The term ‘silent killer’ has been given to ovarian
cancer due to its high mortality rate, which is attributed to the
inapparent tumor growth, delayed onset of symptoms and inadequate
screening, often leading to an advanced-stage diagnosis in the
first instance (5). Furthermore,
ovarian cancer is generally associated with non-specific symptoms.
The symptoms are common and include abdominal bloating, abdominal
pain, frequent urination, early satiety and changes in bowel
habits. However, because these symptoms are often mild or seem
ordinary, patients may not seek medical attention, which can lead
to delays in diagnosis (2). Ovarian
cancer is uncommon, and a general physician may only encounter a
case every 5 years (11). This
rarity, combined with the commonality of the symptoms, creates
notable challenges for both physicians and patients. For example,
physicians frequently misinterpret these symptoms, attributing them
to conditions such as irritable bowel syndrome, stress or
gastritis. At the same time, patients may not recognize the
seriousness of these symptoms, further contributing to delays in
diagnosis (12). As such, the
overlap of ovarian cancer symptoms with more common conditions,
along with a lack of awareness, often results in misdiagnosis and
delayed treatment.
Age is a key predisposing factor for ovarian cancer,
with a higher incidence in women >65 years and a median
diagnosis age of 63 years. Early-onset ovarian cancer, occurring
between 18 and 30 years, accounts for <5% of cases and is
usually diagnosed at a localized stage. By contrast, late-onset
ovarian cancer is often identified at advanced stages with
metastasis, indicating a more favorable prognosis for early-onset
cases (13).
A thorough assessment of family history is key for
identifying hereditary risks, primarily in cases associated with
hereditary breast and ovarian cancer and Lynch syndrome. This
evaluation includes analyzing cancer diagnoses in first- and
second-degree relatives, particularly those with ovarian, breast,
prostate or pancreatic cancer, as well as early-onset or bilateral
malignancy, which may indicate pathogenic variants in BRCA1, BRCA2
or mismatch repair genes. The genes that are highly associated with
ovarian cancer are listed in Table
I. Understanding familial cancer patterns aids risk
stratification, enabling personalized interventions such as
enhanced surveillance, prophylactic surgery, targeted therapies
[such as poly(ADP-ribose) polymerase (PARP) inhibitors] and genetic
counseling (14).
Protective factors include multiparity, advanced
maternal age at first childbirth, contraceptive use and lactation,
which are associated with a reduced risk of ovarian cancer
(5). Factors whose associations
with ovarian cancer remain inconsistent or debated, include age at
menarche and menopause, pregnancy characteristics, pelvic
inflammatory disease, hormone replacement therapy, infertility
treatment, dietary and nutritional patterns, obesity, physical
activity, and the consumption of alcohol, caffeine and tobacco
(5).
The development of ovarian cancer remains to be
elucidated and determining the exact pathophysiology is hampered by
the heterogeneous nature of the disease, which includes a variety
of histological types with distinct behaviors and features. Several
hypotheses have been proposed to explain the etiology of ovarian
cancer, including the incessant ovulation (repeated ovulatory
cycles cause damage to the ovarian epithelium, increasing cancer
risk), inflammation (highlights the role of chronic pelvic
inflammation in stimulating carcinogenesis; androgen/progesterone
and gonadotropin hypotheses, propose that hormonal imbalances may
stimulate abnormal cellular growth (15). The tubal origin hypothesis suggests
that many high-grade serous ovarian cancers may actually originate
from the epithelial cells of the fallopian tubes (16) . However, a definitive scientific
consensus has yet to be established.
Primary ovarian malignancies are classified in three
primary forms (epithelial, germ cell, and sex cord). Of all ovarian
malignancies, ~95% are epithelial cancers. Epithelial cancers are
hypothesized to originate from the single-cell layer covering the
ovary (2). According to histology,
serous [high-grade serous ovarian carcinoma (HGSOC) or low-grade
serous ovarian carcinoma (LGSOC)], clear-cell, endometrioid and
mucinous tumors are the four most prevalent forms of ovarian
cancer, with HGSOC being the most common, accounting for 70–80% of
all epithelial subtypes (2). The
unique biology and responses to different treatments allow further
stratification of the subtypes (17). The remaining 5% of all ovarian
cancers consist of sex and germ cell cancers. Germ cell tumors are
rare, accounting for 3% of cases of ovarian cancer, and they are
most commonly diagnosed in younger patients, typically between the
ages of 10 and 30 years. Sex cord-stromal malignancies are the
rarest ovarian neoplasms, accounting for <2% of primary ovarian
tumors. They are typically benign and frequently identified early
during development (2).
The understanding of ovarian cancer biology has
advanced and is now based on histological characteristics and the
molecular phenotype (19). The
first step in diagnosis is to obtain a thorough medical history
from the patient to determine any family history of ovarian and
other types of cancer (18). If
ovarian cancer is detected early in the development process, the
chances of successful treatment are higher (20). Screening tests must be performed
multiple times to ensure early, cost-effective treatment while
minimizing unnecessary interventions (21). Due to its role in oncogenesis and
metastasis, the cancer antigen-125 (CA-125) test has been widely
used for ovarian cancer screening and differentiation from benign
conditions, making it a key focus in developing antitumor
strategies (18,19).
While >90% of patients with advanced-stage cancer
exhibit elevated CA-125 levels in the blood, 50–60% of patients
with stage I ovarian cancer exhibit elevated CA-125 levels
(6,16). A potential explanation for the low
serum concentration of CA-125 in patients with partial epithelial
ovarian cancer (EOC) may be the development of circulating immune
complexes, which may interfere with detection (19). However, CA-125 testing has
limitations. Studies have reported false positives, with ~1% of
healthy individuals showing elevated CA-125 levels (>35 U/ml)
and 5% of patients with benign conditions also exhibiting increased
levels (20,22). Furthermore, CA-125 levels vary due
to factors such as differences in ethnicity, pregnancy, the
menstrual cycle (particularly the follicular phase), aging and
menopause (20,22). This raises questions regarding the
application of static cut-off points for CA-125 (20) and makes it an unreliable indicator
of ovarian cancer.
Due to its low sensitivity and specificity, research
shows that a single CA-125 measurement is insufficient for
efficient screening. To improve specificity and early-stage disease
detection, an approach with two phases that tracks changes in
CA-125 over time and applies transvaginal ultrasound for abnormal
increases can be adopted (20). It
has previously been demonstrated that the Risk of Ovarian Cancer
Algorithm, which combines age and serial CA-125 measurements, can
increase the identification of cancer during the early stages
(20,23). However, these actions are not
effective in decreasing ovarian cancer-associated mortality and are
expensive (20). The multivariate
index assay, which has been reported to have a lower specificity of
40% and a higher sensitivity of 94% compared with CA-125 assessment
alone, incorporates five different markers [CA-125, transferrin,
transthyretin (prealbumin), apolipoprotein AI and β-2
microglobulin] to generate a score to assess the likelihood of
ovarian cancer in patients with a pelvic mass (19).
In addition to CA-125, >110 other possible
protein biomarkers have been assessed separately and in
combination. Other potential biomarkers include CA15.3,
transthyretin, human epididymal protein 4 (HE4) and CA72.4
(6,16). HE4 is a 124-amino acid glycosylated
protein that is elevated in the serum of 60–75% of patients with
ovarian cancer and can identify a small percentage of cases that
are not detected based on CA-125. Other markers and techniques have
been shown to improve ovarian cancer detection, such as the
presence of autoantibodies, circulating tumor DNA (ctDNA),
microRNAs (miRs/miRNAs), DNA methylation, fallopian tube cytology
and tumor DNA detection in cervical screening tests (6,16).
Furthermore, it has been demonstrated that membrane-spanning mucin
(MUC16) glycoprotein CA-125 is a highly glycosylated protein with a
molecular weight of ~5 MDa (16).
The ovarian cancer cell surface is the site of cleavage of the
extracellular domain of MUC16, which releases CA-125 into the
pericellular space and the blood, where it can be detected using an
immunoassay (16). Extensive
research has demonstrated the association between ovarian cancer
and the MUC16 biomarker (17,18).
Furthermore, novel imaging techniques have been
assessed for the detection of ovarian cancer, including magnetic
resonance imaging (MRI) and relaxometry, superconducting quantum
interference device technology (SQUID), microbubbles and
light-induced endogenous fluorescence (autofluorescence) (6,16).
Although it is more expensive and less widely available, MRI
combined with relaxometry improves tissue characterization and
offers high-resolution, radiation-free imaging. The use of SQUID is
constrained by its technical complexity and requirement for
cryogenic equipment, despite the fact that it provides
ultra-sensitive detection of magnetic signals from cancer-related
biomarkers (24). Although it might
be limited in deep pelvic regions, microbubble-enhanced
ultrasonography increases visualization of tumor vasculature and
aids in early identification in a real-time and cost-effective
manner. Autofluorescence imaging uses variations in natural tissue
fluorescence to detect cancers. It is quick and non-invasive, but
its specificity is low (25).
Comorbidities, previous therapy and the specific
biology of the disease serve a role in guiding treatment decisions.
As 90% of cases of early-stage ovarian cancer are curable, this
highlights the importance of early detection and prompt specialist
treatment. However, most patients are diagnosed at a later stage,
when the effectiveness of targeted agents, such as chemotherapy and
surgery, is limited (26). Since
choices made during the surgical and medical phases of the disease
may impact the prognosis, controlling the cancer and decreasing the
symptoms are the primary goals of treatment (27). Furthermore, although the current
standard of care for the treatment of ovarian cancer is primary
debulking surgery followed by systemic chemotherapy (28,29),
the age at presentation, performance status (PS) and stage at
presentation are prognostic factors that influence the therapeutic
recommendations (30).
Surgical procedures serve a key role in the
management of ovarian cancer, functioning as both a diagnostic and
therapeutic approach (31). The
clinical stage of the disease, histology, specific biology and
clinical characteristics of the patient determine the extent of the
surgery. Surgery is primarily responsible for cytoreduction and
cancer staging. Cytoreductive surgery, which includes primary,
interval, second-look and secondary cytoreductive surgery, can be
performed at various stages during the course of the treatment
(26). The goal of surgeries is to
eradicate all macroscopic illnesses or leave no residual illnesses,
a state known as R0 (27).
There is a clear association between improved
survival results for women with ovarian cancer and complete
cytoreduction (R0), which is the removal of all visible tumor
burden. After surgery, individuals who have no residual disease
have a longer overall survival (OS) and progression-free survival
(PFS) than individuals who still have tumor tissue (32). According to Wright et al
(33), optimal cytoreduction was
associated with a median OS time of up to 60 months in cases of
advanced ovarian cancer; however, sub-optimal debulking
considerably lowered the median OS time to <30 months. These
findings underscore the critical importance of achieving complete
cytoreduction to enhance patient prognosis.
Beyond its direct therapeutic benefits, surgical
intervention also enables the collection of tissue samples for
histopathological evaluation and molecular profiling, which are
crucial for guiding personalized treatment approaches. For example,
BRCA-mutated patients may benefit from PARP inhibitors as part of
their treatment regimen (34,35).
Furthermore, the combination of surgery with systemic therapies,
including chemotherapy and targeted therapies, maximizes treatment
efficacy and improves patient outcomes. Given its pivotal role in
disease management, surgical intervention represents a necessary
component of comprehensive ovarian cancer treatment (36,37).
Of patients with ovarian cancer, ~35% are diagnosed
at early stages (FIGO stage I–II) (38,39).
The typical course of treatment entails extensive surgical staging
to diagnose the condition and determine the extent of the illness
(38). In the initial stages, the
surgery needs to be staged (or stratified) and the following
protocols should be performed: Peritoneal lavage, total
hysterectomy with bilateral salpingo-oophorectomy, biopsy of any
suspicious areas; resection of any adhesions adjacent to the tumor,
infracolic omentectomy and random biopsy of the uterine fundus,
bladder peritoneum, right and left pelvic walls, ovarian fossae,
right and left colic canals and both hemidiaphragms. Additionally,
pelvic lymphadenectomy, along with sampling or dissection of
para-aortic and paracaval lymph nodes, should be performed.
Adjuvant chemotherapy should be used if necessary, and the surgery
should yield sufficient information for prognostication and staging
(27).
In advanced stages, the objective of EOC treatment
is to eradicate all visible macroscopic disease, as this has been
associated with a higher OS and longer time without disease
(27,40). Cytoreductive surgery and
platinum-based chemotherapy are the most commonly used treatments
for advanced ovarian cancer. Since primary cytoreductive surgery is
the gold standard for patients with advanced ovarian cancer and as
this surgical outcome is associated with improved OS, the aim of
the procedure is to stage the tumor and cytoreduce its volume to
the point where there is no gross residual disease >1 cm of
tumor (27,41,42).
An alternative to primary cytoreductive surgery for patients deemed
unsuitable due to insufficient physical fitness or incapacity to
resect disease to <1 cm is interval debulking surgery combined
with platinum-based neoadjuvant chemotherapy (41).
Chemotherapy is a mainstay in the management of
ovarian cancer, showing notable effectiveness in different stages
and clinical settings (43).
Platinum-based agents and taxanes are combined in a standard
first-line regimen, resulting in marked response rates, and
extending OS and PFS, especially in advanced stages of the disease
(37,44). Chemotherapy is important in cases of
recurrence; recurrence of platinum-sensitive tumors typically
responds to treatment with platinum-based regimens, while
non-platinum agents are used in platinum-resistant cancer to
mitigate disease progression (45).
Furthermore, the integration of chemotherapy with novel therapeutic
strategies, such as PARP inhibitors and anti-angiogenic agents, has
enhanced its efficacy, particularly in patients with specific
genetic profiles such as BRCA mutations (46,47).
Clinical trials have demonstrated that these combinations not only
improve response rates but also prolong PFS and, in some cases, OS.
For instance, the addition of bevacizumab to chemotherapy was
associated with a median PFS time improvement of several months in
advanced ovarian cancer (48),
while PARP inhibitors as maintenance therapy post-chemotherapy
reduced the risk of recurrence by up to 70% in BRCA-mutated
patients (46). These advancements
underscore the evolving role of chemotherapy as a foundational
treatment that synergizes with targeted therapies to optimize
outcomes for patients with ovarian cancer.
