Introduction
With the development of immune checkpoint inhibitors
(ICIs) for the treatment of various malignancies, cancer therapy
has entered into a new era of immunotherapy (1). According to statistics released by the
International Agency for Research on Cancer, China had the highest
incidence of nasopharyngeal cancer (NPC) in 2020, with ~60,000 new
cases, accounting for 46.8% of the global cases (2). Treatment regimens for the different
stages of NPC vary. Early-to middle-stage NPC is typically managed
by local radiotherapy or synchronous chemoradiotherapy. In advanced
NPC cases, induction chemotherapy is frequently selected before
chemoradiotherapy to achieve long-term survival (3–7). By
contrast, programmed cell death protein-1 (PD-1) combined with
chemotherapy is the standard first-line treatment for patients with
distant metastases (8,9).
PD-1 is expressed on the cell membrane of various
immune cells, including activated T cells, B cells, dendritic
cells, activated monocytes and tumor-infiltrating lymphocytes. By
contrast, its ligand programmed cell death ligand-1 (PD-L1) is
expressed on the surface of tumor cells and antigen-presenting
cells. PD-1 binds to PD-L1 and prevents the T cells from
recognizing tumor cells, leading to tumor immunosuppression
(10). However, sintilimab, a
monoclonal antibody against PD-1 (11), binds to PD-1 and blocks its
interaction with its ligand, thereby restoring the antitumor
response of T cells (12).
Sintilimab has been successfully used to treat Hodgkin's lymphoma,
non-small cell lung cancer, hepatocellular carcinoma, gastric
cancer and esophageal cancer (12–17).
Although sintilimab has shown efficacy for the treatment of these
tumors, its side effects and potential harm to patients remain an
unavoidable concern. According to a previous study (11), the incidence of all-grade adverse
reactions from sintilimab treatment is >58.0%, whereas the
incidence of grade ≥3 adverse reactions is 13.0%. Among the
immune-related adverse events (irAEs) of ICIs, acute hemorrhagic
gastritis is rare, with only five documented cases (18–22).
For irAEs such as colitis and diarrhea, relevant grading criteria
and treatment guidelines have been published (23,24),
but a lack of consensus concerning upper gastrointestinal adverse
reactions remains.
In the present report, acute erosive hemorrhagic
gastritis occurred 51 weeks after the first sintilimab injection,
where the patient also developed drug-related hypothyroidism and
suspected myocardial injury. The patient achieved a partial tumor
response with sintilimab treatment, and the progression-free
survival (PFS) was 542 days.
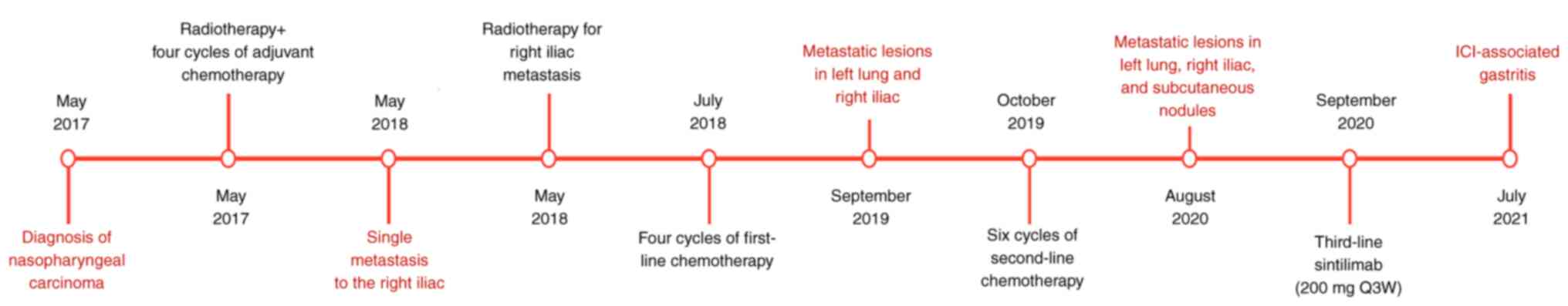
Case report
A 40-year-old man with a mass on the right side of
his neck was first diagnosed with poorly differentiated squamous
cell carcinoma of the nasopharynx and cervical lymph node
metastasis (cT4N3M0; stage IVa) at Mianyang Central Hospital
(Mianyang, China) in May 2017. As a result, radical radiotherapy,
image-guided radiotherapy (IGRT) [planning target volume
(PTV)-gross tumor volume of nasopharyngeal carcinoma, 74 Gy/33 F;
PTV-gross tumor volume of lymph node, 70 Gy/33 F; PTV-clinical
target volume, 60 Gy/33 F] and four cycles of adjuvant chemotherapy
[paclitaxel injection 210 mg on day 1 + cisplatin injection 30 mg
on days 1–4, repeated every 3 weeks (Q3W)] were initiated in May
2017.
Single metastases in the right iliac crest with pain
presented on May 2018, for which the patient received palliative
radiotherapy (IGRT, dose 54 Gy/18 F) followed by four cycles of
first-line chemotherapy starting in July 2018 (gemcitabine 1.2 g on
days 1 and 8 + nedaplatin 100 mg on day 1, repeated Q3W).
In September 2019, the patient showed general
disease progression and metastatic sites in the left lungs and
right iliac crest. Subsequently, 15 days later (October 2019), the
patient was scheduled for six cycles of chemotherapy (gemcitabine
1.6 g on days 1 and 8 + cisplatin 30 mg on days 1–3, repeated Q3W).
The tumor was controlled during this treatment, but metastatic
lesions developed ~11 months later in August 2020.
