Introduction
Liposarcoma is a rare malignant tumor originating
from adipocytes, accounting for 15–20% of soft tissue sarcomas
(1). The differences among its
subtypes primarily depend on immunohistochemical characteristics,
cell morphology, and genetic variations. According to the 2020
World Health Organization Classification of Soft Tissue and Bone
Tumors (2), liposarcoma can be
divided into well-differentiated liposarcoma (atypical lipomatous
tumor), dedifferentiated liposarcoma, myxoid liposarcoma,
pleomorphic liposarcoma, and myxoid pleomorphic liposarcoma, with
well-differentiated liposarcoma being the most common type. Each
subtype exhibits significant differences in clinical presentation,
biological characteristics, and drug sensitivity (3,4).
Esophageal liposarcomas are exceedingly rare, constituting only
1.2–1.5% of gastrointestinal liposarcomas (5). Studies indicate that minimally
invasive procedures, such as transoral and thoracoscopic
techniques, are the preferred surgical methods for treating
esophageal liposarcomas. However, challenges such as risk of
bleeding and high recurrence rates remain for complex giant
esophageal liposarcomas (6,7). Surgical resection is essential for
managing giant, well-differentiated liposarcomas of the esophagus;
however, no standardized protocol exists for selecting the surgical
approach.
Given the rarity of esophageal liposarcomas and the
complexity of their treatment, evaluating the effectiveness of
different surgical methods holds clinical significance. By
discussing the surgical approaches for two cases of giant,
well-differentiated esophageal liposarcoma, through
multidisciplinary collaboration, leveraging the strengths of both
endoscopic techniques and surgical interventions, complete tumor
excision was achieved and both patients demonstrated favorable
