Introduction
Oral diseases rank highly amongst widespread health
conditions worldwide, and impose notable health and economic
burdens, which substantially diminish the quality of life of
patients, impacting overall health and well-being (1). Oral cancer is a generic term used to
refer to malignant tumors in oral organs. Notably, oral cancer
encompasses cancer types of the lip, and all subsites of the oral
cavity and oropharynx (2).
According to the Global Cancer Statistics 2022, oral cancer was the
16th most common malignancy and the 15th leading cause of mortality
worldwide (3), which was similar to
the rankings observed in 2018 (4).
The incidence of oral cancer is 2.72 cases per 100,000 individuals
in China (men, 3.87; women, 1.60). Notably, the aforementioned rate
is lower compared with the global incidence rate (men, 5.8; women,
2.3) (3,5). Oral squamous cell carcinoma (OSCC) is
the most prevalent and extensively researched type of oral cancer,
and the predominant malignancy within the head and neck region
(6,7). In China, a total of 65,400 new cases
of oral and pharyngeal cancer occurred in 2022 (5). The prevalence of oral cancer is
projected to increase by ~40% by 2040 and this may lead to an
increase in mortality rates in the future (5). Thus, numerous studies have focused on
the molecular mechanisms underlying tumor growth, invasion,
migration and distant metastasis, with the aim of identifying novel
therapeutic targets and key tumor markers in OSCC. Previous studies
have demonstrated that deubiquitinating enzymes (DUBs) serve an
important role in the development of OSCC (8,9). The
present review aims to investigate the functional mechanisms,
tumorigenic regulation and therapeutic targets of DUBs in OSCC,
which may provide a novel theoretical basis for the diagnosis and
treatment of oral cancer in the future.
Oral carcinogenesis
In total, ~90% of oral cancer types originate in the
stratified non-keratinized epithelium of the oral mucosa (10). Notably, oral cancer may be caused by
genetic, epigenetic and environmental factors, including tobacco,
alcohol and poor nutrition, which lead to changes in oral
keratinocytessize and shape (10).
Early genetic and molecular alterations of oral keratinocytes occur
in all tissue areas exposed to carcinogens, followed by varying
degrees of damage to the epithelium, which may lead to oral
epithelial carcinoma and metastasis (Fig. 1) (11). A previous study has demonstrated
that the development of OSCC may also be caused by additional
factors, such as autoimmune diseases, infectious diseases,
immunosuppressive disorders and familial cancer syndromes that
modulate the immune system (12).
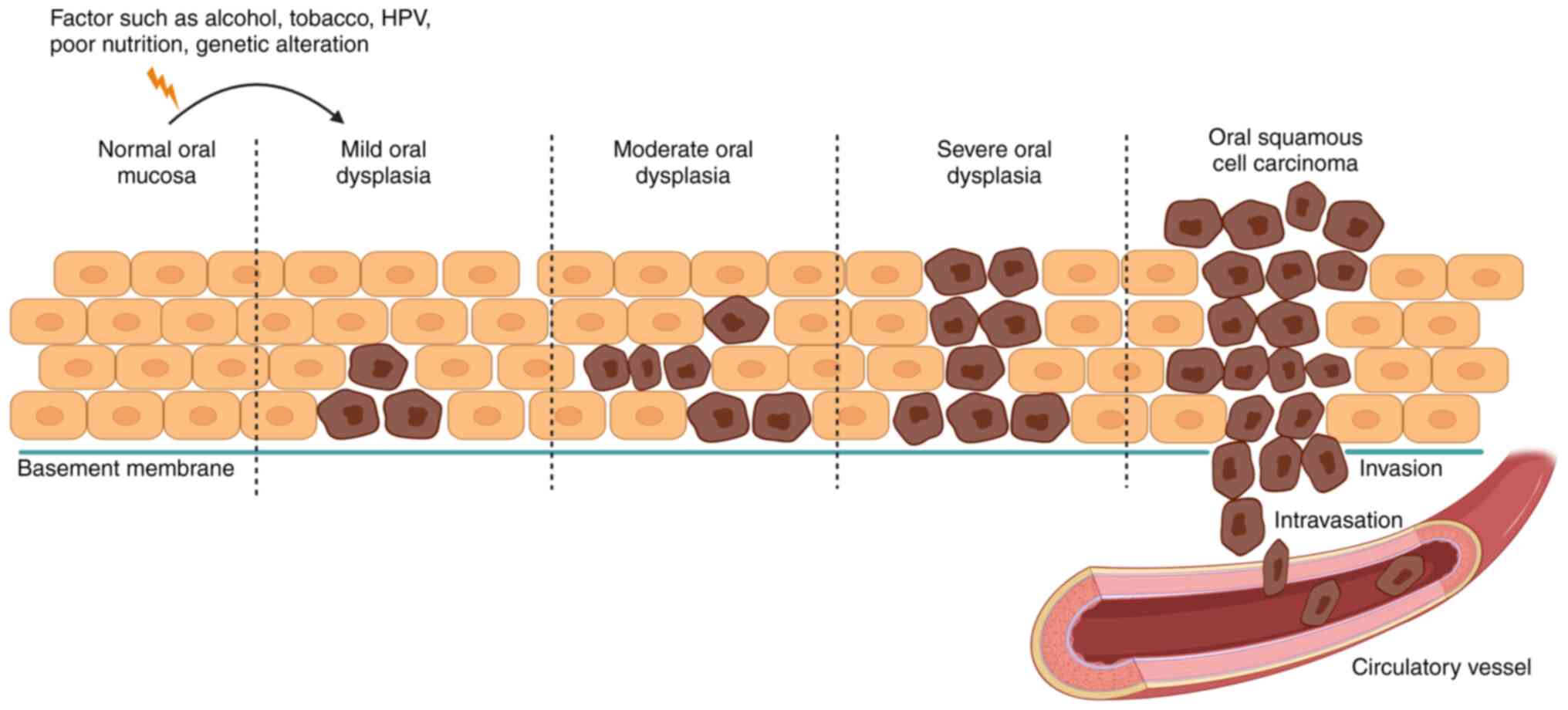 | Figure 1.Schematic diagram of oral
carcinogenesis. The normal oral mucosa is a layer of epithelial
cells arranged on a basement membrane that separates the epithelium
from connective tissue and blood vessels. When the oral mucosa is
stimulated by internal and external factors (genetic alterations,
tobacco, alcohol, poor nutrition), the deepest cells undergo
changes in shape and size, known as oral dysplasia. Oral dysplasia,
which can be classified into mild, moderate and severe, is
considered to precede the development of OSCC and to be a notable
predictor of malignant transformation. During OSCC development,
massive phenotypic changes occur in all epithelial cell layers and
extend towards the tissue boundaries with rupture of the basement
membrane, which invades the connective tissue and binds to the
blood vessels. OSCC, oral squamous cell carcinoma; HPV, human
papillomavirus. |
The key characteristics of oral cancer include
sustained cellular proliferation, resistance to apoptosis, invasion
and metastasis, dysregulation of energy homeostasis, evasion of
growth inhibitory signals, and the ability to circumvent
immunotherapeutic interventions (13). Oral carcinogenesis is complex and
multifactorial, involving genetic mutations, epigenetic
modifications and imbalances in the tumor microenvironment (TME).