In September 2020, intravenous (IV) sintilimab (200
mg on day 1, repeated Q3W) was initiated as the third-line of
regimen. Prior to the ICI injection, the patient was tested for
thyroid hormones, adrenal hormone, myocardial injury marker and
other immunological tests, all of which returned normal result.
Fatigue and bilateral anterior tibial edema developed ~137 days
after sintilimab injection. The thyroid function tests of the
patient showed free triiodothyronine levels of 1.50 pg/ml, free
thyroxine levels of 0.40 ng/dl and thyroid-stimulating hormone
levels of 87.4909 µIU/ml. Based on thyroid reports, the patient was
diagnosed with drug-induced hypothyroidism and was prescribed
thyroxine tablets 25 µg orally once a day in January 2021. Thyroid
hormone levels were monitored during treatment and the dose of
thyroxine tablets was adjusted according to the thyroid hormone
level. The changes in thyroid hormone levels are shown in Fig. S1.
On day 167, the patient's creatine phosphokinase-MB,
a myocardial injury marker, and myohemoglobin levels were 8.33 and
128.8 µg/l, respectively. Echocardiography revealed no
abnormalities and a diagnosis of immune myocarditis was considered.
Since the patient did not have any related symptoms, no additional
treatments were administered at the time. In total, 10 days later,
creatine phosphokinase-MB and myohemoglobin were retested, where
the results were 6.74 and 144.4 µg/l, respectively.
On day 329, the patient experienced subxiphoid pain
and developed melena (treatment protocol detailed in Fig. 1). A gastroenterologist advised
treatment using omeprazole [20 mg orally (p.o.) per day (qd)],
trimebutin maleate [0.2 g p.o. 3 times a day (tid)] and chewable
magnesium aluminum carbonate tablets (1 g p.o. tid). The antitumor
treatment, scheduled for 1 week later, was suspended.
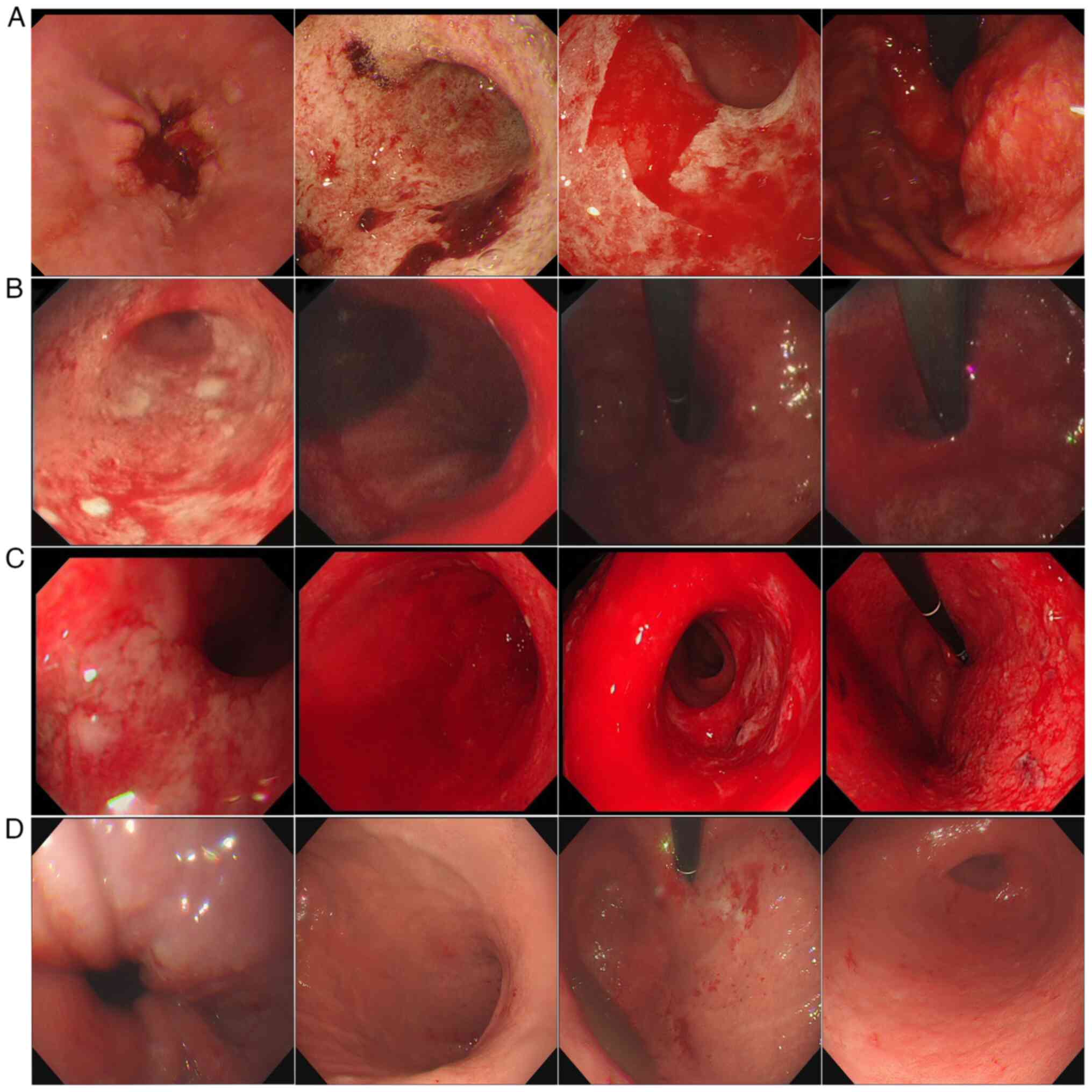
However, 1 month after the initiation of acid
suppression treatment, the symptoms did not improve, and
gastroscopy was performed. Gastroscopy indicated diffuse swelling
with scattered bleeding and a pale pseudomembrane covering the
surface of gastric mucosa (Fig.
