Introduction
Despite significant improvements in colorectal
cancer (CRC) treatment, especially in countries with long-standing
screening programs (1), CRC is
still the third most frequently diagnosed oncologic disease,
ranking second among causes of cancer-related mortality (2,3). A
further rise in CRC incidence is predicted. The burden of CRC is
attributed to increasing early-onset CRC, ageing of the population
and a lifestyle shift toward a Western diet combined with low
physical activity (3,4).
Interestingly, the age-standardized incidence of CRC
is consistently higher in males compared to females, including CRC
cases with early onset (5). CRC is
more often diagnosed in the proximal colon in women, whereas distal
anatomical CRC sites are detected more often in males (6–8). The
most pronounced difference is observed in the incidence of rectal
cancer, which is in 75% of cases diagnosed in males (2,3,9).
Among the possible reasons for this phenomenon are
an unfavourable diet, obesity, alcohol consumption, smoking and low
physical activity (10). These
factors influence CRC progression differently depending on the sex
of the patient and tumour localisation (11–13).
Although sex hormones have been implicated in the
generation of differences in CRC prevalence between males and
females, their precise role has not been completely elucidated
(7,8,14). In
particular, it is assumed that the most potent oestrogen,
17β-oestradiol (E2), influences CRC progression (8,15,16).
E2 signalling is mediated predominantly by two types
of nuclear receptors, ESR1 and ESR2, and the G protein-coupled
membrane receptor Gper1 (previous symbols GPER, GPR30) (15). Among the ligands of ESR1, ESR2 and
Gper1 are, in addition to oestrogens, tamoxifen, raloxifene,
bisphenols, dioxins, phthalates, quercetin, genistein, resveratrol
and others (17,18). The ligands mentioned above can
usually activate both nuclear and membrane-bound receptors,
although their affinity can differ (19). Gper1 has a lower affinity for E2
than ESR1 and ESR2 (20). Direct
binding of E2 to Gper1 was even questioned recently (21,22).
However, there is substantial in vivo and in vitro
evidence that Gper1 is involved in E2-mediated regulation (23). Recently, the binding of E2 to Gper1
was confirmed, and aldosterone was also proposed as a Gper1 agonist
(24).
Nuclear E2 receptors exert most of their effects via
transcriptional gene regulation. ESR1 and ESR2 activate thousands
of genes overlapping by 30% via a specific DNA sequence called
oestrogen response element (ERE) (25).
Compared to nuclear E2 receptors, Gper1 affects
intracellular processes much faster thanks to the prompt activation
of several intracellular signalling pathways. Signalling mediated
by Gper1 includes an increase in cAMP synthesis and consequent
activation of protein kinase A, Src-like nonreceptor tyrosine
kinase (Src) and sphingosine kinase (SphK). Src and SphK can
contribute to the activation of epidermal growth factor receptors
(EGFR). Gper1 signalling can also lead to activation of protein
kinase C and calcium mobilisation (17). Among Gper1-driven regulatory
pathways also belongs Gper1/HIFα activation of NOTCH and consequent
induction of VEGF transcription (26) and some others (18). Tissue-specific distribution and/or
changes in the tissue-specific distribution of E2 receptors in
disease can influence E2 signalling and facilitate its oncostatic
or tumour suppressor effects (17,18,25).
Epidemiological studies of patients using hormone
response therapy (HRT) implicated protective role of E2 in respect
to CRC incidence. It was shown that oestrogen/progestin HRT is
negatively associated with the risk of CRC (27). Similarly, the incidence of CRC in
postmenopausal women exposed to E2 HRT was lower compared to women
of the same age without HRT (28).
Other studies later strengthened the evidence of E2′s protective
role regarding CRC incidence (15,16,29,30).
It is of interest that patients with high levels of endogenous
circulating E2 (31) and those
exposed to E2 therapy whilst diagnosed with CRC demonstrated a
worse disease prognosis (32). On
the other hand, Mori et al (33) did not confirm the association of
high circulating E2 levels with increased incidence and/or
prognosis of CRC in postmenopausal women.
Most of the beneficial effects of E2 for patients
are attributed to ESR2 (15,34–37),
which is far more abundant in the gastrointestinal tract compared
to ESR1 (38–40). The beneficial E2 effect mediated via
ESR2 receptors was proven with the use of several in vivo
experimental models. Apcmin/+mice bearing a mutation in
the Apc gene are prone to developing multiple intestinal
tumours. Ovariectomy (OVX) significantly increased the number of
adenomas detected in the gut compared to control, and this effect
was reversed by E2 administration. E2 treatment was also
accompanied by an increase in ESR2 expression in the intestine
(41). ESR2 deficiency in
Apcmin/+ mice was associated with a higher adenoma
number compared to control (42).
The protective role of E2 executed via ESR2 was also demonstrated
in OVX mice where tumorigenesis was induced by azoxymethane (AOM)
(43), by combined treatment of AOM
and dextran sulphate sodium (44,45)
and in immunodeficient mice implanted with SW480 cells
overexpressing ESR2 (46).
Interestingly, the expression of Gper1 mRNA exceeds
that of ESR2 in healthy gut tissue (40,47).
Similarly, it is the predominant E2 receptor in several colorectal
cell lines (48). Despite that, the
role of the membrane receptor Gper1 in E2-mediated effects on
cancer progression has not been completely elucidated.
The tumour suppressor as well as the oncogenic role
of Gper1 has been demonstrated for several types of tissues.
Perhaps the most promising results were achieved in melanoma
treatment. However, in most cancer types, including CRC, the role
of Gper1 remains inconclusive (17,49,50).
It was shown that G-1, an agonist of Gper1, inhibits
the upregulation of JUN oncogene expression in HT29 cells (48). G-1 administered at a concentration
of 1 µM significantly inhibited the proliferation of HCT-116 and
SW480 cells, increased the number of cells in the G2/M phase and
stimulated apoptosis. The decrease in cell viability induced by G-1
was prevented by the administration of the ROS scavenger NAC.
Growth of tumour xenografts in nude mice was inhibited by G-1
administration (51).
On the other hand, Gper1 silencing prevented
chromosomal instability induced by diethylstilbestrol and the
occurrence of supernumerary centrosomes in HCT116 and control CCD
841 CoN cell lines. In these cell lines, Gper1 activation led to
the presence of multipolar mitotic spindles, an increased number of
cells with lagging chromosomes and aneuploidy. Interestingly,
manipulation of Gper1 levels did not influence the rate of
proliferation and cell cycle distribution under in vitro
conditions (52). The oncogenic
role of Gper1 was confirmed in HT-29 cells as Gper1 silencing
prevented an E2-induced decrease in ATM. Under normoxic conditions,
Gper1 activation caused down regulation of VEGF while the opposite
effect was observed in a hypoxic microenvironment and prevented by
Gper1 silencing. Under hypoxic conditions, Gper1 mediated an
E2-induced increase in HT-29 and DLD1 cell migration (53). E2 and its agonist G-1, via Gper1,
induced the expression of fatty acid synthase (FASN), which can
promote CRC progression. FASN upregulation is executed by the
epidermal growth factor receptor EGFR/ERK/AP1 pathway (54). Oncogenic properties of Gper1 were
demonstrated in the CRC cell lines COLO205 and SW480 as the Gper1
antagonist G15 was able to reverse the effect of the E2-related
endocrine disruptor nonylphenol, which induced proliferation, the
expression of cyclin D1, c-myc and ERK1/2. Administration of G15
also inhibited the growth of colon carcinoma xenografts in mice
(55). Disagreement in the results
of performed studies can be partly related to different
concentrations of G-1 used in experiments. In addition to
experimental designs, the biological context seems to be of special
importance in effects mediated by Gper1 (17,34,48,52,53).
In human studies, the results are also inconclusive.
Up- as well as down-regulated Gper1 expression in CRC tissue was
indicated (17), e.g. a strong
trend toward increased Gper1 levels in CRC cancer tissue compared
to adjacent tissue was reported by Gilligan et al (56) while down-regulated expression of
Gper1 was reported by Liu et al (51). High expression of Gper1 was
associated with better survival compared to low expression
(51); by contrast, low expression
of Gper1 was associated with better survival compared to high
expression (56). High expression
of Gper1 was also associated with worse survival by Bustos et
al (53) and Abancens et
al (48) but only in females in
stages 3–4 of CRC. Worse survival of CRC patients exhibiting high
expression of Gper1 is indicated by the Human Protein Atlas
(47).
As there is evidence suggesting that manipulation of
E2 signalling could be effective in clinical use, several agonists
and antagonists of nuclear and Gper1 receptors have been developed,
and some of them have been clinically tested (18,25,57).
The most promising results were obtained in the clinical study
NCT04130516 evaluating the effects of Gper1 agonist LNS8801 that
proved beneficial effects of Gper1 signalling in patients diagnosed
with cutaneous (58) and uveal
(59) melanoma. However, it was
implied that G-1 can cause effects independent of Gper1 signalling
(60–65). Interestingly, several clinically
approved antagonists of nuclear E2 receptors also act as Gper1
agonists (18).
CRC is among the most frequently diagnosed oncologic
diseases worldwide. To facilitate novel CRC treatment strategies,
we focused on Gper1 functioning in CRC tumours to define the
conditions under which Gper1 upregulation could be beneficial for
patients.
Despite ongoing clinical trials aimed at the
manipulation of Gper1 signalling in solid cancers, the involvement
of Gper1 in the generation of sex-dependent CRC incidence is
largely unknown. Similarly, the biological context that determines
the effect of Gper1 has not been described sufficiently. Therefore,
the present study is aimed to analyse the abundance of Gper1 in
healthy and tumour tissues of males and females diagnosed with CRC
according to disease stage and survival. Mutation in p53 has been
identified in more than 50% of CRC patients and at a higher
frequency in males compared to females (66). Therefore, using the CRC cell lines
LoVo and DLD1, which differ in p53 functionality (67), we tested the hypothesis that the
effects of Gper1 depend on the presence of wild-type p53.
Materials and methods
Tumour and adjacent tissues were obtained in
cooperation with the First Surgery Department, University Hospital,
Comenius University, Bratislava. The study included patients who
had to undergo surgery for CRC treatment (for details see Table SI) and agreed to sign an informed
consent. Patients were not subjected to any CRC treatment before
surgery and during hospitalization were exposed to a standard
hospital practice with lights on from 6:00 a.m. to 9:00 p.m.
Surgery was performed in the morning hours (before noon). Adjacent
tissues were collected ≥10 cm proximally and ≥2 cm distally from
the tumour. After tissue excision, samples were promptly frozen in
liquid nitrogen and stored at −70°C until analysis. The
experimental protocol was approved by the Ethics Committee of
Comenius University in Bratislava (ECH 19001).
To extract mRNA from tissues and cells, RNAzol RT
(Molecular Research Centre) was used as described previously
(40). One microgram of RNA
template from tissues and 0.02 µg from cells was used to synthesize
cDNA with the ImProm-II Reverse Transcription System and random
hexamers (Promega), according to the manufacturer's
instructions.
To analyse gene expression, the QuantiTect SYBR
Green kit (Qiagen, Germany) was used. Real-time PCR was performed
with the StepOnePlus™ Real-Time PCR System (Applied Biosystems).
The PCR conditions were activation of hot start polymerase at 95°C
for 15 min followed by 40 cycles at 94°C for 15 sec, 53°C for 30
sec and 72°C for 30 sec. As a last step of PCR, samples were
subjected to melting curve analysis. The sequences of primers for
the amplification of particular genes are provided in Table SII. The expression of nuclear
u6 mRNA was used for normalisation.
