Introduction
Immune checkpoint inhibitors (ICIs) have emerged as a powerful class of drugs capable of treating various malignant tumors by reactivating the immune system's ability to recognize and eliminate cancer cells (1). These agents, which target pathways such as programmed cell death 1 (PD-1)/programmed death ligand 1 (PD-L1) or cytotoxic T-lymphocyte associated protein 4, have shown significant clinical benefits in a range of cancers (1). With their increasing use, the concept of ICI rechallenge-reintroducing the same or a different ICI after disease progression or discontinuation-has attracted growing attention (2). Several retrospective studies and case series have explored this approach, suggesting that certain patients may benefit from ICI rechallenge. However, the data remain inconsistent and inconclusive, with reports of both positive and negative outcomes (2–7), highlighting the need for further research.
In recent years, ICIs have also demonstrated efficacy in the treatment of hepatocellular carcinoma, offering new hope for patients with this difficult-to-treat cancer (1). Despite this progress, the role of ICI rechallenge in hepatocellular carcinoma remains elusive and clinical evidence is still limited. In the current study, a case of a patient with advanced hepatocellular carcinoma was presented who responded favorably to tislelizumab treatment after previously receiving other ICIs, including Camrelizumab. This case contributes to the growing body of evidence suggesting that ICI rechallenge may be a treatment strategy for selected patients with hepatocellular carcinoma.
Case report
A 50-year-old man was admitted in August 2020 due to a large mass (11.8 cm in diameter) in the liver discovered during an abdominal CT scan, suggesting the possibility of hepatocellular carcinoma. Alpha-fetoprotein (AFP) levels were 978.5 ng/ml (normal range, <40 ng/ml). The patient does not consume alcohol and tested positive for hepatitis B virus. The patient underwent a radical hepatectomy and postoperative pathology confirmed hepatocellular carcinoma (Fig. 1A); the H&E staining was performed according to standard protocols. CT findings also indicated that the tumor had been successfully removed (Fig. 1B). Following the surgery, the patient received transhepatic arterial chemotherapy and embolization (TACE) in October 2021, January 2021 and April 2021. In April 2021, the patient temporarily relocated to another state, where a CT scan was performed at a local hospital. While there was no access to the imaging files, the report from that hospital indicated a complete response of the primary liver lesion, along with the emergence of multiple small pulmonary nodules consistent with metastatic hepatocellular carcinoma. The largest nodule, measuring 1 cm in diameter, was found in the anterior segment of the left upper lobe. The patient was subsequently treated with Lenvatinib, a multi-kinase inhibitor targeting vascular endothelial growth factor receptor (VEGFR)1-3, fibroblast growth factor receptor 1–4, platelet-derived growth factor receptor-alpha, c-Kit and the RET proto-oncogene. It is used as the first-line therapy for metastatic hepatocellular carcinoma. In October 2021, the patient began second-line treatment with Apatinib (VEGFR2 inhibitor) combined with Camrelizumab (PD-1 inhibitor), as the size of the lung metastasis had increased after Lapatinib therapy. Apatinib combined with Camrelizumab showed a trend of halting the progression of metastasis.