Genetic alterations may lead to the abnormal activation of
oncogenic signaling pathways, including PI3K/AKT/mTOR (14,15),
EGFR (16), Wnt/β-catenin (17), Notch (18) and JAK/STAT (19) pathways, and simultaneously disrupt
tumor suppressor pathways, such as the tumour protein
53/retinoblastoma pathway (20).
Notably, the aforementioned alterations serve a key role in the
progression of OSCC. Furthermore, epigenetic modifications, such as
DNA methylation (21), histone
covalent modifications (22) and
chromatin remodeling (23), are
also implicated in the initiation and progression of OSCC.
Additional factors such as immune suppression (24), hypoxia (25) and imbalances in the oral microbiome
(26) may also contribute to the
dysregulated TME, thus facilitating OSCC progression.
Diagnosis and treatment of oral cancer
In clinical practice, patients with OSCC may present
with early-stage lesions that are painless. However, as OSCC
progresses, lesions may cause ulceration, nodules and tissue
adherence (27). In total, ~50% of
OSCC cases arise in the posterior lateral border of the tongue,
with the remaining cases affecting the floor of the mouth, soft
palate, gingiva, buccal mucosa and hard palate (28). Oral cancer is detected in clinical
examinations; however, >50% of patients with OSCC are diagnosed
during the advanced stages of the disease (stages III and IV) and
>40% of patients with OSCC present with regional metastases at
the time of diagnosis (28).
Furthermore, OSCC may invade the ipsilateral cervical lymph nodes
through lymphatic outflow or invade the contralateral or bilateral
lymph nodes. Notably, the lungs, bones and liver are the main sites
of OSCC metastasis (29).
At present, surgery is the primary treatment option
for OSCC; however, adequate resection margins are difficult to
achieve due to the complex anatomy of the affected area (13). Ionizing radiation (IR),
immunotherapy and chemotherapy may be used to prevent or treat OSCC
(13). Thus, the identification of
novel biomarkers and therapeutic targets in OSCC is necessary. A
recent systematic review has summarized the hallmarks of oral
cancer and highlighted the importance of further studies focused on
OSCC (30). In addition, numerous
mono-antibodies or small molecular compounds that inhibit
tumorigenesis have been developed. PRI-724, a specific inhibitor of
the Wnt/β-catenin signaling pathway, works synergistically with
vismodegib, erlotinib and HS-173 to effectively decrease cell
viability, promote apoptosis and decrease cell migration in OSCC
(31). Cetuximab, an EGFR-targeting
antibody, may be used to enhance the antitumor function of PI3K/AKT
inhibitors (32). Current research
on oral cancer focuses on the role of DUBs, which exhibit potential
as molecular targets in the treatment of oral cancer (8,9).
Ubiquitination and deubiquitination
The sequential enzymatic processes that covalently
attach ubiquitin, a 76-residue polypeptide with a molecular mass of
~8.5 kDa, to target proteins, are known as ubiquitylation.
Ubiquitylation is achieved through a mechanism that involves
several factors, including ubiquitin-activating enzyme (E1),
ubiquitin-binding enzyme (E2) and ubiquitin ligase (E3). In humans,
there are two variants of E1 enzymes, namely, ubiquitin-like
modifier activating enzyme 1 and ubiquitin-like modifier activating
enzyme 6, alongside ~50 distinct E2 enzymes and ~600 different E3
enzymes. Notably, E3 enzymes are pivotal in the selective
identification of target proteins for ubiquitination and operate in
a manner that is both spatially and temporally specific (33). Ubiquitin contains seven lysine
residues and an N-terminal region that function as a site for
ubiquitination, specifically at positions K6, K11, K27, K29, K33,
K48, K63 and M1. Ubiquitin chains bind to substrates by linking the
glycine residue of ubiquitin to a lysine molecule of ubiquitin
(34). Different linkages exhibit
different roles for the target substrate. Notably, K48-linked
chains represent the most prevalent type of ubiquitin linkage
within cellular environments, accounting for >50% of all
ubiquitin linkages (33). The
primary function of K48-linked chains is to facilitate the
targeting of proteins to the proteasome for degradation. By
contrast, K63-linked chains, which are the second most abundant
type of ubiquitin linkages, exhibit a range of non-degradative
functions (33). Ubiquitination
serves a key role in numerous pathological conditions, such as
neurodegenerative diseases, various cancers, aging and metabolic
disorders (35). Alternate atypical
ubiquitin modifications, linked through M1, K6, K11, K27, K29 or
K33, also exhibit unique functions in substrate modification
(36). Variations in the use of
ubiquitin lysine residues may lead to the formation of homotypic
chains, which are linked exclusively through a single type of
residue, or heterotypic and branched chains. The aforementioned
processes are exemplified by K63-linear and K48-K11 hybrid
polymers, respectively (37).
DUBs are a class of proteases that facilitate the
reversal of protein ubiquitination, a critical process for
maintaining healthy cellular homeostasis. DUBs are responsible for
the removal of ubiquitin from target proteins, which enables the
recycling of ubiquitin, mediated by ~100 distinct DUBs (38). Ubiquitin molecules may be conjugated
to the N-terminal amino group or lysine residues on other ubiquitin
molecules, which results in the formation of ubiquitin chains
(39). DUBs possess the ability to
dismantle ubiquitin conjugations by cleaving the linkages between
ubiquitin molecules or processing ubiquitin precursors to produce
free pools of ubiquitin (Fig. 2A)
(39). In total, there are ~100
DUBs that are classified into eight different families, namely,
ubiquitin specific protease (USP), ubiquitin carboxy-terminal
hydrolase, JAB1/MPN/MOV34 metalloenzyme, ovarian tumor protease
(OTU), motif interacting with ubiquitin-containing novel DUB,
monocyte chemotactic protein-induced proteins zinc
finger-containing ubiquitin peptidase 1 and Machado-Joseph disease
(Fig. 2B) (40,41).
Furthermore, DUBs exhibit four distinct mechanisms of action,
namely, processing of ubiquitin precursors, recycling of ubiquitin
molecules during ubiquitination, cleavage of poly-ubiquitin chains
and reversal of ubiquitin conjugation (42). The aforementioned mechanisms are
used to regulate several cellular functions, including cell cycle
progression, vesicle transport, signal transduction and chromosome
segregation (43). DUBs also serve
key roles in various developmental processes of eukaryotic cells,
including apoptosis (44), DNA
damage repair (45), maintenance of
cell stemness (46) and
tumorigenesis (47). Thus, the
association between ubiquitination and DUBs is essential for
cellular homeostasis.
