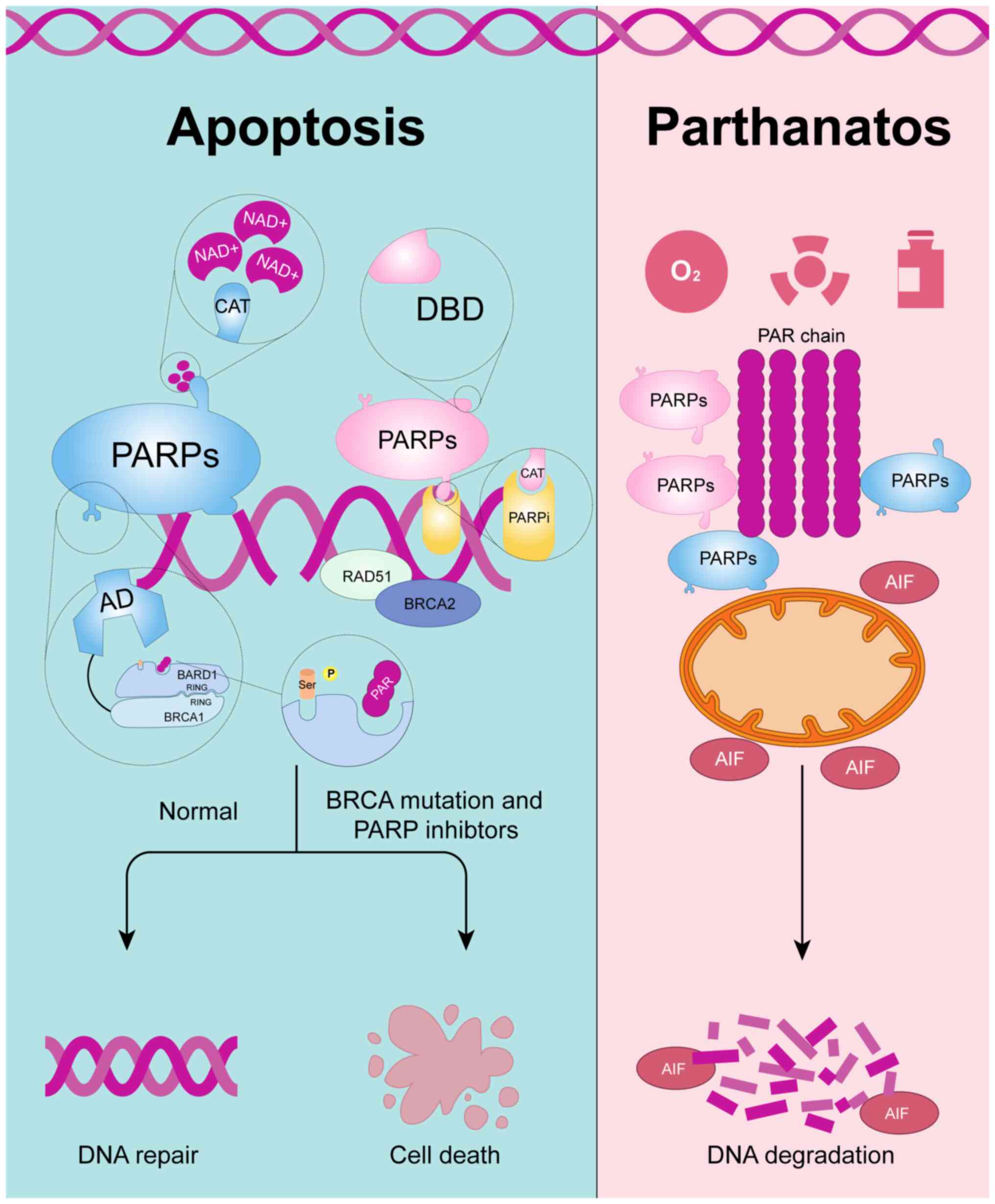
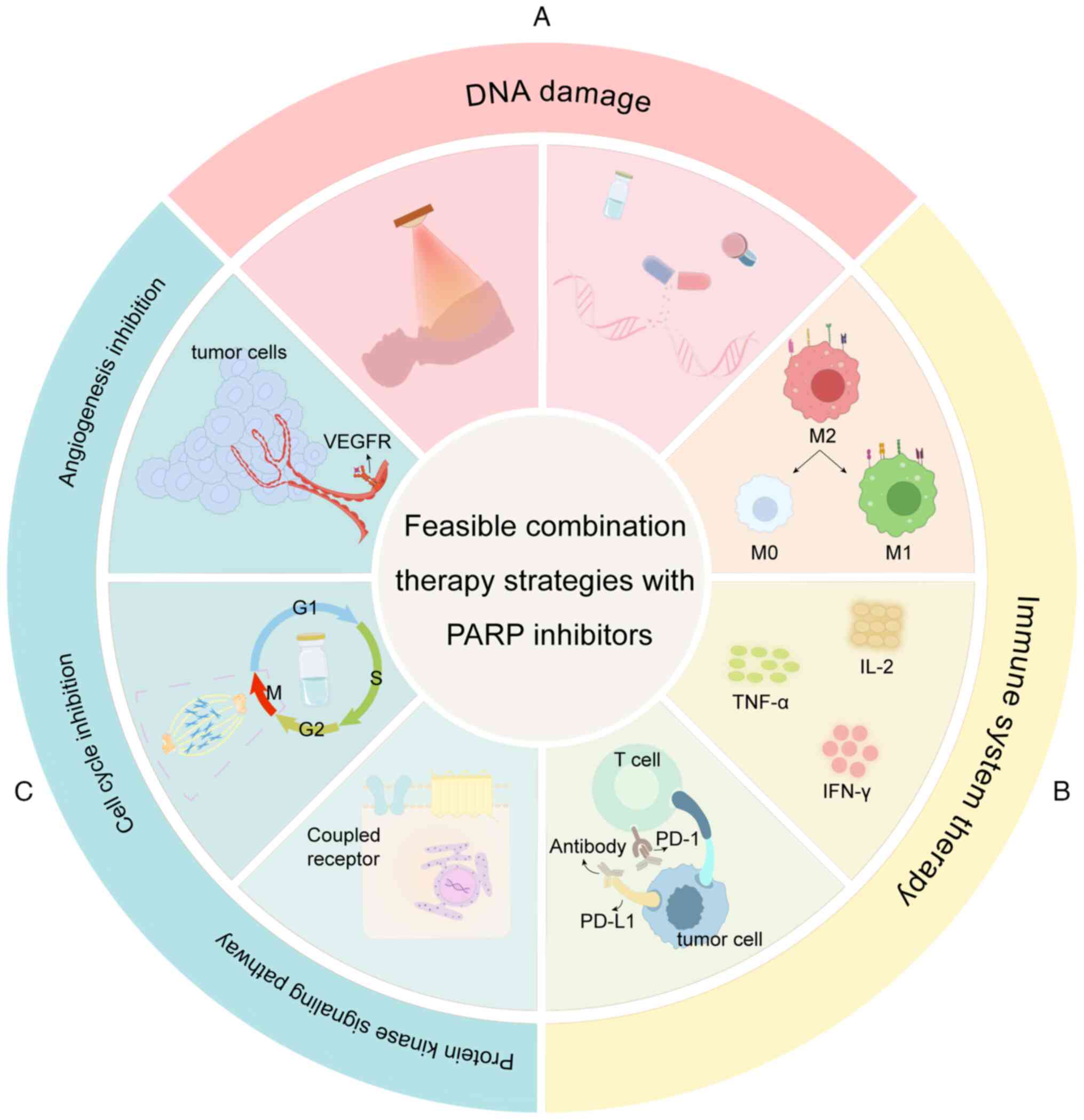
|
1
|
Giaquinto AN, Sung H, Newman LA, Freedman
RA, Smith RA, Star J, Jemal A and Siegel RL: Breast cancer
statistics 2024. CA Cancer J Clin. 74:477–495. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wu SY, Wang H, Shao ZM and Jiang YZ:
Triple-negative breast cancer: New treatment strategies in the era
of precision medicine. Sci China Life Sci. 64:372–388. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yuan P, Ma N and Xu B: Poly (adenosine
diphosphate-ribose) polymerase inhibitors in the treatment of
triple-negative breast cancer with homologous repair deficiency.
Med Res Rev. 44:2774–2792. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Konstantinopoulos PA, Ceccaldi R, Shapiro
GI and D'Andrea AD: Homologous recombination deficiency: Exploiting
the fundamental vulnerability of ovarian cancer. Cancer Discov.
5:1137–1154. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
de Bono J, Mateo J, Fizazi K, Saad F,
Shore N, Sandhu S, Chi KN, Sartor O, Agarwal N, Olmos D, et al:
Olaparib for metastatic castration-resistant prostate cancer. N
Engl J Med. 382:2091–2102. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mateo J, Porta N, Bianchini D, McGovern U,
Elliott T, Jones R, Syndikus I, Ralph C, Jain S, Varughese M, et
al: Olaparib in patients with metastatic castration-resistant
prostate cancer with DNA repair gene aberrations (TOPARP-B): A
multicentre, open-label, randomised, phase 2 trial. Lancet Oncol.
21:162–174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Markham A: Pamiparib: First Approval.
Drugs. 81:1343–1348. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee A: Fuzuloparib: First Approval. Drugs.
