Introduction
Recent epidemiological data confirm increasing
global incidence (13.83 cases per 100,000 individuals) and
mortality (4.54 deaths per 100,000 individuals) rates of cervical
cancer, as evidenced by updated cancer surveillance reports
(1). External beam radiotherapy
(EBRT), often combined with brachytherapy (BT), remains a
cornerstone treatment. Standard EBRT protocols involve ~25
fractions delivered over 4–6 weeks. During this extended treatment
course, anatomical shifts in surrounding organs (e.g., bladder and
rectum) and uterine mobility, coupled with tumor deformation and
volumetric regression, are frequently observed. To mitigate these
dynamic changes and setup uncertainties, conventional RT planning
expands the clinical target volume (CTV) by 7- to 15-mm margins to
create planning target volumes (PTVs), with dose prescriptions
based on PTV contours. However, studies indicate that CTV
displacement in cervical cancer may exceed 20 mm during treatment
(2), suggesting that conventional
PTV margins may inadequately account for such variability.
Furthermore, clinics often apply uniform PTV margins across patient
cohorts, despite significant inter-individual differences in
organ-at-risk (OAR) displacement, uterine motility, tumor
regression rates and daily setup errors. Suboptimal margins risk
geographic miss (underdosing targets) or excessive irradiation of
healthy tissues, contributing to treatment-related toxicities such
as gastrointestinal complications, which affect ~70% of patients
with EBRT (3). While image-guided
RT (IGRT) reduces setup inaccuracies, it cannot compensate for
interfractional organ and tumor variations. Moreover, standard
three-degree-of-freedom treatment couches lack rotational
correction capabilities, further limiting positional accuracy.
Online adaptive RT (oART) was first conceptualized
by Yan et al (4) in 1997 to
address discrepancies between preplanned treatments and real-time
anatomical changes in targets and OARs during RT (5). The core principle of oART lies in its
dynamic closed-loop framework, which integrates daily imaging,
self-responding adjustments and self-correcting adaptations. Recent
advances in imaging, computational power and artificial
intelligence (AI) have enabled its clinical implementation. A
typical oART workflow comprises daily image acquisition, deformable
image registration, automated segmentation of targets and OARs,
adaptive plan generation, dosimetric evaluation, in vivo
quality assurance (QA) and beam delivery (6). This process demands robust AI
algorithms, multidisciplinary team coordination and
