Introduction
Cancer is a global health crisis, which imposes a
notable societal and economic burden, and accounts for 16.8% of
deaths annually worldwide (1).
Despite advancements in targeted therapies and immunotherapy,
challenges such as metastasis, drug resistance and late-stage
diagnosis persist, particularly in prevalent cancer types such as
lung, breast and colorectal carcinomas (2–4).
Further understanding of tumor pathogenesis and the identification
of novel therapeutic targets are essential to improve patient
outcomes.
Insulin-like growth factor (IGF) binding protein 3
(IGFBP3), a key member of the IGFBP family, is encoded by the
IGFBP3 gene located on human chromosome 7p13-12. IGFBP3 comprises
264 amino acids, has a molecular weight of 28.7 kDa, and is
primarily synthesized by hepatic stellate cells and mesenchymal
cells (5–7). The IGFBP3 structure features a highly
conserved NH2 terminus and COOH terminus, along with a central
segment that distinguishes IGFBP3 from other proteins in the IGFBP
family. The other members of this family primarily include IGFBP1,
IGFBP2, IGFBP4, IGFBP5 and IGFBP6 (8). IGFBP3 binds to IGF-1 through its
termini, thereby regulating the half-life and functionality of
IGF-1 (8). Furthermore, the central
segment of IGFBP3 undergoes various post-translational
modifications, including glycosylation, phosphorylation and
protease cleavage, which notably influence its functional
expression (5,9). For instance, in non-small cell lung
cancer (NSCLC), casein kinase 2 (CK2) phosphorylates IGFBP3,
enhances cancer cell resistance to cisplatin, a commonly used
antitumor drug in NSCLC treatment, and promotes anti-apoptotic
capabilities (10). In patients
with melanoma and prostate cancer, the levels of glycosylated and
phosphorylated IGFBP3 in peripheral blood are positively associated
with disease progression (11,12).
Additionally, a disintegrin and metalloprotease 12 (ADAM12)
(13) and pregnancy-associated
plasma protein-A2 (PAPP-A2) (14)
degrade IGFBP3 through proteolysis, thereby reducing the expression
levels of IGFBP3.
The importance of IGFBP3 in cancer is multifaceted
and has been extensively studied. Numerous studies have
demonstrated the close association between IGFBP3 and tumorigenesis
and tumor progression (15–21). In lung adenocarcinoma with brain
metastasis, IGFBP3 stimulates the expression of SMAD4, which leads
to epithelial-mesenchymal transition (EMT) and promotes cancer cell
invasion and migration through the TGF-β1/SMAD4 signaling pathway
(15–17). IGFBP3 expression is closely
associated with different breast cancer subtypes (18–21).
In estrogen receptor (ER)-positive breast cancer cells, IGFBP3
inhibits cell proliferation by preventing the phosphorylation of
cell cycle-related proteins, such as Cyclin D, Cyclin A, Cyclin E,
cyclin-dependent kinases 2 and 4 (CDK2 and CDK4), as well as
retinoblastoma protein (pRB) (18,19).
Conversely, in triple-negative breast cancer, increased IGFBP3
expression enhances cancer cell invasiveness and proliferation
(20,21). Furthermore, IGFBP3 may serve a
potential role in tumor diagnosis and treatment in the future. The
detection of IGFBP3 expression serves as a valuable biomarker in
diagnosis (22–26). Studies have shown a notable
association between IGFBP3 expression, tumor stage and patient
survival rates in esophageal squamous cell carcinoma and pancreatic
cancer (22–26). These findings indicate that IGFBP3
may be a potential indicator for early tumor detection, disease
monitoring and prognosis evaluation. Targeting IGFBP3 and the
related signaling pathways may potentially offer novel
opportunities to develop anticancer therapies with enhanced
efficacy in the future (27–30).
For example, modulating the function of IGFBP3 in clear cell renal
cell carcinoma and prostate cancer may enhance the sensitivity of
tumor cells to chemotherapy drugs or reduce drug resistance and
improve overall treatment outcomes (27,28,30).
In conclusion, IGFBP3 serves a key role in cancer
research. Understanding the role of IGFBP3 in tumor pathogenesis is
essential in the development of targeted and effective strategies
for cancer diagnosis, treatment and prevention. The present review
aimed to comprehensively summarize the current literature on the
changes in IGFBP3 expression in tumors, the impact on cell
signaling pathways and implications for tumor development. Table I illustrates the relationships
between the changes in expression levels of IGFBP3 in several
prevalent cancer types, such as lung cancer (including lung
adenocarcinoma and NSCLC), breast cancer (ER-positive,
HER2-positive, and triple-negative breast cancer), colorectal
cancer, liver cancer (hepatocellular carcinoma), and ovarian
cancer, the associated functions and the signaling pathways
involving IGFBP3.
 | Table I.Expression status and related
functions of IGFBP3 in common tumors. |
Table I.
Expression status and related
functions of IGFBP3 in common tumors.
| First author/s,
year | System | Organ | Cancer type | Expression
status | Cellular
activities | Affected signaling
pathways | (Refs.) |
|---|
| Yang et al,
2019 | Respiratory | Lung | Lung
adenocarcinoma | Up | Invasion,
metastasis | TGF-β1/SMAD4 via
EMT | (16) |
| Sun et al,
2015; |
|
| NSCLC | Up | Invasion,
metastasis, |
IGFBP3-MMP-9/VEGF/MCP-1 | (2,32,33) |
| Luo et al,
2021; |
|
|
|
| promotion of
angiogenesis |
|
|
| Gharib et
al, 2004 |
|
|
|
|
|
|
|
| Kuhn et al,
2023 |
|
| Lung cancer
(unspecified) | Down | Invasion, spheroid
growth | No clear pathway
specified | (22) |
| Bao et al,
2016 |
| Pharynx | Nasopharyngeal
carcinoma | Up | Invasion,
metastasis | No clear pathway
specified | (24) |
| Zhong et al,
2008 | Digestive | Oral cavity | OSCC | Up | Proliferation,
metastasis, drug resistance, promotion of angiogenesis | IGFBP3-Integrin
β1-MEK-ERK; IGFBP3-Integrin β1-FAK/SRC-STAT3-Bcl-2/c-Myc/MMP-9 | (39) |
| Ng et al,
2022 |
|
| TSCC | Up | Invasion,
metastasis, cell cycle arrest (G1/S phase) | IGFBP3-Integrin
β1-FAK-MEK-ERK (hypothetically) | (46) |
| Williams et
al, 2007 |
| Colon | Colorectal
cancer | Down | Apoptosis |
IGFBP3-TRAIL-DISC/NF-κB | (49) |
| Li et al,
2024; |
| Liver | HCC | Down | Tumorigenesis,
metastasis |
WSB2-p53-IGFBP3-PI3K-AKT-mTOR | (52,54,57, |
| Hanafusa et
al, 2005; |
|
|
| (wild-type |
|
| 126-128) |
| Alexia et
al, 2004; |
|
|
| p53) |
|
|
|
| Buckbinder et
al, 1995; |
|
|
|
|
|
|
|
| Whittaker et
al, 2010; |
|
|
|
|
|
|
|
| Subramaniam et
al, 2010 |
|
|
|
|
|
|
|
| Song et al,
2020; |
|
|
| Up | Angiogenesis,
EMT |
Galectin-3-PI3K-AKT-GSK-3β- | (58,59) |
| Playford et
al, 2000 |
|
|
|
|
|
β-catenin-IGFBP3 |
|
| Jang et al,
2023 |
| Pancreas | Pancreatic
cancer | Down | Proliferation |
IGFBP3-IGF-1/IGF-1R-PI3K-mTOR | (23) |
| Kim et al,
2010; | Genitourinary | Breast | ER-positive | Down | Proliferation,
apoptosis, |
IGFBP3-CDK2/4-pRB-E2F; | (18,64,65) |
| Butt et al,
2000; |
|
| breast cancer |
| cell cycle
arrest |
IGFBP3-TMEM219-DISC-caspase 8 |
|
| Jia et al,
2010 |
|
|
|
| (G1/S
phase) |
|
|
| Qiu et al,
2019 |
|
| HER2-positive
breast cancer | Down | Proliferation, drug
resistance |
IGFBP3-IGF-1/IGF-1R-PI3K-AKT-Wnt-β-catenin-TCF7L2 | (62) |
| Martin et
al, 2014; |
|
| TNBC | Up | Invasiveness,
proliferation |
IGFBP3-EGFR-PI3K-AKT; | (20,66) |
| Pitson et
al, 2005 |
|
|
|
|
|
IGFBP3-EGFR-Ras-MEK-ERK |
|
| Chen et al,
2024; |
| Ovary | Ovarian cancer | Down | Angiogenesis,
glycolysis |
miR-19a-3p-IGFBP3-IGF-1/IGF-1R- | (68–70) |
| Shih et al,
2020; |
|
|
|
|
| PI3K-AKT-PKM2 |
|
| Shih et al,
2021 |
|
|
|
|
|
|
|
| Du et al,
2022 |
| Cervix | Cervical
cancer | Up | Lymph
angiogenesis | No clear pathway
specified | (101) |
| Liu et al,
2021 |
| Kidney | Clear cell renal
cell carcinoma | Up | Proliferation,
EMT |
IGFBP3-TGF-β1-SMAD2/4 via EMT;
IGFBP3-PI3K-AKT; IGFBP3-MAPK | (76) |
| Shariat et
al, 2002; |
| Prostate | Prostate
cancer | Down | Cell cycle
arrest |
IGFBP3-IGF-1/IGF-1R-PI3K-AKT-NF-κB; | (78,79) |
| Zhong et al,
2024 |
|
|
|
|
(G0/G1 phase),
induction of apoptosis |
IGFBP3-TMEM219-DISC-caspase 8 |
|
| Vaezi et al,
2022; | Skeletal | Bone | Osteosarcoma | Up | Metastasis |
IGFBP3-PI3K-AKT-c-Jun-VCAM-2 | (99,100) |
| Chao et al,
2021 | system |
|
|
|
|
|
|
| Li et al,
2021; |
|
|
| Down | Invasion,
proliferation |
TRAIP-KANK1-IGFBP3-PI3K-AKT | (96,97) |
| Ressler et
al, 2009 |
|
|
|
|
|
|
|
| Huang et al,
2020 | Other | Thyroid | Papillary thyroid
carcinoma | Up | Metastasis | No clear pathway
specified | (102) |
| Giuliano et
al, 1998 |
| Eye | Retinoblastoma | Down | Apoptosis |
IGFBP3-IGF-1/IGF-1R-PI3K-AKT-p21 | (103) |
| Naspi et al,
2017 |
| Skin | Metastatic
melanoma | Down | Invasion |
IGFBP3-Wnt/β-catenin | (104) |
Changes in the expression levels of IGFBP3
in different tumors and related mechanisms
Lung cancer
According to the Global Cancer Statistics 2022
(1,3), lung cancer remains the most prevalent
and lethal cancer type globally among both men and women, with ~50%
of patients diagnosed at an advanced stage. Due to advancements in
treatment methods and technologies, the median survival period for
patients with advanced lung cancer can be extended to ~1 year;
however, distant metastasis markedly reduces patient survival rates
(3,31). Therefore, managing distant
metastasis is key in primary disease treatment. Numerous studies
have indicated that high IGFBP3 expression may enhance the invasion
and metastasis of lung adenocarcinoma and NSCLC cells (2,16,32,33).