81:1221–1226. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao F, Wang Z, Qiao L, Zhang X, Wu N,
Wang J and Yu X: Application of PARP inhibitors combined with
immune checkpoint inhibitors in ovarian cancer. J Transl Med.
22:7782024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Q, Qian W, Zhang Y, Hu L, Chen S and
Xia Y: A new wave of innovations within the DNA damage response.
Signal Transduct Target Ther. 8:3382023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Afghahi A, Timms KM, Vinayak S, Jensen KC,
Kurian AW, Carlson RW, Chang PJ, Schackmann E, Hartman AR, Ford JM
and Telli ML: Tumor BRCA1 reversion mutation arising during
neoadjuvant platinum-based chemotherapy in triple-negative breast
cancer is associated with therapy resistance. Clin Cancer Res.
23:3365–3370. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
de Bono J, Ramanathan RK, Mina L, Chugh R,
Glaspy J, Rafii S, Kaye S, Sachdev J, Heymach J, Smith DC, et al:
Phase I, dose-escalation, two-part trial of the PARP inhibitor
talazoparib in patients with advanced germline BRCA1/2 mutations
and selected sporadic cancers. Cancer Discov. 7:620–629. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bardia A, Sun S, Thimmiah N, Coates JT, Wu
B, Abelman RO, Spring L, Moy B, Ryan P, Melkonyan MN, et al:
Antibody-drug conjugate sacituzumab govitecan enables a sequential
TOP1/PARP inhibitor therapy strategy in patients with breast
cancer. Clin Cancer Res. 30:2917–2924. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rodler ET, Kurland BF, Griffin M, Gralow
JR, Porter P, Yeh RF, Gadi VK, Guenthoer J, Beumer JH, Korde L, et
al: Phase I study of veliparib (ABT-888) combined with cisplatin
and vinorelbine in advanced triple-negative breast cancer and/or
BRCA mutation-associated breast cancer. Clin Cancer Res.
