Introduction
Cervical cancer is the fourth most common cancer in
women worldwide. Among the pathological types of cervical cancer,
squamous cell carcinoma (SCC) is the most common, accounting for
75–80% of cases (1). Moreover,
cervical adenocarcinoma accounts for 10–25% of cases and
adenosquamous carcinoma accounts for 3–5% of cases (2). Cervical cancer metastasis usually
occurs in the pelvic region, which includes mainly the bladder,
vagina and rectum. Other common sites of occurrence include the
liver, lungs and bones, and metastasis to abdominal organs other
than the liver is rare (3). The
majority of pancreatic tumors are primary ductal adenocarcinomas,
while metastatic pancreatic cancer (mPC) accounts for only 2–5% of
all pancreatic malignancies (4,5). Such
metastases are often accompanied by widespread systemic
dissemination, with isolated pancreatic metastases being
exceptionally rare (~2%) (6).
Tumors with the highest propensity for pancreatic metastasis
include renal cell carcinoma, lung cancer, breast cancer and
colorectal cancer, followed by melanoma and leiomyosarcoma
(7). Notably, cervical cancer
metastases to the pancreas have only been sporadically reported. Of
particular significance is the median latency period of up to 9
years between primary tumor resection and pancreatic metastasis,
with its asymptomatic nature often complicating early diagnosis
(8). Currently, there is no
established clinical treatment for mPC, and the selection of
treatment primarily depends on the pathological type. A
representative example is pancreatic metastasis from renal cell
carcinoma. Compared with that following non-surgical management,
the 10-year survival rate following surgical intervention shows
improvement, suggesting that active surgical intervention may
enable long-term survival in this subgroup (8). This phenomenon may be attributed to
the unique biological behavior of renal cell carcinoma and its
sensitivity to systemic therapies, highlighting the critical
importance of tumor heterogeneity in clinical decision-making
(8). The present report describes
the rare case of metastasis of cervical cancer to the pancreas.
Through a comprehensive search of the medical database, 14
documented cases of cervical carcinoma with pancreatic metastases
were identified. Subsequent systematic review of these clinical
case reports provided valuable insights into the
clinicopathological characteristics of pancreatic metastatic tumors
originating from cervical primary malignancies.
Case report
A 64-year-old woman presented to Peking Union
Medical College Hospital (Beijing, China) 6 years after receiving
radiotherapy and chemotherapy for cervical cancer and 6 months
after a pancreatic head mass was identified.
A total of 6 years prior, the patient underwent a
biopsy for abnormal postmenopausal vaginal bleeding (March 2018;
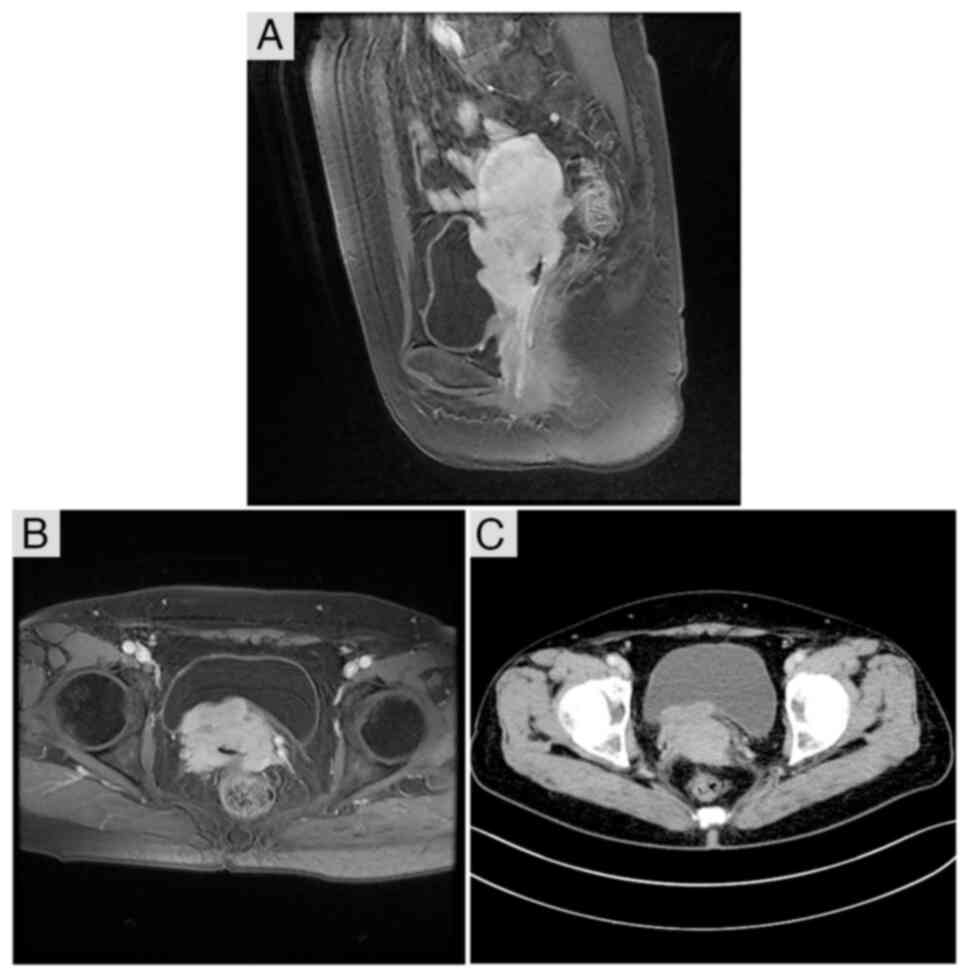
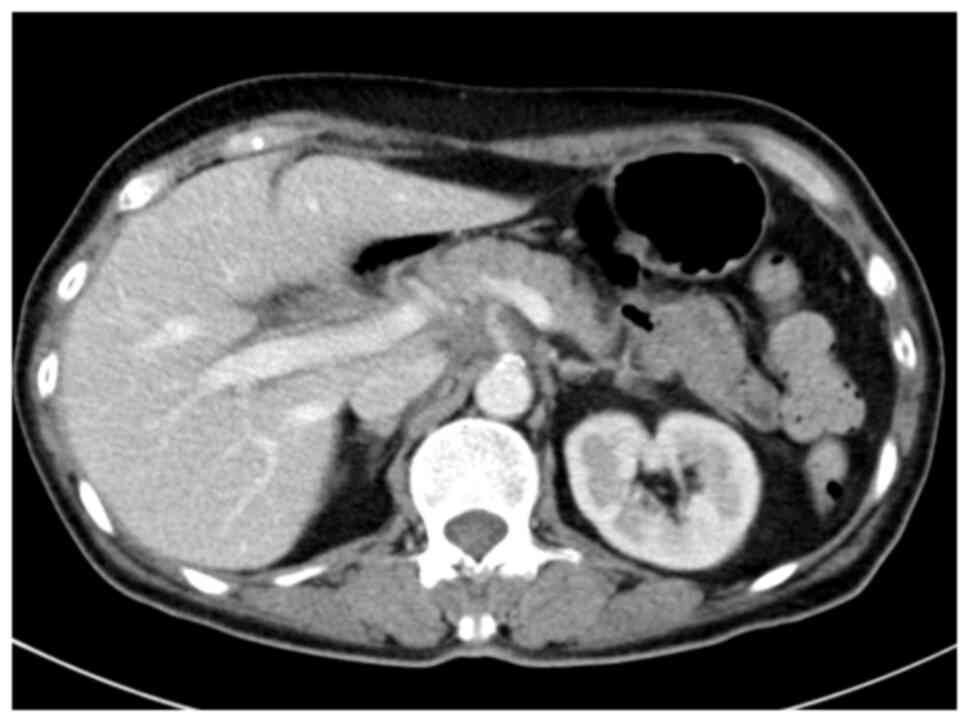
Peking Union Medical College Hospital, Beijing, China). CT and MRI
imaging suggested the presence of an abnormality in the cervical
space (Fig. 1). Pathological
examination (data obtained from medical records) revealed
moderately differentiated SCC in the uterine cervix (data not
shown). The disease was staged as IIIB SCC, per the 2009
International Federation of Gynecology and Obstetrics staging
system (9). Intensity-modulated
radiotherapy was initiated at a dose of 50.4 Gy in 28 fractions for
the whole pelvis, 60.2 Gy for the pelvic lymph nodes, 70 Gy for the
right posterior bladder lesion, and 60.4 Gy for the right
parametrium, along with concurrent weekly paclitaxel treatment. The
treatment regimen consisted of 50 mg/m2 (body surface
area) paclitaxel administered via intravenous infusion once weekly
over 6 consecutive weeks. This was followed by intracavitary
brachytherapy at a dose of 28.5 Gy in 5 fractions, which were
delivered 2 days apart. Regular follow-up (abdominal
contrast-enhanced CT, pelvic MRI and tumor marker analysis) at the
end of treatment revealed no significant abnormalities.
