Introduction
Epithelial ovarian cancer (EOC) is the most common
type of ovarian malignancy. It accounts for 85–90% of all malignant
ovarian tumors, with a higher incidence in middle-aged and elderly
women worldwide (1). Based on
histological differentiation, EOCs are classified into serous,
mucinous and endometrioid carcinoma subtypes. The histological
origins of these tumors are diverse, with high-grade serous ovarian
carcinoma (HGSOC) believed to arise from serous tubal
intraepithelial carcinoma before implantation on the ovarian
surface. It represents the most prevalent histological subtype of
EOC, constituting >70% of cases (1). HGSOC that develops on the ovaries or
fallopian tubes lack anatomical barriers, allowing tumor cells to
disseminate freely within the internal organs of the body (2). Once detached from the primary tumor,
these cells may colonize the retroperitoneum or abdominal cavity,
rapidly forming secondary tumor nodules (3,4). The
present report describes a case of HGSOC presenting as a
retroperitoneal tumor. This is a manifestation that, whilst not
uncommon, presents notable diagnostic and therapeutic challenges
due to its nonspecific symptoms and the anatomical complexity of
the retroperitoneal space.
Case report
A 73-year-old woman presented to the Department of
Urology of The First Affiliated Hospital of Guangxi Medical
University (Nanning, China) in January 2024 with a 2-week history
of right lower abdominal pain. The pain was described as paroxysmal
colic, progressively worsening over time and the patient required
painkillers for sleep. A physical examination revealed no notable
renal tenderness or obvious abdominal mass. The medical history of
the patient included uncontrolled hypertension and the obstetric
history revealed three children, no miscarriages and menopause at
50 years old.
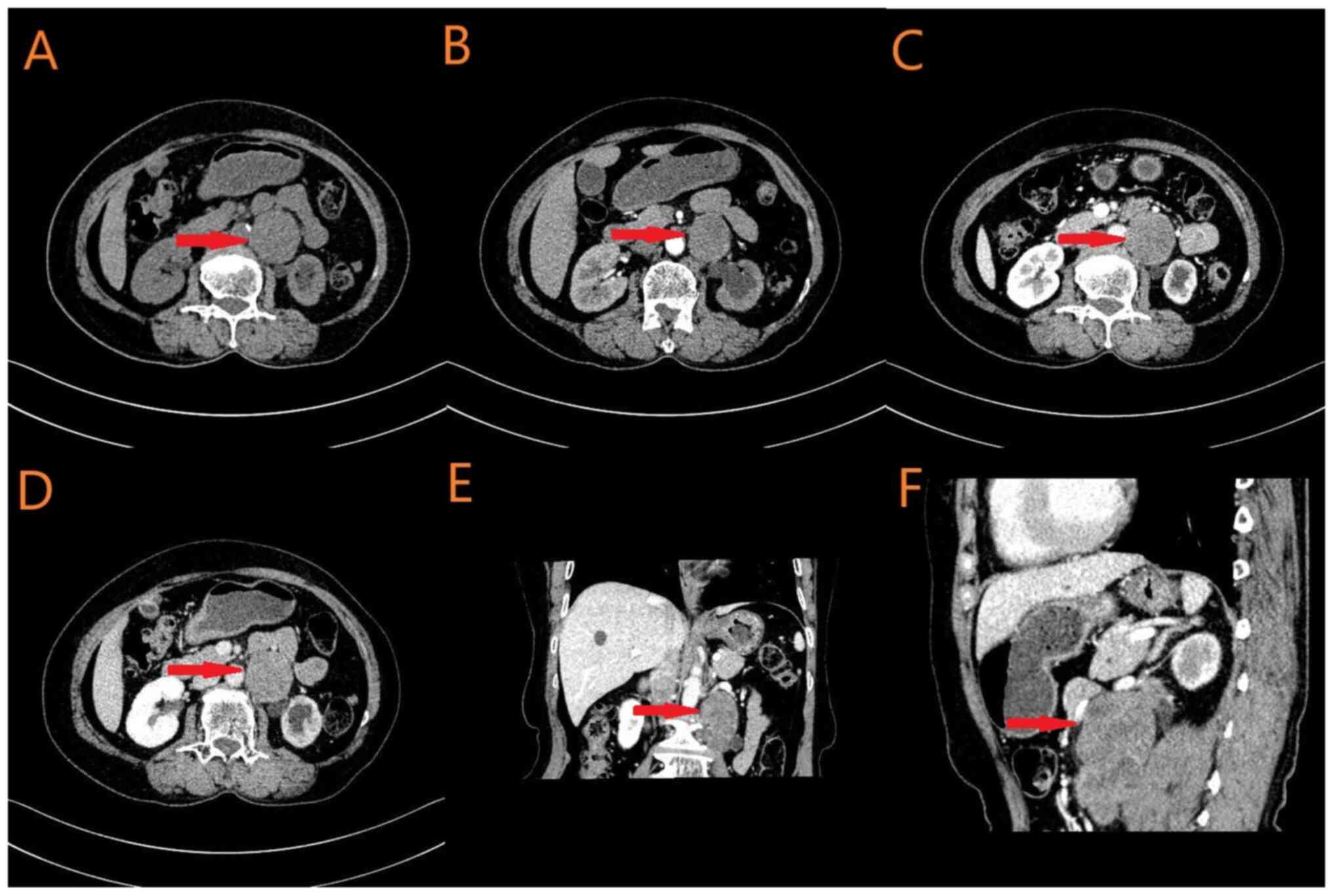
Abdominal CT revealed a 4.2×4.0×8.2 cm mass in the
left posterior abdominal cavity, suspected to be a malignant tumor