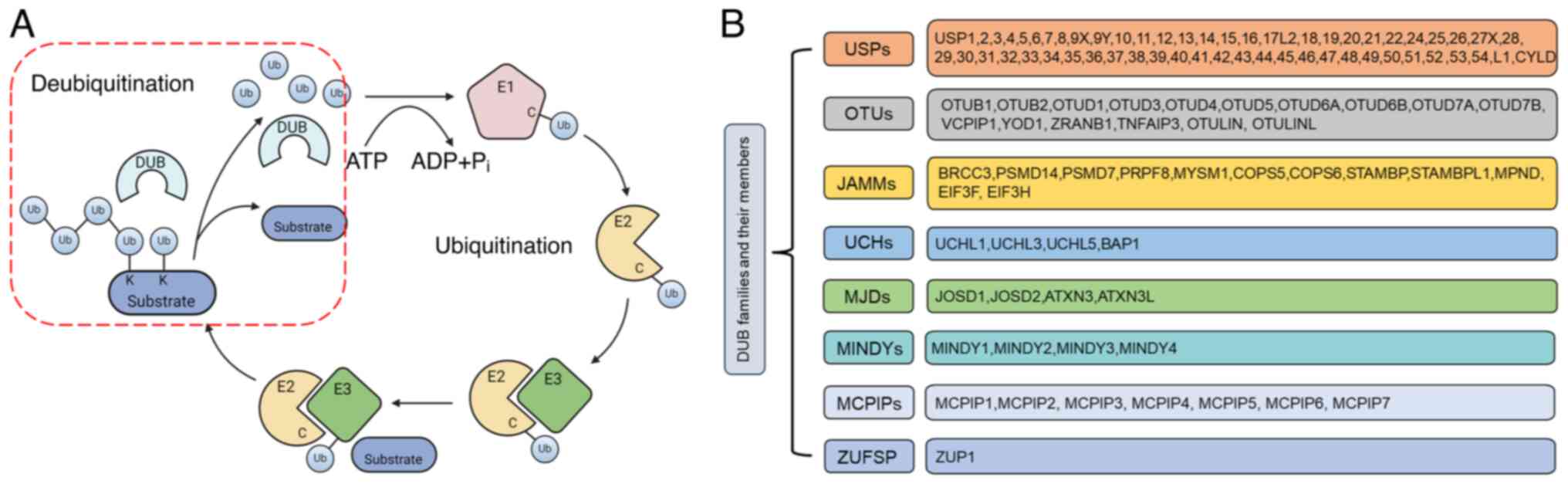 | Figure 2.Ubiquitylation cascade and
classification of DUB families. (A) Schematic diagram of key events
in ubiquitylation and deubiquitylation. Under the condition that
ATP provides energy, ubiquitin binds to the target protein (E1, E2,
E3) through the cascade catalytic reaction. The DUBs cleave the
monoubiquitin or polyubiquitin chains from ubiquitinated proteins.
(B) Classification of DUB families and members. In total, ~100 DUBs
are classified into eight different families. DUBs edit ubiquitin
chains to exert either tumor-suppressive or oncogenic effects on
target substrates, which is dependent on the DUB, context and
substrate. DUB, deubiquitinating enzyme; Ub, ubiquitin; E1,
ubiquitin-activating enzyme; E2, ubiquitin-binding enzyme; E3,
ubiquitin ligase; USP, ubiquitin specific protease; UCH, ubiquitin
carboxy-terminal hydrolase; JAMM, JAB1/MPN/MOV34; OTU, ovarian
tumor protease; MJD, Machado-Joseph disease; MINDY, motif
interacting with ubiquitin-containing novel DUB; MCPIP, monocyte
chemotactic protein-induced proteins; ZUFSP, zinc finger-containing
ubiquitin peptidase 1. |
Deubiquitination in oral cancer
Numerous DUBs may be associated with either
tumor-suppressive or oncogenic activities and exhibit potential as
candidates for therapeutic intervention. Table I highlights key studies that focus
on the regulatory mechanisms of DUBs in oral cancer under reference
summarized.
 | Table I.Summary of previously published
studies on DUBs in oral cancer. |
Table I.
Summary of previously published
studies on DUBs in oral cancer.
| First author/s,
year | Oral cancer
type | DUBs | Substrate | Summary of
results | Prognosis | (Refs.) |
|---|
| Wu et al,
2018 | OSCC | USP9X | PD-L1 | USP9X combined with
PD-L1 induced PD-L1 deubiquitination and stabilized PD-L1 protein
expression in OSCC. | Poor | (8) |
| Sulkshane et
al, 2021 |
|
| MCL1 | USP9X interacted
with and deubiquitinated MCL1, stabilizing MCL1. The upregulation
of USP9X and MCL1 was associated with poor prognosis in patients
with OSCC. | Poor | (72) |
| Shinriki et
al, 2018 | OSCC | CYLD | ALK5 | Knockdown of CYLD
induced stabilization of TGF-β receptor I (ALK5) and promoted TGF-β
signaling in a cell autonomous manner, which was associated with
the clinical features of deep invasion and poor overall survival in
invasive OSCC. | Good | (64) |
| Kanemaru et
al, 2022 |
|
| - | EGFR tyrosine
kinase inhibitor gefitinib decreased the cell survival rate by
inhibiting TGF-β signaling in cisplatin-resistant CYLD-knockdown
OSCC cells. | Good | (65) |
| Suenaga et
al, 2019 |
|
| Cisplatin | Cisplatin
resistance was mediated by CYLD downregulation. Cisplatin
resistance was associated with a decrease in the accumulation of
intracellular cisplatin and the inhibition of cisplatin-induced
apoptosis via hyperactivation of the NF-κB signaling pathway. | Good | (80) |
| Li et al,
2023 | OSCC | OTUB1 | RACK1 | Knockdown of OTUB1
suppressed cell proliferation, invasion, migration and xenograft
tumor growth, and promoted tumor-associated macrophage M1
polarization but suppressed M2 polarization, which inhibited the
survival of OSCC cells. | Poor | (73) |
| Liu et al,
2024 |
|
| SLC7A11 | TCF12 promoted
ubiquitination of SLC7A11 and decreased SLC7A11 protein stability
through transcriptional repression of OTUB1, thereby facilitating
ferroptosis. TCF12 enhanced cisplatin sensitivity in OSCC cells by
promoting ferroptosis, which was achieved by modulating SLC7A11
expression via transcriptional regulation of OTUB1. | Poor | (84) |
| Feng et al,
2020 | OSCC | USP17 | SNAI1 | The direct binding
between LINC02487 and the DUB USP17 inhibited cell migration and
invasion through the USP17-SNAI1 axis in a process that involved
epithelial-mesenchymal transition. | Good | (85) |
| Liu et al,
2024 | OSCC | USP14 | Sox2 | USP14 knockdown
impaired Sox2 stability by increasing its polyubiquitination. USP14
upregulation was associated with progression-free interval in
patients with OSCC. | Poor | (74) |
| Zhang et al,
2024 |
|
| PFKL | PFKL is a key rate
limiting enzyme involved in the glycolytic pathway. The interaction
between USP14 and PFKL improved the stability of PFKL in OSCC
cells, which enhanced PFKL-mediated glycolytic metabolism, and
promoted cellular proliferation, migration and tumorigenesis. | Poor | (75) |
| Xie and Xu,
2021 |
|
| LC3BI/II | USP14 knockdown
promoted IR-induced autophagy via the upregulation of LC3BII and
γH2AX expression levels in IR-treated OSCC cells. | Good | (81) |
| Lu et al,
2021 | OSCC | USP18/USP20 | STING | Knockdown of STING,
a verified substrate of USP18 and USP20, induced the multiplication
of T1012G virus yields in SCC9 cells. The effects of GSK2643943A, a
DUB inhibitor, targeting USP20 on viral replication and tumor death
were evaluated, both in vitro and in vivo. | Poor | (86) |
| Kobayashi et
al, 2019 | OSCC | UCHL1 | LMP1 | UCH-L1 DUB
inhibitors, LDN and LDN-POx, suppressed the motility of metastatic
OSCC and nasopharyngeal cells expressing Epstein-Barr virus
pro-metastatic LMP1 in physiological assays. Furthermore, treatment
with LDN and LDN-POx resulted in decreased levels of pro-metastatic
markers, a decrease in carcinoma cell adhesion, and inhibition of
extracellular vesicle-mediated transfer of the viral invasive
factor LMP1. | Poor | (87) |
| Chen et al,
2024 | OSCC | USP44 | HEXIM1 | Upregulation of
USP44 induced an increase in the stability of the HEXIM1 protein,
which subsequently elevated HEXIM1 expression levels in OSCC. The
silencing of HEXIM1 further exacerbated the malignant
characteristics of OSCC cells. The knockdown of HEXIM1 negated the
antitumor effects associated with USP44. USP44 functions as a
crucial tumor suppressor in OSCC via inhibition of cell
proliferation and metastasis through the stabilization of the
HEXIM1 protein. | Good | (88) |
| Chang et al,
2022 | SCC | OTUB2 | STAT1 | OTUB2 suppressed
development and progression in tongue and esophageal SCCs. OTUB2
promoted the deubiquitination, phosphorylation and dimerization of
STAT1, and induced the activation of
CALML3/Ca2+/phosphatidy lserine signaling. Oral
administration of soybean-derived phosphatidylserine inhibited SCC
initiation and progression, which was associated with low OTUB2
expression. | Good | (89) |
Association between DUBs and the
Wnt/β-catenin pathway in oral cancer
Under healthy conditions, the Wnt family of proteins
bind to Frizzled receptor and related ligands such as LDL
receptor-related protein 5/6 (LRP5/6) on the cell surface to form a
complex that recruits the protein framing protein, Dishevelled,
which leads to the phosphorylation of LRP5/6 and the recruitment
and activation of the Axin protein complex. In turn, the activation
of the Axin protein complex inhibits the phosphorylation and
degradation of β-catenin proteins and leads to stabilization.
