Introduction
The occurrence of squamous-cell carcinoma (SCC) in
the oral cavity, larynx, trachea, lungs, and residual esophagus as
metachronous malignancies after esophageal cancer surgery is
crucial from a clinical perspective. A history of heavy drinking
and/or smoking, presence of oral flora, and preoperative presence
of periodontal disease are reported as strongly relevant factors
(1–3). Conversely, gastric conduit cancer
(GCC) after digestive reconstruction often occurs due to chronic
inflammation caused by preoperative Helicobacter pylori (HP)
infection, patients' lifestyle, postoperative bile and intestinal
juice reflux, and resulting intestinal metaplasia (1–5). Cases
detected in the early stage can be treated via endoscopic
submucosal dissection (ESD); however, in cases with advanced or
widespread cancer, surgical gastric conduit resection with
lymph-node (LN) dissection around the residual stomach and
digestive tract reconstruction are required (6–14).
Robot-assisted minimally invasive esophagectomy
(RAMIE) has recently been gaining global popularity as a
high-definition, high-quality radical surgery that uses robotic
technology for minimally invasive esophagectomy (8). Robot-assisted surgery (RAS) provides
several advantages, such as a three-dimensional (3D) magnified
view, high degree of freedom for surgical maneuvers, and reduced
misses due to hand tremors, allowing easier, more precise, and
gentle manipulation that is required for intrathoracic esophageal
resection and mediastinal LN dissection (15–19).
These advantages may also prove effective for adhesion dissection
that is required for gastric conduit resection, avoidance of
collateral damage to important organs, and accurate recognition of
modified anatomy in reoperation cases.
Here, we report a case with metachronous scirrhous
GCC originating along the entire conduit, which was safely and
radically resected by robot-assisted transthoracic surgery.
Case report
A 69-year-old Japanese man had undergone RAMIE for
thoracic esophageal SCC without synchronous gastric cancer 5 years
ago. The surgical procedure involved complete mediastinal LN
dissection and esophagectomy in the left-lateral position,
abdominal LN dissection, and gastric conduit creation. Cervical LN
dissection was omitted for minimal invasiveness. For
gastrointestinal reconstruction, a gastric conduit was created via
the posterior mediastinal route, and esophagogastric anastomosis
was performed at the cervical site. Postoperatively, pneumonia and
respiratory failure occurred, necessitating temporary tracheotomy.
A pathological diagnosis of multiple early esophageal cancers was
made [1) Mt, 10×10 mm, SCC, pType 0-IIc, INFb, ly1, v0, pIM0,
pT1a-MM; 2) Mt, 5×3 mm, SCC, pType IIb, pTis, pN0 (0/48), M0,
pStage 0, pPM0, pDM0, pRM0, D2, Cur A]. Curative resection was
performed (Fig. 1).
