Introduction
Lung cancer is one of the most frequently diagnosed
cancers and the leading cause of cancer-related deaths worldwide,
with an estimated 2 million new cases and 1.76 million associated
deaths per year (1). As the most
prevalent type of lung cancer, non-small cell lung cancer (NSCLC)
is characterized by high morbidity and mortality rates. Presently,
the arsenal of treatment strategies encompasses surgical resection,
chemotherapy, targeted therapy and radiotherapy. However, despite
these options, the prognosis remains distressingly poor with a low
5-year survival rate (2).
The treatment for stage I or II NSCLC is surgical
resection of the tumor, with adjuvant therapy. By contrast, when
the disease advances to stage III or IV, the treatment shifts
towards chemotherapy or radiotherapy (3). Stage IIIC (TNM-8) NSCLC is an advanced
type of lung cancer (4), with a
5-year survival rate of ~13%. Treatments at this stage often
encompass a multidisciplinary approach, including surgery,
radiation therapy, chemotherapy, targeted therapy and immunotherapy
(5). The identification of
epidermal growth factor receptor (EGFR) gene mutations in up to 50%
of patients with lung adenocarcinoma, and the subsequent
development of highly effective EGFR tyrosine kinase inhibitors
(TKIs), have revolutionized the treatment of this malignancy
(6,7). Targeted therapy is one of the
important treatment modalities for advanced NSCLC (8). Although stage IIIC NSCLC is typically
deemed unresectable, the potential for conversion surgery after
targeted therapy offers hope for improved patient survival
(9). The purpose of this study is
to share the clinical experience of lung cancer targeted therapy
with clinical practitioners through the form of a case report,
thereby enhancing their understanding and application of targeted
therapy in lung cancer treatment.
Case report
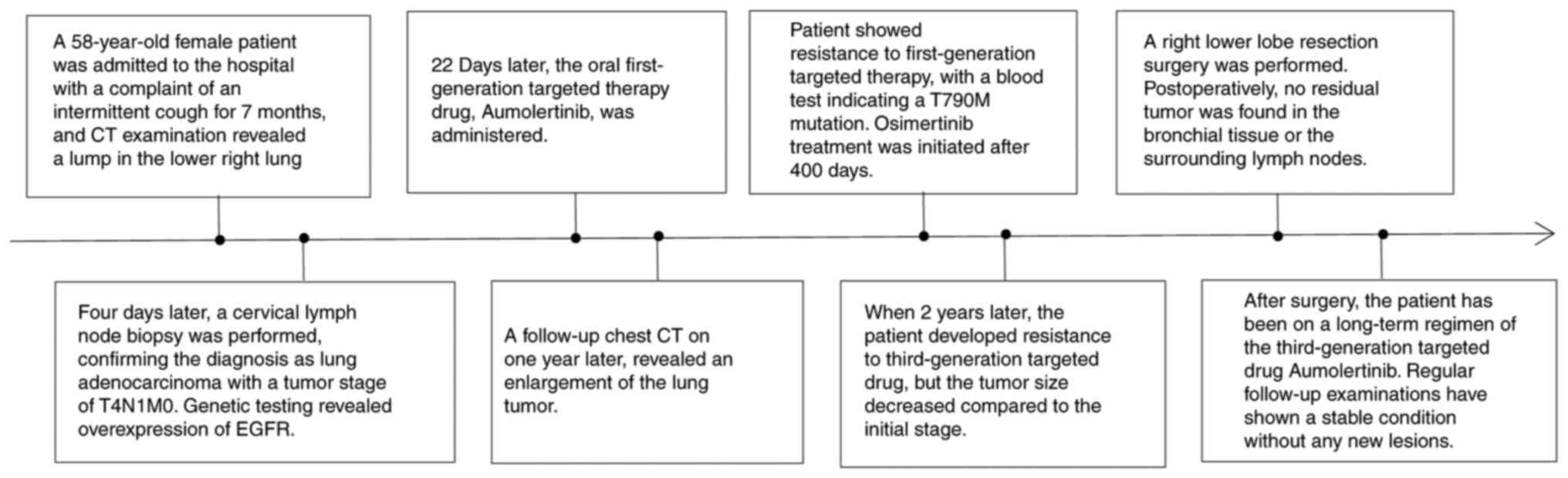
A 58-year-old female patient was admitted to the
Affiliated Hospital of Zunyi Medical University (Zunyi, China)
presenting with an intermittent cough and mass in the right lower
lung for 7 months and 4 days, respectively.
