Introduction
Thyroid cancer is the most common malignancy of the
endocrine system; >90% of the pathological types are papillary
thyroid carcinomas (PTC) and ~80% of patients with PTC have a good
prognosis. However, in patients with invasion of the trachea,
esophagus, recurrent laryngeal nerve and large blood vessels, the
10-year survival rate decreases to 26% (1,2).
Several factors have been used to evaluate the malignancy,
prognosis, possibility of postoperative metastasis and recurrence
of PTC before surgery. Commonly used clinical factors include the
age, grade, tumor invasion and tumor size (AGES), and age and tumor
invasion (AEMS) systems. Additionally, tumor extent, distant
metastasis, tumor size and extrathyroidal extension (ETE) are
considered to be independent risk factors for thyroid cancer
(3–5). An ETE indicates that a tumor is highly
aggressive and has a significant likelihood of spreading to distant
parts of the body (6–8). The TNM staging system for thyroid
cancer has been outlined in the 7 and 8th editions of the American
Joint Committee on Cancer (2,9). ETE
can be categorized as follows: i) Microscopic ETE (histology, band
muscle invasion; and ii) gross ETE, band muscle invasion and more
serious external invasion (subcutaneous tissue, major organs,
nerves and blood vessels) (9).
An accurate preoperative assessment of ETE in PTC is
crucial for clinicians to develop optimal therapeutic strategies
that are both individualized and evidence-based (10–13).
The implementation of appropriate surgical interventions (including
total/subtotal thyroidectomy and thyroid isthmusectomy) combined
with adjuvant therapies (such as thyrotropin suppression,
radioactive iodine therapy, chemotherapy, external beam radiation
and chemical embolization) has been demonstrated to significantly
reduce postoperative recurrence rates, enhance patient survival
outcomes and improve quality of life indicators (14,15).
Currently, imaging methods for the preoperative
diagnosis of ETE in PTC mainly include ultrasonography (US) and
computed tomography (CT) scans. US is the preferred imaging method
for the diagnosis of PTC. US is simple, non-invasive, inexpensive
and reproducible in real-time. However, US fails to accurately
represent the spatial relationship between PTC and the surrounding
trachea and esophagus, and there is still a certain degree of
false-positive and false-negative rates in the preoperative
evaluation of ETE (16). The
radiation-hardening effect in traditional CT imaging causes the
absorption coefficient of X-rays by the nodules to no longer be
constant. This change leads to a change in the average energy of
the X-rays, which in turn causes problems such as information loss,
hardening artifacts and inaccurate CT value measurements (17–19).
Dual-energy CT (DECT) imaging can generate material decomposition
(MD) images using monochromatic images with photon energies varying
from 40 to 140 keV. These images were then used to create spectral
Hounsfield unit (HU) curves. The inclusion of molecular dynamics
images and spectral HU curves is beneficial for identifying ETE of
PTC (20,21). Therefore, the present study aimed to
explore the diagnostic value of DECT combined with US for detecting
ETE in PTC.
Materials and methods
Research participants
Between January 2018 and August 2023, 102 patients
with pathologically confirmed PTC (comprising 136 nodules) who were
hospitalized in Affiliated People's Hospital of Jiangsu University
(Zhenjiang, China) for surgery, were all examined using US and DECT
prior to surgery. The patient cohort included 69 female and 33 male
patients, aged 21-72 years old (mean ± SD age, 47.14±10.73 years).
The male-to-female ratio was ~1:2.09; 62 patients (60.7%) were aged
>45 years old and 40 patients (39.3%) were ≤45 years old.
Asymptomatic physical examination was used in 74 cases,
demonstrating: 12 cases of neck swelling, 4 cases of dysphagia, 5
cases with symptoms of oppression and 7 cases of hoarseness. The
present study was approved by the Institutional Ethical Review
Committee of Jiangsu University Affiliated People's Hospital
(approval no. K-20170104-Y; Zhenjiang, China) and all the patients
provided written informed consent.
The inclusion criteria were as follows: i) Age, ≥18
years; ii) postoperative pathological confirmation of PTC; iii)
absence of any history of neck tumors other than thyroid tumors;
and iv) absence of neck radiation therapy. The exclusion criteria
were as follows: i) Benign thyroid tumors confirmed according to
postoperative pathology; and ii) malignant tumors other than PTC
confirmed using pathology.