After 6 years of follow-up, in December 2023, the
patient was diagnosed with pancreatic space-occupying lesions upon
reexamination at the People's Hospital of Pingluo County
(Shizuishan, China). Subsequently, the patient received treatment
at the Cancer Hospital Chinese Academy of Medical Sciences (CAMS;
Beijing, China). In December 2023, MRI revealed a peritoneal mass
that was located posterior to the pancreas and was considered to
have a high likelihood of malignancy, favoring a retroperitoneal
origin (data not shown). Endoscopic ultrasonography (EUS)-guided
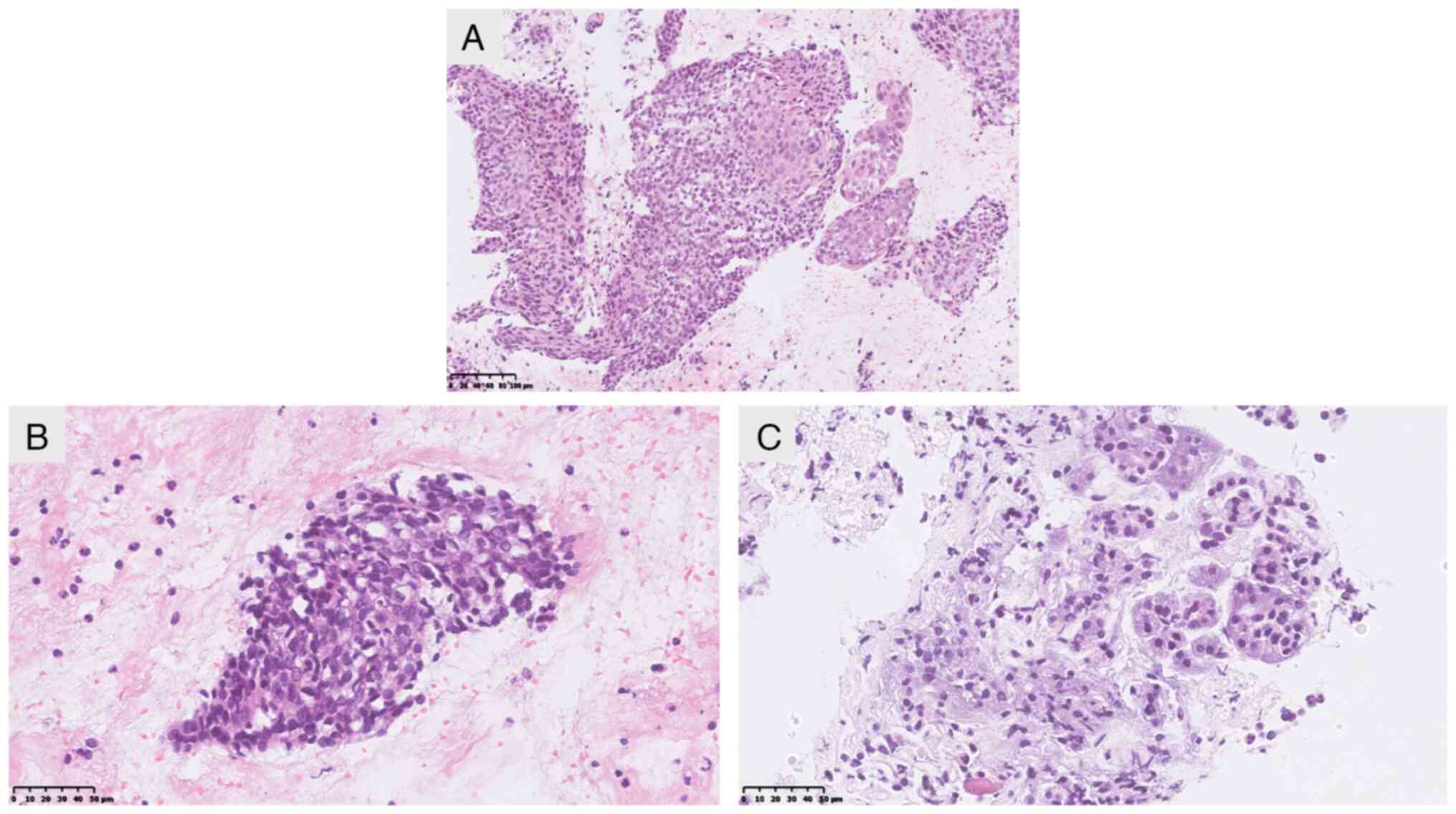
fine-needle aspiration (FNA) biopsy of the pancreatic mass was
performed in January 2024, and pathology (data obtained from
medical records) revealed features suggestive of SCC (Fig. 2). The immunohistochemical results
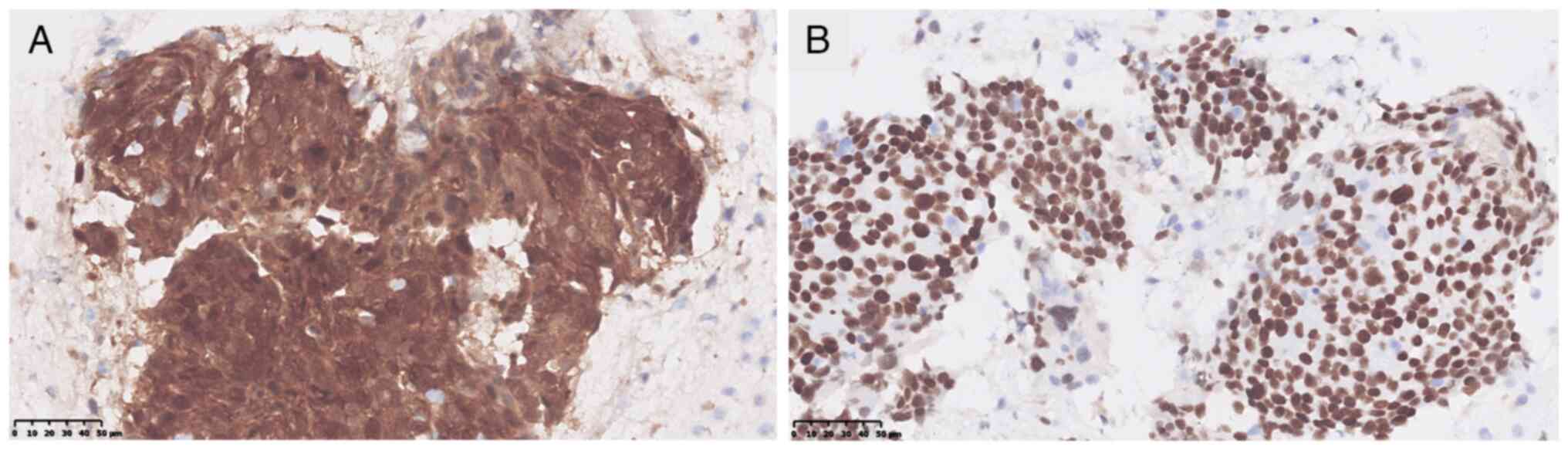
from CAMS (data obtained from medical records) indicated the
following: P16 (3+) and P40 (+) (Fig.
