Introduction
Urachal carcinoma is a rare malignancy originating
from the urachus, a structure that exists during embryonic
development and connects the bladder to the umbilicus (1). Urachal carcinoma accounts for 0.01% of
all malignancies (2), and 0.35–0.7%
of all bladder cancers (3). In the
normal course of development, the urachus usually degenerates into
a fibrous cord, known as the median umbilical ligament, after birth
(4). However, in some cases,
remnants of the urachus can persist, which may potentially undergo
malignant transformation, which leads to the development of urachal
carcinoma (5).
Urachal carcinoma comprises <1% of all bladder
carcinomas (6). The most commonly
encountered histological subtype is adenocarcinoma (7). Owing to its nonspecific symptoms and
clinical manifestation, a large proportion of patients are
diagnosed at an advanced stage, with one-fifth of patients having
distant metastasis at their initial visit (8). The 5-year overall cancer-specific
survival rate is in the range of 43–61%, as per previous studies
(6,9,10).
Currently, there is no standard treatment for
urachal carcinoma. Surgical resection is the preferred treatment
for limited-stage urachal carcinoma (11). Conventional approaches, such as open
and laparoscopic partial cystectomy, have been commonly employed.
However, these methods have lower precision and flexibility
(12), and doctors are prone to
fatigue. In recent years, robot-assisted surgery has been
increasingly extended to urological procedures (13). Robot-assisted laparoscopic surgery
reduces the risk of complications due to its enhanced precision, as
it allows accurate excision of the tumour and does not cause damage
to the surrounding tissues, thereby reducing blood loss. Moreover,
high-definition visualization allows for improved identification
and control of blood vessels (14).
Despite the growing use of robot-assisted surgery in urology, there
are currently few reports of its use in robotic partial cystectomy,
especially in the treatment of primary urachal carcinoma (15).
Case report
A 31-year-old male presented to Guangdong Provincial
People's Hospital (Guangzhou, China) with a 5-day history of
painless, gross haematuria in July 2024. Laboratory test results
demonstrated normal tumor marker levels: Carcinoembryonic antigen
(CEA), 1.46×10−3 ng/l (normal range, 0–5×10−3
ng/l); alpha-fetoprotein, 2.4×10−3 ng/l (normal range,
0–9×10−3 ng/l); and prostate-specific antigen (PSA),
0.93×10−3 ng/l (normal range, 0–4×10−3 ng/l).
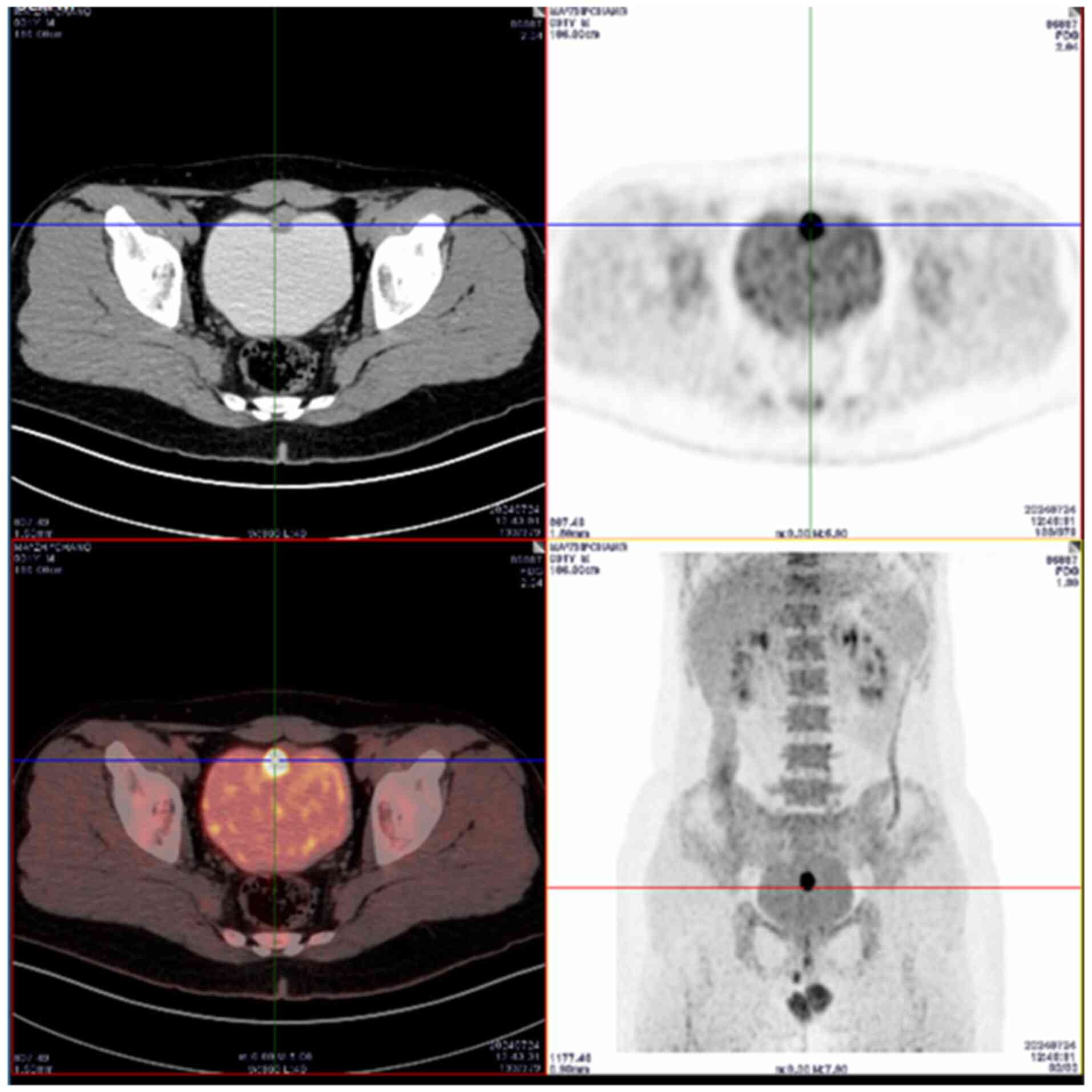
An 18F-FDG-PET/CT scan demonstrated a nodular lesion on
the anterior bladder wall with increased glucose metabolism, which
suggested malignancy and a potential diagnosis of urachal carcinoma
(Fig. 1). No malignant metabolic
lesions were observed in other parts of the body. To further
confirm the diagnosis, a cystoscopy was performed, which
demonstrated a cauliflower-like mass on the bladder dome that was
~3×2 cm in size. The bilateral ureteral orifices appeared normal
and no other bladder wall masses or abnormalities were noted.
