Introduction
Melanoma is a highly malignant and aggressive skin
tumor. According to statistics, the global incidence of malignant
melanoma has increased from 2.01 per 100,000 in 1990 to 3.75 per
100,000 in 2019 (1), and early and
accurate diagnosis is crucial for improving patient prognosis.
However, in the clinic, the pathological features of some pigmented
skin lesions are not clearly differentiated from melanoma, thus
posing a diagnostic challenge. The most common sites of metastasis
for cutaneous malignant melanoma are the brain, lungs, liver and
lymph nodes, and ~90% of patients diagnosed with metastatic
melanoma with three or more metastases die within 1 year (2). Although melanoma metastases can be
found almost anywhere in the body, it is uncommon for multiple
systemic metastases to be detected (3). The present study reports on a case
with unknown diagnosis, but with highly suspected multiple
intracranial, lung, liver, bone and lymph node metastases of
melanoma.
Case report
Patient presentation and background
information
The patient was a 45-year-old woman who presented
with low back pain in June 2024 without any obvious cause, and the
pain was not relieved after independently applying a traditional
Chinese medicine ointment. In mid-July 2024, the pain worsened,
accompanied by coughing and sputum expectoration. The patient was
admitted to The First Affiliated Hospital of Hebei University of
Chinese Medicine at the beginning of August 2024, due to the
presence of low back pain for >2 months and coughing for ~1
month. The patient complained of pigmented skin lesions around
their body at birth, with large patches of hyperpigmentation on the
back and buttocks. There was no evidence of related diseases in the
family and the patient denied that there was a family history of
hereditary disease. At the time of admission, the symptoms were
mainly an intermittent dry cough with little sputum, fatigue and
poor appetite. Written informed consent was obtained from the
patient for the present case report, which was also approved by the
Ethics Committee of The First Affiliated Hospital of Hebei
University of Chinese Medicine (approval no. HBZY2025-KY-004-01;
Shijiazhuang, China).
Clinical examination
The oncologist and the consulting dermatologist
performed a detailed physical examination of the lesions; the
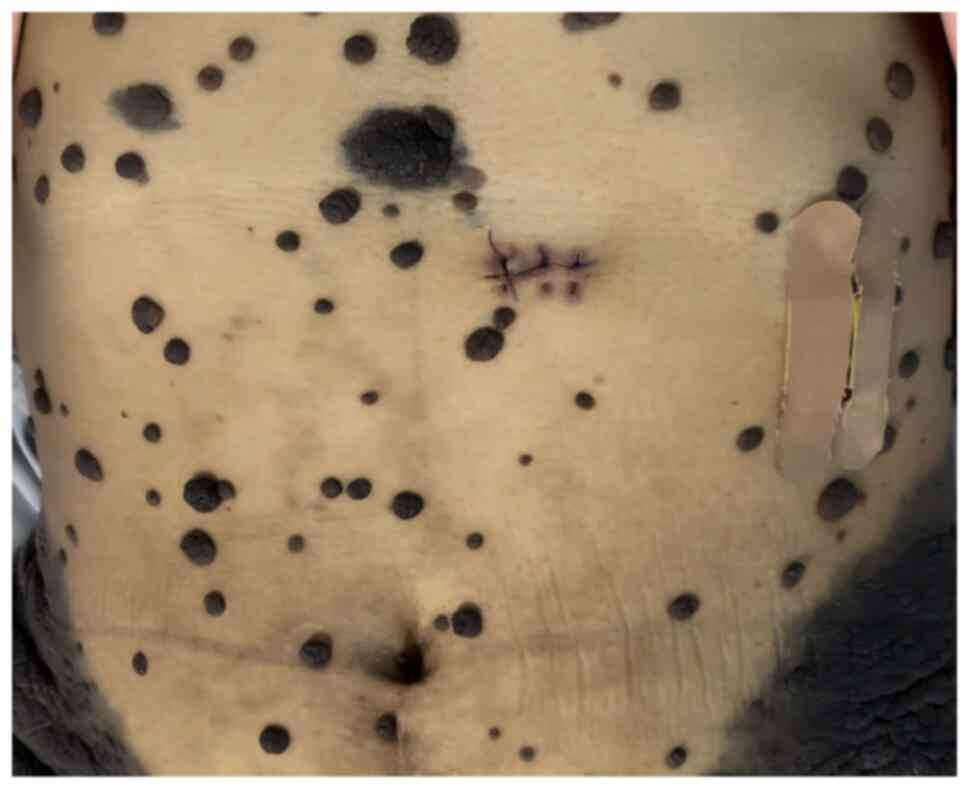
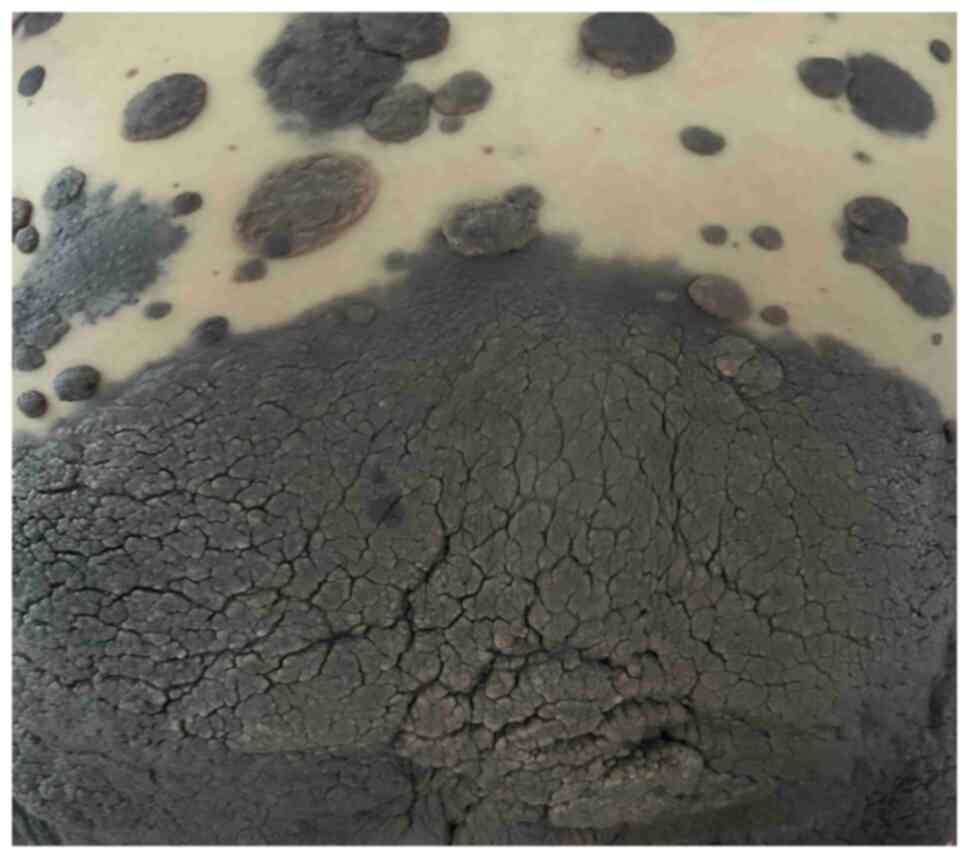
peripheral skin of the patient was seen to have large areas of
hyperpigmentation, some of which were markedly elevated to form
prominent masses, and notable thickening of the skin lesions on the
back and hips was observed, with a thickening of >5 mm (Figs. 1 and 2). No abnormal enlargement of superficial
lymph nodes throughout the body was palpable. Upon observation,
these skin lesions showed a trend of expansion in scope and
thickening of the affected areas within a few days, with the skin
on the back and hips being the most prominent. Such rapid skin
changes were considered to have a certain malignant tendency.
