Introduction
Multiple myeloma (MM), the second most common type
of hematologic malignancy, accounts for approximately 1% of all
cancers and 14% of hematological malignancies globally, with an
estimated 188,000 new cases annually (1). Despite therapeutic advancements,
including proteasome inhibitors and immunomodulatory drugs, nearly
all patients eventually relapse, and relapsed/refractory MM (RRMM)
remains a leading cause of cancer-related mortality, responsible
for over 121,000 deaths worldwide in 2022 (1). Patients with RRMM face a median
overall survival (OS) of 12.4 months (2), which drops sharply in high-risk
subgroups such as those with extramedullary disease (EMD) (3,4). EMD,
characterized by tumor cell infiltration into organs beyond the
bone marrow (such as liver, soft tissues), occurs in 0.5–14% of MM
cases (4) and correlates with
aggressive biology, chemotherapy resistance, and a median OS of
<12 months (3). Chimeric antigen
receptor (CAR) T-cell therapy has revolutionized RRMM treatment,
with pivotal trials (e.g., CARTITUDE-1) reporting overall response
rates of 97%, with a notable 67% of patients achieving stringent
complete remission (sCR) (5).
However, data on CAR-T efficacy in EMD - particularly visceral EMD
- remain sparse due to its exclusion from many clinical trials
(6). Recent studies suggest that
CAR-T therapy may overcome the poor prognosis associated with EMD,
with early data showing promising response rates in this subgroup
(6,7). This case report presents a patient
with RRMM and liver EMD who achieved durable sCR post-CAR-T
therapy, addressing a critical gap in understanding CAR-T's
potential in this high-risk population.
Case report
A 54-year-old male patient presented to the
Department of Orthopedics of Shenzhen Luohu People's Hospital
(Shenzhen, China) with lower back pain following exercise in
December 2019. Lumbar spine MRI revealed a mass, and bone
destruction at the L2-3 vertebral bodies. Biopsy results indicated
plasma cell myeloma, with positive immunohistochemical staining for
the antigens CD138, CD38, MM oncogene 1 and CD56, along with κ
light chain restriction, and the Ki-67 index was 60%. A bone marrow
smear revealed ~5.5% plasma cells, with flow cytometry (FCM)
detecting 3.51% of monoclonal plasma cells restricted to the κ
light chain. The serum IgA level of the patient was 13.32 g/l
(reference range, 0.7–3.5 g/l), whereas levels of β2-microglobulin,
calcium and free light chain levels remained normal. Serum
immunofixation electrophoresis assay yielded a positive IgA-κ
result. Cytogenetics and fluorescence in situ hybridization
assay revealed chromosomal 17p and 13q deletions. Furthermore, a
whole-body positron emission tomography/computed tomography
(PET/CT) scan revealed generalized osteoporosis and multiple sites
of vertebral bone destruction. The patient was subsequently
diagnosed with MM (8) of the IgAκ
subtype, and classified as stage IA according to the Durie-Salmon
staging criteria (9), stage I
according to the International Staging System (ISS) (10) and stage II according to the
revised-ISS (11). Because the
patient had a chromosomal 17p chromosomal deletion, he was also
considered at high risk per the Mayo Stratification of Myeloma and
Risk-Adapted Therapy criteria (12).
Upon diagnosis, the patient promptly underwent
treatment that was in line with the Guidelines for the Diagnosis
and Management of MM in China (2017 revision) (8) and the National Comprehensive Cancer
Network Guidelines for MM, version 1.2020 (13). The specific treatment progress and
response details are presented in Fig.
1. The doctor repeatedly advised the patient to undergo an
autologous stem cell transplant (ASCT); however, the patient
refused the procedure for fear of complications associated with the
transplant. Lenalidomide was discontinued after June 2020 due to
the potential harm it may cause to the patient's hematopoietic stem
cells when used for an extended period. Despite the patient's
persistent opposition to autologous hematopoietic stem cell
transplantation, we, as doctors, were reluctant to entirely close
off this avenue for the patient. In July 2020, bortezomib was
switched to isazomib due to the availability of the drug at that
time, as both belong to the same class of medication. Additionally,
isazomib is an oral medication, making it more convenient to use,
as the patient did not need to frequently visit the hospital.
Henceforth, the reason for the numerous changes in the initial
treatment is that the disease progressed multiple times (Fig. 1).
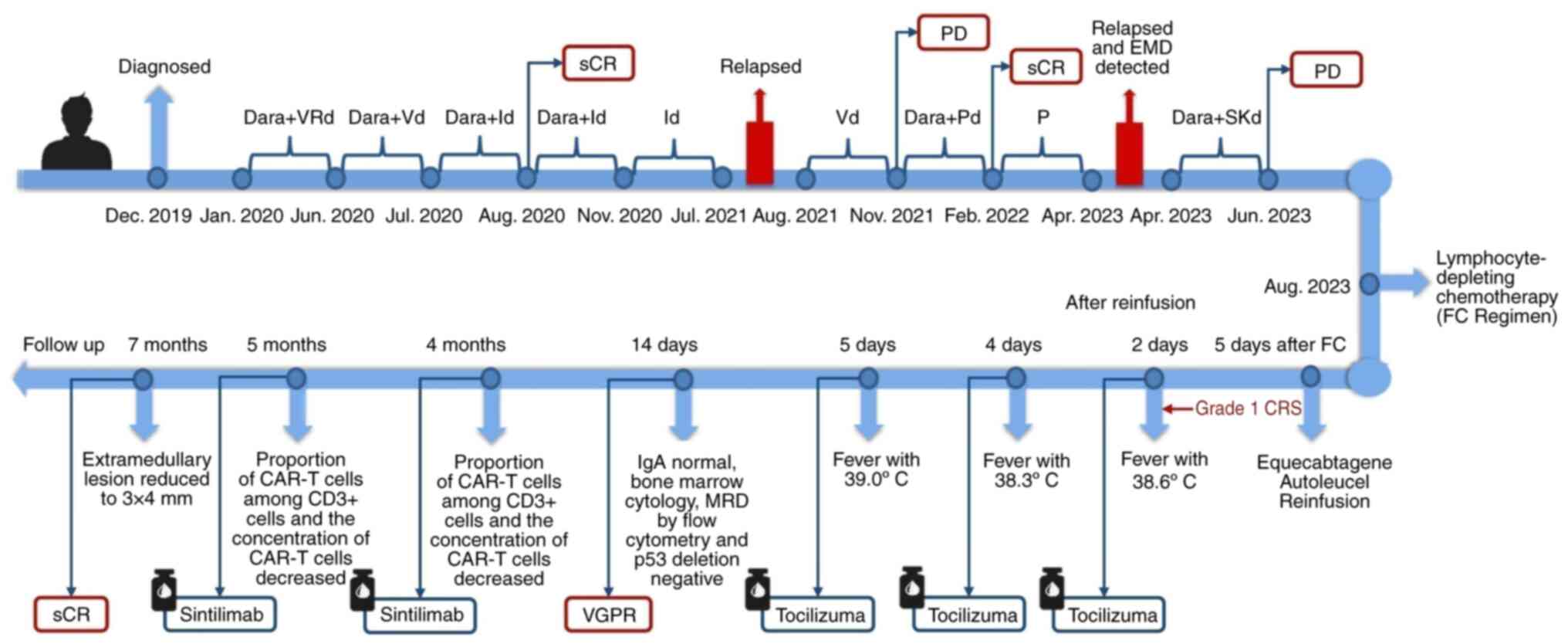 | Figure 1.Treatment progress and response
details. Dara + VRd=Daratumumab 16 mg/kg, once a week for the first
8 weeks, once every 2 weeks from the 9th week, and once every 4
weeks from the 25th week; bortezomib 1.3 mg/m2 on days
1, 4, 8 and 11; lenalidomide 25 mg days 1–21; dexamethasone 20 mg
on days 1–2, 4–5, 8–9 and 11–12; 4 weeks as a treatment cycle.