postoperative recovery. This article aims to provide additional
treatment options and shared experiences to enhance patient
prognosis and quality of life.
Case report
Both patients were admitted due to dysphagia.
Patient 1 was a 62-year-old male who reported ‘dysphagia for 1
month, which worsened 1 week previously’. One month prior to
admission to an external Hospital in May 2024, the patient
underwent laryngoscopy and was diagnosed with pharyngitis. The
condition showed slight improvement following anti-inflammatory
treatment. However, 1 week before admission to the Affiliated
Hospital of Guizhou Medical University (Guiyang, China) in June
2024, the patient's dysphagia worsened, accompanied by difficulty
eating. Of note, the patient was able to expel the tumor from the
esophagus through the mouth and swallow it back into the body. The
patient had a history of varicose vein surgery in both lower limbs
and a 10-year smoking history (20 cigarettes per day, quit 1 month
ago). Upon admission, physical examination revealed a mass in the
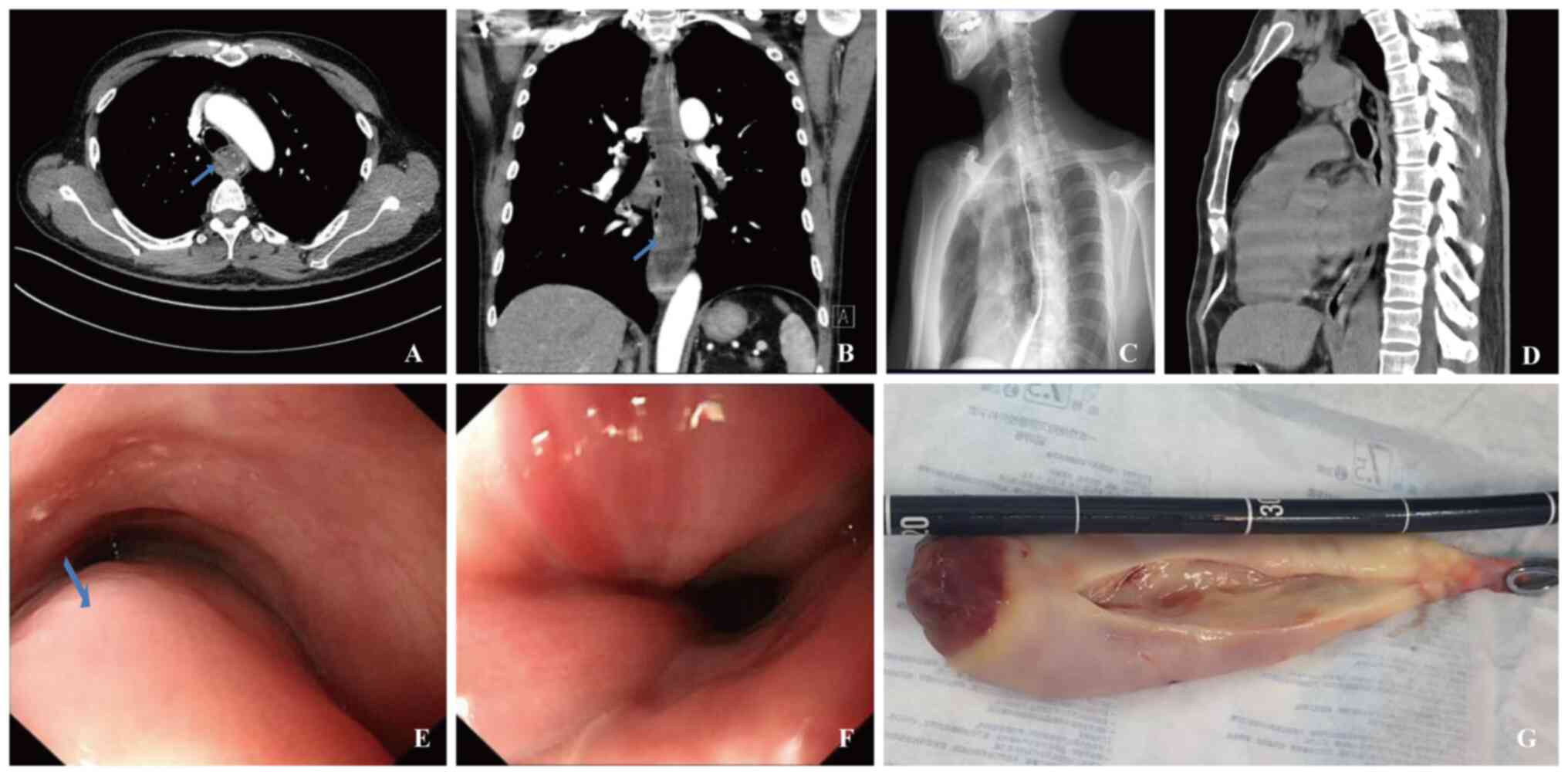
esophagus that could partially be vomited out. Chest computed
tomography (CT; Fig. 1A and B)
revealed esophageal dilation, with a mass-like lesion exhibiting
increased cystic density and no obvious enhancement on the
enhancement scan. The area near the gastroesophageal junction
displayed uneven enhancement, with no fat density components
observed. The esophageal tumor measured ~23 cm in length, with its
base located in the upper segment of the esophagus, parallel to the
level of the T1 vertebra. In addition, multiple lymph node
calcifications were visible in both lung hila and the mediastinum.
Gastroscopy revealed a giant submucosal lesion with expanded blood
vessels and inflammatory exudate (Fig.
1E and F).
Patient 2 was a 41-year-old male who presented to