Instruments and methods
US inspection methods
The equipment used in the present study was a Q5
color Doppler ultrasound diagnostic system (System Software 3.2.1;
Philips Healthcare) equipped with a linear array high-frequency
probe operating at 5-12 MHz. The system was configured with the
pre-set ‘Thyroid’ examination mode for optimal imaging. Before the
examination, the patient assumed a supine position, with the head
removed from the headrest and the neck slightly inclined back to
maximize neck exposure. The conditions preprogrammed into the
thyroid examination instrument were used. Longitudinal and
cross-sectional analyses were performed by positioning the
instrument beneath the thyroid cartilage.
The thyroid diameter was routinely measured in the
superficial thyroid deep muscle group, trachea, esophagus and large
vessels. Additionally, the primary lesion, location, internal echo,
aspect diameter ratio, blood flow signal, morphology, boundary,
calcification, elasticity and cervical lymph node characteristics,
including maximum diameter, shape, edge, calcification, cystic
degeneration, lymphatic hilus structure and blood flow signal, were
measured.
CT examination method
The present study used a third-generation DECT
scanner (Siemens AG) to perform dual-energy scans of the cervical
arteries and veins with the patient's neck extended in the supine
position. The third-generation DECT scanner (Siemens AG) adopted a
dual-source configuration with two X-ray tubes and corresponding
detectors, which could work simultaneously at different energy
levels to collect dual-energy data in a single scan. The present
scan range extended from the cranium base to the aortic arch.
Dual-energy scanning was used to scan the arterial and venous
phases. The fusion coefficients for high and low tube voltage
images were set to 0.3, respectively, with exposure parameters of
89 mAs, collimator width of 128×0.6 mm, matrix dimensions of
256×256 and pitch of 0.7 were the scanning parameters. Iopromide
(Bayer AG) was used as the contrast agent at a concentration of 1.5
ml/kg and an injection flow rate of 3.5 ml/sec. Using contrast
agent bolus monitoring technology, scanning commenced 45 sec
following the injection of iopromide. All images containing data
files were transferred to a workstation to produce spectral HU
curves, iodine-based MD images, and 40, 70 and 100 keV images.
These curves were analyzed by a radiologist with 15 years of
experience in head and neck cancer diagnosis and imaging
measurements. The examination was conducted using gemstone spectral
imaging (GSI) viewer image analysis software (Syngo.via; Siemens
AG).
An additional radiologist with >10 years of
expertise in diagnosing head and neck cancer assessed the CT GSI
parameters by analyzing the 70 keV images. A region of interest
(ROI) was selected over the lesion to avoid capturing images of
necrotic or calcified areas. The parameters were averaged after
three measurements. Iodine concentration (IC), normalized IC (NIC)
and slope of HU curve (λHU) in the ROI, CT attenuation value (HU)
and IC of the common carotid artery on the same image slice were
GSI parameters. The NIC and λHU formulations are expressed as
follows: λHU=[CT value (40 keV)-CT value (100 keV)]/(100–40); and
NIC=ICROI/ICcommon carotid artery. ‘CT value (40 keV)’ and ‘CT
value (100 keV)’ represented the measurements of CT attenuation at
energy levels of 40 and 100 keV, respectively.
Image analysis method
The US and DECT data of all selected PTCs were
analyzed in a blinded manner by two attending or the aforementioned
ultrasound imaging and radiology doctors to determine whether the
PTC contacted the thyroid capsule and whether there was an invasion
of the adjacent organs. In cases of disagreement, consensus was
reached. The final results were based on surgical records and
pathological results.
The image criteria for preoperative US and DECT
diagnosis of ETE were: i) The lesion protruded from the thyroid
capsule, and invaded the sternothyroid muscle and soft tissue
surrounding the thyroid or it contacts the thyroid capsule by
>25%; and ii) in addition to the thyroid capsule, the lesion
spread to the larynx, trachea, esophagus, recurrent laryngeal
nerve, carotid artery and mediastinal blood vessels
(2-4,22-24).
Pathological diagnosis methods
Fresh tissue samples were fixed in formalin at room
temperature and prepared by standardized procedures of gradient
ethanol dehydration, xylene transparency, and paraffin embedding.
The embedded tissue was cut into 4-µm tissue sections using a
microtome. The cells were deparaffinized with xylene and rehydrated
through gradient ethanol. In the staining process, the nuclei were
stained with hematoxylin solution at room temperature for 5-6 min
to make the nuclei blue, and then differentiated with hydrochloric
acid and rinsed with running water to enhance the contrast. The
cytoplasm was then counterstained with eosin staining solution for
another 10 sec at room temperature to give a pink color. Finally,
sections were dehydrated with gradient ethanol, made transparent
with xylene, and sealed with neutral gum for visualization under a
light microscope.
