Introduction
Breast cancer continues to be the most common cancer
and a leading cause of mortality among women (1). A total of >2 million women are
diagnosed with breast cancer globally per year (2) and predicting disease progression has
become a central focus in cancer research (3). Furthermore, it is of great importance
to determine the stage of breast cancer at the first presentation
and enable the treatment plan and the prediction of cancer
prognosis (4).
The diagnosis of invasive breast cancer requires the
evaluation of estrogen receptor (ER) status, progesterone receptor
(PR) status, human epidermal growth factor receptor 2 (HER2)
status, the tumor proliferation index (Ki-67), lymphatic and
vascular invasion status and tumor oncogenesis of the biopsy
specimen. Tumor-node-metastasis (TNM) staging provides a universal
language for clinicians with a collective approach and
interpretation regarding prognosis, and currently, the 8th edition
of the American Joint Cancer Committee is used for breast cancer
staging (5). PR status may be
disregarded as a predictive factor if the ER status is noted to be
positive (6). Another parameter
considered in the histopathological examination is tumor grade,
which evaluates tubule formation, nuclear polymorphism and mitotic
activity together. Elston et al (7) reported that tumors with higher tumor
grades were associated with worse clinical outcomes. This was
further validated by Rakha et al (8). Taken together, a prognostic model was
created with a scoring system based on these clinicopathological
parameters. Using the Neoadjuvant Therapy Outcomes Calculator
software, developed at the MD Anderson Cancer Center, the
pretreatment clinical stage and post-treatment pathologic stage, as
well as ER status and tumor nuclear grade, are incorporated to
assess the prognosis and survival rates of patients with breast
cancer treated with neoadjuvant chemotherapy (NACT) (9,10).
This prediction model indicates that high score rates are
associated with poor prognosis.
The present study aimed to assess the association
between clinical and pathological stage (CPS) and ER status and
histologic grade (EG) scoring with disease-free survival (DFS) and
overall survival (OS) in patients with breast cancer who are
treated with NACT in a Turkish population. Treatment outcomes were
not included as this would require a large-scale randomized
controlled study. The evaluation of the CPS + EG scoring system in
this population group has not been studied yet in Turkey, to the
best of our knowledge.
Materials and methods
The present retrospective study included data from
148 patients diagnosed with breast cancer who were treated with
neoadjuvant systemic chemotherapy followed by surgery in the
Medical Oncology Clinic of Izmir Tepecik Training and Research
Hospital (Bornova, Turkey) between 2013 and 2018. The patients
included had a histopathologically confirmed diagnosis of breast
cancer, were at clinical stage II or III (5), and received neoadjuvant systemic
therapy (chemotherapy and/or anti-HER2 therapy). Patients with
distant metastasis at the time of diagnosis and patients with any
secondary malignancies were excluded from the analysis. Ethics
approval for the present study was obtained from the local ethics
committee of Izmir Tepecik Education and Research Hospital
(approval no. 2023/08).
The following variables were analyzed: Demographic
characteristics, tumor size at presentation, clinical staging with
lymph node involvement and distant metastasis status (clinical TNM
stage), ER status and tumor nuclear grade (NG) in biopsy material,
and postoperative pathological staging after NACT [namely,
pathological TNM stage after treatment (ypTNM)]. Postoperative
tumor pathological response grading was categorized as progression,
stable response, partial response and complete response (CR) to
treatment. A pathological (p)CR was defined as the presence of
residual ductal carcinoma in situ in the breast (ypTisN0) or
the absence of an invasive tumor in both the breast and axillary
lymph nodes (ypT0N0). CPS scores were calculated using the clinical
stage at presentation and pathological stage after NACT. CPS + EG
scores were calculated using the simultaneous ER status and NG, as
implemented in the Neoadjuvant Therapy Outcomes Calculator Software
of the MD Anderson Cancer Center (5,6). As
the CPS-score 4 group included only 1 patient, this was excluded
from the analysis. The scoring criteria for each parameter are
presented in Table I. The 5-year OS
and DFS expectations were recorded as percentages and the 5-year
follow-up of the patients was analyzed.
 | Table I.Points given to each variable in the
clinical and pathological stage + estrogen receptor status and
histologic grade scoring system. |
Table I.
Points given to each variable in the
clinical and pathological stage + estrogen receptor status and
histologic grade scoring system.
| Variable | Points |
|---|
| Clinical stage
(cAJCC) |
|
| I | 0 |
| IIA | 0 |
| IIB | 1 |
| IIIA | 1 |
| IIIB | 2 |
| IIIC | 2 |
| Tumor marker |
|
| ER
negative | 1 |
| Pathologic stage
(pAJCC) |
|
| I | 0 |
| IIA | 1 |
| IIB | 1 |
|
IIIA | 1 |
|
IIIB | 1 |
|
IIIC | 2 |
| Nuclear grade |
|
| 3 | 1 |
The distribution of continuous variables was
assessed for normality using the Kolmogorov-Smirnov test and
skewness and kurtosis. Categorical variables are presented as n
(%), whilst continuous variables are reported as mean ± standard
deviation or median (interquartile range). OS was defined as the
time from disease diagnosis to death, and DFS was defined as the
time from diagnosis to relapse. Statistical calculations were
performed accordingly. OS and DFS were evaluated using the
Kaplan-Meier method. The log-rank test was used to determine
differences in survival. Median follow-up time was calculated using
the reverse Kaplan-Meier method. Univariate and multivariate Cox
proportional regression models were used to identify predictive
variables for survival. SPSS v29.0 (IBM Corp.) and JAMOVI v2.6.2
(www.jamovi.org) were used for statistical
analyses. P<0.05 was considered to indicate a statistically
significant difference. However, in univariate analyses, P<0.10
was considered for inclusion of a variable in the multivariate
model for further analysis. The analysis was computed using the
backward elimination likelihood ratio method.
Results
Patient characteristics and
follow-up
A total of 148 female patients were included in the
present study, with a mean age of 49.3±10.0 years. The
clinicopathological characteristics of patients are presented in
Table II. The median follow-up
period was 76.5 months [95% confidence interval (CI), 67.7–85.4].
The median OS was 104.1 months (95% CI, 97.4–110.9). The median DFS
was 96.0 months (95% CI, 88.5–104.5). The overall pCR rate was
23.6%.
 | Table II.Clinicopathological characteristics
of the patients in the present study. |
Table II.
Clinicopathological characteristics
of the patients in the present study.
