Introduction
According to recent Global Cancer Observatory data,
cervical cancer ranked third in terms of prevalence among women,
with an estimated 661,021 newly diagnosed cases and 348,189 deaths
globally in 2022 (1). Cervical
cancer rates remain significantly high in nations lacking
population-based cervical cancer screening initiatives and
contribute substantially to cancer-related deaths and morbidity
(2).
The staging of cervical cancer has traditionally
been performed at the clinical level. However, since the
introduction of the 2018 International Federation of Gynecology and
Obstetrics (FIGO) staging criteria, surgical and radiological
evaluations have been incorporated into the process (3–5).
Surgical and radiological data provide key information that can
influence treatment (5). According
to the FIGO criteria, stage IB1 disease involves an invasive
carcinoma that invades the stroma to a depth of >5 mm and is ≤2
cm in its greatest dimension, stage IB2 disease involves an
invasive carcinoma that is >2 cm but ≤4 cm in its greatest
dimension and stage IB3 disease involves an invasive carcinoma that
is >4 cm in its greatest dimension (5). Patients diagnosed with stage IB2
cervical cancer typically undergo radical hysterectomy and pelvic
with or without paraaortic lymphadenectomy as standard treatment.
This surgical procedure involves the excision of a substantial
quantity of vaginal tissue, extending up to the upper half, along
with parametrial tissue (6).
Adjuvant therapy is administered in accordance with
the presence of histopathological risk factors (7). Lymph node involvement, positive
surgical margins and parametrial invasion are considered to be
significant risk factors for tumour recurrence (8). It is generally accepted that adjuvant
therapy should be part of the standard care provided to patients
who have these risk factors (8–10).
Nevertheless, there is ongoing debate regarding the
‘intermediate-risk’ factors. Intermediate-risk factors were
established as tumour dimensions of 2–3.99 cm associated with
lymphovascular space invasion (LVSI), deep stromal invasion or
tumour dimensions of ≥4 cm (11).
It is currently unknown whether radical surgery alone or combined
with adjuvant (chemo)radiation is a more effective treatment for
FIGO 2018 stage IB2 cervical cancer (7,9,11,12).
In a previous study, patients with FIGO 2018 stage IB2 who
underwent radical hysterectomy and lymphadenectomy had an 83.3%
5-year overall survival (OS) rate (13). In addition, in patients with FIGO
2018 stage IB2-IIA2 cervical cancer with intermediate-risk factors,
radical surgery alone achieved similar disease-free survival (DFS)
and OS rates as combining radical surgery with adjuvant
(chemo)radiotherapy (14).
The FIGO 2018 staging system currently lacks
sufficient data to accurately predict the overall oncological
outcomes for patients with stage IB2 cancer, particularly those who
have undergone radical surgery. The aim of the present study was to
examine the effect of intermediate-risk factors on the oncological
prognosis of patients with stage FIGO 2018 IB2 cervical cancer who
did not receive adjuvant therapy.
Materials and methods
Patient cohort
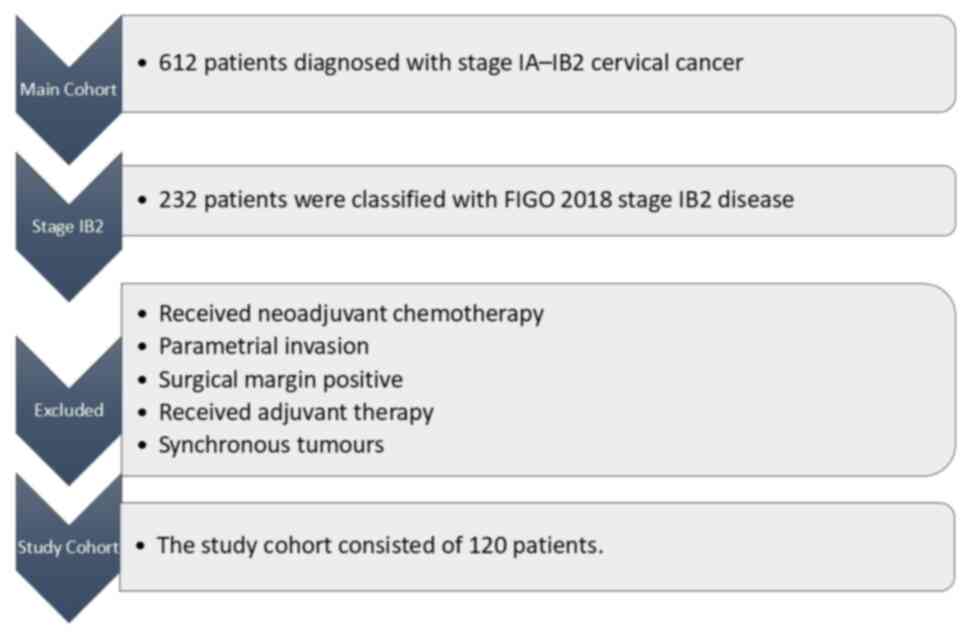
The present multicentric retrospective study
enrolled 612 patients diagnosed with early-stage cervical cancer at
seven tertiary gynaecological oncology centres between 1993 and