Statistical analysis
All data were statistically analyzed using the SPSS
Statistics (version 20.0; IBM Corp.). The measurement data
following a normal distribution were expressed as mean ± standard
deviation, using independent sample t-test. Contrast enhancement,
maximum longitudinal diameter >5 mm, calcification and cystic
changes were analyzed using the χ2 test. Continuous data
were converted into categorical data, and the cut-off values
established by receiver operating characteristic (ROC) curve
analysis were used to analyze the IC, NIC and λHU variables. The
area under the curve (AUC) was calculated and the diagnostic
boundary point of Young's modulus was determined according to the
highest critical point of Youden index. P<0.05 was considered to
indicate a statistically significant difference.
Results
Comparison of clinical characteristics
between ETE and non-ETE cases
Between January 2018 and August 2023, surgical
resection was performed on all 102 PTC cases (comprising 136
nodules) in the present cohort, with pathological confirmation
obtained. DECT and US were performed preoperatively. A total of 136
lesions were observed, comprising 49 lesions on the isthmus, 39 on
the right side and 48 on the left side; diameters ranged from 0.4
to 10.5 cm (mean diameter, 3.57±1.91 cm). A comparison of the
clinical characteristics of the patients with and without ETE is
presented in Table I. Age, sex and
tumor diameter did not significantly differ between patients with
and without ETE (P>0.05).
 | Table I.Comparison of clinical characteristics
of PTC cases in patients without ETE (n=28) and with ETE
(n=108). |
Table I.
Comparison of clinical characteristics
of PTC cases in patients without ETE (n=28) and with ETE
(n=108).
| Characteristics | Non-ETE | ETE | P-value |
|---|
| Mean age,
years | 43.67±14.67 | 47.59±10.06 | 0.069 |
| Sex ratio (Male:
female) | 1:1.88 | 1:2.16 | 0.811 |
| Mean tumor
diameter, cm | 3.35±1.37 | 3.59±1.97 | 0.524 |
| Tumor site |
|
| 0.834 |
|
Isthmus | 9 | 40 |
|
| Left
lobe | 11 | 37 |
|
| Right
lobe | 8 | 31 |
|
Comparison of the diagnostic value of
US and DECT for ETE in PTC
The sensitivity, specificity and accuracy of GSI
parameters (IC, NIC and λHU) and US morphological parameters
(contrast enhancement, maximum longitudinal diameter, calcification
and cystic change) in diagnosing ETE are shown in Tables II and III. Through the analysis of the GSI
parameters (IC, NIC and λHU) of the lesions, the optimal threshold,
sensitivity, specificity and accuracy of GSI parameters for
diagnosing ETE were determined. The IC, NIC and λHU of the ETE
group were significantly increased compared with those of the
non-ETE group (P<0.001).
 | Table II.Diagnostic value of ultrasonography
parameters in diagnosing ETE. |
Table II.
Diagnostic value of ultrasonography
parameters in diagnosing ETE.
| Lesion
morphological characteristics | ETE, n | Non-ETE, n | χ2 | P-value | Sensitivity, % | Specificity, % | Accuracy, % | Positive predictive
value, % | Negative predictive
value, % |
|---|
| Contrast
enhancement | 72 | 14 | 2.65 | 0.125 | 66.7 | 50.0 | 63.2 | 83.7 | 28.0 |
| Maximum
longitudinal diameter >5 mm | 80 | 13 | 7.86 | 0.007 | 74.1 | 53.6 | 69.9 | 86.0 | 34.9 |
| Calcification | 71 | 15 | 1.41 | 0.274 | 65.7 | 46.4 | 61.8 | 82.6 | 26.0 |
| Cystic changes | 54 | 13 | 0.11 | 0.833 | 50.0 | 53.6 | 50.7 | 80.6 | 23.1 |
 | Table III.Diagnostic value of gemstone spectral
imaging parameters in diagnosing ETE. |
Table III.