Id=Ixazomib 4 mg on days 1, 8 and 15; dexamethasone 10 mg on days
1, 8, 15 and 16. Vd=bortezomib 1.3 mg/m2 on days 1, 8,
15 and 22; dexamethasone 20 mg on days 1, 8, 15 and 22; 4 weeks as
a treatment cycle. Dara + Pd=Daratumumab 16 mg/kg, once a week for
the first 8 weeks, once every 2 weeks from the 9th week;
pomalidomide 4 mg on days 1–21; dexamethasone 40 mg on days 1, 8,
15 and 22; 4 weeks as a treatment cycle. Dara + SKd=Daratumumab 16
mg/kg, once a week for the first 8 weeks, once every 2 weeks from
the 9th week; carfilzomib 51 mg on days 1–2, 8–9 and 15–16;
dexamethasone 10 mg on days 1–2, 8–9 and 15–16; 4 weeks as a
treatment cycle. sCR, stringent complete response; PD, progressive
disease; FC, fludarabine and cyclophosphamide; EMD, extramedullary
disease; CRS, cytokine release syndrome; MRD, minimal residual
disease; VGPR, very good partial remission; CAR-T, chimeric antigen
receptor T cell. |
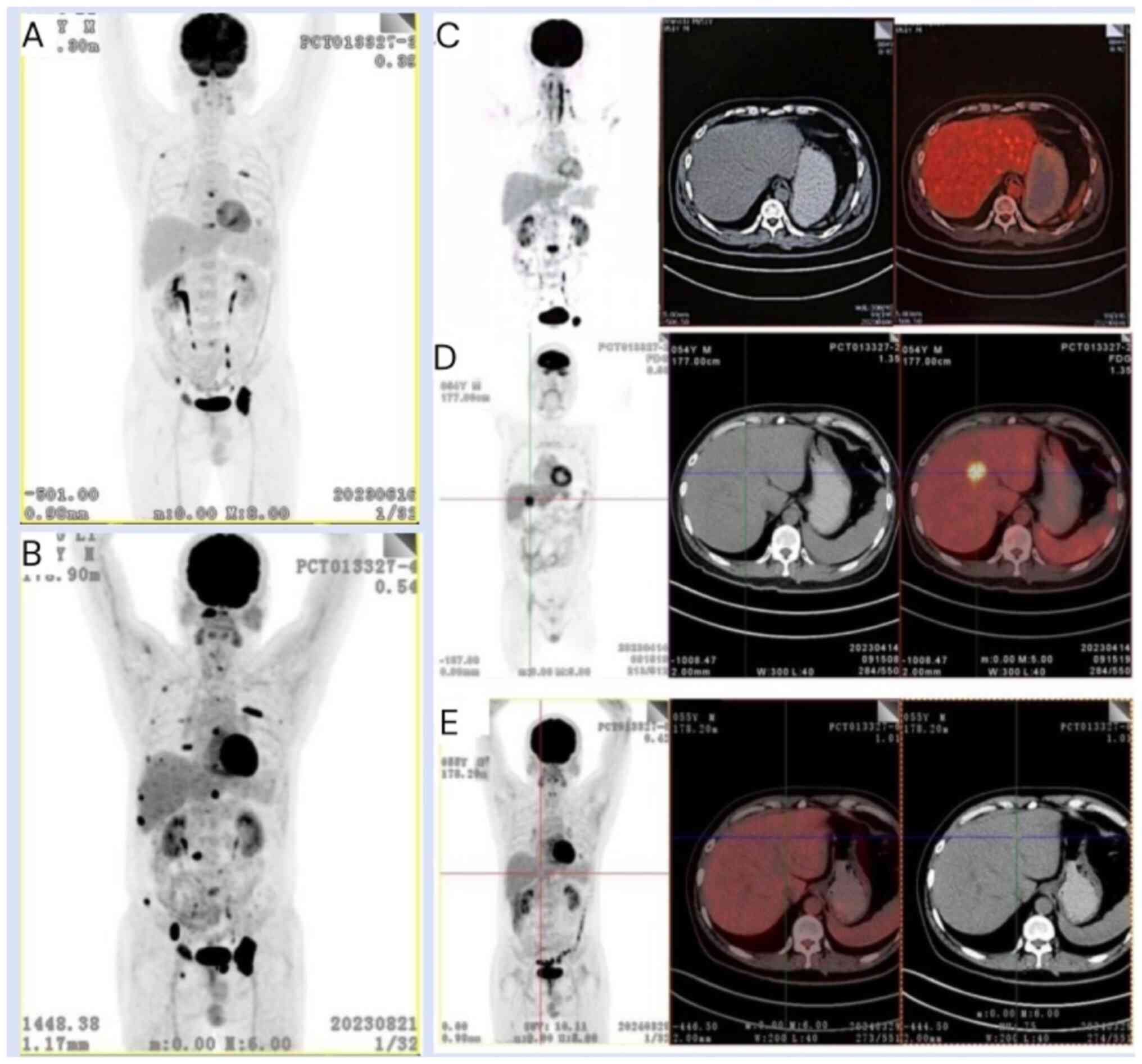
In April 2023, a whole-body PET/CT scan revealed
multiple novel osteolytic bone lesions, including a newly developed
lesion of hypermetabolism in segment S4 of the liver measuring
~25×24 mm compared with the previous results (Fig. 2C and D). The patient declined to
undergo percutaneous biopsy of the lesion. Based on these findings,
the patient was considered to have an aggressive relapse of MM with
concomitant extramedullary involvement in the liver. Between April
2023 and July 2023, the patient underwent four cycles of a Dara +
SKd regimen (daratumumab, 16 mg/kg, once a week for the first 8
weeks, once every 2 weeks from the 9th week; carfilzomib, 51 mg on
days 1–2, 8–9 and 15–16; and dexamethasone, 10 mg on days 1–2, 8–9
and 15–16, 4 weeks as a treatment cycle) as the therapeutic
protocol. Re-evaluation of the results after completing two cycles
of the treatment regimen revealed the presence of an IgA-κ type M
protein on immunofixation electrophoresis, accompanied by a serum
IgA level of 5.84 g/l. The disease status of the patient was
therefore considered to be a partial response (PR).
