Introduction
Hepatocellular carcinoma (HCC) ranks as the fifth
most prevalent carcinoma globally and holds the position of the
third most frequent cause of tumor-associated mortality (1). At present, approximately one-half of
all HCC cases are reported in China, which is largely attributed to
the widespread incidence of chronic hepatitis within the nation
(2,3). Regarding cancer-related mortality in
China, HCC ranks as the second leading cause of cancer-related
death among males and the third leading cause in females (4). A notable aspect of HCC is its tendency
for vascular infiltration, especially in the portal vein. This
often results in portal vein tumor thrombosis (PVTT), affecting
10–40% of individuals at the time of HCC diagnosis. Such malignant
progression detrimentally impacts hepatic functionality and causes
portal hypertension (5). The life
expectancy for patients afflicted by this condition is
significantly shortened, with a median overall survival (OS) time
of ~3 months in the absence of therapeutic intervention (6).
Currently, the use of systemic treatments involving
targeted therapy and immunotherapies for HCC with PVTT has been
relatively accepted and advocated for (7,8).
Research indicates that transarterial chemoembolization (TACE)
enhances survival prospects for HCC sufferers with PVTT (9). The National Comprehensive Cancer
Network (NCCN) and Chinese National Liver Cancer (CNLC) guidelines
also endorse TACE (10,11). Moreover, in China, TACE is
frequently utilized to treat HCC with PVTT, particularly due to
economic factors that restrict access to systemic therapies for
some patients (12).
In the realm of liver cancer treatment, hepatic
arterial infusion chemotherapy (HAIC), building upon the foundation
of the folinic acid, fluorouracil and oxaliplatin (FOLFOX) regimen,
has demonstrated efficacy in managing advanced HCC. Studies have
revealed that HAIC, in comparison to TACE as a sole therapy,
markedly enhances patient OS time (13,14).
It is evident that the integration of HAIC with TACE not only
amplifies the overall response rate (ORR) but also the survival
rates in individuals grappling with inoperable HCC (15). Nevertheless, for patients with HCC
who are afflicted with PVTT, a potent combination therapy remains
unexplored. The present study compares the effectiveness and safety
of a combined regimen, TACE/HAIC with tyrosine kinase inhibitors
(TKIs) and programmed cell death protein 1 (PD-1) inhibitors,
against TACE in isolation, specifically targeting patients with HCC
plus PVTT.
Materials and methods
Study cohort
The clinical data from 251 patients diagnosed with
HCC combined with portal vein tumor thrombosis (PVTT) who were
admitted to Harbin Medical University Cancer Hospital (Harbin,
China) between January 2021 and December 2022 were retrospectively
collected and analyzed. Hepatic cancer diagnoses were confirmed
using imaging techniques, such as computed tomography (CT) and
magnetic resonance imaging (MRI), adhering to guidelines set by the
European Association for the Study of the Liver and the American
Association for the Study of Liver Diseases (16,17).
PVTT severity was subclassified based on established PVTT criteria
(18) as follows: Vp1,
characterized by a tumor thrombus presence distal to the main
portal vein, excluding the secondary branches; Vp2, indicating a
tumor thrombus within secondary branches; Vp3, characterized by a
tumor thrombus in the primary branches; and Vp4, denoting a tumor
thrombus in the main trunk, its opposite counterpart or both.
The following inclusion criteria were applied: i)
Individuals diagnosed with primary HCC combined with PVTT (Vp1-4
stages) who received initial treatment either through TACE or a
combination therapy that included TACE-HAIC along with a TKI or a
PD-1 inhibitor; ii) participants within the age range of 18 to 75
years; iii) patients whose liver function was categorized as
Child-Pugh class A or B; iv) patients with an Eastern Cooperative
Oncology Group (ECOG) performance status score ≤1.
The following exclusion criteria were applied: i)
Individuals diagnosed with severe diseases of the heart, lungs or
kidneys; ii) patients with a prior diagnosis of another distinct
primary cancer; iii) patients with medical records that were not
comprehensive; and iv) patients who were not available for
subsequent follow-up.
Finally, 251 patients were included and divided into
four groups based on the treatment method, with 16 administered
TACE + HAIC + lenvatinib + camrelizumab (Group 1), 90 administered
TACE + lenvatinib + camrelizumab (Group 2), 102 administered HAIC +
lenvatinib + camrelizumab (Group 3) and 43 administered TACE alone
(Group 4). The present study was approved by the Ethics Committee
of Harbin Medical University Cancer Hospital (approval no.
YD2024-11). All patients provided written informed consent for
participation in the study, and all procedures were carried out in
accordance with the Declaration of Helsinki.
Medication protocol
The process of TACE was executed as previously
described (19). This procedure
entailed utilizing a combination of 20–30 mg pirarubicin and 50 mg
oxaliplatin/loplatin, amalgamated with 2–10 ml of an iodized
oil-based agent. The injection of up to 20 ml of this agent
directly into the arteries feeding the tumor was performed to
achieve stasis in blood flow within the target artery. Repeated
sessions of TACE were scheduled every 3–4 weeks.
The TACE-HAIC protocol was performed as previously
described (15). The
chemoembolization process involved combining 30 mg/m2
doxorubicin with 2–10 ml of an iodized oil-based agent, followed by
the administration of the pure agent. A catheter was then inserted
and fixed in the artery supplying the tumor. This was used for
administering FOLFOX-based chemotherapy, comprising 85
mg/m2 oxaliplatin over 2 h, 400 mg/m2 calcium
folinate over the same duration, a 400-mg/m2
fluorouracil (5-FU) bolus, and a choice between a
2,400-mg/m2 continuous 5-FU infusion over 46 h or a
1,200-mg/m2 continuous infusion over 23 h. These
TACE-HAIC sessions were repeated every 3–4 weeks.