Biopsy samples from three different areas of the mass were obtained
and histopathological examination confirmed adenocarcinoma. A
diagnosis of urachal carcinoma was established on the basis of
imaging, cystoscopic findings and pathological results.
Following comprehensive preoperative preparations,
the patient underwent robot-assisted laparoscopic partial
cystectomy, urachal and umbilical resection, and pelvic lymph node
dissection using the da Vinci-Xi robotic system (Intuitive Surgical
Operations, Inc.) in July 2024. After successful induction of
anaesthesia, the patient was placed in the Trendelenburg position
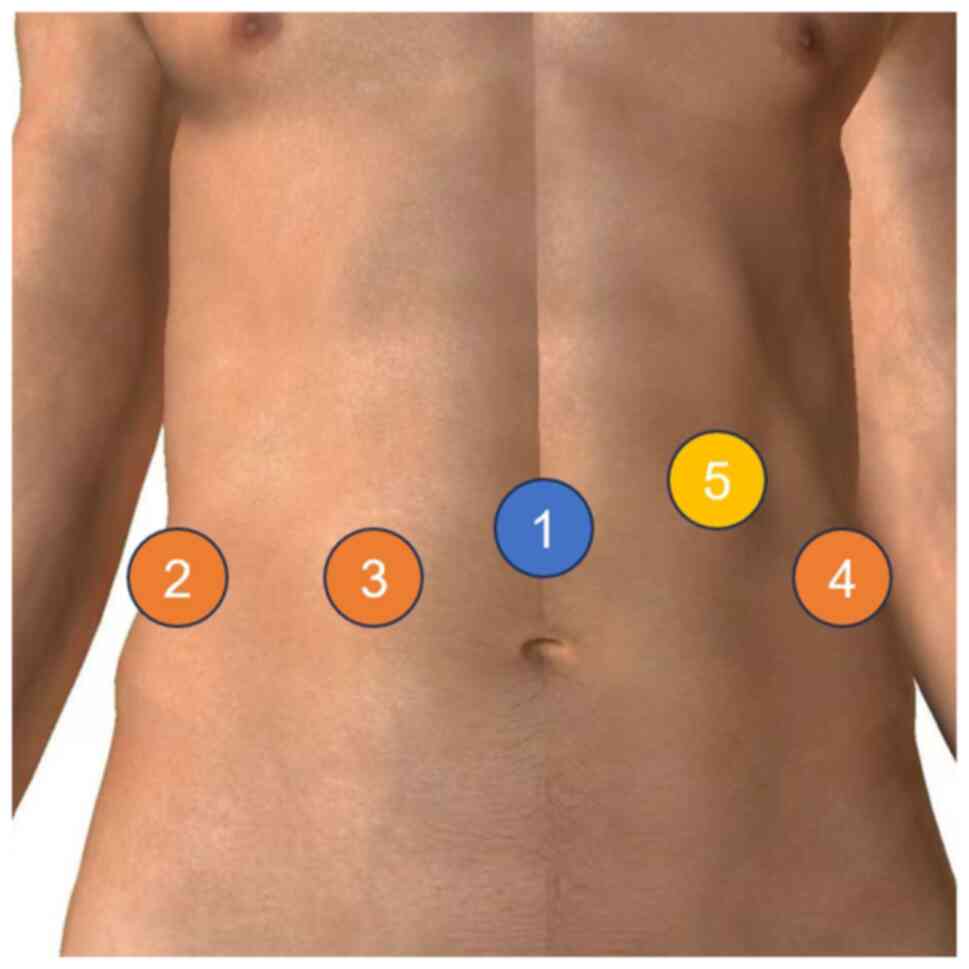
at an angle of ~30°. A small incision was made above the umbilicus.