2023. The seven gynaecological oncology centres were Ankara Bilkent
City Hospital (Ankara, Turkiye), Ankara Etlik City Hospital
(Ankara, Turkiye), Antalya Training and Research Hospital (Antalya,
Turkiye), Etlik Zubeyde Hanim Women's Health Training and Research
Hospital (Ankara, Turkiye), Hacettepe University (Ankara, Turkiye),
Istinye University (Istanbul, Turkiye) and Bahcesehir University
(Istanbul, Turkiye). A total of 232 patients were classified with
FIGO 2018 stage IB2 disease. Patients other than those with FIGO
2018 stage IB2 disease, received neoadjuvant chemotherapy, surgical
margin positive, who had parametrial invasion, received adjuvant
external beam radiotherapy and brachytherapy, received adjuvant
chemoradiotherapy, received adjuvant chemotherapy and had
synchronous tumours were excluded from the present study. The
present study cohort consisted of 120 patients who had undergone
radical hysterectomy and lymphadenectomy (Fig. 1). The clinicopathological data of
the patients were acquired from their patient files or the
hospital's electronic database. Ethical approval in accordance with
the Declaration of Helsinki was obtained from the Institutional
Review Board of Ankara Bilkent City Hospital (approval no.
E2-23-4600; Ankara, Turkiye). Approval was obtained from all
institutions participating in the study. All patients provided
informed consent for the institution to utilise their clinical
data. Gynaecological oncologists were the main professionals
responsible for conducting the surgical procedures.
Tumour specimen analysis
The specimens obtained during the surgical
procedures were examined by gynaecological pathologists. The tumour
size was taken as the maximum diameter of the tumour listed in the
final pathology report. The depth of cervical stromal invasion was
not determined. Tumours that infiltrated >50% of the full
thickness of the cervical stroma are referred to here as showing
‘deep cervical stromal invasion’. LVSI was defined by the presence
of tumour cells or clusters attached to the walls of blood or
lymphatic vessels, as observed using haematoxylin and eosin
staining of pathological sections that included both the tumour and
surrounding healthy tissue. The presence of surgical border
involvement as an indicator of tumour positivity was deemed
acceptable when the tumour was detected within a 5 mm margin of the
pathological specimen. The detection of tumours in other areas of
the vaginal region was referred to as microscopic vaginal
involvement. Uterine invasion was characterised by the extension of
the disease into the endometrium and/or myometrium, beyond the
internal cervical ostium. The histopathological evaluations were
performed following the criteria set by the World Health
Organization in 2014 (15).
Statistical analysis
SPSS statistical software (version 22.0; SPSS Inc.)
was used for the statistical analyses. DFS was calculated as the
time between the surgical procedure and the identification of
disease recurrence or the date of the most recent follow-up. OS was
defined as the time between the initial surgical procedure and
subsequent follow-up visits or death due to the disease. The
Kaplan-Meier method was utilised to determine the survival curves,
and the curves were compared utilising the log-rank test. P<0.05
was considered to indicate a statistically significant
difference.
