Introduction
Bladder cancer is the 10th most common
cancer worldwide, and fourth most common cancer in men as of 2023
(1,2). Annually, it accounts for 3–6% of new
cancer cases, and 2–4% of global cancer deaths (2–4).
According to GLOBOCAN data, approximately 614,298 individuals were
diagnosed with bladder cancer in 2022, and 220,596 patients died
(5). At initial diagnosis, 70–75%
of patients have non-muscle-invasive bladder cancer (NMIBC), which
has a high recurrence rate and requires long-term follow-up; 20–25%
have muscle-invasive bladder cancer (MIBC), which is associated
with a high mortality rate; and 5% have metastatic bladder cancer,
which is the most aggressive type (2,3,6). Among
these, 31–78% of patients with NMIBC relapse (7), and 17–40% progress to MIBC within 5
years (5,7,8).
Therefore, repeated testing is required over a long period,
reducing both cost-effectiveness and quality of life of patients
with bladder cancer (1). NMIBC is
often treated with endoscopic resection and adjuvant intravesical
therapy, whereas MIBC is more aggressively treated with
chemotherapy combined with radical cystectomy or triple combination
therapy, including transurethral resection of a bladder tumor,
radiation therapy, and chemotherapy/immunotherapy (2). After cystectomy, approximately 30% of
patients develop recurrence within 18 months, and half develop
distant metastases within 36 months (2,9–11).
Despite advances in surgical techniques and chemotherapy, the
survival rate of patients with bladder cancer post-cystectomy has
remained constant over the past few decades (12). Owing to the limited number of
approved bladder cancer screening programs and reliable biomarkers
to accurately predict patient outcomes using cytological
assessments or resected bladder cancer tissue (12). To cure or prolong patient survival,
new biomarkers are needed to predict recurrence, prognosis, and
determine when to initiate effective treatment, in addition to
conventional histopathological evaluation of the depth of tumor
invasion.
Our group has demonstrated that AMIGO2 functions as
an inducer of liver metastasis in gastric (13) and colorectal cancers (14,15).
In addition, AMIGO2 functions as a driver molecule of liver
metastasis in cancers with liver metastasis tropism, contributes to
the acceleration of recurrence and malignant progression, and the
exacerbation of patient prognosis in cancer types that rarely
metastasize to the liver such as cervical (16) and ovarian cancers (17). Recently, it has been reported that
AMIGO2 is overexpressed in bladder cancer cell lines and bladder
cancer tissues (18). In this
study, we analyzed AMIGO2 expression in 100 cases of bladder cancer
patients who underwent radical cystectomy using an antibody that
specifically detects AMIGO2 without cross-reacting with other AMIGO
family molecules (15). We further
focused on AMIGO2 expression at the deepest invasive tumor front
and examined whether it could be used to more accurately predict
the prognosis of bladder cancer.
To the best of our knowledge, this study
demonstrated AMIGO2 expression as a prognostic biomarker after
radical cystectomy in patients with bladder cancer. AMIGO2
expression, particularly at the invasive front of bladder cancer,
was identified as the first reliable prognostic factor for both
recurrence-free (RFS) and overall survival (OS).
Materials and methods
Patients and samples
Between January 2010 and December 2017, 100 patients
diagnosed with primary bladder cancer underwent radical cystectomy
and were available for follow-up. Paraffin-embedded specimens were
obtained from the Tottori University Hospital and affiliated
hospitals (Matsue City Hospital, Matsue Red Cross Hospital, Matsue
Seikyo General Hospital, Sanin Rosai Hospital, Tottori Prefectural
Central Hospital, Tottori Red Cross Hospital, and Yonago Medical
Center), and tumors pathologically classified as pT1-pT4 were
included, except for pT0 and pTa. Of 100 tumors, 81 were urothelial
carcinoma, 11 with squamous differentiation, 3 with sarcomatoid
differentiation, 2 with a plasmacytoid variant, 1 with
adenocarcinoma, 1 with small cell carcinoma, and 1 with a
micropapillary variant. The clinicopathological findings were
determined using the Japanese Classification of Bladder Carcinomas
(19). RFS and OS were calculated
from the time of surgery to recurrence or death, respectively.
Tumor recurrence was defined as the time when new bladder cancer
lesions were detected.
Immunohistochemistry
Tumor tissue samples were fixed in formalin and
embedded in paraffin. Serial sections were sliced at 4 µm,
deparaffinized in xylene, and rehydrated using a graded alcohol
series. For AMIGO2 staining, the sections were autoclaved for 10
min in 10 mM citrate buffer (pH 6.0), then the samples were
incubated in 3% hydrogen peroxidase for 15 min to block endogenous
peroxidases, and in 10% normal goat serum (424041; Nichirei
Biosciences, Tokyo, Japan) for 15 min to prevent non-specific
antigen binding. The slides were subsequently incubated with rat
anti-AMIGO2 antibody (rTNK1B012a, 1:1,000 dilution) (15) overnight at 4°C, then incubated with
a goat polyclonal anti-rat IgG horseradish peroxidase-conjugated
antibody (ab98425, 1:200 dilution; Abcam, Cambridge, UK) at 25°C
for 20 min. For Ki-67 staining, the slides were incubated with
mouse anti-human Ki-67 monoclonal antibody (sc-101861, 1:100
dilution; Santa Cruz Biotechnology, Dallas, TX, USA) overnight at
4°C, then incubated with goat polyclonal anti-mouse IgG
peroxidase-conjugated antibody (330; 1:2,000 dilution; Medical
& Biological Laboratories, Nagoya, Japan) at 25°C for 20 min.
After primary antibody treatment, the sections were visualized
using a peroxidase substrate kit (SK-4105, Vector Laboratories,
Burlingame, CA, USA), and counterstained with hematoxylin.
Immunohistochemistry-based classification for AMIGO2 or Ki-67
expression is dependent on the positive rate. A minimum of 2 and a
maximum of 5 fields were randomly selected examined under a
microscope (Leica DM500; Wetzlar, Germany) at ×400 magnification
for ≥500 tumor cells. The evaluation of the cancer tissue was
confirmed by a pathologist. We evaluated AMIGO2 or Ki-67 expression
in bladder cancer tissues, regardless of histological type, in a
blinded manner and identified the optimal cut-off value for AMIGO2
(54.6%) using a receiver operating characteristic (ROC) curve
(Fig. S1). The cut-off value for
Ki-67 positivity was 25% in accordance with a previously published
study (20).
Statistical analysis
All statistical analyses were performed using SPSS
statistics version 28.0.0.0 software (IBM Corp., Armonk, NY, USA).
The Pearson's χ2 test or Fisher's exact test was used to
compare the differences between categorical clinicopathological
variables and AMIGO2 or Ki-67 expression. For the median, the data
were sorted in ascending order, the median was calculated, and then
the Mann-Whitney U test was performed. To evaluate the relationship
between AMIGO2 or Ki-67 expression and RFS and OS rates, hazard
ratios (HRs) and 95% confidence intervals (CIs) were calculated
using the Greenwood formula in the Kaplan-Meier method. HRs and CIs
were also used to estimate the relationships between AMIGO2 or
Ki-67 expression and clinicopathological parameters, including age,
sex, diabetes, pT stage, tumor grade, variant histological
subtypes, ureteric surgical margin, lymph node metastasis, and
neoadjuvant chemotherapy. Associations between the different
expression subtypes and recurrence or prognosis were detected using
univariate and multivariate analyses. Univariate analysis was
performed using the log-rank test. Kaplan-Meier curves were also
generated. Multivariate analysis was performed using Cox
proportional-hazard regression analyses and stratified. A stepwise
selection method was used to determine variables that were
independent predictors of RFS or OS. Survival curves were
calculated using the Kaplan-Meier method and differences between
survival curves were compared using the generalized log-rank test.
The coefficient of determination (R2) was calculated
using a regression analysis model based on sample values.
P<0.05 was considered to indicate a statistically significant
difference.
Results
AMIGO2 expression as a prognostic
factor for OS in patients with bladder cancer
To clarify the relationship between AMIGO2
expression and prognosis of patients with bladder cancer, the
immunohistochemical analysis of tumor tissues was performed using a
human anti-AMIGO2-specific antibody (15). AMIGO2 expression was scored
according to the percentage of positively stained cancer cells.
Because the cut-off value of AMIGO2 expression has not been
consistently confirmed, we first investigated the most appropriate
cut-off value. The area under the receiver operating characteristic
(ROC) curve (AUC) confirmed a cut-off value of 54.6% as the
diagnostic value of AMIGO2 expression in distinguishing the OS rate
of bladder cancer patients (Fig.