involving the left psoas major muscle and left ureter, with
para-aortic lymph node metastasis (Fig.
1). The serum Cancer Antigen 125(CA125 level was mildly
elevated at 39.7 U/ml (normal range, <35 U/ml), while other
tumor markers remained within normal limits, such as cancer antigen
199 (CA199) and CA242. Preoperative renal function tests indicated
renal insufficiency, with a serum creatinine level of 102 µmol/l
(normal range, 50–98 µmol/l). Additionally, adrenal function
abnormalities were noted, with norepinephrine levels elevated at
5,942.7 pmol/l (normal range, 615-3,240 pmol/l). Abdominal
sonography detected a solid mass in the left retroabdominal cavity
(data not shown), whilst renal emission CT (ECT) revealed a notably
reduced left glomerular filtration rate (GFR) of 2.38 ml/min
(normal range, 50–60 ml/min).
Following symptomatic management, including blood
pressure control and analgesia, the patient was evaluated for
surgical intervention. The selected surgical plan included a
laparoscopic retroperitoneal mass resection, left ureteral stent
placement, and if necessary, a left nephrectomy.
Intraoperatively, the left upper ureter was revealed
to be notably narrowed, precluding the successful placement of a
5-mm circumference double-J ureteral stent. Consequently, a left
ureteroscopy was performed, and an external ureteral stent (5 mm in
circumference) was inserted as a ureteral marker. Laparoscopic
exploration revealed a retroperitoneal tumor located inferior to
the left kidney, adherent to the lower pole of the kidney and the
left renal vein, with the invasion of the left psoas major muscle
and left upper ureter. Given the proximity of the tumor to the left
kidney and ureter, and the likelihood of direct invasion, an open
surgical approach was recommended. Following an intraoperative
discussion with the family of the patient, an open left
retroperitoneal tumor resection, left nephrectomy and partial left
ureteral resection were performed to achieve radical tumor
removal.
