Introduction
According to the GLOBOCAN 2020 estimates, as
compiled by the International Agency for Research on Cancer, ~19.3
million new cancer cases and nearly 10 million cancer-related
deaths (excluding non-melanoma skin cancer) occurred globally in
2020. Gastric cancer ranked fifth in terms of incidence and fourth
in terms of mortality worldwide (1). In China, according to the National
Cancer Registry Report, gastric cancer ranked third in terms of
both incidence and mortality among all malignant tumors in 2020.
The incidence of gastric cancer was significantly higher in men
than in women. In 2020, China recorded 480,000 new cases of gastric
cancer and 370,000 associated deaths, with gastric cancer projected
to remain the fourth-most common malignancy in the country by the
year 2022. Although the incidence and mortality rates of gastric
cancer have slightly declined since 2020, the disease continues to
pose a significant threat to public health, with 358,700 new cases
and 260,400 associated deaths reported in China in 2022 (2,3).
Owing to the high heterogeneity of gastric cancer,
traditional treatment modalities, such as chemotherapy, surgery,
radiotherapy, targeted drug therapy and traditional Chinese
medicine therapy (4–6), can only achieve suboptimal clinical
outcomes. In recent years, targeted drugs or other drugs combined
with chemotherapy have become the focus of clinical and basic
research on gastric cancer, and some progress has been made in
reversing chemotherapy resistance and increasing drug efficacy
(7–10). However, immunotherapy, particularly
with programmed cell death protein 1 (PD-1)/programmed death ligand
1 (PD-L1) monoclonal antibodies (MABs), has demonstrated
significant advancements in the treatment of advanced gastric
cancer, irrespective of HER2 expression level. PD-1 MABs combined
with chemotherapy have now become the standard first-line therapy
for advanced gastric cancer (11–16).
With the increasing use of PD-1 MABs, which play a
critical role in improving immune tolerance, the incidence of
immune-related adverse events (irAEs) has also risen. These adverse
events can affect various organs, with the most commonly impacted
ones being the skin, colon, liver, lungs and endocrine organs
(17,18). Endocrine dysfunctions, including
thyroid dysfunction, insulin-deficient diabetes, pituitaritis and
primary adrenal insufficiency, are some of the most common
complications associated with immune checkpoint inhibitors (ICIs)
(19–21). Past studies on non-endocrine tumors,
such as lung cancer and melanoma, have identified an association
between immune-related thyroid dysfunction (irTD) and clinical
outcomes, including increased efficacy and prognosis (22–26).
However, the association between irTD and therapeutic efficacy or
prognosis in gastric cancer remains unclear. Therefore, the present
retrospective study was conducted to investigate the occurrence of
irTD during immunotherapy for gastric cancer and to analyze its
association with the resultant clinical efficacy and patient
prognosis.
Patients and methods
Subjects
The present retrospective study was conducted on
patients with advanced gastric cancer who received PD-1 MAB
combined with chemotherapy in the Department of Oncology of
Changzhou Tumor Hospital (Chongqing, China) between January 2019
and December 2022. The patients were divided into first-line (n=69)
and second-line (n=37) treatment groups. All patients had complete
medical and follow-up records irrespective of their HER-2 status.
The following inclusion criteria were applied: i) Age >18 years;
ii) gastric adenocarcinoma or gastroesophageal junction
adenocarcinoma confirmed by pathology and clinical stage IV
(according to the 8th edition of the American Joint Committee on
Cancer Cancer Staging Manual) (27); iii) administration of at least 2
cycles of chemotherapy combined with PD-1 MAB treatment; iv) no
history of thyroid hormone drug therapy before the treatment; and
v) near doubling of the levels of thyroid hormones
[thyroid-stimulating hormone (TSH), thyroid peroxidase (TPO),
thyroxine (T4)/free T4 (FT4) and triiodothyronine (T3)/free T3
(FT3)] after the initiation of immunotherapy. The exclusion
criteria included: i) A history of thyroid underlying disease; and
ii) having received less than two courses of immunotherapy. The
study protocol was approved by the Ethics Committee of the
Changzhou Tumor Hospital [approval no. 2023 (SR) NO.003].
Research methods
Patient data
The baseline data from patient's medical records
were collected for the following parameters: Age, sex, site of
gastric cancer, site of metastasis, comprehensive treatment line
number and combined chemotherapy scheme, and TSH, FT4, FT3 and TPO
levels.
Outcome measures
For irTD, the time, management and outcome of the
first occurrence of thyroid dysfunction were recorded. Subclinical
hypothyroidism was classified as TSH >5 µIU/ml and a normal
FT4/FT3 level (FT3: 3.1–6.8 pmol/l; FT4: 12–22 pmol/l). Subclinical
hyperthyroidism was classified as TSH <0.4 µIU/ml and a normal
FT4/FT3 level. Hypothyroidism was classified as TSH >5 µIU/ml
and a lower than normal FT4/FT3 level, or TSH ≥10 µIU/ml,
regardless of the FT4/FT3 level. Hyperthyroidism was classified as
TSH <0.4 µIU/ml and an higher than normal FT4/FT3 level.
Clinical efficacy and research
objectives
According to the evaluation criteria listed in
RECIST1.1 guidelines (28), the
efficacy was categorized into progressive disease (PD), stable
disease (SD), partial remission (PR) and complete remission (CR).
The sum of CR + PR proportion statistics was considered as the
objective response rate (ORR), and the sum of CR + PR + SD
proportion statistics was considered as the disease control rate
(DCR). Progression-free survival (PFS) was defined as the time from
initial chemotherapy combined with immunotherapy until PD or death
from any cause. Overall survival (OS) after initial treatment was
assessed by using the follow-up data.
Statistical grouping
Based on the occurrence of thyroid dysfunction after
treatment, the patients were categorized into irTD and non-irTD
groups, and then further divided into a TPO-change group and
no-TPO-change group based on whether a TPO change occurred.
Statistical analysis
SPSS26.0 statistical software (IBM Corp.) was used
for statistical analysis and processing. All measurement data
conforming to a normal distribution are presented as the mean ±
standard deviation, and an independent sample t-test was performed
for comparison among two groups. When three groups were compared,
one-way ANOVA was used for intergroup comparisons. The counting
data are described as n (%), and the differences between the groups
were analyzed by the χ2 test or Fisher's exact
probability method. Single-factor survival analysis was performed
using the Kaplan-Meier method and survival curves were drawn.
Meanwhile, PFS and OS between the groups were compared using a
log-rank non-parametric test. P<0.05 was considered to indicate
a statistically significant difference.
Results
General information
Changes in irTD and TPO levels in patients with
first-line gastric cancer with different clinical
characteristics
Among the 69 patients with advanced gastric cancer
who received first-line treatment, 28 (40.6%) developed irTD. All
cases of irTD were hypothyroidism, with clinical hypothyroidism
being the most prevalent type (25 cases; 89.3%) and subclinical
hypothyroidism occurring in 3 (10.7%) cases. TPO abnormalities were
observed in 34 (49.3%) patients, and all patients with irTD
exhibited TPO abnormalities. However, 6 patients with TPO
abnormalities did not develop irTD. No significant associations
were found between irTD or TPO abnormalities and clinical
characteristics, such as age, sex, tumor location, metastatic
organs or the main first-line treatment drugs, as detailed in
Tables I and II.
 | Table I.Occurrence of immune-related thyroid
dysfunction in patients with gastric cancer with different clinical
characteristics in first-line treatment. |
Table I.
Occurrence of immune-related thyroid
dysfunction in patients with gastric cancer with different clinical
characteristics in first-line treatment.
| Characteristic | Thyroid function
normal group (n=41) | Thyroid function
abnormal group (n=28) | Statistic | P-value |
|---|
| Mean age ± SD,
years | 66.66±10.77 | 67.68±9.89 | −0.40 | 0.6910 |
| Sex, n (%) |
|
|
|
|
|
Male | 27 (65.85) | 19 (67.86) | 0.03 | 0.8624 |
|
Female | 14 (34.15) | 9 (32.14) |
|
|
| Metastatic site, n
(%) |
|
|
|
|
|
Liver | 16 (39.02) | 14 (50.00) | 0.82 | 0.3665 |
|
Others | 25 (60.98) | 14 (50.00) |
|
|
| Treatment, n
(%) |
|
|
|
|
|
Oxaliplatin | 24 (58.54) | 13 (46.43) | 1.53 | 0.4643 |
|
Nab-paclitaxel | 8 (19.51) | 9 (32.14) |
|
|
| Other
treatment | 9 (21.95) | 6 (21.43) |
|
|
| Site of gastric
cancer, n (%) |
|
|
|
|
|
GEJ | 11 (26.83) | 10 (35.71) | 0.62 | 0.4309 |
|
Non-GEJ | 30 (73.17) | 18 (64.29) |
|
|
 | Table II.Occurrence of TPO change in gastric
cancer patients with different clinical characteristics in
first-line treatment. |
Table II.
