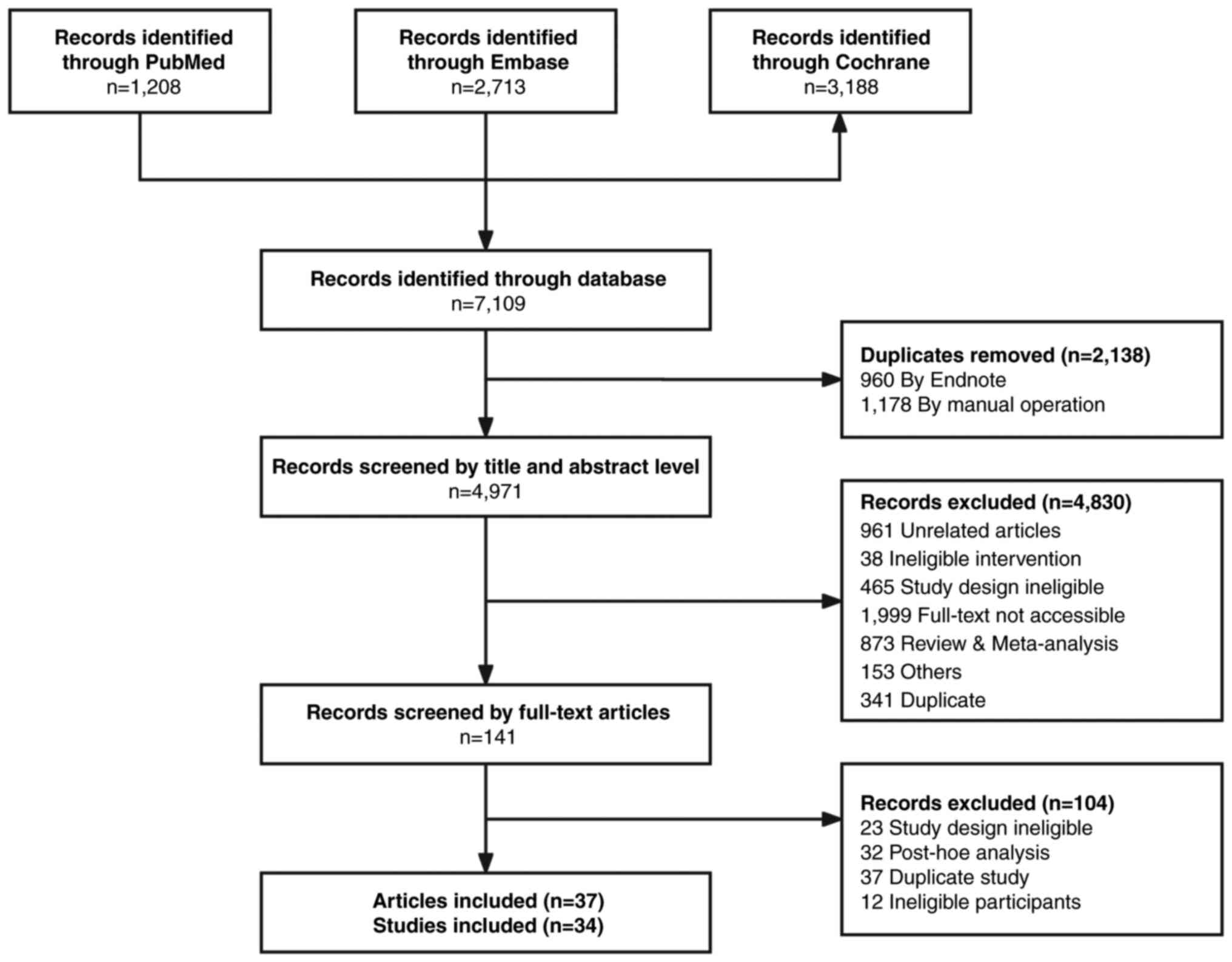
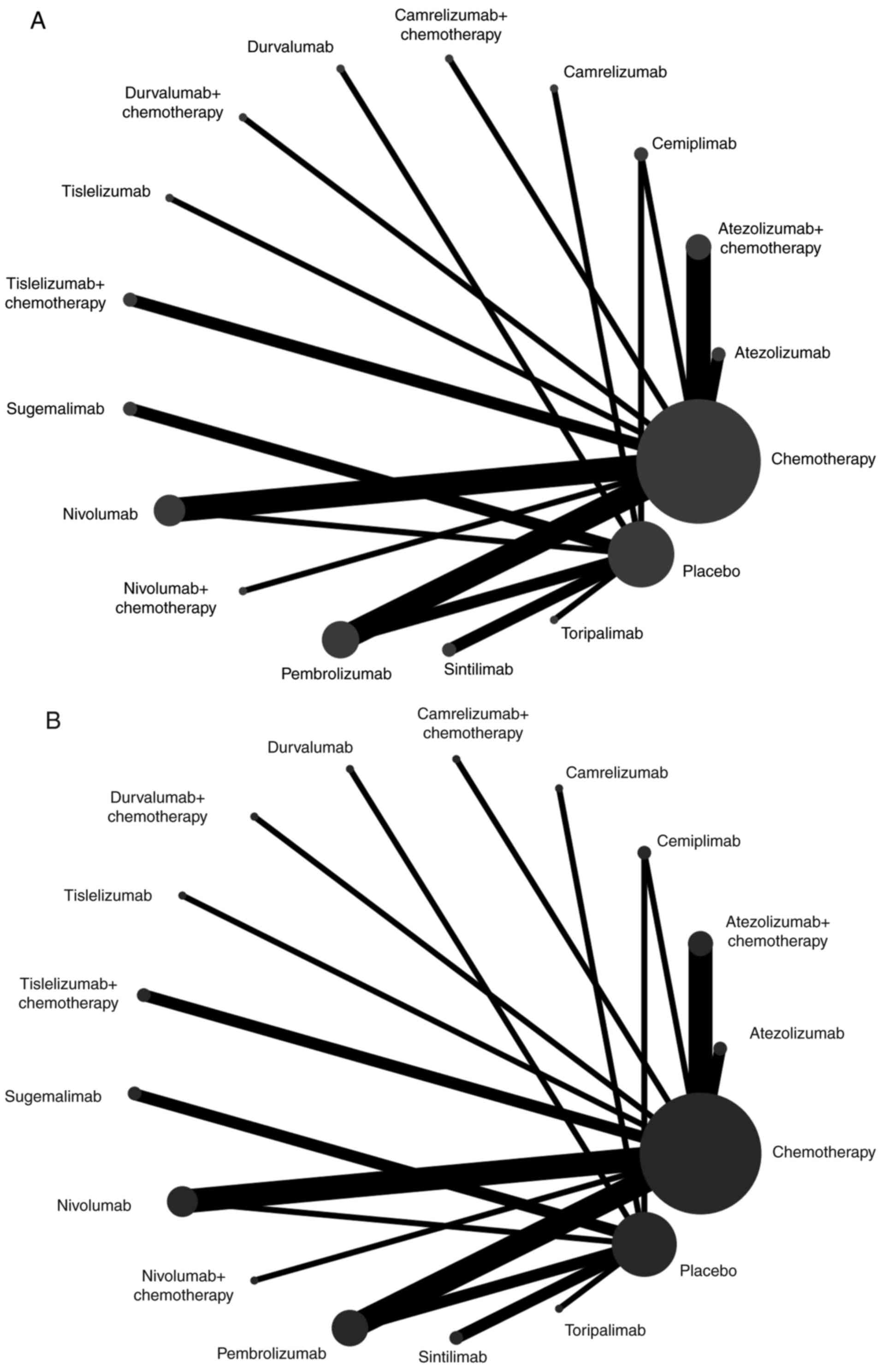
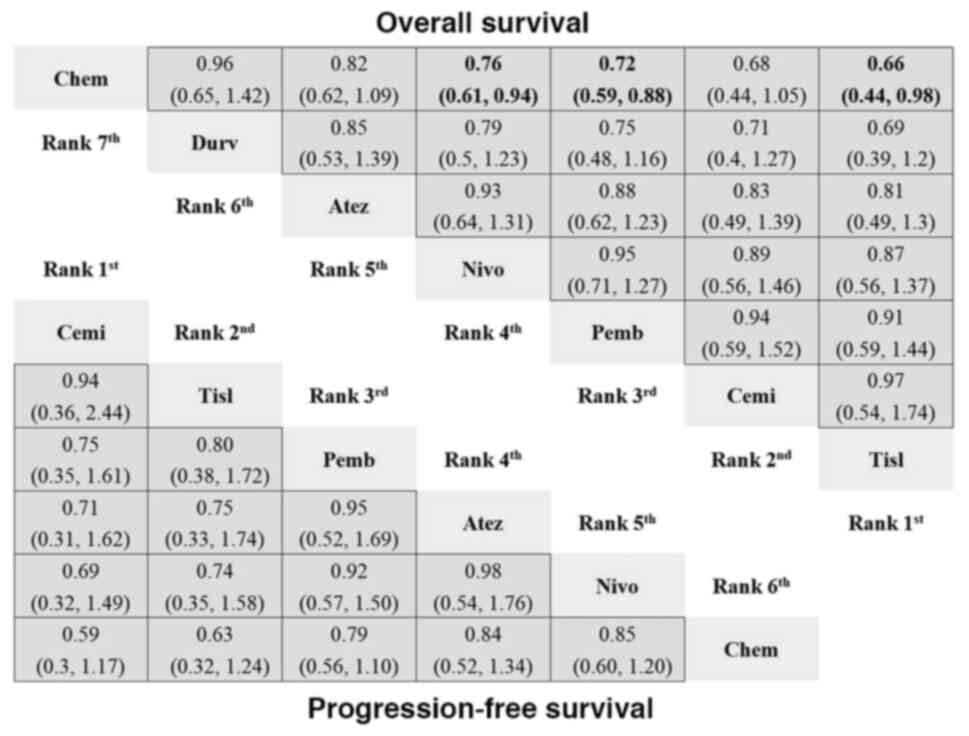
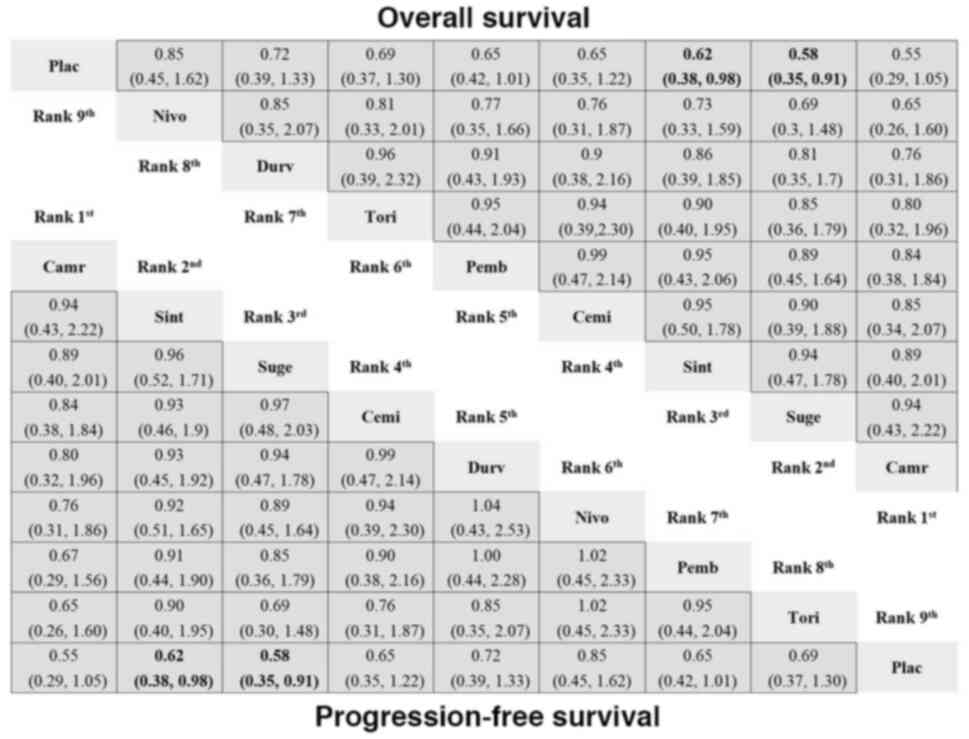
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sequist LV, Rolfe L and Allen AR:
Rociletinib in EGFR-mutated non-small-cell lung cancer. N Engl J
Med. 373:578–579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yin J, Wu Y, Yang X, Gan L and Xue J:
Checkpoint inhibitor pneumonitis induced by anti-PD-1/PD-L1 therapy
in non-small-cell lung cancer: Occurrence and mechanism. Front
Immunol. 13:8306312022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sterne JAC, Savović J, Page MJ, Elbers RG,
Blencowe NS, Boutron I, Cates CJ, Cheng HY, Corbett MS, Eldridge
SM, et al: RoB 2: A revised tool for assessing risk of bias in
randomised trials. BMJ. 366:l48982019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Socinski MA, Nishio M, Jotte RM, Cappuzzo
F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu D,
Moro-Sibilot D, Thomas CA, et al: IMpower150 final overall survival
analyses for atezolizumab plus bevacizumab and chemotherapy in
first-line metastatic nonsquamous NSCLC. J Thorac Oncol.