| Variable | Total (n=148) |
|---|
| Age, years | 49.3±10.0 |
| Pathological
LNs | 1 (0–3) |
| Survival |
|
|
Dead | 38 (25.7) |
|
Survived | 110 (74.3) |
| Hormone status |
|
|
Positive | 102 (68.9) |
|
Negative | 46 (31.1) |
| HER2 status |
|
|
Negative | 87 (58.8) |
|
Positive | 61 (41.2) |
| Menopause
status |
|
|
Premenopausal | 71 (48.0) |
|
Perimenopausal | 12 (8.1) |
|
Postmenopausal | 64 (43.2) |
|
Unknown | 1 (0.7) |
| Pathological
stage |
|
| 0 | 34 (23.0) |
| 1 | 25 (16.9) |
| 2A | 39 (26.4) |
| 2B | 10 (6.8) |
| 3A | 24 (16.2) |
| 3B | 10 (6.8) |
| 3C | 6 (4.1) |
| CPS score |
|
| 0 | 8 (5.4) |
| 1 | 42 (28.4) |
| 2 | 67 (45.3) |
| 3 | 30 (20.3) |
| 4 | 1 (0.7) |
| Tumor diameter,
mm | 15.5±21.9 |
| Operation |
|
|
Lumpectomy | 19 (12.8) |
|
Mastectomy | 129 (87.2) |
| Relapse |
|
|
Yes | 42 (28.4) |
| No | 106 (71.6) |
|
Triple-negative |
|
|
Yes | 22 (14.9) |
| No | 126 (85.1) |