Postoperatively, anastomotic stenosis occurred repeatedly,
requiring multiple endoscopic balloon dilation (EBD) procedures
over the 5-year follow-up period. Although the patient was a
habitual drinker and smoker preoperatively, he abstained from
alcohol and smoking after esophageal cancer surgery. He had already
undergone HP eradication therapy successfully before the primary
RAMIE for esophageal SCC.
Five years after surgery, he presented with the
digestive passage disorder. Esophagogastroduodenoscopy (EGD)
performed at Kanazawa University Hospital (Kanazawa, Japan)
revealed circumferential stenosis at the esophagogastric
anastomosis, for which EBD was repeated. Retrospectively, no
obvious tumor lesion was observed at the esophagogastric
anastomosis and remnant stomach in the annual screening endoscopic
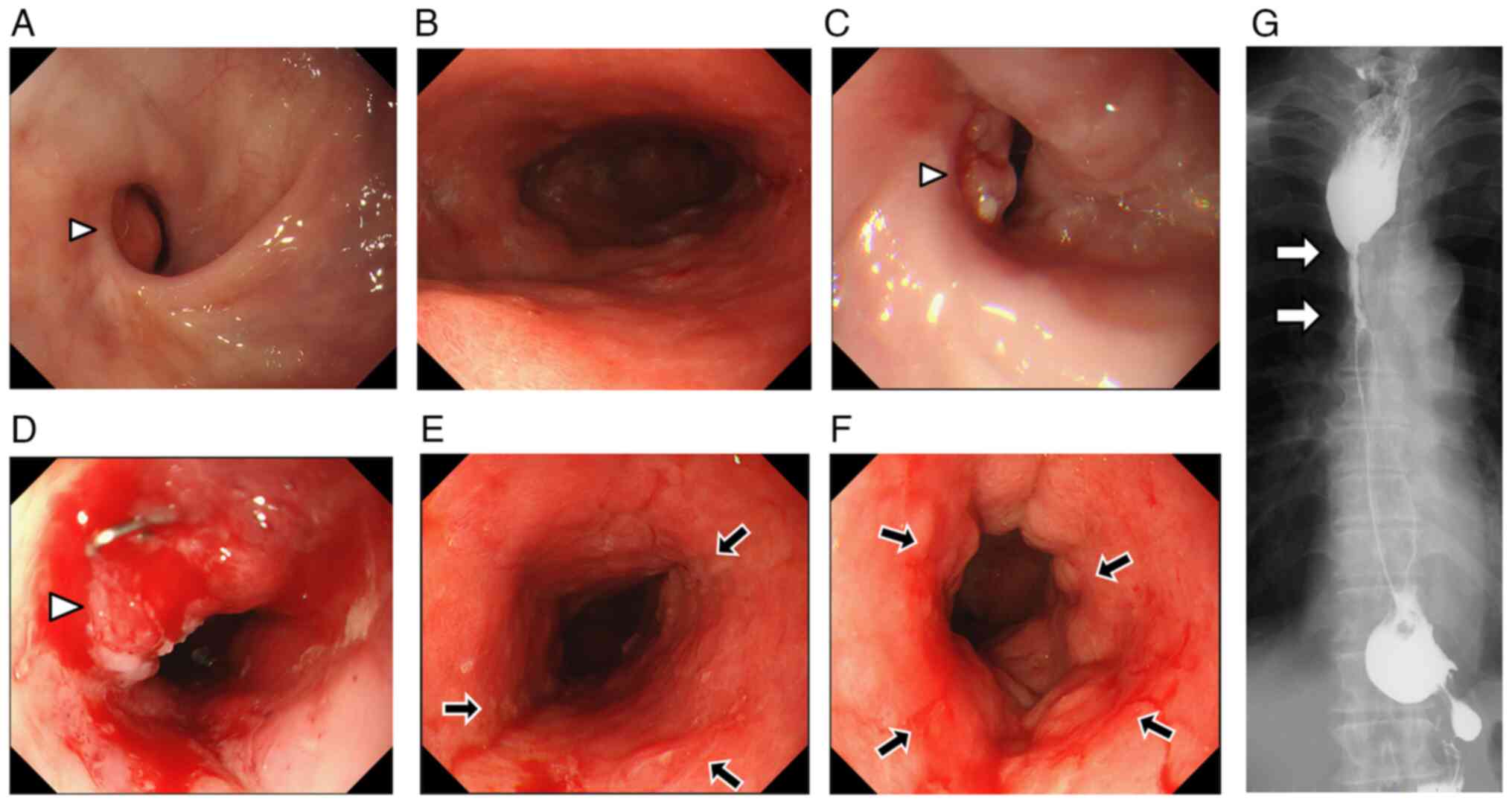
findings from one year before the cancer diagnosis (Fig. 2A and B). An 0-I-type elevated lesion
protruding into the lumen was observed on the posterior wall of the
anastomotic site (Fig. 2C and D);
consequently, a biopsy was performed, indicating a poorly
differentiated carcinoma. The patient was thereafter referred to
our hospital, Kanazawa Medical University Hospital (Kahoku, Japan),
for further examination and treatment. On repeat EGD, we observed
diffuse mucosal hardening, disappearance of folds, and redness from
the upper part to the antrum of the reconstructed gastric conduit
(Fig. 2E and F). Further, specimens
from several sites along the gastric conduit were biopsied, and
histopathological findings revealed poorly differentiated
carcinomas in all specimens. Immunohistochemical staining revealed
strong AE1/3 and carcinoembryonic antigen (CEA) positivity,
supporting a diagnosis of adenocarcinoma (por-tub2). Fluorography
indicated anastomotic stenosis with a wall irregularity. The
expansion of the gastric conduit was poor because of the wall
hardness (Fig. 2G). On
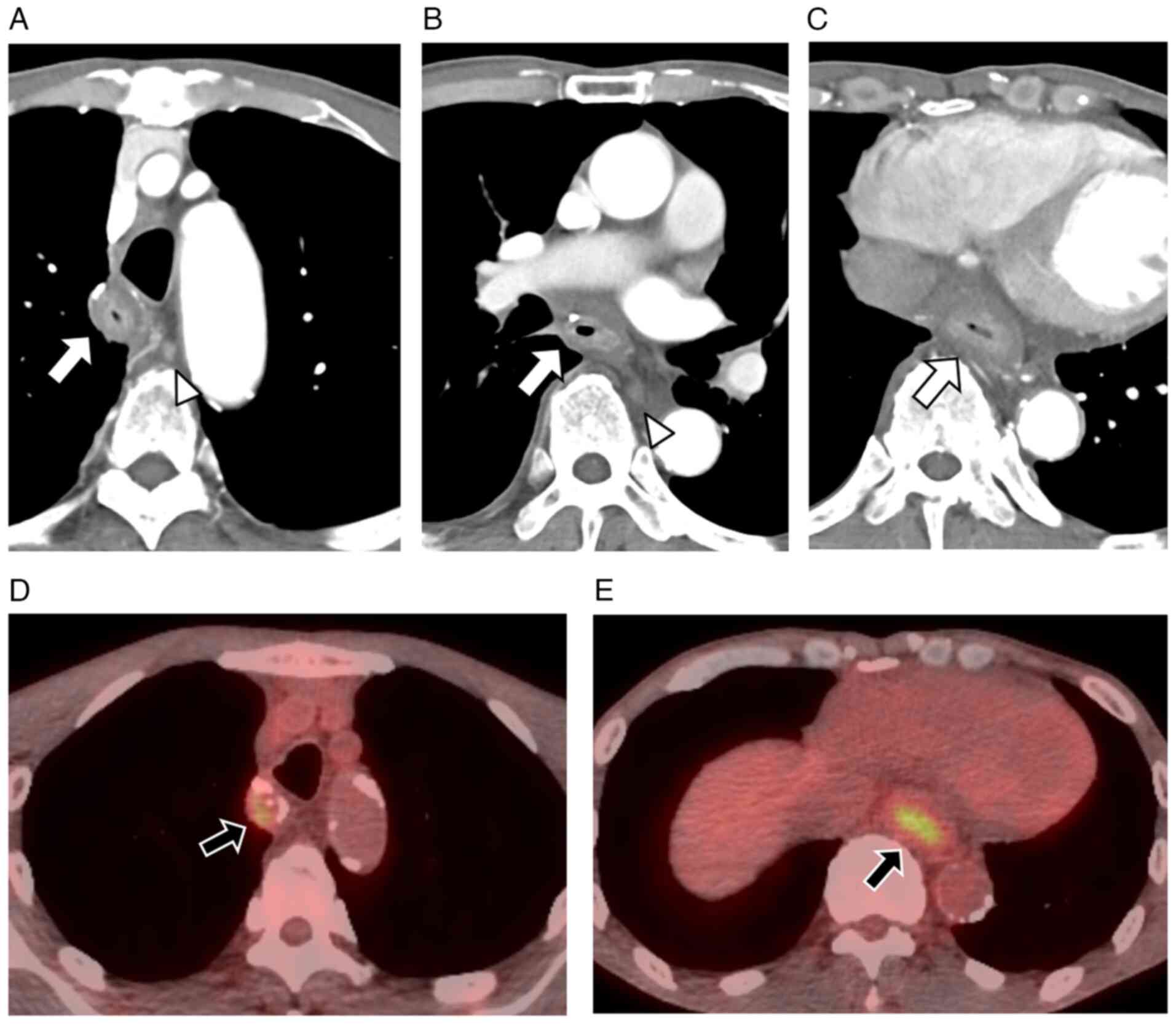
contrast-enhanced computed tomography (CT), diffuse wall thickening
was noted along the entire reconstructed gastric conduit along with
mediastinal LN enlargement (Fig.
3A-C). 18F-fluorodeoxyglucose (18F-FDG)
positron emission tomography indicated faint abnormal
18F-FDG accumulation in the wall of the reconstructed
gastric conduit (Fig. 3D and E). No
obvious distant metastasis, pleural effusion, or ascites was
observed.
According to the 15th edition of the
Japanese classification of gastric carcinoma (20), all the above findings indicated
advanced gastric scirrhous carcinoma [M-5-TE, circ, adenocarcinoma
(tub2-tub1), cType 4, 170 mm, cT3N2M0P0CYX cStage IIIA] originating
along almost the entire reconstructed gastric conduit with
esophageal infiltration. Thereafter, we planned to perform gastric
conduit resection and mediastinal LN dissection through RAS as a
curative treatment.