high-performance RT systems. Owing to rapid technological
innovations, oART has emerged as a pivotal research focus in
contemporary RT, offering the potential to enhance precision and
adaptability in cancer treatment.
Clinical evidence positions cervical cancer as an
optimal candidate for oART. In the present study, oART was applied
to all fractions during the EBRT phase for a patient with cervical
cancer. All aspects of the oART process were observed and studied.
Dosimetric parameters and imaging before and after RT were
compared. More importantly, a result of pathological complete
response (pCR) was achieved in this case only after implementing
external beam oART.
Case report
A previously healthy 54-year-old woman experienced
irregular vaginal bleeding for 1 month and initially presented at
The First Affiliated Hospital of Chengdu Medical College (Chengdu,
China) in June 2023. Magnetic resonance imaging (MRI) showed that
there was a clump of slight hypointensity on T1WI and slight
hyperintensity on T2WI in the cervical cavity, with a size of
~2.2×2.4×1.9 cm. The mass involved the lower one-third of the
uterine body and protruded to the lower part of the uterine cavity,
and the lower edge was not clearly demarcated with the posterior
wall of the upper end of the vagina (Fig. 1A). After gynecological examination
and biopsy, the pathological diagnosis of papillary squamous cell
carcinoma was confirmed by the Department of Pathology. Combined
with laboratory examinations (including complete blood count,
hepatic and renal function tests) and imaging examinations
[including computed tomography (CT) and MRI.], the general
condition of the patient was comprehensively evaluated, and with
the consent of the patient, the treatment regimen of external RT
combined with brachyradiotherapy was adopted, with concurrent
chemotherapy (380 mg albumin-bound paclitaxel on day 1 + 110 mg
nedaplatin on day 1; every 3 weeks). According to the Radiation
Therapy Oncology Group guidelines (7,8), the
primary gross tumor volume (GTVp), CTV (included the tumor, cervix,
uterine corpus, parametrium, upper vaginal portion, and lymph node
regions, including the common iliac, internal iliac, external
iliac, obturator and presacral areas) and PTV (created by expanding
CTV with 5-mm margins) were contoured. Subsequently, 95% of the PTV
received 100% of the prescription dose (50.4 Gy/28 fractions/6
weeks). oART based on fan-beam CT (FBCT) guidance was conducted for
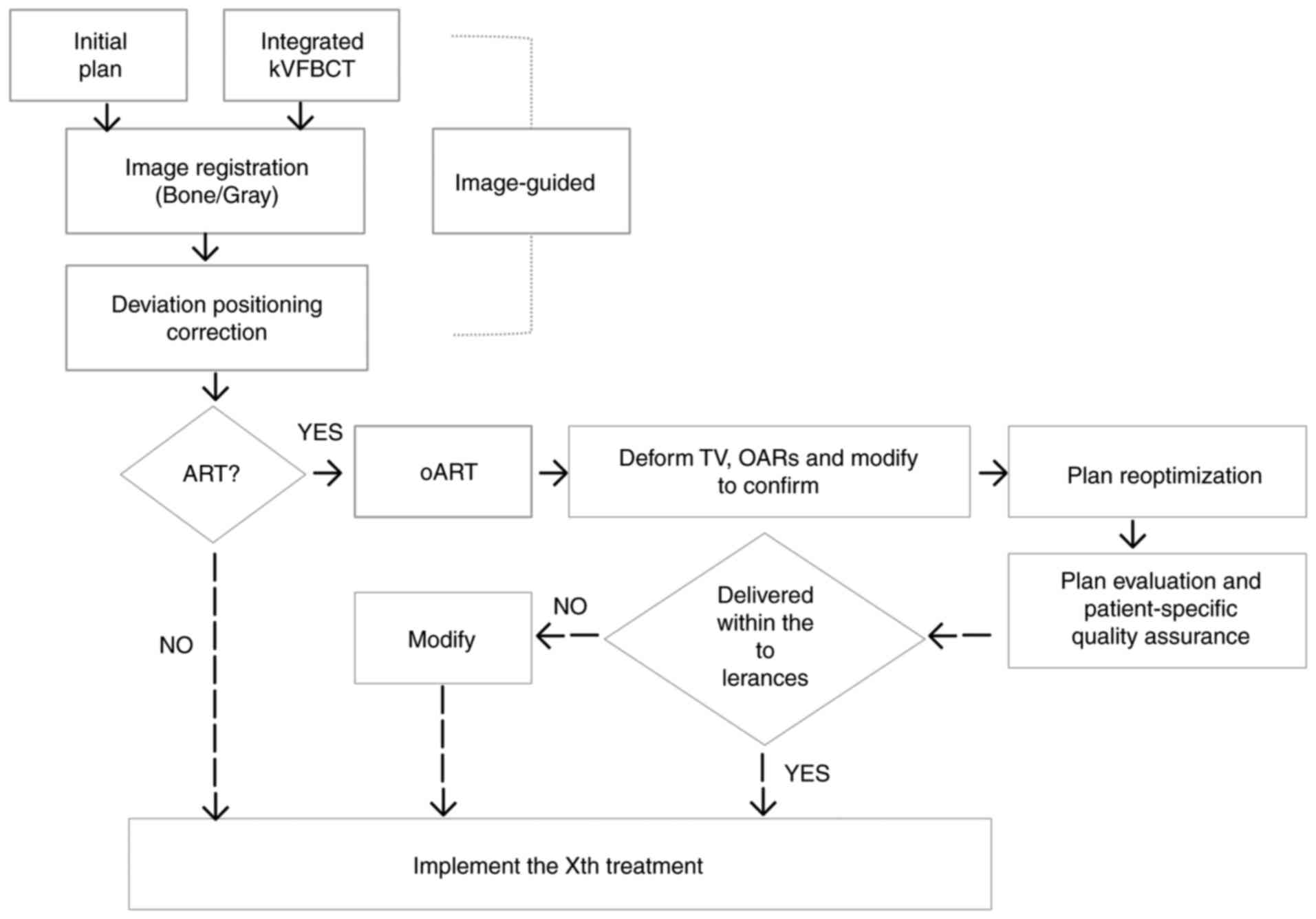
every fraction of EBRT for this patient. A flow chart of the oART
is illustrated in Fig. 2.
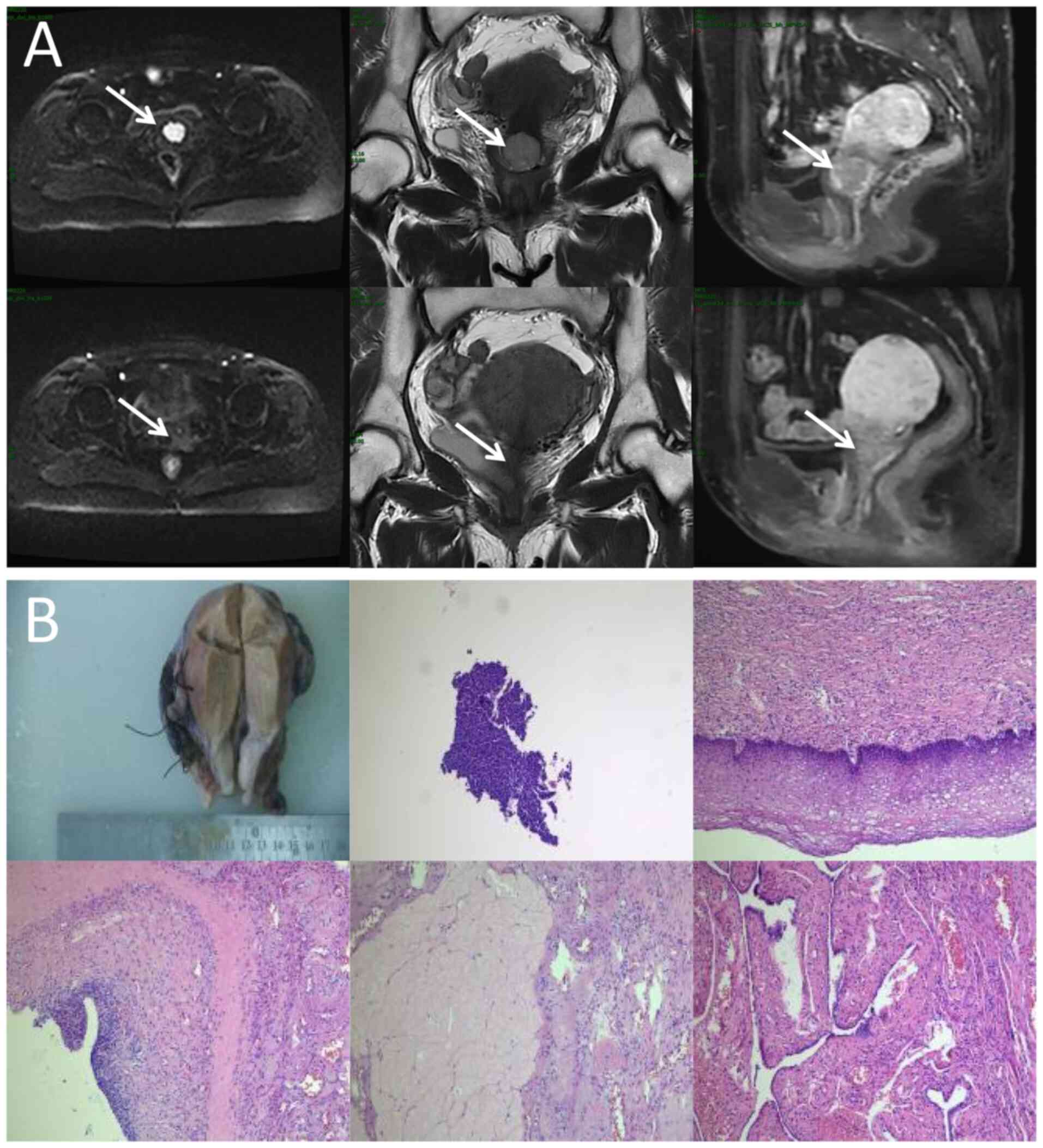 | Figure 1.Locoregional response evaluation. (A)