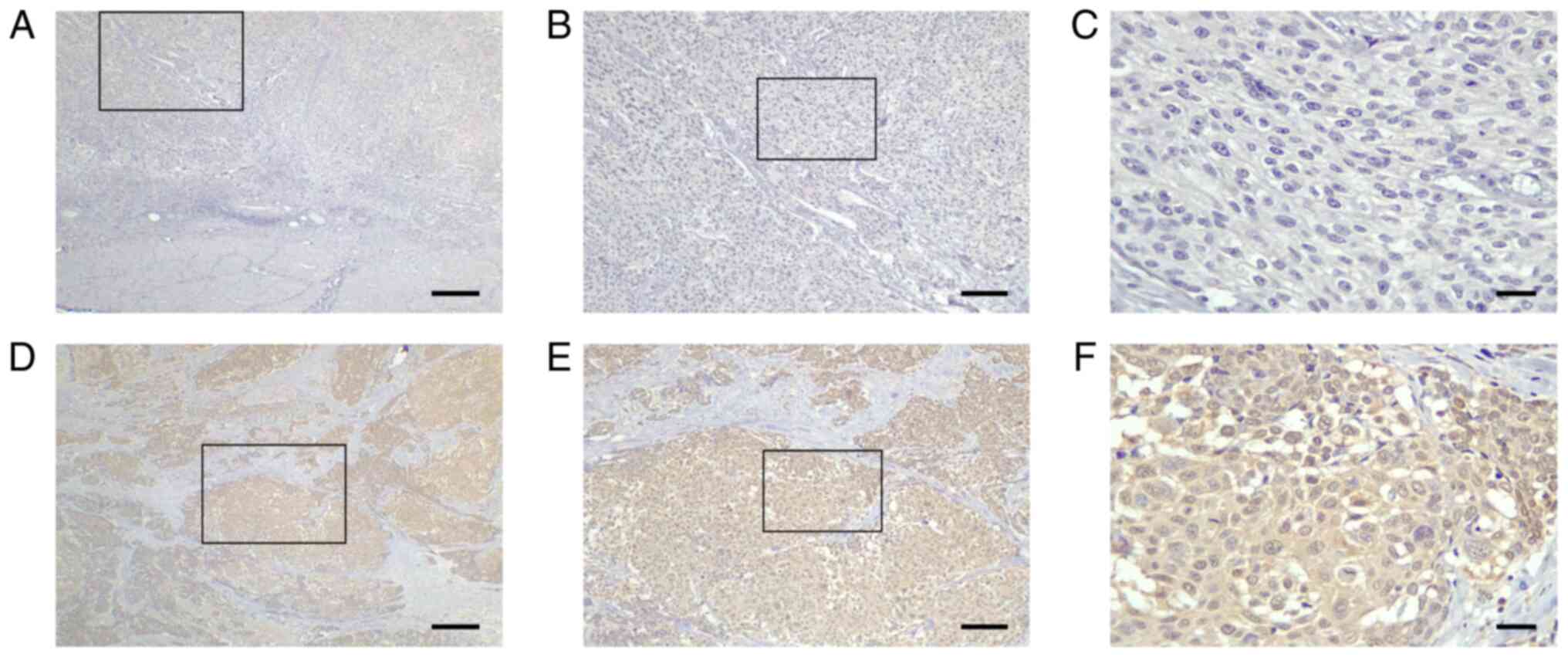
S1, AUC=0.737, P<0.0001). AMIGO2 was primarily expressed in
the cytoplasm and nucleus of bladder cancer cells, and rarely
expressed in stromal cells (Fig.
1). Cases were classified into AMIGO2 low (<54.6%, Fig. 1A-C) and AMIGO2 high (≥54.6%,
Fig. 1D-F) groups. Of 100 evaluated
tumor specimens, 42 showed low AMIGO2 expression, and 58
demonstrated high AMIGO2 expression. No significant differences
were found in the correlation between AMIGO2 expression and
clinicopathological factors (Table
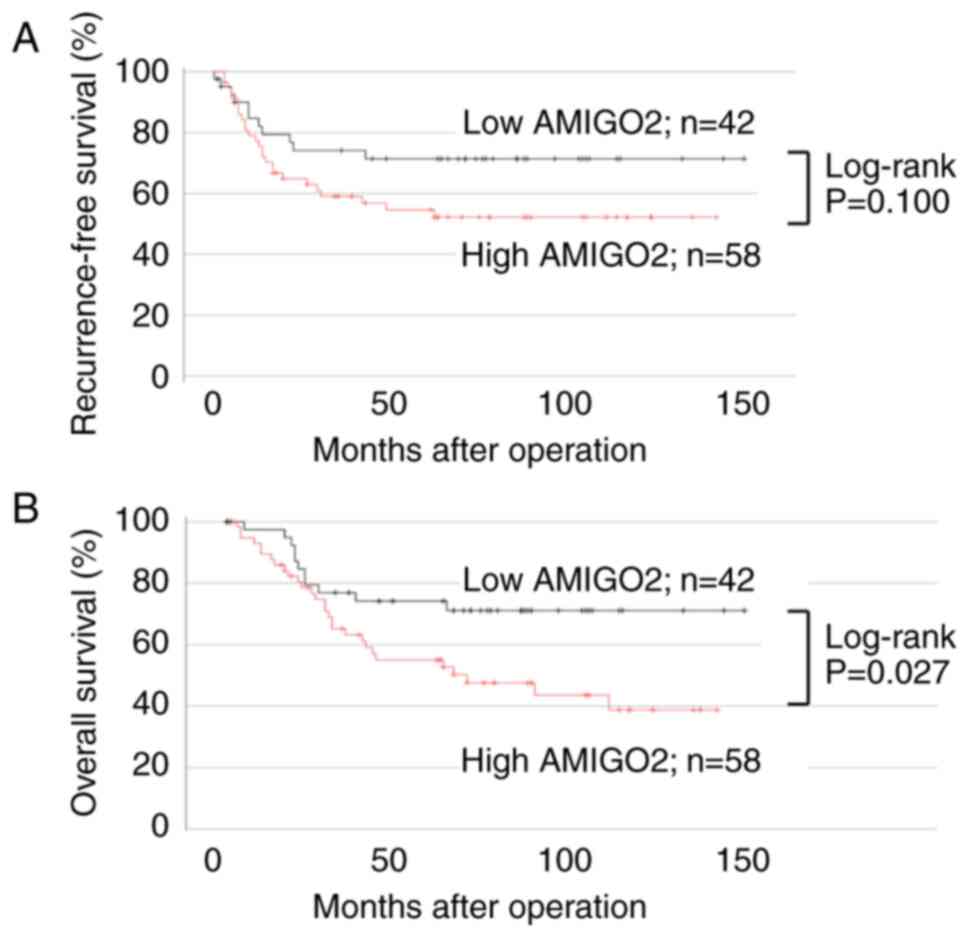
I). Kaplan-Meier analysis and log-rank tests revealed that
compared with low AMIGO2 expression, high AMIGO2 expression did not
affect RFS but reduced OS value (Fig.
2B; 95% CI, 1.009–4.544; P=0.027). In univariate analysis, pT
stage and lymph node metastasis were common prognostic factors for
RFS and OS. Variant histological subtypes were a prognostic factor
for RFS, and AMIGO2 expression was a prognostic factor for OS
(Table II). In multivariate
analysis, AMIGO2 and pT stage were determined as a significantly
independent prognostic factor for OS (P=0.047 and P=0.041,
respectively). Only lymph node metastasis was identified as a
prognostic factor for RFS (P=0.022; Table II).
 | Table I.AMIGO2 expression and
clinicopathological factors in 100 patients with bladder
cancer. |
Table I.
AMIGO2 expression and
clinicopathological factors in 100 patients with bladder
cancer.
|
|
| AMIGO2
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Total | Low (n=42) | High (n=58) |
P-valuea |
|---|
| Median age, years
(range) | 100 | 69.0 (48–85) | 72.5 (46–92) | 0.234 |
| Sex |
|
|
| 0.383 |
|
Male | 86 | 38 (90%) | 48 (83%) |
|
|
Female | 14 | 4 (10%) | 10 (17%) |
|
| Median body mass
index, kg/m2 (range) | 100 | 23.7
(13.5–31.6) | 22.3
(16.3–33.8) | 0.519 |
| Diabetes | 100 | 9 (21%) | 10 (17%) | 0.615 |
| Performance
status |
|
|
| 0.420 |
| 0 | 83 | 33 (79%) | 50 (86%) |
|
| ≥1 | 17 | 9 (21%) | 8 (14%) |
|
| Previous abdominal
surgery |
|
|
| 0.528 |
|
Absent | 65 | 29 (69%) | 36 (62%) |
|
|
Present | 35 | 13 (31%) | 22 (38%) |
|
| Previous NMIBC |
|
|
| >0.999 |
|
Absent | 18 | 8 (19%) | 10 (17%) |
|
|
Present | 82 | 34 (81%) | 48 (83%) |
|
| Tumor size |
|
|
| 0.219 |
| <30
mm | 58 | 21 (50%) | 37 (64%) |
|
| ≥30
mm | 42 | 21 (50%) | 21 (36%) |
|
| cT stage |
|
|
| 0.665 |
|
<cT3 | 68 | 30 (71%) | 38 (66%) |
|
|
≥cT3 | 32 | 12 (29%) | 20 (34%) |
|
| Tumor
multiplicity |
|
|
| >0.999 |
|
Absent | 48 | 20 (48%) | 28 (48%) |
|
|
Present | 52 | 22 (52%) | 30 (52%) |
|
| Variant
histological subtypesb |
|
|
| 0.599 |
|
Absent | 82 | 33 (79%) | 49 (84%) |
|
|
Present | 18 | 9 (21%) | 9 (16%) |
|
| Lymph node
metastasis |
|
|
| 0.127 |
|
Absent | 93 | 37 (88%) | 56 (97%) |
|
|
Present | 7 | 5 (12%) | 2 (3%) |
|
| Neoadjuvant
chemotherapy |
|
|
| 0.139 |
|
Absent | 79 | 30 (71%) | 49 (84%) |
|
|
Present | 21 | 12 (29%) | 9 (16%) |
|
 | Table II.Univariate and multivariate analysis
of AMIGO2 expression and recurrence-free and overall survival. |
Table II.
Univariate and multivariate analysis
of AMIGO2 expression and recurrence-free and overall survival.
|
| Recurrence-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
|
Univariatea |
Multivariatea |
Univariatea |
Multivariatea |
|---|
|
|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
| ≥80 vs.