Occurrence of TPO change in gastric
cancer patients with different clinical characteristics in
first-line treatment.
| Characteristic | TPO normal group
(n=35) | TPO abnormal group
(n=34) | Statistic | P-value |
|---|
| Mean age ± SD,
years | 67.26±10.50 | 66.88±10.37 | 0.15 | 0.8819 |
| Sex, n (%) |
|
|
|
|
|
Male | 22 (62.86) | 24 (70.59) | 0.46 | 0.4958 |
|
Female | 13 (37.14) | 10 (29.41) |
|
|
| Metastatic site, n
(%) |
|
|
|
|
|
Liver | 13 (37.14) | 17 (50.00) | 1.16 | 0.2814 |
|
Others | 22 (62.86) | 17 (50.00) |
|
|
| Treatment, n
(%) |
|
|
|
|
|
Oxaliplatin | 19 (54.29) | 18 (52.94) | 0.14 | 0.9333 |
|
Nab-paclitaxel | 8 (22.86) | 9 (26.47) |
|
|
| Other
treatment | 8 (22.86) | 7 (20.59) |
|
|
| Site of gastric
cancer, n (%) |
|
|
|
|
|
GEJ | 9 (25.71) | 12 (35.29) | 0.75 | 0.3872 |
|
Non-GEJ | 26 (74.29) | 22 (64.71) |
|
|
Association between clinical
characteristics and patient outcomes in first-line gastric cancer
treatment
In patients receiving first-line treatment for
gastric cancer, clinical characteristics, such as age, sex, tumor
site, metastatic organs and the main first-line treatment drugs,
showed no significant associations with the clinical outcomes.
These results are presented in Table
III.
 | Table III.Associations between the best
clinical efficacy in first-line treatment for gastric cancer
patients and different clinical characteristics. |
Table III.
Associations between the best
clinical efficacy in first-line treatment for gastric cancer
patients and different clinical characteristics.
| Characteristic | PD (n=13) | PR (n=29) | SD (n=27) | Statistic | P-value |
|---|
| Mean age ± SD,
years | 68.77±9.47 | 68.31±11.39 | 64.93±9.58 | 0.96 | 0.3882 |
| Sex, n (%) |
|
|
|
|
|
|
Male | 8 (61.54) | 18 (62.07) | 20 (74.07) | 1.10 | 0.5780 |
|
Female | 5 (38.46) | 11 (37.93) | 7 (25.93) |
|
|
| Metastatic site, n
(%) |
|
|
|
|
|
|
Liver | 8 (61.54) | 10 (34.48) | 12 (44.44) | 2.69 | 0.2605 |
|
Others | 5 (38.46) | 19 (65.52) | 15 (55.56) |
|
|
| Treatment, n
(%) |
|
|
|
|
|
|
Oxaliplatin | 5 (38.46) | 14 (48.28) | 18 (66.67) | 4.54 | 0.3418 |
|
Nab-paclitaxel | 3 (23.08) | 9 (31.03) | 5 (18.52) |
|
|
| Other
treatment | 5 (38.46) | 6 (20.69) | 4 (14.81) |
|
|
| Site of gastric
cancer, n (%) |
|
|
|
|
|
|
GEJ | 4 (30.77) | 9 (31.03) | 8 (29.63) | 0.01 | 0.9931 |
|
Non-GEJ | 9 (69.23) | 20 (68.97) | 19 (70.37) |
|
|
| Thyroid function, n
(%) |
|
|
|
|
|
| Normal
group | 5 (38.46) | 17 (58.62) | 19 (70.37) | 3.72 | 0.1558 |
|
Abnormal group | 8 (61.54) | 12 (41.38) | 8 (29.63) |
|
|
Incidence of irTD and TPO
abnormalities in patients receiving second-line treatment for
gastric cancer
Among the 37 patients treated with second-line
therapy for advanced gastric cancer, 17 (45.9%) developed irTD. All
cases of irTD were associated with hypothyroidism, with clinical
hypothyroidism being the most common form (16 cases, 94.1%), while
1 case of subclinical hypothyroidism was observed (5.9%). TPO
abnormalities were present in 22 (59.5%) patients. All patients
with irTD also exhibited TPO abnormalities, although 5 patients had
TPO abnormalities without irTD. The data are presented in Tables IV and V.
 | Table IV.Occurrence of immune-related thyroid
dysfunction in patients with gastric cancer with different clinical
characteristics in second-line treatment. |
Table IV.
Occurrence of immune-related thyroid
dysfunction in patients with gastric cancer with different clinical
characteristics in second-line treatment.
| Characteristic | Thyroid function
normal group (n=22) | Thyroid function
abnormal group (n=17) | Statistic | P-value |
|---|
| Mean age ± SD,
years | 65.75±9.86 | 62.18±9.55 | 1.11 | 0.2726 |
| Sex, n (%) |
|
|
|
|
|
Male | 14 (70.00) | 15 (88.24) | - | 0.2455 |
|
Female | 6 (30.00) | 2 (11.76) |
|
|
| Metastatic site, n
(%) |
|
|
|
|
|
Liver | 10 (50.00) | 8 (47.06) | - | >0.9999 |
|
Others | 10 (50.00) | 9 (52.94) |
|
|
| Treatment, n
(%) |
|
|
|
|
|
Oxaliplatin | 0 (0.00) | 3 (17.65) |
| 0.0648 |
|
Nab-paclitaxel | 4 (20.00) | 6 (35.29) |
|
|
|
Irinotecan | 6 (30.00) | 1 (5.88) |
|
|
| Other
treatment | 10 (50.00) | 7 (41.18) |
|
|
| Site of gastric
cancer, n (%) |
|
|
|
|
|
GEJ | 8 (40.00) | 7 (41.18) | - | >0.9999 |
|
Non-GEJ | 12 (60.00) | 10 (58.82) |
|
|
 | Table V.Occurrence of TPO change in patients
with gastric cancer with different clinical characteristics in
second-line treatment. |
Table V.
Occurrence of TPO change in patients
with gastric cancer with different clinical characteristics in
second-line treatment.
| Characteristic | TPO normal group
(n=15) | TPO abnormal group
(n=22) | Statistic | P-value |
|---|
| Mean age ± SD,
years | 64.60±8.43 | 63.77±10.74 | 0.25 | 0.8040 |
| Sex, n (%) |
|
|
|
|
|
Male | 9 (60.00) | 20 (90.91) | - | 0.0421 |
|
Female | 6 (40.00) | 2 (9.09) |
|
|
| Metastatic site, n
(%) |
|
|
|
|
|
Liver | 7 (46.67) | 11 (50.00) | - | >0.9999 |
|
Others | 8 (53.33) | 11 (50.00) |
|
|
| Treatment, n
(%) |
|
|
|
|
|
Oxaliplatin | 0 (0.00) | 3 (13.64) | - | 0.5138 |
|
Nab-paclitaxel | 4 (26.67) | 6 (27.27) |
|
|
|
Irinotecan | 4 (26.67) | 3 (13.64) |
|
|
| Other
treatment | 7 (46.67) | 10 (45.45) |
|
|
| Site of gastric
cancer, n (%) |
|
|
|
|
|
GEJ | 5 (33.33) | 10 (45.45) | - | 0.5144 |
|
Non-GEJ | 10 (66.67) | 12 (54.55) |
|
|
Association between clinical
characteristics and treatment efficacy in patients with second-line
gastric cancer
In second-line treatment for gastric cancer, no
significant associations were observed between clinical
characteristics, such as age, sex, tumor site, metastatic organs or
the primary second-line treatment drugs, and clinical outcomes.
These findings are summarized in Table
VI.
 | Table VI.Association between the best clinical
efficacy of patients with gastric cancer with different clinical
characteristics in second-line treatment. |
Table VI.