The human colorectal carcinoma cell lines DLD1 and
LoVo obtained from the American Type Culture Collection (ATCC) were
used to reveal how gper1 silencing influences cell migration
and metabolism. DLD1 cells were cultured in RPMI 1640 GlutaMax
medium (Gibco; Thermo Fisher Scientific, Inc.), and LoVo cells were
incubated in F-12K medium (Bioconcept). Both cell lines were
supplemented with 2% or 10% foetal bovine serum (FBS, Biosera)
depending on the experiment; 2% FBS supplementation was used for
the scratch assay while 10% FBS was used for the MTS test. The
culture medium also contained penicillin (50 U/ml) (Gibco),
streptomycin (50 µl/ml) (Gibco, USA) and ampicillin (50 µg/ml)
(Oasis-lab). All experiments were performed in a biological
Celculture® Incubator CCL-050B-8 (Esco Medical) with a
humidified atmosphere containing 5% CO2 at 37°C. The
cells were cultured in 96-well plates coated with 1% sterile
gelatine.
To test the effect of gper1 silencing,
DharmaFECT 1 reagent was used to transfect cells with siGENOME
Human Gper1siRNA-SMART pool or siGENOME non-targeting siRNA Pool #2
(Horizon) at a concentration of 100 nM.
The effect of siGper1 on wound healing was
determined by scratch assay performed when the cell culture reached
a confluence of 80–90% with the use of a 10-ul sterile tip. Images
were taken immediately after scratch and later as indicated in the
figure legends with the use of an inverted fluorescence microscope
NIB-100F (Nanjing Jiangnan Novel Optics Co., Ltd.) and BEL Capture
3.2 software (BEL Engineering s.r.l.). Wound closure was evaluated
with ImageJ software (68).
The rate of metabolism was measured by MTS test
according to the manufacturer's instructions (CellTiter 96 AQueous
Cell Proliferation Assay, Promega) employing a modified tetrazolium
compound to produce water-soluble formazan. The absorbance was
measured at 490 nm using a UV spectrophotometer (Epoch, Agilent
Technologies, Inc.).
Statistical analysis
To evaluate Gper1 mRNA expression in human samples,
the cohort was split into three groups according to TNM
classification. Group 1 included distant metastasis-free patients
without nodal involvement (T1-4N0M0), group 2 consisted of patients
with nodal involvement and without distant metastases (T3-4N1-2M0)
and the 3rd group was composed of patients with distant
metastases (T3-4N0-2M1). Gene expression between three groups was
compared by ANOVA followed by Tukey's post hoc test.
To compare E2 receptor expression between two
groups, a Student's paired t-test was used. ANOVA followed by
Tukey's post hoc multiple comparisons test was used to compare the
expression of three types of oestrogen receptors (ESR1, ESR2 and
Gper1) in human tissues and cells.
To analyse Gper1 mRNA expression with respect to
overall survival, a Kaplan-Meier survival curve and a log-rank test
were performed. Values were split according to the median; high
expression > median, low expression ≤ median. The starting point
for the log-rank test was the day of the surgery. The association
of Gper1 and VEGFA mRNA expression was analysed by correlation
analysis.
Data are provided as mean ± standard error of the
mean (SEM) in relative units (r.u.). The threshold for significance
for all statistical tests was set at P<0.05. Statistical
analyses were performed using GraphPad Prism 6 (GraphPad Software,
Inc.).
Results
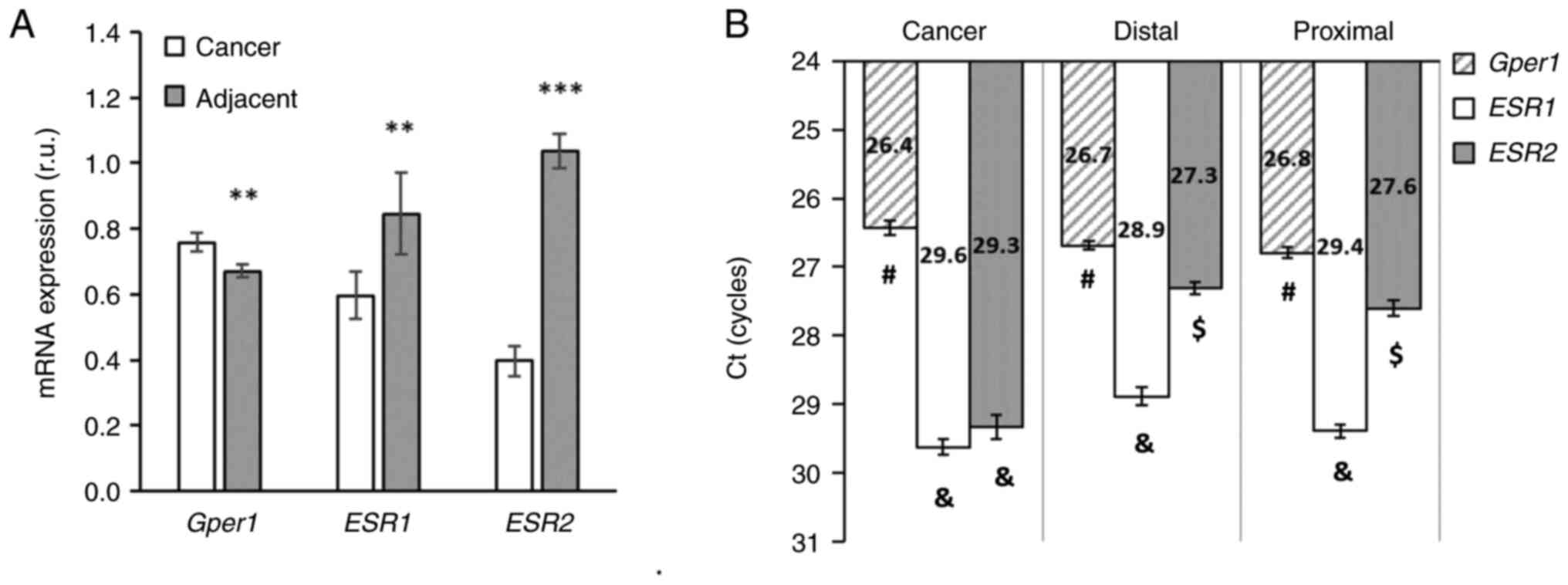
E2 receptors show robust differences in expression
between colorectal tumours and adjacent tissues. In tumour tissue,
the expression of gper1 was higher compared to adjacent
tissue, whereas the opposite pattern was observed in levels of
nuclear E2 receptors. The expression of esr1 and esr2
was significantly lower in cancer tissue compared to adjacent
tissues (Fig. 1A). The expression
of gper1 was higher in cancer tissue compared to adjacent
distal and proximal tissues (Fig.
1B). Comparison of the absolute level of expression based on
the threshold value of the PCR revealed that gper1 is far
most abundant in cancer as well as in the adjacent tissues compared
to esr1 and esr2 mRNA levels (Fig. 1B).
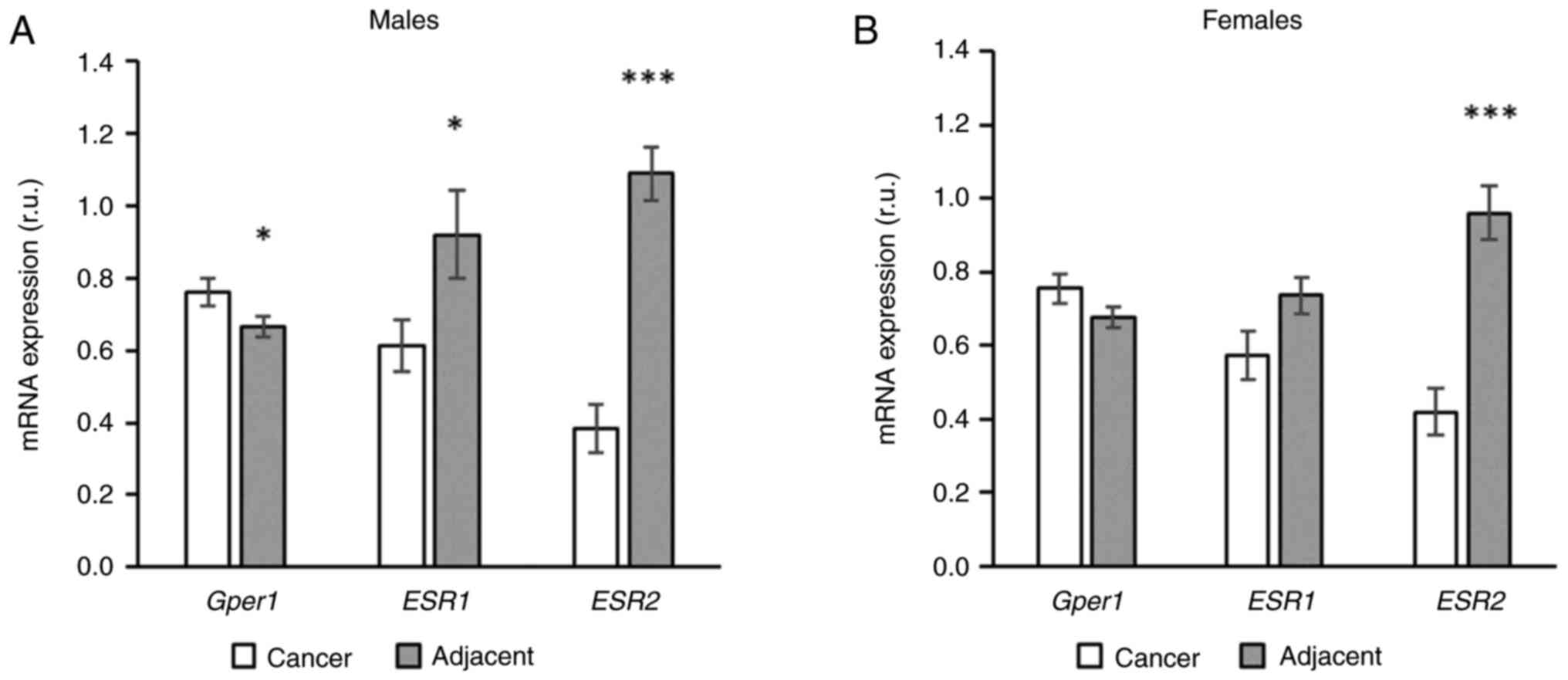
The expression of gper1 was higher in cancer
tissue compared to adjacent tissue in males, but in females this
pattern was observed only as a non-significant trend (Fig. 2). The expression of esr1 was
higher in the adjacent compared to cancer tissue and this
difference, again, achieved a level of significance only in males
(Fig. 2). The most robust
difference in expression between cancer and adjacent tissue was
observed in the expression of esr2. Unlike other receptors,
this pattern was present in both sexes (Fig. 2).