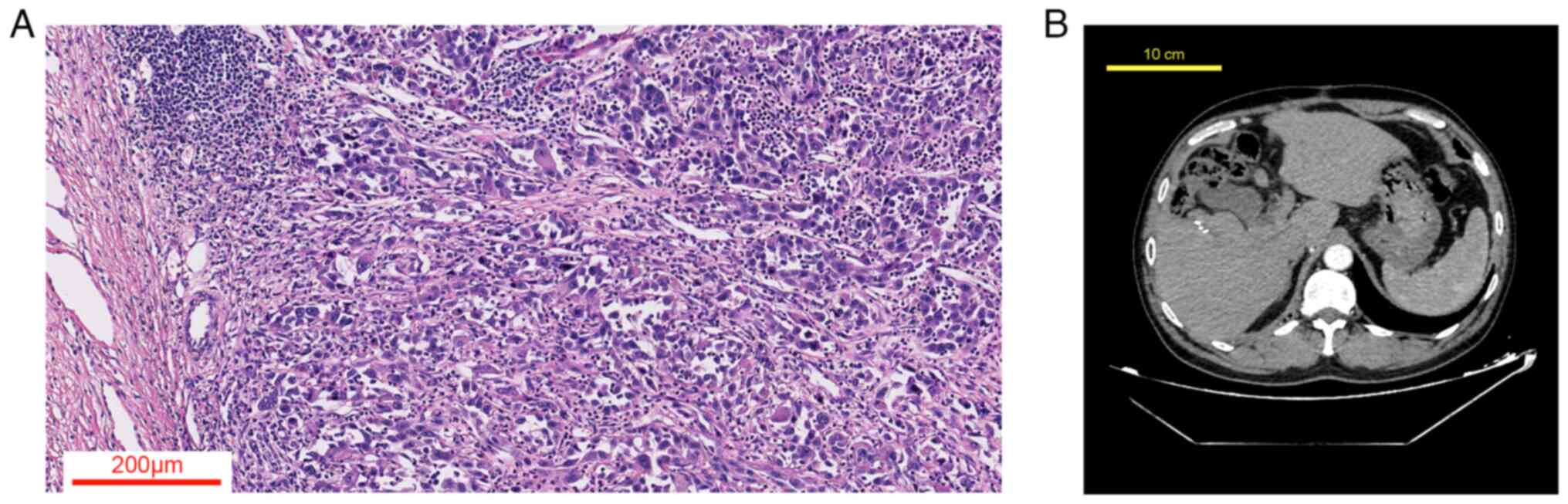 |
Figure 1.
(A) Histological section of hepatocellular carcinoma showing neoplastic cells with marked cellular and nuclear pleomorphism, irregular chromatin, prominent nucleoli and lymphocytic infiltration (H&E; magnification, ×100). (B) Post-hepatectomy CT scan of the patient.
|
In August 2021, the CT scan showed no recurrence of the primary liver tumor and the lung metastasis was regressing/stable (Fig. 2A). The patient's serum AFP level was 110.77 ng/ml. However, in December 2021, the size of the lung metastasis increased, but the primary liver tumor continued to be a complete regression without any recurrence (Fig. 2B). At this point, the patient's serum AFP level had increased to 565.02 ng/ml. Consequently, third-line therapy for lung metastasis with Regorafenib (targets both VEGFR2 and tyrosine kinase with immunoglobulin-like and EGF-like domains 2) was started, which effectively stabilized the lung metastasis, as indicated by a follow-up CT scan in March 2021 (tumor size appeared comparable to that shown in Fig. 2B; image not shown). However, a CT scan in July 2022 confirmed progressive disease (PD) of the lung metastases, which was the third time PD was observed (Fig. 2C). The patient's serum AFP level was high at 1,210 ng/ml. The patient received treatment with Tislelizumab combined with five cycles of Xelox chemotherapy, including Oxaliplatin (130 mg/m2, day 1) and Capecitabine (1,000 mg/m2, twice per day, days 1–14) with three weeks in between cycles. After completing the fourth cycle of Xelox, the lung metastasis had completely regressed (Fig. 2D), and the patient's serum AFP level had decreased to 2.17 ng/ml. However, the patient declined to continue his treatment due to financial reasons and was discharged in January 2023.
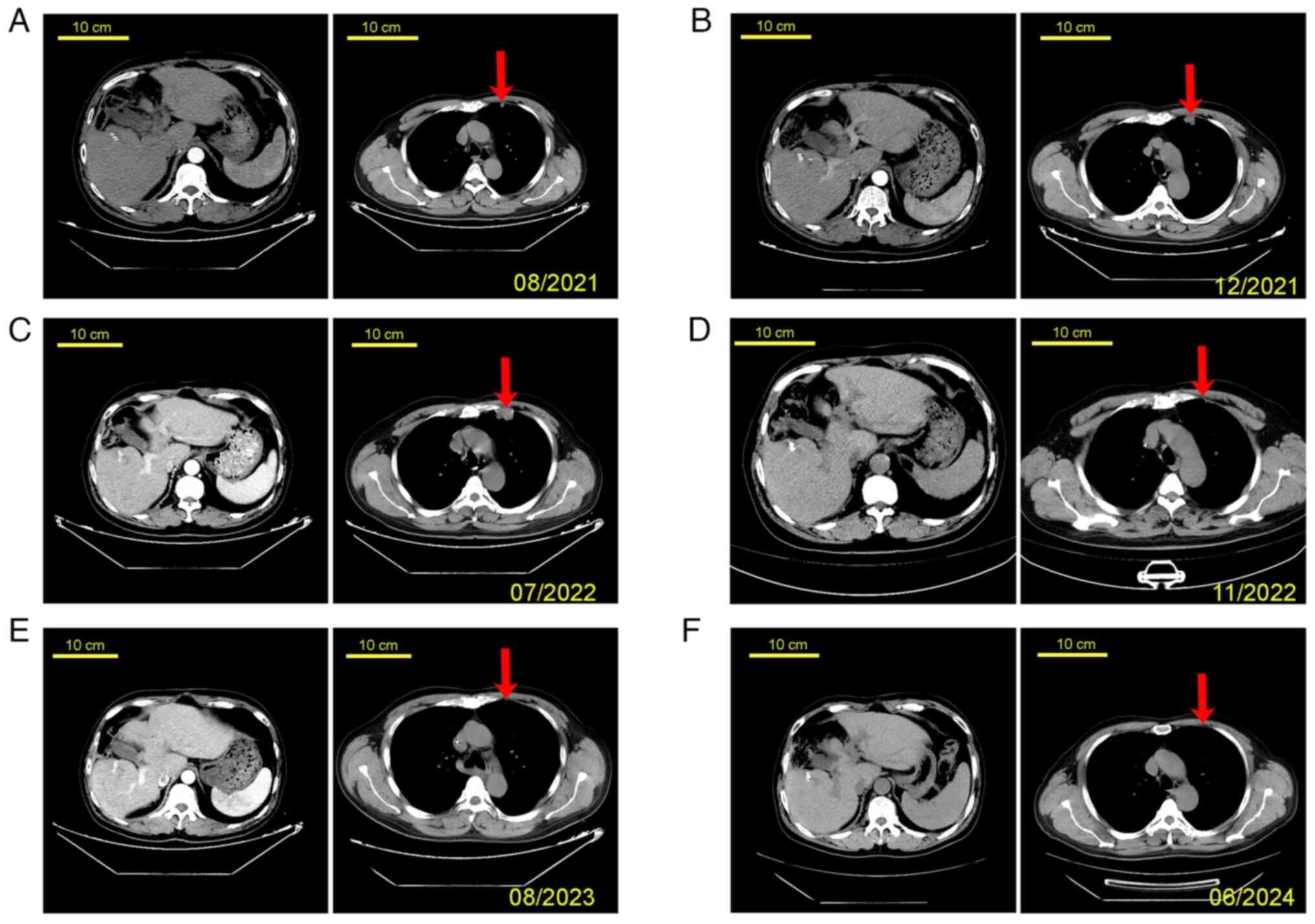 |
Figure 2.
Patient's CT scanning images at different time-points. (A) August 2021; (B) December 2021; (C) July 2022; (D) November 2022; (E) August 2023; and (F) June 2024 (scale bars, 10 cm; the arrows point to the metastatic tumor in the left lung). The images on the left show abdominal CT scans and the images on the right display chest CT scans.