Surgical procedure
We planned to perform a radical surgery using the
DaVinci Xi Surgical System that allows RAS to be conducted with the
patient in a prone position. The first port was carefully inserted
during intrathoracic manipulation, and the extensive intrathoracic
adhesions between the lung surface and chest wall were carefully
dissected via thoracoscopic and robotic manipulation; thereafter,
the remaining ports were inserted and the robot setup was
completed. Cytological examination of the pleural lavage was
negative for cancer cells. However, the patient's respiratory
function was poor because of coexisting chronic obstructive
pulmonary disease; therefore, bilateral lung ventilation was
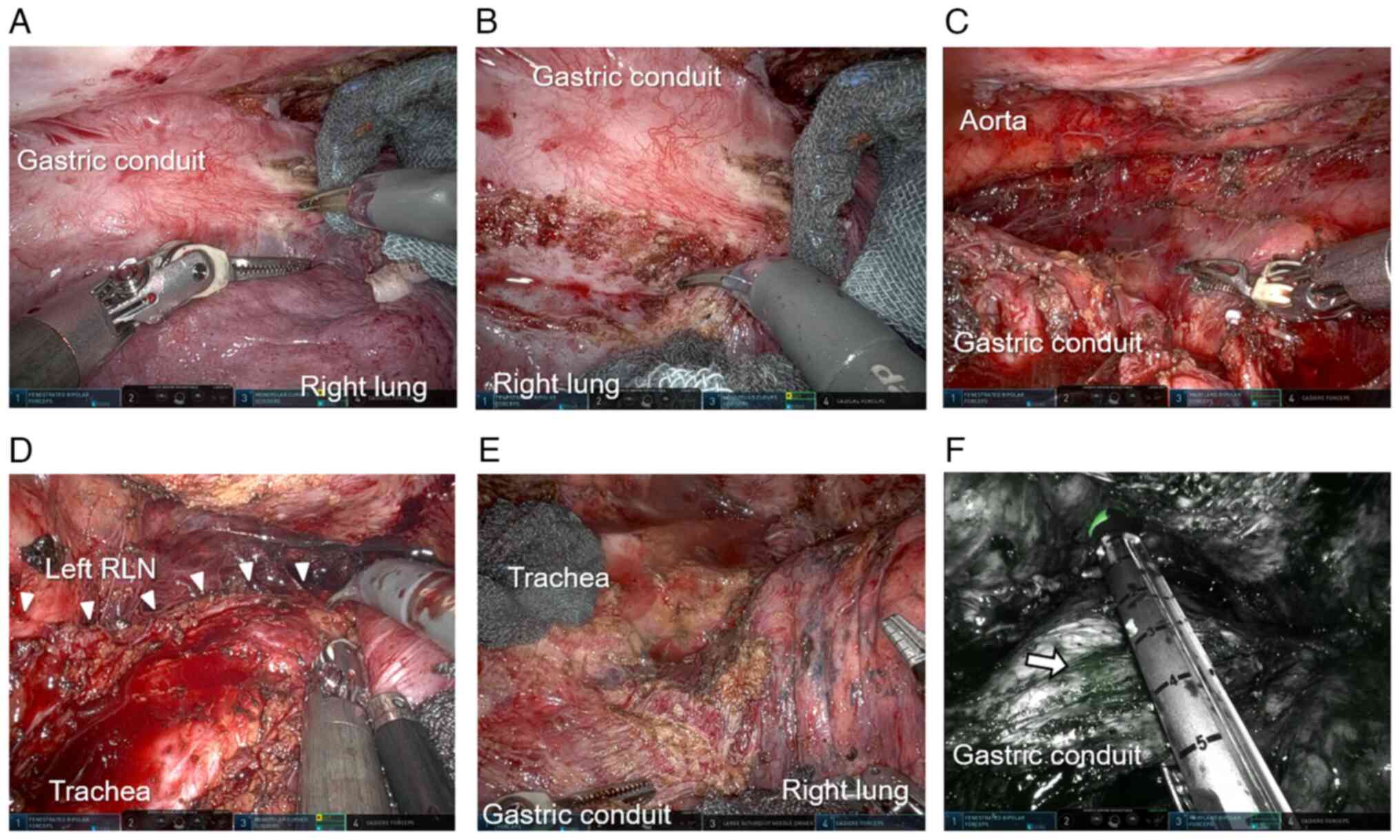
performed. The hard adhesions between the lung surface,
reconstructed gastric conduit, and membranous portion of the
trachea were dissected under 3D magnified observation (Fig. 4A-D). Using the third robotic arm's
forceps to maintain a good surgical field, the right lung was
gently compressed with using gauze (Fig. 4B). The reconstructed gastric conduit
could not be grasped because of diffuse wall hardening and
thickening, so we tried to secure the dissection layer under 3D
magnified observation using an appropriate counter traction with
repeated changing of the third-arm compression points. Minute
traumatic injuries to the lung surface resulting in air leakage
were repaired with sutures each time. Hard scarring also was
observed around the esophagogastric anastomosis, probably due to
repeated EBD procedures; hence, precise tracing of the excisional
line was needed to expose the contours of the surrounding adherent
organs (Fig. 4E). Consequently,
there was no clear exposed cancer tissue on the serosal surface of
the reconstructed gastric conduit and it could be resected without
any visible remnant tumor. This was accompanied by LN dissection
around the right-gastroepiploic artery and right-gastric artery
without injuring the tracheal membranous portion, anterior surface
of the descending aorta, or pericardium. At the upper-mediastinal
level, the ZEOCLIP® (Zeon Medical, Tokyo, Japan) marked