Platinum-based compounds are considered to be the
most effective chemotherapeutic drugs in ovarian cancer (28,49).
Platinum chemotherapy compounds have been used since the mid-1970s.
Cisplatin was the first platinum-based drug, but it had several
undesirable side effects, such as nephrotoxicity, neurotoxicity,
ototoxicity, gastrointestinal tract problems and allergic
reactions. Consequently, the development of second-generation
platinum led to the 1989 launch of carboplatin, which is equally as
effective as cisplatin but has fewer severe side effects,
especially regarding nephrotoxicity (50). The guidance on the use of
platinum-based chemotherapy for relapsed EOC has changed over time,
becoming a limited and occasionally variable time-based approach.
If a relapse occurs >6 months after the conclusion of the
previous platinum-based treatment, the patient is deemed
‘platinum-sensitive’ and eligible for further platinum-based
chemotherapy. If the gap is <6 months, the patients are
considered ‘platinum-resistant’ and not suitable for platinum-based
treatment (44). In the latter
case, non-platinum regimens are typically offered. Single-agent
non-platinum-based chemotherapy, such as weekly administration of
paclitaxel, pegylated liposomal doxorubicin or topotecan, is
typically offered to patients who are not eligible for additional
platinum-based chemotherapy (44,49).
Oral etoposide, tamoxifen, gemcitabine and treosulfan are also
potential non-platinum alternatives; however, there is a limited
probability of these medications being effective because of their
decreased cytotoxic potency and the development of resistance
mechanisms, including enhanced DNA repair and increased drug
efflux, these agents show limited efficacy (51). Moreover, their lack of molecular
specificity and weakened activity against aggressive tumor subtypes
result in lower response rates compared with platinum-based
chemotherapy (52).
Adjuvant chemotherapy using carboplatin (area under
the curve, 5–6) and paclitaxel (175 mg/m2) are
administered following cytoreductive surgery in accordance with
established protocols (53).
Typically, 6–8 cycles are given every 21 days (30). While there is some disagreement
regarding the optimal number of chemotherapy cycles, to the best of
our knowledge, there is no evidence that >6 cycles of
postoperative combination chemotherapy improve outcomes for
patients with advanced ovarian cancer (28,54).
It is advised to start chemotherapy as soon as possible following
surgery, usually within 2–4 weeks; longer wait times are associated
with worse results (30). Notably,
compared with single-agent platinum-based regimens, a combination
of platinum-based drugs (containing paclitaxel, gemcitabine or
pegylated liposomal doxorubicin) is associated with longer PFS and
OS (51). The toxicity profile
should be taken into consideration when choosing therapeutic agent
combinations (55).
Despite high initial response rates (~70%) with
chemotherapy and surgery, recurrence is a notable concern (56). In the 10 years following diagnosis,
80–85% of patients with advanced ovarian cancer experience a
relapse (55). The need for
additional therapy and their performance status should be
considered before beginning treatment for recurrent disease. The
next stage is to determine whether platinum is the best option;
severe side effects, which include fatigue, arthralgia and
neurotoxicity, of chemotherapeutic treatments for ovarian cancer
impede the quality of life (55).
Thus, investigation of the disease mechanisms requires an
understanding of the biology of heterogeneous ovarian cancers.
Intrinsic signaling pathways, angiogenesis, hormone receptors and
immunological factors are among the possible therapeutic targets
being investigated for the treatment of ovarian cancer (56). When chemotherapy is combined with
targeted treatments such as bevacizumab and PARP inhibitors,
patients with homologous recombination (HR) deficiency (HRD) or
BRCA mutations (BRCAms) exhibit improved results once compared with
chemotherapy alone (57).
Certain patients experience relapses following
chemotherapeutic treatments as a result of developing drug
resistance mechanisms (58). In
ovarian cancer, the initial treatment with carboplatin and
paclitaxel as first-line chemotherapy has shown an enhanced
complete response rate compared with single agents or platinum
based regimens (50). However,
recurrence rates are 70–80%, particularly for patients with
advanced-stage cancer (50).
Notably, patients who receive neoadjuvant carboplatin therapy
before surgery are more likely to exhibit platinum resistance.
Matsuo et al demonstrated a markedly elevated incidence of
carboplatin resistance among patients who receive neoadjuvant
therapy (33.3%) compared with those undergoing primary
cytoreductive surgery (9.2%) (59).
Similarly, Rauh-Hain et al identified a substantially higher
prevalence of carboplatin resistance in patients who underwent
neoadjuvant therapy (88.8%) compared with those subjected to
primary cytoreductive surgery (55.3%) (60).
The molecular diversity of tumor cells contributes
to variations in signaling pathways, involving the activation of
oncogenes, inactivation of tumor suppressors and the presence of
pro-survival genetic mutations. Consequently, resistance to
standard chemotherapy regimens is a hurdle in managing the disease
(61) and treating patients
effectively (56). Thus,
understanding of resistance mechanisms is important for the
development of novel therapeutic approaches. There are two primary
types of resistance: Intrinsic and acquired (extrinsic) resistance.
Nevertheless, accurate discrimination between these forms is
difficult (34). Intrinsic
resistance pertains to the inherent capability of cancer cells to
withstand treatment owing to pre-existing characteristics present
before their initial exposure to therapeutic agents. Cell
attributes associated with intrinsic chemo-resistance include the
capacity to decrease drug uptake, increase drug efflux and elevate
the activity of detoxification enzymes such as cytochrome P450 or
glutathione (GSH) transferases. Conversely, acquired
chemoresistance can emerge from genetic and epigenetic alterations
that enable cancer cells to adapt to the effects induced by
chemotherapy, such as stress, DNA damage and apoptosis (22).
In ovarian cancer, a subset of patients possess
germline mutations in BRCA1 and/or BRCA2, which are key
constituents of the HR pathway, essential for repairing DNA
double-strand breaks. Consequently, BRCAms impair the capacity to
rectify DNA damage via HR, potentially accounting for the
heightened sensitivity of this cancer subtype to platinum-based
chemotherapeutic agents (62).
Conversely, the p53 protein, which serves a key role in governing
the cell cycle, is sensitive to DNA damage incurred during
replication, resulting in G1 arrest and/or apoptosis,
thereby inhibiting the generation of defective cells (63). Mutation of the gene responsible for
p53 expression in human cancer can result in the loss of p53
function. This enables uncontrolled cell proliferation and confers
resistance to agents inducing DNA damage. Consequently, a potential
avenue for addressing chemotherapy resistance involves reactivating
mutant p53 (64).
Ovarian cancer resistance to chemotherapy is
markedly influenced by abnormal transmembrane transport, which
includes decreased drug influx and increased drug efflux, leading
to decreased intracellular drug concentrations and treatment
failure. Platinum-resistant ovarian cancer exhibits diminished drug
transport-associated gene and transmembrane transporter expression,
resulting in inadequate intracellular platinum accumulation
(51,65). miRNAs serve a key role in regulating
these transporters by binding to the 3′-untranslated region (UTR)
of target genes, thereby modulating their transcription and
contributing to drug resistance (66). The solute carrier (SLC) superfamily
transporters, such as SLC31A1 and SLC22A1/2/3, are responsible for
drug influx. The transport of cisplatin, carboplatin and
oxaliplatin by SLC31A1 aids intracellular platinum accumulation
(67). Patients with ovarian cancer
and low SLC22A2 expression are more likely to develop resistance to
platinum drugs as they are unable to absorb as much of the drug
(68). While evidence has
demonstrated that dysregulated expression of miRNAs and target
genes serves a critical role in the initiation, proliferation,
survival and chemoresistance of ovarian cancer, the understanding
of how miRNAs contribute to the disease pathology remains limited
(69,70). Studies focusing on how miRNAs
regulate the pathology of ovarian cancer account for <4% of the
total published research, highlighting the need for further
investigation (69).
Key efflux transporters include ATP-binding cassette
subfamily B member 1 (ABCB1), G member 2 and C. miRNAs, such as
miR-27a, miR-451, and miR-298, directly bind to the 3′-UTRs) of ABC
transporter mRNAs, thereby inhibiting their translation or
promoting mRNA degradation by influencing the expression of genes
that encode nuclear receptors, transcription factors and signaling
molecules associated with ABC transporters. ABCB1 is the only
efflux transporter reported to exhibit elevated expression in
resistant ovarian cancer cells, while the expression of other ABC
transporters is markedly decreased (71). P-glycoprotein, an ATP-dependent drug
efflux pump, is encoded by ABCB1 and upregulated in resistant
ovarian cancer cell lines, making it a key factor in resistance to
paclitaxel, doxorubicin, sorafenib and PARP inhibitors (72,73).
miRNAs, including miR-130a/b, miR-186 and miR-495, bind to the
3′-UTR of ABCB1 to degrade the mRNA or limit translation. Although
the exact regulatory mechanism is unknown, upregulated ABCB1
expression decreases miR-21-5p expression (68). In patients with HGSOC who undergo
chemotherapy or targeted treatment, a whole-genome study identified
that an increase in ABCB1 expression is associated with the
transcriptional fusion of ABCB1 and SLC25A40 (74).
Beyond transport mechanisms, drug resistance also
results from drug inactivation by metallothionein (MT) and GSH
(Table III). These
thiol-containing proteins bind platinum-based drugs, rendering them
inactive, allowing for drug resistance beyond transport mechanisms.
Short hairpin RNA targeting MT reverses the well-established
resistance mechanism by decreasing MT binding to cisplatin
(68,75). The GSH S-platinum complex formed by
GSH and cisplatin lowers intracellular platinum levels (76). This platinum inactivation mechanism
is catalyzed by GSH S-transferase and associated with platinum
resistance in ovarian cancer (68,77).
Targeted therapy for ovarian cancer utilizes
treatments that specifically target the pathways essential for the
progression of the disease. By targeting specific proteins, these
treatments minimize the adverse effects of cytotoxic treatment on
healthy cells. Patients with recurrent disease are typically the
first to be assessed for targeted therapy (78). If these treatments demonstrate
potential in clinical studies focusing on recurrent diseases, they
may be considered a primary treatment option for further
investigation. In previous years, targeted therapies, such as PARP
inhibitors, antiangiogenic medications and MAPK inhibitors, have
been acknowledged as notable advancements in treating ovarian
cancer (79,80).
Targeted therapies have revolutionized the treatment
of ovarian cancer by focusing on specific biological pathways that
drive tumor development and resistance. Bevacizumab and PARP
inhibitors are two of the most commonly used targeted treatments
(81). Bevacizumab, a VEGF
inhibitor, prevents the formation of new blood vessels, decreasing
blood supply and slowing tumor growth (82). Bevacizumab can be administered
alongside chemotherapy during initial treatment or as maintenance
therapy, either alone or in combination with olaparib, a PARP
inhibitor. PARP inhibitors block PARP protein, which serves a key
role in DNA repair in cancer cells (46,47).
Initially developed for patients with mutations in BRCA1 or BRCA2,
the use of PARP inhibitors has expanded to patients with other
types of DNA repair deficiencies (such as RAD51C (RAD51 Paralog
C)/RAD51D (RAD51 Paralog D), ATR (Ataxia Telangiectasia and
Rad3-related protein), CHEK1 (Checkpoint Kinase 1)/CHEK2
(Checkpoint Kinase 2), BARD1 (BRCA1 Associated RING Domain 1),
BRIP1 (BRCA1 Interacting Protein C-terminal Helicase 1), ATM
(Ataxia Telangiectasia Mutated) and PALB2 (Partner and Localizer of
BRCA2)) (83). PARP inhibitors
approved for use as ovarian cancer treatment are primarily used as
maintenance therapy following chemotherapy for advanced ovarian
cancer, decreasing the risk of recurrence and tumor progression
(84). Previous research has
evaluated their effectiveness in broader patient populations,
including those with inherited mutations in DNA repair genes, such
as partner and localizer of BRCA2 (PALB2), BRCA1-interacting
protein C-terminal helicase 1 (BRIP1), RAD51 recombinase paralog C
(RAD51C) and RAD51 recombinase paralog D (RAD51D) (85). Additionally, patients without
inherited mutations, but with acquired tumor biomarker mutations in
DNA repair genes may also benefit from PARP inhibitors (86). There is growing interest in
combining PARP inhibitors with immunotherapy or other targeted
agents to enhance treatment outcomes (87,88).
Other targeted therapies have been developed for
ovarian cancer, particularly for recurrent disease or cases where
chemotherapy is ineffective. Mirvetuximab soravtansine (MIRV)-gynx
is approved for recurrent ovarian cancer positive for folate
receptor α (FRα) (89).
Larotrectinib is used to treat metastatic ovarian cancer or cases
that cannot be surgically removed and have progressed despite prior
treatment, especially if the tumor harbors a neurotrophic receptor
tyrosine kinase gene fusion (90).
Selpercatinib is prescribed for ovarian cancer with a RET gene
fusion, as identified by tumor biomarker testing (91). The integration of these therapies
emphasizes the importance of biomarker testing, which can identify
patients most likely to respond to precision medicine approaches.
Ongoing research continues to explore novel targeted therapies and
combination treatment strategies aimed at improving survival and
clinical outcomes for patients with ovarian cancer (92–94).
In 2014, the United States Food and Drug
Administration (FDA) and European Medication Agency authorized the
use of PARP inhibitors for the treatment of ovarian cancer
(95). These drugs target EOC that
cannot repair DNA through HR, which is key for fixing
double-stranded DNA breaks (80,95).
Mutations in BRCA1/2 induce HR repair (HRR) pathway deficiencies in
tumor cells, which prevent DNA double-stranded breaks from being
repaired. PARP inhibitors prevent DNA damage repair in these cells,
inducing apoptosis by synthetic lethality (96). Synthetic lethality describes a
phenomenon where the presence of a mutated gene, such as one
involved in DNA repair, combined with the functional loss or
inhibition of another gene or its product, leads to a synergistic
effect that induces cellular toxicity and cell death (97).
The FDA has approved olaparib as a maintenance
treatment for patients with advanced ovarian cancer with a BRCAm
who respond well to initial platinum-based chemotherapy (98). Notably, olaparib increased PFS
compared with a placebo following a median follow-up of ~41 months.
Furthermore, after 7 years of monitoring, a notable improvement in
OS was noted in the SOLO1 (trial no. NCT01844986) clinical study
(99).
