Introduction
Neurofibromatosis type 2 (NF2) is an autosomal
dominant condition resulting from mutations in the NF2 gene located
on chromosome 22, and it is marked by the presence of multiple
neurological tumors (1). The main
manifestations of NF2 include the presence of bilateral vestibular
schwannomas (also known as bilateral acoustic neuromas),
meningiomas, spinal tumors and various neoplasms affecting both the
central and peripheral nervous systems. These may result in hearing
loss, balance issues, headaches, facial nerve paralysis, impaired
vision and various neurological symptoms (2,3).
The management of NF2 generally requires a
comprehensive strategy, which encompasses the surgical removal of
tumors, radiation treatment, medication-based therapies and
supportive rehabilitation services. The surgical resection method
is frequently employed to remove tumors that are accessible;
however, it entails certain risks, particularly when dealing with
tumors located in sensitive areas. Radiation therapy is effective
in managing tumor growth; however, it can also lead to negative
side effects. At present, there are limited effective treatments
for NF2 available. The effectiveness of pharmaceutical agents in
addressing NF2-associated tumors is constrained, thus surgical
intervention continues to be the primary approach for managing NF2
progression (4,5). For individuals with NF2 who are unable
or opt against surgical intervention, pharmacological treatments
play a vital role in disease management. Currently, there are no
specific medications that demonstrate high efficacy in the
treatment of tumors associated with NF2.
NF2 is linked to various intracellular signaling
pathways, including Hippo, protein kinase A, PI3K/AKT, Rac/p21
activated kinases/JNK, WNT/β-catenin, receptor tyrosine kinase,
Ras, MAPK, Yes-associated protein, p21-activated kinase, CD44 and
Rac/Rho (6,7). Inactivation of NF2 leads to the
activation of these cellular pathways, which are crucial in the
pathological development of NF2-associated tumors. Focusing on
these pathways may prove beneficial in the treatment of the tumors
(8). Protein tyrosine kinase
inhibitors have demonstrated the ability to decelerate tumor growth
and prolong progression-free survival in clinical trials (9,10). The
potential of these inhibitors as a treatment for NF2 tumors
warrants further investigation. Brigatinib represents an advanced
class of tyrosine kinase inhibitors that has received approval for
the treatment of anaplastic lymphoma kinase/ROS proto-oncogene 1
(ALK/ROS1)-positive non-small cell lung cancer (NSCLC). It
effectively inhibits ALK and ROS1 fusion proteins, obstructing
downstream pro-survival pathways including MAPK/ERK and PI3K/AKT.
This combined approach effectively diminishes tumor growth and
spread, particularly in individuals with central nervous system
involvement (11,12). In NF2, the absence of the Merlin
protein resulting from mutations in the NF2 gene causes the
hyperactivation of various tyrosine kinases, such as focal adhesion
kinase 1 (FAK1) and Ephrin type-A receptor 2 (EphA2). The activity
of these kinases is a significant factor in the proliferation of
schwannomas and meningiomas. A preclinical study showed that
brigatinib inhibits FAK1 and EphA2 activity without relying on
ALK/ROS1, leading to a reduction in NF2-deficient tumors in mouse
models (13). This multi-targeted
approach highlights brigatinib as a compelling candidate for tumors
associated with NF2. Currently, there is a limited number of
clinical studies validating its short-term efficacy for NF2. The
present study assessed the immediate efficacy of brigatinib for
NF2, with the goal of introducing a novel therapeutic option for
the condition.
Patients and methods
Study design
Twelve patients with NF2 were retrospectively
identified from the hospital medical information database at The
First Medical Center of Chinese PLA General Hospital (Beijing,
China), covering the period from June 2021 to April 2024. Inclusion
criteria included age ≥6 years, clinical diagnosis of NF2.
Exclusion criteria included medical conditions incompatible with
brigatinib, and tumors not amenable to volumetric MRI analysis. The
present study adhered to the STROBE guidelines (https://www.strobe-statement.org/). All 12
patients received medical consultation and treatment at the First
Medical Center of Chinese PLA General Hospital (Beijing, China).
Their surgical histories involved multiple medical centers,
including the First Medical Center of Chinese PLA General Hospital
(Beijing, China), Xuanwu Hospital of Capital Medical University
(Beijing, China), Beijing Tiantan Hospital (Beijing, China) and
Huashan Hospital Affiliated to Fudan University (Shanghai,
China).
The hypothesis of the present study posits that
brigatinib will lead to a substantial reduction in meningioma
volume and delay disease progression. The primary outcome measure
of this study was the change in meningioma after vs. before
treatment. Secondary outcomes included changes in patients'
hearing, emotional state, pain level and the occurrence of adverse
events.
Participants
Inclusion criteria were defined as patient age >6
years, a clinical diagnosis of NF2 between the ages of 14 and 16
years (14–16) and no prior treatment with tyrosine
kinase inhibitors. Individuals were excluded from the study if they
met any of the following criteria: Prior treatment with tyrosine
kinase inhibitors, surgical procedures necessitated by tumor
progression during the trial, medical conditions that are not
compatible with brigatinib treatment and tumors that are not
amenable to measurement via magnetic resonance imaging (MRI).
Brigatinib was administered for the treatment of NSCLC at a dosage
of 180 mg once daily, following a 7-day lead-in period at 90 mg
once daily (17). To ensure safety
and tolerability in evaluating the potential effects of brigatinib
on NF2, a dose of 90 mg was chosen for the intervention.
Participants received a daily dosage of 90 mg of brigatinib,
provided by Takeda Pharma. Written informed consent was obtained
from the patient's parents or guardians to allow the patient to
receive the treatment.
Variables
The primary outcome was change in meningioma volume
and secondary outcomes included changes in the size of vestibular
schwannomas, as well as hearing, emotional state and pain, measured
before and after treatment. The change in the maximum diameter of
meningiomas on MRI was employed to assess disease progression,
following the RECIST criteria (18). Disease progression was defined as a
≥20% increase in the maximum meningioma diameter on MRI. Patients
without progression by the end of follow-up (censored data) were
those who either completed the 12-month follow-up without meeting
the progression criteria or were lost to follow-up. The time from
starting medication to disease progression was assessed and
patients were divided into two age groups (<18 and ≥18 years) to
compare progression times. Secondary endpoints included changes in
the volume of vestibular schwannomas, changes in hearing, emotional
state and pain. Adverse effects during the study were also observed
and recorded.
Data sources and data measurement
Brain MRIs were performed at baseline, as well as 6
and 12 months during treatment. For scans, sagittal and coronal
positions were used and scanning sequences Spin Echo (SE), Fast SE
(FSE) and Fast Recovery FSE for multi-angle T1-weighted imaging
(T1WI) and T2WI imaging were measured. The MRI scans included
post-contrast images of the internal auditory canal and the entire
brain. The physician selected the largest meningioma and vestibular
schwannoma to assess tumor volume. The measurements in the MRI
examination were carried out by two board-certified
neuroradiologists with >8 years of work experience and these two
neuroradiologists received training before the study was launched.