the Affiliated Hospital of Guizhou Medical University (Guiyang,
China) in August 2024 due to ‘dysphagia and recurrent fever for 1
month’. The patient experienced swallowing discomfort of unknown
causes, with a maximum body temperature of 38.8°C. Self-medication
with anti-inflammatory drugs provided no significant improvement
and the patient had lost 4 kg in weight over the past month. The
patient had a 20-year smoking history (20 cigarettes per day) and
occasionally consumed alcohol, with no other significant health
issues. Physical examination revealed no remarkable findings.
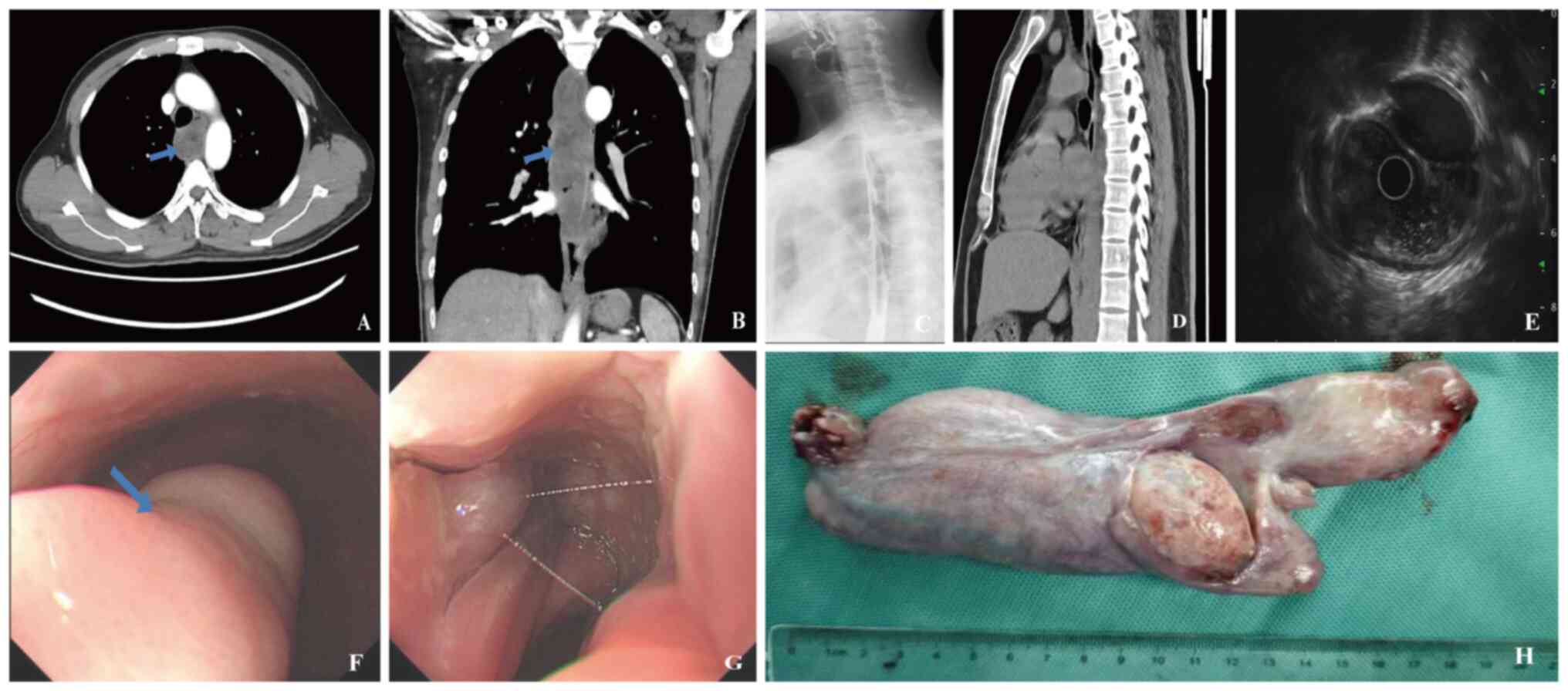
Enhanced chest CT (Fig. 2A and B)
revealed a large strip-like lesion in the esophagus, exhibiting
mixed density comprising both solid and fatty components. The
lesion measured 3.1×6.1×20.2 cm. The mediastinal lymph nodes
appeared normal and the CT also demonstrated prominent vessels
surrounding the tumor. Endoscopic examination (Fig. 2F and G) provided a more direct and
clearer view, revealing a strip-like elevation from the upper
segment of the esophagus (16 cm from the incisors) to the lower
segment (35 cm from the incisors). The upper segment lesion showed
congestion and superficial ulceration, with localized gray-blue
blood vessels. Endoscopic ultrasound examination indicated that the
elevation originated from the third layer and presented as a mixed
echogenic mass (Fig. 2E). In order
to clarify the diagnosis before surgery, two patients underwent a
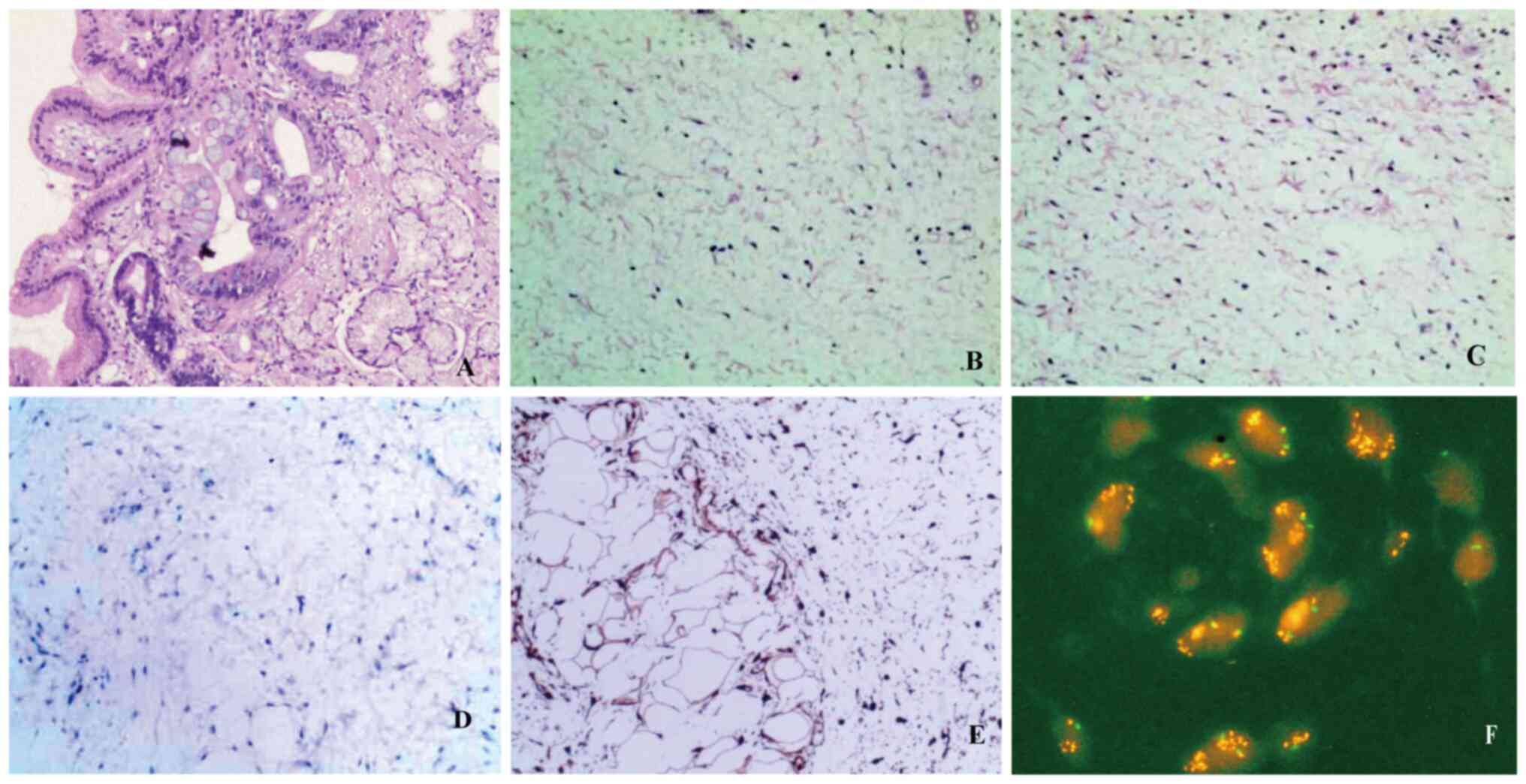
biopsy. The biopsy results of Patient 1 showed inflammatory exudate
and necrosis (Fig. 3A); the biopsy
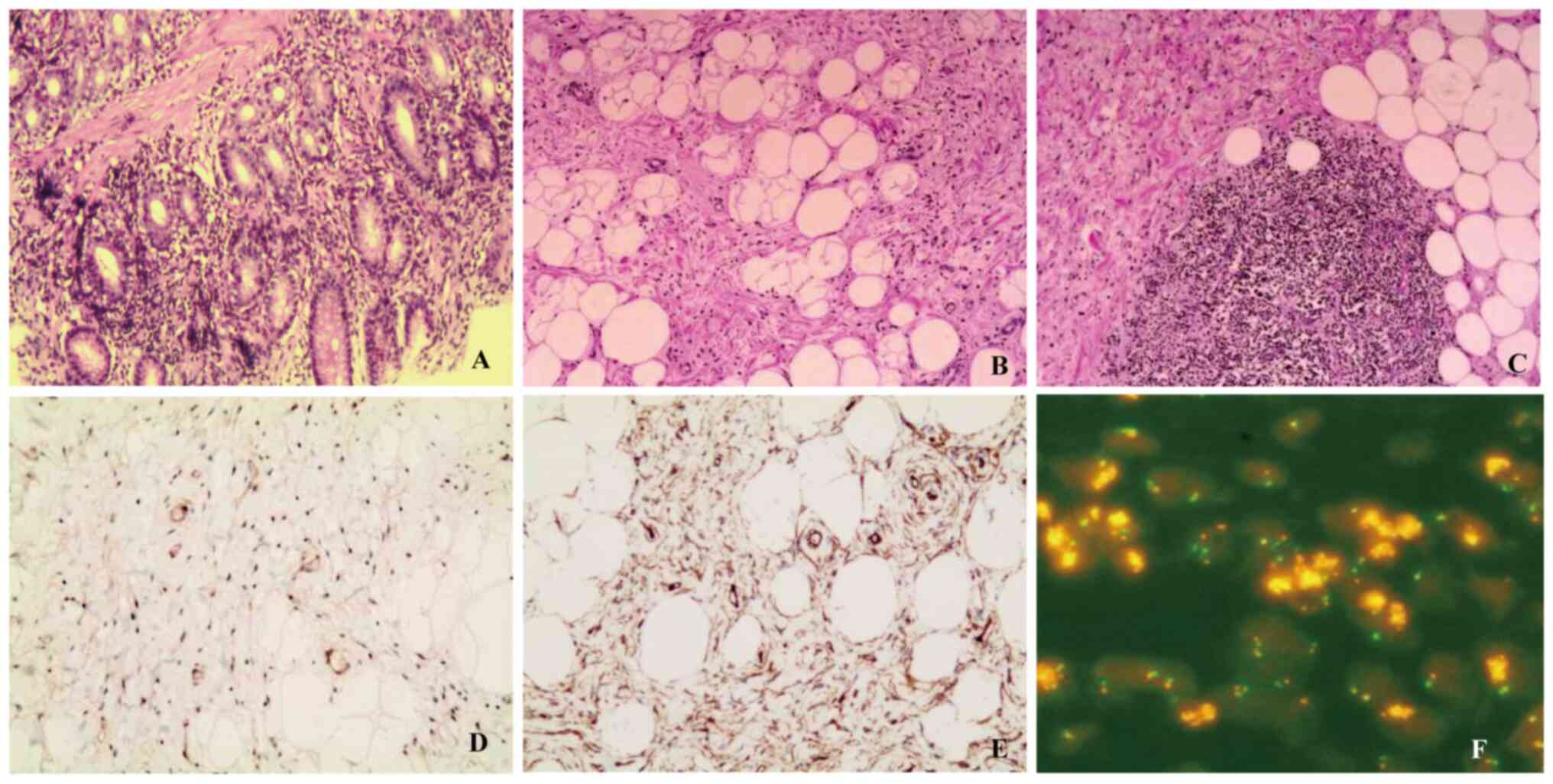
results of Patient 2 exhibited moderate chronic inflammation,
erosion, and lymphoid tissue hyperplasia forming follicles
(Fig. 4A). Both were initially
diagnosed as benign esophageal tumors.