| Adjuvant RT |
|
|
Received | 147 (99.3) |
|
Unknown | 1 (0.7) |
| Lymph nodes at the
diagnose |
|
|
Absent | 7 (4.7) |
| Mobile
LN | 102 (68.9) |
| Fixed
LN | 31 (20.9) |
|
Others | 8 (5.5) |
| Clinical
response |
|
| PD | 12 (8.1) |
| PR | 94 (63.5) |
| CR | 35 (23.6) |
| SD | 7 (4.7) |
| CPS + EG score |
|
| 0 | 3 (2.0) |
| 1 | 22 (14.9) |
| 2 | 39 (26.4) |
| 3 | 49 (33.1) |
| 4 | 28 (18.9) |
| 5 | 7 (4.7) |
Survival and treatment response based
on CPS and CPS+EG scores
Treatment responses based on CPS and CPS + EG
scoring are presented in Tables
III and IV, respectively. OS
and DFS analyses were performed by stratifying patients into five
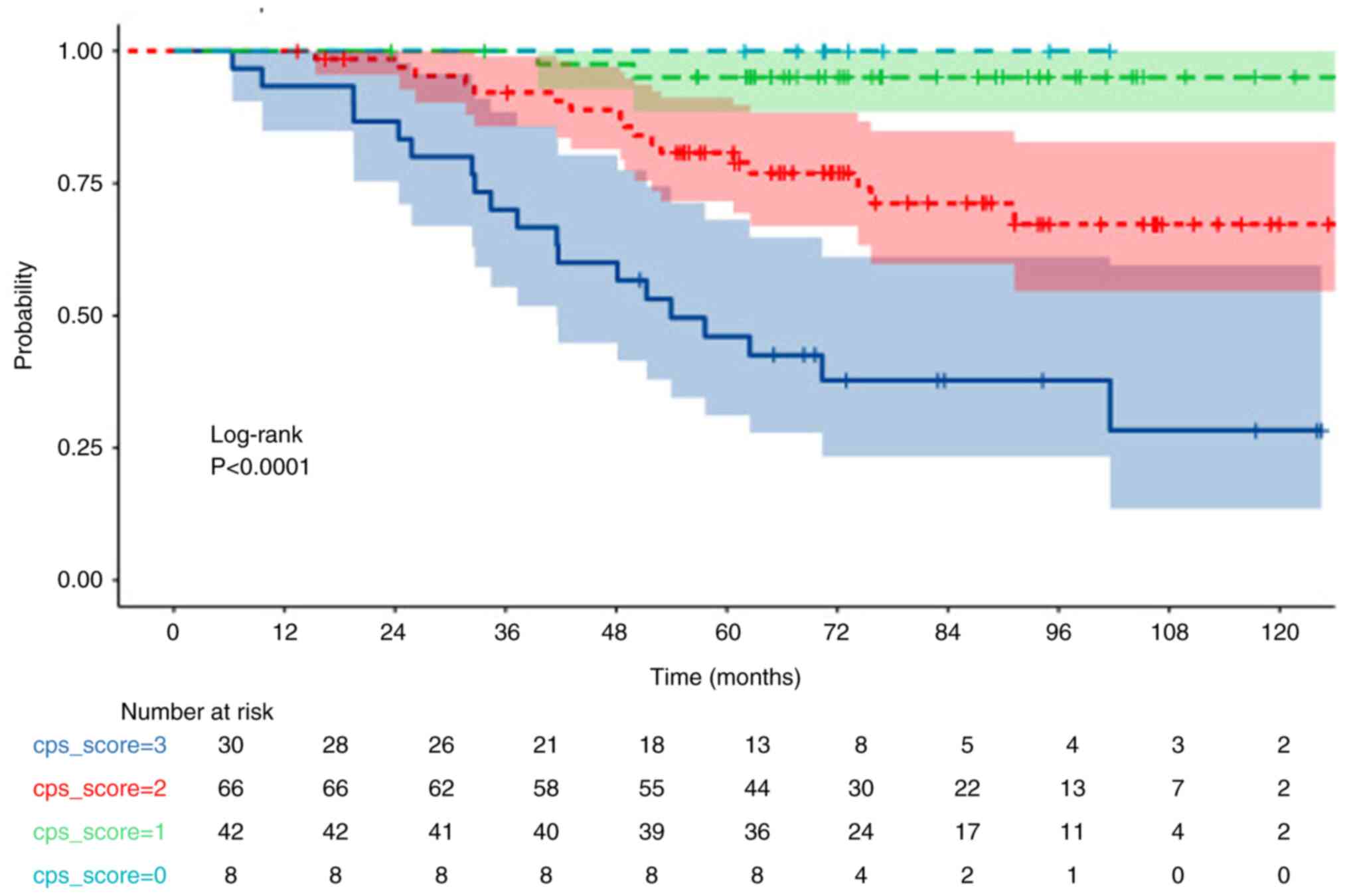
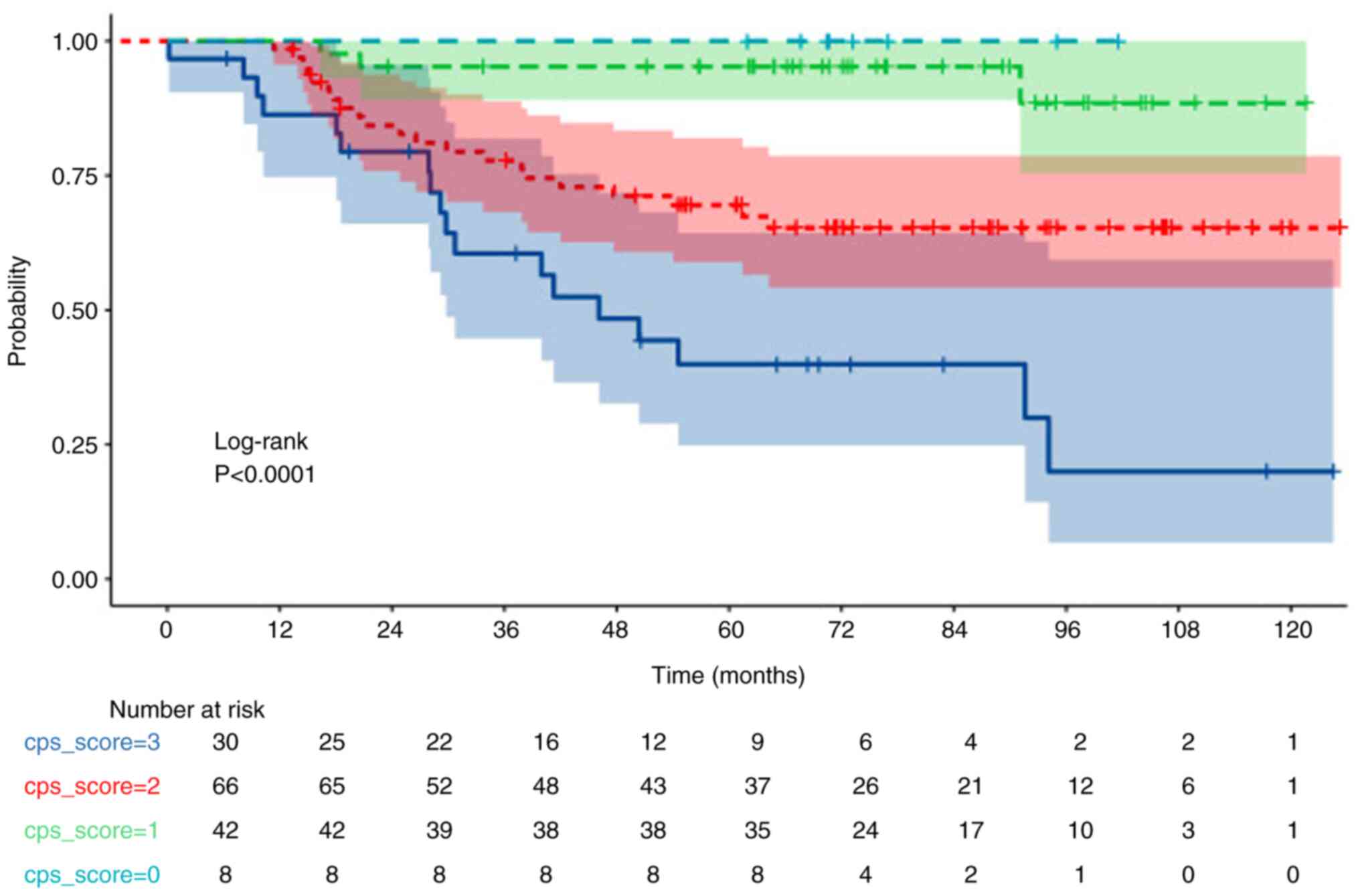
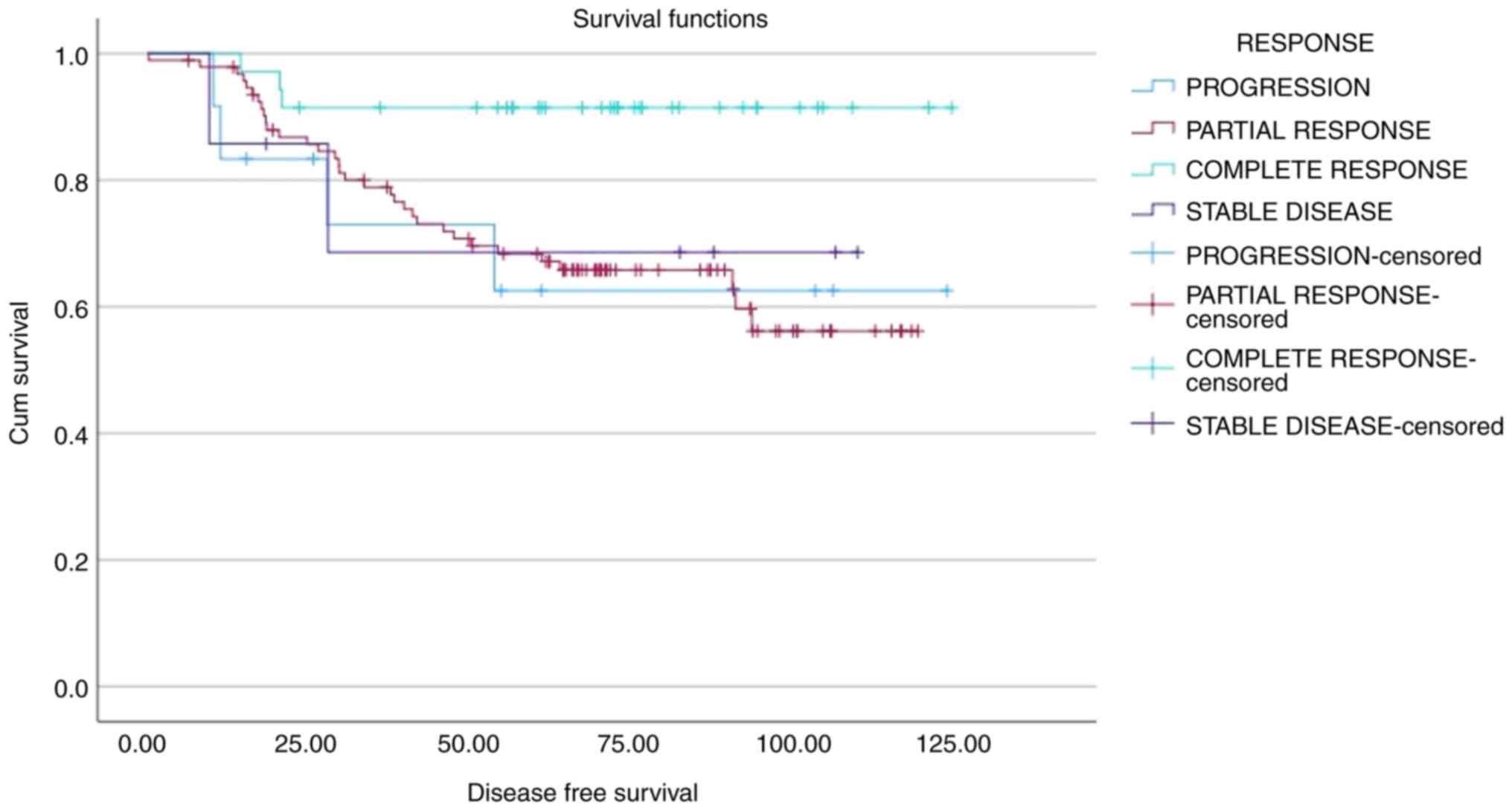
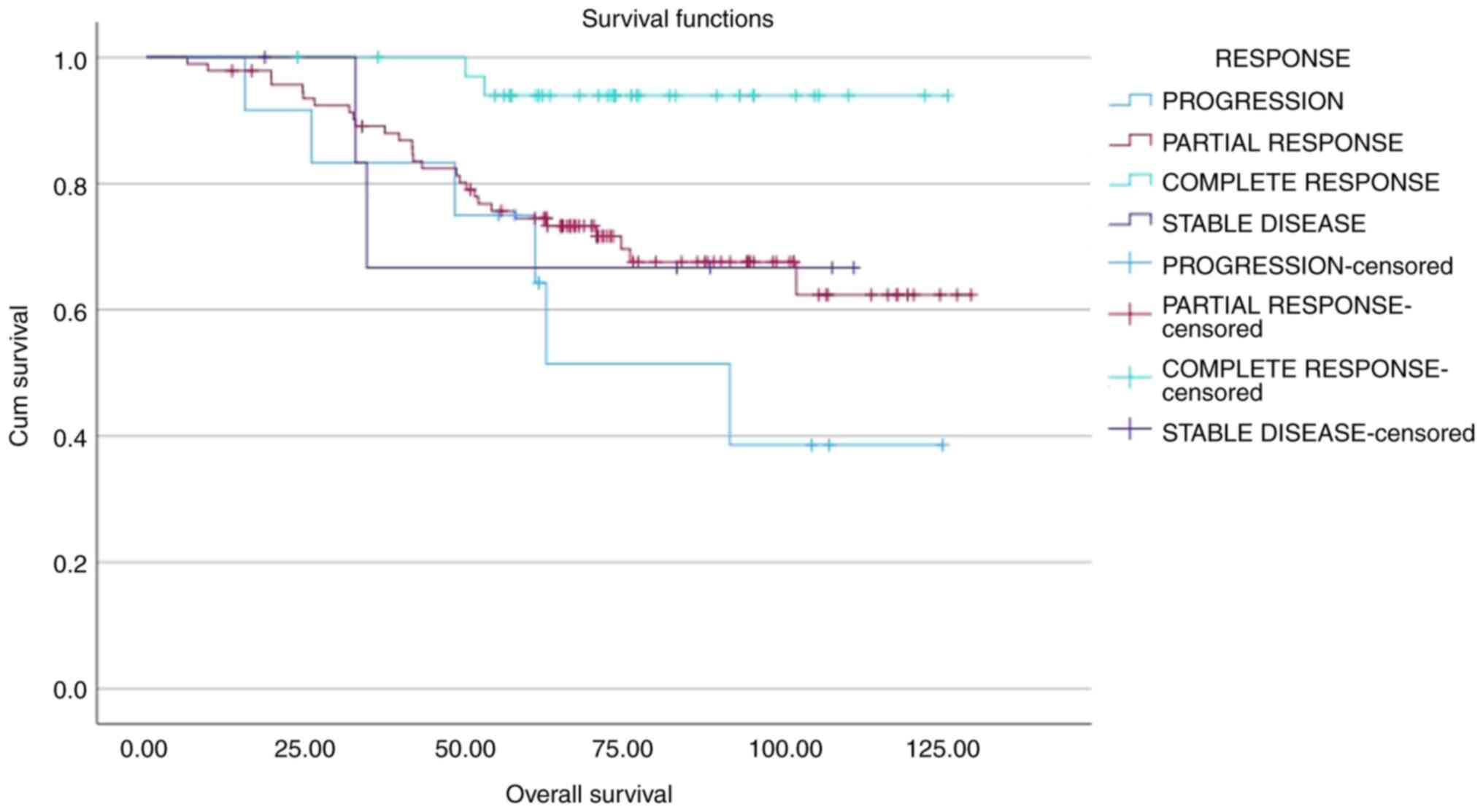
(0–1–2–3–4) groups based on CPS scores. A significant decrease in