3), and CK7 (−), CK20 (−), CK19 (2+), P63 (3+), PAX8 (−), GATA3
(+), CDX-2 (−), AE1/AE3 (3+), programmed death-ligand 1 (PD-L1):
tumor proportion score, 60% and human papillomavirus [HPV (−)]
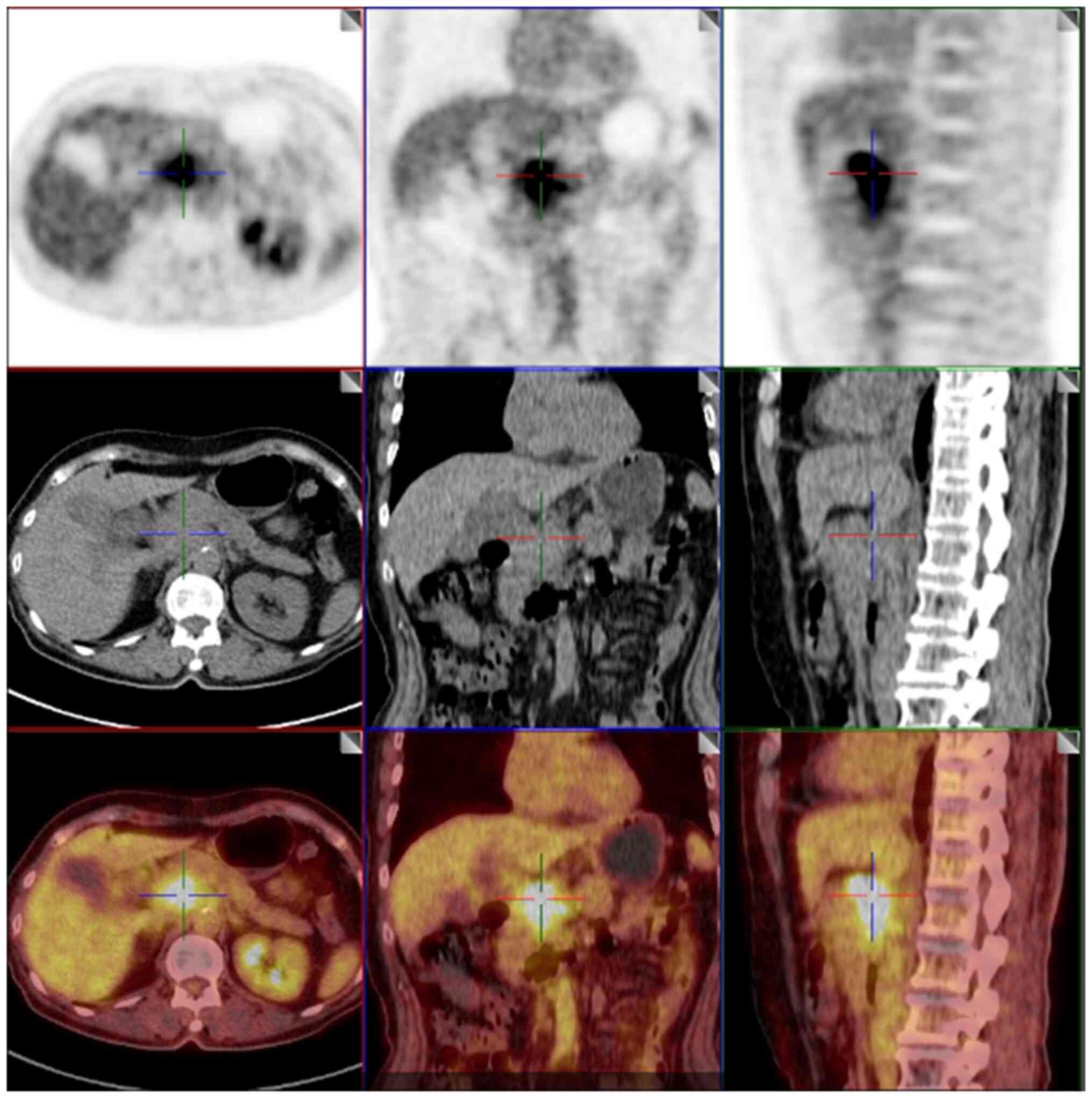
(data not shown). Furthermore, a PET/CT examination in January 2024
revealed refined lymph nodes in the left parietal uterus and
suggested that the mass behind the head of the pancreas may be a
metastatic lesion (Fig. 4). On the
basis of the imaging features and results of immunohistochemical
staining, mPC from the cervical carcinoma was finally
diagnosed.
In the Beijing Sixth People's Hospital (Beijing
China), the patient received six cycles (21-day cycle) of
intravenous infusion therapy consisting of paclitaxel (175
mg/m2), carboplatin (area under the curve=5),
bevacizumab (7.5 mg/kg) and tislelizumab [an anti-programmed cell
death protein 1 (PD-1) antibody; 200 mg], with the last
administration occurring in May 2024. After the completion of
treatment, an abdominal CT scan 16 days later indicated that the
size of the pancreatic lesion had decreased compared with that in
previous assessments, measuring ~32×20 mm (Fig. 5). Follow-up intensity-modulated
radiotherapy was initiated for the pancreatic metastatic lesions
and associated invasion, with a total dose of 45 Gy delivered in 25
treatment fractions. Additionally, the central region of the
metastatic pancreatic lesions received a boost to a cumulative dose
of 55 Gy. Following radiotherapy, the patient continued to receive
200 mg intravenous tislelizumab maintenance immunotherapy at
Pingluo County People's Hospital. Multimodal surveillance
comprising contrast-enhanced abdominal CT, pelvic MRI and serial
serum tumor marker profiling conducted quarterly through November
2024 has yielded negative results across all modalities, confirming
maintained disease-free status.