The surgery was successful, and the patient
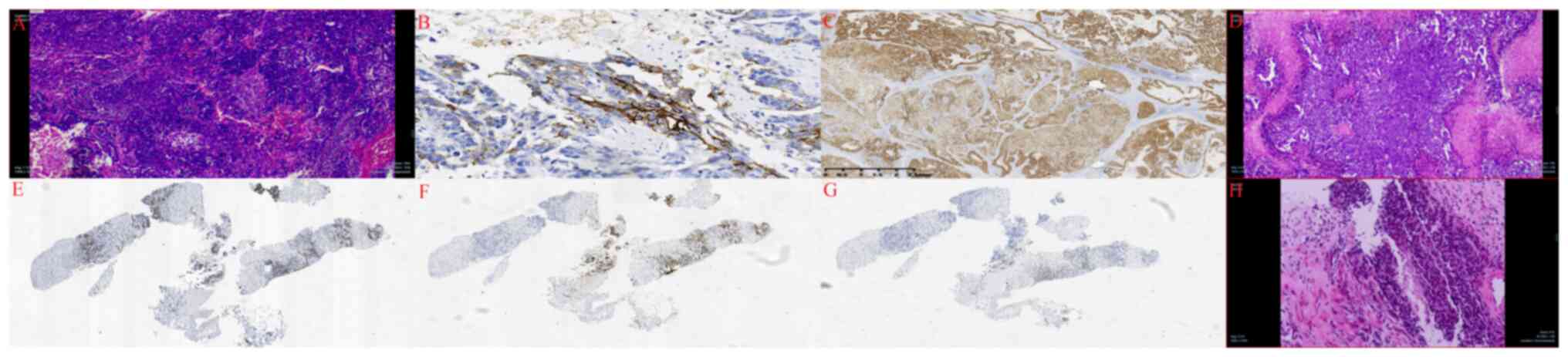
recovered well postoperatively. The pathology report revealed the
following: i) The retroperitoneal mass was identified as a poorly
differentiated adenocarcinoma with immunohistochemical markers
suggestive of ovarian origin (Fig.
2A). Immunohistochemical analysis demonstrated positive CA125
(Fig. 2B) and paired box-8 (PAX-8)
results (Fig. 2C); ii) the tumor
had infiltrated the outer membrane, basal layer and lamina propria
of the ureter; however, the urothelium remained unaffected; iii) no
tumor infiltration was observed in the left renal parenchyma or
perirenal fat (data not shown). For hematoxylin and eosin staining,
tissues were fixed in 4% neutral buffered formalin at room
temperature for ≥24 h, sectioned at 3–5 µm thickness and stained
with hematoxylin (room temperature for 5–10 min) and eosin (room
temperature for 1–3 min). Slides were observed under a light
microscope. For immunohistochemistry, paraffin-embedded tissues
(fixed as aforementioned) were embedded in paraffin and sectioned
at 3–5 µm). Antigen retrieval was performed using citrate buffer
(pH 6.0) at 120°C for 2 min using an autoclave or 95°C for 15 min
using a microwave. Endogenous peroxidase activity was blocked using
3% H2O2. Sections were blocked with 5–10%
Normal Goat Serum (Vector Laboratories) at room temperature for 30
min. Primary antibodies were added to the samples and incubated at
4°C overnight or 37°C for 1–2 h. HRP-conjugated secondary
antibodies were added to samples and incubated at room temperature
for 30 min, followed by DAB chromogen detection.
Given the suspected ovarian origin, further
gynecological examinations were performed post-surgery.
Gynecological sonography revealed a left adnexal hypoechoic mass,
suspected to be metastatic, along with a right adnexal cyst (data
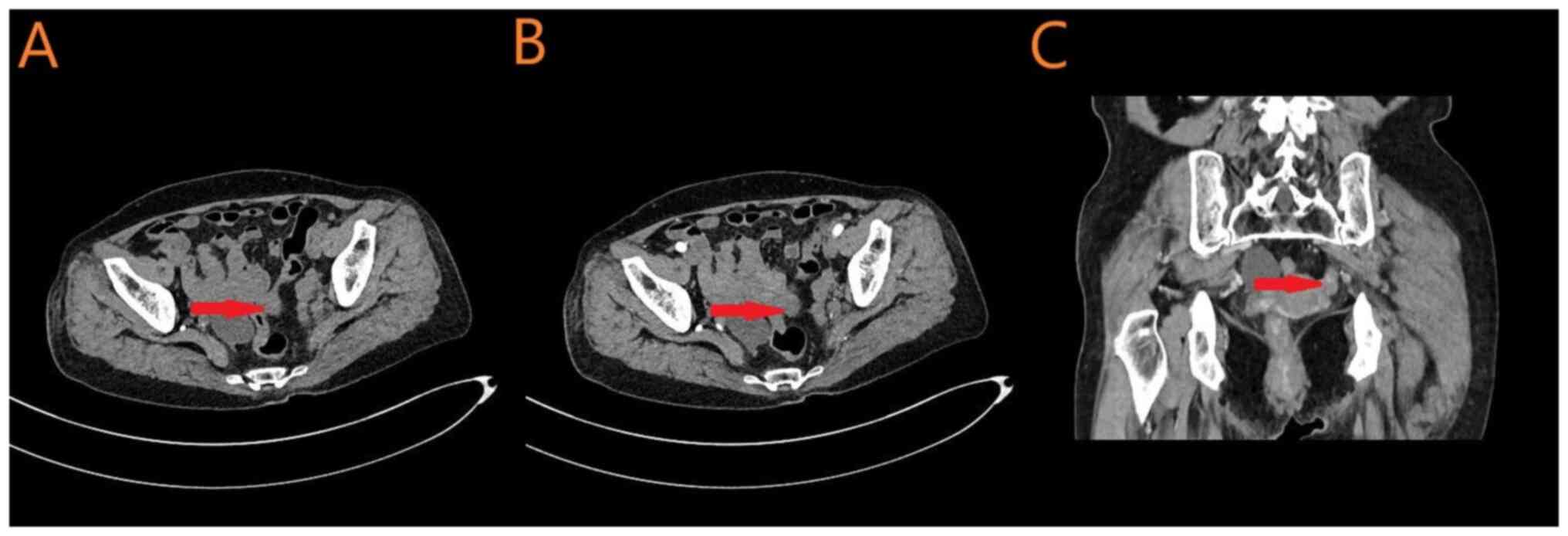
not shown). Furthermore, a pelvic CT (Fig. 3) identified the following: i) A
space-occupying lesion in the left adnexal region and peritoneum,
raising suspicion of malignancy; and ii) A cystic mass in the right
adnexal region, suggesting a benign cystic lesion. Additionally,
PET/CT identified soft tissue metastasis in the left rectouterine
pouch following the retroperitoneal malignant tumor resection (data
not shown). A left adnexal mass of unknown nature prompted a needle
biopsy, which confirmed poorly differentiated adenocarcinoma with