For instance, in lung adenocarcinoma brain metastasis, IGFBP3
stimulates the expression of SMAD4, which leads to EMT by
upregulating N-cadherin and downregulating E-cadherin expression,
thereby promoting cancer cell invasion and metastasis. SMAD4 serves
a key role in TGF-β1-induced EMT. Consequently, IGFBP3 may
contribute to brain metastasis in lung adenocarcinoma by modulating
the TGF-β1/SMAD4 signaling pathway (15–17)
(Fig. 1, Part 1). In NSCLC,
upregulation of IGFBP3 promoted tumor cell metastasis and invasion
by regulating the expression of MMP-9, VEGF and monocyte
chemoattractant protein-1 (2,16,32–35)
(Fig. 1, Part 2). However, several
studies have indicated that the plasma concentration of IGFBP3 in
patients with lung cancer was low and exhibited notable negative
associations with patient survival rates, clinical tumor stage and
tumor grade (22,36–38).
Transient transfection of IGFBP3 has been performed to augment
IGFBP3 expression in H1299 cancer cells (22). IGFBP3 inhibited spheroid growth and
invasion of cancer cells by upregulating MMP-9 expression, while
simultaneously downregulating MMP-1 and total MMP expression in
these cells (22). These findings
contradict previous research conducted by Luo et al
(32). Potential explanations for
these discrepancies include differences in the selected target
populations, cancer types and ethnic backgrounds. Luo et al
(32) included Chinese patients
with NSCLC, while Kuhn et al (22) examined German patients with lung
cancer without specifying the cancer type. Additionally, variations
in the research cell lines and transfection methods were observed;
Luo et al (32) performed
lentiviral transfection to increase IGFBP3 expression levels in
A549 cancer cells, whereas Kuhn et al (22) performed transient transfection in
H1299 cancer cells. Thus, IGFBP3 may exhibit varying effects in
different types of lung cancer.
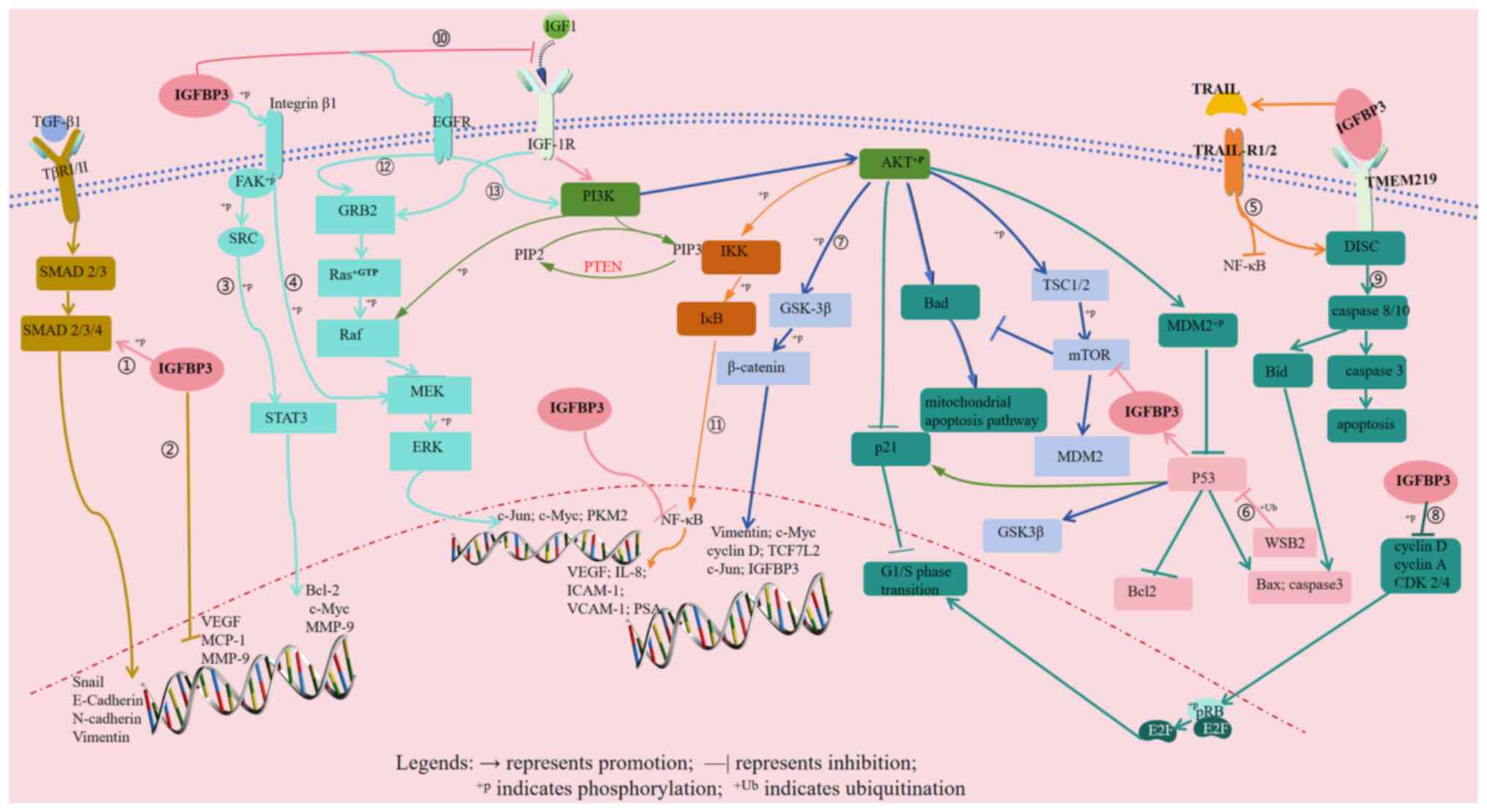 | Figure 1.Key signaling pathways involving
IGFBP3 expression in different tumors. 1, IGFBP3-TGF-β1/SMAD4 → EMT
→ invasion and metastasis (lung adenocarcinoma; clear cell renal
cell carcinoma); 2, IGFBP3-MMP-9/VEGF/MCP-1 → invasion, metastasis
and promotion of angiogenesis (non-small cell lung cancer); 3,
IGFBP3-Integrin β1-FAK/SRC-STAT3-Bcl-2/c-Myc/MMP-9 → proliferation,
metastasis, drug resistance and promotion of angiogenesis (OSCC);
4, IGFBP3-Integrin β1-MEK-ERK→proliferation, metastasis and drug
resistance (OSCC; TSCC); 5, IGFBP3-TRAIL-DISC/NF-κB → apoptosis
(colorectal cancer); 6, WSB2-p53-IGFBP3-PI3K-AKT-mTOR →
tumorigenesis and metastasis [HCC (wild-type p53)]; 7,
PI3K-AKT-GSK-3β → angiogenesis and EMT (HCC; HER2-positive breast
cancer); 8, IGFBP3-CDK2/4-pRB-E2F → proliferation and cell cycle
arrest (G1/S phase) (ER-positive breast cancer); 9,
IGFBP3-TMEM219-DISC-caspase-8 → apoptosis, proliferation and cell
cycle arrest (G1/S phase) (ER-positive breast cancer;
prostate cancer); 10, GFBP3-IGF-1/IGF-1R-PI3K-AKT-PKM2 →
angiogenesis and glycolysis (ovarian cancer; osteosarcoma); 11,
IGFBP3-IGF-1/IGF-1R-PI3K-AKT-NF-κB → cell cycle arrest
(G0/G1 phase) and apoptosis induction
(prostate cancer); 12, IGFBP3-EGFR-Ras/Raf-MEK-ERK → proliferation
and metastasis (triple-negative breast cancer); 13,
IGFBP3-EGFR-PI3K-AKT → proliferation and metastasis
(triple-negative breast cancer). IGFBP3, insulin-like growth factor
binding protein 3; EMT, epithelial-mesenchymal transition; OSCC,
oral squamous cell carcinoma; MCP-1, monocyte chemoattractant
protein-1; SRC, Rous sarcoma virus oncogene homolog; PKM2, pyruvate
kinase M2; TRAIL, TNF-related apoptosis-inducing ligand; DISC,
death-inducing signaling complex; WSB2, WD repeat and SOCS box
containing protein 2; HCC, hepatocellular carcinoma; pRB,
retinoblastoma protein; E2F, E2 promoter binding factor; TMEM219,
transmembrane protein 219; ER, estrogen receptor; IGF-1,
insulin-like growth factor-1; IGF-1R, IGF-1 receptor; PIP2,
phosphatidylinositol 4,5-bisphosphate; PIP3, phosphatidylinositol
3,4,5-trisphosphate; VCAM-1, vascular cell adhesion molecule-1;
ICAM-1, intercellular adhesion molecule-1; PSA, prostate-specific
antigen; TCF7L2, transcription factor 7-like 2; TβR I/II,
transforming growth factor β receptor type I/II; Bid,
BH3-interacting domain death agonist; TSC1/2, tuberous sclerosis
complex 1/2. |
Oral cancer
In oral squamous cell carcinoma (OSCC), the
expression levels of IGFBP3 are markedly elevated compared with
those in normal tissues (39,40).
The upregulation of IGFBP3 facilitates IGFBP3 binding to the cell
membrane integrin β1 receptor (41,42),
which promotes the phosphorylation of MEK and ERK, thereby
contributing to OSCC metastasis (43). Additionally, IGFBP3 induces the
phosphorylation of recombinant focal adhesion kinase, Rous sarcoma
virus oncogene homolog (SRC) and STAT3. Phosphorylated STAT3 is
translocated to the nucleus, which leads to increased expression
levels of anti-apoptotic proteins such as Bcl-2, c-Myc and MMP-9
(Fig. 1, Part 3 and Part 4). Bcl-2
provides resistance to chemotherapy drugs, while c-Myc functions as
an oncogene. MMP-9, a key regulator of cell proliferation,
differentiation and malignant transformation, cleaves various
extracellular matrix (ECM) proteins, such as collagen, laminin, and
fibronectin (44), and influences
ECM remodeling associated with tumor invasion, angiogenesis and
metastasis (45). Consequently,
high levels of IGFBP3 expression may enhance proliferation,
metastasis and drug resistance in OSCC cells. Furthermore, elevated
expression levels of IGFBP3 have been observed in tongue squamous
cell carcinoma (TSCC) (46).