16:1909–1924. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang Y, Sun J, Wang Z, Fang J, Yu Q, Han
B, Cang S, Chen G, Mei X, Yang Z, et al: Updated overall survival
data and predictive biomarkers of sintilimab plus pemetrexed and
platinum as first-line treatment for locally advanced or metastatic
nonsquamous NSCLC in the phase 3 ORIENT-11 study. J Thorac Oncol.
16:2109–2120. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang L, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Stefaniak V, et al: Final overall
survival data of sintilimab plus pemetrexed and platinum as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC in the phase 3 ORIENT-11 study. Lung Cancer. 171:56–60. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sezer A, Kilickap S, Gümüş M, Bondarenko
I, Özgüroğlu M, Gogishvili M, Turk HM, Cicin I, Bentsion D, Gladkov
O, et al: Cemiplimab monotherapy for first-line treatment of
advanced non-small-cell lung cancer with PD-L1 of at least 50%: A
multicentre, open-label, global, phase 3, randomised, controlled
trial. Lancet. 397:592–604. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Rizvi NA, Cho BC, Reinmuth N, Lee KH, Luft
A, Ahn MJ, van den Heuvel MM, Cobo M, Vicente D, Smolin A, et al:
Durvalumab with or without tremelimumab vs standard chemotherapy in
first-line treatment of metastatic non-small cell lung cancer: The
MYSTIC phase 3 randomized clinical trial. JAMA Oncol. 6:661–674.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Carbone DP, Reck M, Paz-Ares L, Creelan B,
Horn L, Steins M, Felip E, van den Heuvel MM, Ciuleanu TE, Badin F,
et al: First-line nivolumab in stage IV or recurrent non-small-cell
lung cancer. N Engl J Med. 376:2415–2426. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Herbst RS, Garon EB, Kim DW, Cho BC,
Gervais R, Perez-Gracia JL, Han JY, Majem M, Forster MD, Monnet I,
et al: Five year survival update from KEYNOTE-010: Pembrolizumab
versus docetaxel for previously treated, programmed death-ligand
1-positive advanced NSCLC. J Thorac Oncol. 16:1718–1732. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
de Castro G Jr, Kudaba I, Wu YL, Lopes G,
Kowalski DM, Turna HZ, Caglevic C, Zhang L, Karaszewska B,
Laktionov KK, et al: Five-year outcomes with pembrolizumab versus
chemotherapy as first-line therapy in patients with non-small-cell
lung cancer and programmed death ligand-1 tumor proportion score
≥1% in the KEYNOTE-042 study. J Clin Oncol. 41:1986–1991. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Reck M, Rodríguez-Abreu D, Robinson AG,
Hui R, Csőszi T, Fülöp A, Gottfried M, Peled N, Tafreshi A, Cuffe
S, et al: Five-year outcomes with pembrolizumab versus chemotherapy
for metastatic non-small-cell lung cancer with PD-L1 tumor