OS was observed as CPS and DFS scores increased (both P<0.001;
Figs. 1 and 2; Table
V). 5-year OS and PFS rates of 46, 80, 95 and 100%, and 40,
69.5, 95 and 100%, respectively, were calculated for CPS scores of
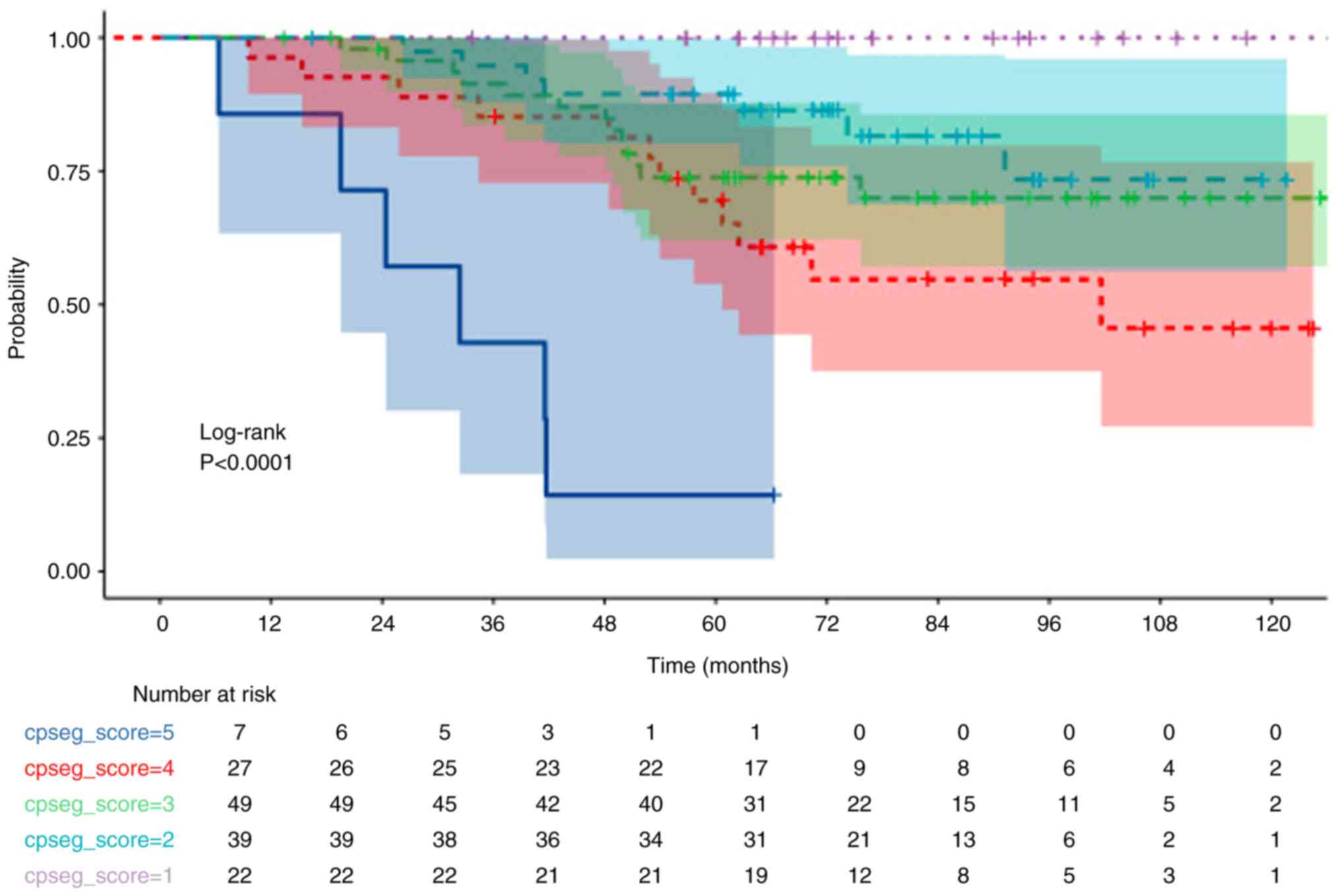
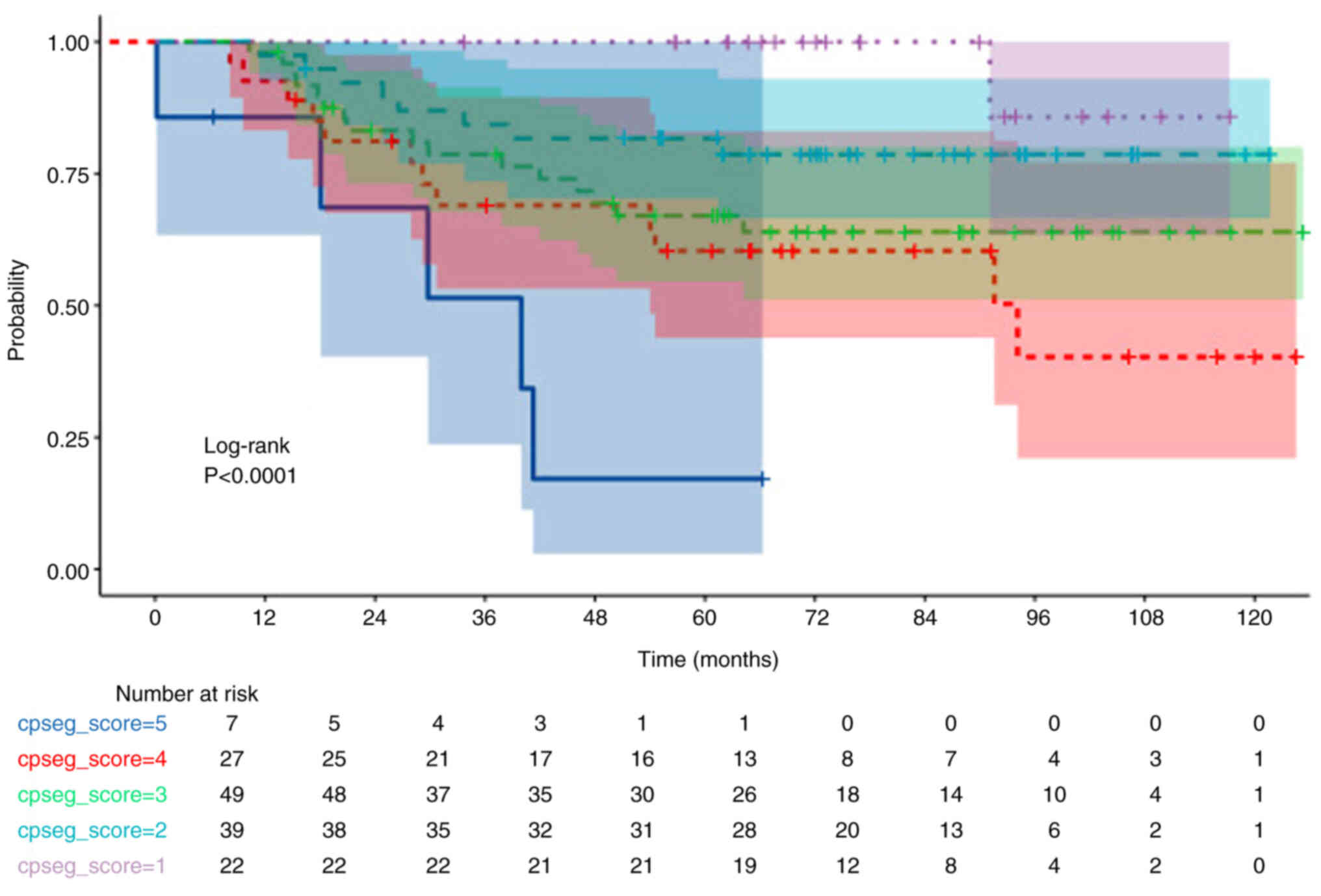
3, 2, 1 and 0, respectively. Similarly, OS and DFS analyses
stratified by CPS + EG scores (0–1–2–3–4–5–6) demonstrated a
significant decrease in OS and DFS as scores increased (both
P<0.001; Figs. 3 and 4; Table V)
Patients with a pCR demonstrated significantly higher DFS rates
compared with that of non-pCR (P=0.038). Moreover, a CPS score of
3–4 (in comparison with 0-1-2) and a CPS + EG score 3-4-5 (in
comparison with 0-1-2) were significantly associated with a worse
OS (both P<0.001; Figs. 1 and
3, Table V).
 | Table III.Distribution of clinical and
pathological stage scores according to responses. |
Table III.