on the oral side of the tumor was identified using indocyanine
green (ICG) fluorescence, and an automatic stapler was used to
resect the remaining esophagus on the oral side of the
ZEOCLIP® (Fig. 4F). The
intrathoracic operation time, console time, and blood loss were 498
min, 439 min, and 520 g, respectively.
Abdominal manipulation involved gastric conduit
resection and LN dissection, with transection of the
right-gastroepiploic and right-gastric arteries and veins, followed
by extraction of the resected specimen. The small intestine and
right colon mesentery was then dissected and mobilized from the
retroperitoneum, securing it such that the pedicled jejunum could
be elevated to the neck. For digestive reconstruction, the pedicled
jejunum was elevated via an anterior-thoracic subcutaneous route in
the anterior chest, and a circular stapler was used to perform
end-to-side esophagojejunal anastomosis. Blood flow evaluation
using ICG fluorescence and Doppler monitoring confirmed that there
was no ischemia or congestion in the reconstructed jejunum;
therefore, additional revascularization procedures, such as
supercharge and superdrainage, were not performed. The overall
intraoperative time was 871 min and the total blood loss was 860
g.
Histological findings of the resected
specimens
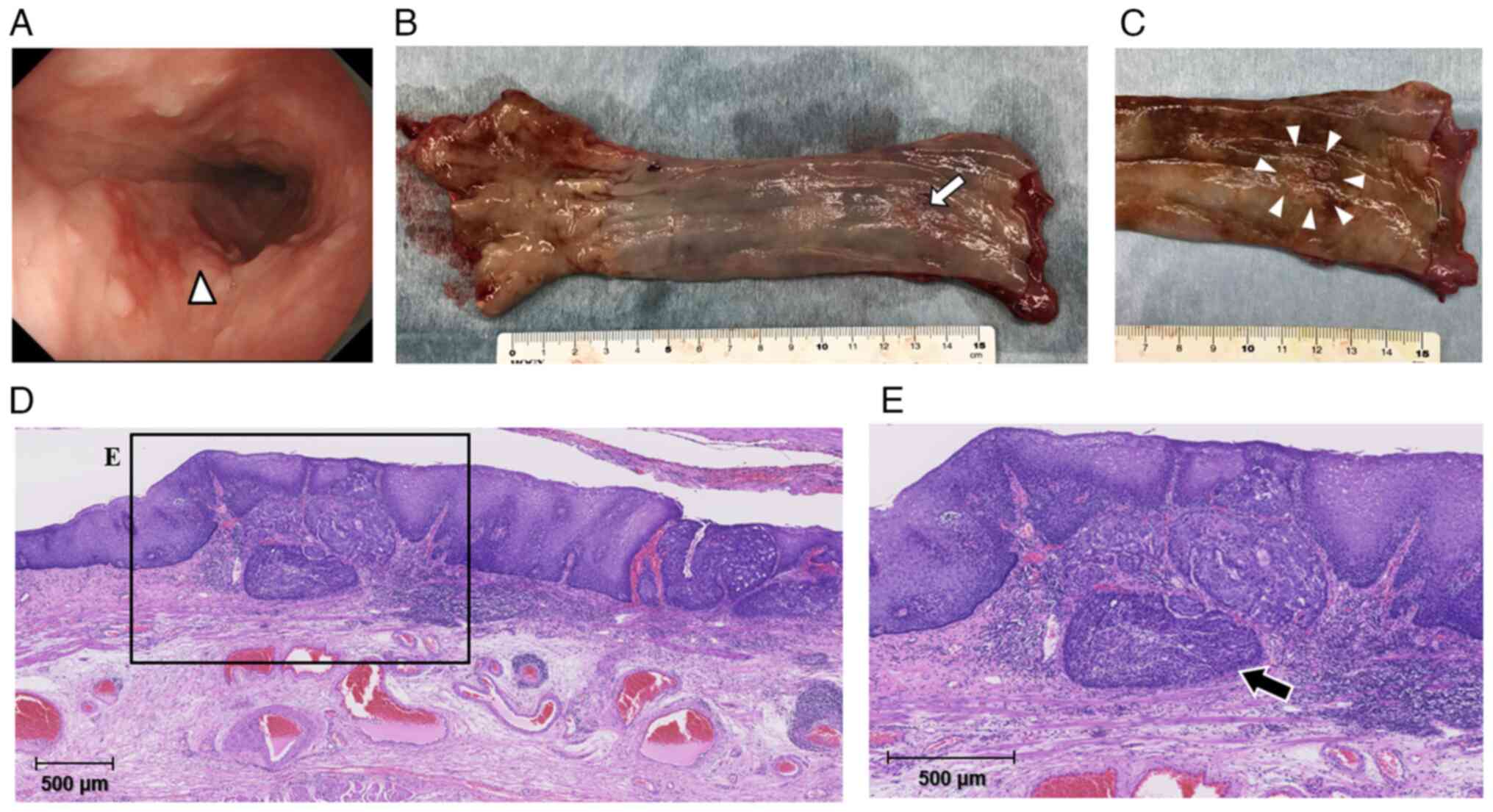
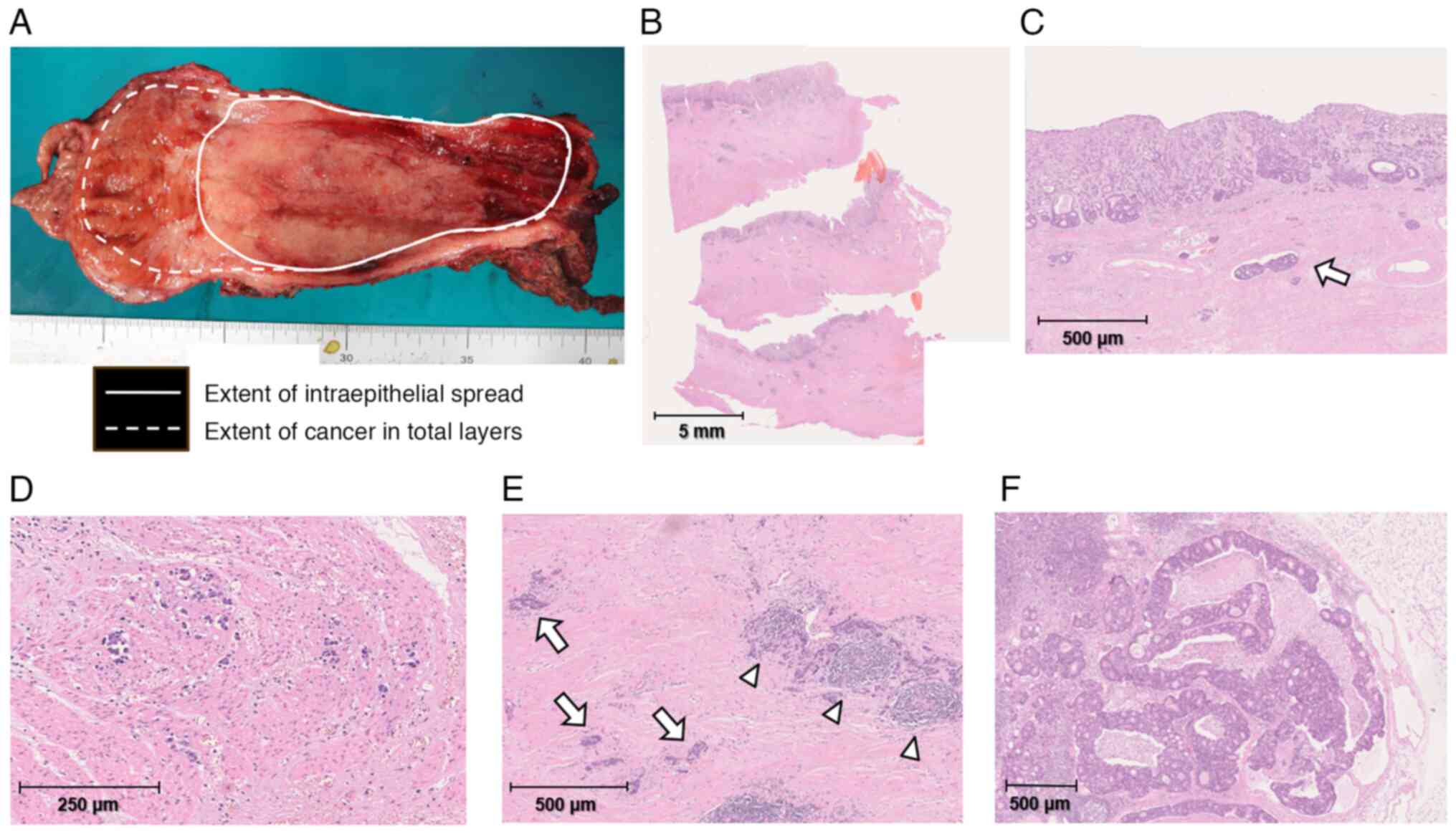
Macroscopically, the resected residual esophagus and
gastric conduit exhibited continuous wall thickening and hardening
from the esophagogastric anastomosis to almost the entire gastric
conduit (Fig. 5A). Histologically,
the gastric conduit exhibited notably broad and thick proliferation
of fibrous scar tissue along most of the stomach wall, except for
the area near the pylorus (Fig.
5B). In addition, adenocarcinoma cells were diffusely and
noncohesively scattered in and under the mucosa along most of the
gastric conduit and had also infiltrated into the resected residual
esophageal wall (Fig. 5C-E) but no
cancer cells were present at the oral resection edge. The serosa of
the resected gastric conduit had been peeled off during the
surgery, but no obvious cancer cells were exposed on the surface.