The TKI applied in the present study was lenvatinib
(daily; 12 mg orally for a body weight ≥60 kg and 8 mg for a body
weight <60 kg). Lenvatinib administration began on the first day
after TACE-HAIC and continued until disease progression or the
onset of severe treatment-associated toxicity. On the first day
after TACE-HAIC, a PD-1 inhibitor was administered intravenously
every 3–4 weeks, consisting of either 200 mg carrelizumab or 200 mg
sindilizumab.
Treatment response evaluation
Between 3 and 4 weeks after treatment, tumor
responses were assessed using enhanced computed tomography (CT) or
magnetic resonance imaging (MRI). Two skilled radiologists examined
the radiological behavior of the tumor, referencing the modified
Response Evaluation Criteria in Solid Tumors (20). The tumor responses were
subclassified into four categories: Complete remission (CR),
partial remission (PR), stable disease (SD) and progressive
disease. The ORR was used to represent the combined CR and PR
rates. Additionally, the disease control rate (DCR) was defined as
the sum of the ORR and the SD rate, reflecting the proportion of
patients with CR, PR, or SD.
Follow-up
Prior to each treatment cycle, patients underwent
liver imaging through CT or MRI, accompanied by various blood
examinations. These included tests for serum α-fetoprotein, liver
function, whole blood profile and coagulation assessments.
Post-surgical follow-ups were scheduled at 1-month intervals
initially, and subsequently once every 3–4 months during the first
2 years. The assessment of any adverse events was conducted in
accordance with the Common Toxicity Criteria version 5.0 set by the
National Cancer Institute (21).
Imaging protocols
To ensure consistency in imaging characteristics,
resolution and sensitivity to tumor features, all patients
underwent contrast-enhanced CT or MRI scans with standardized
protocols. CT was performed with a 64-slice multi-detector scanner
(slice thickness, 1 mm) using a standard contrast agent (iohexol),
while MRI utilized a 1.5T or 3T scanner with gadolinium-based
contrast agents. Tumors were characterized by size, location and
enhancement patterns, with HCC diagnosed based on hypervascularity
and washout patterns. PVTT was evaluated for thrombus involvement
and extension. Imaging data were reviewed by two independent
radiologists to ensure consistency. Disagreements between the
radiologists regarding tumor response assessment were resolved by
following a predefined decision hierarchy: i) Discussion between
the two radiologists to reach a consensus, ii) if no agreement was
reached, a third radiologist, blinded to the initial assessments,
was consulted to provide the final decision. Quality control
measures were implemented across all sites, including regular
calibration and training. These standardized protocols aimed to
minimize variation in tumor assessment and ensure reliable imaging
results.
Statistical analysis
All statistical analyses were performed using SPSS
version 22.0 (IBM Corp.) and R software version 4.3.0 (https://www.r-project.org/). Continuous variables were
assessed for normality using the Shapiro-Wilk test. Normally
distributed continuous variables are presented as the mean ±
standard deviation and were compared using one-way analysis of
variance, followed by Tukey's post hoc test for multiple pairwise
comparisons. Non-normally distributed continuous variables are
presented as the median (interquartile range) and were compared
using the Kruskal-Wallis test, followed by Dunn's test with
Bonferroni's correction for post hoc multiple comparisons.
Categorical variables are expressed as frequencies and percentages.
Intergroup comparisons were initially performed using the
χ2 test. In cases where the expected count in >20% of
the cells was <5, Fisher's exact test was used instead. To
adjust for multiple comparisons among categorical variables,
Bonferroni's correction was applied where appropriate. All
statistical tests were two-sided, and P<0.05 was considered to
indicate a statistically significant difference. Adjusted P-values
following multiple comparisons are reported where applicable.
Results
Baseline characteristics
The baseline characteristics and statistical
comparisons of the patients in the four treatment groups are
summarized in Table I. Significant
differences were observed in several demographic and clinical
variables. The mean age differed significantly among the groups
(P=0.024), although no statistically significant difference was
found in the post hoc analysis. Sex distribution also varied
(P=0.018), with group 4 exhibiting a notably higher proportion of
female patients (34.9%) compared with the other groups. There were
no significant differences in preoperative liver function
parameters, including aspartate aminotransferase, alanine
aminotransferase, total bilirubin and prothrombin time, among the
groups (all P>0.05). However, serum albumin levels differed
significantly (P=0.010), with post hoc analysis indicating that
group 2 had significantly higher albumin levels compared with group
3 (P=0.009). Tumor burden differed markedly between groups. For
example, tumor count (P<0.001) showed significant variation,
with group 3 predominantly comprising patients with ≤3 tumors
(94.1%), whereas group 2 had a majority with >3 tumors (94.4%).
Similarly, maximum tumor diameter significantly varied across
groups (P<0.001), with group 2 including exclusively patients
with tumors ≥100 mm, while the majority of patients in group 4
(76.7%) and group 3 (60.8%) had tumors <100 mm. Post hoc
comparisons revealed significant differences between group 1 and
group 2 (P<0.05 for both tumor count and size). Regarding
medical history, significant differences were found in the
prevalence of hepatitis B and hepatitis C infection (both
P<0.001). Hepatitis B was more common in groups 2, 3 and 4,
while hepatitis C was predominantly seen in group 1 (93.8%). The
prevalence of liver cirrhosis and ascites also differed
significantly among the groups (both P<0.001), with group 1
having the lowest incidence of cirrhosis (25.0%) and the highest
incidence of ascites (81.3%). ECOG performance status and
pretreatment α-fetoprotein levels did not differ significantly
among the groups (P=0.285 and P=0.299, respectively). However,
Child-Pugh classification differed significantly (P<0.001), with
post hoc analysis showing a higher proportion of Class B patients
in group 3 compared with group 2 (P<0.001). Treatment-related
adverse events (AEs) were markedly more frequent and severe in
group 1 compared with other groups. All patients in Group 1
(100.0%) experienced fatigue, diarrhea, nausea and vomiting,
decreased appetite, weight loss, joint pain, and there was a high
incidence of other immune-related AEs such as rash (68.8%), oral
mucositis (93.8%), hand-foot syndrome (75.0%), bleeding events
(93.8%), and immune-related pneumonitis (93.8%). These AEs were
significantly more frequent than those observed in group 2 (all
P<0.001), as confirmed by post hoc analysis with Bonferroni's
correction.
 | Table I.Clinical information of patients with
hepatocellular carcinoma in the four treatment groups. |
Table I.