Distribution of clinical and
pathological stage scores according to responses.
|
| CPS score |
|---|
|
|
|
|---|
| Response | 0 | 1 | 2 | 3 | 4 |
|---|
| Progression | 0 (0.0) | 1 (8.3) | 7 (58.3) | 4 (33.3) | 0 (0.0) |
| Partial
response | 3 (3.2) | 21 (22.3) | 46 (48.9) | 23 (24.5) | 1 (1.1) |
| Complete
response | 5 (14.3) | 20 (57.1) | 10 (28.6) | 0 (0) | 0 (0.0) |
| Stable disease | 0 (0.0) | 0 (0.0) | 4 (57.1) | 3 (42.9) | 0 (0.0) |
 | Table IV.Distribution of clinical and
pathological stage + estrogen receptor status and histologic grade
scores according to responses. |
Table IV.
Distribution of clinical and
pathological stage + estrogen receptor status and histologic grade
scores according to responses.
|
| CPS + EG score |
|---|
|
|
|
|---|
| Response | 0 | 1 | 2 | 3 | 4 | 5 |
|---|
| Progression | 0 (0.0) | 1 (8.3) | 5 (41.7) | 1 (8.3) | 5 (41.7) | 0 (0.0) |
| Partial
response | 1 (1.1) | 12 (12.8) | 24 (25.5) | 32 (34.0) | 18 (19.1) | 7 (7.4) |
| Complete
response | 2 (5.7) | 9 (25.7) | 9 (25.7) | 12 (34.3) | 3 (8.6) | 0 (0.0) |
| Stable disease | 0 (0.0) | 0 (0.0) | 1 (14.3) | 4 (57.1) | 2 (28.6) | 0 (0.0) |
 | Table V.Univariate analyses of several
clinical parameters of patients in terms of overall survival and
disease-free survival. |
Table V.
Univariate analyses of several
clinical parameters of patients in terms of overall survival and
disease-free survival.
| A, Overall
survival |
|---|
|
|---|
| Variable | HR (95% CI) | P-value |
|---|
| Age, years | 1.010
(0.979–1.041) | 0.542 |
| Tumor diameter,
mm | 1.014
(1.002–1.027) | 0.027 |
| Relapse |
| <0.001 |
| No | 1 |
|
|
Yes | 16.247
(7.124–37.258) |
|
| Menopause
status |
| 0.122 |
|
Pre-perimenopausal | 1 |
|
|
Postmenopausal | 1.670
(0.871–3.203) |
|
| Clinical stage |
| 0.006 |
|
2A-2B | 1 |
|
|
3A-3B-3C | 2.576
(1.316–5.043) |
|
| HER2 status |
| 0.019 |
|
Positive | 1 |
|
|
Negative | 2.447
(1.157–5.143) |
|
| CPS score |
| <0.001 |
|
0-1-2 | 1 |
|
|
3-4 | 5.111
(2.699–9.679) |
|
| CPS + EG score |
| <0.001 |
|
0-1-2 | 1 |
|
|
3-4-5 | 4.121
(1.813–9.367) |
|
|
| B, Disease-free
survival |
|
|
Variable | HR (95%
CI) | P-value |
|
| Age, years | 0.996
(0.967–1.027) | 0.809 |
| Tumor diameter,
mm | 1.012
(1.000–1.024) | 0.046 |
| Relapse |
| - |
| No | - |
|
|
Yes | - |
|
| Menopause
status |
| 0.620 |
|
Pre-perimenopausal | 1 |
|
|
Postmenopausal | 1.166
(0.636–2.137) |
|
| Clinical stage |
| <0.001 |
|
2A-2B | 1 |
|
|
3A-3B-3C | 2.696
(1.417–5.128) |
|
| HER2 status |
| 0.024 |
|
Positive | 1 |
|
|
Negative | 2.203
(1.107–4.384) |
|
| CPS score |
| <0.001 |
|
0-1-2 | 1 |
|
|
3-4 | 3.876
(2.095–7.173) |
|
| CPS + EG score |
| <0.001 |
|
0-1-2 | 1 |
|
|
3-4-5 | 3.592
(1.717–7.516) |
|
Recurrence
Patients with recurrence had a shorter median OS
(median, 64.3 months; 95% CI, 52.8–75.8) than patients without
recurrence (median, 118.4 months; 95% CI, 113.5–123.3)
(P<0.001). When patients were stratified according to their pCR
status, median DFS and median OS was significantly lower in
patients with recurrence (P=0.013 and P=0.038, respectively;
Figs. 5 and 6, Table
V).