The deepest part of the cancer tissues invaded the subserous layer
of the stomach. Apparent metastases were evident in multiple
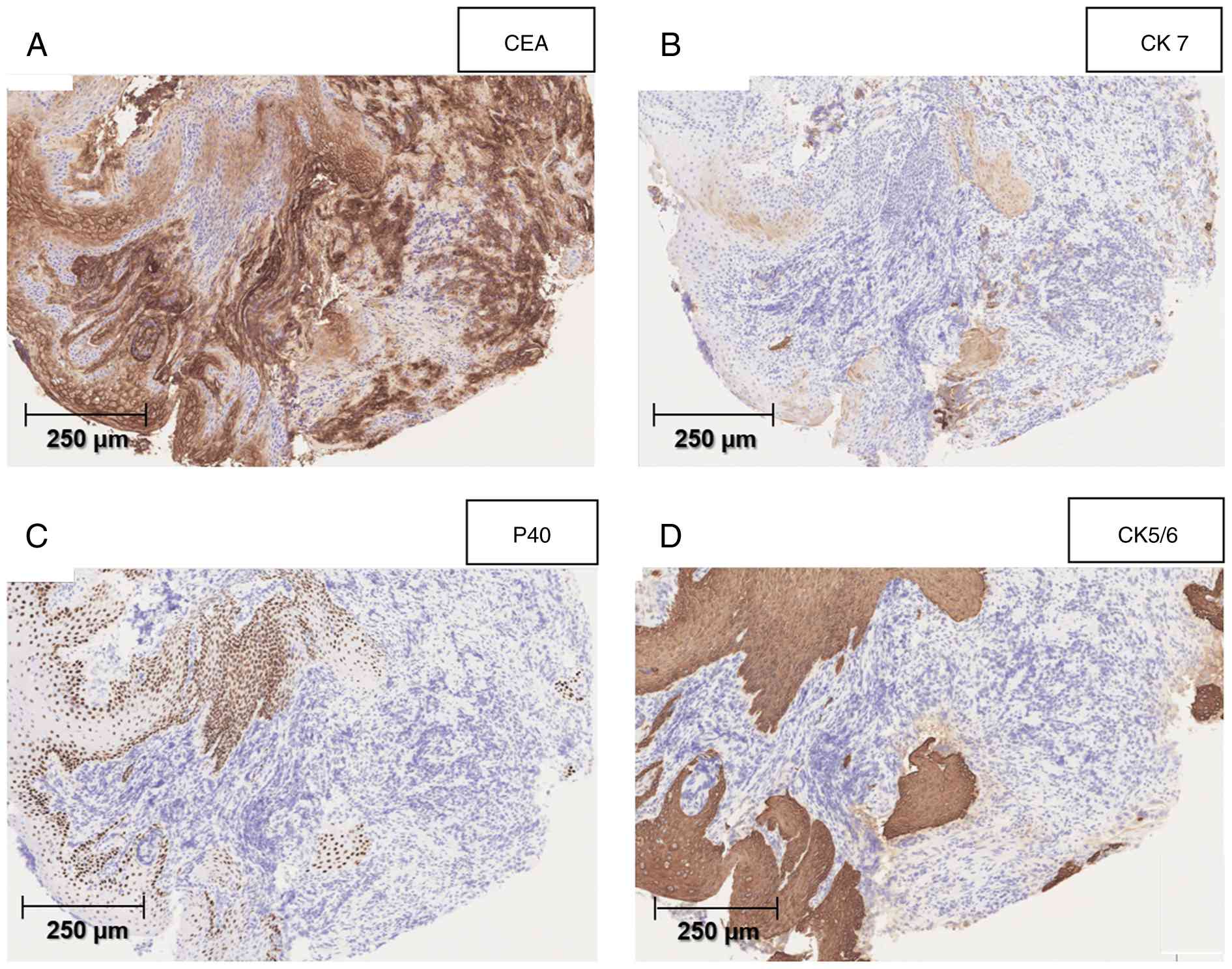
dissected perigastric and intra-abdominal LNs (Fig. 5F). Immunohistochemical staining was
negative for squamous epithelial markers p40 (Fig. 6C) and CK5/6 (Fig. 6D) in the cancerous area but positive
for CEA (Fig. 6A) and CK7 (Fig. 6B); these findings are characteristic
of adenocarcinoma.
Finally, the pathological diagnosis was remnant
scirrhous gastric cancer originating along most of the gastric
conduit [M-5-TAE, circ, 203×76 mm, pType 4,
moderate-to-well-differentiated adenocarcinoma (tub2-tub1), pT3,
INFc, Ly1b, VX, pIM0, pPM0, pDM0, pN3(7/22, #4sax4, #4sbx1, #6×2)
M0, CY0, P0, pStage IIIC, D2, Cur A] (20). Surgical treatment resulted in
pathological curative resection.
Postoperative clinical course
The patient presented with a minor anastomotic
leakage at the esophagojejunostomy and pneumonia; both were
successfully resolved through conservative management.
Subsequently, he was discharged on postoperative day 47. Given the
substantial risk of recurrence, the patient underwent adjuvant
chemotherapy with oral tegafur/gimeracil/oteracil (oral
5-fluorouracil prodrug) and triweekly docetaxel for 4 months after
the prescribed protocol for adjuvant chemotherapy in gastric cancer
(21,22). He remained relapse-free, as shown by
periodic CT examinations, and was alive at postoperative 9
months.
Discussion
To the best of our knowledge, this is the first case
report on the use of minimally invasive robotic procedure for the
successful treatment of scirrhous gastric carcinoma originating in
the reconstructed gastric conduit after esophageal cancer
surgery.
The benefits of RAS include accurate recognition of
microanatomy because of 3D magnification, precise robotic arm
operation through the multi-joint function, image stabilization,
and surgeon-centered surgical-field development using a third arm
(23,24). RAS is also less physically demanding
than laparoscopic surgery, which requires long-term static muscle
activity with a high physical workload for surgeons (25). Studies comparing robotic vs.
laparoscopic gastrectomy for gastric cancer, robotic vs.
laparoscopic colectomy for colon cancer, and RAMIE vs. conventional
thoracoscopic or open esophagectomy for esophageal cancer have
reported that RAS is more useful in the treatment of malignant
tumors in many cases (16,19,23,24,26,27).
Recently, thoracoscopic esophagectomy and RAMIE have
become increasingly popular for the surgical treatment for
resectable esophageal cancer. Warner et al (28) reported that thoracoscopic
esophagectomy is acceptable in resectable cases after neoadjuvant
CRT without evidence of increased morbidity or mortality. Mederos
et al (16) and Tagkalos
et al (29) reported the
usefulness of RAMIE vs. conventional thoracoscopic or open
esophagectomy for esophageal cancer. In addition, Defize et
al (30) reported the
usefulness of RAMIE as a salvage surgery after definitive
chemoradiotherapy for unresectable advanced cancer with
infiltration in other organs. They reported that after definitive
CRT, normal anatomical structures were restored in 75% of
resectable cases, making resection without residual tissue
possible. However, in cases where the border between the fibrosis
and tumor was unclear, curative resection while recognizing the
anatomical structures was challenging. Despite such surgical
results, pathologically curative resection was achieved in 92% of
cases, suggesting that RAMIE can ensure curability even in salvage
surgery after CRT. Alhossaini et al (31) reported that in cases with remnant
gastric cancer, RAS is less invasive than laparoscopic surgery in
terms of the low rate of conversion to open surgery because of
severe adhesions. A review of 10 nonrandomized controlled trials
reported that compared with open gastrectomy, laparoscopic
gastrectomy for remnant gastric cancer can lead to better
short-term outcomes, including lower blood loss, lower
postoperative complication rate, and shorter postoperative hospital
stay. Although laparoscopic gastrectomy for remnant gastric cancer
is technically complex, it is feasible, safe, and is minimally
invasive. However, one report stated that conversion to open
gastrectomy during laparoscopic gastrectomy is unavoidable in many
patients with severe intra-abdominal adhesions and anatomical
abnormalities (32).
In the present case, we opted for robotic remnant
gastrectomy for a thorough and less invasive mediastinal LN
dissection. Although RAMIE was conducted for the previous
esophageal cancer, the right lung was firmly adhered to the chest
wall, trachea, pericardium, and anterior surface of the aorta. The
esophagogastric anastomosis and surrounding organs had developed
hard cicatricial adhesions to the lungs and tracheal membranous
portion after undergoing multiple EBD procedures; however, RAS
enabled the detachment and resection of these adhesions without
severe secondary injury. Moreover, using the robotic forceps to
suture partially injured lung surfaces was also effective in tissue
repair. Using all the advantages offered by RAS and by performing
careful intrathoracic manipulations, the surgery was completed
without any major intraoperative complications, although it was
time-consuming. RAS may enable easy adhesion removal, provide
accurate anatomical recognition, and be less invasiveness in
patients with a history of laparotomy or thoracotomy.