<80 years | 0.831 | 0.680 | 0.295–1.568 | 0.366 | 0.212 | 1.273 | 0.577–2.809 | 0.550 |
| Sex |
|
|
|
|
|
|
|
|
| Male
vs. Female | 0.592 | 0.661 | 0.257–1.698 | 0.390 | 0.942 | 1.073 | 0.395–2.918 | 0.890 |
| Diabetes |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.913 | 0.696 | 0.248–1.950 | 0.491 | 0.273 | 1.462 | 0.623–3.436 | 0.383 |
| pT stage |
|
|
|
|
|
|
|
|
| ≥pT2
vs. <pT2 | 0.020 | 3.275 | 0.930–11.534 | 0.065 | 0.010 | 3.666 | 1.052–12.773 | 0.041 |
| Tumor grade |
|
|
|
|
|
|
|
|
| High
vs. Low | 0.659 | 1.375 | 0.624–3.033 | 0.430 | 0.470 | 1.440 | 0.658–3.151 | 0.362 |
| Variant
histological subtypesb |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.042 | 1.779 | 0.825–3.836 | 0.142 | 0.087 | 1.564 | 0.705–3.471 | 0.271 |
| Ureteric surgical
margin |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.527 | 2.823 | 0.542–14.699 | 0.218 | 0.811 | 1.126 | 0.233–5.447 | 0.882 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.001 | 2.394 | 1.135–5.050 | 0.022 | 0.009 | 1.429 | 0.683–2.990 | 0.343 |
| Neoadjuvant
chemotherapy |
|
|
|
|
|
|
|
|
| Yes vs.
No | 0.928 | 1.525 | 0.653–3.561 | 0.329 | 0.603 | 1.824 | 0.833–3.996 | 0.133 |
| AMIGO2
expression |
|
|
|
|
|
|
|
|
| High
vs. Low | 0.100 | 1.684 | 0.791–3.585 | 0.176 | 0.027 | 2.141 | 1.009–4.544 | 0.047 |
When comparing the expression of a specific protein
using tumor tissue, it is necessary to confirm the stability of the
protein in fixed tissue. The expression of AMIGO2 in formalin-fixed
paraffin-embedded tumor tissue is unlikely to change over time, and
in this study, we verified that stable AMIGO2 expression was
maintained for at least 14 years after fixation (Fig. S2).
AMIGO2 expression at the deepest
invasive tumor front is a prognostic factor for both RFS and
OS
Immunohistochemical analysis found that AMIGO2
expression differed depending on the localization of bladder cancer
in tumor tissues. We investigated AMIGO2 expression in tumor tissue
at the deepest area, that is, the invasive front, and patient
outcome. All cases were classified into two subgroups: those that
showed equal or lower AMIGO2 expression at the tumor invasive front
(Fig. 3D-F) than at the surface
layer (Fig. 3A-C), and those that
showed higher AMIGO2 expression at the tumor invasive front
(Fig. 3J-L) than at the surface
layer (Fig. 3G-I). Of the 100 tumor
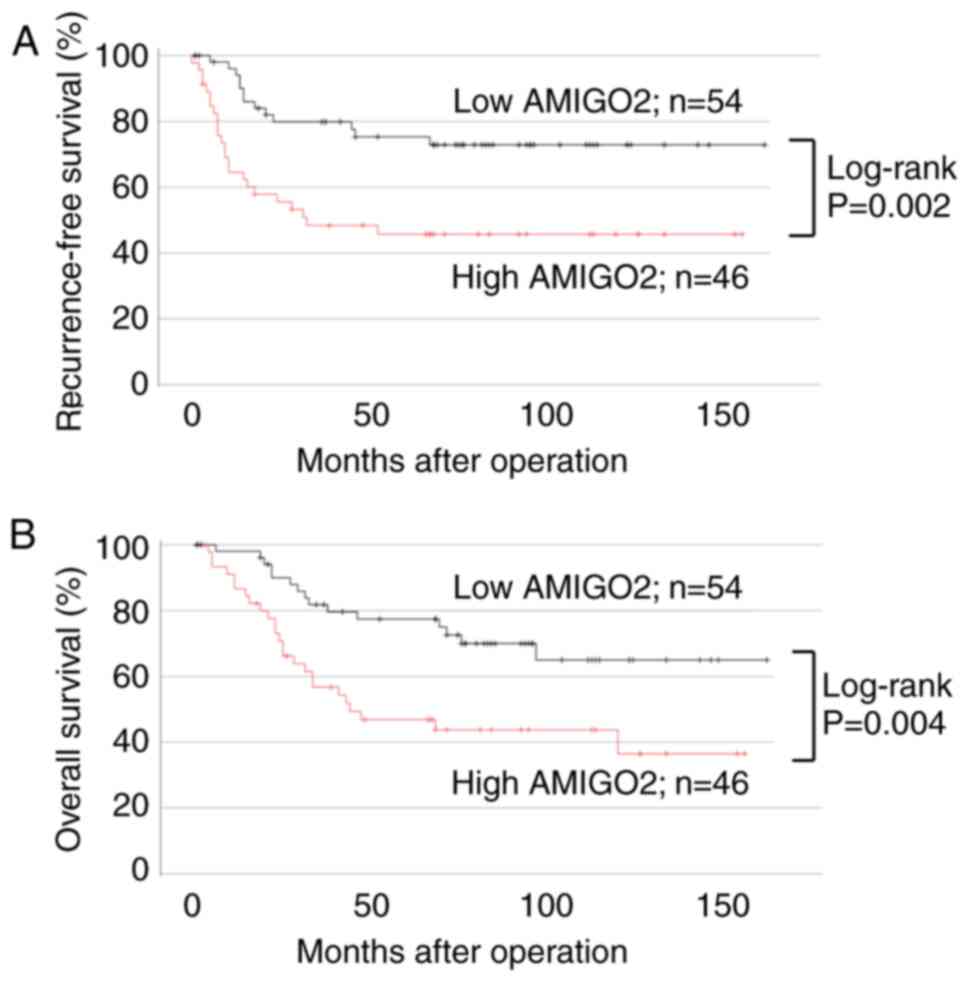
specimens, 46 displayed high AMIGO2 expression at the
tumor-invasive front, and 54 showed low AMIGO2 expression. No
correlation was found between AMIGO2 expression and
clinicopathological factors except for gender differences (Table III). Patients with high AMIGO2
expression at the invasive front had worse RFS (Fig. 4A; 95% CI, 1.283–5.652; P=0.002) and
OS (Fig. 4B; 95% CI, 1.126–4.691;
P=0.004), compared with those with low AMIGO2 expression. In the
univariate analysis, pT stage, lymph node metastasis, and AMIGO2
expression were common prognostic factors for RFS and OS (Table IV). In multivariate analysis,
AMIGO2 expression and lymph node metastasis were independent
prognostic factors for RFS, and only AMIGO2 expression was an
independent prognostic factor for OS (Table IV).
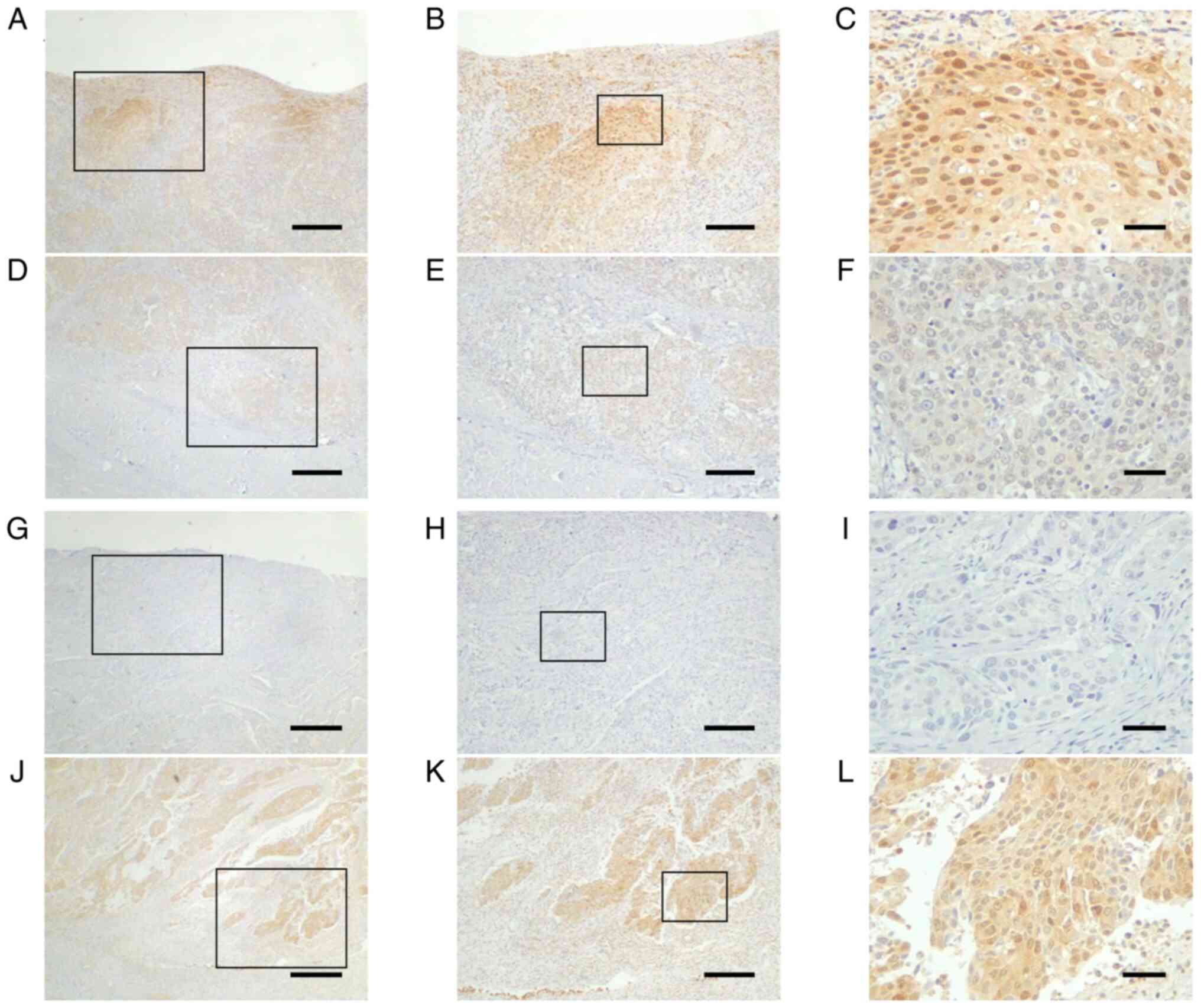 | Figure 3.Differential expression of AMIGO2 in
the surface layer or invasive front of bladder cancer. AMIGO2
expression in bladder cancer can be divided into two major
patterns: (A-C) High expression at the superficial layer and (J-L)
high expression at the invasive front. Images in (A-F) and (G-L)
are from two separate cases. Typical AMIGO2 expression at the (A-C
and G-I) surface layer and (D-F and J-L) invasive front in bladder
cancer. (A-F) In the first case, (A-C) expression at the surface is
equal to or higher than (D-F) expression at the invasive front.