In the surgical management, both patients were
considered to have benign esophageal tumors located in the mucosal
layer. After discussions among experts from the endoscopy center
and thoracic surgery, endoscopic tumor removal was prioritized. In
case the endoscopic procedure could not be completed, the thoracic
surgery team was prepared to perform open resection via a cervical
approach. Both patients ultimately underwent endoscopic tumor
removal. Patient 1 underwent surgery on the third day after
admission, during which a large, strap-like lesion was found on the
left esophageal wall, ~38 cm from the incisors, occupying about
two-thirds of the esophagus. The tumor measured 22×4×4 cm (Fig. 1G) and was successfully removed
endoscopically. Patient 2 underwent surgery on the fifth day after
admission, during which a giant sessile tumor was found, extending
16 to 36 cm from the incisors, with its base located in the neck,
lobulated and occupying two-thirds of the esophagus. The tumor
measured 20×6×3 cm (Fig. 2H) and
exhibited a soft consistency, with two prominent blood vessels
(~0.4 cm in diameter) visible on the surface.
Both procedures followed a similar approach.
Attempts to use a snare and harmonic scalpel were unsuccessful, so
hemostatic forceps were employed to coagulate visible blood vessels
at the tumor's base. The lesion was dissected using a disposable
endoscopic scalpel starting from the mucosa and extending to the
submucosal layer, revealing white fibrous tissue and large blood
vessels. After electrocautery, the tumor was carefully extracted
until significant resistance was encountered at the entrance of the
esophagus; repeated attempts to change angles only resulted in
partial tumor extraction, prompting the use of oval and tongue
forceps to drag the tumor out of the oral cavity. Postoperative
