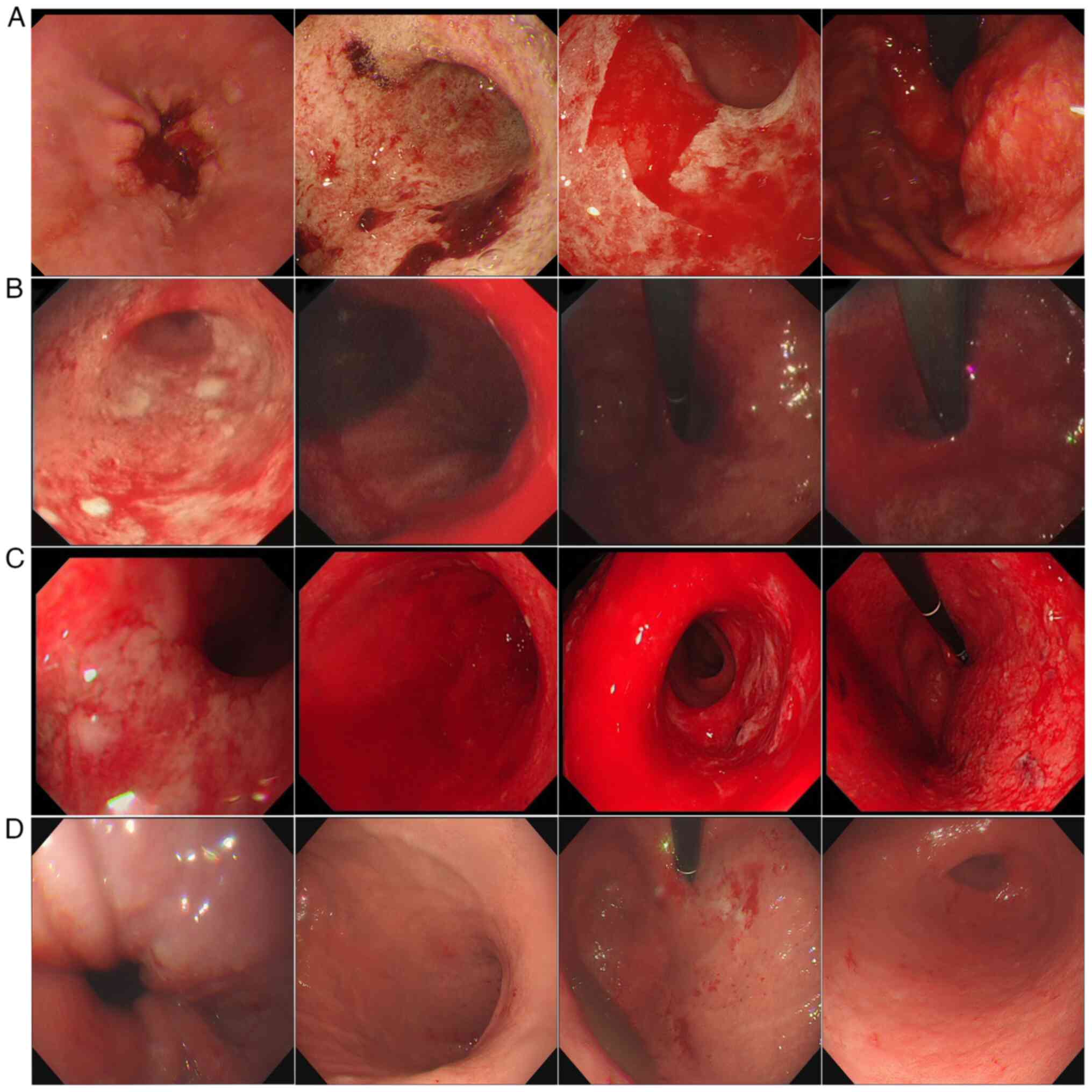
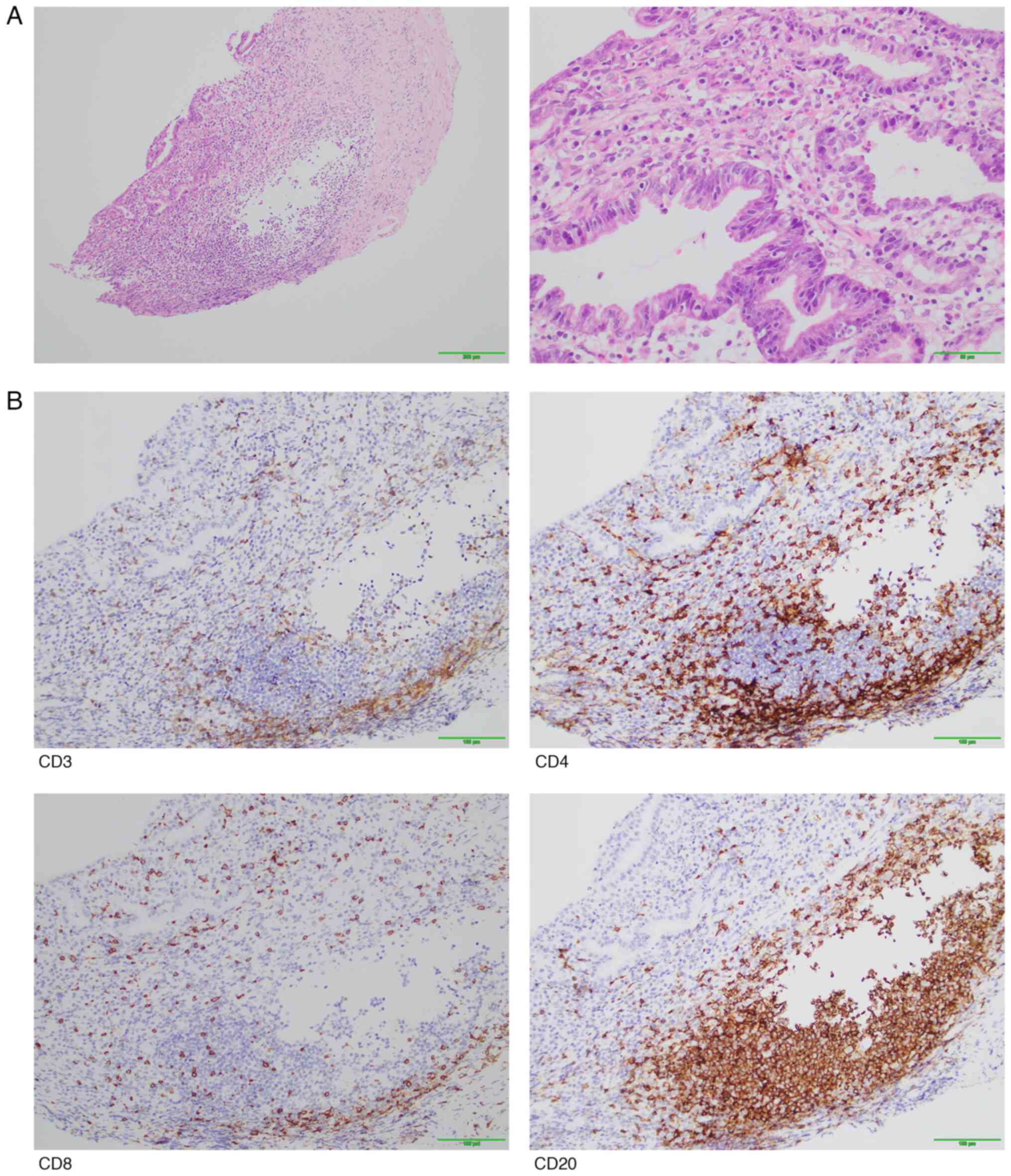
|
1
|
Singh S, Hassan D, Aldawsari HM, Molugulu
N, Shukla R and Kesharwani P: Immune checkpoint inhibitors: A
promising anticancer therapy. Drug Discov Today. 25:223–229. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhang R, He Y, Wei B, Lu Y, Zhang J, Zhang
N, He R, Xue H and Zhu B: Nasopharyngeal carcinoma burden and its
attributable risk factors in China: Estimates and forecasts from
1990 to 2050. Int J Environ Res Public Health. 20:29262023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang L, Miao J, Huang H, Chen B, Xiao X,
Zhu M, Liang Y, Xiao W, Huang S, Peng Y, et al: Long-term
survivals, toxicities and the role of chemotherapy in Early-stage
nasopharyngeal carcinoma patients treated with intensity-modulated
radiation therapy: A retrospective study with 15-Year Follow-up.
