Introduction
Breast cancer is one of the most common cancer types
worldwide, including in China; it has an incidence rate of ~60
cases in 10,000, which ranks second among all cancer types and
mortality rate of 11 in 10,000 cases, which ranks fifth among
female tumors (1,2). The incidence of breast cancer
continues to rise; it is estimated that numerous people will be
diagnosed with breast cancer in the next decades (3). Neoadjuvant chemotherapy (NAC),
initially introduced in the 1970s, has become a widely employed
therapeutic approach for patients with operable and locally
advanced breast cancer (4). NAC has
demonstrated efficacy in improving the rate of breast-conserving
surgery and obviating the need for axillary lymph node dissection
via tumor downstaging, particularly among patients with human
epidermal growth factor receptor 2 (HER2) positive (+) status
(5–7). Furthermore, NAC can provide valuable
information on the drug sensitivity of diverse chemotherapy
regimens, thereby facilitating the guidance of subsequent treatment
strategies (8).
Pathological complete response (pCR) rate is the
standard for evaluating the efficacy of NAC. The pCR rate among
different subtypes of breast cancer ranges from 2 to 68% (9). The prognosis of patients achieving pCR
is significantly improved compared with that of patients with
residual cancer burden, leading to prolonged survival and reduced
risk of distant metastasis (10).
For patients with invasive breast cancer, such as triple-negative
and HER2+ types, attaining a pCR is closely associated
with long-term clinical benefit (11). The prediction of the patients who
will achieve pCR could guide personalized treatment based on
clinical and pathological factors. Numerous studies have explored
the relationship between patient characteristics, clinical tumor
stage, lymph node status, HER2 status and hormonal receptor status
with pCR in patients with breast cancer who have been treated with
NAC (12–14). A previous retrospective study
indicated that age and subtypes of breast cancer were associated
with pCR; following NAC, patients with younger age, luminal B2
HER2+, HER2 upregulation and triple-negative subtype
were more likely to achieve pCR (15). By contrast, another study indicated
that only clinical tumor stage, progesterone receptor (PR) negative
(−) status and HER2+ status were associated with pCR,
while age, clinical lymph node stage, estrogen receptor (ER) status
and Ki-67 index indicated no significant correlation (16). Furthermore, the findings of a
real-world study involving 7,711 patients differed from those
reported in the aforementioned study, as clinical lymph node stage,
ER, PR, HER2 status, Ki-67 index and NAC treatment cycle correlated
with pCR (17). The findings from
various studies regarding the factors influencing pCR exhibit
considerable variability. Therefore, additional research is
required to clarify the factors influencing pCR.
Anthracycline plus taxane regimens serves as the
cornerstone regimen for both neoadjuvant and adjuvant treatment of
breast cancer, effectively reducing recurrence and mortality
(18). Taxanes are antitumor drugs
isolated from plants; it includes mainly paclitaxel, docetaxel,
nanoparticle albumin-bound (Nab)-paclitaxel and paclitaxel
liposome, which are essential agents used in NAC regimens (19). The National Comprehensive Cancer
Network guidelines (version 3; 2024) (20) recommend paclitaxel and docetaxel as
preferred taxane agents and Nab-paclitaxel which may be substituted
with paclitaxel or docetaxel due to medical necessity (such as a
hypersensitivity reaction). Paclitaxel is the standard drug for NAC
used for breast cancer; however, paclitaxel injection can cause
severe allergic reactions in 10–40% of patients (21). Due to these side effects,
Nab-paclitaxel, liposomal paclitaxel and docetaxel have emerged as
alternatives (18,22). Although the toxicity profiles of
Nab-paclitaxel and paclitaxel liposomes are reduced, the
differences in the efficacy of Nab-paclitaxel, paclitaxel liposomes
and paclitaxel and docetaxel in breast cancer are currently
uncertain. Therefore, additional studies are required to confirm
their efficacy in NAC.
The present study aimed to evaluate
clinicopathological data collected from patients with breast cancer
following NAC to analyze the associations of age, tumor location,
clinical stage, lymph node involvement, ER, PR, HER2 status, Ki-67
index and taxane regimen with pCR rate. Furthermore, the efficacy
of Nab-paclitaxel, docetaxel and paclitaxel liposomes was evaluated
in different subtypes of breast cancer following NAC.
Materials and methods
Patient selection
In July 2024, medical records of patients with
breast cancer who were treated with NAC and adjuvant radiotherapy
in Nanchang People's Hospital (Nanchang, China) and Affiliated
Rehabilitation Hospital of Nanchang University (Nanchang, China)
during May 2019 and June 2024 were reviewed. The present study
included a total of 552 patients with invasive breast cancer who
were diagnosed by core needle biopsy and received NAC and
radiotherapy during this period. The clinical stage of all patients
prior to NAC was evaluated using breast ultrasonography, chest
computerized tomography and breast magnetic resonance imaging. All
patients underwent lumpectomy or mastectomy and axillary lymph node
dissection following completion of the planned NAC dosage. Patients
with bilateral breast cancer or prior history of cancer were
excluded from the present study. The present study was approved by
the Institutional Review Board ethics committee of Nanchang
People's Hospital (approval no. K-kt2024005; Nanchang, China) from
which the patients were enrolled. The requirement for patient
approval or written informed consent was waived due to the
retrospective nature of the present study.
NAC regimens
The NAC regimens were selected according to the
subtypes of breast cancer. Breast cancer samples with ≥10% of ER or
PR positive tumor nuclei were defined as ER+ or
PR+, since primary breast cancer with ER 1–9% positivity
shows similar clinical behavior to ER 1% (23). HER2 protein upregulation was
assessed using immunohistochemical analysis; 3 or 2+ with HER2 gene
amplification assessed using in situ hybridization was
defined as HER2+. The treatment options for patients
with HR+HER2− and
HR−HER2− included four cycles of
anthracyclines and cyclophosphamide followed by four cycles of
taxanes (AC-T), six cycles of taxanes, anthracyclines and
cyclophosphamide (TAC) and six cycles of taxanes and carboplatin
(Tcb). The regimens for patients with HER2+ breast
cancer were composed of four cycles of anthracyclines and
cyclophosphamide followed by four cycles of taxanes with
trastuzumab or trastuzumab and pertuzumab (AC-TH/AC-THP), six
cycles of taxanes and carboplatin with trastuzumab or trastuzumab
and pertuzumab (TcbH/TcbHP) and six cycles of taxanes with
trastuzumab and pertuzumab (THP). Regarding taxane-based regimens,
liposomal paclitaxel was administered in both weekly and tri-weekly
regimens.
Assessment of pathological
response
pCR was defined as the absence of invasive disease
in both primary tumor and lymph nodes and the presence of in
situ cancer following treatment in the absence of residual
invasive disease (ypT0/is ypN0), as per the 8th Edition of American
Joint Committee on Cancer Staging Manual (24).
Statistical analysis
The data were analyzed using the SPSS software
(version 23; IBM Corp.). Categorical variables age, tumor location,
clinical tumor stage, Ki-67 index, molecular subtypes of breast
cancer, chemotherapy regimens, taxanes and lymph node, ER, PR and
HER2 status were described by percentage. The differences in pCR
rate for these variables was analyzed using the χ2 or
Fisher's exact tests. With the exception of the subtypes of breast
cancer and the chemotherapeutic regimens, the other variables were
considered as alternative variables with P<0.05 in the
univariate analysis. In multivariate analysis, binary regression
analysis was used to analyze the role of these variables in
predicting pCR. A prediction model was established using the
results of the multivariate logistic regression analysis, and
receiver operating characteristic (ROC) curve analysis was used to
assess the prediction power of the model. P<0.05 was considered
to indicate a statistically significant difference. All charts were
generated using the GraphPad Prism software (version 9.5;
Dotmatics).