A Veress pneumoperitoneum needle was subsequently employed to
establish a stable pneumoperitoneum; four 8-mm and one 12-mm
trocars were inserted at predetermined positions (Fig. 2) and then connected to the robotic
system. The bladder was first emptied, after which a routine pelvic
lymph node dissection was precisely performed. The peritoneal
reflection was opened and the bladder was dissected anteriorly.
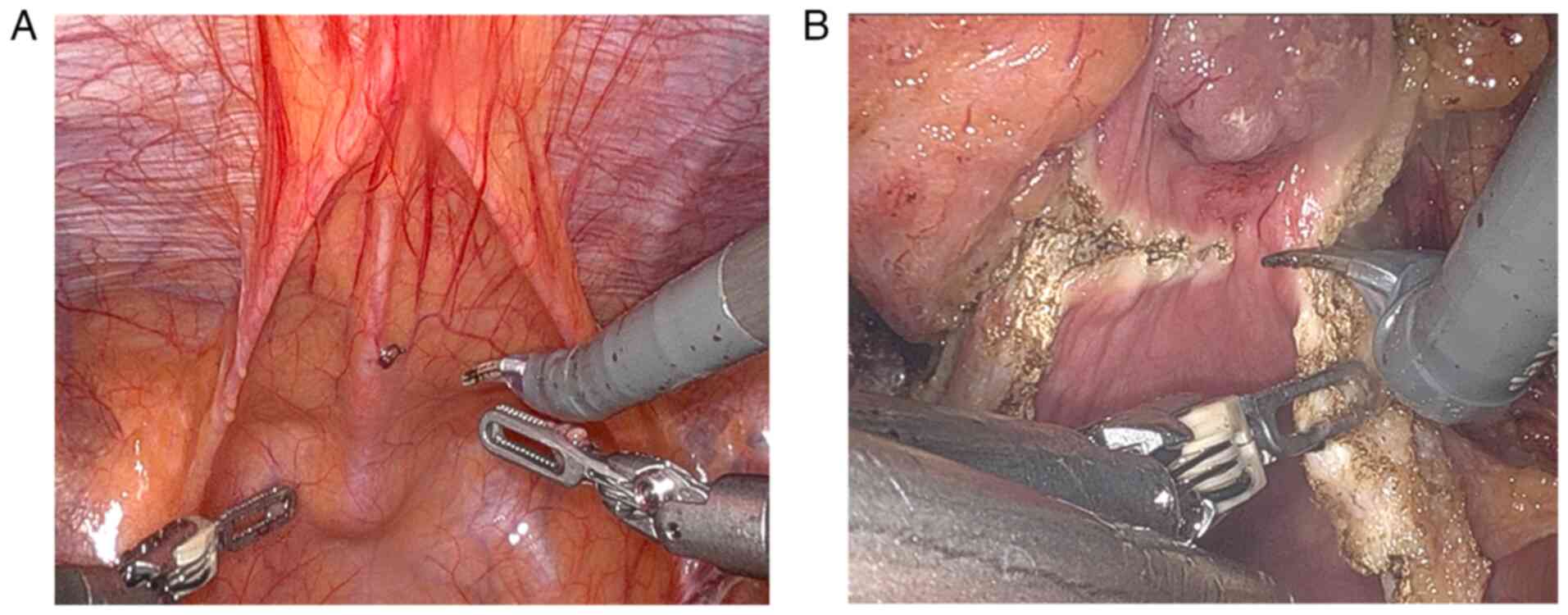
Dissection was performed along the urachus until the linea alba was
reached. A 3×3 cm mass was visible at the junction of the urachus
and the bladder (Fig. 3A). The mass
was firm and protruded into the bladder. The bladder was incised
around the mass, and the tumour along with a part of the bladder
wall was completely resected (Fig.
3B). The urachus was further dissected up to the umbilicus. The
umbilical incision was extended, and the umbilicus, urachus,
bladder wall and mass were completely removed. The estimated
intraoperative blood loss was ~20 ml.
Postoperative tissues were fixed in 10% neutral
buffered formalin for 24 h at room temperature, embedded in
paraffin and sectioned into 4 µm slices. Hematoxylin and eosin
staining was performed using standard protocols (hematoxylin for 5
min at room temperature and eosin for 2 min at room temperature),
visualized using a light microscope (×10 and ×20 magnification),
revealing low-grade adenocarcinoma of the urachus and part of the
bladder, with areas showing mucinous adenocarcinoma (Fig. 4A and B). The surgical margins were
negative and no cancer metastasis was observed in the bilateral
iliac lymph nodes.