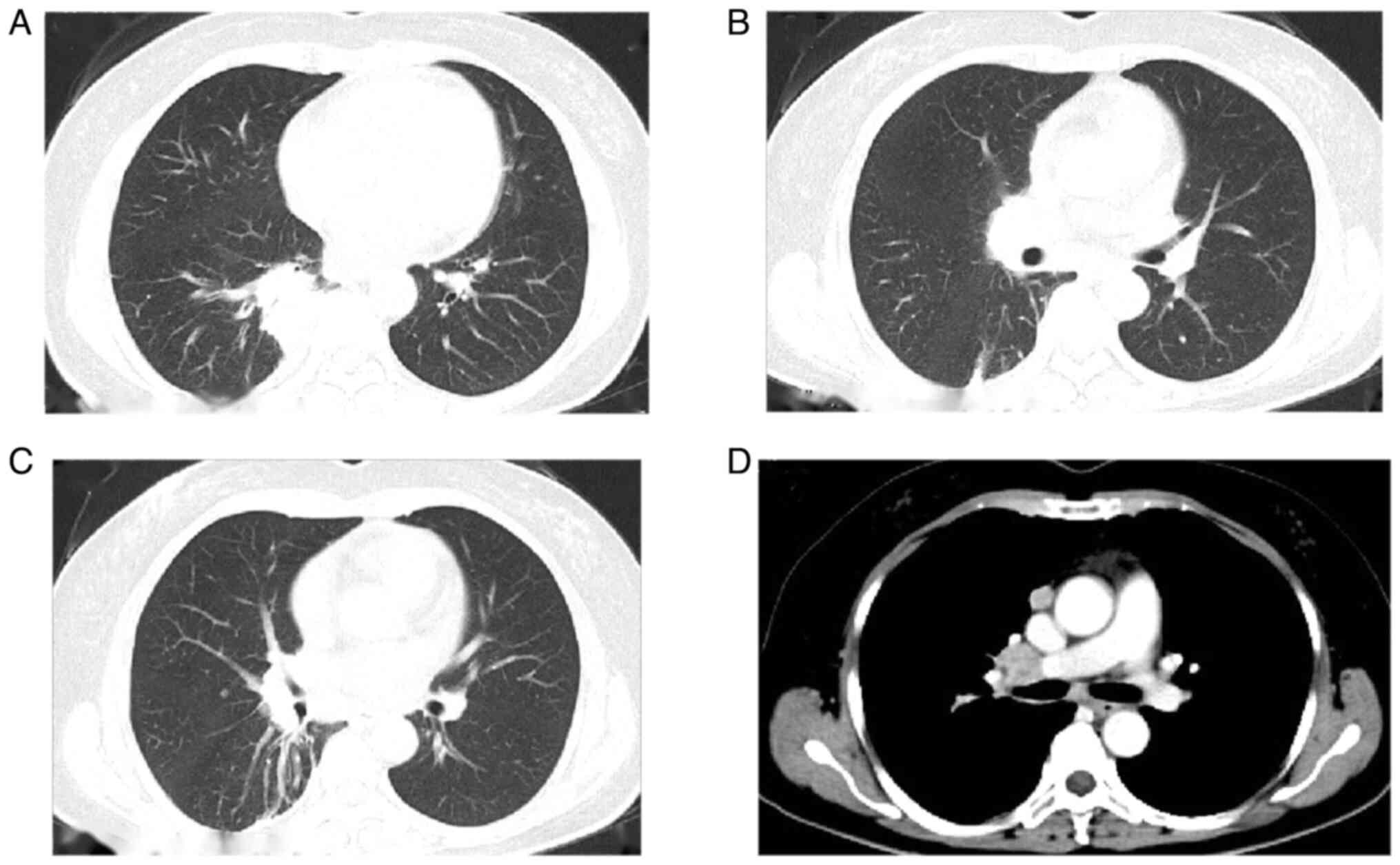
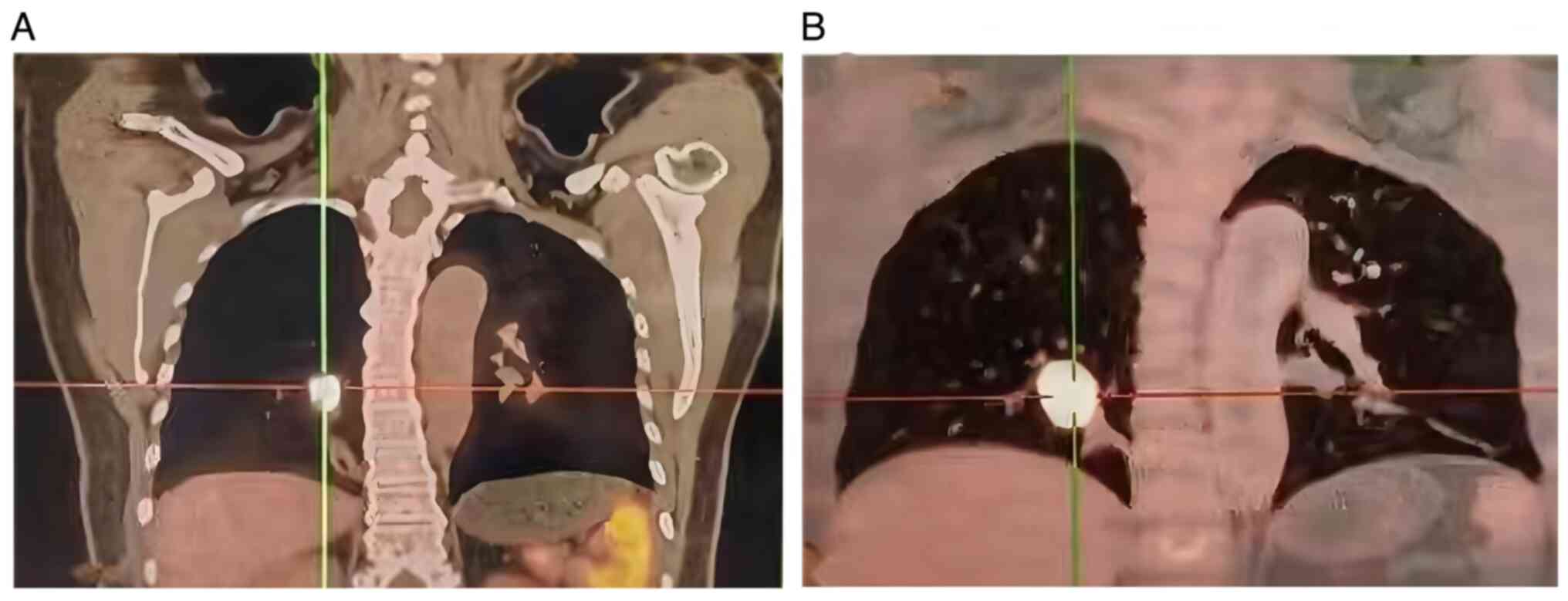
Computed tomography (CT) revealed a primary mass in
the lower right lung lobe measuring ~40×37×67 mm, along with
nodules suspected to be metastatic tumors in the lateral segment of
the middle right lung lobe (~5.5 mm) and the upper lobe of the left
lung (~4 mm). Multiple lymph node enlargements were also observed
in the mediastinum and right hilum (Fig. 1). The patient underwent positron
emission tomography-CT (PET-CT) at an external hospital, revealing
centrally located lung cancer accompanied by atelectasis of the
right lower lobe measuring 4.28×3.72 cm (Fig. 2A). Abnormal glucose metabolism was
observed with increased fluorodeoxyglucose (FDG) uptake, as
indicated by a maximum standardized uptake value
(SUVmax) of 8.84. Additionally, multiple lymph nodes
were found in the right hilum, mediastinum and supraclavicular
region, with the largest measuring 2.21 cm in diameter and
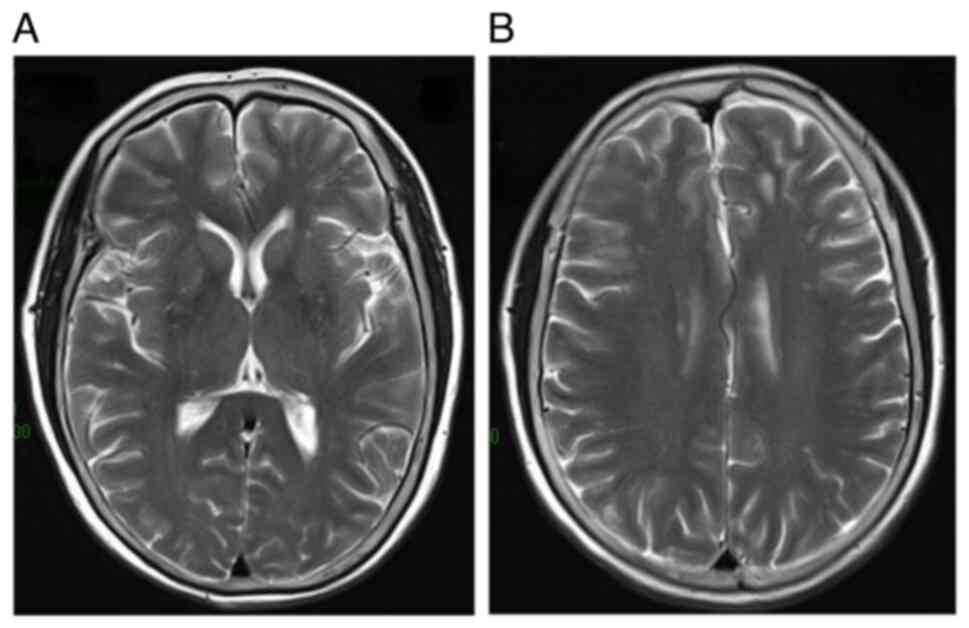
exhibiting an SUVmax of 5.68. Contrast-enhanced brain
MRI showed no signs of intracranial metastasis (Fig. 3).
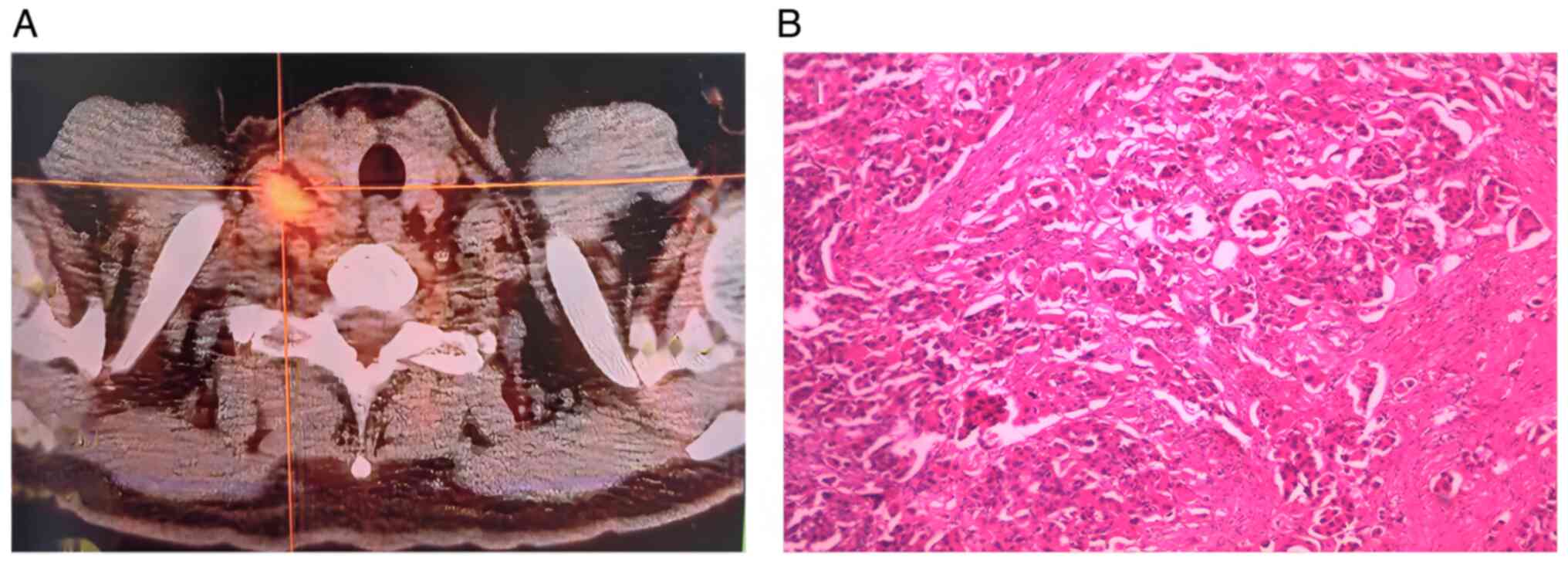
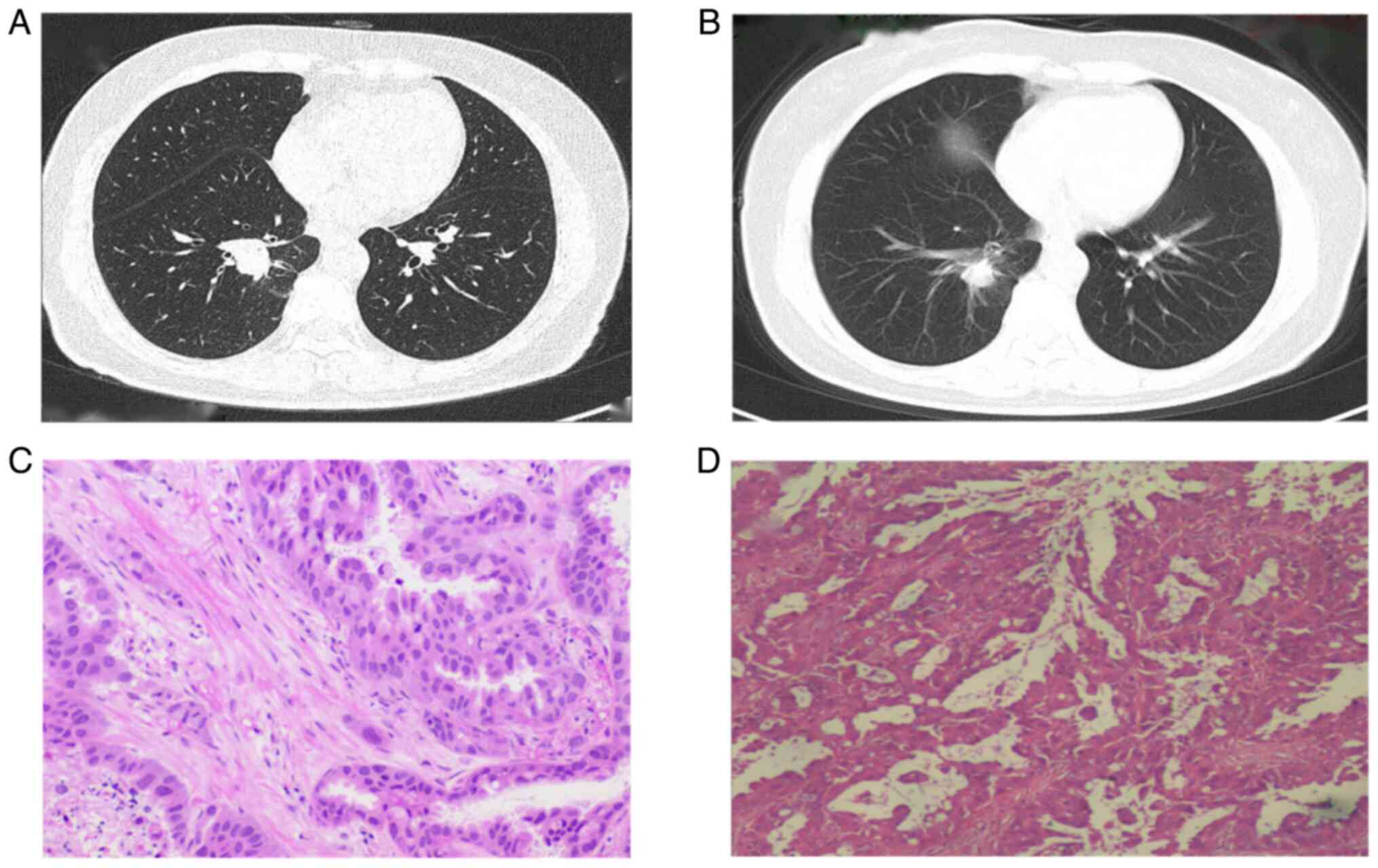
Subsequent biopsy of the right supraclavicular lymph
node confirmed the diagnosis of adenocarcinoma metastasis through
postoperative pathological examination (Fig. 2B). Tissue specimens were fixed in