22:2855–2864. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gelmon KA, Tischkowitz M, Mackay H,
Swenerton K, Robidoux A, Tonkin K, Hirte H, Huntsman D, Clemons M,
Gilks B, et al: Olaparib in patients with recurrent high-grade
serous or poorly differentiated ovarian carcinoma or
triple-negative breast cancer: A phase 2, multicentre, open-label,
non-randomised study. Lancet Oncol. 12:852–861. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu S, Wu Y, Song B, Yi M, Yan Y, Mei Q
and Wu K: Recent advances in targeted strategies for
triple-negative breast cancer. J Hematol Oncol. 16:1002023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Subhan MA, Parveen F, Shah H, Yalamarty
SSK, Ataide JA and Torchilin VP: Recent advances with precision
medicine treatment for breast cancer including triple-negative
sub-type. Cancers (Basel). 15:22042023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Won KA and Spruck C: Triple-negative
breast cancer therapy: Current and future perspectives (Review).
Int J Oncol. 57:1245–1261. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kraus WL: PARPs and ADP-ribosylation: 50
Years … and counting. Mol Cell. 58:902–910. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Schreiber V, Dantzer F, Ame JC and de
Murcia G: Poly(ADP-ribose): Novel functions for an old molecule.
Nat Rev Mol Cell Biol. 7:517–528. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Duma L and Ahel I: The function and
regulation of ADP-ribosylation in the DNA damage response. Biochem
Soc Trans. 51:995–1008. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rouleau-Turcotte É and Pascal JM:
ADP-ribose contributions to genome stability and PARP enzyme
trapping on sites of DNA damage; paradigm shifts for a
coming-of-age modification. J Biol Chem. 299:1053972023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tutt A, Robson M, Garber JE, Domchek SM,
Audeh MW, Weitzel JN, Friedlander M, Arun B, Loman N, Schmutzler
RK, et al: Oral poly(ADP-ribose) polymerase inhibitor olaparib in
patients with BRCA1 or BRCA2 mutations and advanced breast cancer:
A proof-of-concept trial. Lancet. 376:235–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim D and Nam HJ; PARP inhibitors, :
Clinical limitations and recent attempts to overcome them. Int J
Mol Sci. 23:84122022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim MY, Mauro S, Gévry N, Lis JT and Kraus
WL: NAD+-dependent modulation of chromatin structure and
transcription by nucleosome binding properties of PARP-1. Cell.
119:803–814. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kun E, Kirsten E, Mendeleyev J and Ordahl
CP: Regulation of the enzymatic catalysis of poly(ADP-ribose)
polymerase by dsDNA, polyamines, Mg2+, Ca2+, histones H1 and H3,
and ATP. Biochemistry. 43:210–216. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kraus WL: Transcriptional control by
PARP-1: Chromatin modulation, enhancer-binding, coregulation, and
insulation. Curr Opin Cell Biol. 20:294–302. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Baek SH, Bae ON, Kim EK and Yu SW:
Induction of mitochondrial dysfunction by poly(ADP-ribose) polymer:
Implication for neuronal cell death. Mol Cells. 36:258–266. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tuo QZ, Zhang ST and Lei P: Mechanisms of
neuronal cell death in ischemic stroke and their therapeutic
implications. Med Res Rev. 42:259–305. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wang Z, Li Y, Yang J, Sun Y, He Y, Wang Y,
Liang Y, Chen X, Chen T, Han D, et al: CircCFL1 promotes TNBC
stemness and immunoescape via deacetylation-mediated c-Myc
deubiquitylation to facilitate mutant TP53 transcription. Adv Sci
(Weinh). 11:e24046282024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
De Souza C, Madden JA, Minn D, Kumar VE,
Montoya DJ, Nambiar R, Zhu Z, Xiao WW, Tahmassebi N, Kathi H, et
al: The P72R polymorphism in R248Q/W p53 mutants modifies the
mutant effect on epithelial to mesenchymal transition phenotype and
cell invasion via CXCL1 expression. Int J Mol Sci. 21:80252020.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Macdonald FH, Yao D, Quinn JA and
Greenhalgh DA: PTEN ablation in Ras(Ha)/Fos skin carcinogenesis
invokes p53-dependent p21 to delay conversion while p53-independent
p21 limits progression via cyclin D1/E2 inhibition. Oncogene.
33:4132–4143. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Marvalim C, Datta A and Lee SC: Role of
p53 in breast cancer progression: An insight into p53 targeted
therapy. Theranostics. 13:1421–1442. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Deng CX: Tumorigenesis as a consequence of
genetic instability in Brca1 mutant mice. Mutat Res. 477:183–189.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Du Y, Luo L, Xu X, Yang X, Yang X, Xiong
S, Yu J, Liang T and Guo L: Unleashing the power of synthetic
lethality: Augmenting treatment efficacy through synergistic
integration with chemotherapy drugs. Pharmaceutics. 15:24332023.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Anders CK, Winer EP, Ford JM, Dent R,
Silver DP, Sledge GW and Carey LA: Poly(ADP-Ribose) polymerase
inhibition: ‘Targeted’ therapy for triple-negative breast cancer.
Clin Cancer Res. 16:4702–4710. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Bryant HE, Schultz N, Thomas HD, Parker
KM, Flower D, Lopez E, Kyle S, Meuth M, Curtin NJ and Helleday T:
Specific killing of BRCA2-deficient tumours with inhibitors of
poly(ADP-ribose) polymerase. Nature. 434:913–917. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Farmer H, McCabe N, Lord CJ, Tutt AN,
Johnson DA, Richardson TB, Santarosa M, Dillon KJ, Hickson I,
Knights C, et al: Targeting the DNA repair defect in BRCA mutant
cells as a therapeutic strategy. Nature. 434:917–921. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Tarsounas M and Sung P: The
antitumorigenic roles of BRCA1-BARD1 in DNA repair and replication.
Nat Rev Mol Cell Biol. 21:284–299. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Peña-Guerrero J, Fernández-Rubio C,
García-Sosa AT and Nguewa PA: BRCT domains: Structure, functions,
and implications in disease-new therapeutic targets for innovative
drug discovery against infections. Pharmaceutics. 15:18392023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Li M and Yu X: Function of BRCA1 in the
DNA damage response is mediated by ADP-ribosylation. Cancer Cell.