(G-L) In the second case, (J-L) expression at the invasive front is
higher than (G-I) expression at the surface. Boxed areas in A, D, G
and J are enlarged in B, E, H and K, respectively, and boxed areas
in B, E, H and K are enlarged in C, F, I and L, respectively. Scale
bars, (A, D, G and J) 500 µm, (B, E, H and K) 200 µm and (C, F, I
and L) 50 µm. AMIGO2, amphoterin-induced gene and open reading
frame 2. |
 | Table III.Expression of AMIGO2 at the invasive
front of bladder cancer and clinicopathological factors. |
Table III.
Expression of AMIGO2 at the invasive
front of bladder cancer and clinicopathological factors.
|
|
| AMIGO2
expressiona |
|
|---|
|
|
|
|
|
|---|
| Variable | Total | Low (n=54) | High (n=46) |
P-valueb |
|---|
| Median age, years
(range) | 100 | 72.5 (49–86) | 69.5 (46–92) | 0.760 |
| Sex |
|
|
| 0.047 |
|
Male | 86 | 50 (93%) | 36 (78%) |
|
|
Female | 14 | 4 (7%) | 10 (22%) |
|
| Median body mass
index, kg/m2 (range) | 100 | 23.2
(13.5–31.6) | 22.3
(16.3–33.8) | 0.974 |
| Diabetes | 100 | 13 (24%) | 6 (13%) | 0.205 |
| Performance
status |
|
|
| 0.791 |
| 0 | 83 | 44 (81%) | 39 (85%) |
|
| ≥1 | 17 | 10 (19%) | 7 (15%) |
|
| Previous abdominal
surgery |
|
|
| 0.141 |
|
Absent | 65 | 39 (72%) | 26 (57%) |
|
|
Present | 35 | 15 (28%) | 20 (43%) |
|
| Previous NMIBC |
|
|
| 0.195 |
|
Absent | 18 | 7 (13%) | 11 (24%) |
|
|
Present | 82 | 47 (87%) | 35 (76%) |
|
| Tumor size |
|
|
| 0.546 |
| <30
mm | 58 | 33 (61%) | 25 (54%) |
|
| ≥30
mm | 42 | 21 (39%) | 21 (46%) |
|
| cT stage |
|
|
| 0.669 |
|
<cT3 | 68 | 38 (70%) | 30 (65%) |
|
|
≥cT3 | 32 | 16 (30%) | 16 (35%) |
|
| Tumor
multiplicity |
|
|
| 0.224 |
|
Absent | 48 | 23 (43%) | 25 (54%) |
|
|
Present | 52 | 31 (57%) | 21 (46%) |
|
| Variant
histological subtypesc |
|
|
| 0.796 |
|
Absent | 82 | 45 (83%) | 37 (80%) |
|
|
Present | 18 | 9 (17%) | 9 (20%) |
|
| Lymph node
metastasis |
|
|
| 0.700 |
|
Absent | 93 | 51 (94%) | 42 (91%) |
|
|
Present | 7 | 3 (6%) | 4 (9%) |
|
| Neoadjuvant
chemotherapy |
|
|
| 0.225 |
|
Absent | 79 | 40 (74%) | 39 (85%) |
|
|
Present | 21 | 14 (26%) | 7 (15%) |
|
 | Table IV.Univariate and multivariate analysis
of AMIGO2 expression at the invasive front of tumors and
recurrence-free and overall survival. |
Table IV.
Univariate and multivariate analysis
of AMIGO2 expression at the invasive front of tumors and
recurrence-free and overall survival.
|
| Recurrence-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
|
Univariatea |
Multivariatea |
Univariatea |
Multivariatea |
|---|
|
|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
| ≥80 vs.
<80 years | 0.831 | 0.563 | 0.236–1.343 | 0.195 | 0.212 | 1.260 | 0.580–2.737 | 0.410 |
| Sex |
|
|
|
|
|
|
|
|
| Male
vs. Female | 0.592 | 0.687 | 0.268–1.764 | 0.436 | 0.942 | 1.167 | 0.435–3.127 | 0.759 |
| Diabetes |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.913 | 0.826 | 0.285–2.398 | 0.724 | 0.273 | 1.744 | 0.720–4.224 | 0.218 |
| pT stage |
|
|
|
|
|
|
|
|
| ≥pT2
vs. <pT2 | 0.020 | 2.769 | 0.775–9.889 | 0.117 | 0.010 | 3.249 | 0.926–11.404 | 0.066 |
| Tumor grade |
|
|
|
|
|
|
|
|
| High
vs. Low | 0.659 | 1.389 | 0.622–3.101 | 0.422 | 0.470 | 1.303 | 0.598–2.839 | 0.505 |
| Variant
histological subtypesb |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.042 | 1.710 | 0.759–3.850 | 0.195 | 0.087 | 1.383 | 0.611–3.132 | 0.437 |
| Ureteric surgical
margin |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.527 | 2.175 | 0.407–11.636 | 0.364 | 0.811 | 0.879 | 0.176–4.397 | 0.875 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.001 | 2.570 | 1.192–5.539 | 0.016 | 0.009 | 1.459 | 0.681–3.128 | 0.331 |
| Neoadjuvant
chemotherapy |
|
|
|
|
|
|
|
|
| Yes vs.
No | 0.928 | 1.674 | 0.704–3.985 | 0.244 | 0.603 | 1.763 | 0.563–3.827 | 0.151 |
| AMIGO2
expressionc |
|
|
|
|
|
|
|
|
| High
vs. Low | 0.002 | 2.693 | 1.283–5.652 | 0.009 | 0.004 | 2.298 | 1.126–4.691 | 0.022 |
Ki-67 expression positivity rate, a
dependent prognostic factor for bladder cancer, and a prognostic
factor when combined with AMIGO2 expression
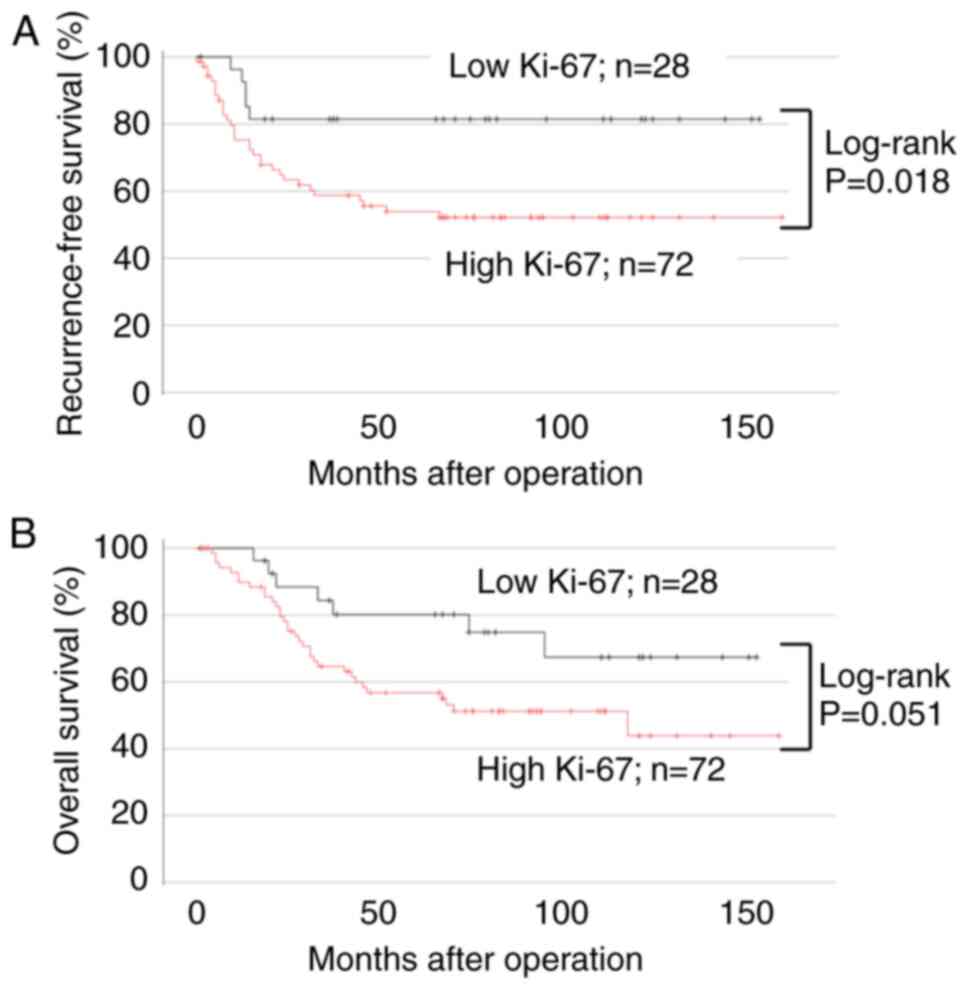
Ki-67 expression was reported to be a useful marker
of cell proliferation and prognostic marker for bladder cancer