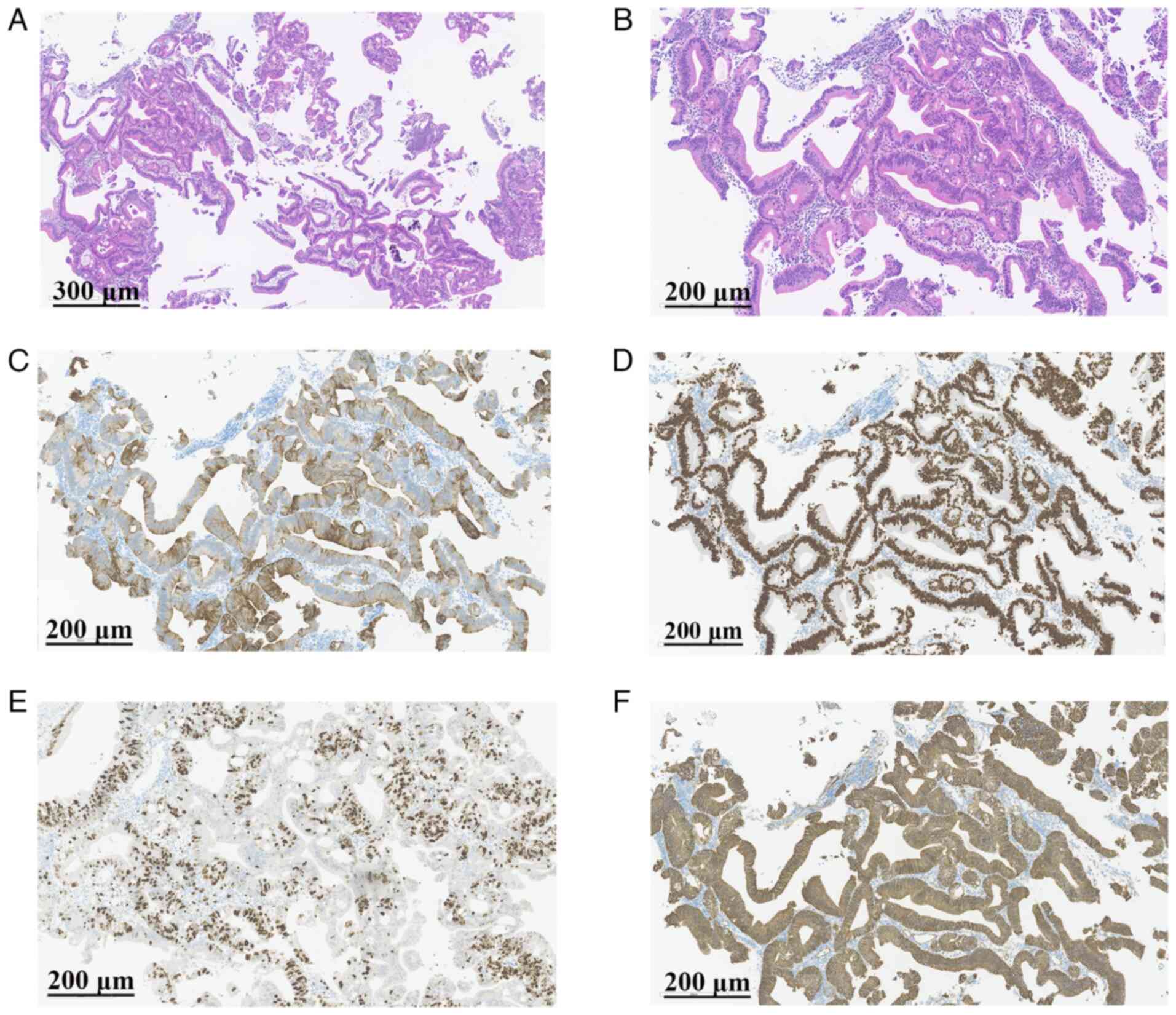 | Figure 4.Representative images of
immunohistochemistry results. (A) H&E staining (magnification,
×10; scale bar, 300 µm). (B) H&E staining (magnification, ×20;
scale bar, 200 µm). (C) Positive expression of CK20 (magnification,
×20; scale bar, 200 µm). (D) Positive expression of caudal type
homeobox 2 (magnification, ×20; scale bar, 200 µm). (E) Ki-67
staining demonstrates ~50% positive cells in the hot-spot area
(magnification, ×20; scale bar, 200 µm). (F) Positive
membrane-bound expression of β-catenin (magnification, ×20; scale
bar, 200 µm). H&E, hematoxylin and eosin. |
Paraffin-embedded tissues were sectioned at 4 µm
thickness, microwave-treated in sodium citrate buffer (pH 6) for 10
min for antigen retrieval and processed for immunohistochemistry.
After permeabilization with 0.1% Triton X-100 and blocking with 5%
goat serum (cat. no. ab7481; Abcam) at room temperature for 30 min.
Subsequently, sections were incubated overnight at 4°C with rabbit
primary antibodies against CK20 (cat. no. ab64090; 1:100; Abcam),
caudal type homeobox 2 (CDX2; cat. no. ab101532; 1:100; Abcam),
β-catenin (cat. no. ab32572; 1:500; Abcam) and Ki-67 (cat. no.
ab15580; 1:100; Abcam). Biotinylated goat anti-rabbit IgG secondary
antibodies (cat. no. ab6720; 1:2,000; Abcam) was applied for 30 min
at room temperature, followed by diaminobenzidine chromogen
detection. Sections were counterstained with hematoxylin for 5 min
at room temperature and staining scores were independently
evaluated by two pathologists in a blinded manner. Images were
captured using a light microscope at ×20 magnification. The results
were as follows: CK20 (positive, +), CDX2 (negative, -), CK7 (−),
CK34BE12 (−), β-catenin (membrane +) and Ki-67 (hot spot area,
50%+; Fig. 4C-F). Based on these
findings, the patient was diagnosed with Sheldon stage IIIa urachal
carcinoma. Postoperatively, the patient was transferred to the
oncology department for chemotherapy with a gemcitabine and
cisplatin regimen (gemcitabine 1,000 mg/m2 intravneously
on days 1 and 8; cisplatin 70 mg/m2 intravenously on day
1; 21-day cycle, 6 cycles total). At the latest follow-up (March
2025), which was 5 months after surgery, the patient was monitored
with physical examinations and pelvic CT scans. No signs of tumour
recurrence were observed.