Association between the best clinical
efficacy of patients with gastric cancer with different clinical
characteristics in second-line treatment.
| Characteristic | PD (n=8) | PR (n=14) | SD (n=15) | Statistic | P-value |
|---|
| Mean age ± SD,
years | 62.25±12.36 | 63.71±8.78 | 65.47±9.61 | 0.29 | 0.7499 |
| Sex, n (%) |
|
|
|
|
|
|
Male | 6 (75.00) | 11 (78.57) | 12 (80.00) | - | >0.9999 |
|
Female | 2 (25.00) | 3 (21.43) | 3 (20.00) |
|
|
| Metastatic site, n
(%) |
|
|
|
|
|
|
Liver | 4 (50.00) | 8 (57.14) | 6 (40.00) | - | 0.6426 |
|
Others | 4 (50.00) | 6 (42.86) | 9 (60.00) |
|
|
| Treatment, n
(%) |
|
|
|
|
|
|
Oxaliplatin | 0 (0.00) | 1 (7.14) | 2 (13.33) | - | 0.4722 |
|
Nab-paclitaxel | 3 (37.50) | 5 (35.71) | 2 (13.33) |
|
|
|
Irinotecan | 1 (12.50) | 1 (7.14) | 5 (33.33) |
|
|
| Other
treatment | 4 (50.00) | 7 (50.00) | 6 (40.00) |
|
|
| Site of gastric
cancer, n (%) |
|
|
|
|
|
|
GEJ | 1 (12.50) | 7 (50.00) | 7 (46.67) | - | 0.2209 |
|
Non-GEJ | 7 (62.50) | 7 (50.00) | 8 (53.33) |
|
|
| Thyroid function, n
(%) |
|
|
|
|
|
| Normal
group | 4 (50.00) | 6 (42.86) | 10 (66.67) | - | 0.4765 |
|
Abnormal group | 4 (50.00) | 8 (57.14) | 5 (33.33) |
|
|
Efficacy evaluation and survival
analysis
Association between irTD and PFS/OS in patients
with first-line gastric cancer
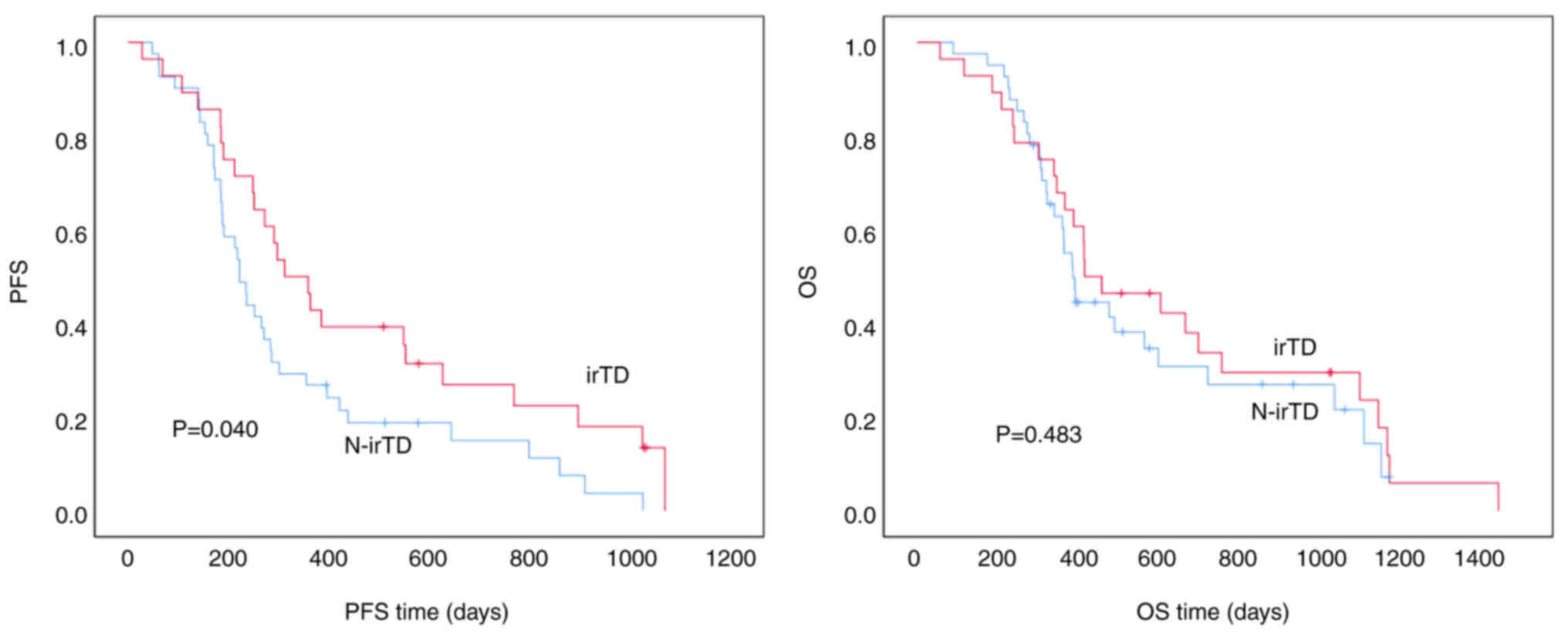
In patients with first-line gastric cancer, those
with irTD had a median PFS time of 312.0±47.6 days compared to
222.0±14.7 days in patients without irTD. This difference was
statistically significant (P=0.040), indicating that patients with
irTD experienced longer PFS times. However, there was no
statistically significant difference in the median OS time, which
was 417.0±121.5 days for patients with irTD vs. 388.0±17.7 days for
those without irTD (P=0.483). While a trend toward longer OS time
was indicated in patients with irTD, the difference did not reach
statistical significance (Fig.
1).
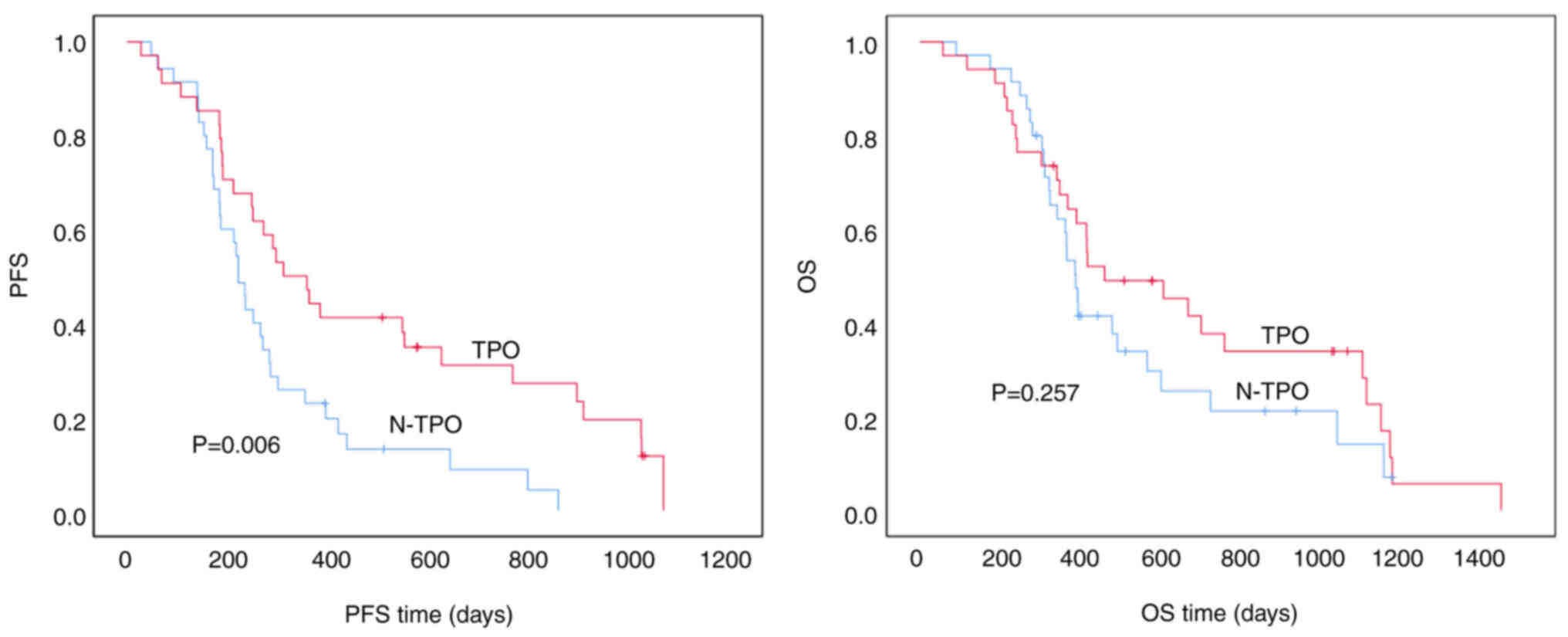
Association between TPO abnormalities
and PFS/OS in patients with first-line gastric cancer
In patients with first-line gastric cancer with TPO
abnormalities, the median PFS time was 312.0±52.5 days compared
with 222.0±13.6 days in patients without TPO abnormalities. This
difference was statistically significant (P=0.006), suggesting that
patients with TPO abnormalities had significantly longer PFS times.