Following knockdown of IGFBP3 in SAS cells, cell cycle analysis was
performed using the Fucci reporter system, which revealed that SAS
cells were arrested in the G1 phase. Subsequently,
western blot analyses were conducted to validate the results
observed in SAS cells after IGFBP3 knockout and MEK inhibitor
treatment. The findings indicated that IGFBP3 enhanced the
migration of cancer cells by promoting the phosphorylation of MEK
and ERK. Furthermore, the direct addition of exogenous IGFBP3 did
not affect the migration of the cells (46). Therefore, the elevated expression
levels of IGFBP3 in TSCC modulated cancer cell migration through
the MEK/ERK signaling pathway (Fig. 1,
Part 4). In conclusion, the upregulation of IGFBP3 in both OSCC
and TSCC cells can stimulate tumor cell proliferation, invasion and
metastasis.
Colorectal cancer
In colorectal cancer, the methylation of the IGFBP3
gene promoter frequently results in reduced IGFBP3 expression
(47,48). TNF-related apoptosis-inducing ligand
(TRAIL) binds to the membrane receptors TRAIL-R1 and TRAIL-R2,
forming a death-inducing signaling complex with Fas-associated
protein with death domain and procaspase-8, which ultimately
activates caspase-8 and initiates cell death (5). Williams et al (49) demonstrated that upregulation of
IGFBP3 expression in colon cancer HT29 cells via lentiviral
transfection enhanced the effects of TRAIL, inhibited the
activation of the NF-κB signaling pathway and suppressed colon
cancer development. Furthermore, exogenous IGFBP3 promoted cell
apoptosis by inhibiting the NF-κB signaling pathway (Fig. 1, Part 5). Therefore, regulating
IGFBP3 expression in colorectal cancer may potentially represent a
promising therapeutic approach for colorectal cancer in the
future.
Liver cancer
Hepatocellular carcinoma (HCC) is the most prevalent
form of liver cancer, and accounts for ~90% of all liver cancer
cases (50). Early symptoms of HCC
are often subtle and HCC is frequently diagnosed at an advanced
stage, when treatment becomes exceedingly challenging. The 5-year
survival rate for patients diagnosed with HCC is <20% (51). Li et al (52) performed co-immunoprecipitation and
ubiquitin amino acid substitution techniques. Findings from the
study demonstrated that in HCC with wild-type p53, the elevated
expression of WD repeat and SOCS box containing protein 2 (WSB2)
induced polyubiquitination and degradation of p53, while
simultaneously downregulating the expression of Bax and IGFBP3, and
promoted the phosphorylation of AKT and mTOR, thereby facilitating
tumor progression. Furthermore, when Li et al (52) targeted and inhibited mTOR expression
with everolimus, a blockade of tumorigenesis and metastasis of HCC
mediated by WSB2 was observed in vivo. These results further
suggested that WSB2 upregulation contributed to HCC tumorigenesis
and metastasis via the ubiquitin-mediated degradation of wild-type
p53 and the activation of the IGFBP3-AKT/mTOR axis (Fig. 1, Part 6). However, in HCC cells
harboring mutant p53, high WSB2 expression did not markedly impact
the IGFBP3-AKT-mTOR signaling pathway (53). Additionally, Hanafusa et al
(54) reported that the reduction
in IGFBP3 expression in human HCC cells was due to hypermethylation
of the IGFBP3 gene promoter. The decrease in IGFBP3 expression
resulted in a relative increase in IGF-1 levels. Upon binding to
IGF-1 receptor (IGF-1R), the PI3K-AKT signaling pathway was
activated, which promoted the occurrence and metastasis of HCC.
Conversely, the introduction of exogenous IGFBP3 inhibited the
activation of the PI3K-AKT signaling pathway, thereby suppressing
the proliferation of HCC cells (55–57).
On the contrary, studies have indicated that galectin-3 activated
the PI3K-AKT-GSK-3β-β-catenin signaling pathway in HCC cases
characterized by elevated galectin-3 expression (58). Activated β-catenin directly enhanced
the expression of IGFBP3 and vimentin, which induced angiogenesis
and EMT-driven tumor metastasis, and ultimately impacted patient
survival (58,59) (Fig. 1,
Part 7). The dual role of IGFBP3 in both inhibition of
tumorigenesis and promotion of cancer within the same HCC tumor may
be attributed to several factors. First, the effects of IGFBP3 vary
depending on its localization. Previous studies by Li et al
(52) and Alexia et al
(57) have demonstrated that
extracellular IGFBP3 primarily inhibited tumorigenesis by
preventing the binding of IGF-1 to IGF-1R. Conversely, the studies
by Song et al (58) have
focused on the intracellular effects of IGFBP3. Second, Li et
al (52) and Song et al
(58) have identified distinct key
pathways influenced by IGFBP3. In the study conducted by Li et
al (52), IGFBP3 was primarily
involved in the PI3K-AKT-mTOR pathway. Conversely, Song et
al (58) found that IGFBP3
mainly participates in the regulation of the
PI3K-Akt-GSK-3β-β-catenin -IGFBP3 pathway in HCC. The human body
functions as a complex system and tumor development may entail the
activation of multiple signaling pathways. Consequently, IGFBP3 may
interact with various pathways at different stages of tumor
development and lead to divergent outcomes.
Breast cancer
Breast cancer is a prevalent type of tumor among
women and is the second most common cause of cancer-related
mortality globally (60). Based on
histological characteristics, breast cancer can be classified into
three subtypes: ER-positive, HER2-positive and triple-negative.
Currently, chemotherapy, hormonal therapies and targeted therapies
represent the primary treatment options for breast cancer. While
these methods have demonstrated effectiveness in reducing mortality
rates-e.g., due to these treatments, in 2016, the breast cancer
mortality rate in the European Union decreased by ~8%-notable
challenges such as drug toxicity and resistance remain (61). Therefore, further research is
warranted to explore novel treatments and enhance patient outcomes.
Previous studies have indicated that the expression levels of
IGFBP3 were reduced in both ER-positive and HER2-positive breast
cancer cells (18,19,62).
For instance, in ER-positive breast cancer cells, IGFBP3 expression
prevents the phosphorylation of cell cycle-related proteins,
including Cyclin D, Cyclin A, Cyclin E, CDK2, CDK4 and pRB
(18,19). Unphosphorylated pRB forms a
heterodimer with E2 promoter binding factor (E2F) in cells, which
suppresses E2F activity and obstructs the G1/S
transition of the cell cycle, and consequently exerts a tumor
suppressor effect (18). However,
CDKs counteract the effect by phosphorylating pRB, which leads to
the dissociation of pRB from the pRB/E2F heterodimer, thereby
abolishing the inhibitory effect on E2F, and ultimately promoting
the G1/S transition of the cell cycle and cell
proliferation (18,19). Consequently, IGFBP3 inhibits the
activity of CDK2 and CDK4, reduces the phosphorylation of pRB and
induces cell cycle arrest in the G1 phase of ER-positive
breast cancer cells, and inhibits cell proliferation (Fig. 1, Part 8). Additionally, IGFBP3
interacts with the transmembrane protein 219 (TMEM219) receptor
located on the cell surface. The interaction results in the
phosphorylation and activation of procaspase-8, which binds to
TMEM219 during the resting phase and initiates the death
receptor-mediated apoptotic pathway (63). This regulation also impacts the
expression of Bax and Bcl-2, which promotes apoptosis in breast
cancer cells (30,64,65)
(Fig. 1, Part 9). In
trastuzumab-resistant HER2-positive breast cancer cells, the
upregulation of Cullin7 had dual effects (62). On one hand, Cullin7 promoted the
degradation of insulin receptor substrate-1 (IRS-1) phosphorylated
at the serine position and induced the accumulation of
phosphorylated tyrosine IRS-1 within the cells. The accumulation
subsequently phosphorylated and activated downstream proteins with
SRC homolog 2 domains such as PI3K. On the other hand, Cullin7
inhibited the expression of IGFBP3, which activated the IGF-1R
signaling pathway. IGFBP3 is known as a Wnt signaling inhibitor
that suppresses the transcription of β-catenin and downstream
factors such as transcription factor 7-like 2 (TCF7L2). TCF7L2
binds to the TCF/lymphoid enhancer-binding factor-1 binding motif
within the Cullin7 promoter, and enhances Cullin7 transcription.
Consequently, in trastuzumab-resistant HER2-positive breast cancer
cells, high expression levels of Cullin7 activated the PI3K-AKT
signaling pathway by suppressing IGFBP3 expression, which promoted
resistance to trastuzumab treatment (62) (Fig. 1,
Part 7). By contrast, in triple-negative breast cancer, the
expression levels of both IGFBP3 and sphingosine kinase-1 (SphK1)
are elevated. The increased activity of SphK1 catalyzes the
conversion of sphingosine in the cell membrane to
sphingosine-1-phosphate (S1P). S1P subsequently mediates the
enhancement of the EGFR signaling pathway by IGFBP3 in breast
cancer cells, which leads to the phosphorylation of ERK1/2 and AKT.
The process enhances the invasiveness and proliferative capacity of
breast cancer cells, ultimately affecting patient prognosis
(20,21,66)
(Fig. 1, Part 12 and 13). In
summary, the processes of tumorigenesis and progression are
determined by the activation of key signaling pathways within
cells. In breast cancer, the diverse functions of IGFBP3 may be
significantly affected by tumor cell phenotypes and genetic
markers. For example, in ER-positive breast cancer cells, IGFBP3
inhibits cell proliferation through regulating cell cycle-related
proteins. In triple-negative breast cancer, however, it promotes
cancer cell invasiveness and proliferation.
Ovarian cancer
In ovarian cancer, the expression levels of IGFBP3
are notably reduced (67,68). High expression levels of IGFBP3
inhibit tumor cell proliferation and angiogenesis. In the absence
of blood vessels, tumors become hypoxic, which activates
hypoxia-inducible factor-1α (HIF-1α) in the early stages of
hypoxia. HIF-1α promotes angiogenesis and stimulates the synthesis
of IGFBP3. Increased IGFBP3 levels subsequently upregulate
thrombospondin-1 (69), which in
turn inhibits tumor angiogenesis and exacerbates tumor hypoxia.
Prolonged hypoxia leads to methylation and silencing of the IGFBP3
promoter, which results in the activation of hypoxia-inducible
factor-2α (HIF-2α). This cascade accelerates the progression of
ovarian cancer, increases resistance to chemotherapy, and enhances
tumor invasion and metastasis (68–72).
Concurrently, previous studies (67,73)
have demonstrated that microRNA (miR)-19a-3p was markedly
upregulated in ovarian cancer. miR-19a-3p binds to the 3′
untranslated region of IGFBP3, thereby inhibiting IGFBP3 expression
(67). The inhibitory effect of
miR-19a-3p further obstructs the binding of IGFBP3 to IGF-1 and
activates the IGF-1/IGF-1R/PI3K-AKT signaling pathway. The
activation of the IGF-1/IGF-1R/PI3K-AKT signaling pathway promotes
the expression of enzymes associated with aerobic glycolysis, such
as pyruvate kinase M2, lactate dehydrogenase A, glucose transporter
1 and glucose transporter 3, which facilitates the proliferation
and metastasis of tumor cells, and promotes the progression of
ovarian cancer (Fig. 1, Part 10).