In July 2023, after having completed four cycles of
the Dara + SKd regimen, a PET/CT scan revealed an increase in
osteolytic bone destruction lesions (Fig. 2A and B). Additionally, a significant
increase in the patient's serum IgA concentration to 8.27 g/l was
observed, with a slight reduction in the size of the lesion located
in hepatic segment S4, now measuring ~17×10 mm. Bone marrow
cytological analysis indicated that plasma cells comprised ~1.5% of
the total cells, and FCM analysis detected a population of 0.1%
monoclonal plasma cells with an aberrant immunophenotype. The
patient was considered to have progressive disease. At this point,
the doctor again recommended that the patient undergo an ASCT, and
the patient continued to refuse. In addition, at this time, the
first Chinese anti-BCMA CAR-T cell product (FUCASO®;
also known as ‘Equecabtagene Autoleucel’; IASO BioTherapeutics,
Ltd. and Innovent Biologics, Inc.) was approved by the China
National Medical Products Administration (NMPA). After discussion
with the care team, the patient selected the anti-BCMA CAR-T cell
therapy.
A lymphocyte-depleting chemotherapy protocol
consisting of fludarabine (50 mg/m2 on days 1–3) and
cyclophosphamide (500 mg/m2 on days 1–3; namely, an
fludarabine and cyclophosphamide regimen) was administered in
August 2023. Subsequently, 5 days afterwards (day 0), the patient
received a reinfusion of anti-BCMA CAR-T cell therapy
(FUCASO®; Equecabtagene Autoleucel) provided by IASO
BioTherapeutics, Ltd.
After CAR-T cell infusion, the patient's vital
signs, such as body temperature, blood pressure, oxygen saturation
and heart rate, were closely monitored, and calculation,
orientation and reading abilities were assessed. On day 1 of
treatment, the patient had a fever that peaked at 38.6°C, although
his blood pressure and oxygen saturation remained normal. A
preliminary diagnosis of grade 1 cytokine release syndrome (CRS)
was made, and tocilizumab, antipyretics, valacyclovir,
levofloxacin, entecavir and cefoperazone sodium/sulbactam sodium
were prescribed to prevent infection. Subsequently, the patient's
temperature gradually returned to normal, and an increasing trend
in the level of interleukin-6 (IL-6) was observed. On day 4 of
treatment, the patient's temperature increased again to 38.3°C, and
tocilizumab (8 mg/kg) was administered for the second time. On day
5 of treatment, the patient's temperature increased to 39.0°C, and
the IL-6 level rose to a peak of 3,809.06 pg/ml. In response to
these observations, treatment with tocilizumab (8 mg/kg) was
restarted, and imipenem/cilastatin was also added to the regimen as
an adjunctive therapy. As a result, both a significant reduction in
the level of IL-6 and a gradual stabilization of the patient's
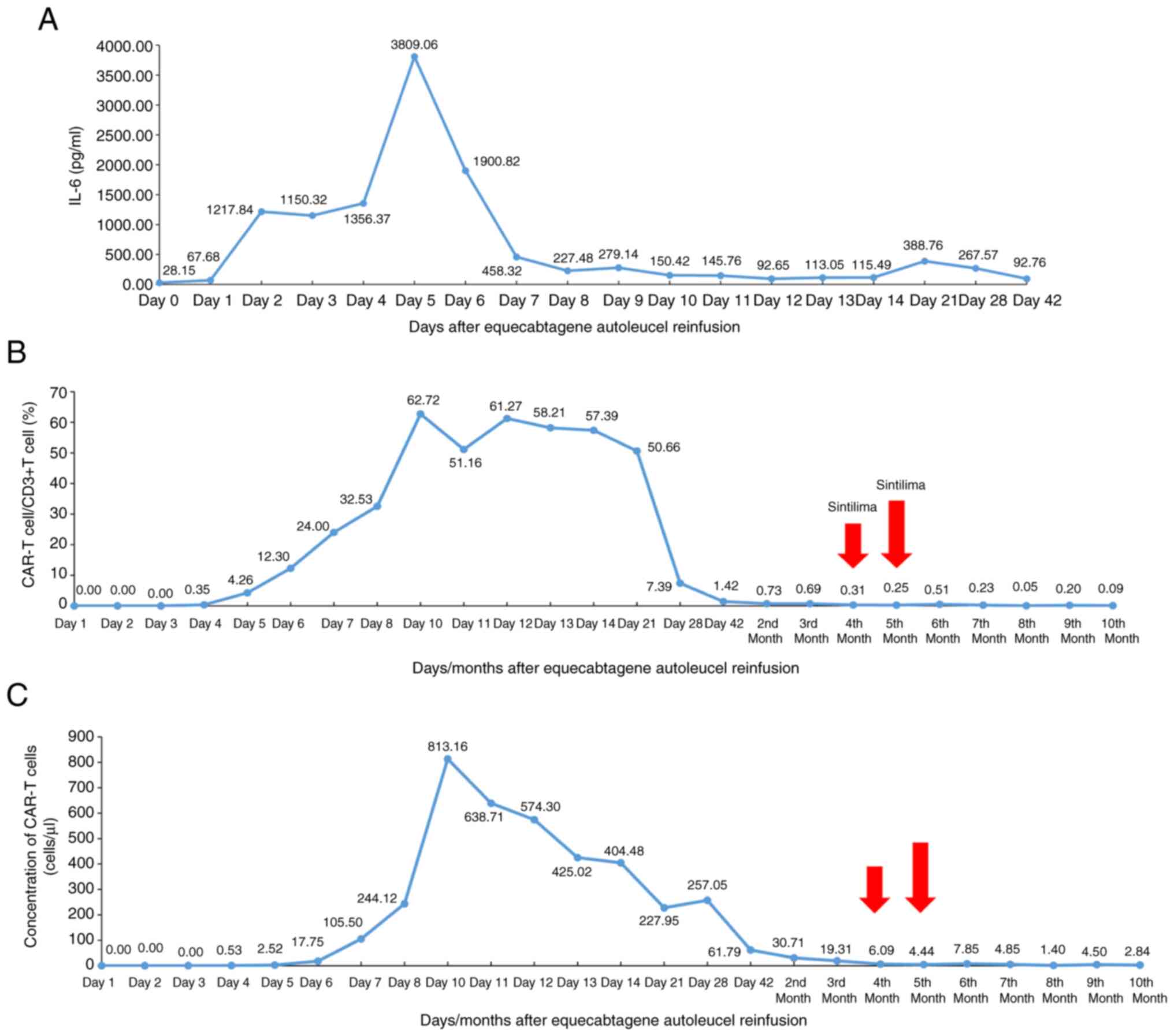
temperature were observed (Fig.