Results
Study population and treatment regimen
distribution
The clinicopathological and treatment
characteristics of the enrolled 552 patients were analyzed
(Table I). Overall, 274 (49.6%)
patients were ER- and 366 (66.3%) patients were PR-. A total of 433
(78.4%) patients exhibited a Ki-67 index >30% and 254 (46.0%)
patients were HER2+. The distribution of the molecular
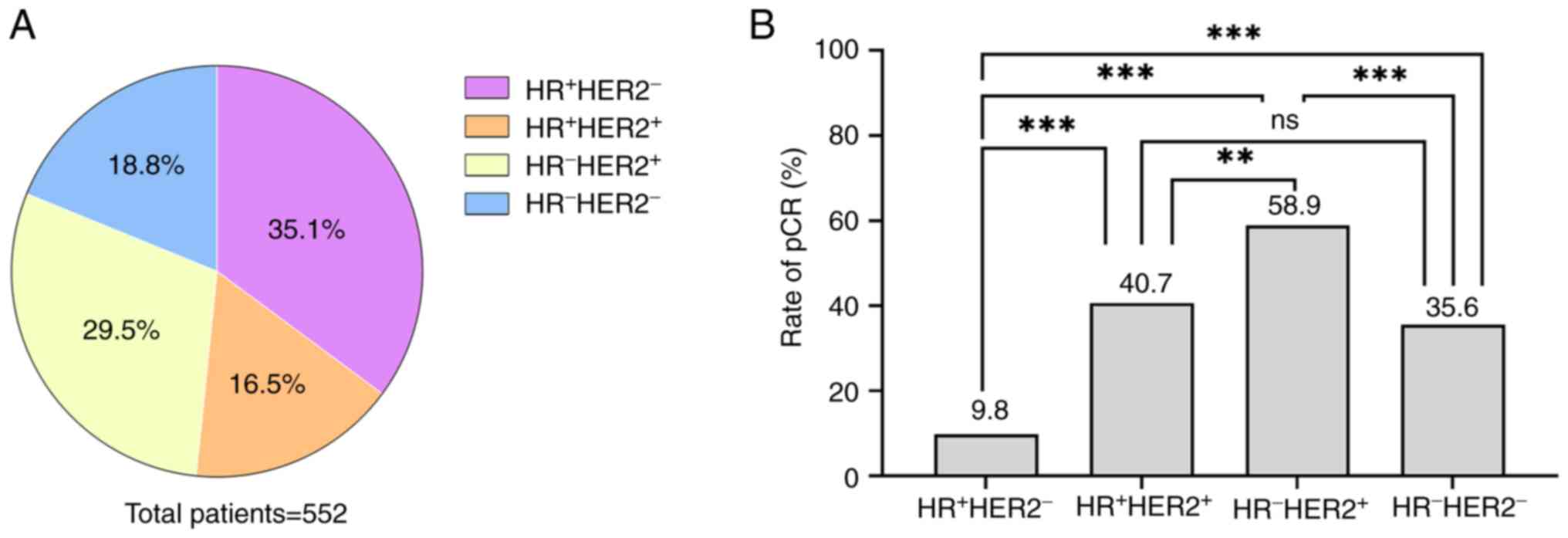
subtypes among the enrolled patients is shown in Fig. 1A. A total of 200 (36.2%) patients
received the AC-T regimen, 82 (14.9%) patients received the TAC
regimen, 16 patients (2.9%) received the Tcb regimen, 48 (8.7%)
patients received the THP regimen, 23 (4.2%) patients received the
AC-TH/TcbH regimen, 31 (5.6%) patients received the AC-THP regimen
and 152 (27.5%) patients received the TcbHP regimen. Among the
taxane-based regimens, 167 (30.3%) patients received
Nab-paclitaxel, 178 (32.2%) patients received docetaxel and 207
(37.5%) patients received paclitaxel liposomes.
 | Table I.Baseline characteristics of 552
patients who achieved pCR (n=189) and non-pCR (n=363). |
Table I.
Baseline characteristics of 552
patients who achieved pCR (n=189) and non-pCR (n=363).
| Variable | Non-pCR, n (%) | pCR, n (%) | Total, n | P-value |
|---|
| Age, years |
|
|
| 0.477 |
|
≤35 | 48 (69.6) | 21 (30.4) | 69 |
|
|
>35 | 315 (65.2) | 168 (34.8) | 483 |
|
| Tumor location |
|
|
| 0.795 |
|
Left | 179 (66.3) | 91 (33.7) | 270 |
|
|
Right | 184 (65.2) | 98 (34.8) | 282 |
|
| Tumor location,
quadrant |
|
|
| 0.872 |
| Upper
outer | 198 (65.1) | 106 (34.9) | 304 |
|
| Lower
outer | 42 (63.6) | 24 (36.4) | 66 |
|
| Upper
inner | 69 (69.7) | 30 (30.3) | 99 |
|
| Lower
inner | 17 (60.7) | 11 (39.3) | 28 |
|
|
Center | 37 (67.3) | 18 (32.7) | 55 |
|
| cT stage |
|
|
| 0.755 |
|
cT1 | 13 (65.0) | 7 (35.0) | 20 |
|
|
cT2 | 222 (65.3) | 118 (34.7) | 340 |
|
|
cT3 | 82 (69.5) | 36 (30.5) | 118 |
|
|
cT4 | 46 (62.2) | 28 (37.8) | 74 |
|
| Lymph node
status |
|
|
| 0.104 |
|
Negative | 26 (78.8) | 7 (21.2) | 33 |
|
|
Positive | 337 (64.9) | 182 (35.1) | 519 |
|
| ER status |
|
|
| <0.001 |
|
Negative | 139 (50.7) | 135 (49.3) | 274 |
|
|
Positive | 224 (80.6) | 54 (19.4) | 278 |
|
| PR status |
|
|
| <0.001 |
|
Negative | 208 (56.8) | 158 (43.2) | 366 |
|
|
Positive | 155 (83.3) | 31 (16.7) | 186 |
|
| Ki-67 index |
|
|
| <0.001 |
|
<30% | 107 (89.9) | 12 (10.1) | 119 |
|
|
≥30% | 256 (59.1) | 177 (40.9) | 433 |
|
| HER2 status |
|
|
| <0.001 |
|
Negative | 242 (81.2) | 56 (18.8) | 298 |
|
|
Positive | 121 (47.6) | 133 (52.4) | 254 |
|
| Molecular
subtype |
|
|
| <0.001 |
|
HR+HER2− | 175 (90.2) | 19 (9.8) | 194 |
|
|
HR−HER2+ | 67 (41.1) | 96 (58.9) | 163 |
|
|
HR+HER2+ | 54 (59.3) | 37 (40.7) | 91 |
|
|
HR−HER2− | 67 (64.4) | 37 (35.6) | 104 |
|
| NAC regimen |
|
|
| <0.001 |
|
AC-T | 164 (82.0) | 36 (18.0) | 200 |
|
|
TAC | 66 (80.5) | 16 (19.5) | 82 |
|
|
Tcb | 12 (75.0) | 4 (25.0) | 16 |
|
|
THP | 24 (50.0) | 24 (50.0) | 48 |
|
| AC-TH +
TcbH | 16 (69.6) | 7 (30.4) | 23 |
|
|
AC-THP | 15 (48.4) | 16 (51.6) | 31 |
|
|
TcbHP | 66 (43.4) | 86 (56.6) | 152 |
|
| Taxanes |
|
|
| 0.002 |
|
Paclitaxel liposome | 155 (74.9) | 52 (25.1) | 207 |
|
|
Nab-paclitaxel | 98 (58.7) | 69 (41.3) | 167 |
|
|
Docetaxel | 110 (61.8) | 68 (38.2) | 178 |
|
Overall and subtype-specific pCR
rates
Overall, 189 of 552 (34.2%) patients achieved pCR.
