Introduction
Lung cancer, a common fatal malignancy, contributes
to 2.21 million new cancer cases and 1.80 million new
cancer-related deaths annually worldwide (1). In China, 0.83 million new lung cancer
cases and 0.66 million new lung cancer-related deaths are recorded
every year (2), thus causing heavy
burdens to the patients and society (3,4).
Non-small cell lung cancer (NSCLC) accounts for ~85% of lung cancer
cases in China, thus giving an opportunity for research and
development (5). Along with the
advances in molecular detection technology and big-data gathering
and analysis, biomarker-based precision therapy is considered an
effective approach to improve prognosis and reduce disease burden
in patients with NSCLC (6–8). However, the detection of a large
proportion of predictive biomarkers requires the isolation of
tissue lesions, which is not always feasible, particularly in
patients who cannot undergo surgery.
Mucosa-associated lymphoid tissue lymphoma
translocation protein-1 (MALT1), acting as a scaffolding
gene/protein that triggers the activation of NF-κB transcription
factors and as a protease to modify immune activation-related
signaling via diversified substrate cleavage, was recently
discovered to facilitate cancer development via both cancer
cell-intrinsic and -extrinsic mechanisms (9,10).
MALT1 has been reported to promote cancer cell viability,
migration, invasion and drug resistance, and particularly
resistance to immune checkpoint inhibitors, in several
malignancies, such as breast cancer, prostate cancer,
hepatocellular carcinoma and lymphomas (11–15).
Previous studies have also demonstrated that MALT1 was upregulated
and associated with poor prognosis in patients with colorectal
cancer, malignant melanoma and lymphomas (16–18).
In terms of lung cancer, MALT1 could promote cancer progression via
interacting with caspase recruitment domain-coiled-coil complexes
and NF-κB activation (19,20). Apart from the aforementioned direct
regulation of cancer by MALT1, other studies indicated that MALT1
could regulate cancer immunity to promote cancer progression
(15,21). These findings supported the
potential of MALT1 expression levels as a prognostic biomarker in
patients with NSCLC.
Therefore, the present study aimed to investigate
the utility of the expression levels of MALT1 in blood samples from
patients with NSCLC and their association with the clinical
features, treatment options and survival outcomes for these
patients.
Patients and methods
Subjects
In the present study, a total of 125 patients with
NSCLC who underwent tumor resection surgery between April 2019 and
January 2022 at North Sichuan Medical University affiliated
Nanchong Central Hospital (Nanchong, China) were enrolled. The
inclusion criteria were as follows: i) Patients diagnosed with
NSCLC by pathological examination; ii) aged ≥18 years; iii)
received tumor resection surgery; and iv) followed the normal
follow-up requirements. In addition, the exclusion criteria were as
follows: i) Patients whose NSCLC was accompanied by other primary
types of cancer; ii) suffering from hematological malignancies;
iii) with needle or blood phobia; and iv) pregnant women or
lactating mothers. Additionally, a total of 20 healthy individuals
undergoing physical examination between February and March 2024
were enrolled as the control group. The recruitment criteria for
individuals into the control group were as follows: i) Subjects
without any abnormalities in recent physical examinations; ii) aged
>18 years; and iii) those who were willing to cooperate with
blood collection. The exclusion criteria of patients with NSCLC
were also applied to individuals in the healthy control group. The
present study was approved by the Ethics Committee of North Sichuan
Medical University affiliated Nanchong Central Hospital (approval
no. 2024018; Nanchong, China), and most patients with NSCLC and the
controls had provided written informed consent, while a waiver was
obtained for certain patients with NSCLC who had not provided
informed consent.
Data collection and sample
detection
The clinical characteristics of patients with NSCLC,
including demographic, and disease- and therapy-related data, were
collected. The tumor-node-metastasis (TNM) stage was defined
according to the guidelines provided by the International
Association for the Study of Lung Cancer (22). Prior to treatment initiation,
neoadjuvant therapy or tumor resection surgery, 2 ml peripheral
blood (PB) was collected from all patients with NSCLC. In addition,
PB from healthy controls was collected immediately after
enrollment. For MALT1 detection, PB mononuclear cells (PBMCs) were
separated from PB, and MALT1 levels were detected by reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
assay. For qPCR, the thermocycling was as follows: 95°C for 60 sec,
1 cycle; 95°C for 15 sec and 61°C for 60 sec, 40 cycles. GAPDH was
used as the internal reference and MALT1 levels were quantified
using the 2−ΔΔCq method (23). The primer sequences were as follows:
MALT1 forward (F), 5′-TCTTGGCTGGACAGTTTGTGA-3′ and reverse (R),
5′-GCTCTCTGGGATGTCGCAA-3′; GAPDH F, 5′-TGACCACAGTCCATGCCATCAC-3′
and R, 5′-GCCTGCTTCACCACCTTCTTGA-3′. The corresponding kits used
for RT-qPCR are listed in Table SI
and the kits were used according to the manufacturer's
instructions.
Grading of MALT1 levels
MALT1 expression levels in patients with NSCLC were
divided into four different grades. The quartile (Q) values for
each grade were as follows: i) Q1, MALT1 ranged from the minimum
value, 0.690, to the 1st quartile value, 2.190 (Q1, 0.690–2.190);
ii) Q2, MALT1 ranged from the 1st quartile value to the 2nd
quartile value, 3.600 (2.190–3.600); iii) Q3, MALT1 ranged from the
2nd quartile value to the 3rd quartile value, 5.945 (Q3,
3.600–5.945); and iv) Q4, MALT1 ranged from the 3rd quartile value
to the maximum value, 12.770 (Q4, 5.945–12.770).