2A). Contrast-enhanced abdominal CT revealed that a large area
of the stomach wall was thickened and edematous (Fig. S2). The gastroenterologist adjusted
the patient's treatment regimen with somatostatin [3 mg IV every 12
h (q12 h)] and esomeprazole sodium (40 mg IV q12h) during
hospitalization. At 2 weeks after this infusion treatment,
gastroscopy showed diffuse mucosal swelling with a pale
pseudomembrane on the surface and scattered blood exudation,
similar to the previous gastroscopy results (Fig. 2B). The gastroenterologist did not
adjust the treatment plan and continued with the original infusion
treatment for another week. The subsequent gastroscopy suggested
that the gastric mucosal bleeding had worsened (Fig. 2C).
Since the patient's symptoms persisted, the
gastroenterologist could not explain the relationship between the
treatment and gastroscopy results. After consultation with an
oncologist, the patient was considered to have immune-related
gastritis and was recommended to be treated with
methylprednisolone, an immunosuppressive agent (40 mg IV twice a
day). On day 5 of treatment, the patient's abdominal discomfort was
significantly relieved, and his feces turned yellow. Therefore, the
dosage was reduced. In total, 10 days after the entire treatment
course, the regimen was changed to oral prednisone acetate tablets
(30 mg qd), which were gradually withdrawn.
On day 428, gastroscopy revealed a smooth gastric
mucosa, reduced mucosal congestion and edema, with no signs of
active exudation (Fig. 2D). During
the exacerbation of gastric mucosal hemorrhage, the pathological
examination of the gastric antrum revealed moderate chronic
inflammation of the mucosa with erosion, glandular atrophy and
partial dilation of glandular lumens. In addition, multiple
lymphoid aggregates, plasma cells and scattered neutrophil
infiltration were observed in the lamina propria. There was also
intraepithelial lymphocytic infiltration accompanied by mucosal
erosion (Fig. 3A).
Immunohistochemical staining showed infiltration of CD3+
T cells and CD8+ T cells in the gastric mucosal
epithelium, with significant infiltration of CD4+ T
cells, CD8+ T cells, and CD20+ B cells in the
lamina propria. The lymphoid aggregates were predominantly composed
of CD20+ B cells, whilst CD8+ T cells
infiltrated the epithelium and CD4+ T cells were mainly
distributed between the glands. The ratio of CD4+ T
cells to CD8+ T cells in the lamina propria was 1.5–2:1
(Fig. 3B). Based on the
classification criteria and treatment guidelines for irAEs
(25), the patient experienced a
grade 3 or higher adverse events. Therefore, further sintilimab
therapy was stopped. Longitudinal changes in CT imaging findings
reflecting tumor progression and treatment response throughout the
therapeutic course are illustrated in Fig. S3. Following drug withdrawal, the
patient was prospectively monitored until February 2022 through
serial 6-month imaging evaluations-including contrast-enhanced MRI
of the nasopharynx and CT scans of the chest/abdomen/neck-to
continuously assess treatment response, with radiographic
progression in the lung and mediastinal lymph nodes documented in
Fig. S4. Sintilimab therapy
achieved a PFS of 18 months. Subsequently, the patient ultimately
succumbed to disease progression in September 2023. The patient
provided written informed consent for publication prior to their
death.
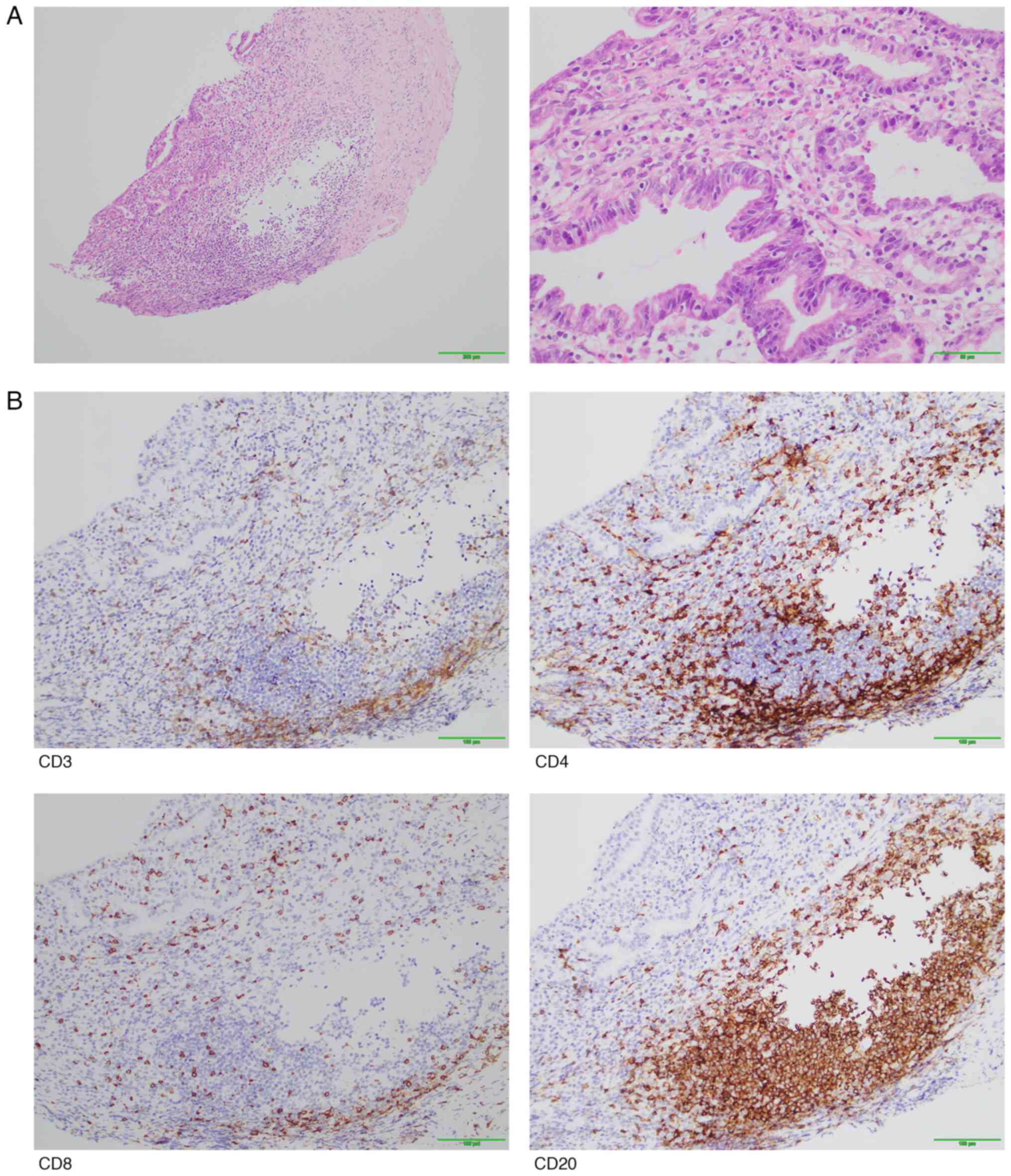 | Figure 3.Pathological staining results. (A)
H&E staining pictures of the gastric mucosa of the patient.