The pCR rates of different subtypes varied as follows: 58.9%
(96/163), 40.7% (37/91), 35.6% (37/104) and 9.8% (19/298) for the
patient groups of HR−HER2+,
HR+HER2+, HR−HER2− and
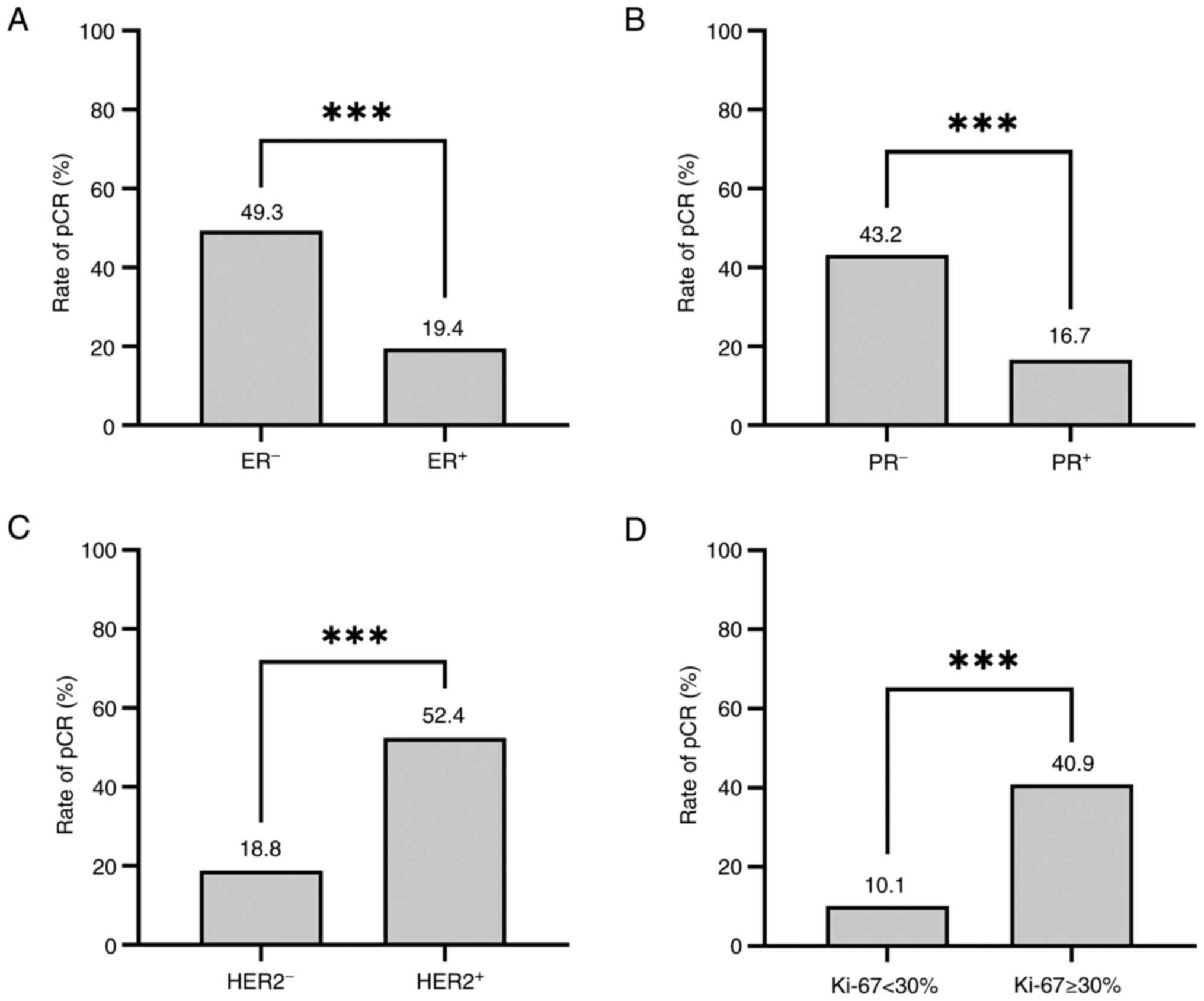
HR+HER2−, respectively (Fig. 1B; P<0.001). Following univariate
analysis, the results indicated that pCR was more likely to be
achieved in patients with ER− [49.3 vs. 19.7%; odds
ratio (OR)=4.029; 95% confidence interval (CI), 2.755–5.891;
P<0.001] and PR− breast cancer (43.2 vs. 16.7%;
OR=3.798; 95% CI, 2.452–5.883; P<0.001). Compared with patients
with HER2− breast cancer, the HER2+ group
exhibited a significantly increased pCR rate (52.4 vs. 18.8%;
OR=4.750; 95% CI, 3.245–6.952; P<0.001). In addition, the Ki-67
≥30% group also exhibited a significantly increased pCR rate
compared with the Ki-67 <30% group (40.9 vs. 10.1%; OR=6.165;
95% CI, 3.294–11.537; P<0.001; Fig.