Results
The present study involved 120 patients with a
median age of 50 years (range, 26–76 years). The median tumour size
was 30 mm (range, 21–40 mm) and the median lymph node removal count
was 45 (range, 14–113). In terms of the tumour types found, 89
(74.2%) patients had squamous cell cancer, 18 (15.0%) had
adenocarcinoma, 2 (1.7%) had a mixed type tumour consisting of
squamous cell cancer and adenocarcinoma and 11 (9.1%) had other
types of tumours (adenosquamous cancer and glassy cell cancer). All
patients presented with negative surgical border involvement. LVSI
was found in 43 (35.8%) patients, while microscopic vaginal
involvement was present in 5 (4.2%) patients. Deep cervical stromal
invasion was found in 68 (56.7%) patients. Uterine invasion was
present in 7 (5.8%) patients. The present study cohort
characteristics are shown in Table
I.
 | Table I.Characteristics of present study group
(n=120). |
Table I.
Characteristics of present study group
(n=120).
| Characteristics | Values |
|---|
| Age at initial
diagnosis, years |
|
| Mean ±
SD | 50.9±10.6 |
| Median,
(range) | 50 (26–76) |
| Tumor size, mm |
|
| Mean ±
SD | 31.3±5.5 |
| Median,
(range) | 30 (21–40) |
| No. of removed lymph
nodes |
|
| Mean ±
SD | 47±18.6 |
| Median,
(range) | 45 (14–113) |
| Tumor
typea, n (%) |
|
|
Squamous cell cancer | 89 (74.2) |
|
Adenocarcinoma | 18 (15.0) |
| Mixed
typeb | 2 (1.7) |
|
Othersc | 11 (9.1) |
| Microscopic vaginal
involvement, n (%) |
|
|
Negative | 115 (95.8) |
|
Positive | 5 (4.2) |
| Lymphovascular
space invasion, n (%) |
|
|
Negative | 69 (57.5) |
|
Positive | 43 (35.8) |
| Not
reported | 8 (6.7) |
| Depth of cervical
stromal invasion, n (%) |
|
|
≤50% | 48 (40.0) |
|
>50% | 68 (56.7) |
| Not
reported | 4 (3.3) |
| Uterine invasion, n
(%) |
|
|
Negative | 112 (93.3) |
|
Positive | 7 (5.8) |
| Not
reported | 1 (0.8) |
| Ovarian
transposition, n (%) |
|
| Not
performed | 88 (73.3) |
|
Performedd | 32 (26.7) |
| Ovarian metastasis,
n (%) |
|
|
Negativee | 91 (75.8) |
|
Positive | 0 (0.0) |
The duration of patient follow-up varied from 1 to
246 months and the median was 36 months. Recurrence was observed
between 9–42 months in 6 patients (5%; Table II). In 3 (50%) of the patients with
recurrence, the disease was located at pelvic sites and in the
other 3 (50%) patients, the disease was located at extra-pelvic
with or without pelvic sites. Of the 6 patients with recurrence, 1
patient (0.8%) succumbed to the disease. The 3-year OS rate was 99%
and the 3-year DFS rate was 94%. Overall, 1 patient had microscopic
vaginal involvement. LVSI was observed in 4 patients. All patients
with recurrence had deep cervical stromal invasion.
 | Table II.Features of 6 patients with
recurrence. |
Table II.
Features of 6 patients with
recurrence.