However, the median OS time was 460.0±160.2 days for patients with
TPO abnormalities vs. 388.0±20.2 days for those without, and this
difference was not statistically significant (P=0.257). While
patients with TPO abnormalities exhibited a trend toward longer OS
time, the difference was not statistically significant (Fig. 2).
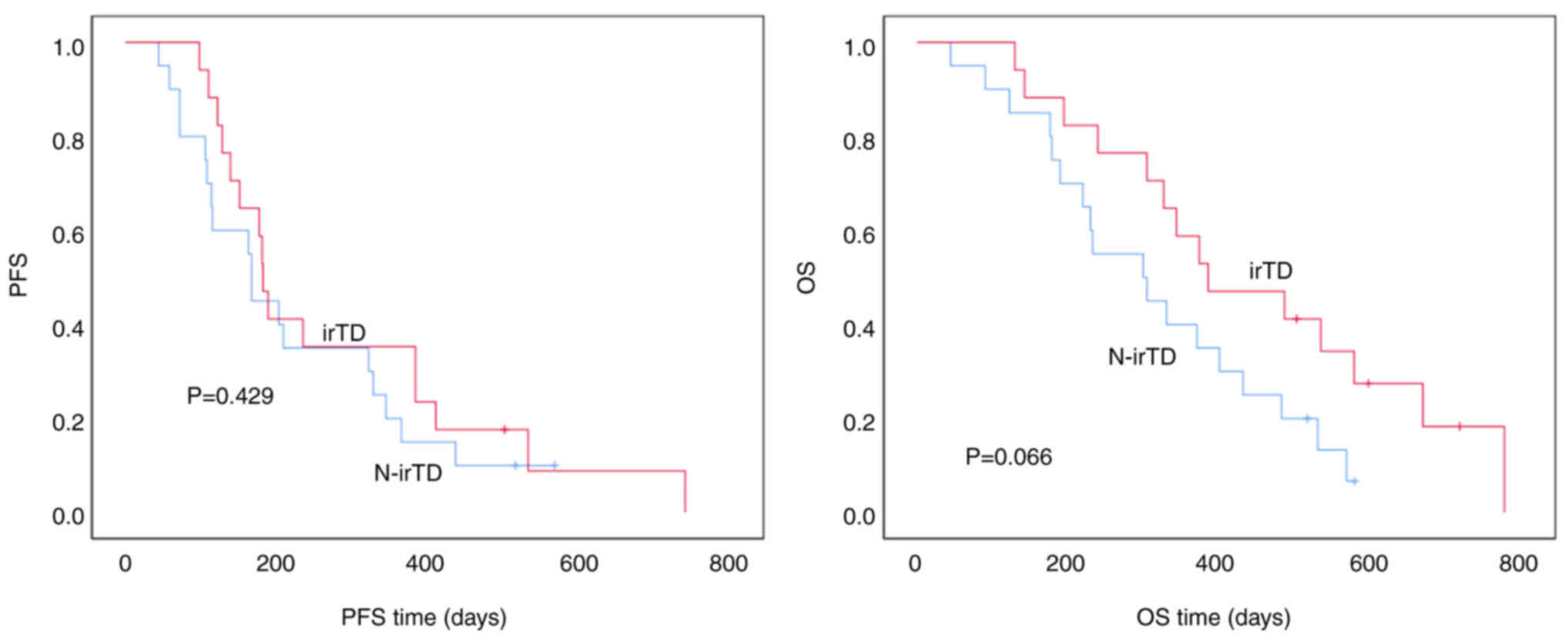
Association between irTD and PFS/OS in
patients with second-line gastric cancer
In patients with second-line gastric cancer, those
with irTD had a median PFS time of 182.0±8.2 days compared with
167.0±4.5 days in patients without irTD. There was no statistically
significant difference between the two groups (P=0.429). Similarly,
the median OS time was 385.0±98.1 days for patients with irTD vs.
299.0±80.5 days for those without, and although there was a trend
toward longer OS time in patients with irTD, the difference was not
statistically significant (P=0.066) (Fig. 3).
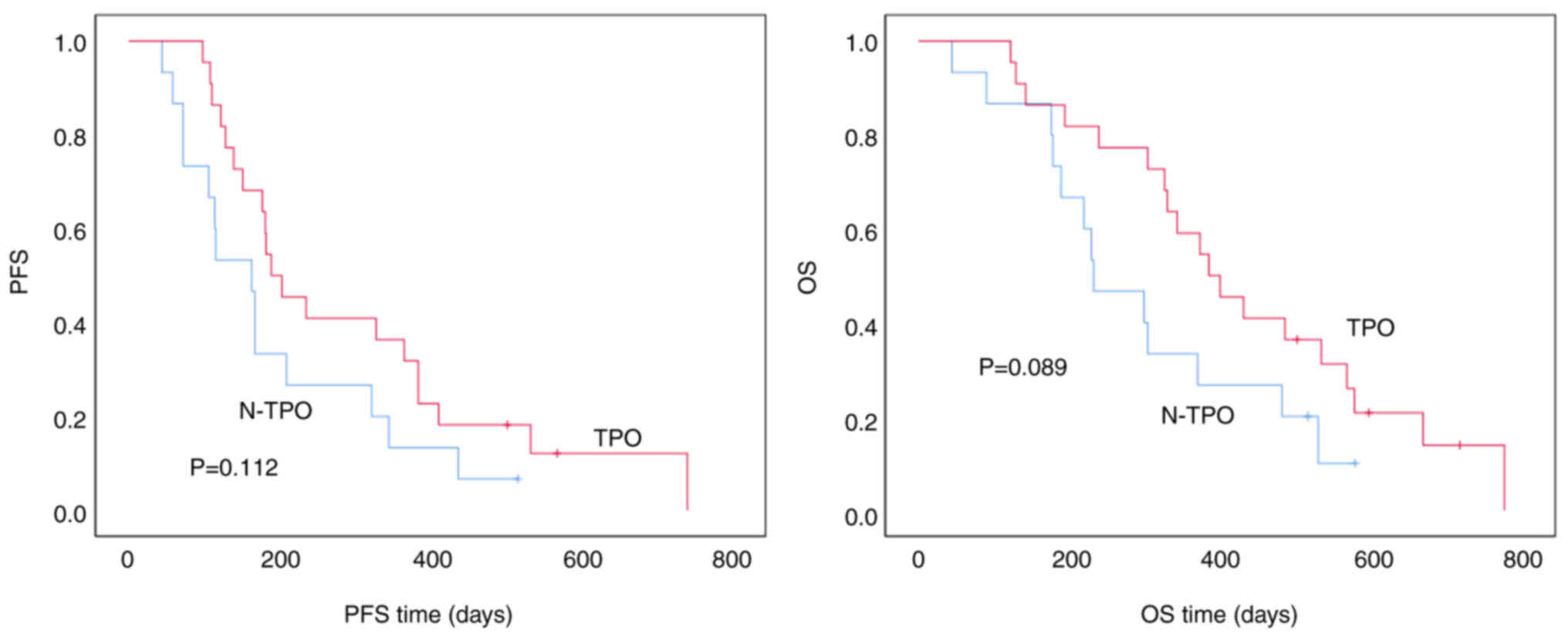
Association between TPO abnormalities
and PFS/OS in patients with second-line gastric cancer
Among patients with second-line gastric cancer,
those with TPO abnormalities had a median PFS time of 189.0±31.7
days when compared with 163.0±25.6 days in patients without TPO
abnormalities. Although there was a trend toward longer PFS time in
patients with TPO abnormalities, the difference was not
statistically significant (P=0.112). The median OS time was
385.0±51.6 days for patients with TPO abnormalities vs. 232.0±51.5
days for those without. While patients with TPO abnormalities
showed a trend toward longer OS, the difference was not
statistically significant (P=0.089) (Fig. 4).
Discussion
Gastric cancer is one of the most prevalent
malignant tumors globally, with its morbidity and mortality rates
ranking among the highest for all malignancies both in China and
worldwide (1–3). Before 2018, treatment options were
primarily limited to chemotherapy drugs, with only a few targeted
therapies, such as herceptin and ramucirumab, available for
controlling the disease. However, due to the spatial and temporal
heterogeneity of gastric cancer, patients often face short survival
times and a poor prognosis. The development of immunotherapy in
recent years has brought new hope, particularly for advanced
gastric cancer. Immunotherapy combined with chemotherapy has
demonstrated the potential to extend survival and improve clinical
outcomes (11–16). However, irAEs frequently occur
during the use of immunotherapy, commonly affecting the skin,
colon, liver, lungs and endocrine organs (17). Endocrine complications, particularly
thyroid dysfunction, are among the most prevalent irAEs associated
with ICIs, alongside diabetes, pituitaritis and primary adrenal
insufficiency (19,20). Notably, PD-1/PD-L1 inhibitors have
been found to frequently induce thyroid dysfunction (21,29).
