Introduction
Renal cell carcinoma (RCC) is a significant
malignancy that arises from the epithelial lining of the renal
tubules. Within the spectrum of urinary tract malignancies
encountered in clinical practice, RCC accounts for 3–5% of all
adult cancer diagnoses. Major risk factors include smoking, obesity
and hypertension (1). According to
data from the Centers for Disease in the United States, patients
with localized RCC have a 80–90% 5-year survival rate, contrasting
sharply with the rates for recurrent or metastatic disease (10–40%)
(2). In China, the incidence of
kidney cancer ranks third among urological tumors, following
bladder cancer and prostate cancer (3). In patients with kidney cancer, ~30%
present with distant metastases at the time of initial diagnosis
(4). Therefore, the implementation
of effective treatment strategies is critical for improving patient
outcomes.
With the success of the CLEAR, KEYNOTE and CheckMate
series of studies, the management of recurrent or metastatic RCC
has entered an era of combined targeted and immune therapy
(target-immunotherapy). In the treatment guidelines for kidney
cancer from the National Comprehensive Cancer Network (5), the European Association of Urology
(6) and the Chinese Society of
Clinical Oncology (7), the
combination of axitinib and programmed cell death protein 1 (PD-1)
inhibitors is recommended as one of the first-line therapeutic
options for recurrent or metastatic RCC. This combination
significantly prolongs the median progression-free survival (PFS)
time compared with targeted therapy alone (15.4 vs. 11.1 months;
P<0.0001) (8). Toripalimab, a
PD-1 monoclonal antibody developed in China, has demonstrated
promising results in the RENOTORCH study (axitinib combined with
toripalimab), achieving a median PFS time of 18 months in patients
with advanced RCC (9). In April
2024, the combination of axitinib and toripalimab was officially
approved by the National Medical Products Administration of China
as a first-line treatment for patients with recurrent or metastatic
RCC.
Despite these advances, most patients experience
disease progression within 1–2 years of initiating combined
targeted and immunotherapy (9–13).
Extending overall survival (OS) time for patients with advanced RCC
remains a notable challenge. The potential of incorporating lesion
radiotherapy to improve PFS warrants further exploration.
Previous studies have demonstrated that RCC is not
particularly sensitive to conventional fractionated radiotherapy
(14,15). However, previous evidence suggests
that stereotactic ablative body radiotherapy (SABR), characterized
by high single-dose fractions, high biological efficacy and fewer
treatment sessions, can markedly enhance the radiosensitivity of
RCC (16). SABR has demonstrated
notable efficacy and safety in treating recurrent and metastatic
RCC lesions (17–19), with reported 1-year local control
(LC) rates >90% (20). Higher
biological effective dose (BED) values are associated with improved
LC rates, with 1-year LC rates reaching 100% when the BED is 117 Gy
(21).
Based on these findings, a single-center,
prospective study was initiated to evaluate the combination of
axitinib and toripalimab with SABR for the treatment of recurrent
or metastatic RCC.
Materials and methods
Patient selection
The present prospective, single center, single-arm,
phase II trial was reviewed and approved by the Ethics Committee of
Peking University First Hospital (Beijing, China; approval no.
2019-345). The patient recruitment commenced on 01-04-2020 and
concluded on 31-07-2024. The patients provided written informed
consent before treatment. Eligible patients possessed
histopathologically confirmed recurrent or metastatic RCC with
radiologically evaluable lesions demonstrating either ≤5 completely
irradiable metastases or >5 lesions with ≥3 amenable to
radiotherapy planning, while excluding those with prior
anti-PD-1/programmed death-ligand 1 therapy or radiotherapy
history. The detailed enrollment protocols are shown in Data S1. The present trial was
retrospectively registered at clinicaltrials.gov (registration no.
NCT06889649; 03-03-2025).
Treatment protocol
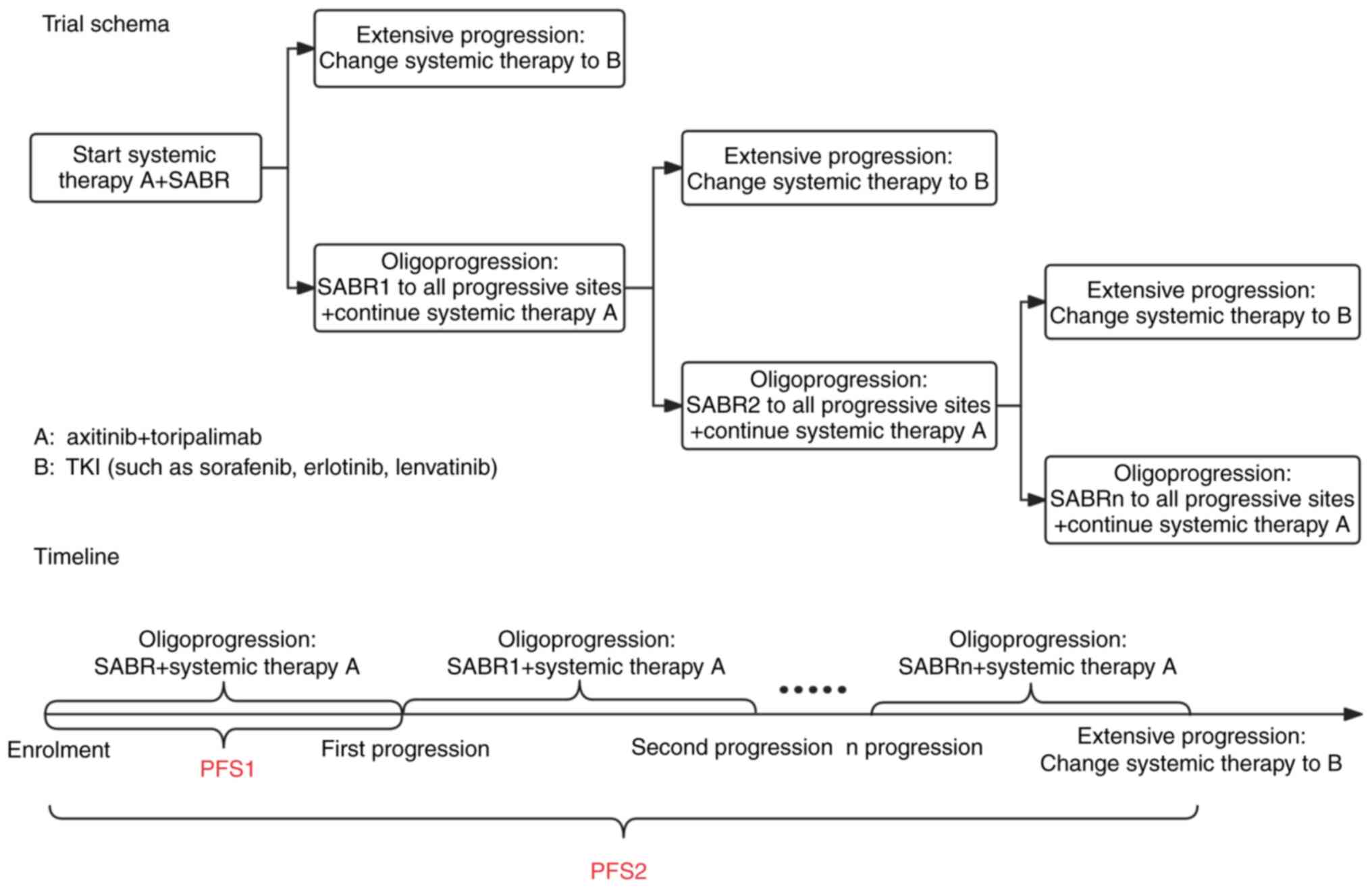
All patients received a combination therapy of
targeted therapy and immunotherapy plus radiotherapy. The trial
schema and timeline are shown in Fig.