Cancer Res Treat. 54:118–129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bongiovanni A, Vagheggini A, Fausti V,
Mercatali L, Calpona S, Di Menna G, Miserocchi G and Ibrahim T:
Induction chemotherapy plus concomitant chemoradiotherapy in
nasopharyngeal carcinoma: An updated network meta-analysis. Crit
Rev Oncol Hematol. 160:1032442021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petit C, Lee A, Ma J, Lacas B, Ng WT, Chan
ATC, Hong RL, Chen MY, Chen L, Li WF, et al: Role of chemotherapy
in patients with nasopharynx carcinoma treated with radiotherapy
(MAC-NPC): An updated individual patient data network
meta-analysis. Lancet. 24:611–623. 2023. View Article : Google Scholar
|
|
6
|
Liu X, Zhang Y, Yang KY, Zhang N, Jin F,
Zou GR, Zhu XD, Xie FY, Liang XY, Li WF, et al:
Induction-concurrent chemoradiotherapy with or without sintilimab
in patients with locoregionally advanced nasopharyngeal carcinoma
in China (CONTINUUM): A multicentre, open-label, parallel-group,
randomised, controlled, phase 3 trial. Lancet. 403:2720–2731. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zhang Y, Chen L, Hu GQ, Zhang N, Zhu XD,
Yang KY, Jin F, Shi M, Chen YP, Hu WH, et al: Gemcitabine and
cisplatin induction chemotherapy in nasopharyngeal carcinoma. N
Engl J Med. 381:1124–1135. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mai HQ, Chen QY, Chen D, Hu C, Yang K, Wen
J, Li J, Shi YR, Jin F, Xu R, et al: Toripalimab or placebo plus
chemotherapy as first-line treatment in advanced nasopharyngeal
carcinoma: A multicenter randomized phase 3 trial. Nat Med.
9:1536–1543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yang Y, Qu S, Li J, Hu C, Xu M, Li W, Zhou
T, Shen L, Wu H, Lang J, et al: Camrelizumab versus placebo in
combination with gemcitabine and cisplatin as first-line treatment
for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st):
A multicentre, randomised, double-blind, phase 3 trial. Lancet
Oncol. 22:1162–1174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nishimura CD, Pulanco MC, Cui W, Lu L and
Zang X: PD-L1 and B7-1 Cis-Interaction: New mechanisms in immune
checkpoints and immunotherapies. Trends Mol Med. 27:207–219. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang L, Mai W, Jiang W and Geng Q:
Sintilimab: A promising Anti-tumor PD-1 antibody. Front Oncol.
10:5945582020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu X and Yi Y: Recent updates on
Sintilimab in solid tumor immunotherapy. Biomark Res. 8:692020.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ren Z, Xu J, Bai Y, Xu A, Cang S, Du C, Li
Q, Lu Y, Chen Y, Guo Y, et al: Sintilimab plus a bevacizumab
biosimilar (IBI305) versus sorafenib in unresectable hepatocellular
carcinoma (ORIENT-32): A randomised, open-label, phase 2–3 study.
Lancet Oncol. 22:977–990. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang Y, Wang Z, Fang J, Yu Q, Han B, Cang
S, Chen G, Mei X, Yang Z, Ma R, et al: Efficacy and safety of
sintilimab plus pemetrexed and platinum as First-line treatment for
locally advanced or metastatic nonsquamous NSCLC: A randomized,
Double-blind, phase 3 study (Oncology pRogram by InnovENT
anti-PD-1-11). J Thorac Oncol. 15:1636–1646. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou C, Wu L, Fan Y, Wang Z, Liu L, Chen
G, Zhang L, Huang D, Cang S, Yang Z, et al: Sintilimab plus
platinum and gemcitabine as First-line treatment for advanced or
metastatic squamous NSCLC: Results from a randomized, Double-blind,
phase 3 trial (ORIENT-12). J Thorac Oncol. 16:1501–1511. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lu Z, Wang J, Shu Y, Liu L, Kong L, Yang
L, Wang B, Sun G, Ji Y, Cao G, et al: Sintilimab versus placebo in
combination with chemotherapy as first line treatment for locally
advanced or metastatic oesophageal squamous cell carcinoma
(ORIENT-15): multicentre, randomised, double blind, phase 3 trial.