Past studies on the incidence of irTD have yielded
inconsistent findings. In a retrospective study conducted in South
Korea, Yoon et al (29)
reported that 50.5% (164/325) of the patients with cancer treated
with PD-1/PD-L1 MABs experienced at least one type of thyroid
dysfunction. Chilelli et al (30) studied 75 patients with
non-small-cell lung cancer (NSCLC) and found that 25.3% of the
participants developed irTD. Meanwhile, Ferreira et al
(25) reported an irTD incidence of
18% during immunotherapy, while Li et al (31) found a 13.2% incidence of thyroid
dysfunction, predominantly hypothyroidism, in patients with NSCLC.
Zhou et al (32) also
observed thyroid dysfunction in 11.2% (27/241) of patients with
NSCLC following immunotherapy. Horesh et al (33) conducted a retrospective analysis of
patients with NSCLC treated with PD-1 MABs and found that 34.6%
(37/107) developed thyroid dysfunction. These studies highlight the
relatively common occurrence of thyroid dysfunction associated with
immunotherapy, although the specific incidence rates vary across
studies.
Similarly, patients with gastric cancer experience a
high incidence of irTD following immunotherapy. According to
several first-line clinical studies on gastric cancer (13–16),
hypothyroidism is the most frequently observed irTD, with reported
incidences of 11.0–13.6%, whereas hyperthyroidism is much less
common, with an incidence of 4.0–6.1%. However, the true incidence
in real-world settings remains unclear. In the present study, it
was observed that the incidence of thyroid dysfunction in patients
with gastric cancer was 40.6% (28/69) during first-line treatment
and 45.9% (17/37) during second-line treatment, with hypothyroidism
being the predominant presentation. These findings align with the
incidence rates reported in the aforementioned previous literature,
thereby further emphasizing the significance of irTD in patients
with gastric cancer undergoing immunotherapy.
Regarding the timing of irTD induced by
immunotherapy, Zhou et al (32) found that thyroid dysfunction
typically occurs within 3–6 months after initiating ICI treatment,
although, in some cases, it may occur even after the
discontinuation of ICIs. Similarly, the study by Ferreira et
al (25) reported a median time
of irTD onset of 10.6 weeks (range, 6.1–31.1 weeks), with irTD most
often arising after the fourth cycle of treatment. Horesh et
al (33) observed a marked
increase in irTD incidence following the administration of PD-1 or
PD-L1 MABs, with the median time to thyroid dysfunction in patients
with NSCLC being 45.0 days (interquartile range, 29.5–91.0 days).
In the present study, the median time for thyroid dysfunction was
82 days (range, 21–302 days), which aligns with the previous
literature.
The pathogenesis of thyroid dysfunction under ICIs
remains unclear. However, it has been noted that PD-L1 and PD-L2
are expressed in normal thyroid tissues. As a result, blocking PD-1
in normal thyroid tissues may reduce immune tolerance and promote
the development of thyroid inflammation (34). In addition, PD-1 is believed to
regulate autoimmune balance and may trigger thyroid inflammation
from latent autoimmune exposure or induce anti-thyroid
antibody-mediated thyroid inflammation through PD-1-regulated
humoral immunity (35). Thyroid
antibodies are common autoantibodies found in the serum of patients
with autoimmune thyroid diseases and may have predictive value for
irAEs induced by immunosuppressive therapies. Yoon et al
(29) reported a significant
association between hypothyroidism and the presence of anti-TPO
antibodies at the baseline in patients treated with PD-1/PD-L1
inhibitors. However, different studies have reported conflicting
conclusions, and the matter remains controversial. Some studies
have demonstrated that baseline thyroid antibodies are
significantly associated with an increased risk of thyroid
dysfunction, while others have found no such association (36–39).
Currently, the prevailing view is that comprehensive measurement of
autoimmune-related antibodies before ICI therapy has limited
predictive value for irAEs. Nonetheless, TPO antibodies are
considered a risk factor for the development of ICI-induced thyroid
dysfunction and can be used to monitor thyroid function changes
(40). Recent studies have
suggested that the positive conversion of thyroid antibodies during
PD-1/PD-L1 inhibitor treatment serves as a potential marker for
thyroid irAEs (41). Furthermore,
hypothyroidism is significantly associated with the presence of
anti-thyroglobulin antibodies during treatment (29). In the present study, it was observed
that 34 patients who had normal TPO levels before treatment
experienced a positive conversion of TPO after treatment, with 28
of these patients developing thyroid dysfunction. This finding
suggests that TPO conversion is a predictor of thyroid irAEs. In
addition, when thyroid dysfunction improves, either through hormone
replacement therapy or spontaneously, TPO conversion tends to
resolve more slowly than changes in the FT3, FT4 and TSH
levels.
Thyroid irAEs can manifest as hypothyroidism,
hyperthyroidism or fluctuations in thyroid function. Most cases of
thyrotoxicosis associated with PD-1/PD-L1 inhibitors are mild and
typically present as painless thyroiditis. Only a few instances of
PD-1/PD-L1 inhibitor-induced autoimmune hyperthyroidism, such as
Graves' disease, have been documented (42,43).
Following treatment, some patients may experience a restoration of
normal thyroid function, while others require lifelong thyroid
hormone replacement therapy due to severe thyroid failure (44,45).
In the present study, only one case of hyperthyroidism was
identified among the thyroid dysfunction cases in irAEs, with the
remaining 27 cases classified as hypothyroidism or subclinical
hypothyroidism. Thyroid hormone replacement therapy was initiated
when T3/T4 levels fell below normal, and this therapy was continued
for 6 months following the conclusion of immunotherapy. Meanwhile,
hormone replacement therapy did not reduce the patient's quality of
life and the clinical symptoms worsened during the treatment
process. irAE-induced thyroid failure is often irreversible,
necessitating long-term thyroid hormone replacement therapy.
Notably, none of the present patients had to discontinue
immunotherapy due to hypothyroidism. Additionally, 14 cases of
thyroid dysfunction accompanied by hypopituitarism were found.
Immunotherapy was continued under active treatment with thyroid
hormone and glucocorticoid supplementation; however, subsequent
immunotherapy was frequently suspended or delayed due to the
patient's abnormal pituitary function.
Thyroid irAEs induced by PD-1/PD-L1 inhibitors are
associated with prolonged OS and PFS times (46–49).
Hussaini et al (50)
retrospectively studied patients with major malignancies, including
melanoma, and lung, kidney, and head and neck tumors, and found
that patients experiencing irAEs had higher ORRs and longer PFS/OS
times compared with those without irAEs. This finding suggests that
irAEs have predictive implications for the efficacy of ICIs, with
thyroid dysfunction being the most prevalent irAE supported by
objective evidence. In a retrospective study of 75 patients with
NSCLC, immunological thyroid dysfunction occurred in 25.3% of
patients treated with ICIs. The ORR and DCR for patients with irTD
were 42.1 vs. 7.1% and 78.9 vs. 32.1%, respectively, when compared
with those for patients without irTD. The median PFS time was 15.7
months for patients with irTD vs. 3.6 months for patients without
irTD, while the median OS time was 18.6 vs. 5.1 months, indicating
a significant improvement in ORR and DCR, alongside a reduced risk
of PD and mortality (30).
Similarly, a retrospective study involving PD-1 MAB
in advanced solid tumors revealed that the thyroid dysfunction
group achieved longer median PFS (66 vs. 27 weeks) and median OS
(156 vs. 59 weeks) times compared with the non-thyroid dysfunction
group. Subsequent multivariate analysis identified thyroid
dysfunction as an independent prognostic factor for OS, associated
with a 58% reduction in the risk of death, highlighting its
significant survival benefit (51).