10% neutral-buffered formalin and then sections were prepared at a
thickness of 5 µm, and subsequently stained with hematoxylin at
room temperature for a duration of 10 min, and eosin staining for 2
min at 60°C. Bronchoscopy further confirmed the presence of cancer
cells (NSCLC) in the lower right lobe of the lung. Blood
biochemistry results were with normal ranges. Therefore, the
clinical diagnosis was stage IIIC NSCLC (T4N3M0) localized in the
right lower lung. Next-generation sequencing (NGS) was performed by
Genecast Biotechnology Co., Ltd. The genetic analysis (Data S1) utilized the HG19 (UCSC) and
769-gene NGS panel genomic platforms and the human EGFR/ALK/ROS1
Gene Mutation Combined Detection Kit. Genetic testing revealed an
exon 19 deletion in the EGFR gene. Consequently, the patient was
initiated on treatment with the first-generation EGFR-TKI icotinib
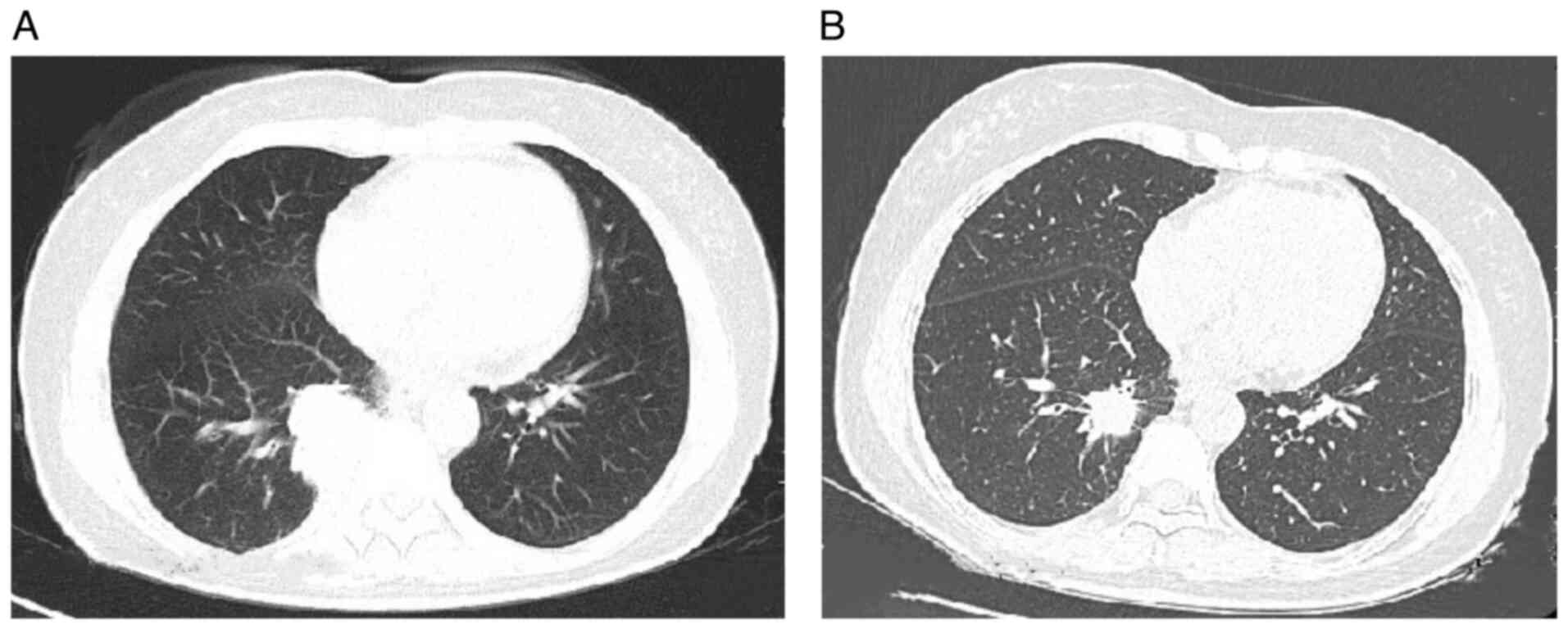
(125 mg, three times a day). After 3 weeks of treatment, a chest CT
demonstrated a significant reduction in the lesion size (Fig. 4), accompanied by notable alleviation
of the patient's cough symptoms. At 11 months after the initiation
of icotinib, the patient presented with a cough, increased sputum
production and chest discomfort. Chest CT re-examination revealed
significant enlargement of the lesion in the right lower lobe of
the lung, while the small nodules in both lungs remained unchanged.