A previous study compared the efficacy of
maintenance niraparib with a placebo in patients with advanced
ovarian cancer (100,101). In the PRIMA study (trial no.
NCT02655016), 733 patients with newly diagnosed advanced ovarian
cancer were randomly assigned to receive either maintenance
niraparib or a placebo for up to 36 months, or until disease
progression. After 3.5 years of follow-up, the improvement in
progression-free survival (PFS) with niraparib was significant,
confirming PFS as the primary and durable outcome of the trial
(101,102). As a first line of maintenance
treatment, 384 patients with advanced ovarian cancer were
randomized to receive niraparib [individualized starting dose
(ISD)] or a placebo in the PRIME trial (NCT03709316). Following a
median observation period of 27.5 months, there was a marked
increase in PFS with the niraparib (ISD) regimen compared with the
placebo (100).
Similarly, the ATHENA-MONO trial (trial no.
NCT03522246) compared maintenance rucaparib with a placebo
(103). Rucaparib maintenance
therapy markedly increased the median PFS compared with the placebo
in the HRD-positive patient group after a median follow-up of ~26
months (103,104). Initial research demonstrated that
administration of rucaparib resulted in improved PFS outcomes in
patients with BRCAm, non-BRCAm/loss of heterozygosity-high cancer
and malignancies that test negative for HRD compared with a placebo
(103). Preservation therapy with
PARP inhibitors is effective when administered initially to
patients with BRCAm or tumors exhibiting HRD (103).
While the PARP inhibitor veliparib is in the
advanced stages of clinical testing, the FDA has approved four PARP
inhibitors to date: Olaparib, rucaparib, niraparib and talazoparib
(105). Talazoparib may exert its
therapeutic effect by blocking PARP enzyme activity, which leads to
PARP1/2 being trapped on damaged DNA (thereby inhibiting DNA
repair) (106). The clinical
efficacy of olaparib, an oral PARP inhibitor, or cediranib, an oral
VEGF inhibitor, in conjunction with durvalumab, has been evaluated
in a phase I dose-escalation trial (107). After determining that chemotherapy
with avelumab exhibits antitumor activity and acceptable safety,
the JAVELIN OVARIAN PARP100 trial (trial no. NCT03642132) proceeded
with maintenance treatment combining the two drugs (108). The selective PARP inhibitor
saruparib markedly inhibited tumor growth in preclinical models of
breast, ovarian, pancreatic and prostate cancer with HRD mutations
(109,110). Notably, saruparib exhibited lower
toxicity compared with other PARP inhibitors, allowing
administration at higher doses (111).
Chemotherapeutic drugs, such as carboplatin and
paclitaxel, along with angiogenesis inhibitors, such as
bevacizumab, have demonstrated synergistic effects with PARP
inhibitors (35,46). Clinical trials evaluate these
combinations to optimize treatment strategies (112–114). Although PARP inhibitors have
markedly improved the treatment of ovarian cancer, particularly in
patients with BRCAm and HRD, their use is hindered. A major
challenge is drug resistance (Table
III). Adverse effects, including hematological toxicity
(anemia, neutropenia and thrombocytopenia) and gastrointestinal
problems (nausea, vomiting and diarrhea), further limit treatment
adherence (115,116). Furthermore, the high cost of PARP
inhibitors restricts access, particularly in low-resource settings,
making affordability a concern (117). Another serious risk is the
potential development of secondary malignancy during and after
treatment with PARP inhibitors (118). To address these challenges,
research is exploring combination therapy, novel biomarkers for
patient selection and strategies to overcome resistance and enhance
PARP inhibitor efficacy (119–121).
Although angiogenesis inhibitors have demonstrated
promising efficacy in ovarian cancer treatment, patients exhibit
adverse effects such as hypertension, proteinuria and
gastrointestinal perforation, necessitating careful patient
selection (131,132). Therapeutic strategies that combine
angiogenesis inhibitors with PARP inhibitors or immunotherapy have
been explored to improve clinical outcomes and overcome resistance
mechanisms (96).
Several miRNAs are dysregulated in numerous types
of cancer, and this dysregulated expression is associated with
resistance to chemotherapy (141).
miR-139-5p serves a crucial role in ovarian cancer, and its
expression levels are decreased in ovarian cancer tissues from
cisplatin-resistant patients (142). As such, upregulation of miR-139-5p
can impede proliferation, decrease resistance to cisplatin and
enhance apoptosis in ovarian cancer cells. Furthermore, the
combined use of miR-139-5p and MAPK inhibitors decreases cisplatin
resistance in ovarian cancer (143). Thus, upregulating miR-139-5p
expression may be a promising treatment approach for ovarian cancer
(144).
Immunotherapy, which is designed to encourage the
immune system to identify and eradicate cancerous cells, has become
a promising therapeutic option for ovarian cancer;
immunotherapeutic strategies, such as immune checkpoint inhibitors
(ICIs) and customized vaccinations, improve outcomes in certain
patients with ovarian cancer (145).
Dendritic cells are specialized antigen-presenting
cells that are essential for initiating and guiding the development
of numerous subsets of CD4+ T cells. They activate
immune cells to fight against invading pathogens or cancer cells,
and the presence of tumors hinders their function (146). TGF-b is a protein released by
tumor cells that impedes the ability of cytotoxic CD8 T lymphocytes
to eradicate cancer cells (147).
Programmed death ligand 1 (PD-L1) is an immunosuppressive ligand,
which is produced by tumor cells. PD-L1 induces immunological
tolerance by inhibiting T cells through binding to their receptor,
programmed cell death protein 1 (PD-1). Additionally, PD-L1
inhibits IL-2 release by interacting with PD-1, inducing T-cell
immunity. PD-L1 expression on monocytes in blood samples of
patients with ovarian cancer is associated with a poor prognosis
(148,149). Antigen-presenting cells have
another immunological checkpoint known as cytotoxic T
lymphocyte-associated protein 4 (CTLA-4). CTLA-4 binds to CD80, a
co-stimulatory factor, and prevents T-cell activation, resulting in
cell cycle arrest (150).
Anti-PD-1 and anti-CTLA-4 ICI therapies were first
authorized for use in 2011 (such as ipilimumab) to treat malignant
melanoma and non-small and renal cell carcinoma. Official
authorization for other anti-PD1 therapies, such as nivolumab use
was obtained in 2014 (151). In a
phase I trial (trial no. NCT00729664), 17 patients with ovarian
cancer were treated with a PD-L1 blocking antibody (BMS-936559); 1
patient exhibited a partial response, while disease stability was
reported in 2 patients (152). A
total of 10% of patients with platinum-resistant ovarian cancer
exhibited a sustained complete response in a phase II trial (trial
no. UMIN000005714) utilizing nivolumab (anti-PD-1) (153). In the KEYNOTE-028 multicohort
phase Ib trial (trial no. NCT02054806), patients with
platinum-resistant ovarian cancer treated with pembrolizumab
achieved an objective response rate of 11.5%, while 23% of patients
experienced stable disease (154).
Ipilimumab, a CTLA-4 blocker, was given as a monotherapy in a phase
II trial including patients with platinum-sensitive ovarian cancer
(trial no. NCT01611558) (155);
95% of the patients did not survive the induction phase due to
disease progression, medication toxicity, mortality or undiscovered
factors.
Due to the limited efficacy of immunotherapies that
target the PD-1/PD-L1 pathway in ovarian cancer, there is interest
in combination treatments that target additional immune
checkpoints, such as the T cell immunoreceptor with Ig and ITIM
domains (TIGIT)/CD155/DNAX accessory molecule-1 (DNAM-1) pathway.
This dual blockade approach may boost T cell and natural killer
(NK) cell activity against cancer cells, improve tumor antigen
expression and overcome immunosuppression in the tumor
microenvironment (156,157). However, more research is required
to understand the mechanisms and synergistic benefits of targeting
both the PD-1/PD-L1 and TIGIT/CD155/DNAM-1 pathways in ovarian
cancer (156). Although
immunotherapy has potential in the treatment of ovarian cancer,
individual outcomes can vary, and certain patients may not
experience notable improvements. Immunotherapy may also result in
immune-associated side effects, such as autoimmune responses and
inflammation (158).
Researchers have investigated various combination
treatment approaches to enhance clinical outcomes (26,159).
Integrating multiple treatment modalities effectively treats
ovarian cancer by enhancing the efficacy of each approach (160). These approaches utilize the
increasing accessibility of therapeutic drugs and comprehension of
the disease biology. The combinations target multiple cancer
pathways simultaneously by utilizing DNA-damaging medications,
targeted therapy impacting signaling pathways and immunotherapies.
Immunotherapy is effective in patients with hypercalcemic small
cell carcinoma with high PD-L1 expression and severe ovarian cancer
due to their active immune environment (161). A review of 15 clinical trials,
involving 945 patients with advanced ovarian cancer, found that
PD-1/PD-L1 inhibitors achieve an overall response rate (ORR) of
19%. These inhibitors were significantly more effective when
combined with chemotherapy (36% ORR) compared to when used alone
(9% ORR) (162). Additionally,
patients with platinum-sensitive ovarian cancer responded better to
these inhibitors (31% ORR) than those with platinum-resistant
disease (19%) ORR (162).
Checkpoint-blocking medications have not been successful in
treating ovarian cancer despite their efficacy in solid tumors such
as melanoma, lung cancer and renal cell carcinoma (163).
It has been demonstrated that elevated
intracellular enzyme indoleamine 2,3 dioxygenase (IDO) levels
suppress the immunological response (164). Due to its toxic nature, IDO
converts tryptophan into kynurenine, enhancing regulatory T cell
levels and reducing NK cell levels (165). This results in a weakened immune
response, allowing cancer cells to evade immune detection and
continue to proliferate. In a phase I trial (trial no.
NCT01191216), 41% of patients with various metastatic solid tumors
achieved disease stability. By comparison, 18% had a partial
response when given a combination of docetaxel and the IDO
inhibitor indoximod (146,166). Phase II research on using an IDO1
inhibitor + tamoxifen to treat recurrent EOC and primary peritoneal
and fallopian tube carcinoma was discontinued because there was no
notable difference in responses between the treatment and control
groups (167).
Combining ICIs with cytotoxic medications is a
rational approach to enhance tumor immunogenicity and improve the
effectiveness of ICIs. For example, a phase II trial investigated
the efficacy of combining nivolumab with bevacizumab in recurrent
ovarian cancer (168). The
combination demonstrated clinical effectiveness, with an overall
response rate (ORR) of 28.9% and a PFS of 8.1 months. Atezolizumab
is being evaluated in various cancer types in ongoing phase III
trials that combine it with chemotherapy and/or bevacizumab. OS and
investigator-assessed PFS are co-primary outcomes in the IMagyn050
study (NCT03038100) (169,170). Evaluation of atezolizumab
effectiveness in combination with platinum-based chemotherapy with
concurrent and maintenance bevacizumab is the primary goal of the
ATALANTE study (NCT02891824) (170).
Additionally, ICIs have been studied in combination
with PARP inhibitors, as reported in the TOPACIO/KEYNOTE-162 trial
(trial no. NCT02657889). Niraparib + pembrolizumab achieved a 25%
ORR and 68% disease control rate (DCR) in patients with
platinum-resistant recurrent ovarian cancer, with patients with
BRCAm showing higher responses (ORR, 45%; DCR, 73%). In
platinum-sensitive recurrent ovarian cancer, adding atezolizumab to
carboplatin and niraparib maintenance does not improve PFS,
regardless of BRCA status or PD-L1 expression (171). The DUO-O trial (NCT03737643)
demonstrated that triplet therapy (Durvalumab with chemotherapy and
Bevacizumab, followed by maintenance Durvalumab, Bevacizumab and
Olaparib) extended the median PFS by 5 months overall and 14.3
months in HRD-positive patients compared with bevacizumab alone,
offering notable benefits for non-BRCAm ovarian cancer (172). Furthermore, ICIs have been studied
with antibody-drug conjugates in the FORWARD II trial
(NCT02606305). MIRV combined with pembrolizumab had promising
efficacy in 14 patients with FRα-platinum-resistant recurrent
ovarian cancer, achieving a 43% ORR, a median duration of response
of 6.9 months and a median PFS of 5.2 months, with no severe
adverse events (145).
The FDA has approved olaparib in combination with
bevacizumab as a maintenance treatment for patients with advanced
ovarian cancer who show improvement after receiving first-line
platinum-based chemotherapy and whose tumors are positive for HRD
(173). The primary endpoint of
investigator-assessed PFS was markedly longer with olaparib +
bevacizumab compared with the placebo + bevacizumab after a median
follow-up of 22.9 months. The combination of olaparib and
bevacizumab resulted in a markedly improved PFS for patients with
BRCAm tumors and tumors positive for HRD compared with bevacizumab
alone. The combined group receiving olaparib and bevacizumab had a
median OS of 56.5 months, while the placebo + bevacizumab group had
a median OS of 51.6 months, according to a randomized controlled
trial (174). Patients whose
tumors were positive for HRD or for BRCAm had the longest median
PFS and the highest rates of PFS at 18 months (175,176). Furthermore, OVARIO (phase II), has
examined the use of niraparib with bevacizumab as a first-line
maintenance regimen for patients with recently diagnosed advanced
ovarian cancer demonstrated promising progression-free survival
(PFS) outcomes. The safety profile was consistent with the
established adverse effect patterns of niraparib and bevacizumab
when administered as monotherapies. In the triplet combination of
niraparib, dostarlimab and bevacizumab, the OPAL-A trial
(NCT03574779) observed limited ORR in patients with
platinum-resistant ovarian cancer, although the median PFS of 7.9
months and OS of 22.1 months were favorable compared with
historical data. Notably, the majority of responders (85.7%) were
bevacizumab-naïve. Exploratory biomarker analysis from paired pre-
and post-treatment samples indicated immune activation, warranting
further investigation into whether these biomarkers predict the
clinical efficacy of triplet therapy (177).
Combining therapeutic modalities can improve the
efficacy of ovarian cancer treatment, but it also makes treatment
more complicated and raises the possibility of adverse effects,
such as immunological, hematological and gastrointestinal toxicity
(178).