3A). The CRS was therefore resolved following treatment, and
there was no evidence of immune effector cell-associated
neurotoxicity syndrome (ICANS) by assessing the calculative,
orientational and reading abilities of the patient.
Both the proportion of CAR-T cells among
CD3+ cells (CAR-T cells/CD3+ T cells) and the
concentration of CAR-T cells began to significantly increase
beginning on day 4, reaching a plateau on day 10 (62.72 and 813.16
cells/µl, respectively), before gradually decreasing to 1.42% and
61.79 cells/µl, respectively, on day 42 (Fig. 3B and C).
On day 14, the patient's test results indicated that
the level of IgA had returned to normal, and the concentration of
IgA-κ type M protein was 0.4 g/l. Bone marrow cytology, FCM for
minimal residual disease and p53 deletion all elicited negative
results. Furthermore, a significant reduction (>90%) in the
liver involvement lesion was observed compared with its previous
size by ultrasonogram. The patient's response was considered to be
very good partial remission (VGPR). At 1-month post-infusion, no
monoclonal protein was detected for the patient, and the
normalization of free light chains was observed.
Follow-up examinations at 2 and 3 months following
CAR-T cell infusion revealed further decreases in the patient's
CAR-T cell/CD3+ T cell ratio, which decreased to 0.73
and 0.69%, respectively, and decreases in the CAR-T cell
concentration to 30.71 and 19.31 cells/µl, respectively, were also
observed. The patient subsequently received sintilimab (200 mg),
which is a PD-1 blocker, once a month to enhance CAR-T cell
function from December 2023 (4 months following CAR-T cell
infusion) onwards. Subsequently, 5 months following CAR-T cell
infusion, both the CAR-T cell/CD3+ T-cell ratio and the
concentration of CAR-T cells dropped to 0.25% and 4.44 cells/µl,
respectively. The 6-month follow-up data indicated that the
percentage ratio of CAR-T cells/CD3+ T cells and the
concentration of CAR-T cells increased to 0.51% and 7.85 cells/µl,
respectively, before gradually decreasing up to the 10th month
(Fig. 3B and C). The decay rate of
CAR-T cells was decreasing. The lesion located in the S4 liver
segment also showed a significant reduction to ~3×4 mm, and no
hypermetabolism was observed in the PET/CT scan results at 7 months
following reinfusion (scan taken in March 2024) (Fig. 2E). In addition, bone marrow biopsy
confirmed the absence of clonal plasma cells, and the patient was
classified as having achieved sCR.
Discussion
The present case report presents a successful case
of anti-BCMA CAR-T cell therapy in a patient with penta-refractory
MM who also presented with extramedullary involvement. The
successful treatment in this case has offered novel therapeutic
insights for patients with penta-refractory MM, also providing
valuable information for treating patients with extramedullary soft
tissue involvement. Furthermore, the prompt and protocolized
management of adverse events, in addition to the innovative
strategy of enhancing CAR-T cell functionality with programmed cell
death 1 (PD-1) blockade during periods of lymphopenia, has yielded
important empirical evidence, which may contribute to advancements
in CAR-T cell therapy management and maintenance in clinical
settings, highlighting the importance of such strategies in
improving treatment safety and efficacy.
The management of MM is becoming more complex due to
an increasing array of treatment options, including
immunomodulatory drugs (IMiDs), proteasome inhibitors (PIs), and
anti-CD38 monoclonal antibodies (mAbs), which are now standard for
newly diagnosed and relapsed/refractory MM. However, even with
these advancements, dealing with triple-class and penta-refractory
MM poses ongoing challenges (2,14–17).
The term ‘penta-refractory’ describes a therapeutically challenging
subset of MM characterized by resistance to at least two different
IMiDs, two PIs and a single anti-CD38 mAb (18). Patients with penta-refractory MM
exhibit poor outcomes, with a median overall survival (OS) time of
5.6 months. This trend of diminishing therapeutic efficacy is
further underscored by a decrease in the response rates for stable
disease, PR and VGPR with each subsequent treatment line (18). In the present case, the patient
underwent a variety of treatment regimens, including Dara + VRd,
Dara + Vd, Dara + Id, Id, Vd, Dara + Pd and Dara + SKd (Fig. 1), all of which failed to achieve
satisfactory outcomes, and were followed by inevitable relapses;
therefore, novel therapeutic interventions are urgently
required.
Currently, multiple BCMA CAR-T cell therapies have
been approved, including Ciltacabtagene Autoleucel and Idecabtagene
Vicleucel, which have been approved by the U.S. Food and Drug
Administration (FDA), and Equecabtagene Autoleucel and
Zevorcabtagene Autoleucel, which has been approved by the China
NMPA, and also by the FDA. Clinical investigations up to this time
pertaining to the aforementioned approved therapies have
demonstrated that, in the treatment of heavily pretreated MM, BCMA
CAR-T cell therapy achieves an overall response rate (ORR) ranging
from 75–100%, with a composite endpoint of VGPR or greater [sCR +
complete response (CR) + VGPR] reaching 50–100%. The median
progression-free survival ranges from 8.8 months to not being
reached, the median duration of response extends from 10.7 months
to not being reached, and the median OS varies from 19.4 months to
not being reached (Table I)
(19–25). Furthermore, the majority of cases of
CRS and neurotoxicity are of lower than grade 3 toxicities, and
grade 5 toxicity (resulting in death) accounts for only 0.8–1.0 and
1.0%, respectively (Table I)
(19–25). Therefore, for patients who have been
heavily pretreated, BCMA CAR-T cell therapy is indeed an effective
and safe treatment option, offering a novel therapeutic strategy
for patients with RRMM who have become resistant to multiple prior
treatment modalities.
 | Table I.Efficacy and safety data for approved
BCMA CAR-T therapies. |
Table I.