(21,22). We compared its efficacy with that of
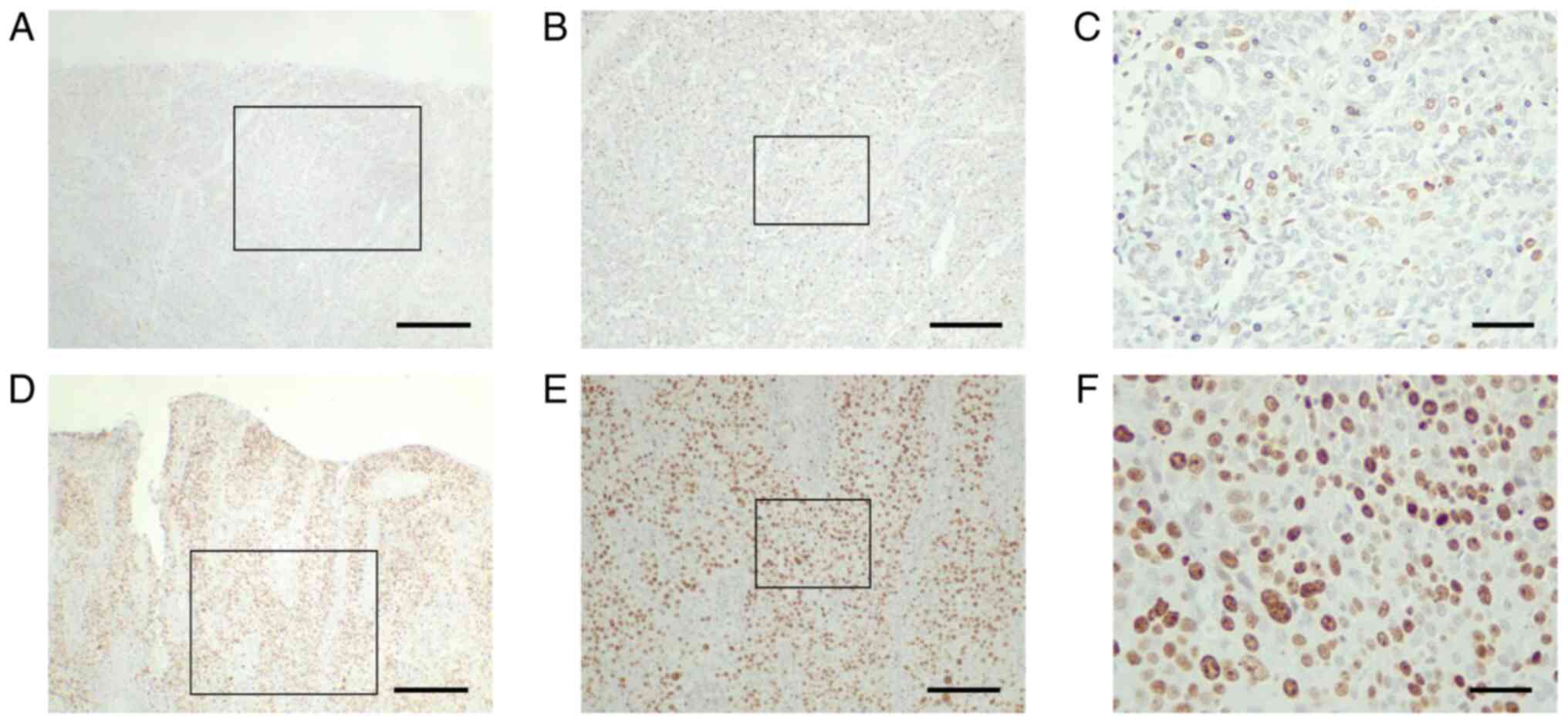
AMIGO2 expression. Based on the Ki-67 positive cell rate, 28 of 100
tumor tissues had low Ki-67 expression (<25%, Fig. 5A-C); and 72 had high Ki-67
expression (≥25%, Fig. 5D-F). No
significant differences were found in the correlation between Ki-67
expression and clinicopathological factors, except for performance
status (Table V). Compared with low
Ki-67 expression, high Ki-67 expression was associated with lower
RFS (Fig. 6A; 95% CI, 0.878–6.557;
P=0.018) but not significantly lower OS (Fig. 6B; 95% CI, 0.773–4.310; P=0.051).
Univariate analysis of Ki-67 expression and patient prognosis
showed that pT stage and lymph node metastasis were independent
prognostic factors for RFS and OS (Table VI). In contrast, multivariate
analysis showed that lymph node metastasis was prognostic factors
for RFS; however, none of the other clinicopathological factors,
including Ki-67 expression, were independent prognostic factors for
OS (Table VI).
 | Table V.Ki-67 expression and
clinicopathological factors. |
Table V.
Ki-67 expression and
clinicopathological factors.
|
|
| Ki-67
expression |
|
|---|
|
|
|
|
|
|---|
| Variable | Total | Low (n=28) | High (n=72) |
P-valuea |
|---|
| Median age, years
(range) | 100 | 70 (48–86) | 72 (46–92) | 0.750 |
| Sex |
|
|
| 0.338 |
|
Male | 86 | 26 (93%) | 60 (83%) |
|
|
Female | 14 | 2 (7%) | 12 (17%) |
|
| Median body mass
index, kg/m2 (range) | 100 | 22.2
(14.7–29.4) | 22.9
(13.5–33.8) | 0.252 |
| Diabetes | 100 | 7 (25%) | 12 (17%) | 0.397 |
| Performance
status |
|
|
| 0.035 |
| 0 | 83 | 27 (96%) | 56 (78%) |
|
| ≥1 | 17 | 1 (4%) | 16 (22%) |
|
| Previous abdominal
surgery |
|
|
| >0.999 |
|
Absent | 65 | 18 (64%) | 47 (65%) |
|
|
Present | 35 | 10 (36%) | 25 (35%) |
|
| Previous NMIBC |
|
|
| 0.573 |
|
Absent | 18 | 6 (21%) | 12 (17%) |
|
|
Present | 82 | 22 (79%) | 60 (83%) |
|
| Tumor size |
|
|
| 0.116 |
| <30
mm | 58 | 20 (71%) | 38 (53%) |
|
| ≥30
mm | 42 | 8 (29%) | 34 (47%) |
|
| cT stage |
|
|
| 0.812 |
|
<cT3 | 68 | 20 (71%) | 48 (67%) |
|
|
≥cT3 | 32 | 8 (29%) | 24 (33%) |
|
| Tumor
multiplicity |
|
|
| 0.117 |
|
Absent | 48 | 17 (61%) | 31 (43%) |
|
|
Present | 52 | 11 (39%) | 41 (57%) |
|
| Variant
histological subtypesb |
| |
| 0.384 |
|
Absent | 82 | 25 (89%) | 57 (79%) |
|
|
Present | 18 | 3 (11%) | 15 (21%) |
|
| Lymph node
metastasis |
|
|
| >0.999 |
|
Absent | 93 | 26 (93%) | 67 (93%) |
|
|
Present | 7 | 2 (7%) | 5 (7%) |
|
| Neoadjuvant
chemotherapy |
|
|
| 0.415 |
|
Absent | 79 | 24 (86%) | 55 (76%) |
|
|
Present | 21 | 4 (14%) | 17 (24%) |
|
 | Table VI.Univariate and multivariate analysis
of Ki-67 expression and recurrence-free and overall survival. |
Table VI.
Univariate and multivariate analysis
of Ki-67 expression and recurrence-free and overall survival.
|
| Recurrence-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
|
Univariatea |
Multivariatea |
Univariatea |
Multivariatea |
|---|
|
|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
| ≥80 vs.
<80 years | 0.831 | 0.765 | 0.341–1.712 | 0.514 | 0.212 | 1.437 | 0.677–3.047 | 0.345 |
| Sex |
|
|
|
|
|
|
|
|
| Male
vs. Female | 0.592 | 0.370 | 0.259–1.654 | 0.370 | 0.942 | 0.991 | 0.373–2.630 | 0.986 |
| Diabetes |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.913 | 0.892 | 0.317–2.511 | 0.829 | 0.273 | 1.534 | 0.663–3.550 | 0.318 |
| pT stage |
|
|
|
|
|
|
|
|
| ≥pT2
vs. <pT2 | 0.020 | 2.787 | 0.786–9.880 | 0.112 | 0.010 | 3.428 | 0.987–11.900 | 0.052 |
| Tumor grade |
|
|
|
|
|
|
|
|
| High
vs. Low | 0.659 | 1.186 | 0.532–2.645 | 0.676 | 0.470 | 1.296 | 0.592–2.837 | 0.516 |
| Variant
histological subtypesb |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.042 | 1.564 | 0.730–3.351 | 0.250 | 0.087 | 1.236 | 0.572–2.671 | 0.590 |
| Ureteric surgical
margin |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.527 | 2.624 | 0.514–13.397 | 0.246 | 0.811 | 1.208 | 0.252–5.786 | 0.813 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
| Present
vs. Absent | 0.001 | 2.588 | 1.250–5.356 | 0.010 | 0.009 | 1.766 | 0.877–3.555 | 0.111 |
| Neoadjuvant
chemotherapy |
|
|
|
|
|
|
|
|
| Yes vs.