In summary, current research suggests that IGFBP3 serves a key role
in inhibiting the occurrence and progression of ovarian cancer and
is closely linked to the tumor hypoxic microenvironment and the
activation of the IGF-1/IGF-1R/PI3K-AKT signaling pathway. Further
research on IGFBP3 may potentially provide valuable insights for
the prevention and treatment of ovarian cancer in the future.
Clear cell renal cell carcinoma
IGFBP3 has been reported to be upregulated in clear
cell renal cell carcinoma (74–76).
Liu et al (76) demonstrated
that IGFBP3 enhanced the activation of the PI3K/AKT and MAPK
signaling pathways, which led to the downregulation of E-cadherin,
while upregulating N-cadherin and vimentin. This modulation
influenced processes such as proliferation, angiogenesis and
apoptosis, which ultimately promoted the progression of clear cell
renal cell carcinoma (Fig. 1, Part
1). Similarly, Rosendahl et al (77) showed that IGFBP3 promoted tumor cell
proliferation in Caki-2 cells derived from renal cell carcinoma by
triggering the TGF-β1 signaling pathway. Therefore, targeting
IGFBP3 expression in clear cell renal cell carcinoma and inhibiting
the activation of these pathways could potentially provide a
promising therapeutic approach for the management of these tumors
in the future.
Prostate cancer
The expression levels of IGFBP3 are reduced in the
serum of patients with prostate cancer, with a gradual decrease
observed as the tumor progresses (78,79). A
study conducted by Boyle et al (80) reported that 1,25-hydroxyvitamin D
(vitamin D) impedes the proliferation of prostate cancer cells.
Vitamin D induces biological functions such as inhibiting the
proliferation, invasion and metastasis of tumor cells (81,82)
through the vitamin D receptor (81). Upon binding with vitamin D, this
receptor activates target genes, including IGFBP3, which is known
to contain a vitamin D receptor element. The activation of target
genes led to the upregulation of cell cycle regulatory proteins
such as p21/WAF1, ultimately halting cell proliferation in the
G0/G1 phase (80). Short-term exposure to vitamin D
resulted in sustained secretion of IGFBP3 (28,83,84).
Furthermore, the expression of NK3 homeobox 1 (NKX3.1), a
prostate-specific homeobox gene, key for prostate development and
maturation, is often downregulated in primary prostate cancer
tissues (85). Upregulation of
NKX3.1 in prostate cancer cells increased IGFBP3 expression, which
in turn hindered the phosphorylation of IGF-1R, IRS-1, PI3K and AKT
induced by IGF-1, and inhibited prostate cancer cell proliferation.
Conversely, inhibition of IGFBP3 expression in prostate cancer
cells could reverse the inhibitory effect of NKX3.1 on IGF-1R
signaling (86,87). The results suggested that the
inhibitory effect of NKX3.1 on tumor growth was mediated through
the activation of IGFBP3 expression (87). Additionally, previous studies have
demonstrated that the NF-κB signaling pathway is implicated in
prostate cancer. The activation of NF-κB and its translocation to
the nucleus upregulated the expression of various factors,
including VEGF, IL-8, intercellular adhesion molecule-1, vascular
cell adhesion molecule-1 (VCAM-1) and prostate-specific antigen,
and impacted the survival, adhesion, inflammation, proliferation
and angiogenesis of prostate cancer cells (88–90)
(Fig. 1, Part 11). Furthermore,
IGFBP3 expression triggers the apoptotic pathway by binding to the
TMEM219 receptor on the cell surface. Elevated expression levels of
caspase-3 facilitate the degradation of IκBα and NF-κB, inhibiting
the activation of the NF-κB signaling pathway and impeding the
progression of prostate cancer (91,92)
(Fig. 1, Part 9). In summary,
IGFBP3 acts as a tumor suppressor in prostate cancer by hindering
the binding of IGF-1 to IGF-1R, blocking the activation of the
PI3K-AKT-NF-κB signaling pathway, or directly engaging with the
cell membrane receptor TMEM219 to initiate the apoptotic pathway,
which restrains tumor initiation and progression.
Osteosarcoma
Osteosarcoma, a highly aggressive and metastatic
malignant bone tumor, predominantly affects adolescents and
children, with >70% of cases occurring within this demographic
(93). The tumor typically arises
in the metaphysis of long bones. At the time of diagnosis, 15–20%
of patients exhibit visible metastatic lesions, with lung
metastasis being the most prevalent symptom (94). Previous studies (95–97)
have indicated that in children and adolescents with osteosarcoma,
the expression levels of IGFBP3 and KNF motif and ankyrin repeat
domains 1 (KANK1) are markedly lower compared with those in normal
tissues, while TRAF interacting protein (TRAIP) expression is
upregulated. Elevated expression levels of TRAIP have been
associated with decreased metastasis-free survival and overall
survival rates in patients with osteosarcoma. TRAIP functions as an
E3 ubiquitin ligase in osteosarcoma, which facilitates the
degradation of KANK1 through ubiquitination and leads to reduced
expression levels of IGFBP3, the downstream target gene of KANK1.
This mechanism results in the phosphorylation of AKT and the
subsequent activation of the AKT signaling pathway, which promotes
osteosarcoma invasion and metastasis (96,97)
(Fig. 1, Part 10). Additionally,
Schedlich et al (98)
reported that elevated expression levels of TGF-β1 in the initial
phases of osteosarcoma can enhance IGFBP3 expression. Elevated
IGFBP3 expression, in turn, inhibit the activation of the MAPK
pathway and the phosphorylation of SMAD2, induced by TGF-β1, and
hinder the proliferation and invasion of tumor cells. However,
previous studies have also demonstrated that the expression levels
of IGFBP3 in osteosarcoma tissues were notably higher compared with
those in normal tissues (99,100).
For example, Chao et al (100) identified that elevated expression
levels of IGFBP3 could facilitate the nuclear translocation of the
transcription activator c-Jun via the PI3K-AKT signaling pathway,
which led to increased VCAM-1 expression and myeloma cell
metastasis, and impacted patient prognosis. The contrasting
outcomes may be attributed to two main factors: i) In the study
conducted by Chao et al (100), the osteosarcoma cell lines MG63
and U2OS were treated with exogenous IGFBP3. The effects of IGFBP3
on cell migration were evaluated using Transwell assays, wound
healing assays and western blot analysis. Conversely, Li et
al (96) utilized small
interfering RNA (siRNA) to target and inhibit the expression of
endogenous IGFBP3 in MG63 and MNNG/HOS cells. They assessed the
expression and phosphorylation levels of the AKT protein, which are
associated with cell invasion and proliferation, through western
blotting techniques; and ii) IGFBP3 plays a critical role in
regulating tumor cell functions in osteosarcoma through various
signaling pathways. For instance, Chao et al (100) demonstrated that IGFBP3 can
activate the PI3K-AKT-c-Jun-VCAM-2 signaling pathway, which
promotes tumor cell metastasis and consequently impacts patient
prognosis. Conversely, research by Li et al (96) revealed that IGFBP3, as a key protein
in the TRAIP-KANK1-IGFBP3-PI3K-AKT signaling pathway, has its
expression inhibited by the TRAIP protein, ultimately leading to
tumor cell invasion and metastasis. The detailed signaling pathways
involved are illustrated in Fig.
1.
Other tumors
Previous studies have demonstrated that IGFBP3
notably contributes to the occurrence and progression of other
tumors. For instance, in cervical cancer and papillary thyroid
carcinoma, elevated expression levels of IGFBP3 enhanced tumor cell
metastasis by facilitating the formation of lymphatic vessels
(101,102). In nasopharyngeal carcinoma, the
upregulation of IGFBP3 led to the downregulation of E-cadherin and
the upregulation of N-cadherin, thereby promoting metastasis and
invasion (24). Conversely, IGFBP3
is downregulated in certain tumors, such as pancreatic cancer,
retinoblastoma and diffuse metastatic melanoma. In pancreatic
cancer, increased IGFBP3 expression inhibited the PI3K-mTOR
signaling pathway, and impeded cancer cell proliferation (23). Similarly, the upregulation of IGFBP3
in retinoblastoma Y79 cells counteracted the anti-apoptotic effects
of IGF-1 (103). Additionally,
previous studies (104–106) have shown that in diffuse
metastatic melanoma, IGFBP3 dephosphorylated GSK-3β, which led to
the degradation of cytoplasmic β-catenin and subsequent inhibition
of the Wnt signaling pathway, and resulted in reduced tumor cell
migration and invasion (104).
Post-translational modifications of IGFBP3:
Impacts on tumorigenesis and development
Current research has indicated that IGFBP3 serves a
key role in the initiation and progression of tumors. However, the
functional efficacy of IGFBP3 is influenced by variations in
expression levels and post-translational modifications, which
notably affect IGFBP3 functionality. The primary post-translational
modifications of IGFBP3 include glycosylation, phosphorylation and
proteolysis. Glycosylation, an important modification, enhances the
stability of IGFBP3; however, its role varies across different
tumor types (11,107–113). In melanoma, glycosylated IGFBP3 is
cleaved by proteases, which results in decreased affinity for IGF-1
and increased release of free IGF-1, and ultimately promotes tumor
cell proliferation (11). In
patients that succumbed to breast cancer, glycosylated IGFBP3
retained its binding capacity to IGF-1; however, the ability to
bind to the cell surface was inferior compared with that of the
non-glycosylated form, which potentially affected the localization
and function of IGFBP3 within the tumor microenvironment (107). Phosphorylation also serves a role
in regulating the function of IGFBP3. For example, in head and neck
squamous cell carcinoma and prostate cancer, the phosphorylation
and subsequent degradation of IGFBP3 mediated by protein kinase C
and CK2 inhibits tumor cell apoptosis, thereby promoting tumor
progression (12,114). In NSCLC, CK2 phosphorylates
IGFBP3, which obstructs the interaction between IGFBP3 and
hyaluronic acid, and activates the hyaluronic acid-CD44 signaling
pathway, leading to increased tumor cell survival and enhanced
resistance to cisplatin (107). In
terms of proteolytic modification, specific proteases such as
ADAM12 and PAPP-A2 degrade IGFBP3 through proteolysis, thereby
reducing IGFBP3 expression levels and releasing IGF-1, which
promotes fetal or child growth and development (113,115–117). Additionally, previous studies have
demonstrated that in colitis-associated colorectal cancer and
breast cancer, serine proteases and matrix metalloproteinase-7
facilitated the proteolysis of IGFBP3, which inhibited IGFBP3
function, and consequently promoted tumor initiation and
progression (108–113).
In summary, post-translational modifications of
IGFBP3 notably influence tumor initiation and development. Various
types of post-translational modifications affect the function of
IGFBP3 through complex and diverse mechanisms, exerting notable
effects on the biological behaviors of tumor cells. For instance,
in NSCL, CK2 can induce the phosphorylation of IGFBP3, thereby
inhibiting its interaction with hyaluronic acid. This process
activates the HA-CD44 pathway, leading to tumor cell proliferation
and the development of cisplatin resistance (107). In melanoma, glycosylated IGFBP3
can be cleaved by proteases, resulting in decreased levels of
IGFBP3, which activates the Akt-GSK3β axis and ultimately promotes
cell proliferation (10). However,
current research is focusing on the post-translational
modifications of IGFBP3, with numerous specific molecular
mechanisms yet to be fully elucidated. Further research is
necessary to investigate the detailed mechanisms of IGFBP3
post-translational modifications in different tumors, as well as
the relationships between these modifications, the clinical
characteristics and prognosis of tumors, to provide a more robust
theoretical basis and potential therapeutic targets for the precise
diagnosis and treatment of tumors in the future.