Clinical information of patients with
hepatocellular carcinoma in the four treatment groups.
| Characteristic | Group 1 | Group 2 | Group 3 | Group 4 | P-value | Between-group
variations |
|---|
| Age, years | 53.38±7.24 | 54.07±10.02 | 57.49±8.59 | 53.58±9.35 | 0.024 | NS |
| Sex, n (%) |
|
|
|
| 0.018 | NS |
|
Male | 15 (93.75) | 77 (85.6) | 87 (85.3) | 28 (65.12) |
|
|
|
Female | 1 (6.25) | 13 (14.4) | 15 (14.7) | 15 (34.88) |
|
|
| Preoperative
indices |
|
|
|
|
|
|
| AST,
U/l | 54.00 | 52.00 | 53.00 | 54.00 | 0.926 | NS |
|
| (42.25,
102.50) | (35.50, 91.50) | (38.00, 82.25) | (39.00, 83.00) |
|
|
| ALT,
U/l | 40.50 | 35.00 | 36.50 | 37.00 | 0.955 | NS |
|
| (25.00, 53.25) | (26.00, 63.00) | (24.00, 57.75) | (26.00, 52.00) |
|
|
| TB,
µmol/l | 20.30 | 20.40 | 20.35 | 20.10 | 0.961 | NS |
|
| (13.40, 26.25) | (13.90, 26.60) | (13.68, 28.38) | (16.90, 28.10) |
|
|
| PT,
sec | 12.20 | 12.00 | 12.30 | 12.50 | 0.167 | NS |
|
| (11.65, 12.88) | (11.35, 12.95) | (11.78, 13.00) | (11.90, 13.10) |
|
|
|
Albumin, g/l | 40.90 | 39.45 | 37.65 | 39.50 | 0.01 | Group 2 vs. group
3, P=0.009 |
|
| (36.93, 42.10) | (35.80, 43.30) | (34.28, 40.80) | (36.00, 42.30) |
|
|
| Tumor count, n
(%) |
|
|
|
| <0.001 | Group 1 vs. group
2, P=0.0047 |
| ≤3 | 6 (37.5) | 5 (5.6) | 96 (94.1) | 27 (62.8) |
|
|
|
>3 | 10 (62.5) | 85 (94.4) | 6 (5.9) | 16 (37.2) |
|
|
| Maximum tumor
diameter, n (%) |
|
|
|
| <0.001 | Group 1 vs. group
2, P<0.001 |
| <100
mm | 8 (50.0) | 0 (0.0) | 62 (60.8) | 33 (76.7) |
|
|
| ≥100
mm | 8 (50.0) | 90 (100.0) | 40 (39.2) | 10 (23.3) |
|
|
| Medical history, n
(%) |
|
|
|
|
|
|
|
Hepatitis B | 4 (25.0) | 70 (77.8) | 73 (71.6) | 34 (79.1) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Hepatitis C | 15 (93.8) | 4 (4.4) | 15 (14.7) | 3 (7.00) | <0.001 | Group 1 vs. group
2. P<0.001 |
|
Cirrhosis | 4 (25.0) | 67 (74.4) | 75 (73.5) | 33 (76.7) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Ascites | 13 (81.3) | 18 (20.0) | 34 (33.3) | 2 (4.7) | <0.001 | Group 1 vs. group
2, P<0.001 |
| ECOG, n (%) |
|
|
|
| 0.285 | NS |
|
<1 | 12 (75.0) | 51 (56.7) | 51 (50.0) | 32 (74.4) |
|
|
| ≥1 | 4 (25.0) | 39 (43.3) | 51 (50.0) | 11 (25.6) |
|
|
| Child-Pugh
classification |
|
|
|
| <0.001 | Group 2 vs. group
3, P<0.001 |
| A | 14 (87.5) | 90 (100.0) | 80 (78.4) | 39 (90.7) |
|
|
| B | 2 (12.5) | 0 (0.0) | 22 (21.6) | 4 (9.3) |
|
|
| Pre-treatment AFP,
n (%) |
|
|
|
| 0.299 | NS |
| <400
ng/ml | 7 (43.8) | 46 (51.1) | 54 (52.9) | 16 (38.1) |
|
|
| ≥400
ng/ml | 9 (56.2) | 44 (48.9) | 48 (47.1) | 27 (61.9) |
|
|
|
Treatment-associated adverse events, n
(%) |
|
|
|
|
|
|
|
Fatigue | 16 (100.0) | 6 (6.7) | 46 (45.1) | 5 (11.6) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Abdominal pain | 15 (93.8) | 0 (0.0) | 42 (41.2) | 2 (4.7) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Diarrhea | 16 (100.0) | 7 (7.8) | 39 (38.2) | 2 (4.7) | <0.001 | Group 1 vs. group
2, P<0.001 |
| Nausea