The tumor's maximum diameter was measured in the coronal plane (a),
axial plane (b) and sagittal plane (c) using the Siemens Picture
Archiving and Communication System (version 4.5; Siemens
Healthineers), with the tumor volume calculated as a × b × c.
Hearing levels were assessed using pure-tone
audiometry (PTA) for air conduction thresholds. Initially, patients
were guided into the auditory evaluation chamber to ensure a
tranquil testing setting for accurate results. Using standard
headphones, audiologists gradually increased the sound volume until
the participants could hear it and then decreased it until they
could no longer hear it. This meticulous procedure allowed accurate
recording of the minimum sound thresholds at which participants
could hear different frequencies. PTA was measured at the
frequencies 0.25, 0.5, 1.0, 2.0, 4.0 and 8.0 kHz in each ear and
the average of the thresholds at four frequencies was used to
assess hearing loss (19).
Quantitative variables
The patients' emotional state was assessed using the
Symptom Checklist-90 (SCL-90) (20). A total score of 150 indicated a poor
emotional state. The SCL-90 is a widely used psychological
appraisal instrument to assess an individual's psychological
well-being and emotional disposition. This measure includes 90
items that explore various psychological symptoms and emotions,
including but not limited to anxiety, depression, hostility,
obsession and fear. Participants rate each item based on their
experiences over the past month, providing a detailed view of their
psychological landscape.
A visual analog scale (VAS) was used to measure the
patient's pain levels (21). The
VAS is a common tool for assessing the intensity of pain or other
attributes based on individual experiences. The VAS consists of a
line of adjustable length, with numerical values at each end
representing diametrically opposed sensations, denoted as 0 and 10.
Patients indicate their pain levels along this continuum to create
a gradient of ratings. Types and severity of adverse reactions are
carefully recorded. Regular follow-ups were conducted through
outpatient clinics or by phone for 12 months.
Research studies assessed patients' overall health
status and functional capacity using the Eastern Cooperative
Oncology Group (ECOG) performance score (22).
The hearing levels, pain and emotional states of the
patients were evaluated by the same two board-certified
neuroradiologists who conducted the MRI imaging assessment.
Statistical analysis
Data were statistically analyzed using SPSS software
(version 25.0; IBM Corp.) and the R programming language (version
4.3.2; The R Project for Statistical Computing; www.r-project.org). Continuous variables were
expressed using means and standard deviations and categorical data
were presented as counts and percentages. Repeated-measures ANOVA
was used to compare the changes over time (pre- and
post-medication). The normality of the data distribution was tested
using the Shapiro-Wilk test before conducting the analysis.
Non-parametric tests, like the Friedman non-parametric
repeated-measures ANOVA test, were used to compare the change
before and after the medication if the data didn't follow a normal
distribution assumption. Kaplan-Meier curves were generated to