23:693–704. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Becker JR, Clifford G, Bonnet C, Groth A,
Wilson MD and Chapman JR: BARD1 reads H2A lysine 15 ubiquitination
to direct homologous recombination. Nature. 596:433–437. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Liu C, Wu J, Paudyal SC, You Z and Yu X:
CHFR is important for the first wave of ubiquitination at DNA
damage sites. Nucleic Acids Res. 41:1698–1710. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wu W, Zhao J, Xiao J, Wu W, Xie L, Xie X,
Yang C, Yin D and Hu K: CHFR-mediated degradation of RNF126 confers
sensitivity to PARP inhibitors in triple-negative breast cancer
cells. Biochem Biophys Res Commun. 573:62–68. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Djerir B, Marois I, Dubois JC, Findlay S,
Morin T, Senoussi I, Cappadocia L, Orthwein A and Maréchal A: An E3
ubiquitin ligase localization screen uncovers DTX2 as a novel
ADP-ribosylation-dependent regulator of DNA double-strand break
repair. J Biol Chem. 300:1075452024. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yan Q, Xu R, Zhu L, Cheng X, Wang Z, Manis
J and Shipp MA: BAL1 and its partner E3 ligase, BBAP, link
Poly(ADP-ribose) activation, ubiquitylation, and double-strand DNA
repair independent of ATM, MDC1, and RNF8. Mol Cell Biol.
33:845–857. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Kolas NK, Chapman JR, Nakada S, Ylanko J,
Chahwan R, Sweeney FD, Panier S, Mendez M, Wildenhain J, Thomson
TM, et al: Orchestration of the DNA-damage response by the RNF8
ubiquitin ligase. Science. 318:1637–1640. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Marteijn JA, Bekker-Jensen S, Mailand N,
Lans H, Schwertman P, Gourdin AM, Dantuma NP, Lukas J and Vermeulen
W: Nucleotide excision repair-induced H2A ubiquitination is
dependent on MDC1 and RNF8 and reveals a universal DNA damage
response. J Cell Biol. 186:835–847. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Tian H, Luan P, Liu Y and Li G:
Tet-mediated DNA methylation dynamics affect chromosome
organization. Nucleic Acids Res. 52:3654–3666. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Messner S and Hottiger MO: Histone
ADP-ribosylation in DNA repair, replication and transcription.
Trends Cell Biol. 21:534–542. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hottiger MO: ADP-ribosylation of histones
by ARTD1: An additional module of the histone code? FEBS Lett.
585:1595–1599. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Messner S, Altmeyer M, Zhao H, Pozivil A,
Roschitzki B, Gehrig P, Rutishauser D, Huang D, Caflisch A and
Hottiger MO: PARP1 ADP-ribosylates lysine residues of the core
histone tails. Nucleic Acids Res. 38:6350–6362. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Rouleau M, Patel A, Hendzel MJ, Kaufmann
SH and Poirier GG: PARP inhibition: PARP1 and beyond. Nat Rev
Cancer. 10:293–301. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Zhou Y, Feng X and Koh DW: Enhanced DNA
accessibility and increased DNA damage induced by the absence of
poly(ADP-ribose) hydrolysis. Biochemistry. 49:7360–7366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Martin BJE, Ablondi EF, Goglia C, Mimoso
CA, Espinel-Cabrera PR and Adelman K: Global identification of
SWI/SNF targets reveals compensation by EP400. Cell.
186:5290–5307.e26. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Wanior M, Krämer A, Knapp S and Joerger
AC: Exploiting vulnerabilities of SWI/SNF chromatin remodelling
complexes for cancer therapy. Oncogene. 40:3637–3654. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Kolthur-Seetharam U, Dantzer F, McBurney
MW, de Murcia G and Sassone-Corsi P: Control of AIF-mediated cell
death by the functional interplay of SIRT1 and PARP-1 in response
to DNA damage. Cell Cycle. 5:873–877. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Sobotka AA and Tempera I: PARP1 as an
epigenetic modulator: Implications for the regulation of host-viral
dynamics. Pathogens. 13:1312024. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Ahmad M, Weiswald LB, Poulain L, Denoyelle
C and Meryet-Figuiere M: Involvement of lncRNAs in cancer cells
migration, invasion and metastasis: Cytoskeleton and ECM crosstalk.
J Exp Clin Cancer Res. 42:1732023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Bartonicek N, Maag JL and Dinger ME: Long
noncoding RNAs in cancer: Mechanisms of action and technological
advancements. Mol Cancer. 15:432016. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Jiang J, Lu Y, Zhang F, Huang J, Ren XL
and Zhang R: The emerging roles of long noncoding RNAs as hallmarks
of lung cancer. Front Oncol. 11:7615822021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Zhang C, Zhou B, Gu F, Liu H, Wu H, Yao F,
Zheng H, Fu H, Chong W, Cai S, et al: Micropeptide PACMP inhibition
elicits synthetic lethal effects by decreasing CtIP and
poly(ADP-ribosyl)ation. Mol Cell. 82:1297–1312.e8. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Siegel C and McCullough LD: NAD+ depletion
or PAR polymer formation: Which plays the role of executioner in
ischaemic cell death? Acta Physiol (Oxf). 203:225–234. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
De P, Sun Y, Carlson JH, Friedman LS,
Leyland-Jones BR and Dey N: Doubling down on the PI3K-AKT-mTOR
pathway enhances the antitumor efficacy of PARP inhibitor in triple
negative breast cancer model beyond BRCA-ness. Neoplasia. 16:43–72.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ding JH, Xiao Y, Yang F, Song XQ, Xu Y,
Ding XH, Ding R, Shao ZM, Di GH and Jiang YZ: Guanosine
diphosphate-mannose suppresses homologous recombination repair and
potentiates antitumor immunity in triple-negative breast cancer.