Discussion
Urachal carcinoma, a rare and highly invasive
malignancy, was first described by Begg in 1931 (16). Urachal carcinoma predominantly
affects middle-aged and elderly adults, particularly males >50
years old. The most prevalent symptom is macroscopic haematuria,
followed by lower urinary tract symptoms, abdominal masses,
mucusuria and abdominal pain (5).
Currently, the diagnostic criteria for urachal carcinoma remain
controversial. The modified diagnostic criteria for urachal
carcinoma proposed in studies such as that by Gopalan et al
(17) are as follows: i) A tumour
located at the anterior or apical wall of the bladder; ii) the main
body of the tumour is within the bladder wall; iii) there is no
extensive cystic or glandular cystitis outside the anterior or
apical walls of the bladder; and iv) there is no known primary
tumour in other organs. The patient in the present study met these
diagnostic criteria. At present, there are two main distinct
staging standards for urachal carcinoma: i) The Sheldon staging
system (18); and ii) the Mayo
staging system (19), both of which
can predict the survival time of patients with urachal carcinoma.
The Sheldon tumour-node-metastasis staging system (18) was first proposed in 1984, is widely
recognized and used in clinical practice as a staging diagnostic
criterion (17,20). The Sheldon staging system classifies
urachal carcinoma as follows: Stage I, the tumour is confined to
the urachal mucosa; stage II, tumour with invasion confined to the
urachus itself; stage IIIA, tumour with local extension to the
bladder; stage IIIB, tumour with local extension to the abdominal
wall; stage IIIC, tumour invading the peritoneum; stage IIID,
tumour invading local viscera other than the bladder; stage IVA,
urachal cancer with metastasis to the lymph nodes; and stage IVB,
urachal cancer with distant metastases (18). In the present study, the patient was
diagnosed with stage IIIA urachal carcinoma.
Urachal cancer is primarily diagnosed on the basis
of the results of serum tumour marker tests, imaging examinations
and cystoscopy (21). Clinically
significant serum tumour markers in patients with urachal cancer
primarily include CEA, CA724, CA199 and CA125. The expression
levels of tumour markers are usually positively correlated with
tumour stage, and these markers can significantly decrease after
surgery or chemotherapy (22).
Typically, one-half of all patients with urachal cancer of have
elevated levels of CEA, CA724, CA19-9 and CA125, and all of these
markers may increase in the event of tumour metastasis or
recurrence (7,23). While serum tumour markers may assist
in the diagnosis and monitoring of urachal cancer, they lack
specificity and some case reports have documented instances where
serum tumour marker levels do not increase (24–26).
In the present study, the serum tumour marker levels of the
patients were also normal. The imaging methods used for examining
urachal cancer mainly include contrast-enhanced CT, MRI, FDG-PET/CT
and ultrasound imaging (18).
Pelvic ultrasound typically demonstrates a complex cystic mass
above the bladder, which may be considered an echo-enhancing lesion
and suggests calcification (27).
On CT and MRI scans, urachal cancer usually presents as a
cystic-solid mass in the midline of the urachal tract, extending
from the umbilicus to the top of the bladder, with visible central
or peripheral calcification (28,29).
FDG-PET/CT imaging has demonstrated variability among patients with
urachal cancer, possibly because a large proportion of cases of
urachal cancer are adenocarcinomas, some of which are mucin-rich,
which leads to poor FDG uptake and visualization (30,31).
Cystoscopy serves a key role in the visualization and histological
diagnosis of urachal cancer, with numerous lesions appearing as
cauliflower-like neoplasms at the top or anterior wall of the
bladder (21).
The most common pathological type of urachal cancer
is adenocarcinoma, with mucinous adenocarcinoma being the most
common; urachal adenocarcinoma accounts for ~20-39% of all types of
primary bladder adenocarcinoma (18). Primary bladder adenocarcinomas share
immunohistochemical similarities with colorectal adenocarcinomas
that invade the bladder, which may be due to the common
embryological origin of the urachus, bladder and colon, which leads
to a tendency for intestinal metaplasia in the urachus (17). In a study by Wang et al
(32), the expression levels of
CK7, CK20, CDX2 and nuclear β-catenin in urachal adenocarcinoma
were 50, 100, 85 and 6%, respectively. In mucinous urachal
adenocarcinoma, CDX2 is highly expressed, whereas nuclear β-catenin
is not expressed. In the present study, the patient's tumour
markers CK20, CDX2 and membrane β-catenin were positive, whereas
CK7 and CK34BE12 were negative, which is consistent with the
immunohistochemical features of urachal cancer.