|
In August 2023, the patient returned for a follow-up visit. The disease remained in complete remission (Fig. 2E), with an AFP level of 0.97 ng/ml, and maintenance therapy with Tislelizumab was continued. The patient is being managed by 3-month follow-ups. As of June 2024, at the conclusion of the study, the patient has been progression-free for 23 months (Fig. 2F) with AFP levels at 0.80 ng/ml.
Discussion
The present case study reported on a patient who was initially diagnosed with hepatocellular carcinoma. After undergoing surgery and TACE treatment, the primary liver lesion showed complete regression, but the patient later developed lung metastasis. Although the metastatic tumor in the lungs progressed despite three different therapies, including Lenvatinib, Apatinib combined with Camrelizumab and Regorafenib, a combination of immune rechallenge with chemotherapy (Xelox) led to a progression-free survival (PFS) of 23 months.
Immune rechallenge is a treatment approach that involves re-administering the same therapeutic class of medication to patients with progressive disease after they have previously shown a clinical benefit from treatment for unresectable or metastatic cancer. This strategy aims to help select patients achieve additional tumor regression, particularly those who may still have responsive cancer cells remaining after the initial treatment period (8).
In the present study, immune rechallenge therapy combined with Xelox was selected for several reasons. Firstly, Tislelizumab offers unique biological advantages; studies have shown that tislelizumab demonstrates effective anti-tumor activity in the posterior line therapy of hepatocellular carcinoma (9). Secondly, the Xelox regimen has promising efficacy and safety in treating hepatocellular carcinoma (10). Lastly, PD-1 inhibitors combined with oxaliplatin-based chemotherapy exhibit effective anti-tumor activity in treating advanced hepatocellular carcinoma (11).