Follow-up and evaluation
Routine follow-up was conducted, with a median
follow-up time of 18.1 months (range, 2.8–39.1 months). In the
first year after surgery, follow-up was conducted once every 3
months and once every 3–6 months thereafter. If the patients
experienced disease recurrence, a follow-up was performed once
every 2 months. During the follow-up, the disease progression
status, death and the corresponding periods were recorded. The
accumulating disease-free survival (DFS) and overall survival (OS)
rates were calculated.
Bioinformatics
The association of MALT1 gene expression levels with
TNM stage, DFS and OS in patients with NSCLC using publicly data
was performed as well, which was based on the Gene Expression
Profiling Interactive Analysis (GEPIA) database (http://gepia.cancer-pku.cn/). Correlation analysis of
MALT1 gene expression with immune infiltrates in various types of
cancer and NSCLC specifically using publicly available data was
performed using the TIMER database (http://timer.comp-genomics.org/).
Statistical analyses
Data analyses were performed using SPSS (version
26.0; IBM Corp.). Categorical data were expressed as number
(percentage), normally distributed continuous data were expressed
as the mean ± standard deviation and skewedly distributed
continuous data were expressed as the median [interquartile range
(IQR)]. To compare the clinical characteristics between different
grades of MALT1, the Mann-Whitney U-test, χ2 test or
Fisher's exact test was carried out. In addition, to compare
accumulating DFS/OS rates between patients with NSCLC with
different grades of MALT1, a log-rank test was performed. The
results were displayed by Kaplan-Meier curves. Enter-method Cox
regression models were used to identify the factors that were
independently associated with DFS or OS. P<0.05 was considered
to indicate a statistically significant difference.
Results
Characteristics of patients with
NSCLC
A total of 125 patients with NSCLC were included in
the present study. The mean age of the patients with NSCLC was
57.1±12.3 years. Among them, 27.2 and 72.8% were ≥65 and <65
years old, respectively. A total of 54.4% of patients were males
and 45.6% were females (Table I),
while 61.6, 28.8 and 9.6% of the patients were categorized as lung
adenocarcinoma, lung squamous cell carcinoma and others,
respectively. In addition, 36.0% of the cases had poor
differentiation, and 8.0, 8.8, 20.0, 39.2, 21.6 and 2.4% of
patients were of TNM stage of IA, IB, IIA, IIB, IIIA and IIIB,
respectively. The mean age of the healthy control subjects was
55.5±5.2 years, consisting of 50% females and 50% males. Age
(P=0.384) and sex (P=0.714) were not significantly different
between the healthy controls and patients with NSCLC (Table SII).
 | Table I.Clinical characteristics of patients
with NSCLC. |
Table I.
Clinical characteristics of patients
with NSCLC.
|
Characteristics | Total (n=125) | Q1-MALT1
(n=31) | Q2-MALT1
(n=32) | Q3-MALT1
(n=31) | Q4-MALT1
(n=31) | P-value |
|---|
| Age, years |
|
|
|
|
| 0.245 |
|
<65 | 91 (72.8) | 24 (26.4) | 21 (23.1) | 26 (28.6) | 20 (22.0) |
|
|
≥65 | 34 (27.2) | 7 (20.6) | 11 (32.4) | 5 (14.7) | 11 (32.4) |
|
| Sex |
| |
|
|
| 0.793 |
|
Female | 57 (45.6) | 15 (26.3) | 13 (22.8) | 16 (28.1) | 13 (22.8) |
|
|
Male | 68 (54.4) | 16 (23.5) | 19 (27.9) | 15 (22.1) | 18 (26.5) |
|
| Smoking status |
|
|
|
|
| 0.463 |
| Never