Diagnostic value of gemstone spectral
imaging parameters in diagnosing ETE.
| Spectral imaging
parameters | ETE (n=108) | Non-ETE (n=28) | t | P-value |
|---|
| IC, mg/ml | 3.09±1.24 | 2.53±0.88 | −2.3 | <0.001 |
| NIC | 0.41±0.21 | 0.27±0.20 | −3.3 | 0.001 |
| λHU, HU/keV | 3.94±1.61 | 2.87±0.93 | −3.4 | <0.001 |
ROC curve analysis of DECT parameters
and US combined diagnosis of ETE
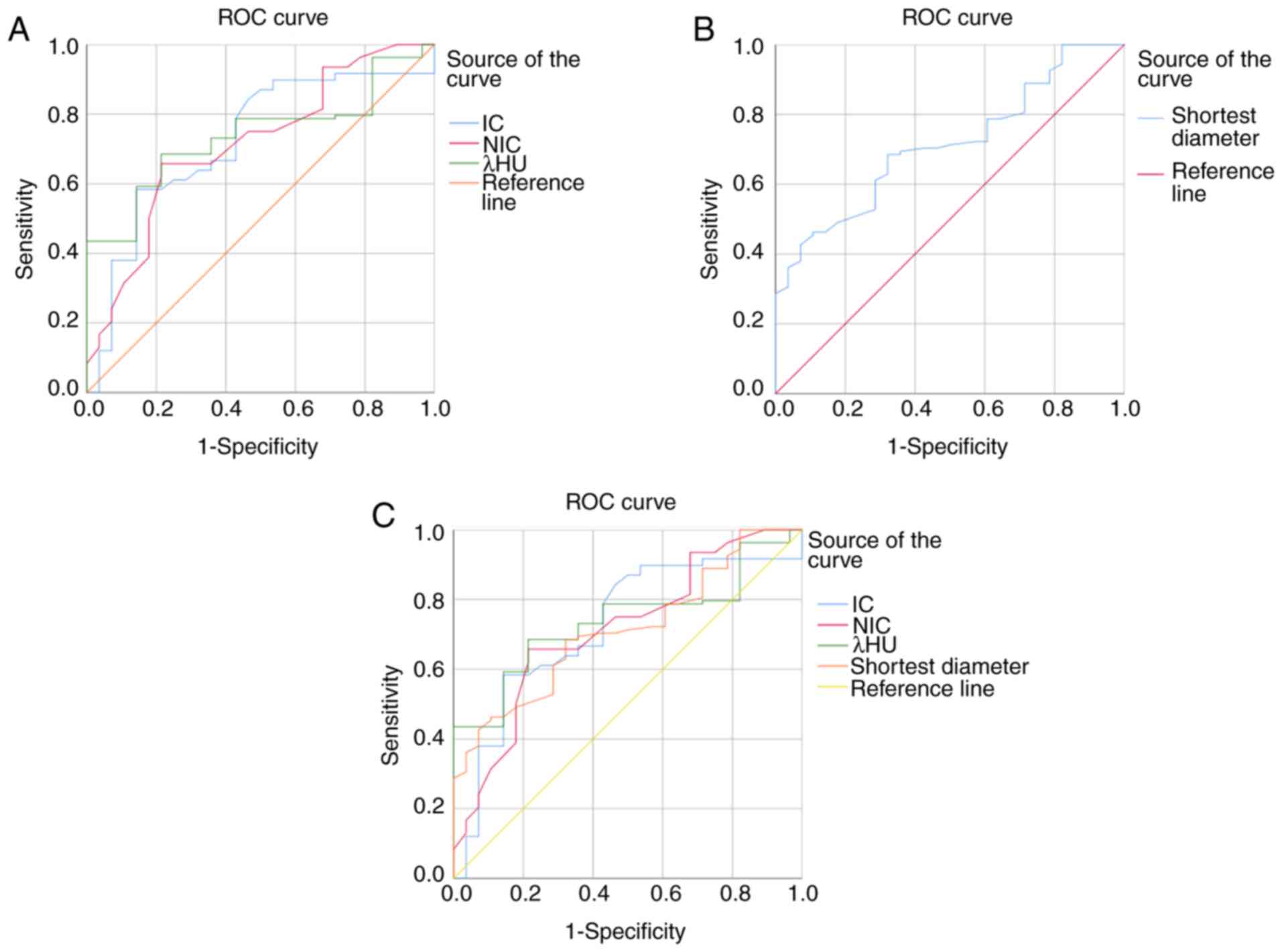
The AUC for IC in identifying ETE was 0.722, with
the highest accuracy when 2.88 mg/ml was used as the diagnostic
threshold. The corresponding sensitivity and specificity were 58.3
and 85.6%, respectively, with a Youden index of 0.44. The AUC of
NIC in identifying ETE was 0.713, with the highest accuracy when
0.285 was used as the diagnostic threshold. The corresponding
sensitivity and specificity were 65.7 and 78.6%, respectively, with
a Youden index of 0.443. The AUC of λHU in identifying ETE was
0.738, with the highest accuracy when 3.4 was used as a diagnostic
threshold. The corresponding sensitivity and specificity were 68.5
and 78.6%, respectively, with a Youden index of 0.471 (Fig. 1A). The AUC of US (maximum
longitudinal diameter >5 mm) morphological parameters for
identifying ETE was 0.712, with the highest accuracy when 3.845 cm
was used as the diagnostic threshold. The corresponding sensitivity
and specificity were 46.3 and 89.3%, respectively, with a Youden
index of 0.356 (Fig. 1B). The
differences between the two groups were statistically significant
(Table IV).