Laboratory tests
The patient underwent blood tests, including routine
blood, liver function, renal function and tumor marker tests. Among
them, alanine aminotransferase (ALT), aspartate aminotransferase
(AST), alkaline phosphatase, lactate dehydrogenase (LDH),
γ-glutamyl transferase (GGT) and neuron-specific enolase (NSE) were
all elevated compared with the normal levels, the Risk of Ovarian
Malignancy Algorithm (4) was
slightly decreased compared with the normal levels, and the levels
of tumor markers, such as carcinoembryonic antigen, α-fetoprotein,
cancer antigen (CA)19-9, CA72-4, CA125, CA15-3, squamous cell
carcinoma-associated antigen and cytokeratin 19 fragment antigen
21-1, were within the normal range (Table I). Notably, all other indexes did
not suggest abnormalities.
 | Table I.Laboratory test results. |
Table I.
Laboratory test results.
| Characteristic | Result | Normal value |
|---|
| ALT, U/l | 230.9 | 7-40 |
| AST, U/l | 226.9 | 13-35 |
| ALP, U/l | 295 | 35-100 |
| LDH, U/l | 4,450 | 120-150 |
| GGT, U/l | 183 | 7-45 |
| NSE, ng/ml | >300 | <16.3 |
| ROMAI, % | 6.61 | <11.4 |
| CEA, ng/ml | 0.70 | <3.4 |
| AFP, IU/ml | 2.28 | ≤5.8 |
| CA19-9, U/ml | 3.38 | <27 |
| CA72-4, U/ml | 0.96 | ≤6.9 |
| CA12-5, U/ml | 26.30 | ≤35 |
| CA15-3, U/ml | 7.72 | 0-25 |
| SCC, ng/ml | 0.97 | 0-2.7 |
| CYFRA21-1,