In another retrospective study of metastatic renal cancer, ~67.4%
of patients experienced irAEs, with thyroid dysfunction being the
most common. The median PFS time for patients with thyroid
dysfunction was notably longer following treatment initiation, and
multifactorial analysis confirmed thyroid dysfunction as an
independent prognostic factor. PFS time was significantly prolonged
in patients with thyroid dysfunction (P=0.028). The median PFS time
for the group with normal thyroid function was 121 days
(interquartile range, 92–305 days), while the median PFS time for
the group with thyroid dysfunction was not reached. Patients with
irTD exhibited significant benefits in terms of PFS time (52). Yu et al (53) retrospectively analyzed 425 patients
with advanced NSCLC treated with anti-PD-L1 monotherapy and divided
them into the irAE group (n=127) and irAE group (n=298). The
occurrence of overall irAEs was significantly associated with
higher PFS (11.2 vs. 3.4 months; P<0.001) and OS (31.4 vs. 14.0
months; P<0.001) times. For organ-specific irAEs, patients with
skin, thyroid and liver-related irAEs demonstrated significantly
improved survival times when compared with the group without irAEs,
while those with pneumonia-related irAEs did not (53). Similarly, in another retrospective
study of 244 patients with NSCLC, 140 (57.4%) had irAEs. Patients
with irAEs had higher ORRs (73.6 vs. 52.9%; P<0.001) and DCRs
(97.9 vs. 79.8%; P<0.001), as well as longer median PFS (8.8 vs.
4.5 months; P<0.001) and OS (23.2 vs. 21.6 months; P<0.05)
times. Among the different types of irAEs, thyroid dysfunction,
rash and pneumonia were the most powerful indicators of PFS
improvement (54). A few studies
have investigated whether thyroid dysfunction in patients with
gastric cancer treated with ICIs has a similar prognostic effect
(13–16). Multivariable Cox regression analysis
of organ-specific irAEs found in gastric cancer studies has shown
favorable survival outcomes in patients with thyroid, adrenal and
cutaneous irAEs when compared with patients without these irAEs
(21). In the present study, 45 out
of 106 patients developed irTD, yielding an incidence rate of
42.5%. Among these, 28 out of 69 patients with first-line gastric
cancer and 17 out of 37 patients with second-line gastric cancer
were analyzed based on the presence or absence of irAEs. Patients
with first-line gastric cancer who experienced irAEs received more
effective treatment than those without irAEs. The irAE group
demonstrated a better PFS time (P=0.04), although there was no
significant difference in the OS time between the two groups
(P=0.483), which may be related to subsequent treatments.
In patients with gastric cancer receiving
second-line therapy, while the PFS and OS times in the irAE group
were higher than those in the non-irAE group in the present study,
the two survival curves exhibited a divergent trend without a
statistically significant difference, possibly due to the small
sample size. Meanwhile, since PD-1 MABs were included in the
medical insurance reimbursement scope in 2023, patients with
advanced gastric cancer often use PD-1 MABs in the first-line
treatment. The same type of PD-1 MABs are no longer used for the
subsequent second-line treatment. Therefore, it is difficult to
increase the sample size for the second-line treatment. Moreover,
since TPO changes are closely associated with irTD, the present
study found that 56 out of 106 patients had positive TPO
conversion, indicating an incidence of 52.8%. Among these, 34 out
of 69 patients with first-line gastric cancer and 22 out of 37
patients with second-line gastric cancer were analyzed based on TPO
changes. In the first-line treatment group, patients with TPO
changes displayed a better PFS time than those without TPO changes
(P=0.006), although there was no significant difference in OS
between the two groups (P=0.483). Similarly, in patients receiving
second-line treatment for gastric cancer, the group with TPO
changes exhibited higher PFS and OS times than the group without
changes; however, this difference was not statistically
significant.
The association between irAE and efficacy after the
use of ICIs is unclear. The underlying mechanism may be as follows:
i) irAE and antitumor mechanisms have some common pathways related
to cytotoxic memory CD4+ T cell markers, which are
activated by PD-1 blockers (55).
ii) irAEs caused by PD-(L)1 inhibitor activation of
autoantigen-specific T cells may indirectly reflect the killing
ability of tumor-specific T cells (56). ICIs reactivate depleted T cells that
are cross-reactive to tumor and normal tissue antigens, thereby
enhancing antitumor immunity and irAEs (57). ICIs can enable tissue-resident
memory T cells in tumors to be reactivated and proliferated.
Killing of tumor cells is achieved by the secretion of effector
molecules such as interferon-γ (IFN-γ), and this event also has
some off-target effects (58). iii)
The relevant immune response induced by ICIs is non-specific, which
makes the attack on tumor and non-tumor cells indistinguishable
(59). iv) Inflammatory cytokines
involved in the occurrence of irAEs affect the treatment rate in
patients with cancer. For example, patients with melanoma with risk
allelic mutations in the interleukin 7 gene may have both a higher
incidence of irAEs and improved survival (60). Changes in another cytokine, IFN-γ,
are associated with the occurrence and clinical outcome
expectations of irAEs in patients with liver cancer/NSCLC (58,61–63).
Currently, the comprehensive treatment of gastric
cancer has achieved substantial progress, yet the overall
effectiveness remains at only 50–65% due to the considerable
heterogeneity of the disease. Therefore, further screening for
biomarkers related to the efficacy of PD-1 MABs is clinically
significant for patient selection, assessing treatment response and
predicting potential outcomes. Based on the present findings,
changes in irTD and TPO may serve as additional predictors of the
efficacy of immunotherapy, similar to combined positive score of
PD-L1, microsatellite instability status and Epstein-Barr virus
infection. Thyroid dysfunction occurring during chemotherapy
combined with immunotherapy for gastric cancer is a relatively safe
adverse reaction, allowing the continuation of immunotherapy under
careful monitoring and active treatment. Moreover, thyroid irAEs
are mainly hypothyroidism, and, under the premise of hormone
replacement therapy, this irAE has little impact on the patient's
quality of life. However, due to the limitations of this
retrospective study, including a small sample size and
single-center design, further prospective studies are warranted to
validate its clinical significance. At the same time, more basic
research is needed to confirm the mechanism of the association
between irTD and efficacy.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
MX and ZYB contributed to the conception and design
of the study. LLQ analyzed data. QZ, JM, QXL and QL collected data
and performed some data analysis. MX wrote and revised the
manuscript. MX and ZYB confirm the authenticity of all the raw
data. All authors have read and approved the final manuscript.
Ethics approval and consent to
participate
The study protocol was in accordance with the
Declaration of Helsinki of the World Medical Association. The study
was approved by the Ethical Committee of Changzhou Tumor Hospital
[Changzhou, China; approval no. 2023 (SR) NO.003]. The requirement
for informed consent was waived for this study, as the research was
retrospective and conducted on anonymized data
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
MAB
|
monoclonal antibody
|
|
TPO
|
thyroid peroxidase
|
|
PFS
|
progression-free survival
|
|
OS
|
overall survival
|
|
irTD
|
immune-related thyroid dysfunction
|
|
irAE
|
immune-related adverse event
|
|
ICI
|
immune checkpoint inhibitor
|
|
PD
|
progressive disease
|
|
SD
|
stable disease
|
|
PR
|
partial remission
|
|
CR
|
complete remission
|
|
NSCLC
|
non-small cell lung cancer
|
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cao W, Chen HD, Yu YW, Li N and Chen WQ:
Changing profiles of cancer burden worldwide and in China: A
secondary analysis of the global cancer statistics 2020. Chin Med J
(Engl). 134:783–791. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zheng RS, Chen R, Han BF, Wang SM, Li L,
Sun KX, Zeng HM, Wei WW and He J: Cancer incidence and mortality in
China, 2022. Zhonghua Zhong Liu Za Zhi. 46:221–231. 2024.(In
Chinese). PubMed/NCBI
|
|
4
|
Riely GJ, Wood DE, Ettinger DS, Aisner DL,
Akerley W, Bauman JR, Bharat A, Bruno DS, Chang JY, Chirieac LR, et
al: Non-small cell lung cancer, version 4.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:249–274. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lordick F, Carneiro F, Cascinu S, Fleitas
T, Haustermans K, Piessen G, Vogel A and Smyth EC; ESMO Guidelines
Committee. Electronic address, : simpleclinicalguidelines@esmo.org:
Gastric cancer: ESMO clinical practice guideline for diagnosis,
treatment and follow-up. Ann Oncol. 33:1005–1020. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang FH, Zhang XT, Tang L, Wu Q, Cai MY,
Li YF, Qu XJ, Qiu H, Zhang YJ, Ying JE, et al: The Chinese society
of clinical oncology (CSCO): Clinical guidelines for the diagnosis
and treatment of gastric cancer, 2023. Cancer Commun (Lond).