and vomiting | 16 (100.0) | 5 (5.6) | 37 (36.3) | 5 (11.6) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Decreased appetite | 16 (100.0) | 7 (7.8) | 42 (41.2) | 8 (18.6) | <0.001 | Group 1 vs. group
2, P<0.001 |
| Weight
loss | 16 (100.0) | 7 (7.8) | 49 (48.0) | 8 (18.6) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Rash | 11 (68.8) | 8 (8.9) | 26 (25.5) | 0 (0.0) | <0.001 | Group 1 vs. group
2, P<0.001 |
| Oral
mucositis | 15 (93.8) | 6 (6.7) | 12 (11.8) | 0 (0.0) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Hand-foot syndrome | 12 (75.0) | 2 (2.2) | 14 (13.7) | 0 (0.0) | <0.001 | Group 1 vs. group
2, P<0.001 |
| Joint
pain | 16 (100.0) | 2 (2.2) | 9 (8.8) | 0 (0.0) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Bleeding events | 15 (93.8) | 4 (4.4) | 30 (29.4) | 0 (0.0) | <0.001 | Group 1 vs. group
2, P<0.001 |
|
Immune-related
pneumonitis | 15 (93.8) | 2 (2.2) | 9 (8.8) | 0 (0.0) | <0.001 | Group 1 vs. group
2, P<0.001 |
Comparison between the patients with
HCC treated with TACE + HAIC + lenvatinib + camrelizumab (group 1)
and those treated with TACE (group 4)
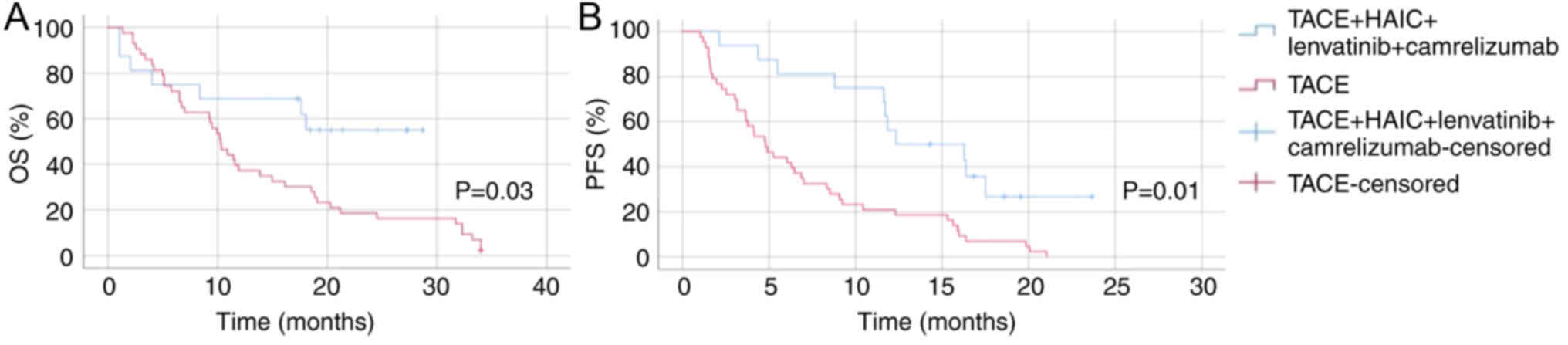
The Kaplan-Meier survival curves for OS and
progression-free survival (PFS) in patients with HCC and PVTT
treated with TACE-HAIC combined with lenvatinib and camrelizumab
(group 1) vs. TACE alone (group 4) are shown in Fig. 1. For OS, patients in group 1
exhibited a significantly longer survival time compared with those
in group 4 (P=0.03). Regarding PFS time, group 1 also showed marked
improvement over group 4 (P=0.01). The survival analysis
underscores the enhanced benefit of adding lenvatinib and
camrelizumab to TACE-HAIC in terms of extending the
progression-free interval. These results collectively suggest that
TACE-HAIC combined with lenvatinib and camrelizumab significantly
improves both OS and PFS times in patients with HCC and PVTT,
compared with TACE alone, highlighting the potential of this
combination therapy as a more effective treatment strategy.
Comparison between the patients with
HCC treated with TACE + lenvatinib + camrelizumab (group 2) and
those treated with TACE (group 4)
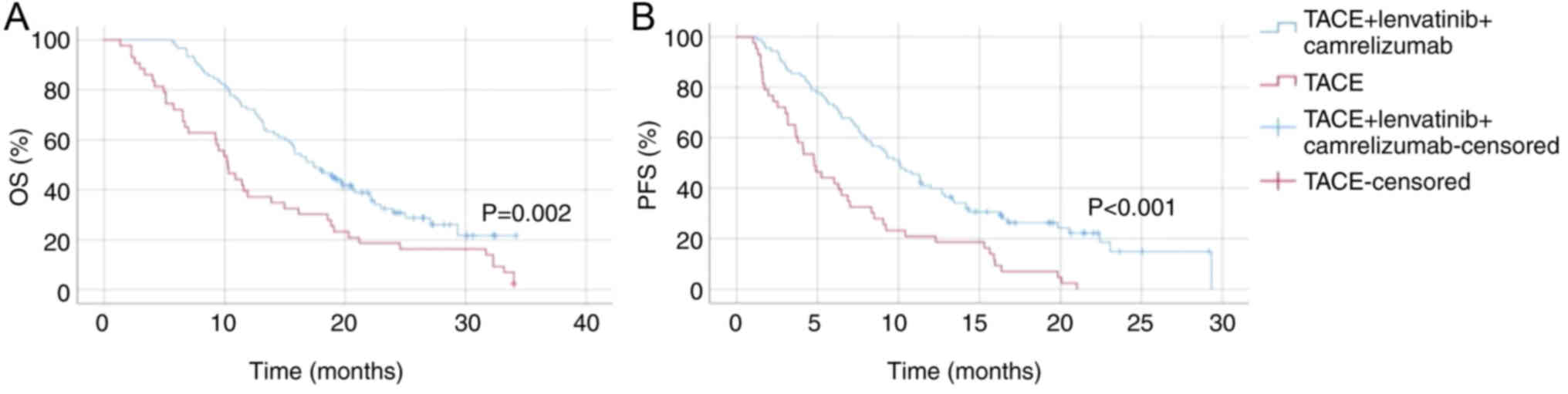
Group 2 demonstrated a significantly longer OS time
compared with group 4 (P=0.002; Fig.
2). The median OS time for group 2, which was ~18 months,
extended beyond the observed period, while the median OS time for
group 4 was ~10 months. The 12-month survival rate was notably
higher in group 2, underscoring the improved efficacy of the
combined treatment. In terms of PFS time, group 2 also showed a
substantial improvement over group 4 (P<0.001; Fig. 2). The median PFS for group 2 was
markedly longer, with a higher proportion of patients remaining
progression-free at 12 months compared with group 4, which had a
median PFS time of ~5 months. These findings indicate that TACE
combined with lenvatinib and camrelizumab significantly enhances
both OS and PFS time in patients with HCC and PVTT, compared with
TACE alone.