Discussion
The histopathology of cervical cancer is
predominantly SCC, and the highest incidence is in the 40–59 year
age group. In high-income nations, women aged 40–59 years exhibit
stable age-standardized incidence rates, maintaining a consistently
low range of 6.5–7.5 per 100,000 annually (10). This pattern sharply contrasts with
the pronounced geographical disparities observed in low- and
middle-income countries. India exemplifies this divergence, where
incidence rates in this demographic have surged to 18–25 per
100,000, with rural regions experiencing even higher levels of
24–32 per 100,000 (11,12). Sub-Saharan Africa remains the global
epicenter of disease burden, where countries such as Malawi and
Zimbabwe report high rates persistently ranging between 40–56 per
100,000. Particularly in rural settings, these figures increase
beyond 60 cases per 100,000 individuals (13). Irregular vaginal bleeding and
abdominal pain are the main clinical symptoms, but certain patients
have no symptoms at all (14).
Cervical cancer metastasis typically occurs in the pelvic area,
with the bladder, vagina and rectum being frequent sites of
metastasis. Other common sites include the liver, lungs and bones.
Early-stage cervical cancer is usually treated by means of surgery,
with chemoradiotherapy used for inoperable lesions (15). The patient in the present case was
58 years old, presented with abnormal vaginal bleeding, diagnosed
with stage IIIB SCC and was treated with concurrent
chemoradiotherapy. Pancreatic metastases were found 6 years after
treatment.
Pancreatic cancers (PCs) tend to be primary
pancreatic ductal adenocarcinomas. mPC is rare, constituting 2–5%
of all pancreatic malignancies. Moreover, renal cell carcinoma is
the most likely tumor to metastasize to the pancreas (4). Patients with mPC present with symptoms
related to the site of involvement: Obstructive jaundice may occur
if the lesion is in the head of the pancreas, whereas there may not
be any obvious symptoms in the early stages if the lesion is in the
tail. The most common clinical symptoms are abdominal pain,
jaundice and emaciation (16). In
cases of cervical cancer, metastasis to the pancreas is very
rare.
Following the Preferred Reporting Items for
Systematic Reviews and Meta-Analyses guidelines (17), a literature search was performed
using the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Metstr (https://www.metstr.com) and CNKI (https://www.cnki.net/) databases to identify all
eligible articles published from January 1, 1964 to December 31,
2024. The following search strategy was used: ‘Uterine Cervical
Neoplasms’ OR ‘cervical cancer’ OR ‘cervix carcinoma’) AND
(‘Pancreatic Neoplasms/secondary’ OR ‘pancreatic metastasis’ OR
‘metastases to pancreas’ OR ‘pancreatic metastasis’). In total, 173
studies (169 in English and 4 in Chinese) were retrieved for
review, including 51 case reports. After the removal of duplicates,
the titles and abstracts of the remaining papers were carefully
screened, and 20 case reports were identified as being inconsistent
with the findings presented in the present article. Finally, a
total of 14 studies (12 articles in English and 2 articles in
Chinese), published from 1966–2024, were included in the review
(Table I) (6,17–30).
Out of the studies, seven cases were histologically classified as
SCC, two cases as adenocarcinoma, four cases as neuroendocrine
carcinoma, and one case as small cell carcinoma. The median age of
the patients was 49 years, ranging from 36–70 years. Most of the
patients had heterochronic metastases (13/14), and one patient was
found to have pancreatic metastases at the same time as the
diagnosis of cervical cancer, which is rare (26). Among the remaining patients, the
time interval between the initial diagnosis and metastasis ranged
from 2 months to 8 years, with a mean interval of 46 months. In
these patients, back pain and weight loss were the most common
symptoms, and only two patients had no obvious symptoms. In
addition, 11 patients had a single metastasis, and 3 patients had
multiple metastases. The exact process through which cervical
cancer spreads to the pancreas remains unclear. Typically, cervical
cancer spreads locally and can metastasize to other organs once the
lymphatic and vascular systems become involved (31). Among the documented cases of
pancreatic metastasis, only four presented evidence of lymph node
involvement; therefore, we hypothesize that hematogenous
dissemination is a common route for the spread of cervical cancer
to the pancreas.
 | Table I.Comparative analysis of
literature-reported patient profiles. |
Table I.
Comparative analysis of
literature-reported patient profiles.
| Case | First author/s,
year | Age at diagnosis of
PT, year | Age at discovery of
metastatic disease, year | Time period between
PT and metastasis, months | Histology | Stage | PT treatment | Metastasis
symptoms | Other site | Lymph nodes | Diagnostic
means | Metastasis
treatment | Survival,
months | (Refs.) |
|---|
| 1 | Wastell et
al, 1966 | 66 | 71 | 60 | SCC | II | RT | Back pain; weight
loss; anorexia; dark urine; and pale stools | - | - | Postoperative
pathology | Surgery | 0.5 | (18) |
| 2 | Chung et al,
2007 | 45 | 53 | 90 | SCC | IB | Surgery and
CCRT | Acute renal
failure; nausea; vomiting; and anorexia | Liver; lumbar
spine; and scalp | - | CT | - | 3 | (19) |
| 3 | Kuwatani et
al, 2008 | 38 | 39 | 11 | Small cell
carcinoma | IIB | Chemotherapy | No symptoms | - | - | EUS-FNA | Chemotherapy | 5 | (20) |
| 4 | Ogawa et al,
2011 | 43 | 45 | 24 | SCC | Not mentioned | Not mentioned | Back pain and
weight loss | - | - | Postoperative
pathology and IHC | Surgery and RT | 3 | (6) |
| 5 | Mahajan et
al, 2017 | 54 | 57 | 36 |
Adeno-carcinoma | IIIA | Surgery and
CCRT | No symptoms | - | - | EUS-FNA | Chemotherapy | - | (21) |
| 6 | Lee et al,
2019 | 36 | 36 | 2 | SCC | IB2 | CCRT | Nausea and
vomiting | Both breasts; both
adrenal glands; and peritoneum | + | EUS-FNA | Chemotherapy | - | (22) |
| 7 | Gupta et al,
2019 | 55 | 58 | 36 | SCC | IIIB | CCRT | Hematemesis
melena | - | + | Postoperative
pathology | Surgery | - | (23) |
| 8 | Kim et al,
2019 | 68 | 70 | 20 |
Adeno-carcinoma | IIB | CCRT | Back pain | - | - | EUS-FNA | - | - | (24) |
| 9 | Datta et al,
2022 | 51 | 53 | 24 | SCC | IIIC | CCRT | Back pain;
anorexia; and weight loss | - | - | EUS-FNA | Chemotherapy | - | (25) |
| 10 | Kopke Túlio et
al, 2018 | 56 | 56 | 0 | SCENC | IVB | Chemotherapy | Epigastric
pain | Liver and bilateral
retroperitonea | + | EUS-FNA | Chemotherapy | - | (26) |
| 11 | Nishimura et
al, 2013 | 36 | 44 | 96 | MANEC | IB | Surgery and
CCRT | Back pain | - | - | EUS-FNA | Surgery | - | (27) |
| 12 | Liu et al,
2018 | 49 | 51 | 22 | SCENC | IB | CCRT | No symptoms | - | - | EUS-FNA | RT | - | (28) |
| 13 | Nakajima et
al, 2023 | 50 | 48 | 24 | SCC | IIB | CCRT | Abdominal pain | - | - | EUS-FNA | RT | - | (29) |
| 14 | Ye et al,
2022 | 51 | 63 | 91 | SCC | IIIB | CCRT | Obstructive
jaundice | - | + | Postoperative
pathology | Surgery and
chemotherapy | - | (30) |
mPC is difficult to distinguish from primary
pancreatic lesions. The diagnosis of pancreatic metastases usually
includes ultrasound, CT, MRI, EUS, PET and magnetic resonance
cholangiography (27). On CT
images, primary PC and mPC have similar enhancement patterns,
except in cases of metastatic renal cell carcinoma. In a previous
study, pancreatic metastases were observed on multi-slice CT images
in 75% of patients with nonrenal cell carcinoma, presenting as
solitary, heterogeneous, ill-defined nodules with persistent low
attenuation, indistinguishable from primary PC (32). The increased use of EUS-FNA has made
histopathological diagnosis possible. EUS-FNA is widely used for
the evaluation of pancreatic lesions due to its higher accuracy in
detecting small lesions and the higher availability of samples for
cytological/histological diagnosis than for CT or MRI. The
sensitivity of EUS-FNA for the diagnosis of pancreatic metastases
has been reported to be 93.8%, with a specificity of 60% and a
positive diagnosis rate of 89% (33). mPC was diagnosed by EUS-FNA in 9/14
patients previously reported (3,5,6,8–13).