The main treatment for localized urachal cancer is
surgery, although there is no consensus on the specific surgical
approach. Surgical options include radical cystectomy (RC), partial
cystectomy (PC) and en bloc resection of the urachus and umbilicus
(33). A study by Knoedler et
al (34) demonstrated no
significant differences in survival between patients who underwent
RC and those who underwent PC; however, PC may offer a better
quality of life due to bladder preservation, making it more
commonly utilized in clinical practice (35,36). A
previous study reported that umbilical resection is an independent
prognostic factor for overall survival in patients with urachal
cancer (37). Thus, complete
resection of the umbilicus is recommended. There was no significant
difference in survival between patients who underwent pelvic lymph
node dissection and those who did not (33,35).
However, patients with pathologically positive lymph nodes (without
distant metastasis) have a poor prognosis similar to that of
patients with distant metastasis, suggesting that pelvic lymph node
dissection may be beneficial (6).
According to the expert consensus reached by the Canadian
Urological Association in 2016, the preferred intervention for
localized urachal cancer was surgery involving resection of the
umbilicus, urachus and partial cystectomy, combined with pelvic
lymph node dissection. In cases where partial cystectomy cannot
achieve negative surgical margins, RC with en bloc removal of the
urachus and umbilicus should be considered (36). Surgical approaches can include open
surgery, laparoscopic surgery or robot-assisted laparoscopic
surgery. In 2006, Milhoua et al (38) performed laparoscopic partial
cystectomy with en bloc resection of the urachus and umbilicus for
patients with urachal cancer, with no signs of recurrence at the
1.5-year follow-up. Compared with open surgery, laparoscopic
surgery reduces intraoperative blood loss, shortens the hospital
stay and accelerates recovery (39). In the field of urology, robotic
surgery is more commonly used in prostate and kidney surgeries,
with fewer reports of robot-assisted laparoscopic partial
cystectomy. In 2015, James et al (40) reported the use of robot-assisted
laparoscopic partial cystectomy in 8 patients with urachal cancer
and achieved similar tumour resection results as those of open
surgery, but with a lower incidence of perioperative complications.
At a median follow-up of 32 months, there was no sign of
recurrence, which indicated that robot-assisted partial cystectomy
is a feasible and safe approach. In the present study, the patient
underwent robot-assisted laparoscopic extended partial cystectomy
without difficulty or postoperative complications, resulting in
good surgical outcomes. Compared with open and laparoscopic
surgery, robotic surgery offers notable advantages, including
improved mobility and flexibility, high-definition visualization,
stability and precision, as well as improved suturing capabilities
(41). However, robot-assisted
laparoscopic surgery also has disadvantages. The most notable of
these is the high cost, which impedes the distribution of the
technology in numerous countries (42). Additionally, device malfunctions,
such as instrument breakage, electrical arcing or system errors
(43) and a steep learning curve
requiring extensive training are significant drawbacks of this
method (44). In teaching
hospitals, the cost of the procedure may also deprive junior
residents of the opportunity to gain laparoscopic experience.
For patients with positive lymph nodes, peritoneal
involvement, positive surgical margins or a high likelihood of
recurrence, postoperative chemotherapy is recommended (1). Owing to the rarity of urachal cancer,
there is no standard chemotherapy regimen. Commonly used regimens
include platinum-based therapies or 5-FU, with gemcitabine being
increasingly included in treatment plans (45). A previous study demonstrated that
platinum-based chemotherapy is more effective compared with
nonplatinum chemotherapy for disease control (median
progression-free survival, 8.23 vs. 3.80 months) (46). In the present study, the patient was
treated with a cisplatin and gemcitabine regimen, and no tumour
recurrence was detected at the latest follow-up in March 2025.
Limitations of the present study should be noted.
First, the lack of a cystoscopic images due to non-collection
during the cystoscopy procedure limits the visual demonstration of
the tumour. Second, a single patient may not fully represent the
overall situation of patients with urachal carcinoma. Third, the
relatively short follow-up period (5 months) may not be sufficient
to evaluate the long-term recurrence rate and survival rate
accurately.
Urachal cancer is a rare and aggressive malignancy
with a poor prognosis. For localized urachal cancer, surgery
remains the primary treatment modality, which should completely
resect part of the bladder, urachus and umbilicus. The present
study described a case of a 31-year-old urachal carcinoma patient
successfully treated using robot-assisted laparoscopic modified
partial cystectomy. Such a case is rare in the literature and
therefore provides important clinical experience. Future research
should aim to include larger sample sizes and long-term follow-up
studies to comprehensively evaluate the efficacy, safety and
potential broader application of robot-assisted laparoscopic
partial cystectomy as a minimally invasive treatment for urachal
carcinoma.
Acknowledgements
Not applicable.