smoked | 84 (67.2) | 24 (28.6) | 22 (26.2) | 19 (22.6) | 19 (22.6) |
|
| Former
smoker | 15 (12.0) | 3 (20.0) | 5 (33.3) | 5 (33.3) | 2 (13.3) |
|
| Current
smoker | 26 (20.8) | 4 (15.4) | 5 (19.2) | 7 (26.9) | 10 (38.5) |
|
| Histological
type |
|
|
|
|
| 0.263 |
| Lung
adenocarcinoma | 77 (61.6) | 16 (20.8) | 17 (22.1) | 21 (27.3) | 23 (29.9) |
|
| Lung
squamous cell carcinoma | 36 (28.8) | 9 (25.0) | 13 (36.1) | 8 (22.2) | 6 (16.7) |
|
|
Other | 12 (9.6) | 6 (50.0) | 2 (16.7) | 2 (16.7) | 2 (16.7) |
|
| Poor
differentiation | 45 (36.0) | 9 (20.0 | 9 (20.0) | 10 (22.2) | 17 (37.8) | 0.091 |
| T stage |
|
|
|
|
| 0.058 |
| 1 | 14 (11.2) | 6 (42.9) | 4 (28.6) | 1 (7.1) | 3 (21.4) |
|
| 2 | 68 (54.4) | 19 (27.9) | 19 (27.9) | 20 (29.4) | 10 (14.7) |
|
| 3 | 34 (27.2) | 5 (14.7) | 6 (17.6) | 8 (23.5) | 15 (44.1) |
|
| 4 | 9 (7.2) | 1 (11.1) | 3 (33.3) | 2 (22.2) | 3 (33.3) |
|
| N stage |
|
|
|
|
| 0.026 |
| 0 | 66 (52.8) | 21 (31.8) | 14 (21.2) | 14 (21.2) | 17 (25.8) |
|
| 1 | 54 (43.2) | 9 (16.7) | 18 (33.3) | 17 (31.5) | 10 (18.5) |
|
| 2 | 5 (4.0) | 1 (20.0) | 0 (0.0) | 0 (0.0) | 4 (80.0) |
|
| M stage |
|
|
|
|
|
|
| 0 | 125 (100.0) | 31 (24.8) | 32 (25.6) | 31 (24.8) | 31 (24.8) | (−) |
| TNM detailed
stage |
|
|
|
|
| 0.036 |
| IA | 10 (8.0) | 4 (40.0) | 3 (30.0) | 1 (10.0) | 2 (20.0) |
|
| IB | 11 (8.8) | 2 (18.2) | 3 (27.3) | 3 (27.3) | 3 (27.3) |
|
|
IIA | 25 (20.0) | 13 (52.0) | 5 (20.0) | 4 (16.0) | 3 (12.0) |
|
|
IIB | 49 (39.2) | 7 (14.3) | 14 (28.6) | 18 (36.7) | 10 (20.40 |
|
|
IIIA | 27(21.6) | 5 (18.5) | 7 (25.9) | 5 (18.5) | 10 (37.0) |
|
|
IIIB | 3 (2.4) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) |
|
| Neoadjuvant
chemotherapy |
|
|
|
|
| 0.188 |
| No | 102 (81.6) | 27 (26.5) | 25 (24.5) | 28 (27.5) | 22 (21.6) |
|
|
Yes | 23 (18.4) | 4 (17.4) | 7 (30.4) | 3 (13.0) | 9 (39.1) |
|
| Neoadjuvant
TKI |
|
|
|
|
| 0.754 |
| No | 121 (96.8) | 31 (25.6) | 31 (25.6) | 30 (24.8) | 29 (24.0) |
|
|
Yes | 4 (3.2) | 0 (0.0) | 1 (25.0) | 1 (25.0) | 2 (50.0) |
|
| Neoadjuvant
ICI |
|
|
|
|
| 0.571 |
| No | 120 (96.0) | 31 (25.8) | 31 (25.8) | 29 (24.2) | 29 (24.2) |
|
|
Yes | 5 (4.0) | 0 (0.0) | 1 (20.0) | 2 (40.0) | 2 (40.0) |
|
| Surgical type |
|
|
|
|
| 0.817 |
|
Thoracoscopic surgery | 19 (15.2) | 5 (26.3) | 6 (31.6) | 5 (26.3) | 3 (15.8) |
|
|
Thoracotomy | 106 (84.8) | 26 (24.5) | 26 (24.5) | 26 (24.5) | 28 (26.4) |
|
| Adjuvant
chemotherapy |
|
|
|
|
| 0.157 |
| No | 42 (33.6) | 15 (35.7) | 9 (21.4) | 11 (26.2) | 7 (16.7) |
|
|
Yes | 83 (66.4) | 16 (19.3) | 23 (27.7) | 20 (24.1) | 24 (28.9) |
|
| Adjuvant TKI |
|
|
|
|
| 0.406 |
| No | 102 (81.6) | 25 (24.5) | 29 (28.4) | 23 (22.5) | 25 (24.5) |
|
|
Yes | 23 (18.4) | 6 (26.1) | 3 (13.0) | 8 (34.8) | 6 (26.1) |
|
| Adjuvant ICI |
|
|
|
|
| 0.737 |
| No | 110 (88.0) | 29 (26.4) | 28 (25.5) | 26 (23.6) | 27 (24.5) |
|
|
Yes | 15 (12.0) | 2 (13.3) | 4 (26.7) | 5 (33.3) | 4 (26.7) |
|
MALT1 is aberrantly expressed in the
blood and is associated with the clinical characteristics of
patients with NSCLC
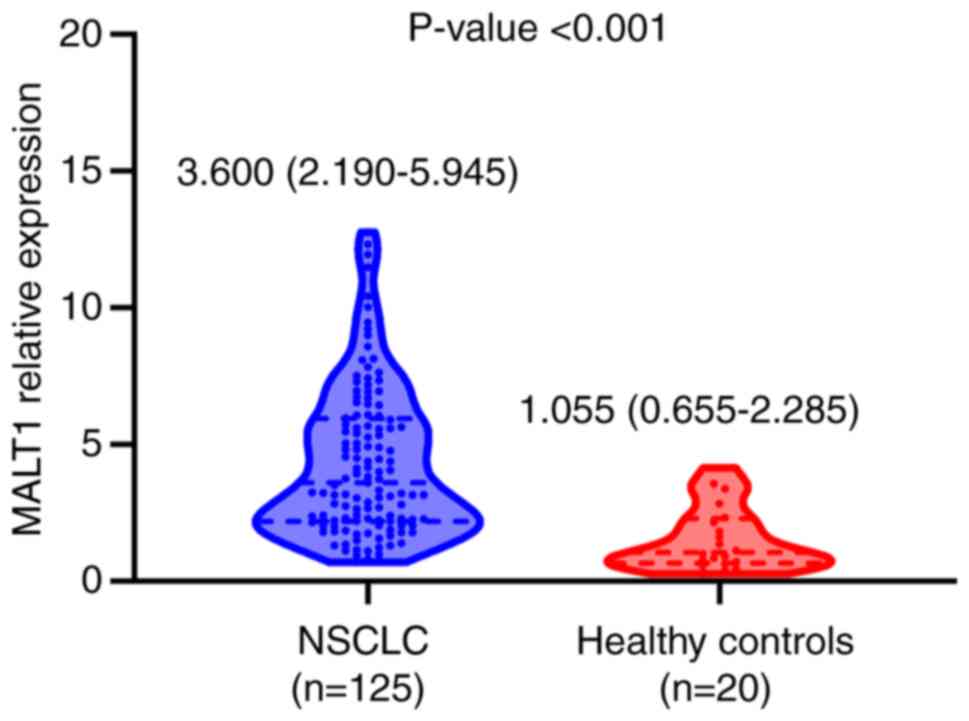
The blood MALT1 mRNA expression levels were
quantified in the 20 healthy subjects and patients with NSCLC. The
relative expression levels of MALT1 mRNA were increased in the
blood samples of patients with NSCLC (median, 3.60; IQR, 2.19–5.95)
compared with the healthy controls (median, 1.06; IQR, 0.66–2.29;
P<0.001; Fig. 1), suggesting
MALT1 was aberrantly expressed in the blood of patients with NSCLC.