proportion score ≥50. J Clin Oncol. 39:2339–2349. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ren S, Feng J, Ma S, Chen H, Ma Z, Huang
C, Zhang L, He J, Wang C, Zhou J, et al: KEYNOTE-033: Randomized
phase 3 study of pembrolizumab vs docetaxel in previously treated,
PD-L1-positive, advanced NSCLC. Int J Cancer. 153:623–634. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu YL, Lu S, Cheng Y, Zhou C, Wang J, Mok
T, Zhang L, Tu HY, Wu L, Feng J, et al: Nivolumab versus docetaxel
in a predominantly chinese patient population with previously
treated advanced NSCLC: CheckMate 078 randomized phase III clinical
trial. J Thorac Oncol. 14:867–875. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Borghaei H, Paz-Ares L, Horn L, Spigel DR,
Steins M, Ready NE, Chow LQ, Vokes EE, Felip E, Holgado E, et al:
Nivolumab versus docetaxel in advanced nonsquamous non-small-cell
lung cancer. N Engl J Med. 373:1627–1639. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Brahmer J, Reckamp KL, Baas P, Crinò L,
Eberhardt WE, Poddubskaya E, Antonia S, Pluzanski A, Vokes EE,
Holgado E, et al: Nivolumab versus docetaxel in advanced
squamous-cell non-small-cell lung cancer. N Engl J Med.
373:123–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou C, Huang D, Fan Y, Yu X, Liu Y, Shu
Y, Ma Z, Wang Z, Cheng Y, Wang J, et al: Tislelizumab versus
docetaxel in patients with previously treated advanced NSCLC
(RATIONALE-303): A phase 3, open-label, randomized controlled
trial. J Thorac Oncol. 18:93–105. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fehrenbacher L, von Pawel J, Park K,
Rittmeyer A, Gandara DR, Ponce Aix S, Han JY, Gadgeel SM, Hida T,
Cortinovis DL, et al: Updated efficacy analysis including secondary
population results for OAK: A randomized phase iii study of
atezolizumab versus docetaxel in patients with previously treated
advanced non-small cell lung cancer. J Thorac Oncol. 13:1156–1170.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jassem J, de Marinis F, Giaccone G,
Vergnenegre A, Barrios CH, Morise M, Felip E, Oprean C, Kim YC,
Andric Z, et al: Updated overall survival analysis from IMpower110:
Atezolizumab versus platinum-based chemotherapy in treatment-naive
programmed death-ligand 1-selected NSCLC. J Thorac Oncol.
16:1872–1882. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen
G, Zhang L, Huang D, Cang S, Yang Z, et al: Sintilimab plus
platinum and gemcitabine as first-line treatment for advanced or
metastatic squamous NSCLC: Results from a randomized, double-blind,
phase 3 trial (ORIENT-12). J Thorac Oncol. 16:1501–1511. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ren S, Chen J, Xu X, Jiang T, Cheng Y,
Chen G, Pan Y, Fang Y, Wang Q, Huang Y, et al: Camrelizumab plus
carboplatin and paclitaxel as first-line treatment for advanced
squamous NSCLC (CameL-Sq): A phase 3 trial. J Thorac Oncol.
