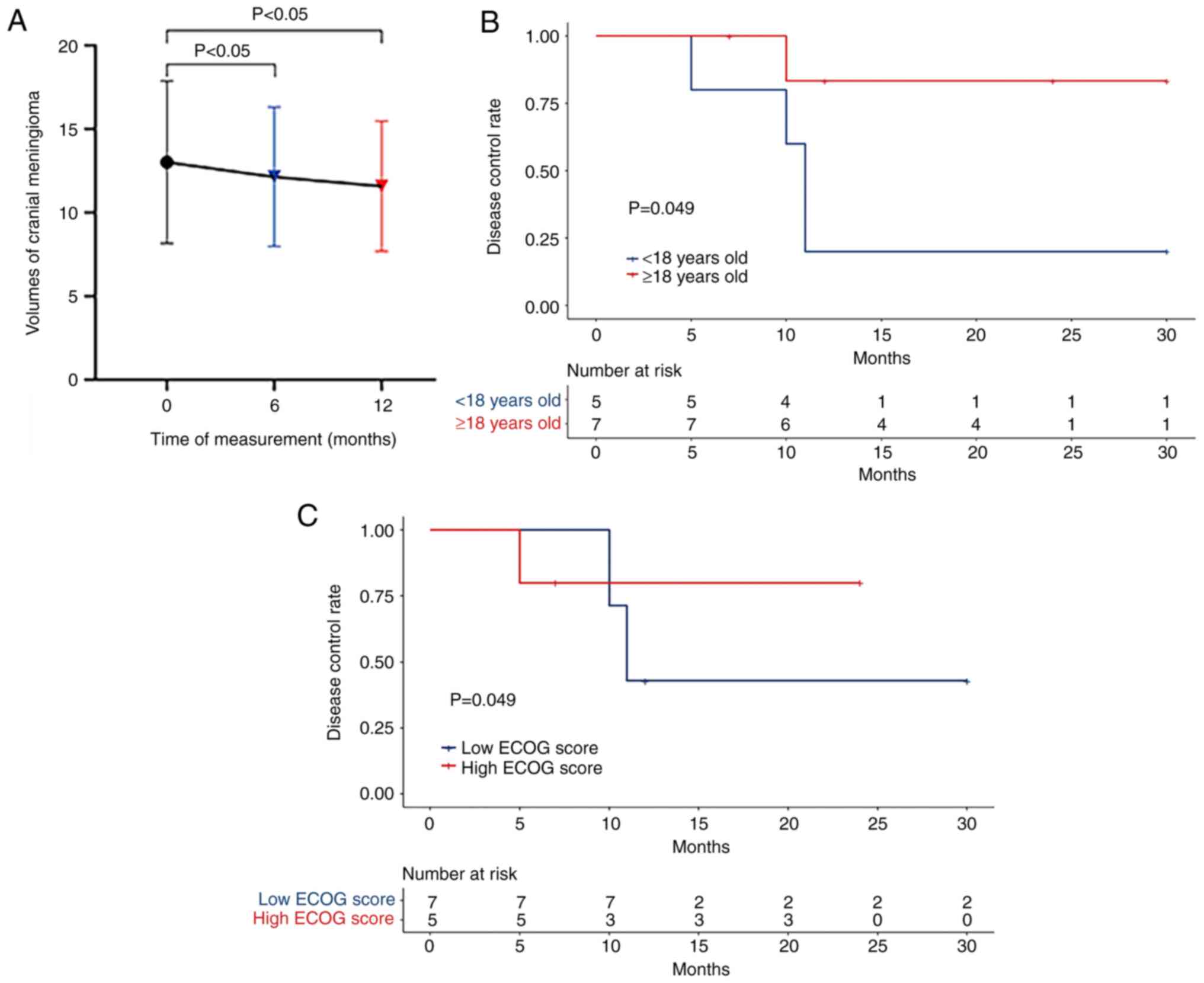
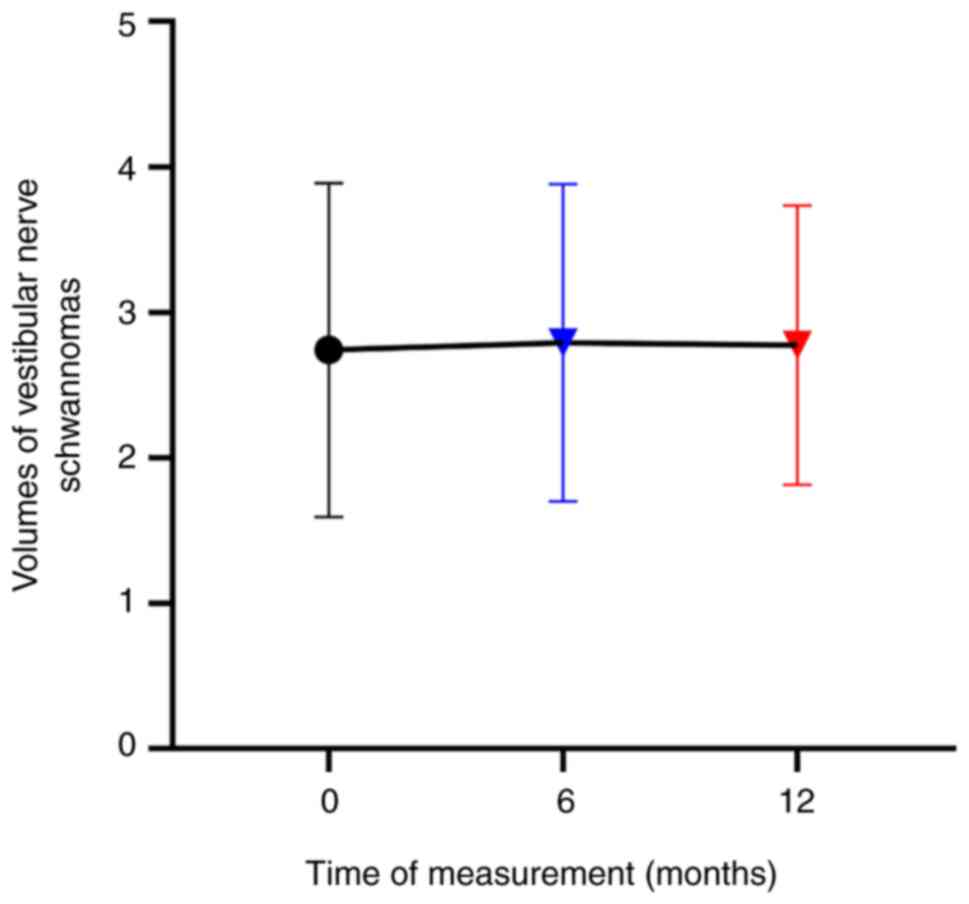
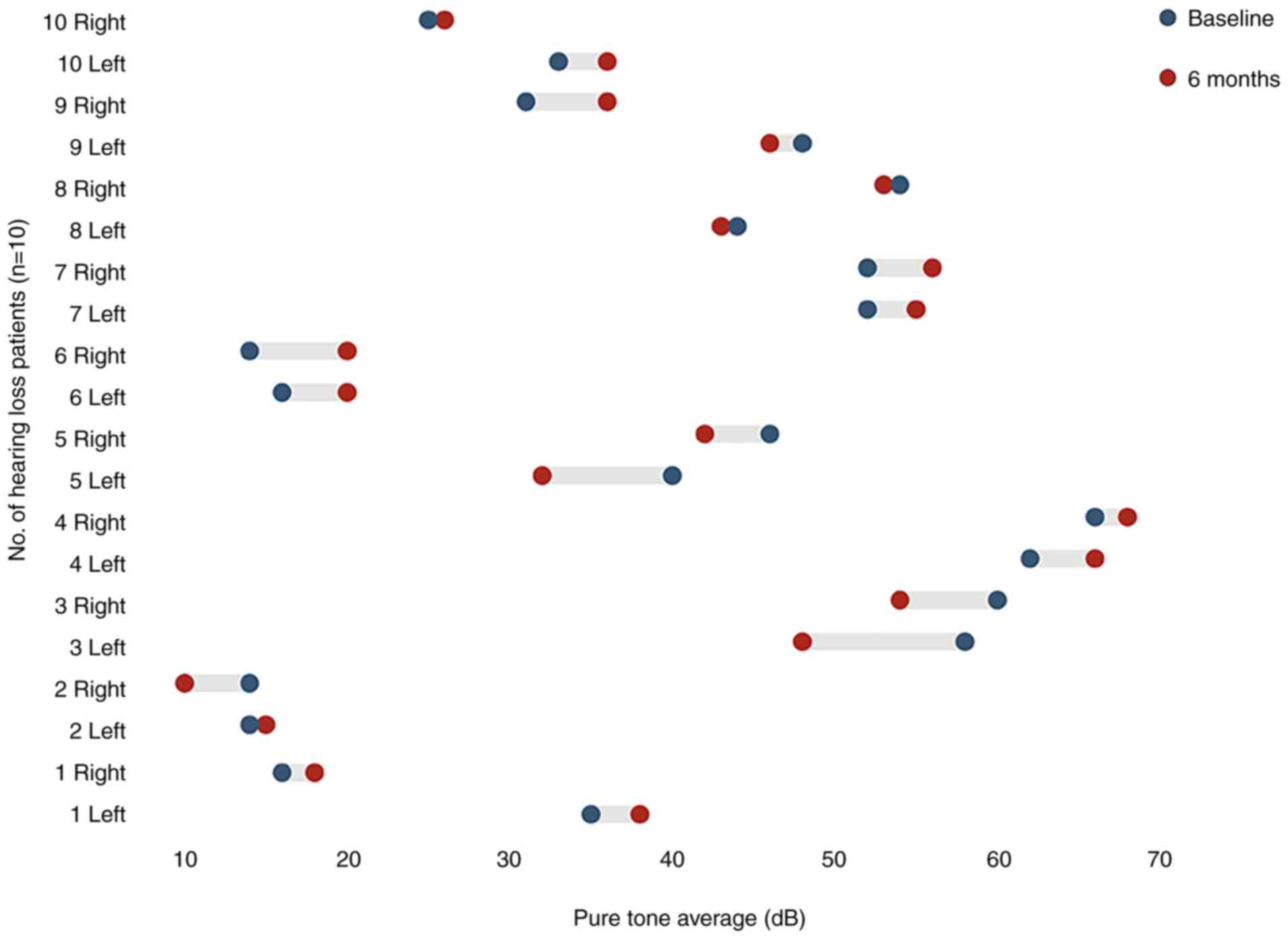
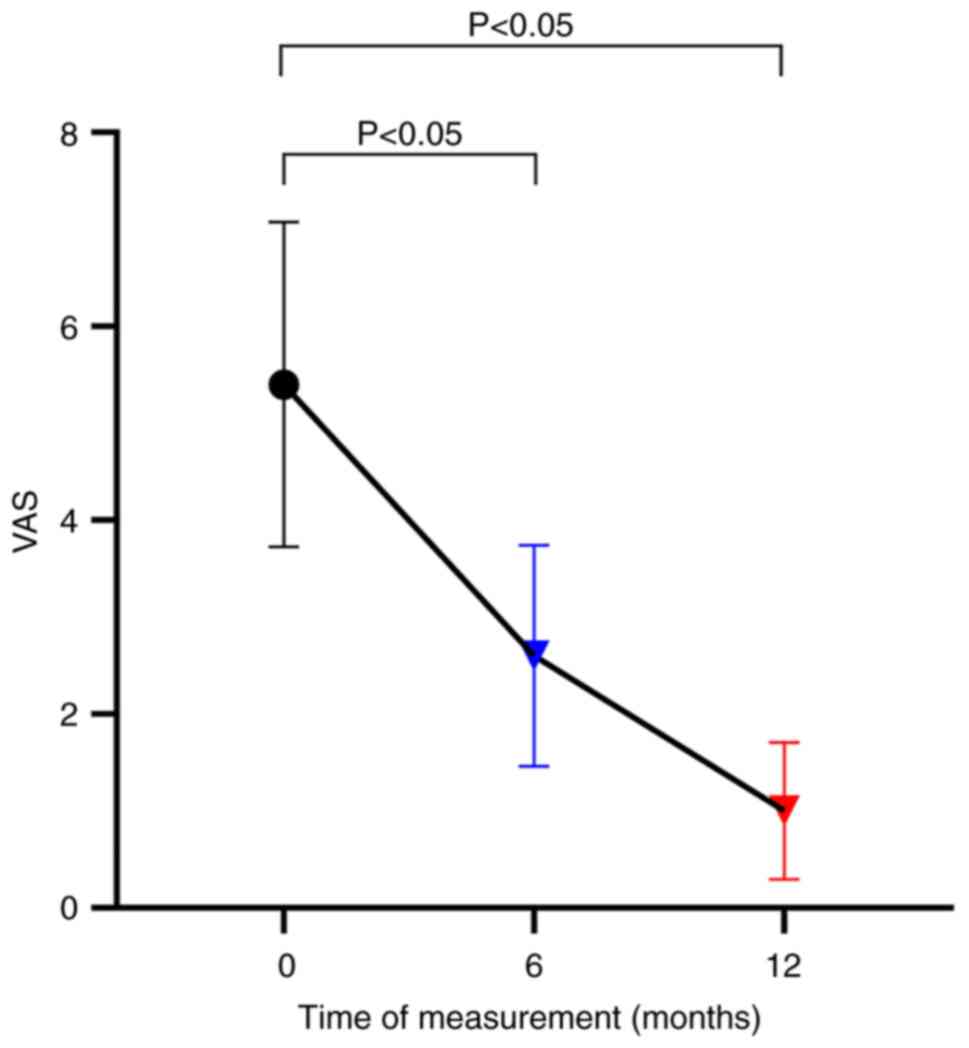
|
1
|
Rouleau GA, Merel P, Lutchman M, Sanson M,
Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C,
Plougastel B, et al: Alteration in a new gene encoding a putative
membrane-organizing protein causes neuro-fibromatosis type 2.
Nature. 363:515–521. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asthagiri AR, Parry DM, Butman JA, Kim HJ,
Tsilou ET, Zhuang Z and Lonser RR: Neurofibromatosis type 2.