Pathological findings of moderate chronic inflammation of the
mucosa with erosion, glandular atrophy and partial dilation of
glandular lumens. Multiple lymphoid aggregates, plasma cells and
scattered neutrophil infiltration were observed in the lamina
propria. There was also intraepithelial lymphocytic infiltration
accompanied by mucosal erosion. Representative H&E staining of
gastric mucosa (left: magnification, ×100; scale bar, 200 µm;
right: magnification, ×400; scale bar, 50 µm). (B)
Immunohistochemical staining showed infiltration of CD3+
T cells and CD8+ T cells in the gastric mucosal
epithelium, with significant infiltration of CD4+ T
cells, CD8+ T cells and CD20+ B cells in the
lamina propria. The lymphoid aggregates were predominantly composed
of CD20+ B cells, whilst CD8+ T cells
infiltrated the epithelium and CD4+ T cells were mainly
distributed between the glands. The ratio of CD4+ T
cells to CD8+ T cells in the lamina propria was 1.5–2:1
(magnification, ×200; Scale bar, 100 µm). H&E, hematoxylin and
eosin. |
Discussion
ICIs have changed the course of treatment for
various malignant tumors. Blocking PD-1/PD-L1 binding using ICIs
restores T cell-mediated antitumor immune responses and enhances
tumor cell death. At present, clinically used ICIs include PD-1,
PD-L1 and cytotoxic T lymphocyte-associated antigen 4 antibodies
(26). The present case reports a
case of sintilimab-induced hemorrhagic gastritis observed in a
patient receiving third-line anti-PD-1 monoclonal antibodies. The
patient experienced multi-system irAEs during drug treatment,
including hypothyroidism, suspected myocardial injury and
hemorrhagic gastritis. After active treatment, the patient
recovered completely from hemorrhagic gastritis, where all the
irAEs were well controlled. The patient achieved partial response
after anti-PD-1 treatment, where the toxicity and side effects were
controllable. This suggests that hemorrhagic gastritis as a result
of sintilimab for the treatment of advanced NPC should be added to
its list of potential irAEs.
The incidence of irAEs during immunotherapy is ~20%,
where the combination of ≥2 immunotherapeutic agents or the
presence of an autoimmune disease increases the risk of irAEs
(27). The common manifestations of
irAEs include dermatitis, fatigue, endocrine system injury,
colitis, pneumonia, arthritis, musculoskeletal injury and liver
injury. Rarer irAEs include gastrointestinal bleeding,
cardiovascular injury, pancreatic injury, ocular injury, and
neurological injury (28–30). However, the majority of irAEs are
not severe, where favorable remission is frequently achieved after
the discontinuation of immunotherapeutic drugs and symptomatic
treatment with steroids. IrAEs may occur during any period of
immunotherapy, even after the final treatment. In the present case,
hypothyroidism developed 19 weeks after the initiation of
immunotherapy and immune-associated hemorrhagic gastritis developed
at 51 weeks.
The hallmark endoscopic features of ICI-associated
hemorrhagic gastritis include diffuse gastric mucosal erythema and
edema. Three distinctive patterns warrant particular diagnostic
attention: i) Reticular erosions/ulcers in the antrum; ii) diffuse
mucosal congestion covered by thick yellowish-white plaques; and
iii) contact-induced hemorrhages upon minimal instrumentation.
Patterns i) and iii) show high specificity for ICI-associated
gastritis. By contrast, pattern ii) requires differentiation from
H. pylori-induced gastritis, since heavy purulent exudates
may also occur in severe infectious cases (31,32).
Endoscopic maneuvers (insufflation pressure and scope contact) may
artifactually exacerbate exudate formation, necessitating cautious
interpretation (33).
The symptoms of ICI-associated hemorrhagic gastritis
are non-specific and may mimic those of other etiologies.
Consequently, clinical symptoms alone are insufficient for
diagnosing ICI-gastritis. Definitive diagnosis requires integration
with characteristic endoscopic findings, particularly mucosal
vulnerability and reticular erosions in the antrum. The
histopathology of ICI-associated hepatitis and colitis has been
extensively studied (34,35). However, to the best of our
knowledge, the histopathological features of gastric involvement
remain poorly defined (36).