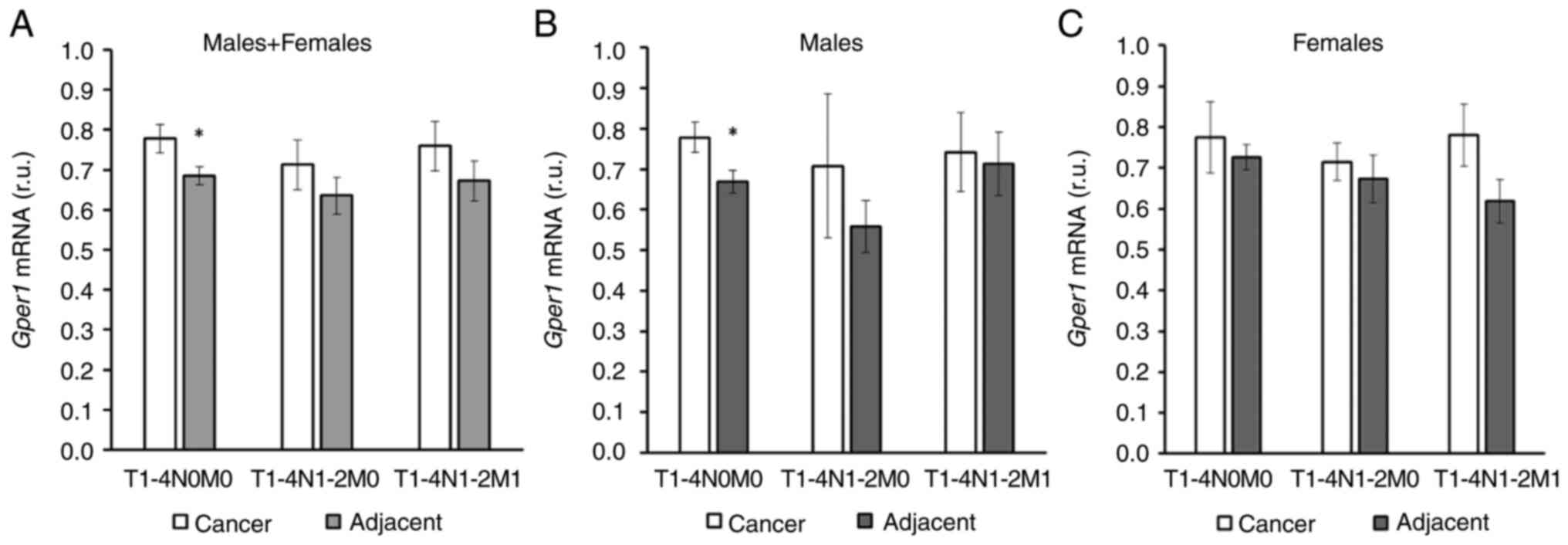
In the whole cohort an increase in gper1
expression in cancer tissue compared to adjacent tissue was
observed in patients without nodal involvement and distal
metastases (Fig. 3A). However, when
the cohort was split into male and female patients, this difference
was observed only in males (Fig. 3B and
C). gper1 expression did not differ between cancer and
adjacent tissues in more advanced stages of disease (Fig. 3).
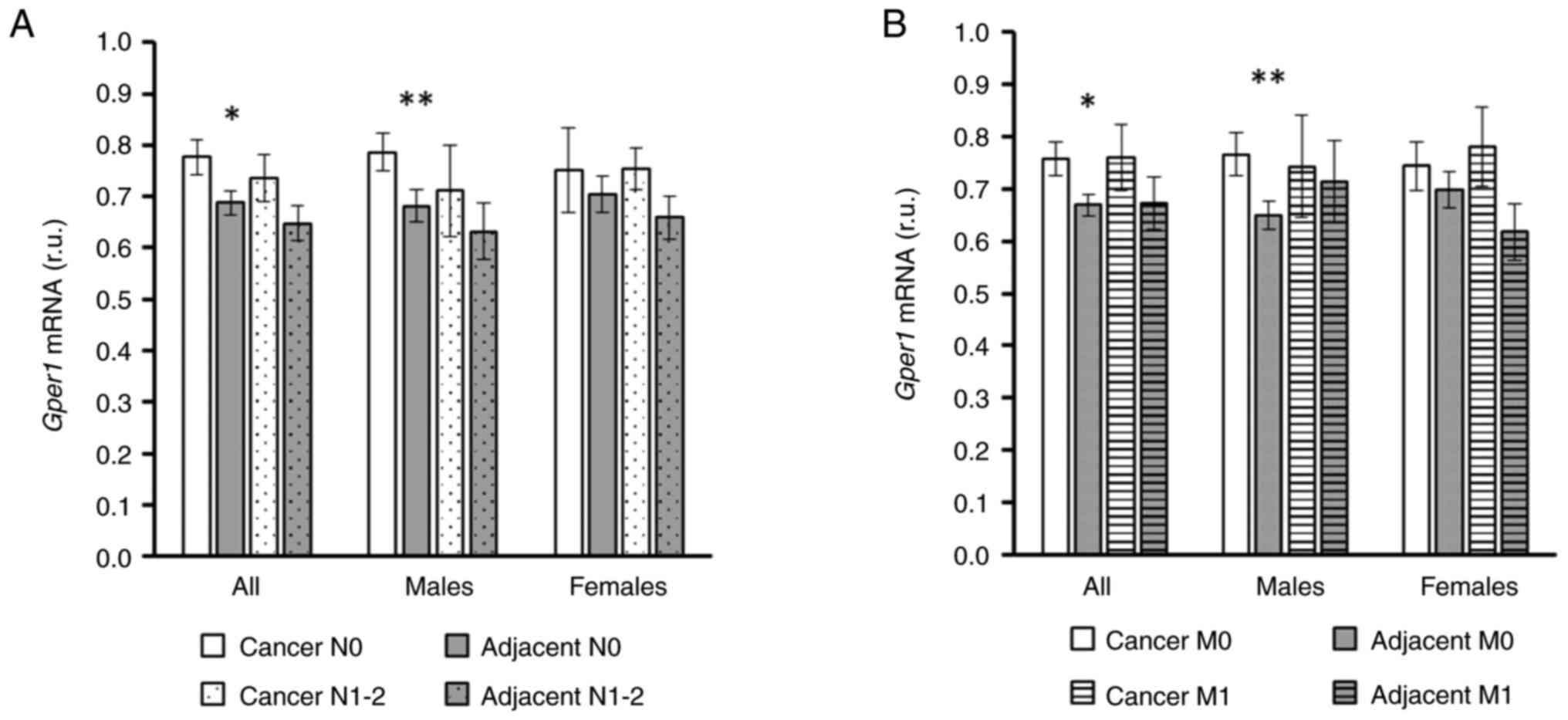
When the cohort was split according to nodal
involvement only (without considering TNM staging as a whole) the
sex-dependent difference in gper1 expression became even
more pronounced, and there was a highly significant increase in
gper1 expression in cancer tissue compared to adjacent
tissue in males without nodal involvement (Fig. 4A). Similarly, male patients without
distant metastases displayed a pronounced increase in gper1
expression in cancer tissue compared to adjacent tissue that was
not observed in females (Fig.
4B).
In accordance with the sex-dependent expression of
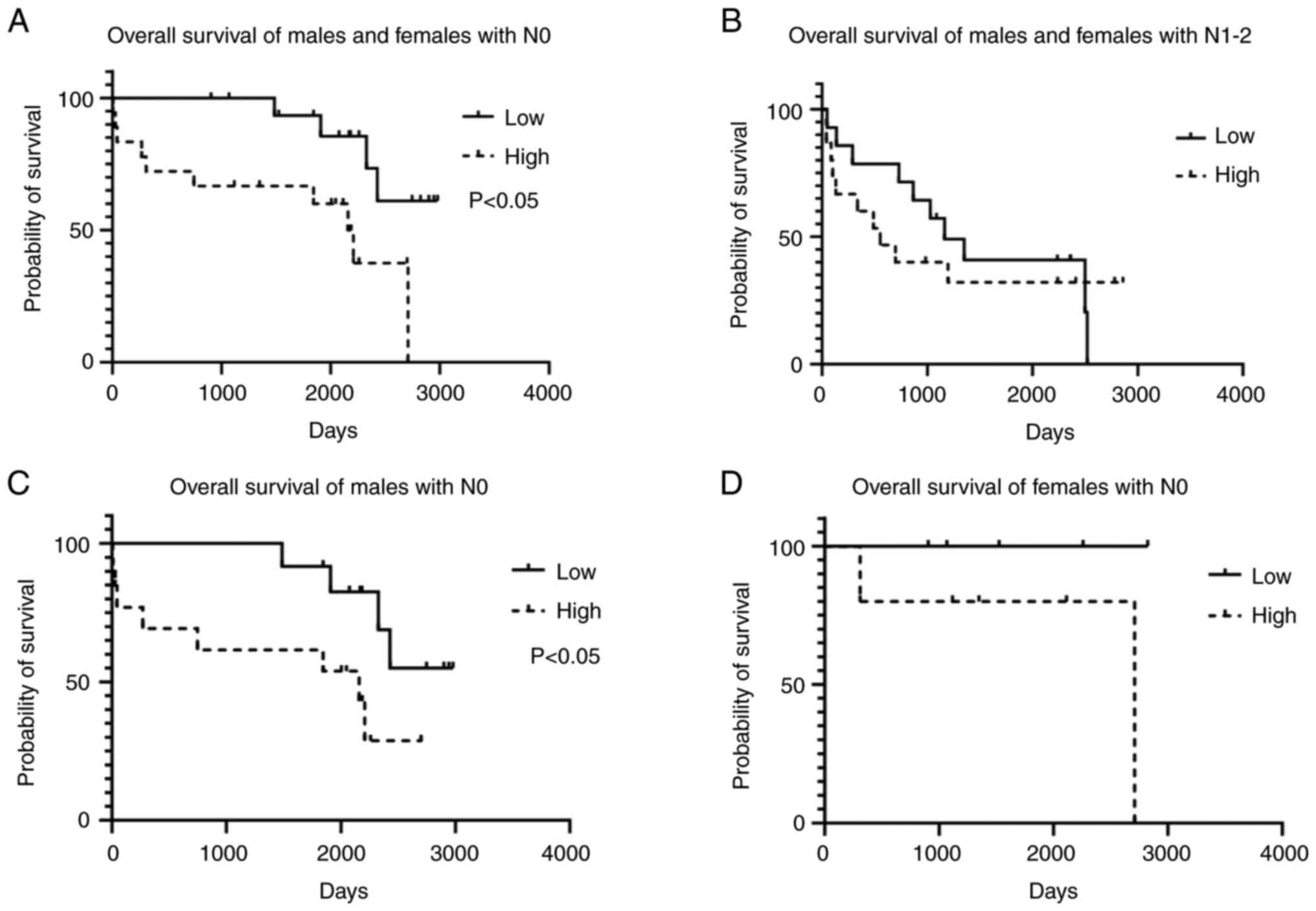
gper1 in CRC patients, the association of overall survival
and gper1 expression exerted a sex-dependent pattern. In the
whole cohort, better survival was correlated with low gper1
expression in patients without nodal involvement (Fig. 5A) but not in patients in higher
stages of disease (Fig. 5B). When
the cohort was split according to sex, a log-rank test revealed
that gper1 association with survival was generated by the
male subcohort (Fig. 5C). In
females the correlation between survival and gper1
expression did not reach significance (Fig. 5D).
In human samples from our cohort, the expression of
gper1 significantly correlated with the mRNA expression of
VEGFA in cancer tissue. This relationship was not observed in
proximal and distal adjacent tissues (Fig. S1).
Transfection of siRNA targeting gper1
expression successfully inhibited Gper1 mRNA expression in DLD1 as
well as LoVo cells compared to control (Fig. S2A and B, respectively).