| Patient no. | Age, years | Tumor type | Tumor size, mm | Microscopic vaginal
involvement | Cervical stromal
invasion, % | Spread of
endometrium | Over
transposition | Lymphovascular
space invasion | Recurrence time,
months | Recurrence
site | Succumbed to
disease |
|---|
| 1 | 76 | SCC | 22 | + | >50 | - | - | - | 42 | Pelvic | No |
| 2 | 42 | SCC | 30 | - | >50 | - | + | + | 14 |
Extra-pelvica | Yes |
| 3 | 70 | SCC | 35 | - | >50 | - | - | + | 9 |
Extra-pelvicb | No |
| 4 | 41 | SCC | 40 | - | >50 | - | + | + | 12 | Pelvic | No |
| 5 | 41 | SCC | 40 | - | >50 | - | + | - | 28 |
Extra-pelvicc | No |
| 6 | 58 | AC | 40 | - | >50 | + | - | + | 15 | Pelvic | No |
The patient and clinicopathological factors analysed
in relation to 3-year DFS are shown in Table III. Patient age, histopathology,
tumour size, vaginal metastasis, uterine invasion, LVSI, number of
total lymph nodes removed and bilateral salpingo-oophorectomy were
not statistically significantly associated with 3-year DFS. In the
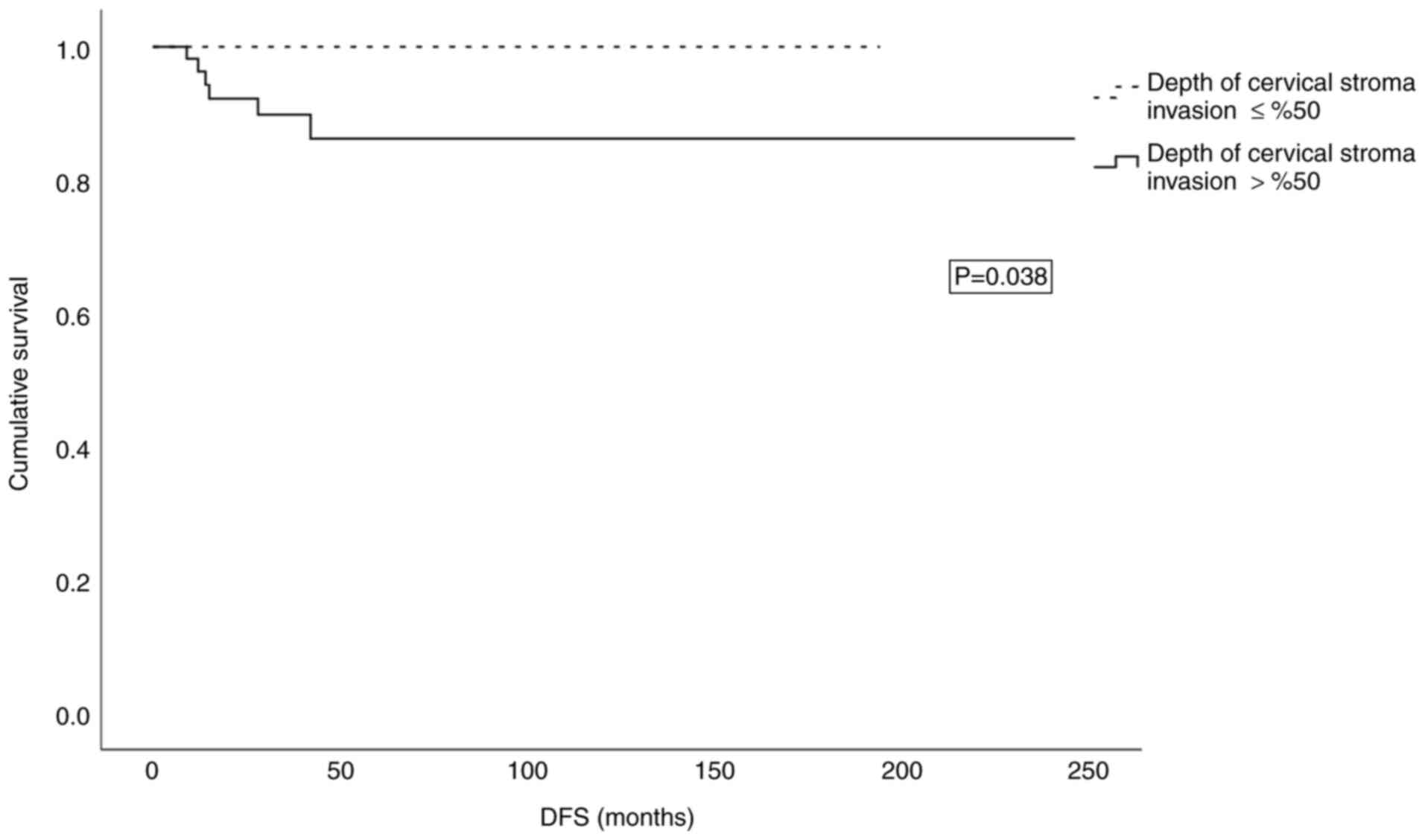
present study, deep cervical stromal invasion was determined as
>50% of stromal invasion. Deep cervical stromal invasion was
significantly associated with 3-year DFS (P=0.038; Fig. 2).
 | Table III.Factors related to 3-year DFS in the
study cohort (n=120). |
Table III.