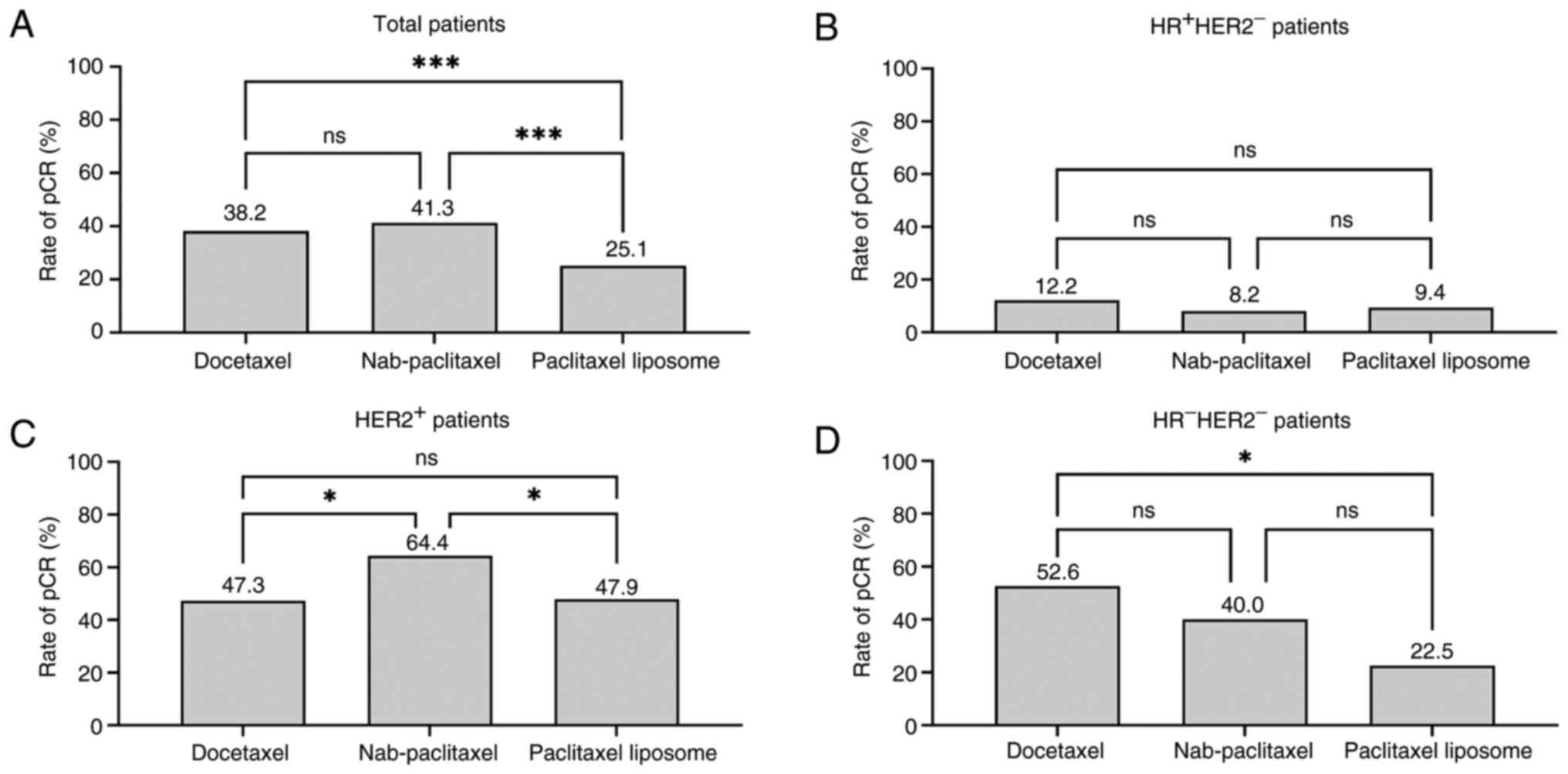
2). The pCR rates were compared among different types of
taxanes. The pCR rates of Nab-paclitaxel, docetaxel and paclitaxel
liposomes were 41.3, 38.2 and 25.1%, respectively (P=0.002;
Fig. 3). The pCR rates of
Nab-paclitaxel (OR=2.099; 95% CI, 1.352–3.258; P=0.001) and
docetaxel (OR=1.843; 95% CI, 1.192–2.850; P=0.001) were
significantly increased compared with those who received paclitaxel
liposomes. No significant differences were observed in the pCR
rates when patients were stratified by age, tumor location,
clinical T stage and lymph node status (Table II).
 | Table II.Univariate and multivariate analysis
for pathologic complete response. |
Table II.
Univariate and multivariate analysis
for pathologic complete response.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | OR | 95% CI | P-value | OR | 95% CI | P-value |
|---|
| Age, years |
|
| 0.477 |
|
|
|
|
≤35 | Reference | - |
|
|
|
|
|
>35 | 1.219 | 0.705–2.104 |
|
|
|
|
| Tumor location |
|
| 0.795 |
|
|
|
|
Left | Reference | - |
|
|
|
|
|
Right | 1.048 | 0.737–1.489 |
|
|
|
|
| Tumor location,
quadrant |
|
| 0.872 |
|
|
|
| Upper
outer | Reference | - |
|
|
|
|
| Lower
outer | 1.067 | 0.613–1.858 |
|
|
|
|
| Upper
inner | 0.812 | 0.498–1.325 |
|
|
|
|
| Lower
inner | 1.209 | 0.546–2.674 |
|
|
|
|
|
Center | 0.909 | 0.493–1.674 |
|
|
|
|
| cT stage |
|
| 0.755 |
|
|
|
|
cT1 | Reference | - |
|
|
|
|
|
cT2 | 0.987 | 0.383–2.541 |
|
|
|
|
|
cT3 | 0.815 | 0.300–2.214 |
|
|
|
|
|
cT4 | 1.130 | 0.403–3.173 |
|
|
|
|
| Lymph node
status |
|
| 0.104 |
|
|
|
|
Negative | Reference |
|
|
|
|
|
|
Positive | 2.006 | 0.854–4.711 |
|
|
|
|
| ER status |
|
| <0.001 |
|
|
|
|
Negative | 4.029 | 2.755–5.891 |
| 2.161 | 1.238–3.77 | 0.007 |
|
Positive | Reference | - |
| Reference | - |
|
| PR status |
|
| <0.001 |
|
|
|
|
Negative | 3.798 | 2.452–5.883 |
| 1.386 | 0.727–2.643 | 0.322 |
|
Positive | Reference | - |
| Reference | - |
|
| Ki-67 index |
|
| <0.001 |
|
|
|
|
<30% | Reference | - |
| Reference | - |
|
|
≥30% | 6.165 | 3.294–11.537 |
| 3.741 | 1.917–7.301 | <0.001 |
| HER2 status |
|
| <0.001 |
|
|
|
|
Negative | Reference | - |
| Reference | - |
|
|
Positive | 4.750 | 3.245–6.952 |
| 3.662 | 2.419–5.545 | <0.001 |
| Taxane |
|
| 0.002 |
|
|
|
|
Paclitaxel liposome | Reference | - |
| Reference | - |
|
|
Nab-paclitaxel | 2.099 | 1.352–3.258 | 0.001 | 1.647 | 1.007–2.695 | 0.047 |
|
Docetaxel | 1.843 | 1.192–2.850 | 0.006 | 1.093 | 0.666–1.795 | 0.725 |
Comparative efficacy of taxane-based
regimens
The pCR rates of different taxanes were analyzed
among different molecular subtypes of breast cancer. The results
demonstrated no significant difference in the pCR rate among the
paclitaxel liposomes, Nab-paclitaxel and docetaxel (9.4 vs. 8.2 vs.
12.2%; P=0.78) in HR+HER2 group −. For
patients that were HER2+, Nab-paclitaxel (64.4%) had the
highest pCR rate compared with patients who received docetaxel
(47.3%; P=0.024) or paclitaxel liposomes (47.89%; P=0.047). In the
HR−HER2− group, no significant difference was
observed in the pCR rates between docetaxel and Nab-paclitaxel
treatments (52.63 vs. 40.00%; P=0.354); however, the pCR rate of
the docetaxel group was significantly increased compared with that
the paclitaxel liposomes group (52.63 vs. 22.50%; P=0.024; Table III and Fig. 3). Overall, both Nab-paclitaxel and
docetaxel subgroups demonstrated increased pCR rates among patients
that were HR−HER2−, while Nab-paclitaxel
demonstrated the highest pCR rate in patients that were
HER2+. By contrast, the paclitaxel liposome group had
the lowest pCR rate overall, and there was no efficacy advantage in
any subtypes of breast cancer.
 | Table III.Pathological complete response rates
of types of taxane among molecular subtypes of breast cancer. |
Table III.