17:544–557. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Makharadze T, Gogishvili M, Melkadze T,
Baramidze A, Giorgadze D, Penkov K, Laktionov K, Nemsadze G,
Nechaeva M, Rozhkova I, et al: Cemiplimab plus chemotherapy versus
chemotherapy alone in advanced NSCLC: 2-Year follow-up from the
phase 3 EMPOWER-lung 3 part 2 trial. J Thorac Oncol. 18:755–768.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Ma R, et al: Efficacy and safety of
sintilimab plus pemetrexed and platinum as first-line treatment for
locally advanced or metastatic nonsquamous NSCLC: A randomized,
double-blind, phase 3 study (Oncology pRogram by InnovENT
anti-PD-1-11). J Thorac Oncol. 15:1636–1646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spigel DR, Faivre-Finn C, Gray JE, Vicente
D, Planchard D, Paz-Ares L, Vansteenkiste JF, Garassino MC, Hui R,
Quantin X, et al: Five-year survival outcomes from the PACIFIC
trial: Durvalumab after chemoradiotherapy in stage iii
non-small-cell lung cancer. J Clin Oncol. 40:1301–1311. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugawara S, Lee JS, Kang JH, Kim HR, Inui
N, Hida T, Lee KH, Yoshida T, Tanaka H, Yang CT, et al: Nivolumab
with carboplatin, paclitaxel, and bevacizumab for first-line
treatment of advanced nonsquamous non-small-cell lung cancer. Ann
Oncol. 32:1137–1147. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Novello S, Kowalski DM, Luft A, Gümüş M,
Vicente D, Mazières J, Rodríguez-Cid J, Tafreshi A, Cheng Y, Lee
KH, et al: Pembrolizumab plus chemotherapy in squamous
non-small-cell lung cancer: 5-year update of the phase III
KEYNOTE-407 study. J Clin Oncol. 41:1999–2006. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Garassino MC, Gadgeel S, Speranza G, Felip
E, Esteban E, Dómine M, Hochmair MJ, Powell SF, Bischoff HG, Peled
N, et al: Pembrolizumab plus pemetrexed and platinum in nonsquamous
non-small-cell lung cancer: 5-Year outcomes from the phase 3
KEYNOTE-189 study. J Clin Oncol. 41:1992–1998. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zhou Q, Chen M, Jiang O, Pan Y, Hu D, Lin
Q, Wu G, Cui J, Chang J, Cheng Y, et al: Sugemalimab versus placebo
after concurrent or sequential chemoradiotherapy in patients with
locally advanced, unresectable, stage III non-small-cell lung
cancer in China (GEMSTONE-301): Interim results of a randomised,
double-blind, multicentre, phase 3 trial. Lancet Oncol. 23:209–219.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou C, Wang Z, Sun Y, Cao L, Ma Z, Wu R,
Yu Y, Yao W, Chang J, Chen J, et al: Sugemalimab versus placebo, in
combination with platinum-based chemotherapy, as first-line
treatment of metastatic non-small-cell lung cancer (GEMSTONE-302):
Interim and final analyses of a double-blind, randomised, phase 3
clinical trial. Lancet Oncol. 23:220–233. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Z, Wu L, Li B, Cheng Y, Li X, Wang X,
Han L, Wu X, Fan Y, Yu Y, et al: Toripalimab plus chemotherapy for
patients with treatment-naive advanced non-small-cell lung cancer:
A multicenter randomized phase III trial (CHOICE-01). J Clin Oncol.
41:651–663. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jotte R, Cappuzzo F, Vynnychenko I,
Stroyakovskiy D, Rodríguez-Abreu D, Hussein M, Soo R, Conter HJ,
Kozuki T, Huang KC, et al: Atezolizumab in combination with
carboplatin and nab-paclitaxel in advanced squamous NSCLC
(IMpower131): Results from a randomized phase III trial. J Thorac
Oncol. 15:1351–1360. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
West H, McCleod M, Hussein M, Morabito A,
Rittmeyer A, Conter HJ, Kopp HG, Daniel D, McCune S, Mekhail T, et
al: Atezolizumab in combination with carboplatin plus
nab-paclitaxel chemotherapy compared with chemotherapy alone as
first-line treatment for metastatic non-squamous non-small-cell
lung cancer (IMpower130): A multicentre, randomised, open-label,
phase 3 trial. Lancet Oncol. 20:924–937. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Reck M, Mok TSK, Nishio M, Jotte RM,
Cappuzzo F, Orlandi F, Stroyakovskiy D, Nogami N, Rodríguez-Abreu