ng/ml | 1.47 | <3.3 |
Imaging
In the course of diagnosis and treatment, the
patient underwent systematic CT examination of the important organs
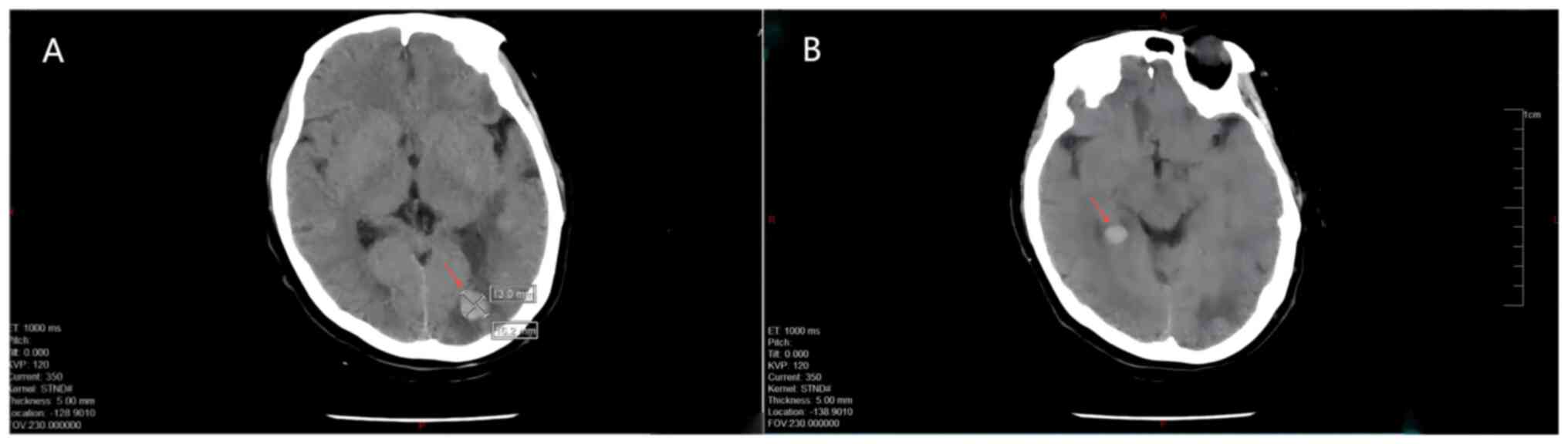
of the body, and the specific results were as follows: CT of the
skull and brain showed multiple metastatic tumors in the skull, and
multiple soft tissue nodular shadows in the soft tissues of the
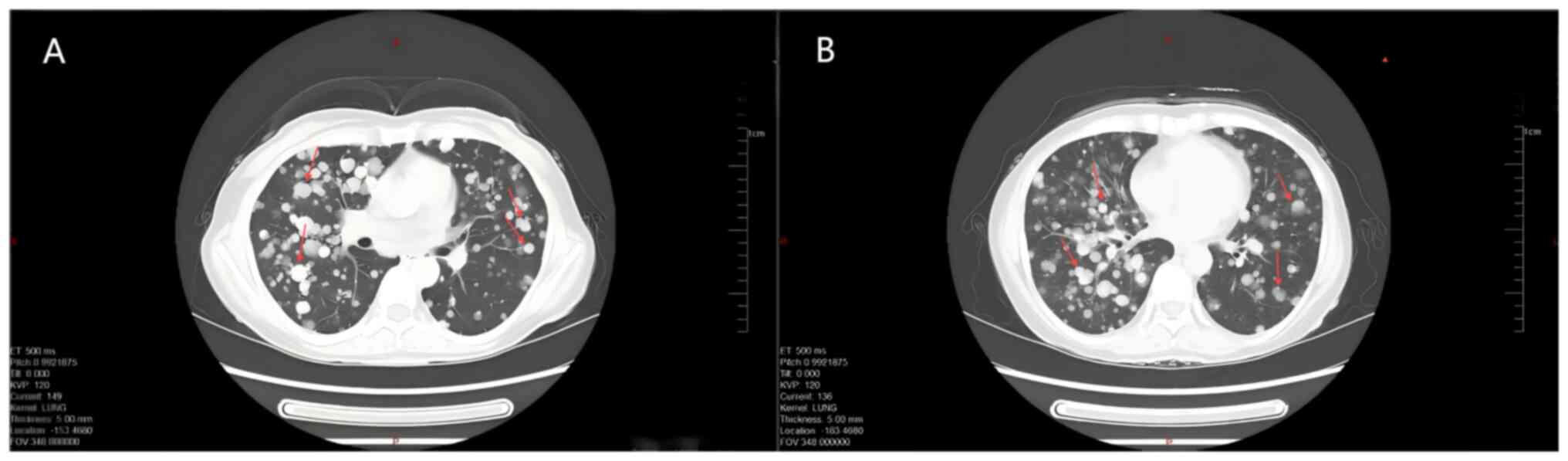
scalp (Fig. 3). Enhanced CT of the
chest and abdomen showed multiple solid nodular shadows in both
lungs (the larger one was located in the lower lobe of the left
lung, with a long diameter of ~3.6 cm), indicating a pulmonary
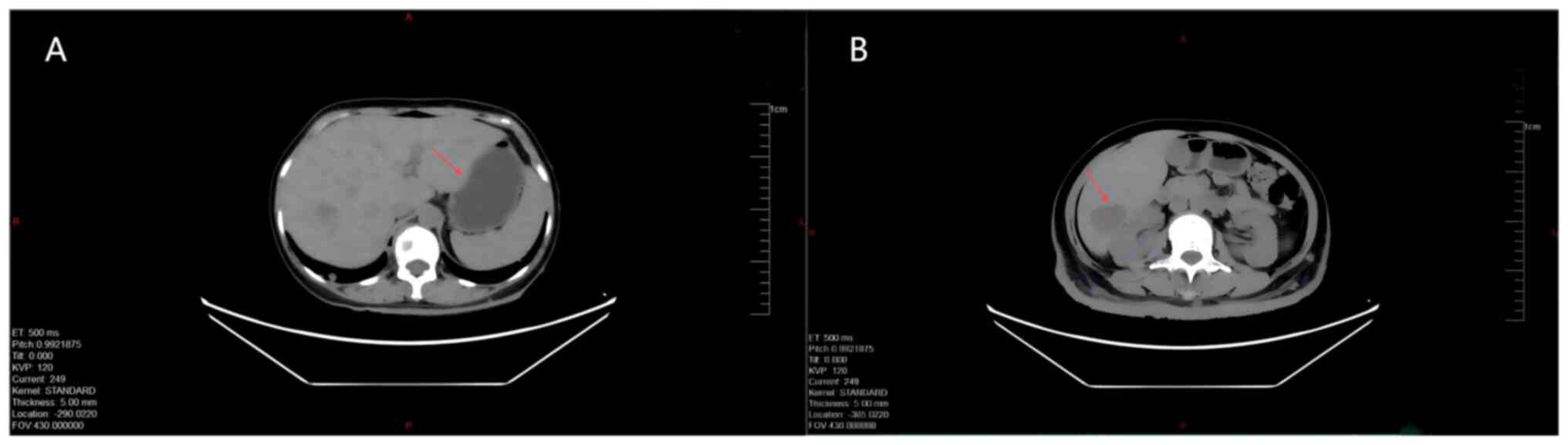
metastatic lesion (Fig. 4).
Multiple slightly low-density shadows were observed in the liver
(the larger one with a long diameter of ~3.5 cm), indicating a
hepatic metastatic lesion.; and increased soft-tissue shadows were
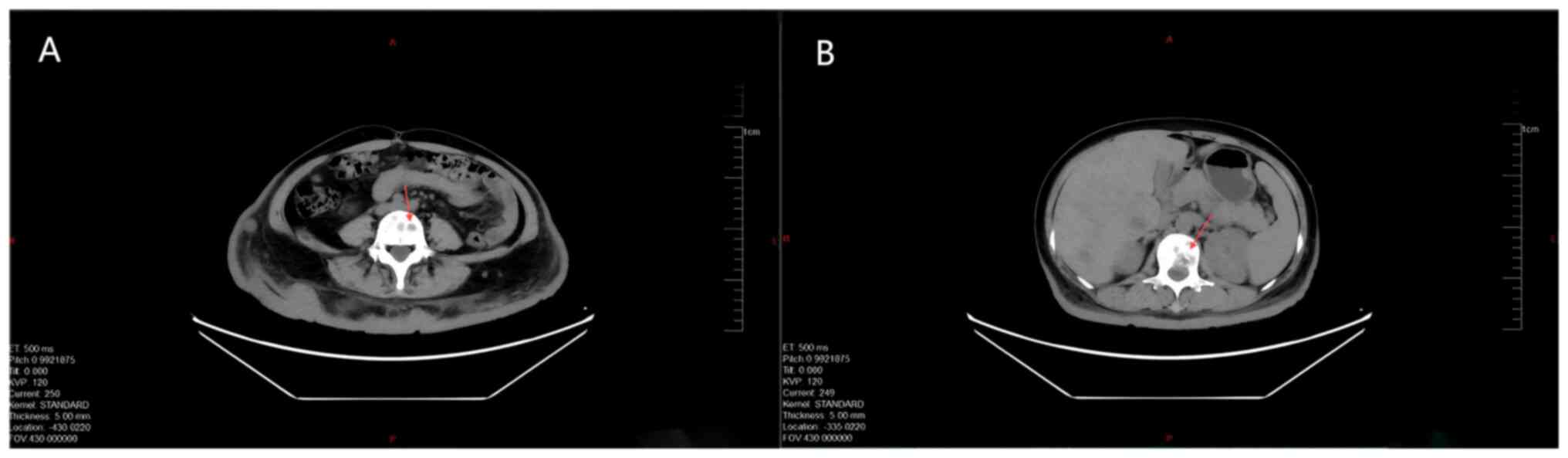
detected in the subcutaneous soft tissues of the back (Fig. 5). Chest CT and frontal and lateral
X-ray imaging of the spine revealed right-sided comminuted fracture
of thoracic 12 vertebra; formation of a large Schmorl's node in the
laryngeal region of lumbar 1 vertebra, accompanied by detachment
and displacement of bone fragments; and multiple hypodense foci in
the body of lumbar vertebrae (Fig.