Magnetic resonance imaging of the patient pre-radiotherapy (upper)
and post-radiotherapy (lower). From left to right:
Diffusion-weighted imaging (b=1,000 sec/mm2) axial view,
T2-weighted coronal plane and T1-weighted sagittal plane. Arrows
indicate the mass. (B) Representative imaging from the
postoperative histopathological diagnosis report, comprising both
the gross surgical specimen and photomicrographs (obtained from the
cervix, surgical margins, ovaries, fallopian tubes and pelvic lymph
nodes; hematoxylin and eosin staining, with magnifications of ×40,
×100, ×200, ×200 and ×400, respectively). |
After completing EBRT, the image review of MRI was
performed and the results showed that the lesions in the cervix
region had basically regressed (Fig.
1A). No significant radiotherapy-related toxicity or side
effects occurred throughout the process for the patient. At 1-month
post-EBRT, the patient asked for a change in treatment and opted
for surgery instead of BT. There were no significant intestinal
adhesions and parametrial tissue thickening during the operation.
The surgery was successfully completed, no cancer cells were
observed in the postoperative pathological examination, and a
result of pCR was achieved. In the follow-up of nearly 1 year
(final assessment in October 2024), the patient did not complain of
obvious discomfort, and there was no obvious abnormality in the
results of various examinations.
Discussion
RT is a cornerstone of cervical cancer treatment.
However, the proximity of the cervix to the bladder and rectum
introduces challenges: During successive treatment fractions,
variations in the morphology and position of these organs occur to
varying degrees, leading to discrepancies between the delivered
dose and the planned dose. In the present case, the bladder and
rectum exhibited significant and irregular morphological and
volumetric changes. Concurrently, the volume of GTVp progressively
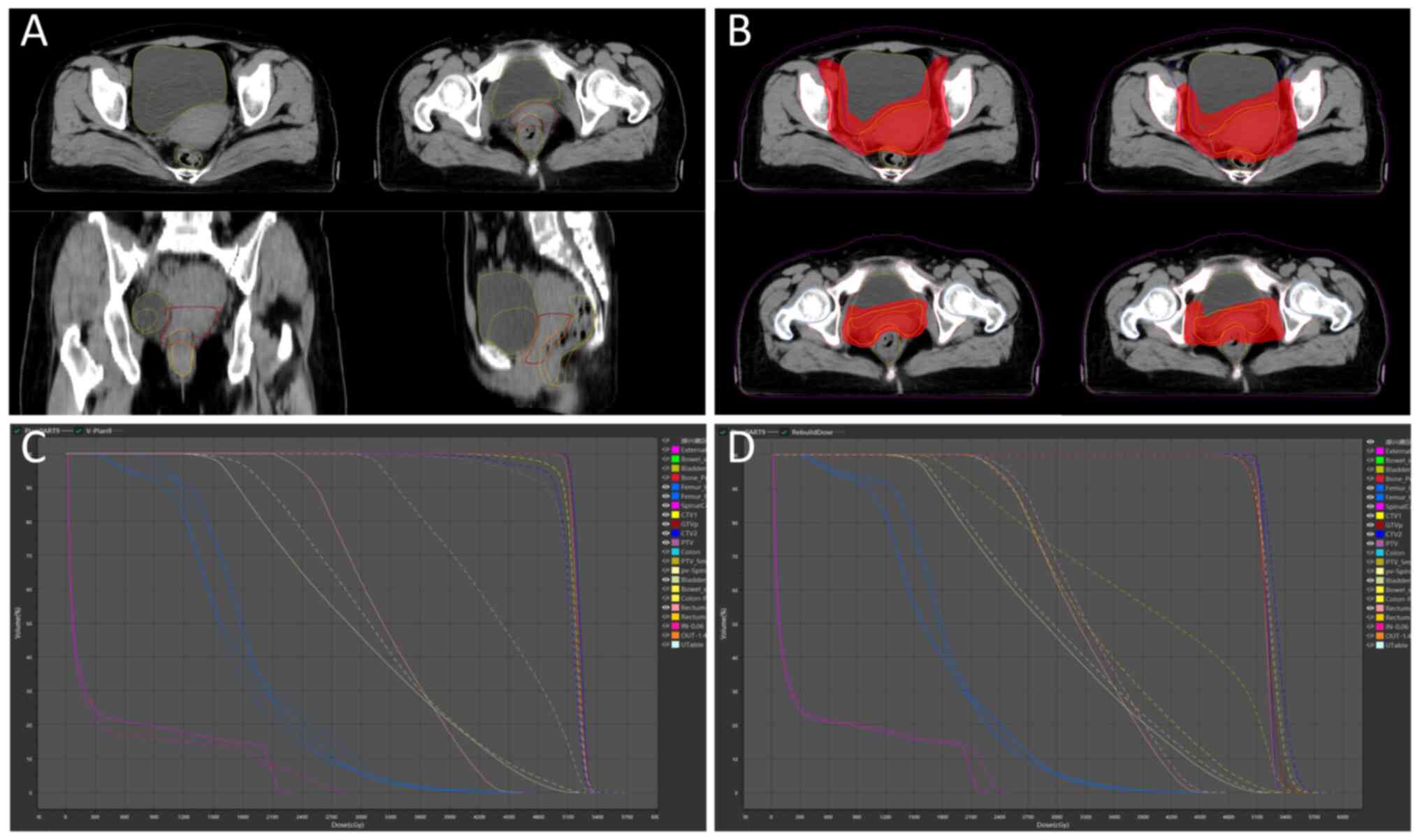
decreased (Fig. 3A), directly
altering the actual dose distribution of the initial plan. oART
addressed these issues by reoptimizing the dose distribution to
conform to the target anatomy. This approach not only mitigated the
impact of inter-fractional anatomical shifts and rotational
deviations but also allowed for a reduced CTV to PTV expansion
margin. Consequently, both target coverage and OARs dose
constraints were optimized (Fig.
3B). Compared with the IGRT plan (Fig. 3C), the ART plan demonstrated
superior dosimetric outcomes for target coverage and OAR sparing
(9,10) (Table