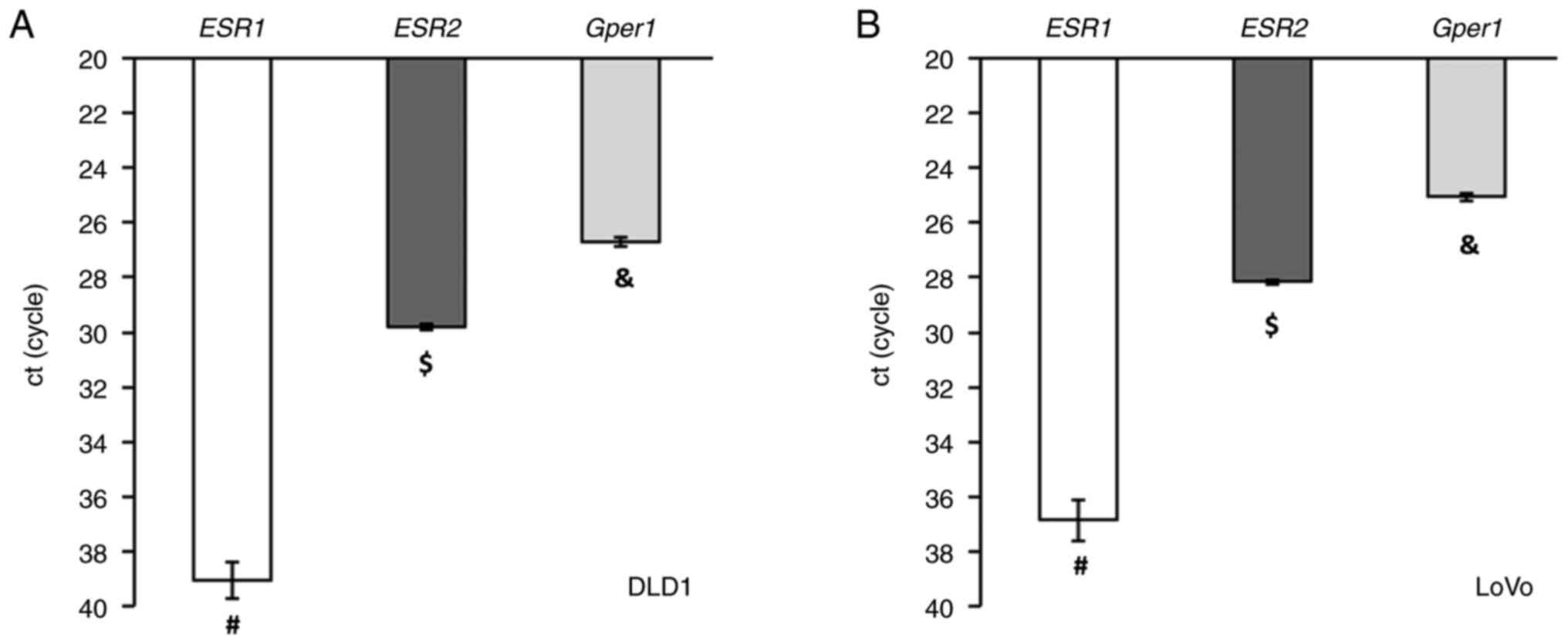
The distribution of E2 receptors in CRC cell lines
LoVo and DLD1 resembled that of human cancer tissue. The expression
of the membrane gper1 receptor was much higher compared to
mRNA levels of nuclear E2 receptors, and the expression of ESR1
mRNA was nearly undetectable (Fig.
6).
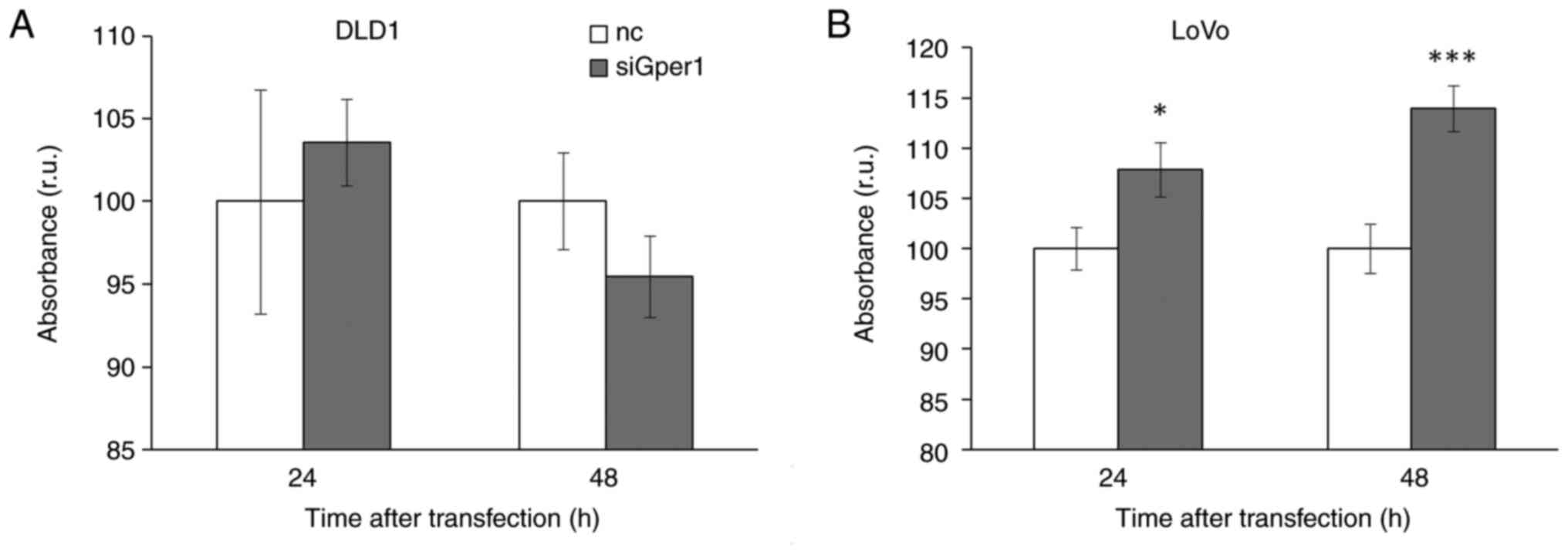
Silencing of gper1 expression significantly
stimulated metabolism in LoVo cells in a time-dependent manner that
implicates the tumour-suppressor capacity of Gper1. In DLD1 cells
we did not observe this effect (Fig.
7).
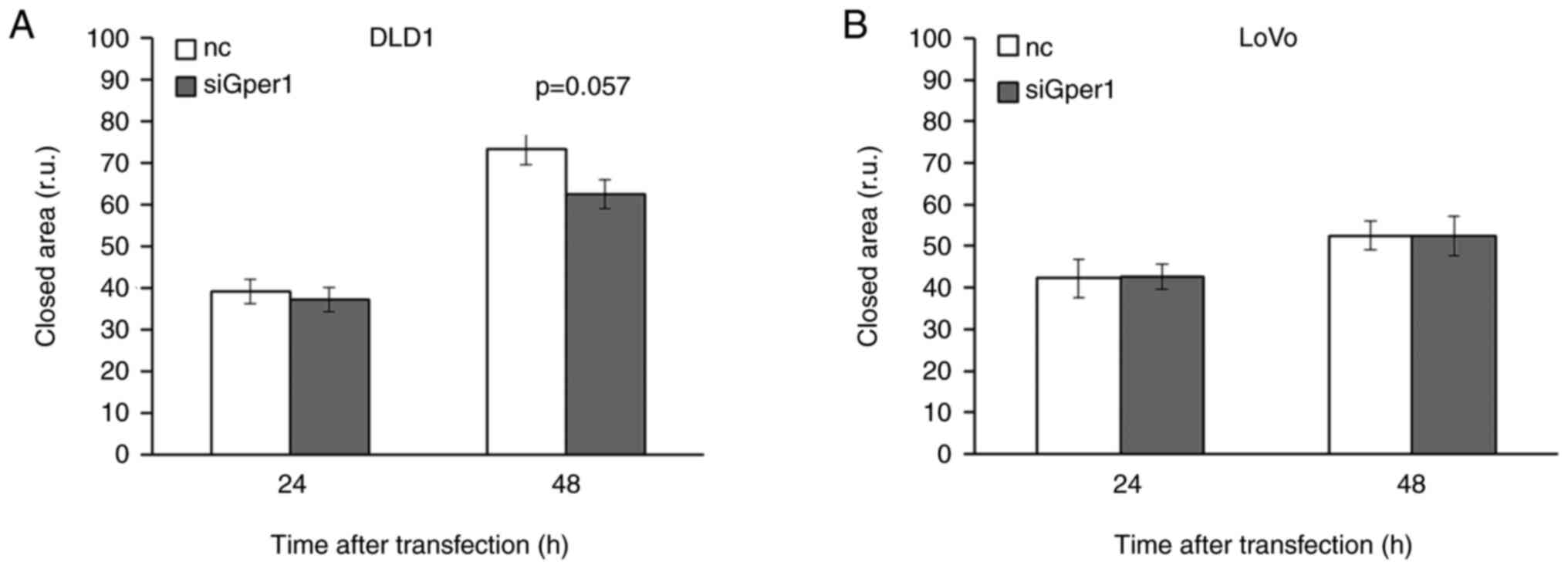
Evaluation by the scratch test demonstrated a
time-dependent decrease in wound closure in DLD1 cells with
silenced Gper1 expression compared to control. Whereas 24h after
Gper1 silencing there was no effect on the rate of wound closure,
48 h after transfection the width of the closed area was different
between control and transfected cells with P=0.057 (Fig. 8A). Inhibition of wound healing by
Gper1 silencing implicates the oncogenic potential of Gper1 in DLD1
cells.
Unlike in DLD1 cells, the administration of siRNA
interfering with Gper1 expression did not influence the rate of
wound closure in LoVo cells (Fig.
8B).
The expression of p53 mRNA did not differ
significantly between DLD1 and LoVo cells. However, as DLD1 cells
generate a mutated form of the p53 protein, expression of
p53-inducible gene p21 was significantly lover in DLD1 cells
compared to LoVo cells (Fig.
S3).
The expression of gper1 and tp53
showed a significant correlation in males (A) but not in females
(B) undergoing surgery for CRC treatment (Fig. S4) (TCGA, Colorectal Adenocarcinoma,
Nature 2012).
Discussion
The relative ratio of E2 receptors in CRC tissue
undergoes remodelling. While the expression of nuclear E2 receptors
decreases in cancer tissue compared to adjacent tissue, the
opposite pattern is observed in Gper1 mRNA expression. Therefore,
Gper1 is by far the most abundant E2 receptor in the CRC tumour
followed by the ESR2 and very low ESR1 mRNA expression. The
increase in gper1 expression in tumours is most pronounced
in males in the lower stages of disease. A higher expression of
Gper1 mRNA was associated with worse survival in the whole cohort
and in males without metastases in nodes. This dependency was not
detected in males with nodal involvement and females. We suppose
that sex-dependent differences in E2 receptor expression can
contribute to sex-dependent features of CRC progression.
In accordance with our data, Gilligan et al
(56) showed an increase in Gper1
expression in CRC tissue compared to adjacent tissue. Several
factors can induce an increase in Gper1 mRNA levels in cancer
tissue. Firstly, Gper1 belongs to HIF-1 targeted genes that are
expressed in response to hypoxia (69). The stimulatory effect of hypoxia on
Gper1 mRNA expression was also demonstrated in the colon cancer
cell line HT-29 and the rectal cancer cell line C80 (53). The oncogenic role of Gper1 was
implicated as it was demonstrated that Gper1 cooperated with HIF-1α
in the activation of VEGF expression in hypoxia (26,70).
In line with this finding, we detected a positive correlation
between Gper1 and VEGFA mRNA expression in cancer but not in the
adjacent tissues.
We observed a decrease in ESR2 mRNA expression in
CRC tumours compared to proximal and distal parts of the gut, which
is consistent with previous studies (71–77)
that reported lower ESR2 expression in CRC tissue compared to
adjacent tissues at the protein and mRNA levels.
According to our results, the expression of ESR1
mRNA is lower in cancer tissue compared to adjacent tissues, and
this difference is more pronounced in males than in females.
Previously, a decrease was observed in ESR1 expression in
colorectal cancer tissue, which was attributed to CpG island
methylation (78). No significant
differences between cancer and adjacent tissue have also been
reported (38). According to Jiang
et al (79), the expression
of the dominant ESR1 isoform does not differ in its mRNA levels
between tumour and matched normal tissues. However, the ER-α46
isoform that shares most of its sequence with the dominant isoform
was down-regulated in cancer tissue compared to adjacent tissue. On
the other hand, an increase in esr1 mRNA in cancer compared
to adjacent healthy tissue has been reported lately (80). Although data referring to ESR1
expression in CRC seem to be inconclusive, there is a consensus on
the decrease in nuclear ESR2 and increase in Gper1 expression in
cancer compared to adjacent tissue.