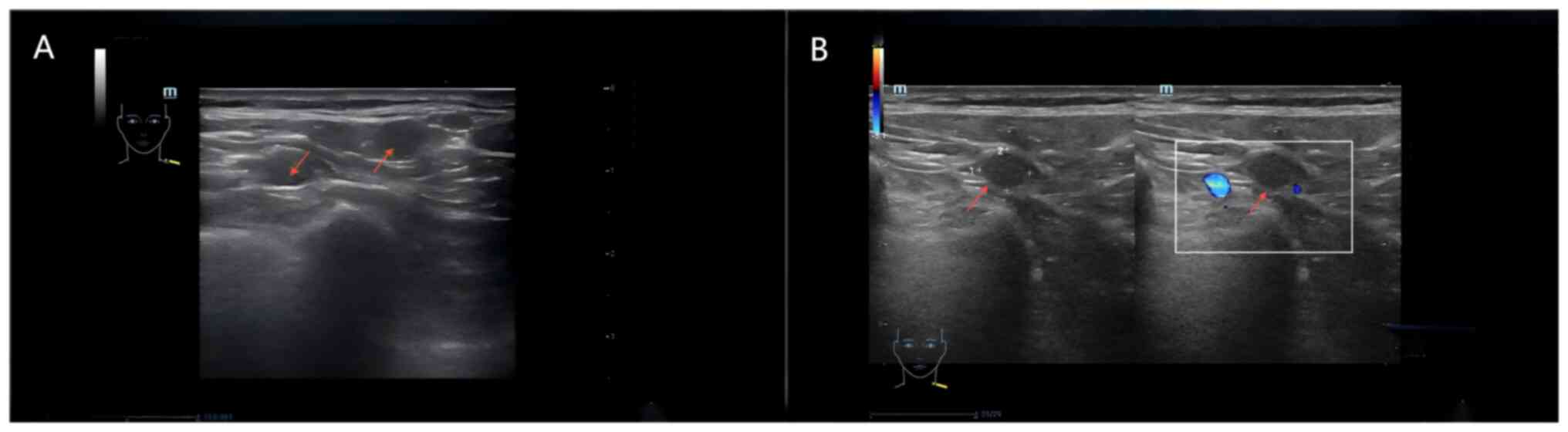
6). Lymph node ultrasound showed multiple abnormal lymph nodes
in both sides of the supraclavicular fossa, and in conjunction with
the medical history, lymph node metastasis was suspected (Fig. 7). Imaging suggested that the patient
may have multiple metastases to the cranium, lungs, liver, bone and
lymph nodes, but the source was not yet clear. Notably, the patient
refused to undergo dermoscopy.
Histopathological examination
The patient underwent a biopsy of an abdominal skin
mass at The Fourth Hospital of Hebei Medical University in July
2024, and the pathology showed a mixed nevus with cut margins (no
tumor cells were found around the surgical incision). In August
2024, a subcutaneous soft-tissue nodule was taken from the left hip
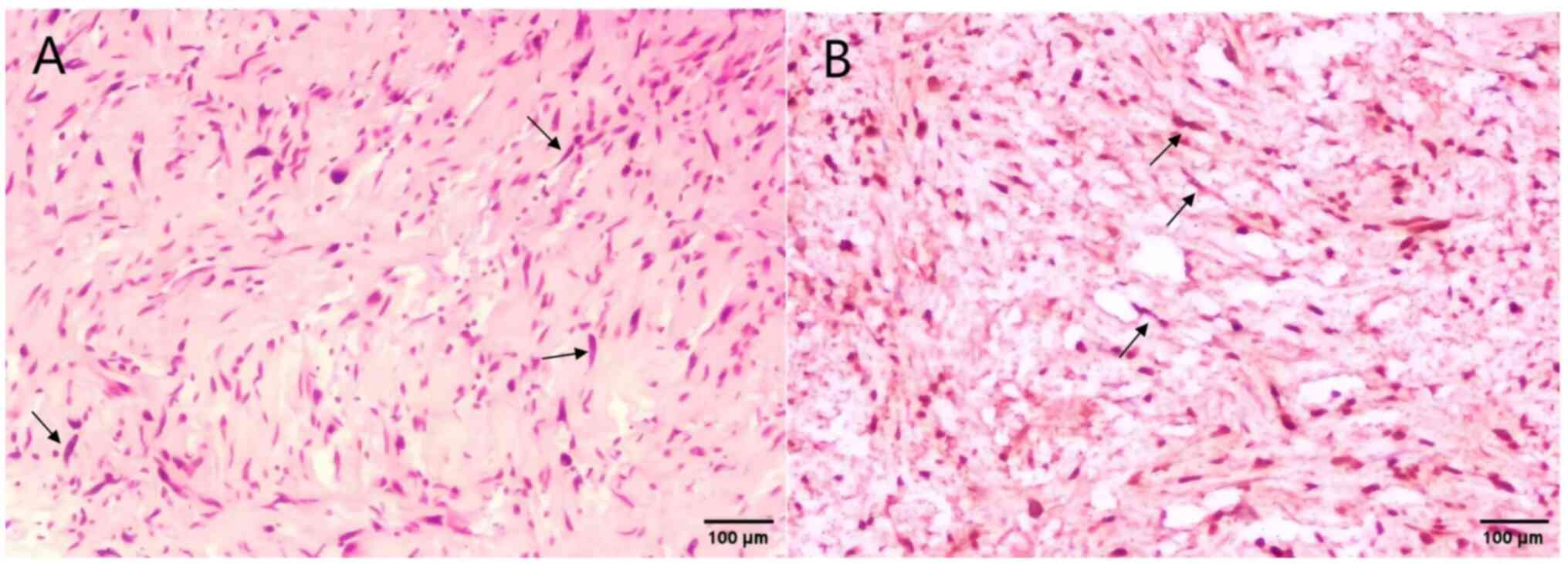
and sent to the hospital for another pathological examination, and
the results showed that spindle-shaped tumor cells resembling
fibroblasts could be seen within the collagen bundles of the
tissue, and the spindle tumor cells were arranged in bundles, with
atypical cellular properties. The histological pattern and
immunohistochemical expression were not specific (Data S1). Combined with the clinical and
medical history, a differential diagnosis of neurofibroma and
fibroproliferative melanoma was suggested (Fig. 8). The immunohistochemical findings
were as follows: Pan-cytokeratin (−), Vimentin (+), Ki-67 (1%),
S-100 (+), Melan-A (−), HMB45 (−), P53 (no mutation suggested) and
SMA (−). The pathology results were inconclusive; the pathologist
considered the sample to be consistent with some features of
melanoma, but the evidence was insufficient. Multiple
out-of-hospital consultations or genetic testing were recommended
for further analysis.