The most prevalent pathological type of PC is
adenocarcinoma. By contrast, primary SCC is very rare, constituting
~0.28% of all PCs (34). Most
patients diagnosed with pancreatic SCC are aged >65 years and
are predominantly male (35). Given
its rarity, a diagnosis of primary pancreatic SCC should be
considered only after ruling out the presence of a primary site for
SCC elsewhere. P40 is one of the isoforms of the P63 protein, whose
specificity in differentiating between SCC from adenocarcinoma is
high. The sensitivity and specificity for SCC are 100 and 90%,
respectively (36). Furthermore,
P40 is rarely expressed in the pancreas, as this organ is
predominantly affected by adenocarcinoma. The P16 gene is located
at the chromosome 9p21 locus and functions as an oncogene. Diffuse
positive immunostaining for p16 serves as a reliable surrogate
marker for high-risk HPV positive cervical cancer. Notably, even
among patients who are negative for HPV, the majority still
demonstrate positive p16 expression (37). As early as 2012, the American
Society for Colposcopy and Cervical Pathology recommended p16 as a
diagnostic marker for cervical cancerous lesions (38). Furthermore, a study (39) has reported a notable association
between the degree of squamous intraepithelial lesions of the
cervix and both the distribution and intensity of p16 staining.
Additionally, strong positive expression of p16 has been observed
in distant metastatic lesions, indicating its specificity. By
contrast, primary PC has reduced expression of p16. Research
indicates that the level of p16 in PC is markedly lower than that
in adjacent normal tissues, particularly in advanced-stage patients
(40,41).
There is no standard treatment for mPC, and
chemotherapy is the most common treatment. 5-fluorouracil,
leucovorin, irinotecan and oxaliplatin (FOLFIRINOX) and
nab-paclitaxel + gemcitabine (AG) are frequently recommended as
first-line treatment regimens for mPC; however, there is currently
no international consensus on the progression-free survival and
overall survival (OS) of patients receiving these two chemotherapy
regimens (42). Notably, FOLFIRINOX
is associated with a greater toxic response and is not recommended
for patients with an Eastern Cooperative Oncology Group performance
status of 2 or with comorbidities, where the risk of complications
due to chemotherapy outweighs the expected benefit of prolonging OS
(43,44). Therefore, when deciding on the
first-line chemotherapy, clinicians must consider not only the
extent of mPC but also the general condition of the patient and the
presence of comorbidities. Moreover, immunotherapy has changed the
treatment landscape for numerous solid tumors. In a clinical trial
using toripalimab (anti-PD-1) + AG as a first-line treatment for
patients with locally advanced PC or mPC, a favorable response and
manageable toxicity were reported (45). Multiple clinical studies have also
reported that the combination of anti-PD-1/PD-L1 antibodies with
chemotherapy can improve mPC outcomes, with a manageable safety
profile (46,47). Thus, immunotherapy may be effective
in the treatment of PC but can be used as part of a multiagent
strategy rather than as monotherapy.
Radiotherapy always requires histological (or
cytological) confirmation of the pathology of the tumor, and the
sensitivity and complete remission rate of SCC to radiotherapy are
generally higher than those of other histological types (48). In a previous study, a combination of
radiotherapy and PD-L1 blockade improved survival and reduced tumor
volume in patients with PC, compared with a single modality. PD-L1
expression was also reported to be increased in tumor cells
following radiotherapy (49).
Moreover, a phase II randomized study by Chen et al
(50) reported that the disease
control rates of radiotherapy combined with nabumab/ipilizumab and
nabumab/ipilizumab alone were 37.2 and 17.1%, respectively,
indicating that stereotactic body radiation therapy combined with
nabumab/ipilizumab has a good safety profile and antitumor
activity.
With respect to targeted therapies, the randomized,
double-blind study, Pancreas Cancer Olaparib Ongoing, reported that
the addition of olaparib to first-line platinum-based chemotherapy
improved the outcomes of patients with germline breast cancer gene
1 and 2 mutations and mPC (51).
The use of entrectinib was included in the American Society of
Clinical Oncology guidelines for the first time in 2020 (52) and studies have reported that this
drug induces durable and clinically significant responses in
patients with neurotrophic tyrosine receptor kinase fusion-positive
solid tumors (53,54). Furthermore, according to the
National Comprehensive Cancer Network 2023 guidelines, individuals
with mPC who have distinctive KRAS gene mutations (KRAS G12C) may
be able to extend their survival through the use of molecular
therapeutics, including sotorasib or adagrasib (55).
Previous studies have reported the prospective
benefits of pancreatic metastasectomy, including improved patient
survival and quality of life (56).
Akashi et al (57) analyzed
the surgical outcomes of 15 patients with mPC and reported that
surgical resection of the pancreas resulted in longer survival in
those with primary renal cell carcinoma, whereas those with primary
nonrenal cell carcinoma had a worse prognosis. Thus, surgery may be
justified for localized metastasis to the pancreas in the absence
of broad metastatic disease if the surgical risk is tolerable and
resection with no remaining malignancy can be performed. A total of
1/13 of the aforementioned patients with cervical mPC underwent
concurrent surgery, chemotherapy and radiotherapy; nonetheless, the
patient developed liver metastases 3 months after the operation and
died 8 months after surgery. Among the remaining patients, three
underwent surgery alone; one died 16 days after the procedure from
an abdominal infection; and the other two patients were still alive
at 6 and 7 months of follow-up, with no evidence of local
progression. After receiving chemotherapy, five patients had no
signs of cancer progression at 4–16 months of follow-up; however,
one patient developed numerous brain metastases at 16 months of
follow-up. After receiving radiotherapy in addition to surgery or
chemotherapy, two patients developed liver metastases 9 months
after treatment, and one patient died 7 months after treatment due
to brain metastases. A total of one patient underwent surgery
combined with chemotherapy and recovered well. Furthermore, one
patient received no treatment, whilst the course of treatment of
one patient was unknown. In the absence of a consensus treatment
model, the patient described in the current report was treated with
multiple adjuvant therapies.