IGFBP3 and cancer: Novel perspectives on
precision treatment and clinical challenges
IGFBP3 plays a complex and diverse role in the
processes of tumorigenesis and tumor development. It can influence
various aspects of cancer cells, including proliferation,
apoptosis, invasion and metastasis, through different mechanisms.
This complexity offers new opportunities for developing targeted
cancer therapies. The expression and function of IGFBP3 vary
significantly among different tumor types. These differences can be
utilized to design patient-stratified treatment strategies. For
instance, in clear cell renal carcinoma, elevated IGFBP3 expression
is associated with increased invasion and metastasis of tumor cells
(74–76). Liu et al (27), through spatial conformation
analysis, demonstrated that cyclovirobuxines formed hydrogen bonds
with two amino acid residues, GLU-64 and GLU-86, in the IGFBP3
molecule. These hydrogen bonds inhibited IGFBP3 function. The
interaction disrupted the IGFBP3-AKT/STAT3/MAPK-Snail signaling
pathway, which ultimately hindered the progression of clear cell
renal carcinoma. In the context of glioblastoma, Chen et al
(118) utilized
convection-enhanced delivery to administer IGFBP3 small interfering
RNA into the brains of mice, which successfully inhibited
intracranial tumor growth and extended the survival of the mice.
For patients exhibiting high IGFBP3 expression, targeted inhibitors
can be developed to mitigate IGFBP3 function and effectively
prevent tumor progression. Conversely, for patients with low IGFBP3
expression, treatment strategies aimed at enhancing IGFBP3
expression, such as gene therapy or pharmacological activation of
relevant signaling pathways, like the vitamin D signaling pathway
and the p53 signaling pathway, can be explored to amplify the
antitumor effects of IGFBP3 (28,52,83,84).
For instance, in the research on gastric cancer, it has been found
that infecting gastric cancer cells with lentiviruses carrying
IGFBP3 can effectively upregulate the expression level of IGFBP3 in
the cells. The upregulated IGFBP3 can inhibit the activity of NF-κB
in gastric cancer cells, thereby significantly enhancing the cell
growth inhibition effect induced by etoposide (119).
Findings from previous studies (78,120,121) regarding IGFBP3-related signaling
pathways hold notable promise for clinical translation. The
detection of IGFBP3 and its downstream signaling molecules in tumor
tissues or blood is key for the diagnosis and prognostic evaluation
of patients with cancer. Previous studies have indicated that in
the peripheral blood of patients with tumors, such as prostate
cancer and colorectal cancer, the concentration of IGFBP3 was
reduced compared with that in healthy individuals. This reduction
was associated with a higher metastasis rate and poorer prognosis
(78,120,121). Conversely, some studies have
suggested that in certain tumors, elevated expression levels of
IGFBP3 may promote tumor progression and decrease patient survival
rates. For instance, in the peripheral blood of individuals with
OSCC and glioblastoma, IGFBP3 expression levels were markedly
higher compared with those in the control group (43,122).
Thus, in OSCC and glioblastoma, IGFBP3 expression can serve as a
biomarker for the assessment of tumor development stages and
patient prognosis (43,122). Regarding drug development for
cancer treatment, although some drugs have been utilized to target
tumors by directly or indirectly modulating IGFBP3 expression, the
variable effects and associated signaling pathways of IGFBP3 across
different tumors pose challenges for clinical translation and drug
selection. For example, drug specificity is key to ensure that
drugs precisely target IGFBP3-related signaling pathways while
minimizing adverse effects on normal cells. Additionally, prolonged
use of targeted therapies may lead to the development of drug
resistance in tumor cells, thereby diminishing treatment efficacy
(123–125). Therefore, comprehensive research
on the aforementioned issues and the pursuit of effective solutions
are essential to potentially achieve clinical translation in the
future.
IGFBP3 exhibits dual functions across various cancer
types, such as ovarian cancer and HCC. These cancers which are
influenced by the tumor microenvironment and genetic background.
Factors such as hypoxia and inflammation within the tumor
microenvironment modulate IGFBP3 activity (68–72,126).
In ovarian cancer, during the initial hypoxic phase, elevated
levels of HIF-1α promote IGFBP3 synthesis, which inhibits tumor
angiogenesis and hinders tumor progression. However, as hypoxia
intensifies, increased HIF-2α induces methylation of the IGFBP3
gene promoter and adversely affects IGFBP3 function (68–72).
From a genetic standpoint, mutations in genes across different
tumor cells can alter the role of IGFBP3. For example, in liver
cancer cells with wild-type p53, high WSB2 expression enhances the
polyubiquitination and degradation of p53, which leads to the
downregulation of IGFBP3 expression, and promotion of AKT and mTOR
phosphorylation, and ultimately accelerates HCC progression.
Conversely, in HCC with mutant p53, WSB2 expression does not
promote the progression of HCC as it does in the case of wild-type
p53 (126–128). The aforementioned differences
underline the importance of developing personalized treatment plans
to potentially improve therapeutic outcomes in the future.
In summary, a comprehensive exploration of the
mechanisms underlying IGFBP3 expression in tumors, the clinical
translation of the associated signaling pathways and the factors
influencing the dual function of IGFBP3 is key to further the
current understanding of tumorigenesis and progression, and provide
theoretical bases for the development of more effective treatment
strategies in the future. Future research on integrating research
with clinical practice is warranted to potentially enhance the
application of IGFBP3-related research in cancer therapy in the
future.
Summary and outlook
IGFBP3, an important protein in the human body, has
garnered notable attention in recent years due to its role in
various diseases, particularly in tumorigenesis and tumor
development. Numerous studies have demonstrated that alterations in
IGFBP3 expression and its post-translational modifications are
closely associated with the progression, treatment efficacy and
prognosis of multiple tumors. For instance, in OSCC and TSCC, the
upregulated expression of IGFBP3 promotes the proliferation,
invasion and metastasis of tumor cells through signaling pathways
such as the MEK/ERK pathway. In colorectal cancer, the methylation
of the IGFBP3 gene promoter leads to a decrease in its expression.
Upregulating its expression can enhance the effect of TRAIL,
inhibit the NF-κB signaling pathway, and suppress tumor development
(43,46–48).
The present review systematically summarizes the role of IGFBP3 in
the occurrence and development of different tumors, as well as the
cellular signaling pathways involving IGFBP3. Although the present
review has reported changes in IGFBP3 expression in tumors such as
clear cell renal cell carcinoma, ovarian cancer and colorectal
cancer, and the impact on tumor cell metastasis, studies on the
underlying molecular mechanisms and signaling pathways are
considerably less comprehensive compared with studies on liver and
breast cancer. The disparity in research depth primarily stems from
the availability of existing data and the varying clinical
significance of different tumors. To achieve a more comprehensive
understanding of the role of IGFBP3 in tumorigenesis and
development, and to establish a theoretical foundation for targeted
therapies, future research is warranted to address the current gaps
and investigate the mechanisms underlying the role of IGFBP3 in
understudied cancer types.
In conclusion, conducting in-depth research on the
expression level changes of IGFBP3 in tumors and other diseases, as
well as IGFBP3 regulation via post-translational modifications,
holds notable scientific and clinical value. From a scientific
perspective, the present review provides further understanding of
the specific mechanisms by which IGFBP3 contributes to the onset
and progression of cancer. Clinically, the present review
highlights the identification of novel diagnostic markers, the
discovery of potential therapeutic targets and the development of
innovative strategies for disease prevention. Therefore, the
present review facilitates the establishment of a more
comprehensive disease management system, which offers novel
insights and methodologies to address related diseases.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
Not applicable.
Authors' contributions
YZ wrote the first draft. XL reviewed and revised
the draft. Data authentication is not applicable. All authors have
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IGFBP3
|
insulin-like growth factor binding
protein 3
|
|
EMT
|
epithelial-mesenchymal transition
|
|
SRC
|
Rous sarcoma virus oncogene
homolog
|
|
TRAIL
|
TNF-related apoptosis-inducing
ligand
|
|
WSB2
|
WD repeat and SOCS box containing
protein 2
|
|
pRB
|
retinoblastoma protein
|
|
E2F
|
E2 promoter binding factor
|
|
TMEM219
|
transmembrane protein 219
|
|
HIF
|
hypoxia-inducible factor
|
|
miR-19a-3p
|
microRNA-19a-3p
|
|
IGF-1
|
insulin-like growth factor-1
|
|
IGF-1R
|
IGF-1 receptor
|
|
VCAM-1
|
vascular cell adhesion molecule-1
|
|
TCF7L2
|
transcription factor 7-like 2
|
|
KANK1
|
KNF motif and ankyrin repeat domains
1
|
|
TRAIP
|
TRAF-interacting protein
|
References
|
1
|
Bray F, Laversanne M, Sung H, Ferlay J,
Siegel RL, Soerjomataram I and Jemal A: Global cancer statistics
2022: Globocan estimates of incidence and mortality worldwide for
36 cancers in 185 countries. CA Cancer J Clin. 74:229–263. 2024.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sun Y, Ai X, Shen S, Gu L and Lu S:
Detection and correlation analysis of serum cytokines in
non-small-cell lung cancer patients with bone and non-bone
metastases. Patient Prefer Adherence. 9:1165–1169. 2015.PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2020. CA Cancer J Clin. 70:7–30. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Soerjomataram I, Cabasag C, Bardot A,
Fidler-Benaoudia MM, Miranda-Filho A, Ferlay J, Parkin DM,
Ranganathan R, Piñeros M, Znaor A, et al: Cancer survival in
Africa, Central and South America, And Asia (survcan-3): A
population-based benchmarking study in 32 countries. Lancet Oncol.