Diagnostic considerations
The National Comprehensive Cancer Network (NCCN)
guidelines state that cutaneous melanomas tend to develop from
nevi, with melanomas originating from giant congenital nevi and
melanomas from neurocutaneous melanocytosis (NCM) being rare
histological types (3).
Giant congenital melanocytic nevus, a specific type
of giant congenital nevus, is closely related to genetic factors or
gene mutations (5,6). The size of giant congenital nevus is
usually >20 cm in diameter, the border is usually irregular, the
shape may be round, oval or irregular, the surface may be rough and
uneven with hair growth, the color may be black, brown or tan, and
the color is often uneven (2).
Giant congenital nevi may be distributed in any part of the body,
and are commonly found on the head and neck, trunk area and limbs
(1). Dermoscopy and pathological
examination are important auxiliary examinations, of which
pathological examination is the gold standard for the diagnosis of
giant congenital melanocytic nevus, which can accurately determine
the nature of the lesion and whether there is a tendency toward
malignant changes (7). Giant
congenital melanocytic nevus is large in size and the nevus cells
are often distributed in deeper tissues, sometimes even extending
to the borders of muscles or other internal organs (5). Due to the wide distribution and deep
location of the nevus cells, it is difficult to detect signs of
malignant changes in the early stages through conventional
examinations, thus making early diagnosis difficult (5). In the present case, the patient
complained of multiple giant nevus lesions on the skin at birth,
which did not receive sufficient attention and diagnosis at the
early stage. Furthermore, the area of the skin lesions was
extensive, and upon examination, the pathological features were
atypical, which complicated the condition and indicated a
potentially severe prognosis.
NCM is a relatively rare congenital disorder. Some
cases of NCM are associated with genetic factors, and there may be
mutations in genes such as GNAQ, BRAF and NRAS or chromosomal
abnormalities, which lead to abnormal distribution of melanocytes
in the nerves and skin (8–10). In addition, abnormal embryonic
development is a common cause, with abnormal differentiation and
migration of neural crest cells being the main cause of NCM
(11). Neural crest cells are
pluripotent stem cells that can differentiate into various cell
types, such as melanocytes and neural cells (11). NCM can be triggered by errors in the
migration and differentiation of neural crest cells into the skin
and the nervous system, resulting in the accumulation of excessive
melanocytes in the skin and the central nervous system; this is
similar to the patient described in the present case, who had
multiple large nevi of different sizes and shapes that appeared at
birth or shortly after birth, often on the head, neck and trunk
(9,10). The size and shape of the nevi vary,
the border is irregular, and the color can be black, brown or tan
(11). Neurological symptoms may
appear gradually in childhood or adolescence, and the severity
varies with individual differences, including headache, vomiting,
seizures, movement disorders and intellectual disabilities
(12). Although the patient did not
exhibit typical symptoms of neurological involvement, the NSE level
of the patient was >300 ng/ml and cranial CT imaging showed
multiple metastatic intracranial tumors, suggesting the existence
of neurological lesions. Dermatopathological examination is an
important basis for the diagnosis of NCM skin lesions. Pathological
biopsy reveals increased melanocytes in the epidermis, with a
nested or diffuse distribution, and the cell morphology may show
some degree of heterogeneity (10).
Neuroimaging may show melanin deposits or space-occupying lesions
in the brain parenchyma, commonly in the cerebral hemispheres,
cerebellum and brainstem (11). In
addition, some patients may have abnormalities in the cerebrospinal
fluid, such as elevated protein levels and increased cell counts,
with melanoma cells sometimes detected (9,10).
Patients are often not diagnosed until they develop significant
neurological symptoms or tumor metastasis, and early diagnosis is
challenging (9).
The NCCN guidelines summarize the symptoms of early
nevus malignancy as the ‘ABCDE’ rule: Asymmetry, border
irregularity, color variation, diameter and elevation (7). The clinical symptoms of the patient in
the present study fully conformed to the ‘ABCDE’ rule. The
guidelines state that a shortcoming of this rule is that it does
not take into account the speed of development of melanoma, such as
the tendency of notable changes in weeks or months. In the present
case report, the skin lesions on the back and both hip areas
exhibited an obvious trend of enlargement and thickening over a few
days, which had a certain malignant tendency.
The current gold standard for diagnosing melanoma is
histopathology combined with immunohistochemistry, with adjunctive
diagnostic modalities including visualization, dermoscopy, skin
confocal technology, skin CT and artificial intelligence-assisted
diagnosis. In the present case, the histopathological pattern and
immunohistochemical expression of the patient were not specific,
and the patient and their family refused further genetic testing
and consultation with outside hospitals, which made it difficult to
make a definitive diagnosis. The pathological report of the present
patient suggested that the diagnosis needed to distinguish between
neurofibroma and pro-fibroproliferative melanoma. The spindle cells
in melanoma may be indistinguishable from those of neurofibroma,
but features such as marked fibroproliferative growth, poor lateral
borders, and diffuse infiltration of subcutaneous tissues and
lymphoid aggregates may be useful information for the diagnosis
(13). Specific immunohistochemical
markers include S-100, SOX-10, HMB45, Melan-A, PNL2, tyrosinase,
MITF and Vimentin; however, there is a lack of objective, highly
reproducible immunohistochemical markers for all melanoma (14,15).