According to previous studies, platinum (cisplatin
or carboplatin) combined with paclitaxel remains the first-line
protocol for advanced cervical cancer and can effectively reduce
the risk of metastasis (58,59).
The emergence of pancreatic metastases in patients with cervical
cancer is typically associated with systemic spread, and
chemotherapy can systematically eliminate potential
micrometastases, thereby delaying disease progression (60). As indicated by prior studies, the
use of anti-PD-1/PD-L1 antibodies in combination with chemotherapy
has improved therapeutic outcomes in patients with mPC (49,50).
Therefore, tislelizumab was incorporated into the treatment regimen
in the present case. Given that VEGF-driven angiogenesis is a
primary driver of cervical cancer progression, antiangiogenic
therapy has emerged as a promising strategy for treating
persistent, metastatic or recurrent cervical cancer. Bevacizumab
blocks the VEGF signaling pathway, inhibits tumor angiogenesis and
reduces the blood supply to metastatic lesions. Additionally, it
enhances vascular permeability within the tumor microenvironment,
improving chemotherapy drug penetration and immune cell
infiltration, thus synergizing with chemotherapy and immunotherapy
(61). A phase III trial (GOG240)
evaluating the efficacy of chemotherapy (topotecan/paclitaxel or
cisplatin/paclitaxel) with or without bevacizumab reported that
this targeted therapy markedly improved OS (62). The pathological type of the patient
reported in the present study was SCC, which is sensitive to
radiotherapy. Radiotherapy can effectively control local disease
progression and induce the release of tumor antigens and
anti-inflammatory factors, thereby improving the immune
microenvironment and enhancing the immune response (63).
The present study describes a unique case of
cervical SCC metastasizing to the pancreas. Treatment for a
58-year-old patient, who was in the high-incidence age category for
cervical cancer, involved concurrent radiotherapy and chemotherapy.
Irregular vaginal bleeding led to the diagnosis of stage IIIB SCC
of the cervix, and 6 years after treatment, a review of a pelvic
MRI revealed a pancreatic mass without any clinical signs.
Pancreatic tissue was obtained through EUS-FNA, and
immunohistochemical analysis revealed P16 (3+) and p40 (+). This,
in conjunction with the patient's history of SCC, led to a
diagnosis of secondary pancreatic adenocarcinoma originating from
cervical cancer. A novel therapeutic approach combining
chemotherapy, immunotherapy, targeted therapy and radiotherapy,
which is previously unreported in the literature, to the best of
our knowledge, may prove beneficial in enhancing patient
outcomes.
In conclusion, for patients with a previous history
of cervical cancer, when imaging suggests the presence of a
pancreatic mass, even if there are no clinical symptoms, the
possibility of mPC should not be ignored, and EUS-FNA is feasible
for definitive diagnosis. Currently, there is no uniform standard
for the treatment of mPC of cervical origin. Classical chemotherapy
has been shown to be the baseline method for improving the survival
rate among patients with mPC, and early genetic testing and
appropriate supplementation with immune and targeted therapies may
further prolong the survival period. However, at present, it is
difficult to evaluate the prognosis and survival time of patients,
as there are few cases of cervical cancer complicated with
pancreatic metastasis, resulting in the lack of large-sample
clinical studies. The existing evidence is mostly based on case
reports or small-sample retrospective analysis, and the statistical
efficacy is insufficient. Furthermore, the biological behavior of
the tumor (such as pathological type and differentiation degree),
the number of metastases (single or multiple) and whether it is
combined with metastasis of other organs (such as liver and lung)
are notably different among different patients, making it difficult
to establish a unified prognostic model. In addition, there is
currently no guideline for the first-line treatment of this
metastatic site, and the treatment mainly depends on case
experience, and the efficacy of different programs varies
significantly. Therefore, more data are required for patients with
cervical cancer and pancreatic metastasis.
Acknowledgements
Not applicable.
Funding
The present work was supported by the National Key R&D
Program of China, Ministry of Science and Technology of the
People's Republic of China (grant nos. 2022YFC2407100 and
2022YFC2407101).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
HL, XC, XH and FZ contributed to the study
conception and design. Material preparation, data collection and
analysis were performed by XC. The first draft of the manuscript
was written by HL and XC, and all authors commented on previous
versions of the manuscript. XH and FZ confirm the authenticity of
all the raw data. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present case report was approved by the Ethics
Committee of Peking Union Medical College Hospital Chinese Academy
of Medical Sciences & Peking Union Medical College (Beijing,
China; approval no. K24C3515).
Patient consent for publication
The patient provided written consent for the
publication of the data and images included in this case
report.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Xie H, Kong B and Duan T: Obstetrics and
Gynaecology. 1st edition. People's Health Press; Beijing: pp.
p2982018
|
|
2
|
Gadducci A, Guerrieri ME and Cosio S:
Adenocarcinoma of the uterine cervix: Pathologic features,
treatment options, clinical outcome and prognostic variables. Crit
Rev Oncol Hematol. 135:103–114. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Shen J, Feng XS, Wen H, Zhou C, Mo M, Wang
Z, Yuan J, Wu X and Zheng Y: Metastatic characteristics and
survival analysis of 572 patients with distant metastases of
cervical cancer: A hospital-based real-world study. Chin J Cancer.
34:361–367. 2024.
|
|
4
|
Ballarin R, Spaggiari M, Cautero N, De
Ruvo N, Montalti R, Longo C, Pecchi A, Giacobazzi P, De Marco G,
D'Amico G, et al: Pancreatic metastases from renal cell carcinoma:
The state of the art. World J Gastroenterol. 17:4747–4756. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tsitouridis I, Diamantopoulou A,
Michaelides M, Arvanity M and Papaioannou S: Pancreatic metastases:
CT and MRI findings. Diagn Interv Radiol. 16:45–51. 2010.PubMed/NCBI
|
|
6
|
Ogawa H, Tsujie M, Miyamoto A, Yasui M,
Ikenaga M, Hirao M, Fujitani K, Mishima H, Tsujinaka T and Nakamori
S: Isolated pancreatic metastasis from uterine cervical cancer: A
case report. Pancreas. 40:797–798. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Geraizadeh B, Kashkooe A, Nikeghbalian S
and Malek-Hosseini SA: Metastatic tumors to the pancreas: A single
center study. Arch Iran Med. 22:50–52. 2019.PubMed/NCBI
|
|
8
|
Reddy S, Edil BH, Cameron JL, Pawlik TM,
Herman JM, Gilson MM, Campbell KA, Schulick RD, Ahuja N and
Wolfgang CL: Pancreatic resection of isolated metastases from
nonpancreatic primary cancers. Ann Surg Oncol. 15:3199–3206. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva, cervix, and endometrium. Int J Gynecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
National Cancer Institute, . SEER: Cancer
Stat Facts: Cervical Cancer. https://seer.cancer.gov/statfacts/html/cervix.html4–5.