visualize time-to-progression differences between age groups, as
previously described in studies analyzing tumor growth dynamics
(17). For the comparison of the
time to disease progression between patients aged <18 and ≥18
years, the log-rank test was initially used. However, considering
the potential issue of survival curves crossing, an alternative
analysis using the Fleming-Harrington test family was also
conducted. Categorical variables were compared with Fisher's exact
test. In this study, multiple hypotheses were tested. To address
the potential increase in Type I error probability, the Bonferroni
correction method was applied for the post-hoc tests. P<0.05 was
considered to indicate statistical significance.
Results
Patient characteristics
A total of 12 participants were enrolled with a
median age of 22 years (range, 14–32 years) and a median body mass
index of 21.02 kg/m2 (range, 17.6–26.8
kg/m2). The cohort included nine men (75%) and three
women (25%). The median follow-up time was 20.58 months (range,
12–40 months) (Table I). A total of
nine participants had previous surgeries. The median time from
diagnosis to receiving brigatinib was 13 months (range, 3–30
months). All participants had bilateral vestibular nerve
schwannomas and spinal meningiomas; nine participants had cranial
meningiomas and seven participants had subcutaneous tumors. The
number of participants reporting hearing loss, pain, impaired motor
function, visual impairment and poor emotional states was,
respectively, 10, 5, 6, 7 and 6 (Table
I).
 | Table I.Demographics and clinical
characteristics of participants at baseline. |
Table I.
Demographics and clinical
characteristics of participants at baseline.
| Participant
no. | Age, years | Sex | BMI,
kg/m2 | Previous surgical
excision | Time before
brigatinib, years | Follow-up time,
months | Type of
tumora |
Symptomsb |
|---|
| 1 | 30 | Male | 23.1 | Yes | 20 | 24 | 1, 2, 3 | 1, 3, 4, 5 |
| 2 | 32 | Male | 19.4 | Yes | 30 | 24 | 1, 2, 3, 4 | 1, 2, 3, 4, 5 |
| 3 | 14 | Female | 17.8 | Yes | 13 | 16 | 1, 2, 3 | 1, 5 |
| 4 | 19 | Male | 22.7 | Yes | 3 | 38 | 1, 2, 3 | 1, 2, 4, 5 |
| 5 | 26 | Female | 20.4 | Yes | 21 | 18 | 1, 2, 3 | 1, 2, 4, 5 |
| 6 | 32 | Male | 22.3 | Yes | 17 | 24 | 1, 2, 3 | 1, 3, 4, 5 |
| 7 | 17 | Male | 20.1 | No | 3 | 13 | 1, 3 |
|
| 8 | 15 | Male | 19.1 | No | 3 | 12 | 1, 2, 3 | 1, 2 |
| 9 | 30 | Male | 17.6 | Yes | 10 | 13 | 1, 3 | 1, 2 |
| 10 | 15 | Male | 24.2 | Yes | 12 | 40 | 1, 3, 4 | 3, 4 |
| 11 | 14 | Female | 18.7 | No | 10 | 13 | 1, 2, 3, 4 | 1, 3 |
| 12 | 20 | Male | 26.8 | Yes | 14 | 12 | 1, 2, 3, 4 | 1, 3, 4 |
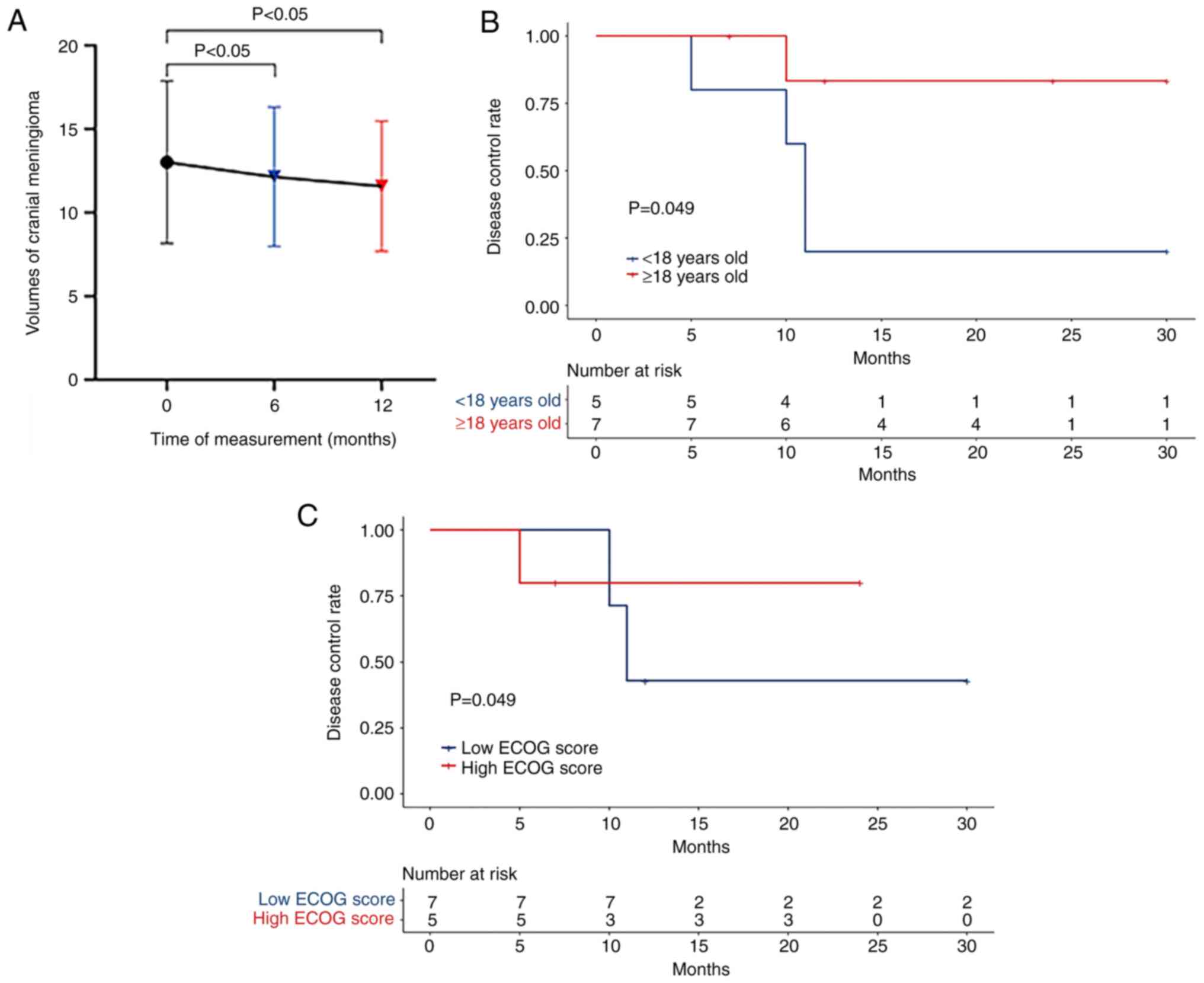
Tumor size
The average size of the meningioma in the brain was
13.00±4.86 mm3 at the start, 12.14±4.12 mm3
at 6 months and 11.58±3.89 mm3 at 12 months, indicating
a significant decrease (P<0.05; Fig.
1A). The time to disease progression was significantly
different between patients aged <18 years and those aged ≥18
years (P=0.049; Fig. 1B), but there
was no significant difference between patients with a low ECOG
score (<2) and high ECOG score (>2) (P>0.05 according to
the log-rank test) (Fig. 1C). By
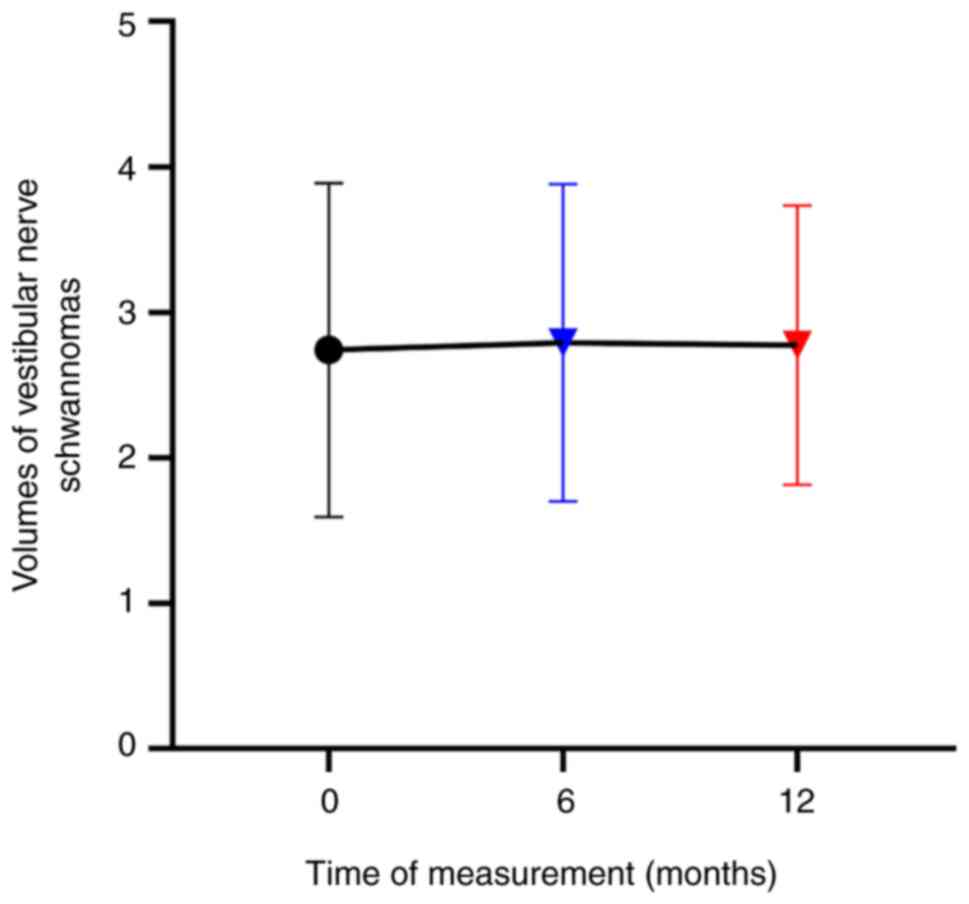
contrast, the volume of vestibular nerve schwannomas showed little
change during drug treatment (Fig.