Factors related to 3-year DFS in the
study cohort (n=120).
| Factors | 3-year DFS, % | P-value |
|---|
| Median age,
years |
| 0.967 |
|
≤50 | 93 |
|
|
>50 | 95 |
|
|
Histopathologya |
| 0.825 |
|
Squamous cell | 94 |
|
|
Non-squamous cell cancer | 94 |
|
| Median tumor size,
mm |
| 0.169 |
|
≤30 | 98 |
|
|
>30 | 88 |
|
| Vaginal
metastasis |
| 0.122 |
|
Negative | 94 |
|
|
Positive | 67 |
|
| Uterine
invasion |
| 0.096 |
|
Negative | 95 |
|
|
Positive | 67 |
|
| Lymphovascular
space invasion |
| 0.141 |
|
Negative | 98 |
|
|
Positive | 88 |
|
| Depth of cervical
stromal invasion, % |
| 0.038 |
|
≤50 | 100 |
|
|
>50 | 90 |
|
| Median no. of total
lymph nodes removed |
| 0.200 |
|
≤45 | 91 |
|
|
>45 | 96 |
|
| Bilateral
salpingo-oophorectomy |
| 0.132 |
| Not
performed | 85 |
|
|
Performed | 97 |
|
Discussion
The survival of patients with cervical carcinoma is
influenced by several prognostic factors: Stage, tumour volume,
depth of cervical stromal invasion, LVSI, lymph node involvement,
parametrium involvement and surgical margin involvement (11,16).
The present study involved 120 patients with FIGO 2018 stage IB2
cervical cancer who did not have any high-risk prognostic factors
and did not receive any adjuvant treatment. For the present patient
group, the mean duration of follow-up was 36 months, the 3-year DFS
rate was 94% and the recurrence rate was 5%. Deep cervical stromal
invasion was present in 56.7% of the patients and was found to be
statistically significantly related to DFS in this group.
It is recommended that patients with early-stage
cervical cancer who have had primary surgery and whose risk of
disease recurrence is established by the final pathology report
receive adjuvant treatment (17).
Patients are considered to be at high-risk of recurrence when the
pathological findings show that the parametrium is microscopically
involved, there is involvement of the pelvic lymph nodes and the
surgical margins are positive (17). For patients at intermediate-risk,
the Sedlis criteria are employed to classify the disease (11). A comprehensive retrospective
analysis of 861 patients with intermediate-risk stage IB1-IIA2
disease reported that there were no substantial disparities in DFS
and disease-specific survival between groups that received adjuvant
therapy and a group that did not receive adjuvant therapy (18). In a previous study with 765 patients
with intermediate-risk FIGO 2018 stage IB disease, adjuvant
radiotherapy with or without ± chemotherapy administered after
radical hysterectomy and pelvic lymphadenectomy did not provide a
survival advantage (19).
Kissel et al (20) conducted a study with 145 patients
with FIGO 2018 IB2 cervical cancer who had undergone radical
hysterectomy with lymph node staging and found that the 5-year DFS
rate for these patients was 74.4%. It was also found that the risk
of recurrence increased when the depth of stromal invasion was
>10 mm. In the present study, the 3-year DFS rate was 94% and
the presence of deep cervical stromal invasion was significantly
associated with DFS. Deep cervical stromal invasion was present in
all cases of recurrence. The present study group did not include
high-risk patients; thus, the present study differs from Kissel
et al (20) study in terms
of DFS. Chen et al (21)
included 4,065 patients with 2018 FIGO stages IB1, IB2 and IIA1
cervical cancer in their study. The authors found deep cervical
stromal invasion was an independent prognostic factor for DFS. This
study included patients with stage IB1, IB2 and IIA1 cervical
cancer, but the present study included only patients with stage
IB2. By contrast, DFS was determined by deep cervical stromal
invasion, which was similar to the present research. Zhu et
al (22) included 3,298
patients with cervical cancer undergoing radical hysterectomy. It
found, similar to the present study, that DFS was independently
associated with the deep cervical stromal invasion; the authors
also demonstrated that postoperative radiotherapy is an independent
prognostic factor for DFS (22).