Efficacy and safety data for approved
BCMA CAR-T therapies.
| Clinical trial
(Trial ID) | Site | Therapy | n | Median previous
LOT, no. (range) | Triple class
refractory, no. (%) | Penta-drug
refractory, no. (%) | High-risk
cytogenetic profile, no. (%) | ORR, % (95%
CI) | CR or greater (sCR
+ CR), n % | VGPR or greater
(sCR + CR +, VGPR) n % | Median PFS,
months | Median DOR,
months | Median OS,
months | CRS, n (%) | Neurotoxicity, n
(%) |
|
|---|
|
|
|
|---|
| Any grade | Grade 3 or 4 | Grade 5 | Any grade | Grade 3 or 4 | Grade 5 | (Refs.) |
|---|
| CARTIT-UDE-1
(NCT03-548207) | USA | Cilta-cel
(JNJ-68-284528) | 97 | 6 (4–8) | 85 (87.6) | 41 (42.3) | 23 (23.7) | 97.9
(92.7–99.7) | 65 (67.0) | 90 (92.8) | NR | NR | NR | 92 (94.8) | 4 (4.1) | 1 (1.0) | 21 (21.6) | 11 (11.3) | 1 (1.0) | (19) |
| CARTIT-UDE-1
(NCT03-548207) | Japan | Cilta-cel
(JNJ-68-284528) | 9 | 5 (3–7) | 8 (88.9) | 2 (22.2) | 5 (55.6) | 100.0
(66.4–100.0) | 9 (100.0) | 7 (87.5) | NR | NR | NR | 8 (88.9) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | (20) |
| KarMMa
(NCT03-361748) | / | Ide-cel
(bb2121) | 128 | 6 (3–16) | 108 (84.4) | 33 (25.8) | 45 (35.2) | 73.4 (66–81) | 42 (32.8) | 67 (52.3) | 8.8 (5.6–11.6) | 10.7
(9.0–11.3) | 19.4 (18.2-NE) | 107 (83.6) | 7 (5.5) | 1 (0.8) | 23 (18.0) | 4 (3.1) | 0 (0.0) | (21) |
| KarMMa
(NCT03-361748) | Japan | Ide-cel
(bb2121) | 9 | 4 (3–15) | 3 (33.3) | 0 (0.0) | 2 (2.2) | 88.9 | 5 (55.6) | 8 (88.9) | NR (4.9-NR) | NR (3.9-NR) | NR (3.3-NR) | 9 (100.0) | 0 (0.0) | 0 (0.0) | 2 (22.2) | 0 (0.0) | 0 (0.0) | (22) |
|
| Switzerland | Ide-cel
(bb2121) | 16 | 6 (3–12) | 16 (100.0) | - | 6 (37.5) | 75.0 | 11 (68.8) | 8 (50.0) | - | - | - | 15 (93.8) | 0 (0.0) | 0 (0.0) | 1 (6.3) | 0 (0.0) | 0 (0.0) | (23) |
| FUMA-NBA-1
(CTR20-192510, NCT05-066646) | China | Equecab tagene
Autoleucel (CT10-3A) | 105 | 4 (3–23) | - | - | 73 (69.5) | 96.1 | 80 (77.7) | 94 (91.3) | NR | NR | - | 98 (93.3) | 1 (0.9) |
| 2 (1.9) | 0 (0.0) | 0 (0.0) | (24) |
| LUMM-ICAR STUDY1
(NCT03975907) | China | Zevor-cabta-gene
autole ucel (CT053) | 102 | 4 (3–15) | 23 (22.5) | - | 46 (45.1%) | 92.8
(84.9–97.3) | 43(42.2) | 83 (81.4) | NR | NR | - | 92 (90.2) | 7 (6.9) | 0 (0.0) | 2 (2.0) | 0 (0.0) | 0 (0.0) | (25) |
Equecabtagene autoleucel (CT103A) is a novel CAR-T
cell therapy that specifically targets BCMA, incorporating a fully
human aspect through the utilization of a single-chain variable
fragment derived from a human antibody. This humanized approach
potentially offers advantages in terms of reduced immunogenicity
and potentially improving the persistence of CAR-T cells within the
body, thereby enhancing therapeutic efficacy (26). It was approved by the NMPA in China
on 30th June 2023 for the treatment of adult patients with RRMM,
whose disease has progressed after having received at least three
lines of prior therapy (including at least one PI and an IMiD)
(27). FUMANBA-1, a phase 1b/2
study, evaluated the therapeutic efficacy and safety profile of
Equecabtagene Autoleucel (28).
This study revealed a median ORR of 96.1% among the 103
participants, with 91.3% of them achieving a VGPR (or greater)
response. In addition, the combined rate of sCR and CR was 77.7%,
demonstrating significant clinical benefit to patients, especially
patients with MM who had been heavily pretreated (24). The patients described in this study
were patients with penta-refractory MM. In the present case report,
following anti-BCMA CAR-T cell therapy (FUCASO®;
Equecabtagene Autoleucel), at 7 months post-reinfusion, the patient
was classified as having achieved sCR.
CAR-T cells, although they are engineered to target
specific cancer cells, may have their efficacy compromised within
the tumor microenvironment where, for example, programmed cell
death ligand 1 (PD-L1) on cancer cells may interact with PD-1 on
CAR-T cells, thereby impeding their antitumor activity (28). In fact, the interaction between
PD-L1 molecules expressed on cancer cells and PD-1 proteins
expressed on CAR-T cells provides a prime example of this. This
interaction weakens the ability of CAR-T cells to effectively
destroy cancer cells (28).
Blocking the PD-1/PDL-1 molecular interaction enables CAR-T cells
to maintain their activity, thereby targeting tumor cells more
effectively (29). In the present
patient, the CAR T-cell ratio and the CAR T-cell concentration were
both decreased at 28 days post-reinfusion, which had a negative
impact on the efficacy of the treatment. Therefore, a PD-1 blocker
(sintilimab) was administered when the number of CAR-T cells had
decreased, providing valuable real-world evidence for CAR-T
maintenance therapy.
The safety of CAR-T cell treatment requires vigilant
monitoring, given the possibility of severe adverse reactions,
which may aggravate the condition or even lead to a fatal outcome
(30). The incidence of CRS in
patients with heavily pretreated MM who receive approved CAR-T cell
therapies ranges from 83.6–100.0%, depending on the specific
therapy administered (Table I)
(19–25). In the FUMANBA-1 study, which
featured the CAR-T cell therapeutic agent that was administered to
the patient in the current study, a CRS incidence rate of 93.3%
(98/105) was reported. The vast majority of patients experienced
CRS grades 1–2, with only one patient having a CRS grade ≥3.