immunohistochemical features consistent with HGSOC origin (Fig. 2D). The immunohistochemical markers,
CA125 (Fig. 2E), PAX-8 (Fig. 2F) and Wilms tumor protein 1 (WT1;
Fig. 2G), were positive.
Based on the aforementioned findings, the patient
was diagnosed with left ovarian HGSOC and poorly differentiated
adenocarcinoma in the retroperitoneal space. Following surgical
resection of the retroperitoneal tumor, the patient recovered well
and was transferred to the Department of Gynecology in March 2024
for further management of the left ovarian malignancy. During
hospitalization, the patient underwent a laparoscopic radical
procedure for ovarian cancer, which included a total hysterectomy,
bilateral adnexa, greater omentum, resection of masses in the
Douglas fossa and cecal surface, and pelvic lymph node dissection.
Postoperative histopathology confirmed HGSOC of the left fallopian
tube (Fig. 2H) with tumor invasion
extending through the entire layer of the fallopian tube and into
local mesangial fiber tissue. Metastatic fibrovascular tissue was
identified in the rectouterine pouch mass. However, no metastatic
involvement was detected in the appendages, pelvic lymph nodes,
intestinal wall masses or right-sided omental tissue (data not
shown).
The patient recovered well after surgery and was
treated with postoperative chemotherapy consisting of intravenous
paclitaxel (210 mg/m2) and intravenous carboplatin (360
mg/m2; TC regimen). To date, the patient has completed
six cycles of TC chemotherapy and two cycles of bevacizumab
maintenance therapy. The TC chemotherapy regimen followed a 3-week
cycle. After chemotherapy, bevacizumab maintenance therapy was
administered in 3-week cycles via intravenous injection at a dose
of 15 mg/kg. After completion of the aforementioned treatments, the
following surveillance protocol was recommended: Quarterly
follow-up visits for the first 2 years post-surgery, semiannual
evaluations from the years 3–5 and annual assessments thereafter.
Surveillance components included gynecological examination, blood
tests, pelvic ultrasound and either PET-CT or tumor-specific CT.
Currently, the patient remains in good health and is
self-sufficient without discomfort.
Discussion
Ovarian cancer is a significant public health
concern. According to the World Health Organization, there are
~225,500 new cases and 140,200 deaths annually, making it the
seventh most common cancer globally. HGSOC accounts for ~90% of
these cases (5,6).