The domain of cancer nanomedicine is experiencing
notable growth, with a range of nanoparticle systems investigated
through various targeting strategies, suggesting potential for
reshaping cancer therapeutics (179,180). Nanomedicine may confer notable
advantages over traditional chemotherapeutic agents (181). Nanotechnology-based therapeutics
are associated with improved efficacy, decreased toxicity
experienced by healthy tissues and improved patient adherence
(182). Furthermore, the
encapsulation of drugs within nanocarriers offers control over
pharmacokinetic properties, including drug release kinetics,
prolonged circulation half-life and interaction with healthy
tissues (58,180). As such, numerous materials,
including carbon- and metal-based nanomaterials, liposomal
formulations, cubosomes, lipid and polymeric nanoparticles,
micelles (179,182), as well as viral and cell
membrane-coated nanoconstructs (179), have been investigated.
Notably, investigations have examined the
application of nanomaterials in the encapsulation and concurrent
delivery of not only pharmaceutical agents but also imaging agents
and genetic material, as well as in the recognition of neoplastic
cells through receptor-specific binding mechanisms (179,183). Additionally, these nanostructures
yield synergistic effects by amalgamating imaging techniques, such
as Ultrasound, Computed Tomography (CT), Magnetic Resonance Imaging
(MRI), Positron Emission Tomography (PET), fluorescence Imaging,
and photoacoustic Imaging, with one or multiple therapeutic
approaches, such as chemotherapy, photodynamic and photothermal
therapy, radiotherapy, and immunotherapy (179).
Two principal tumor targeting strategies, passive
and active targeting, have been investigated (180,182). The aberrant vascular architecture
resulting from rapid tumor vascularization, coupled with inadequate
lymphatic drainage, facilitates the enhanced permeability and
retention (EPR) effect, which is key for the enrichment of
proliferating malignant tumors (180–182). Nevertheless, passive targeting is
associated with non-discriminatory accumulation in both healthy and
diseased tissue, akin to conventional chemotherapeutic regimens
(181). Additionally, the EPR
effect presents challenges, as macromolecules or nanoparticles must
evade reticuloendothelial system clearance and renal filtration to
infiltrate tumor tissue (184).
Furthermore, to exploit the EPR effect, a drug must remain in
circulation for ≥6 h to accumulate in neoplastic tissues (180). By contrast, active targeting
strategies leverage the strong binding affinity of targeting
ligands or agents to tumor cell surfaces, facilitating
receptor-mediated endocytosis (180,181). Targeted nanocarriers offer
advantages over non-targeted counterparts by enhancing efficacy at
the delivery site while mitigating potential adverse effects
(181). Several active targeting
ligands, including folate receptors, monoclonal antibodies, nucleic
acids and polypeptides, have been employed to modify nanocarriers,
thereby promoting cell uptake (185).
Patients with ovarian cancer often initially
respond favorably to conventional therapeutic approaches, but
develop resistance over time (182,186). An avenue to enhance the
effectiveness and specificity of chemotherapeutic agents involves
nanotechnology-based formulations, encompassing encapsulated,
conjugated or entrapped/loaded forms within nanocarriers or drug
delivery vectors (58,182). The integration of nanotechnology
in ovarian cancer management has garnered increasing attention due
to its promising attributes in molecular imaging, tumor targeting
and drug delivery (187,188). Furthermore, the use of
nanotechnology in ovarian cancer extends beyond the delivery of
therapeutic agents, including the incorporation of imaging and
diagnostic materials (189),
rendering such systems ‘theranostic’ nanotechnology (190,191). Various types of nanoparticles have
been employed in ovarian cancer therapeutics to facilitate the
delivery of drugs, including liposomes, nanoparticles, micelles,
dendrimers and polymers (187,189). Notably, certain formulations
loaded with chemotherapeutic agents have gained approval from the
FDA for ovarian cancer treatment (58). Examples of approved nanoparticles
include Doxil®, Genexol-PM® (192) and Abraxane® (190).
Several clinical studies have incorporated drugs
into nanoparticles for the treatment of ovarian cancer. Although
the majority of studies focused on applying nanoparticles with
chemotherapeutic agents, primarily paclitaxel (trial nos.
NCT03304210, NCT00499252, NCT00825201, NCT00666991 and
NCT00989131), docetaxel (trial no. NCT03742713) and doxorubicin
(trial no. NCT01489371), other clinical studies demonstrated the
effectiveness of applying nanoparticles with other treatment
options such as PARP inhibitors [olaparib (trial no. NCT04669002)]
or angiogenesis inhibitors [bevacizumab (trial no. NCT01652079)].
Furthermore, the application of nanoparticles in ovarian cancer
extends to studies of the application of combination therapy with
nanoparticles such as irinotecan with bevacizumab (trial no.
NCT04753216), sargramostim with paclitaxel (trial no. NCT00466960)
(193), apatinib and paclitaxel
(trial no. NCT03942068), and lapatinib and paclitaxel (trial no.
NCT00313599) (186).
Although nanoparticles have potential, there are
drawbacks, including toxicity, issues with biocompatibility and
immunological reactions that can cause accumulation in organs
(194). Concerns regarding
scalability, quality control and environmental effects are
associated with the complicated and expensive production process
(195). Numerous types of
treatment based on nanoparticles are in the experimental stage and
have had minimal clinical success despite continuous research
(196,197).
HRR pathway germline or somatic mutations are
responsible for 20–30% of ovarian cancer cases (198). BRCA1/2, RAD51C, RAD51D, BRIP1,
PALB2 and BRCA1 associated RING domain 1 are key proteins in the
HRR pathway. According to recommendations by National Comprehensive
Cancer Network (NCCN) (9,199,200), American Society of Clinical
Oncology and Society of Gynecologic Oncology, genetic testing is
recommended for all newly diagnosed cases of EOC (200). Furthermore, BRCA1/2 mutation
testing is crucial because it can inform the potential efficacy of
PARP inhibitors (9,200). Despite the clinical advantages of
genetic testing in ovarian cancer treatment, such as detection of
hereditary cancer syndromes, guiding treatment decisions,
facilitating risk assessment, early intervention and preventive
measures for patients and their family members, it remains
underused due to insufficient awareness among clinicians and
patients, financial and insurance-related barriers, and limited
availability of genetic counseling resources (9). Genetic testing for hereditary ovarian
cancer includes whole-exome/genome sequencing, multigene panels or
single-gene tests, with next-generation sequencing enabling
high-throughput analysis (201).
Variant interpretation follows American College of Medical Genetics
and Genomics guidelines, with in silico tools, databases
such as ClinVar and tumor testing used to differentiate germline
from somatic mutations (202). A
key part of the genetic testing process is genetic counseling,
particularly for individuals with hereditary cancer syndrome.
Professional genetic counselors or adequately qualified oncologists
should provide counseling, in accordance with regional regulatory
requirements (9).
The process of correcting a mutated gene to treat
an underlying disorder is known as gene therapy (203). Several gene therapy approaches
have been investigated in preclinical studies focused on the
management of ovarian cancer (96,204):
These strategies include the replacement of tumor suppressor genes
to reestablish cellular regulation (such as TP53), oncogene
inhibition (such as EGFR), suicide gene therapy involving the
introduction of toxin-encoding genes (such as herpes simplex virus
thymidine kinase), genetic immunopotentiation to enhance the immune
response against tumor cells (such as IL-12A/B), antiangiogenic
gene therapy (such as collagen type XVIII α1 chain), methods to
restore pharmacological sensitivity (such as survivin) and cancer
virotherapy (such as vesicular stomatitis virus). Notably, several
of these approaches have been tested in clinical trials; although
they showed promising results, most of them are in phase I
(96,204).
It is well established that estrogen stimulates the
proliferation of ovarian cancer cells (205–207). Estrogen signaling, which is
mediated by estrogen receptor (ER)α and ERβ and their various
isoforms, is further amplified by G protein-coupled ER 1 (208). Both in vitro and in
vivo studies have demonstrated that estrogen, through its
interaction with ERα, influences cell motility and survival by
promoting ovarian cancer cell proliferation and migration and
triggering epithelial-mesenchymal transition (205,209,210). Several clinical studies have
demonstrated that EOCs, which express ERα, respond well to hormonal
therapy such as tamoxifen and aromatase inhibitors (such as
letrozole) (211–213). When binding to ER, tamoxifen
competes with estrogen, but aromatase inhibitors work by preventing
the synthesis of estrogen (96).
Patients with platinum-resistant and recurrent
ovarian cancer may consider hormonal therapy as an alternative
treatment option according to the 2019 guidelines from the European
Society for Medical Oncology-European Society of Gynecological
Oncology (214) and the 2021
guidelines from NCCN (version 2.2021) (215). To the best of our knowledge, the
clinical effectiveness of hormonal therapy in the treatment of
ovarian cancer has not been systematically evaluated in large-scale
clinical trials, in spite of these recommendations. Notable trials
have assessed fulvestrant, an ER degrader (trial no. NCT00617188),
tamoxifen (trial nos. NCT02728622 and NCT00041080), arzoxifene, an
ER modulator (trial no. NCT00003670) and mifepristone, a
progesterone receptor modulator (trial nos. NCT00459290 and
NCT02046421) (96).
For patients undergoing surgery, adjuvant treatment
options such as intraperitoneal and HIPEC therapy should be
considered (216). Following
cytoreductive surgery, HIPEC is the administration of
chemotherapeutic agents directly into the peritoneal cavity to
improve patient outcomes through more efficient removal of residual
disease. The sensitivity of the tumor to treatment is increased by
the hyperthermic environment, which also improves chemotherapeutic
drug penetration at the peritoneal surface (96). Notably, when several ovarian cancer
treatments, such as carboplatin, paclitaxel and docetaxel, are
administered intraperitoneally compared with intravenously, their
effective drug concentration in the abdominal cavity is increased
several fold and their clearance from the peritoneal cavity is
notably slower (34). Although
intraperitoneal therapy has been demonstrated to markedly improve
the OS (216), other studies have
found that paclitaxel and cisplatin intraperitoneal therapy do not
extend OS or PFS in patients with stage III ovarian cancer
(217,218).
HIPEC is effective in treating gastric and
colorectal cancer and primary peritoneal carcinomatosis (92). Uncertainties exist regarding patient
selection, drug delivery protocol, treatment timing,
chemotherapeutic regimen and possibility of complications (96). The current NCCN guidelines state
that patients with ovarian cancer with peritoneal carcinomatosis
(FIGO stage III) who show improvement or stable disease following
neoadjuvant chemotherapy should be considered for HIPEC (96). Additionally, intraperitoneal
injection application has extended to include the administration of
low-dose bevacizumab after the drainage of malignant ascites and
has shown efficacy and a tolerable safety profile, especially in
symptomatic patients with chemotherapy-resistant ovarian, fallopian
tube or primary peritoneal cancer (219).
The metabolism of folate is key for cellular
functions such as DNA synthesis, methylation and repair (220). Folate and its derivatives enter
cells via endocytosis, which is aided by the transmembrane
glycoprotein FRα (221). In most
cases, FRα expression is limited to specific tissue, such as the
kidney, retina, lung, choroid plexus and placenta. FRα is markedly
upregulated in several types of cancer, such as those that impact
the ovaries, breast, lung and endometrium (222,223). Due to the capacity to enter the
cell post-ligand binding and its selective upregulation, FRα is a
desirable target for cancer drug delivery schemes (96). FRα is typically upregulated in
ovarian carcinoma, while this receptor is absent in normal ovarian
epithelium. Notably, the levels of soluble FRα (sFRα) in
circulation are associated with tumor FRα expression, disease
progression and treatment outcomes in patients with EOC. As a
result, sFRα shows improved diagnostic accuracy compared with serum
CA-125 levels, suggesting it may be a useful biomarker for early
EOC detection (224).
Approaches that target FRα have become increasingly
attractive in the treatment for ovarian cancer (96,225,226). Antibody-drug conjugates are a
specialized class of drugs to deliver chemotherapeutics to tumor
sites in a targeted and selective manner. A common antibody-drug
conjugate that targets FRα, MIRV, combines an anti-FRα antibody
with DM4, a strong tubulin-targeting agent. This compound functions
by binding FRα and allowing for the targeted delivery of DM4 to
tumor cells, which optimizes the balance of beneficial to side
effects (225). Treatment of
platinum-resistant EOC using MIRV has been evaluated (225). Following encouraging results from
the phase III trial SORAYA (trial no. NCT04296890), the FDA granted
accelerated approval in 2022 for use in patients with FRα-positive,
platinum-resistant EOC who have previously received systemic
anticancer therapy (225,227). The humanized monoclonal antibody
farletuzumab is another treatment strategy involving FRα.
Preclinical research has demonstrated that farletuzumab may hinder
FRα-expressing ovarian cancer cell proliferation (228,229). However, clinical trials evaluating
farletuzumab in combination with other therapies for
platinum-sensitive EOC (trial nos. NCT00318370 and NCT02289950)
have yielded conflicting outcomes (96,226).
STRO-002 is an innovative antibody-drug conjugate
targeting FRα, currently under clinical investigation for ovarian
and endometrial cancer. Preclinical studies have demonstrated that
a single dose of STRO-002 markedly inhibited tumor growth in
FRα-expressing xenograft and patient-derived models, with enhanced
efficacy when combined with carboplatin or bevacizumab (230,231). These findings underscore its
potential as a promising therapeutic option for FRα-expressing
cancer, including ovarian, endometrial and non-small cell lung
cancer (226).