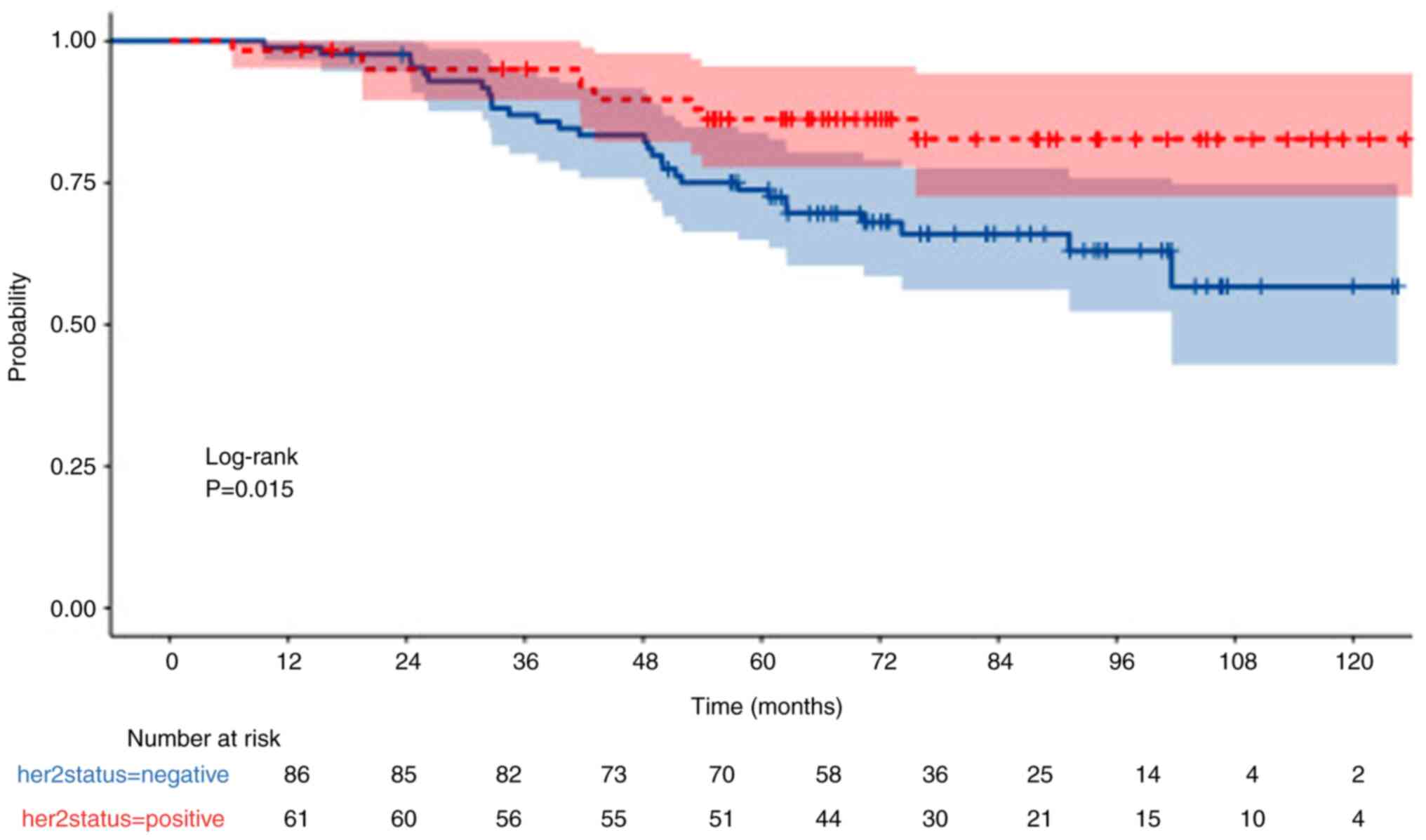
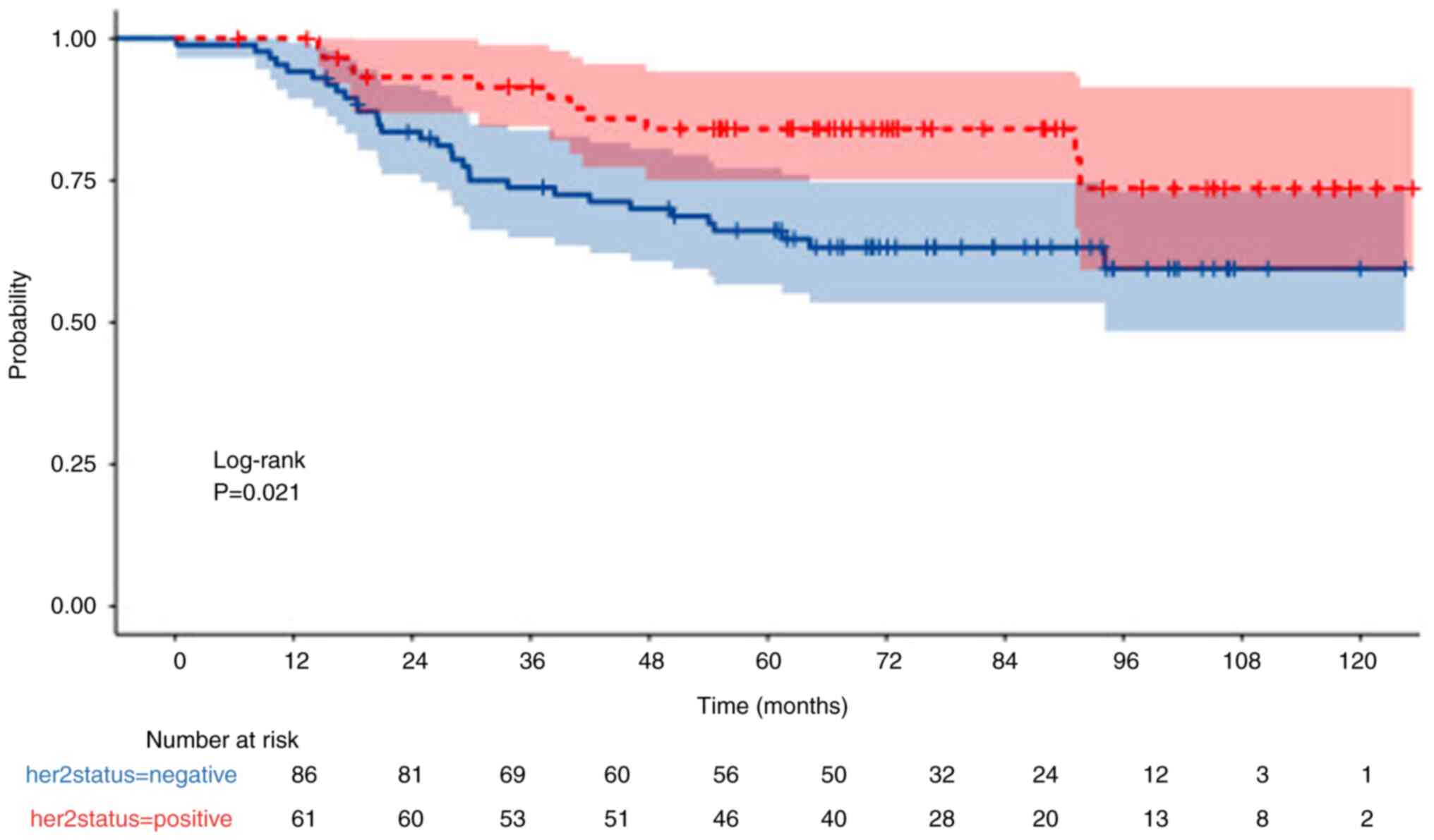
HER2 status
OS was also significantly higher in HER2-positive
patients compared with HER2-negative patients (P=0.015; Fig. 7). Furthermore, DFS was significantly
higher in HER2-positive patients compared with HER2-negative
patients (P=0.021; Fig. 8).
Moreover, the 5-year OS rates for HER2-positive and -negative
patients was 86 and 74%, respectively, whereas the 5-year PFS was
84 and 66%, respectively.
Univariate and Cox regression
analysis
Univariate analyses of the variables are presented
in Table V. Cox regression analysis
revealed that HER2-negative status and higher CPS scores (3–4) were
significantly associated with worse OS and DFS rates. HER2-negative
patients had a higher risk of mortality [hazard ratio (HR), 2.447;
P=0.038] and recurrence (HR, 2.203; P=0.033) compared with
HER2-positive patients. Similarly, patients with higher CPS scores
had a significantly increased risk of poor OS (HR, 5.111; P=0.007)
and DFS (HR, 3.876; P=0.025). Furthermore, a higher CPS + EG score
(3–4–5) was predictive of a worse DFS (HR, 3.592; P=0.028), but its
association with OS did not reach statistical significance (HR,
4.121; P=0.058). Age and tumor diameter also did not demonstrate a
significant impact on survival outcomes. These findings suggest
that CPS and CPS + EG scores are valuable prognostic indicators,
particularly for disease recurrence. Notably, the greatest increase
in risk was observed in OS for higher CPS scores (HR, 5.111),
indicating a more than five-fold increase in mortality risk, whilst
HER2-negative status was associated with a two-fold increase in
both mortality and recurrence (Table
VI).
 | Table VI.Multivariate analyses of several
clinical parameters of patients in terms of overall survival and
disease-free survival. |
Table VI.
Multivariate analyses of several
clinical parameters of patients in terms of overall survival and
disease-free survival.
| A, Overall
survival. |
|---|
|
|---|
| Variable | HR (95% CI) | P-value |
|---|
| Age, years | 1.010
(0.979–1.041) | 0.826 |
| Tumor diameter,
mm | 1.014
(1.002–1.027) | 0.348 |
| HER2 status |
| 0.038 |
|
Positive | 1 |
|
|
Negative | 2.447
(1.157–5.143) |
|
| CPS score |
| 0.007 |
|
0-1-2 | 1 |
|
|
3-4 | 5.111
(2.699–9.679) |
|
| CPS + EG score |
| 0.058 |
|
0-1-2 | 1 |
|
|
3-4-5 | 4.121
(1.813–9.367) |
|
|
| B, Disease-free
survival |
|
|
Variable | HR (95%
CI) | P-value |
|
| Age, years | 0.996
(0.967–1.027) | 0.528 |
| Tumor diameter,
mm | 1.012
(1.000–1.024) | 0.561 |
| HER2 status |
| 0.033 |
|
Positive | 1 |
|
|
Negative | 2.203
(1.107–4.384) |
|
| CPS score |
| 0.025 |
|
0-1-2 | 1 |
|
|
3-4 | 3.876
(2.095–7.173) |
|
| CPS + EG score |
| 0.028 |
|
0-1-2 | 1 |
|
|
3-4-5 | 3.592
(1.717–7.516) |
|
Discussion
Determination of the prognosis in patients with
breast cancer is important for several reasons: It has an important
role in establishing the treatment decision and frequency of
follow-ups. Moreover, sharing the prognosis with patients and their
relatives is an important reinforcement regarding quality of life.
