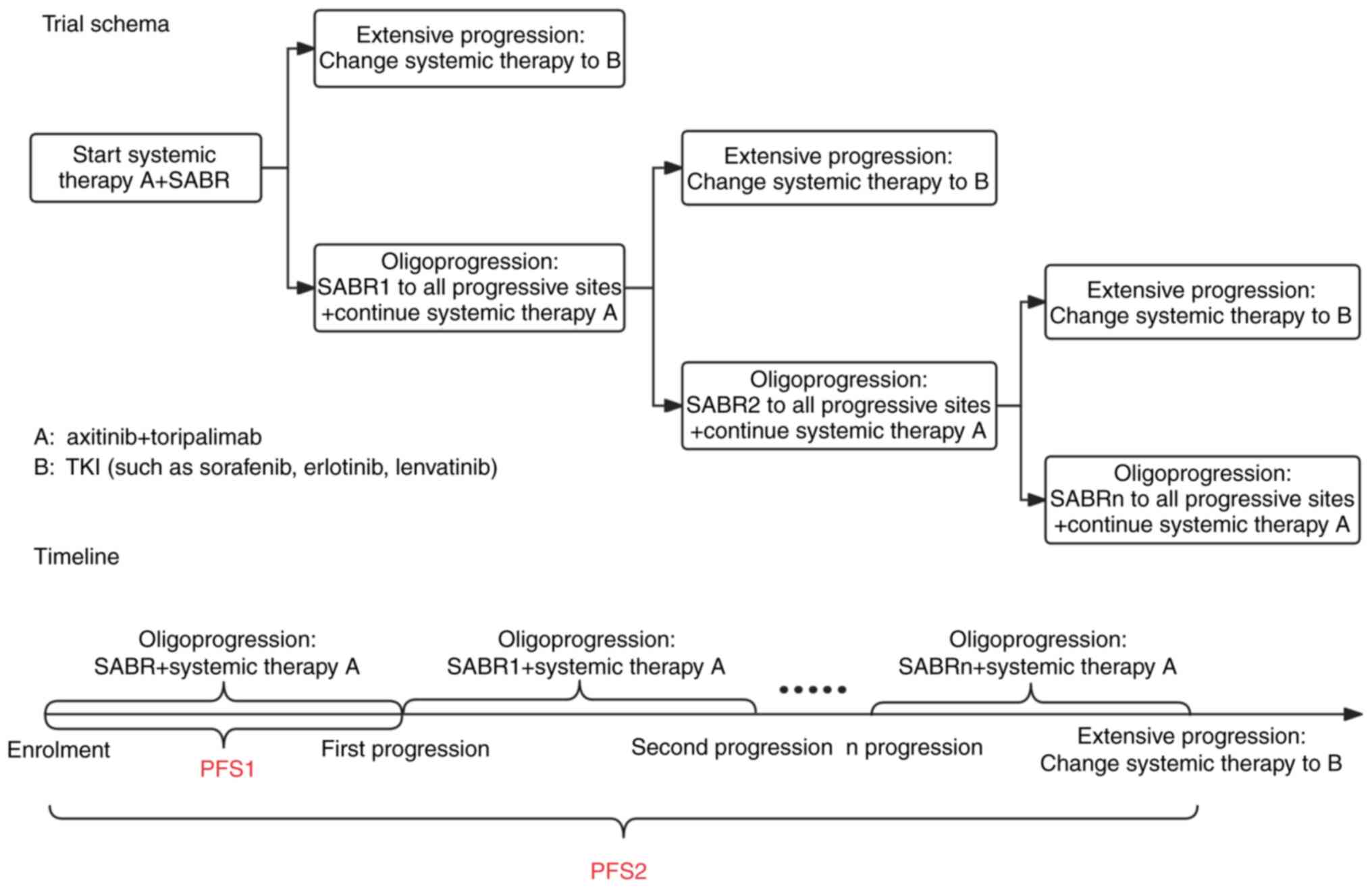
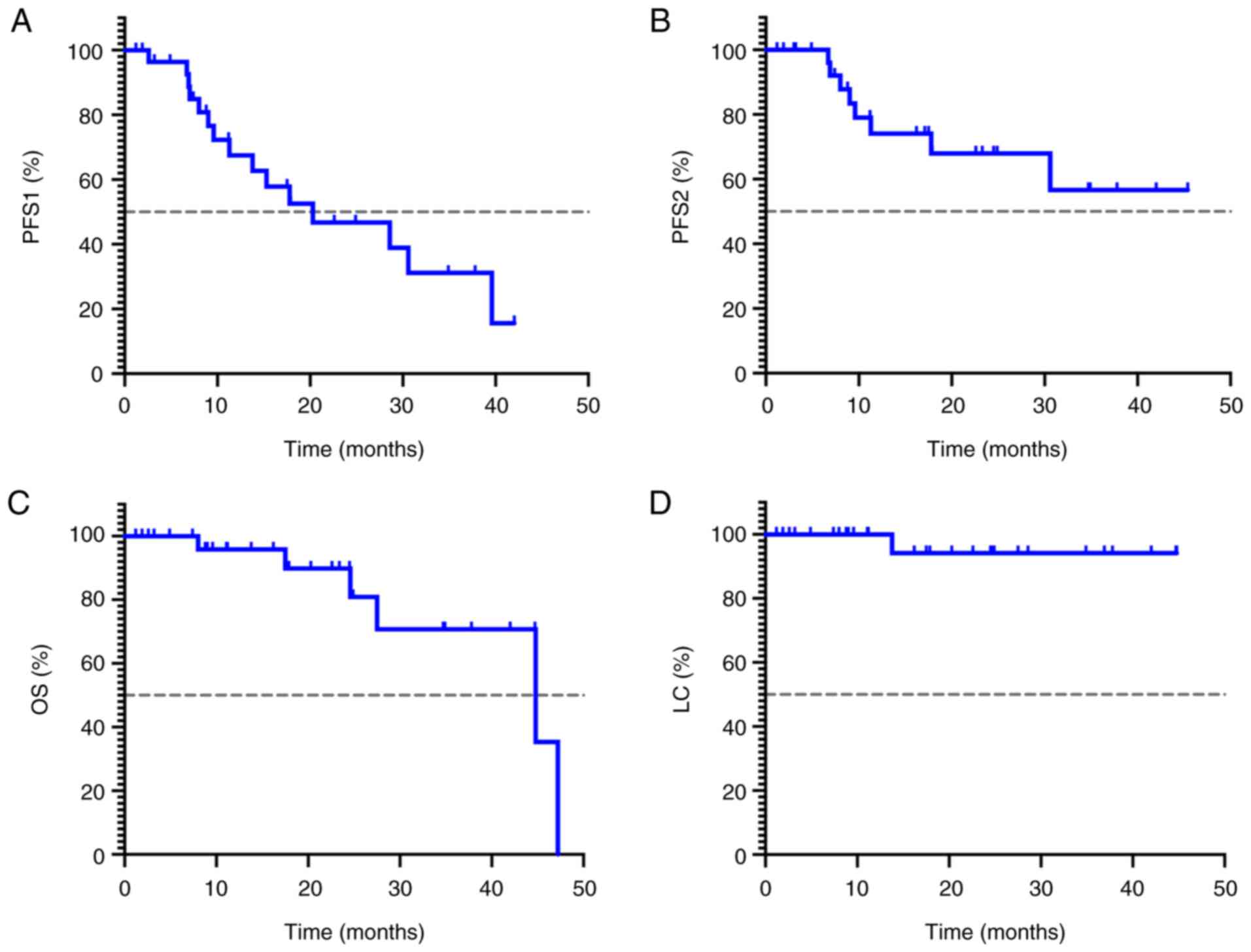
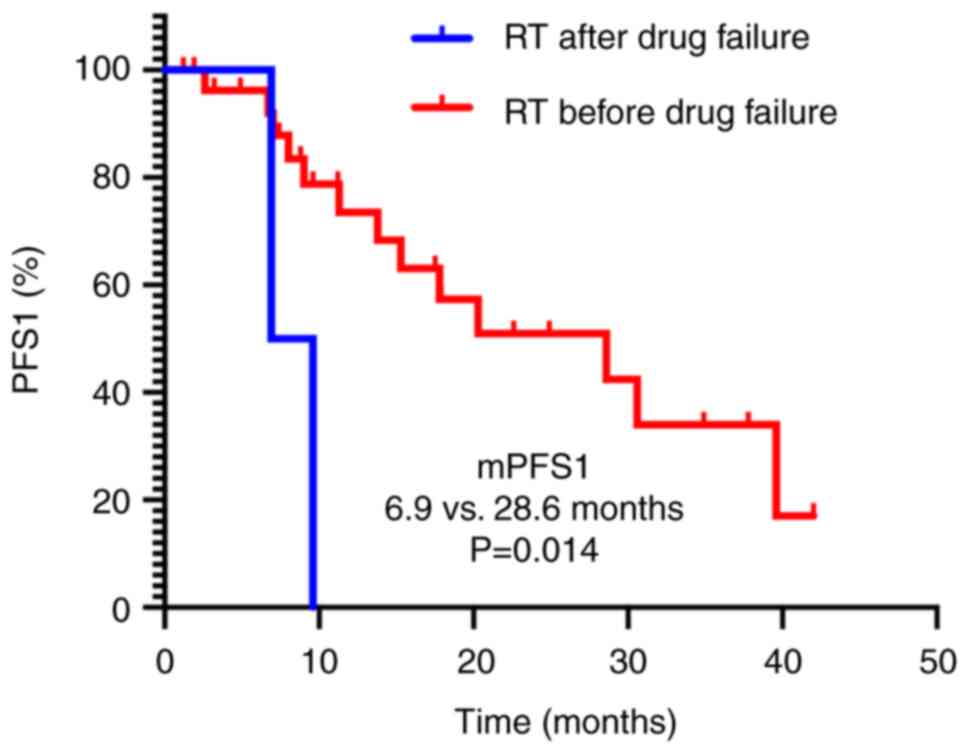
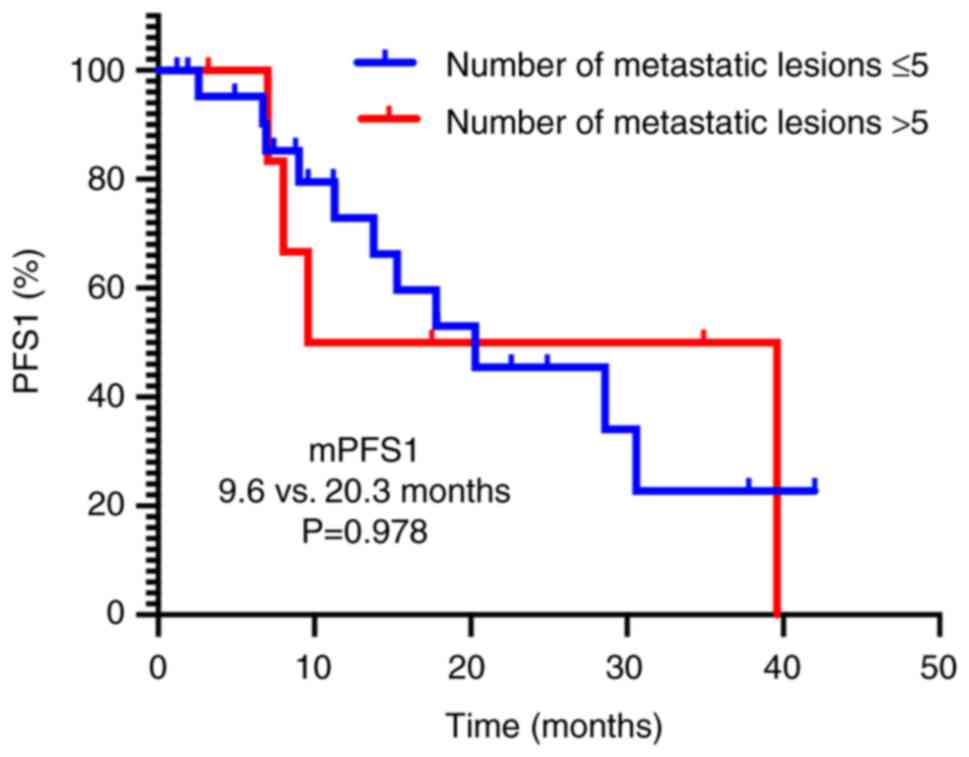
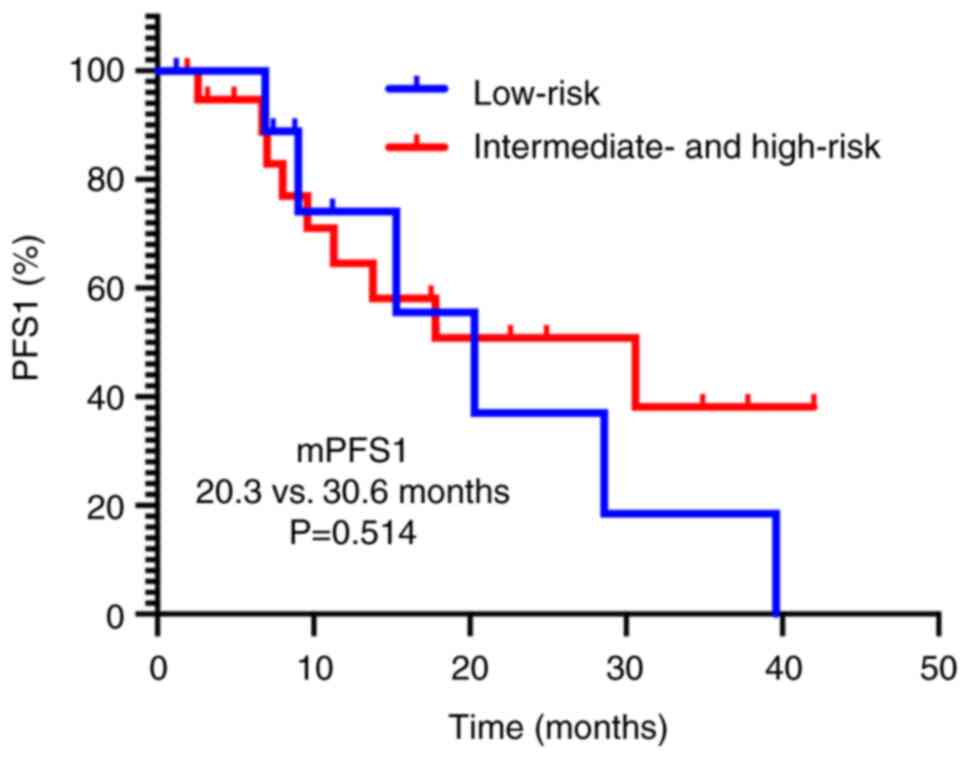
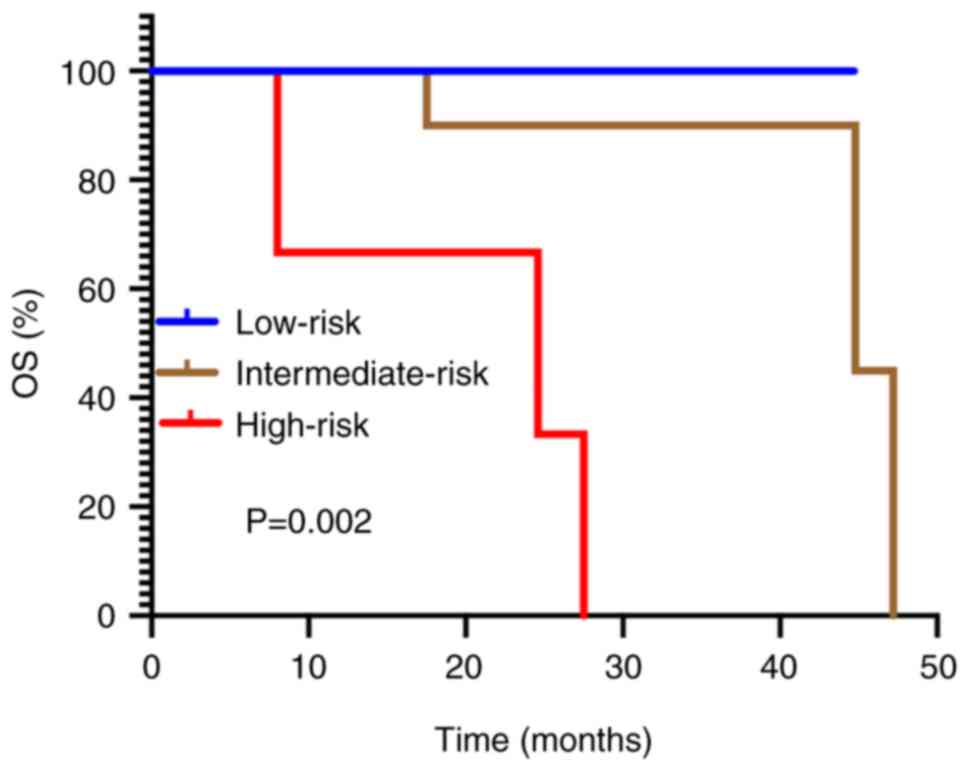
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Centers for Disease Control and
Prevention, . Male urologic cancers. USCS Data Brief, no 21.
Centers for Disease Control and Prevention. US Department of Health
and Human Services; Atlanta GA: 2020, Available from. https://dx.doi.org/10.15620/cdc:105242