It is known that the biomarkers associated with the efficacy of immunotherapy include PD-L1 expression levels, microsatellite instability-high or mismatch repair defect and tumor mutational burden. In other types of cancer, the relationship between the effectiveness of PD-1/PD-L1 inhibitors and biomarkers has been established (12,13). However, there is currently no suitable biomarker to identify the most beneficial recipients for treating hepatocellular carcinoma Although numerous biomarkers have been identified for prognosis or treatment choice (14), the coexistence of chronic liver disease and inflammation limits the accuracy of several tumor biomarkers, making them unsuitable for daily practice, except for AFP. Other biomarkers have shown associations with poor prognosis in various hepatocellular carcinoma stages and post-treatment assessments. However, selecting candidates for therapy based solely on these biomarkers is not yet clinically applicable. Ideal biomarkers should allow for early diagnosis and better candidate identification for each tumor stage and treatment modality (15).
It is to be noted that PD-L1 expression was not detected in this case due to economic constraints. The cost of biomarker analysis and limited access to advanced tests can hinder personalized treatment, particularly in resource-limited settings. Economic factors significantly influence treatment choices, as patients may be unable to afford necessary tests. Consequently, clinicians often make decisions based on available resources.
The development of primary and acquired resistance to ICIs involves various intricate factors. Tumor-related factors include low immunogenicity, loss of new antigens due to immune editing, abnormal antigen presentation, resistance to immune effector molecules, formation of vascular barriers to impede CD8+ T-cell infiltration and epithelial-mesenchymal transition. Host-related factors involve T-cell inhibition due to severe exhaustion, co-inhibitory receptors, metabolites, reduced formation of memory T cells and the influence of enteric microbiota (16,17).
Tislelizumab, a unique PD-1 antibody, offers four distinct pharmaceutical advantages (18): i) Its more significant Fab segment binding site to PD-1 allows for complete blocking of PD-1 to PD-L1 binding, with a slower dissociation rate and higher affinity to PD-1; ii) Tislelizumab's Fc modification removes its ability to bind to FcγR, thereby eliminating antibody-dependent cell-mediated phagocytosis and avoiding T-cell count reduction, which would otherwise affect its antitumor efficacy; and iii) Tislelizumab boasts low IC50 and EC50 values and a longer half-life of 26 days, the longest among similar drugs. It promotes T-cell activation and demonstrates potent antitumor activity.
The median PFS for hepatocellular carcinoma in first-line or second-line treatment is less than nine months (19). Specifically, the median PFS of chemotherapy alone in hepatocellular carcinoma is less than five months (20). However, the PFS data of immune rechallenge combined with chemotherapy in this case greatly surpassed the median data of other studies, indicating its potential as an alternative for posterior-line therapy for hepatocellular carcinoma.
In conclusion, as presented in this case, immune rechallenge combined with chemotherapy showed great potential for curing metastatic hepatocellular carcinoma.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
XT, LK, HC, XL and CD contributed to the preparation of this case report, they were responsible for the clinical management of the patient and drafted the manuscript. XT and CD reviewed the manuscript and confirm the authenticity of all raw data. CD supervised the project. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
This project was approved by the Ethics Committee of Dujiangyan People's Hospital (Chengdu, China).
Patient consent for publication
The patient has provided written consent for the publication of this study, including case information and images.
Competing interests
The authors declare that they have no competing interests.