HGSOC is associated with a high mortality rate.
According to the American Cancer Society, the overall 5-year
survival rate for HGSOC across all stages is ~44%, which decreases
to ~25% in advanced-stage cases. The majority of patients are
diagnosed only after metastasis, with only ~13% diagnosed at an
early stage (7). This presents a
critical challenge in ensuring timely treatment and improving
survival outcomes.
In most tumors, metastatic dissemination requires
cells to typically undergo a series of cellular transformations by
crossing the basement membrane, migrating and invading the
vasculature, surviving in circulation and extravasating before
forming a mass in a distant organ or tissue. However, HGSOC
primarily originates from the fallopian tube epithelium, which
lacks a physical anatomical barrier to prevent its spread. As a
result, it disseminates mainly through direct peritoneal extension
rather than through the blood or lymph. Consequently, its symptoms
are often non-specific and predominantly gastrointestinal due to
tumor burden in the abdominal cavity or retroperitoneal space.
Patients frequently present with abdominal pain, distension,
nausea, constipation, anorexia, diarrhea and acid reflux,
complicating early diagnosis and delaying treatment (1).
Currently, histopathological examination remains the
gold standard for diagnosing HGSOC, whereas imaging serves
primarily as a screening tool. Key imaging features of HGSOC
include complex pelvic or peritoneal masses rich in blood vessels,
though these findings are nonspecific (6). Identifying the precise origin of the
tumor is often challenging, leading to frequent misdiagnoses and
delayed treatment. In cases involving retroperitoneal tumors,
comprehensive abdominal and pelvic imaging is essential to exclude
tumors originating from other organs, facilitating earlier and more
accurate diagnosis and treatment of HGSOC (8,9).
Clinical evidence from laboratory tests also serves
a critical role in diagnosing HGSOC. CA125, a glycoprotein derived
from embryonic coelomic epithelium, is absent in normal ovary
tissue but frequently expressed in serous tumors. Elevated CA125
levels are observed in ~90% of patients with advanced HGSOC, often
reaching 500–1,000 U/ml (10).
However, in early-stage HGSOC, only ~50% of patients exhibit
elevated CA125 levels, limiting its use as an early diagnostic
marker. Furthermore, CA125 lacks specificity as elevated levels are
also seen in menopausal women, and in conditions such as
endometriosis, pregnancy and pelvic inflammatory disease. These
limitations restrict its effectiveness as a screening tool for
early-stage HGSOC (11). A total of
two large-scale screening studies, the UKCTOCS trial in the UK
(12) and the PLCO trial in the US
(13), investigated the combined
use of CA125 and gynecological ultrasound for early detection.
Although these studies detected more cases of ovarian cancer, most
patients were diagnosed at an advanced stage, and screening did not
markedly improve overall survival (OS). Consequently, the American
Congress of Obstetricians and Gynecologists recommends against
using CA125 for routine early detection, citing the high risk of
false positives, increased patient anxiety and stress, and
unnecessary healthcare costs (13).
Despite its limitations in early diagnosis, CA125 serves as a
valuable biomarker for monitoring treatment response and disease
recurrence in HGSOC. In patients with advanced ovarian cancer, a
decrease in serum CA125 levels following treatment is associated
with a favorable prognosis and treatment efficacy (14). Additionally, recurrent HGSOC is
often asymptomatic, and CA125 monitoring is the primary method for
detecting relapse. A CA125 level that exceeds twice the upper limit
of normal is commonly used as a threshold for diagnosing recurrence
(15). Therefore, whilst CA125 is
not a reliable marker for early detection, it serves a crucial role
in predicting chemotherapy response and assessing the likelihood of
tumor recurrence in HGSOC (16).
In immunohistochemistry (IHC), CA125 exhibits
specific expression patterns that make it useful for the auxiliary
diagnosis, classification and differential diagnosis of HGSOC.