Sci Transl Med. 16:eadg77402024. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhong Y, Le H, Zhang X, Dai Y, Guo F, Ran
X, Hu G, Xie Q, Wang D and Cai Y: Identification of restrictive
molecules involved in oncolytic virotherapy using genome-wide
CRISPR screening. J Hematol Oncol. 17:362024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen LM, Yang PP, Al Haq AT, Hwang PA, Lai
YC, Weng YS, Chen MA and Hsu HL: Oligo-Fucoidan supplementation
enhances the effect of Olaparib on preventing metastasis and
recurrence of triple-negative breast cancer in mice. J Biomed Sci.
29:702022. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Rudolph J, Jung K and Luger K: Inhibitors
of PARP: Number crunching and structure gazing. Proc Natl Acad Sci
USA. 119:e21219791192022. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Anders C, Deal AM, Abramson V, Liu MC,
Storniolo AM, Carpenter JT, Puhalla S, Nanda R, Melhem-Bertrandt A,
Lin NU, et al: TBCRC 018: phase II study of iniparib in combination
with irinotecan to treat progressive triple negative breast cancer
brain metastases. Breast Cancer Res Treat. 146:557–566. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Diéras V, Han HS, Kaufman B, Wildiers H,
Friedlander M, Ayoub JP, Puhalla SL, Bondarenko I, Campone M,
Jakobsen EH, et al: Veliparib with carboplatin and paclitaxel in
BRCA-mutated advanced breast cancer (BROCADE3): A randomised,
double-blind, placebo-controlled, phase 3 trial. Lancet Oncol.
21:1269–1282. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Domchek SM, Postel-Vinay S, Im SA, Park
YH, Delord JP, Italiano A, Alexandre J, You B, Bastian S, Krebs MG,
et al: Olaparib and durvalumab in patients with germline
BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label,
multicentre, phase 1/2, basket study. Lancet Oncol. 21:1155–1164.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Shroff RT, Hendifar A, McWilliams RR, Geva
R, Epelbaum R, Rolfe L, Goble S, Lin KK, Biankin AV, Giordano H, et
al: Rucaparib monotherapy in patients with pancreatic cancer and a
known deleterious BRCA mutation. JCO Precis Oncol.
2018.PO.17.00316. 2018. View Article : Google Scholar
|
|
74
|
Zhao M, Qiu S, Wu X, Miao P, Jiang Z, Zhu
T, Xu X, Zhu Y, Zhang B, Yuan D, et al: Efficacy and safety of
niraparib as first-line maintenance treatment for patients with
advanced ovarian cancer: Real-world data from a multicenter study
in China. Target Oncol. 18:869–883. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Savill KMZ, Ivanova J, Asgarisabet P,
Falkenstein A, Balanean A, Niyazov A, Ryan JC, Kish J, Gajra A and
Mahtani RL: Characteristics, treatment, and outcomes of real-world
talazoparib-treated patients with germline BRCA-mutated advanced
HER2-negative breast cancer. Oncologist. 28:414–424. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Xu B, Yin Y, Dong M, Song Y, Li W, Huang
X, Wang T, He J, Mu X, Li L, et al: Pamiparib dose escalation in
Chinese patients with non-mucinous high-grade ovarian cancer or
advanced triple-negative breast cancer. Cancer Med. 10:109–118.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
O'Shaughnessy J, Osborne C, Pippen JE,
Yoffe M, Patt D, Rocha C, Koo IC, Sherman BM and Bradley C:
Iniparib plus chemotherapy in metastatic triple-negative breast
cancer. N Engl J Med. 364:205–214. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
O'Shaughnessy J, Schwartzberg L, Danso MA,
Miller KD, Rugo HS, Neubauer M, Robert N, Hellerstedt B, Saleh M,
Richards P, et al: Phase III study of iniparib plus gemcitabine and
carboplatin versus gemcitabine and carboplatin in patients with
metastatic triple-negative breast cancer. J Clin Oncol.
32:3840–3847. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Mateo J, Ong M, Tan DS, Gonzalez MA and de
Bono JS: Appraising iniparib, the PARP inhibitor that never
was-what must we learn? Nat Rev Clin Oncol. 10:688–696. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Liu X, Shi Y, Maag DX, Palma JP, Patterson
MJ, Ellis PA, Surber BW, Ready DB, Soni NB, Ladror US, et al:
Iniparib nonselectively modifies cysteine-containing proteins in
tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res.
18:510–523. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Wu HL, Luo ZY, He ZL, Gong Y, Mo M, Ming
WK and Liu GY: All HER2-negative breast cancer patients need gBRCA
testing: Cost-effectiveness and clinical benefits. Br J Cancer.
128:638–646. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Kaufman B, Shapira-Frommer R, Schmutzler
RK, Audeh MW, Friedlander M, Balmaña J, Mitchell G, Fried G,
Stemmer SM, Hubert A, et al: Olaparib monotherapy in patients with
advanced cancer and a germline BRCA1/2 mutation. J Clin Oncol.
33:244–250. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Dent RA, Lindeman GJ, Clemons M, Wildiers
H, Chan A, McCarthy NJ, Singer CF, Lowe ES, Watkins CL and
Carmichael J: Phase I trial of the oral PARP inhibitor olaparib in
combination with paclitaxel for first- or second-line treatment of
patients with metastatic triple-negative breast cancer. Breast
Cancer Res. 15:R882013. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu JF, Tolaney SM, Birrer M, Fleming GF,
Buss MK, Dahlberg SE, Lee H, Whalen C, Tyburski K, Winer E, et al:
A Phase 1 trial of the poly(ADP-ribose) polymerase inhibitor
olaparib (AZD2281) in combination with the anti-angiogenic
cediranib (AZD2171) in recurrent epithelial ovarian or
triple-negative breast cancer. Eur J Cancer. 49:2972–2978. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Lee JM, Hays JL, Annunziata CM, Noonan AM,
Minasian L, Zujewski JA, Yu M, Gordon N, Ji J, Sissung TM, et al:
Phase I/Ib study of olaparib and carboplatin in BRCA1 or BRCA2
mutation-associated breast or ovarian cancer with biomarker
analyses. J Natl Cancer Inst. 106:dju0892014. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Del Conte G, Sessa C, von Moos R, Viganò
L, Digena T, Locatelli A, Gallerani E, Fasolo A, Tessari A,
Cathomas R and Gianni L: Phase I study of olaparib in combination
with liposomal doxorubicin in patients with advanced solid tumours.
Br J Cancer. 111:651–659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Loap P, Loirat D, Berger F, Ricci F,
Vincent-Salomon A, Ezzili C, Mosseri V, Fourquet A, Ezzalfani M and
Kirova Y: Combination of olaparib and radiation therapy for triple
negative breast cancer: Preliminary results of the RADIOPARP phase
1 trial. Int J Radiat Oncol Biol Phys. 109:436–440. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Loap P, Loirat D, Berger F, Cao K, Ricci
F, Jochem A, Raizonville L, Mosseri V, Fourquet A and Kirova Y:
Combination of Olaparib with radiotherapy for triple-negative
breast cancers: One-year toxicity report of the RADIOPARP Phase I
trial. Int J Cancer. 149:1828–1832. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Loap P, Loirat D, Berger F, Rodrigues M,
Bazire L, Pierga JY, Vincent-Salomon A, Laki F, Boudali L,
Raizonville L, et al: Concurrent Olaparib and radiotherapy in
patients with triple-negative breast cancer: The phase 1 Olaparib
and radiation therapy for triple-negative breast cancer trial. JAMA
Oncol. 8:1802–1808. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Ganz PA, Bandos H, Španić T, Friedman S,
Müller V, Kuemmel S, Delaloge S, Brain E, Toi M, Yamauchi H, et al:
Patient-reported outcomes in OlympiA: A phase III, randomized,
placebo-controlled trial of adjuvant Olaparib in gBRCA1/2 mutations
and high-risk human epidermal growth factor receptor 2-negative
early breast cancer. J Clin Oncol. 42:1288–1300. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Plummer R, Jones C, Middleton M, Wilson R,
Evans J, Olsen A, Curtin N, Boddy A, McHugh P, Newell D, et al:
Phase I study of the poly(ADP-ribose) polymerase inhibitor,
AG014699, in combination with temozolomide in patients with
advanced solid tumors. Clin Cancer Res. 14:7917–7923. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Drew Y, Ledermann J, Hall G, Rea D,
Glasspool R, Highley M, Jayson G, Sludden J, Murray J, Jamieson D,
et al: Phase 2 multicentre trial investigating intermittent and
continuous dosing schedules of the poly(ADP-ribose) polymerase
inhibitor rucaparib in germline BRCA mutation carriers with
advanced ovarian and breast cancer. Br J Cancer. 114:723–730. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Chopra N, Tovey H, Pearson A, Cutts R,
Toms C, Proszek P, Hubank M, Dowsett M, Dodson A, Daley F, et al:
Homologous recombination DNA repair deficiency and PARP inhibition
activity in primary triple negative breast cancer. Nat Commun.
11:26622020. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Kristeleit R, Leary A, Oaknin A, Redondo
A, George A, Chui S, Seiller A, Liste-Hermoso M, Willis J, Shemesh
CS, et al: PARP inhibition with rucaparib alone followed by
combination with atezolizumab: Phase Ib COUPLET clinical study in
advanced gynaecological and triple-negative breast cancers. Br J
Cancer. 131:820–831. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Sandhu SK, Schelman WR, Wilding G, Moreno
V, Baird RD, Miranda S, Hylands L, Riisnaes R, Forster M, Omlin A,
et al: The poly(ADP-ribose) polymerase inhibitor niraparib (MK4827)
in BRCA mutation carriers and patients with sporadic cancer: A
phase 1 dose-escalation trial. Lancet Oncol. 14:882–892. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
96
|
van Andel L, Rosing H, Zhang Z, Hughes L,
Kansra V, Sanghvi M, Tibben MM, Gebretensae A, Schellens JHM and
Beijnen JH: Determination of the absolute oral bioavailability of
niraparib by simultaneous administration of a (14)C-microtracer and
therapeutic dose in cancer patients. Cancer Chemother Pharmacol.
81:39–46. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Mirza MR, Monk BJ, Herrstedt J, Oza AM,
Mahner S, Redondo A, Fabbro M, Ledermann JA, Lorusso D, Vergote I,
et al: Niraparib maintenance therapy in platinum-sensitive,
recurrent ovarian cancer. N Engl J Med. 375:2154–2164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Scott LJ: Niraparib: First global
approval. Drugs. 77:1029–1034. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Wang L, Mason KA, Ang KK, Buchholz T,
Valdecanas D, Mathur A, Buser-Doepner C, Toniatti C and Milas L:
MK-4827, a PARP-1/-2 inhibitor, strongly enhances response of human
lung and breast cancer xenografts to radiation. Invest New Drugs.
30:2113–2120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Shen Y, Rehman FL, Feng Y, Boshuizen J,
Bajrami I, Elliott R, Wang B, Lord CJ, Post LE and Ashworth A: BMN
673, a novel and highly potent PARP1/2 inhibitor for the treatment
of human cancers with DNA repair deficiency. Clin Cancer Res.
19:5003–5015. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Litton JK, Beck JT, Jones JM, Andersen J,
Blum JL, Mina LA, Brig R, Danso M, Yuan Y, Abbattista A, et al:
Neoadjuvant talazoparib in patients with germline BRCA1/2
mutation-positive, early-stage triple-negative breast cancer:
Results of a phase II study. Oncologist. 28:845–855. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Li Y, Li L, Fu H, Yao Q, Wang L and Lou L:
Combined inhibition of PARP and ATR synergistically potentiates the
antitumor activity of HER2-targeting antibody-drug conjugate in
HER2-positive cancers. Am J Cancer Res. 13:161–175. 2023.PubMed/NCBI
|
|
103
|
Kozono DE, Stinchcombe TE, Salama JK,
Bogart J, Petty WJ, Guarino MJ, Bazhenova L, Larner JM, Weiss J,
DiPetrillo TA, et al: Veliparib in combination with
carboplatin/paclitaxel-based chemoradiotherapy in patients with
stage III non-small cell lung cancer. Lung Cancer. 159:56–65. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
O'Reilly EM, Lee JW, Zalupski M, Capanu M,
Park J, Golan T, Tahover E, Lowery MA, Chou JF, Sahai V, et al:
Randomized, multicenter, phase II trial of gemcitabine and
cisplatin with or without veliparib in patients with pancreas
adenocarcinoma and a germline BRCA/PALB2 mutation. J Clin Oncol.
38:1378–1388. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hussain M, Carducci MA, Slovin S, Cetnar
J, Qian J, McKeegan EM, Refici-Buhr M, Chyla B, Shepherd SP,
Giranda VL and Alumkal JJ: Targeting DNA repair with combination
veliparib (ABT-888) and temozolomide in patients with metastatic
castration-resistant prostate cancer. Invest New Drugs. 32:904–912.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Gojo I, Beumer JH, Pratz KW, McDevitt MA,
Baer MR, Blackford AL, Smith BD, Gore SD, Carraway HE, Showel MM,
et al: A phase 1 study of the PARP inhibitor veliparib in
combination with temozolomide in acute myeloid leukemia. Clin
Cancer Res. 23:697–706. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhang Q, Shao B, Tong Z, Ouyang Q, Wang Y,
Xu G, Li S and Li H: A phase Ib study of camrelizumab in
combination with apatinib and fuzuloparib in patients with
recurrent or metastatic triple-negative breast cancer. BMC Med.
20:3212022. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Liu Y, Wang W, Yin R, Zhang Y, Zhang Y,
Zhang K, Pan H, Wang K, Lou G, Li G, et al: A phase 1 trial of
fuzuloparib in combination with apatinib for advanced ovarian and
triple-negative breast cancer: Efficacy, safety, pharmacokinetics
and germline BRCA mutation analysis. BMC Med. 21:3762023.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Friedlander M, Meniawy T, Markman B,
Mileshkin L, Harnett P, Millward M, Lundy J, Freimund A, Norris C,
Mu S, et al: Pamiparib in combination with tislelizumab in patients
with advanced solid tumours: Results from the dose-escalation stage
of a multicentre, open-label, phase 1a/b trial. Lancet Oncol.
20:1306–1315. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Lord CJ and Ashworth A: Mechanisms of
resistance to therapies targeting BRCA-mutant cancers. Nat Med.
19:1381–1388. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Murai J, Huang SY, Das BB, Renaud A, Zhang
Y, Doroshow JH, Ji J, Takeda S and Pommier Y: Trapping of PARP1 and
PARP2 by clinical PARP inhibitors. Cancer Res. 72:5588–5599. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Damale MG, Pathan SK, Shinde DB, Patil RH,
Arote RB and Sangshetti JN: Insights of tankyrases: A novel target
for drug discovery. Eur J Med Chem. 207:1127122020. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Yu M, Yang Y, Sykes M and Wang S:
Small-molecule inhibitors of tankyrases as prospective therapeutics
for cancer. J Med Chem. 65:5244–5273. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Smith S, Giriat I, Schmitt A and de Lange
T: Tankyrase, a poly(ADP-ribose) polymerase at human telomeres.
Science. 282:1484–1487. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Rottenberg S, Jaspers JE, Kersbergen A,
van der Burg E, Nygren AO, Zander SA, Derksen PW, de Bruin M,
Zevenhoven J, Lau A, et al: High sensitivity of BRCA1-deficient
mammary tumors to the PARP inhibitor AZD2281 alone and in
combination with platinum drugs. Proc Natl Acad Sci USA.
105:17079–17084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Abkevich V, Timms KM, Hennessy BT, Potter
J, Carey MS, Meyer LA, Smith-McCune K, Broaddus R, Lu KH, Chen J,
et al: Patterns of genomic loss of heterozygosity predict
homologous recombination repair defects in epithelial ovarian
cancer. Br J Cancer. 107:1776–1782. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang S, Chang CW, Huang J, Zeng S, Zhang
X, Hung MC and Hou J: Gasdermin C sensitizes tumor cells to PARP
inhibitor therapy in cancer models. J Clin Invest. 134:e1668412024.
View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Stringer-Reasor EM, May JE, Olariu E,
Caterinicchia V, Li Y, Chen D, Della Manna DL, Rocque GB, Vaklavas
C, Falkson CI, et al: An open-label, pilot study of veliparib and
lapatinib in patients with metastatic, triple-negative breast
cancer. Breast Cancer Res. 23:302021. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Ray Chaudhuri A, Callen E, Ding X, Gogola
E, Duarte AA, Lee JE, Wong N, Lafarga V, Calvo JA, Panzarino NJ, et
al: Replication fork stability confers chemoresistance in
BRCA-deficient cells. Nature. 535:382–387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Tan Q and Xu X: PTIP UFMylation promotes
replication fork degradation in BRCA1-deficient cells. J Biol Chem.