2).
Hearing function, emotional state and
pain
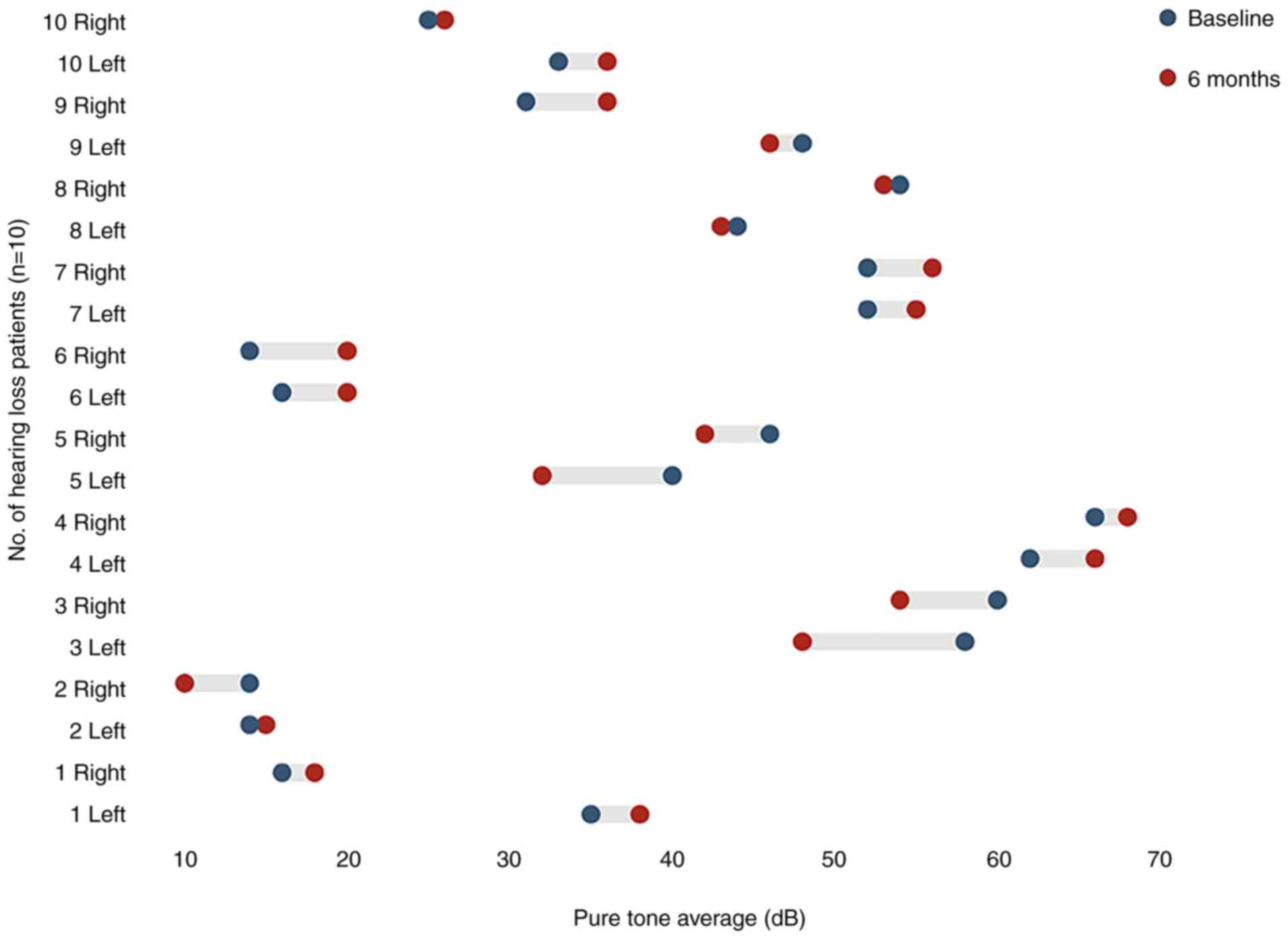
Median PTA thresholds of participants who had
hearing loss (n=10) were 39.90±15.34 dB (left ear) and 38.30±19.37
dB (right ear). After 12 months of treatment, the thresholds were
40.2±16.19 dB (left ear) and 37.80±20.10 dB (right ear). Hearing
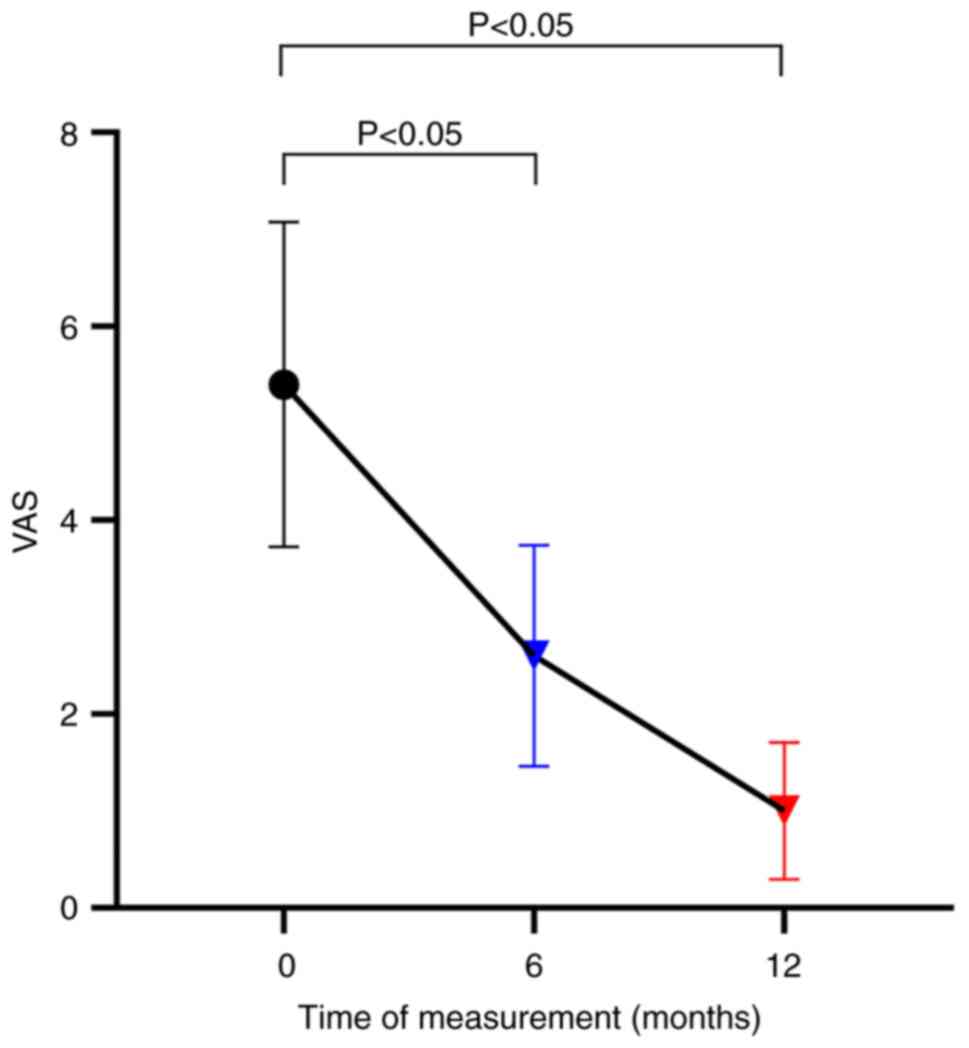
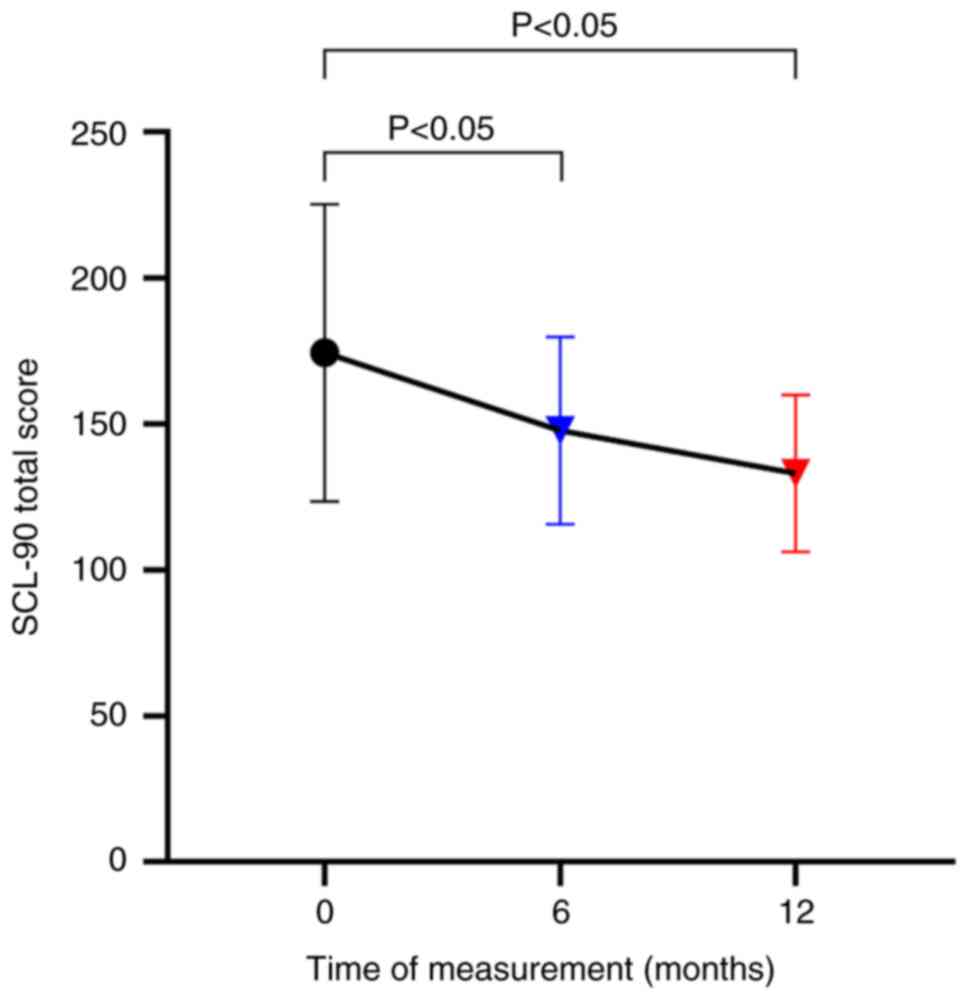
did not significantly improve (P>0.05; Fig. 3). The VAS (Fig. 4) and SCL-90 (Fig. 5) scores were significantly lower at
6 and 12 months compared to the baseline (P<0.05).
Safety
At the 6 months, 17 adverse events were reported:
Hypertension (n=1), diarrhea (n=5), liver dysfunction (n=1),
arrhythmia (n=1), skin rash (n=4) and fatigue (n=5). At 12 months,
19 adverse events were reported: Hypertension (n=2), diarrhea
(n=4), liver dysfunction (n=1), arrhythmia (n=2), skin rash (n=5)
and fatigue (n=5) (Table II).
 | Table II.Adverse events occurring in patients
receiving brigatinib. |
Table II.
Adverse events occurring in patients
receiving brigatinib.
| Adverse Event | Total no. of events
(n=36) | 6 months
(n=17) | 12 months
(n=19) |
|---|
| Hypertension | 3 | 1 | 2 |
| Diarrhoea | 9 | 5 | 4 |
| Liver
dysfunction | 2 | 1 | 1 |
| Arrhythmia | 3 | 1 | 2 |
| Skin rash | 9 | 4 | 5 |
| Fatigue | 10 | 5 | 5 |
Discussion
NF2 is marked by the presence of numerous tumors
within the nervous system that progressively deteriorate over time.
Even though tumors may be classified as histologically benign, they
can lead to significant complications, including hearing and vision
loss, motor function impairment, paralysis, pain and epilepsy,
which are substantial contributors to mortality and disability