I). For instance, anatomical deformations were observed in the
bladder, rectum and GTVp during the ninth treatment fraction in the
present case. Marked clinical concerns arose when applying IGRT
based on the original plan, including an increased irradiated
rectal volume and a partial target miss in the ventral tumor
region. After adaptive plan optimization, the volume ratio of 40 Gy
covering the rectum (V40Gy) was significantly reduced
from 67.57 to 13.21%, and the PTV coverage (V100%)
increased from 77.63 to 99.00%. The PTV coverage (V100%)
values for the 28 ART plans and IGRT plans were 98.51±0.82 and
88.99±4.40%, respectively. Although this case study did not include
statistical analyses to account for random variability, the ART
plans demonstrated clear dosimetric superiority, evidenced by
exceptional consistency and clinical target achievement.
 | Table I.Comparison of dosimetric parameters of
PTV and organs at risk. |
Table I.
Comparison of dosimetric parameters of
PTV and organs at risk.
| Parameter | Initial-Plan | IGRT-Plan | ART-Plan |
|---|
| PTV |
|
|
|
|
Dmax, cGy | 5,375.14 | 5,815.58±106.01 | 5,356.02±21.25 |
|
D98, cGy | 5,011.73 | 4,422.74±669.05 | 5,053.52±19.89 |
|
D95, cGy | 5,047.39 | 4,825.80±191.45 | 5,087.15±15.04 |
|
D50, cGy | 5,171.68 | 5,195.35±23.82 | 5,198.88±22.32 |
|
D2, cGy | 5,279.86 | 5,334.00±16.63 | 5,304.06±26.12 |
|
CIa | 0.92 | 0.79±0.05 | 0.92±0.01 |
|
HIb | 0.05 | 0.18±0.13 | 0.05±0.00 |
|
V100%, % | 95.94 | 88.99±4.40 | 98.51±0.82 |
| Bladder-PTV |
|
|
|
|
V40, % | 25.66 | 22.29±12.12 | 31.54±12.15 |
| Rectum-PTV |
|
|
|
|
Dmax, cGy | 4576.36 | 5076.71±248.57 | 4566.73±45.61 |
|
V40, % | 16.94 | 33.03±19.05 | 16.96±3.39 |
| Small bowel-PTV |
|
|
|
|
Dmax, cGy | 4757.92 | 5368.87±208.65 | 4734.66±46.63 |
|
V40, % | 4.68 | 5.06±2.00 | 4.46±1.06 |
| Left femur head |
|
|
|
|
V30, % | 5.88 | 6.81±1.01 | 6.00±0.81 |
|
V50, % | 0.00 | 0.00±0.00 | 0.00±0.00 |
| Right femur head |
|
|
|
|
V30, % | 4.83 | 4.78±1.06 | 5.18±0.48 |
|
V50, % | 0.00 | 0.00±0.00 | 0.00±0.00 |
Achieving efficient and high-quality oART requires
addressing several critical factors. First, high-quality imaging is
critical for oART, as target contouring and dose planning depend on
linear accelerator (linac)-acquired images. Conventional linacs use
kilovoltage (kV) cone-beam CT (CBCT), but the low soft-tissue
contrast (due to scattering artifacts) limits tumor visualization
and forces reliance on bony landmarks for registration. CBCT also
lacks sufficient resolution for precise target/OAR delineation and
fails to provide accurate Hounsfield unit-based electron density
data for dose calculation (11–14).