|
|
3
|
Zheng R, Zhang S, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2016. J Natl Cancer Cent. 2:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lam JS, Shvarts O, Leppert JT, Figlin RA
and Belldegrun AS: Renal cell carcinoma 2005: New frontiers in
staging, prognostication and targeted molecular therapy. J Urol.
173:1853–1862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Jonasch E, Agarwal N, Alva A,
Bagshaw H, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello
BA, et al: NCCN guidelines® insights: Kidney cancer,
version 2.2024. J Natl Compr Canc Netw. 22:4–16. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bex A, Ghanem YA, Albiges L, Bonn S, Campi
R, Capitanio U, Dabestani S, Hora M, Klatte T, Kuusk T, et al:
European association of urology guidelines on renal cell carcinoma:
The 2025 update. Eur Urol. Mar 10–2025.(Epub ahead of print).
View Article : Google Scholar
|
|
7
|
Atlas of Genetics and Cytogenetics in
Oncology and Haematology, . 2024.Available from. http://atlasgeneticsoncology.org/teaching/209251/csco
|
|
8
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan XQ, Ye MJ, Zou Q, Chen P, He ZS, Wu B,
He DL, He CH, Xue XY, Ji ZG, et al: Toripalimab plus axitinib
versus sunitinib as first-line treatment for advanced renal cell
carcinoma: RENOTORCH, a randomized, open-label, phase III study.
Ann Oncol. 35:190–199. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motzer RJ, Powles T, Burotto M, Escudier
B, Bourlon MT, Shah AY, Suárez C, Hamzaj A, Porta C, Hocking CM, et
al: Nivolumab plus cabozantinib versus sunitinib in first-line
treatment for advanced renal cell carcinoma (CheckMate 9ER):
Long-term follow-up results from an open-label, randomised, phase 3
trial. Lancet Oncol. 23:888–898. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choueiri TK, Motzer RJ, Rini BI, Haanen J,
Campbell MT, Venugopal B, Kollmannsberger C, Gravis-Mescam G,
Uemura M, Lee JL, et al: Updated efficacy results from the JAVELIN
Renal 101 trial: First-line avelumab plus axitinib versus sunitinib
in patients with advanced renal cell carcinoma. Ann Oncol.