Strong CA125 expression is observed in 80–90% of HGSOC cases, with
diffuse staining of the cell membrane and cytoplasm. In the present
case, the pathological examination of retroperitoneal tumors,
ovarian biopsy tissues and ovarian cancer lesions demonstrated
positive or strongly positive CA125 expression. CA125 can be used
to distinguish primary ovarian cancer from metastatic cancers of
gastrointestinal or breast origin, as these tumors typically lack
CA125 expression in IHC. However, CA125 alone lacks sufficient
specificity for a definitive diagnosis of HGSOC. When necessary, it
should be used in combination with other immune markers, such as
WT1 and PAX-8, along with morphological assessment, to improve the
diagnostic accuracy of HGSOC (17).
Most patients with HGSOC are diagnosed at an
advanced stage, limiting the effectiveness of radical surgery to a
small subset of stage I patients, where the tumor remains confined
to the ovary or fallopian tube. Due to the proximity of tumors to
adjacent organs, even when surgical separation is technically
feasible, concerns about tumor invasion into surrounding tissues
pose significant challenges for surgeons. Therefore, cytoreductive
surgery remains the primary surgical approach for HGSOC and is
crucial for improving patient prognosis (6). Complete tumor resection is associated
with improved long-term outcomes and certain patients may achieve
clinical remission with chemotherapy. Standard cytoreductive
surgery involves the removal of all visible tumors, reproductive
organs and adjacent tissues such as the cecum, sigmoid colon and
greater omentum. Lymph node resection is performed based on nodal
involvement, with the primary aim of achieving optimal
cytoreduction, defined as residual tumor <1 cm in diameter
(5). In the present case,
preoperative renal ECT revealed a left GFR of 2.38 ml/min,
indicating severe functional impairment of the left kidney and
ureter due to tumor infiltration affecting both renal function and
urinary drainage. During the attempt to place a left ureteral
internal stent intraoperatively, complete luminal obstruction was
encountered at an undetermined level of the left ureter, which
precluded passage of a 5-mm circumference stent. Consequently,
ureteral dimensions were not estimated based on the stenotic
segment and smaller-caliber internal stents were not used.
Ultimately, an external ureteral stent (5-mm circumference) was
placed at a non-stenotic portion of the left ureter for anatomical
marking. Notably, external stent placement was not the initial
approach due to its inherent risk of dislodgement. Upon initiating
retroperitoneal tumor resection, surgical findings confirmed the
preoperative assessment: The retroperitoneal tumor demonstrated
complete encasement of the left kidney and proximal ureter,
rendering safe dissection impossible whilst ensuring tumor-free
margins. Therefore, an en bloc resection of the retroperitoneal
mass was performed with the involved left kidney and proximal
ureter. This complete cytoreductive procedure provided optimal
oncological management, markedly improving the prognostic outlook
of the patient.
Following successful cytoreductive surgery, most
patients with HGSOC undergo adjuvant chemotherapy, except for a
small number of stage I patients who may not require further
treatment (5). The standard
chemotherapy regimen, consisting of six consecutive cycles of
paclitaxel plus carboplatin, has remained the standard treatment
for ovarian cancer for the past two decades (18). For patients unable to undergo
surgery or with extensive metastases, neoadjuvant chemotherapy
(NACT) is a viable alternative. This approach typically consists of
three cycles of chemotherapy before surgery, followed by
cytoreductive surgery and an additional three cycles
postoperatively. A total of two randomized trials have reported
that NACT is a non-inferior option to surgery followed by
chemotherapy in terms of both progression-free survival (PFS) and
OS (19,20). Furthermore, advances in
immunotherapy and targeted therapies have expanded treatment
options for HGSOC. WT1 functions as a tumor suppressor protein and
its inactivation has been implicated in the development of
urogenital or renal embryonic tumors (21). SELLAS Life Sciences Group has
performed clinical trials using a WT1-targeted vaccine for the
treatment of ovarian cancer, malignant pleural mesothelioma and
several hematologic and solid tumors (21). The WT1 vaccine is administered in
combination with an adjuvant and an immune modulator,
granulocyte-macrophage colony-stimulating factor, to enhance the
immune response. Early-stage clinical trials in acute myelogenous
leukemia and mesothelioma have reported promising improvements in
both PFS and OS (22). P16, another
tumor suppressor gene, is implicated in HGSOC pathogenesis.