Pathological complete response rates
of types of taxane among molecular subtypes of breast cancer.
| Variable | Non-pCR, n (%) | pCR, n (%) | Total, n | Odds ratio | 95% CI | P-value |
|---|
|
HR+HER2− |
|
|
|
|
|
|
|
Paclitaxel liposome | 87 (90.6) | 9 (9.4) | 96 | Reference |
| 0.781 |
|
Nab-paclitaxel | 45 (91.8) | 4 (8.2) | 49 | 0.85 | 0.25–2.94 | 0.809 |
|
Docetaxel | 43 (87.8) | 6 (12.2) | 49 | 1.34 | 0.45–4.03 | 0.592 |
|
HER2+ |
|
|
|
|
|
|
|
Nab-paclitaxel | 26 (35.6) | 47 (64.4) | 73 | Reference |
| 0.054 |
|
Docetaxel | 58 (52.7) | 52 (47.3) | 110 | 0.496 | 0.270–0.911 | 0.024 |
|
Paclitaxel liposome | 37 (52.1) | 34 (47.9) | 71 | 0.508 | 0.261–0.991 | 0.047 |
|
HR−HER2− |
|
|
|
|
|
|
|
Docetaxel | 9 (47.4) | 10 (52.6) | 19 | Reference |
| 0.062 |
|
Nab-paclitaxel | 27 (60.0) | 18 (40.0) | 45 | 0.6 | 0.203–1.767 | 0.353 |
|
Paclitaxel liposome | 31 (77.5) | 9 (22.5) | 40 | 0.261 | 0.081–0.839 | 0.024 |
Development and validation of a pCR
predictive model
Based on the results of univariate analysis,
variables with statistical significance were included, such as ER
status, PR status, HER2 status, Ki-67 index and taxane regimen were
used to establish a model to predict pCR. A multivariate regression
analysis was performed on the variables with P<0.05 from the
univariate analysis. The results indicated that the patients that
achieved pCR were associated with the following characteristics:
ER−, Ki-67 ≥30% and HER2+ with regard to
tumor features, and taxane regimen with Nab-paclitaxel with regard
to treatment administration (Table
II). Based on the clinically and statistically significant
variables, a model was constructed to predict pCR for patients with
breast cancer treated with NAC. The area under the curve value of
the ROC curve of the model was 0.774 (95% CI, 0.735–0.813; Fig. 4), which indicated that the model
exhibited acceptable discriminatory power in predicting pCR. The
cut-off value of this model was 0.5, with a sensitivity of 50.26%
and a specificity of 82.92% (Table
IV). The positive predictive value was 60.51%, negative
predictive value was 76.20% and correction rate was 71.74%.
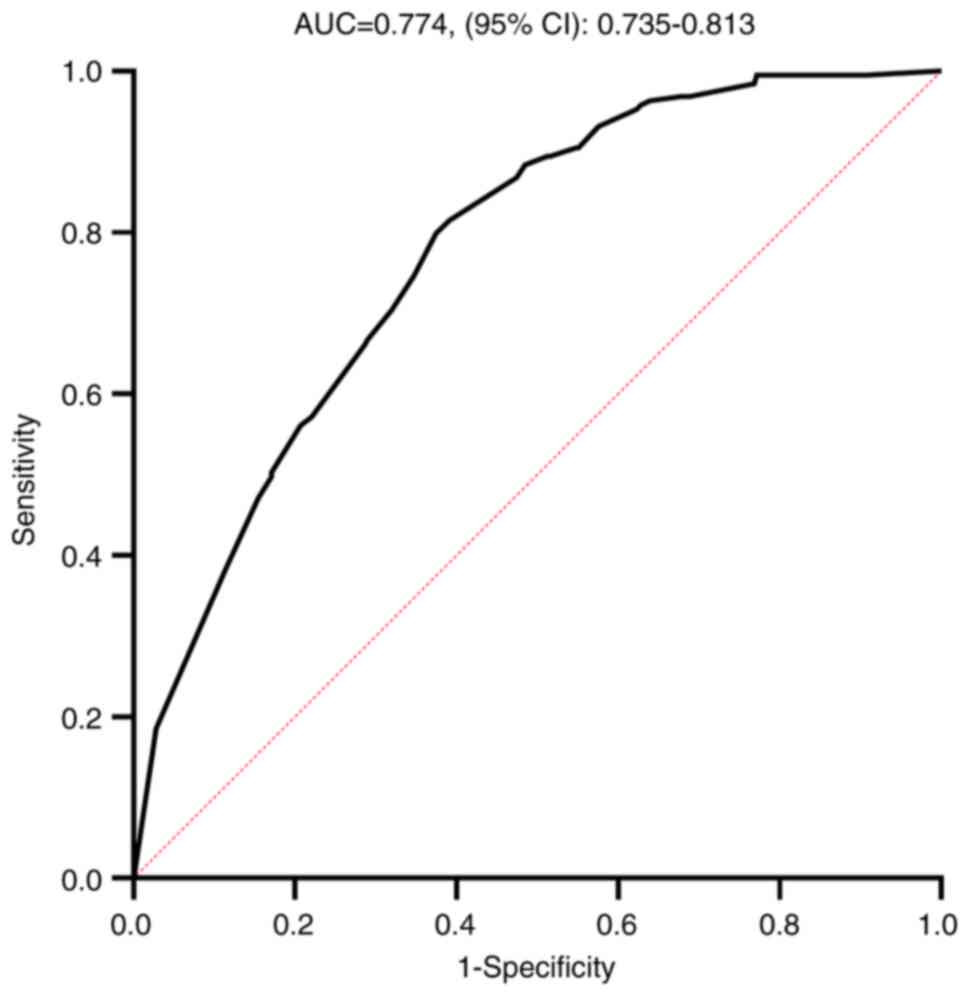 | Figure 4.ROC curve of the prediction model
pCR. The variables ER+, PR+,
HER2−, Ki-67 ≤30% and liposomal paclitaxel were used as
references to produce the ROC curve. ROC, receiver operating
characteristic; AUC, area under curve; pCR, pathological complete
response; HR, hormone receptor; HER, human epidermal growth factor
receptor; ER, estrogen receptor; PR, progesterone receptor. |
 | Table IV.Sensitivity and specificity of the
model for pCR. |
Table IV.
Sensitivity and specificity of the
model for pCR.
| Classification | Predicted non-pCR,
n | Predicted pCR,
n | Total, n |
|---|
| Observed
non-pCR | 301 | 62 | 363 |
| Observed pCR | 94 | 95 | 189 |
| Total | 395 | 157 | 552 |
Discussion
NAC has become a widely employed therapeutic
approach for patients with operable and locally advanced breast
cancer (25). pCR has been applied
to evaluate the efficacy of NAC. Breast cancer is a heterogeneous
malignancy with distinct subtypes exhibiting varied responses to
NAC. The pCR rate among different breast cancer subtypes varies
significantly. The patients who achieve pCR exhibit an optimal
prognosis compared with those with residual cancer burden (26). However, the prediction of pCR is
challenging.
To assess the predictive value of the
clinicopathological factors and different types of taxanes in
predicting pCR following NAC in breast cancer, the present study
conducted a retrospective analysis on patients with breast cancer
who received NAC. A predictive model was developed based on the
clinicopathological characteristics to estimate the rates of pCR.