24:22–32. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cai Q, Dozmorov M and Oh Y:
IGFBP-3/IGFBP-3 receptor system as an anti-tumor and
anti-metastatic signaling in cancer. Cells. 9:12612020. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
MacParland SA, Liu JC, Ma XZ, Innes BT,
Bartczak AM, Gage BK, Manuel J, Khuu N, Echeverri J, Linares I, et
al: Single cell RNA sequencing of human liver reveals distinct
intrahepatic macrophage populations. Nat Commun. 9:43832018.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yaqoob U, Luo F, Greuter T, Sakrikar NJ,
Sehrawat TS, Lu J, Hu X, Gao J, Kostallari E, Chen J, et al:
GIPC-regulated IGFBP-3 promotes HSC migration in vitro and portal
hypertension in vivo through a β1-integrin pathway. Cell Mol
Gastroenterol Hepatol. 10:545–559. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fujimoto M, Andrew M and Dauber A:
Disorders caused by genetic defects associated with GH-dependent
genes: Pappa2 defects. Mol Cell Endocrinol. 518:1109672020.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yamada PM and Lee KW: Perspectives in
mammalian IGFBP-3 biology: Local vs. systemic action. Am J Physiol
Cell Physiol. 296:C954–C976. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Coleman KL, Chiaramonti M, Haddad B,
Ranzenberger R, Henning H, Al Khashali H, Ray R, Darweesh B,
Guthrie J, Heyl D and Evans HG: Phosphorylation of IGFBP-3 by
casein kinase 2 blocks its interaction with hyaluronan, enabling
HA-CD44 signaling leading to increased NSCLC cell survival and
cisplatin resistance. Cells. 12:4052023. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Panasiti V, Naspi A, Devirgiliis V, Curzio
M, Roberti V, Curzio G, Gobbi S, Calvieri S and Londei P:
Correlation between insulin-like growth factor binding protein-3
serum level and melanoma progression. J Am Acad Dermatol.
64:865–872. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cobb LJ, Mehta H and Cohen P: Enhancing
the apoptotic potential of insulin-like growth factor-binding
protein-3 in prostate cancer by modulation of CK2 phosphorylation.
Mol Endocrinol. 23:1624–1633. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bernstein HG, Keilhoff G, Dobrowolny H,
Lendeckel U and Steiner J: From putative brain tumor marker to high
cognitive abilities: Emerging roles of a disintegrin and
metalloprotease (ADAM) 12 in the brain. J Chem Neuroanat.
109:1018462020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fujimoto M, Hwa V and Dauber A: Novel
modulators of the growth hormone-insulin-like growth factor axis:
Pregnancy-associated plasma protein-A2 and stanniocalcin-2. J Clin
Res Pediatr Endocrinol. 9 (Suppl 2):S1–S8. 2017.
|
|
15
|
Cheng H, Fertig EJ, Ozawa H, Hatakeyama H,
Howard JD, Perez J, Considine M, Thakar M, Ranaweera R, Krigsfeld G
and Chung CH: Decreased SMAD4 expression is associated with
induction of epithelial-to-mesenchymal transition and cetuximab
resistance in head and neck squamous cell carcinoma. Cancer Biol
Ther. 16:1252–1258. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yang L, Li J, Fu S, Ren P, Tang J, Wang N,
Shi X, Wu J and Lin S: Up-regulation of insulin-like growth factor
binding protein-3 is associated with brain metastasis in lung
adenocarcinoma. Mol Cells. 42:321–332. 2019.PubMed/NCBI
|
|
17
|
Liu A, Yu C, Qiu C, Wu Q, Huang C, Li X,
She X, Wan K, Liu L, Li M, et al: PRMT5 methylating SMAD4 activates
TGF-β signaling and promotes colorectal cancer metastasis.
Oncogene. 42:1572–1584. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kim HS, Lee WJ, Lee SW, Chae HW, Kim DH
and Oh Y: Insulin-like growth factor binding protein-3 induces G1
cell cycle arrest with inhibition of cyclin-dependent kinase 2 and
4 in MCF-7 human breast cancer cells. Horm Metab Res. 42:165–172.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sheldon LA: Inhibition of E2F1 activity
and cell cycle progression by arsenic via retinoblastoma protein.
Cell Cycle. 16:2058–2072. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Martin JL, de Silva HC, Lin MZ, Scott CD
and Baxter RC: Inhibition of insulin-like growth factor-binding
protein-3 signaling through sphingosine kinase-1 sensitizes
triple-negative breast cancer cells to EGF receptor blockade. Mol
Cancer Ther. 13:316–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Nagahashi M and Miyoshi Y: Targeting
sphingosine-1-phosphate signaling in breast cancer. Int J Mol Sci.
25:33542024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kuhn H, Frille A, Petersen MA,
Oberhuber-Kurth J, Hofmann L, Gläser A, Taubenheim S, Klagges S,
Kraemer S, Broschewitz J, et al: IGFBP3 inhibits tumor growth and
invasion of lung cancer cells and is associated with improved
survival in lung cancer patients. Transl Oncol. 27:1015662023.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jang HJ, Lee S, Hong E, Yim KJ, Choi YS,
Jung JY and Kim ZH: Mychonastes sp. 246 suppresses human pancreatic
cancer cell growth via IGFBP3-PI3K-mtor signaling. J Microbiol
Biotechnol. 33:449–462. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Bao L, Liu H, You B, Gu M, Shi S, Shan Y,
Li L, Chen J and You Y: Overexpression of IGFBP3 is associated with
poor prognosis and tumor metastasis in nasopharyngeal carcinoma.
Tumour Biol. 37:15043–15052. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kim D, Park YJ and Hwang YS: Regulation of
tumor growth and invasion in oral squamous cell carcinoma by the
tumor microenvironment through the enhancement of IGFBP-3
expression. Anticancer Res. 44:5337–5349. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Luo Y, Hong CQ, Huang BL, Ding TY, Chu LY,
Zhang B, Qu QQ, Li XH, Liu CT, Peng YH, et al: Serum insulin-like
growth factor binding protein-3 as a potential biomarker for
diagnosis and prognosis of oesophageal squamous cell carcinoma. Ann
Med. 54:2153–2166. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Liu Y, Lv H, Li X, Liu J, Chen S, Chen Y,
Jin Y, An R, Yu S and Wang Z: Erratum: Cyclovirobuxine inhibits the
progression of clear cell renal cell carcinoma by suppressing the
IGFBP3-AKT/STAT3/MAPK-snail signalling pathway: Erratum. Int J Biol
Sci. 20:6279–6280. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Igarashi K, Yui Y, Watanabe K, Kumai J,
Nishizawa Y, Miyaura C, Inada M and Sasagawa S: Molecular evidence
of IGFBP-3 dependent and independent VD3 action and its nonlinear
response on IGFBP-3 induction in prostate cancer cells. BMC Cancer.
20:8022020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yoshino K, Motoyama S, Koyota S, Shibuya
K, Usami S, Maruyama K, Saito H, Minamiya Y, Sugiyama T and Ogawa
J: IGFBP3 and BAG1 enhance radiation-induced apoptosis in squamous
esophageal cancer cells. Biochem Biophys Res Commun. 404:1070–1075.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Mofid MR, Gheysarzadeh A and Bakhtiyari S:
Insulin-like growth factor binding protein 3 chemosensitizes
pancreatic ductal adenocarcinoma through its death receptor.
Pancreatology. 20:1442–1450. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
He Y, Luo W, Liu Y, Wang Y, Ma C, Wu Q,
Tian P, He D, Jia Z, Lv X, et al: IL-20RB mediates tumoral response
to osteoclastic niches and promotes bone metastasis of lung cancer.
J Clin Invest. 132:e1579172022. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo Q, Shi W, Dou B, Wang J, Peng W, Liu
X, Zhao D, Tang F, Wu Y, Li X, et al: XBP1-IGFBP3 signaling pathway
promotes NSCLC invasion and metastasis. Front Oncol. 11:6549952021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Gharib TG, Chen G, Huang CC, Misek DE,
Iannettoni MD, Hanash SM, Orringer MB and Beer DG: Genomic and
proteomic analyses of vascular endothelial growth factor and
insulin-like growth factor-binding protein 3 in lung
adenocarcinomas. Clin Lung Cancer. 5:307–312. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Xia T, Tong S, Fan K, Zhai W, Fang B, Wang
SH and Wang JJ: XBP1 induces MMP-9 expression to promote
proliferation and invasion in human esophageal squamous cell
carcinoma. Am J Cancer Res. 6:2031–2040. 2016.PubMed/NCBI
|
|
35
|
Chen S, Chen J, Hua X, Sun Y, Cui R, Sha J
and Zhu X: The emerging role of XBP1 in cancer. Biomed
Pharmacother. 127:1100692020. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Han JY, Choi BG, Choi JY, Lee SY and Ju
SY: The prognostic significance of pretreatment plasma levels of
insulin-like growth factor (IGF)-1, IGF-2, and IGF binding
protein-3 in patients with advanced non-small cell lung cancer.
Lung Cancer. 54:227–234. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Meek CL, Wallace AM, Forrest LM and
McMillan DC: The relationship between the insulin-like growth
factor-1 axis, weight loss, an inflammation-based score and
survival in patients with inoperable non-small cell lung cancer.
Clin Nutr. 29:206–209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Z L: Analysis of the levels of IGF1,
IGFBP3 and VEGF in the serum of patients with non-small cell lung
cancer and their correlation with clinicopathological features.
Soochow university. 2017.
|
|
39
|
Zhong LP, Yang X, Zhang L, Wei KJ, Pan HY,
Zhou XJ, Li J, Chen WT and Zhang ZY: Overexpression of insulin-like
growth factor binding protein 3 in oral squamous cell carcinoma.
Oncol Rep. 20:1441–1447. 2008.PubMed/NCBI
|
|
40
|
Xu HY, Zhu DW, Zhong LP, Zhang ZY, Yang
CZ, Yang X and Zhang P: Effects of insulin-like growth factor
binding protein 3 on cell growth and tumorigenesis in oral squamous
cell carcinoma. Transl Cancer Res. 8:1709–1717. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Huttenlocher A and Horwitz AR: Integrins
in cell migration. Cold Spring Harb Perspect Biol. 3:a0050742011.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Burrows C, Holly JM, Laurence NJ, Vernon
EG, Carter JV, Clark MA, McIntosh J, McCaig C, Winters ZE and Perks
CM: Insulin-like growth factor binding protein 3 has opposing
actions on malignant and nonmalignant breast epithelial cells that
are each reversible and dependent upon cholesterol-stabilized
integrin receptor complexes. Endocrinology. 147:3484–3500. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Yen YC, Hsiao JR, Jiang SS, Chang JS, Wang
SH, Shen YY, Chen CH, Chang IS, Chang JY and Chen YW: Insulin-like
growth factor-independent insulin-like growth factor binding
protein 3 promotes cell migration and lymph node metastasis of oral
squamous cell carcinoma cells by requirement of integrin β1.
Oncotarget. 6:41837–41855. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mondal S, Adhikari N, Banerjee S, Amin SA
and Jha T: Matrix metalloproteinase-9 (MMP-9) and its inhibitors in
cancer: A minireview. Eur J Med Chem. 194:1122602020. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang D, Gao J, Zhao C, Li S, Zhang D, Hou
X, Zhuang X, Liu Q and Luo Y: Cyclin G2 inhibits oral squamous cell
carcinoma growth and metastasis by binding to IGFBP3 and regulating
the FAK-SRC-STAT signaling pathway. Front Oncol. 10:5605722020.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ng EFY, Kaida A, Nojima H and Miura M:
Roles of IGFBP-3 in cell migration and growth in an endophytic
tongue squamous cell carcinoma cell line. Sci Rep. 12:115032022.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Kumar A, Singh P, Pandey A and Gosipatala
SB: IGFBP3 gene promoter methylation analysis and its association
with clinicopathological characteristics of colorectal carcinoma.