Anti-aging therapy or tumor immunization vaccines targeting P16 may
serve a potential role in treating HGSOC, as aging and the abnormal
retinoblastoma pathway contribute to P16 overexpression (23). Additionally, the formation of
malignant tumors can result from accumulated genetic mutations in
breast cancer (BRCA)1 or BRCA2. Patients with BRCA1- or
BRCA2-positive HGSOC are more responsive to poly-ADP ribose
polymerase inhibitors, which have shown efficacy in inhibiting
tumor recurrence and progression (24).
A total of ~70% of patients with ovarian cancer
initially respond well to platinum-based chemotherapy, with ≥50%
showing no residual cancer on imaging and serum markers at 5 months
post-treatment. However, recurrence rates are >80%, emphasizing
the necessity for long-term follow-up and continuous monitoring
(2). Based on clinical experience
and the 2023 National Comprehensive Cancer Network guidelines
(25), the authors of the present
study recommend the following follow-up protocol: Imaging and
tumor-marker assessments every 3 months for the first 2 years
post-chemotherapy, every 6 months from years 3–5, and then an
annual evaluation thereafter. High-risk patients with HGSOC,
particularly those with retroperitoneal metastasis, should receive
individualized treatment plans and close follow-up, with active
symptom monitoring to promote early detection, diagnosis and timely
treatment.
In conclusion, the present study assessed the
mechanisms through which HGSOC metastasizes to the retroperitoneal
space. It also discussed the symptoms, diagnosis and treatment
strategies through a case initially diagnosed as a retroperitoneal
tumor. The findings suggest that retroperitoneal metastasis of
HGSOC may be more common in clinical practice than previously
recognized. However, most patients with HGSOC are diagnosed at an
advanced stage, often presenting with non-specific symptoms.
Therefore, it is recommended that patients presenting with
nonspecific symptoms of retroperitoneal tumor undergo timely
abdominal and pelvic imaging and tumor marker tests to rule out
metastases from other primary sites. Elevated serum CA125 levels
may suggest an ovarian tumor. If other primary lesions are
confirmed, a multidisciplinary approach including biopsy should be
implemented to ensure accurate diagnosis and optimal treatment
planning, ultimately improving patient outcomes.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PH and WP conceived and designed the study. HM
provided administrative support. PH and WP supplied study materials
and patient data. PH and JY collected and assembled the data. PH
and WP confirm the authenticity of all the raw data. HM and JY made
substantial contributions to the conception, design of the work,
and data acquisition and interpretation. JY and WP drafted and
revised the work for important intellectual content. PH and HM
approved the final version to be published and agreed to be
accountable for all aspects of the work, ensuring proper resolution
of integrity-related issues. The authors are accountable for all
aspects of the work in ensuring that questions related to the
accuracy or integrity of any part of the work are appropriately
investigated and resolved. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was performed in accordance with
the Declaration of Helsinki (as revised in 2013). The study was
approved by the First Affiliated Hospital of Guangxi Medical
University ethics board (approval no. 2024-E275-01).
Patient consent for publication
Written informed consent was obtained from the
patient to publish the present report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lisio MA, Fu L, Goyeneche A, Gao ZH and
Telleria C: High-grade serous ovarian cancer: Basic sciences,
clinical and therapeutic standpoints. Int J Mol Sci. 20:9522019.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bast RC Jr, Hennessy B and Mills GB: The
biology of ovarian cancer: New opportunities for translation. Nat
Rev Cancer. 9:415–428. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lengyel E: Ovarian cancer development and
metastasis. Am J Pathol. 177:1053–1064. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primers. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kurman RJ, Carcangiu ML, Herrington CS and
Young RH: WHO Classification of Tumours of Female Reproductive
Organs. 4th edition. WHO; Geneva, Switzerland: 2014
|
|
7
|
Ohman AW, Hasan N and Dinulescu DM:
Advances in tumor screening, imaging, and avatar technologies for
high-grade serous ovarian cancer. Front Oncol. 4:3222014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
National Cancer Institute Surveillance,
Epidemiology and End Results Program, . Cancer Stat Facts: Ovarian
Cancer. https://seer.cancer.gov/statfacts/html/ovary.htmlMay
26–2018
|
|
9
|
Gilbert L, Basso O, Sampalis J, Karp I,
Martins C, Feng J, Piedimonte S, Quintal L, Ramanakumar AV,
Takefman J, et al: Assessment of symptomatic women for early
diagnosis of ovarian cancer: Results from the prospective DOvE
pilot project. Lancet Oncol. 13:285–291. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Narod S: Can advanced-stage ovarian cancer
be cured? Nat Rev Clin Oncol. 13:255–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
The American College of Obstetricians and
Gynecologists, . Five Things Physicians and Patients Should
Question. Choosing Wisely; 2013
|
|
12
|
Menon U, Gentry-Maharaj A, Hallett R, Ryan
A, Burnell M, Sharma A, Lewis S, Davies S, Philpott S, Lopes A, et
al: Sensitivity and specificity of multimodal and ultrasound
screening for ovarian cancer, and stage distribution of detected
cancers: Results of the prevalence screen of the UK Collaborative
Trial of Ovarian Cancer Screening (UKCTOCS). Lancet Oncol.