In the present study, distinct sensitivities were observed with
regard to NAC among various subtypes of breast cancer. The highest
rate of pCR was achieved in the HER2+ group, followed by
those in the HR−HER2− group and patients with
HR+HER2− who exhibited the lowest pCR rate,
which was just 9.8% indicating that HR+HER2−
subtype breast cancer was not sensitive to chemotherapy. It was
reported that patients with the HR+HER2−
subtype of breast cancer demonstrated a low response rate to NAC
regimens containing taxanes and anthracyclines with pCR rates
ranging from 0 to 15%, consistent with the findings of the present
study (27–29). In addition, the present univariate
regression analysis found that ER, PR and HER2 status, Ki-67 index,
and taxane regimen were significantly associated with pCR.
Targeted therapy combined with chemotherapy is the
standard treatment for patients with HER2+ breast cancer
(20). Clinical trials, such as
NeoSphere, NeoALTTO and KRISTINE have demonstrated that the
integration of trastuzumab and pertuzumab with chemotherapy yields
a high response rate, achieving a pCR rate of up to 55% (6,10,30).
The present study found that patients with HER2+ breast
cancer were associated with a high pCR rate, especially patients
with HR−HER2+ breast cancer, which reached
58.9%.
HR−HER2− breast cancer was
also more sensitive to chemotherapy compared with the
HR+HER2− subtypes, as reported by the CALGB
40603 study, with a pCR rate of 44% for patients who received the
TAC regimen; the I-SPY2 study reported a pCR rate of 26% in
patients who received the T-AC regimen and the BrighTNess study
reported a pCR rate of 58% in patients who received the Tcb regimen
(15,31,32).
In the present study, AC-T, TAC and Tcb were used as NAC regimens,
with an overall pCR rate of 35.6%, which was similar to that
reported by aforementioned studies. Immunotherapy may also improve
pCR rate in TNBCs (33). However,
only 1 in 104 patients that were HR−HER2−
used pembrolizumab in combination with Nab-paclitaxel and
carboplatin in the present cohort (treated May 2019 and June 2024)
as local health insurance policies did not cover the treatment
until late 2023 in the present center and the patient achieved pCR.
As it was only an individual case in the present cohort, the case
was not analyzed separately, which reflects a limitation of the
present study.
Dou et al conducted a study of 879 breast
cancer cases treated with NAC, reporting a significantly increased
rate of pCR in patients who were ER−/PR−
compared with that of patients with ER+/PR+
(64.6 vs. 21.5%; P<0.001) (34).
The findings of the present study also indicated that patients with
ER− or PR− breast cancer exhibited a higher
likelihood of achieving pCR. Although univariate analysis indicated
a notable association between PR and pCR, the multivariate analysis
did not demonstrate any significant associations.
A previous study reports that 15–20% of breast
cancer cases exhibit amplification of HER2, resulting in an
upregulation of HER2 (35). The
homo- or hetero-dimerization of HER2 with one of the other three
receptors (HER1 or EGFR, HER3 and HER4) triggers the activation of
signaling pathways which promote cancer cell proliferation,
invasion and survival (36). HER2
status is positively correlated with the pCR rate in breast cancer
cases treated with NAC (37). The
present study demonstrated a significantly higher pCR rate in
patients with HER2+ breast cancer.
The Ki-67 index also exhibits important predictive
value in breast cancer cases treated with NAC. Denkert et al
reported pCR rates of 4.2, 12.8 and 29.0% in patients with a Ki-67
index of ≤15, 15.1–35 and >35%, respectively (38). Another predictive model that
examined the response to NAC in breast cancer also demonstrated an
association between Ki-67 status and the pCR rate (39). Consistent with these findings, the
results of the present study demonstrated that patients with Ki-67
≥30% expression demonstrated an increased pCR rate compared with
those with Ki-67 <30% expression, which highlighted the key
predictive value of Ki-67 index in NAC treatment for breast
cancer.
Taxanes containing regimens are widely used as NAC
for breast cancer. The toxicity profiles of different paclitaxel
have been reported in previous publications (40–42).
Generally, paclitaxel liposome and Nab-paclitaxel showed relatively
mild side effects, especially hypersensitivity reaction. The
response rate of breast cancer to NAC may be influenced by
different types of taxanes (43).
Zhang et al (44) conducted
a retrospective study on 235 patients with breast cancer treated
with NAC and indicated that Nab-paclitaxel demonstrated an
advantage in improving the total and axillary-only pCR rate over
liposomal paclitaxel. An additional study retrospectively analyzed
the efficacy of solvent-based paclitaxel, liposomal paclitaxel,
Nab-paclitaxel and docetaxel, which also indicated that the
Nab-paclitaxel group exhibited the highest total pCR cases and
breast pCR rates (43). The present
study demonstrated that the Nab-paclitaxel group achieved the
highest pCR rate across all patient cohorts analyzed. Subgroup
analyses demonstrated that the Nab-paclitaxel group also exhibited
the highest pCR rate in patients with HER2+ breast
cancer. By contrast, the docetaxel group demonstrated the highest
pCR rate among patients HR−HER2− breast
cancer. However, this difference was not statistically significant
compared with that of the Nab-paclitaxel group. Notably, in the
HR+HER2− group, no significant differences in
pCR rates were observed among the three treatment groups, which may
be due to their inherent insensitivity to chemotherapy. Although
paclitaxel liposome exhibits low toxicity profiles, it does not
show an advantage in improving pCR in any subtypes of breast cancer
(44). Therefore, the selection of
taxanes according to breast cancer subtype may improve the pCR rate
after NAC.
Based on these findings, a multivariate regression
model was used to predict pCR. The model revealed that patients
characterized by ER−, Ki-67 index ≥30%, HER2+
and taxane regimen with Nab-paclitaxel were more likely to achieve
pCR. Moreover, the present findings demonstrated that
Nab-paclitaxel and docetaxel exhibited a significantly increased
pCR rate compared with that of paclitaxel liposomes in patients
with HER2+ and HR−HER2− breast
cancer. Consequently, patients with HER2+ and
HR−HER2− breast cancer are potentially more
suitable for NAC compared with those with
HR+HER2− breast cancer, especially the
HR−HER2+ subgroup. The patients included in
the present study were predominantly residents of the Jiangxi
province, which may represent a limitation in regard to the
generalization of the findings to a global population. Cancer cell
differentiation degree may affect the pCR rate, however, not all of
the patients enrolled in the present study had differentiation data
available from the pathology reports, particularly those with core
needle biopsy samples; therefore, and differentiation data were not
used to analyze separate subgroups. The HR status among the
HER2+ group were not further differentiated due to the
low number of cases after subdivision. Furthermore, external
validation of the predictive model was not conducted in the present
study. The sensitivity and the specificity of the present model was
50.26 and 82.92% respectively, which indicated that the model
exhibited a certain level of deficiency. Due to these limitations,
further study regarding the different paclitaxel regimens effects
on pCR rates are warranted in future.
In summary, the present study demonstrated that ER,
PR and HER2 status, Ki-67 index and different types of taxanes are
independent predictive factors for pCR in patients with breast
cancer who receive NAC. Patients with ER−,
PR−, HER2+, Ki-67 index ≥30% breast cancer
were more sensitive to NAC. Patients who received Nab-paclitaxel or
docetaxel were more likely to achieve pCR compared with those who
received paclitaxel liposomes, notably in the HER2+ and
HR−HER2− breast cancer subgroups. These
findings indicated that molecular subtypes and taxane choice may
significantly influence the likelihood of achieving pCR.