pathology confirmed well-differentiated liposarcoma of the
esophagus. In Patient 1, the esophageal lesion presented as a
mucosal ulcer, with spindle-like cells and vascular proliferation
observed beneath the ulcer, and certain areas showing mucinous
degeneration (Fig. 3B and C).
Immunohistochemical results showed negative Cytokeratin (CK), with
positive Vimentin (Vim), cyclin-dependent kinase inhibitor 2A (P16;
Fig. 3E), CDK4 and CD34 and
negative mouse double minute 2 homolog (MDM2; Fig. 3D), SRY-box transcription factor 10
(Sox10), desmin, smooth muscle actin (SMA), caldesmon, melanocyte
antigen (Melan-A), human melanoma black 45 (HMB45), CD117 and
discovered on GIST-1 (DOG-1); Ki-67 antigen (Ki-67) was ~3%
positive. Fluorescence in situ hybridization (FISH)
(Fig. 3F) analysis indicated
positive amplification of the MDM2 gene (12q15). In Patient
2, there was significant proliferation of fibrofatty tissue in the
submucosal layer of the esophageal lesion, forming fat lobules of
varying sizes, accompanied by thick-walled blood vessels and focal
lymphoid tissue hyperplasia (Fig. 4B
and C). Immunohistochemical results showed atypical cells
positive for Vim, S100, CD34, desmin, P16 (Fig. 4E), MDM2 (Fig. 4D) and CDK4 and negative for SMA,
caldesmon, DOG-1 and CD117; and Ki-67 was ~5% positive (primary
antibody details may be viewed in Table SI). FISH analysis (Fig. 4F) indicated positive amplification
of the MDM2 gene (for detailed pathological methods, please
refer to Data S1). Following
endoscopic surgical resection, neither patient experienced
significant discomfort or complications. On the first postoperative
day, imaging (Figs. 1C and 2D) confirmed a normal esophageal lumen. A
follow-up chest CT 1 month after surgery showed that the esophageal
lumen remained unobstructed, suggesting a good prognosis (Figs. 1D and 2E).