Funding
The present research was supported by the Guangdong Finance
Department Project (grant no. KS0120220271).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
CY, QX and XP drafted the manuscript and
conceptualized the present study. ZL and KW participated in the
analysis, collection and interpretation of data. FZ obtained the
PET/CT images and analysed patient data. FZ, WK and ZL confirm the
authenticity of all the raw data. All authors read and approved the
final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
The authors obtained the patient's written informed
consent. The patient was informed of privacy protection and the
potential uses of the published content. A copy of the signed
consent form is retained and ethical and legal standards adhered to
during the publication process.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siefker-Radtke A: Urachal adenocarcinoma:
A clinician's guide for treatment. Semin Oncol. 39:619–624. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liang L, Zhou N, Xu H, Liu D, Lu Y, Li F
and Guo J: Urachal mucinous adenocarcinoma with pseudomyxoma
peritonei: A case report. Medicine (Baltimore). 96:e75482017.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Paras FA Jr and Maclennan GT: Urachal
adenocarcinoma. J Urol. 180:7202008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Das JP, Vargas HA, Lee A, Hutchinson B,
O'Connor E, Kok HK, Torreggiani W, Murphy J, Roche C, Bruzzi J and
McCarthy P: The urachus revisited: Multimodal imaging of benign
& malignant urachal pathology. Br J Radiol. 93:201901182020.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ashley RA, Inman BA, Routh JC, Rohlinger
AL, Husmann DA and Kramer SA: Urachal anomalies: A longitudinal
study of urachal remnants in children and adults. J Urol.
178:1615–1618. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bruins HM, Visser O, Ploeg M,
Hulsbergen-van De Kaa CA, Kiemeney LALM and Witjes JA: The clinical
epidemiology of urachal carcinoma: Results of a large, population
based study. J Urol. 188:1102–1107. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Siefker-Radtke AO, Gee J, Shen YU, Wen S,
Daliani D, Millikan RE and Pisters LL: Multimodality management of
urachal carcinoma: The M. D. Anderson cancer center experience. J
Urol. 169:1295–1298. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Shao G, Xu C, Liu J, Li X, Li L, Li X,
Zhang X, Fan Y and Zhou L: Clinical, pathological, and prognostic
analysis of urachal carcinoma. Urol Int. 106:199–208. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wright JL, Porter MP, Li CI, Lange PH and
Lin DW: Differences in survival among patients with urachal and
nonurachal adenocarcinomas of the bladder. Cancer. 107:721–728.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Molina JR, Quevedo JF, Furth AF,
Richardson RL, Zincke H and Burch PA: Predictors of survival from
urachal cancer: A Mayo Clinic study of 49 cases. Cancer.
110:2434–2440. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Collins DC, Velázquez-Kennedy K, Deady S,
Brady AP, Sweeney P and Power DG: National incidence, management
and survival of urachal carcinoma. Rare Tumors. 8:62572016.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zihni A, Gerull WD, Cavallo JA, Ge T, Ray
S, Chiu J, Brunt LM and Awad MM: Comparison of precision and speed
in laparoscopic and robot-assisted surgical task performance. J
Surg Res. 223:29–33. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franco A, Ditonno F, Manfredi C, Johnson
AD, Mamgain A, Feldman-Schultz O, Feng CL, Pellegrino AA, Mir MC,
Porpiglia F, et al: Robot-assisted surgery in the field of urology:
The most pioneering approaches 2015–2023. Res Rep Urol. 15:453–470.
2023.PubMed/NCBI
|
|
14
|
Spiess PE and Correa JJ: Robotic assisted
laparoscopic partial cystectomy and urachal resection for urachal
adenocarcinoma. Int Braz J Urol. 35:6092009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Suartz CV, Martinez LM, Brito PH, Neto CV,
Cordeiro MD, Botelho LAA, Gallucci FP, Mota JM, Nahas WC and
Ribeiro-Filho LA: Robotic-assisted approaches to urachal carcinoma:
A comprehensive systematic review of the safety and efficacy
outcomes. BJUI Compass. 5:327–333. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Begg RC: The urachus: Its anatomy,
histology and development. J Anat. 64:170–183. 1930.PubMed/NCBI
|
|
17
|
Gopalan A, Sharp DS, Fine SW, Tickoo SK,
Herr HW, Reuter VE and Olgac S: Urachal carcinoma: A
clinicopathologic analysis of 24 cases with outcome correlation. Am
J Surg Pathol. 33:659–668. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sheldon CA, Clayman RV, Gonzalez R,
Williams RD and Fraley EE: Malignant urachal lesions. J Urol.
131:1–8. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ashley RA, Inman BA, Sebo TJ, Leibovich
BC, Blute ML, Kwon ED and Zincke H: Urachal carcinoma:
Clinicopathologic features and long-term outcomes of an aggressive
malignancy. Cancer. 107:712–720. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Duan F, Zhai W, Zhang B and Guo S: Urachal
carcinoma: Impact of recurrence pattern and lymphadenectomy on
long-term outcomes. Cancer Med. 9:4166–4174. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang J and Wu J: Options for diagnosis
and treatment of urachal carcinoma. Asia Pac J Clin Oncol.
9:117–122. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ke C, Hu Z and Yang C: Preoperative
accuracy of diagnostic evaluation of urachal carcinoma. Cancer Med.
12:9106–9115. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reis H, Krafft U, Niedworok C, Módos O,
Herold T, Behrendt M, Al-Ahmadie H, Hadaschik B, Nyirady P and
Szarvas T: Biomarkers in urachal cancer and adenocarcinomas in the
bladder: A comprehensive review supplemented by own data. Dis
Markers. 2018:73081682018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tiutiucă RC, Năstase Pușcașu AI, Țarcă E,
Stoenescu N, Cojocaru E, Trandafir LM, Țarcă V, Scripcariu DV and
Moscalu M: Urachal carcinoma, an unusual possibility of hematuria;
case report and literature review. Diagnostics (Basel).