(23). Bilateral vestibular
schwannomas are the most prevalent tumors associated with NF2,
occurring in ~90% of cases. Furthermore, it is observed that 45–58%
of individuals with NF2 present with intracranial meningiomas,
while 20% exhibit spinal meningiomas. Meningiomas frequently
present as multiple benign tumors that exert pressure on
surrounding brain tissue and cranial nerves, potentially leading to
seizures and cerebral edema (24).
To date, no effective treatment for NF2 has been
established. Surgery and stereotactic radiosurgery are employed in
the management of tumors; however, their effectiveness is typically
limited to short-term relief. The long-term outlook for patients
continues to be unfavorable (25).
Currently, there is no medication authorized for the treatment of
NF2 tumors. Investigations indicate that bevacizumab, a vascular
endothelial growth inhibitor, has the potential to impede the
growth of NF2-induced acoustic neuromas and may lead to some
enhancement in hearing (26–29).
However, the impact on meningiomas and cutaneous neurofibromas
remains ambiguous.
Chang et al (13) discovered that cells from NF2-related
meningioma and schwannoma do not exhibit ALK expression. In
contrast to NSCLC, where ALK inhibition is applied, brigatinib
demonstrates the ability to inhibit multiple tyrosine kinases, such
as EphA2 and FAK1. Brigatinib has demonstrated the ability to
inhibit the activity of NF2 gene knockout cells and decrease the
volume of meningioma and schwannoma in mice, suggesting its
potential as a treatment for NF2 tumors (13). Building upon the foundation
established by Chang et al (13), the present investigation involved a
follow-up of 12 patients receiving oral therapy with brigatinib.