Discussion
The incidence of esophageal liposarcomas is low,
with most cases originating from the mucosal layer; mesenchymal
tumors account for only 5% (8). The
two cases reported in the present study were both
well-differentiated liposarcomas, characterized by slow growth and
low metastatic potential but with a risk of local recurrence and
resistance to chemotherapy and radiotherapy (9). Research indicates that the development
of well-differentiated liposarcomas is associated with repeated
amplification of chromosome 12, which provides an important
direction for targeted therapy (10). Related studies indicate that
esophageal liposarcomas predominantly occur in males, with an
average onset age of 58.4 years (range, 38–73 years). The tumor
typically extends from the cervical segment to the thoracic
segment. However, its nonspecific clinical manifestations pose
challenges for accurate diagnosis (11–14).
In the present study, in the context of esophageal
liposarcoma, preoperative chest enhanced CT and esophagogastroscopy
were particularly important. These two diagnostic methods not only
provided a clear delineation of the tumor and surrounding
structures but also effectively assessed the tumors' vascular
supply. Chest enhanced CT has a unique advantage in visualizing the
vessels around the tumor; the use of contrast agents allows for
effective differentiation between the tumor and adjacent tissues,
as well as clarifying the blood supply status at the tumor margins.
This imaging technique provided crucial information for evaluating
the tumors' vascularity. By contrast, endoscopic examination offers
a more direct and clearer observational perspective. Through
endoscopy, physicians can view the detailed structures of the tumor
and its surrounding vessels in real-time, enabling direct
assessment and, when necessary, performing biopsies or other
interventions to obtain comprehensive diagnostic information.
Typically, preoperative endoscopy reveals a pedunculated tumor that
could easily be mistaken for a benign lesion, with a definitive
diagnosis relying on postoperative pathological analysis. The
occurrence of high-grade dedifferentiated liposarcoma is closely
related to the amplification of the 12q13-15 chromosomal region,
while the immunohistochemical triad of MDM2, CDK4 and p16 serves as
its core diagnostic markers (15).
MDM2 gene amplification testing is considered the most
reliable method for distinguishing well-differentiated liposarcomas
from benign lipomas (16).
Currently, there is no standardized treatment
protocol for esophageal liposarcomas and surgical resection remains
the primary treatment. Achieving a negative margin during complete
resection is essential for a curative outcome and for effectively
relieving tumor-induced obstruction. Surgical approaches vary and
include total esophagectomy, subtotal esophagectomy and minimally
invasive techniques. The choice of surgical approach typically
depends on tumor location and size, with options including
transcervical, thoracoscopic or endoscopic access routes.
Historically, open surgery has been the gold standard (6,17–19).
However, with advancements in minimally invasive techniques, these
procedures have become the preferred treatment due to their
benefits, including reduced postoperative pain, shorter hospital
stays and faster recovery. Endoscopic submucosal dissection (ESD)
has emerged as a promising, less invasive alternative, particularly
for smaller tumors with a stalk and no involvement of large blood
vessels. A recent study reported successful endoscopic resection of
well-differentiated esophageal liposarcomas, including a tumor
measuring 8.3×4.2×2.3 cm, demonstrating the feasibility of ESD
(20). In cases where the tumor
volume is large and complete endoscopic resection is not possible,
or if large blood vessels are involved, cervical incision for
resection is a viable option.
The present study reported two cases of giant
esophageal tumors, each exceeding 20 cm in length and 4 cm in
diameter. Gastroscopy findings revealed visible expanded blood
vessels on the surface, with one tumor exhibiting a lobulated
appearance, complicating endoscopic removal. After
multidisciplinary discussions among thoracic surgery and endoscopy
experts, both tumors were presumed to be benign based on
gastroscopy and endoscopic ultrasound results. As their stalks were
located in the cervical region and confined to the mucosal layer
without muscular invasion, endoscopic resection was considered
feasible. However, given the large tumor sizes and vascular
involvement, the risk of bleeding was high, and limited working
space during endoscopy increased the potential for incomplete
resection and recurrence. Therefore, thoracic surgery support was
necessary to manage potential complications. Following informed
consent from the patients and their families, a multidisciplinary
surgical approach was adopted. Under general anesthesia with
tracheal intubation, the endoscopy team performed ESD, while the
thoracic surgery team provided standby support for rapid
intervention in case of significant bleeding or other complications
beyond endoscopic management. This collaborative approach ensured
patient safety while minimizing surgical trauma. Ultimately, both
tumors were successfully removed endoscopically without
complications and both patients had favorable postoperative
outcomes without requiring any lymph node clearance or adjuvant
therapy.