Accumulation of β-catenin in the cytoplasm will lead to entry into
the nucleus spontaneously, where β-catenin binds to T cell
factor/lymphoid enhancer factor family proteins, which promotes the
transcription and expression of Wnt target genes, including Axin2,
c-Myc and Cyclin D1. The expression levels of Wnt target genes
serve a key role in cell proliferation, cycle regulation and
differentiation. In the absence of Wnt activation, β-catenin is
phosphorylated by the Axin protein complex, where β-catenin binds
to ubiquitin E3 ligase (β-Trcp) (48). β-Trcp is subsequently presented to
the proteasome for ubiquitination (48). To date, numerous studies have
focused on the role of Wnt in OSCC and demonstrated that components
of the Wnt/β-catenin signaling pathway, including Wnt ligands, Wnt
inhibitors, membrane receptors and intracellular mediators, serve a
key role in the inhibition of OSCC (11,49,50).
A previous study has demonstrated that USP14
activates the Wnt downstream pathway by regulating the
deubiquitination and subsequent phosphorylation of Dishevelled
(51). In OSCC tissues, USP14
expression levels are markedly upregulated (52). Furthermore, in vitro cellular
experiments and investigations using mice transplantation tumor
models have demonstrated that the proliferation, invasion and
migration of OSCC were inhibited following USP14 knockdown
(52).
Association between DUBs and the NF-κB
pathway in oral cancer
NF-κB is a transcription factor that is often
located in the cytoplasm and NF-κB regulates the expression of
various genes, impacting cellular physiology and pathology.
Activation of the NF-κB signaling pathway is often achieved through
IκB protein degradation and nuclear translocation of NF-κB
proteins, which serve key roles in inflammatory responses, immune
responses and cell survival. In the inactive state, the IκB protein
forms a complex with NF-κB, which leads to the prevention of NF-κB
nuclear translocation (53). When
inflammatory factors or cytokines stimulate the cell, the IκB
protein is ubiquitinated and degraded, which allows the release of
NF-κB protein into the nucleus to regulate the transcription of
target genes (IL-6, inducible nitric oxide synthase) (53). Activated NF-κB promotes OSCC
migration, invasion and resistance to radiotherapy (54).
DUBs regulate the NF-κB signaling pathway, which
leads to oncogenic and anti-oncogenic activity.
Receptor-interacting protein 1 (RIP1) may be modified by K63-linked
polyubiquitination, which leads to TNF-α-induced NF-κB activation,
increased expression levels of anti-apoptotic proteins [cellular
inhibitor of apoptosis protein-1/2, (cIAP1/2), Bcl-2] and the
promotion of cell survival. The DUB USP4 exerts a regulatory effect
on RIP1 and a previous study has demonstrated that USP4 was
upregulated in OSCC (55). USP4
inhibits NF-κB activation and promotes apoptosis via cleavage of
the K63 ubiquitin chain of RIP1, which leads to oncogenic activity
(56). In addition, cylindromatosis
lysine 63 deubiquitinase (CYLD) is a key negative regulator of
NF-κB. CYLD specifically removes the K63 ubiquitin chain and the M1
linear ubiquitin chain, and inhibits NF-κB signaling within
different pathways. Mutations or low expression levels of CYLD in
OSCC result in abnormal activation of NF-κB and inhibition of TGF-β
(57). Previous studies have also
demonstrated that CYLD upregulation inhibited the invasion and
metastasis of the SCC15 OSCC cell line (58,59).
Association between DUBs and the TGF-β
pathway in oral cancer
Members of the TGF-β family exert cellular effects
through the formation of heterotetrameric complexes, comprising
type I and type II serine/threonine kinase transmembrane receptors.
To date, five type II receptors and seven type I receptors,
referred to as activin receptor-like kinases (ALKs), have been
characterized. TGF-β and bone morphogenetic protein dimers induce
the formation of a heterotetrameric complex between a specific type
II receptor and a type I receptor, which leads to the
transphosphorylation and subsequent activation of the type I
receptor. Furthermore, type I receptors propagate signals into the
cell through the phosphorylation of receptor-regulated SMADs, which
form heteromeric complexes with SMAD4 (Co-SMAD) (60,61).
Co-SMAD translocates to the nucleus and interacts with other
transcription factors (p300/CBP, Snail), which leads to the
regulation of gene transcription responses (60,61).
TGF-β signal transduction pathways may elicit a variety of cellular
responses, which serve a key role in embryonic development,
maintenance of tissue homeostasis and the process of tumorigenesis
(62,63).
Notably, CYLD knockdown induced stabilization of
TGF-β receptor I (ALK5), which promoted TGF-β signaling in OSCC.
Low CYLD expression levels may lead to increased phosphorylation of
SMAD3, which is a key indicator for the activation of the TGF-β
signaling pathway. Low CYLD expression was associated with poor
overall survival of patients with invasive OSCC (64). In addition, results from a previous
study have demonstrated that cell survival was markedly increased
in cisplatin-resistant OSCC cells with CYLD knockdown, which was
associated with activation of the TGF-β signaling pathway. EGFR
tyrosine kinase inhibitors, such as gefitinib, may be used to
decrease cell survival via inhibition of TGF-β (65).