Treatment options for ovarian cancer either in the
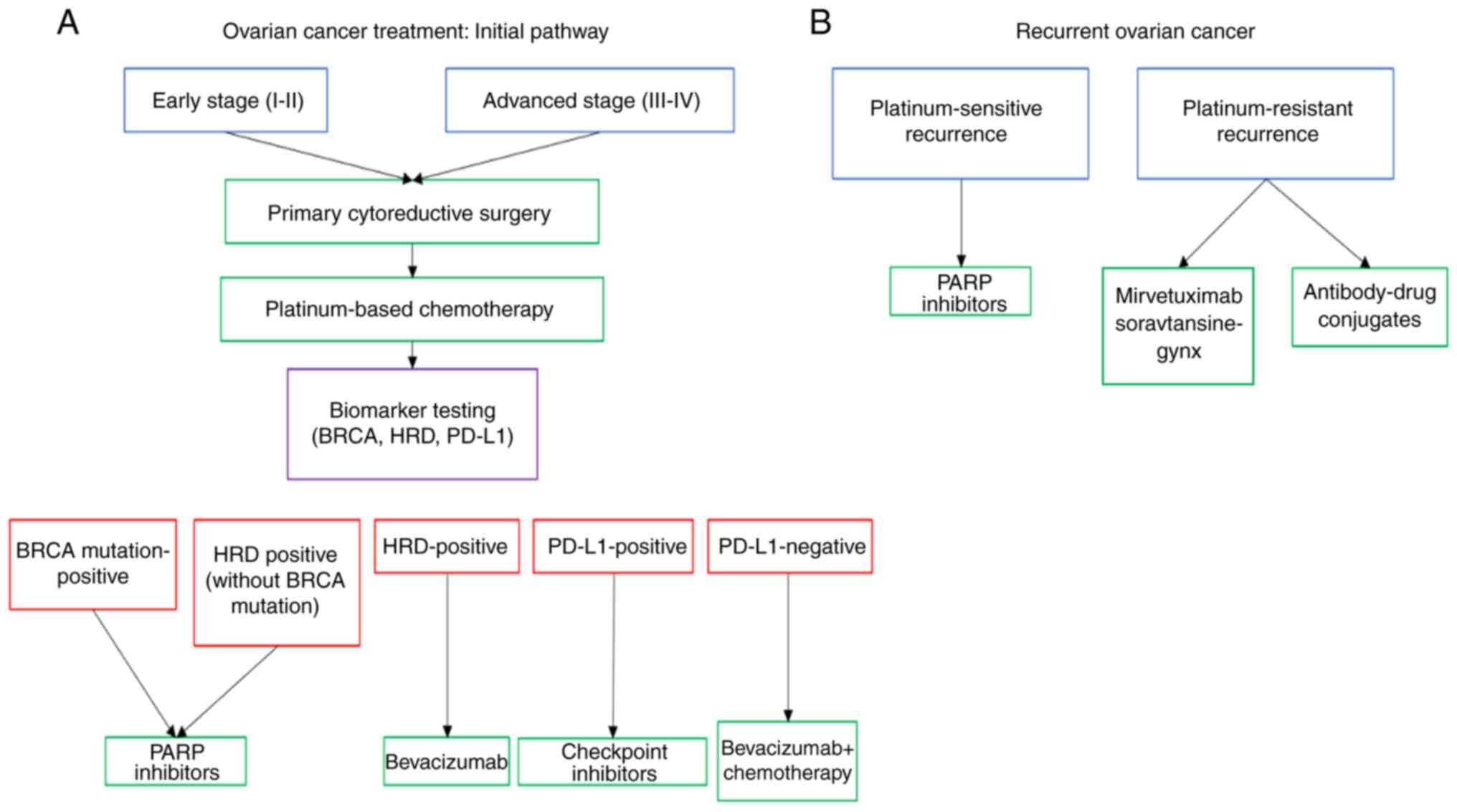
initial stages or if recurrence occurs are presented in Fig. 1.
Drug repurposing is the process of finding novel
therapeutic uses for approved medications outside of their initial
indications (Table IV) (232). For example, vitamin D and its
analogs, are being studied for the treatment of ovarian cancer
(233–237). These steroid-like compounds have
demonstrated antitumor activity in preclinical models, in addition
to their typical physiological roles. In particular, they suppress
cell proliferation and the potential for metastasis while causing
tumor cell differentiation and apoptosis (238). As a result, synthetic vitamin D
analogs, designed to mitigate the risk of hypercalcemia, have been
developed for targeting malignant disease, such as breast,
colorectal and prostate cancer. By contrast, the effect of vitamin
D and its analogs on ovarian cancer remains unclear (96,239).
Vitamin D-based treatment may improve the effectiveness of PARP
inhibitors and chemotherapeutics (240). The active form of vitamin D,
calcitriol, has been shown to inhibit PARP1 activity in both
cell-free and cellular assays. This suggests that vitamin D
supplementation may enhance the efficacy of pharmacologic PARP1
inhibitors through a synergistic inhibitory effect (240). Furthermore, combining vitamin D
with immunotherapy may be advantageous due to its immunomodulatory
effects (241,242). To the best of our knowledge, there
are few clinical studies that have evaluated the effectiveness of
vitamin D-based treatment for ovarian cancer (96,239).
Venous thromboembolism (VTE), which has an
incidence rate of 10–30%, is a common diagnosis in patients with
ovarian tumors (243). For
patients with cancer, this thrombotic event is the second most
common cause of mortality. Notably, most patients with cancer show
signs of hypercoagulability even in the absence of VTE (244). Within the tumor microenvironment,
cancer cells produce tissue factor (TF) independently and promote
TF production by normal cells. The pro-tumorigenic functions of TF
include tumor cell proliferation, maintenance of cancer stemness,
angiogenesis, immune evasion and metastasis through both
clotting-dependent and -independent mechanisms (245–247). In several tumor types, including
ovarian cancer, upregulation of TF is associated with a poor
prognosis (96).
The FDA recently approved tisotumab vedotin
(Tivdak™), a human antibody-drug conjugate specific to TF and
associated with the tubulin-targeting agent monomethyl auristatin
E, for the treatment of recurrent or metastatic cervical cancer
(248). In patients with
platinum-resistant ovarian cancer, the drug exhibits a favorable
safety profile and notable antitumor activity, according to the
phase I/II innovaTV-201 trial (trial no. NCT02001623) (249). These findings support the
continued investigation of tisotumab vedotin in this patient
cohort.
Despite notable advancements in targeted therapy,
ICIs and biomarker-driven treatment, several gaps remain in the
treatment of ovarian cancer. These gaps include underserved patient
populations, the role of precision medicine and the integration of
comprehensive molecular profiling in clinical decision-making.
While biomarker-driven treatment strategies have
improved outcomes for certain groups, disparities persist,
particularly for ethnic minorities, elderly patients and those with
rare ovarian cancer subtypes. Clinical trials have predominantly
included Caucasian populations, leading to limited data on Black,
Hispanic and Asian patients (102,113). Studies have indicated that African
American and Hispanic patients experience higher mortality rates
and lower enrollment in clinical trials (250), which may limit access to novel
therapies such as PARP inhibitors and immunotherapy (251). Additionally, several clinical
trials exclude older patients (aged ≥70 years) or those with
multiple comorbidities, despite the fact that ovarian cancer
predominantly affects postmenopausal patients (252–254). Accordingly, trial findings may not
be generalizable to the broader patient population. This is
revealed by the limited number of trials, such as ROSiA,
ENGOT-OV16/NOVA, and GOG-182 trials, that were conducted on
patients aged 70 or more (252–254). The impact of aggressive
treatments, such as PARP inhibitors, ICIs and combination regimens,
on older or frail patients requires further investigation.
Another underserved subgroup is patients with rare
ovarian cancer subtypes, such as LGSOC, clear cell and mucinous
ovarian cancer. Most phase III clinical trials focus on HGSOC,
which represents the majority of cases, while rarer subtypes
exhibit distinct molecular alterations that may render standard
therapies less effective (46,113,126,255,256). For example, LGSOC often harbors
KRAS or BRAF mutations, suggesting that MEK inhibitors may be a
more effective approach compared with traditional platinum-based
chemotherapy (134). However,
these alternatives remain underexplored, emphasizing the need for
histology-specific clinical trials.
While precision medicine has transformed ovarian
cancer treatment, current biomarker-based strategies remain
incomplete. Presently, treatment decisions are primarily guided by
BRCAm status and HRD testing, but these biomarkers do not fully
capture the complexity of ovarian cancer biology. A large
proportion of HRD-negative tumors respond to PARP inhibitors,
suggesting that improved stratification tools are needed to
identify the true PARP-sensitive patient population. Additionally,
certain HRD-positive tumors exhibit intrinsic resistance to PARP
inhibitors, highlighting the limitations of genomic testing alone
(257).
Beyond HRD testing, the role of other emerging
biomarkers, such as tumor mutation burden (TMB), microsatellite
instability (MSI) and PD-L1 expression, is uncertain in ovarian
cancer. While high TMB and MSI are used as predictive biomarkers
for checkpoint inhibitor therapy in several types of cancer, their
utility in ovarian cancer has not been well established (258,259). Similarly, PD-L1 expression, a key
biomarker for ICIs in lung and breast cancer, has shown limited
predictive value in ovarian cancer trials (260–262). The lack of validated predictive
biomarkers for immunotherapy is a major limitation, contributing to
the low success of ICIs in ovarian cancer compared with other solid
tumors.
Another gap in precision medicine is the reliance
on genomic HRD assays, which assess DNA repair deficiencies at a
static point in time, but may not accurately predict treatment
response. Some researchers argue that functional HRD testing, which
directly measures the ability of a tumor to repair DNA damage, may
be a more reliable biomarker for PARP inhibitor sensitivity
(263,264). As some HRD-negative tumors benefit
from PARP inhibitors, the development of more comprehensive
functional assays may improve patient selection and maximize
treatment efficacy (265).
The integration of genomic, transcriptomic and
proteomic data may transform ovarian cancer treatment by
identifying novel drug targets and guiding therapy selection
(266). However, challenges remain
in implementing comprehensive molecular profiling in routine
clinical practice. A notable limitation is the lack of real-time,
actionable molecular data. Most genomic profiling methods provide
retrospective insight, but real-time molecular testing is necessary
to dynamically adjust treatment based on tumor evolution (267). The integration of liquid biopsy,
which analyzes ctDNA or circulating tumor cells, may offer a
minimally invasive way to track treatment response and resistance
mechanisms in real-time (268).
Another challenge is the integration of multi-omic
data to create a holistic view of tumor biology. Several targeted
therapies focus on a single pathway, yet ovarian cancer is
heterogeneous, and adaptive resistance mechanisms often emerge. For
example, while PARP inhibitors target DNA repair defects,
resistance can develop through secondary BRCA reversion mutation or
upregulation of drug efflux transporters. The ability to combine
genomic, epigenomic, transcriptomic and proteomic insight may
facilitate more precise, patient-specific treatment strategies
(269). Additionally, targetable
mutations in TP53, cyclin E1, KRAS and PI3K/AKT pathways remain
underexplored in ovarian cancer, highlighting the need for novel
drug development beyond BRCA/PARP inhibitors.
Targeted therapies have replaced surgery and
chemotherapy as the mainstays of ovarian cancer treatment, notably
improving patient outcomes. However, optimal patient selection,
resistance mechanisms and the long-term effectiveness of these
medicines are key concerns. Although targeted treatments provide
improved control of ovarian cancer, issues with cost-effectiveness,
accessibility and treatment-associated toxicity exist in practical
application. Furthermore, due to the possibility of medication
resistance and secondary malignancy, it is still unclear how long
the response lasts and how it affects long-term survival. Future
studies should concentrate on improving biomarker-driven strategies
for treatment, identifying combination treatments to overcome
resistance and developing affordable models for greater
accessibility globally.
Not applicable.
Funding: No funding was received.
Not applicable.
KA designed and performed the narrative review, and
drafted and proofread the article critically. AZA helped in writing
the section on poly(ADP-ribose) polymerase inhibitors,
immunotherapy, angiogenesis inhibitors and targeted therapy, and
critically revised the manuscript. GBH helped in writing the
combination therapy, MEK inhibitors and microRNAs sections, and
revised the article critically for intellectual content. AFA
contributed to writing of the section on hyperthermic
intraperitoneal chemotherapy. OSG, AA, SM and MJA revised the
manuscript. Data authentication is not applicable. All authors have
read and approved the final version of the manuscript.
Not applicable.
Not applicable.
The authors declare that they have no competing
interests.
|
1
|
Reid BM, Permuth JB and Sellers TA:
Epidemiology of ovarian cancer: A review. Cancer Biol Med. 14:9–32.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Stewart C, Ralyea C and Lockwood S:
Ovarian cancer: An integrated review. Semin Oncol Nurs. 35:151–156.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gaona-Luviano P, Medina-Gaona LA and
Magaña-Pérez K: Epidemiology of ovarian cancer. Chin Clin Oncol.
9:472020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Khanlarkhani N, Azizi E, Amidi F,
Khodarahmian M, Salehi E, Pazhohan A, Farhood B, Mortezae K,
Goradel NH and Nashtaei MS: Metabolic risk factors of ovarian
cancer: A review. JBRA Assist Reprod. 26:335–347. 2022.PubMed/NCBI
|
|
5
|
Momenimovahed Z, Tiznobaik A, Taheri S and
Salehiniya H: Ovarian cancer in the world: Epidemiology and risk
factors. Int J Womens Health. 11:287–299. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nebgen DR, Lu KH and Bast RC: Novel
approaches to ovarian cancer screening. Curr Oncol Rep. 21:752019.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang J, Chan WC, Ngai CH, Lok V, Zhang L,
Lucero-Prisno DE III, Xu W, Zheng ZJ, Elcarte E, Withers M, et al:
Worldwide burden, risk factors, and temporal trends of ovarian
cancer: A global study. Cancers (Basel). 14:22302022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sambasivan S: Epithelial ovarian cancer:
Review article. Cancer Treat Res Commun. 33:1006292022. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gajjar K, Ogden G, Mujahid MI and Razvi K:
Symptoms and risk factors of ovarian cancer: A survey in primary
care. ISRN Obstet Gynecol. 2012:7541972012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Goff B: Symptoms associated with ovarian
cancer. Clin Obstet Gynecol. 55:36–42. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Horackova K, Janatova M, Kleiblova P,
Kleibl Z and Soukupova J: Early-onset ovarian cancer <30 years:
What do we know about its genetic predisposition? Int J Mol Sci.
24:170202023. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Norquist BM, Harrell MI, Brady MF, Walsh
T, Lee MK, Gulsuner S, Bernards SS, Casadei S, Yi Q, Burger RA, et
al: Inherited mutations in women with ovarian carcinoma. JAMA
Oncol. 2:482–490. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Choi JH, Wong AST, Huang HF and Leung PCK:
Gonadotropins and ovarian cancer. Endocr Rev. 28:440–461. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Elias KM, Guo J and Bast RC Jr: Early
detection of ovarian cancer. Hematol Oncol Clin North Am.