Subsequently, to analyze the association between blood MALT1 levels
and clinical features, treatment options and prognosis in patients
with NSCLC, the mRNA expression levels of MALT1 were divided into
four groups, according to the quartiles (Q1, Q2, Q3 and Q4). The
results demonstrated that the blood MALT1 quartile was markedly
positively associated with the N (P=0.026) and TNM (P=0.036) stages
in patients with NSCLC. Additionally, MALT1 expression levels
displayed a tendency to be positively associated with poor
differentiation (P=0.091) and T stage (P=0.058; Table I), but this did not reach
statistical significance. By contrast, the blood MALT1 quartile was
not associated with other clinical characteristics or with
neoadjuvant therapy, surgical type or adjuvant therapy.
Prognostic value of blood MALT1 levels
in patients with NSCLC
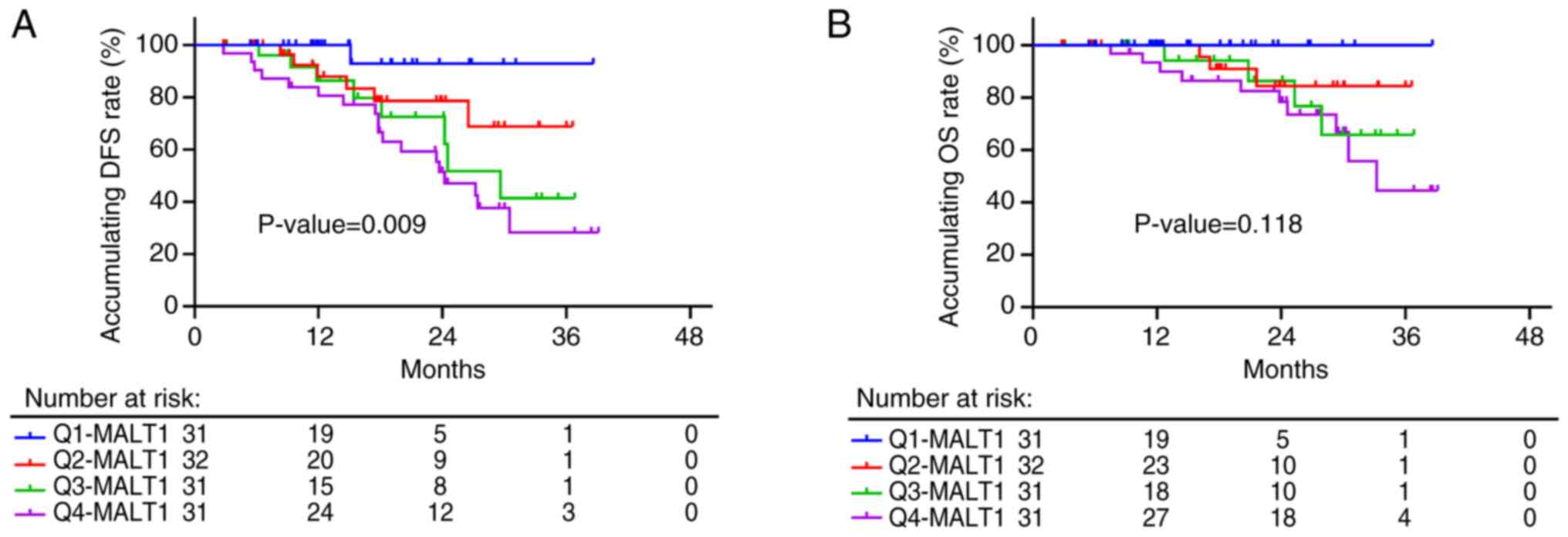
The blood MALT1 quartile was associated with shorter
DFS (P=0.009), with 3-year accumulating DFS rates of 92.9, 68.8,
41.4 and 28.2% in patients with MALT1 levels at Q1, Q2, Q3 and Q4,
respectively (Fig. 2A). However,
the blood MALT1 quartile only showed a tendency to be associated
with poor OS in these patients (P=0.118), but it did not reach
statistical significance; 3-year accumulating OS rates of 100.0,
84.4, 65.7 and 44.5% were recorded in patients with MALT1 levels at
Q1, Q2, Q3 and Q4, respectively (Fig.
2B). The aforementioned data suggested that blood
MALT1expression levels may potentially predict DFS; however, they
lacked efficiency in OS estimations of patients with NSCLC.