Their findings indicated that extra-pelvic recurrence occurred in
the majority of patients exhibiting full-thickness cervical stromal
invasion following radical surgery. Postoperative radiotherapy in
patients with full-thickness cervical stromal invasion may reduce
recurrence and enhance survival, which suggests that this
population may be suitable for adjuvant radiation therapy (22). The depth of cervical stromal
invasion correlated with survival and was proportional to
postoperative adjuvant therapy (23–25).
Li et al (26) conducted a
study comparing the DFS of postoperative adjuvant chemotherapy and
adjuvant radiation in patients with deep cervical stromal invasion.
Their findings indicated that patients demonstrated worse responses
to chemotherapy compared with that radiotherapy, and those with
full-thickness invasion had an increased risk of recurrence. Moon
et al (27) found that
postoperative radiotherapy may improve DFS in patients with FIGO
IB-IIA cervical carcinoma with cervical stromal invasion compared
with no adjuvant treatment.
In terms of the location at which cervix cancer
recurs, significant variation has been observed. In a previous
study that only included patients with FIGO 2018 stage IB2 cervical
cancer, recurrence occurred in 14.4% of the patients; 33% of the
recurrences were in the central pelvic area, 33% were nodal and 33%
were distant metastases (20). In
another study conducted with 274 patients with FIGO 2018 stage
IB2-IIA2 cervical cancer, 67.4% of the patients had recurrences in
the pelvic region and 14% of the patients had a recurrence in
another location (14). In the
present study, 5% of the patients experienced recurrence, and half
of those had recurrence at extra-pelvic with or without pelvic
sites.
The main limitation of the present study was its
retrospective design. Cervical cancer has been classified as a
human papillomavirus-based disease since 2020 (28). Since the present study was
retrospective, this new classification was not used and a central
pathological review was not conducted. The factors that determined
the DFS could not be evaluated in a multivariate analysis, as there
were only 6 patients with recurrence. The present study's
advantages are its multicentre design and the sample size of
participants. Gynaecological oncologists performed all the surgical
procedures and specialised gynaecological pathologists evaluated
all the surgical specimens.
In conclusion, patients with FIGO 2018 stage IB2
disease who did not receive adjuvant therapy were found to have a
3-year DFS rate of 94%. Deep cervical stromal invasion was found to
be associated with recurrence. Therefore, the presence of deep
cervical stromal invasion may be a significant indicator of the
need for adjuvant therapy in these patients.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study are not
publicly available due to privacy or ethical restrictions but may
be requested from the corresponding author.
Authors' contributions
AAT designed the study, drafted the manuscript and
interpreted the data. OO, OA, AA, AK and VE acquired the data. FK,
BE, FC and HEKY performed formal data analysis. OA and OO confirm
the authenticity of all the raw data. CC, DY, CK and IS interpreted
the data. GKC, NB, DB and IU were responsible for the design of the
study. TTo, VK, AK, TTa, OMT, YU, and NO interpreted the data and
reviewed the study critically for important intellectual content.
TTu analyzed and interpreted the data, critically reviewing it for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study approved from Ankara Bilkent City
Hospital institutional review board (approval no. E2-23-4600;
Ankara, Turkiye). Approval was obtained from all institutions
participating in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Dr. Abdurrahman Alp Tokalioglu
(ORCID:0000-0002-1776-2744), Dr. Okan Oktar
(ORCID:0000-0002-9696-7886), Dr. Okan Aytekin
(ORCID:0000-0002-6430-4607), Dr. Aysun Alci
(ORCID:0000-0002-7912-7375), Dr. Alper Kahraman
(ORCID:0000-0002-1689-2782), Dr. Volkan Ege
(ORCID:0000-0002-4056-9037), Dr. Fatih Kilic (ORCID:
0000-0002-7333-4883), Dr. Burak Ersak (ORCID: 0000-0003-3301-062X),
Dr. Fatih Celik (ORCID: 0000-0002-9523-180X), Dr. Hande Esra Koca
Yildirim (ORCID: 0000-0002-3715-9424), Dr. Caner Cakir (ORCID:
0000-0003-2559-9104), Dr. Dilek Yuksel (ORCID:
0000-0002-2366-8412), Dr. Cigdem Kilic (ORCID:
0000-0002-4433-8068), Dr. Ilker Selcuk (ORCID:
0000-0003-0499-5722), Dr. Gunsu Kimyon Comert (ORCID:
0000-0003-0178-4196), Professor Nurettin Boran (ORCID:
0000-0002-0367-5551), Dr. Derman Basaran (ORCID:
0000-0002-2689-1417), Professor Isin Ureyen (ORCID:
0000-0002-3491-4682), Professor Tayfun Toptas (ORCID:
0000-0002-6706-6915), Professor Vakkas Korkmaz (ORCID:
0000-0001-8895-6864) Professor Alper Karalok (ORCID:
0000-0002-0059-8773), Professor Tolga Tasci (ORCID:
0000-0001-8645-4385), Professor Ozlem Moraloglu Tekin (ORCID:
0000-0001-8167-3837), Professor Yaprak Ustun (ORCID:
0000-0002-1011-3848), Professor Nejat Ozgul (ORCID:
0000-0002-4257-9431), Professor Taner Turan (ORCID:
0000-0001-8120-1143).