Lancet. 373:1974–1986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slattery WH: Neurofibromatosis type 2.
Otolaryngol Clin North Am. 48:443–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans DG, Baser ME, O'Reilly B, Rowe J,
Gleeson M, Saeed S, King A, Huson SM, Kerr R, Thomas N, et al:
Management of the patient and family with neurofibromatosis 2: A
consensus conference statement. Br J Neurosurg. 19:5–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plotkin SR, Messiaen L, Legius E, Pancza
P, Avery RA, Blakeley JO, Babovic-Vuksanovic D, Ferner R, Fisher
MJ, Friedman JM, et al: Updated diagnostic criteria and
nomenclature for neurofibromatosis type 2 and schwannomatosis: An
international consensus recommendation. Genet Med. 24:1967–1977.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okada T, You L and Giancotti FG: Shedding
light on Merlin's wizardry. Trends Cell Biol. 17:222–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Bai H, David KK, Dong J, Zheng Y,
Cai J, Giovannini M, Liu P, Anders RA and Pan D: The Merlin/NF2
tumor suppressor functions through the YAP oncoprotein to regulate
tissue homeostasis in mammals. Dev Cell. 19:27–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Apra C, Peyre M and Kalamarides M: Current
treatment options for meningioma. Expert Rev Neurother. 18:241–249.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Angus SP, Oblinger JL, Stuhlmiller TJ,
DeSouza PA, Beauchamp RL, Witt L, Chen X, Jordan JT, Gilbert TSK,
Stemmer-Rachamimov A, et al: EPH receptor signaling as a novel
therapeutic target in NF2-deficient meningioma. Neuro Oncol.