 | Table IV.Comparison of IC, NIC and λHU
parameter diagnostic ETE AUC curves. |
Table IV.
Comparison of IC, NIC and λHU
parameter diagnostic ETE AUC curves.
| Test result
variables | Area | Standard
errora | Asymptotic
sigb | Asymptotic 95%
confidence interval |
|---|
| IC | 0.722 | 0.054 | 0.000 | 0.616-0.828 |
| NIC | 0.713 | 0.054 | 0.001 | 0.607-0.819 |
| λHU | 0.738 | 0.044 | 0.000 | 0.652-0.824 |
The AUC of the GSI parameters combined with the US
morphological parameters to identify ETE was 0.782, with the
highest accuracy when 0.762 was used as the diagnostic threshold.
The corresponding sensitivity and specificity were 80.6 and 85.7%,
respectively, with a Youden index of 0.663 (Fig. 1C).
The GSI and US morphological parameters were
combined to diagnose ETE in patients with PTC differentially. Among
them, IC >2.88 mg/ml, NIC >0.285, λHU >3.4, and a lesion
that contacts the thyroid capsule >25% and protrudes from the
thyroid capsule indicated ETE (Fig.
2).
Discussion
ETE is an important factor affecting PTC prognosis;
the American Thyroid Association guidelines (25) indicate that for patients with PTC
without clinical evidence of ETE, only lobectomy and isthmusectomy
are necessary. Therefore, an accurate preoperative assessment of
the presence of ETE can guide surgeons in choosing a reasonable and
standardized treatment plan that is of significant clinical
importance.
US is the preferred imaging modality for diagnosing
PTC (26,27). US offers high resolution and is
radiation-free, allowing clear visualization of PTC boundaries.
However, US is highly dependent on the operator's skill, which can
lead to reduced objectivity in diagnosis owing to human factors.
Additionally, US has certain limitations in displaying lymph nodes
in the retropharyngeal and parapharyngeal spaces and the thyroid
capsule. However, DECT can generate quantitative parameters such as
monoenergetic and MD images based on the differing X-ray absorption
coefficients of various substances. These findings provide a
theoretical basis for the disease diagnosis. DECT offers enhanced
tissue contrast information, accurately displaying the size,
extent, location, potential extrathyroidal extension, lymph node
metastasis and the relationship with the surrounding tissues and
organs of PTC (17,28–30).
Consequently, the application of DECT for thyroid lesions has
increased in recent years (30).
The findings of the present study indicated that a
maximum longitudinal diameter >5 mm in ultrasound features has
significant clinical implications for predicting ETE in PTC. This
phenomenon may be attributed to differences in the biological
behavior of PTC. During the early stages of PTC, cancer cells in
the anterior-posterior direction are in the proliferative phase,
whereas those in other directions remain relatively quiescent. This
results in a larger diameter in the anteroposterior direction
compared with that in the lateral direction. Additionally, when the
maximum longitudinal diameter of a PTC >5 mm, the tumor is more
likely to exhibit a vertical growth pattern; this growth pattern
increases the risk of capsular invasion in PTC (31,32).
Vaish et al (33) used histological results as the gold
standard to calculate the sensitivity, specificity, negative and
positive predictive value, and accuracy of US, CT and US + CT in
detecting the overall, lateral compartment and central compartment
regional metastasis. It was found that CT has higher sensitivity in
detecting lymph node metastasis. CT can be used to complement US to
address the issue of low specificity. The present study used
pathological results as the ‘gold standard’ and combined DECT with
ultrasound image features to diagnose ETE in PTC. Compared with
traditional CT, which mainly relies on morphological features, DECT
uses two different energies of X-rays simultaneously to analyze the
attenuation differences of substances at different energies,
thereby accurately distinguishing tissue components. Meanwhile,
compared with the study by Vaish et al (33), the present study added two
quantitative indicators, IC and NIC, which objectively reflect the
uptake of iodinated contrast agents by PTC and indirectly evaluate
the angiogenesis status of the lesion area. The present study also
included the characteristic parameter λHU of the organization,
which represents the differences in physical density and chemical
composition among different tissues (34). Therefore, IC, NIC and λHU can help
diagnose PTC with or without ETE.