1.
The treatment protocols were as follows: i) Systemic
therapy, where patients received 5 mg oral axitinib twice daily and
240 mg intravenous toripalimab every 3 weeks; maintenance therapy
continued post-oligoprogression (defined as progression at ≤5
sites) with additional local radiotherapy to oligoprogressing
lesions, until further treatment modification [inferior-line
tyrosine kinase inhibitor (TKI) such as sorafenib, erlotinib and
lenvatinib] due to extensive disease progression or intolerance to
adverse reactions; and ii) radiotherapy, for primary and metastatic
lesions distant from organs at risk, where SABR was administered
with peripheral doses of 6–8 Gy/fractions in 5 fractions and tumor
doses of 8–10 Gy/fraction in 5 fractions. For lesions adjacent to
organs at risk, partial-SABR (p-SABR) was used with edge doses
≥2-2.5 Gy/fraction in 20–25 fractions and central tumor doses of
8–12 Gy/fraction in 3 fractions. If neither SABR nor p-SABR were
feasible, moderate hypofractionated radiotherapy (MHFRT) with
curative doses was applied. Patients with oligoprogression
post-radiotherapy could receive additional radiotherapy to
progressing lesions. For patients demonstrating extensive
progression who had switched systemic therapy (inferior-line TKI
such as sorafenib, erlotinib and lenvatinib), while all-sites
radiotherapy was attempted, complete irradiation of all progressing
sites could not be ensured due to the high tumor burden
characterized by numerous metastatic foci, their widespread
anatomical distribution and compromised patient tolerance
thresholds. The frequency of radiotherapy (1–3 courses) was
determined by the time to disease progression and the tolerance of
the patient to treatment.
Endpoints
The primary endpoint was PFS1, defined as the time
from the start of radiotherapy to the first disease progression.
Secondary endpoints included PFS2 (defined as the time from the
start of radiotherapy to the need for a second-line systemic
treatment due to disease progression), OS (defined as the time from
the start of radiotherapy to death from any cause), LC rate of
irradiated lesions, objective response rate (ORR) [complete
response (CR) + partial response (PR)] and disease control rate
(DCR) [CR + PR + stable disease (SD)]. Adverse events were assessed
using the Common Terminology Criteria for Adverse Events version
5.0 (22). Toxicities associated
with radiotherapy, targeted therapy and immunotherapy were
attributed based on temporal relationships and known toxicity
profiles. Serological tests and imaging examinations were also
utilized to differentiate the attribution of adverse reactions. The
specific standards are shown in Data
S1. The evaluation of efficacy and adverse events continued
until disease progression, initiation of subsequent antitumor
treatments, study completion or patient mortality.
Follow-up
Patients were followed up via outpatient visits and
telephone calls every 3 months for 2 years, and then every 6 months
beyond 2 years or until tumor progression or mortality. Follow-up
evaluations included hematological tests, enhanced chest CT,
enhanced abdominal and pelvic CT or MRI and bone scans. Additional
brain MRI or positron emission tomography/CT exams were performed
as necessary. The follow-up examinations included both the primary
tumor and oligometastatic sites.
Statistical analysis
Statistical analyses were performed using SPSS 26.0
software (IBM Corp.). Continuous data are expressed as median
(range), and categorical data as frequency and percentage. The
Kaplan-Meier method was used to calculate PFS and OS, with group
differences assessed by the log-rank test. P<0.05 was considered
to indicate a statistically significant difference.
Results
General characteristics
The present study included 30 patients with
recurrent or metastatic RCC who received combination therapy with
axitinib, toripalimab and SABR at Peking University First Hospital
between May 2020 and June 2024. The median age was 57 years, with
66.7% of patients classified as intermediate to high risk according
to the International Metastatic Renal Cell Carcinoma Database
Consortium (IMDC) criteria (23).
Clear cell carcinoma was the predominant histological type,
accounting for 70% of the cases. The baseline characteristics of
the patients are detailed in Table
I.
 | Table I.Baseline characteristics of patients
(n=30). |
Table I.
Baseline characteristics of patients
(n=30).
| Characteristic | Value |
|---|
| Median age (range),
years | 57 (36–81) |
| Sex, n (%) |
|
|
Male | 24 (80.0) |
|
Female | 6 (20.0) |
| IMDC risk
classification at enrolment, n (%) |
|
| Low
risk | 10 (33.3) |
|
Intermediate risk | 15 (50.0) |
| High
risk | 5 (16.7) |
| Surgical
intervention for primary tumor, n (%) |
|
| Radical
nephrectomy | 20 (66.7) |
| Partial
nephrectomy | 4 (13.3) |
| No
surgery | 6 (20.0) |
| Histology, n
(%) |
|
|
ccRCC | 21 (70.0) |
|
Papillary | 3 (10.0) |
| XP11.2
translocation/TFE3 fusion RCC | 2 (6.7) |
|
Chromophobe | 1 (3.3) |
|
Other | 3 (10.0) |
| Number of
metastatic lesions, n (%) |
|
|
1-5 | 23 (76.7) |
| ≥6 | 7 (23.3) |
| Sites of
metastasis, n (%) |
|
| Lymph
nodes | 13 (20.0) |
|
Bone | 13 (20.0) |
|
Lung | 12 (19.1) |
|
Liver | 5 (7.5) |
|
Muscle | 3 (4.5) |
|
Pancreas | 2 (3.0) |
| Seminal
vesicle | 1 (1.5) |
|
Vagina | 1 (1.5) |
|
Ovary | 1 (1.5) |
|
Breast | 1 (1.5) |
|
Peritoneum | 1 (1.5) |
| Renal
fossa/residual kidney recurrence | 6 (9.2) |
| Primary
tumor | 6 (9.2) |
| Prior systemic
therapy, n (%) |
|
|
None | 26 (86.7) |
|
Sorafenib | 2 (6.7) |
|
Pazopanib | 1 (3.3) |
|
Lenvatinib | 1 (3.3) |
Treatment details
All 30 patients received combination therapy with
axitinib, toripalimab and radiotherapy. Post-radiotherapy, 7
patients (16.7%) developed new oligoprogressive lesions, each of
whom completed two or three courses of radiotherapy. A total of 20
patients (66.7%) underwent all-sites radiotherapy (all metastatic
sites of the patient were treated with radiotherapy). Among the 30
patients, there were a total of 114 metastatic lesions, 88 of which
(77.2%) received radiotherapy. Of these patients, 85% underwent
SABR or p-SABR treatment.
As of the follow-up in September 2024, 16 out of 30
patients were still undergoing treatment. The cycles of toripalimab
received by these patients are detailed in Table II.
 | Table II.Treatment details (n=30). |
Table II.