20:1185–1196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohanty A, Pharaon RR, Nam A, Salgia S,
Kulkarni P and Massarelli E: FAK-targeted and combination therapies
for the treatment of cancer: An overview of phase I and II clinical
trials. Expert Opin Investig Drugs. 29:399–409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Markham A: Brigatinib: First global
approval. Drugs. 77:1131–1135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoy SM: Brigatinib: A review in
ALK-inhibitor Naïve advanced ALK-positive NSCLC. Drugs. 81:267–275.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang LS, Oblinger JL, Smith AE, Ferrer M,
Angus SP, Hawley E, Petrilli AM, Beauchamp RL, Riecken LB, Erdin S,
et al: Brigatinib causes tumor shrinkage in both NF2-deficient
meningioma and schwannoma through inhibition of multiple tyrosine
kinases but not ALK. PLoS One. 16:e02520482021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mulvihill JJ, Parry DM, Sherman JL, Pikus
A, Kaiser-Kupfer MI and Eldridge R: NIH conference.
Neurofibromatosis 1 (Recklinghausen disease) and neurofibromatosis
2 (bilateral acoustic neurofibromatosis). An update. Ann Intern
Med. 113:39–52. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans DG, Huson SM, Donnai D, Neary W,
Blair V, Newton V and Harris R: A clinical study of type 2
neurofibromatosis. Q J Med. 84:603–618. 1992.PubMed/NCBI
|
|
16
|
Baser ME, Friedman JM, Wallace AJ, Ramsden
RT, Joe H and Evans DGR: Evaluation of clinical diagnostic criteria
for neurofibromatosis 2. Neurology. 59:1759–1765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han
JY, Hochmair MJ, Lee KH, Delmonte A, Garcia Campelo MR, Kim DW, et
al: Brigatinib versus crizotinib in ALK inhibitor-naive advanced
ALK-positive NSCLC: Final results of phase 3 ALTA-1L trial. J
Thorac Oncol. 16:2091–2108. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walia A, Tuia J and Prasad V:
Progression-free survival, disease-free survival and other
composite end points in oncology: Improved reporting is needed. Nat
Rev Clin Oncol. 20:885–895. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carl AC, Hohman MH and Cornejo J:
Audiology pure tone evaluation. [2023 Mar 1]. StatPearls [Internet]
Treasure Island (FL): StatPearls Publishing; 2025
|
|
20
|
Kostaras P, Martinaki S, Asimopoulos C,
Maltezou M and Papageorgiou C: The use of the symptom checklist
90-R in exploring the factor structure of mental disorders and the
neglected fact of comorbidity. Psychiatry Res. 294:1135222020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCormack HM, Horne DJ and Sheather S:
Clinical applications of visual analogue scales: A critical review.
Psychol Med. 18:1007–1019. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (eastern cooperative oncology group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grossen A, Gavula T, Chrusciel D, Evans A,
McNall-Knapp R, Taylor A, Fossey B, Brakefield M, Carter C,
Schwartz N, et al: Multidisciplinary neurocutaneous syndrome
clinics: A systematic review and institutional experience.
Neurosurg Focus. 52:E22022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mautner VF, Lindenau M, Baser ME, Hazim W,
Tatagiba M, Haase W, Samii M, Wais R and Pulst SM: The neuroimaging
and clinical spectrum of neurofibromatosis 2. Neurosurgery.