44:127–172. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nakayama I, Qi C, Chen Y, Nakamura Y, Shen
L and Shitara K: Claudin 18.2 as a novel therapeutic target. Nat
Rev Clin Oncol. 21:354–369. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hu HH, Wang SQ, Zhao H, Chen ZS, Shi X and
Chen XB: HER2+ advanced gastric cancer: Current state and
opportunities (Review). Int J Oncol. 64:362024. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yao Y, Zhou J, Song J and Chen C:
Ruxolitinib enhances gastric cancer to chemotherapy by suppressing
JAK/STAT3 and inducing mitochondrial dysfunction and oxidative
stress. Immunopharmacol Immunotoxicol. 26:1–9. 2025.
|
|
10
|
Babaei S, Nikbakht M, Majd A and Mousavi
SA: Comparative effects of arsenic trioxide and chemotherapy on
Chk1 and CDC25 gene expression in gastric cancer cells AGS and
MKN45: A potential therapeutic strategy. Mol Biol Rep. 52:1982025.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Janjigian YY, Shitara K, Moehler M,
Garrido M, Salman P, Shen L, Wyrwicz L, Yamaguchi K, Skoczylas T,
Bragagnoli AC, et al: First-line nivolumab plus chemotherapy versus
chemotherapy alone for advanced gastric, gastro-oesophageal
junction, and oesophageal adenocarcinoma (CheckMate 649): A
randomised, open-label, phase 3 trial. Lancet. 398:27–40. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kang YK, Chen LT, Ryu MH, Oh DY, Oh SC,
Chung HC, Lee KW, Omori T, Shitara K, Sakuramoto S, et al:
Nivolumab plus chemotherapy versus placebo plus chemotherapy in
patients with HER2-negative, untreated, unresectable advanced or
recurrent gastric or gastrooesophageal junction cancer
(ATTRACTION-4): A randomised, multicentre, double-blind,
placebo-controlled, phase 3 trial. Lancet Oncol. 23:234–247. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Xu J, Jiang H, Pan Y, Gu K, Cang S, Han L,
Shu Y, Li J, Zhao J and Pan H: LBA53 Sintilimab plus chemotherapy
(chemo) versus chemo as. first-line treatment for advanced gastric
or gastroesophageal junction (G/GEJ) adenocarcinoma (ORIENT16):
First results of a randomized, double-blind, phase III study. Anna
Oncol. 32:S13312021. View Article : Google Scholar
|
|
14
|
Moehler MH, Kato K, Arkenau HT, Oh DY,
Tabernero J, Cruz-Correa M, Wang H, Xu H, Li J, Yang S and Xu RH:
RATIONALE-305: Phase 3 study of tislelizumab plus chemotherapy vs.
placebo plus chemotherapy as first-line treatment (1L) of advanced
gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). 2023
ASCO GI. Abstract#286.
|
|
15
|
Cruz-Correa M, Xu RH, Moehler MH, Oh DY,
Kato K, Spigel DR, Arkenau HT, Tabernero J, Zimina AV, Bai Y, et
al: Tislelizumab (TIS) plus chemotherapy (Chemo) vs. placebo (PBO)
plus chemo as first-line (1L) treatment of advanced gastric or
gastroesophageal junction adenocarcinoma (GC/GEJC): Final analysis
results of the RATIONALE-305 study. 2023, ESMO. Abstract LBA80.
|
|
16
|
Janjigian YY, Kawazoe A, Bai Y, Xu J,
Lonardi S, Metges JP, Yanez P, Wyrwicz LS, Shen L, Ostapenko Y, et
al: Pembrolizumab plus trastuzumab and chemotherapy for
HER2-positive gastric or gastro-oesophageal junction
adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811
randomised placebo-controlled trial. Lancet. 402:2197–2208. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Michot JM, Bigenwald C, Champiat S,
Collins M, Carbonnel F, Postel-Vinay S, Berdelou A, Varga A,
Bahleda R, Hollebecque A, et al: Immune-related adverse events with
immune checkpoint blockade: A comprehensive review. Eur J Cancer.
54:139–148. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yang J, Lyu M, Feng X, Liu F, Zeng R, Sun
X, Bao Z, Zhou L, Gao B, Ni L and Xiang Y: The predict factors and
clinical prognosis value of immune-related pneumonia of receiving
PD-1 inhibitor in advanced non-small cell lung cancer: A
retrospective study. Int Immunopharmacol. 2142:1131402024.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jhaveri KD and Wanchoo R: Adverse events
associated with immune checkpoint inhibitors. JAMA. 321:1218–1219.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang LS, Barroso-Sousa R, Tolaney SM,
Hodi FS, Kaiser UB and Min L: Endocrine toxicity of cancer
immunotherapy targeting immune checkpoints. Endocr Rev. 40:17–65.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang T, Lv H, Li J, Zhang S, Zhang J,
Wang S, Wang Y and Guo Z: The impact of immune-related adverse
events on the outcome of advanced gastric cancer patients with
immune checkpoint inhibitor treatment. Front Immunol.
15:15033162024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Grangeon M, Tomasini P, Chaleat S, Jeanson
A, Souquet-Bressand M, Khobta N, Bermudez J, Trigui Y, Greillier L,
Blanchon M, et al: Association between immune-related adverse
events and efficacy of immune checkpoint inhibitors in
non-small-cell lung cancer. Clin Lung Cancer. 20:201–207. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Koyama J, Horiike A, Yoshizawa T, Dotsu Y,
Ariyasu R, Saiki M, Sonoda T, Uchibori K, Nishikawa S, Kitazono S,
et al: Correlation between thyroid transcription factor-1
expression, immune-related thyroid dysfunction, and efficacy of
anti-programmed cell death protein-1 treatment in non-small cell
lung cancer. J Thorac Dis. 11:1919–1928. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhou Y, Xia R, Xiao H, Pu D, Long Y, Ding
Z, Liu J and Ma X: Thyroid function abnormality induced by PD-1
inhibitors have a positive impact on survival in patients with
non-small cell lung cancer. Int Immunopharmacol. 91:1072962021.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ferreira JL, Costa C, Marques B, Castro S,
Victor M, Oliveira J, Santos AP, Sampaio IL, Duarte H, Marques AP
and Torres I: Improved survival in patients with thyroid function
test abnormalities secondary to immune-checkpoint inhibitors.
Cancer Immunol Immunother. 70:299–309. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kotwal A and Ryder M: Survival benefit of
endocrine dysfunction following immune checkpoint inhibitors for
nonthyroidal cancers. Curr Opin Endocrinol Diabetes Obes.
28:517–524. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Amin MB, Edge SB, Greene FL, Byrd DR,
Brookland RK, Washington MK, Gershenwald JE, Compton CC, Hess KR,
Sullivan DC, et al: AJCC Cancer Staging Manual. 8th Edition.
Springer; New York: 2017
|
|
28
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoon JH, Hong AR, Kim HK and Kang HC:
Characteristics of immune-related thyroid adverse events in
patients treated with PD-1/PD-L1 inhibitors. Endocrinol Metab
(Seoul). 36:413–423. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chilelli MG, Signorelli C, Berrios JR,
Onorato A, Nelli F, Fabbri MA, Primi F, Marrucci E, Virtuoso A,
Schirripa M, et al: Immune-related thyroid dysfunction (irTD) in
non-small cell lung cancer (NSCLC) correlates with response and
survival. Cancer Diagn Progn. 2:55–63. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li Z, Xia Y, Xia M, Liu C, Wang T, Liu Y
and Ren Y: Immune-related thyroid dysfunction is associated with
improved long-term prognosis in patients with non-small cell lung
cancer treated with immunotherapy: A systematic review and
meta-analysis. J Thorac Dis. 15:690–700. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou N, Velez MA, Bachrach B, Gukasyan J,
Fares CM, Cummings AL, Lind-Lebuffe JP, Akingbemi WO, Li DY,
Brodrick PM, et al: Immune checkpoint inhibitor induced thyroid
dysfunction is a frequent event post-treatment in NSCLC. Lung
Cancer. 161:34–41. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Horesh A, Pollack R, Nechushtan H,
Dresner-Pollak R and Neuman T: Tumor PD-L1 expression and molecular
profiling are not associated with immune checkpoint
inhibitor-induced thyroid dysfunction in advanced NSCLC patients.
Pathol Oncol Res. 29:16109512023. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yamauchi I, Sakane Y, Fukuda Y, Fujii T,
Taura D, Hirata M, Hirota K, Ueda Y, Kanai Y, Yamashita Y, et al:
Clinical features of nivolumab-induced thyroiditis: A case series
study. Thyroid. 27:894–901. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Osorio JC, Ni A, Chaft JE, Pollina R,
Kasler MK, Stephens D, Rodriguez C, Cambridge L, Rizvi H, Wolchok
JD, et al: Antibody-mediated thyroid dysfunction during T-cell
checkpoint blockade in patients with non-small-cell lung cancer.