No | 0.928 | 1.235 | 0.532–2.867 | 0.624 | 0.603 | 1.471 | 0.684–3.164 | 0.323 |
| Ki-67
expression |
|
|
|
|
|
|
|
|
| High
vs. Low | 0.018 | 2.399 | 0.878–6.557 | 0.088 | 0.051 | 1.825 | 0.773–4.310 | 0.170 |
AMIGO2 and Ki-67 co-expression was investigated to
determine if it could predict prognosis (Fig. 7). Patients with high AMIGO2 and
Ki-67 co-expression tended to have a lower RFS (Fig. 7A) and significantly lower OS
(Fig. 7B) than patients with other
expression pattern combinations (AMIGO2 high vs Ki-67 low, AMIGO2
low vs Ki-67 high, and AMIGO2 low vs Ki-67 low), indicating that
the prognosis of patient OS could be evaluated. Univariate analysis
showed that pT stage, lymph node metastasis, and co-expression of
AMIGO2 and Ki-67 were independent prognostic factors for RFS and OS
(Table VII). Multivariate
analysis showed that lymph node metastasis was a prognostic factor
for RFS, while high co-expression of AMIGO2 and Ki67 and pT stage
were prognostic factors for OS (Table
VII).
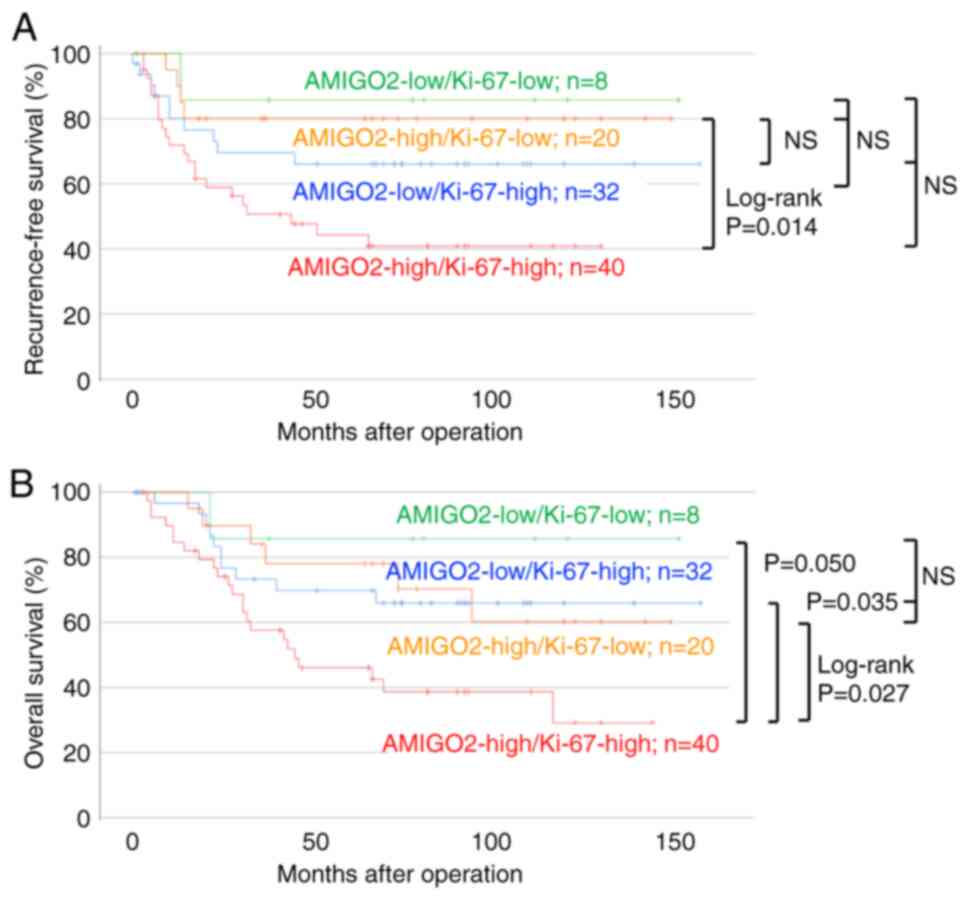 | Figure 7.High co-expression of AMIGO2 and
Ki-67 reduced overall survival more than other expression
combinations. The association between (A) recurrence-free and (B)
overall survival, and the combinations of AMIGO2 high and Ki-67
high (red, n=40), AMIGO2 high and Ki-67 low (yellow, n=20), AMIGO2
low and Ki-67 high (blue, n=32), and AMIGO2 low and Ki-67 low
(green, n=8) expression was analyzed using the Kaplan-Meier
survival analysis and log-rank test. AMIGO2, amphoterin-induced
gene and open reading frame 2; NS, not significant. |
 | Table VII.Univariate and multivariate analysis
of co-expression of AMIGO2 and Ki-67 expression and recurrence-free
and overall survival. |
Table VII.
Univariate and multivariate analysis
of co-expression of AMIGO2 and Ki-67 expression and recurrence-free
and overall survival.
|
| Recurrence-free
survival | Overall
survival |
|---|
|
|
|
|
|---|
|
|
Univariatea |
Multivariatea |
Univariatea |
Multivariatea |
|---|
|
|
|
|
|
|
|---|
| Variable | P-value | HR | 95% CI | P-value | P-value | HR | 95% CI | P-value |
|---|
| Age |
|
|
|
|
|
|
|
|
| ≥80 vs.
<80 years | 0.876 | 0.731 | 0.332–2.039 | 0.437 | 0.123 | 1.478 | 1.024–2.134 | 0.594 |
| Sex |
|
|
|
|
|
|
|
|
| Male
vs. Female | 0.748 | 0.800 | 0.314–2.039 | 0.640 | 0.636 | 1.281 | 0.476–3.442 | 0.624 |
| pT stage |
|
|
|
|
|
|
|
|
| ≥cT2
vs. <cT2 | <0.001 | 2.219 | 0.881–10.733 | 0.078 | <0.001 | 3.445 | 1.005–11.804 | 0.049 |
| Lymph node
metastasis |
|
|
|
|
|
|
|
|
| Present
vs. Absent | <0.001 | 2.219 | 1.099–4.480 | 0.026 | 0.003 | 1.566 | 0.784–3.127 | 0.204 |
| Neoadjuvant
chemotherapy |
|
|
|
|
|
|
|
|
| Yes vs.
No | 0.564 | 1.499 | 0.655–3.428 | 0.338 | 0.558 | 1.617 | 0.760–3.441 | 0.212 |
| AMIGO2 & Ki-67
expression |
|
|
|
|
|
|
|
|
| High
vs. Low | <0.001 | 1.396 | 0.958–2.034 | 0.083 | <0.001 | 1.478 | 1.024–2.134 | 0.037 |
Discussion
The present study demonstrates that high AMIGO2
expression is a prognostic factor for bladder cancer, and is an
indicator for predicting OS in patients with bladder cancer.
Notably, AMIGO2 expression at the invasive front of tumor tissues
is significantly associated with both OS and RFS in patients who
underwent radical cystectomy. To the best of our knowledge, this is
the first study to demonstrate that AMIGO2 expression is a
biomarker for predicting the risk of recurrence and reduced
survival in patients with bladder cancer.
Identifying biomarkers that can predict recurrence
and survival in patients with bladder cancer allows for early
diagnosis, identifying patients at risk of recurrence, and guiding
the selection of optimal treatment and timing (23). Considering the organ characteristics
of the bladder, attempts have been made to identify biomarkers in
urine, blood, and bladder tissue. In urine and blood liquid
biopsies, proteins (cytokines), gene mutations, microsatellites,
DNA methylation sites, mRNA, miRNA, and extracellular vesicles
containing these have been investigated as potential sources of
promising biomarkers (23–25). Tumor tissue tests include
immunohistochemical staining to detect specific antigen expression
and in situ hybridization to recognize chromosomal aneuploidy or
loss of gene loci (26). Such tests
have their advantages and disadvantages, including frequent false
positive of urine and blood tests due to contamination from
hematuria or inflammation and their low sensitivity for the
detection of low-grade and small lesions (26,27).
Moreover, a limitation of nucleic acid-based assays is their
difficulty in collecting sufficient nucleotide to obtain reliable
analytical results (28,29). In contrast, histological examination
has a high sensitivity for detecting small tumors (30) but is highly invasive for tumor
resection, and antigen expression differs depending on the
histological type and tumor progression stage (21,31).
Although many potential biomarker studies have been reported, no
single marker has been adopted in clinical practice for urine or
blood test (32). A relatively
reliable diagnostic tool for bladder cancer is urine cytology
combined with cystoscopy and evaluation of the pathological
invasion depth of the resected tumor tissue (33). Immunohistochemical staining was
conducted to determine the characteristics of various bladder
cancer lesions (21). For instance,
NMIBC is frequently positive for CD44, CK20, Ki-67, and p53
(21,34,35),
whereas MIBC is often positive for cadherin 17, CD44, CDX2, CK5/6,
CK7, CK20, GATA3, Ki-67, p63, thrombomodulin, and UroII (21,34,36–38);
however, no antigens have been determined to identify metastatic
bladder cancer (34). This study
suggests that AMIGO2 may be included as a new candidate antigen in
bladder cancer biomarker panels.