Treatment details (n=30).
| Treatment | n (%) |
|---|
| Number of
radiotherapy courses |
|
| 1 | 25 (83.3) |
| 2 | 3 (10.0) |
| 3 | 2 (6.7) |
| All sites
radiotherapy |
|
|
Yes | 20 (66.7) |
| No | 10 (33.3) |
| Radiotherapy
technique for metastases |
|
|
SABR | 54 (61.4) |
|
p-SABR | 19 (21.6) |
|
MHFRT | 15 (17.0) |
| Cycles of
toripalimab |
|
|
1-8 | 14 (46.7) |
|
9-17 | 9 (30.0) |
|
18-34 | 6 (20.0) |
|
≥34 | 1 (3.3) |
Efficacy
As of September 2024, the clinical efficacy of the
treatment was evaluated in 30 patients. The outcomes were as
follows: CR in 3 patients, PR in 15 patients, SD in 6 patients and
progressive disease (PD) in 6 patients. The ORR rate was 60.0%
(18/30) and the disease control rate DCR was 80.0% (24/30). The DCR
for irradiated lesions was 96.7% (29/30).
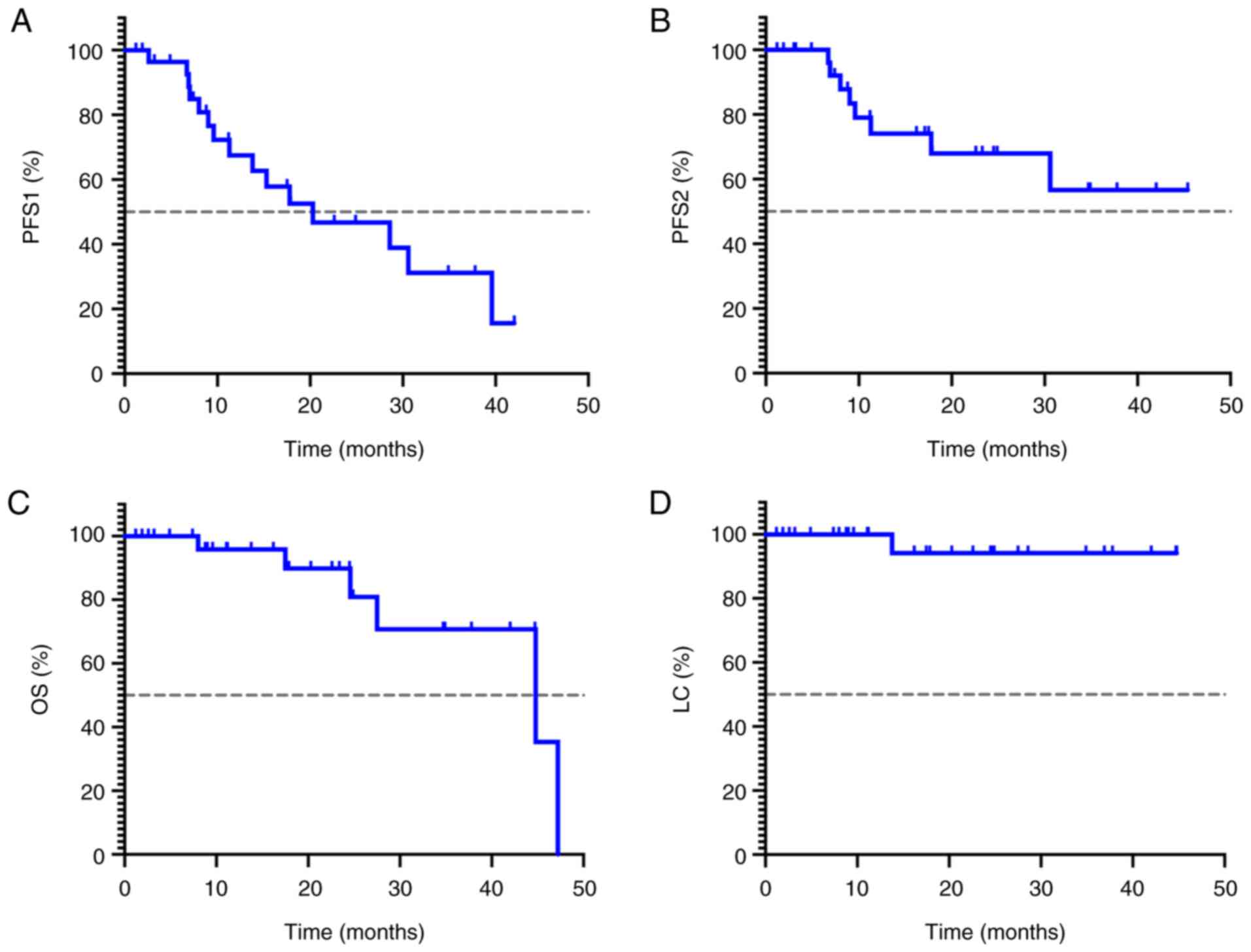
The median follow-up duration for the 30 patients
was 17.8 months (range, 1.2–47.7 months). The median PFS1 was 20.3
months (95% CI, 5.2–35.4 months), with 1- and 2-year PFS1 rates of
67.3 and 46.7%, respectively. The median PFS2 was not reached; the
1 and 2-year PFS2 rates were 74.1 and 56.6%, respectively. The
median OS time was 44.8 months (95% CI, 20.0–69.6 months), with 1-
and 2-year OS rates of 95.3 and 89.6%, respectively. The 1- and
2-year LC rates were 100.0 and 96.7%, respectively. The survival
curves are presented in Fig. 2. By
the end of the follow-up period, the LC rate of all irradiated
lesions was 96.7%. Only 1 patient experienced local recurrence at
the edge of the radiotherapy field 13.8 months after radiotherapy
due to the inability to deliver sufficient SABR dose to lesions
adjacent to the bowel.
As of September 2024, 24 patients were still alive,
and 6 patients had died. Of these, 5 mortalities were due to tumor
progression and 1 mortality was due to an unknown infection leading
to systemic inflammatory response syndrome. A total of 15 patients
experienced disease progression, with 7 patients exhibiting
oligoprogression and 8 patients showing wide progression. Among the
7 patients with oligoprogression, 6 underwent radiotherapy for the
progressive lesions without altering the systemic therapy regimen.
However, 1 patient did not receive a second course of radiotherapy
due to local recurrence at the edge of the radiotherapy field.