Association between DUBs and the
tumorigenesis of oral cancer
P53 is one of the most commonly mutated proteins in
various cancer types and exhibits oncogenic activity in tumors.
Notably, P53 is activated following cellular stress, which leads to
the inhibition of cell cycle progression and induction of
pro-apoptotic signaling (66).
Murine double minute 2 (MDM2) is an E3 ubiquitin ligase that
specifically binds to P53, which leads to ubiquitination and
degradation of P53 proteins. Under healthy conditions, MDM2
regulates the stability of P53, which limits P53 activity and
maintains low levels of protein expression. Following DNA damage,
MDM2 is inhibited by DUBs, which induces the release of accumulated
P53 and promotes P53 activity. In turn, P53 induces MDM2 gene
expression, which forms a negative feedback loop known as the
P53-MDM2 signaling pathway (67).
In OSCC, P53 gene mutations result in a loss of the oncogenic
function of P53 and the upregulation of P53 and MDM2, which are
associated with poor prognosis in patients (68).
DUBs serve a key role in stabilizing P53. Notably,
CYLD inhibits tumor growth by cleaving the K63 ubiquitination chain
on P53, which indirectly removes the K48 chain and inhibits the
ubiquitination degradation of P53 (69). Findings from previous studies have
demonstrated that USP28 effectively removed the MDM2-catalyzed K48
ubiquitin chain from P53, which led to the stabilization of P53.
However, transcription of USP28 was notably upregulated in OSCC.
Another study has demonstrated that OSCC is often associated with
mutations or genetic variations in P53 (70,71).
Thus, USP28-mediated stabilization of P53 may be detrimental to
patients with OSCC (70,71).
Notably, alternative mechanisms may also serve a
role in DUB-regulated tumorigenesis. Programmed cell death-ligand 1
(PD-L1) is upregulated in OSCC and acts as an oncogene (8). Results from a previous study have
demonstrated that ubiquitin-specific peptidase 9 X-linked (USP9X)
interacted with PD-L1, which facilitated deubiquitination of PD-L1
and thereby enhanced the stability of protein expression, which may
promote OSCC tumorigenesis (8).
Furthermore, myeloid cell leukemia-1 (MCL1), an anti-apoptosis
protein, is markedly upregulated in OSCC. MCL1 is also
deubiquitinated by USP9X. Notably, pharmacological inhibition of
USP9X may decrease MCL1 expression and induce cell death in OSCC
(72). Another study also
demonstrated that OTU deubiquitinase, ubiquitin aldehyde binding 1
(OTUB1) was positively associated with OSCC tumor stage. OTUB1
knockdown leads to the suppression of OSCC cell proliferation,
invasion and migration, and promotes tumor-associated macrophage M1
polarization. However, OTUB1 knockdown leads to the suppression of
M2 polarization, which, in turn, inhibits the survival of OSCC
cells (73). Furthermore, USP14
knockdown suppresses OSCC cell proliferation in vitro and
tumor growth in vivo, due to impaired Sox2 stability
mediated by polyubiquitination. Additionally, USP14 interacts with
phosphofructokinase-1 liver type (PFKL), a key rate-limiting enzyme
in the glycolytic pathway, which enhances PFKL-mediated glycolytic
metabolism, and ultimately promotes cellular proliferation,
migration and tumorigenesis (74,75).
Association between DUBs and the
treatment of oral cancer
Treatment of OSCC requires a multidisciplinary
approach, which often consists of surgical resection of the primary
lesion, followed by post-operative radiotherapy (76). Molecular targeted therapy is a novel
therapeutic strategy, and at present, two types of drugs are
approved by the Food and Drug Administration for the treatment of
OSCC, namely, cetuximab and nabulizumab (77). A key determinant of mortality in
patients with OSCC is the elevated incidence of recurrence
following treatment. Numerous studies have indicated that the
overall recurrence rate ranges from 28 to 44.5% (26,78,79).
Cisplatin resistance is a major obstacle in the treatment of
middle- and late-stage OSCC, which leads to recurrence, metastasis
and a poor prognosis. Cisplatin resistance mediated by decreased
CYLD expression is associated with the diminished accumulation of
intracellular cisplatin and the inhibition of cisplatin-induced
apoptosis, which occurs as a result of hyperactivation of the NF-κB
signaling pathway (80). The
tolerance of OSCC to radiotherapy also affects patient prognosis
and IR may induce the apoptosis of tumor cells. In a previous
study, USP14 was knocked down in nude mice bearing OSCC tumors.
USP14 knockdown facilitated IR-induced autophagy via upregulation
of LC3BII and γH2AX expression levels in OSCC cells subjected to IR
(81).
Conclusions
Aberrant activation and expression of signaling
pathway components are commonly observed in OSCC, which may promote
tumor cell proliferation, invasion and metastasis, and inhibit
apoptosis (82). Regulation of DUBs
in OSCC is considered to be an important factor in the abnormal
activation of signaling pathways (83–89).
Notably, DUBs operate through four distinct mechanisms: i) The
processing of ubiquitin protein precursors; ii) the retrieval of
ubiquitin molecules during the ubiquitination process; iii) the
cleavage of ubiquitin protein chains; and iv) the disassociation of
ubiquitin proteins from substrate targets. According to the
aforementioned functions, DUBs may reverse the ubiquitination of
target proteins, thereby contributing to the equilibrium between
ubiquitination and deubiquitination of substrate proteins (90). While DUBs are known to be involved
in the initiation and progression of OSCC, the specific mechanisms
and downstream effects of DUBs remain poorly understood. DUBs have
a dual role in oral cancer. The upregulation of some DUBs, such as
USP14 and USP9X, promotes tumor development, while the
downregulation of others like CYLD is linked to tumor invasion and
drug resistance. By regulating key pathways (Wnt/β-catenin, NF-κB,
TGF-β and P53), DUBs influence tumor progression. Their expression
levels correlate with patient prognosis, suggesting a potential as
therapeutic targets. Clinically, DUBs can indicate the prognosis of
invasive OSCC patients. Targeting DUBs may overcome treatment
resistance, and some DUBs inhibitors might enhance therapeutic
effects when combined with other treatments. Given their potential
as therapeutic targets in the treatment of OSCC, further research
is warranted to elucidate the regulatory mechanisms associated with
DUBs and to assess the potential side effects of targeted
therapies.
Acknowledgements
Not applicable.
Funding
This work was supported by Tianjin Beichen Hospital (Beichen
District Health System Technology Project; grant no.
SHGY-2023005).
Availability of data and materials
Not applicable.
Authors' contributions
WY and FL conceived and organized the manuscript.
ZW, SC and JW wrote the manuscript. JH revised the manuscript for
important intellectual content. Data authentication is not
applicable. All authors have read and approved the final version of
the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Peres MA, Macpherson LMD, Weyant RJ, Daly
B, Venturelli R, Mathur MR, Listl S, Celeste RK, Guarnizo-Herreño
CC, Kearns C, et al: Oral diseases: A global public health
challenge. Lancet. 394:249–260. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Inchingolo F, Santacroce L, Ballini A,
Topi S, Dipalma G, Haxhirexha K, Bottalico L and Charitos I: Oral
cancer: A historical review. Int J Environ Res Public Health.