The present study employed the uRT-linac 506c (United Imaging
Healthcare Co., Ltd.), a CT-integrated linac with a 16-slice kV
FBCT scanner. The FBCT delivers diagnostic-quality images
comparable to planning CTs, enabling superior soft-tissue contrast
for target/OAR delineation and reliable electron density mapping.
By contrast, systems like Ethos enhance CBCT through iterative
reconstruction with scatter correction, while Unity uses predefined
electron density maps for synthetic CT generation. The second
critical factor is an efficient and intelligent workflow.
Anatomical shifts often occur within minutes or tens of minutes;
thus, timely execution is essential for successful oART. The oART
workflow relies on seamless integration of software and hardware,
requiring multidisciplinary collaboration among radiotherapists,
physicists and therapists. Key components include rapid contouring
tools, efficient treatment planning systems and high-precision
delivery equipment. Three oART platforms are currently implemented
in clinical practice: The FBCT-guided uRT-Linac 506c (United
Imaging Healthcare Co., Ltd.), CBCT-guided Ethos (Varian; Siemens
Healthineers) and MR-guided Unity (Elekta Instrument AB). Empirical
studies have validated the feasibility and time efficiency of these
systems (15–17). Specifically, the uRT-Linac 506c and
Ethos workflows require ~20 min for a complete oART cycle, whereas
Unity necessitates >30 min. Efficiency in oART workflows is
critically dependent on AI. Advances in AI have accelerated
contouring and planning processes, primarily through automated
segmentation of targets and OARs, and AI-driven treatment planning.
To date, studies have demonstrated the utility of AI-based
segmentation models and dose-prediction algorithms for automated
planning (18–21). These tools reduce labor and time
demands while enhancing the precision, efficiency and consistency
of target delineation and dose distribution. In the present case, a
dedicated AI segmentation model for cervical cancer based on