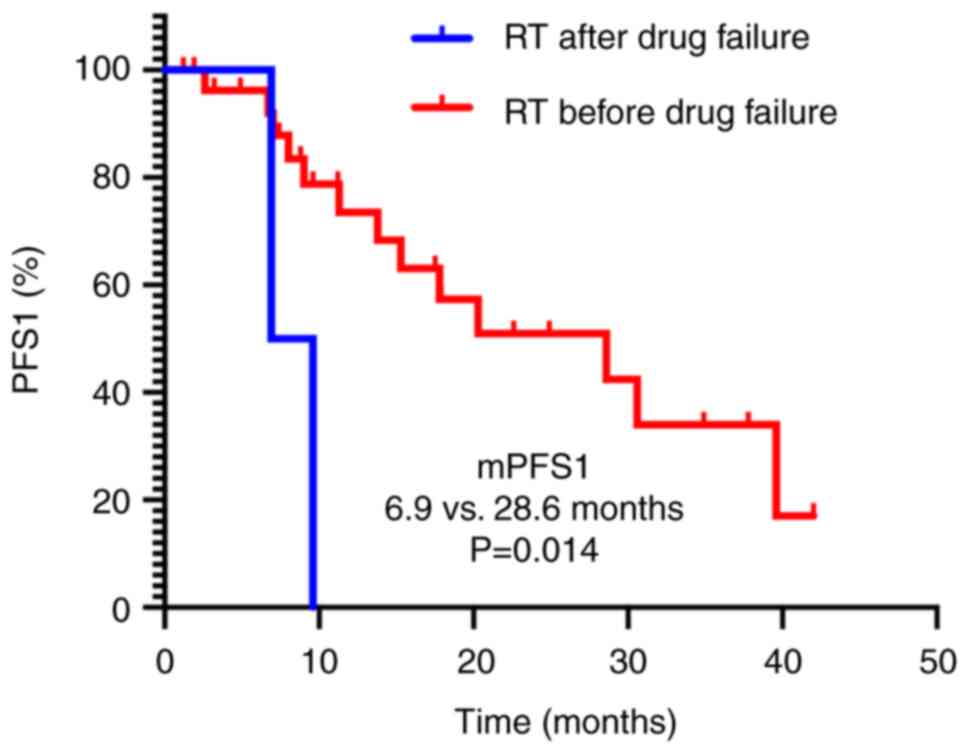
Subgroup analysis revealed that patients who
received radiotherapy before systemic treatment failure had a
significantly longer median PFS1 time compared with those who
received radiotherapy after treatment failure (28.6 vs. 6.9 months;
P=0.014; Fig. 3). However, no
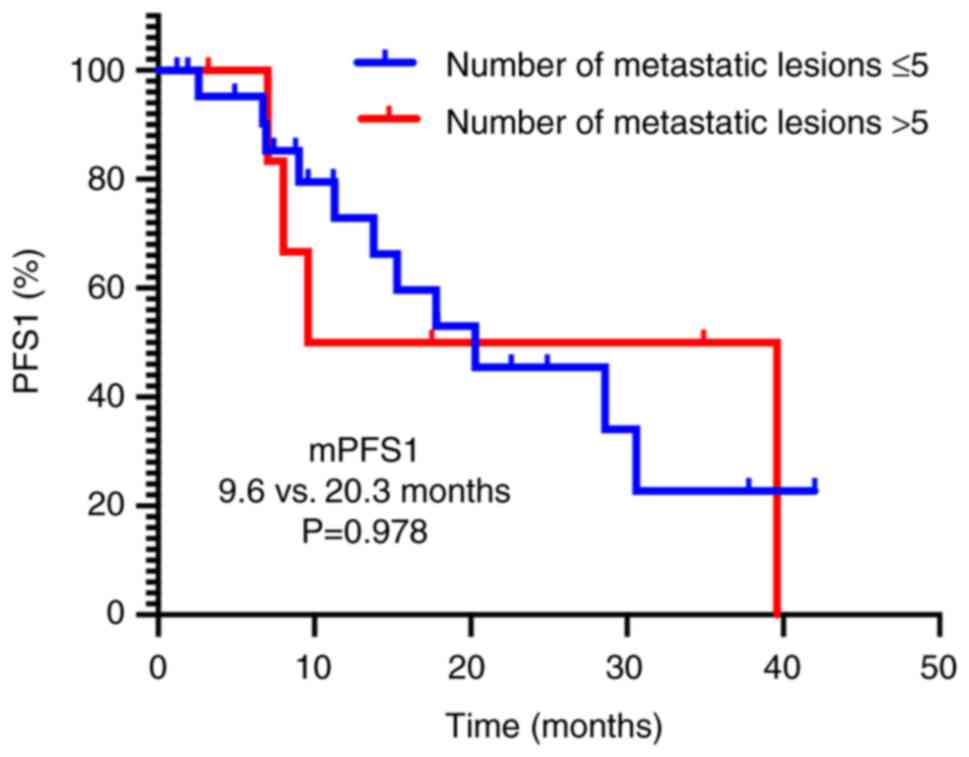
significant differences in median PFS1 time were observed, neither
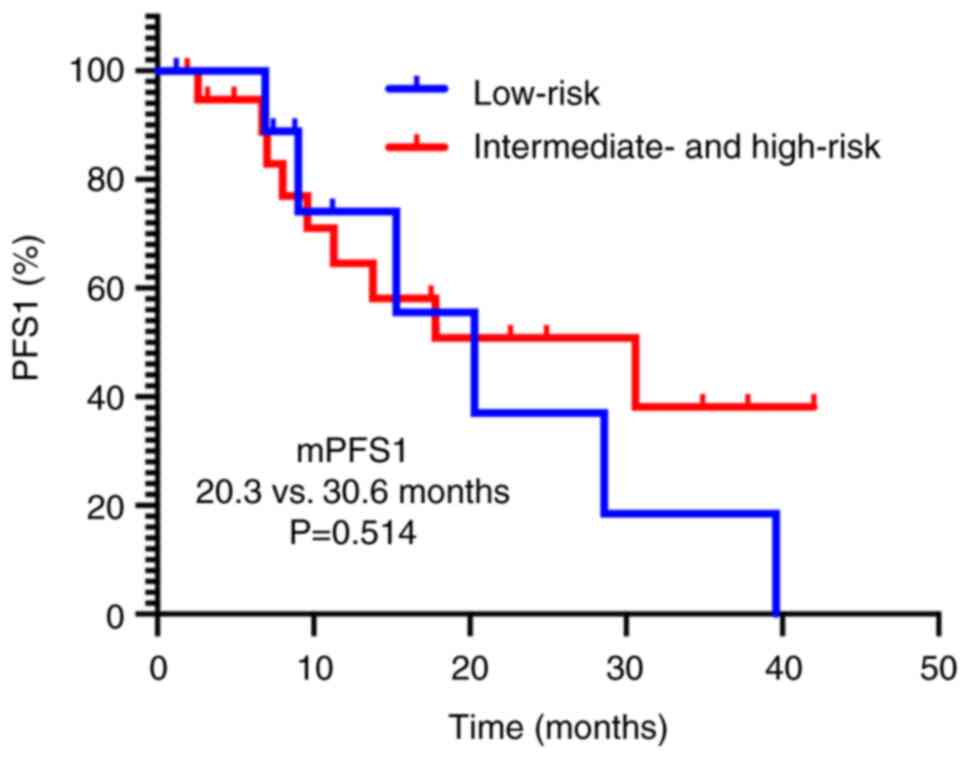
based on metastatic burden (≤5 vs. >5 lesions, 9.6 vs. 20.3
months; P=0.978; Fig. 4) nor IMDC
risk categories (low-risk vs. intermediate- and high-risk, 20.3 vs.