Comparison between the patients with
HCC treated with HAIC + lenvatinib + camrelizumab (group 3) and
those treated with TACE (group 4)
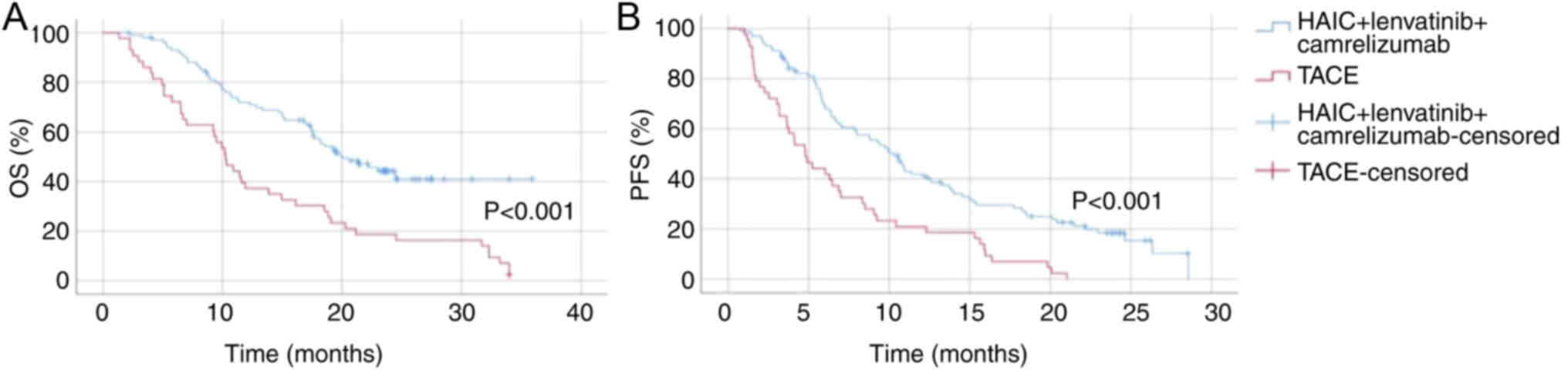
Group 3 exhibited a significantly prolonged OS time
compared with group 4 (P<0.001; Fig.
3). Regarding PFS, group 3 also showed a marked improvement
over group 4 (P<0.001; Fig. 3).
The median PFS time for group 3 was markedly longer, with a higher
proportion of patients remaining progression-free at 12 months
compared with group 4, which had a median PFS time of ~5 months.
These results demonstrate that HAIC combined with lenvatinib and
camrelizumab significantly enhances both OS and PFS times in
patients with HCC and PVTT compared with TACE alone, supporting the
efficacy of this combination therapy.
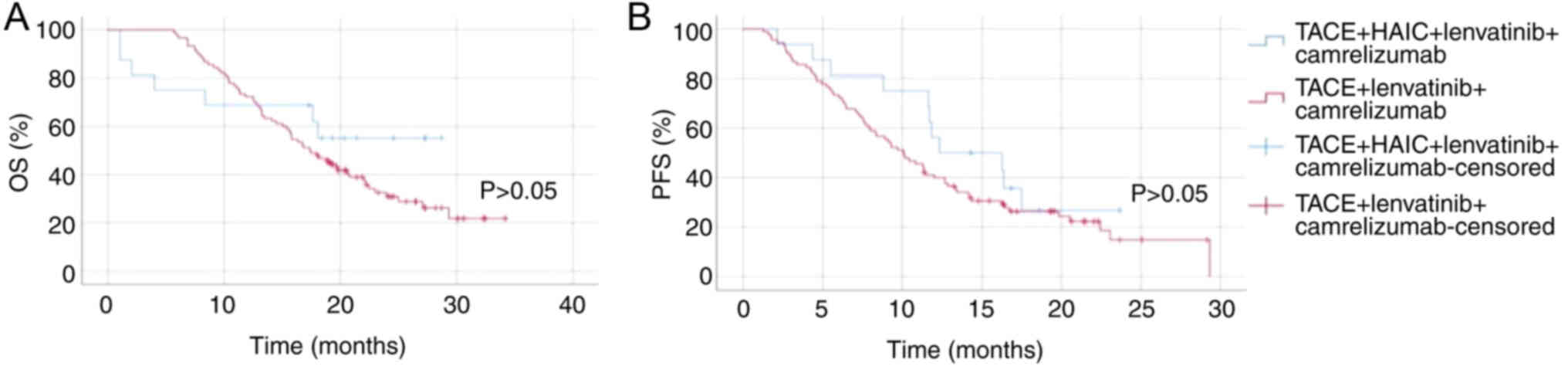
Comparison between the patients with
HCC treated with TACE + HAIC + lenvatinib + camrelizumab (group 1)
and those treated with HAIC + lenvatinib + camrelizumab (group
3)
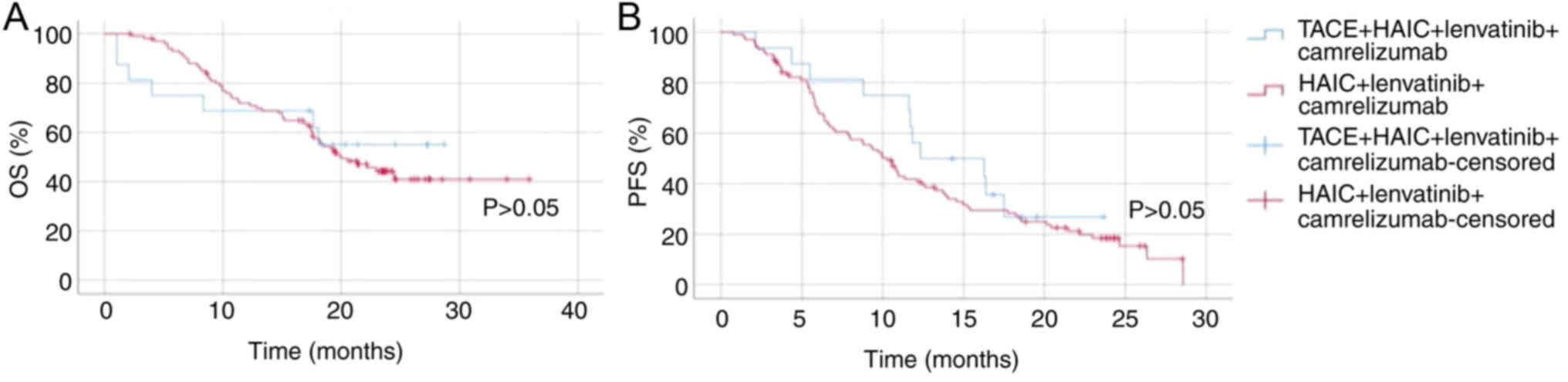
For OS time, there was no significant difference
between group 1 and group 3 (P>0.05; Fig. 4). Both groups exhibited similar
survival rates over the observed period, indicating that the
addition of TACE to HAIC combined with lenvatinib and camrelizumab
did not significantly impact OS. In terms of PFS, group 1
demonstrated a slight improvement over group 3, but there was no
significant difference (P>0.05; Fig.