Given the disease progression, a PET-CT scan was conducted,
revealing a 1.8×2.2×3.2-cm tumor in the lower right region, with an
SUVmax of 21.7 (Fig. 5).
The highest SUV for lymph node metastasis was 3.9. Peripheral blood
tests confirmed the persistent absence of EGFR exon 19 but the
presence of an acquired T790M mutation in exon 20, indicating
sensitivity to third-generation EGFR-TKIs.
The patient's treatment was consequently switched to
the third-generation TKI aumolertinib (110 mg/day). Over the next 5
months, the primary tumor in the chest decreased in size to 10×13
mm. However, during a follow-up chest CT scan 6 months after
initiating aumolertinib, a soft-tissue density shadow measuring
18×16 mm was observed in the right lower lobe, indicating an
increase in size compared with that after the initial drug
administration. Although metastasis was not detected in other body
parts, tumor growth may have been attributed to the development of
resistance to the third-generation targeted drug. Despite tumor
growth, the chest lesion decreased from stage T4N3M0 IIIC to stage
T2N0M0 IB. Subsequently, the patient underwent video-assisted
thoracoscopic surgery for right lower lobe resection and systematic
lymph node dissection under general anesthesia. No pleural
involvement was observed during the surgery. Postoperative
pathological examination showed no lymph node involvement or
metastasis in the fourth group of lymph nodes. There was no tumor
involvement in the residual bronchus or regional lymph node
metastasis. The lung tumor was successfully resected with clear
margins (R0 resection: Figs. 6 and
S1). Postoperatively, the patient
exhibited no signs of respiratory distress, infection, pulmonary
embolism, atrial fibrillation or other adverse events. Pathological
examination revealed an invasive adenocarcinoma in the right lower
lung (moderate grade, predominantly acinar type). There was no
evidence of tumor involvement at the bronchial stump, and no
metastatic spread was observed in the lymph nodes surrounding the
bronchial stump. Since the patient remained sensitive to
third-generation targeted therapy drugs, aumolertinib treatment was
continued after surgery. The patient was administered ametinib at a
daily dose of 55 mg for 2 years postoperatively. During the
medication period, no adverse events were observed. Furthermore, in
the 2-year postoperative follow-up, with imaging assessments
conducted every 3 months, no recurrence or metastasis was
detected.
The patient underwent regular postoperative
follow-up, with no signs of recurrence or metastasis. To date,
follow-up has been performed for 26 months and the patient's
condition has remained stable, with no new lesions detected
(Fig. 7). Informed consent was
obtained from the patient for the publication of this case report
and the accompanying images.