17:31682020. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: GLOBOCAN estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferlay J, Colombet M, Soerjomataram I,
Mathers C, Parkin DM, Piñeros M, Znaor A and Bray F: Estimating the
global cancer incidence and mortality in 2018: GLOBOCAN sources and
methods. Int J Cancer. 144:1941–1953. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Irfan M, Delgado RZR and Frias-Lopez J:
The oral microbiome and cancer. Front Immunol. 11:5910882020.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mody MD, Rocco JW, Yom SS, Haddad RI and
Saba NF: Head and neck cancer. Lancet. 398:2289–2299. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Guo W, Wen D, Hou G, Zhou A and Wu
W: Deubiquitination and stabilization of programmed cell death
ligand 1 by ubiquitin-specific peptidase 9, X-linked in oral
squamous cell carcinoma. Cancer Med. 7:4004–4011. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Apoorva CC, Ananthaneni A, Kumar AJ,
Guduru VS and Puneeth HK: Evaluation of USP22 and Ki-67 expression
in oral squamous cell carcinoma: An immunohistochemical study. J
Oral Maxillofac Pathol. 27:679–684. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Barsouk A, Aluru JS, Rawla P, Saginala K
and Barsouk A: Epidemiology, risk factors, and prevention of head
and neck squamous cell carcinoma. Med Sci (Basel).
11:422023.PubMed/NCBI
|
|
11
|
Reyes M, Flores T, Betancur D,
Pena-Oyarzun D and Torres VA: Wnt/β-catenin signaling in oral
carcinogenesis. Int J Mol Sci. 21:46822020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tarle M and Luksic I: Pathogenesis and
therapy of oral carcinogenesis. Int J Mol Sci. 25:63432024.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tan Y, Wang Z, Xu M, Li B, Huang Z, Qin S,
Nice EC, Tang J and Huang C: Oral squamous cell carcinomas: State
of the field and emerging directions. Int J Oral Sci. 15:442023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wang J, Jiang C, Li N, Wang F, Xu Y, Shen
Z, Yang L, Li Z and He C: The circEPSTI1/mir-942-5p/LTBP2 axis
regulates the progression of OSCC in the background of OSF via EMT
and the PI3K/Akt/mTOR pathway. Cell Death Dis. 11:6822020.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhang X, Dong Y, Zhao M, Ding L, Yang X,
Jing Y, Song Y, Chen S, Hu Q and Ni Y: ITGB2-mediated metabolic
switch in CAFs promotes OSCC proliferation by oxidation of NADH in
mitochondrial oxidative phosphorylation system. Theranostics.
10:12044–12059. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Huang Z, Rui X, Yi C, Chen Y, Chen R,
Liang Y, Wang Y, Yao W, Xu X and Huang Z: Silencing LCN2 suppresses
oral squamous cell carcinoma progression by reducing EGFR signal
activation and recycling. J Exp Clin Cancer Res. 42:602023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pena-Oyarzun D, Flores T, Torres VA, Quest
AFG, Lobos-González L, Kretschmar C, Contreras P, Maturana-Ramírez
A, Criollo A and Reyes M: Inhibition of PORCN blocks wnt signaling
to attenuate progression of oral carcinogenesis. Clin Cancer Res.
30:209–223. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen Y, Chen Y and Liu W: Chaperonin
containing TCP1 subunit 6A may activate Notch and Wnt pathways to
facilitate the malignant behaviors and cancer stemness in oral
squamous cell carcinoma. Cancer Biol Ther. 25:22871222024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang S, Wang X, Sun J, Yang J, Wu D, Wu F
and Zhou H: Down-regulation of DNA key protein-FEN1 inhibits OSCC
growth by affecting immunosuppressive phenotypes via
IFN-gamma/JAK/STAT-1. Int J Oral Sci. 15:172023. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang J, Chen T, Yang X, Cheng H, Späth
SS, Clavijo PE, Chen J, Silvin C, Issaeva N, Su X, et al:
Attenuated TRAF3 fosters activation of alternative NF-ĸB and
reduced expression of antiviral interferon, TP53, and RB to promote
HPV-positive head and neck cancers. Cancer Res. 78:4613–4626. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu Y, Sun Y, Yang J, Wu D, Yu S, Liu J,
Hu T, Luo J and Zhou H: DNMT1-targeting remodeling global DNA
hypomethylation for enhanced tumor suppression and circumvented
toxicity in oral squamous cell carcinoma. Mol Cancer. 23:1042024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang X, Li R, Wu L, Chen Y, Liu S, Zhao H,
Wang Y, Wang L and Shao Z: Histone methyltransferase KMT2D
cooperates with MEF2A to promote the stem-like properties of oral
squamous cell carcinoma. Cell Biosci. 12:492022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Oh SY, Kim J, Lee KY, Lee HJ, Kwon TG, Kim
JW, Lee ST, Kim DG, Choi SY and Hong SH: Chromatin
remodeling-driven autophagy activation induces cisplatin resistance
in oral squamous cell carcinoma. Cell Death Dis. 15:5892024.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hu S, Lu H, Xie W, Wang D, Shan Z, Xing X,
Wang XM, Fang J, Dong W, Dai W, et al: TDO2+ myofibroblasts mediate
immune suppression in malignant transformation of squamous cell
carcinoma. J Clin Invest. 132:e1576492022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Fang K, Sun M, Leng Z, Chu Y, Zhao Z, Li
Z, Zhang Y, Xu A, Zhang Z, Zhang L, et al: Targeting IGF1R
signaling enhances the sensitivity of cisplatin by inhibiting
proline and arginine metabolism in oesophageal squamous cell
carcinoma under hypoxia. J Exp Clin Cancer Res. 42:732023.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lyu WN, Lin MC, Shen CY, Chen LH, Lee YH,
Chen SK, Lai LC, Chuang EY, Lou PJ and Tsai MH: An oral microbial
biomarker for early detection of recurrence of oral squamous cell
carcinoma. ACS Infect Dis. 9:1783–1792. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bagan J, Sarrion G and Jimenez Y: Oral
cancer: Clinical features. Oral Oncol. 46:414–417. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Harada H, Kikuchi M, Asato R, Hamaguchi K,
Tamaki H, Mizuta M, Hori R, Kojima T, Honda K, Tsujimura T, et al:
Characteristics of oral squamous cell carcinoma focusing on cases
unaffected by smoking and drinking: A multicenter retrospective
study. Head Neck. 45:1812–1822. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jerjes W, Upile T, Petrie A, Riskalla A,
Hamdoon Z, Vourvachis M, Karavidas K, Jay A, Sandison A, Thomas GJ,
et al: Clinicopathological parameters, recurrence, locoregional and
distant metastasis in 115 T1-T2 oral squamous cell carcinoma
patients. Head Neck Oncol. 2:92010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gonzalez-Moles MA, Warnakulasuriya S,
Lopez-Ansio M and Ramos-Garcia P: Hallmarks of cancer applied to
oral and oropharyngeal carcinogenesis: A scoping review of the
evidence gaps found in published systematic reviews. Cancers
(Basel). 14:38342022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kleszcz R, Frackowiak M, Dorna D and
Paluszczak J: Combinations of PRI-724 Wnt/beta-catenin pathway
inhibitor with vismodegib, erlotinib, or HS-173 synergistically
inhibit head and neck squamous cancer cells. Int J Mol Sci.