The NCCN guidelines state that S-100 is the most
sensitive marker and is a screening indicator for melanoma. In
addition, it is recommended that two to three of the aforementioned
markers be used in conjunction with S-100 when differential
diagnosis is needed to improve the detection rate of melanoma.
Although LDH is not a sensitive indicator for detecting metastasis
of melanoma, it is an effective guide to prognosis of melanoma
(7). The patient had positive
expression of the immunohistochemical markers S-100 and Vimentin;
of which, positive expression of S-100 suggested the possible
presence of a neurogenic or melanocytic origin. However, Melan-A
and HMB45 negativity did not support the typical melanoma
manifestations, which made differential diagnosis difficult, and
the markers S-100 and Vimentin are not specific in distinguishing
between neurofibroma and pro-fibroproliferative melanoma. However,
it should be noted that the S-100 protein is usually positive in
melanocytes and their tumors, and it is also a marker for neural
tissues and tumors of neural origin, and can thus be expressed in
tumors with neural differentiation; therefore, S-100 may be
positive when NCM develops into melanoma. Vimentin (waveform
protein) is a marker for tumors of mesenchymal origin, and melanoma
originates from melanocytes of neural crest origin, which are of
mesenchymal origin, and thus Vimentin is usually positive in
melanoma.
Notably, the LDH levels of the patient in the
present case were >10 times higher than the normal range, and
LDH has a role in distinguishing neurofibromas from melanomas
(7,16). Studies have shown that serum LDH
levels are often associated with disease progression in patients
with melanoma, and the serum markers LDH and S-100B independently
predict recurrent metastasis and disease prognosis in these
patients (16,17). When melanoma metastasizes or is in
advanced stages, tumor cells proliferate rapidly and are
metabolically active, leading to increased LDH release (17). High levels of LDH are usually
indicative of a poor prognosis, shorter survival and faster disease
progression in patients with melanoma (7). By contrast, neurofibroma is a benign
tumor with slow growth and relatively inactive metabolism, and thus
serum LDH levels are generally within the normal range in cases of
neurofibroma (16,17). Considering that the present patient
was suspected to have melanoma and had a history of congenital
giant nevus, the RAS gene of the patient should be tested for
mutations. Studies have shown that RAS gene mutations serve an
important role in the development of congenital nevi into melanoma
(1,7,15).
However, the patient and their family refused further pathological
consultations and genetic testing, which made it difficult to
clearly diagnose the disease, and to accurately determine the
molecular typing and genetic status of the disease. In addition, to
a certain extent, this affected the accuracy of the diagnosis and
optimization of the treatment plan. Subsequently, the skin lesions
rapidly expanded and thickened during the hospitalization period.
Taking into account the symptoms, signs, and test and examination
results of the patient, a preliminary diagnosis of melanoma with
multiple metastatic foci that developed from a giant congenital
melanocytic nevus or NCM was finally made.
Treatment
The lack of further genetic testing to clearly
diagnose and identify the molecular typing of the disease and
potential therapeutic targets, as well as the preference of the
patient and their family for conservative treatment, limited the
optimization of the treatment plan to a certain extent. The
treatment strategy was actively adjusted and the clinical team
communicated with the patient and their family, explaining in
detail the positives and negatives of conservative treatment, and
the possible risks of disease progression. According to the
symptoms of the patient, a personalized symptomatic treatment plan
was formulated. Due to obvious lung infection, wheezing and
shortness of breath, the patient was given 4.5 g piperacillin
sodium and sulbactam sodium by intravenous drip and 30 mg
methylprednisolone sodium succinate by intravenous injection for 8
days, and received ceftazidime (2 g) by intravenous drip for 11
days. Due to multiple metastatic tumors in the liver combined with
hepatic insufficiency, 20 ml magnesium isoglycyrrhizinate injection
and an intravenous drip of 1.2 g glutathione were administered to
protect the liver and lower the enzyme levels, such as ALT, AST and
GGT, for 13 days. Due to the multiple bone metastases and
neuropathy, the patient was administered one tablet of the oral
analgesic paracetamol (500 mg) and dihydrocodeine tartrate (10 mg)
tablets every 6 h along with other symptomatic treatments, such as
oxygen inhalation, cough suppression, laxative administration and
nutritional supplementation. After 2 weeks of conservative
treatment, the condition of the patient progressed rapidly, and
they developed respiratory failure, which could not be improved by
mask oxygenation. Approximately 1 week later, the patient entered a
shallow comatose state and the family requested an automatic
discharge. Through subsequent telephone follow-up, it was confirmed
that the patient had succumbed in September 2024.
Discussion
The histopathological recommendation for the
diagnosis of cutaneous melanoma is excisional biopsy, although
partial biopsies (scraping and puncture) are also frequently used
(18). In the present patient,
excisional biopsy of the abdominal and hip skin was selected
separately because the lesions were very extensive in terms of body
surface area, with near circumferential involvement, which created
some uncertainty in the accuracy of the diagnosis. In the present
case, the patient was suspected to have developed melanoma from a
congenital nevus, in which RAS mutation was a potential factor. The
RAS gene family includes NRAS, HRAS and KRAS, of which the NRAS
mutation is the most common, which leads to continuous activation
of the RAS protein and activation of downstream signaling pathways,
such as MAPK and PI3K/AKT. This activation promotes cell
proliferation, survival, migration and invasion, ultimately
promoting melanoma development (1,19). If
the RAS gene status can be clarified, it is considered to be of
value for the diagnosis and treatment of the case (7,15,19).
In diagnosis, it can be used as additional evidence for a melanoma
diagnosis, particularly in cases with atypical immunohistochemistry
and pathology results; in treatment, targeted therapeutic drugs or
combination therapies targeting RAS mutations may provide more
effective therapeutic choices for patients (7,15). In
addition, RAS mutation status is important in assessing the
prognosis of patients, as patients with these mutations may have a
poorer prognosis and a higher risk of disease progression (19). Since the present patient and their
family refused further genetic testing and opted for conservative
treatment, this affected the accuracy of the diagnosis and
optimization of the treatment plan to a certain extent, and posed a
number of challenges for the medical team regarding diagnosis and
treatment.