Discussion
NSCLC is a prevalent form of cancer worldwide, and
its impact on cancer-related fatalities cannot be overstated
(10). Surgical therapy is the
primary course of action for treating early stage lung cancer
(11). First-line treatment for
unresectable advanced lung cancer with gene mutations typically
involves targeted therapy (4).
Compared with platinum-based chemotherapy, targeted therapy has
considerably decreased the incidence of adverse reactions while
simultaneously enhancing progression-free survival (PFS) (12). This treatment method targets tumors
more accurately, reduces damage to normal cells and minimizes toxic
side effects (13). The field of
neoadjuvant targeted therapy for EGFR mutations is currently in its
nascent stage, yet it holds significant potential for widespread
application (14). However,
targeted therapies are not suitable for all patients with lung
adenocarcinoma. Targeted therapy typically targets specific
molecular targets; if a patient's tumor does not have mutations in
these targets, targeted therapy may be ineffective. Therefore,
before undergoing targeted therapy, genetic testing of the
patient's tumor is necessary to determine suitability for targeted
treatment.
Neoadjuvant targeted therapy can be employed for
patients with mutations in specific genes, facilitating tumor
downstaging and thus improving the feasibility of surgical
intervention. This approach increases the likelihood of achieving
complete resection or the potential for minimally invasive surgical
procedures (15). Patients who
undergo neoadjuvant targeted therapy present with fewer surgical
challenges for the operating surgeons and are associated with more
favorable surgical outcomes (16).
Patients who are EGFR-positive and responsive to
first- and second-generation targeted therapies can achieve
noteworthy improvement in their overall response rate (ORR) and PFS
times. Despite this, after 9 to 12 months of treatment, ~60% of
these patients will experience the EGFRT790 mutation and develop
acquired resistance to EGFR TKIs. Consequently, tumors ultimately
become resistant to therapy, and acquired resistance will develop
(17,18). The APOLLO study of advanced lung
cancer revealed that multiple treatments could effectively prolong
the lives of patients. In the study, 244 patients with
EGFR-positive exon 19 deletions and L858R mutations were enrolled
and treated with aumolertinib as a second- or third-line treatment.
The study found that these patients had median PFS times of 12.4
and 12.3 months, respectively. Successful treatment was achieved
using the third-generation drug, aumolertinib, with the study
demonstrating a disease control rate and ORR of 93.4 and 68.9%,
respectively (19). Furthermore,
the median PFS times of patients with locally advanced and
metastatic disease were 17.0 and 12.0 months, respectively
(20). A study involving 26
patients evaluated the efficacy of aumolertinib in treating lung
cancer (21). The results inferred
that the use of aumolertinib is safe and effective in treating
NSCLC (22). First- and
third-generation targeted drugs have significantly improved PFS
rates. Compared to first-generation targeted drugs,
third-generation drugs result in significantly improved PFS times
(23). However, it is worth noting
that >60% of patients are diagnosed with advanced lung cancer
that is not amenable to resection (24). Conventional chemotherapy has a
limited ability to prolong PFS time and is associated with
significant adverse effects (25,26).
For example, in a study on advanced gastric cancer, the PFS was
extended by only ~3 months through optimization of the existing
chemotherapy regimen. This indicates that conventional chemotherapy
may not achieve the desired long-term effects in extending PFS
(27). In a retrospective study, 9
patients with lung adenocarcinoma underwent targeted therapy
followed by conversion surgery. After a mean follow-up time of 18.9
months, the median event-free survival, PFS and overall survival
times were 14, 17 and 25 months, respectively (28). These studies provide evidence for
postoperative treatment with targeted therapies. Studies have
demonstrated that patients exhibit good tolerance to preoperative
targeted therapy, which can significantly improve the prognosis of
individuals with advanced non-small cell lung cancer
(16,29-31).
In the present case, a patient with advanced lung
adenocarcinoma was diagnosed with unresectable stage IIIC cancer
based on the latest National Comprehensive Cancer Network (NCCN)
guidelines. Genetic testing revealed the absence of EGFR exon 19,
and EGFR TKIs are the recommended first-line therapy for patients
with EGFR mutation-positive tumors (32). Despite drug resistance, the tumor
was still effectively suppressed, which distinguishes it from
previous findings (33,34). According to the latest NCCN
guidelines, certain stage IIIB and IIIC NSCLC cases are classified
as unresectable. In the present study, after ~1 year of targeted
therapy combined with transformative surgical treatment, inoperable
stage IIIC NSCLC was successfully converted to stage IB NSCLC
(ypT2N0M0). The patient had not been eligible for surgical
resection at the initial diagnosis, but EGFR TKI-targeted therapy
had been employed to convert the patient to a potentially operable
disease state. In this case, new insights can be gained, leading to
improvements in the treatment plan. For patients with advanced,
unresectable NSCLC, initial diagnostic evaluations should include
enhanced chest and upper abdominal CT, PET-CT and contrast-enhanced
brain MRI to rule out the metastatic spread of lung cancer.
Endoscopic procedures, such as ultrasound bronchoscopy or
mediastinoscopy, should be utilized to assess the status of
mediastinal lymph nodes. Additionally, pulmonary function tests are
necessary to evaluate the patient's ability to tolerate subsequent
treatments. Moreover, genetic testing of both primary and
metastatic lesions should be conducted to identify driver
mutations. When formulating treatment plans for these patients,
targeted therapy should be prioritized for those with positive
driver gene mutations. Through genetic testing, these mutations can
be accurately identified, thereby facilitating the selection of
appropriate targeted therapies for individual patients. This study
provides a practical case study of a specific treatment method that
can aid in the development of personalized and effective treatment
plans. This may demonstrate significant therapeutic effects in
certain patients, enabling clinical trials with a broader patient
population.