D, Moro-Sibilot D, et al: Atezolizumab plus bevacizumab and
chemotherapy in non-small-cell lung cancer (IMpower150): Key
subgroup analyses of patients with EGFR mutations or baseline liver
metastases in a randomised, open-label phase 3 trial. Lancet Respir
Med. 7:387–401. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishio M, Barlesi F, West H, Ball S,
Bordoni R, Cobo M, Longeras PD, Goldschmidt J Jr, Novello S,
Orlandi F, et al: Atezolizumab plus chemotherapy for first-line
treatment of nonsquamous NSCLC: Results from the randomized phase 3
IMpower132 trial. J Thorac Oncol. 16:653–664. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Zhou C, Chen G, Huang Y, Zhou J, Lin L,
Feng J, Wang Z, Shu Y, Shi J, Hu Y, et al: Camrelizumab plus
carboplatin and pemetrexed as first-line treatment for advanced
nonsquamous NSCLC: Extended follow-up of CameL phase 3 trial. J
Thorac Oncol. 18:628–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johnson ML, Cho BC, Luft A,
Alatorre-Alexander J, Geater SL, Laktionov K, Kim SW, Ursol G,
Hussein M, Lim FL, et al: Durvalumab with or without tremelimumab
in combination with chemotherapy as first-line therapy for
metastatic non-small-cell lung cancer: The phase III POSEIDON
study. J Clin Oncol. 41:1213–1227. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Forde PM, Spicer J, Lu S, Provencio M,
Mitsudomi T, Awad MM, Felip E, Broderick SR, Brahmer JR, Swanson
SJ, et al: Neoadjuvant nivolumab plus chemotherapy in resectable
lung cancer. N Engl J Med. 386:1973–1985. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu S, Wu L, Jian H, Chen Y, Wang Q, Fang
J, Wang Z, Hu Y, Sun M, Han L, et al: Sintilimab plus bevacizumab
biosimilar IBI305 and chemotherapy for patients with EGFR-mutated
non-squamous non-small-cell lung cancer who progressed on EGFR
tyrosine-kinase inhibitor therapy (ORIENT-31): First interim
results from a randomised, double-blind, multicentre, phase 3
trial. Lancet Oncol. 23:1167–1179. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lu S, Wang J, Yu Y, Yu X, Hu Y, Ai X, Ma
Z, Li X, Zhuang W, Liu Y, et al: Tislelizumab plus chemotherapy as
first-line treatment for locally advanced or metastatic nonsquamous
NSCLC (RATIONALE 304): A randomized phase 3 trial. J Thorac Oncol.
16:1512–1522. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wang J, Lu S, Yu X, Hu Y, Sun Y, Wang Z,
Zhao J, Yu Y, Hu C, Yang K, et al: Tislelizumab plus chemotherapy
vs chemotherapy alone as first-line treatment for advanced squamous
non-small-cell lung cancer: A phase 3 randomized clinical trial.
JAMA Oncol. 7:709–717. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wang DD, Shaver LG, Shi FY, Wei JJ, Qin
TZ, Wang SZ and Kong YJ: Comparative efficacy and safety of
PD-1/PD-L1 immunotherapies for non-small cell lung cancer: A
network meta-analysis. Eur Rev Med Pharmacol Sci. 25:2866–2884.
2021.PubMed/NCBI
|
|
44
|
Jiang M, Liu C, Ding D, Tian H and Yu C:
Comparative efficacy and safety of anti-PD-1/PD-L1 for the
treatment of non-small cell lung cancer: A network meta-analysis of
13 randomized controlled studies. Front Oncol. 12:8270502022.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Li H, Han H, Li C, Wu R, Wang Z, Wang Y,
Zhan P, Lv T, Zhang F, Song Y and Lu H: Efficacy and safety of
first-line PD-1/PD-L1 inhibitor combinations for extensive-stage
small-cell lung cancer: A Bayesian network meta-analysis. Ther Adv
Med Oncol. 15:175883592311894302023. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee J, Sun JM, Lee SH, Ahn JS, Park K and
Ahn MJ: Are there any ethnic differences in the efficacy and safety
of immune checkpoint inhibitors for treatment of lung cancer? J
Thorac Dis. 12:3796–3803. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Ayers KL, Mullaney T, Zhou X, Liu JJ, Lee
K, Ma M, Jones S, Li L, Redfern A, Jappe W, et al: Analysis of
real-world data to investigate the impact of race and ethnicity on
response to programmed cell death-1 and programmed cell
death-ligand 1 inhibitors in advanced non-small cell lung cancers.