Nab-paclitaxel and docetaxel were identified as effective taxanes,
highlighting their potential clinical preference, especially in
HER2+ and HR−HER2− breast
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by grants from the National
Natural Science Foundation of China (grant no. 82060482) and the
Natural Science Foundation of Jiangxi Province (grant no.
20171BAB205057).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
JD and YC contributed to the conception and design
of the manuscript. JK, HX, XJ, ZH and YG were responsible for the
acquisition, analysis and interpretation of data. XJ, YG and JD
undertook the editing, drafting and writing of the manuscript. All
authors confirm the authenticity of all the raw data, and read and
approved the final manuscript.
Ethics approval and consent to
participate
The present study was approved by the review board
ethics committee of Nanchang People's Hospital (approval no.
K-kt2024005; Nanchang, China). The requirement for patient approval
or written informed consent was waived due to the retrospective
nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Han B, Zheng R, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2022. J Natl Cancer Cent. 4:47–53. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Giaquinto AN and Jemal A:
Cancer statistics, 2024. CA Cancer J Clin. 74:12–49. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Song W, Gao P, Guan X, Wang B, Zhang
L, Yao Y, Guo Y, Wang Y, Jiang S and Sun S: Global, regional, and
national burden of breast, cervical, uterine, and ovarian cancer
and their risk factors among women from 1990 to 2021, and
projections to 2050: Findings from the global burden of disease
study 2021. BMC Cancer. 25:3302025. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Freitas AJA, Causin RL, Varuzza MB,
Hidalgo Filho CMT, Silva VDD, Souza CP and Marques MMC: Molecular
biomarkers predict pathological complete response of neoadjuvant
chemotherapy in breast cancer patients: Review. Cancers (Basel).
13:54772021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dubsky P, Pinker K, Cardoso F, Montagna G,
Ritter M, Denkert C, Rubio IT, de Azambuja E, Curigliano G,
Gentilini O, et al: Breast conservation and axillary management
after primary systemic therapy in patients with early-stage breast
cancer: the Lucerne toolbox. Lancet Oncol. 22:e18–e28. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hurvitz SA, Martin M, Symmans WF, Jung KH,
Huang CS, Thompson AM, Harbeck N, Valero V, Stroyakovskiy D,
Wildiers H, et al: Neoadjuvant trastuzumab, pertuzumab, and
chemotherapy versus trastuzumab emtansine plus pertuzumab in
patients with HER2-positive breast cancer (KRISTINE): A randomised,
open-label, multicentre, phase 3 trial. Lancet Oncol. 19:115–126.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim HJ, Dominici L, Rosenberg SM, Zheng Y,
Pak LM, Poorvu PD, Ruddy KJ, Tamimi R, Schapira L, Come SE, et al:
Surgical treatment after neoadjuvant systemic therapy in young
women with breast cancer: Results from a prospective cohort study.
Ann Surg. 276:173–179. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
von Minckwitz G, Blohmer JU, Costa SD,
Denkert C, Eidtmann H, Eiermann W, Gerber B, Hanusch C, Hilfrich J,
Huober J, et al: Response-guided neoadjuvant chemotherapy for
breast cancer. J Clin Oncol. 31:3623–3630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Conforti F, Pala L, Sala I, Oriecuia C, De
Pas T, Specchia C, Graffeo R, Pagan E, Queirolo P, Pennacchioli E,
et al: Evaluation of pathological complete response as surrogate
endpoint in neoadjuvant randomised clinical trials of early stage
breast cancer: Systematic review and meta-analysis. BMJ.
375:e0663812021. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
de Azambuja E, Holmes AP, Piccart-Gebhart
M, Holmes E, Di Cosimo S, Swaby RF, Untch M, Jackisch C, Lang I,
Smith I, et al: Lapatinib with trastuzumab for HER2-positive early
breast cancer (NeoALTTO): Survival outcomes of a randomised,
open-label, multicentre, phase 3 trial and their association with
pathological complete response. Lancet Oncol. 15:1137–1146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortazar P, Zhang L, Untch M, Mehta K,
Costantino JP, Wolmark N, Bonnefoi H, Cameron D, Gianni L,
Valagussa P, et al: Pathological complete response and long-term
clinical benefit in breast cancer: The CTNeoBC pooled analysis.
Lancet. 384:164–172. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng Q, Yan H, He Y, Wang J, Zhang N, Huo
L, Liu Y, Wang L, Xu L and Fan Z: An ultrasound-based nomogram for
predicting axillary node pathologic complete response after
neoadjuvant chemotherapy in breast cancer: Modeling and external
validation. Cancer. 130:1513–1523. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Değerli E, Şentürk Öztaş N, Alkan G, Bedir
Ş, Derin S, Valıkhanova N, Saraç B, Kacar E, Demirci NS, Demirelli
HF and Turna H: Relationship between pathological response and
molecular subtypes in locally advanced breast cancer patients
receiving neoadjuvant chemotherapy. J Chemother. 35:29–38. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang T, Liu Y and Tian T: Predicting
pathological complete response after neoadjuvant chemotherapy in
breast cancer by clinicopathological indicators and ultrasound
parameters using a nomogram. Sci Rep. 14:163482024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sikov WM, Berry DA, Perou CM, Singh B,
Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER,
et al: Impact of the addition of carboplatin and/or bevacizumab to
neoadjuvant once-per-week paclitaxel followed by dose-dense
doxorubicin and cyclophosphamide on pathologic complete response
rates in stage II to III triple-negative breast cancer: CALGB 40603
(Alliance). J Clin Oncol. 33:13–21. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi HJ, Ryu JM, Kim I, Nam SJ, Kim SW, Yu
J, Lee JE and Lee SK: Nomogram for accurate prediction of breast
and axillary pathologic response after neoadjuvant chemotherapy in
node positive patients with breast cancer. Ann Surg Treat Res.
96:169–176. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Maimaitiaili A, Li Y, Chai N, Liu Z, Ling
R, Zhao Y, Yang H, Liu Y, Liu K, Zhang J, et al: A nomogram for
predicting pathologic node negativity after neoadjuvant
chemotherapy in breast cancer patients: A nationwide, multicenter
retrospective cohort study (CSBrS-012). Front Oncol.
14:13263852024. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Anthracycline-containing and
taxane-containing chemotherapy for early-stage operable breast
cancer: A patient-level meta-analysis of 100 000 women from 86
randomised trials. Lancet. 401:1277–1292. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Nižnanský Ľ, Osinová D, Kuruc R, Hengerics
Szabó A, Szórádová A, Masár M and Nižnanská Ž: Natural taxanes:
From plant composition to human pharmacology and toxicity. Int J
Mol Sci. 23:156192022. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gradishar WJ, Moran MS, Abraham J,
Abramson V, Aft R, Agnese D, Allison KH, Anderson B, Bailey J,
Burstein HJ, et al: Breast cancer, version 3.2024, NCCN clinical
practice guidelines in oncology. J Natl Compr Canc Netw.