Ann Oncol. 28:583–589. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Mazarico I, Capel I, Giménez-Palop O,
Albert L, Berges I, Luchtenberg F, García Y, Fernández-Morales LA,
De Pedro VJ, Caixàs A and Rigla M: Low frequency of positive
antithyroid antibodies is observed in patients with thyroid
dysfunction related to immune check point inhibitors. J Endocrinol
Invest. 42:1443–1450. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Okada N, Iwama S, Okuji T, Kobayashi T,
Yasuda Y, Wada E, Onoue T, Goto M, Sugiyama M, Tsunekawa T, et al:
Anti-thyroid antibodies and thyroid echo pattern at baseline as
risk factors for thyroid dysfunction induced by anti-programmed
cell death-1 antibodies: A prospective study. Br J Cancer.
122:771–777. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kobayashi T, Iwama S, Yasuda Y, Okada N,
Tsunekawa T, Onoue T, Takagi H, Hagiwara D, Ito Y, Morishita Y, et
al: Patients with antithyroid antibodies are prone to develop
destructive thyroiditis by nivolumab: A prospective study. J Endocr
Soc. 2:241–251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kimbara S, Fujiwara Y, Iwama S, Ohashi K,
Kuchiba A, Arima H, Yamazaki N, Kitano S, Yamamoto N and Ohe Y:
Association of antithyroglobulin antibodies with the development of
thyroid dysfunction induced by nivolumab. Cancer Sci.
109:3583–3590. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Izawa N, Shiokawa H, Onuki R, Hamaji K,
Morikawa K, Saji H, Ohashi H, Kasugai S, Hayakawa N, Ohara T and
Sunakawa Y: The clinical utility of comprehensive measurement of
autoimmune disease-related antibodies in patients with advanced
solid tumors receiving immune checkpoint inhibitors: A
retrospective study. ESMO Open. 7:1004152022. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
de Moel EC, Rozeman EA, Kapiteijn EH,
Verdegaal EME, Grummels A, Bakker JA, Huizinga TWJ, Haanen JB, Toes
REM and van der Woude D: Autoantibody development under treatment
with immune-checkpoint inhibitors. Cancer Immunol Res. 7:6–11.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Brancatella A, Viola N, Brogioni S,
Montanelli L, Sardella C, Vitti P, Marcocci C, Lupi I and Latrofa
F: Graves' disease induced by immune checkpoint inhibitors: A case
report and review of the literature. Eur Thyroid J. 8:192–195.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yajima K and Akise Y: A case report of
graves' disease induced by the anti-human programmed cell death-1
monoclonal antibody pembrolizumab in a bladder cancer patient. Case
Rep Endocrinol. 17:23140322019.PubMed/NCBI
|
|
44
|
Al Mushref M, Guido PA, Collichio FA,
Moore DT and Clemmons DR: Thyroid dysfunction, recovery, and
prognosis in melanoma patients treated with immune checkpoint
inhibitors: A RETROSPECTIVE REVIEW. Endocr Pract. 26:36–42. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Iyer PC, Cabanillas ME, Waguespack SG, Hu
MI, Thosani S, Lavis VR, Busaidy NL, Subudhi SK, Diab A and Dadu R:
Immune-Related thyroiditis with immune checkpoint inhibitors.
Thyroid. 28:1243–1251. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Basak EA, van der Meer JWM, Hurkmans DP,
Schreurs MWJ, Oomen-de Hoop E, van der Veldt AAM, Bins S, Joosse A,
Koolen SLW, Debets R, et al: Overt thyroid dysfunction and
anti-thyroid antibodies predict response to anti-PD-1 immunotherapy
in cancer patients. Thyroid. 30:966–973. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kotwal A, Kottschade L and Ryder M: PD-L1
inhibitor-induced thyroiditis is associated with better overall
survival in cancer patients. Thyroid. 30:177–184. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Peiró I, Palmero R, Iglesias P, Díez JJ,
Simó-Servat A, Marín JA, Jiménez L, Domingo-Domenech E, Mancho-Fora
N, Nadal E and Villabona C: Thyroid dysfunction induced by
nivolumab: searching for disease patterns and outcomes. Endocrine.
64:605–613. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yamauchi I, Yasoda A, Matsumoto S,
Sakamori Y, Kim YH, Nomura M, Otsuka A, Yamasaki T, Saito R,
Kitamura M, et al: Incidence, features, and prognosis of
immune-related adverse events involving the thyroid gland induced
by nivolumab. PLoS One. 14:e02169542019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hussaini S, Chehade R, Boldt RG, Raphael
J, Blanchette P, Vareki SM and Fernandes R: Association between
immune-related side effects and efficacy and benefit of immune
checkpoint inhibitors-A systematic review and meta-analysis. Cancer
Treat Rev. 92:1021342021. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sakakida T, Ishikawa T, Uchino J, Chihara
Y, Komori S, Asai J, Narukawa T, Arai A, Kobayashi T, Tsunezuka H,
et al: Clinical features of immune-related thyroid dysfunction and
its association with outcomes in patients with advanced
malignancies treated by PD-1 blockade. Oncol Lett. 18:2140–2147.
2019.PubMed/NCBI
|
|
52
|
Paderi A, Giorgione R, Giommoni E, Mela
MM, Rossi V, Doni L, Minervini A, Carini M, Pillozzi S and
Antonuzzo L: Association between immune related adverse events and
outcome in patients with metastatic renal cell carcinoma treated
with immune checkpoint inhibitors. Cancers (Basel). 13:8602021.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Yu Y, Chen N, Yu S, Shen W, Zhai W, Li H
and Fan Y: Association of immune-related adverse events and the
efficacy of anti-PD-(L)1 monotherapy in non-small cell lung cancer:
Adjusting for immortal-time bias. Cancer Res Treat. 56:751–764.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang J, Gao A, Wang S and Sun Y, Wu J,
Wang D, Ge Y, Li J, Sun H, Cheng Q and Sun Y: Correlation between
immune-related adverse events and efficacy of PD-(L)1 inhibitors in
small cell lung cancer: A multi-center retrospective study. Respir
Res. 25:2562024. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Yasuda Y, Iwama S, Sugiyama D, Okuji T,
Kobayashi T, Ito M, Okada N, Enomoto A, Ito S, Yan Y, et al: CD4+ T
cells are essential for the development of destructive thyroiditis
induced by anti-PD-1 antibody in thyroglobulin-immunized mice. Sci
Transl Med. 13:eabb74952021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Shimozaki K, Sukawa Y, Beppu N, Kurihara
I, Suzuki S, Mizuno R, Funakoshi T, Ikemura S, Tsugaru K, Togasaki
K, et al: Multiple immune-related adverse events and anti-tumor
efficacy: Real-world data on various solid tumors. Cancer Manag
Res. 12:4585–4593. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Nishimura T, Fujimoto H, Fujiwara T, Ito
K, Fujiwara A, Yuda H, Itani H, Naito M, Kodama S, Furuhashi K, et
al: Impact of immune-related adverse events on survival outcomes in
extensive-stage small cell lung cancer patients treated with immune
checkpoint inhibitors. Cancer Med. 13:e71882024. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Reschke R, Deitert B, Enk AH and Hassel
JC: The role of tissue-resident memory T cells as mediators for
response and toxicity in immunotherapy-treated melanoma-two sides
of the same coin? Front Immunol. 15:13857812024. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Postow MA, Sidlow R and Hellmann MD:
Immune-Related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Taylor CA, Watson RA, Tong O, Ye W,
Nassiri I, Gilchrist JJ, de Los Aires AV, Sharma PK, Koturan S,
Cooper RA, et al: IL7 genetic variation and toxicity to immune
checkpoint blockade in patients with melanoma. Nat Med.
28:2592–2600. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Song Y, Pan S, Tian J, Yu Y, Wang S, Qiu
Q, Shen Y, Yang L, Liu X, Luan J, et al: Activation of CD14+
monocytes via the IFN-γ signaling pathway is associated with
immune-related adverse events in hepatocellular carcinoma patients
receiving PD-1 inhibition combination therapy. Biomedicines.
12:11402024. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Kashiwada T, Takano R, Ando F, Kuroda S,
Miyabe Y, Owada R, Miyanaga A, Asatsuma-Okumura T, Hashiguchi M,
Kanazawa Y, et al: Lysosomal degradation of PD-L1 is associated
with immune-related adverse events during anti-PD-L1 immunotherapy
in NSCLC patients. Front Pharmacol. 15:13847332024. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Foldi J, Blenman KRM, Marczyk M,
Gunasekharan V, Polanska A, Gee R, Davis M, Kahn AM, Silber A and
Pusztai L: Peripheral blood immune parameters, response, and
adverse events after neoadjuvant chemotherapy plus durvalumab in
early-stage triple-negative breast cancer. Breast Cancer Res Treat.
208:369–377. 2024. View Article : Google Scholar : PubMed/NCBI
|