Additionally, only 1.9% (2/105) of patients developed ICANS, with
one case each of grade 1 and grade 2 ICANS, and no cases of grade
≥3 ICANS (Table I) (24). Following the guidelines of the
product manual, after CAR-T cell infusion, the patient's vital
signs, including body temperature, blood, oxygen saturation and
heart rate, were closely monitored. In addition, the patient's
calculative, orientational and reading abilities were assessed.
Apart from fever, no other adverse events were detected. On this
basis, the patient was diagnosed with grade 1 CRS, which was
successfully treated with tocilizumab. Notably, the levels of IL-6
closely paralleled the progression of the fever, reaching a plateau
on day 5, before subsequently tapering off. Concurrently, the CAR-T
cells reached their peak expansion on day 10, and gradually
decreased thereafter. During grade 1 CRS, to prevent infection, the
patient was prescribed antipyretics, valacyclovir, levofloxacin,
entecavir and cefoperazone sodium/sulbactam sodium. Owing to our
timely and standardized adverse event management, our patient
experienced only grade 1 CRS within the designated treatment
duration, and subsequently, no ICANS was observed following the
infusion of Equecabtagene Autoleucel. These findings were
consistent with the results of the FUMANBA-1 study (24).
In addition to bone marrow lesions, extramedullary
lesions are also an important issue for patients with RRMM. There
are two main types of extramedullary disease (EMD) in MM,
reflecting the proliferation of malignant plasma cells outside the
bone marrow, namely paraskeletal disease and non-paraskeletal (NPS)
spread (31). Our patient was a
patient with heavily pretreated MM who presented with NPS spread
and concomitant liver involvement. This condition, characterized by
increased aggressiveness, generally leads to poorer survival
outcomes, making patient treatment more challenging. Although the
majority of published studies have attested to the safety of
anti-BCMA CAR-T cell therapy in the treatment of RRMM, a notable
deficiency persists in the research literature concerning its
efficacy in patients with extramedullary MM, who are frequently
excluded from clinical trials (6,7,32).
Table II presents a
compilation of the findings from selected BCMA CAR-T cell clinical
trials wherein patients with EMD were enrolled (21,26,32–37).
Analysis of the data in Table II
revealed an ORR for patients with EMD between 57.1 and 100.0%, with
a CR or greater rate ranging from 28.6 to 66.7% and a VGPR or
greater rate ranging from 57.1 to 80.0%. For patients without EMD,
the ORR ranged from 44.4 to 100.0%, the CR or greater rate ranged
from 0.0 to 84.6%, and the VGPR or greater rate ranged from 5.6 to
92.3%. However, these data do not capture the full extent of the
impact of this therapy due to the limited number of patients with
EMD who were included; moreover, there were inconsistencies in the
study designs. Consequently, there is an urgent need for more
extensive and methodologically consistent studies to be performed
that also include patients with EMD to accurately assess the
efficacy and safety profile of BCMA CAR-T cell therapy in this
specific patient group. Building upon the discussed research, the
present case study has presented a compelling example of the
effectiveness of anti-BCMA CAR-T cell therapy. Specifically, our
patient, who had a lesion located in the liver, achieved notable
control post-treatment, highlighting this as a successful
therapeutic option.
 | Table II.Anti-BCMA CAR-T cell products used in
clinical trials involving patients with EMD. |
Table II.
Anti-BCMA CAR-T cell products used in
clinical trials involving patients with EMD.
|
|
|
| EMD | Non-EMD |
|
|---|
|
|
|
|
|
|
|
|---|
| Clinical trial
(Trial ID) | Therapy | EMD, n (total
patients) | ORR, % (95%
CI) | CR or greater (sCR
+ CR), n % | VGPR or greater
(sCR + CR + VGPR), n % | ORR, % VGPR), n
% | CR or greater (sCR
+ CR), n % | VGPR or greater
(sCR + CR + VGPR), n % | (Refs.) |
|---|
| CRB-401
(NCT02658929) | bb2121 | 9 (33) | 88.9
(51.8–99.7) | 4 (44.4) | 6 (66.7) | 83.3
(62.6–95.3) | 11 (45.8) | 18 (75.0) | (34) |
| KarMMa
(NCT03361748) | Idecabtagene
vicleucel (bb2121) | 50 (128) | NAV | NAV | NAV | NAV | NAV | NAV | (21) |
| LEGEND-2
(NCT03090659) | LCAR-B38M | 5 (17) | 80.0 | 3 (60.0) | 4 (80.0) | 91.7 | 9 (75.0) | 11 (91.7) | (33) |
| N/A
(NCT02546167) | Anti-CART-BCMA
cells | 7 (25) | 57.1 | 2 (28.6) | 4 (57.1) | 44.4 | 0 (0.0) | 1 (5.6) | (32) |
| N/A
(ChiCTR1800018137) | CT103A | 5 (18) | 100.0 | 2 (40.0) | 4 (80.0) | 100.0 | 11 (84.6) | 12 (92.3) | (26) |
| N/A
(ChiCTR-OPC-16009113) | Anti-CART-BCMA
cells | 9 (30) | 88.9 | 6 (66.7) | 7 (77.8) | 90.5 | 7 (33.3) | 9 (42.9) | (35) |
| N/A
(ChiCTR1800017404) | Anti-CART-BCMA
cells | 28 (61) | 100.0 | 16 (59.3) | 21 (77.8) | 96.8 | 25 (80.6) | 26 (83.9) | (36) |
| N/A
(ChiCTR1800017051 and ChiCTR2000033925) | Anti-CART-BCMA
cells | 12 (21) | 100.0 | 5 (41.7) | 8 (66.7) | 88.9 | 4 (44.4) | 7 (77.8) | (37) |
In conclusion, CAR-T cell therapy has shown
significant promise in the treatment of RRMM, especially in
patients who have exhausted other therapeutic options. This case
has demonstrated the potential of CAR-T therapy to induce
long-lasting responses for a patient with EMD, resulting in only
mild adverse effects, thereby underscoring the potential of CAR-T
cell therapy to achieve sCR in patients with RRMM. Further studies
and clinical trials, however, are required to optimize the efficacy
and safety of this therapy, and to improve understanding of the
long-term outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
LZ, DL, FD, JC, WL, HX and HC were involved in
management of the patient and contributed to the preparation of
this manuscript. All authors read and approved the final
manuscript. LZ, DL, FD, JC, WL, HX and HC confirm the authenticity
of all the raw data.
Ethics approval and consent to
participate
This report was reviewed and approved by the
Scientific Ethics Committee of Shenzhen Luohu People's Hospital
(approval no. 2024-LHQRMYY-KYLL-088). Data collection and
manuscript writing were carried out by following the guidelines of
Declaration of Helsinki. The consent letter was signed by the
patient.