BMJ. 377:e0687142022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang H, Yu X, Li N, Kong M, Ma Z, Zhou D,
Wang W, Wang H, Wang H, He K, et al: Efficacy and safety of
neoadjuvant sintilimab, oxaliplatin and capecitabine in patients
with locally advanced, resectable gastric or gastroesophageal
junction adenocarcinoma: Early results of a phase 2 study. J
Immunother Cancer. 10:e0036352022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ai Q, Chen W, Li Y and Li G: Upper
gastrointestinal tract IrAEs: A case report about
Sintilimab-induced acute erosive hemorrhagic gastritis. Front
Immunol. 13:8409162022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Elmasry M, Dong B, Rios C, Breaux A and
Miller D: Delayed hemorrhagic gastritis caused by immunotherapy in
a patient With metastatic melanoma. Am J Med Sci. 364:343–346.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi M, Yamaguchi O, Nagata K, Nonaka
K and Ryozawa S: Acute hemorrhagic gastritis after nivolumab
treatment. Gastrointest Endosc. 86:915–916. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rao BB, Robertson S and Philpott J:
Checkpoint Inhibitor-induced hemorrhagic gastritis with
pembrolizumab. Am J Gastroenterol. 114:1962019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bazarbashi AN, Dolan RD, Yang J and
Perencevich ML: Combination checkpoint inhibitor-induced
hemorrhagic gastritis. ACG Case Rep J. 7:e004022020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brahmer JR, Abu-Sbeih H, Ascierto PA,
Brufsky J, Cappelli LC, Cortazar FB, Gerber DE, Hamad L, Hansen E,
Johnson DB, et al: Society for immunotherapy of cancer (SITC)
clinical practice guideline on immune checkpoint inhibitor-related
adverse events. J Immunother Cancer. 9:e0024352021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Elia G, Ferrari SM, Galdiero MR, Ragusa F,
Paparo SR, Ruffilli I, Varricchi G, Fallahi P and Antonelli A: New
insight in endocrine-related adverse events associated to immune
checkpoint blockade. Best Pract Res Clin Endocrinol Metab.
34:1013702020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haanen J, Obeid M, Spain L, Carbonnel F,
Wang Y, Robert C, Lyon AR, Wick W, Kostine M, Peters S, et al:
Management of toxicities from immunotherapy: ESMO Clinical Practice
Guideline for diagnosis, treatment and follow-up. Ann Oncol.
33:1217–1238. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng W, Kang K, Zhao A and Wu Y: Dual
blockade immunotherapy targeting PD-1/PD-L1 and CTLA-4 in lung
cancer. J Hematol Oncol. 17:542024. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Brown TJ, Mamtani R and Bange EM:
Immunotherapy adverse effects. JAMA Oncol. 7:19082021. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Samimi M: Immune checkpoint inhibitors and
beyond: An overview of Immune-based therapies in merkel cell
carcinoma. Am J Clin Dermatol. 20:391–407. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Darnell EP, Mooradian MJ, Baruch EN,
Yilmaz M and Reynolds KL: Immune-related adverse events (irAEs):
Diagnosis, management, and clinical pearls. Curr Oncol Rep.
22:392020. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Russo L, Avesani G, Gui B, Trombadori CML,
Salutari V, Perri MT, Di Paola V, Rodolfino E, Scambia G and
Manfredi R: Immunotherapy-related imaging findings in patients with
gynecological malignancies: What radiologists need to know. Korean
J Radiol. 22:1310–1322. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Sugiyama Y, Tanabe H, Matsuya T, Kobayashi
Y, Murakami Y, Sasaki T, Kunogi T, Takahashi K, Ando K, Ueno N, et
al: Severe immune checkpoint inhibitor-associated gastritis: A case
series and literature review. Endosc Int Open. 10:E982–E989. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sugiyama Y, Tanabe H and Fujiya M: Immune
checkpoint inhibitor-related gastritis in a patient with metastatic
melanoma. JGH Open. 5:1218–1219. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hayashi Y, Hosoe N, Takabayashi K, Limpias
Kamiya KJL, Tsugaru K, Shimozaki K, Hirata K, Fukuhara K, Fukuhara
S, Mutaguchi M, et al: Clinical, endoscopic, and pathological
characteristics of immune checkpoint Inhibitor-induced
gastroenterocolitis. Dig Dis Sci. 66:2129–2134. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Johncilla M, Misdraji J, Pratt DS, Agoston
AT, Lauwers GY, Srivastava A and Doyle LA: Ipilimumab-associated
hepatitis: Clinicopathologic characterization in a series of 11
cases. Am J Surg Pathol. 39:1075–1084. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Oble DA, Mino-Kenudson M, Goldsmith J,
Hodi FS, Seliem RM, Dranoff G, Mihm M, Hasserjian R and Lauwers GY:
Alpha-CTLA-4 mAb-associated panenteritis: A histologic and
immunohistochemical analysis. Am J Surg Pathol. 32:1130–1137. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang ML and Deshpande V: Histopathology
of gastrointestinal Immune-related adverse events: A practical
review for the practicing pathologist. Am J Surg Pathol.
46:e15–e26. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Lin J, Lin ZQ, Zheng SC and Chen Y: Immune
checkpoint inhibitor-associated gastritis: Patterns and management.