While brigatinib is mainly focused on targeting tyrosine kinases in
NSCLC, emerging evidence indicates its possible application in NF2.
A phase II trial indicated a 25% tumor response rate and a 35%
improvement in hearing among patients with NF2 receiving brigatinib
at a dosage of 180 mg daily (30).
However, the effectiveness regarding the meningioma volume and
non-auditory symptoms is still uncertain, necessitating additional
research. The present investigation built upon previous findings by
showcasing its efficacy against meningiomas, underscoring a wider
scope of therapeutic possibilities.
NF2 represents an autosomal dominant genetic
disorder resulting from mutations in the NF2 gene. The gene is
responsible for encoding and expressing the Merlin protein in
vivo, predominantly located in Schwann cells, meningeal cells,
lens fiber cells and nerve cells (30). Merlin plays a crucial role in
inhibiting cell proliferation through the regulation of cell-cell
adhesion. Consequently, the loss of Merlin due to mutations in the
NF2 gene results in tumor growth. Merlin engages with various
intracellular signaling pathways, such as receptor tyrosine
kinases. Certain tyrosine kinase inhibitors have shown efficacy in
NF2-deficient mice (31),
indicating that these inhibitors may represent a viable treatment
option for NF2.
Brigatinib demonstrates inhibitory effects on
several receptor tyrosine kinases, such as ALK, ROS1, insulin-like
growth factor-1 receptor and EGFR. However, there is a lack of
studies regarding its application in the treatment of NF2. The
study by Plotkin et al (30)
evaluated the efficacy and safety of brigatinib in the treatment of
NF2-related schwannomatosis. Their results showed that with
brigatinib, significant radiological responses were achieved in
multiple types of tumors. Of the target tumors, 10% (95% CI, 3–24%)
showed shrinkage and 23% (95% CI, 16–30%) of all tumors showed a
response. In addition, ~35% (95% CI, 20–53%) of patients
experienced improved hearing and there was a reduction in
self-reported pain severity. This present study involved the
observation and evaluation of brigatinib's short-term effectiveness
in treating NF2, revealing its potential to reduce the volume of
meningioma. Meningiomas present a range of histological features
and genetic irregularities. Research shows that between 45 and 58%
of individuals with NF2 present with intracranial meningiomas,
while ~20% exhibit spinal meningiomas (32). NF2-related meningiomas predominantly
arise in the supratentorial areas, encompassing the frontal,
parietal and temporal lobes, along with the cerebral falx. The MRI
scans conducted on the patients in this study revealed the presence
of meningiomas in the conventional cranial regions. The mean volume
decreased from baseline. Although the volume change was not
significant, the patient's pain and emotional state improved
significantly. Younger patients (aged <18 years) exhibited a
shorter disease progression time in comparison to their older
counterparts (≥18 years). This may be associated with accelerated
tumor growth in younger individuals (15).
NF2 may lead to peripheral neuropathy, ophthalmic
abnormalities and cutaneous lesions. Patients with NF2 frequently
present with cutaneous lesions; however, these lesions are
generally less pronounced compared to those seen in patients with
NF1. Schwannomas cause significant pain in individuals with NF2.
While the precise mechanisms underlying NF2-related pain remain
incompletely understood, it is suggested that this pain may be
linked to tumor activation and/or the sensitization of primary
sensory afferents through several pathways, such as mechanical
compression of nerves, direct cell-cell signaling and the release
of secretory factors (33).
Currently, surgical resection is recognized as the most effective
and safe approach for addressing pain associated with NF2
peripheral nerve schwannoma (34).
Nonetheless, pain associated with schwannomas frequently continues
after tumor removal and does not seem to have a direct relationship
with the size of the tumor. This indicates that pain could arise
from factors beyond nerve compression (35).
The present investigation evaluated the short-term
effectiveness of brigatinib in the treatment of NF2. An important
advantage of brigatinib is its ability to enhance patients' pain
management and emotional well-being. Follow-up feedback revealed
notable pain relief within 24 h of initiating brigatinib treatment.
Brigatinib primarily focuses on tumor cells by engaging multiple
pathways to impede their growth and dissemination, particularly in
the context of NSCLC. This investigation indicates that brigatinib
may also alleviate tumor-associated pain symptoms through a
reduction in tumor burden. Furthermore, brigatinib has the
potential to offer swift pain relief via additional mechanisms,
warranting further exploration.
The findings suggest that blocking tyrosine kinase
activity may serve as an effective strategy for managing NF2
symptoms and enhancing clinical outcomes. This facilitates further
investigation into tyrosine kinase inhibitors as a possible
treatment for NF2. Nonetheless, there was no observed enhancement
in acoustic neuroma and hearing, likely attributable to the
challenges of addressing all symptoms in patients with NF2 with a
singular pharmacological intervention.
The majority of individuals diagnosed with
vestibular schwannomas demonstrate a reduction in Merlin
expression. Investigations into the associated cellular pathways
triggered by the loss of Merlin have examined these as possible
therapeutic targets for addressing vestibular schwannomas. Merlin's
interaction with ErbB2 facilitates the activation of downstream
MAPK/ERK and PI3K/Akt signaling pathways (36). Activation of ErbB receptors occurs
in both sporadic and NF2-related vestibular schwannomas, with EGF
expression showing an increase in NF2-related cases. This indicates
the potential effectiveness of EGFR inhibitors in the treatment of
NF2-related vestibular schwannomas. Clinical studies indicate that
the EGFR inhibitor erlotinib does not have a significant impact on
hearing or tumor size in patients with progressive vestibular
schwannoma (37).
Tyrosine kinase inhibitors that target EGFR/ErbB2
show variable efficacy in reducing tumor size and enhancing hearing
in NF2-related vestibular schwannomas, presenting challenges in
their role as effective treatment options. The levels of VEGF and
its receptors are increased in schwannomas, showing an association
with tumor growth and volume. VEGF inhibitors, such as bevacizumab,
have demonstrated encouraging outcomes in vestibular schwannomas,
notably enhancing hearing and decreasing tumor size. Nonetheless,
the administration of bevacizumab poses several challenges, such as
the requirement for regular parenteral delivery, potential side
effects, noticeable drug resistance and the possibility of rebound
tumor progression (38). Numerous
investigations into the use of bevacizumab for vestibular
schwannomas depend on brief follow-up periods and are deficient in
long-term findings. Brigatinib engages in distinct pathways
compared to those that have demonstrated efficacy in reducing tumor
size and enhancing hearing in NF2-related vestibular schwannomas.