32:903–914. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zamwar UM and Anjankar AP: Aetiology,
epidemiology, histopathology, classification, detailed evaluation,
and treatment of ovarian cancer. Cureus. 14:e305612022.PubMed/NCBI
|
|
18
|
Berek JS, Renz M, Kehoe S, Kumar L and
Friedlander M: Cancer of the ovary, fallopian tube, and peritoneum:
2021 update. Int J Gynecol Obstet. 155 (Suppl 1):S61–S85. 2021.
View Article : Google Scholar
|
|
19
|
Zhang M, Cheng S, Jin Y, Zhao Y and Wang
Y: Roles of CA125 in diagnosis, prediction, and oncogenesis of
ovarian cancer. Biochim Biophys Acta Rev Cancer. 1875:1885032021.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gupta KK, Gupta VK and Naumann RW: Ovarian
cancer: Screening and future directions. Int J Gynecol Cancer.
29:195–200. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jacobs IJ, Menon U, Ryan A, Gentry-Maharaj
A, Burnell M, Kalsi JK, Amso NN, Apostolidou S, Benjamin E,
Cruickshank D, et al: Ovarian cancer screening and mortality in the
UK collaborative trial of ovarian cancer screening (UKCTOCS): A
randomised controlled trial. Lancet. 387:945–956. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hu X, Zhang J and Cao Y: Factors
associated with serum CA125 level in women without ovarian cancer
in the United States: A population-based study. BMC Cancer.
22:5442022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Skates SJ, Menon U, MacDonald N, Rosenthal
AN, Oram DH, Knapp RC and Jacobs IJ: Calculation of the risk of
ovarian cancer from serial CA-125 values for preclinical detection
in postmenopausal women. J Clin Oncol. 21 (10 Suppl):206s–210s.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adolphi NL, Butler KS, Lovato DM, Tessier
TE, Trujillo JE, Hathaway HJ, Fegan DL, Monson TC, Stevens TE,
Huber DL, et al: Imaging of Her2-targeted magnetic nanoparticles
for breast cancer detection: Comparison of SQUID-detected magnetic
relaxometry and MRI. Contrast Media Mol Imaging. 7:308–319. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wan W, Liu H, Zou J, Xie T, Zhang G, Ying
W and Zou X: The optimization and application of photodynamic
diagnosis and autofluorescence imaging in tumor diagnosis and
guided surgery: Current status and future prospects. Front Oncol.
14:15034042025. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lheureux S, Braunstein M and Oza AM:
Epithelial ovarian cancer: Evolution of management in the era of
precision medicine. CA Cancer J Clin. 69:280–304. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hernandez-Lopez LA and Elizalde-Mendez A:
How far should we go in optimal cytoreductive surgery for ovarian
cancer? Chin Clin Oncol. 9:702020. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haghighat S: New treatment of advanced
ovarian cancer: A literature review. J Obstet Gynecol Cancer Res.
4:131–134. 2019. View Article : Google Scholar
|
|
29
|
Lukanović D, Kobal B and Černe K: Ovarian
cancer: Treatment and resistance to pharmacotherapy. Reprod Med.
3:127–140. 2022. View Article : Google Scholar
|
|
30
|
Orr B and Edwards RP: Diagnosis and
treatment of ovarian cancer. Hematol Oncol Clin North Am.
32:943–964. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ghirardi V, Fagotti A, Ansaloni L, Valle
M, Roviello F, Sorrentino L, Accarpio F, Baiocchi G, Piccini L, De
Simone M, et al: Diagnostic and therapeutic pathway of advanced
ovarian cancer with peritoneal metastases. Cancers (Basel).
15:4072023. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Chang SJ, Hodeib M, Chang J and Bristow
RE: Survival impact of complete cytoreduction to no gross residual
disease for advanced-stage ovarian cancer: A meta-analysis. Gynecol
Oncol. 130:493–498. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wright AA, Bohlke K, Armstrong DK, Bookman
MA, Cliby WA, Coleman RL, Dizon DS, Kash JJ, Meyer LA, Moore KN, et
al: Neoadjuvant chemotherapy for newly diagnosed, advanced ovarian
cancer: Society of gynecologic oncology and American society of
clinical oncology clinical practice guideline. Gynecol Oncol.
143:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Armstrong DK and Walker JL: Role of
intraperitoneal therapy in the initial management of ovarian
cancer. J Clin Oncol. 37:2416–2419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Matulonis UA, Sood AK, Fallowfield L,
Howitt BE, Sehouli J and Karlan BY: Ovarian cancer. Nat Rev Dis
Primer. 2:160612016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Vergote I, Tropé CG, Amant F, Kristensen
GB, Ehlen T, Johnson N, Verheijen RHM, van der Burg MEL, Lacave A,
Panici PB, et al: Neoadjuvant chemotherapy or primary surgery in
stage IIIC or IV ovarian cancer. N Engl J Med. 363:943–953. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
du Bois A, Reuss A, Pujade-Lauraine E,
Harter P, Ray-Coquard I and Pfisterer J: Role of surgical outcome
as prognostic factor in advanced epithelial ovarian cancer: A
combined exploratory analysis of 3 prospectively randomized phase 3
multicenter trials: By the Arbeitsgemeinschaft Gynaekologische
Onkologie Studiengruppe Ovarialkarzinom (AGO-OVAR) and the Groupe
d'Investigateurs Nationaux Pour les Etudes des Cancers de l'Ovaire
(GINECO). Cancer. 115:1234–1244. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Gallotta V, Certelli C, Oliva R, Rosati A,
Federico A, Loverro M, Lodoli C, Foschi N, Lathouras K, Fagotti A
and Scambia G: Robotic surgery in ovarian cancer. Best Pract Res
Clin Obstet Gynaecol. 90:1023912023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Siegel RL, Miller KD, Fuchs HE and Jemal
A: Cancer statistics, 2022. CA Cancer J Clin. 72:7–33. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Finch L and Chi DS: An overview of the
current debate between using minimally invasive surgery versus
laparotomy for interval cytoreductive surgery in epithelial ovarian
cancer. J Gynecol Oncol. 34:e842023. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Kumar A and Cliby WA: Advanced ovarian
cancer: Weighing the risks and benefits of surgery. Clin Obstet
Gynecol. 63:74–79. 2019. View Article : Google Scholar
|
|
42
|
Ramirez PT: Standardizing ovarian cancer
surgery and peri-operative care: A European society of
gynecological oncology (ESGO) consensus statement. Int J Gynecol
Cancer. 31:1207–1208. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
McGuire WP III and Markman M: Primary
ovarian cancer chemotherapy: Current standards of care. Br J
Cancer. 89 (Suppl 3):S3–S8. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum-resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mikuła-Pietrasik J, Witucka A, Pakuła M,
Uruski P, Begier-Krasińska B, Niklas A, Tykarski A and Książek K:
Comprehensive review on how platinum- and taxane-based chemotherapy
of ovarian cancer affects biology of normal cells. Cell Mol Life
Sci. 76:681–697. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Moore K, Colombo N, Scambia G, Kim BG,
Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A, Sonke
GS, et al: Maintenance olaparib in patients with newly diagnosed
advanced ovarian cancer. N Engl J Med. 379:2495–2505. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Mirza MR, Lundqvist EA, Birrer MJ, dePont
Christensen R, Nyvang GB, Malander S, Anttila M, Werner TL, Lund B,
Lindahl G, et al: Niraparib plus bevacizumab versus niraparib alone
for platinum-sensitive recurrent ovarian cancer
(NSGO-AVANOVA2/ENGOT-ov24): A randomised, phase 2, superiority
trial. Lancet Oncol. 20:1409–1419. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Oza AM, Cook AD, Pfisterer J, Embleton A,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, et al: Standard chemotherapy with or without
bevacizumab for women with newly diagnosed ovarian cancer (ICON7):
Overall survival results of a phase 3 randomised trial. Lancet
Oncol. 16:928–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Baert T, Ferrero A, Sehouli J, O'Donnell
DM, González-Martín A, Joly F, van der Velden J, Blecharz P, Tan
DSP, Querleu D, et al: The systemic treatment of recurrent ovarian
cancer revisited. Ann Oncol. 32:710–725. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Dasari S and Tchounwou PB: Cisplatin in
cancer therapy: Molecular mechanisms of action. Eur J Pharmacol.
740:364–378. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Bowtell DD, Böhm S, Ahmed AA, Aspuria PJ,
Bast RC, Beral V, Berek JS, Birrer MJ, Blagden S, Bookman MA, et
al: Rethinking ovarian cancer II: Reducing mortality from
high-grade serous ovarian cancer. Nat Rev Cancer. 15:668–679. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Vergote I, Denys H, Greve JD, Gennigens C,
de Vijver V, Kerger J, Vuylsteke P and Baurain JF: Treatment
algorithm in patients with ovarian cancer. Facts Views Vis Obgyn.
12:227–239. 2020.PubMed/NCBI
|
|
54
|
Coada CA, Dondi G, Ravegnini G, Di
Costanzo S, Tesei M, Fiuzzi E, Di Stanislao M, Giunchi S, Zamagni
C, Bovicelli A, et al: Optimal number of neoadjuvant chemotherapy
cycles prior to interval debulking surgery in advanced epithelial
ovarian cancer: A systematic review and meta-analysis of
progression-free survival and overall survival. J Gynecol Oncol.
34:e822023. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Redondo A, Guerra E, Manso L,
Martin-Lorente C, Martinez-Garcia J, Perez-Fidalgo JA, Varela MQ,
Rubio MJ, Barretina-Ginesta MP and Gonzalez-Martin A: SEOM clinical
guideline in ovarian cancer (2020). Clin Transl Oncol. 23:961–968.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Akter S, Rahman MA, Hasan MN, Akhter H,
Noor P, Islam R, Shin Y, Rahman MDH, Gazi MS, Huda MN, et al:
Recent advances in ovarian cancer: Therapeutic strategies,
potential biomarkers, and technological improvements. Cells.
11:6502022. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Faraoni I and Graziani G: Role of BRCA
mutations in cancer treatment with Poly(ADP-ribose) polymerase
(PARP) inhibitors. Cancers (Basel). 10:4872018. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Levit SL and Tang C: Polymeric
nanoparticle delivery of combination therapy with synergistic
effects in ovarian cancer. Nanomaterials (Basel). 11:10482021.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Matsuo K, Eno ML, Im DD and Rosenshein NB:
Chemotherapy time interval and development of platinum and taxane
resistance in ovarian, fallopian, and peritoneal carcinomas. Arch
Gynecol Obstet. 281:325–328. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Rauh-Hain JA, Nitschmann CC, Worley MJ Jr,
Bradford LS, Berkowitz RS, Schorge JO, Campos SM, del Carmen MG and
Horowitz NS: Platinum resistance after neoadjuvant chemotherapy
compared to primary surgery in patients with advanced epithelial
ovarian carcinoma. Gynecol Oncol. 129:63–68. 2025. View Article : Google Scholar
|
|
61
|
Pokhriyal R, Hariprasad R, Kumar L and
Hariprasad G: Chemotherapy resistance in advanced ovarian cancer
patients. Biomark Cancer. 11:1179299X19860812019. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Basourakos SP, Li L, Aparicio AM, Corn PG,
Kim J and Thompson TC: Combination platinum-based and DNA damage
response-targeting cancer therapy: Evolution and future directions.
Curr Med Chem. 24:1586–1606. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Luqmani YA: Mechanisms of drug resistance
in cancer chemotherapy. Med Princ Pract. 14 (Suppl 1):S35–S48.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Bykov VJN, Eriksson SE, Bianchi J and
Wiman KG: Targeting mutant p53 for efficient cancer therapy. Nat
Rev Cancer. 18:89–102. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Yang L, Xie HJ, Li YY, Wang X, Liu XX and
Mai J: Molecular mechanisms of platinum-based chemotherapy
resistance in ovarian cancer (Review). Oncol Rep. 47:822022.
View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Mihanfar A, Fattahi A and Nejabati HR:
MicroRNA-mediated drug resistance in ovarian cancer. J Cell
Physiol. 234:3180–3191. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Puris E, Fricker G and Gynther M: The role
of solute carrier transporters in efficient anticancer drug
delivery and therapy. Pharmaceutics. 15:3642023. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Wang L, Wang X, Zhu X, Zhong L, Jiang Q,
Wang Y, Tang Q, Li Q, Zhang C, Wang H and Zou D: Drug resistance in
ovarian cancer: from mechanism to clinical trial. Mol Cancer.
23:662024. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Alshamrani AA: Roles of microRNAs in
ovarian cancer tumorigenesis: Two decades later, what have we
learned? Front Oncol. 10:10842020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Nguyen VHL, Yue C, Du KY, Salem M, O'Brien
J and Peng C: The role of microRNAs in epithelial ovarian cancer
metastasis. Int J Mol Sci. 21:70932020. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Vaidyanathan A, Sawers L, Gannon AL,
Chakravarty P, Scott AL, Bray SE, Ferguson MJ and Smith G: ABCB1
(MDR1) induction defines a common resistance mechanism in
paclitaxel- and olaparib-resistant ovarian cancer cells. Br J
Cancer. 115:431–441. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Rottenberg S, Jaspers JE, Kersbergen A,
van Der Burg E, Nygren AOH, Zander SAL, Derksen PW, de Bruin M,
Zevenhoven J, Lau A, et al: High sensitivity of BRCA1-deficient
mammary tumors to the PARP inhibitor AZD2281 alone and in
combination with platinum drugs. Proc Natl Acad Sci USA.
105:17079–17084. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang B, Kang Z, Zhang J, Kang Y, Liang L,
Liu Y, Liu Y and Wang Q: Simultaneous binding mechanism of multiple
substrates for multidrug resistance transporter P-glycoprotein.
Phys Chem Chem Phys. 23:4530–4543. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kazmierczak D, Jopek K, Sterzynska K,
Nowicki M, Rucinski M and Januchowski R: The profile of MicroRNA
expression and potential role in the regulation of drug-resistant
genes in cisplatin- and paclitaxel-resistant ovarian cancer cell
lines. Int J Mol Sci. 23:5262022. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lee JH, Chae JW, Kim JK, Kim HJ, Chung JY
and Kim YH: Inhibition of cisplatin-resistance by RNA interference
targeting metallothionein using reducible oligo-peptoplex. J
Control Release. 215:82–90. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Boušová I and Skálová L: Inhibition and
induction of glutathione S-transferases by flavonoids: Possible
pharmacological and toxicological consequences. Drug Metab Rev.