multi-decoder and semi-supervised learning was implemented to
enable automated target delineation during the patient's oART
workflow (22). The dice similarity
coefficient (DSC) for GTVp, CTV1 and CTV2 were determined to be
0.81±0.03, 0.90±0.02 and 0.92±0.01, respectively. Manual
modification of the target during oART based on the
auto-segmentation efficiently reduced time consumption, which was
measured to be 4.31±1.43 min. For autosegmentation of OARs, a
multiresolution VB-Net convolutional neural network was used, in
which a cascade coarse-to-fine strategy was utilized to accelerate
the contouring process (23), and
all the contouring of OARs were clinically accepted without any
modification. For the online adaptive plan, an adaptive
optimization algorithm based on dose prediction was adopted. In the
adaptive optimization algorithm, dose prediction and the original
plan's clinical goal were utilized as inputs, allowing the
algorithm to take into account patient-specific geometry, while
clinical priorities contained in the clinical goal sheet provide
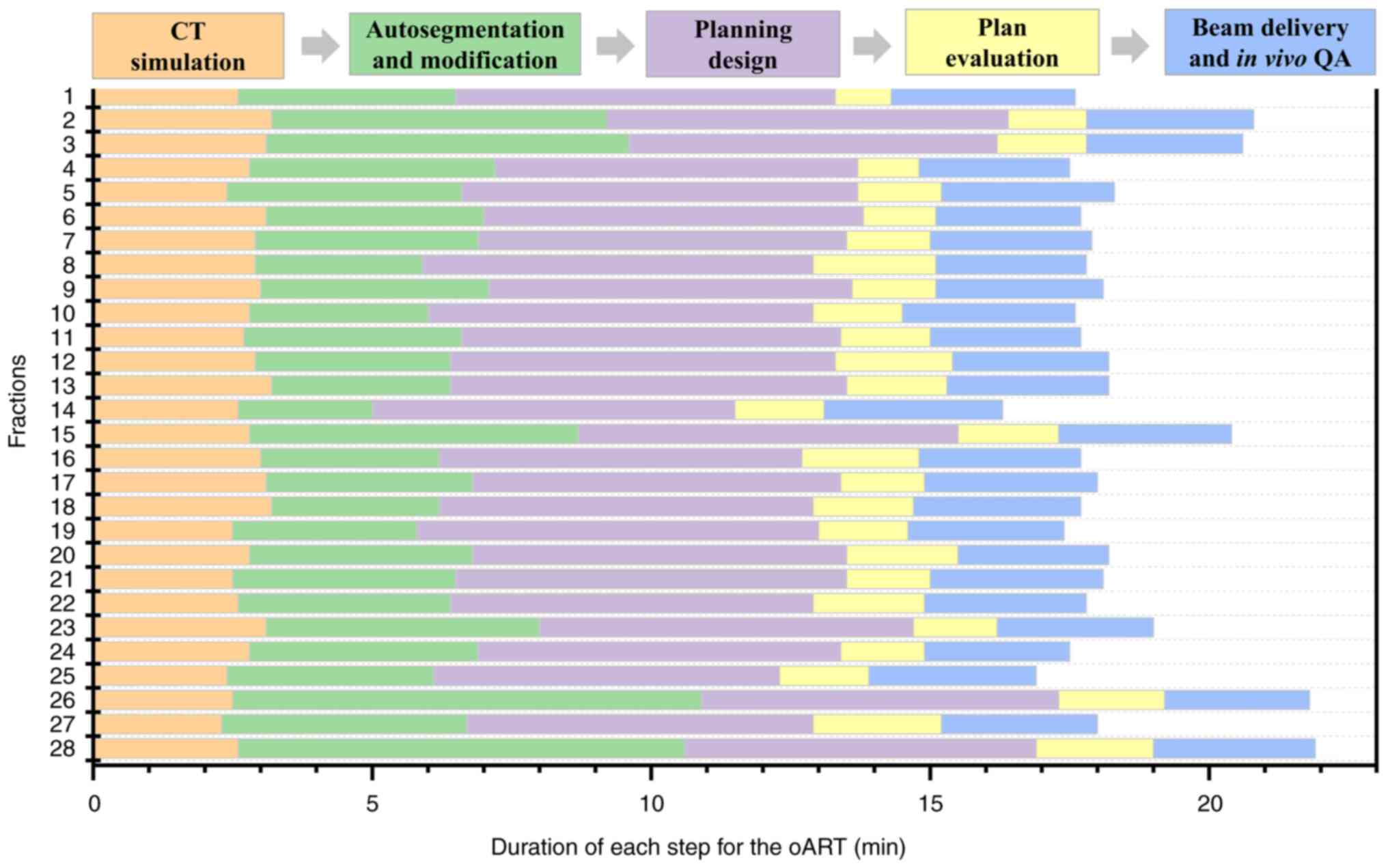
explicit guidance. Notably, the first-pass rate for the adaptive
plan was 100%. The whole process of oART for this patient was
recorded as 18.38±1.40 min (Fig.
4). The third critical requirement for oART is robust online
QA. In oART, the patient remains positioned on the treatment couch
throughout the session, precluding conventional pre-treatment plan
verification. Consequently, rapid and precise online QA systems are
essential to validate treatment plans while minimizing overall
workflow duration. In the present study, the electronic portal
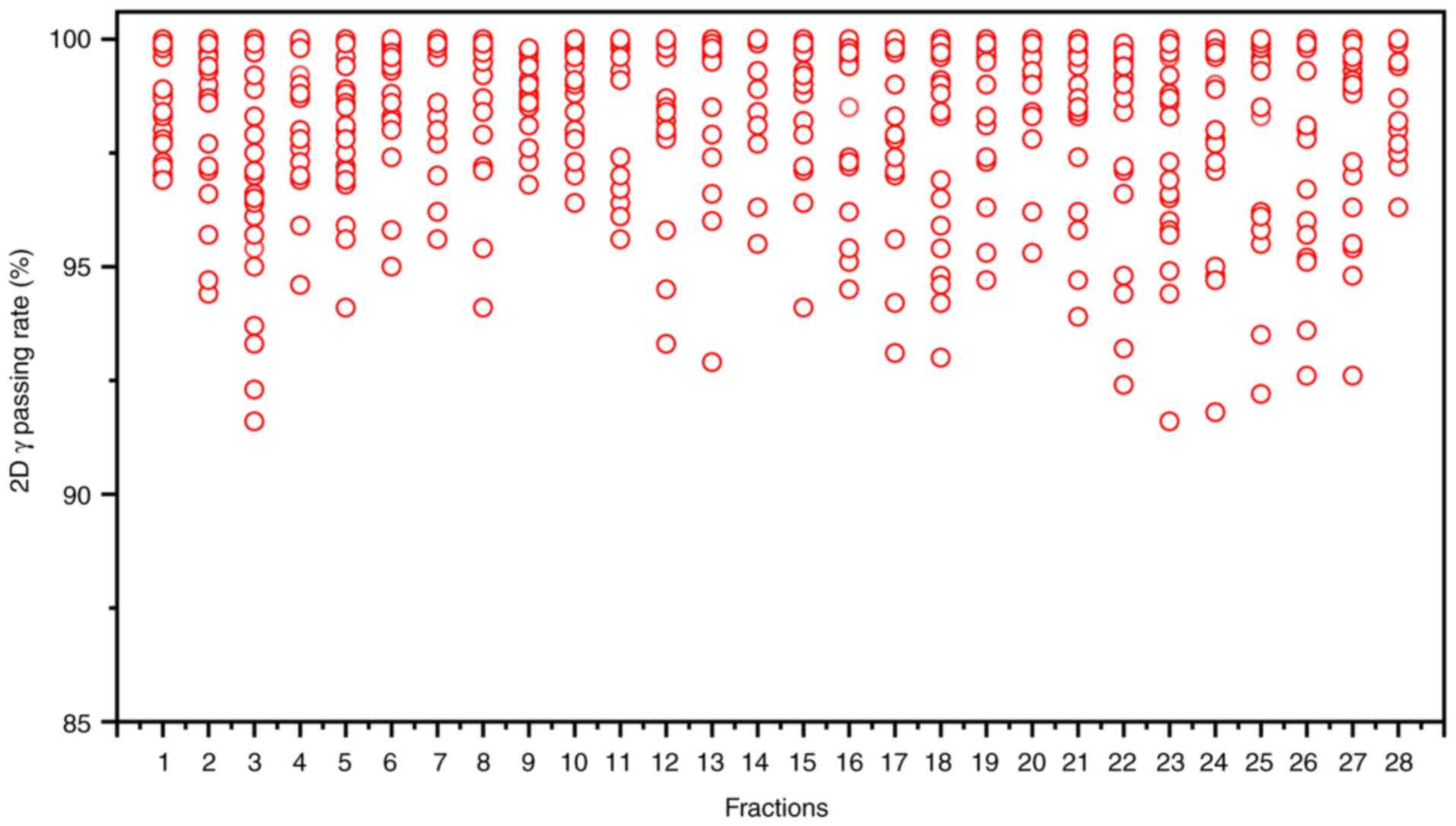
imaging device integrated into the linac facilitated in vivo
QA. During beam delivery, the two-dimensional γ index was assessed
at 30° intervals to verify beam accuracy, yielding a mean γ passing
rate of 98.52±0.55% and a minimum γ passing rate of 91.60%
(criteria: 3%/3 mm, 10% threshold; Fig.