24:104482023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tathineni P, Joshi N and Jelinek MJ:
Current state and future directions of EGFR-Directed therapy in
head and neck cancer. Curr Treat Options Oncol. 24:680–692. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Swatek KN and Komander D: Ubiquitin
modifications. Cell Res. 26:399–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Harrigan JA, Jacq X, Martin NM and Jackson
SP: Deubiquitylating enzymes and drug discovery: Emerging
opportunities. Nat Rev Drug Discov. 17:57–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Song L and Luo ZQ: Post-translational
regulation of ubiquitin signaling. J Cell Biol. 218:1776–1786.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
van Wijk SJ, Fulda S, Dikic I and
Heilemann M: Visualizing ubiquitination in mammalian cells. EMBO
Rep. 20:e465202019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yau RG, Doerner K, Castellanos ER,
Haakonsen DL, Werner A, Wang N, Yang XW, Martinez-Martin N,
Matsumoto ML, Dixit VM and Rape M: Assembly and function of
heterotypic ubiquitin chains in cell-cycle and protein quality
control. Cell. 171:918–933.e20. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Loix M, Zelcer N, Bogie JFJ and Hendriks
JJA: The ubiquitous role of ubiquitination in lipid metabolism.
Trends Cell Biol. 34:416–429. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
De Cesare V, Carbajo Lopez D, Mabbitt PD,
Fletcher AJ, Soetens M, Antico O, Wood NT and Virdee S:
Deubiquitinating enzyme amino acid profiling reveals a class of
ubiquitin esterases. Proc Natl Acad Sci USA. 118:e20069471182021.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Abdul Rehman SA, Kristariyanto YA, Choi
SY, Nkosi PJ, Weidlich S, Labib K, Hofmann K and Kulathu Y: MINDY-1
Is a member of an evolutionarily conserved and structurally
distinct new family of deubiquitinating enzymes. Mol Cell.
63:146–155. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kwasna D, Abdul Rehman SA, Natarajan J,
Matthews S, Madden R, De Cesare V, Weidlich S, Virdee S, Ahel I,
Gibbs-Seymour I and Kulathu Y: Discovery and characterization of
ZUFSP/ZUP1, a distinct deubiquitinase class important for genome
stability. Mol Cell. 70:150–164. e62018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tsuchida S and Nakayama T: Ubiquitination
and deubiquitination in oral disease. Int J Mol Sci. 22:54882021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Trulsson F, Akimov V, Robu M, van Overbeek
N, Berrocal DAP, Shah RG, Cox J, Shah GM, Blagoev B and Vertegaal
ACO: Deubiquitinating enzymes and the proteasome regulate
preferential sets of ubiquitin substrates. Nat Commun. 13:27362022.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Schwickart M, Huang X, Lill JR, Liu J,
Ferrando R, French DM, Maecker H, O'Rourke K, Bazan F,
Eastham-Anderson J, et al: Deubiquitinase USP9X stabilizes MCL1 and
promotes tumour cell survival. Nature. 463:103–107. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Chen Y, Zhao Y, Yang X, Ren X, Huang S,
Gong S, Tan X, Li J, He S, Li Y, et al: USP44 regulates
irradiation-induced DNA double-strand break repair and suppresses
tumorigenesis in nasopharyngeal carcinoma. Nat Commun. 13:5012022.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ling S, Shan Q, Zhan Q, Ye Q, Liu P, Xu S,
He X, Ma J, Xiang J, Jiang G, et al: USP22 promotes hypoxia-induced
hepatocellular carcinoma stemness by a HIF1α/USP22 positive
feedback loop upon TP53 inactivation. Gut. 69:1322–1334. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Deng L, Meng T, Chen L, Wei W and Wang P:
The role of ubiquitination in tumorigenesis and targeted drug
discovery. Signal Transduct Target Ther. 5:112020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: Function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Xie J, Huang L, Lu YG and Zheng DL: Roles
of the wnt signaling pathway in head and neck squamous cell
carcinoma. Front Mol Biosci. 7:5909122020. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Moon JH, Lee SH and Lim YC:
Wnt/β-catenin/Slug pathway contributes to tumor invasion and lymph
node metastasis in head and neck squamous cell carcinoma. Clin Exp
Metastasis. 38:163–174. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Jung H, Kim BG, Han WH, Lee JH, Cho JY,
Park WS, Maurice MM, Han JK, Lee MJ, Finley D and Jho EH:
Deubiquitination of dishevelled by Usp14 is required for Wnt
signaling. Oncogenesis. 2:e642013. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chen X, Wu J, Chen Y, Ye D, Lei H, Xu H,
Yang L, Wu Y and Gu W: Ubiquitin-specific protease 14 regulates
cell proliferation and apoptosis in oral squamous cell carcinoma.
Int J Biochem Cell Biol. 79:350–359. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Guo Q, Jin Y, Chen X, Ye X, Shen X, Lin M,
Zeng C, Zhou T and Zhang J: NF-kappaB in biology and targeted
therapy: New insights and translational implications. Signal
Transduct Target Ther. 9:532024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Chiu HW, Lee HL, Lee HH, Lu HW, Lin KY,
Lin YF and Lin CH: AIM2 promotes irradiation resistance, migration
ability and PD-L1 expression through STAT1/NF-ĸB activation in oral
squamous cell carcinoma. J Transl Med. 22:132024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Weinlich R and Green DR: The two faces of
receptor interacting protein kinase-1. Mol Cell. 56:469–480. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Hou X, Wang L, Zhang L, Pan X and Zhao W:
Ubiquitin-specific protease 4 promotes TNF-α-induced apoptosis by
deubiquitination of RIP1 in head and neck squamous cell carcinoma.
FEBS Lett. 587:311–316. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhao Y, Thornton AM, Kinney MC, Ma CA,
Spinner JJ, Fuss IJ, Shevach EM and Jain A: The deubiquitinase CYLD
targets Smad7 protein to regulate transforming growth factor β
(TGF-β) signaling and the development of regulatory T cells. J Biol
Chem. 286:40520–40530. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Morgan EL, Chen Z and Van Waes C:
Regulation of NFĸB signalling by ubiquitination: A potential
therapeutic target in head and neck squamous cell carcinoma?
Cancers (Basel). 12:28772020. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Ge WL, Xu JF and Hu J: Regulation of oral
squamous cell carcinoma proliferation through crosstalk between
SMAD7 and CYLD. Cell Physiol Biochem. 38:1209–1217. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Deng Z, Fan T, Xiao C, Tian H, Zheng Y, Li
C and He J: TGF-β signaling in health, disease, and therapeutics.
Signal Transduct Target Ther. 9:612024. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Derynck R, Turley SJ and Akhurst RJ: TGFβ
biology in cancer progression and immunotherapy. Nat Rev Clin
Oncol. 18:9–34. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Tauriello DVF, Sancho E and Batlle E:
Overcoming TGFβ-mediated immune evasion in cancer. Nat Rev Cancer.