44:267–286. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Zhang J, Xie S, Zhou L, Tang X, Guan X,
Deng M, Zheng H, Wang Y, Lu R and Guo L: Up-regulation of GSTT1 in
serous ovarian cancer associated with resistance to
TAXOL/carboplatin. J Ovarian Res. 14:1222021. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Tagawa T, Morgan R, Yen Y and Mortimer J:
Ovarian cancer: Opportunity for targeted therapy. J Oncol.
2012:6824802012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Duan P, Fan L, Gao Q, Silwal M, Ren M,
Shen Y and Qu W: Targeted therapy of ovarian cancer with
angiogenesis inhibitors. Curr Drug Targets. 18:1171–1178. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Pulla P, Lakshmanan K, Byran G, Rajagopal
K, Krishnamurthy PT and Palati DJ: A review on recent PARP
inhibitor advancements in cancer therapy. Curr Enzyme Inhib. 18:
View Article : Google Scholar : 2022.
|
|
81
|
Suh YJ, Lee B, Kim K, Jeong Y, Choi HY,
Hwang SO and Kim YB: Bevacizumab versus PARP-inhibitors in women
with newly diagnosed ovarian cancer: A network meta-analysis. BMC
Cancer. 22:3462022. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Ferrara N, Hillan KJ and Novotny W:
Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody
for cancer therapy. Biochem Biophys Res Commun. 333:328–335. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Chen A: PARP inhibitors: Its role in
treatment of cancer. Chin J Cancer. 30:463–471. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
O'Malley DM, Krivak TC, Kabil N, Munley J
and Moore KN: PARP inhibitors in ovarian cancer: A review. Target
Oncol. 18:471–503. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Sato K, Koyasu M, Nomura S, Sato Y, Kita
M, Ashihara Y, Adachi Y, Ohno S, Iwase T, Kitagawa D, et al:
Mutation status of RAD51C, PALB2 and BRIP1 in 100 Japanese familial
breast cancer cases without BRCA1 and BRCA2 mutations. Cancer Sci.
108:2287–2294. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lee JM, Ledermann JA and Kohn EC: PARP
Inhibitors for BRCA1/2 mutation-associated and BRCA-like
malignancies. Ann Oncol. 25:32–40. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Domchek SM, Postel-Vinay S, Im SA, Park
YH, Delord JP, Italiano A, Alexandre J, You B, Bastian S, Krebs MG,
et al: Olaparib and durvalumab in patients with germline
BRCA-mutated metastatic breast cancer (MEDIOLA): An open-label,
multicentre, phase 1/2, basket study. Lancet Oncol. 21:1155–1164.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Drew Y, Kaufman B, Banerjee S, Lortholary
A, Hong SH, Park YH, Zimmermann S, Roxburgh P, Ferguson M, Alvarez
RH, et al: Phase II study of olaparib + durvalumab (MEDIOLA):
Updated results in germline BRCA-mutated platinum-sensitive
relapsed (PSR) ovarian cancer (OC). Ann Oncol. 30:v485–v486. 2019.
View Article : Google Scholar
|
|
89
|
Dilawari A, Shah M, Ison G, Gittleman H,
Fiero MH, Shah A, Hamed SS, Qiu J, Yu J, Manheng W, et al: FDA
approval summary: Mirvetuximab soravtansine-Gynx for FRα-positive,
platinum-resistant ovarian cancer. Clin Cancer Res. 29:3835–3840.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Orbach D, Carton M, Khadir SK, Feuilly M,
Kurtinecz M, Phil D, Vokuhl C, Koscielniak E, Pierron G, Lemelle L
and Sparber-Sauer M: Therapeutic benefit of larotrectinib over the
historical standard of care in patients with locally advanced or
metastatic infantile fibrosarcoma (EPI VITRAKVI study). ESMO Open.
9:1030062024. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Gouda MA and Subbiah V: Precision oncology
with selective RET inhibitor selpercatinib in RET-rearranged
cancers. Ther Adv Med Oncol. 15:175883592311770152023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Dinkins K, Barton W, Wheeler L, Smith HJ,
Mythreye K and Arend RC: Targeted therapy in high grade serous
ovarian cancer: A literature review. Gynecol Oncol Rep.
54:1014502024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Luvero D, Angioli R, Celoro F, Plotti F,
Terranova C, Guzzo F, Cundari GB, Liparulo F, Verdone C and Montera
R: Tailored treatment strategies in first line therapy for ovarian
cancer patients: A critical review of the literature.
Pharmaceuticals (Basel). 17:7782024. View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Hillmann J, Maass N, Bauerschlag DO and
Flörkemeier I: Promising new drugs and therapeutic approaches for
treatment of ovarian cancer-targeting the hallmarks of cancer. BMC
Med. 23:102025. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Zeimet A, Wieser V, Knoll K, Reimer D and
Marth C: PARP inhibitors in the treatment of ovarian cancer. Memo
Mag Eur Med Oncol. 13:198–201. 2020.
|
|
96
|
Tavares V, Marques IS, de Melo IG, Assis
J, Pereira D and Medeiros R: Paradigm shift: A comprehensive review
of ovarian cancer management in an era of advancements. Int J Mol
Sci. 25:18452024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Kolesnichenko M and Scheidereit C:
Synthetic lethality by PARP inhibitors: New mechanism uncovered
based on unresolved transcription-replication conflicts. Signal
Transduct Target Ther. 9:1792024. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Ledermann JA and Pujade-Lauraine E:
Olaparib as maintenance treatment for patients with
platinum-sensitive relapsed ovarian cancer. Ther Adv Med Oncol.
11:17588359198497532019. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Bradley W, Moore K, Colombo N, Scambia G,
Kim BG, Oaknin A, Friedlander M, Lisyanskaya A, Floquet A, Leary A,
et al: Maintenance olaparib for patients with newly diagnosed,
advanced ovarian cancer and a BRCA mutation: 5-year follow-up from
SOLO1. Gynecol Oncol. 162:S25–S26. 2021. View Article : Google Scholar
|
|
100
|
Li N, Zhu J, Yin R, Wang J, Pan L, Kong B,
Zheng H, Liu J, Wu X, Wang L, et al: Efficacy and safety of
niraparib as maintenance treatment in patients with newly diagnosed
advanced ovarian cancer using an individualized starting dose
(PRIME Study): A randomized, double-blind, placebo-controlled,
phase 3 trial (LBA 5). Gynecol Oncol. 166:S50–S51. 2022. View Article : Google Scholar
|
|
101
|
Tyszka M and Stec R: Niraparib maintenance
in newly diagnosed advanced ovarian cancer-review and case series.
Oncol Clin Pract. 19:62022.
|
|
102
|
O'Cearbhaill R, Perez-Fidalgo JA, Monk B,
Tusquets I, McCormick C, Fuentes J, Moore RG, Vulsteke C, Shahin
MS, Forget F, et al: Efficacy of niraparib by time of surgery and
postoperative residual disease status: A post hoc analysis of
patients in the PRIMA/ENGOT-OV26/GOG-3012 study. Gynecol Oncol.
166:36–43. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Monk B, Parkinson C, Lim M, O'Malley D,
Oaknin A, Wilson M, Coleman RL, Lorusso D, Bessette P, Ghamande S,
et al: A randomized, phase III trial to evaluate rucaparib
monotherapy as maintenance treatment in patients with newly
diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin
Oncol. 381:JCO.22.01003. 2022.
|
|
104
|
Ledermann J, Oza A, Lorusso D, Aghajanian
C, Oaknin A, Dean A, Colombo N, Weberpals JI, Clamp AR, Scambia G,
et al: Rucaparib for patients with platinum-sensitive, recurrent
ovarian carcinoma (ARIEL3): Post-progression outcomes and updated
safety results from a randomised, placebo-controlled, phase 3
trial. Lancet Oncol. 21:710–722. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Mirza MR, Pignata S and Ledermann JA:
Latest clinical evidence and further development of PARP inhibitors
in ovarian cancer. Ann Oncol. 29:1366–1376. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Boussios S, Abson C, Moschetta M, Rassy E,
Karathanasi A, Bhat T, Ghumman F, Sheriff M and Pavlidis N: Poly
(ADP-Ribose) polymerase inhibitors: Talazoparib in ovarian cancer
and beyond. Drugs R D. 20:55–73. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zimmer A, Nichols E, Cimino-Mathews A,
Peer C, Cao L, Lee MJ, Kohn EC, Annunziata CM, Lipkowitz S, Trepel
JB, et al: A phase I study of the PD-L1 inhibitor, durvalumab, in
combination with a PARP inhibitor, olaparib, and a VEGFR1-3
inhibitor, cediranib, in recurrent women's cancers with biomarker
analyses. J Immunother Cancer. 7:1972019. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Eskander R, Ledermann J, Birrer M,
Fujiwara K, Gaillard S, Richardson G, Wei C, Baig MA, Zohren F and
Monk BJ: JAVELIN ovarian PARP 100 study design: Phase III trial of
avelumab + chemotherapy followed by avelumab + talazoparib
maintenance in previously untreated epithelial ovarian cancer. J
Clin Oncol. 37:TPS9. 2019. View Article : Google Scholar
|
|
109
|
Herencia-Ropero A, Llop-Guevara A,
Staniszewska AD, Domènech-Vivó J, García-Galea E, Moles-Fernández
A, Pedretti F, Domènech H, Rodríguez O, Guzmán M, et al: The PARP1
selective inhibitor saruparib (AZD5305) elicits potent and durable
antitumor activity in patient-derived BRCA1/2-associated cancer
models. Genome Med. 16:1072024. View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Muzzana M, Broggini M and Damia G: The
landscape of PARP inhibitors in solid cancers. Onco Targets Ther.
18:297–317. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
111
|
American Association for Cancer Research
(AACR), . Next-generation PARP inhibitor demonstrates clinical
benefit in patients with homologous recombination repair-deficient
breast cancer [Internet]. AACR, Philadelphia. 2024.https://www.aacr.org/about-the-aacr/newsroom/news-releases/next-generation-parp-inhibitor-demonstrates-clinical-benefit-in-patients-with-homologous-recombination-repair-deficient-breast-cancer/February
22–2025
|
|
112
|
MERCK, . LYNPARZA® (olaparib)
Phase 3 PAOLA-1 trial significantly increased progression-free
survival as first-line maintenance treatment with bevacizumab for
newly-diagnosed advanced ovarian cancer [Internet]. Merck &
Co., Inc., Rahway, NJ. 2019.https://www.merck.com/news/lynparza-olaparib-phase-3-paola-1-trial-significantly-increased-progression-free-survival-as-first-line-
maintenance-treatment-with-bevacizumab-for-newly-diagnosed-
advanced-ovarian-cancer/May 4–2025
|
|
113
|
Ray-Coquard I, Leary A, Pignata S, Cropet
C, González-Martín A, Marth C, Nagao S, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab first-line maintenance
in ovarian cancer: Final overall survival results from the
PAOLA-1/ENGOT-ov25 trial. Ann Oncol. 34:681–692. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Paclitaxel and Carboplatin and Veliparib
in Ovarian Cancer and Ovarian Neoplasm-Clinical Trials Registry-ICH
GCP [Internet], . https://ichgcp.net/clinical-trials-registry/NCT02470585?utm_source=chatgpt.comMay
4–2025
|
|
115
|
Mirza MR, Benigno B, Dørum A, Mahner S,
Bessette P, Barceló IB, Berton-Rigaud D, Ledermann JA, Rimel BJ,
Herrstedt J, et al: Long-term safety in patients with recurrent
ovarian cancer treated with niraparib versus placebo: Results from
the phase III ENGOT-OV16/NOVA trial. Gynecol Oncol. 159:442–448.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Fong PC, Boss DS, Yap TA, Tutt A, Wu P,
Mergui-Roelvink M, Mortimer P, Swaisland H, Lau A, O'Connor MJ, et
al: Inhibition of poly(ADP-Ribose) polymerase in tumors from BRCA
mutation carriers. N Engl J Med. 361:123–134. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Chan VKY, Yang R, Wong ICK and Li X:
Cost-effectiveness of poly ADP-ribose polymerase inhibitors in
cancer treatment: A systematic review. Front Pharmacol.
13:8911492022. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
European Society for Medical Oncology
(ESMO), . Elucidating a risk of developing second primary
malignancy during and after treatment with PARP inhibitors
[Internet]. ESMO; Lugano: 2021, https://www.esmo.org/oncology-news/elucidating-a-risk-of-developing-second-primary-malignancy-during-and-after-treatment-with-parp-inhibitorsFebruary
11–2025
|
|
119
|
Desai C, Pathak A, Limaye S, Maniar V and
Joshi A: A review on mechanisms of resistance to PARP inhibitors.
Indian J Cancer. 59 (Suppl):S119–S129. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Wang N, Yang Y, Jin D, Zhang Z, Shen K,
Yang J, Chen H, Zhao X, Yang L and Lu H: PARP inhibitor resistance
in breast and gynecological cancer: Resistance mechanisms and
combination therapy strategies. Front Pharmacol. 13:9676332022.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Dana-Farber Cancer Institute, .