References
|
1
|
Llovet JM, Castet F, Heikenwalder M, Maini MK, Mazzaferro V, Pinato DJ, Pikarsky E, Zhu AX and Finn RS: Immunotherapies for hepatocellular carcinoma. Nat Rev Clin Oncol. 19:151–172. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gobbini E, Charles J, Toffart AC, Leccia MT, Moro-Sibilot D and Giaj Levra M: Current opinions in immune checkpoint inhibitors rechallenge in solid cancers. Crit Rev Oncol Hematol. 144:1028162019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Inno A, Roviello G, Ghidini A, Luciani A, Catalano M, Gori S and Petrelli F: Rechallenge of immune checkpoint inhibitors: A systematic review and meta-analysis. Crit Rev Oncol Hematol. 165:1034342021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kagohashi K, Miyazaki K, Shiozawa T and Satoh H: Pseudoprogression during successful rechallenge of immune checkpoint inhibitor in a NSCLC patient. Adv Respir Med. 89:316–319. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Plazy C, Hannani D and Gobbini E: Immune checkpoint inhibitor rechallenge and resumption: A systematic review. Curr Oncol Rep. 24:1095–1106. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Takemura K, Yamanaka T, Hayashida M, Kizawa R, Yamaguchi T, Tanabe Y, Sakaguchi K, Suyama K, Urakami S and Miura Y: Bell's palsy during rechallenge of immune checkpoint inhibitor. IJU Case Rep. 6:144–146. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Xu M, Hao Y, Shi Z and Song Z: Efficacy of rechallenge immunotherapy after immune monotherapy resistance in patients with advanced non-small cell lung cancer. J Cancer Res Clin Oncol. 149:17987–17995. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zaremba A, Eggermont AMM, Robert C, Dummer R, Ugurel S, Livingstone E, Ascierto PA, Long GV, Schadendorf D and Zimmer L: The concepts of rechallenge and retreatment with immune checkpoint blockade in melanoma patients. Eur J Cancer. 155:268–280. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ren Z, Ducreux M, Abou-Alfa GK, Merle P, Fang W, Edeline J, Li Z, Wu L, Assenat E, Hu S, et al: Tislelizumab in patients with previously treated advanced hepatocellular carcinoma (RATIONALE-208): A multicenter, non-randomized, open-label, phase 2 trial. Liver Cancer. 12:72–84. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Boige V, Raoul JL, Pignon JP, Bouche O, Boucher E, Blanc JF, Seitz JF, Dupouy BN and Ducreux M; For the Fédération Francophone de Cancérologie Digestive, : Preliminary results of capecitabine-oxaliplatin (XELOX) in hepatocellular carcinoma (HCC): A phase II trial of the Fédération Francophone de Cancérologie Digestive (FFCD 2003-03 trial). J Clin Oncol. 23 (16 Suppl):S41282005. View Article : Google Scholar
|
|
11
|
Qin S, Chen Z, Liu Y, Xiong J, Ren Z, Meng Z, Gu S, Wang L and Zou J: A phase II study of anti-PD-1 antibody camrelizumab plus FOLFOX4 or GEMOX systemic chemotherapy as first-line therapy for advanced hepatocellular carcinoma or biliary tract cancer. J Clin Oncol. 37 (15 Suppl):S40742019. View Article : Google Scholar
|
|
12
|
Bie F, Tian H, Sun N, Zang R, Zhang M, Song P, Liu L, Peng Y, Bai G, Zhou B and Gao S: Research progress of Anti-PD-1/PD-L1 immunotherapy related mechanisms and predictive biomarkers in NSCLC. Front Oncol. 12:7691242022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin X, Zong C, Zhang Z, Fang W and Xu P: Progresses in biomarkers for cancer immunotherapy. MedComm (2020). 4:e3872023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chan YT, Zhang C, Wu J, Lu P, Xu L, Yuan H, Feng Y, Chen ZS and Wang N: Biomarkers for diagnosis and therapeutic options in hepatocellular carcinoma. Mol Cancer. 23:1892024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Piñero F, Dirchwolf M and Pessôa MG: Biomarkers in hepatocellular carcinoma: Diagnosis, prognosis and treatment response assessment. Cells. 9:13702020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
O'Donnell JS, Long GV, Scolyer RA, Teng MWL and Smyth MJ: Resistance to PD1/PDL1 checkpoint inhibition. Cancer Treat Rev. 52:71–81. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Syn NL, Teng MWL, Mok TSK and Soo RA: De-novo and acquired resistance to immune checkpoint targeting. Lancet Oncol. 18:e731–e741. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Geng Z, Hao B and Geng Q: Tislelizumab: A modified anti-tumor programmed death receptor 1 antibody. Cancer Control. 29:107327482211112962022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Vogel A, Meyer T, Sapisochin G, Salem R and Saborowski A: Hepatocellular carcinoma. Lancet. 400:1345–1362. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu L, Zheng YH, Han L and Qin SK: Efficacy and safety of the oxaliplatin-based chemotherapy in the treatment of advanced primary hepatocellular carcinoma: A meta-analysis of prospective studies. Medicine (Baltimore). 95:e49932016. View Article : Google Scholar : PubMed/NCBI
|
