The insights from this report are as follows: When
patients present with swallowing difficulties, a chest CT scan
should be considered, and if necessary, endoscopy should be
performed to prevent excessive tumor growth and associated
treatment challenges. Prior to endoscopic resection of esophageal
liposarcomas, it is crucial to determine tumor malignancy and stalk
location to determine whether esophageal resection is necessary.
Ensuring that the tumor is confined to the mucosal layer without
muscular invasion helps minimize the risk of esophageal perforation
(21). For large tumors or cases
with significant vascular involvement, procedures should be
conducted under general anesthesia in an operating room equipped
for emergency open surgery if needed in order to handle unexpected
situations and ensure surgical safety. The surgeon should have
extensive experience and blood vessels should be pre-coagulated to
minimize intraoperative bleeding during tumor resection.
Superficial excision should be avoided to prevent recurrence, while
overly deep excision could increase the risk of esophageal
perforation (22). Large tumors may
be difficult to extract through the oropharynx, so it is
recommended to use sterile surgical forceps guided by an endoscope
to grasp the tumor through the oral cavity, ensuring the safety and
effectiveness of the procedure. Postoperatively, patients should
refrain from eating for 3 days and receive jejunal or parenteral
nutrition (23). Follow-up imaging,
including chest CT or upper gastrointestinal studies, should be
conducted promptly to monitor for esophageal fistulas. A follow-up
chest CT or endoscopy is recommended 6 months to 1 year
post-surgery to detect any potential local recurrence (24).
In conclusion, for giant and complex
well-differentiated liposarcomas of the esophagus, endoscopic
surgery combined with multidisciplinary collaboration is a safe and
effective treatment strategy. This approach enables complete tumor
removal while minimizing surgical trauma and ensuring favorable
patient outcomes. Future studies should continue to accumulate case
data to refine treatment strategies, improve endoscopic surgery
success rates and strengthen clinical evidence. In addition,
further exploration of minimally invasive techniques in esophageal
tumor management will be crucial for enhancing patient prognosis
and quality of life.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JM was responsible for the conceptualization of the
study and wrote the manuscript. LL collected data and organized
images. XD participated in the conception and design of the study,
was responsible for data analysis and interpretation and guided the
revision of the content. JM, LL and XD confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript..
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Both patients have provided written informed consent
regarding the publication of this case report and related
images.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability, and subsequently, the authors
revised and edited the content produced by the AI tools as
necessary, taking full responsibility for the ultimate content of
the present manuscript.
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
ESD
|
endoscopic submucosal dissection
|
|
FISH
|
fluorescence in situ hybridization
|
|
P16
|
cyclin-dependent kinase inhibitor
2A
|
|
Vim
|
Vimentin
|
|
CK4
|
cytokeratin 4
|
|
MDM2
|
mouse double minute 2 homolog
|
|
S100
|
S100 calcium-binding protein
|
|
Sox10
|
SRY-box transcription factor 10
|
|
SMA
|
smooth muscle actin
|
|
Melan-A
|
melanocyte antigen
|
|
HMB45
|
human melanoma black 45
|
|
DOG-1
|
discovered on GIST-1
|
|
Ki-67
|
Ki-67 antigen
|
|
P53
|
tumor protein P53
|
References
|
1
|
Ducimetière F, Lurkin A, Ranchère-Vince D,
Decouvelaere AV, Péoc'h M, Istier L, Chalabreysse P, Muller C,
Alberti L, Bringuier PP, et al: Incidence of sarcoma histotypes and
molecular subtypes in a prospective epidemiological study with
central pathology review and molecular testing. PLoS One.
6:e202942011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Anderson WJ and Doyle LA: Updates from the
2020 world health organization classification of soft tissue and
bone tumours. Histopathology. 78:644–657. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Resag A, Toffanin G, Benešová I, Müller L,
Potkrajcic V, Ozaniak A, Lischke R, Bartunkova J, Rosato A, Jöhrens
K, et al: The immune contexture of liposarcoma and its clinical
implications. Cancers (Basel). 14:45782022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Somaiah N and Tap W: MDM2-p53 in
liposarcoma: The need for targeted therapies with novel mechanisms
of action. Cancer Treat Rev. 122:1026682024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Thway K: Well-differentiated liposarcoma
and dedifferentiated liposarcoma: An updated review. Semin Diagn
Pathol. 36:112–121. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ferrari D, Bernardi D, Siboni S, Lazzari
V, Asti E and Bonavina L: Esophageal lipoma and liposarcoma: A
systematic review. World J Surg. 45:225–234. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ng YA, Lee J, Zheng XJ, Nagaputra JC, Tan
SH and Wong SA: Giant pedunculated oesophageal liposarcomas: A
review of literature and resection techniques. Int J Surg Case Rep.