30.6 months; P=0.514; Fig. 5). By
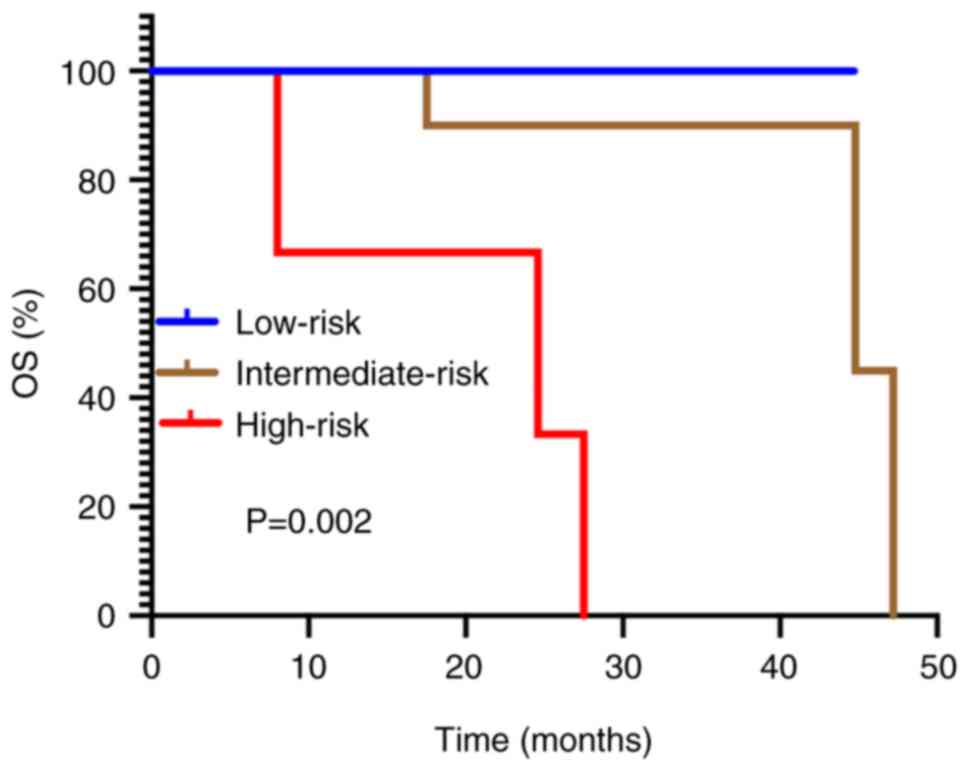
contrast, subgroup analysis of OS demonstrated significant
disparities across IMDC risk categories (low-risk vs.
intermediate-risk vs. high-risk, not reached vs. 47.2 vs. 24.6
months; P=0.002; Fig. 6).
Adverse reactions
Targeted therapy and immunotherapy-related
adverse reactions
Among the 30 patients, 15 (50.0%) experienced grade
1–2 adverse reactions, while 15 (50.0%) experienced grade 3–4
adverse reactions (Table III).
All adverse reactions improved following symptomatic treatment,
dose reduction or discontinuation of therapy.
 | Table III.Incidence of targeted therapy- and
immunotherapy-related adverse reactions (n=30). |
Table III.
Incidence of targeted therapy- and
immunotherapy-related adverse reactions (n=30).
| Adverse
reaction | Grade 1–2, n
(%) | Grade 3–4, n
(%) |
|---|
| Diarrhea | 12 (40.0) | 7 (23.3) |
| Fatigue | 8 (26.7) | 9 (30.0) |
| Hypertension | 13 (43.3) | 4 (13.3) |
| Liver
dysfunction | 11 (36.7) | 5 (16.7) |
| Rash or hand-foot
syndrome | 15 (50.0) | 1 (3.3) |
| Kidney
dysfunction | 12 (40.0) | 1 (3.3) |
| Decreased
appetite | 11 (36.7) | 1 (3.3) |
| Hypothyroidism | 12 (40.0) | 0 (0.0) |
| Esophageal or
throat pain, hoarseness | 11 (36.7) | 0 (0.0) |
| Proteinuria | 10 (33.3) | 0 (0.0) |
| Pain | 3 (10.0) | 6 (20.0) |
| Nausea and
vomiting | 9 (30.0) | 0 (0.0) |
| Pneumonia or
respiratory distress | 6 (20.0) | 3 (10.0) |
| Anemia | 9 (30.0) | 0 (0.0) |
| Leukopenia or
neutropenia | 7 (23.3) | 0 (0.0) |
|
Thrombocytopenia | 6 (20.0) | 0 (0.0) |
| Myositis | 5 (16.7) | 0 (0.0) |
| Myocarditis | 4 (13.3) | 0 (0.0) |
| Infection | 3 (10.0) | 0 (0.0) |
| Edema | 3 (10.0) | 0 (0.0) |
| Pancreatitis | 1 (3.3) | 0 (0.0) |
Radiotherapy-related adverse
reactions
Among the 30 patients, 9 experienced grade 1–2
adverse reactions (30.0%). The most common reactions included
fatigue (16.7%), diarrhea and decreased appetite (16.7%), skin
reactions (3.3%), leukopenia (3.3%) and radiation pneumonitis
(3.3%) (Table IV). All adverse
reactions improved following symptomatic treatment. Notably, there
were no occurrences of grade ≥3 radiotherapy-related adverse
reactions.
 | Table IV.Incidence of radiotherapy-related
adverse reactions (n=30). |
Table IV.
Incidence of radiotherapy-related
adverse reactions (n=30).
| Adverse
reaction | Grade 1–2, n
(%) | Grade 3–4, n
(%) |
|---|
| Fatigue | 5 (16.7) | 0 (0.0) |
| Diarrhea and
decreased appetite | 5 (16.7) | 0 (0.0) |
| Skin reactions | 1 (3.3) | 0 (0.0) |
| Leukopenia | 1 (3.3) | 0 (0.0) |
| Radiation
pneumonitis | 1 (3.3) | 0 (0.0) |
Discussion
The present study explored the application of SABR
in conjunction with targeted therapy using axitinib and
immunotherapy with toripalimab for patients with recurrent or
metastatic RCC. To the best of our knowledge, the present study is
the only prospective study to date that integrates targeted
therapy, immunotherapy and radiotherapy in a triple-modality
approach for recurrent or metastatic RCC, and preliminary results
indicate promising efficacy.
The treatment landscape for recurrent or metastatic
RCC has entered the era of combined targeted therapy and
immunotherapy. Since 2018, multiple large-scale, international,
multicenter clinical trials have demonstrated that immunotherapy
with PD-1/L1 monoclonal antibodies, when combined with targeted
therapy, markedly improves first-line treatment outcomes for
advanced RCC compared with the TKI sunitinib. Notable randomized
controlled trials such as JAVELIN Renal 101, KEYNOTE-426, CheckMate
9ER and CLEAR have reported median PFS times ranging from 13.3 to
23.3 months, which is markedly superior to those for sunitinib
monotherapy (10–13). However, the application of combined
targeted therapy and immunotherapies remains limited in China due
to the lack of regulatory approval.
Toripalimab, the first approved fully human
monoclonal antibody targeting the PD-1 receptor in China, exhibits
higher affinity for PD-1 compared with pembrolizumab and nivolumab,
and possesses a unique IgG4 binding site that does not rely on
glycosylation modifications (24).