Several reports have been published on the
epidemiology, etiology, diagnostic methods, and treatment of GCC
after esophageal cancer surgery. Lee et al (7) reported that the incidence rate of
reconstructed GCC is 2.4% per 5 years and 5.7% per 10 years, and
the average occurrence-to-diagnostic time is 55.8 months (4–236
months) after esophagectomy, with 92% histological types being
adenocarcinoma. A nationwide study in Japan by Ota et al
(13) reported that the proportion
of early cancer (T1) was ~60% in primary gastric cancer but 81.5%
in GCC, which is high. This may be because of the continued
postoperative follow-up for patients with esophageal cancer and the
accessibility of EGD in Japan (33,34).
The median interval between esophagectomy and GCC diagnosis was 6
years, with ~25% of patients being diagnosed >10 years later.
The 5-year overall survival (OS) rates after endoscopic and
surgical treatments for GCC were 75.9 and 52.7%, respectively. In a
study by Gentile et al (10), 41.6% of GCC cases were treated via
endoscopic resection while avoiding gastric conduit resection.
Close monitoring and long-term follow-up may be useful in the early
detection of GCC and appropriate therapeutic intervention. Yearly
endoscopic follow-up >10 years after esophageal cancer surgery
has been recommended (9,10,13).
However, in cases of resectable advanced cancer where early
detection through meticulous screening is not possible, surgery is
the only curative treatment.
The etiology of GCC includes chronic gastritis
because of HP infection and subsequent intestinal metaplasia
(35,36). Various other factors, such as
relaxation of the pylorus ring due to vagotomy, decreased
peristalsis of the gastric conduit, and intestinal metaplasia due
to bile reflux caused by negative intrathoracic pressure, may be
involved. Histologically, primary gastric cancer caused by chronic
inflammation can be intestinal and well-differentiated (37). In this case, HP had been eradicated
before the initial surgery, and the patient had chronic gastritis
along with moderate mucosal atrophy; therefore, GCC onset at 5
years was probably earlier than the average. Palmes et al
(38) reported that bile reflux can
cause chronic inflammation and intestinal metaplasia in the
reconstructed gastric conduit; therefore, pyloric drainage
(pyloroplasty or pyloromyotomy) after esophagectomy with
esophagogastrostomy and vagotomy are not recommended. In fact, in
the present case, clear bile reflux was observed in the
reconstructed gastric conduit during the annual screening EGD up to
the previous year. Shirakawa et al (9) and Ota et al (13) reported that ~60% of GCC cases
occurred in the lower third portion of the gastric conduit. In
cases where GCC was of detected early through appropriate
screening, endoscopic treatment in accordance with the indications
for ESD for normal early gastric cancer can be expected to cure GCC
in a less invasive manner (8,11,14,39).
Furthermore, abstinence from alcohol and smoking after treatment
for initial esophageal cancer significantly reduces the incidence
of pharynx and laryngeal, oral, and residual esophageal cancers
(1,2,4);
however, GCC occurred in this case despite strict abstinence from
alcohol and smoking since the initial esophageal cancer
diagnosis.
Gastric cancer with histological findings of signet
ring-cell carcinoma or poorly differentiated adenocarcinoma tends
to have an unclear border, exhibits diffuse infiltration, and is
often accompanied by significant fibrosis within the wall;
therefore, it is called scirrhous gastric cancer. Macroscopically,
it is often classified as type 4 and is characterized by hardening
and thickening of the wall (40).
Compared with other macroscopic types, type-4 gastric cancer is
more likely to disseminate peritoneally. This type of cancer is
also characterized by the absence of protuberances, depressions, or
ulcers in the shape of the tumor, wall hardening, and slow
progression, making a qualitative or extensive diagnosis difficult.
Histologically, scirrhous gastric cancer is often diffuse and
poorly differentiated (por, sig), and chronic gastritis and
intestinal metaplasia may not necessarily be present in the
background (26,32). In the present case, EGD was
performed to examine the wall hardening and the esophagogastric
conduit anastomosis stenosis, which indicated the presence of a
lesion. However, it cannot be denied that GCC did not exist at that
time. Therefore, it is necessary to be fully aware that scirrhous
GCC presents as a stenotic lesion with an unclear border, and to
consider performing a biopsy if necessary. The primary lesion in
the gastric conduit extended not only to the upper stomach but was
also accompanied by extensive infiltration of most of the gastric
conduit. However, we could not accurately diagnose the extent of
the lesion preoperatively. Furthermore, the resected specimen did
not include signet ring cells, and the histological type was a
mixture of tubular and poorly differentiated adenocarcinoma. At
Kanazawa University Hospital where the initial histological
diagnosis was made, the diagnosis was not definitively made as
adenocarcinoma, but was confirmed by immunostaining in Kanazawa
Medica University Hospital. In fact, because only a small portion
of the tumor was collected in the biopsy tissue, it is possible
that the cancer cells scattered within the fibrous tissue were
misdiagnosed as poorly differentiated cancer. Moreover, EBD had
been performed multiple times for anastomotic stenosis before the
diagnosis of GCC. No obvious tumor lesion was observed at the
esophagogastric anastomosis on annual endoscopic screening from the
year before the cancer diagnosis (Fig.
2A and B). However, it cannot be confirmed if GCC was present
at that time. Therefore, it is necessary to consider that scirrhous
GCC can present as a stenotic lesion with an unclear border, and a
biopsy should be conducted if necessary. Preoperative images
suggested advanced GCC with invasion deeper than the muscular
propria layer, and multiple perigastric LN metastases were
suspected in the area of the right-gastroepiploic artery, the
nutrient vessel of the reconstructed gastric conduit. A nationwide
study in Japan reported that the degree of LN metastasis in GCC was
strongly associated with prognosis (13). This finding suggests that adequate
LN dissection of the gastric conduit basin is necessary in curative
GCC surgery. As a result, total resection of the residual esophagus
and reconstructed gastric conduit and a thorough regional LN
dissection is unavoidable for radical resection. However, given the
need for thorough perigastric LN dissection, we consider RAS to be
extremely useful in this case.
The pathological diagnosis of the resected specimen
was T3N3M0 pStage IIIC. Although robotic resection was considered
to be curative, the risk of recurrence was high, leading to DS
therapy with docetaxel and S-1 being administered as adjuvant
chemotherapy. The JACCRO GC-07 study was a randomized phase-III
study that aimed to determine whether adjuvant chemotherapy with DS
therapy in pStage-II–III advanced gastric cancer was superior to
S-1 alone (21,22,41).
DS therapy resulted in a significantly better 3-year relapse-free
survival [hazard ratio (HR), 0.632; S-1 plus docetaxel, 65.9%; S-1
alone, 49.5%; P<0.001], and is a commonly used regimen in Japan
(22). In addition, the JACCRO
GC-07 study reported a better 5-year OS in patients with
pathological stage-III gastric cancer treated with DS therapy (HR,
0.752; S-1 plus docetaxel, 67.9%; S-1 alone, 60.3%; P=0.0059)
(41). In this case, DS therapy was
discontinued at the patient's request after 4 months of treatment.
Strict follow-up is necessary as the risk of peritoneal
dissemination, pleural dissemination, and LN recurrence is very
high as in scirrhous gastric cancer.
