424. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seidlitz T, Chen YT, Uhlemann H, Schölch
S, Kochall S, Merker SR, Klimova A, Hennig A, Schweitzer C, Pape K,
et al: Mouse models of human gastric cancer subtypes with
stomach-specific CreERT2-mediated pathway alterations.
Gastroenterology. 157:1599–1614.e2. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lyons K, Le LC, Pham YTH, Borron C, Park
JY, Tran CTD, Tran TV, Tran HT, Vu KT, Do CD, et al: Gastric
cancer: epidemiology, biology, and prevention: A mini review. Eur J
Cancer Prev. 28:397–412. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ferlay J, Steliarova-Foucher E,
Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D and
Bray F: Cancer incidence and mortality patterns in Europe:
Estimates for 40 countries in 2012. Eur J Cancer. 49:1374–1403.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yao Y, Sun S, Gu J, Ni H, Zhong K, Xu Q,
Zhou D, Wang X, Gao L and Zhu X: Roux-en-Y reconstruction
alleviates radical gastrectomy-induced colitis via down-regulation
of the butyrate/NLRP3 signaling pathway. EBioMedicine.
86:1043472022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lee SD, Yu D, Lee DY, Shin HS, Jo JH and
Lee YC: Upregulated microRNA-193a-3p is responsible for cisplatin
resistance in CD44(+) gastric cancer cells. Cancer Sci.
110:662–673. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim SH, Jin H, Meng RY, Kim DY, Liu YC,
Chai OH, Park BH and Kim SM: Activating Hippo pathway via rassf1 by
ursolic acid suppresses the tumorigenesis of gastric cancer. Int J
Mol Sci. 20:47092019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Malloy J, Zhuang D, Kim T, Inskeep P, Kim
D and Taylor K: Single and multiple dose evaluation of a novel
MetAP2 inhibitor: Results of a randomized, double-blind,
placebo-controlled clinical trial. Diabetes Obes Metab.
20:1878–1884. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kamin Mukaz D, Gergi M, Koh I, Zakai NA,
Judd SE, Sholzberg M, Baumann Kreuziger L, Freeman K, Colovos C,
Olson NC and Cushman M: Thrombo-inflammatory biomarkers and D-dimer
in a biracial cohort study. Res Pract Thromb Haemost. 5:e126322021.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ho-Tin-Noé B, Goerge T and Wagner DD:
Platelets: Guardians of tumor vasculature. Cancer Res.
69:5623–6526. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Elyamany G, Alzahrani AM and Bukhary E:
Cancer-associated thrombosis: An overview. Clin Med Insights Oncol.
8:129–137. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Rickles FR and Falanga A: Activation of
clotting factors in cancer. Cancer Treat Res. 148:31–41. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Demers M, Krause DS, Schatzberg D,
Martinod K, Voorhees JR, Fuchs TA, Scadden DT and Wagner DD:
Cancers predispose neutrophils to release extracellular DNA traps
that contribute to cancer-associated thrombosis. Proc Natl Acad Sci
USA. 109:13076–13081. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fukumoto K, Taniguchi T, Usami N,
Kawaguchi K, Fukui T, Ishiguro F, Nakamura S and Yokoi K:
Preoperative plasma D-dimer level is an independent prognostic
factor in patients with completely resected non-small cell lung
cancer. Surg Today. 45:63–37. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu L, Zhang X, Yan B, Gu Q, Zhang X, Jiao
J, Sun D, Wang N and Yue X: Elevated plasma D-dimer levels
correlate with long term survival of gastric cancer patients. PLoS
One. 9:e905472014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Guan Y, Xu B, Sui Y, Chen Z, Luan Y, Jiang
Y, Wei L, Long W, Zhao S, Han L, et al: Pan-cancer analysis and
validation reveals that D-dimer-related genes are prognostic and
downregulate CD8+ T cells via TGF-beta signaling in
gastric cancer. Front Mol Biosci. 9:7907062022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dai H, Zhou H, Sun Y, Xu Z, Wang S, Feng T
and Zhang P: D-dimer as a potential clinical marker for predicting
metastasis and progression in cancer. Biomed Rep. 9:453–457.
2018.PubMed/NCBI
|
|
25
|
Suzuki T, Shimada H, Nanami T, Oshima Y,
Yajima S, Ito M, Washizawa N and Kaneko H: Hyperfibrinogenemia is
associated with inflammatory mediators and poor prognosis in
patients with gastric cancer. Surg Today. 46:1394–1401. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Go SI, Lee MJ, Lee WS, Choi HJ, Lee US,
Kim RB, Kang MH, Kim HG, Lee GW, Kang JH, et al: D-dimer can serve
as a prognostic and predictive biomarker for metastatic gastric
cancer treated by chemotherapy. Medicine (Baltimore). 94:e9512015.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kim EY and Song KY: Prognostic value of
D-dimer levels in patients with gastric cancer undergoing
gastrectomy. Surg Oncol. 37:1015702021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liang Y, He D, Wu L, Ding X, Wang X, Wang
B, Zhang R and Liang H: Elevated preoperative plasma D-dimer dose
not adversely affect survival of gastric cancer after gastrectomy
with curative intent: A propensity score analysis. Chin J Cancer
Res. 30:254–262. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Edge SB and Compton CC: The american joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Coccolini F, Montori G, Ceresoli M, Cima
S, Valli MC, Nita GE, Heyer A, Catena F and Ansaloni L: Advanced
gastric cancer: What we know and what we still have to learn. World
J Gastroenterol. 22:1139–1159. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Shen L, Huang Y, Sun M, Xu H, Wei W and Wu
W: Clinicopathological features associated with lymph node
metastasis in early gastric cancer: Analysis of a
single-institution experience in China. Can J Gastroenterol.
23:353–356. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Asakawa Y, Ohtaka M, Maekawa S, Fukasawa
M, Nakayama Y, Yamaguchi T, Inoue T, Uetake T, Sakamoto M, Sato T,
et al: Stratifying the risk of lymph node metastasis in
undifferentiated-type early gastric cancer. World J Gastroenterol.
21:2683–2692. 2015. View Article : Google Scholar : PubMed/NCBI
|