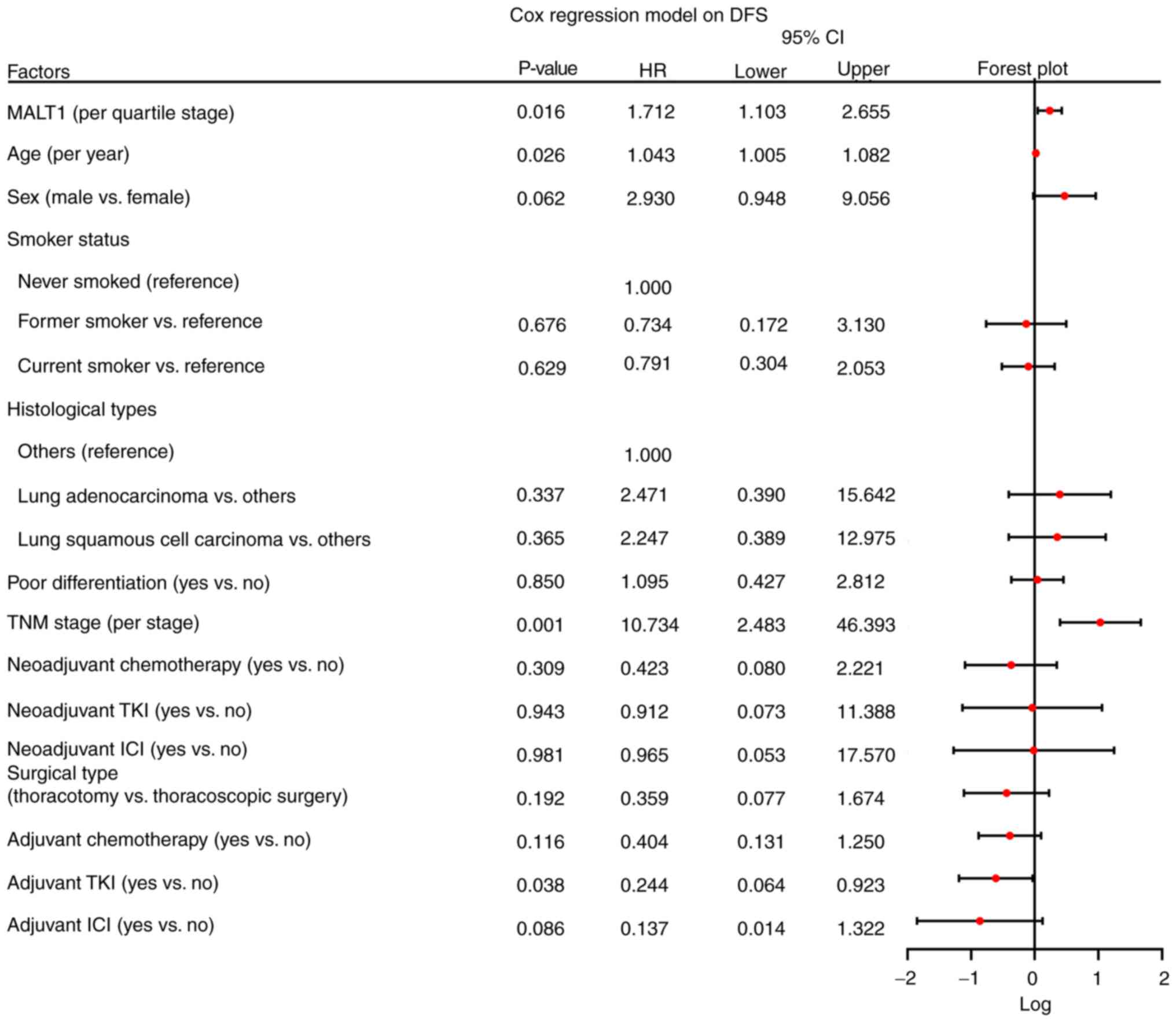
Furthermore, multivariate Cox analysis was performed to identify
the factors that could affect DFS and OS in patients with NSCLC.
Increased MALT1 quartile [P=0.016; hazard ratio (HR), 1.712], age
(P=0.026; HR, 1.043) and TNM stage (P=0.001; HR, 10.734) had a
negative impact on DFS independently, while adjuvant tyrosine
kinase inhibitor therapy (P=0.038; HR, 0.244) was independently
associated with satisfied DFS (Fig.
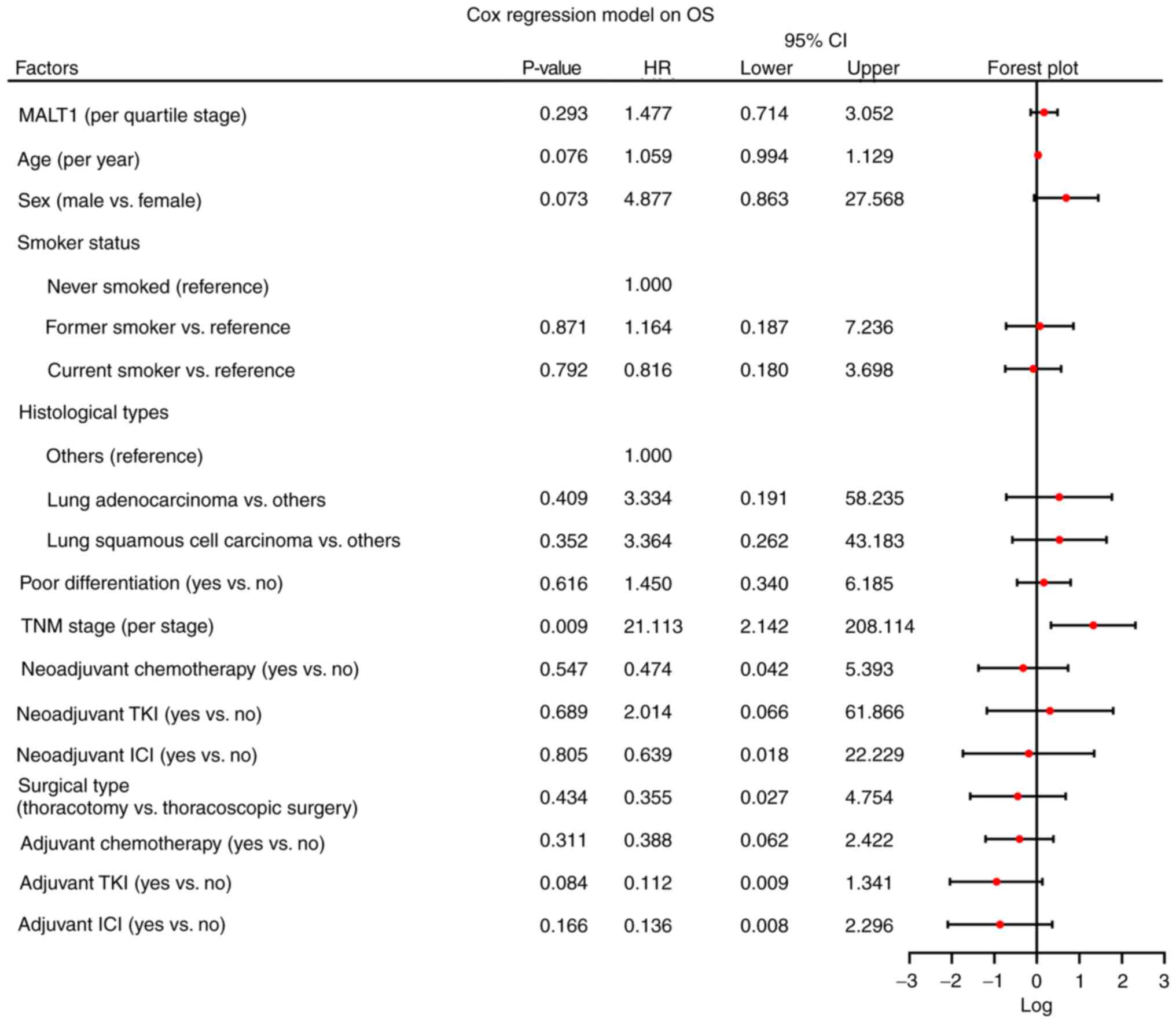
3). In terms of OS, a higher MALT1 quartile (P=0.293; HR,
1.477) was not significantly associated with OS. However, a higher
TNM stage (P=0.009; HR, 21.113) was significantly associated with
lower OS rates (Fig. 4).