The reconstructive route used in prior esophagectomy
may influence the success of radical resection for GCC. Koyanagi
et al (42) reported a
resection rate of 50% in cases where the posterior mediastinal
route was used, 77% for those using the retrosternal route, and 93%
for those using the anterior-thoracic route. In general, secondary
cancers were detected easily in cases with anterior-thoracic route
reconstructions, and the operative procedure was less difficult
than in those by other routes. Thus, the resection rate was better
with the anterior-thoracic reconstruction route than for the other
routes (42). Fujisawa et al
(43) recently reported a study
where transabdominal gastric conduit resection was conducted for
metachronous GCC in a gastric conduit reconstructed via a
retrosternal route. RAS should be considered to be a useful option,
even in cases with severe scar changes, postoperative adhesions,
and modified anatomy in reoperation cases.
In summary, we report a rare case of scirrhous GCC
originating in the reconstructed gastric conduit after esophageal
cancer surgery that was successfully treated through minimally
invasive RAS with radical transthoracic and gastric conduit
resection and regional LN dissection. Even in cases of reoperation
for GCC, RAS can be safely used to perform appropriate radical
surgery while minimizing secondary damage, making it a useful
option that improves the feasibility of difficult surgeries.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
KO conceived this case presentation and drafted the
manuscript. KO, KF, KM, YS, HN and AH performed the surgery and
therapeutic management of the patient. KO, TT, NI and IN managed
the previous esophageal cancer surgery of the patient. DK, TM, HF,
SK and HT contributed to the acquisition of data, interpretation of
data and editing of the report. KO and HT confirm the authenticity
of all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for the publication of this case report and the
accompanying images.
Competing interests
The authors declare that they have no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were
used to improve the readability and language of the manuscript or
to generate images, and subsequently, the authors revised and
edited the content produced by the AI tools as necessary, taking
full responsibility for the ultimate content of the present
manuscript.
Authors' information
KO, NI and IN are specialists and instructors of the
Japanese Society of Gastroenterological Surgery and the Japanese
Esophageal Society, and serve as councilors of the Japanese
Esophageal Society.
Glossary
Abbreviations
Abbreviations:
|
SCC
|
squamous-cell carcinoma
|
|
GCC
|
gastric conduit cancer
|
|
CT
|
computed tomography
|
|
EBD
|
endoscopic balloon dilation
|
|
ESD
|
endoscopic submucosal dissection
|
|
HP
|
Helicobacter pylori
|
|
ICG
|
indocyanine green
|
|
LN
|
lymph-node
|
|
OS
|
overall survival
|
|
RAMIE
|
robot-assisted minimally invasive
esophagectomy
|
References
|
1
|
Chen SC, Teng CJ, Hu YW, Yeh CM, Hung MH,
Hu LY, Ku FC, Tzeng CH, Chiou TJ, Chen TJ and Liu CJ: Secondary
primary malignancy risk among patients with esophageal cancer in
Taiwan: A nationwide population-based study. PLoS One.
10:e01163842015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cui Y, Ren W, Du X, Yang L and Tan B:
Research progress of multiple primary malignancies associated with
esophageal cancer. Cancer Control. 30:107327482311766412023.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Baba Y, Ishimoto T, Kurashige J, Iwatsuki
M, Sakamoto Y, Yoshida N, Watanabe M and Baba H: Epigenetic field
cancerization in gastrointestinal cancers. Cancer Lett.
375:360–366. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen D, Fan N, Mo J, Wang W, Wang R, Chen
Y, Hu J and Wen Z: Multiple primary malignancies for squamous cell
carcinoma and adenocarcinoma of the esophagus. J Thorac Dis.
11:3292–3301. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Maeda M, Moro H and Ushijima T: Mechanisms
for the induction of gastric cancer by Helicobacter pylori
infection: Aberrant DNA methylation pathway. Gastric Cancer.
20:8–15. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oki E, Morita M, Toh Y, Kimura Y, Ohgaki
K, Sadanaga N, Egashira A, Kakeji Y, Tsujitani S and Maehara Y:
Gastric cancer in the reconstructed gastric tube after radical
esophagectomy: A single-center experience. Surg Today. 41:966–969.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lee GD, Kim YH, Choi SH, Kim HR, Kim DK
and Park SI: Gastric conduit cancer after oesophagectomy for
oesophageal cancer: Incidence and clinical implications. Eur J
Cardiothorac Surg. 45:899–903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mukasa M, Takedatsu H, Matsuo K, Sumie H,
Yoshida H, Hinosaka A, Watanabe Y, Tsuruta O and Torimura T:
Clinical characteristics and management of gastric tube cancer with
endoscopic submucosal dissection. World J Gastroenterol.
21:919–925. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Shirakawa Y, Noma K, Maeda N, Ninomiya T,
Tanabe S, Kikuchi S, Kuroda S, Nishizaki M, Kagawa S, Kawahara Y,
et al: Clinical characteristics and management of gastric tube
cancer after esophagectomy. Esophagus. 15:180–189. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gentile D, Riva P, Da Roit A, Basato S,
Marano S and Castoro C: Gastric tube cancer after esophagectomy for
cancer: A systematic review. Dis Esophagus. 32:doz0492019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hirayama Y, Fujisaki J, Yoshimizu S,
Horiuchi Y, Yoshio T, Ishiyama A, Hirasawa T, Imamura Y, Mine S,
Watanabe M and Tsuchida T: Efficacy and safety of endoscopic
resection for gastric tube cancer after surgical resection of
esophageal squamous cell carcinoma. Esophagus. 16:194–200. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Urabe M, Haruta S, Tanaka M, Yago A,
Ohkura Y, Tanaka T, Hoteya S, Ueno M and Udagawa H: Management and
outcomes of resectable gastric conduit cancer: A retrospective
comparative study of 51 cases. Langenbecks Arch Surg.
406:1433–1441. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ota M, Morita M, Ikebe M, Nakashima Y,
Yamamoto M, Matsubara H, Kakeji Y, Doki Y and Toh Y:
Clinicopathological features and prognosis of gastric tube cancer
after esophagectomy for esophageal cancer: A nationwide study in
Japan. Esophagus. 19:384–392. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Toyoshima Y, Narumiya K, Kudo K, Egawa H
and Hosoda K: Comparative analysis of the outcomes of gastrectomy
vs. endoscopic mucosal resection or endoscopic submucosal
dissection for the treatment of gastric tube cancer after
esophagectomy. Glob Health Med. 5:40–46. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
de Groot EM, van der Horst S, Kingma BF,
Goense L, van der Sluis PC, Ruurda JP and van Hillegersberg R:
Robot-assisted minimally invasive thoracolaparoscopic esophagectomy
versus open esophagectomy: Long-term follow-up of a randomized
clinical trial. Dis Esophagus. 33:doaa0792020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mederos MA, de Virgilio MJ, Shenoy R, Ye
L, Toste PA, Mak SS, Booth MS, Begashaw MM, Wilson M, Gunnar W, et
al: Comparison of clinical outcomes of robot-assisted,
video-assisted, and open esophagectomy for esophageal cancer: A
systematic review and meta-analysis. JAMA Netw Open.
4:e21292282021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ninomiya I, Okamoto K, Yamaguchi T, Saito
H, Terai S, Moriyama H, Kinoshita J and Fushida S: Optimization of
robot-assisted thoracoscopic esophagectomy in the lateral decubitus
position. Esophagus. 18:482–488. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Fujita T, Sato K, Ozaki A, Akutsu T,
Fujiwara H, Kojima T and Daiko H: Propensity-matched analysis of
the short-term outcome of robot-assisted minimally invasive
esophagectomy versus conventional thoracoscopic esophagectomy in
thoracic esophageal cancer. World J Surg. 46:1926–1933. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang Y, Dong D, Cao Y, Huang M, Li J,
Zhang J, Lin J, Sarkaria IS, Toni L, David R, et al: Robotic versus
conventional minimally invasive esophagectomy for esophageal
cancer: A meta-analysis. Ann Surg. 278:39–50. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Japanese Gastric Cancer Association, .