4). Overall, these results indicate that while there is a
slight non-significant improvement in PFS with the addition of TACE
to HAIC combined with lenvatinib and camrelizumab, the overall
survival benefit remains similar between the two treatment
strategies.
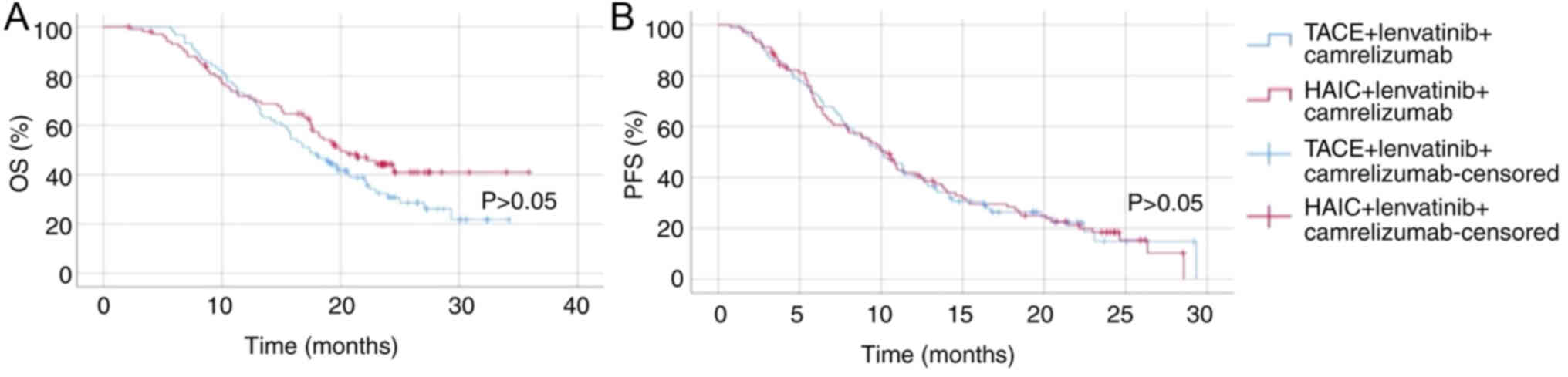
Comparison between the patients with
HCC treated with TACE + lenvatinib + camrelizumab (group 2), those
treated with both TACE + HAIC + lenvatinib + camrelizumab (group 1)
and those treated with HAIC + lenvatinib + camrelizumab (group
3)
In terms of OS time, there was no significant
difference between group 2 (TACE + lenvatinib + camrelizumab) and
group 3 (HAIC + lenvatinib + camrelizumab) (P>0.05; Fig. 5). Both groups demonstrated
comparable survival profiles throughout the observation period,
suggesting that substituting TACE with HAIC in combination with
lenvatinib and camrelizumab did not result in a marked improvement
in OS time. Regarding PFS time, no statistically significant
difference was observed between group 2 and group 3 either
(P>0.05; Fig. 5), further
indicating similar clinical efficacy between the two approaches.
Similarly, when comparing group 1 (TACE + HAIC + lenvatinib +
camrelizumab) with group 2, no significant difference in OS time
was identified (P>0.05; Fig. 6).
Both regimens yielded parallel survival trends, implying that the
addition of HAIC to the TACE-based regimen did not significantly
enhance OS time. Although a slight numerical improvement in PFS
time was noted in group 1 compared with group 2, it did not reach
statistical significance (P>0.05; Fig. 6). Collectively, these findings
suggest that the incorporation of either TACE or HAIC, or both,
into a systemic regimen with lenvatinib and camrelizumab offers
comparable survival outcomes in this patient population.
Discussion
The present study highlighted the potential benefits
of an integrated therapeutic approach for patients with HCC and
PVTT, a subgroup traditionally associated with a poor prognosis
(18). Despite advancements in
systemic therapies for HCC, the specific challenges posed by PVTT
require more nuanced treatment strategies. The results from the
present study indicated that combination therapy of TACE-HAIC with
tyrosine kinase inhibitors (TKIs) and PD-1 inhibitors can
significantly improve survival outcomes compared with TACE
alone.