The present study further demonstrated that the PTC
group with ETE exhibits significantly increased IC, NIC and λHU
values in both the arterial and venous phases compared with that
the non-ETE group, which suggested that the PTC group with ETE has
an increased iodine uptake. This could be attributed to the ability
of DECT to depict characteristic spectral curves based on CT values
at different unit quantities and to reflect local lesions and
microvascular perfusion through the slope of the spectral curve.
Because the tissue structures of PTC with metastatic and
non-metastatic ETE differ, their X-ray absorption coefficients
vary, leading to different slopes of the spectral curve. The amount
and capacity of iodine uptake by cells are closely related to the
blood supply (16,34). PTC with ETE contained more
neovascularization and a richer blood supply (35), resulting in a higher iodine uptake
rate and significant enhancement in the early phase of contrast,
whereas the PTC group without ETE had a lower iodine uptake rate.
Additionally, the diagnostic efficacy of the quantitative
parameters in both the arterial and venous phases of ETE was high,
further confirming the significant diagnostic value of DECT
quantitative parameters in the ETE of PTC.
In the present study, the AUC of DECT combined with
GSI parameters and ultrasound morphological parameters for
distinguishing ETE was 0.782, which was higher in comparison with
the AUC of IC, NIC and λHU. The diagnostic efficiency of DECT was
increased compared with that of single-parameter diagnosis. As
ultrasound can clearly display the boundaries of the PTC, DECT has
excellent soft-tissue contrast and quantitative analysis
capabilities, and is advantageous in evaluating the thyroid
envelope and deep tissue (36,37).
DECT and ultrasound could complement each other in evaluating the
ETE of PTC, and their combined use can significantly improve
diagnostic accuracy and comprehensively evaluate the invasiveness
of PTC.
The present study had limitations: i) this was a
single-center study with small sample size; and ii) the recurrence
rate after PTC surgery was not included in the present study.
Further research includes the incorporation of the recurrence rate
of PTC and future large-scale clinical studies to provide more data
for the diagnosis of ETE in PTC.
In conclusion, the diagnostic accuracy of GSI
parameters combined with ultrasound morphology parameters was
superior to that of solitary DECT and ultrasound morphology
examination when identifying ETE in PTC. Integrating dual-source
DECT quantitative parameters and ultrasound image features can
improve the diagnostic accuracy of extraglandular invasion in PTC.
Clinicians can potentially enhance preoperative staging and
treatment planning for patients diagnosed with PTC using
sophisticated imaging methodologies, artificial intelligence
algorithms and molecular markers. Such advancements could
potentially result in improved patient outcomes and individualized
management approaches in the future.
Acknowledgements
Not applicable.
Funding
The present study received contributions from the Jiangsu
Provincial Health Commission Scientific Research Project (grant no.
Z2021071), Zhenjiang City Key R&D Plan-Social Development
(grant no. SH2023049), Jiangsu University Medical Education
Collaborative Innovation Fund (grant no. JDYY2023015) and Jiangsu
University's 22nd batch of college student scientific research
projects (grant no. 22A485).
Availability of data and materials
The data generated in the present study are not
publicly available due to confidential patient information and
privacy concerns but may be requested from the corresponding
author.
Authors' contributions
MAI, NFM and HZ designed the study framework and
wrote the original manuscript. JH and HS conducted data collection
and data analysis. DP and XW analyzed and interpreted data results,
and critically modified the manuscript for important intellectual
content. All authors collaborated in writing the manuscript and
critically reviewed its content. DP and XW confirm the authenticity
of all the raw data. All authors read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
The present study was conducted in compliance with
ethical standards and was approved by the Ethics Committee of
Jiangsu University Affiliated People's Hospital (approval no.
K-20170104-Y; Zhenjiang, China). All participants provided written
informed consent before their inclusion in the present study.
Patient consent for publication
Written informed consent for the publication of
their anonymized data was obtained from all patients involved in
the present study. No identifiable patient information is included
in the present study.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2019. CA Cancer J Clin. 69:7–34. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tran B, Roshan D, Abraham E, Wang L,
Garibotto N, Wykes J, Campbell P and Ebrahimi A: An analysis of the
american joint committee on cancer 8th edition T staging system for
papillary thyroid carcinoma. J Clin Endocrinol Metab.