25
|
|
11
|
Sathishkumar K, Chaturvedi M, Das P,
Stephen S and Mathur P: Cancer incidence estimates for 2022 and
projection for 2025: Result from national cancer registry
programme, India. Indian J Med Res. 156:598–607. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Budukh AM, Dikshit R and Chaturvedi P:
Outcome of the randomized control screening trials on oral, cervix
and breast cancer from India and way forward in COVID-19 pandemic
situation. Int J Cancer. 149:1619–1620. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stelzle D, Tanaka LF, Lee KK, Khalil AI,
Baussano I, Shah ASV, McAllister DA, Gottlieb SL, Klug SJ, Winkler
AS, et al: Estimates of the global burden of cervical cancer
associated with HIV. Lancet Glob Health. 9:e161–e169. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han SY and Kong WM: Pathological
characteristics and changes of cervical cancer. Hebei Medical
Journal. 42:1414–1417+1421. 2020.(In Chinese).
|
|
15
|
Crafton SM, Venkat PS and Salani R: A
review of the state of cervical cancer: Updates from prevention to
recurrent disease. Curr Opin Obstet Gyencol. 36:28–33. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jaén-Torrejimeno I, López-Guerra D,
Rojas-Holguín A, De-Armas-Conde N and Blanco-Fernández G: Resection
of isolated pancreatic metastases from pulmonary neoplasia: A
systematic review. Updates Surg. 74:1817–1825. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Page MJ, McKenzie JE, Bossuyt PM, Boutron
I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan
SE, et al: The PRISMA 2020 statement: An updated guideline for
reporting systematic reviews. BMJ. 372:n712021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wastell C: A solitary secondary deposit in
the pancreas from a carcinoma of the cervix. Postgrad Med J.
42:59–61. 1966. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chung JJ, Namiki T and Johnson DW:
Cervical cancer metastasis to the scalp presenting as alopecia
neoplastica. Int J Dermatol. 46:188–189. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kuwatani M, Kawakami H, Asaka M, Marukawa
K, Matsuno Y and Hosaka M: Pancreatic metastasis from small cell
carcinoma of the uterine cervix demonstrated by endoscopic
ultrasonography-guided fine needle aspiration. Diagn Cytopathol.
36:840–842. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mahajan S and Pandit-Taskar N: Uncommon
metastasis to the pancreas from adenocarcinoma of the cervix
detected on surveillance 18F-FDG PET/CT imaging. Clin Nucl Med.
42:e511–e512. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee EJ, Hwang J and Kim DW: Small-cell
neuroendocrine carcinoma of the uterine cervix with pancreatic
metastasis: A case report and a review of the literature. J Obstet
Gynaecol. 39:573–575. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gupta PK, Lal P and Tiwari A: A case
report of carcinoma of uterine cervix throwing heterochronous
metastasis to the skin, spleen, and pancreas: The role of
multimodality treatment approach. J Egypt Natl Cancer Inst.
31:82019. View Article : Google Scholar
|
|
24
|
Kim DJ, Park JM, Kim JH, Nam K, Kang CD,
Lee SJ, Lee K and Jeon YH: Pancreatic metastasis from
adenocarcinoma of the uterine cervix. Korean J Gastroenterol.
73:182–185. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Datta D, Aggarwal D, Balakrishnan S,
Varshney VK and Kumar R: Metastasis from cervical cancer presenting
as a pancreatic head mass-an unexpected diagnosis! J Gastrointest
Canc. 54:300–303. 2022. View Article : Google Scholar
|
|
26
|
Kopke Túlio MACB, Horta MSF, BispoMC S,
Costa TSNBE and Chagas CMDBR: Pancreatic Metastases as the Initial
Manifestation of a Neuroendocrine Carcinoma of the Uterine Cervix.
Pancreas. 47:e4–e5. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nishimura C, Naoe H, Hashigo S, Tsutsumi
H, Ishii S, Konoe T, Watanabe T, Shono T, Sakurai K, Takaishi K, et
al: Pancreatic metastasis from mixed adenoneuroendocrine carcinoma
of the uterine cervix: A case report. Case Rep Oncol. 6:256–262.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu AL, Feng Y and Zhao Y: Pancreatic
metastasis of complex small cell neuroendocrine carcinoma of
cervix: A case report and literature review. Gastroenterology.
23:638–640. 2018.
|
|
29
|
Nakajima Y, Iwasaki E, Kayashima A,
Machida Y, Kawasaki S, Horibe M, Kawaida M, Masugi Y, Iwata T and
Kanai T: Successful radiotherapy for recurrent obstructive
pancreatitis secondary to pancreatic metastasis from cervical
squamous-cell carcinoma. Clin J Gastroenterol. 16:755–760. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ye H, Yi XL and Li XH: A case report of
obstructive jaundice due to pancreatic metastasis from squamous
cervical carcinoma. J Clin Hep Dis. 38:646–648. 2022.
|
|
31
|
Akers A, Read S, Feldman J, Gooden C and
English DP: Diagnostic challenges and individualized treatment of
cervical adenocarcinoma metastases to the breast: A case report.
World J Clin Cases. 12:412–417. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Choi TW, Kim SH, Shin CI, Han JK and Choi
BI: MDCT findings of pancreatic metastases according to primary
tumors. Abdom Imaging. 40:1595–1607. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ardengh JC, Lopes CV, Kemp R, Venco F, de
Lima-Filho ER and dos Santos JS: Accuracy of endoscopic
ultrasound-guided fine-needle aspiration in the suspicion of
pancreatic metastases. BMC Gastroenterol. 13:632013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tella SH, Kommalapati A, Yadav S,
Bergquist JR, Truty MJ, Durgin L, Ma WW, Cleary SP, McWilliams RR
and Mahipal A: Survival and prognostic factors in patients with
pancreatic squamous cell carcinoma. Eur J Surg Oncol. 45:1700–1705.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Makarova-Rusher OV, Ulahannan S, Greten TF
and Duffy A: Pancreatic squamous cell carcinoma: A population-based
study of epidemiology, clinicopathologic characteristics and
outcomes. Pancreas. 45:1432–1437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim NI and Lee JS: Greater specificity of
p40 compared with p63 in distinguishing squamous cell carcinoma
from adenocarcinoma in effusion cellblocks. Cytojournal. 17:132020.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bao H, Zhao Y, Zhang X, Bi H, Cong S, Fang
L, Wang HJ and Wang L: HPV-negative high-grade cervical
precancerous lesions or invasive cancer in China: A post hoc
analysis of a multicentric clinical study. Int J Gynecol Obstet.
161:159–167. 2022. View Article : Google Scholar
|
|
38
|
Paya A, Alenda C, Perez-Carbonell L, Rojas
E, Soto JL, Guillén C, Castillejo A, Barberá VM, Carrato A,
Castells A, et al: Utjlity Of p16 immunohistochemistry for the
identification of Lynch syndrome. Clin Cancer Res. 15:3156–3162.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Shafique M, Shoaib I, Aslam B, Khalid R,
Tanvir I, Rasool MH, Shah TA, Almaary KS, Bourhia M and Qamar MU:
Detection of high-risk human papillomavirus infected cervical
biopsies samples by immunohistochemical expression of the p16 tumor
marker. Arch Microbiol. 206:172023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Mou H, Yu L, Zheng X, Liao Q, Hou X and Wu
Y: p16 gene expression in pancreatic cancer tissue and its
importance in diagnosis. J Biol Regul Homeost Agents. 31:1043–1047.