It has been reported that AMIGO2 is overexpressed in
bladder cancer cell lines and bladder cancer tissues that underwent
radical cystectomy (18). However,
since that paper examined 16 cases, we used tissue from 100 bladder
cancer patients who had undergone radical cystectomy to demonstrate
that AMIGO2 expression is a universal factor that worsens patient
prognosis. Moreover, we revealed for the first time that patients
with high AMIGO2 expression in the deepest invasive bladder cancer
had a worse prognosis in both RFS and OS compared to patients with
low AMIGO2 expression. The phenomenon of high AMIGO2 expression in
cancer cells with high growth/migration/invasive potential has been
observed not only in bladder cancer but also in colorectal cancer
(39). The cell biological
mechanism by which AMIGO2-highly expressing cancer cells are at the
invasive front is that AMIGO2-expressing cancer cells undergo
epithelial-mesenchymal transition, including through activation of
the TGF-ß/Smad signaling pathway, to acquire motility and invasive
ability (39). Furthermore,
AMIGO2-expressing cancer cells that have migrated to the invasive
front have been shown to have AMIGO2 expression localized to the
nucleus (39). These findings
suggest that increased expression of AMIGO2 accelerates the
malignancy of cancer cells, and AMIGO2 expression is expected to
become a comprehensive marker for the progression of cancer cells.
In the future, we plan to not only further investigate the
correlation between AMIGO2 expression and malignancy in bladder
cancer, as well as to examine the relationship with localization
changes in AMIGO2 expression focusing on nuclear translocation.
In Japan, nivolumab, a programmed cell death 1
monoclonal antibody, was approved in 2017 as an adjuvant therapy
for patients at high risk of recurrence after radical cystectomy
(40). When assessing recurrence
risk, we routinely used tumor stage and lymph node metastasis as
the most important histopathological prognostic variables after
radical cystectomy and lymph node dissection, as described in the
European Association of Urology guidelines (11). In addition, AMIGO2 expression
predicts recurrence and OS in patients who underwent radical
cystectomy; therefore, AMIGO2 may be a new indicator for initiating
immunotherapy.
Ki-67 is a DNA-binding nuclear protein expressed in
proliferating cells during the cell cycle except in the quiescent
phase (20,41). Ki-67 was reported to correlate with
poor prognosis in bladder carcinoma (22), breast cancer (42), non-small cell lung cancer (43), and renal cell carcinoma (44); however, Ki-67 expression in bladder
cancer remains controversial. No correlation has been found between
recurrence, progression, or tumor-related mortality in pT1 tumors
(45), and Ki-67 has been reported
to correlate with favorable survival in MIBC (46). In contrast, Ki-67 is an independent
predictor of NMIBC recurrence and progression as well as
progression alone (47–49). Herein, using multivariate analysis,
Ki-67 expression alone did not predict the prognosis of patients
with bladder cancer (Table VI).
This result is consistent with a previous report that determined
that Ki-67 positivity cannot predict the prognosis of Asian
patients with bladder cancer (20).
However, it is an indicator of poor prognosis in non-Asians
patients (20). The difference in
Ki-67 expression and prognosis between Asian and non-Asian Western
patients may reflect differences in detection antibodies, cut-off
values, race, age, inflammatory reaction, or chemotherapy agents
used (20,31,50).
Although further validation is required, these data suggest
national differences in the biological characteristics of bladder
cancer between Asian and non-Asian Western patients (20). AMIGO2 and Ki-67 co-expression
predicted a worse OS in our study (Fig.
7; Table VII). Similar
results were observed when Ki-67 expression and tumor suppressor
TP53 expression were combined to predict NMIBC recurrence, although
the mechanism of their molecular interaction remains unclear
(51).
There are several limitations to this study. First,
although clinical data were obtained from multiple institutions,
but only from a limited area of two neighboring prefectures in
Western Japan, with a small number of cases. Second, the clinical
significance of AMIGO2 expression needs to be validated in a large
cohort of Asian and non-Asian Western patients. Third, it is
currently unclear why AMIGO2 and Ki-67 co-expression can predict
the prognosis of patients with bladder cancer, possibly because the
role and clinical significance of Ki-67 expression in bladder
cancer has not yet been fully elucidated (52). Fourth, this study was evaluated only
by immunohistochemical staining using an antibody specific to human
AMIGO2 (15), but the AMIGO2
expression in tumor tissues should also be verified at another
protein (western blot) or mRNA (reverse transcription-quantitative
PCR) levels.
In conclusion, this study revealed that AMIGO2
expression is an independent prognostic factor for bladder cancer
and its RFS and OS, especially patient with bladder cancer at the
invasive front who underwent radical cystectomy.
Supplementary Material
Supporting Data
Acknowledgments
The authors would like to thank Dr Sumiyo Toji
(Sanin Rosai Hospital), Dr Kuniyasu Muraika (Tottori Prefectural
Central Hospital), Dr Hirofumi Ohno (Matsue Red Cross Hospital), Dr
Manabu Shiono (Matsue Seikyo General Hospital), Dr Tadahiro Isoyama
(Yonago Medical Center), Dr Koji Ono (Tottori Red Cross Hospital),
and Dr Takehiro Sejima (Matsue City Hospital) for their help in
collecting tumor tissue specimens and clinical data.
Funding
This work was partially supported by a Grant-in-Aid for
Scientific Research to FO (grant nos. 20K07447 and 23K06482) and
Grant-in-Aid for Research Activity Start-up (grant no. 23K19538) to
RI from the Japanese Ministry of Education, Culture, Sports,
Science, and Technology.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
AY performed immunohistochemistry and
quantification. AY, SM, MH, AT and FO contributed to the
formulation of the experimental design. AY, RS, RN, YK, NY, SM, KH,
and MH performed statistical analyses. AY, RI, HKS, RS, RN, YK, NY,
SM, KH, MH, AT, and FO contributed to the interpretation and
discussion of the results. AY wrote the original draft. FO designed
and arranged all experiments, and wrote the manuscript. AY and FO
confirm the authenticity of all the raw data. All authors read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Informed consent was waived due to the retrospective
design of the study and the use of an opt-out approach for subject
inclusion. The experimental protocol was conducted in accordance
with the guidelines of The Declaration of Helsinki, and was
approved by the Tottori University Hospital Institutional Review
Board (approval nos. 22A062 and 21A210).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
AMIGO2
|
amphoterin-induced gene and open
reading frame 2
|
|
MIBC
|
muscle-invasive bladder cancer
|
|
NMIBC
|
non-muscle-invasive bladder cancer
|
|
OS
|
overall survival
|
|
pT
|
pathological T
|
|
RFS
|
recurrence-free survival
|
|
ROC
|
receiver operating characteristic
|
References
|
1
|
Gill E and Perks CM: Mini-review: Current
bladder cancer treatment-The need for improvement. Int J Mol Sci.
25:15572024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lopez-Beltran A, Cookson MS, Guercio BJ
and Cheng L: Advances in diagnosis and treatment of bladder cancer.
BMJ. 384:e0767432024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu L, Lin N, Ye Y, Zhou S, Xu Y, Chen J,
Zhuang W and Wang Q: Prognostic and chemotherapeutic response
prediction by proliferation essential gene signature: Investigating
POLE2 in bladder cancer progression and cisplatin resistance. J
Cancer. 15:1734–1749. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Richters A, Aben KKH and Kiemeney LALM:
The global burden of urinary bladder cancer: An update. World J
Urol. 38:1895–1904. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Harsanyi S, Novakova ZV, Bevizova K,
Danisovic L and Ziaran S: Biomarkers of bladder cancer: Cell-free
DNA, epigenetic modifications and non-coding RNAs. Int J Mol Sci.
23:132062022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lenis AT, Lec PM, Chamie K and Mshs MD:
Bladder cancer: A review. JAMA. 324:1980–1991. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Castaneda PR, Theodorescu D, Rosser CJ and
Ahdoot M: Identifying novel biomarkers associated with bladder
cancer treatment outcomes. Front Oncol. 13:11142032023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hautmann RE, Volkmer BG and Gust K:
Quantification of the survival benefit of early versus deferred
cystectomy in high-risk non-muscle invasive bladder cancer (T1G3).
World J Urol. 27:347–351. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cheng L, Lopez-Beltran A and Bostwick DG:
Bladder pathology. John Wiley & Sons, Inc.; Hoboken, NJ: pp.
1–733. 2012
|
|
10
|
Soukup V, Babjuk M, Bellmunt J, Dalbagni
G, Giannarini G, Hakenberg OW, Herr H, Lechevallier E and Ribal MJ:
Follow-up after surgical treatment of bladder cancer: A critical
analysis of the literature. Eur Urol. 62:290–302. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Witjes JA, Bruins HM, Carrion A, Cathomas
R, Comperat E, Efstathiou JA, Fietkau R, Gakis G, Lorch A, Martini
A, et al: European association of urology guidelines on
muscle-invasive and metastatic bladder cancer: Summary of the 2023
guidelines. Eur Urol. 85:17–31. 2024. View Article : Google Scholar
|
|
12
|
Dobruch J and Oszczudłowski M: Bladder
cancer: Current challenges and future directions. Medicina
(Kaunas). 57:7492021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goto K, Morimoto M, Osaki M, Tanio A,
Izutsu R, Fujiwara Y and Okada F: The impact of AMIGO2 on prognosis
and hepatic metastasis in gastric cancer patients. BMC Cancer.
22:2802022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tanio A, Saito H, Amisaki M, Hara K,
Sugezawa K, Uejima C, Tada Y, Kihara K, Yamamoto M, Nosaka K, et
al: AMIGO2 as a novel indicator of liver metastasis in patients
with colorectal cancer. Oncol Lett. 21:2782021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goto K, Osaki M, Izutsu R, Tanaka H,
Sasaki R, Tanio A, Satofuka H, Kazuki Y, Yamamoto M, Kugoh H, et
al: Establishment of an antibody specific for AMIGO2 improves
immunohistochemical evaluation of liver metastases and clinical
outcomes in patients with colorectal cancer. Diagn Pathol.