The expression of all three types of receptors in
one set of samples has rarely been studied. There is a strong
evidence that the expression of ESR2 in the gut is more abundant
compared to that of ESR1 (71,76,81).
The assumption that ESR2 is the predominant form of the E2 receptor
in the gut can, to some extent, be caused by later identification
of the Gper1 receptor compared to nuclear E2 receptors (82). Previously, it has been shown that
the expression of Gper1 mRNA is more abundant compared to that of
both ESR1 and ESR2 in the rat colon (40). According to datasets available from
the Human Protein Atlas, the expression of E2 receptors is
Gper1>ESR2>>ESR1 (47),
which is in line with Harvey and Harvey (83).
The density of E2 receptors changes in CRC tumour
tissue. In CRC the abundance of E2 receptors shows the pattern
Gper1>>ESR2>ESR1. The order of E2 receptor density in the
healthy colon is similar, but there is an abrupt decrease in ESR2
and an increase in Gper1 expression in the tumour compared to the
healthy gut. Changes in the abundance of E2 receptors in CRC are
more pronounced in males than in females.
As CRC occurs to a lesser extent in females compared
to males (83), and sex-dependent
differences in nuclear E2 receptor expression have been revealed
(72,74–76,84),
female sex hormones were suggested to be involved in the regulation
of CRC progression (85).
Beneficial effects of E2 mediated via ESR2 receptors have been
convincingly demonstrated (76,86);
however, contradictory reports are available concerning the role of
Gper1 in CRC progression (34,53,56,83).
Similarly, little is known about sex-related differences in Gper1
expression, although they have been implied (70,83).
According to our results, there are more pronounced differences in
Gper1 expression in cancer tissue compared to adjacent tissue in
males than in females. This difference is noticeable mainly in
males without nodal metastases.
In our cohort poor survival correlated with high
Gper1 mRNA expression more significantly in males without nodal
involvement than in males in higher stages of disease or females.
These results are in accordance with Gilligan et al
(56), who reported an association
of worse survival in patients with high Gper1 expression.
Information about the correlation in males and females separately
was not provided. Bustos et al (53) reported sex-dependent differences in
relapse-free survival and Gper1 expression; however, as we do not
have data allowing this type of analysis, a comparison was not
possible. Our results are in accordance with the Human Protein
Atlas (47), according to which
there is a stronger association between Gper1 and survival in males
compared to females and worse survival in patients with high Gper1
expression. The association reaches significance only in males in
stage I–II and not in patients with higher stages of disease.
Interestingly, when the survival of males and females together are
correlated with Gper1 expression, the association does not reach
significance, which also implicates a sex-specific dichotomy in
regulation.
There are reports implicating Gper1 as a tumour
suppressor in pancreatic cancer, melanoma and adrenocortical cancer
and as a tumour promoter in glioblastoma and endometrial and
ovarian cancers, whereas in the case of lung, prostate, breast and
colorectal cancers, information about the regulatory impact of
Gper1 is inconclusive (17,18,34,87,88).
Gper1-mediated effects are dependent on the biological context. Our
results imply that intracellular conditions are vital for the
interpretation of Gper1 signalling, even at the level of one type
of solid cancer.
Gper1 is known to induce signalisation mediated via
the EGFR receptor with tyrosine kinase activity (17,89,90). A
deregulated EGFR pathway has been associated with the progression
of many types of cancer, including CRC (91,92).
Therefore, several tyrosine kinase inhibitors and EFGR antibodies
have been developed and introduced into clinical practice (92). Inhibition of EGFR signalling was
shown to benefit patients diagnosed with CRC, and the EFGR
monoclonal antibodies Cetuximab and Panitumumab are now routinely
used to treat this type of cancer (93). In vitro studies elucidated
the molecular mechanisms of cancer inhibition caused by EFGR
repression in several cancer cell lines, including DLD1 (94–97).
Gper1 signalling mediated via the Gs-coupled cAMP/PKA/CREB pathway
has also been shown to promote cell proliferation (98). Therefore, as Gper1 is known to
induce both EGFR- and cAMP-mediated regulation and is reciprocally
involved in VEGFA release, presumably, an oncogenic role of Gper1
might be expected in CRC.
However, signalling mediated by Gper1 is highly
complex. The same group that discovered the connection between
Gper1 and EGFR two years later revealed that Gper1 inhibits EGFR
signalling via the cAMP/PKA pathway by Raf-1 inactivation (89). To further elucidate the ambiguous
effects of Gper1 on CRC progression, we investigated the effect of
Gper1 silencing in CRC cell lines LoVo and DLD1.
According to our results, the distribution of E2
receptors in the CRC cell lines DLD1 and LoVo resembles that
observed in human tissues: the highest mRNA expression is that of
gper1, and the expression of ESR1 is nearly undetectable.
Gper1 silencing in the DLD1 cell line, which expresses mutated
tp53, inhibited the wound closure, implicating the oncogenic
potential of Gper1. By contrast, the decrease in Gper1 availability
caused an increase in the metabolic rate in LoVo cells that
preserved p53 functionality (99).
The p53 protein, encoded by the gene tp53, is
a well-known tumour suppressor that inhibits cell cycle progression
and initiates DNA repair and/or apoptosis and autophagy, in
response to DNA damage, hypoxia, nutrition deficiency, oxidative
stress and some hormones. Cell cycle inhibition is executed by
induction of p21 expression. p21 inhibits the activity of several
types of cyclin/CDK complexes that release repression of
retinoblastoma protein (RB). As cyclin/CDK complexes are
inactivated by p21 and cannot phosphorylate RB, hypophosphorylated
RB inhibits E2F-induced transcription, which is necessary for cell
proliferation (100,101).
The p53 protein is frequently mutated in many types
of cancer, including CRC. According to TCGA, more than 50% of CRC
patients carry a mutated form of tp53. The presence of a
mutated form of p53 is accompanied by worse survival than that of
wild-type tp53. Interestingly, tp53 mutations are
more frequent in males compared to females, which may also
contribute to the higher CRC incidence in males compared to females
(66).
We hypothesize that the signalling pathway
downstream of p53 influences the outcome of Gper1 signalling.
Previously, it was shown that Gper1 expression in the
triple-negative breast cancer cell lines MDA-MB-231 and MDA-MB-468,
which express a mutated form of tp53, had been induced by
γ-radiation, whereas in MCF-7 cells expressing wild-type
tp53, the opposite pattern was observed. Therefore, a tumour
suppressor role dependent on p53 was attributed to Gper1 (102). Our results agree with this
statement as Gper1 was associated with oncostatic functions in LoVo
but not in DLD1 cell lines. Previously, the oncogenic potential of
Gper1 has been described in the CRC cell lines HT-29, DLD1, COLO205
and SW480 (53,55) carrying mutations in the tp53
gene (66,103–106).
Although there are reports implicating a functional
relationship between p53 and oestradiol, the exact mechanism has
either not been completely elucidated or, more likely, comprises
several ways by which E2 and p53 signalling interfere (107). These interferences can differ in a
tissue-dependent manner, e.g. E2 administration decreases p53
expression in lung cancer cells by induction of methyltransferase 1
expression, which increases methylation of the tp53 promoter
(108). On the other hand, a
protective role of E2 in non-malignant colonocytes executed via
p53-mediated regulation has been implicated (109).
The effects of E2 on p53 expression have been
previously investigated mainly with respect to the effects of ERα
on breast cancer progression. ERα interacts with tp53 and
influences its expression. Most of studies report an increase in
tp53 expression in response to ERα binding (107,110–113). On the other hand, there are also
results implicating ERα-induced suppression of p53-regulated gene
expression (114,115). However, in DLD1 cells, ESR1
expression is nearly undetectable; therefore, we do not suppose
that a substantial increase in p53 expression can be induced via
ERα. The results are inconclusive with respect to ERβ signalling
and p53 expression. Although induction of p53 activity in response
to ERβ activation has been reported (107,113), a stimulatory effect of ERβ
interacting with the tp53 promoter on tp53 expression
has not been detected (111).
In silico analysis showed a positive
correlation between Gper1 and tp53 expression in CRC tissues
of males (TCGA) (116). The E2
receptor ligands bisphenol A (117) and G-1 (102,118,119) have also been shown to induce
tp53 expression. Silencing of Gper1 caused a decrease in
tp53 expression in uveal melanoma cell lines (88). The exact mechanism of how Gper1
executes its effect on p53 transcription is unknown. A cAMP
response element (CREB) was identified in the human tp53
gene (120,121) and CREB binding protein plays a key
role in p53 activation (122).
However, whether Gper1 executes its stimulatory effect on
tp53 expression via this region remains to be
elucidated.
To conclude, the expression of E2 receptors in
healthy tissue follows the descending order
Gper1>ESR2>>ESR1 with nearly undetectable expression of
ESR1 mRNA. In CRC patients the ratio of receptors changes more in
males than in females; the expression of Gper1 mRNA increases while
the expression of ESR2 and ESR1 decreases, resulting in the
descending order Gper1>>ESR2>ESR1. Therefore, under
conditions of cancer progression, most E2 signalling is mediated
via the membrane E2 receptor Gper1. High expression of Gper1 is
associated with worse survival in males without nodal involvement
in comparison with other subcohorts (Fig. 9).