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bhatla N, Aoki D, Sharma DN and
Sankaranarayanan R: Cancer of the cervix uteri. Int J Gynecol
Obstet. 143:22–36. 2018. View Article : Google Scholar
|
|
4
|
Benedet J, Pecorelli S, Ngan H and Hacker
N: Staging classifications and clinical practice guidelines for
gynaecological cancers. Int J Gynecol Obstet. 70:207–312. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pecorelli S, Zigliani L and Odicino F:
Revised FIGO staging for carcinoma of the cervix. Int J Gynecol
Obstet. 105:107–108. 2009. View Article : Google Scholar
|
|
6
|
Querleu D, Cibula D and Abu-Rustum NR:
2017 update on the Querleu-Morrow classification of radical
hysterectomy. Ann Surg Oncol. 24:3406–3412. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Cibula D, Pötter R, Planchamp F,
Avall-Lundqvist E, Fischerova D, Haie-Meder C, Köhler C, Landoni F,
Lax S, Lindegaard JC, et al: The European Society of Gynaecological
Oncology/European Society for Radiotherapy and Oncology/European
Society of Pathology guidelines for the management of patients with
cervical cancer. Virchows Arch. 472:919–936. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Peters III WA, Liu P, Barrett II RJ, Stock
RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr and
Alberts DS: Concurrent chemotherapy and pelvic radiation therapy
compared with pelvic radiation therapy alone as adjuvant therapy
after radical surgery in high-risk early-stage cancer of the
cervix. J Clin Oncol. 18:1606–1613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dostalek L, Åvall-Lundqvist E, Creutzberg
CL, Kurdiani D, Ponce J, Dostalkova I and Cibula D: ESGO survey on
current practice in the management of cervical cancer. Int J
Gynecol Cancer. 28:1226–1231. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Monk BJ, Wang J, Im S, Stock RJ, Peters WA
III, Liu PY, Barrett RJ II, Berek JS, Souhami L, Grigsby PW, et al:
Rethinking the use of radiation and chemotherapy after radical
hysterectomy: A clinical-pathologic analysis of a gynecologic
oncology Group/Southwest oncology Group/Radiation therapy oncology
group trial. Gynecol Oncol. 96:721–728. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sedlis A, Bundy BN, Rotman MZ, Lentz SS,
Muderspach LI and Zaino RJ: A randomized trial of pelvic radiation
therapy versus no further therapy in selected patients with stage
IB carcinoma of the cervix after radical hysterectomy and pelvic
lymphadenectomy: A gynecologic oncology group study. Gynecol Oncol.
73:177–183. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cibula D: Early-stage
intermediate-risk-the group with the most heterogenous management
among patients with cervical cancer. Int J Gynecol Cancer.