ICI-associated gastritis includes chronic active
gastritis, idiopathic lymphocytic gastritis and granulomatous
gastritis, with periglandular inflammation (37). Chronic active gastritis, the most
common type of gastric injury caused by ICI therapy, is
characterized by increased lymphocytic infiltration of the lamina
propria and increased intraepithelial lymphocytes and neutrophils,
with or without neutrophilic gland abscesses. In ~80% cases of
chronic active gastritis caused by ICI therapy, intraepithelial
lymphocytes are increased, where these epithelial cells are
predominantly CD8+ T cells. Although chronic active
gastritis is superficially similar to H. pylori gastritis in
the majority of cases of ICI-associated gastritis, the former is
typically more destructive and ulcerous, whilst chronic active
gastritis has milder lymphatic infiltration of the lamina propria,
less lymphocyte aggregation and increased intraepithelial
lymphocytes. Syphilitic gastritis, although extremely rare, can
manifest as full-thickness chronic gastritis with dense plasma cell
infiltration. However, this diagnosis can be easily ruled out by
the lack of high-risk clinical features, endarteritis and poorly
formed granulomas (38). Idiopathic
lymphocytic gastritis is a disease with an increase in
intraepithelial lymphocytes, mainly CD8+ T lymphocytes.
Unlike chronic active gastritis with intraepithelial lymphocytosis,
idiopathic lymphocytic gastritis is not associated with active
gastritis. Foci of active neutrophilic inflammation can be
seen in idiopathic lymphocytic gastritis, from which it may be
almost indistinguishable. However, lymphocytosis is rarely
associated with marked neutrophilic inflammation and apoptosis in
biopsies obtained from patients with idiopathic lymphocytic
gastritis. Viral-associated gastritis can also be shown with
intraepithelial lymphocytosis on gastric biopsy. However, in the
context of immunotherapy, the majority of common infections, such
as cytomegalovirus, herpes simplex virus, adenovirus and
Epstein-Barr virus, can be typically ruled out by prebiopsy
microbiological testing (39).
Focal-enhancing gastritis is characterized by a small collection of
lymphocytes and histiocytes surrounding a small group of actively
inflamed gastric pits or glands, forming focal-enhancing lesions.
Periglandular inflammation is characterized by inflammation of the
pit, isthmus and neck of the stomach, which is predominantly
comprised of lymphocytes and granulomatous inflammation and is not
associated with the rupture of the gastric glands. Focal-enhancing
or perigenoidal gastritis, especially when combined with
granulomas, may resemble granulomatous gastritis in infection,
sarcoidosis or inflammatory bowel disease (Crohn's disease).
However, in ICI-associated gastritis, histiocytes within
focal-enhancing lesions are typically loosely intermingled with
neutrophils and eosinophils, rather than the discrete epithelioid
granulomas frequently seen in infections involving the stomach,
sarcoidosis, or Crohn's disease (40,41).
Finally, the diagnosis of ICI-associated gastritis is effectively a
diagnosis of exclusion. On the basis of excluding other causes of
gastritis, recognizing the gastroscopic manifestations and
histopathological types of ICI-associated gastritis and before
associating them with the time course of symptoms after treatment
will be key for pathologists to make a diagnosis of ICI-associated
gastritis.
To the best of our knowledge, ICI-associated
hemorrhagic gastritis has been rarely reported, where there are no
relevant grading criteria or treatment guidelines. The gastroscopy
results of the present patient indicated an acute inflammatory
reaction in the stomach, which is more frequently caused by heavy
drinking or long-term drug use (42). However, the patient in the present
case had no history of peptic ulcers and was not taking long-term
nonsteroidal anti-inflammatory drugs or alcohol. Only sintilimab
was administered during the period of hemorrhagic gastritis, making
hemorrhagic gastritis caused by sintilimab highly likely. In
addition, cytomegalovirus infection may also lead to hemorrhagic
gastritis (43). However, the
present patient did not have a cytomegalovirus infection, because
viral inclusion bodies were not found in the tissue sections
(44). The patient was initially
treated with a proton pump inhibitor, but there was no improvement
in the bleeding symptoms. Subsequently, methylprednisolone sodium
succinate was administered, leading to rapid alleviation of the
symptoms. This further indicates that the patient's hemorrhagic
gastritis was indeed induced by ICIs rather than other causes. Due
to the current lack of diagnostic criteria for ICI-associated
gastritis, the diagnosis of this condition was essentially a
process of elimination. After ruling out other potential causes of
gastritis and considering the patient's clinical symptoms,
endoscopic findings, pathological biopsy, immunohistochemical
results, medical history and treatment course, it was concluded
that the diagnosis of gastrointestinal bleeding induced by
sintilimab occurred in the current case.
A previous study found that irAEs are associated
with patient prognosis (45) and
that patients who develop irAEs have longer overall survival (OS),
PFS, and objective remission rates (46–50).
In non-small cell lung cancer, patients presenting with multiple
irAEs have prolonged OS and PFS compared with those presenting with
single irAEs (51). In the present
case, the patient also showed initial multi-system adverse
reactions during immunotherapy. Severe adverse reactions may be
associated with a superior prognosis. Gemcitabine combined with
cisplatin (GP), as the standard first-line treatment for recurrent
or metastatic NPC, has a median PFS of 7.0 months and a median OS
of 29.1 months (52). The median
PFS was 11.7 months in the toripalimab plus GP group and 8.0 months
in the GP alone group [hazard ratio (HR), 0.52; P=0.0003] (8). The median PFS was 9.7 months in the
combination group and 6.9 months in the GP group (HR, 0.54;
P=0.0002) (9). In the present case,
the PFS3 was >1.5 years and the patient remains alive. The PFS
time may be associated with superior benefits for patients with
ICI-related multi-system damage, as reported in the literature.
After the occurrence of hemorrhagic gastritis,
according to the treatment principles of ICI-associated colitis,
patients are not recommended for PD-1 antibody treatment again for
irAEs grade ≥3. The present patient was followed-up regularly after
improvement of the hemorrhagic gastritis. The patient's right iliac
and subcutaneous nodules remained stable for 1 year whilst he was
off sintilimab. However, right lung and mediastinal lymph node
enlargement were present. To determine whether the patient could
undergo ICI treatment again, the literature was investigated.
According to a previous study, 25–33% patients who discontinued ICI
therapy after their first irAE experienced recurrence of the same
irAE after reinitiation of the concerned ICI. Therefore, ICI reuse
may be beneficial for patients who develop irAEs and progress again
after ICI discontinuation (53). In
the present case, the efficacy was satisfactory and the PFS was
achieved for ~1.5 years after the third-line treatment with
single-drug sintilimab. However, reactivating PD-1 monoclonal
antibodies was considered too high a risk for the present patient,
where after the multi-disciplinary discussion, IGRT was scheduled
to shrink the mediastinal lymph nodes.
In conclusion, the present report highlights a rare
case of ICI-associated hemorrhagic gastritis, whereas with other
irAEs, recognition and appropriate treatment of ICI-associated
gastritis cannot be underestimated. When clinicians suspect
ICI-associated gastritis, immunosuppressive agents, such as
glucocorticoids, should be used immediately. It is hoped that the
present case will increase clinician awareness of ICI-associated
hemorrhagic gastritis and provide a reference for physicians in
similar situations.
The present study has several limitations. The
diagnosis of suspected immune myocarditis remains uncertain;
although transient elevations in creatine phosphokinase-MB and
myohemoglobin were observed, the absence of clinical symptoms,
normal echocardiography and spontaneous resolution without
intervention weaken the causal association with sintilimab.
Additionally, while the management of ICI-associated hemorrhagic
gastritis achieved symptomatic relief through endoscopic mucosal
evaluation and glucocorticoid step-up therapy, the underlying
immune mechanisms remain unclear due to the lack of systematic
immunomonitoring (dynamic profiling of cytokines such as
interleukin-6 and interferon-γ). It is critical to emphasize that
endomyocardial biopsy-the gold standard for diagnosing immune
myocarditis-is clinically underutilized due to its invasiveness.
Future studies should optimize non-invasive diagnostic parameters
(cardiac biomarkers, electrocardiography, echocardiography, cardiac
MRI and PET/CT) to improve sensitivity and specificity.
Concurrently, elucidating the immunopathology of ICI-associated
hemorrhagic gastritis requires longitudinal analysis of cytokine
dynamics and multi-omics investigations to characterize the
evolving immune microenvironment of gastric mucosa, thereby
comprehensively mapping disease pathogenesis and progression.
Ultimately, constructing toxicity surveillance systems, developing
personalized therapeutic strategies, and achieving precise immune
homeostasis modulation will be critical to advancing immune therapy
management paradigms.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present case report was funded by the Key Laboratory
Foundation of The Sciences and Technology on Plasma Physics
Laboratory (grant no. 6142A04210109), the Key Laboratory of Nuclear
Technology and Medical Transformation, National Health Commission
(grant no. 2021HYX021) and the Natural Science Foundation of
Sichuan Province (grant no. 2022NSFSC0849).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
WT, XD and FG conceptualized the study. WT, YL, XY,
KG, GF, JL, LN and YB collected the data. WT and YL wrote the
original draft preparation. XD and FG wrote, reviewed and edited
the manuscript. FG supervised. FG acquired funding. XD and FG
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Ethical review and approval were not required for
the report on human participants in accordance with the local
legislation and institutional requirements. The
patients/participants provided their written informed consent to
participate in this study.
Patient consent for publication
Written informed consent was obtained from the
individual(s) for the publication of any potentially identifiable
images or data included in this article.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Singh S, Hassan D, Aldawsari HM, Molugulu
N, Shukla R and Kesharwani P: Immune checkpoint inhibitors: A
promising anticancer therapy. Drug Discov Today. 25:223–229. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang R, He Y, Wei B, Lu Y, Zhang J, Zhang
N, He R, Xue H and Zhu B: Nasopharyngeal carcinoma burden and its
attributable risk factors in China: Estimates and forecasts from
1990 to 2050. Int J Environ Res Public Health. 20:29262023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Miao J, Huang H, Chen B, Xiao X,
Zhu M, Liang Y, Xiao W, Huang S, Peng Y, et al: Long-term
survivals, toxicities and the role of chemotherapy in Early-stage
nasopharyngeal carcinoma patients treated with intensity-modulated
radiation therapy: A retrospective study with 15-Year Follow-up.