Mol Biol Rep. 47:6919–6927. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Shalmashi H, Safaei S, Zarredar H, Kermani
TA, Hashemzadeh S and Navaz AM: Diagnostic significance of
hypomethylated IGFBP3 and TWIST1 genes in patients with colorectal
cancer. Adv Biomed Res. 12:2412023. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Williams AC, Smartt H, H-Zadeh AM,
Macfarlane M, Paraskeva C and Collard TJ: Insulin-like growth
factor binding protein 3 (IGFBP-3) potentiates trail-induced
apoptosis of human colorectal carcinoma cells through inhibition of
NF-kappaB. Cell Death Differ. 14:137–145. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Chan YT, Zhang C, Wu J, Lu P, Xu L, Yuan
H, Feng Y, Chen ZS and Wang N: Biomarkers for diagnosis and
therapeutic options in hepatocellular carcinoma. Mol Cancer.
23:1892024. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Li X, Zhang CC, Lin XT, Zhang J, Zhang YJ,
Yu HQ, Liu ZY, Gong Y, Zhang LD and Xie CM: Elevated expression of
WSB2 degrades P53 and activates the IGFBP3-AKT-mTOR-dependent
pathway to drive hepatocellular carcinoma. Exp Mol Med. 56:177–191.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Wu D, Yu HQ, Xiong HJ, Zhang YJ, Lin XT,
Zhang J, Wu W, Wang T, Liu XY and Xie CM: Elevated sodium pump α3
subunit expression promotes colorectal liver metastasis via the
P53-PTEN/IGFBP3-AKT-mTOR axis. Front Oncol. 11:7438242021.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Hanafusa T, Shinji T, Shiraha H, Nouso K,
Iwasaki Y, Yumoto E, Ono T and Koide N: Functional promoter
upstream p53 regulatory sequence of IGFBP3 that is silenced by
tumor specific methylation. BMC Cancer. 5:92005. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Aishima S, Basaki Y, Oda Y, Kuroda Y,
Nishihara Y, Taguchi K, Taketomi A, Maehara Y, Hosoi F, Maruyama Y,
et al: High expression of insulin-like growth factor binding
protein-3 is correlated with lower portal invasion and better
prognosis in human hepatocellular carcinoma. Cancer Sci.
97:1182–1190. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Liu QW, Li JY, Zhang XC, Liu Y, Liu QY,
Xiao L, Zhang WJ, Wu HY, Deng KY and Xin HB: Human amniotic
mesenchymal stem cells inhibit hepatocellular carcinoma in
tumour-bearing mice. J Cell Mol Med. 24:10525–10541. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Alexia C, Fallot G, Lasfer M,
Schweizer-Groyer G and Groyer A: An evaluation of the role of
insulin-like growth factors (IGF) and of type-I IGF receptor
signalling in hepatocarcinogenesis and in the resistance of
hepatocarcinoma cells against drug-induced apoptosis. Biochem
Pharmacol. 68:1003–1015. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Song M, Pan Q, Yang J, He J, Zeng J, Cheng
S, Huang Y, Zhou ZQ, Zhu Q, Yang C, et al: Galectin-3 favours
tumour metastasis via the activation of β-catenin signalling in
hepatocellular carcinoma. Br J Cancer. 123:1521–1534. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Playford MP, Bicknell D, Bodmer WF and
Macaulay VM: Insulin-like growth factor 1 regulates the location,
stability, and transcriptional activity of beta-catenin. Proc Natl
Acad Sci USA. 97:12103–12108. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Larkin L: Breast cancer genetics and risk
assessment: An overview for the clinician. Climacteric. 26:229–234.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Harbeck N and Gnant M: Breast cancer.
Lancet. 389:1134–1150. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Qiu N, He YF, Zhang SM, Zhan YT, Han GD,
Jiang M, He WX, Zhou J, Liang HL, Ao X, et al: Cullin7 enhances
resistance to trastuzumab therapy in Her2 positive breast cancer
via degrading IRS-1 and downregulating IGFBP-3 to activate the
PI3K/AKT pathway. Cancer Lett. 464:25–36. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Ingermann AR, Yang YF, Han J, Mikami A,
Garza AE, Mohanraj L, Fan L, Idowu M, Ware JL, Kim HS, et al:
Identification of a novel cell death receptor mediating
IGFBP-3-induced anti-tumor effects in breast and prostate cancer. J
Biol Chem. 285:30233–30246. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Butt AJ, Firth SM, King MA and Baxter RC:
Insulin-like growth factor-binding protein-3 modulates expression
of Bax and Bcl-2 and potentiates p53-independent radiation-induced
apoptosis in human breast cancer cells. J Biol Chem.
275:39174–39181. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Jia Y, Lee KW, Swerdloff R, Hwang D, Cobb
LJ, Hikim AS, Lue YH, Cohen P and Wang C: Interaction of
insulin-like growth factor-binding protein-3 and BAX in
mitochondria promotes male germ cell apoptosis. J Biol Chem.
285:1726–1732. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Pitson SM, Xia P, Leclercq TM, Moretti PA,
Zebol JR, Lynn HE, Wattenberg BW and Vadas MA:
Phosphorylation-dependent translocation of sphingosine kinase to
the plasma membrane drives its oncogenic signalling. J Exp Med.
201:49–54. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Bai R, Cui Z, Ma Y, Wu Y, Wang N, Huang L,
Yao Q and Sun J: The NF-κB-modulated miR-19a-3p enhances malignancy
of human ovarian cancer cells through inhibition of IGFBP-3
expression. Mol Carcinog. 58:2254–2265. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Chen X, Shao C, Liu J, Sun H, Yao B, Ma C,
Xu H and Zhu W: ULK2 suppresses ovarian cancer cell migration and
invasion by elevating IGFBP3. PeerJ. 12:e176282024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Shih HJ, Chen CL and Torng PL: IGFBP3
inhibits angiogenesis through intracellular regulation of thbs1
expression. Am J Cancer Res. 10:1728–1744. 2020.PubMed/NCBI
|
|
70
|
Shih HJ, Chang HF, Chen CL and Torng PL:
Differential expression of hypoxia-inducible factors related to the
invasiveness of epithelial ovarian cancer. Sci Rep. 11:229252021.
View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Feldser D, Agani F, Iyer NV, Pak B,
Ferreira G and Semenza GL: Reciprocal positive regulation of
hypoxia-inducible factor 1alpha and insulin-like growth factor 2.
Cancer Res. 59:3915–3918. 1999.PubMed/NCBI
|
|
72
|
Yan Y, He M, Zhao L, Wu H, Zhao Y, Han L,
Wei B, Ye D, Lv X, Wang Y, et al: A novel HIF-2α targeted inhibitor
suppresses hypoxia-induced breast cancer stemness via
SOD2-mtROS-PDI/GPR78-UPRER axis. Cell Death Differ.
29:1769–1789. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Du L, Dou K, Zhang D, Xia H, Liang N, Wang
N, Sun J and Bai R: MiR-19a-3p promotes aerobic glycolysis in
ovarian cancer cells via IGFBP3/PI3K/AKT pathway. Folia Biol
(Praha). 69:163–172. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Chuang ST, Patton KT, Schafernak KT,
Papavero V, Lin F, Baxter RC, Teh BT and Yang XJ: Over expression
of insulin-like growth factor binding protein 3 in clear cell renal
cell carcinoma. J Urol. 179:445–449. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Braczkowski R, Białożyt M, Plato M,
Mazurek U and Braczkowska B: Expression of insulin-like growth
factor family genes in clear cell renal cell carcinoma. Contemp
Oncol (Pozn). 20:130–136. 2016.PubMed/NCBI
|
|
76
|
Liu Y, Lv H, Li X, Liu J, Chen S, Chen Y,
Jin Y, An R, Yu S and Wang Z: Cyclovirobuxine inhibits the
progression of clear cell renal cell carcinoma by suppressing the
IGFBP3-AKT/STAT3/MAPK-snail signalling pathway. Int J Biol Sci.
17:3522–3537. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Rosendahl AH and Forsberg G: IGF-I and
IGFBP-3 augment transforming growth factor-beta actions in human
renal carcinoma cells. Kidney Int. 70:1584–1590. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Shariat SF, Lamb DJ, Kattan MW, Nguyen C,
Kim J, Beck J, Wheeler TM and Slawin KM: Association of
preoperative plasma levels of insulin-like growth factor I and
insulin-like growth factor binding proteins-2 and −3 with prostate
cancer invasion, progression, and metastasis. J Clin Oncol.
20:833–841. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhong K, Luo W, Li N, Tan X, Li Y, Yin S,
Huang Y, Fang L, Ma W, Cai Y and Yin Y: CDK12 regulates
angiogenesis of advanced prostate cancer by IGFBP3. Int J Oncol.
64:202024. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Boyle BJ, Zhao XY, Cohen P and Feldman D:
Insulin-like growth factor binding protein-3 mediates 1
alpha,25-dihydroxyvitamin d(3) growth inhibition in the LNCaP
prostate cancer cell line through p21/WAF1. J Urol. 165:1319–1324.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Carlberg C and Muñoz A: An update on
vitamin D signaling and cancer. Semin Cancer Biol. 79:217–230.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Jeon SM and Shin EA: Exploring vitamin D
metabolism and function in cancer. Exp Mol Med. 50:1–14. 2018.
View Article : Google Scholar
|
|
83
|
Baxter RC: Nuclear actions of insulin-like
growth factor binding protein-3. Gene. 569:7–13. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Lee KW and Cohen P: Nuclear effects:
Unexpected intracellular actions of insulin-like growth factor
binding protein-3. J Endocrinol. 175:33–40. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Bowen C, Ostrowski MC, Leone G and Gelmann
EP: Loss of PTEN accelerates NKX3.1 degradation to promote prostate
cancer progression. Cancer Res. 79:4124–4134. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Zhang PJ, Hu XY, Liu CY, Chen ZB, Ni NN,
Yu Y, Yang LN, Huang ZQ, Liu QW and Jiang AL: The inhibitory
effects of NKX3.1 on IGF-1R expression and its signalling pathway
in human prostatic carcinoma PC3 cells. Asian J Androl. 14:493–498.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Muhlbradt E, Asatiani E, Ortner E, Wang A
and Gelmann EP: NKX3.1 activates expression of insulin-like growth
factor binding protein-3 to mediate insulin-like growth factor-I
signaling and cell proliferation. Cancer Res. 69:2615–2622. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Chen CD and Sawyers CL: NF-kappa B
activates prostate-specific antigen expression and is upregulated
in androgen-independent prostate cancer. Mol Cell Biol.
22:2862–2870. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Al-Rashidi RR, Noraldeen SAM, Kareem AK,
Mahmoud AK, Kadhum WR, Ramírez-Coronel AA, Iswanto AH, Obaid RF,
Jalil AT, Mustafa YF, et al: Malignant function of nuclear
factor-kappaB axis in prostate cancer: Molecular interactions and
regulation by non-coding RNAs. Pharmacol Res. 194:1067752023.