Combination ATR and PARP Inhibitor (CAPRI) trial with AZD6738 and
olaparib in recurrent ovarian cancer. Dana-Farber Cancer Institute,
Inc.; Boston MA: https://www.dana-farber.org/clinical-trials/20-320May
4–2025
|
|
122
|
Yang Y, Yang Y, Yang J, Zhao X and Wei X:
Tumor microenvironment in ovarian cancer: Function and therapeutic
strategy. Front Cell Dev Biol. 8:7582020. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mei C, Gong W, Wang X, Lv Y, Zhang Y, Wu S
and Zhu C: Anti-angiogenic therapy in ovarian cancer: Current
understandings and prospects of precision medicine. Front
Pharmacol. 14:11477172023. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Park SA, Jeong MS, Ha KT and Jang SB:
Structure and function of vascular endothelial growth factor and
its receptor system. BMB Rep. 51:73–78. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Burger RA, Sill MW, Monk BJ, Greer BE and
Sorosky JI: Phase II trial of bevacizumab in persistent or
recurrent epithelial ovarian cancer or primary peritoneal cancer: A
gynecologic oncology group study. J Clin Oncol. 25:5165–5171. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Perren T, Swart AM, Pfisterer J, Ledermann
JA, Lortholary A, Kristensen G, Carey MS, Beale P, Cervantes A and
Oza A: ICON7: A phase iii randomised gynaecologic cancer intergroup
trial of concurrent bevacizumab and chemotherapy followed by
maintenance bevacizumab, versus chemotherapy alone in women with
newly diagnosed epithelial ovarian (EOC), primary peritoneal (PPC)
or fallopian tube cancer (FTC). Ann Oncol. 21:2010.PubMed/NCBI
|
|
127
|
Achtari C, Fink D, Günthert AR, Huober J,
Pestalozzi B, Petignat P, von Moos R and Sessa C: Bevacizumab in
the primary treatment of epithelial ovarian cancer-some comments on
the latest results. chweizer Krebs-Bulletin = Bulletin Suisse du
Cancer. 35:pp35–38. 2011.
|
|
128
|
Sfakianos GP, Numnum TM, Halverson CB,
Panjeti D, Kendrick JE IV and Straughn JM Jr: The risk of
gastrointestinal perforation and/or fistula in patients with
recurrent ovarian cancer receiving bevacizumab compared to standard
chemotherapy: A retrospective cohort study. Gynecol Oncol.
114:424–426. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Psyrri A, Kassar M, Yu Z, Bamias A,
Weinberger PM, Markakis S, Kowalski D, Camp RL, Rimm DL and
Dimopoulos MA: Effect of epidermal growth factor receptor
expression level on survival in patients with epithelial ovarian
cancer. Clin Cancer Res. 11:8637–8643. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Konner J, Schilder RJ, DeRosa FA, Gerst
SR, Tew WP, Sabbatini PJ, Hensley ML, Spriggs DR and Aghajanian CA:
A phase II study of cetuximab/paclitaxel/carboplatin for the
initial treatment of advanced-stage ovarian, primary peritoneal, or
fallopian tube cancer. Gynecol Oncol. 110:140–145. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Liang XJ and Shen J: Adverse events risk
associated with angiogenesis inhibitors addition to therapy in
ovarian cancer: A meta-analysis of randomized controlled trials.
Eur Rev Med Pharmacol Sci. 20:2701–2790. 2016.PubMed/NCBI
|
|
132
|
Watson N and Al-Samkari H: Thrombotic and
bleeding risk of angiogenesis inhibitors in patients with and
without malignancy. J Thromb Haemost. 19:1852–1863. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Moujaber T, Balleine R, Gao B, Madsen I,
Harnett P and DeFazio A: New therapeutic opportunities for
low-grade serous ovarian cancer. Endocr Relat Cancer. 29:R1–R16.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
134
|
Gershenson DM, Miller A, Brady WE, Paul J,
Carty K, Rodgers W, Millan D, Coleman RL, Moore KN, Banerjee S, et
al: Trametinib versus standard of care in patients with recurrent
low-grade serous ovarian cancer (GOG 281/LOGS): An international,
randomised, open-label, multicentre, phase 2/3 trial. Lancet.
399:541–553. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
135
|
Grisham RN, Iyer G, Garg K, DeLair D,
Hyman DM, Zhou Q, Iasonos A, Berger MF, Dao F, Spriggs DR, et al:
BRAF Mutation is associated with early stage disease and improved
outcome in patients with low-grade serous ovarian cancer. Cancer.
119:548–554. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
136
|
Hendrikse C, Theelen P, van der Ploeg P,
Westgeest H, Boere I, Thijs AMJ, Ottevanger PB, van de Stolpe A,
Lambrechts S, Bekkers RLM and Piek JMJ: The potential of
RAS/RAF/MEK/ERK (MAPK) signaling pathway inhibitors in ovarian
cancer: A systematic review and meta-analysis. Gynecol Oncol.
171:83–94. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
137
|
Chen X, Chen Y, Lin X, Su S, Hou X, Zhang
Q and Tian Y: The drug combination of SB202190 and SP600125
significantly inhibit the growth and metastasis of
olaparib-resistant ovarian cancer cell. Curr Pharm Biotechnol.
19:506–513. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
138
|
Cicenas J, Zalyte E, Rimkus A, Dapkus D,
Noreika R and Urbonavicius S: JNK, p38, ERK, and SGK1 inhibitors in
cancer. Cancers (Basel). 10:12017. View Article : Google Scholar : PubMed/NCBI
|
|
139
|
Santiago-O'Farrill J, Essien S, Figueroa
M, Pang L, Amaravadi R, Lu Z and Bast RC: Abstract 3317: Autophagy
protects ovarian cancer cells from olaparib-induced toxicity.
Cancer Res. 77:33172017. View Article : Google Scholar
|
|
140
|
Welsh SJ and Corrie PG: Management of BRAF
and MEK inhibitor toxicities in patients with metastatic melanoma.
Ther Adv Med Oncol. 7:122–136. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
141
|
Rupaimoole R and Slack FJ: MicroRNA
therapeutics: Towards a new era for the management of cancer and
other diseases. Nat Rev Drug Discov. 16:203–222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
142
|
Langhe R: microRNA and ovarian cancer. Adv
Exp Med Biol. 889:119–151. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
143
|
Chen Y, Cao XY, Li YN, Qiu YY, Li YN, Li W
and Wang H: Reversal of cisplatin resistance by
microRNA-139-5p-independent RNF2 downregulation and MAPK inhibition
in ovarian cancer. Am J Physiol Cell Physiol. 315:C225–C235. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
144
|
Wu Y, Wang T, Xia L and Zhang M: LncRNA
WDFY3-AS2 promotes cisplatin resistance and the cancer stem cell in
ovarian cancer by regulating hsa-miR-139-5p/SDC4 axis. Cancer Cell
Int. 21:2842021. View Article : Google Scholar : PubMed/NCBI
|
|
145
|
Chen K, Wang J, Yang M, Deng S and Sun L:
Immunotherapy in recurrent ovarian cancer. Biomedicines.
13:1682025. View Article : Google Scholar : PubMed/NCBI
|
|
146
|
Turner T, Buchsbaum D, Straughn J, Randall
T and Arend R: Ovarian cancer and the immune system-The role of
targeted therapies. Gynecol Oncol. 142:349–356. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
147
|
Wang L, Zhang C, Yue D and Dong J: Tumor
specific TGF-β insensitive CD8 + T cells augments the antitumor
effect through inhibition of epithelial-mesenchymal transition in
CD 105 + renal carcinoma stem cells. 2024. View Article : Google Scholar
|
|
148
|
Han Y, Liu D and Li L: PD-1/PD-L1 pathway:
Current researches in cancer. Am J Cancer Res. 10:727–742.
2020.PubMed/NCBI
|
|
149
|
Zhu J, Yan L and Wang Q: Efficacy of
PD-1/PD-L1 inhibitors in ovarian cancer: A single-arm
meta-analysis. J Ovarian Res. 14:1122021. View Article : Google Scholar : PubMed/NCBI
|
|
150
|
Conway J, Kofman E, Mo S, Elmarakeby H and
Van Allen E: Genomics of response to immune checkpoint therapies
for cancer: Implications for precision medicine. Genome Med.
10:932018. View Article : Google Scholar : PubMed/NCBI
|
|
151
|
Hargadon KM, Johnson CE and Williams CJ:
Immune checkpoint blockade therapy for cancer: An overview of
FDA-approved immune checkpoint inhibitors. Int Immunopharmacol.
62:29–39. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
152
|
Brahmer J, Tykodi S, Chow L, Hwu WJ,
Topalian S, Hwu P, Drake CG, Camacho LH, Kauh J, Odunsi K, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
153
|
Inayama Y, Hamanishi J, Matsumura N,
Murakami R, Abiko K, Yamaguchi K, Baba T, Horie K, Konishi I and
Mandai M: Antitumor effect of nivolumab on subsequent chemotherapy
for platinum-resistant ovarian cancer. Oncologist. 23:1382–1384.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
154
|
Varga A, Piha-Paul S, Ott P, Mehnert J,
Berton-Rigaud D, Morosky A, Zhao GQ, Rangwala RA and Matei D:
Pembrolizumab in patients (pts) with PD-L1-positive (PD-L1 +)
advanced ovarian cancer: Updated analysis of KEYNOTE-028. J Clin
Oncol. 35:55132017. View Article : Google Scholar
|
|
155
|
Demircan N, Boussios S, Tasci T and Öztürk
MA: Current and future immunotherapy approaches in ovarian cancer.
Ann Transl Med. 8:17142020. View Article : Google Scholar : PubMed/NCBI
|
|
156
|
Pawłowska A, Skiba W, Suszczyk D, Kuryło
W, Jakubowicz-Gil J, Paduch R and Wertel I: The dual blockade of
the TIGIT and PD-1/PD-L1 pathway as a new hope for ovarian cancer
patients. Cancers (Basel). 14:57572023. View Article : Google Scholar
|
|
157
|
Chauvin JM and Zarour HM: TIGIT in cancer
immunotherapy. J Immunother Cancer. 8:e0009572020. View Article : Google Scholar : PubMed/NCBI
|
|
158
|
Postow MA, Sidlow R and Hellmann MD:
Immune-related adverse events associated with immune checkpoint
blockade. N Engl J Med. 378:158–168. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
159
|
Sanmamed MF and Chen L: A paradigm shift
in cancer immunotherapy: From enhancement to normalization. Cell.
175:313–326. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
160
|
Liao T, Li L and Wang L: Bevacizumab
combined with chemotherapy for ovarian cancer: A protocol for
systematic review and meta-analysis. Medicine (Baltimore).
100:e283762021. View Article : Google Scholar : PubMed/NCBI
|
|
161
|
Tischkowitz M, Huang S, Banerjee S, Hague
J, Hendricks W, Huntsman D, Lang JD, Orlando KA, Oza AM, Pautier P,
et al: Small-cell carcinoma of the ovary, hypercalcemic
type-genetics, new treatment targets, and current management
guidelines. Clin Cancer Res. 26:3908–3917. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
162
|
Zhu J, Yan L and Wang Q: Efficacy of
PD-1/PD-L1 inhibitors in ovarian cancer: A single-arm
meta-analysis. J Ovarian Res. 14:1122021. View Article : Google Scholar : PubMed/NCBI
|
|
163
|
Pawłowska A, Rekowska A, Kuryło W,
Pańczyszyn A, Kotarski J and Wertel I: Current understanding on why
ovarian cancer is resistant to immune checkpoint inhibitors. Int J
Mol Sci. 24:108592023. View Article : Google Scholar : PubMed/NCBI
|
|
164
|
Wu H, Gong J and Liu Y: Indoleamine 2,
3-dioxygenase regulation of immune response (Review). Mol Med Rep.
17:4867–4873. 2018.PubMed/NCBI
|
|
165
|
Liu X, Newton RC, Friedman SM and Scherle
PA: Indoleamine 2,3-dioxygenase, an emerging target for anti-cancer
therapy. Curr Cancer Drug Targets. 9:938–952. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
166
|
Rogala E, Nowicka A, Bednarek W,
Barczyński B, Piekarczyk W, Klimek K, Zakrzewski M and Kotarski J:
Evaluation of the expression of the immunosuppressive
enzyme-indoleamine 2,3-dioxygenase in ovarian cancer tissue.
Menopause Review/Przegląd Menopauzalny. 3:223–227. 2013.(In
Polish).
|
|
167
|
Safdarian A, Farhangnia P and Rezaei N:
Indoleamine 2,3-Dioxygenase (IDO) and cancerous cells.
2023.1–23
|
|
168
|
Liu JF, Herold C, Luo W, Penson R,
Horowitz N, Konstantinopoulos P, Castro C, Curtis J, Matulonis UA,
Cannistra S and Dizon DS: 937PD - A phase II trial of combination
nivolumab and bevacizumab in recurrent ovarian cancer. Ann Oncol.
29:viii334–viii335. 2018. View Article : Google Scholar
|
|
169
|
Moore K, Bookman M, Sehouli J, Miller A,
Anderson C, Scambia G, Myers T, Taskiran C, Robison K, Mäenpää J,
et al: Atezolizumab, bevacizumab, and chemotherapy for newly
diagnosed stage III or IV ovarian cancer: Placebo-controlled
randomized phase III trial (IMagyn050/GOG 3015/ENGOT-OV39). J Clin
Oncol. 39:1842–1855. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
170
|
Kurtz JE, Pujade-Lauraine E, Oaknin A,
Belin L, Leitner K, Cibula D, Denys H, Rosengarten O, Rodrigues M,
de Gregorio N, et al: Atezolizumab combined with bevacizumab and
platinum-based therapy for platinum-sensitive ovarian cancer:
Placebo-Controlled randomized phase III ATALANTE/ENGOT-ov29 trial.
J Clin Oncol. 41:4768–4778. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
171
|
González-Martín A, Rubio MJ, Heitz F,
Christensen RD, Colombo N, Van Gorp T, Romeo M, Ray-Coquard I, Gaba
L, Leary A, et al: Atezolizumab combined with platinum and
maintenance niraparib for recurrent ovarian cancer with a
platinum-free interval >6 months: ENGOT-OV41/GEICO 69-O/ANITA
phase III trial. J Clin Oncol. 42:4294–4304. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
172
|
Okamoto A, Kim JW, Yin R, Trillsch F,
Reuss A, Aghajanian C, Rubio-Pérez MJ, Vardar MA, Scambia G,
Floquet A, et al: A randomized Phase III trial of durvalumab with
chemotherapy and bevacizumab, followed by maintenance durvalumab,
bevacizumab and olaparib in newly diagnosed advanced ovarian cancer
(DUO-O): Updated trial endpoint and inclusion of China cohort
(329). Gynecol Oncol. 166 (Suppl 1):S1702022. View Article : Google Scholar
|
|
173
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Berger R, Fujiwara K, Vergote I, Colombo N,
Mäenpää J, et al: Olaparib plus bevacizumab as first-line
maintenance in ovarian cancer. N Engl J Med. 381:2416–2428. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
174
|
Harter P, