Introduction
Venetoclax, an orally administered B-cell lymphoma
(BCL-2) inhibitor plays a crucial role in regulating cell survival
by inhibiting apoptosis. Overexpression of BCL-2 in cancer cells
can promote the survival and proliferation of such cells (1–4).
Venetoclax selectively binds to BCL-2, restoring the apoptotic
process within cancer cells and inhibiting tumor growth (5). Studies have demonstrated the notable
therapeutic efficacy of venetoclax in various malignancies,
including acute myeloid leukemia (AML), chronic lymphocytic
leukemia, multiple myeloma and mantle cell lymphoma (6–20).
In hematological-oncological treatment, antifungal
agents are commonly used either for therapeutic or prophylactic
purposes alongside anticancer medications (21). Specific antifungal agents, such as
posaconazole and voriconazole, act as cytochrome P450 inhibitors
(22,23), and since venetoclax is metabolized
through cytochrome P450, their co-administration can result in drug
interactions, necessitating a reduction in venetoclax dosage to
prevent potential adverse effects (24).
The literature emphasizes the need for dose
adjustments when combining venetoclax with cytochrome P450
inhibitors in patients with AML. However, drug interactions
affecting venetoclax blood concentrations are non-linear (25,26).
Currently, research on clinical outcomes in patients with AML
receiving reduced venetoclax doses due to such interactions is
limited. Furthermore, venetoclax has a restricted duration of
coverage under Taiwan's National Health Insurance system.
Therefore, to alleviate the financial burden or extend treatment
duration, combining venetoclax with antifungal agents may be a
viable option for patients with AML who cannot tolerate intensive
chemotherapy.
This single-center study retrospectively
investigated progression-free survival (PFS), overall survival (OS)
and drug-related adverse events (AEs) in patients with AML who
received adjusted venetoclax doses in combination with antifungal
agents compared with those receiving standard treatment.
Materials and methods
This retrospective observational single-center study
included patients who had been treated within a 7-year period
between January 2015 and December 2021, and who met the following
inclusion criteria: i) Age >20 years; ii) diagnosis of non-M3
AML classified according to the French-American-British
classification system (27); iii)
AML treatment with venetoclax and a cytochrome P450 inhibitor
(posaconazole or voriconazole); iv) induction therapy with
idarubicin + cytarabine and high-dose cytarabine consolidation
therapy; and v) treatment with low-dose cytarabine (LDAC). Patient
demographic and clinical characteristics, namely age, sex, Eastern
Cooperative Oncology Group (ECOG) performance status (28), cytogenetic risk category (29), somatic mutations, bone marrow blast
count, PFS, OS and optimal treatment response dose, were collected
retrospectively. Ethical approval was granted by the Chi-Mei
Medical Center Institutional Review Board (approval no. 11107-L01)
on August 2, 2022, and the study commenced on August 8, 2022, and
was conducted in accordance with the Helsinki Declaration. The
requirement for informed patient consent was exempted by the
Institutional Review Board. Patients were enrolled from the
Division of Hematology-Oncology, Department of Internal Medicine,
Chi-Mei Medical Center (Tainan, Taiwan). To ensure patient
confidentiality, all data were anonymized and were accessible only
through unique codes. In accordance with legal regulations, only
the principal investigator was authorized access and allowed to
manage the complete dataset.
The primary outcomes of the study were OS (defined
as the time from the start of drug treatment to death) and PFS
(defined as the time from treatment initiation to disease
progression or treatment change). For patients alive at the study
endpoint, data were based on the most recent confirmation of
survival date.
Secondary outcomes included the evaluation of
treatment-related grade 3/4 AEs, based on the National Cancer
Institute's Common Terminology Criteria for Adverse Events version
5.0 (30), and treatment response
rates, based on the criteria outlined by Döhner et al
(31).
AML treatment regimens included induction therapy
with idarubicin and cytarabine (12 mg/m2 idarubicin on
days 1–3 + 100 mg/m2 cytarabine on days 1–7 or 12
mg/m2 idarubicin on days 1–2 + 100 mg/m2
cytarabine on days 1–5). Consolidation therapy involved 1–2
g/m2 cytarabine on days 1, 3 and 5. Venetoclax-based
regimens included 100 mg venetoclax on days 1–28 + 300 mg
posaconazole on days 1–28 + 100 mg azacitidine on days 1–7 (28 days
per cycle) or 100 mg venetoclax on days 1–28 + 300 mg posaconazole
on days 1–28 or 20 mg/m2 LDAC on days 1–10 + 100 mg
venetoclax on days 1–28 + 300 mg posaconazole on days 1–28 or 200
mg voriconazole twice daily on days 1–28 (28 days per cycle). The
cytarabine-based regimen involved 20 mg/m2 LDAC on days
1–10 (28 days per cycle). Treatment continued until disease
progression or until the patient could no longer tolerate drug side
effects.
Statistical analysis
Statistical analysis involved descriptive statistics
to analyze retrospective patient data. Baseline clinical
characteristics were compared among the four groups using
appropriate statistical methods. Age was analyzed using the
Kruskal-Wallis test, as it did not follow a normal distribution.
Categorical variables were analyzed using the Fisher-Freeman-Halton
Exact Test. The overall response rate and AEs were also analyzed
using the Fisher-Freeman-Halton Exact Test to assess statistical
differences across groups. Associations with OS and PFS were
assessed using a Cox proportional hazards regression model. The
Kaplan-Meier product-limit method was used to calculate PFS and OS
curves, with differences between subgroups assessed using the
log-rank test. P<0.05 was considered to indicate a statistically
significant difference. All analyses were performed using MedCalc
version 20.007 (MedCalc Software Ltd.) or R software version 4.4.2
(The R Foundation for Statistical Computing).
Results
Patients
Between January 2015 and December 2021, 45 patients
with AML were retrospectively included in the study. These patients
were stratified into the following four groups based on their
first-line treatment regimen: The I3A7, LDAC and I2A5 groups, which
received conventional chemotherapy as standard therapy, and the
venetoclax group, which received an adjusted low-dose venetoclax
regimen combined with antifungal agents. The I3A7 group were those
patients who received idarubicin on days 1–3 + cytarabine on days
1–7 (n=19); the venetoclax group included patients who received one
of the three venetoclax-based regimens, namely venetoclax +
azacitidine + antifungal agents, venetocalx + LDAC + antifungal
agents or venetocalx+antifungal agents (n=14). All patients in the
venetoclax group received 100 mg venetoclax daily alone with
antifungal agents. The LDAC group received 20 mg/m2 LDAC
on days 1–10 (n=9); and the I2A5 group were those who received
idarubicin on days 1–2 + cytarabine on days 1–5 (n=3). Demographic
characteristics are summarized in Table
I, and the groupings are illustrated in Fig. 1. In the I3A7 group, the median age
was 52 years (range, 46–75 years), with only 1 patient (5%) older
than 75 years. In the venetoclax group, the median age was 71 years
(range, 50–82 years), with 5 patients (36%) older than 75 years. In
the LDAC group, the median age was 79 years (range, 71–90 years),
with 7 patients (78%) older than 75 years. In the I2A5 group, the
median age was 69 years (range, 36–79 years), with 1 patient (33%)
older than 75 years. Regarding sex distribution, 10 patients (53%)
in the I3A7 group, 7 patients (50%) in the venetoclax group, 6
patients (67%) in the LDAC group, and 2 patients (67%) in the I2A5
group were male. Most patients (58%) in the I3A7 group had an ECOG
performance score of 0, while most patients in the other groups had
a performance status score of 1. Bone marrow blast counts were
predominantly <30% across all groups. The cytogenetic risk
category was mostly intermediate, and somatic mutations were most
prevalent in the I3A7 group.
 | Table I.Baseline demographic and clinical
characteristics of the study patients. |
Table I.
Baseline demographic and clinical
characteristics of the study patients.
| Characteristic | I3A7 group
(n=19) | Venetoclax group
(n=14) | LDAC group
(n=9) | I2A5 group
(n=3) | P-value |
|---|
| Age, years |
|
|
|
| <0.01 |
| Median
(range) | 52 (46–75) | 71 (50–82) | 79 (71–90) | 69 (36–79) |
|
| ≥75, n
(%) | 1 (5) | 5 (36) | 7 (78) | 1 (33) |
|
| Sex |
|
|
|
| 0.88 |
| Male, n (%) | 10 (53) | 7 (50) | 6 (67) | 2 (67%) |
|
| ECOG performance
status score, n (%) |
|
|
|
| 0.17 |
| 0 | 11 (58) | 6 (43) | 2 (22) | 1 (33) |
|
| 1 | 7 (37) | 8 (57) | 4 (44) | 2 (67) |
|
| 2 | 1 (5) | 0 (0) | 3 (33) | 0 (0) |
|
| Bone marrow blast
count, n (%) |
|
|
|
| 0.34 |
|
<30% | 14 (74) | 6 (43) | 7 (78) | 2 (67) |
|
|
30-50% | 2 (11) | 2 (14) | 1 (11) | 1 (33) |
|
|
>50% | 3 (16) | 6 (43) | 1 (11) | 0 (0) |
|
| Cytogenetic risk
category, n (%) |
|
|
|
| 0.80 |
|
Favorable | 4 (21) | 2 (14) | 0 (0) | 0 (0) |
|
|
Intermediate | 12 (63) | 11 (79) | 8 (89) | 3 (100) |
|
|
Poor | 3 (16) | 1 (7) | 1 (11) | 0 (0) |
|
| Somatic mutations,
n (%) |
|
|
|
| 0.12 |
|
ASXL1 | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
|
| BCR
ABL1 | 0 (0) | 0 (0) | 1 (11) | 0 (0) |
|
|
CBFB-MYH11 | 0 (0) | 2 (14) | 0 (0) | 0 (0) |
|
|
FLT3&NPM1 | 1 (5) | 0 (0) | 1 (11) | 0 (0) |
|
|
IDH2 | 0 (0) | 1 (7) | 0 (0) | 0 (0) |
|
|
NPM1 | 2 (11) | 0 (0) | 0 (0) | 0 (0) |
|
|
RUNX1 CBFB-MYH11 | 3 (16) | 0 (0) | 0 (0) | 0 (0) |
|
|
TP53 | 1 (5) | 1 (7) | 0 (0) | 0 (0) |
|
|
abn(17p) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
|
|
del(5q) | 1 (5) | 0 (0) | 0 (0) | 0 (0) |
|
Baseline clinical characteristics showed a
statistically significant age difference across the four groups
(P<0.01). However, no significant differences were found for sex
(P=0.88), ECOG (P=0.17), bone marrow blast count (P=0.34),
cytogenetic risk category (P=0.80) or somatic mutations (P=0.12)
(Table I). Additionally, age was
found to be significantly associated with OS (P<0.05), but no
significant association was found for PFS (Table SI).
Efficacy
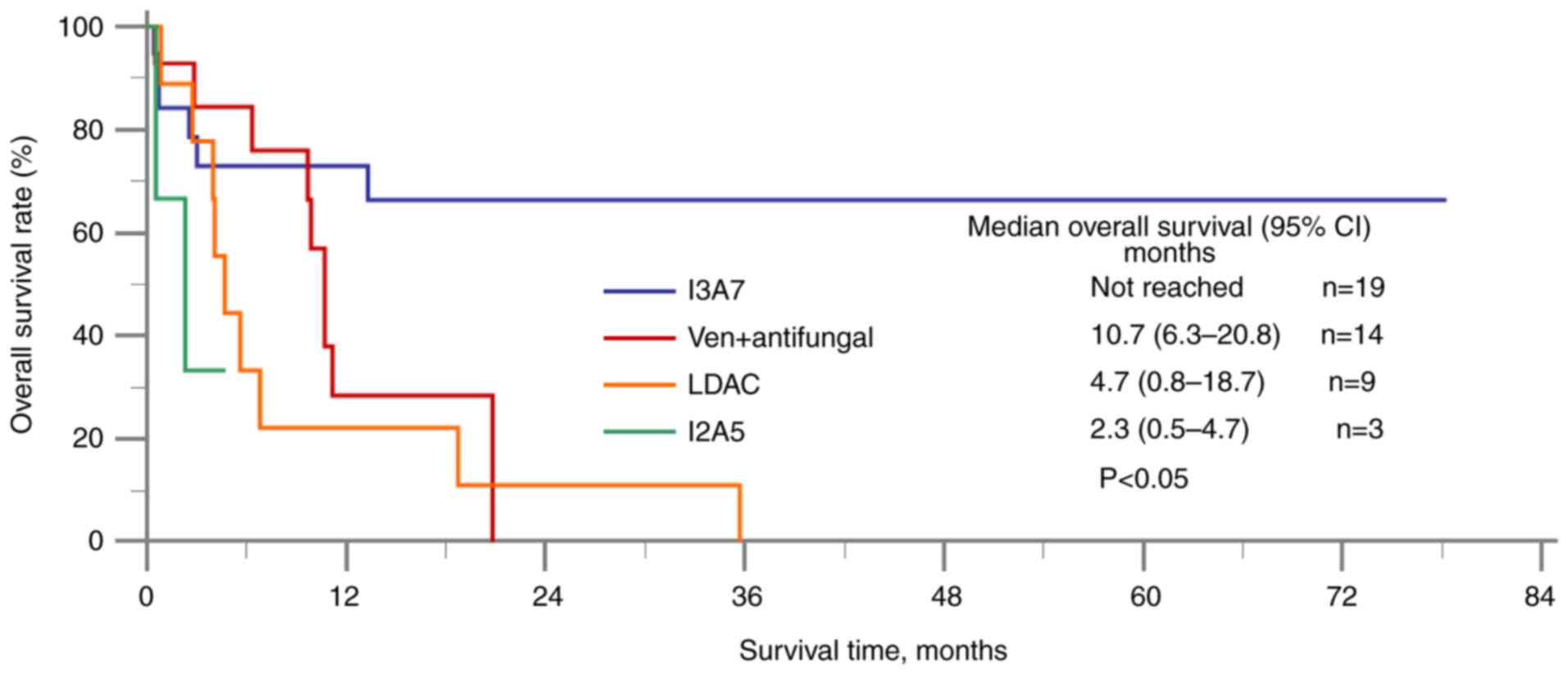
The primary OS outcomes are shown in Fig. 2. The I3A7 group was found to have a
significantly longer median OS time (median not reached) compared
with the venetoclax + antifungal group [10.7 months; 95% confidence
interval (CI), 6.3–20.8], the LDAC group (4.7 months; 95% CI,
0.8–18.7) and the I2A5 group (2.3 months; 95% CI, 0.5–2.3). For
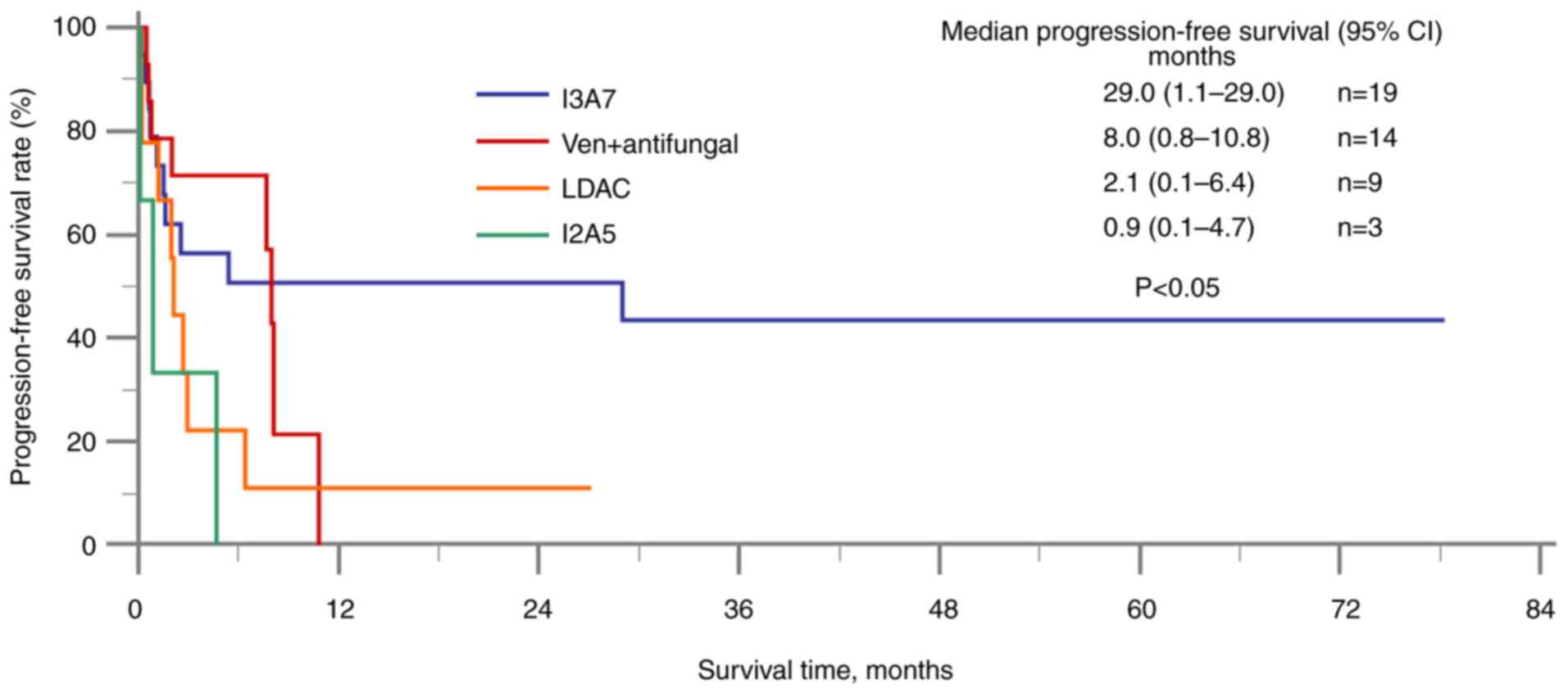
PFS, the I3A7 group had a significantly longer median PFS time
(29.0 months; 95% CI, 1.1–29.0) compared with the venetoclax group
(8.0 months; 95% CI, 0.8–10.8), the LDAC group (2.1 months; 95% CI,
0.1–6.4) and the I2A5 group (0.9 months; 95% CI, 0.1–4.7) (Fig. 3). The response rates of all the
study patients are detailed in Table
II, with a statistically significant difference in overall
response rate observed across the groups (P<0.05). In the I3A7
group, 4 patients (21%) achieved complete remission, while 4
patients (21%) also achieved complete remission, but with
incomplete hematological recovery. In the venetoclax group, 1
patient (7%) achieved complete remission with incomplete
hematological recovery. Partial response was observed in 2 patients
in both the venetoclax (14%) and I2A5 (67%) groups. Stable disease
was reported in 6 patients (32%) in the I3A7 group, 6 patients
(43%) in the venetoclax group and 3 patients (33%) in the LDAC
group. Progressive disease occurred in 5 patients (26%) in the I3A7
group, 5 patients (36%) in the venetoclax group, 6 patients (67%)
in the LDAC group and 1 patient (33%) in the I2A5 group. Among the
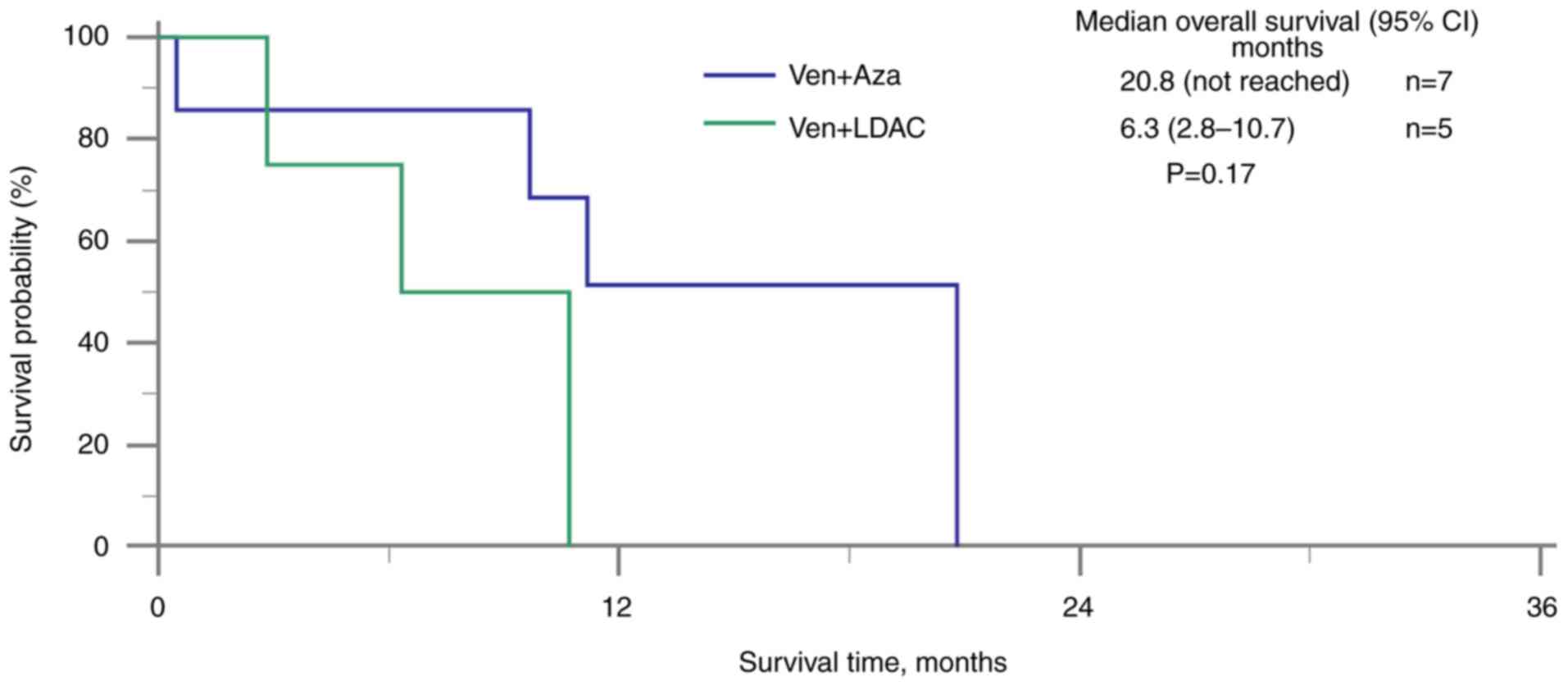
patients treated with venetoclax with antifungal agents, those who
also received azacitidine had a longer median OS time (20.8 months;
95% CI not reached) compared with those who received LDAC (6.3
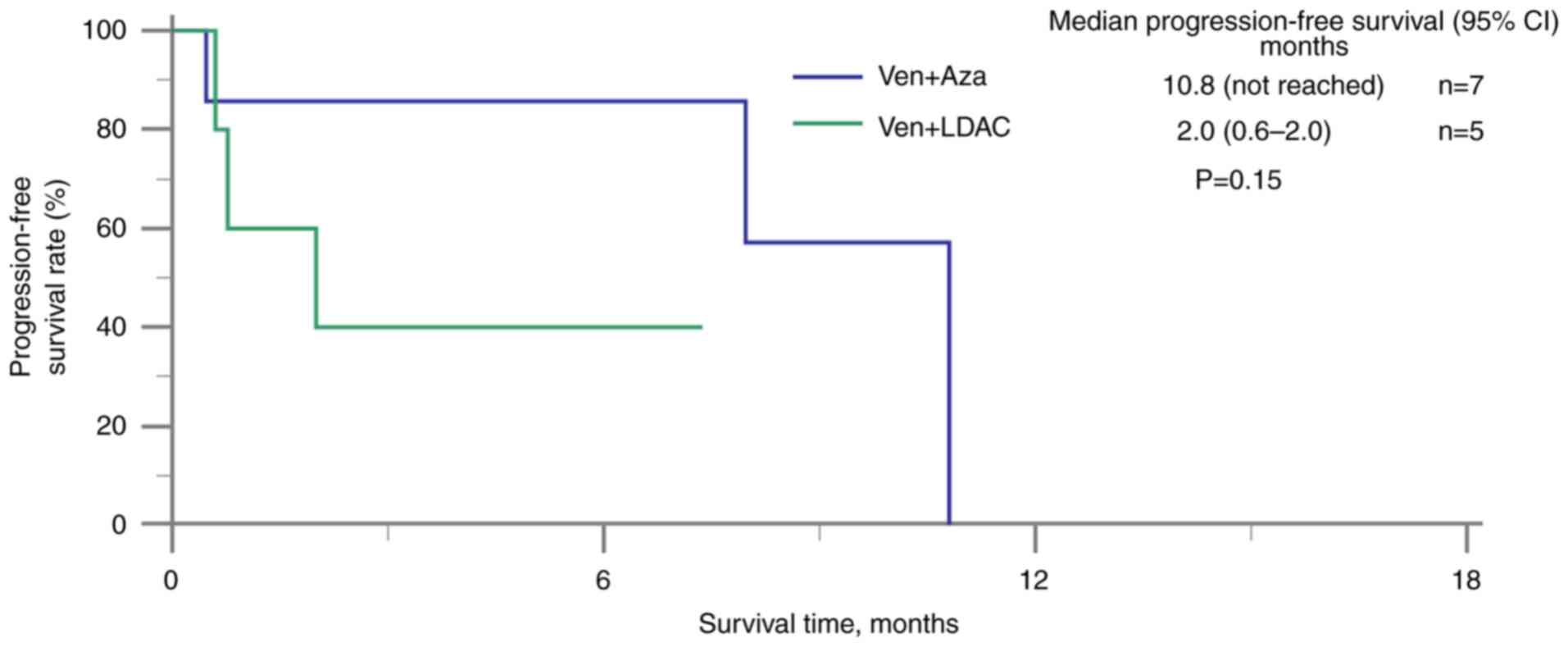
months; 95% CI, 2.8–10.7) (Fig. 4).
Patients receiving venetoclax, antifungal agents and azacitidine
had a longer median PFS time (10.8 months; 95% CI not reached)
compared with those receiving venetoclax, antifungal agents and
LDAC (2.0 months; 95% CI, 0.6–2.0) (Fig. 5). Additionally, patients in the I3A7
group exhibited a longer median RFS time (not reached) compared
with those in the venetoclax plus antifungal agents group (2.4
months, 95% CI: NA) (Fig. S1).
However, none of these differences were statistically
significant.
 | Table II.Response rates for all patients. |
Table II.
Response rates for all patients.
| Response | I3A7 group
(n=19) | Venetoclax group
(n=14) | LDAC group
(n=9) | I2A5 group
(n=3) | P-value |
|---|
| CR, n (%) | 4 (21) | 0 (0) | 0 (0) | 0 (0) |
|
| CRi, n (%) | 4 (21) | 1 (7) | 0 (0) | 0 (0) |
|
| PR, n (%) | 0 (0) | 2 (14) | 0 (0) | 2 (67) |
|
| SD, n (%) | 6 (32) | 6 (43) | 3 (33) | 0 (0) |
|
| PD, n (%) | 5 (26) | 5 (36) | 6 (67) | 1 (33) |
|
| ORR, n (%) | 8 (42) | 3 (21) | 0 (0) | 2 (67) | P<0.05 |
Safety
The safety analysis comprised 45 patients (19 in the
I3A7 group, 14 in the venetoclax group, 9 in the LDAC group and 3
in the I2A5 group), with the most common AEs summarized in Table III. Grade 3 or higher
hematological AEs were frequently reported in the groups, including
thrombocytopenia (89, 71, 78 and 67% in the I3A7, venetoclax, LDAC
and I2A5 groups, respectively), neutropenia (100, 79, 67 and 100%,
respectively) and anemia (37, 57, 44 and 67%, respectively). No
statistically significant differences in AEs were observed among
the groups, including anemia, neutropenia, thrombocytopenia, fever,
sepsis, increased ALT levels, pneumonia, other infections,
increased AST levels, increased Scr levels, cardiovascular events
and tumor lysis syndrome. Grade 3 or higher infection-related AEs
were most frequently reported in the I3A7 group and included fever
(21, 7, 0 and 0%, in the I3A7, venetoclax, LDAC and I2A5 groups,
respectively), pneumonia (11, 21, 33 and 33%, respectively), sepsis
(63, 36, 11 and 67%, respectively) and other infections (21, 14, 22
and 0%, respectively). Finally, cardiovascular events and grade 3
or higher TLS (14 and 7%, respectively) were only observed in the
venetoclax group.
 | Table III.Patient AEs. |
Table III.
Patient AEs.
|
| I3A7 group
(n=19) | Venetoclax group
(n=14) | LDAC group
(n=9) | I2A5 group
(n=3) |
|
|---|
|
|
|
|
|
|
|
|---|
| Event | All grades | ≥ Grade 3 | All grades | ≥ Grade 3 | All grades | ≥ Grade 3 | All grades | ≥ Grade 3 | P-value |
|---|
| All AEs | 19 (100) | 19 (100) | 14 (100) | 13 (93) | 9 (100) | 8 (89) | 3 (100) | 3 (100) | >0.99 |
| Anemia | 18 (95) | 7 (37) | 12 (86) | 8 (57) | 8 (89) | 4 (44) | 3 (100) | 2 (67) | 0.85 |
| Neutropenia | 19 (100) | 19 (100) | 12 (86) | 11 (79) | 7 (78) | 6 (67) | 3 (100) | 3 (100) | 0.16 |
|
Thrombocytopenia | 19 (100) | 17 (89) | 13 (93) | 10 (71) | 8 (89) | 7 (78) | 3 (100) | 2 (67) | 0.39 |
| Fever | 13 (68) | 4 (21) | 6 (43) | 1 (7) | 5 (56) | 0 (0) | 3 (100) | 0 (0) | 0.27 |
| Pneumonia | 2 (11) | 2 (11) | 3 (21) | 3 (21) | 3 (33) | 3 (33) | 1 (33) | 1 (33) | 0.41 |
| Sepsis | 12 (63) | 12 (63) | 5 (36) | 5 (36) | 2 (22) | 1 (11) | 2 (67) | 2 (67) | 0.14 |
| Other
infections | 5 (26) | 4 (21) | 6 (43) | 2 (14) | 4 (44) | 2 (22) | 0 (0) | 0 (0) | 0.51 |
| AST increased | 8 (42) | 1 (5) | 5 (36) | 0 (0) | 0 (0) | 0 (0) | 1 (33) | 0 (0) | 0.12 |
| ALT increased | 10 (53) | 1 (5) | 8 (57) | 0 (0) | 1 (11) | 0 (0) | 1 (33) | 0 (0) | 0.11 |
| Scr increased | 5 (26) | 2 (11) | 7 (50) | 1 (7) | 4 (44) | 0 (0) | 0 (0) | 0 (0) | 0.33 |
| CV event | 0 (0) | 0 (0) | 2 (14) | 2 (14) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.26 |
| TLS | 0 (0) | 0 (0) | 1 (7) | 1 (7) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0.58 |
Discussion
For elderly patients with AML or those unfit for
intensive chemotherapy, venetoclax combined with hypomethylating
agents or LDAC has become the gold-standard treatment in numerous
clinical settings (13,15). However, in Taiwan, financial
constraints and limited health insurance coverage restrict the
widespread clinical application of this treatment approach. During
the period covered by the present study, venetoclax was not
reimbursed under Taiwan's National Health Insurance system.
According to current regulations, its coverage is restricted to
frail patients and limited to a maximum of four cycles. As a
result, patients requiring venetoclax must bear a portion of the
medication cost themselves. Considering that not all patients have
the financial capacity to afford the treatment, a potential
solution to this issue is using a reduced dosage of venetoclax in
conjunction with antifungal agents, which can lower medication
costs while maintaining efficacy and safety. This approach aims to
optimize treatment outcomes while alleviating the financial burden
on patients.
Elderly patients with AML often have reduced
tolerance to intensive chemotherapy and face an increased risk of
complications. Consequently, low-intensity treatment regimens are
advisable to balance efficacy and tolerability. Previous research
has highlighted that advanced age is associated with a poorer
prognosis in AML, emphasizing the need to consider physiological
age and overall health when making treatment decisions (32). Although no statistically significant
differences were observed in the cytogenetic risk categories
(P=0.80) and somatic mutations (P=0.12) across treatment groups in
our study (which may reflect the small sample size or patient
heterogeneity), these factors remain important prognostic markers
in AML (33) and should be
considered in future studies that aim to tailor treatment
strategies more effectively.
The present study uniquely focused on the efficacy
and safety of reduced-dose venetoclax (primarily 100 mg daily)
combined with antifungal agents such as voriconazole and
posaconazole. In this retrospective analysis, the I3A7 group
demonstrated superior OS and PFS times compared with the
venetoclax, LDAC and I2A5 groups, consistent with current AML
treatment guidelines (34).
Additionally, among the patients receiving venetoclax combined with
antifungal agents, those treated with azacitidine exhibited
numerically longer median OS and PFS times compared with those
treated with LDAC. While this trend suggests a potential benefit of
combining azacitidine with venetoclax over LDAC, the differences
were not statistically significant. The I3A7 group also
demonstrated a numerically longer median RFS time compared with the
venetoclax plus antifungal agents group, although this analysis was
limited to patients achieving complete remission (8 in the I3A7
group and 1 in the venetoclax group), reducing the statistical
power and with a lack of statistically significant differences,
thereby limiting the generalizability of the findings. Larger
studies are needed to validate these results and explore
venetoclax-based regimens in AML treatment. While the venetoclax
group exhibited slightly lower OS and PFS times than the I3A7
group, patients also experienced fewer hematological and
infection-related AEs. Additionally, despite poorer survival
outcomes compared with the more intensive I3A7 chemotherapy
regimen, venetoclax achieved more favorable outcomes than
traditional treatments such as LDAC and I2A5, particularly when
combined with hypomethylating agents. Patients in the venetoclax
group also exhibited a lower risk of infection compared with the
I3A7 and I2A5 groups, and patients who were treated with
hypomethylating agents showed better survival outcomes than those
treated with the LDAC regimen. Accordingly, venetoclax-based
combination regimens should be considered a superior treatment
option to LDAC or I2A5, particularly for elderly or frail AML
patients.
Regarding antifungal prophylaxis, 3 patients in the
I3A7 group and 7 patients in the LDAC group did not receive
prophylactic antifungal therapy. However, no cases of fungal
infections were observed in any treatment group throughout the
study. This aligns with recent findings (35) that suggest a low incidence of
invasive fungal infections among patients with AML treated with
venetoclax and hypomethylating agents, even without routine
antifungal prophylaxis. This study (35) and others, suggest that while
antifungal prophylaxis can be beneficial, its necessity should be
determined based on individual patient risk factors and specific
treatment regimens. Similarly, another previous study (36) found that antifungal prophylaxis did
not significantly reduce fungal infections in patients receiving
azacitidine alone, further indicating that routine prophylaxis may
not be necessary for all patients. These findings imply that the
risk of fungal infections in certain AML treatment regimens may be
lower than expected, and the decision to implement antifungal
prophylaxis should be tailored to the specific treatment context
and patient risk profile. The present clinical observations,
supported by the extant literature, suggest that the absence of
antifungal prophylaxis in specific patient groups, including the
LDAC group, did not lead to an increased incidence of fungal
infections. In the venetoclax group, antifungal agents were, in
fact, primarily administered to increase drug concentration not to
prevent infections.
The observed statistical difference in overall
response rate (P<0.05) in the present study provides valuable
insights into the clinical implications of the treatment regimens.
This finding suggests that certain regimens may have better
efficacy, but their safety profiles should still be carefully
evaluated to ensure a favorable risk-benefit balance. By contrast,
no statistically significant differences in AEs were observed among
the groups. However, common hematological toxicities, including
anemia, neutropenia and thrombocytopenia, remain critical concerns
in AML treatment and necessitate careful monitoring and supportive
care, such as transfusions when required. Similarly,
infection-related events, including fever and sepsis, should be
actively managed through early detection and prophylactic
strategies to mitigate potential complications. While hepatic and
renal toxicities did not differ significantly between groups,
routine liver and kidney function assessments remain essential in
clinical practice. While no statistically significant differences
in AEs were found, continued monitoring remains essential to ensure
patient safety and optimize treatment outcomes.
Notably, in the venetoclax group, two grade 3 or
higher cardiovascular AEs were identified. Literature regarding
venetoclax-related cardiovascular reactions is limited (12,14,20),
with only one such publication suggesting a potential mechanism
where venetoclax induces cardiovascular toxicity through the NF-κB
and BCL-2 pathways, which regulate oxidative stress-mediated
cardiac inflammation and apoptosis (37). However, further analysis is needed
to better understand the origins of these adverse cardiovascular
reactions.
Another grade 3 or higher adverse reaction
identified in the present study was TLS, with incidence rates
comparable to those reported in previous clinical trials (11,12,20).
Venetoclax is metabolized by the liver enzyme cytochrome P450 3A4
(24), therefore the present study
conducted a subgroup analysis in the venetoclax group to assess the
combined effects of multiple cytochrome P450 (CYP) inhibitors and
chemotherapy drugs on OS and PFS time. However, the analysis
concluded that this did not significantly influence OS or PFS time,
which suggests that a 75% reduction in venetoclax dosage with CYP
inhibitors may not affect treatment efficacy (24). This dose reduction could potentially
lower medication costs and reduce adverse reactions compared with
normal doses of venetoclax. Previous studies have shown that strong
CYP inhibitors such as ritonavir (38), ketoconazole (39), itraconazole (26,40,41)
and posaconazole (25,42) can significantly increase venetoclax
levels in the bloodstream.
The unique aspect of the present study was the use
of low-dose venetoclax in combination with potent CYP inhibitors.
Compared with standard chemotherapy, the venetoclax plus antifungal
agents regimen resulted in lower PFS time, OS time and overall
response rate. However, it also had a lower infection risk and
reduced drug costs, offering potential savings for patients.
The present study also had several limitations
primarily stemming from its retrospective design, which may have
resulted in small sample sizes in certain groups, potentially
introducing statistical bias. For instance, the venetoclax plus
azacitidine and antifungal agents group was not represented
separately in Fig. 1, Fig. 2, Fig.
3, 4 due to the small sample
size, which limited the statistical power for meaningful
comparisons. However, aggregated data for this subgroup showed
promising trends in terms of efficacy and safety. While these
findings are encouraging, they should be interpreted with caution,
and further studies with larger sample sizes are necessary to
validate these observations. Moreover, venetoclax blood
concentrations were not monitored during the study period, although
it has previously been suggested that body surface area is
negatively correlated with venetoclax levels, implying that
monitoring these blood concentrations could improve personalized
treatment strategies (43). A final
limitation of the present study is the absence of patients with
relapsed AML and those receiving standard venetoclax therapy, which
is a key area for future research.
In conclusion, the present study suggests that
low-dose venetoclax combined with antifungal agents may be inferior
to standard chemotherapy treatment, but more effective than
regimens involving LDAC or I2A5. In addition, venetoclax
demonstrated a relatively low risk of infection, but vigilant
monitoring for adverse cardiovascular events and TLS, particularly
in patients with relevant medical histories, is crucial. Further
studies are necessary to validate these findings.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by Chi Mei Medical Center,
Liouying, Tainan, Taiwan (grant nos. CLFHR11133 and
CLFHR11106).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
SYH and KYW were responsible for designing the
research and extracting the data. WTH, CYL and KYC conducted the
statistical analysis and handled data visualization and
interpretation. TSW contributed to data analysis and
interpretation, particularly in assessing statistical outcomes and
refining subgroup classifications. SYH and TSW prepared the initial
draft of the manuscript, performed critical revisions to the
manuscript, focusing on essential intellectual content and reviewed
the data analysis. TSW shaped the study's conclusions and aligned
them with the statistical findings. SYH and TSW confirm the
authenticity of all the raw data. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
This study was conducted in accordance with the
Declaration of Helsinki guidelines and received approval from the
Ethics Committee of Chi Mei Medical Center, Liouying (Tainan,
Taiwan; approval no. 11107-L01). The requirement for informed
patient consent was exempted by the Institutional Review Board.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lessene G, Czabotar PE and Colman PM:
Bcl-2 family antagonists for cancer therapy. Nat Rev Drug Discov.
7:989–1000. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Letai AG: Diagnosing and exploiting
cancer's addiction to blocks in apoptosis. Nat Rev Cancer.
8:121–132. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Marzo I and Naval J: Bcl-2 family members
as molecular targets in cancer therapy. Biochem Pharmacol.
76:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Scarfò L and Ghia P: Reprogramming cell
death: Bcl2 family inhibition in hematological malignancies.
Immunol Lett. 155:36–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Anderson MA, Deng J, Seymour JF, Tam CK,
Kim SY, Fein J, Yu L, Brown JR, Westerman D, Si EG, et al: The bcl2
selective inhibitor venetoclax induces rapid onset apoptosis of cll
cells in patients via a tp53-independent mechanism. Blood.
127:3215–3224. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Robak T, Smolewski P, Robak P and Dreyling
M: Mantle cell lymphoma: Therapeutic options in
transplant-ineligible patients. Leuk Lymphoma. 60:2622–2634. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bhatt P, Kloock C and Comenzo R:
Relapsed/refractory multiple myeloma: A review of available
therapies and clinical scenarios encountered in myeloma relapse.
Curr Oncol. 30:2322–2347. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
El-Cheikh J, Bidaoui G, Saleh M, Moukalled
N, Dalle IA and Bazarbachi A: Venetoclax: A new partner in the
novel treatment era for acute myeloid leukemia and myelodysplastic
syndrome. Clin Hematol Int. 5:143–154. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Laurenti L, Scarfò L, Frustaci AM, Sanna
A, Iannella E, Caira M, Finsinger P, Schifano S, Neri B, Molica S
and Mauro FR: Real-world evidence on venetoclax in chronic
lymphocytic leukemia: The Italian experience. Hematol Oncol.
41:621–630. 2023. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Cramer P, von Tresckow J, Bahlo J,
Robrecht S, Langerbeins P, Al-Sawaf O, Engelke A, Fink AM, Fischer
K, Tausch E, et al: Bendamustine followed by obinutuzumab and
venetoclax in chronic lymphocytic leukaemia (cll2-bag): Primary
endpoint analysis of a multicentre, open-label, phase 2 trial.
Lancet Oncol. 19:1215–1228. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Seymour JF, Kipps TJ, Eichhorst B, Hillmen
P, D'Rozario J, Assouline S, Owen C, Gerecitano J, Robak T, De la
Serna J, et al: Venetoclax-rituximab in relapsed or refractory
chronic lymphocytic leukemia. N Engl J Med. 378:1107–1120. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Al-Sawaf O, Zhang C, Tandon M, Sinha A,
Fink AM, Robrecht S, Samoylova O, Liberati AM, Pinilla-Ibarz J,
Opat S, et al: Venetoclax plus obinutuzumab versus chlorambucil
plus obinutuzumab for previously untreated chronic lymphocytic
leukaemia (cll14): Follow-up results from a multicentre,
open-label, randomised, phase 3 trial. Lancet Oncol. 21:1188–1200.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
DiNardo CD, Jonas BA, Pullarkat V, Thirman
MJ, Garcia JS, Wei AH, Konopleva M, Döhner H, Letai A, Fenaux P, et
al: Azacitidine and venetoclax in previously untreated acute
myeloid leukemia. N Engl J Med. 383:617–629. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kumar SK, Harrison SJ, Cavo M, de la Rubia
J, Popat R, Gasparetto C, Hungria V, Salwender H, Suzuki K, Kim I,
et al: Venetoclax or placebo in combination with bortezomib and
dexamethasone in patients with relapsed or refractory multiple
myeloma (bellini): A randomised, double-blind, multicentre, phase 3
trial. Lancet Oncol. 21:1630–1642. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wei AH, Montesinos P, Ivanov V, DiNardo
CD, Novak J, Laribi K, Kim I, Stevens DA, Fiedler W, Pagoni M, et
al: Venetoclax plus ldac for newly diagnosed aml ineligible for
intensive chemotherapy: A phase 3 randomized place-bo-controlled
trial. Blood. 135:2137–2145. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
DiNardo CD, Lachowiez CA, Takahashi K,
Loghavi S, Xiao L, Kadia T, Daver N, Adeoti M, Short NJ, Sasaki K,
et al: Venetoclax combined with flag-ida induction and
consolidation in newly diagnosed and relapsed or refractory acute
myeloid leukemia. J Clin Oncol. 39:2768–2778. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wei AH, Panayiotidis P, Montesinos P,
Laribi K, Ivanov V, Kim I, Novak J, Stevens DA, Fiedler W, Pagoni
M, et al: 6-month follow-up of viale-c demonstrates improved and
durable efficacy in patients with untreated aml ineligible for
intensive chemotherapy (141/150). Blood Cancer J. 11:1632021.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wierda WG, Allan JN, Siddiqi T, Kipps TJ,
Opat S, Tedeschi A, Badoux XC, Kuss BJ, Jackson S, Moreno C, et al:
Ibrutinib plus venetoclax for first-line treatment of chronic
lymphocytic leukemia: Primary analysis results from the minimal
residual disease cohort of the randomized phase ii captivate study.
J Clin Oncol. 39:3853–3865. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yamamoto K, Shinagawa A, DiNardo CD, Pratz
KW, Ishizawa K, Miyamoto T, Komatsu N, Nakashima Y, Yoshida C,
Fukuhara N, et al: Venetoclax plus azacitidine in Japanese patients
with untreated acute myeloid leukemia ineligible for intensive
chemotherapy. Jpn J Clin Oncol. 52:29–38. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eichhorst B, Niemann CU, Kater AP,
Fürstenau M, von Tresckow J, Zhang C, Robrecht S, Gregor M,
Juliusson G, Thornton P, et al: First-line venetoclax combinations
in chronic lymphocytic leukemia. N Engl J Med. 388:1739–1754. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Azanza JR, Mensa J, Barberán J, Vázquez L,
de Oteyza JP, Kwon M, Yáñez L, Aguado JM, Gracian AC, Solano C, et
al: Recommendations on the use of azole antifungals in
hematology-oncology patients. Rev Esp Quimioter. 3:236–258. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
U.S. Food and Drug Administration (FDA), .
Drug development and drug interactions: Table of substrates,
inhibitors and inducers. FDA, Silver Spring, MD. 2024.https://www.fda.gov/drugs/drug-interactions-labeling/drug-development-and-drug-interactions-table-substrates-inhibitors-and-inducers#table2-2November
7–2024
|
|
23
|
Brüggemann RJ, Alffenaar JW, Blijlevens
NM, Billaud EM, Kosterink JG, Verweij PE and Burger DM: Clinical
relevance of the pharmacokinetic interactions of azole antifungal
drugs with other coadministered agents. Clin Infect Dis.
48:1441–1458. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
24
|
U.S. Food and Drug Administration (FDA), .
Venetoclax prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/208573s000lbl.pdfNovember
7–2024
|
|
25
|
Agarwal SK, DiNardo CD, Potluri J, Dunbar
M, Kantarjian HM, Humerickhouse RA, Wong SL, Menon RM, Konopleva MY
and Salem AH: Management of venetoclax-posaconazole interaction in
acute myeloid leukemia patients: Evaluation of dose adjustments.
Clin Ther. 39:359–367. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De la Garza-Salazar F, Colunga-Pedraza PR,
Gómez-Almaguer D, García-Zárate VA and Gómez-De León A: Low dose
venetoclax plus itraconazole outpatient induction in newly
diagnosed acute myeloid leukemia: A phase 2 study. Leuk Res.
133:1073732023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bennett JM, Catovsky D, Daniel MT,
Flandrin G, Galton DA, Gralnick HR and Sultan C: Proposals for the
classification of the acute leukaemias. French-American-British
(FAB) co-operative group. Br J Haematol. 33:451–458. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and response
criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Grimwade D, Hills RK, Moorman AV, Walker
H, Chatters S, Goldstone AH, Wheatley K, Harrison CJ and Burnett
AK; National Cancer Research Institute Adult Leukaemia Working
Group, : Refinement of cytogenetic classification in acute myeloid
leukemia: Determination of prognostic significance of rare
recurring chromosomal abnormalities among 5876 younger adult
patients treated in the UK MRC trials. Blood. 116:354–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
National Cancer Institute, . Terminology
Criteria for Adverse Events (CTCAE). Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_5×7.pdfNovember
7–2024
|
|
31
|
Döhner H, Estey E, Grimwade D, Amadori S,
Appelbaum FR, Büchner T, Dombret H, Ebert BL, Fenaux P, Larson RA,
et al: Diagnosis and management of aml in adults: 2017 eln
recommendations from an international expert panel. Blood.
129:424–447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Estey E and Döhner H: Acute myeloid
leukaemia. Lancet. 368:1894–1907. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Klepin HD, Geiger AM, Tooze JA,
Kritchevsky SB, Williamson JD, Pardee TS, Ellis LR and Powell BL:
Geriatric assessment predicts survival for older adults receiving
induction chemotherapy for acute myelogenous leukemia. Blood.
121:4287–4294. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
National comprehensive cancer network
(NCCN), . Clinical Practice Guidelines in Oncology (NCCN
Guidelines®): Acute myeloid leukemia, Version (3.2024).
NCCN, Plymouth Meeting, PA. 2024.https://www.nccn.org/professionals/physician_gls/pdf/aml.pdfNovember
7–2024
|
|
35
|
Aldoss I, Dadwal S, Zhang J, Tegtmeier B,
Mei M, Arslan S, Al Malki MM, Salhotra A, Ali H, Aribi A, et al:
Invasive fungal infections in acute myeloid leukemia treated with
venetoclax and hypomethylating agents. Blood Adv. 10:4043–4049.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang A, Johnson T, Abbott D, Phupitakphol
T, Gutman JA, Pollyea DA and Koullias Y: Incidence of invasive
fungal infections in patients with previously untreated acute
myeloid leukemia receiving venetoclax and azacitidine. Open Forum
Infect Dis. 9:ofac4862022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
AlAsmari AF, Alghamdi A, Ali N, Almeaikl
MA, Hakami HM, Alyousef MK, AlSwayyed M, Alharbi M, Alqahtani F,
Alasmari F and Alsaleh N: Venetoclax induces cardiotoxicity through
modulation of oxidative-stress-mediated cardiac inflammation and
apoptosis via NF-κB and BCL-2 pathway. Int J Mol Sci. 23:62602022.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Freise KJ, Hu B and Salem AH: Impact of
ritonavir dose and schedule on cyp3a inhibition and venetoclax
clinical pharmacokinetics. Eur J Clin Pharmacol. 74:413–421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Agarwal SK, Salem AH, Danilov AV, Hu B,
Puvvada S, Gutierrez M, Chien D, Lewis LD and Wong SL: Effect of
ketoconazole, a strong cyp3a inhibitor, on the pharmacokinetics of
venetoclax, a BCL-2 inhibitor, in patients with non-hodgkin
lymphoma. Br J Clin Pharmacol. 83:846–854. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
De la Garza-Salazar F, Peña-Lozano SP and
Gómez-Almaguer D and Gómez-Almaguer D: Orbital myeloid sarcoma
treated with low-dose venetoclax and a potent cytochrome p450
inhibitor. J Oncol Pharm Pract. 29:493–497. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
De la Garza-Salazar F, Colunga-Pedraza PR
and Gómez-Almaguer D: Cytochrome p450 inhibition to decrease dosage
and costs of venetoclax and ibrutinib: A proof-of-concept case
study. Br J Clin Pharmacol. 89:898–902. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Bhatnagar S, Mukherjee D and Salem AH:
Dose adjustment of venetoclax when co-administered with
posaconazole: Clinical drug-drug interaction predictions using a
pbpk approach. Cancer Chemother Pharmacol. 87:465–474. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kobayashi M, Yasu T, Suzaki K and Kosugi
N: Utility of therapeutic drug monitoring of venetoclax in acute
myeloid leukemia. Oncology. 39:1–5. 2022.
|