View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Mu HQ, He YH, Wang SB, Yang S, Wang YJ,
Nan CJ, Bao YF, Xie QP and Chen YH: Mir-130b/TNF-α/NF-κB/VEGFA loop
inhibits prostate cancer angiogenesis. Clin Transl Oncol.
22:111–121. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Han J, Jogie-Brahim S, Harada A and Oh Y:
Insulin-like growth factor-binding protein-3 suppresses tumor
growth via activation of caspase-dependent apoptosis and cross-talk
with NF-κB signaling. Cancer Lett. 307:200–210. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Huang S, Pettaway CA, Uehara H, Bucana CD
and Fidler IJ: Blockade of NF-kappaB activity in human prostate
cancer cells is associated with suppression of angiogenesis,
invasion, and metastasis. Oncogene. 20:4188–4197. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Smeland S, Bielack SS, Whelan J, Bernstein
M, Hogendoorn P, Krailo MD, Gorlick R, Janeway KA, Ingleby FC,
Anninga J, et al: Survival and prognosis with osteosarcoma:
Outcomes in more than 2000 patients in the EURAMOS-1 (European and
American Osteosarcoma Study) cohort. Eur J Cancer. 109:36–50. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Isakoff MS, Bielack SS, Meltzer P and
Gorlick R: Osteosarcoma: Current treatment and a collaborative
pathway to success. J Clin Oncol. 33:3029–3035. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Tan Y, Chen L, Li S, Hao H and Zhang D:
Mir-384 inhibits malignant biological behavior such as
proliferation and invasion of osteosarcoma by regulating IGFBP3.
Technol Cancer Res Treat. 19:15330338209091252020. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Li M, Wu W, Deng S, Shao Z and Jin X:
TRAIP modulates the IGFBP3/AKT pathway to enhance the invasion and
proliferation of osteosarcoma by promoting KANK1 degradation. Cell
Death Dis. 12:7672021. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Ressler S, Radhi J, Aigner T, Loo C,
Zwerschke W and Sergi C: Insulin-like growth factor-binding
protein-3 in osteosarcomas and normal bone tissues. Anticancer Res.
29:2579–2587. 2009.PubMed/NCBI
|
|
98
|
Schedlich LJ, Yenson VM and Baxter RC:
TGF-β-induced expression of IGFBP-3 regulates IGF1R signaling in
human osteosarcoma cells. Mol Cell Endocrinol. 377:56–64. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Vaezi MA, Eghtedari AR, Safizadeh B,
Babaheidarian P, Salimi V, Adjaminezhad-Fard F, Yarahmadi S,
Mirzaei A, Rahbar M and Tavakoli-Yaraki M: Evaluating the local
expression pattern of IGF-1R in tumor tissues and the circulating
levels of IGF-1, IGFBP-1, and IGFBP-3 in the blood of patients with
different primary bone tumors. Front Oncol. 12:10964382022.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Chao CC, Lee WF, Yang WH, Lin CY, Han CK,
Huang YL, Fong YC, Wu MH, Lee IT, Tsai YH, et al: Igfbp-3
stimulates human osteosarcoma cell migration by upregulating VCAM-1
expression. Life Sci. 265:1187582021. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Du Q, Liu P, Zhang C, Liu T, Wang W, Shang
C, Wu J, Liao Y, Chen Y, Huang J, et al: FASN promotes lymph node
metastasis in cervical cancer via cholesterol reprogramming and
lymphangiogenesis. Cell Death Dis. 13:4882022. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Huang Y, Chang A, Zhou W, Zhao H and Zhuo
X: IGFBP3 as an indicator of lymph node metastasis and unfavorable
prognosis for papillary thyroid carcinoma. Clin Exp Med.
20:515–525. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Giuliano M, Lauricella M, Vassallo E,
Carabillò M, Vento R and Tesoriere G: Induction of apoptosis in
human retinoblastoma cells by topoisomerase inhibitors. Invest
Ophthalmol Vis Sci. 39:1300–1311. 1998.PubMed/NCBI
|
|
104
|
Naspi A, Zingariello M, Sancillo L,
Panasiti V, Polinari D, Martella M, Alba RR and Londei P: IGFBP-3
inhibits wnt signaling in metastatic melanoma cells. Mol Carcinog.
56:681–693. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Naspi A, Panasiti V, Abbate F, Roberti V,
Devirgiliis V, Curzio M, Borghi M, Lozupone F, Carotti S, Morini S,
et al: Insulin-like-growth-factor-binding-protein-3 (IGFBP-3)
contrasts melanoma progression in vitro and in vivo. PLoS One.
9:e986412014. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wu N, Sun Q, Yang L, Sun H, Zhou Z, Hu Q,
Li C, Wang D, Zhang L, Hu Y and Cong X: HDAC3 and SNAIL2 complex
promotes melanoma metastasis by epigenetic repression of IGFBP3.
Int J Biol Macromol. 300:1403102025. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Baricević I, Masnikosa R, Lagundzin D,
Golubović V and Nedić O: Alterations of insulin-like growth factor
binding protein 3 (IGFBP-3) glycosylation in patients with breast
tumours. Clin Biochem. 43:725–731. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Miyamoto S, Yano K, Sugimoto S, Ishii G,
Hasebe T, Endoh Y, Kodama K, Goya M, Chiba T and Ochiai A: Matrix
metalloproteinase-7 facilitates insulin-like growth factor
bioavailability through its proteinase activity on insulin-like
growth factor binding protein 3. Cancer Res. 64:665–671. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Helle SI, Geisler S, Aas T, Paulsen T,
Holly JM and Lønning PE: Plasma insulin-like growth factor binding
protein-3 proteolysis is increased in primary breast cancer. Br J
Cancer. 85:74–77. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Blat C, Villaudy J and Binoux M: In vivo
proteolysis of serum insulin-like growth factor (IGF) binding
protein-3 results in increased availability of IGF to target cells.
J Clin Invest. 93:2286–2290. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Cai Q, Kim M, Harada A, Idowu MO,
Sundaresan G, Zweit J and Oh Y: Alpha-1 antitrypsin inhibits
tumorigenesis and progression of colitis-associated colon cancer
through suppression of inflammatory neutrophil-activated serine
proteases and IGFBP-3 proteolysis. Int J Mol Sci. 23:137372022.
View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Maile LA, Gill ZP, Perks CM and Holly JM:
The role of cell surface attachment and proteolysis in the
insulin-like growth factor (IGF)-independent effects of IGF-binding
protein-3 on apoptosis in breast epithelial cells. Endocrinology.
140:4040–4045. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Mitsui Y, Mochizuki S, Kodama T, Shimoda
M, Ohtsuka T, Shiomi T, Chijiiwa M, Ikeda T, Kitajima M and Okada
Y: Adam28 is overexpressed in human breast carcinomas: Implications
for carcinoma cell proliferation through cleavage of insulin-like
growth factor binding protein-3. Cancer Res. 66:9913–9920. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Oh SH, Whang YM, Min HY, Han SH, Kang JH,
Song KH, Glisson BS, Kim YH and Lee HY: Histone deacetylase
inhibitors enhance the apoptotic activity of insulin-like growth
factor binding protein-3 by blocking PKC-induced IGFBP-3
degradation. Int J Cancer. 131:2253–2263. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Overgaard MT, Boldt HB, Laursen LS,
Sottrup-Jensen L, Conover CA and Oxvig C: Pregnancy-associated
plasma protein-A2 (PAPP-A2), a novel insulin-like growth
factor-binding protein-5 proteinase. J Biol Chem. 276:21849–21853.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Koistinen H, Paju A, Koistinen R, Finne P,
Lövgren J, Wu P, Seppälä M and Stenman UH: Prostate-specific
antigen and other prostate-derived proteases cleave IGFBP-3, but
prostate cancer is not associated with proteolytically cleaved
circulating IGFBP-3. Prostate. 50:112–118. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Fujimoto M, Khoury JC, Khoury PR, Kalra B,
Kumar A, Sluss P, Oxvig C, Hwa V and Dauber A: Anthropometric and
biochemical correlates of PAPP-A2, free IGF-I, and IGFBP-3 in
childhood. Eur J Endocrinol. 182:363–374. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Chen CH, Chen PY, Lin YY, Feng LY, Chen
SH, Chen CY, Huang YC, Huang CY, Jung SM, Chen LY and Wei KC:
Suppression of tumor growth via IGFBP3 depletion as a potential
treatment in glioma. J Neurosurg. 132:168–179. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Kim MS and Lee DY: Insulin-like growth
factor binding protein-3 enhances etoposide-induced cell growth
inhibition by suppressing the NF-κB activity in gastric cancer
cells. Mol Cell Biochem. 403:107–113. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Rhodes DR, Barrette TR, Rubin MA, Ghosh D
and Chinnaiyan AM: Meta-analysis of microarrays: Interstudy
validation of gene expression profiles reveals pathway
dysregulation in prostate cancer. Cancer Res. 62:4427–4433.
2002.PubMed/NCBI
|
|
121
|
Hou YL, Luo P, Ji GY and Chen H: Clinical
significance of serum IGFBP-3 in colorectal cancer. J Clin Lab
Anal. 33:e229122019. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Abdolhoseinpour H, Mehrabi F, Shahraki K,
Khoshnood RJ, Masoumi B, Yahaghi E and Goudarzi PK: Investigation
of serum levels and tissue expression of two genes IGFBP-2 and
IGFBP-3 act as potential biomarker for predicting the progression
and survival in patients with glioblastoma multiforme. J Neurol
Sci. 366:202–206. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Chen X, Wang M, Wang H, Yang J, Li X,
Zhang R, Ding X, Hou H, Zhou J and Wu M: Mettl3 inhibitor
suppresses the progression of prostate cancer via IGFBP3/AKT
pathway and synergizes with PARP inhibitor. Biomed Pharmacother.
179:1173662024. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Huang H, Qin J, Wen Z, Liu Y, Chen C, Wang
C, Li H and Yang X: Effects of natural extract interventions in
prostate cancer: A systematic review and network meta-analysis.
Phytomedicine. 129:1555982024. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Singh RP, Tyagi AK, Dhanalakshmi S,
Agarwal R and Agarwal C: Grape seed extract inhibits advanced human
prostate tumor growth and angiogenesis and upregulates insulin-like
growth factor binding protein-3. Int J Cancer. 108:733–740. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Buckbinder L, Talbott R, Velasco-Miguel S,
Takenaka I, Faha B, Seizinger BR and Kley N: Induction of the
growth inhibitor IGF-binding protein 3 by p53. Nature. 377:646–649.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Whittaker S, Marais R and Zhu AX: The role
of signaling pathways in the development and treatment of
hepatocellular carcinoma. Oncogene. 29:4989–5005. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Subramaniam K, Ooi LL and Hui KM:
Transcriptional down-regulation of IGFBP-3 in human hepatocellular
carcinoma cells is mediated by the binding of TIA-1 to its AT-rich
element in the 3′-untranslated region. Cancer Lett. 297:259–268.
2010. View Article : Google Scholar : PubMed/NCBI
|