Cancer Res Treat. 54:118–129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bongiovanni A, Vagheggini A, Fausti V,
Mercatali L, Calpona S, Di Menna G, Miserocchi G and Ibrahim T:
Induction chemotherapy plus concomitant chemoradiotherapy in
nasopharyngeal carcinoma: An updated network meta-analysis. Crit
Rev Oncol Hematol. 160:1032442021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petit C, Lee A, Ma J, Lacas B, Ng WT, Chan
ATC, Hong RL, Chen MY, Chen L, Li WF, et al: Role of chemotherapy
in patients with nasopharynx carcinoma treated with radiotherapy
(MAC-NPC): An updated individual patient data network
meta-analysis. Lancet. 24:611–623. 2023. View Article : Google Scholar
|
|
6
|
Liu X, Zhang Y, Yang KY, Zhang N, Jin F,
Zou GR, Zhu XD, Xie FY, Liang XY, Li WF, et al:
Induction-concurrent chemoradiotherapy with or without sintilimab
in patients with locoregionally advanced nasopharyngeal carcinoma
in China (CONTINUUM): A multicentre, open-label, parallel-group,
randomised, controlled, phase 3 trial. Lancet. 403:2720–2731. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD,
Yang KY, Jin F, Shi M, Chen YP, Hu WH, et al: Gemcitabine and
cisplatin induction chemotherapy in nasopharyngeal carcinoma. N
Engl J Med. 381:1124–1135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen
J, Li J, Shi YR, Jin F, Xu R, et al: Toripalimab or placebo plus
chemotherapy as first-line treatment in advanced nasopharyngeal
carcinoma: A multicenter randomized phase 3 trial. Nat Med.
9:1536–1543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou
T, Shen L, Wu H, Lang J, et al: Camrelizumab versus placebo in
combination with gemcitabine and cisplatin as first-line treatment
for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st):
A multicentre, randomised, double-blind, phase 3 trial. Lancet
Oncol. 22:1162–1174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimura CD, Pulanco MC, Cui W, Lu L and
Zang X: PD-L1 and B7-1 Cis-Interaction: New mechanisms in immune
checkpoints and immunotherapies. Trends Mol Med. 27:207–219. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Mai W, Jiang W and Geng Q:
Sintilimab: A promising Anti-tumor PD-1 antibody. Front Oncol.
10:5945582020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X and Yi Y: Recent updates on
Sintilimab in solid tumor immunotherapy. Biomark Res. 8:692020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li
Q, Lu Y, Chen Y, Guo Y, et al: Sintilimab plus a bevacizumab
biosimilar (IBI305) versus sorafenib in unresectable hepatocellular
carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study.
Lancet Oncol. 22:977–990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Ma R, et al: Efficacy and safety of
sintilimab plus pemetrexed and platinum as First-line treatment for
locally advanced or metastatic nonsquamous NSCLC: A randomized,
Double-blind, phase 3 study (Oncology pRogram by InnovENT
anti-PD-1-11). J Thorac Oncol. 15:1636–1646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen
G, Zhang L, Huang D, Cang S, Yang Z, et al: Sintilimab plus
platinum and gemcitabine as First-line treatment for advanced or
metastatic squamous NSCLC: Results from a randomized, Double-blind,
phase 3 trial (ORIENT-12). J Thorac Oncol. 16:1501–1511. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): multicentre, randomised, double blind, phase 3 trial.
BMJ. 377:e0687142022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D,
Wang W, Wang H, Wang H, He K, et al: Efficacy and safety of
neoadjuvant sintilimab, oxaliplatin and capecitabine in patients
with locally advanced, resectable gastric or gastroesophageal
junction adenocarcinoma: Early results of a phase 2 study. J
Immunother Cancer. 10:e0036352022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ai Q, Chen W, Li Y and Li G: Upper
gastrointestinal tract IrAEs: A case report about
Sintilimab-induced acute erosive hemorrhagic gastritis. Front
Immunol. 13:8409162022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elmasry M, Dong B, Rios C, Breaux A and
Miller D: Delayed hemorrhagic gastritis caused by immunotherapy in
a patient With metastatic melanoma. Am J Med Sci. 364:343–346.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi M, Yamaguchi O, Nagata K, Nonaka
K and Ryozawa S: Acute hemorrhagic gastritis after nivolumab
treatment. Gastrointest Endosc. 86:915–916. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao BB, Robertson S and Philpott J:
Checkpoint Inhibitor-induced hemorrhagic gastritis with
pembrolizumab. Am J Gastroenterol. 114:1962019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bazarbashi AN, Dolan RD, Yang J and
Perencevich ML: Combination checkpoint inhibitor-induced
hemorrhagic gastritis. ACG Case Rep J. 7:e004022020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brahmer JR, Abu-Sbeih H, Ascierto PA,
Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E,
Johnson DB, et al: Society for immunotherapy of cancer (SITC)
clinical practice guideline on immune checkpoint inhibitor-related
adverse events. J Immunother Cancer. 9:e0024352021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elia G, Ferrari SM, Galdiero MR, Ragusa F,
Paparo SR, Ruffilli I, Varricchi G, Fallahi P and Antonelli A: New
insight in endocrine-related adverse events associated to immune
checkpoint blockade. Best Pract Res Clin Endocrinol Metab.
34:1013702020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, et al:
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng W, Kang K, Zhao A and Wu Y: Dual
blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung
cancer. J Hematol Oncol. 17:542024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown TJ, Mamtani R and Bange EM:
Immunotherapy adverse effects. JAMA Oncol. 7:19082021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samimi M: Immune checkpoint inhibitors and
beyond: An overview of Immune-based therapies in merkel cell
carcinoma. Am J Clin Dermatol. 20:391–407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22:392020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Russo L, Avesani G, Gui B, Trombadori CML,
Salutari V, Perri MT, Di Paola V, Rodolfino E, Scambia G and
Manfredi R: Immunotherapy-related imaging findings in patients with
gynecological malignancies: What radiologists need to know. Korean
J Radiol. 22:1310–1322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugiyama Y, Tanabe H, Matsuya T, Kobayashi
Y, Murakami Y, Sasaki T, Kunogi T, Takahashi K, Ando K, Ueno N, et
al: Severe immune checkpoint inhibitor-associated gastritis: A case
series and literature review. Endosc Int Open. 10:E982–E989. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugiyama Y, Tanabe H and Fujiya M: Immune
checkpoint inhibitor-related gastritis in a patient with metastatic
melanoma. JGH Open. 5:1218–1219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hayashi Y, Hosoe N, Takabayashi K, Limpias
Kamiya KJL, Tsugaru K, Shimozaki K, Hirata K, Fukuhara K, Fukuhara
S, Mutaguchi M, et al: Clinical, endoscopic, and pathological
characteristics of immune checkpoint Inhibitor-induced
gastroenterocolitis. Dig Dis Sci. 66:2129–2134. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johncilla M, Misdraji J, Pratt DS, Agoston
AT, Lauwers GY, Srivastava A and Doyle LA: Ipilimumab-associated
hepatitis: Clinicopathologic characterization in a series of 11
cases. Am J Surg Pathol. 39:1075–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oble DA, Mino-Kenudson M, Goldsmith J,
Hodi FS, Seliem RM, Dranoff G, Mihm M, Hasserjian R and Lauwers GY:
Alpha-CTLA-4 mAb-associated panenteritis: A histologic and
immunohistochemical analysis. Am J Surg Pathol. 32:1130–1137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang ML and Deshpande V: Histopathology
of gastrointestinal Immune-related adverse events: A practical
review for the practicing pathologist. Am J Surg Pathol.