The enhanced efficacy of the combination therapy is
evidenced by the markedly better survival metrics. For instance,
patients treated with TACE-HAIC + lenvatinib + camrelizumab (group
1) exhibited significantly higher OS and PFS times compared with
those treated with TACE alone (group 4), with P-values of 0.03 and
0.01, respectively. Additionally, group 2 (TACE + lenvatinib +
camrelizumab) and group 3 (HAIC + lenvatinib + camrelizumab) both
demonstrated improved OS and PFS times compared with group 4, with
statistically significant differences (both P<0.05). Consistent
with these findings, a number of previous studies have investigated
both the effectiveness and the safety aspects of localized
therapies (either TACE or HAIC individually) and their combination
in treating HCC (22,23). The current research reinforces the
effectiveness of combining TACE-HAIC, targeted therapy and
immunotherapy for treating patients with HCC, particularly those
with PVTT (24,25). These findings align with the
hypothesis that an aggressive and multifaceted approach can be
beneficial in this patient subset, corroborating earlier research
that advocates for the use of multimodal therapies in advanced HCC
with vascular invasion. Combination therapy in HCC with PVTT
provides comprehensive antitumor effects. Primarily,
chemotherapeutic agents trigger tumor cell apoptosis via
jeopardizing DNA, alongside inducing immunogenic cell death. Such
actions provoke a defensive antitumor immune reaction, thus
boosting the potency of immunotherapy (26). Furthermore, TKIs harbor
anti-proliferative and anti-angiogenic properties that effectively
oppose hypoxia-driven angiogenesis via TACE-HAIC (27). Moreover, TKI therapy, when merged
with anti-PD-1 treatment, has shown synergistic effects. This
combination alters the immune environment of the tumor and enhances
T cell penetration within it (28,29).
In addition, anti-PD-1 treatment intensifies the ability of the
immune system to combat tumors, thereby strengthening the overall
immune assault on cancer cells (30,31).
It should be noted that the lack of statistical significance
between the quadruple and triple therapy groups may be due to the
sample size.
The notable improvements in therapeutic outcomes
must be balanced against the higher incidence of adverse events
observed in the combination therapy group. While most events were
manageable, the presence of adverse effects such as abdominal
discomfort, diarrhea and appetite reduction in nearly all patients
receiving combination therapy in the present study cannot be
overlooked. This underscores the need for a careful selection of
patients who are likely to tolerate and benefit from this intensive
treatment regimen, considering the marked demands it places on
patient quality of life.
While the current study presents promising results,
it is imperative to acknowledge its limitations, including its
retrospective nature and the relatively small sample size, which
may influence the generalizability of the findings.
In conclusion, the present study successfully
revealed that TACE-HAIC combined with lenvatinib and camrelizumab
significantly improves both OS and PFS times in patients with HCC
and PVTT compared with TACE alone, despite a higher incidence of
adverse events. This combination therapy represents a promising
treatment strategy for this patient population, offering enhanced
survival benefits.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
RL was responsible for conceptualization,
methodology, resources, supervision and funding acquisition. DH was
responsible for checking the data collection and follow-up data,
writing (reviewing and editing), retrospective collection of
clinopathological data and follow-up data aquisition and formal
analysis. XH and QX checked the data collection and follow-up data,
wrote the original draft and performed formal analysis. LY and HW
were responsible for retrospective collection of clinopathological
data and follow-up data aquisition, checking the data collection
and follow-up data and writing (review and editing). JW and BL
performed the retrospective collection of clinopathological data
and follow-up data aquisition and formal analysis. XH and QX
confirm the authenticity of all the raw data. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The study was approved by the Ethics Committee of
the Harbin Medical University Cancer Hospital (Harbin, China;
approval no. YD2024-11). The procedures used in this study were
performed in accordance with the ethical standards as laid down in
the 1964 Declaration of Helsinki and its later amendments or
comparable ethical standards. Written informed consent was obtained
from all patients.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
HCC
|
hepatocellular carcinoma
|
|
PVTT
|
portal vein tumor thrombosis
|
|
TACE
|
transarterial chemoembolization
|
|
HAIC
|
hepatic arterial infusion
chemotherapy
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
ORR
|
overall response rate
|
|
TKI
|
tyrosine kinase inhibitor
|
|
ECOG
|
Eastern Cooperative Oncology Group
|
|
CR
|
complete remission
|
|
PR
|
partial remission
|
|
SD
|
stable disease
|
References
|
1
|
Singal A, Kanwal F and Llovet J: Global
trends in hepatocellular carcinoma epidemiology: Implications for
screening, prevention and therapy. Nat Rev Clin Oncol. 20:864–884.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baecker A, Liu X, La Vecchia C and Zhang
ZF: Worldwide incidence of hepatocellular carcinoma cases
attributable to major risk factors. Eur J Cancer Prev. 27:205–212.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Konyn P, Ahmed A and Kim D: Current
epidemiology in hepatocellular carcinoma. Expert Rev Gastroenterol
Hepatol. 15:1295–1307. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tao ZW, Cheng BQ, Zhou T and Gao YJ:
Management of hepatocellular carcinoma patients with portal vein
tumor thrombosis: A narrative review. Hepatobiliary Pancreat Dis
Int. 21:134–144. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forner A, Llovet JM and Bruix J:
Hepatocellular carcinoma. Lancet. 379:1245–1255. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tella SH, Kommalapati A and Mahipal A:
Systemic therapy for advanced hepatocellular carcinoma: Targeted
therapies. Chin Clin Oncol. 10:102021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fan Y, Xue H and Zheng H: Systemic therapy
for hepatocellular carcinoma: Current updates and outlook. J
Hepatocell Carcinoma. 9:233–263. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lau WY, Wang K, Zhang XP, Li LQ, Wen TF,
Chen MS, Jia WD, Xu L, Shi J, Guo WX, et al: A new staging system
for hepatocellular carcinoma associated with portal vein tumor
thrombus. Hepatobiliary Surg Nutr. 10:782–795. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chung GE, Lee JH, Kim HY, Hwang SY, Kim
JS, Chung JW, Yoon JH, Lee HS and Kim YJ: Transarterial
chemoembolization can be safely performed in patients with
hepatocellular carcinoma invading the main portal vein and may
improve the overall survival. Radiology. 258:627–634. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang YF, Guo RP, Zou RH, Shen JX, Wei W,
Li SH, OuYang HY, Zhu HB, Xu L, Lao XM and Shi M: Efficacy and
safety of preoperative chemoembolization for resectable
hepatocellular carcinoma with portal vein invasion: A prospective
comparative study. Eur Radiol. 26:2078–2088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao XH, Yuan H, Xia WL, Zhang LL, Li Z,
Cao GS, Li HL, Fan WJ, Li HL, Guo CY, et al: Prospective study of
TACE combined with sorafenib vs TACE combined with 125I
seed implantation in the treatment of hepatocellular carcinoma with
portal vein tumor thrombus and arterioportal fistulas. Front Oncol.