Clinical studies across various disease areas have demonstrated its
efficacy (25–27), and it is currently approved for the
treatment of advanced melanoma, nasopharyngeal carcinoma and
urothelial carcinoma. Phase I clinical trials have reported
effective treatment responses in patients with advanced RCC
(28). The results of the
randomized controlled phase III trial, RENOTORCH, presented at the
2023 European Society for Medical Oncology Congress, indicated a
median PFS time of 18.0 months for patients with advanced RCC
treated with the combination of axitinib and toripalimab compared
with sunitinib, with a favorable safety profile (9). Notably, the patients included in the
RENOTORCH study were categorized as intermediate- to high-risk, and
comparisons with high-risk cohorts from the JAVELIN Renal 101,
KEYNOTE-426, CheckMate 9ER and CLEAR studies revealed longer median
PFS times for the axitinib plus toripalimab combination compared
with sunitinib, with PFS remaining a critical focus in the
comprehensive treatment of advanced RCC.
SABR has emerged as a principal non-surgical
treatment modality for patients with recurrent or metastatic RCC in
conjunction with targeted therapy and immunotherapy, gaining
increasing recognition in current clinical guidelines (5,6).
However, the current guidelines primarily position SABR as a means
to enhance LC rates, without fully acknowledging its potential role
in improving OS as part of a combination treatment strategy.
Previous studies have reported positive outcomes for
the combination of targeted therapy and SABR in patients with
oligometastatic RCC. Preliminary results from a prospective phase
II clinical trial presented at the 2023 American Society of
Clinical Oncology meeting indicated that the combination of
sunitinib and SABR in treatment-naïve patients with oligometastatic
RCC achieved a significantly longer median PFS time of 18.6 months
compared with the sunitinib monotherapy group (7.1 months;
P=0.003), with no notable difference in the incidence of
treatment-related adverse events between the two groups (29). A retrospective analysis by Ma et
al (30) of patients receiving
TKI therapy combined with SABR for oligometastatic RCC from 2015 to
2020, involving 100 lesions with 90% classified as intermediate to
high-risk, indicated a median PFS time of 22 months for those
receiving combined treatment during first-line therapy. Notably,
preclinical evidence suggests that SABR can synergistically enhance
the sensitivity of associated pathways inhibited by targeted
therapy, including AKT and mTOR pathways, which provides a further
biological basis for clinical research (31–33).
Additionally, accumulating evidence has demonstrated that
SABR-induced immunogenic cell death facilitates the release of
tumor-associated antigens, which subsequently triggers dendritic
cell maturation and initiates systemic cytotoxic T-cell responses,
thereby remodeling the immunosuppressive tumor microenvironment
(34,35). Based on these research outcomes,
previous studies have investigated the efficacy of immunotherapy
combined with SABR; however, the median PFS time reported (5.6 to
15.6 months) has not been optimal, possibly related to insufficient
radiation dose and delayed treatment lines (36–38).
The present study preliminarily explored the
efficacy and safety of combining axitinib with toripalimab, an
anti-PD-1 monoclonal antibody developed in China, and SABR for
patients with recurrent or metastatic RCC. The present study
included patients who had received second-line or subsequent
therapies, with a high proportion of intermediate to high-risk
patients (66.7%) and 30.0% being non-clear cell RCC. Preliminary
results indicated a median PFS1 time of 20.3 months, with patients
receiving first-line targeted therapy and immunotherapy combined
with radiotherapy achieving a PFS1 time of 28.6 months. This
outcome surpasses data from studies that utilized axitinib combined
solely with immunotherapy (median PFS time ranging from 13.8 to
18.0 months) (9,11,13).
The median OS time reached 44.8 months, showing no notable
difference compared with previous studies (9,11,13),
potentially due to insufficient follow-up duration.
Subgroup analyses revealed that PFS1 time was
significantly longer for patients who received radiotherapy before
treatment failure compared with those who received it afterward.
Early radiotherapy intervention before first-line treatment failure
may lead to better PFS due to lower tumor burden, a more favorable
immune microenvironment and the opportunity to proactively control
disease progression (20,31). The OS subgroup analysis revealed a
significant survival gradient across IMDC risk strata: Median OS
was not reached in the low-risk group, while median OS time was
47.2 months in the intermediate-risk group and declined to 24.6
months in the high-risk group, which implies that combining
radiation with systemic therapy may amplify benefits in less
aggressive disease states, while high-risk tumors likely harbor
intrinsic resistance mechanisms that diminish therapeutic
impact.
In terms of safety, the observed 50.0% incidence of
grade ≥3 adverse events with the axitinib-toripalimab-SABR
triple-modality therapy were below the rates reported in
contemporary TKI/immune-checkpoint inhibitor combinations (67–70%
in CheckMate 9ER/KEYNOTE-426) (10,13).
There were no occurrences of grade ≥3 radiotherapy-associated
adverse reactions, suggesting that the triple-modality regimen may
not inherently exacerbate the toxicity profile of the underlying
targeted therapy-immunotherapy combination. The most common reasons
for discontinuation of targeted therapy and immunotherapy were
hepatic dysfunction, diarrhea and gastrointestinal bleeding. These
findings indicate the need for heightened vigilance regarding liver
function and gastrointestinal responses when administering the
axitinib and toripalimab combination.
Furthermore, challenges arose in administering
adequate SABR doses due to the presence of large metastatic lesions
or their proximity to critical organs. The present study introduced
the p-SABR technique, which allowed for dose escalation at the
tumor center while maintaining a total dose of 50–60 Gy, thereby
optimizing tumor targeting while protecting adjacent critical
structures. The institutional protocol prioritizes SABR as the
primary radiotherapy modality. The p-SABR approach (combining SABR
for tumor core with MHFRT for peripheral regions) is reserved for
larger tumors with notable adjacent normal tissue involvement,
while MHFRT is typically employed for smaller tumors surrounded by
critical structures. For example, in the present study cohort, one
patient's tumor was located in the abdominal wall and near the
bowel, but the patient chose SABR technology for personal reasons.
A controlled dose compromise was strategically accepted in the
peripheral target area to maximize SABR coverage of the predominant
tumor volume while maintaining bowel sparing. However, the patient
experienced a recurrence due to marginal underdosage in the tumor
target area. This outcome indicated that choosing p-SABR technology
for suitable patients may provide greater benefits compared with
traditional SABR technology.
The limitations of the present study include its
single-arm design, small sample size and short follow-up for OS
endpoints. Due to the exploratory trial design and the ongoing
nature of this research, the present publication presents
preliminary findings based on a limited sample size (n=30), which
inherently restricts the statistical power of the analyses. To
enhance the validity of these observations, patient enrollment is
actively being expanded and extended follow-up protocols will be
implemented in subsequent phases of the investigation. The
single-arm design introduces potential confounding factors such as
heterogeneous disease burden, variability in prior therapies and
IMDC risk stratification, which may compromise outcome
interpretation. While propensity score weighting could
theoretically mitigate some confounding factors, its applicability
remains limited in small single-arm cohorts (n<100). Future
randomized trials stratified by confounding factors are warranted
to validate the present findings. Future studies will also
investigate potential biomarkers associated with the
immune-activating effects of SABR in metastatic RCC, such as
circulating free DNA and microRNAs, which have been identified as
predictors of immunotherapy response in patients with
oligoprogressive lung cancer receiving combined SABR and
immunotherapy (39). These
investigations will aim to further evaluate the efficacy of the
triple combination therapeutic regimen.