Oncologist. 26:e1226–e1239. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang J, He X, Lv Q, Jing J and Shi H:
Management of adverse events in cancer patients treated with
PD-1/PD-L1 blockade: Focus on Asian populations. Front Pharmacol.
10:7262019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Peravali M, Gomes-Lima C, Tefera E, Baker
M, Sherchan M, Farid S, Burman K, Constantinescu F and Veytsman I:
Racial disparities in immune-related adverse events of immune
checkpoint inhibitors and association with survival based on
clinical and biochemical responses. World J Clin Oncol. 12:103–114.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Lang W, Ai Q, He Y, Pan Y, Jiang Q, Ouyang
M and Sun T: Cost-effectiveness analysis of tislelizumab plus
chemotherapy versus standard chemotherapy in first-line treatment
for extensive-stage small cell lung cancer: Perspectives from the
United States and China. Int J Clin Pharm. 46:1536–1545. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Peravali M, Ahn J, Chen K, Rao S, Veytsman
I, Liu SV and Kim C: Safety and efficacy of first-line
pembrolizumab in black patients with metastatic non-small cell lung
cancer. Oncologist. 26:694–700. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Guo Y, Jia J, Hao Z and Yang J:
Tislelizumab plus chemotherapy versus pembrolizumab plus
chemotherapy for the first-line treatment of advanced non-small
cell lung cancer: Systematic review and indirect comparison of
randomized trials. Front Pharmacol. 14:11729692023. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hu J, Li M, Xie Z and Chen J: Comparison
of the efficacy and safety of domestically produced tislelizumab,
camrelizumab, and imported pembrolizumab in the treatment of
advanced NSCLC: A real-world retrospective study. Clin Transl
Oncol. Jun 27–2024.(Epub ahead of print). View Article : Google Scholar
|
|
54
|
Paz-Ares L, Ciuleanu TE, Cobo M, Schenker
M, Zurawski B, Menezes J, Richardet E, Bennouna J, Felip E,
Juan-Vidal O, et al: First-line nivolumab plus ipilimumab combined
with two cycles of chemotherapy in patients with non-small-cell
lung cancer (CheckMate 9LA): An international, randomised,
open-label, phase 3 trial. Lancet Oncol. 22:198–211. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Lei J, Zhao J, Gong L, Ni Y, Zhou Y, Tian
F, Liu H, Gu Z, Huang L, Lu Q, et al: Neoadjuvant camrelizumab plus
platinum-based chemotherapy vs chemotherapy alone for chinese
patients with resectable stage IIIA or IIIB (T3N2) non-small cell
lung cancer: The TD-FOREKNOW randomized clinical trial. JAMA Oncol.
9:1348–1355. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Markham A and Keam SJ: Camrelizumab: First
global approval. Drugs. 79:1355–1361. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Chen P, Li Y, Jing X, Chen J, Chen S and
Yang Q: Cost-effectiveness analysis of sugemalimab in combination
with chemotherapy as first-line treatment in Chinese patients with
metastatic NSCLC. Lung Cancer. 174:157–164. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Blum SM, Rouhani SJ and Sullivan RJ:
Effects of immune-related adverse events (irAEs) and their
treatment on antitumor immune responses. Immunol Rev. 318:167–178.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Kuchroo JR, Hafler DA, Sharpe AH and Lucca
LE: The double-edged sword: Harnessing PD-1 blockade in tumor and
autoimmunity. Sci Immunol. 6:eabf40342021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Huang YT, Chen YP, Lin WC, Su WC and Sun
YT: Immune checkpoint inhibitor-induced myasthenia gravis. Front
Neurol. 11:6342020. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22:392020. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Li Y, Liang X, Li H and Chen X: Efficacy
and safety of immune checkpoint inhibitors for advanced non-small
cell lung cancer with or without PD-L1 selection: A systematic
review and network meta-analysis. Chin Med J (Engl). 136:2156–2165.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chen W, Chen J, Zhang L, Cheng S and Yu J:
Network meta-analysis of first-line immune checkpoint inhibitor
therapy in advanced non-squamous non-small cell lung cancer
patients with PD-L1 expression ≥ 50. BMC Cancer. 23:7912023.
View Article : Google Scholar : PubMed/NCBI
|