5). Additionally, post-treatment three-dimensional dose
reconstruction quantified the delivered dose, achieving a mean γ
passing rate of 96.28±1.05% under the same criteria (Fig. 3D). These results confirm the
precision and reliability of dose delivery during the oART
process.
Notably, this patient showed an almost complete
response on imaging with only the dose of external beam
irradiation, and no cancer cells were even detected by
postoperative pathology, as reported by the Department of Pathology
(Fig. 1B). Throughout treatment and
follow-up, the patient experienced no marked radiation-related
toxicities, likely attributable to the precision of oART. While
oART demonstrated efficacy in this case, its routine application
across all treatment fractions remains impractical under current
clinical workflows. Further research is warranted to establish
standardized patient-specific criteria for initiating oART.
Notably, histopathological confirmation in this case was only made
possible due to treatment protocol modification (replacing BT with
surgery) requested by the patient following EBRT, which is not
routinely attainable under standard therapeutic guidelines.
Consequently, the generalizability of these findings to broader
patient populations remains uncertain. Further validation through
additional clinical investigations is warranted, such as
comparative assessments utilizing anatomical and functional imaging
modalities to evaluate local tumor response between oART and
non-oART cohorts post-EBRT, along with longitudinal monitoring of
treatment-related toxicities and survival outcomes. Whether the BT
dosage and frequency can be reduced or even entirely omitted
following online adaptive EBRT in cervical cancer management
remains a critical question that warrants rigorous clinical
investigation. Comparative studies evaluating de-escalated BT
protocols against standard-of-care regimens, with longitudinal
assessment of local control rates and toxicity profiles, are
imperative to validate this therapeutic paradigm shift.
In conclusion, the present case demonstrated the
feasibility, safety and efficacy of FBCT-guided oART for cervical
cancer. Notably, the workflow exhibited not only dosimetric
superiority but also favorable tumor response and clinically
tolerable toxicities. These findings advocate for the broader
clinical adoption of oART in cervical cancer management to optimize
local tumor control while mitigating radiation-induced toxicity to
adjacent organs and systemic effects.
Acknowledgements
Not applicable.
Funding
This study was financially supported by Chengdu Medical Research
Project Funding (grant no. 2023566), the Clinical Science Research
Funding of Chengdu Medical College (grant no. 24LHLNYX1-19), the
Clinical Research Special Program on Science and Technology Project
of Sichuan Provincial Health Commission (grant no. 23LCYJ049) and
Wu Jieping Medical Funding (grant no. 320.6750.2021-22-17).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HP and DG drafted the manuscript. NX and HT
collected data. HP, NX, DG, HT and TR participated in the study
design, and read and approved the final manuscript. HP and TR
confirm the authenticity of all the raw data. TR supervised this
study. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study was conducted according to the guidelines
of the Declaration of Helsinki and was approved by the
Institutional Review Board of The First Affiliated Hospital of
Chengdu Medical College (Chengdu, China; approval no.
2023CYFYIRB-SQ-04).
Patient consent for publication
The patient in the present report provided written
informed consent for publication.
Competing interests
Dong Gao is employed by United Imaging (equipment
manufacturer) but participated solely in study design and initial
drafting, with no involvement in data collection, analysis or
interpretation. All other authors declare no competing
interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools
(DeepSeek) were used to improve the readability and language of the
manuscript, and subsequently, the authors revised and edited the
content produced by the AI tools as necessary, taking full
responsibility for the ultimate content of the present
manuscript.
References
|
1
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In
Chinese). PubMed/NCBI
|
|
2
|
van de Bunt L, Jürgenliemk-Schulz IM, de
Kort GAP, Roesink JM, Tersteeg RJHA and van der Heide UA: Motion
and deformation of the target volumes during IMRT for cervical
cancer: What margins do we need? Radiother Oncol. 88:233–240. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klopp AH, Yeung AR, Deshmukh S, Gil KM,
Wenzel L, Westin SN, Gifford K, Gaffney DK, Small W Jr, Thompson S,
et al: Patient-reported toxicity during pelvic intensity-modulated
radiation therapy: NRG oncology-RTOG 1203. J Clin Oncol.