38:880–886. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coy S, Rashid R, Stemmer-Rachamimov A and
Santagata S: An update on the CNS manifestations of
neurofibromatosis type 2. Acta Neuropathol. 139:643–665. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blakeley JO, Ye X, Duda DG, Halpin CF,
Bergner AL, Muzikansky A, Merker VL, Gerstner ER, Fayad LM, Ahlawat
S, et al: Efficacy and biomarker study of bevacizumab for hearing
loss resulting from neurofibromatosis type 2-associated vestibular
schwannomas. J Clin Oncol. 34:1669–1675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu VM, Ravindran K, Graffeo CS, Perry A,
Van Gompel JJ, Daniels DJ and Link MJ: Efficacy and safety of
bevacizumab for vestibular schwannoma in neurofibromatosis type 2:
A systematic review and meta-analysis of treatment outcomes. J
Neurooncol. 144:239–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamura R, Fujioka M, Morimoto Y, Ohara K,
Kosugi K, Oishi Y, Sato M, Ueda R, Fujiwara H, Hikichi T, et al: A
VEGF receptor vaccine demonstrates preliminary efficacy in
neurofibromatosis type 2. Nat Commun. 10:57582019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plotkin SR, Allen J, Dhall G, Campian JL,
Clapp DW, Fisher MJ, Jain RK, Tonsgard J, Ullrich NJ, Thomas C, et
al: Multicenter, prospective, phase II study of maintenance
bevacizumab for children and adults with NF2-related
schwannomatosis and progressive vestibular schwannoma. Neuro Oncol.
25:1498–1506. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plotkin SR, Yohay KH, Nghiemphu PL, Dinh
CT, Babovic-Vuksanovic D, Merker VL, Bakker A, Fell G, Trippa L and
Blakeley JO; INTUITT-NF2 Consortium, : Brigatinib in NF2-related
schwannomatosis with progressive tumors. N Engl J Med.
390:2284–2294. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
den Bakker MA, Vissers KJ, Molijn AC, Kros
JM, Zwarthoff EC and van der Kwast TH: Expression of the
neurofibromatosis type 2 gene in human tissues. J Histochem
Cytochem. 47:1471–1480. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paldor I, Abbadi S, Bonne N, Ye X,
Rodriguez FJ, Rowshanshad D, Itzoe M, Vigilar V, Giovannini M, Brem
H, et al: The efficacy of lapatinib and nilotinib in combination
with radiation therapy in a model of NF2 associated peripheral
schwannoma. J Neurooncol. 135:47–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coy S, Rashid R, Stemmer-Rachamimov A and
Santagata S: Correction to: An update on the CNS manifestations of
neurofibromatosis type 2. Acta Neuropathol. 139:6672020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kukutla P, Ahmed SG, DuBreuil DM,
Abdelnabi A, Cetinbas M, Fulci G, Aldikacti B, Stemmer-Rachamimov
A, Plotkin SR, Wainger B, et al: Transcriptomic signature of
painful human neurofibromatosis type 2 schwannomas. Ann Clin Transl
Neurol. 8:1508–1514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peyre M, Tran S, Parfait B, Bernat I,
Bielle F and Kalamarides M: Surgical management of peripheral nerve
pathology in patients with neurofibromatosis type 2. Neurosurgery.
92:317–328. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohammed N, Hung YC, Xu Z, Chytka T,
Liscak R, Tripathi M, Arsanious D, Cifarelli CP, Perez Caceres M,
Mathieu D, et al: Neurofibromatosis type 2-associated meningiomas:
An international multicenter study of outcomes after Gamma Knife
stereotactic radiosurgery. J Neurosurg. 136:109–114. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fernandez-Valle C, Tang Y, Ricard J,
Rodenas-Ruano A, Taylor A, Hackler E, Biggerstaff J and Iacovelli
J: Paxillin binds schwannomin and regulates its density-dependent
localization and effect on cell morphology. Nat Genet. 31:354–562.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Plotkin SR, Halpin C, McKenna MJ, Loeffler
JS, Batchelor TT and Barker FG II: Erlotinib for progressive
vestibular schwannoma in neurofibromatosis 2 patients. Otol
Neurotol. 31:1135–1143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tamura R, Tanaka T, Miyake K, Yoshida K
and Sasaki H: Bevacizumab for malignant gliomas: Current
indications, mechanisms of action and resistance, and markers of
response. Brain Tumor Pathol. 34:62–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Camidge DR, Kim HR, Ahn MJ, Yang JC, Han
JY, Lee JS, Hochmair MJ, Li JY, Chang GC, Lee KH, et al: Brigatinib
versus crizotinib in ALK-positive non-small-cell lung cancer. N
Engl J Med. 379:2027–2039. 2018. View Article : Google Scholar : PubMed/NCBI
|