Japanese Classification of Gastric Carcinoma. Kanehara & Co.,
Ltd.; Tokyo: pp. 10–24. 2017
|
|
21
|
Yoshida K, Kodera Y, Kochi M, Ichikawa W,
Kakeji Y, Sano T, Nagao N, Takahashi M, Takagane A, Watanabe T, et
al: Addition of docetaxel to oral fluoropyrimidine improves
efficacy in patients with stage III gastric cancer: Interim
analysis of JACCRO GC-07, a randomized controlled trial. J Clin
Oncol. 37:1296–1304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kakeji Y, Yoshida K, Kodera Y, Kochi M,
Sano T, Ichikawa W, Lee SW, Shibahara K, Shikano T, Kataoka M, et
al: Three-year outcomes of a randomized phase III trial comparing
adjuvant chemotherapy with S-1 plus docetaxel versus S-1 alone in
stage III gastric cancer: JACCRO GC-07. Gastric Cancer. 25:188–196.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
van der Sluis PC, van der Horst S, May AM,
Schippers C, Brosens LAA, Joore HCA, Kroese CC, Haj Mohammad N,
Mook S, Vleggaar FP, et al: Robot-assisted minimally invasive
thoracolaparoscopic esophagectomy versus open transthoracic
esophagectomy for resectable esophageal cancer: A randomized
controlled trial. Ann Surg. 269:621–630. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Perry R, Barbosa JP, Perry I and Barbosa
J: Short-term outcomes of robot-assisted versus conventional
minimally invasive esophagectomy for esophageal cancer: A
systematic review and meta-analysis of 18,187 patients. J Robot
Surg. 18:1252024. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dalager T, Jensen PT, Eriksen JR, Jakobsen
HL, Mogensen O and Sogaard K: Surgeons' posture and muscle strain
during laparoscopic and robotic surgery. Br J Surg. 107:756–766.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zong L, Seto Y, Aikou S and Takahashi T:
Efficacy evaluation of subtotal and total gastrectomies in robotic
surgery for gastric cancer compared with that in open and
laparoscopic resections: A meta-analysis. PLoS One. 9:e1033122014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sheng S, Zhao T and Wang X: Comparison of
robot-assisted surgery, laparoscopic-assisted surgery, and open
surgery for the treatment of colorectal cancer: A network
meta-analysis. Medicine. 97:e118172018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Warner S, Chang YH, Paripati H, Ross H,
Ashman J, Harold K, Day R, Stucky CC, Rule W and Jaroszewski D:
Outcomes of minimally invasive esophagectomy in esophageal cancer
after neoadjuvant chemoradiotherapy. Ann Thorac Surg. 97:439–445.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Tagkalos E, van der Sluis PC, Berlth F,
Poplawski A, Hadzijusufovic E, Lang H, van Berge Henegouwen MI,
Gisbertz SS, Müller-Stich BP, Ruurda JP, et al: Robot-assisted
minimally invasive thoraco-laparoscopic esophagectomy versus
minimally invasive esophagectomy for resectable esophageal
adenocarcinoma, a randomized controlled trial (ROBOT-2 trial). BMC
Cancer. 21:10602021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Defize IL, van der Horst S, Ruurda JP and
van Hillegersberg R: ASO author reflections: Preoperative selection
of cT4b esophageal cancer patients who benefit from a salvage
robot-assisted minimally invasive esophagectomy (RAMIE). Ann Surg
Oncol. 28:2739–2740. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Alhossaini RM, Altamran AA, Cho M, Roh CK,
Seo WJ, Choi S, Son T, Kim HI and Hyung WJ: Lower rate of
conversion using robotic-assisted surgery compared to laparoscopy
in completion total gastrectomy for remnant gastric cancer. Surg
Endosc. 34:847–852. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ma FH, Liu H, Ma S, Li Y and Tian YT:
Current controversies in treating remnant gastric cancer: Are
minimally invasive approaches feasible? World J Clin Cases.
7:3384–3393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Toh Y, Kitagawa Y, Kuwano H, Kusano M,
Oyama T, Muto M, Kato H, Takeuchi H, Doki Y, Naomoto Y, et al: A
nation-wide survey of follow-up strategies for esophageal cancer
patients after a curative esophagectomy or a complete response by
definitive chemoradiotherapy in Japan. Esophagus. 13:173–181. 2015.
View Article : Google Scholar
|
|
34
|
Nakanoko T, Morita M, Nakashima Y, Ota M,
Ikebe M, Yamamoto M, Booka E, Takeuchi H, Kitagawa Y, Matsubara H,
et al: Nationwide survey of the follow-up practices for patients
with esophageal carcinoma after radical treatment: Historical
changes and future perspectives in Japan. Esophagus. 19:69–76.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nonaka S, Oda I, Sato C, Abe S, Suzuki H,
Yoshinaga S, Hokamura N, Igaki H, Tachimori Y, Taniguchi H, et al:
Endoscopic submucosal dissection for gastric tube cancer after
esophagectomy. Gastrointest Endosc. 79:260–270. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kise Y, Kijima H, Shimada H, Tanaka H,
Kenmochi T, Chino O, Tajima T and Makuuchi H: Gastric tube cancer
after esophagectomy for esophageal squamous cell cancer and its
relevance to Helicobacter pylori. Hepatogastroenterology.
50:408–411. 2003.PubMed/NCBI
|
|
37
|
Dixon MF, Mapstone NP, Neville PM,
Moayyedi P and Axon AT: Bile reflux gastritis and intestinal
metaplasia at the cardia. Gut. 51:351–355. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Palmes D, Weilinghoff M, Colombo-Benkmann
M, Senninger N and Bruewer M: Effect of pyloric drainage procedures
on gastric passage and bile reflux after esophagectomy with gastric
conduit reconstruction. Langenbecks Arch Surg. 392:135–141. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bamba T, Kosugi S, Takeuchi M, Kobayashi
M, Kanda T, Matsuki A and Hatakeyama K: Surveillance and treatment
for second primary cancer in the gastric tube after radical
esophagectomy. Surg Endosc. 24:1310–1317. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Agnes A, Estrella JS and Badgwell B: The
significance of a nineteenth century definition in the era of
genomics: linitis plastica. World J Surg Oncol. 15:1232017.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kodera Y, Yoshida K, Kochi M, Sano T,
Ichikawa W, Kakeji Y, Sunakawa Y, Takeuchi M and Fujii M: Addition
of docetaxel to S-1 results in significantly superior 5-year
survival outcomes in stage III gastric cancer: A final report of
the JACCRO GC-07 study. Gastric Cancer. 26:1063–1068. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Koyanagi K, Ozawa S, Ando N, Shih CH,
Nakamura E, Takeuchi H, Hayashi K and Kitajima M: Case report:
Metachronous early gastric carcinoma in a reconstructed gastric
tube after radical operation for oesophageal carcinoma. J
Gastroenterol Hepatol. 13:311–315. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Fujisawa K, Ueno M, Okamoto K, Shimoyama
H, Ohkura Y, Haruta S and Udagawa H: Successful robot-assisted
surgery for advanced metachronous cancer in a gastric conduit after
esophagectomy: A case report. Ann Thorac Cardiovasc Surg.
30:23–00202. 2024. View Article : Google Scholar : PubMed/NCBI
|