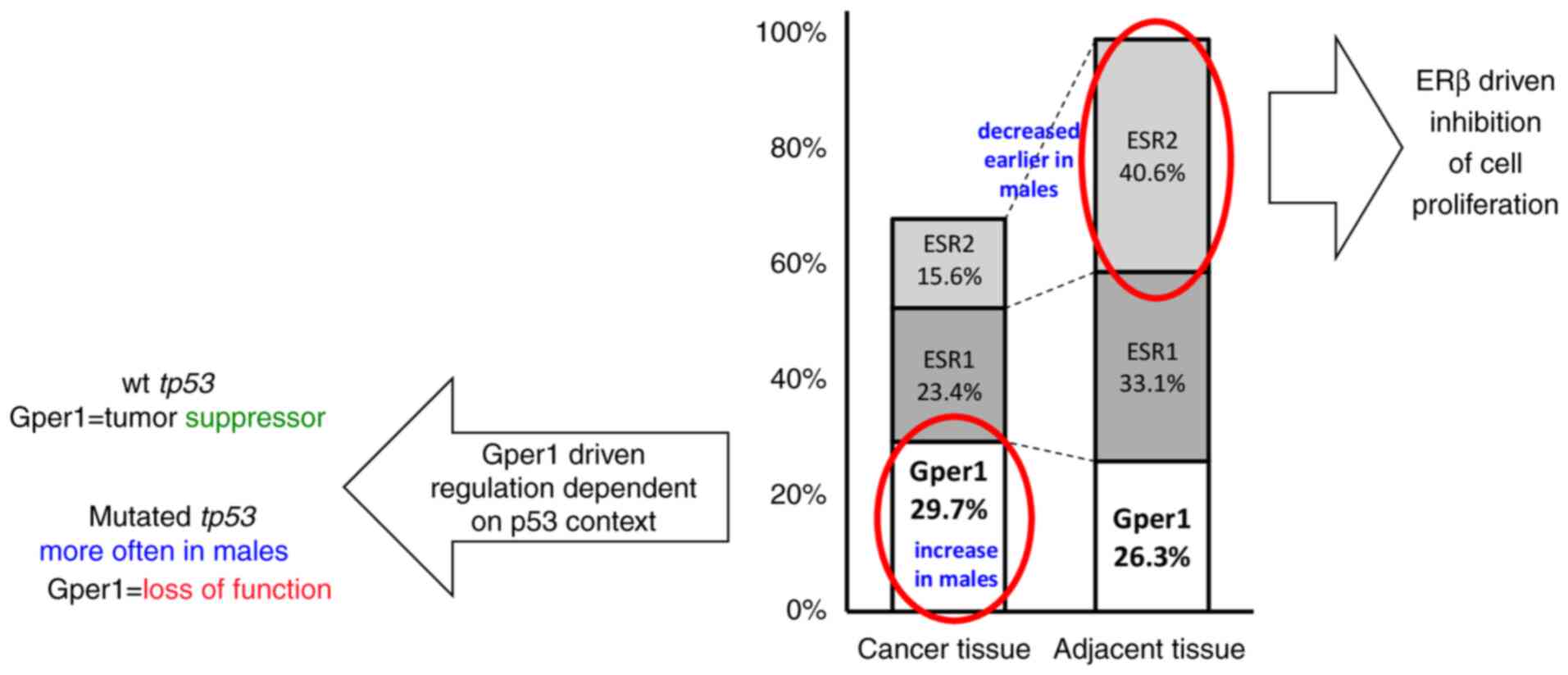 | Figure 9.Possible mechanism of how Gper1
signalling contributes to sex-dependent differences in CRC. In
cancer tissue, the expression of ESR2, which inhibits CRC
progression, decreases in the earlier stages of disease in males
compared to females (74). E2
signalling is further modulated by an increase in Gper1 expression
in cancer tissue in males in the early stages of disease, which is
not observed in females. Therefore, in the CRC tumour
Gper1-mediated regulation strongly influences how E2 signalling
will be interpreted by the cell. Gper1 demonstrates an oncostatic
effect in the LoVo cell line carrying wild-type tp53, which
is not observed in DLD1 cells with mutated tp53. It was
concluded that Gper1 functioning is dependent on the p53
intracellular context. As the mutated form of the gene is more
frequent in males compared to females, and the two most important
gut receptors, Gper1 and ESR2, show a sex-dependent pattern of
expression, we suppose that sex-dependent interpretation of E2
signalling and the availability of a functional p53 pathway
contribute cooperatively to the differences in CRC progression
observed between sexes. ESR, oestrogen receptor; Gper1, G
protein-coupled oestrogen receptor 1; E2, 17β-oestradiol; CRC,
colorectal cancer; wt, wild-type. |
The relative expression of E2 receptors in DLD1 and
LoVo cells is similar to that observed in human CRC tumours. In
LoVo cells with wild-type tp53, a tumour suppressor effect
of Gper1 was observed. This effect was not detected in DLD1 cells
with the mutated form of tp53. We suppose that Gper1
activates the EFGR/RAS oncogenic pathway as well as the p53
pathway. In case the p53 pathway is not functional, the oncogenic
potential of Gper1 overwhelms its tumour suppressor effects. As the
frequency of tp53 mutations as well as changes in Gper1
expression are more robust in males compared to females, we suppose
that these effects can contribute to sex-dependent differences in
CRC incidence.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
The authors would like to thank Dr. Soňa Olejarova
(Department of Animal Physiology and Ethology, Faculty of Natural
Sciences, Comenius University in Bratislava, Bratislava, Slovak
Republic) for help with laboratory analyses.
Funding
The research was supported by projects APVV-16-0209 and
APVV-20-0241 provided by The Slovak Research and Development Agency
and project VEGA 1/0455/23 provided by grant agency of the Ministry
of Education, Science, Research and Sport of the Slovak
Republic.
Availability of data and materials
The datasets are available from the corresponding
author on reasonable request.
Authors' contributions
IH and RR designed and administered the human
study. IH and RR confirm the authenticity of all the raw data. RR
obtained samples and organized their transport to the laboratory.
IH performed analysis of human samples. IH and DV performed cell
culture experiments. IH performed in silico analysis,
interpreted results, wrote the manuscript and prepared the figures.
IH, RR and DV reviewed and revised the manuscript for the
scientific content. All authors have read and approved the final
manuscript.
Ethics approval and consent to
participate
The design of the study describing the sampling of
human tissues was approved by the Ethics Committee of the Comenius
University in Bratislava (approval no. ECH 19001). All patients
included in the study agreed to sign an informed consent. The
manuscript does not contain experiments using animals or embryonic
cells.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Professor Iveta Herichová, ORCID:
0000-0002-0475-0461.
References
|
1
|
Cardoso R, Guo F, Heisser T, Hackl M, Ihle
P, De Schutter H, Van Damme N, Valerianova Z, Atanasov T, Májek O,
et al: Colorectal cancer incidence, mortality, and stage
distribution in European countries in the colorectal cancer
screening era: An international population-based study. Lancet
Oncol. 22:1002–1013. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2021. CA Cancer J Clin. 71:7–33. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xi Y and Xu P: Global colorectal cancer
burden in 2020 and projections to 2040. Transl Oncol.
14:1011742021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murphy N, Ward HA, Jenab M, Rothwell JA,
Boutron-Ruault MC, Carbonnel F, Kvaskoff M, Kaaks R, Kühn T, Boeing
H, et al: Heterogeneity of colorectal cancer risk factors by
anatomical subsite in 10 european countries: Amultinational cohort
study. Clin Gastroenterol Hepatol. 17:1323–1331.e6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gausman V, Dornblaser D, Anand S, Hayes
RB, O'Connell K, Du M and Liang PS: Risk factors associated with
early-onset colorectal cancer. Clin Gastroenterol Hepatol.
18:2752–2759.e2. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jacobs ET, Thompson PA and Martínez ME:
Diet, gender, and colorectal neoplasia. J Clin Gastroenterol.
41:731–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Quirt JS, Nanji S, Wei X, Flemming JA and
Booth CM: Is there a sex effect in colon cancer? Disease
characteristics, management, and outcomes in routine clinical
practice. Curr Oncol. 24:e15–e23. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lopes-Ramos CM, Quackenbush J and DeMeo
DL: Genome-wide sex and gender differences in cancer. Front Oncol.
10:5977882020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Høydahl Ø, Edna TH, Xanthoulis A, Lydersen
S and Endreseth BH: Long-term trends in colorectal cancer:
Incidence, localization, and presentation. BMC Cancer. 20:10772020.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Johnson CM, Wei C, Ensor JE, Smolenski DJ,
Amos CI, Levin B and Berry DA: Meta-analyses of colorectal cancer
risk factors. Cancer Causes Control. 24:1207–1222. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Murphy N, Ward HA, Jenab M, Rothwell JA,
Boutron-Ruault MC, Carbonnel F, Kvaskoff M, Kaaks R, Kühn T, Boeing
H, et al: Heterogeneity of colorectal cancer risk factors by
anatomical subsite in 10 European countries: A multinational cohort
study. Clin Gastroenterol Hepatol. 17:1323–1331.e6. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murphy N, Moreno V, Hughes DJ, Vodicka L,
Vodicka P, Aglago EK, Gunter MJ and Jenab M: Lifestyle and dietary
environmental factors in colorectal cancer susceptibility. Mol
Aspects Med. 69:2–9. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wele P, Wu X and Shi H: Sex-dependent
differences in colorectal cancer: With a focus on obesity. Cells.
11:36882022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Barzi A, Lenz AM, Labonte MJ and Lenz HJ:
Molecular pathways: Estrogen pathway in colorectal cancer. Clin
Cancer Res. 19:5842–5848. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nie X, Xie R and Tuo B: Effects of
estrogen on the gastrointestinal tract. Dig Dis Sci. 63:583–596.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Labadie JD, Harrison TA, Banbury B, Amtay
EL, Bernd S, Brenner H, Buchanan DD, Campbell PT, Cao Y, Chan AT,
et al: Postmenopausal hormone therapy and colorectal cancer risk by
molecularly defined subtypes and tumor location. JNCI Cancer
Spectr. 4:pkaa0422020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qiu YA, Xiong J and Yu T: Role of G
Protein-coupled estrogen receptor in digestive system carcinomas: A
minireview. Onco Targets Ther. 14:2611–2622. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Prossnitz ER and Barton M: The G
protein-coupled oestrogen receptor GPER in health and disease: An
update. Nat Rev Endocrinol. 19:407–424. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Heldring N, Pike A, Andersson S, Matthews
J, Cheng G, Hartman J, Tujague M, Ström A, Treuter E, Warner M and
Gustafsson JA: Estrogen receptors: How do they signal and what are
their targets. Physiol Rev. 87:905–931. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Fuentes N and Silveyra P: Estrogen
receptor signaling mechanisms. Adv Protein Chem Struct Biol.
116:135–170. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Otto C, Rohde-Schulz B, Schwarz G, Fuchs
I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R
and Fritzemeier KH: G protein-coupled receptor 30 localizes to the
endoplasmic reticulum and is not activated by estradiol.
Endocrinology. 149:4846–4856. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ahmadian Elmi M, Motamed N and Picard D:
Proteomic analyses of the G Protein-coupled estrogen receptor GPER1
reveal constitutive links to endoplasmic reticulum, glycosylation,
trafficking, and calcium signaling. Cells. 12:25712023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mauvais-Jarvis F, Lange CA and Levin ER:
Membrane-initiated estrogen, androgen, and progesterone receptor
signaling in health and disease. Endocr Rev. 43:720–742. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ding Q, Chorazyczewski J, Gros R, Motulsky
HJ, Limbird LE and Feldman RD: Correlation of functional and
radioligand binding characteristics of GPER ligands confirming
aldosterone as a GPER agonist. Pharmacol Res Perspect.
10:e009952022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Leitman DC, Paruthiyil S, Vivar OI,
Saunier EF, Herber CB, Cohen I, Tagliaferri M and Speed TP:
Regulation of specific target genes and biological responses by
estrogen receptor subtype agonists. Curr Opin Pharmacol.
10:629–636. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
De Francesco EM, Lappano R, Santolla MF,
Marsico S, Caruso A and Maggiolini M: HIF-1α/GPER signaling
mediates the expression of VEGF induced by hypoxia in breast cancer
associated fibroblasts (CAFs). Breast Cancer Res. 15:R642013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Rennert G, Rennert HS, Pinchev M, Lavie O
and Gruber SB: Use of hormone replacement therapy and the risk of
colorectal cancer. J Clin Oncol. 27:4542–4547. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Johnson JR, Lacey JV Jr, Lazovich D,
Geller MA, Schairer C, Schatzkin A and Flood A: Menopausal hormone
therapy and risk of colorectal cancer. Cancer Epidemiol Biomarkers
Prev. 18:196–203. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Symer MM, Wong NZ, Abelson JS, Milsom JW
and Yeo HL: Hormone replacement therapy and colorectal cancer
incidence and mortality in the prostate, lung, colorectal, and
ovarian cancer screening trial. Clin Colorectal Cancer.
17:e281–e288. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jang YC, Huang HL and Leung CY:
Association of hormone replacement therapy with mortality in
colorectal cancer survivor: A systematic review and meta-analysis.
BMC Cancer. 19:11992019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Hang D, He X, Kværner AS, Chan AT, Wu K,
Ogino S, Hu Z, Shen H, Giovannucci EL and Song M: Plasma sex
hormones and risk of conventional and serrated precursors of
colorectal cancer in postmenopausal women. BMC Med. 19:182021.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Foster PA: Oestrogen and colorectal
cancer: Mechanisms and controversies. Int J Colorectal Dis.