103:2199–2206. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hay ID, Johnson TR, Thompson GB, Sebo TJ
and Reinalda MS: Minimal extrathyroid extension in papillary
thyroid carcinoma does not result in increased rates of either
cause-specific mortality or postoperative tumor recurrence.
Surgery. 159:11–19. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Haugen BR: 2015 American thyroid
association management guidelines for adult patients with thyroid
nodules and differentiated thyroid cancer: What is new and what has
changed? Cancer. 123:372–381. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Youngwirth LM, Adam MA, Scheri RP, Roman
SA and Sosa JA: Extrathyroidal extension is associated with
compromised survival in patients with thyroid cancer. Thyroid.
27:626–331. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chu KP, Baker S, Zenke J, Morad A, Ghosh
S, Morrish DW, McEwan AJBS, Williams DC, Severin D and McMullen
TPW: Low-activity radioactive iodine therapy for thyroid carcinomas
exhibiting nodal metastases and extrathyroidal extension may lead
to early disease recurrence. Thyroid. 28:902–912. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Liu L, Oh C, Heo JH, Park HS, Lee K, Chang
JW, Jung SN and Koo BS: Clinical significance of extrathyroidal
extension according to primary tumor size in papillary thyroid
carcinoma. Eur J Surg Oncol. 44:1754–1759. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Edge SB and Compton CC: The American joint
committee on cancer: The 7th edition of the AJCC cancer staging
manual and the future of TNM. Ann Surg Oncol. 17:1471–1474. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lee CY, Kim SJ, Ko KR, Chung KW and Lee
JH: Predictive factors for extrathyroidal extension of papillary
thyroid carcinoma based on preoperative sonography. J Ultrasound
Med. 33:231–238. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gweon HM, Son EJ, Youk JH, Kim JA and Park
CS: Preoperative assessment of extrathyroidal extension of
papillary thyroid carcinoma: comparison of 2- and 3-dimensional
sonography. J Ultrasound Med. 33:819–825. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Seo YL, Yoon DY, Lim KJ, Cha JH, Yun EJ,
Choi CS and Bae SH: Locally advanced thyroid cancer: Can CT help in
prediction of extrathyroidal invasion to adjacent structures? AJR
Am J Roentgenol. 195:W240–W244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Y, Li X, Li L, Wang X, Lin M, Zhao X,
Luo D and Li J: Preliminary study on the diagnostic value of
single-source dual-energy CT in diagnosing cervical lymph node
metastasis of thyroid carcinoma. J Thorac Dis. 9:4758–4766. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tian T, Qi Z, Huang S, Wang H and Huang R:
Radioactive iodine therapy decreases the recurrence of
intermediate-risk PTC with low thyroglobulin levels. J Clin
Endocrinol Metab. 108:2033–2041. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Byrd JK, Yawn RJ, Wilhoit CS, Sora ND,
Meyers L, Fernandes J and Day T: Well differentiated thyroid
carcinoma: Current treatment. Curr Treat Options Oncol. 13:47–57.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chen M, Jiang Y, Zhou X, Wu D and Xie Q:
Dual-energy computed tomography in detecting and predicting lymph
node metastasis in malignant tumor patients: A comprehensive
review. Diagnostics (Basel). 14:3772024. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
García-Figueiras R and Baleato-González S:
Quantitative multi-energy CT in oncology: State of the art and
future directions. Eur J Radiol. 182:1118402025. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
García-Figueiras R, Oleaga L, Broncano J,
Tardáguila G, Fernández-Pérez G, Vañó E, Santos-Armentia E, Méndez
R, Luna A and Baleato-González S: What to expect (and what not)
from dual-energy CT imaging now and in the future? J Imaging.
10:1542024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haase V, Hahn K, Schöndube H, Stierstorfer
K, Maier A and Noo F: Single-material beam hardening correction via
an analytical energy response model for diagnostic CT. Med Phys.
49:5014–5037. 2022. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Su GY, Xu XQ, Zhou Y, Zhang H, Si Y, Shen
MP and Wu FY: Texture analysis of dual-phase contrast-enhanced CT
in the diagnosis of cervical lymph node metastasis in patients with
papillary thyroid cancer. Acta Radiol. 62:890–896. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Deng Y, Soule E, Samuel A, Shah S, Cui E,
Asare-Sawiri M, Sundaram C, Lall C and Sandrasegaran K: CT texture
analysis in the differentiation of major renal cell carcinoma
subtypes and correlation with Fuhrman grade. Eur Radiol.