31:1030–1039. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choueiri TK, Eto M, Motzer R, De Giorgi U,
Buchler T, Basappa NS, Méndez-Vidal MJ, Tjulandin S, Hoon Park S,
Melichar B, et al: Lenvatinib plus pembrolizumab versus sunitinib
as first-line treatment of patients with advanced renal cell
carcinoma (CLEAR): Extended follow-up from the phase 3, randomised,
open-label study. Lancet Oncol. 24:228–238. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rini BI, Plimack ER, Stus V, Waddell T,
Gafanov R, Pouliot F, Nosov D, Melichar B, Soulieres D,
Borchiellini D, et al: Pembrolizumab (pembro) plus axitinib (axi)
versus sunitinib as first-line therapy for advanced clear cell
renal cell carcinoma (ccRCC): Results from 42-month follow-up of
KEYNOTE-426. J Clin Oncol. 39 (Suppl 15):S45002021. View Article : Google Scholar
|
|
14
|
Tolia BM and Whitmore WF Jr: Solitary
metastasis from renal cell carcinoma. J Urol. 114:836–838. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaeth JM: Proceedings: Cancer of the
kidney-radiation therapy and its indications in non-Wilms' tumors.
Cancer. 32:1053–1055. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Francolini G, Detti B, Ingrosso G,
Desideri I, Becherini C, Carta G, Pezzulla D, Caramia G, Dominici
L, Maragna V, et al: Stereotactic body radiation therapy (SBRT) on
renal cell carcinoma, an overview of technical aspects, biological
rationale and current literature. Crit Rev Oncol Hematol.
131:24–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wersäll PJ, Blomgren H, Lax I, Kälkner KM,
Linder C, Lundell G, Nilsson B, Nilsson S, Näslund I, Pisa P and
Svedman C: Extracranial stereotactic radiotherapy for primary and
metastatic renal cell carcinoma. Radiother Oncol. 77:88–95. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Svedman C, Sandström P, Pisa P, Blomgren
H, Lax I, Kälkner KM, Nilsson S and Wersäll P: A prospective phase
II trial of using extracranial stereotactic radiotherapy in primary
and metastatic renal cell carcinoma. Acta Oncol. 45:870–875. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaorsky NG, Lehrer EJ, Kothari G, Louie AV
and Siva S: Stereotactic ablative radiation therapy for
oligometastatic renal cell carcinoma (SABR ORCA): A meta-analysis
of 28 studies. Eur Urol Oncol. 2:515–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ali M, Mooi J, Lawrentschuk N, McKay RR,
Hannan R, Lo SS, Hall WA and Siva S: The role of stereotactic
ablative body radiotherapy in renal cell carcinoma. Eur Urol.
82:613–622. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyer E, Pasquier D, Bernadou G, Calais G,
Maroun P, Bossi A, Theodore C, Albiges L, Stefan D, de Crevoisier
R, et al: Stereotactic radiation therapy in the strategy of
treatment of metastatic renal cell carcinoma: A study of the Getug
group. Eur J Cancer. 98:38–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katayama H, Kurokawa Y, Nakamura K, Ito H,
Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H and
Sasako M: Extended Clavien-Dindo classification of surgical
complications: Japan clinical oncology group postoperative
complications criteria. Surg Today. 46:668–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heng DYC, Xie W, Regan MM, Harshman LC,
Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan
MH, et al: External validation and comparison with other models of
the international metastatic renal-cell carcinoma database
consortium prognostic model: A population-based study. Lancet
Oncol. 14:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Guo L, Zhang J, Zhou Y, Zhou J, Yao
J, Wu H, Yao S, Chen B, Chai Y, et al: Glycosylation-independent
binding of monoclonal antibody toripalimab to FG loop of PD-1 for
tumor immune checkpoint therapy. MAbs. 11:681–690. 2019.PubMed/NCBI
|
|
25
|
Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen
J, Song X, Wang W, Dong L, Song H, et al: Safety, efficacy, and
biomarker analysis of toripalimab in previously treated advanced
melanoma: Results of the POLARIS-01 multicenter phase II trial.
Clin Cancer Res. 26:4250–4259. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu
XC, Liao W, Jiang Y, Lin XY, Zhang QY, et al: Efficacy, safety, and
correlative biomarkers of toripalimab in previously treated
recurrent or metastatic nasopharyngeal carcinoma: A phase II
clinical trial (POLARIS-02). J Clin Oncol. 39:704–712. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li LC, Chen XW, Fang L, Jian CL, Yu YX,
Liao XY and Sun JG: YAP1 as a novel negative biomarker of immune
checkpoint inhibitors for EGFR-mutant non-small-cell lung cancer.