Patient consent for publication
Written informed consent was obtained from the
patient of this case report and accompanying images.
Competing interests
The authors declare they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
ASCT
|
autologous stem cell transplant
|
|
BCMA
|
B cell maturation antigen
|
|
CAR
|
chimeric antigen receptor
|
|
CR
|
complete response
|
|
CRS
|
cytokine release syndrome
|
|
EMD
|
extramedullary disease
|
|
FCM
|
flow cytometry
|
|
FISH
|
fluorescence in situ hybridization
|
|
ICANS
|
immune effector cell-associated
neurotoxicity syndrome
|
|
IL-6
|
interleukin-6
|
|
ImiDs
|
immunomodulatory drugs
|
|
ISS
|
International Staging System
|
|
MM
|
multiple myeloma
|
|
NMPA
|
National Medical Products
Administration
|
|
NPS
|
non-paraskeletal
|
|
ORR
|
overall response rate
|
|
OS
|
overall survival
|
|
PD-1
|
programmed cell death 1
|
|
PD-L1
|
programmed cell death ligand 1
|
|
PET/CT
|
positron emission tomography/computed
tomography
|
|
PIs
|
proteasome inhibitors
|
|
PR
|
partial response
|
|
sCR
|
stringent complete response
|
|
VGPR
|
very good partial remission
|
References
|
1
|
Mafra A, Laversanne M, Marcos-Gragera R,
Chaves HVS, McShane C, Bray F and Znaor A: The global multiple
myeloma incidence and mortality burden in 2022 and predictions for
2045. J Natl Cancer Inst. 117:907–914. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mateos MV, Weisel K, De Stefano V,
Goldschmidt H, Delforge M, Mohty M, Cavo M, Vij R, Lindsey-Hill J,
Dytfeld D, et al: LocoMMotion: A prospective, noninterventional,
multinational study of real-life current standards of care in
patients with relapsed and/or refractory multiple myeloma.
Leukemia. 36:1371–1376. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ho M, Paruzzo L, Minehart J, Nabar N, Noll
JH, Luo T, Garfall A and Zanwar S: Extramedullary multiple myeloma:
challenges and opportunities. Current Oncology. 32:1822025.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bladé J, Beksac M, Caers J, Jurczyszyn A,
von Lilienfeld-Toal M, Moreau P, Rasche L, Rosiñol L, Usmani SZ, et
al: Extramedullary disease in multiple myeloma: a systematic
literature review. Blood Cancer J. 12:452022. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hu Y, Xie Y, Wang X, Yang L, Geng H, Yi Z,
Zhang Y, Ma L and Chen F: Targeting BCMA in multiple myeloma:
designs, challenges, and future directions. Cancer Immunol
Immunother. 74:772025. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Que Y, Xu M, Xu Y, Almeida VDF, Zhu L,
Wang Z, Wang Y, Liu X, Jiang L, et al: Anti-BCMA CAR-T Cell Therapy
in Relapsed/Refractory Multiple Myeloma Patients With
Extramedullary Disease: A Single Center Analysis of Two Clinical
Trials. Frontiers in Immunology. 12:7558662021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dima D, Abdallah AO, Davis JA, Awada H,
Goel U, Rashid A, DeJarnette S, Anwer F, Shune L, et al: Impact of
Extraosseous Extramedullary Disease on Outcomes of Patients with
Relapsed-Refractory Multiple Myeloma receiving Standard-of-Care
Chimeric Antigen Receptor T-Cell Therapy. Blood Cancer J.
14:902024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Chinese Hematology Association, . Chinese
Society of Hematology, Chinese Myeloma Committee-Chinese Hematology
Association. The guidelines for the diagnosis and management of
multiple myeloma in China (2017 revision). Zhonghua Nei Ke Za Zhi.
56:866–870. 2017.(In Chinese). PubMed/NCBI
|
|
9
|
Durie BG and Salmon SE: A clinical staging
system for multiple myeloma. Correlation of measured myeloma cell
mass with presenting clinical features, response to treatment, and
survival. Cancer. 36:842–854. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Greipp PR, San Miguel J, Durie BG, Crowley
JJ, Barlogie B, Bladé J, Boccadoro M, Child JA, Avet-Loiseau H,
Kyle RA, et al: International staging system for multiple myeloma.
J Clin Oncol. 23:3412–3420. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Palumbo A, Avet-Loiseau H, Oliva S,
Lokhorst HM, Goldschmidt H, Rosinol L, Richardson P, Caltagirone S,
Lahuerta JJ, Facon T, et al: Revised international staging system
for multiple myeloma: A report from international myeloma working
group. J Clin Oncol. 33:2863–2869. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gonsalves WI, Buadi FK, Ailawadhi S,
Bergsagel PL, Chanan Khan AA, Dingli D, Dispenzieri A, Fonseca R,
Hayman SR, Kapoor P, et al: Utilization of hematopoietic stem cell
transplantation for the treatment of multiple myeloma: A mayo
stratification of myeloma and risk-adapted therapy (mSMART)
consensus statement. Bone Marrow Transplant. 54:353–367. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
National Comprehensive Cancer Network
(NCCN), . NCCN Guidelines Multiple Myeloma. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1445April
18–2024
|
|
14
|
Holstein SA, Grant SJ and Wildes TM:
Chimeric antigen receptor T-cell and bispecific antibody therapy in
multiple myeloma: Moving into the future. J Clin Oncol.
41:4416–4429. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bal S, Malek E, Kansagra A, Usmani SZ, Vij
R, Godby KN, Cornell RF, Kang Y, Umyarova E, Giri S, et al:
Treatment outcomes of triple class refractory multiple myeloma: A
benchmark for new therapies. Leukemia. 36:877–880. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gill SK, Unawane R, Wang S, Ahn J, Aleman
A, Siegel DS, Vesole DH, Parmar H, Phull P and Biran N: I-OPen:
Inferior outcomes of Penta-refractory compared to Penta-exposed
multiple myeloma patients. Blood Cancer J. 12:1382022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Goel U, Charalampous C, Kapoor P, Binder
M, Buadi FK, Dingli D, Dispenzieri A, Fonder A, Gertz MA, Gonsalves
WI, et al: Defining drug/drug class refractoriness vs lines of
therapy in relapsed/refractory multiple myeloma. Blood Cancer J.