29:6922–6929. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu X, Ouyang D, Li H, Zhang R, Lv Y, Yang
A and Xie C: Papillary thyroid cancer: dual-energy spectral CT
quantitative parameters for preoperative diagnosis of metastasis to
the cervical lymph nodes. Radiology. 275:167–176. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim E, Park JS, Son KR, Kim JH, Jeon SJ
and Na DG: Preoperative diagnosis of cervical metastatic lymph
nodes in papillary thyroid carcinoma: Comparison of ultrasound,
computed tomography, and combined ultrasound with computed
tomography. Thyroid. 18:411–418. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cho SJ, Suh CH, Baek JH, Chung SR, Choi YJ
and Lee JH: Diagnostic performance of CT in detection of metastatic
cervical lymph nodes in patients with thyroid cancer: A systematic
review and meta-analysis. Eur Radiol. 29:4635–4647. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haugen BR, Alexander EK, Bible KC, Doherty
GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM,
Schlumberger M, et al: 2015 American thyroid association management
guidelines for adult patients with thyroid nodules and
differentiated thyroid cancer: The American thyroid association
guidelines task force on thyroid nodules and differentiated thyroid
cancer. Thyroid. 26:1–133. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Alhassan R, Al Busaidi N, Al Rawahi AH, Al
Musalhi H, Al Muqbali A, Shanmugam P and Ramadhan FA: Features and
diagnostic accuracy of fine needle aspiration cytology of thyroid
nodules: Retrospective study from Oman. Ann Saudi Med. 42:246–251.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang H, Zhao S, Yao J, Yu X and Xu D:
Factors influencing extrathyroidal extension of papillary thyroid
cancer and evaluation of ultrasonography for its diagnosis: A
retrospective analysis. Sci Rep. 13:183442023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guerrini S, Bagnacci G, Perrella A, Meglio
ND, Sica C and Mazzei MA: Dual energy CT in oncology: benefits for
both patients and radiologists from an emerging quantitative and
functional diagnostic technique. Semin Ultrasound CT MR.
44:205–213. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han A, Liu Y, Cai L, Xie J and Hu S: The
diagnostic performance of dual-energy CT imaging in cervical lymph
node metastasis of papillary thyroid cancer: A meta-analysis. Front
Med (Lausanne). 11:14573072024. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tunlayadechanont P and Sananmuang T:
Dual-energy CT in head and neck applications. Neuroradiol J.
19714009251313507. Jan 8–2025.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhou L, Zhu Q, Yao J, Yang C and Xu D:
Correlation analysis of nodular sonographic parameters with
cervical lymph node metastases in papillary thyroid carcinoma.
Biomed Res Int. 2022:46800642022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Ren J, Liu B, Zhang LL, Li HY, Zhang F, Li
S and Zhao LR: A taller-than-wide shape is a good predictor of
papillary thyroid carcinoma in small solid nodules. J Ultrasound
Med. 34:19–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Vaish R, Mahajan A, Sable N, Dusane R,
Deshmukh A, Bal M and D'cruz AK: Role of computed tomography in the
evaluation of regional metastasis in well-differentiated thyroid
cancer. Front Radiol. 3:12430002023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Geng D, Zhou Y, Shang T, Su GY, Lin SS, Si
Y, Wu FY and Xu XQ: Effect of Hashimoto's thyroiditis on the
dual-energy CT quantitative parameters and performance in
diagnosing metastatic cervical lymph nodes in patients with
papillary thyroid cancer. Cancer Imaging. 24:102024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Zhang L, Li Y, Chen Z, Dai X, Gao H and
Chen Y: Diagnostic performance of dual-energy computed tomography
(DECT) quantitative parameters for detecting metastatic cervical
lymph nodes in patients with papillary thyroid cancer: A systematic
review and meta-analysis. Eur J Radiol. 183:1119172025. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yoon J, Choi Y, Jang J, Shin NY, Ahn KJ
and Kim BS: Preoperative assessment of cervical lymph node
metastases in patients with papillary thyroid carcinoma:
Incremental diagnostic value of dual-energy CT combined with
ultrasound. PLoS One. 16:e02612332021. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Santos Armentia E, Martín Noguerol T,
Silva Priegue N, Delgado Sánchez-Gracián C, Trinidad López C and
Prada González R: Strengths, weaknesses, opportunities, and threat
analysis of dual-energy CT in head and neck imaging. Radiologia
(Engl Ed). 64:333–347. 2022. View Article : Google Scholar : PubMed/NCBI
|