17:162022. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Iida Y, Sato S, Izutsu R, Seong HK, Okawa
M, Osaku D, Komatsu H, Osaki M, Taniguchi F and Okada F: AMIGO2
expression as a predictor of recurrence in cervical cancer with
intermediate risk. Mol Clin Oncol. 19:562023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Iida Y, Osaki M, Sato S, Izutsu R, Seong
H, Komatsu H, Taniguchi F and Okada F: AMIGO2 is involved in the
spread of peritoneal metastasis in serous ovarian cancer via
promoting adhesion to the peritoneal mesothelial cells. Int J Clin
Oncol. 29:1354–1363. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Han D, Xiong B, Zhang X, Chen C, Yao Z, Wu
H, Cao J, Li J, Li P, Wang Z and Tian J: Knockdown of AMIGO2
suppresses proliferation and migration through regulating PPAR-γ in
bladder cancer. Hereditas. 161:212024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
The Japanese Urothelial Association, The
Japanese Society of Pathology, Japan Radiological Society and
Japanese Society of Medical Oncology (eds), . The general rule for
clinical and pathological studies on renal pelvic, ureteral, and
bladder cancer. 2nd Edition. Igakutosho Shuppan; Tokyo: pp. 1–180.
2021
|
|
20
|
Tian Y, Ma Z, Chen Z, Li M, Wu Z, Hong M,
Wang H, Svatek R, Rodriguez R and Wang Z: Clinicopathological and
prognostic value of Ki-67 expression in bladder cancer: A
systematic review and meta-analysis. PLoS One. 11:e01588912016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Akgul M, MacLennan GT and Cheng L: The
applicability and utility of immunohistochemical biomarkers in
bladder pathology. Human Pathol. 98:32–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Krabbe LM, Bagrodia A, Haddad AQ, Kapur P,
Khalil D, Hynan LS, Wood CG, Karam JA, Weizer AZ, Raman JD, et al:
Multi-institutional validation of the predictive value of Ki-67 in
patients with high grade urothelial carcinoma of the upper urinary
tract. J Urol. 193:1486–1493. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nováková ZV, Kuniaková M, Žiaran S and
Harsányi Š: Molecular biomarkers of bladder cancer: A mini-review.
Physiol Res. 72:S247–S256. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
O'Sullivan P, Sharples K, Dalphin M,
Davidson P, Gilling P, Cambridge L, Harvey J, Toro T, Giles N,
Luxmanan C, et al: A multigene urine test for the detection and
stratification of bladder cancer in patients presenting with
hematuria. J Urol. 188:741–747. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sugeeta SS, Sharma A, Ng K, Nayak A and
Vasdev N: Biomarkers in bladder cancer surveillance. Front Surg.
8:7358682021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Flores Monar GV, Reynolds T, Gordon M,
Moon D and Moon C: Molecular markers for bladder cancer screening:
An insight into bladder cancer and FDA-approved biomarkers. Int J
Mol Sci. 24:143742023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pycha A, Lodde M, Comploj E, Negri G,
Egarter-Vigl E, Vittadello F, Lusuardi L, Palermo S and Mian C:
Intermediate-risk urothelial carcinoma: An unresolved problem?
Urology. 63:472–475. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wallace E, Higuchi R, Satya M, McCann L,
Sin MLY, Bridge JA, Wei H, Zhang J, Wong E, Hiar A, et al:
Development of a 90-minute integrated noninvasive urinary assay for
bladder cancer detection. J Urol. 199:655–662. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Henning GM, Barashi NS and Smith ZL:
Advances in biomarkers for detection, surveillance, and prognosis
of bladder cancer. Clin Genitouri Cancer. 19:194–198. 2021.
View Article : Google Scholar
|
|
30
|
Lokeshwar VB, Habuchi T, Grossman HB,
Murphy WM, Hautmann SH, Hemstreet GP III, Bono AV, Getzenberg RH,
Goebell P, Schmitz-Dräger BJ, et al: Bladder tumor markers beyond
cytology: International Consensus Panel on bladder tumor markers.
Urology. 66 (Suppl 1):35–63. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng L, MacLennan GT and Bostwick DG:
Urologic surgical pathology. Elsevier Inc.; Philadelphia, PA: pp.
1–944. 2019
|
|
32
|
Smith ZL and Guzzo TJ: Urinary markers for
bladder cancer. F1000Prime Rep. 5:212013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Maas M, Todenhöfer T and Black PC: Urine
biomarkers in bladder cancer-current status and future
perspectives. Nat Rev Urol. 20:597–614. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Amin MB, Trpkov K, Lopez-Beltran A and
Grignon D; Members of the IIiDUPG, : Best practices recommendations
in the application of immunohistochemistry in the bladder lesions:
Report from the International Society of Urologic Pathology
consensus conference. Am J Surg Pathol. 38:e20–e34. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
McKenney JK, Desai S, Cohen C and Amin MB:
Discriminatory immunohistochemical staining of urothelial carcinoma
in situ and non-neoplastic urothelium: An analysis of cytokeratin
20, p53, and CD44 antigens. Am J Surg Pathol. 25:1074–1078. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Moch H, Cubilla AL, Humphrey PA, Reuter VE
and Ulbright TM: The 2016 WHO classification of tumours of the
urinary system and male genital organs-part A: Renal, penile, and
testicular tumours. Eur Urol. 70:93–105. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rao Q, Williamson SR, Lopez-Beltran A,
Montironi R, Huang W, Eble JN, Grignon DJ, Koch MO, Idrees MT,
Emerson RE, et al: Distinguishing primary adenocarcinoma of the
urinary bladder from secondary involvement by colorectal
adenocarcinoma: Extended immunohistochemical profiles emphasizing
novel markers. Mod Pathol. 26:725–732. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Margulis V, Shariat SF, Ashfaq R,
Sagalowsky AI and Lotan Y: Ki-67 is an independent predictor of
bladder cancer outcome in patients treated with radical cystectomy
for organ-confined disease. Clin Cancer Res. 12:7369–7373. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Izutsu R, Osaki M, Seong H, Ogata S, Sato
R, Hamada JI and Okada F: AMIGO2 enhances the invasive potential of
colorectal cancer by inducing EMT. Cancer Gene Ther. 31:1786–1795.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Bajorin DF, Witjes JA, Gschwend JE,
Schenker M, Valderrama BP, Tomita Y, Bamias A, Lebret T, Shariat
SF, Park SH, et al: Adjuvant nivolumab versus placebo in
muscle-invasive urothelial carcinoma. N Engl J Med. 384:2102–2114.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Schlüter C, Duchrow M, Wohlenberg C,
Becker MH, Key G, Flad HD and Gerdes J: The cell
proliferation-associated antigen of antibody Ki-67: A very large,
ubiquitous nuclear protein with numerous repeated elements,
representing a new kind of cell cycle-maintaining proteins. J Cell
Biol. 123:513–522. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Shui R, Yu B, Bi R, Yang F and Yang W: An
interobserver reproducibility analysis of Ki67 visual assessment in
breast cancer. PLoS One. 10:e01251312015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Wen S, Zhou W, Li CM, Hu J, Hu XM, Chen P,
Shao GL and Guo WH: Ki-67 as a prognostic marker in early-stage
nonsmall cell lung cancer in Asian patients: A meta-analysis of
published studies involving 32 studies. BMC Cancer. 15:5202015.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gayed BA, Youssef RF, Bagrodia A, Darwish
OM, Kapur P, Sagalowsky A, Lotan Y and Margulis V: Ki67 is an
independent predictor of oncological outcomes in patients with
localized clear-cell renal cell carcinoma. BJU Int. 113:668–673.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Acikalin D, Oner U, Can C, Acikalin MF and
Colak E: Predictive value of maspin and Ki-67 expression in
transurethral resection specimens in patients with T1 bladder
cancer. Tumori. 98:344–350. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tanabe K, Yoshida S, Koga F, Inoue M,
Kobayashi S, Ishioka J, Tamura T, Sugawara E, Saito K, Akashi T, et
al: High Ki-67 expression predicts favorable survival in
muscle-invasive bladder cancer patients treated with
chemoradiation-based bladder-sparing protocol. Clin Genitourin
Cancer. 13:e243–e251. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Chen JX, Deng N, Chen X, Chen LW, Qiu SP,
Li XF and Li JP: A novel molecular grading model: Combination of
Ki67 and VEGF in predicting tumor recurrence and progression in
non-invasive urothelial bladder cancer. Asian Pac J Cancer Prev.
13:2229–2234. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Makboul R, Refaiy AE, Badary FA, Abdelkawi
IF, Merseburger AS and Mohammed RA: Expression of surviving in
squamous cell carcinoma and transitional cell carcinoma of the
urinary bladder: A comparative immunohistochemical study. Korean J
Urol. 56:31–40. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Gontero P, Gillo A, Fiorito C, Oderda M,
Pacchioni D, Casetta G, Peraldo F, Zitella A, Tizzani A and Ricceri
F: Prognostic factors of ‘high-grade’ Ta bladder cancers according
to the WHO 2004 classification: Are these equivalent to ‘high-risk’
non-muscle-invasive bladder cancer? Urol Int. 92:136–142. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Mallofré C, Castillo M, Morente V and Solé
M: Immunohistochemical expression of CK20, p53, and Ki-67 as
objective markers of urothelial dysplasia. Mod Pathol. 16:187–191.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang L, Feng C, Ding G, Ding Q, Zhou Z,
Jiang H and Wu Z: Ki67 and TP53 expressions predict recurrence of
non-muscle-invasive bladder cancer. Tumour Biol. 35:2989–2995.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bryan RT, Zeegers MP, James ND, Wallace DM
and Cheng KK: Biomarkers in bladder cancer. BJU Int. 105:608–613.
2010. View Article : Google Scholar : PubMed/NCBI
|