13:112023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gandhi UH, Cornell RF, Lakshman A, Gahvari
ZJ, McGehee E, Jagosky MH, Gupta R, Varnado W, Fiala MA, Chhabra S,
et al: Outcomes of patients with multiple myeloma refractory to
CD38-targeted monoclonal antibody therapy. Leukemia. 33:2266–2275.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Berdeja JG, Madduri D, Usmani SZ,
Jakubowiak A, Agha M, Cohen AD, Stewart AK, Hari P, Htut M,
Lesokhin A, et al: Ciltacabtagene autoleucel, a B-cell maturation
antigen-directed chimeric antigen receptor T-cell therapy in
patients with relapsed or refractory multiple myeloma
(CARTITUDE-1): A phase 1b/2 open-label study. Lancet. 398:314–324.
2021.PubMed/NCBI
|
|
20
|
Ri M, Suzuki K, Ishida T, Kuroda J,
Tsukamoto T, Teshima T, Goto H, Jackson CC, Sun H, Pacaud L, et al:
Ciltacabtagene autoleucel in patients with relapsed/refractory
multiple myeloma: CARTITUDE-1 (phase 2) Japanese cohort. Cancer
Sci. 113:4267–4276. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Munshi NC, Anderson LD Jr, Shah N, Madduri
D, Berdeja J, Lonial S, Raje N, Lin Y, Siegel D, Oriol A, et al:
Idecabtagene vicleucel in relapsed and refractory multiple myeloma.
N Engl J Med. 384:705–716. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Minakata D, Ishida T, Ando K, Suzuki R,
Tanaka J, Hagiwara S, Ananthakrishnan R, Kuwayama S, Nishio M,
Kanda Y and Suzuki K: Phase 2 results of idecabtagene vicleucel
(ide-cel, bb2121) in Japanese patients with relapsed and refractory
multiple myeloma. Int J Hematol. 117:729–737. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sanoyan DA, Seipel K, Bacher U, Kronig MN,
Porret N, Wiedemann G, Daskalakis M and Pabst T: Real-life
experiences with CAR T-cell therapy with idecabtagene vicleucel
(ide-cel) for triple-class exposed relapsed/refractory multiple
myeloma patients. BMC Cancer. 23:3452023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
IASO Biotherapeutics, . IASO Bio Presents
updated long-term follow-up data for BCMA CAR-T FUCASO®
(Equecabtagene Autoleucel) at IMS 2023. 2023.https://en.iasobio.com/info.php?id=225April
18–2024
|
|
25
|
Chen W, Fu C, Fang B, Liang A, Xia Z, He
Y, Lu J, Liu H, Hou M, Cai Z, et al: Phase II study of fully human
BCMA-Targeting CAR-T Cells (Zevorcabtagene Autoleucel) in patients
with relapsed/refractory multiple myeloma. Blood. 140
(Suppl):1:S4564–S4565. 2022. View Article : Google Scholar
|
|
26
|
Wang D, Wang J, Hu G, Wang W, Xiao Y, Cai
H, Jiang L, Meng L, Yang Y, Zhou X, et al: A phase 1 study of a
novel fully human BCMA-targeting CAR (CT103A) in patients with
relapsed/refractory multiple myeloma. Blood. 137:2890–2901. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
National Medical Products Administration
(NMPA), . Equecabtagene autoleucel injection approved with
conditions by China NMPA. 2023.https://english.nmpa.gov.cn/2023-06/30/c_940315.htmApril
19–2024
|
|
28
|
Rafiq S, Yeku OO, Jackson HJ, Purdon TJ,
van Leeuwen DG, Drakes DJ, Song M, Miele MM, Li Z, Wang P, et al:
Targeted delivery of a PD-1-blocking scFv by CAR-T cells enhances
anti-tumor efficacy in vivo. Nat Biotechnol. 36:847–856. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zolov SN, Rietberg SP and Bonifant CL:
Programmed cell death protein 1 activation preferentially inhibits
CD28.CAR-T cells. Cytotherapy. 20:1259–1266. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yan Z, Cao J, Cheng H, Qiao J, Zhang H,
Wang Y, Shi M, Lan J, Fei X, Jin L, et al: A combination of
humanized anti-CD19 and anti-BCMA CAR T cells in patients with
relapsed or refractory multiple myeloma: A single-arm, phase 2
trial. Lancet Hematol. 6:e521–e529. 2019. View Article : Google Scholar
|
|
31
|
Rosiñol L, Beksac M, Zamagni E, Van de
Donk NWCJ, Anderson KC, Badros A, Caers J, Cavo M, Dimopoulos MA,
Dispenzieri A, et al: Expert review on soft-tissue plasmacytomas in
multiple myeloma: Definition, disease assessment and treatment
considerations. Br J Hematol. 194:496–507. 2021. View Article : Google Scholar
|
|
32
|
Cohen AD, Garfall AL, Stadtmauer EA,
Melenhorst JJ, Lacey SF, Lancaster E, Vogl DT, Weiss BM, Dengel K,
Nelson A, et al: B-cell maturation antigen-specific CAR T cells are
clinically active in multiple myeloma. J Clin Invest.
129:2210–2221. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Xu J, Chen LJ, Yang SS, Sun Y, Wu W, Liu
YF, Xu J, Zhuang Y, Zhang W, Weng XQ, et al: Exploratory trial of a
biepitopic CAR T-targeting B-cell maturation antigen in
relapsed/refractory multiple myeloma. Proc Natl Acad Sci USA.
116:9543–9551. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Raje N, Berdeja J, Lin Y, Siegel D,
Jagannath S, Madduri D, Liedtke M, Rosenblatt J, Maus MV, Turka A,
et al: Anti-BCMA CAR T-Cell therapy bb2121 in relapsed or
refractory multiple myeloma. N Engl J Med. 380:1726–1737. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li C, Cao W, Que Y, Wang Q, Xiao Y, Gu C,
Wang D, Wang J, Jiang L, Xu H, et al: A phase I study of anti-BCMA
CAR T-cell therapy in relapsed/refractory multiple myeloma and
plasma cell leukemia. Clin Transl Med. 11:e3462021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang M, Zhou L, Zhao H, Zhang Y, Wei G,
Hong R, Wu W, Xu H, Wang L, Ni F, et al: Risk factors associated
with durable progression-Free survival in patients with relapsed or
refractory multiple myeloma treated with Anti-BCMA CAR T-cell
therapy. Clin Cancer Res. 27:6384–6392. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li W, Liu M, Yuan T, Yan L, Cui R and Deng
Q: Efficacy and follow-up of humanized anti-BCMA CAR-T-cell therapy
in relapsed/refractory multiple myeloma patients with
extramedullary-extraosseous, extramedullary-bone related, and
without extramedullary disease. Hematol Oncol. 40:223–232. 2022.
View Article : Google Scholar : PubMed/NCBI
|