Can Respir J. 2023:46890042023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang B, Yan X, Sheng X, Si L, Cui C, Kong
Y, Mao L, Lian B, Bai X, Wang X, et al: Safety and clinical
activity with an anti-PD-1 antibody JS001 in advanced melanoma or
urologic cancer patients. J Hematol Oncol. 12:72019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Wei W, Zhang Z, Liu R, Gao J, Guo
S, Han H, Dong P, He L and Zhou H: Preliminary results from a phase
II study comparing sunitinib alone or with stereotactic body
radiotherapy (SBRT) for newly diagnosed oligometastatic renal cell
carcinoma. J Clin Oncol. 41 (Suppl 16):S45332023. View Article : Google Scholar
|
|
30
|
Ma MW, Li HZ, Gao XS, Liu MZ, Yin H, Yang
KW, Chen JY, Ren XY and Wang D: Outcomes of high-dose stereotactic
ablative radiotherapy to all/multiple sites for oligometastatic
renal cell cancer patients. Curr Oncol. 29:7832–7841. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura JL, Karlsson A, Arvold ND,
Gottschalk AR, Pieper RO, Stokoe D and Haas-Kogan DA: PKB/Akt
mediates radiosensitization by the signaling inhibitor LY294002 in
human malignant gliomas. J Neurooncol. 71:215–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frémin C and Meloche S: From basic
research to clinical development of MEK1/2 inhibitors for cancer
therapy. J Hematol Oncol. 3:82010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shinohara ET, Cao C, Niermann K, Mu Y,
Zeng F, Hallahan DE and Lu B: Enhanced radiation damage of tumor
vasculature by mTOR inhibitors. Oncogene. 24:5414–5422. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wersäll PJ, Blomgren H, Pisa P, Lax I,
Kälkner KM and Svedman C: Regression of non-irradiated metastases
after extracranial stereotactic radiotherapy in metastatic renal
cell carcinoma. Acta Oncol. 45:493–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dybal EJ, Haas GP, Maughan RL, Sud S,
Pontes JE and Hillman GG: Synergy of radiation therapy and
immunotherapy in murine renal cell carcinoma. J Urol.
148:1331–1337. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hammers HJ, Vonmerveldt D, Ahn C, Nadal
RM, Drake CG, Folkert MR, Laine AM, Courtney KD, Brugarolas J, Song
DY, et al: Combination of dual immune checkpoint inhibition (ICI)
with stereotactic radiation (SBRT) in metastatic renal cell
carcinoma (mRCC) (RADVAX RCC). J Clin Oncol. 38 (Suppl 6):S6142020.
View Article : Google Scholar
|
|
37
|
Masini C, Iotti C, De Giorgi U, Bellia RS,
Buti S, Salaroli F, Zampiva I, Mazzarotto R, Mucciarini C, Vitale
MG, et al: Nivolumab in combination with stereotactic body
radiotherapy in pretreated patients with metastatic renal cell
carcinoma. Results of the phase II NIVES study. Eur Urol.
81:274–282. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siva S, Bressel M, Wood ST, Shaw MG, Loi
S, Sandhu SK, Tran B, A Azad A, Lewin JH, Cuff KE, et al:
Stereotactic radiotherapy and short-course pembrolizumab for
oligometastatic renal cell carcinoma-The RAPPORT trial. Eur Urol.
81:364–372. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zafra J, Onieva JL, Oliver J,
Garrido-Barros M, González-Hernández A, Martínez-Gálvez B, Román A,
Ordóñez-Marmolejo R, Pérez-Ruiz E, Benítez JC, et al: Novel blood
biomarkers for response prediction and monitoring of stereotactic
ablative radiotherapy and immunotherapy in metastatic
oligoprogressive lung cancer. Int J Mol Sci. 25:45332024.
View Article : Google Scholar : PubMed/NCBI
|