64:113–119. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Temes R, Quinn P, Davis M, Endara S,
Follis F, Pett S and Wernly J: Endoscopic resection of esophageal
liposarcoma. J Thorac Cardiovasc Surg. 116:365–367. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Goldblum JR, Folpe AL and Weiss SW:
Enzinger and Weiss's soft tissue tumors. 7th ed. Elsevier;
Philadelphia: 2020
|
|
10
|
Lee ATJ, Thway K, Huang PH and Jones RL:
Clinical and molecular spectrum of liposarcoma. J Clin Oncol.
36:151–159. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Valiuddin HM, Barbetta A, Mungo B,
Montgomery EA and Molena D: Esophageal liposarcoma:
Well-differentiated rhabdomyomatous type. World J Gastrointest
Oncol. 8:835–839. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lin ZC, Chang XZ, Huang XF, Zhang CL, Yu
GS, Wu SY, Ye M and He JX: Giant liposarcoma of the esophagus: A
case report. World J Gastroenterol. 21:9827–9832. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Graham RP, Yasir S, Fritchie KJ, Reid MD,
Greipp PT and Folpe AL: Polypoid fibroadipose tumors of the
esophagus: ‘Giant fibrovascular polyp’ or liposarcoma? A
clinicopathological and molecular cytogenetic study of 13 cases.
Mod Pathol. 31:337–342. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Caceres M, Steeb G, Wilks SM and Garrett
HE Jr: Large pedunculated polyps originating in the esophagus and
hypopharynx. Ann Thorac Surg. 81:393–396. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kiełbowski K, Ruszel N, Skrzyniarz SA,
Wojtyś ME, Becht R, Ptaszyński K, Gajić D and Wójcik J:
Clinicopathological features of intrathoracic liposarcoma-A
systematic review with an illustrative case. J Clin Med.
11:73532022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen C, He X, Jing WY, Qiu Y, Chen M, Luo
TY, Liu XY, Chen HJ, Zhang HY and Bu H: Diagnostic value of MDM2
RNA in situ hybridization in atypical lipomatous
tumor/well-differentiated liposarcoma and dedifferentiated
liposarcoma. Zhonghua Bing Li Xue Za Zhi. 51:190–195.
2022.PubMed/NCBI
|
|
17
|
Takiguchi G, Nakamura T, Otowa Y, Tomono
A, Kanaji S, Oshikiri T, Suzuki S, Ishida T and Kakeji Y:
Successful resection of giant esophageal liposarcoma by endoscopic
submucosal dissection combined with surgical retrieval: A case
report and literature review. Surg Case Rep. 2:902016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ye YW, Liao MY, Mou ZM, Shi XX and Xie YC:
Thoracoscopic resection of a huge esophageal dedifferentiated
liposarcoma: A case report. World J Clin Cases. 8:1698–1704. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bernardi D, Ferrari D, Siboni S, Porta M,
Bruni B and Bonavina L: Minimally invasive approach to esophageal
lipoma. J Surg Case Rep. 2020:rjaa1232020. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lee MJ, Lee MW, Joo DC, Hong SM, Baek DH,
Lee BE, Kim GH and Song GA: Effective endoscopic submucosal
dissection of a huge esophageal liposarcoma: A case report. Korean
J Gastroenterol. 83:243–246. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ogami T, Bozanich N, Richter JE and
Velanovich V: Giant esophageal liposarcoma. J Gastrointest Surg.
22:368–370. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodríguez Pérez A, Pérez Serrano N, García
Tejero A, Escudero Nalda B and Gil Albarellos R: Giant esophageal
liposarcoma in asymptomatic young patient. Cir Esp (Engl Ed).
96:381–383. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Fontes F, Fernandes D, Almeida A, Sá I and
Dinis-Ribeiro M: Patient-reported outcomes after surgical,
endoscopic, or radiological techniques for nutritional support in
esophageal cancer patients: A systematic review. Curr Oncol.
31:6171–6190. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ajani JA, D'Amico TA, Bentrem DJ, Cooke D,
Corvera C, Das P, Enzinger PC, Enzler T, Farjah F, Gerdes H, et al:
Esophageal and esophagogastric junction cancers, Version 2.2023,
NCCN clinical practice guidelines in oncology. J Natl Compr Canc
Netw. 21:393–422. 2023. View Article : Google Scholar : PubMed/NCBI
|