In conclusion, the combination of axitinib,
toripalimab and SABR for the treatment of recurrent or metastatic
RCC demonstrates manageable adverse effects and shows preliminary
efficacy in clinical practice. Whether this combination ultimately
improves PFS and OS will require further validation through
long-term follow-up results. In the future, a two-arm randomized
trial of axitinib and toripalimab vs. the triple-modality of
axitinib, toripalimab and SABR will be performed, to confirm the
efficacy and safety of the triple combination therapy and explore
the correlative biomarkers.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by Capital's Funds for Health
Improvement and Research (grant no. 2024-4-40710), National High
Level Hospital Clinical Research Funding (High Quality Clinical
Research Project of Peking University First Hospital; grant no.
2023HQ11) and National High-Level Hospital Clinical Research
Funding (Interdepartmental Clinical Research Project of Peking
University First Hospital; grant no. 2022CR29).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
KH wrote the original manuscript and contributed to
methodology design, data curation, formal analysis and
investigation. MWM participated in methodology design,
conceptualization, data validation, formal analysis, software
development and revision of the manuscript. XSG was responsible for
project administration, methodology design, funding acquisition and
supervision, and participated in the review and editing of the
manuscript. HZL was responsible for supervision and methodology
design. JYC participated in data curation and visualization. XYL
participated in formal analysis, data validation and resource
provision. SBQ participated in formal analysis and validation. XYR
participated in data validation and software development. KH and
XSG confirm the authenticity of all the raw data. All authors read
and approved the final version of the manuscript.
Ethics approval and consent to
participate
The present study received ethics approval from the
Ethics Committee of Peking University First Hospital (Beijing,
China; approval no. 2019-345). All procedures performed in studies
involving human participants were in accordance with the ethical
standards of the Ethics Committee of Peking University First
Hospital and with the 1964 Helsinki Declaration and its later
amendments or comparable ethical standards. The patients provided
written informed consent before treatment.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD, Wagle NS and Jemal
A: Cancer statistics, 2023. CA Cancer J Clin. 73:17–48. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Centers for Disease Control and
Prevention, . Male urologic cancers. USCS Data Brief, no 21.
Centers for Disease Control and Prevention. US Department of Health
and Human Services; Atlanta GA: 2020, Available from. https://dx.doi.org/10.15620/cdc:105242
|
|
3
|
Zheng R, Zhang S, Zeng H, Wang S, Sun K,
Chen R, Li L, Wei W and He J: Cancer incidence and mortality in
China, 2016. J Natl Cancer Cent. 2:1–9. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lam JS, Shvarts O, Leppert JT, Figlin RA
and Belldegrun AS: Renal cell carcinoma 2005: New frontiers in
staging, prognostication and targeted molecular therapy. J Urol.
173:1853–1862. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Motzer RJ, Jonasch E, Agarwal N, Alva A,
Bagshaw H, Baine M, Beckermann K, Carlo MI, Choueiri TK, Costello
BA, et al: NCCN guidelines® insights: Kidney cancer,
version 2.2024. J Natl Compr Canc Netw. 22:4–16. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bex A, Ghanem YA, Albiges L, Bonn S, Campi
R, Capitanio U, Dabestani S, Hora M, Klatte T, Kuusk T, et al:
European association of urology guidelines on renal cell carcinoma:
The 2025 update. Eur Urol. Mar 10–2025.(Epub ahead of print).
View Article : Google Scholar
|
|
7
|
Atlas of Genetics and Cytogenetics in
Oncology and Haematology, . 2024.Available from. http://atlasgeneticsoncology.org/teaching/209251/csco
|
|
8
|
Powles T, Plimack ER, Soulières D, Waddell
T, Stus V, Gafanov R, Nosov D, Pouliot F, Melichar B, Vynnychenko
I, et al: Pembrolizumab plus axitinib versus sunitinib monotherapy
as first-line treatment of advanced renal cell carcinoma
(KEYNOTE-426): Extended follow-up from a randomised, open-label,
phase 3 trial. Lancet Oncol. 21:1563–1573. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yan XQ, Ye MJ, Zou Q, Chen P, He ZS, Wu B,
He DL, He CH, Xue XY, Ji ZG, et al: Toripalimab plus axitinib
versus sunitinib as first-line treatment for advanced renal cell
carcinoma: RENOTORCH, a randomized, open-label, phase III study.
Ann Oncol. 35:190–199. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motzer RJ, Powles T, Burotto M, Escudier
B, Bourlon MT, Shah AY, Suárez C, Hamzaj A, Porta C, Hocking CM, et
al: Nivolumab plus cabozantinib versus sunitinib in first-line
treatment for advanced renal cell carcinoma (CheckMate 9ER):
Long-term follow-up results from an open-label, randomised, phase 3
trial. Lancet Oncol. 23:888–898. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Choueiri TK, Motzer RJ, Rini BI, Haanen J,
Campbell MT, Venugopal B, Kollmannsberger C, Gravis-Mescam G,
Uemura M, Lee JL, et al: Updated efficacy results from the JAVELIN
Renal 101 trial: First-line avelumab plus axitinib versus sunitinib
in patients with advanced renal cell carcinoma. Ann Oncol.
31:1030–1039. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Choueiri TK, Eto M, Motzer R, De Giorgi U,
Buchler T, Basappa NS, Méndez-Vidal MJ, Tjulandin S, Hoon Park S,
Melichar B, et al: Lenvatinib plus pembrolizumab versus sunitinib
as first-line treatment of patients with advanced renal cell
carcinoma (CLEAR): Extended follow-up from the phase 3, randomised,
open-label study. Lancet Oncol. 24:228–238. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rini BI, Plimack ER, Stus V, Waddell T,
Gafanov R, Pouliot F, Nosov D, Melichar B, Soulieres D,
Borchiellini D, et al: Pembrolizumab (pembro) plus axitinib (axi)
versus sunitinib as first-line therapy for advanced clear cell
renal cell carcinoma (ccRCC): Results from 42-month follow-up of
KEYNOTE-426. J Clin Oncol. 39 (Suppl 15):S45002021. View Article : Google Scholar
|
|
14
|
Tolia BM and Whitmore WF Jr: Solitary
metastasis from renal cell carcinoma. J Urol. 114:836–838. 1975.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vaeth JM: Proceedings: Cancer of the
kidney-radiation therapy and its indications in non-Wilms' tumors.
Cancer. 32:1053–1055. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Francolini G, Detti B, Ingrosso G,
Desideri I, Becherini C, Carta G, Pezzulla D, Caramia G, Dominici
L, Maragna V, et al: Stereotactic body radiation therapy (SBRT) on
renal cell carcinoma, an overview of technical aspects, biological
rationale and current literature. Crit Rev Oncol Hematol.