This may clarify the absence of observed improvements in acoustic
schwannoma size or hearing in the present study. Besides, the study
by Plotkin et al (30)
observed a certain degree of hearing improvement, but in the
present study, there was no significant improvement in the volume
of acoustic neuroma or the average hearing threshold (P>0.05).
This difference may be, to a certain extent, attributed to the
variation in sample size.
The small cohort of patients with NF2 and the
elevated expense of brigatinib constrained the sample size of the
present study, complicating the evaluation of brigatinib's impact
on spinal tumors in NF2. This may clarify the absence of progress
in vestibular schwannomas and auditory function. In the interim,
additional clinical observation is required to assess the long-term
efficacy of brigatinib in the treatment of NF2.
The main adverse effects noted during the trial
included fatigue, skin rash and diarrhea, none of which resulted in
the cessation of the medication. No significant adverse reactions
were observed; however, one patient experienced severe anemia.
Given the patient's history of anemia, which showed improvement
following treatment, anemia may not be associated with brigatinib.
No instances of anemia were reported in other studies involving
brigatinib (39,40).
Of note, the present study has several limitations.
NF2 exhibits a wide range of clinical manifestations that differ
significantly from one individual to another, complicating the
development of a universal treatment that can effectively address
all patient symptoms. Tailored approaches that involve
collaboration across various disciplines could prove to be a
promising strategy in the management of NF2. In addition, further
clinical drug studies are essential to generate evidence and
explore new therapeutic possibilities for NF2. The limited sample
size (n=12) restricts the statistical power of the findings.
Although selection bias was minimized by continuously enrolling all
eligible patients, as this study was based on a retrospective
dataset, selection bias may still exist. Certain patients may not
have been included in the study due to milder or more severe
conditions, which may result in the sample of the present study not
fully representing the entire NF2 patient population. It is
essential to validate these findings through larger, multicenter
trials. Although both the log-rank test and Cox model indicated a
significant relationship between age and progression risk, it is
essential to interpret these findings with caution and validate
them in larger cohorts.
In conclusion, the present findings indicate that
brigatinib has a notable impact on reducing meningioma volume in
individuals with NF2, while also enhancing their emotional health
and relieving pain. Nonetheless, the effect on vestibular
schwannoma volume and hearing thresholds is not significant.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
ZL and WY were involved in the conception and design
of the study and analyzed data. FJ and ZX collected the data and
helped with the data analysis. ML and SJ helped with the data
analysis, drafted and proofread the manuscript, performed final
editing and are guarantors of the study. ML and SJ confirm the
authenticity of the raw data. All authors have checked and approved
the final version of the manuscript.
Ethics approval and consent to
participate
The study was conducted in accordance with the
Declaration of Helsinki (2013) and approved by the Institutional
Review Board (IRB) of The First Medical Center of Chinese PLA
General Hospital (Beijing, China; IRB approval no. S2021-409-01).
Informed consent was obtained from all adult participants and
written parental consent was secured for minors, consistent with
ethical guidelines for pediatric research.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Rouleau GA, Merel P, Lutchman M, Sanson M,
Zucman J, Marineau C, Hoang-Xuan K, Demczuk S, Desmaze C,
Plougastel B, et al: Alteration in a new gene encoding a putative
membrane-organizing protein causes neuro-fibromatosis type 2.
Nature. 363:515–521. 1993. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Asthagiri AR, Parry DM, Butman JA, Kim HJ,
Tsilou ET, Zhuang Z and Lonser RR: Neurofibromatosis type 2.
Lancet. 373:1974–1986. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Slattery WH: Neurofibromatosis type 2.
Otolaryngol Clin North Am. 48:443–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Evans DG, Baser ME, O'Reilly B, Rowe J,
Gleeson M, Saeed S, King A, Huson SM, Kerr R, Thomas N, et al:
Management of the patient and family with neurofibromatosis 2: A
consensus conference statement. Br J Neurosurg. 19:5–12. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plotkin SR, Messiaen L, Legius E, Pancza
P, Avery RA, Blakeley JO, Babovic-Vuksanovic D, Ferner R, Fisher
MJ, Friedman JM, et al: Updated diagnostic criteria and
nomenclature for neurofibromatosis type 2 and schwannomatosis: An
international consensus recommendation. Genet Med. 24:1967–1977.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Okada T, You L and Giancotti FG: Shedding
light on Merlin's wizardry. Trends Cell Biol. 17:222–229. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang N, Bai H, David KK, Dong J, Zheng Y,
Cai J, Giovannini M, Liu P, Anders RA and Pan D: The Merlin/NF2
tumor suppressor functions through the YAP oncoprotein to regulate
tissue homeostasis in mammals. Dev Cell. 19:27–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Apra C, Peyre M and Kalamarides M: Current
treatment options for meningioma. Expert Rev Neurother. 18:241–249.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Angus SP, Oblinger JL, Stuhlmiller TJ,
DeSouza PA, Beauchamp RL, Witt L, Chen X, Jordan JT, Gilbert TSK,
Stemmer-Rachamimov A, et al: EPH receptor signaling as a novel
therapeutic target in NF2-deficient meningioma. Neuro Oncol.
20:1185–1196. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohanty A, Pharaon RR, Nam A, Salgia S,
Kulkarni P and Massarelli E: FAK-targeted and combination therapies
for the treatment of cancer: An overview of phase I and II clinical
trials. Expert Opin Investig Drugs. 29:399–409. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Markham A: Brigatinib: First global
approval. Drugs. 77:1131–1135. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hoy SM: Brigatinib: A review in
ALK-inhibitor Naïve advanced ALK-positive NSCLC. Drugs. 81:267–275.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chang LS, Oblinger JL, Smith AE, Ferrer M,
Angus SP, Hawley E, Petrilli AM, Beauchamp RL, Riecken LB, Erdin S,
et al: Brigatinib causes tumor shrinkage in both NF2-deficient
meningioma and schwannoma through inhibition of multiple tyrosine
kinases but not ALK. PLoS One. 16:e02520482021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mulvihill JJ, Parry DM, Sherman JL, Pikus
A, Kaiser-Kupfer MI and Eldridge R: NIH conference.