46:e15–e26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin J, Lin ZQ, Zheng SC and Chen Y: Immune
checkpoint inhibitor-associated gastritis: Patterns and management.
World J Gastroenterol. 30:1941–1948. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johncilla M, Grover S, Zhang X, Jain D and
Srivastava A: Morphological spectrum of immune check-point
inhibitor therapy-associated gastritis. Histopathology. 76:531–539.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei H, Sun W, Liu X and Wang C: Clinical
characteristics and outcomes of pembrolizumab induced gastritis: A
systematic review of the literature. J Gastrointestinal Cancer.
55:1–8. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Irshaid L, Robert ME and Zhang X: Immune
checkpoint Inhibitor-induced upper gastrointestinal tract
inflammation shows morphologic similarities to, but is
immunologically distinct from, helicobacter pylori gastritis and
celiac disease. Arch Pathol Lab Med. 145:191–200. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patil PA and Zhang X: Pathologic
manifestations of gastrointestinal and hepatobiliary injury in
immune checkpoint inhibitor therapy. Arch Pathol Lab Med.
145:571–582. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pennelli G, Grillo F, Galuppini F,
Ingravallo G, Pilozzi E, Rugge M, Fiocca R, Fassan M and Mastracci
L: Gastritis: Update on etiological features and histological
practical approach. Pathologica. 112:153–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H, Ha SY, Kim J, Kang M and Lee J:
Severe cytomegalovirus gastritis after pembrolizumab in a patient
with melanoma. Curr Oncol. 27:e436–e439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yeh PJ, Wu RC, Chen CL, Chiu CT, Lai MW,
Chen CC, Chiu CH, Pan YB, Lin WR and Le PH: Cytomegalovirus
diseases of the gastrointestinal tract in immunocompetent patients:
A narrative review. Viruses. 16:3462024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kfoury M, Najean M, Lappara A, Voisin AL,
Champiat S, Michot JM, Laghouati S, Robert C, Besse B, Soria JC, et
al: Analysis of the association between prospectively collected
immune-related adverse events and survival in patients with solid
tumor treated with immune-checkpoint blockers, taking into account
immortal-time bias. Cancer Treat Rev. 110:1024522022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barron CC, Stefanova I, Cha Y, Elsolh K,
Zereshkian A, Gaafour N and McWhirter E: Chronic immune-related
adverse events in patients with cancer receiving immune checkpoint
inhibitors: A systematic review. J Immunother Cancer.
11:e0065002023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou Y, Xia R, Xiao H, Pu D, Long Y, Ding
Z, Liu J and Ma X: Thyroid function abnormality induced by PD-1
inhibitors have a positive impact on survival in patients with
non-small cell lung cancer. Int Immunopharmacol. 91:1072962021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Daher R, Ruplin A, Gupta S, Spiess PE,
Kamat AM, Cigliola A, Tateo V, Mercinelli C, Grivas P and Necchi A:
The spectrum of cutaneous toxicities related to novel genitourinary
cancer therapies. Crit Rev Oncol Hematol. 200:1044202024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pang X, Qian J, Jin H, Zhang L, Lin L,
Wang Y, Lei Y, Zhou Z, Li M and Zhang H: Durable benefit from
immunotherapy and accompanied lupus erythematosus in pancreatic
adenocarcinoma with DNA repair deficiency. J Immunother Cancer.
8:e0004632020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chan L, Hwang SJE, Byth K, Kyaw M, Carlino
MS, Chou S and Fernandez-Penas P: Survival and prognosis of
individuals receiving programmed cell death 1 inhibitor with and
without immunologic cutaneous adverse events. J Am Acad Dermatol.
82:311–316. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shankar B, Zhang J, Naqash AR, Forde PM,
Feliciano JL, Marrone KA, Ettinger DS, Hann CL, Brahmer JR,
Ricciuti B, et al: Multisystem Immune-related adverse events
associated with immune checkpoint inhibitors for treatment of
Non-small cell lung cancer. JAMA Oncol. 6:1952–1956. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang L, Huang Y, Hong S, Yang Y, Yu G,
Jia J, Peng P, Wu X, Lin Q, Xi X, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin in recurrent or metastatic
nasopharyngeal carcinoma: A multicentre, randomised, open-label,
phase 3 trial. Lancet. 388:1883–1892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dolladille C, Ederhy S, Sassier M, Cautela
J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF,
et al: Immune checkpoint inhibitor rechallenge after Immune-related
adverse events in patients with cancer. JAMA Oncol. 6:865–871.
2020. View Article : Google Scholar : PubMed/NCBI
|