22:25–44. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Wen B, Liao H, Lin W, Li Z, Ma X, Xu Q and
Yu F: The Role of TGF-β during pregnancy and pregnancy
complications. Int J Mol Sci. 24:168822023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Shinriki S, Jono H, Maeshiro M, Nakamura
T, Guo J, Li JD, Ueda M, Yoshida R, Shinohara M, Nakayama H, et al:
Loss of CYLD promotes cell invasion via ALK5 stabilization in oral
squamous cell carcinoma. J Pathol. 244:367–379. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Kanemaru A, Shinriki S, Kai M, Tsurekawa
K, Ozeki K, Uchino S, Suenaga N, Yonemaru K, Miyake S, Masuda T, et
al: Potential use of EGFR-targeted molecular therapies for tumor
suppressor CYLD-negative and poor prognosis oral squamous cell
carcinoma with chemoresistance. Cancer Cell Int. 22:3582022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Hassin O and Oren M: Drugging p53 in
cancer: One protein, many targets. Nat Rev Drug Discov. 22:127–144.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Zhu H, Gao H, Ji Y, Zhou Q, Du Z, Tian L,
Jiang Y, Yao K and Zhou Z: Targeting p53-MDM2 interaction by
small-molecule inhibitors: Learning from MDM2 inhibitors in
clinical trials. J Hematol Oncol. 15:912022. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Hong A, Zhang X, Jones D, Veillard AS,
Zhang M, Martin A, Lyons JG, Lee CS and Rose B: Relationships
between p53 mutation, HPV status and outcome in oropharyngeal
squamous cell carcinoma. Radiother Oncol. 118:342–349. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Fernandez-Majada V, Welz PS, Ermolaeva MA,
Schell M, Adam A, Dietlein F, Komander D, Büttner R, Thomas RK,
Schumacher B and Pasparakis M: The tumour suppressor CYLD regulates
the p53 DNA damage response. Nat Commun. 7:125082016. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Muller I, Strozyk E, Schindler S, Beissert
S, Oo HZ, Sauter T, Lucarelli P, Raeth S, Hausser A, Al Nakouzi N,
et al: Cancer cells employ nuclear caspase-8 to overcome the
p53-Dependent G2/M checkpoint through cleavage of USP28. Mol Cell.
77:970–984. e72020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Prieto-Garcia C, Tomaskovic I, Shah VJ,
Dikic I and Diefenbacher M: USP28: Oncogene or tumor suppressor? A
unifying paradigm for squamous cell carcinoma. Cells. 10:26522021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Sulkshane P, Pawar SN, Waghole R, Pawar
SS, Rajput P, Uthale A, Oak S, Kalkar P, Wani H, Patil R, et al:
Elevated USP9X drives early-to-late-stage oral tumorigenesis via
stabilisation of anti-apoptotic MCL-1 protein and impacts outcome
in oral cancers. Br J Cancer. 125:547–560. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Li Y, Li R, Qin H, He H and Li S: OTUB1′s
role in promoting OSCC development by stabilizing RACK1 involves
cell proliferation, migration, invasion, and tumor-associated
macrophage M1 polarization. Cell Signal. 110:1108352023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Liu C, Zhou S and Tang W: USP14 promotes
the cancer stem-like cell properties of OSCC via promoting SOX2
deubiquitination. Oral Dis. 30:4255–4265. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Zhang X, Geng L, Tang Y, Wang Y, Zhang Y,
Zhu C, Lei H, Xu H, Zhu Q, Wu Y and Gu W: Ubiquitin-specific
protease 14 targets PFKL-mediated glycolysis to promote the
proliferation and migration of oral squamous cell carcinoma. J
Transl Med. 22:1932024. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Millen R, De Kort WWB, Koomen M, van Son
GJF, Gobits R, Penning de Vries B, Begthel H, Zandvliet M,
Doornaert P, Raaijmakers CPJ, et al: Patient-derived head and neck
cancer organoids allow treatment stratification and serve as a tool
for biomarker validation and identification. Med. 4:290–310.
e122023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Li H, Zhang Y, Xu M and Yang D: Current
trends of targeted therapy for oral squamous cell carcinoma. J
Cancer Res Clin Oncol. 148:2169–2186. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Wang W, Adeoye J, Thomson P and Choi SW:
Multiple tumour recurrence in oral, head and neck cancer:
Characterising the patient journey. J Oral Pathol Med. 50:979–984.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Blatt S, Kruger M and Sagheb K, Barth M,
Kämmerer PW, Al-Nawas B and Sagheb K: Tumor recurrence and
follow-up intervals in oral squamous cell carcinoma. J Clin Med.
11:70612022. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Suenaga N, Kuramitsu M, Komure K, Kanemaru
A, Takano K, Ozeki K, Nishimura Y, Yoshida R, Nakayama H, Shinriki
S, et al: Loss of tumor suppressor CYLD expression triggers
cisplatin resistance in oral squamous cell carcinoma. Int J Mol
Sci. 20:51942019. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Xie W and Xu L: Ubiquitin-specific
protease 14 promotes radio-resistance and suppresses autophagy in
oral squamous cell carcinoma. Exp Cell Res. 398:1123852021.
View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Patni AP, Harishankar MK, Joseph JP,
Sreeshma B, Jayaraj R and Devi A: Comprehending the crosstalk
between Notch, Wnt and Hedgehog signaling pathways in oral squamous
cell carcinoma-clinical implications. Cell Oncol (Dordr).
44:473–494. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Sun T, Liu Z and Yang Q: The role of
ubiquitination and deubiquitination in cancer metabolism. Mol
Cancer. 19:1462020. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Liu Y, Bai Q, Pang N and Xue J: TCF12
induces ferroptosis by suppressing OTUB1-mediated SLC7A11
deubiquitination to promote cisplatin sensitivity in oral squamous
cell carcinoma. Cell Biol Int. 48:1649–1663. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Feng L, Zhang J, Sun M, Qiu F, Chen W and
Qiu W: Tumor Suppressor LINC02487 inhibits oral squamous cell
carcinoma cell migration and invasion through the USP17-SNAI1 Axis.
Front Oncol. 10:5598082020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lu R, Wu G, Chen M, Ji D, Liu Y, Zhou GG
and Fu W: USP18 and USP20 restrict oHSV-1 replication in resistant
human oral squamous carcinoma cell line SCC9 and affect the
viability of SCC9 cells. Mol Ther Oncolytics. 23:477–487. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Kobayashi E, Hwang D, Bheda-Malge A,
Whitehurst CB, Kabanov AV, Kondo S, Aga M, Yoshizaki T, Pagano JS,
Sokolsky M and Shakelford J: Inhibition of UCH-L1 deubiquitinating
activity with two forms of LDN-57444 has anti-invasive effects in
metastatic carcinoma cells. Int J Mol Sci. 20:37332019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen S, Wu K, Zong Y, Hou Z, Deng Z and
Xia Z: USP44 regulates HEXIM1 stability to inhibit tumorigenesis
and metastasis of oral squamous cell carcinoma. Biol Direct.
19:1432024. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Chang W, Luo Q, Wu X, Nan Y, Zhao P, Zhang
L, Luo A, Jiao W, Zhu Q, Fu Y and Liu Z: OTUB2 exerts
tumor-suppressive roles via STAT1-mediated CALML3 activation and
increased phosphatidylserine synthesis. Cell Rep. 41:1115612022.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Dewson G, Eichhorn PJA and Komander D:
Deubiquitinases in cancer. Nat Rev Cancer. 23:842–862. 2023.
View Article : Google Scholar : PubMed/NCBI
|
