Bioinformatic analyses based on public
database
GEPIA is a developed interactive web server for
analyzing the RNA sequencing expression data from the The Cancer
Genome Atlas and the Genotype-Tissue Expression projects, using a
standard processing pipeline (24).
With the use of GEPIA, it was observed that MALT1 was not related
to TNM stage, DFS or OS in patients with lung adenocarcinoma
(Fig. S1A), nor in patients with
lung squamous cell carcinoma (Fig.
S1B). MALT1 showed a notable association with DFS in lung
squamous cell carcinoma but this was not of statistical
significance.
The TIMER database is a comprehensive resource for
the systematic analysis of immune infiltrates across diverse types
of cancer (25). The TIMER database
was used for immune analysis and demonstrated that MALT1 expression
levels were correlated with macrophages and CD8+ T cells
in various types of cancer (Fig. S1C
and D). Regarding NSCLC, MALT1 was closely correlated with
macrophages (particularly the M1 type) and CD8+ T cells
in lung adenocarcinoma, but the correlation was lower in lung
squamous cell carcinoma.
Discussion
It has been suggested that MATL1 is a significant
carcinogenetic factor that promotes the initiation and progression
of several types of cancer (11–21).
More specifically, the role of MALT1 inhibitor as a candidate
treatment option for hepatocellular carcinoma, colorectal cancer,
chronic lymphocytic leukemia and glioblastoma both in vivo
and in vitro, has been recently investigated (13,26–28).
Due to these functions, MALT1 has attracted increasing attention as
a biomarker in different types of cancer. Previous studies
demonstrated that MALT1 was upregulated in colorectal cancer,
melanoma and lymphomas (16–18).
However, the aforementioned studies detected the expression levels
of MALT1 in cancerous tissues. Therefore, data on the expression
levels of MALT1 in blood samples are lacking. The present study
demonstrated that MALT1 was upregulated in blood samples from
patients with NSCLC compared with the control group (~3.4-fold).
This finding could be due to the possible promoting role of MALT1
in lung cancer (19,20) and its effects on CD8+
T-cell functions, immune escape and anticancer immunity (29–31).
Subsequently, the present study also demonstrated
that blood MALT1 expression levels were positively associated with
N and TNM stages in patients with NSCLC, and showed a notable
positive association with poor differentiation and T stage, thus
supporting the possible role of MALT1 in predicting tumor
progression or disease burden in patients with NSCLC. These
findings could be for the following reasons: i) Previous studies
demonstrated that MALT1 could directly activate NF-κB to promote
NSCLC progression, while blood MALT1 levels could also reflect the
expression levels of MALT1 in tumor tissues, to a certain extent
(32,33); ii) in addition to the NF-κB
signaling pathway, bioinformatics analysis in the Kyoto
Encyclopedia of Genes and Genomes predicted that MALT1 could also
regulate the C-type lectin, T-cell and B-cell receptor signaling
pathways, thus promoting NSCLC progression (34,35);
and ii) other studies also suggested that the T-cell receptor
signaling pathway, a notable downstream pathway of MALT1, could
affect tumor immunity and immune escape to promote NSCLC
progression (36–38).
The present study also suggested that blood MALT1
levels may predict the prognosis of patients with NSCLC.
Kaplan-Meier curves predicted that the expression levels of MALT1
in the blood of patients with NSCLC were associated with a shorter
DFS, while they showed a notable association with worse OS. Further
adjustment by multivariate Cox analysis demonstrated that the
expression levels of MALT1 could independently predict unsatisfied
DFS. The prognostic value of blood MALT1 levels could be due to the
following: i) Correlation analysis revealed that MALT1 was
associated with advanced tumor conditions, such as N and TNM
stages, thus possibly affecting the prognosis of patients with
NSCLC; and ii) a large proportion of patients with NSCLC were
treated with adjuvant therapies. It has been reported that MALT1
regulates the sensitivity of patients to adjuvant therapies
(19,21,39).
Therefore, the aforementioned findings could affect the prognosis
of patients with NSCLC. Furthermore, the lack of significance
regarding the association between MALT1 levels and OS could be due
to the fact that OS could be affected by several factors, such as
treatment after tumor recurrence, comorbidity and supportive
treatment. These factors may weaken the predictive value of MALT1
regarding the prognosis of patients with NSCLC. Furthermore, GEPIA
database analysis revealed that MALT1 was not correlated with DFS
or OS in lung cancer and the difference between the aforementioned
GEPIA data and the present findings may have been due to the fact
that tumor tissue was used in GEPIA, while blood samples were used
in the present study.