In accordance with the principles of comprehensive
treatment, including neoadjuvant chemotherapy, surgery and
radiotherapy, supraclavicular lymph node metastasis in patients
without distant metastasis is classified as regional rather than
distant metastasis. In the case of the patient in question, the
initial treatment plan was modified after the patient declined
chemotherapy and radiotherapy. Therefore, the therapeutic objective
focused on targeting the supraclavicular lymph node metastasis
through neoadjuvant targeted therapy. While surgical resection of
supraclavicular lymph nodes may improve prognosis in certain cases,
it is not always effective in achieving complete tumor eradication.
Residual disease following surgery is associated with a poorer
prognosis. Furthermore, surgical resection of supraclavicular lymph
nodes may lead to complications, such as nerve damage and
lymphedema, with no discernible survival benefit for the patient.
Consequently, surgical intervention was not selected for the
patient's supraclavicular lymph node metastasis and targeted
therapy was continued. Follow-up imaging revealed a reduction in
the size of the metastasis in the right supraclavicular lymph node,
suggesting a favorable treatment response.
The present study has some limitations. Firstly, due
to patient-related reasons, a biopsy of the right lower lobe
pulmonary lesion was not performed at the initial diagnosis.
However, PET-CT showed increased FDG uptake, with an
SUVmax of 8.84. Secondly, the sample size was small.
In conclusion, this case indicates that initially
unresectable advanced NSCLC may transform into resectable NSCLC
through effective TKI-targeted therapy. Neoadjuvant EGFR-TKIs in
combination with radical surgery may extend the postoperative PFS
time and improve the clinical benefits in unresectable advanced
EGFR-mutant NSCLC.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The sequencing data generated in the present study
are not publicly available due to a privacy request from the
patient.
Author's contributions
YW, XK and CC conceptualized the case report. YW
wrote the manuscript and performed additional data analysis. LL,
YT, YS and GX were involved in the treatment and follow-up in this
case. XK and CC critically revised the manuscript and provided
valuable feedback, and provided supervision and approved the final
manuscript for publication. XK and CC confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent to publish the clinical
details and images were obtained from the patient.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Thai AA, Solomon BJ, Sequist LV, Gainor JF
and Heist RS: Lung cancer. Lancet. 398:535–554. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Yan B and He S: Advances and
challenges in the treatment of lung cancer. Biomed Pharmacother.
169:1158912023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wathoni N, Puluhulawa LE, Joni IM,
Muchtaridi M, Mohammed AFA, Elamin KM, Milanda T and Gozali D:
Monoclonal antibody as a targeting mediator for nanoparticle
targeted delivery system for lung cancer. Drug Deliv. 29:2959–2970.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Carter BW, Lichtenberger JP III,
Benveniste MK, de Groot PM, Wu CC, Erasmus JJ and Truong MT:
Revisions to the TNM staging of lung cancer: Rationale,
significance, and clinical application. Radiographics. 38:374–391.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Evison M and AstraZeneca UK Limited: The
current treatment landscape in the UK for stage III NSCLC. Br J
Cancer. 123:3–9. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Du X, Yang B, An Q, Assaraf YG, Cao X and
Xia J: Acquired resistance to third-generation EGFR-TKIs and
emerging next-generation EGFR inhibitors. Innovation (Camb).
2:1001032021.PubMed/NCBI
|
|
7
|
Cheng Y, He Y, Li W, Zhang HL, Zhou Q,
Wang B, Liu C, Walding A, Saggese M, Huang X, et al: Osimertinib
versus comparator EGFR TKI as first-line treatment for EGFR-mutated
advanced NSCLC: FLAURA China, A randomized study. Target Oncol.
16:165–176. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wang Z, Cai G, Zhu J, Wang J and Zhang Y:
Treatment of advanced-stage non-small cell lung cancer: Current
progress and a glimpse into the future (Review). Mol Clin Oncol.
22:422025. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aoki M, Ueda K, Umehara T, Kamimura GO,
Tokunaga T, Harada-Takeda A, Maeda K, Nagata T, Yokomakura N,
Kariatsumari K, et al: Targeted therapy followed by cytotoxic
chemotherapy in preoperative patients with locally advanced lung
adenocarcinoma. Anticancer Res. 40:2911–2916. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shukla AA, Podder S, Chaudry SR, Benn BS
and Kurman JS: Non-small cell lung cancer: Epidemiology, screening,
diagnosis, and treatment. AIMS Med Sci. 9:348–361. 2022. View Article : Google Scholar
|
|
11
|
Chen KN: The diagnosis and treatment of
lung cancer presented as ground-glass nodule. Gen Thorac Cardiovasc
Surg. 68:697–702. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tan AC, Teh YL, Lai GGY and Tan DSW: Third
generation EGFR TKI landscape for metastatic EGFR mutant non-small
cell lung cancer (NSCLC). Expert Rev Anticancer Ther. 19:431–435.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Villaruz LC, Burns TF, Ramfidis VS and
Socinski MA: Personalizing therapy in advanced non-small cell lung
cancer. Semin Respir Crit Care Med. 34:822–836. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grant C and Nagasaka M: Neoadjuvant
EGFR-TKI therapy in non-small cell lung cancer. Cancer Treat Rev.
126:1027242024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sorin M, Huynh C, Rokah M, Dubé LR, Rayes
R, Ofiara L, Shieh B, Owen S, Fiset PO, et al: Neoadjuvant targeted
therapy in non-small cell lung cancer and its impact on surgical
outcomes. Ann Thorac Surg Short Rep. 1:102–106. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang K, Yi H, Lv Z, Jin D, Fu L and Mao Y:
Analysis of surgical complexity and short-term prognostic
indicators in NSCLC patients: Neoadjuvant targeted therapy versus
neoadjuvant chemoimmunotherapy. Ther Adv Med Oncol.