Neurofibromatosis 1 (Recklinghausen disease) and neurofibromatosis
2 (bilateral acoustic neurofibromatosis). An update. Ann Intern
Med. 113:39–52. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Evans DG, Huson SM, Donnai D, Neary W,
Blair V, Newton V and Harris R: A clinical study of type 2
neurofibromatosis. Q J Med. 84:603–618. 1992.PubMed/NCBI
|
|
16
|
Baser ME, Friedman JM, Wallace AJ, Ramsden
RT, Joe H and Evans DGR: Evaluation of clinical diagnostic criteria
for neurofibromatosis 2. Neurology. 59:1759–1765. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Camidge DR, Kim HR, Ahn MJ, Yang JCH, Han
JY, Hochmair MJ, Lee KH, Delmonte A, Garcia Campelo MR, Kim DW, et
al: Brigatinib versus crizotinib in ALK inhibitor-naive advanced
ALK-positive NSCLC: Final results of phase 3 ALTA-1L trial. J
Thorac Oncol. 16:2091–2108. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Walia A, Tuia J and Prasad V:
Progression-free survival, disease-free survival and other
composite end points in oncology: Improved reporting is needed. Nat
Rev Clin Oncol. 20:885–895. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Carl AC, Hohman MH and Cornejo J:
Audiology pure tone evaluation. [2023 Mar 1]. StatPearls [Internet]
Treasure Island (FL): StatPearls Publishing; 2025
|
|
20
|
Kostaras P, Martinaki S, Asimopoulos C,
Maltezou M and Papageorgiou C: The use of the symptom checklist
90-R in exploring the factor structure of mental disorders and the
neglected fact of comorbidity. Psychiatry Res. 294:1135222020.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
McCormack HM, Horne DJ and Sheather S:
Clinical applications of visual analogue scales: A critical review.
Psychol Med. 18:1007–1019. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Azam F, Latif MF, Farooq A, Tirmazy SH,
AlShahrani S, Bashir S and Bukhari N: Performance status assessment
by using ECOG (eastern cooperative oncology group) score for cancer
patients by oncology healthcare professionals. Case Rep Oncol.
12:728–736. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Grossen A, Gavula T, Chrusciel D, Evans A,
McNall-Knapp R, Taylor A, Fossey B, Brakefield M, Carter C,
Schwartz N, et al: Multidisciplinary neurocutaneous syndrome
clinics: A systematic review and institutional experience.
Neurosurg Focus. 52:E22022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mautner VF, Lindenau M, Baser ME, Hazim W,
Tatagiba M, Haase W, Samii M, Wais R and Pulst SM: The neuroimaging
and clinical spectrum of neurofibromatosis 2. Neurosurgery.
38:880–886. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Coy S, Rashid R, Stemmer-Rachamimov A and
Santagata S: An update on the CNS manifestations of
neurofibromatosis type 2. Acta Neuropathol. 139:643–665. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Blakeley JO, Ye X, Duda DG, Halpin CF,
Bergner AL, Muzikansky A, Merker VL, Gerstner ER, Fayad LM, Ahlawat
S, et al: Efficacy and biomarker study of bevacizumab for hearing
loss resulting from neurofibromatosis type 2-associated vestibular
schwannomas. J Clin Oncol. 34:1669–1675. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lu VM, Ravindran K, Graffeo CS, Perry A,
Van Gompel JJ, Daniels DJ and Link MJ: Efficacy and safety of
bevacizumab for vestibular schwannoma in neurofibromatosis type 2:
A systematic review and meta-analysis of treatment outcomes. J
Neurooncol. 144:239–248. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tamura R, Fujioka M, Morimoto Y, Ohara K,
Kosugi K, Oishi Y, Sato M, Ueda R, Fujiwara H, Hikichi T, et al: A
VEGF receptor vaccine demonstrates preliminary efficacy in
neurofibromatosis type 2. Nat Commun. 10:57582019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Plotkin SR, Allen J, Dhall G, Campian JL,
Clapp DW, Fisher MJ, Jain RK, Tonsgard J, Ullrich NJ, Thomas C, et
al: Multicenter, prospective, phase II study of maintenance
bevacizumab for children and adults with NF2-related
schwannomatosis and progressive vestibular schwannoma. Neuro Oncol.
25:1498–1506. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Plotkin SR, Yohay KH, Nghiemphu PL, Dinh
CT, Babovic-Vuksanovic D, Merker VL, Bakker A, Fell G, Trippa L and
Blakeley JO; INTUITT-NF2 Consortium, : Brigatinib in NF2-related
schwannomatosis with progressive tumors. N Engl J Med.
390:2284–2294. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
den Bakker MA, Vissers KJ, Molijn AC, Kros
JM, Zwarthoff EC and van der Kwast TH: Expression of the
neurofibromatosis type 2 gene in human tissues. J Histochem
Cytochem. 47:1471–1480. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Paldor I, Abbadi S, Bonne N, Ye X,
Rodriguez FJ, Rowshanshad D, Itzoe M, Vigilar V, Giovannini M, Brem
H, et al: The efficacy of lapatinib and nilotinib in combination
with radiation therapy in a model of NF2 associated peripheral
schwannoma. J Neurooncol. 135:47–56. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Coy S, Rashid R, Stemmer-Rachamimov A and
Santagata S: Correction to: An update on the CNS manifestations of
neurofibromatosis type 2. Acta Neuropathol. 139:6672020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kukutla P, Ahmed SG, DuBreuil DM,
Abdelnabi A, Cetinbas M, Fulci G, Aldikacti B, Stemmer-Rachamimov
A, Plotkin SR, Wainger B, et al: Transcriptomic signature of
painful human neurofibromatosis type 2 schwannomas. Ann Clin Transl
Neurol. 8:1508–1514. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Peyre M, Tran S, Parfait B, Bernat I,
Bielle F and Kalamarides M: Surgical management of peripheral nerve
pathology in patients with neurofibromatosis type 2. Neurosurgery.
92:317–328. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mohammed N, Hung YC, Xu Z, Chytka T,
Liscak R, Tripathi M, Arsanious D, Cifarelli CP, Perez Caceres M,
Mathieu D, et al: Neurofibromatosis type 2-associated meningiomas:
An international multicenter study of outcomes after Gamma Knife
stereotactic radiosurgery. J Neurosurg. 136:109–114. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fernandez-Valle C, Tang Y, Ricard J,
Rodenas-Ruano A, Taylor A, Hackler E, Biggerstaff J and Iacovelli
J: Paxillin binds schwannomin and regulates its density-dependent
localization and effect on cell morphology. Nat Genet. 31:354–562.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Plotkin SR, Halpin C, McKenna MJ, Loeffler
JS, Batchelor TT and Barker FG II: Erlotinib for progressive
vestibular schwannoma in neurofibromatosis 2 patients. Otol
Neurotol. 31:1135–1143. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Tamura R, Tanaka T, Miyake K, Yoshida K
and Sasaki H: Bevacizumab for malignant gliomas: Current
indications, mechanisms of action and resistance, and markers of
response. Brain Tumor Pathol. 34:62–77. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Camidge DR, Kim HR, Ahn MJ, Yang JC, Han
JY, Lee JS, Hochmair MJ, Li JY, Chang GC, Lee KH, et al: Brigatinib
versus crizotinib in ALK-positive non-small-cell lung cancer. N
Engl J Med. 379:2027–2039. 2018. View Article : Google Scholar : PubMed/NCBI
|