2017.PubMed/NCBI
|
|
41
|
Zińczuk J, Zaręba K, Guzińska-Ustymowicz
K, Kędra B, Kemona A and Pryczynicz A: p16, p21, and p53 proteins
play an important role in development of pancreatic intraepithelial
neoplastic. Ir J Med Sci. 187:629–637. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Pacheco-Barcia V, Custodio-Cabello S,
Carrasco-Valero F, Palka-Kotlowska M, Mariño-Mendez A,
Carmona-Bayonas A, Gallego J, Martín AJM, Jimenez-Fonseca P and
Cabezon-Gutierrez L: Systemic inflammation response index and
weight loss as prognostic factors in metastatic pancreatic cancer:
A concept study from the PANTHEIA-SEOM trial. World J Gastrointest
Oncol. 16:386–397. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yang L, Su J, Wang W and Zhou F: The
efficacy and safety of Nab-paclitaxel plus gemcitabine versus
mFOLFIRINOX in the first-line treatment of metastatic pancreatic
cancer: A retrospective study. World J Surg Oncol. 21:192023.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wainberg ZA, Melisi D, Macarulla T, Cid
RP, Chandana SR, De La Fouchardière C, Dean A, Kiss I, Lee WJ,
Goetze TO, et al: NALIRIFOX versus nab-paclitaxel and gemcitabinein
treatment-naive patients with metastaticpancreatic ductal
adenocarcinoma (NAPOLI 3): A randomised, open-label, phase 3 trial.
Lancet. 402:1272–1281. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shui L, Cheng K, Li X, Shui P, Zhou X, Li
J, Yi C and Cao D: Study protocol for an open-label, single-arm,
phase Ib/II study of combination of toripalimab, nab-paclitaxel,
and gemcitabine as the first-line treatment for patients with
unresectable pancreatic ductal adenocarcinoma. BMC Cancer.
20:6362020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wainberg ZA, Hochster HS, Kim EJ, George
B, Kaylan A, Chiorean EG, Waterhouse DM, Guiterrez M, Parikh A,
Jain R, et al: Open-label, phase I study of nivolumab combined with
paclitaxel plus gemcitabine in advanced pancreatic cancer. Clin
Cancer Res. 26:4814–4822. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Cheng K, Lv WR, LI X, Tian B and Cao D:
Toripalimab with nabpaclitaxel/gemcitabine as first line treatment
for advanced pancreatic adenocarcinoma: Updated results of a single
arm, open label, phase Ib/II clinical study. J Clin Oncol. 39
(Suppl 15):e162132021. View Article : Google Scholar
|
|
48
|
Perez CA and Brady LW: Perez and Brady's
Principles and Practice of Radiation Oncology. 7th edition.
Lippincott Williams & Wilkins; Philadelphia, PA: 2018
|
|
49
|
Azad A, Lim SY, D'Costa Z, Jones K, Diana
A, Sansom OJ, Kruger P, Liu S, McKenna WG, Dushek O, et al: PD-L1
blockade enhances response of pancreatic ductal adenocarcinoma to
radiotherapy. EMBO Mol Med. 9:167–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chen IM, Johansen JS, Theile S, Hjaltelin
JX, Novitski SI, Brunak S, Hasselby JP, Willemoe GL, Lorentzen T,
Madsen K, et al: Randomized phase II study of nivolumab with or
without ipilimumab combined with stereotactic body radiotherapy for
refractory metastatic pancreatic cancer (CheckPAC). J Clin Oncol.
40:3180–3189. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Golan T, Hammel P, Reni M, Van Cutsem E,
Macarulla T, Hall MJ, Park JO, Hochhauser D, Arnold D, Oh DY, et
al: Maintenance olaparib for germline BRCA-mutated metastatic
pancreatic cancer. N Engl J Med. 381:317–327. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
National Comprehensive Cancer
Network-NCCN, . Guidelines for Non-Small Cell Lung Cancer. Version
2.2020. Available from:. https://www.nccn.orgJuly 15–2024
|
|
53
|
Yılmaz F, Yaşar S, Mandel NM, Kaçan T,
Özdemir M, Doğu GG, Şengül N, Meydan N, Başal FB, Tolunay PK, et
al: Real-Life experience with entrectinib in neurotrophic tyrosine
receptor kinase fusion-positive solid tumors: A multicenter
retrospective trial. Target Oncol. 19:957–964. 2023. View Article : Google Scholar
|
|
54
|
Yue S, Zhang Y and Zhang W: Recent
advances in immunotherapy for advanced biliary tract cancer. Curr
Treat Option Oncol. 25:1089–1111. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Pajewska M, Partyka O, Czerw A, Deptała A,
Cipora E, Gąska I, Wojtaszek M, Sygit K, Sygit M, Krzych-Fałta E,
et al: Management of metastatic pancreatic cancer-comparison of
global guidelines over the last 5 years. Cancers (Basel).
15:44002023. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Sperti C, Pasquali C, Liessi G, Pinciroli
L, Decet G and Pedrazzoli S: Pancreatic resection for metastatic
tumorsto the pancreas. J Surg Oncol. 83:161–166. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Akashi Y, Saiura A, Kishi Y, Koga R,
Morimura R, Yoshioka R, Yamamoto J and Yamaguchi T: Outcome after
surgical resection of isolated metastases to the pancreas.
Hepatogastroenterology. 57:1549–1552. 2010.PubMed/NCBI
|
|
58
|
Lou H, Cai H, Huang X, Li G, Wang L, Liu
F, Qin W, Liu T, Liu W, Wang ZM, et al: Cadonilimab combined with
chemotherapy with or without bevacizumab as first-line treatment in
recurrent or metastatic cervical cancer (COMPASSION-13): A phase 2
study. Clin Cancer Res. 30:1501–1508. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Lan C, Lu H, Zhou L, Liao K, Liu J, Xie Z,
Liang H, Zou G, Yang T, Xu Q and Huang X: Long-term survival
outcomes and immune checkpoint inhibitor retreatment in patients
with advanced cervical cancer treated with camrelizumab plus
apatinib in the phase II CLAP study. Cancer Commun (Lond).
44:654–669. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Wlodarczyk JR and Lee SW: New frontiers in
management of early and advanced rectal cancer. Cancers (Basel).
14:9382022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Kumar L, Harish P, Malik PS and Khurana S:
Chemotherapy and targeted therapy in the management of cervical
cancer. Curr Probl Cancer. 42:120–128. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Godoy-Ortiz A, Plata Y, Alcaide J, Galeote
A, Pajares B, Saez E, Alba E and Sánchez-Muñoz A: Bevacizumab for
recurrent, persistent or advanced cervical cancer: Reproducibility
of GOG 240 study results in ‘real world’ patients. Clin Transl
Oncol. 20:922–927. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Choi C, Yoo GS, Cho WK and Park HC:
Optimizing radiotherapy with immune checkpoint blockade in
hepatocellular carcinoma. World J Gastroenterol. 25:2416–2429.
2019. View Article : Google Scholar : PubMed/NCBI
|