16:175883592412652142024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Reguart N and Remon J: Common EGFR-mutated
subgroups (Del19/L858R) in advanced non-small-cell lung cancer:
Chasing better outcomes with tyrosine kinase inhibitors. Future
Oncol. 11:1245–1257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chaft JE, Shyr Y, Sepesi B and Forde PM:
Preoperative and postoperative systemic therapy for operable
non-small-cell lung cancer. J Clin Oncol. 40:546–555. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lu S, Wang Q, Zhang G, Dong X, Yang CT,
Song Y, Chang GC, Lu Y, Pan H, Chiu CH, et al: Efficacy of
Aumolertinib (HS-10296) in patients with advanced EGFR T790M+
NSCLC: Updated post-national medical products administration
approval results from the Apollo registrational trial. J Thorac
Oncol. 17:411–422. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu L, Zhong W, Li A, Qiu Z, Xie R, Shi H
and Lu S: Successful treatment of EGFR T790M-mutant non-small cell
lung cancer with almonertinib after osimertinib-induced
interstitial lung disease: A case report and literature review. Ann
Transl Med. 9:9502021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou C, Xie L, Liu W, Zhang L, Zhou S,
Wang L, Chen J, Li H, Zhao Y, Zhu B, et al: Absorption, metabolism,
excretion, and safety of [(14)C]almonertinib in healthy chinese
subjects. Ann Transl Med. 9:8672021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang JC, Camidge DR, Yang CT, Zhou J, Guo
R, Chiu CH, Chang GC, Shiah HS, Chen Y, Wang CC, et al: Safety,
efficacy, and pharmacokinetics of almonertinib (HS-10296) in
pretreated patients with EGFR-mutated advanced NSCLC: A
multicenter, open-label, phase 1 trial. J Thorac Oncol.
15:1907–1918. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Arbour KC and Riely GJ: Systemic therapy
for locally advanced and metastatic non-small cell lung cancer: A
review. JAMA. 322:764–774. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li K, Cao X, Ai B, Xiao H, Huang Q, Zhang
Z, Chu Q, Zhang L, Dai X and Liao Y: Salvage surgery following
downstaging of advanced non-small cell lung cancer by targeted
therapy. Thorac Cancer. 12:2161–2169. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fukuoka M, Wu YL, Thongprasert S,
Sunpaweravong P, Leong SS, Sriuranpong V, Chao TY, Nakagawa K, Chu
DT, Saijo N, et al: Biomarker analyses and final overall survival
results from a phase III, randomized, open-label, first-line study
of gefitinib versus carboplatin/paclitaxel in clinically selected
patients with advanced non-small-cell lung cancer in Asia (IPASS).
J Clin Oncol. 29:2866–2874. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shang Z and Li J: Comparison of clinical
efficacy between chrono-chemotherapy and conventional chemotherapy
in patients with non-small cell lung cancer. Am J Cancer Res.
13:4277–4287. 2023.PubMed/NCBI
|
|
27
|
Randon G, Lonardi S, Fassan M, Palermo F,
Tamberi S, Giommoni E, Ceccon C, Di Donato S, Fornaro L, Brunetti
O, et al: Ramucirumab plus paclitaxel as switch maintenance versus
continuation of first-line oxaliplatin-based chemotherapy in
patients with advanced HER2-negative gastric or gastro-oesophageal
junction cancer (ARMANI): A randomised, open-label, multicentre,
phase 3 trial. Lancet Oncol. 25:1539–1550. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Song W, Di S, Liu J, Fan B, Zhao J, Zhou
S, Chen S, Dong H, Yue C and Gong T: Salvage surgery for advanced
non-small cell lung cancer after targeted therapy: A case series.
Thorac Cancer. 11:1061–1067. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lengel HB, Zheng J, Tan KS, Liu CC, Park
BJ, Rocco G, Adusumilli PS, Molena D, Yu HA, Riely GJ, et al:
Clinicopathologic outcomes of preoperative targeted therapy in
patients with clinical stage I to III non-small cell lung cancer. J
Thorac Cardiovasc Surg. 165:1682–1693. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bian D, Ji S, Liu Y, Huang Z, Jiang L, Liu
M, Bao X, Yang J, Zhou Y, Hu J, et al: Neoadjuvant Aumolertinib for
unresectable stage III EGFR-mutant non-small cell lung cancer: A
single-arm phase II trial. Nat Commun. 16:31432025. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bian D, Sun L, Hu J, Duan L, Xia H, Zhu X,
Sun F, Zhang L, Yu H, Xiong Y, et al: Neoadjuvant Afatinib for
stage III EGFR-mutant non-small cell lung cancer: A phase II study.
Nat Commun. 14:46552023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lu S, Dong X, Jian H, Chen J, Chen G, Sun
Y, Ji Y, Wang Z, Shi J, Lu J, et al: Aeneas: A randomized phase III
trial of aumolertinib versus gefitinib as first-line therapy for
locally advanced or metastatic non-small-cell lung cancer with EGFR
exon 19 deletion or L858R mutations. J Clin Oncol. 40:3162–3171.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan CY, Zhao ML, Wei YN and Zhao XH:
Mechanisms of drug resistance in breast cancer liver metastases:
Dilemmas and opportunities. Mol Ther Oncolytics. 28:212–229. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Huang K and Liu H: Identification of
drug-resistant individual cells within tumors by semi-supervised
transfer learning from bulk to single-cell transcriptome. Commun
Biol. 8:5302025. View Article : Google Scholar : PubMed/NCBI
|