10:327–340. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Buys SS, Partridge E, Black A, Johnson CC,
Lamerato L, Isaacs C, Reding DJ, Greenlee RT, Yokochi LA, Kessel B,
et al: Effect of screening on ovarian cancer mortality: The
prostate, lung, colorectal and ovarian (PLCO) cancer screening
randomized controlled trial. JAMA. 305:2295–2302. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
van Altena AM, Kolwijck E, Spanjer MJ,
Hendriks JC, Massuger LF and de Hullu JA: CA125 nadir concentration
is an independent predictor of tumor recurrence in patients with
ovarian cancer: A population-based study. Gynecol Oncol.
119:265–269. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jayson GC, Kohn EC, Kitchener HC and
Ledermann JA: Ovarian cancer. Lancet. 384:1376–1388. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Piatek S, Panek G, Lewandowski Z, Piatek
D, Kosinski P and Bidzinski M: Nadir CA-125 has prognostic value
for recurrence, but not for survival in patients with ovarian
cancer. Sci Rep. 11:181902021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bates M, Mohamed BM, Lewis F, O'Toole S
and O'Leary JJ: Biomarkers in high grade serous ovarian cancer.
Biochim Biophys Acta Rev Cancer. 1879:1892242024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Markman M: Optimizing primary chemotherapy
in ovarian cancer. Hematol Oncol Clin North Am. 17:957–968. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RH, van der Burg ME, Lacave AJ,
Panici PB, et al: Neoadjuvant chemotherapy or primary surgery in
stage IIIC or IV ovarian cancer. N Engl J Med. 363:943–953. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kehoe S, Hook J, Nankivell M, Jayson GC,
Kitchener H, Lopes T, Luesley D, Perren T, Bannoo S, Mascarenhas M,
et al: Primary chemotherapy versus primary surgery for newly
diagnosed advanced ovarian cancer (CHORUS): An open-label,
randomised, controlled, non-inferiority trial. Lancet. 386:249–257.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Denkert C, Loibl S, Müller B, Eidtmann H,
Schmitt W, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, et
al: Ki67 levels as predictive and prognostic parameters in
pretherapeutic breast cancer core biopsies: A translational
investigation in the neoadjuvant GeparTrio trial. Ann Oncol.
24:2786–2793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi X, Zhang F, Wu H, Liu J, Zong B, Xu C
and Jiang J: Wilms' tumor 1 (WT1) expression and prognosis in solid
cancer patients: A systematic review and meta-analysis. Sci Rep.
5:89242015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Reuschenbach M, Rafiyan M, Karbach J,
Pauligk C, Faulstich F, Sauer M, Prigge ES, Kloor M, Al-Batran S,
Kaufmann AM, et al: Phase I/IIa study of therapeutic p16INK4a
vaccination in patients with HPV-associated cancers. J Clin Oncol.
32:3092–3097. 2014. View Article : Google Scholar
|
|
24
|
Rice JC, Ozcelik H, Maxeiner P, Andrulis I
and Futscher BW: Methylation of the BRCA1 promoter is associated
with decreased BRCA1 mRNA levels in clinical breast cancer
specimens. Carcinogenesis. 21:1761–1765. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
National Comprehensive Cancer Network.
NCCN Clinical Practice Guidelines in Oncology, . Ovarian Cancer,
version 1.2023. National Comprehensive Cancer Network; 2023,
https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1453February
15–2025
|