131:24–29. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wersäll PJ, Blomgren H, Lax I, Kälkner KM,
Linder C, Lundell G, Nilsson B, Nilsson S, Näslund I, Pisa P and
Svedman C: Extracranial stereotactic radiotherapy for primary and
metastatic renal cell carcinoma. Radiother Oncol. 77:88–95. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Svedman C, Sandström P, Pisa P, Blomgren
H, Lax I, Kälkner KM, Nilsson S and Wersäll P: A prospective phase
II trial of using extracranial stereotactic radiotherapy in primary
and metastatic renal cell carcinoma. Acta Oncol. 45:870–875. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zaorsky NG, Lehrer EJ, Kothari G, Louie AV
and Siva S: Stereotactic ablative radiation therapy for
oligometastatic renal cell carcinoma (SABR ORCA): A meta-analysis
of 28 studies. Eur Urol Oncol. 2:515–523. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ali M, Mooi J, Lawrentschuk N, McKay RR,
Hannan R, Lo SS, Hall WA and Siva S: The role of stereotactic
ablative body radiotherapy in renal cell carcinoma. Eur Urol.
82:613–622. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Meyer E, Pasquier D, Bernadou G, Calais G,
Maroun P, Bossi A, Theodore C, Albiges L, Stefan D, de Crevoisier
R, et al: Stereotactic radiation therapy in the strategy of
treatment of metastatic renal cell carcinoma: A study of the Getug
group. Eur J Cancer. 98:38–47. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Katayama H, Kurokawa Y, Nakamura K, Ito H,
Kanemitsu Y, Masuda N, Tsubosa Y, Satoh T, Yokomizo A, Fukuda H and
Sasako M: Extended Clavien-Dindo classification of surgical
complications: Japan clinical oncology group postoperative
complications criteria. Surg Today. 46:668–685. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Heng DYC, Xie W, Regan MM, Harshman LC,
Bjarnason GA, Vaishampayan UN, Mackenzie M, Wood L, Donskov F, Tan
MH, et al: External validation and comparison with other models of
the international metastatic renal-cell carcinoma database
consortium prognostic model: A population-based study. Lancet
Oncol. 14:141–148. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu H, Guo L, Zhang J, Zhou Y, Zhou J, Yao
J, Wu H, Yao S, Chen B, Chai Y, et al: Glycosylation-independent
binding of monoclonal antibody toripalimab to FG loop of PD-1 for
tumor immune checkpoint therapy. MAbs. 11:681–690. 2019.PubMed/NCBI
|
|
25
|
Tang B, Chi Z, Chen Y, Liu X, Wu D, Chen
J, Song X, Wang W, Dong L, Song H, et al: Safety, efficacy, and
biomarker analysis of toripalimab in previously treated advanced
melanoma: Results of the POLARIS-01 multicenter phase II trial.
Clin Cancer Res. 26:4250–4259. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang FH, Wei XL, Feng J, Li Q, Xu N, Hu
XC, Liao W, Jiang Y, Lin XY, Zhang QY, et al: Efficacy, safety, and
correlative biomarkers of toripalimab in previously treated
recurrent or metastatic nasopharyngeal carcinoma: A phase II
clinical trial (POLARIS-02). J Clin Oncol. 39:704–712. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li LC, Chen XW, Fang L, Jian CL, Yu YX,
Liao XY and Sun JG: YAP1 as a novel negative biomarker of immune
checkpoint inhibitors for EGFR-mutant non-small-cell lung cancer.
Can Respir J. 2023:46890042023. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tang B, Yan X, Sheng X, Si L, Cui C, Kong
Y, Mao L, Lian B, Bai X, Wang X, et al: Safety and clinical
activity with an anti-PD-1 antibody JS001 in advanced melanoma or
urologic cancer patients. J Hematol Oncol. 12:72019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Liu Y, Wei W, Zhang Z, Liu R, Gao J, Guo
S, Han H, Dong P, He L and Zhou H: Preliminary results from a phase
II study comparing sunitinib alone or with stereotactic body
radiotherapy (SBRT) for newly diagnosed oligometastatic renal cell
carcinoma. J Clin Oncol. 41 (Suppl 16):S45332023. View Article : Google Scholar
|
|
30
|
Ma MW, Li HZ, Gao XS, Liu MZ, Yin H, Yang
KW, Chen JY, Ren XY and Wang D: Outcomes of high-dose stereotactic
ablative radiotherapy to all/multiple sites for oligometastatic
renal cell cancer patients. Curr Oncol. 29:7832–7841. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nakamura JL, Karlsson A, Arvold ND,
Gottschalk AR, Pieper RO, Stokoe D and Haas-Kogan DA: PKB/Akt
mediates radiosensitization by the signaling inhibitor LY294002 in
human malignant gliomas. J Neurooncol. 71:215–222. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Frémin C and Meloche S: From basic
research to clinical development of MEK1/2 inhibitors for cancer
therapy. J Hematol Oncol. 3:82010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shinohara ET, Cao C, Niermann K, Mu Y,
Zeng F, Hallahan DE and Lu B: Enhanced radiation damage of tumor
vasculature by mTOR inhibitors. Oncogene. 24:5414–5422. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wersäll PJ, Blomgren H, Pisa P, Lax I,
Kälkner KM and Svedman C: Regression of non-irradiated metastases
after extracranial stereotactic radiotherapy in metastatic renal
cell carcinoma. Acta Oncol. 45:493–497. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Dybal EJ, Haas GP, Maughan RL, Sud S,
Pontes JE and Hillman GG: Synergy of radiation therapy and
immunotherapy in murine renal cell carcinoma. J Urol.
148:1331–1337. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hammers HJ, Vonmerveldt D, Ahn C, Nadal
RM, Drake CG, Folkert MR, Laine AM, Courtney KD, Brugarolas J, Song
DY, et al: Combination of dual immune checkpoint inhibition (ICI)
with stereotactic radiation (SBRT) in metastatic renal cell
carcinoma (mRCC) (RADVAX RCC). J Clin Oncol. 38 (Suppl 6):S6142020.
View Article : Google Scholar
|
|
37
|
Masini C, Iotti C, De Giorgi U, Bellia RS,
Buti S, Salaroli F, Zampiva I, Mazzarotto R, Mucciarini C, Vitale
MG, et al: Nivolumab in combination with stereotactic body
radiotherapy in pretreated patients with metastatic renal cell
carcinoma. Results of the phase II NIVES study. Eur Urol.
81:274–282. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Siva S, Bressel M, Wood ST, Shaw MG, Loi
S, Sandhu SK, Tran B, A Azad A, Lewin JH, Cuff KE, et al:
Stereotactic radiotherapy and short-course pembrolizumab for
oligometastatic renal cell carcinoma-The RAPPORT trial. Eur Urol.
81:364–372. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Zafra J, Onieva JL, Oliver J,
Garrido-Barros M, González-Hernández A, Martínez-Gálvez B, Román A,
Ordóñez-Marmolejo R, Pérez-Ruiz E, Benítez JC, et al: Novel blood
biomarkers for response prediction and monitoring of stereotactic
ablative radiotherapy and immunotherapy in metastatic
oligoprogressive lung cancer. Int J Mol Sci. 25:45332024.
View Article : Google Scholar : PubMed/NCBI
|