Surgical resection is the first choice of treatment
for early-stage melanoma. Since the present patient had a large
peripheral area of involved skin and multiple organ metastases, it
was recommended that systemic treatment options, such as
immunotherapy and targeted therapy, be considered. Immunotherapy
mainly includes immunosuppressants, cellular immunotherapy and
tumor vaccines (7,15,20).
The more widely used immunosuppressants include PD-1 inhibitors,
such as pembrolizumab and nivolumab, PD-L1 inhibitors, such as
atezolizumab, and CTLA-4 inhibitors, such as ipilimumab (7,15).
Cellular immunotherapy involves extracting immune cells from the
body of the patient, culturing, transforming or modifying them
in vitro to make them more capable of recognizing and
attacking tumor cells, and then infusing them back into the body to
exert antitumor effects (20). For
example, tumor-infiltrating lymphocyte (TIL) therapy has been shown
to achieve good results in some patients with advanced melanoma
(21). Furthermore, tumor vaccines,
such as the MAGE-A3 vaccine, although still in the research and
development stage, provide novel potential options for the
treatment of melanoma (22).
Commonly used drugs for targeted therapy include
BRAF inhibitors and MEK inhibitors (7,15). For
patients with distant metastases or localized metastases that
cannot be radically resected, BRAF V600 gene testing is important
for guiding subsequent treatment (1,7,15). For
patients with wild-type BRAF, immunotherapy with PD-1 inhibitors
alone or in combination with a CTLA-4 antibody should be considered
(7,15). For patients with concomitant BRAF
V600 mutations, BRAF inhibitors such as vemurafenib and dabrafenib
may be selected as first-line treatment options for patients with
melanoma (7,15). MEK inhibitors, such as trametinib,
are often used in combination with BRAF inhibitors to improve
efficacy and delay resistance (7,15).
Notably, the lack of genetic test results in the
present case report made it difficult to identify potential
therapeutic targets and optimize the treatment plan. The only
option was for a multidisciplinary team to develop a personalized
symptomatic treatment plan, and to closely monitor the condition of
the patient during the course of conservative treatment. However,
conservative treatment could not stop the progression of the
disease, and the patient eventually developed severe respiratory
failure and entered a shallow coma.
Notably, progress is being made in clinical trials
for advanced melanoma. In terms of immunotherapy, new immune
checkpoint targets are being explored, such as TIM-3 and LAG-3
inhibitors, for which clinical trials are ongoing (23). Several studies have shown that
inhibitors of these new targets may further improve therapeutic
efficacy when used in combination with established
immunotherapeutic agents (24,25).
In the field of targeted therapies, the development of targeted
drugs against mutations other than BRAF is also advancing. For
example, clinical trials of drugs targeting NRAS mutations have
made some breakthroughs (19). As
for cell therapy, in addition to TIL therapy, chimeric antigen
receptor (CAR)-T cell therapy and CAR-natural killer therapy also
have some potential in advanced melanoma (26,27).
In addition, clinical trials of combination therapies are
increasing; for example, immunotherapy and radiotherapy, targeted
therapy and chemotherapy, and the combination of anti-angiogenic
drugs and immunotherapy, in order to identify more optimal
therapeutic combinations and regimens (7,15,24).
There are some new research advances worth noting:
Cui et al (28) conducted a
trial evaluating the safety and efficacy of OrienX010, a modified
herpes simplex virus 1 oncolytic virus, for the treatment of
patients with unresectable stage IIIC-IV melanoma in China. The
results showed an objective remission rate of 19.2%, a disease
control rate of 53.8%, a median duration of remission of 6.0
months, a median progression-free survival time of 2.9 months and
an overall survival time of 19.2 months. Preliminary evidence
indicates that OrienX010 oncolytic viral therapy has a tolerable
safety profile and antitumor effects in both the injected
metastases and other non-injected metastatic sites in patients.
Overall, clinical trials in advanced melanoma continue to bring new
promise and breakthroughs, offering more treatment options and
better prognosis for such patients.
In conclusion, the present case provides valuable
experience and considerations. Firstly, patients with congenital
skin lesions, particularly when the lesions are extensive and
rapidly developing, should be alerted to the possibility of
malignant transformation to melanoma. These patients should be
examined comprehensively as early as possible to achieve a timely
diagnosis and treatment, and to improve their prognosis. Secondly,
accurate pathological and genetic test results are crucial to the
diagnosis and treatment of the disease. In the future, the
communication with patients and their families should be
strengthened, and they should be fully informed of the importance
of genetic testing, so as to obtain more comprehensive information
about the disease, and to ensure accurate diagnosis and treatment.
In addition, the active search for immunohistochemical markers of
melanoma with high specificity is an urgent clinical issue. The
choices of patients and their families are often influenced by a
variety of factors, such as cultural background, economic status
and psychological factors; therefore, healthcare professionals
should provide comprehensive information and support, so that even
in cases where patients refuse certain key tests and therapeutic
treatments, the professionals can still work closely with
multidisciplinary diagnostic and therapeutic teams to optimize
treatment strategies. The aim of this case report is to encourage
more medical personnel and patients to pay attention to early and
accurate diagnoses, so as to avoid any delay, and to improve the
prevention and treatment of such complex diseases.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YH, HF and ZD designed the study. HF and DL advised
on patient treatment. YH, ZD, YG and ZL acquired the data. HF, DL,
YH and ZD analyzed and interpreted data for the work. YH, HF, ZD,
DL, YG and ZL confirm the authenticity of all the raw data. All
authors agree to be accountable for all aspects of the work. All
authors read and approved the final version of the manuscript, and
agreed on the journal to which the article has been submitted.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
The First Affiliated Hospital of Hebei University of Chinese
Medicine and was conducted in accordance with the 1964 Helsinki
Declaration and its subsequent amendments or comparable ethical
standards.