36:2538–2544. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yan D, Vicini F, Wong J and Martinez A:
Adaptive radiation therapy. Phys Med Biol. 42:123–132. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lim K, Stewart J, Kelly V, Xie J, Brock
KK, Moseley J, Cho YB, Fyles A, Lundin A, Rehbinder H, et al:
Dosimetrically triggered adaptive intensity modulated radiation
therapy for cervical cancer. Int J Radiat Oncol Biol Phys.
90:147–154. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim-Reinders S, Keller BM, Al-Ward S,
Sahgal A and Kim A: Online adaptive radiation therapy. Int J Radiat
Oncol Biol Phys. 99:994–1003. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jhingran A, Winter K, Portelance L, Miller
B, Salehpour M, Gaur R, Souhami L, Small W Jr, Berk L and Gaffney
D: A phase II study of intensity modulated radiation therapy to the
pelvis for postoperative patients with endometrial carcinoma:
Radiation therapy oncology group trial 0418. Int J Radiat Oncol
Biol Phys. 84:e23–e28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim K, Small W Jr, Portelance L,
Creutzberg C, Jürgenliemk-Schulz IM, Mundt A, Mell LK, Mayr N,
Viswanathan A, Jhingran A, et al: Consensus guidelines for
delineation of clinical target volume for intensity-modulated
pelvic radiotherapy for the definitive treatment of cervix cancer.
Int J Radiat Oncol Biol Phys. 79:348–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Peng H, Zhang J, Xu N, Zhou Y, Tan H and
Ren T: Fan beam CT-guided online adaptive external radiotherapy of
uterine cervical cancer: A dosimetric evaluation. BMC Cancer.
23:5882023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nierer L, Eze C, da Silva Mendes V, Braun
J, Thum P, von Bestenbostel R, Kurz C, Landry G, Reiner M, Niyazi
M, et al: Dosimetric benefit of MR-guided online adaptive
radiotherapy in different tumor entities: Liver, lung, abdominal
lymph nodes, pancreas and prostate. Radiat Oncol. 17:532022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jaffray DA, Siewerdsen JH, Wong JW and
Martinez AA: Flat-panel cone-beam computed tomography for
image-guided radiation therapy. Int J Radiat Oncol Biol Phys.
53:1337–1349. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhu L, Xie Y, Wang J and Xing L: Scatter
correction for cone-beam CT in radiation therapy. Med Phys.
36:2258–2268. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Létourneau D, Wong JW, Oldham M, Gulam M,
Watt L, Jaffray DA, Siewerdsen JH and Martinez AA: Cone-beam-CT
guided radiation therapy: Technical implementation. Radiother
Oncol. 75:279–286. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Morin O, Gillis A, Chen J, Aubin M, Bucci
MK, Roach M III and Pouliot J: Megavoltage cone-beam CT: System
description and clinical applications. Med Dosim. 31:51–61. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang G, Wang Z, Guo Y, Zhang Y, Qiu J, Hu
K, Li J, Yan J and Zhang F: Evaluation of PTV margins with daily
iterative online adaptive radiotherapy for postoperative treatment
of endometrial and cervical cancer: A prospective single-arm phase
2 study. Radiat Oncol. 19:22024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ma CY, Guo J, Jiang H, Li ZB, Wang SM and
Li BH: Clinical practice of online adaptive radiotherapy guided by
fan-beam CT in cervical cancer. Chin J Radiol Med Prot. 44:262–271.
2024.(In Chinese).
|
|
17
|
Kerkmeijer LGW, Valentini V, Fuller CDD
and Slotman BJ: Editorial: Online adaptive MR-guided radiotherapy.
Front Oncol. 11:7486852021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bibault JE and Giraud P: Deep learning for
automated segmentation in radiotherapy: A narrative review. Br J
Radiol. 97:13–20. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Krayenbuehl J, Norton I, Studer G and
Guckenberger M: Evaluation of an automated knowledge based
treatment planning system for head and neck. Radiat Oncol.
10:2262015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Paganetti H, Botas P, Sharp GC and Winey
B: Adaptive proton therapy. Phys Med Biol.
66:10.1088/1361–6560/ac344f. 2021. View Article : Google Scholar
|
|
21
|
Zhan B, Xiao J, Cao C, Peng X, Zu C, Zhou
J and Wang Y: Multi-constraint generative adversarial network for
dose prediction in radiotherapy. Med Image Anal. 77:1023392022.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Peng H, Liu T, Li P, Yang F, Luo X, Sun X,
Gao D, Lin F, Jia L, Xu N, et al: Automatic delineation of cervical
cancer target volumes in small samples based on multi-decoder and
semi-supervised learning and clinical application. Sci Rep.
14:269372024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shi F, Hu W, Wu J, Han M, Wang J, Zhang W,
Zhou Q, Zhou J, Wei Y, Shao Y, et al: Deep learning empowered
volume delineation of whole-body organs-at-risk for accelerated
radiotherapy. Nat Commun. 13:65662022. View Article : Google Scholar : PubMed/NCBI
|