22:331–357. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Symons R, Heath F, Duggan J, Bui KT, Byun
L, Friedlander M and Lee YC: Rates of paclitaxel hypersensitivity
reactions using a modified Markman's infusion protocol as primary
prophylaxis. Support Care Cancer. 32:2922024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bi Z, Chen P, Liu YB, Zhao T, Sun X, Song
XR and Wang YS: Efficacy and safety analysis of paclitaxel,
docetaxel and liposomal paclitaxel after neoadjuvant therapy in
breast cancer. Breast Cancer Res Treat. 184:397–405. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dieci MV, Griguolo G, Bottosso M,
Tsvetkova V, Giorgi CA, Vernaci G, Michieletto S, Angelini S,
Marchet A, Tasca G, et al: Impact of estrogen receptor levels on
outcome in non-metastatic triple negative breast cancer patients
treated with neoadjuvant/adjuvant chemotherapy. NPJ Breast Cancer.
7:1012021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Giuliano AE, Edge SB and Hortobagyi GN:
Eighth Edition of the AJCC cancer staging manual: Breast cancer.
Ann Surg Oncol. 25:1783–1785. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kerr AJ, Dodwell D, McGale P, Holt F,
Duane F, Mannu G, Darby SC and Taylor CW: Adjuvant and neoadjuvant
breast cancer treatments: A systematic review of their effects on
mortality. Cancer Treat Rev. 105:1023752022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Spring LM, Fell G, Arfe A, Sharma C,
Greenup R, Reynolds KL, Smith BL, Alexander B, Moy B, Isakoff SJ,
et al: Pathologic complete response after neoadjuvant chemotherapy
and impact on breast cancer recurrence and survival: A
comprehensive Meta-analysis. Clin Cancer Res. 26:2838–2848. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ortmann O, Blohmer JU, Sibert NT, Brucker
S, Janni W, Wöckel A, Scharl A, Dieng S, Ferencz J, Inwald EC, et
al: Current clinical practice and outcome of neoadjuvant
chemotherapy for early breast cancer: Analysis of individual data
from 94,638 patients treated in 55 breast cancer centers. J Cancer
Res Clin Oncol. 149:1195–1209. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Guan D, Jie Q, Wu Y, Xu Y, Hong W and Meng
X: Real-world data on breast pathologic complete response and
disease-free survival after neoadjuvant chemotherapy for hormone
receptor-positive, human epidermal growth factor
receptor-2-negative breast cancer: A multicenter, retrospective
study in China. World J Surg Oncol. 20:3262022. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Villegas SL, Nekljudova V, Pfarr N, Engel
J, Untch M, Schrodi S, Holms F, Ulmer HU, Fasching PA, Weber KE, et
al: Therapy response and prognosis of patients with early breast
cancer with low positivity for hormone receptors-An analysis of
2765 patients from neoadjuvant clinical trials. Eur J Cancer.
148:159–170. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gianni L, Pienkowski T, Im YH, Roman L,
Tseng LM, Liu MC, Lluch A, Staroslawska E, de la Haba-Rodriguez J,
Im SA, et al: Efficacy and safety of neoadjuvant pertuzumab and
trastuzumab in women with locally advanced, inflammatory, or early
HER2-positive breast cancer (NeoSphere): A randomised multicentre,
open-label, phase 2 trial. Lancet Oncol. 13:25–32. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Loibl S, O'Shaughnessy J, Untch M, Sikov
WM, Rugo HS, McKee MD, Huober J, Golshan M, von Minckwitz G, Maag
D, et al: Addition of the PARP inhibitor veliparib plus carboplatin
or carboplatin alone to standard neoadjuvant chemotherapy in
triple-negative breast cancer (BrighTNess): A randomised, phase 3
trial. Lancet Oncol. 19:497–509. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai
L, Wallace A, Chien AJ, Forero-Torres A, Ellis E, Han H, et al:
Effect of pembrolizumab plus neoadjuvant chemotherapy on pathologic
complete response in women with early-stage breast cancer: An
analysis of the ongoing phase 2 adaptively randomized I-SPY2 trial.
JAMA Oncol. 6:676–684. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Schmid P, Cortes J, Pusztai L, McArthur H,
Kümmel S, Bergh J, Denkert C, Park YH, Hui R, Harbeck N, et al:
Pembrolizumab for early Triple-negative breast cancer. N Engl J
Med. 382:810–821. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dou H, Li F, Wang Y, Chen X, Yu P, Jia S,
Ba Y, Luo D, Gao T, Li Z and Xiao M: Estrogen
receptor-negative/progesterone receptor-positive breast cancer has
distinct characteristics and pathologic complete response rate
after neoadjuvant chemotherapy. Diagn Pathol. 19:52024. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ahn S, Woo JW, Lee K and Park SY: HER2
status in breast cancer: Changes in guidelines and complicating
factors for interpretation. J Pathol Transl Med. 54:34–44. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Roskoski R Jr: The ErbB/HER family of
protein-tyrosine kinases and cancer. Pharmacol Res. 79:34–74. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Baumgartner A, Tausch C, Hosch S,
Papassotiropoulos B, Varga Z, Rageth C and Baege A:
Ultrasound-based prediction of pathologic response to neoadjuvant
chemotherapy in breast cancer patients. Breast. 39:19–23. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Denkert C, Loibl S, Müller BM, Eidtmann H,
Schmitt WD, Eiermann W, Gerber B, Tesch H, Hilfrich J, Huober J, et
al: Ki67 levels as predictive and prognostic parameters in
pretherapeutic breast cancer core biopsies: A translational
investigation in the neoadjuvant GeparTrio trial. Ann Oncol.
24:2786–2793. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim SY, Cho N, Choi Y, Lee SH, Ha SM, Kim
ES, Chang JM and Moon WK: Factors affecting pathologic complete
response following neoadjuvant chemotherapy in breast cancer:
Development and validation of a predictive nomogram. Radiology.
299:290–300. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li Y, Chen X, Zhu Q, Chen R, Xu L, Li S,
Shi X, Xu H, Xu Y, Zhang W, et al: Retrospective comparisons of
nanoparticle albumin-bound paclitaxel and docetaxel neoadjuvant
regimens for breast cancer. Nanomedicine (Lond). 16:391–400. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wu X, Ye C, Wang X, Cai R, Yang J, Yu X,
Zhou Y, Shen L, Zhu Y and Liu X: The efficacy and toxicity of
neoadjuvant chemotherapy regimens of epirubicin plus
cyclophosphamide followed by docetaxel or paclitaxel in female
breast cancer patients. Cancer Manag Res. 13:1517–1527. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jivani A and Shinde RK: A comprehensive
review of taxane treatment in breast cancer: Clinical perspectives
and toxicity profiles. Cureus. 16:e592662024.PubMed/NCBI
|
|
43
|
Zhang W, Wang Y, He J, Xu Y, Chen R, Wan
X, Shi W, Huang X, Xu L, Wang J and Zha X: Efficacy comparisons of
solvent-based paclitaxel, liposomal paclitaxel, nanoparticle
albumin-bound paclitaxel, and docetaxel after neoadjuvant systemic
treatment in breast cancer. Nanomedicine. 54:1027072023. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Zhang W, Xu Y, Shi X, Huang X, Chen R, Xu
H, Shi W, Wan X, Wang Y, He J, et al: Nanoparticle albumin-bound
paclitaxel is superior to liposomal paclitaxel in the neoadjuvant
treatment of breast cancer. Nanomedicine (Lond). 17:683–694. 2022.
View Article : Google Scholar : PubMed/NCBI
|