World J Gastroenterol. 30:1941–1948. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Johncilla M, Grover S, Zhang X, Jain D and
Srivastava A: Morphological spectrum of immune check-point
inhibitor therapy-associated gastritis. Histopathology. 76:531–539.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lei H, Sun W, Liu X and Wang C: Clinical
characteristics and outcomes of pembrolizumab induced gastritis: A
systematic review of the literature. J Gastrointestinal Cancer.
55:1–8. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Irshaid L, Robert ME and Zhang X: Immune
checkpoint Inhibitor-induced upper gastrointestinal tract
inflammation shows morphologic similarities to, but is
immunologically distinct from, helicobacter pylori gastritis and
celiac disease. Arch Pathol Lab Med. 145:191–200. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Patil PA and Zhang X: Pathologic
manifestations of gastrointestinal and hepatobiliary injury in
immune checkpoint inhibitor therapy. Arch Pathol Lab Med.
145:571–582. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pennelli G, Grillo F, Galuppini F,
Ingravallo G, Pilozzi E, Rugge M, Fiocca R, Fassan M and Mastracci
L: Gastritis: Update on etiological features and histological
practical approach. Pathologica. 112:153–165. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Kim H, Ha SY, Kim J, Kang M and Lee J:
Severe cytomegalovirus gastritis after pembrolizumab in a patient
with melanoma. Curr Oncol. 27:e436–e439. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yeh PJ, Wu RC, Chen CL, Chiu CT, Lai MW,
Chen CC, Chiu CH, Pan YB, Lin WR and Le PH: Cytomegalovirus
diseases of the gastrointestinal tract in immunocompetent patients:
A narrative review. Viruses. 16:3462024. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Kfoury M, Najean M, Lappara A, Voisin AL,
Champiat S, Michot JM, Laghouati S, Robert C, Besse B, Soria JC, et
al: Analysis of the association between prospectively collected
immune-related adverse events and survival in patients with solid
tumor treated with immune-checkpoint blockers, taking into account
immortal-time bias. Cancer Treat Rev. 110:1024522022. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Barron CC, Stefanova I, Cha Y, Elsolh K,
Zereshkian A, Gaafour N and McWhirter E: Chronic immune-related
adverse events in patients with cancer receiving immune checkpoint
inhibitors: A systematic review. J Immunother Cancer.
11:e0065002023. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Zhou Y, Xia R, Xiao H, Pu D, Long Y, Ding
Z, Liu J and Ma X: Thyroid function abnormality induced by PD-1
inhibitors have a positive impact on survival in patients with
non-small cell lung cancer. Int Immunopharmacol. 91:1072962021.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Daher R, Ruplin A, Gupta S, Spiess PE,
Kamat AM, Cigliola A, Tateo V, Mercinelli C, Grivas P and Necchi A:
The spectrum of cutaneous toxicities related to novel genitourinary
cancer therapies. Crit Rev Oncol Hematol. 200:1044202024.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pang X, Qian J, Jin H, Zhang L, Lin L,
Wang Y, Lei Y, Zhou Z, Li M and Zhang H: Durable benefit from
immunotherapy and accompanied lupus erythematosus in pancreatic
adenocarcinoma with DNA repair deficiency. J Immunother Cancer.
8:e0004632020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chan L, Hwang SJE, Byth K, Kyaw M, Carlino
MS, Chou S and Fernandez-Penas P: Survival and prognosis of
individuals receiving programmed cell death 1 inhibitor with and
without immunologic cutaneous adverse events. J Am Acad Dermatol.
82:311–316. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Shankar B, Zhang J, Naqash AR, Forde PM,
Feliciano JL, Marrone KA, Ettinger DS, Hann CL, Brahmer JR,
Ricciuti B, et al: Multisystem Immune-related adverse events
associated with immune checkpoint inhibitors for treatment of
Non-small cell lung cancer. JAMA Oncol. 6:1952–1956. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Zhang L, Huang Y, Hong S, Yang Y, Yu G,
Jia J, Peng P, Wu X, Lin Q, Xi X, et al: Gemcitabine plus cisplatin
versus fluorouracil plus cisplatin in recurrent or metastatic
nasopharyngeal carcinoma: A multicentre, randomised, open-label,
phase 3 trial. Lancet. 388:1883–1892. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Dolladille C, Ederhy S, Sassier M, Cautela
J, Thuny F, Cohen AA, Fedrizzi S, Chrétien B, Da-Silva A, Plane AF,
et al: Immune checkpoint inhibitor rechallenge after Immune-related
adverse events in patients with cancer. JAMA Oncol. 6:865–871.
2020. View Article : Google Scholar : PubMed/NCBI
|