12:18922022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kanamaru T, Iguchi T, Yukimatsu N, Shimizu
Y, Kohyama Y, Tachibana H, Kato M, Yamasaki T, Tamada S and
Nakatani T: A case of metastatic urachal carcinoma treated with
FOLFIRI (irinotecan and 5-fluorouracil/leucovorin) plus
bevacizumab. Urol Case Rep. 3:9–11. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kumar R, Harilal S, Abdelgawad MA, Ghoneim
MM, Kumar A and Mathew B: Urachal carcinoma: The journey so far and
the road ahead. Pathol Res Pract. 243:1543792023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Monteiro V and Cunha TM: Urachal
carcinoma: Imaging findings. Acta Radiol Short Rep.
1:arsr.2011.110018. 2012.PubMed/NCBI
|
|
28
|
Machida H, Ueno E, Nakazawa H, Fujimura M
and Kihara T: Computed tomographic appearance of urachal carcinoma
associated with urachal diverticulum misdiagnosed by cystoscopy.
Abdom Imaging. 33:363–366. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li S, Meng X, Liang P, Feng C, Shen Y, Hu
D and Li Z: Clinical and radiological features of urachal carcinoma
and infection. Front Oncol. 11:7021162021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kapoor R, Darasani N and Bansal A: Rare
presentation of urachal adenocarcinoma with skip metastasis to
colon. Indian J Urol. 32:244–246. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Stokkel LE, Stokkel MPM, Donswijk ML,
Lahaye MJ, Bekers EM, Van Rhijn BWG and Mertens LS: The diagnostic
value of FDG-PET/CT for urachal cancer. Clin Genitourin Cancer.
19:373–380. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang HL, Lu DW, Yerian LM, Alsikafi N,
Steinberg G, Hart J and Yang XJ: Immunohistochemical distinction
between primary adenocarcinoma of the bladder and secondary
colorectal adenocarcinoma. Am J Surg Pathol. 25:1380–1387. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Herr HW, Bochner BH, Sharp D, Dalbagni G
and Reuter VE: Urachal carcinoma: Contemporary surgical outcomes. J
Urol. 178:74–78. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Knoedler JJ, Boorjian SA, Kim SP, Weight
CJ, Thapa P, Tarrell RF, Cheville JC and Frank I: Does partial
cystectomy compromise oncologic outcomes for patients with bladder
cancer compared to radical cystectomy? A matched case-control
analysis. J Urol. 188:1115–1119. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dursun F, Lim K, Svatek RS, Xu J,
El-Zaatari ZM, Wenker EP, Klaassen ZW, Mansour AM, Muhammad T,
Efstathiou E, et al: Clinical outcomes and patterns of
population-based management of urachal carcinoma of the bladder: An
analysis of the national cancer database. Cancer Med. 11:4273–4282.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamilou Z, North S, Canil C, Wood L, Hotte
S, Sridhar SS, Soulières D, Latour M, Taussky D, Kassouf W and
Blais N: Management of urachal cancer: A consensus statement by the
canadian urological association and genitourinary medical
oncologists of Canada. Can Urol Assoc J. 14:E57–E64.
2020.PubMed/NCBI
|
|
37
|
Jia Z, Chang X, Li X, Wang B and Zhang X:
Urachal carcinoma: Are lymphadenectomy and umbilectomy necessary?
Med Sci Monit. 26:e9279132020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Milhoua PM, Knoll A, Bleustein CB and
Ghavamian R: Laparoscopic partial cystectomy for treatment of
adenocarcinoma of the urachus. Urology. 67:423.e15–423.e17. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shi Z: Laparoscopic vs open surgery: A
comparative analysis of wound infection rates and recovery
outcomes. Int Wound J. 21:e144742024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
James K, Vasdev N, Mohan-S G, Lane T and
Adshead JM: Robotic partial cystectomy for primary urachal
adenocarcinoma of the urinary bladder. Curr Urol. 8:183–188. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Brassetti A, Ragusa A, Tedesco F, Prata F,
Cacciatore L, Iannuzzi A, Bove AM, Anceschi U, Proietti F,
D'Annunzio S, et al: Robotic Surgery in Urology: History from
PROBOT® to HUGOTM. Sensors (Basel).
23:71042023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Schwaibold H, Wiesend F and Bach C: The
age of robotic surgery-is laparoscopy dead? Arab J Urol.
16:262–269. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Alemzadeh H, Raman J, Leveson N,
Kalbarczyk Z and Iyer RK: Adverse events in robotic surgery: A
retrospective study of 14 years of FDA data. PLoS One.
11:e01514702016. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Kirkpatrick T and LaGrange C: Robotic
surgery: Risks vs rewards. 2023.
|
|
45
|
Mylonas KS, O'Malley P, Ziogas IA,
El-Kabab L and Nasioudis D: Malignant urachal neoplasms: A
population-based study and systematic review of literature. Urol
Oncol. 35:33.e11–33.e19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chen M, Xue C, Huang R, Ni MQ, Li L, Li
HF, Yang W, Hu AQ, Zheng ZS, An X and Shi Y: Treatment outcome of
different chemotherapy in patients with relapsed or metastatic
malignant urachal tumor. Front Oncol. 11:7391342021. View Article : Google Scholar : PubMed/NCBI
|