Relieving anxiety reduces depression and anxiety disorders along
with increasing treatment compliance (11). Inclusion and stratification in
clinical trials are also based on prognosis determination (12).
Prognostic acuity of the universally used TNM
classification, established by the American Cancer Joint Committee
has been defined. This system requires biopsy-based information,
including ER, PR and HER2 status, and tumor grade and imaging for
clinical staging (13). As an
individualized approach is becoming more widespread in daily
practice, prognostic models are needed to evaluate prognosis.
According to the report published by the National Comprehensive
Cancer Network, markers that carry descriptive, complementary and
prognostic expressions of the disease should be able to express
general terminology including analytical validity, clinical
validity and clinical utility (14).
The utility and validity of the prognostic models
are tested with validation studies (12). Prediction models should be validated
using large cohorts from diverse ethnic groups to ensure that their
impact on clinical decision-making does not interfere with the
implementation of life-saving measures. Several prognostic models
have been identified for predicting the outcomes of breast cancer;
however, validation studies are insufficient. Their performances in
independent populations are also suboptimal (15). Occasionally, internal validation
works well but external validation may not (16). The Nottingham Prognostic Index is
the oldest and easiest model in this field which was validated
following different modifications (17–19).
Determining the validity of the CPS + EG scoring
system will expand the use of this easily accessible and
cost-effective system. It has been used in the stratification of
other clinical trials for prognosis determination. Furthermore, it
has been emphasized that this scoring system is valuable in the
evaluation of local recurrence: The addition of postmastectomy
radiotherapy to the treatment of a high-risk group with a CPS + EG
score of >3 was reported to be associated with reduced
recurrence rates (20). The
findings of the study by Marmé et al (21) of the CPS + EG scores following NACT
in HR-positive and HER2-negative breast cancer groups were also
consistent with the findings of the present study. However, the
study assessed 5-year DFS and OS following NACT in patients with
triple negative breast cancer, and reported that the prognostic
value of the scoring was ‘insufficient’.
To assess prognosis, key factors such as patient
age, tumor size, degree of invasion, receptor status and
histological grade are typically evaluated. In the case of locally
advanced hormone-positive breast cancer, genomic profiling tools,
including Oncotype DX risk scoring, EndoPredict, PAM50, the Breast
Cancer Index and MammaPrint Genomic profiling assist in
distinguishing between in high-risk and low-risk tumors (22). However, the high costs associated
with these tests have led many authorities to impose restrictions
on their use. Moreover, the lack of insurance coverage of these
tests presents a notable challenge, particularly for economically
disadvantaged patients (23). In
this context, the incorporation of cost-free and non-invasive
prognostic alternatives could offer additional advantages in
clinical decision-making.
Mortality rates of patients with HER2-positive
breast cancer are lower than those of patients with HER2-negative
breast cancer in both hormone-positive and -negative subgroups
(24), and anti-HER2 treatments
serve a marked role in these outcomes. A previous study reported
that the hazard ratios of patients with HER2-positive breast cancer
gradually decreased in the hormone-positive subgroup compared with
the hormone-negative subgroup, whereas the hormone-negative
subgroup hazard ratios did not change over time (25).
NACT has become an integral part of breast cancer
management, particularly in recent decades. In addition to
shrinking the tumor and reducing the extent of surgery, NACT serves
a crucial role in tumor downstaging and holds prognostic
significance. Whilst radiological imaging remains an important tool
for assessing treatment response, pathological evaluation continues
to be the gold standard (26).
Treatment response and tumor histological subtype are key factors
in predicting the effectiveness of NACT, particularly as achieving
a pCR notably improves PFS and OS (27,28).
However, whilst pCR is a well-established prognostic marker in
HR-negative tumors, a German study reported that achieving pCR was
not a surrogate marker for prognosis in patients with Luminal A and
Luminal B/HER2 positive breast cancer (29,30).
This finding underscores the notion that tumor biology is a more
significant determinant of prognosis than the response to NACT.
There is currently insufficient evidence to support
treatment modifications based solely on the CPS + EG score, to the
best of our knowledge. In patients with low-risk breast cancer,
radiotherapy omission has been recommended in selected cases
(31). However, its association
with the CPS + EG score has not been clearly established. Although
the role of treatment escalation in patients with high CPS + EG
scores is a matter of debate, no standardized approach has been
established (20,32). Therefore, whilst the CPS + EG score
is a well-validated prognostic tool, its utility in guiding
specific therapeutic decisions remains unclear.
The present study has certain limitations. First,
age was not considered a distinguishing criterion. Given that
breast cancer tends to exhibit a more aggressive course in younger
(<35 years) and elderly (>65 years) patients, the predictive
value of the CPS + EG scoring system may be less reliable in these
age groups (33,34). Second, the present study did not
incorporate genetic data, which may provide additional prognostic
and predictive insights. Third, the relatively small sample size
limits the generalizability of the findings. The small number of
patients in subgroup analyses further reduced statistical power,
making definitive conclusions more challenging. Additionally,
heterogeneity in treatment regimens and clinical characteristics
among patients may introduce variability in outcomes. Standardized
treatment protocols in future prospective studies could help
clarify the true impact of CPS + EG scoring in clinical
decision-making. Finally, integrating CPS + EG scoring with
emerging molecular and imaging biomarkers could further improve
risk stratification and facilitate personalized treatment
strategies in breast cancer. Future research should explore these
aspects to optimize patient outcomes.
In conclusion, although the clinical stage at the
first presentation of the disease may provide information on the
prognosis of the patient, incorporating biological markers to the
equation refines the outcomes in patients treated with NACT.
Prognostic models that are incorporated with biological markers
with several parameters facilitate an individualized approach. In
the present study, the CPS + EG scoring system effectively
predicted the prognosis of patients with non-metastatic breast
cancer treated with NACT. Estimating prognosis at the initial
presentation may help to identify the escalation and
intensification of the chemotherapy treatment needed during the
neoadjuvant therapy period.
The present study is the first to assess the CPS +
EG scoring system in real-life data from patients with hormone
positive breast cancer in Turkey, to the best of our knowledge.
Furthermore, it provides a valuable opportunity to provide
comparisons with other data sets on mortality and OS rates. The
findings of the present study demonstrate a clear association
between the CPS + EG score and survival. As the score increases,
both the probability of recovery and the OS decrease. The CPS + EG
scoring system is an easy-to calculate, cost-effective and
accessible tool for clinical follow-up, survival estimation and
patient stratification in NACT trials along with adjuvant
chemotherapy choices.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
EYY collected and analyzed the data, and drafted the
manuscript. SA planned the study and wrote the paper. OUU developed
the study concept and reviewed the paper. EYY, SA and OUU confirm
the authenticity of all the raw data. All authors read and approved
the final version of the manuscript.
Ethics approval and consent to
participate
Ethics approval for the present study was obtained
from the local ethics committee of Izmir Tepecik Education and
Research Hospital (approval no: 2023/08). As the present study was
retrospective, informed patient consent was not required.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
The International Agency for Research on
Cancer, . Breast Fact Sheet. 2020.
|
|
3
|
Urut DU, Karabulut D, Hereklioglu S,
Özdemir G, Cicin BA, Hacıoglu B, Süt N and Tunçbilek N: Diffusion
tensor imaging: Survival analysis prediction in breast cancer
patients. Radiologie (Heidelb). 64 (Suppl 1):S54–S59. 2024.