However, the present study had certain limitations.
First, the present study only included surgical patients with NSCLC
to avoid interference, since the prognosis of advanced cases was
far from surgical ones, which limited the generalizability of the
study findings. More patients with different types of lung cancer
would have been needed to be included to obtain more comprehensive
conclusions. Second, the sample size of the present study was
relatively small, which may limit the reliability and
generalizability of the results. Therefore, more large
population-based validation studies are needed to verify the
results of the present study. Third, the follow-up time of the
present study was relatively short and it was difficult to assess
the correlation between blood MALT1 and long-term prognosis.
Therefore, longer follow-up studies are needed to verify the
long-term prognostic value of blood MALT1 expression levels.
Finally, no investigation into the mechanistic relationship between
blood MALT1 levels and the development and prognosis of NSCLC was
performed, which could be further evaluated in future studies.
In conclusion, the results of the present study
suggested that blood MALT1 levels could potentially reflect the
stage of lymph node metastasis and predict DFS in patients with
NSCLC. This suggests the potential of blood MALT1 to be a
prognostic biomarker in patients with NSCLC, which could help
facilitate personalized treatment via early identifying prognostic
risk stratification. However, future large-scale studies with a
longer follow-up duration are needed to further validate the
results of the present study.
Supplementary Material
Supporting Data
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by Nanchong City School
Cooperation Special Project (grant no. 19SXH0353).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XZ contributed to the study conception and design.
Data collection and analysis were performed by FZ and BW. XP was
responsible for the interpretation of data for the present study.
XZ and FZ confirm the authenticity of all the raw data. All authors
contributed to drafting of article and revising it critically for
important intellectual content. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The Ethics Committee of North Sichuan Medical
University (Nanchong, China) approved the present study (approval
no. 2024018). Most NSCLC patients and the controls had provided
written informed consent, while a waiver was obtained for certain
patients with NSCLC who had not provided informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zheng RS, Zhang SW, Sun KX, Chen R, Wang
SM, Li L, Zeng HM, Wei WW and He J: Cancer statistics in China,
2016. Zhonghua Zhong Liu Za Zhi. 45:212–220. 2023.(In Chinese).
PubMed/NCBI
|
|
3
|
Leiter A, Veluswamy RR and Wisnivesky JP:
The global burden of lung cancer: Current status and future trends.
Nat Rev Clin Oncol. 20:624–639. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi J, Li M, Wang L, Hu Y, Liu W, Long Z,
Zhou Z, Yin P and Zhou M: National and subnational trends in cancer
burden in China, 2005-20: An analysis of national mortality
surveillance data. Lancet Public Health. 8:e943–e955. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen P, Liu Y, Wen Y and Zhou C: Non-small
cell lung cancer in China. Cancer Commun (Lond). 42:937–970. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang SR, Schultheis AM, Yu H, Mandelker D,
Ladanyi M and Buttner R: Precision medicine in non-small cell lung
cancer: Current applications and future directions. Semin Cancer
Biol. 84:184–198. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Restrepo JC, Duenas D, Corredor Z and
Liscano Y: Advances in genomic data and biomarkers: Revolutionizing
NSCLC diagnosis and treatment. Cancers (Basel). 15:34742023.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tostes K, Siqueira AP, Reis RM, Leal LF
and Arantes L: Biomarkers for immune checkpoint inhibitor response
in NSCLC: Current developments and applicability. Int J Mol Sci.
24:118872023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
O'Neill TJ, Tofaute MJ and Krappmann D:
Function and targeting of MALT1 paracaspase in cancer. Cancer Treat
Rev. 117:1025682023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gomez Solsona B, Schmitt A,
Schulze-Osthoff K and Hailfinger S: The paracaspase malt1 in
cancer. Biomedicines. 10:3442022. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhou B, Mo Z, Lai G, Chen X, Li R, Wu R,
Zhu J and Zheng F: Targeting tumor exosomal circular RNA cSERPINE2
suppresses breast cancer progression by modulating MALT1-NF-.
B-IL-6 axis of tumor-associated macrophages. J Exp Clin Cancer Res.
42:482023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Vanneste D, Staal J, Haegman M, Driege Y,
Carels M, Van Nuffel E, De Bleser P, Saeys Y, Beyaert R and Afonina
IS: CARD14 signalling ensures cell survival and cancer associated
gene expression in prostate cancer cells. Biomedicines.
10:20082022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kurden-Pekmezci A, Cakiroglu E, Eris S,
Mazi FA, Coskun-Deniz OS, Dalgic E, Oz O and Senturk S: MALT1
paracaspase is overexpressed in hepatocellular carcinoma and
promotes cancer cell survival and growth. Life Sci. 323:1216902023.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Minderman M, Lantermans HC, Gruneberg LJ,
Cillessen SAGM, Bende RJ, van Noesel CJM, Kersten MJ, Pals ST and
Spaargaren M: MALT1-dependent cleavage of CYLD promotes NF-κB
signaling and growth of aggressive B-cell receptor-dependent
lymphomas. Blood Cancer J. 13:372023. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Di Pilato M, Gao Y, Sun Y, Fu A, Grass C,
Seeholzer T, Feederle R, Mazo I, Kazer SW, Litchfield K, et al:
Translational studies using the MALT1 inhibitor (S)-Mepazine to
induce treg fragility and potentiate immune checkpoint therapy in
cancer. J Immunother Precis Oncol. 6:61–73. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li C, Yu F and Xu W: Early low blood MALT1
expression levels forecast better efficacy of PD-1 inhibitor-based
treatment in patients with metastatic colorectal cancer. Oncol
Lett. 26:3292023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Zhang G, Jin J, Degan S, Tameze Y
and Zhang JY: MALT1 promotes melanoma progression through JNK/c-Jun
signaling. Oncogenesis. 6:e3652017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tibiletti MG, Martin V, Bernasconi B, Del
Curto B, Pecciarini L, Uccella S, Pruneri G, Ponzoni M,
Mazzucchelli L, Martinelli G, et al: BCL2, BCL6, MYC, MALT 1, and
BCL10 rearrangements in nodal diffuse large B-cell lymphomas: A
multicenter evaluation of a new set of fluorescent in situ
hybridization probes and correlation with clinical outcome. Hum
Pathol. 40:645–652. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pan D, Jiang C, Ma Z, Blonska M, You MJ
and Lin X: MALT1 is required for EGFR-induced NF-κB activation and
contributes to EGFR-driven lung cancer progression. Oncogene.