Patient consent for publication
The patient provided written informed consent for
the publication of their data before they succumbed to the
disease.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
WU Na-Ming, LI Jun and TAO Juan:
Diagnostic hotspots of malignant melanoma. Diagn Theory Pract
(Chinese). 2:215–220. 2023.
|
|
2
|
Wang JY, Wang EB and Swetter SM: What is
melanoma? JAMA. 329:9482023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Haydu LE, Lo SN, McQuade JL, Amaria RN,
Wargo J, Ross MI, Cormier JN, Lucci A, Lee JE, Ferguson SD, et al:
Cumulative incidence and predictors of CNS metastasis for patients
with American joint committee on cancer 8th edition stage iii
melanoma. J Clin Oncol. 38:1429–1441. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
National Comprehensive Cancer Network, .
NCCN Guidelines for Ovarian Cancer. NCCN; Philadelphia: 2025
|
|
5
|
Etchevers HC: Hiding in plain sight:
Molecular genetics applied to giant congenital melanocytic nevi. J
Invest Dermatol. 134:879–882. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gerami P and Paller AS: Making a mountain
out of a molehill: NRAS, mosaicism, and large congenital nevi. J
Invest Dermatol. 133:2127–2130. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
National Comprehensive Cancer Network, .
NCCN Guidelines for Melanoma. NCCN; Philadelphia: 2025
|
|
8
|
Gessi M, Hammes J, Lauriola L, Dörner E,
Kirfel J, Kristiansen G, Zur Muehlen A, Denkhaus D, Waha A and
Pietsch T: GNA11 and N-RAS mutations: Alternatives for MAPK pathway
activating GNAQ mutations in primary melanocytic tumours of the
central nervous system. Neuropathol Appl Neurobiol. 4:417–425.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen HC, Hsu TI, Chao TY and Yang ST:
Neurocutaneous melanosis with meningeal melanocytosis: A rare case
of intracranial hypertension and cutaneous manifestations. Life
(Basel). 14:1392024.PubMed/NCBI
|
|
10
|
Kinsler VA, Thomas AC, Ishida M, Bulstrode
NW, Loughlin S, Hing S, Chalker J, McKenzie K, Abu-Amero S, Sater
O, et al: Multiple congenital melanocytic nevi and neurocutaneous
melanosis are caused by postzygotic mutations in codon 61 of NRAS.
J Invest Dermatol. 9:2229–2236. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jakchairoongruang K, Khakoo Y, Beckwith M
and Barkovich AJ: New insights into neurocutaneous melanosis.
Pediatr Radiol. 48:1786–1796. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vanood A, Lee YA, Leleszi E and Krishnan
A: Symptomatic neurocutaneous melanosis: Mild clinical onset in a
teenager. BMJ Case Rep. 13:e2357722020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gerami P, Kim D, Zhang B, Compres EV, Khan
AU, Yazdan P, Guitart J and Busam K: Desmoplastic melanomas
mimicking neurofibromas. Am J Dermatopathol. 42:916–922. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kashani-Sabet M: Molecular markers in
melanoma. Br J Dermatol. 170:31–35. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lee BM and Yang YG: Interpretation of the
European expert consensus-based multidisciplinary melanoma
diagnosis and treatment guideline 2022 edition. J Prac Dermatol
(Chinese). 16:153–155. 2023.
|
|
16
|
Várvölgyi T, Janka EA, Szász I, Koroknai
V, Toka-Farkas T, Szabó IL, Ványai B, Szegedi A, Emri G and Balázs
M: Combining biomarkers for the diagnosis of metastatic melanoma. J
Clin Med. 13:1742023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Janka EA, Várvölgyi T, Sipos Z, Soós A,
Hegyi P, Kiss S, Dembrovszky F, Csupor D, Kéringer P, Pécsi D, et
al: Predictive performance of serum S100B versus LDH in melanoma
patients: A systematic review and meta-analysis. Front Oncol.
11:7721652021. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Doolan BJ, Robinson AJ, Wolfe R, Kelly JW,
McLean C, McCormack C, Henderson MA and Pan Y: Accuracy of partial
biopsies in the management of cutaneous melanoma. Australas J
Dermatol. 60:209–213. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Randic T, Kozar I, Margue C, Utikal J and
Kreis S: NRAS mutant melanoma: Towards better therapies. Cancer
Treat Rev. 99:1022382021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valpione S and Campana LG: Immunotherapy
for advanced melanoma: Future directions. Immunotherapy. 8:199–209.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klobuch S, Seijkens TTP, Schumacher TN and
Haanen JBAG: Tumour-infiltrating lymphocyte therapy for patients
with advanced-stage melanoma. Nat Rev Clin Oncol. 21:173–184. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Russo V, Lunghi F, Fontana R and Bregni M:
A clinical study of a cell-based MAGE-A3 active immunotherapy in
advanced melanoma patients. J Cancer. 2:329–330. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lu C and Tan Y: Promising immunotherapy
targets: TIM3, LAG3, and TIGIT joined the party. Mol Ther Oncol.
32:2007732024. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ascierto PA, Agarwala SS, Blank C, Caracò
C, Carvajal RD, Ernstoff MS, Ferrone S, Fox BA, Gajewski TF, Garbe
C, et al: Perspectives in melanoma: Meeting report from the
melanoma bridge (December 2nd-4th, 2021, Italy). J Transl Med.
20:3912022. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ma B, Akosman B, Kamle S, Lee CM, He CH,
Koo JS, Lee CG and Elias JA: CHI3L1 regulates PD-L1 and
anti-CHI3L1-PD-1 antibody elicits synergistic antitumor responses.
J Clin Invest. 131:e1377502021. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shah PD, Huang AC, Xu X, Orlowski R,
Amaravadi RK, Schuchter LM, Zhang P, Tchou J, Matlawski T, Cervini
A, et al: Phase I trial of autologous RNA-electroporated
cMET-directed CAR T cells administered intravenously in patients
with melanoma and breast carcinoma. Cancer Res Commun. 3:821–829.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hibler W, Merlino G and Yu Y: CAR NK cell
therapy for the treatment of metastatic melanoma: Potential &
prospects. Cells. 12:27502023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cui C, Wang X, Lian B, Ji Q, Zhou L, Chi
Z, Si L, Sheng X, Kong Y, Yu J, et al: OrienX010, an oncolytic
virus, in patients with unresectable stage IIIC-IV melanoma: A
phase Ib study. J Immunother Cancer. 10:e0043072022. View Article : Google Scholar : PubMed/NCBI
|