28:737–749. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Mori N, Keski-Rahkonen P, Gicquiau A,
Rinaldi S, Dimou N, Harlid S, Harbs J, Van Guelpen B, Aune D, Cross
AJ, et al: Endogenous circulating sex hormone concentrations and
colon cancer risk in postmenopausal women: A prospective study and
meta-analysis. JNCI Cancer Spectr. 5:pkab0842021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Das PK, Saha J, Pillai S, Lam AK, Gopalan
V and Islam F: Implications of estrogen and its receptors in
colorectal carcinoma. Cancer Med. 12:4367–4379. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Mal R, Magner A, David J, Datta J,
Vallabhaneni M, Kassem M, Manouchehri J, Willingham N, Stover D,
Vandeusen J, et al: Estrogen receptor beta (ERβ): A ligand
activated tumor suppressor. Front Oncol. 10:5873862020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mahbub AA, Aslam A, Elzubier ME, El-Boshy
M, Abdelghany AH, Ahmad J, Idris S, Almaimani R, Alsaegh A,
El-Readi MZ, et al: Enhanced anti-cancer effects of oestrogen and
progesterone co-therapy against colorectal cancer in males. Front
Endocrinol (Lausanne). 13:9418342022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Refaat B, Aslam A, Idris S, Almalki AH,
Alkhaldi MY, Asiri HA, Almaimani RA, Mujalli A, Minshawi F, Alamri
SA, et al: Profiling estrogen, progesterone, and androgen receptors
in colorectal cancer in relation to gender, menopausal status,
clinical stage, and tumour sidedness. Front Endocrinol (Lausanne).
14:11872592023. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Campbell-Thompson M, Lynch IJ and Bhardwaj
B: Expression of estrogen receptor (ER) subtypes and ERbeta
isoforms in colon cancer. Cancer Res. 61:632–640. 2001.PubMed/NCBI
|
|
39
|
Maingi JW, Tang S, Liu S, Ngenya W and Bao
E: Targeting estrogen receptors in colorectal cancer. Mol Biol Rep.
47:4087–4091. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Herichová I, Jendrisková S, Pidíková P,
Kršková L, Olexová L, Morová M, Stebelová K and Štefánik P: Effect
of 17β-estradiol on the daily pattern of ACE2, ADAM17, TMPRSS2 and
estradiol receptor transcription in the lungs and colon of male
rats. PLoS One. 17:e02706092022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Weyant MJ, Carothers AM, Mahmoud NN,
Bradlow HL, Remotti H, Bilinski RT and Bertagnolli MM: Reciprocal
expression of ERalpha and ERbeta is associated with
estrogen-mediated modulation of intestinal tumorigenesis. Cancer
Res. 61:2547–2551. 2001.PubMed/NCBI
|
|
42
|
Giroux V, Lemay F, Bernatchez G,
Robitaille Y and Carrier JC: Estrogen receptor beta deficiency
enhances small intestinal tumorigenesis in ApcMin/+ mice. Int J
Cancer. 123:303–311. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Weige CC, Allred KF and Allred CD:
Estradiol alters cell growth in nonmalignant colonocytes and
reduces the formation of preneoplastic lesions in the colon. Cancer
Res. 69:9118–9124. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Song CH, Kim N, Lee SM, Nam RH, Choi SI,
Kang SR, Shin E, Lee DH, Lee HN and Surh YJ: Effects of
17β-estradiol on colorectal cancer development after
azoxymethane/dextran sulfate sodium treatment of ovariectomized
mice. Biochem Pharmacol. 164:139–151. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Son HJ, Sohn SH, Kim N, Lee HN, Lee SM,
Nam RH, Park JH, Song CH, Shin E, Na HY, et al: Effect of estradiol
in an Azoxymethane/Dextran sulfate Sodium-treated mouse model of
colorectal cancer: Implication for sex difference in colorectal
cancer development. Cancer Res Treat. 51:632–648. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hartman J, Edvardsson K, Lindberg K, Zhao
C, Williams C, Ström A and Gustafsson JA: Tumor repressive
functions of estrogen receptor beta in SW480 colon cancer cells.
Cancer Res. 69:6100–6106. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Uhlén M, Fagerberg L, Hallström BM,
Lindskog C, Oksvold P, Mardinoglu A, Sivertsson Å, Kampf C,
Sjöstedt E, Asplund A, et al: Proteomics. Tissue-based map of the
human proteome. Science. 347:12604192015. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Abancens M, Harvey BJ and McBryan J: GPER
agonist G1 prevents Wnt-induced JUN upregulation in HT29 colorectal
cancer cells. Int J Mol Sci. 23:125812022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Muller C, Chaney MF, Cohen JV, Garyantes
T, Lin JJ, LoRusso P, Mita AC, Mita MM, Natale C, Orloff MM, et al:
Phase 1b study of the novel first-in-class G protein-coupled
estrogen receptor (GPER) agonist, LNS8801, in combination with
pembrolizumab in patients with immune checkpoint inhibitor
(ICI)-relapsed and refractory solid malignancies and dose
escalation update. J Clinical Oncol. 40 (16_suppl):S2574. 2022.
View Article : Google Scholar
|
|
50
|
Hall KA and Filardo EJ: The G
Protein-coupled estrogen receptor (GPER): A critical therapeutic
target for cancer. Cells. 12:24602023. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Liu Q, Chen Z, Jiang G, Zhou Y, Yang X,
Huang H, Liu H, Du J and Wang H: Epigenetic down regulation of G
protein-coupled estrogen receptor (GPER) functions as a tumor
suppressor in colorectal cancer. Mol Cancer. 16:872017. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bühler M, Fahrländer J, Sauter A, Becker
M, Wistorf E, Steinfath M and Stolz A: GPER1 links estrogens to
centrosome amplification and chromosomal instability in human colon
cells. Life Sci Alliance. 6:e2022014992022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bustos V, Nolan ÁM, Nijhuis A, Harvey H,
Parker A, Poulsom R, McBryan J, Thomas W, Silver A and Harvey BJ:
GPER mediates differential effects of estrogen on colon cancer cell
proliferation and migration under normoxic and hypoxic conditions.
Oncotarget. 8:84258–84275. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Santolla MF, Lappano R, De Marco P, Pupo
M, Vivacqua A, Sisci D, Abonante S, Iacopetta D, Cappello AR, Dolce
V, et al: G protein-coupled estrogen receptor mediates the
up-regulation of fatty acid synthase induced by 17β-estradiol in
cancer cells and cancer-associated fibroblasts. J Biol Chem.
287:43234–43245. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Xie M, Liang JL, Huang HD, Wang MJ, Zhang
T and Yang XF: Low doses of nonylphenol promote growth of colon
cancer cells through activation of ERK1/2 via G Protein-coupled
receptor 30. Cancer Res Treat. 51:1620–1631. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Gilligan LC, Rahman HP, Hewitt AM, Sitch
AJ, Gondal A, Arvaniti A, Taylor AE, Read ML, Morton DG and Foster
PA: Estrogen activation by steroid sulfatase increases colorectal
cancer proliferation via GPER. J Clin Endocrinol Metab.
102:4435–4447. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Rouhimoghadam M, Lu AS, Salem AK and
Filardo EJ: Therapeutic perspectives on the modulation of G-protein
coupled estrogen receptor, GPER, Function. Front Endocrinol
(Lausanne). 11:5912172020. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Rodon J, Chaney M, Cohen J, Garyantes TK,
Ishizuka J, Lin JJ, Lorusso P, Mita A, Mita M, Muller C, et al: The
effect of LNS8801 in combination with pembrolizumab in patients
with treatment-refractory cutaneous melanoma. J Immuno Ther Cancer.
11 (Suppl 1):A6272023.
|
|
59
|
Shoushtari AN, Chaney MF, Cohen JV,
Garyantes T, Lin JJ, Ishizuka JJ, Mita AC, Mita MM, Muller C,
Natale C, et al: The effect of LNS8801 alone and in combination
with pembrolizumab in patients with metastatic uveal melanoma. J
Clin Oncol. 41 (16_Suppl):S9543. 2023. View Article : Google Scholar
|
|
60
|
Holm A, Grände PO, Ludueña RF, Olde B,
Prasad V, Leeb-Lundberg LM and Nilsson BO: The G protein-coupled
oestrogen receptor 1 agonist G-1 disrupts endothelial cell
microtubule structure in a receptor-independent manner. Mol Cell
Biochem. 366:239–249. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Wang C, Lv X, Jiang C and Davis JS: The
putative G-protein coupled estrogen receptor agonist G-1 suppresses
proliferation of ovarian and breast cancer cells in a
GPER-independent manner. Am J Transl Res. 4:390–402.
2012.PubMed/NCBI
|
|
62
|
Gui Y, Shi Z, Wang Z, Li JJ, Xu C, Tian R,
Song X, Walsh MP, Li D, Gao J, et al: The GPER agonist G-1 induces
mitotic arrest and apoptosis in human vascular smooth muscle cells
independent of GPER. J Cell Physiol. 230:885–895. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Mori T, Ito F, Matsushima H, Takaoka O,
Tanaka Y, Koshiba A, Kusuki I and Kitawaki J: G protein-coupled
estrogen receptor 1 agonist G-1 induces cell cycle arrest in the
mitotic phase, leading to apoptosis in endometriosis. Fertil
Steril. 103:1228–1235.e1. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Lv X, He C, Huang C, Hua G, Wang Z,
Remmenga SW, Rodabough KJ, Karpf AR, Dong J, Davis JS, et al: G-1
Inhibits breast cancer cell growth via targeting Colchicine-binding
site of tubulin to interfere with microtubule assembly. Mol Cancer
Ther. 16:1080–1091. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Torres-López L, Olivas-Aguirre M,
Villatoro-Gómez K and Dobrovinskaya O: The G-protein-coupled
estrogen receptor agonist G-1 inhibits proliferation and causes
apoptosis in leukemia cell lines of T lineage. Front Cell Dev Biol.
10:8114792022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Haupt S, Caramia F, Herschtal A, Soussi T,
Lozano G, Chen H, Liang H, Speed TP and Haupt Y: Identification of
cancer sex-disparity in the functional integrity of p53 and its X
chromosome network. Nat Commun. 10:53852019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Liu Y and Bodmer WF: Analysis of P53
mutations and their expression in 56 colorectal cancer cell lines.
Proc Natl Acad Sci USA. 103:976–981. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Schneider CA, Rasband WS and Eliceiri KW:
NIH Image to ImageJ: 25 years of image analysis. Nat Methods.
9:671–675. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Recchia AG, De Francesco EM, Vivacqua A,
Sisci D, Panno ML, Andò S and Maggiolini M: The G protein-coupled
receptor 30 is up-regulated by hypoxia-inducible factor-1alpha
(HIF-1alpha) in breast cancer cells and cardiomyocytes. J Biol
Chem. 286:10773–10782. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Jacenik D, Beswick EJ, Krajewska WM and
Prossnitz ER: G protein-coupled estrogen receptor in colon
function, immune regulation and carcinogenesis. World J
Gastroenterol. 25:4092–4104. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Foley EF, Jazaeri AA, Shupnik MA, Jazaeri
O and Rice LW: Selective loss of estrogen receptor beta in
malignant human colon. Cancer Res. 60:245–248. 2000.PubMed/NCBI
|
|
72
|
Jassam N, Bell SM, Speirs V and Quirke P:
Loss of expression of oestrogen receptor beta in colon cancer and
its association with Dukes' staging. Oncol Rep. 14:17–21.
2005.PubMed/NCBI
|
|
73
|
Mostafaie N, Kállay E, Sauerzapf E, Bonner
E, Kriwanek S, Cross HS, Huber KR and Krugluger W: Correlated
downregulation of estrogen receptor beta and the circadian clock
gene Per1 in human colorectal cancer. Mol Carcinog. 48:642–647.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Herichova I, Reis R, Hasakova K, Vician M
and Zeman M: Sex-dependent regulation of estrogen receptor beta in
human colorectal cancer tissue and its relationship with clock
genes and VEGF-A expression. Physiol Res. 68 (Suppl 3):S297–S305.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hasakova K, Vician M, Reis R, Zeman M and
Herichova I: Sex-dependent correlation between survival and
expression of genes related to the circadian oscillator in patients
with colorectal cancer. Chronobiol Int. 35:1423–1434. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Williams C, DiLeo A, Niv Y and Gustafsson
JÅ: Estrogen receptor beta as target for colorectal cancer
prevention. Cancer Lett. 372:48–56. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Ya G, Wang H, Ma Y, Hu A, Ma Y, Hu J and
Yu Y: Serum miR-129 functions as a biomarker for colorectal cancer
by targeting estrogen receptor (ER) β. Pharmazie. 72:107–112.