35:919–928. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Israel L, Gluck A, Berger M, Coral M, Ceci
M, Unterreiner A, Rubert J, Bardet M, Ginster S, Golding-Ochsenbein
AM, et al: CARD10 cleavage by MALT1 restricts lung carcinoma growth
in vivo. Oncogenesis. 10:322021. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mempel TR and Krappmann D: Combining
precision oncology and immunotherapy by targeting the MALT1
protease. J Immunother Cancer. 10:e0054422022. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Goldstraw P, Chansky K, Crowley J,
Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, Groome P,
Mitchell A, Bolejack V, et al: The IASLC lung cancer staging
project: Proposals for revision of the TNM stage groupings in the
forthcoming (eighth) edition of the TNM classification for lung
cancer. J Thorac Oncol. 11:39–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Y, Huang Q and He F: Aberrant blood
MALT1 and its relevance with multiple organic dysfunctions, T
helper cells, inflammation, and mortality risk of sepsis patients.
J Clin Lab Anal. 36:e243312022. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang Z, Li C, Kang B, Gao G, Li C and
Zhang Z: GEPIA: A web server for cancer and normal gene expression
profiling and interactive analyses. Nucleic Acids Res. 45:W98–W102.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Li T, Fu J, Zeng Z, Cohen D, Li J, Chen Q,
Li B and Liu XS: TIMER2.0 for analysis of tumor-infiltrating immune
cells. Nucleic Acids Res. 48:W509–W514. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Qian R, Niu X, Wang Y, Guo Z, Deng X, Ding
Z, Zhou M and Deng H: Targeting MALT1 suppresses the malignant
progression of colorectal cancer via miR-375/miR-365a-3p/NF-κB
axis. Front Cell Dev Biol. 10:8450482022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Saba NS, Wong DH, Tanios G, Iyer JR,
Lobelle-Rich P, Dadashian EL, Liu D, Fontan L, Flemington EK,
Nichols CM, et al: MALT1 inhibition is efficacious in both naive
and ibrutinib-resistant chronic lymphocytic leukemia. Cancer Res.
77:7038–7048. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Liu X, Yue C, Shi L, Liu G, Cao Q, Shan Q,
Wang Y, Chen X, Li H, Wang J, et al: MALT1 is a potential
therapeutic target in glioblastoma and plays a crucial role in
EGFR-induced NF-κB activation. J Cell Mol Med. 24:7550–7562. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Flynn SM, Chen C, Artan M, Barratt S,
Crisp A, Nelson GM, Peak-Chew SY, Begum F, Skehel M and de Bono M:
MALT-1 mediates IL-17 neural signaling to regulate C. elegans
behavior, immunity and longevity. Nat Commun. 11:20992020.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yu JW, Hoffman S, Beal AM, Dykon A,
Ringenberg MA, Hughes AC, Dare L, Anderson AD, Finger J, Kasparcova
V, et al: MALT1 protease activity is required for innate and
adaptive immune responses. PLoS One. 10:e01270832015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng L, Deng N, Yang N, Zhao X and Lin X:
Malt1 protease is critical in maintaining function of regulatory T
cells and may be a therapeutic target for antitumor immunity. J
Immunol. 202:3008–3019. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kingeter LM and Schaefer BC: Malt1 and
cIAP2-Malt1 as effectors of NF-kappaB activation: Kissing cousins
or distant relatives? Cell Signal. 22:9–22. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen W, Li Z, Bai L and Lin Y: NF-kappaB
in lung cancer, a carcinogenesis mediator and a prevention and
therapy target. Front Biosci (Landmark Ed). 16:1172–1185. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pathan J, Mondal S, Sarkar A and
Chakrabarty D: Daboialectin, a C-type lectin from Russell's viper
venom induces cytoskeletal damage and apoptosis in human lung
cancer cells in vitro. Toxicon. 127:11–21. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Luo B, Chu X, Yu P and Tian J: The TCR
repertoire diversity and its application in the prevention and
treatment of lung cancer. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi.
38:939–943. 2022.(In Chinese). PubMed/NCBI
|
|
36
|
Che T, You Y, Wang D, Tanner MJ, Dixit VM
and Lin X: MALT1/paracaspase is a signaling component downstream of
CARMA1 and mediates T cell receptor-induced NF-kappaB activation. J
Biol Chem. 279:15870–15876. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Shah K, Al-Haidari A, Sun J and Kazi JU: T
cell receptor (TCR) signaling in health and disease. Signal
Transduct Target Ther. 6:4122021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Taniuchi I: CD4 Helper and CD8 Cytotoxic T
Cell Differentiation. Annu Rev Immunol. 36:579–601. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li Y, Chen X, Wang Z, Zhao D, Chen H, Chen
W, Zhou Z, Zhang J, Zhang J, Li H and Chen C: The HECTD3 E3
ubiquitin ligase suppresses cisplatin-induced apoptosis via
stabilizing MALT1. Neoplasia. 15:39–48. 2013. View Article : Google Scholar : PubMed/NCBI
|