32:1227–1228. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wright JD, Matsuo K, Huang Y, Tergas AI,
Hou JY, Khoury-Collado F, St Clair CM, Ananth CV, Neugut AI and
Hershman DL: Prognostic performance of the 2018 International
Federation of Gynecology and Obstetrics cervical cancer staging
guidelines. Obstet Gynecol. 134:49–57. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cibula D, Akilli H, Jarkovsky J, van
Lonkhuijzen L, Scambia G, Meydanli MM, Ortiz DI, Falconer H,
Abu-Rustum NR, Odetto D, et al: Role of adjuvant therapy in
intermediate-risk cervical cancer patients-Subanalyses of the SCCAN
study. Gynecol Oncol. 170:195–202. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kurman RJ, Carcangiu ML and Herrington CS:
WHO Classification of Tumours of Female Reproductive Organs. 4th
Edition. IARC Publications; Lyon: 2014
|
|
16
|
Rotman M, Sedlis A, Piedmonte MR, Bundy B,
Lentz SS, Muderspach LI and Zaino RJ: A phase III randomized trial
of postoperative pelvic irradiation in Stage IB cervical carcinoma
with poor prognostic features: Follow-up of a gynecologic oncology
group study. Int J Radiat Oncol Biol Phys. 65:169–176. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peters WA III, Liu P, Barrett II RJ, Stock
RJ, Monk BJ, Berek JS, Souhami L, Grigsby P, Gordon W Jr and
Alberts DS: Concurrent chemotherapy and pelvic radiation therapy
compared with pelvic radiation therapy alone as adjuvant therapy
after radical surgery in high-risk early-stage cancer of the
cervix. J Clin Oncol. 18:1606–1613. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cao L, Wen H, Feng Z, Han X, Zhu J and Wu
X: Role of adjuvant therapy after radical hysterectomy in
intermediate-risk, early-stage cervical cancer. Int J Gynecol
Cancer. 31:52–58. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nasioudis D, Latif NA, Giuntoli II RL,
Haggerty AF, Cory L, Kim SH, Morgan MA and Ko EM: Role of adjuvant
radiation therapy after radical hysterectomy in patients with stage
IB cervical carcinoma and intermediate risk factors. Int J Gynecol
Cancer. 31:829–834. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kissel M, Balaya V, Guani B, Magaud L,
Mathevet P and Lécuru F; SENTICOL group, : Impact of preoperative
brachytherapy followed by radical hysterectomy in stage IB2 (FIGO
2018) cervical cancer: An analysis of SENTICOL I–II trials. Gynecol
Oncol. 170:309–316. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen X, Duan H, Liu P, Lin L, Ni Y, Li D,
Dai E, Zhan X, Li P, Huo Z, et al: Development and validation of a
prognostic nomogram for 2018 FIGO stages IB1, IB2, and IIA1
cervical cancer: A large multicenter study. Ann Transl Med.
10:1212022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhu J, Cao L, Wen H, Bi R, Wu X and Ke G:
The clinical and prognostic implication of deep stromal invasion in
cervical cancer patients undergoing radical hysterectomy. J Cancer.
11:7368–7377. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim SI, Cho JH, Seol A, Kim YI, Lee M, Kim
HS, Chung HH, Kim JW, Park NH and Song YS: Comparison of survival
outcomes between minimally invasive surgery and conventional open
surgery for radical hysterectomy as primary treatment in patients
with stage IB1-IIA2 cervical cancer. Gynecol Oncol. 153:3–12. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ryu S, Kim M, Nam B, Lee TS, Song ES, Park
CY, Kim JW, Kim YB and Ryu HS: Intermediate-risk grouping of
cervical cancer patients treated with radical hysterectomy: A
Korean Gynecologic Oncology Group study. Br J Cancer. 110:278–285.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Takeshima N, Umayahara K, Fujiwara K,
Hirai Y, Takizawa K and Hasumi K: Treatment results of adjuvant
chemotherapy after radical hysterectomy for intermediate-and
high-risk stage IB-IIA cervical cancer. Gynecol Oncol. 103:618–622.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Li L, Song X, Liu R, Li N, Zhang Y, Cheng
Y, Chao H and Wang L: Chemotherapy versus radiotherapy for FIGO
stages IB1 and IIA1 cervical carcinoma patients with postoperative
isolated deep stromal invasion: A retrospective study. BMC Cancer.
16:4032016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Moon SH, Wu HG, Ha SW, Lee HP, Kang SB,
Song YS, Park NH, Kim JW, Park IA and Kim BH: Isolated
full-thickness cervical stromal invasion warrants post-hysterectomy
pelvic radiotherapy in FIGO stages IB-IIA uterine cervical
carcinoma. Gynecol Oncol. 104:152–157. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herrington CS: WHO Classification of
Tumours Female Genital Tumours. IARC Publications; Lyon: 2020
|