2017.PubMed/NCBI
|
|
78
|
Issa JP, Ottaviano YL, Celano P, Hamilton
SR, Davidson NE and Baylin SB: Methylation of the oestrogen
receptor CpG island links ageing and neoplasia in human colon. Nat
Genet. 7:536–540. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Jiang H, Teng R, Wang Q, Zhang X, Wang H,
Wang Z, Cao J and Teng L: Transcriptional analysis of estrogen
receptor alpha variant mRNAs in colorectal cancers and their
matched normal colorectal tissues. J Steroid Biochem Mol Biol.
112:20–24. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Topi G, Ghatak S, Satapathy SR, Ehrnström
R, Lydrup ML and Sjölander A: Combined estrogen alpha and beta
receptor expression has a prognostic significance for colorectal
cancer patients. Front Med (Lausanne). 9:7396202022. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Kennelly R, Kavanagh DO, Hogan AM and
Winter DC: Oestrogen and the colon: Potential mechanisms for cancer
prevention. Lancet Oncol. 94:385–391. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Barton M, Filardo EJ, Lolait SJ, Thomas P,
Maggiolini M and Prossnitz ER: Twenty years of the G
protein-coupled estrogen receptor GPER: Historical and personal
perspectives. J Steroid Biochem Mol Biol. 176:4–15. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Harvey BJ and Harvey HM: Sex differences
in colon cancer: Genomic and nongenomic signalling of oestrogen.
Genes (Basel). 14:22252023. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Nüssler NC, Reinbacher K, Shanny N,
Schirmeier A, Glanemann M, Neuhaus P, Nussler AK and Kirschner M:
Sex-specific differences in the expression levels of estrogen
receptor subtypes in colorectal cancer. Gend Med. 5:209–217. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Deli T, Orosz M and Jakab A: Hormone
replacement therapy in cancer survivors-review of the literature.
Pathol Oncol Res. 26:63–78. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Topi G, Satapathy SR, Dash P, Fred Mehrabi
S, Ehrnström R, Olsson R, Lydrup ML and Sjölander A:
Tumour-suppressive effect of oestrogen receptor β in colorectal
cancer patients, colon cancer cells, and a zebrafish model. J
Pathol. 251:297–309. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Tirado-Garibay AC, Falcón-Ruiz EA,
Ochoa-Zarzosa A and López-Meza JE: GPER: An estrogen receptor key
in metastasis and tumoral microenvironments. Int J Mol Sc.
24:149932023. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ambrosini G, Natale CA, Musi E, Garyantes
T and Schwartz GK: The GPER agonist LNS8801 induces mitotic arrest
and apoptosis in uveal melanoma cells. Cancer Res Commun.
3:540–547. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Filardo EJ, Quinn JA, Bland KI and
Frackelton AR Jr: Estrogen-induced activation of Erk-1 and Erk-2
requires the G protein-coupled receptor homolog, GPR30, and occurs
via trans-activation of the epidermal growth factor receptor
through release of HB-EGF. Mol Endocrinol. 14:1649–1660. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Hsu LH, Chu NM, Lin YF and Kao SH:
G-Protein coupled estrogen receptor in breast cancer. Int J Mol
Sci. 20:3062019. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Rubin Grandis J, Melhem MF, Gooding WE,
Day R, Holst VA, Wagener MM, Drenning SD and Tweardy DJ: Levels of
TGF-alpha and EGFR protein in head and neck squamous cell carcinoma
and patient survival. J Natl Cancer Inst. 90:824–832. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Uribe ML, Marrocco I and Yarden Y: EGFR in
cancer: Signaling mechanisms, drugs, and acquired resistance.
Cancers (Basel). 13:27482021. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Janani B, Vijayakumar M, Priya K, Kim JH,
Prabakaran DS, Shahid M, Al-Ghamdi S, Alsaidan M, Othman Bahakim N,
Hassan Abdelzaher M and Ramesh T: EGFR-based targeted therapy for
colorectal cancer-promises and challenges. Vaccines (Basel).
10:4992022. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Giannopoulou E, Antonacopoulou A, Floratou
K, Papavassiliou AG and Kalofonos HP: Dual targeting of EGFR and
HER-2 in colon cancer cell lines. Cancer Chemother Pharmacol.
63:973–981. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Yuan HH, Han Y, Bian WX, Liu L and Bai YX:
The effect of monoclonal antibody cetuximab (C225) in combination
with tyrosine kinase inhibitor gefitinib (ZD1839) on colon cancer
cell lines. Pathology. 44:547–551. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Palumbo I, Piattoni S, Valentini V, Marini
V, Contavalli P, Calzuola M, Vecchio FM, Cecchini D, Falzetti F and
Aristei C: Gefitinib enhances the effects of combined radiotherapy
and 5-fluorouracil in a colorectal cancer cell line. Int J
Colorectal Dis. 29:31–41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Chen X, Liu Y, Yang HW, Zhou S, Cheng C,
Zheng MW, Zhong L, Fu XY, Pan YL, Ma S, et al: SKLB-287, a novel
oral multikinase inhibitor of EGFR and VEGFR2, exhibits potent
antitumor activity in LoVo colorectal tumor model. Neoplasma.
61:514–22. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Chuang SC, Chen CH, Chou YS, Ho ML and
Chang JK: G Protein-coupled estrogen receptor mediates cell
proliferation through the cAMP/PKA/CREB pathway in murine bone
marrow mesenchymal stem cells. Int J Mol Sci. 21:64902020.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Berg KCG, Eide PW, Eilertsen IA,
Johannessen B, Bruun J, Danielsen SA, Bjørnslett M, Meza-Zepeda LA,
Eknæs M, Lind GE, et al: Multi-omics of 34 colorectal cancer cell
lines-a resource for biomedical studies. Mol Cancer. 16:1162017.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Moulder DE, Hatoum D, Tay E, Lin Y and
McGowan EM: The roles of p53 in mitochondrial dynamics and cancer
metabolism: The pendulum between survival and death in breast
cancer? Cancers (Basel). 10:1892018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Engeland K: Cell cycle regulation:
P53-p21-RB signaling. Cell Death Differ. 29:946–960. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Weißenborn C, Ignatov T, Ochel HJ, Costa
SD, Zenclussen AC, Ignatova Z and Ignatov A: GPER functions as a
tumor suppressor in triple-negative breast cancer cells. J Cancer
Res Clin Oncol. 140:713–23. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Rochette PJ, Bastien N, Lavoie J, Guérin
SL and Drouin R: SW480, a p53 double-mutant cell line retains
proficiency for some p53 functions. J Mol Biol. 352:44–57. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Berglind H, Pawitan Y, Kato S, Ishioka C
and Soussi T: Analysis of p53 mutation status in human cancer cell
lines: A paradigm for cell line cross-contamination. Cancer Biol
Ther. 7:699–708. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Hassin O, Nataraj NB, Shreberk-Shaked M,
Aylon Y, Yaeger R, Fontemaggi G, Mukherjee S, Maddalena M, Avioz A,
Iancu O, et al: Different hotspot p53 mutants exert distinct
phenotypes and predict outcome of colorectal cancer patients. Nat
Commun. 13:28002022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Leroy B, Girard L, Hollestelle A, Minna
JD, Gazdar AF and Soussi T: Analysis of TP53 mutation status in
human cancer cell lines: A reassessment. Hum Mutat. 35:756–765.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Berger C, Qian Y and Chen X: The
p53-estrogen receptor loop in cancer. Curr Mol Med. 13:1229–1240.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Chen YC, Young MJ, Chang HP, Liu CY, Lee
CC, Tseng YL, Wang YC, Chang WC and Hung JJ: Estradiol-mediated
inhibition of DNMT1 decreases p53 expression to induce
M2-macrophage polarization in lung cancer progression. Oncogenesis.
11:252022. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Weige CC, Allred KF, Armstrong CM and
Allred CD: P53 mediates estradiol induced activation of apoptosis
and DNA repair in non-malignant colonocytes. J Steroid Biochem Mol
Biol. 128:113–120. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Qin C, Nguyen T, Stewart J, Samudio I,
Burghardt R and Safe S: Estrogen up-regulation of p53 gene
expression in MCF-7 breast cancer cells is mediated by calmodulin
kinase IV-dependent activation of a nuclear factor
kappaB/CCAAT-binding transcription factor-1 complex. Mol
Endocrinol. 16:1793–809. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Berger CE, Qian Y, Liu G, Chen H and Chen
X: p53, a target of estrogen receptor (ER) α, modulates DNA
damage-induced growth suppression in ER-positive breast cancer
cells. J Biol Chem. 287:30117–30127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Swetzig WM, Wang J and Das GM: Estrogen
receptor alpha (ERα/ESR1) mediates the p53-independent
overexpression of MDM4/MDMX and MDM2 in human breast cancer.
Oncotarget. 7:16049–16069. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mancini F, Giorgini L, Teveroni E,
Pontecorvi A and Moretti F: Role of sex in the therapeutic
targeting of p53 circuitry. Front Oncol. 11:6989462021. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Konduri SD, Medisetty R, Liu W,
Kaipparettu BA, Srivastava P, Brauch H, Fritz P, Swetzig WM,
Gardner AE, Khan SA, et al: Mechanisms of estrogen receptor
antagonism toward p53 and its implications in breast cancer
therapeutic response and stem cell regulation. Proc Natl Acad Sci
USA. 107:15081–6. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Lu W and Katzenellenbogen BS: Estrogen
receptor-β modulation of the ERα-p53 loop regulating gene
expression, proliferation, and apoptosis in breast cancer. Horm
Cancer. 8:230–242. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Cerami E, Gao J, Dogrusoz U, Gross BE,
Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, et
al: The cBio cancer genomics portal: An open platform for exploring
multidimensional cancer genomics data. Cancer Discov. 2:401–404.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Bilancio A, Bontempo P, Di Donato M, Conte
M, Giovannelli P, Altucci L, Migliaccio A and Castoria G: Bisphenol
A induces cell cycle arrest in primary and prostate cancer cells
through EGFR/ERK/p53 signaling pathway activation. Oncotarget.
8:115620–115631. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wei W, Chen ZJ, Zhang KS, Yang XL, Wu YM,
Chen XH, Huang HB, Liu HL, Cai SH, Du J, et al: The activation of G
protein-coupled receptor 30 (GPR30) inhibits proliferation of
estrogen receptor-negative breast cancer cells in vitro and in
vivo. Cell Death Dis. 5:e14282014. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Morelli E, Hunter ZR, Fulciniti M, Gullà
A, Perrotta ID, Zuccalà V, Federico C, Juli G, Manzoni M, Ronchetti
D, et al: Therapeutic activation of G protein-coupled estrogen
receptor 1 in Waldenström Macroglobulinemia. Exp Hematol Onco.
11:542022. View Article : Google Scholar
|
|
120
|
Giebler HA, Lemasson I and Nyborg JK: p53
recruitment of CREB binding protein mediated through phosphorylated
CREB: A novel pathway of tumor suppressor regulation. Mol Cell
Biol. 20:4849–4858. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Okoshi R, Ando K, Suenaga Y, Sang M, Kubo
N, Kizaki H, Nakagawara A and Ozaki T: Transcriptional regulation
of tumor suppressor p53 by cAMP-responsive element-binding
protein/AMP-activated protein kinase complex in response to glucose
deprivation. Genes Cells. 14:1429–1440. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Lee CW, Ferreon JC, Ferreon AC, Arai M and
Wright PE: Graded enhancement of p53 binding to CREB-binding
protein (CBP) by multisite phosphorylation. Proc Natl Acad Sci USA.
107:19290–19295. 2010. View Article : Google Scholar : PubMed/NCBI
|