View Article : Google Scholar
|
|
4
|
van der Meer DJ, Kramer I, van Maaren MC,
van Diest PJ, C Linn S, Maduro JH, J A Strobbe L, Siesling S,
Schmidt MK and Voogd AC: Comprehensive trends in incidence,
treatment, survival and mortality of first primary invasive breast
cancer stratified by age, stage and receptor subtype in the
Netherlands between 1989 and 2017. Int J Cancer. 148:2289–2303.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Simon RM, Paik S and Hayes DF: Use of
archived specimens in evaluation of prognostic and predictive
biomarkers. J Natl Cancer Inst. 101:1446–1452. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Elston CW and Ellis IO: Pathological
prognostic factors in breast cancer. I. The value of histological
grade in breast cancer: Experience from a large study with
long-term follow-up. Histopathology. 19:403–410. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Rakha EA, El-Sayed ME, Lee AH, Elston CW,
Grainge MJ, Hodi Z, Blamey RW and Ellis IO: Prognostic significance
of Nottingham histologic grade in invasive breast carcinoma. J Clin
Oncol. 26:3153–3158. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeruss JS, Mittendorf EA, Tucker SL,
Gonzalez-Angulo AM, Buchholz TA, Sahin AA, Cormier JN, Buzdar AU,
Hortobagyi GN and Hunt KK: Staging of breast cancer in the
neoadjuvant setting. Cancer Res. 68:6477–6481. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mittendorf EA, Jeruss JS, Tucker SL, Kolli
A, Newman LA, Gonzalez-Angulo AM, Buchholz TA, Sahin AA, Cormier
JN, Buzdar AU, et al: Validation of a novel staging system for
disease-specific survival in patients with breast cancer treated
with neoadjuvant chemotherapy. J Clin Oncol. 29:1956–1962. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pollak KI, Arnold RM, Jeffreys AS,
Alexander SC, Olsen MK, Abernethy AP, Sugg Skinner C, Rodriguez KL
and Tulsky JA: Oncologist communication about emotion during visits
with patients with advanced cancer. J Clin Oncol. 25:5748–5752.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Altman DG and Royston P: What do we mean
by validating a prognostic model? Stat Med. 19:453–473. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hortobagyi GN, Edge SB and Giuliano A: New
and important changes in the TNM staging system for breast cancer.
Am Soc Clin Oncol Educ Book. 38:457–467. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Febbo PG, Ladanyi M, Aldape KD, De Marzo
AM, Hammond ME, Hayes DF, Iafrate AJ, Kelley RK, Marcucci G, Ogino
S, et al: NCCN Task Force report: Evaluating the clinical utility
of tumor markers in oncology. J Natl Compr Canc Netw. 9 (Suppl
5):S1–S32. 2011. View Article : Google Scholar
|
|
15
|
Phung MT, Tin Tin S and Elwood JM:
Prognostic models for breast cancer: A systematic review. BMC
Cancer. 19:2302019. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Clift AK, Dodwell D, Lord S, Petrou S,
Brady M, Collins GS and Hippisley-Cox J: Development and
internal-external validation of statistical and machine learning
models for breast cancer prognostication: Cohort study. BMJ.
381:e0738002023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rakha EA, Soria D, Green AR, Lemetre C,
Powe DG, Nolan CC, Garibaldi JM, Ball G and Ellis IO: Nottingham
prognostic index plus (NPI+): A modern clinical decision making
tool in breast cancer. Br J Cancer. 110:1688–1697. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gray E, Donten A, Payne K and Hall PS:
Survival estimates stratified by the Nottingham Prognostic Index
for early breast cancer: A systematic review and meta-analysis of
observational studies. Syst Rev. 7:1422018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hawkins RA, Tesdale AL, Prescott RJ,
Forster T, McIntyre MA, Baker P, Jack WJ, Chetty U, Dixon JM,
Killen ME, et al: Outcome after extended follow-up in a prospective
study of operable breast cancer: Key factors and a prognostic
index. Br J Cancer. 87:8–14. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vila J, Teshome M, Tucker SL, Woodward WA,
Chavez-MacGregor M, Hunt KK and Mittendorf EA: Combining clinical
and pathologic staging variables has prognostic value in predicting
Local-regional recurrence following neoadjuvant chemotherapy for
breast cancer. Ann Surg. 265:574–580. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Marmé F, Solbach C, Michel L, Schneeweiss
A, Blohmer JU, Huober J, Fasching PA, Jackisch C, Nekljudova V,
Link T, et al: Utility of the CPS+ EG scoring system in
triple-negative breast cancer treated with neoadjuvant
chemotherapy. Eur J Cancer. 153:203–212. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Song R, Lee DE, Lee EG, Lee S, Kang HS,
Han JH, Lee KS, Sim SH, Chae H, Kwon Y, et al: Clinicopathological
factors associated with oncotype DX risk group in patients with
ER+/HER2-Breast cancer. Cancers (Basel). 15:44512023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yabroff KR, Sylvia Shi K, Zhao J, Freedman
AN, Zheng Z, Nogueira L and de Moor JS: Importance of patient
health ınsurance coverage and Out-of-Pocket costs for genomic
testing in oncologists' Treatment decisions. JCO Oncol Pract.
20:429–437. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Taylor C, McGale P, Probert J, Broggio J,
Charman J, Darby SC, Kerr AJ, Whelan T, Cutter DJ, Mannu G and
Dodwell D: Breast cancer mortality in 500 000 women with early
invasive breast cancer diagnosed in England, 1993–2015: Population
based observational cohort study. BMJ. 381:e0746842023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Blows FM, Driver KE, Schmidt MK, Broeks A,
van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO,
Blomqvist C, et al: Subtyping of breast cancer by
immunohistochemistry to investigate a relationship between subtype
and short and long term survival: A collaborative analysis of data
for 10,159 cases from 12 studies. PLoS Med. 7:e10002792010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kulturoglu MO, Aydın F, Aslan F, Sagdic
MF, Coskun O and Dogan L: The rush to evaluate response at the end
of treatment in breast cancer patients who received neoadjuvant
treatment: Which tests are effective in the selection of surgical
technique? Asian Pac J Cancer Prev. 26:619–624. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tayebi A, TizMaghz A, Gorjizad M, Tavasol
A, Tajaddini A, Rashnoo F, Vakili K, Behmanesh M, Olamaeian F and
Ashoori M: Evaluating the effect of neoadjuvant chemotherapy on
surgical outcomes in breast cancer patients: A systematic review
study. J Chemother. February 28;(Epub ahead of print). PubMed/NCBI
|
|
29
|
Roussot N, Constantin G, Desmoulins I,
Bergeron A, Arnould L, Beltjens F, Mayeur D, Kaderbhai C, Hennequin
A, Jankowski C, et al: Prognostic stratification ability of the
CPS+EG scoring system in HER2-low and HER2-zero early breast cancer
treated with neoadjuvant chemotherapy. Eur J Cancer.
202:1140372024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
von Minckwitz G, Untch M, Blohmer JU,
Costa SD, Eidtmann H, Fasching PA, Gerber B, Eiermann W, Hilfrich
J, Huober J, et al: Definition and impact of pathologic complete
response on prognosis after neoadjuvant chemotherapy in various
intrinsic breast cancer subtypes. J Clin Oncol. 30:1796–804. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Rhodes S, Miller DG and Chino F: ‘When
Less is More’: Paradigm shifts in radiation treatment for
Early-stage breast cancer. Curr Treat Options Oncol. 25:1495–1505.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Michel LL, Sommer L, González Silos R,
Lorenzo Bermejo J, von Au A, Seitz J, Hennigs A, Smetanay K,
Golatta M, Heil J, et al: Locoregional risk assessment after
neoadjuvant chemotherapy in patients with primary breast cancer:
Clinical utility of the CPS + EG score. Breast Cancer Res Treat.
177:437–446. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fredholm H, Eaker S, Frisell J, Holmberg
L, Fredriksson I and Lindman H: Breast cancer in young women: Poor
survival despite intensive treatment. PLoS One. 4:e76952009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bastiaannet E, Liefers GJ, de Craen AJ,
Kuppen PJ, van de Water W, Portielje JE, van der Geest LG,
Janssen-Heijnen ML, Dekkers OM, van de Velde CJ and Westendorp RG:
Breast cancer in elderly compared to younger patients in the
Netherlands: Stage at diagnosis, treatment and survival in 127,805
unselected patients. Breast Cancer Res Treat. 124:801–807. 2010.
View Article : Google Scholar : PubMed/NCBI
|