300:1073122024. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tian T, Chen J, Zhao H, Li Y, Xia F, Huang
J, Han J and Liu T: UFL1 triggers replication fork degradation by
MRE11 in BRCA1/2-deficient cells. Nat Chem Biol. 20:1650–1661.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Tutt ANJ, Garber JE, Kaufman B, Viale G,
Fumagalli D, Rastogi P, Gelber RD, de Azambuja E, Fielding A,
Balmaña J, et al: Adjuvant olaparib for patients with BRCA1- or
BRCA2-mutated breast cancer. N Engl J Med. 384:2394–2405. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Gelmon KA, Fasching PA, Couch FJ, Balmaña
J, Delaloge S, Labidi-Galy I, Bennett J, McCutcheon S, Walker G and
O'Shaughnessy J; Collaborating Investigator, : Clinical
effectiveness of olaparib monotherapy in germline BRCA-mutated,
HER2-negative metastatic breast cancer in a real-world setting:
Phase IIIb LUCY interim analysis. Eur J Cancer. 152:68–77. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Pusztai L, Yau C, Wolf DM, Han HS, Du L,
Wallace AM, String-Reasor E, Boughey JC, Chien AJ, Elias AD, et al:
Durvalumab with olaparib and paclitaxel for high-risk HER2-negative
stage II/III breast cancer: Results from the adaptively randomized
I-SPY2 trial. Cancer Cell. 39:989–998.e5. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Batalini F, Xiong N, Tayob N, Polak M,
Eismann J, Cantley LC, Shapiro GI, Adalsteinsson V, Winer EP,
Konstantinopoulos PA, et al: Phase 1b clinical trial with alpelisib
plus olaparib for patients with advanced triple-negative breast
cancer. Clin Cancer Res. 28:1493–1499. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Geyer CE Jr, Garber JE, Gelber RD, Yothers
G, Taboada M, Ross L, Rastogi P, Cui K, Arahmani A, Aktan G, et al:
Overall survival in the OlympiA phase III trial of adjuvant
olaparib in patients with germline pathogenic variants in BRCA1/2
and high-risk, early breast cancer. Ann Oncol. 33:1250–1268. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Robson ME, Im SA, Senkus E, Xu B, Domchek
SM, Masuda N, Delaloge S, Tung N, Armstrong A, Dymond M, et al:
OlympiAD extended follow-up for overall survival and safety:
Olaparib versus chemotherapy treatment of physician's choice in
patients with a germline BRCA mutation and HER2-negative metastatic
breast cancer. Eur J Cancer. 184:39–47. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Yamauchi H, Toi M, Takayama S, Nakamura S,
Takano T, Cui K, Campbell C, De Vos L, Geyer C Jr and Tutt A:
Adjuvant olaparib in the subset of patients from Japan with BRCA1-
or BRCA2-mutated high-risk early breast cancer from the phase 3
OlympiA trial. Breast Cancer. 30:596–605. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Senkus E, Delaloge S, Domchek SM, Conte P,
Im SA, Xu B, Armstrong A, Masuda N, Fielding A, Robson M and Tung
N: Olaparib efficacy in patients with germline BRCA-mutated,
HER2-negative metastatic breast cancer: Subgroup analyses from the
phase III OlympiAD trial. Int J Cancer. 153:803–814. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Ring A, Kilburn LS, Pearson A, Moretti L,
Afshari-Mehr A, Wardley AM, Gurel B, Macpherson IR, Riisnaes R,
Baird RD, et al: Olaparib and ceralasertib (AZD6738) in patients
with triple-negative advanced breast cancer: Results from cohort E
of the plasmaMATCH trial (CRUK/15/010). Clin Cancer Res.
29:4751–4759. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Balmaña J, Fasching PA, Couch FJ, Delaloge
S, Labidi-Galy I, O'Shaughnessy J, Park YH, Eisen AF, You B,
Bourgeois H, et al: Clinical effectiveness and safety of olaparib
in BRCA-mutated, HER2-negative metastatic breast cancer in a
real-world setting: Final analysis of LUCY. Breast Cancer Res
Treat. 204:237–248. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Tan TJ, Sammons S, Im YH, She L, Mundy K,
Bigelow R, Traina TA, Anders C, Yeong J, Renzulli E, et al: Phase
II DORA study of olaparib with or without durvalumab as a
chemotherapy-free maintenance strategy in platinum-pretreated
advanced triple-negative breast cancer. Clin Cancer Res.
30:1240–1247. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Abraham JE, Pinilla K, Dayimu A, Grybowicz
L, Demiris N, Harvey C, Drewett LM, Lucey R, Fulton A, Roberts AN,
et al: The PARTNER trial of neoadjuvant olaparib with chemotherapy
in triple-negative breast cancer. Nature. 629:1142–1148. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Litton JK, Rugo HS, Ettl J, Hurvitz SA,
Gonçalves A, Lee KH, Fehrenbacher L, Yerushalmi R, Mina LA, Martin
M, et al: Talazoparib in patients with advanced breast cancer and a
germline BRCA mutation. N Engl J Med. 379:753–763. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Ettl J, Quek RGW, Lee KH, Rugo HS, Hurvitz
S, Gonçalves A, Fehrenbacher L, Yerushalmi R, Mina LA, Martin M, et
al: Quality of life with talazoparib versus physician's choice of
chemotherapy in patients with advanced breast cancer and germline
BRCA1/2 mutation: Patient-reported outcomes from the EMBRACA phase
III trial. Ann Oncol. 29:1939–1947. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Litton JK, Scoggins ME, Hess KR, Adrada
BE, Murthy RK, Damodaran S, DeSnyder SM, Brewster AM, Barcenas CH,
Valero V, et al: Neoadjuvant talazoparib for patients with operable
breast cancer with a germline BRCA pathogenic variant. J Clin
Oncol. 38:388–394. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Litton JK, Hurvitz SA, Mina LA, Rugo HS,
Lee KH, Gonçalves A, Diab S, Woodward N, Goodwin A, Yerushalmi R,
et al: Talazoparib versus chemotherapy in patients with germline
BRCA1/2-mutated HER2-negative advanced breast cancer: Final overall
survival results from the EMBRACA trial. Ann Oncol. 31:1526–1535.
2020. View Article : Google Scholar : PubMed/NCBI
|