12:9774622022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sidaway P: FOLFOX-HAIC active in large
HCC. Nat Rev Clin Oncol. 19:52022. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu SJ, Xu X, Chen MP, Zhao ZY, Wang Y,
Yin X, Zhang L, Ge NL, Chen Y, Wang YH, et al: Hepatic arterial
infusion chemotherapy with modified FOLFOX as an alternative
treatment option in advanced hepatocellular carcinoma patients with
failed or unsuitability for transarterial chemoembolization. Acad
Radiol. 28 (Suppl 1):S157–S166. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li B, Qiu J, Zheng Y, Shi Y, Zou R, He W,
Yuan Y, Zhang Y, Wang C, Qiu Z, et al: Conversion to resectability
using transarterial chemoembolization combined with hepatic
arterial infusion chemotherapy for initially unresectable
hepatocellular carcinoma. Ann Surg Open. 2:e0572021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
European Association for the Study of the
Liver, . EASL-ILCA clinical practice guidelines on the management
of intrahepatic cholangiocarcinoma. J Hepatol. 79:181–208. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Marrero JA, Kulik LM, Sirlin CB, Zhu AX,
Finn RS, Abecassis MM, Roberts LR and Heimbach JK: Diagnosis,
staging, and management of hepatocellular carcinoma: 2018 Practice
guidance by the american association for the study of liver
diseases. Hepatology. 68:723–750. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Lu J, Zhang XP, Zhong BY, Lau WY, Madoff
DC, Davidson JC, Qi X, Cheng SQ and Teng GJ: Management of patients
with hepatocellular carcinoma and portal vein tumour thrombosis:
Comparing east and west. Lancet Gastroenterol Hepatol. 4:721–730.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yang Z, Zou R, Zheng Y, Qiu J, Shen J,
Liao Y, Zhang Y, Wang C, Wang Y, Yuan Y, et al: Lipiodol deposition
in portal vein tumour thrombus predicts treatment outcome in HCC
patients after transarterial chemoembolisation. Eur Radiol.
29:5752–5762. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
National Cancer Institute, . Cancer
Therapy Evaluation Program. Common Toxicity Criteria for Adverse
Events. Version 5.0, 2017. Available from:. http://ctep.cancer.govMay 10–2021
|
|
22
|
Park JW, Kim YJ, Kim DY, Bae SH, Paik SW,
Lee YJ, Kim HY, Lee HC, Han SY, Cheong JY, et al: Sorafenib with or
without concurrent transarterial chemoembolization in patients with
advanced hepatocellular carcinoma: The phase III STAH trial. J
Hepatol. 70:684–691. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
He MK, Liang RB, Zhao Y, Xu YJ, Chen HW,
Zhou YM, Lai ZC, Xu L, Wei W, Zhang YJ, et al: Lenvatinib,
toripalimab, plus hepatic arterial infusion chemotherapy versus
lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv
Med Oncol. Mar 25–2021.(Epub ahead of print). View Article : Google Scholar
|
|
24
|
Yuan Y, He W, Yang Z, Qiu J, Huang Z, Shi
Y, Lin Z, Zheng Y, Chen M, Lau WY, et al: TACE-HAIC combined with
targeted therapy and immunotherapy versus TACE alone for
hepatocellular carcinoma with portal vein tumour thrombus: A
propensity score matching study. Int J Surg. 109:1222–1230. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu BJ, Gao S, Zhu X, Guo JH, Kou FX, Liu
SX, Zhang X, Wang XD, Cao G, Chen H, et al: Combination therapy of
chemoembolization and hepatic arterial infusion chemotherapy in
hepatocellular carcinoma with portal vein tumor thrombosis compared
with chemoembolization alone: A propensity score-matched analysis.
Biomed Res Int. 2021:66703672021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheu JWSC and Wong CCL: Mechanistic
rationales guiding combination hepatocellular carcinoma therapies
involving immune checkpoint inhibitors. Hepatology. 74:2264–2276.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Cai M, Huang W, Huang J, Shi W, Guo Y,
Liang L, Zhou J, Lin L, Cao B, Chen Y, et al: Transarterial
chemoembolization combined with lenvatinib plus PD-1 inhibitor for
advanced hepatocellular carcinoma: A retrospective cohort study.
Front Immunol. 13:8483872022. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhang Z, Jiao T, Li J, Hu B, Zhang W, Wang
Z, Wan T, Wang Y and Lu S: Efficacy of treatment based on TKIs in
combination with PD-1 inhibitors for unresectable recurrent
hepatocellular carcinoma. World J Surg Oncol. 21:532023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhu XD, Huang C, Shen YH, Ji Y, Ge NL, Qu
XD, Chen L, Shi WK, Li ML, Zhu JJ, et al: Downstaging and resection
of initially unresectable hepatocellular carcinoma with tyrosine
kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer.
10:320–329. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Pinato D, Guerra N, Fessas P, Murphy R,
Mineo T, Mauri FA, Mukherjee SK, Thursz M, Wong CN, Sharma R and
Rimassa L: Immune-based therapies for hepatocellular carcinoma.
Oncogene. 39:3620–3637. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He X and Xu C: Immune checkpoint signaling
and cancer immunotherapy. Cell Res. 30:660–669. 2020. View Article : Google Scholar : PubMed/NCBI
|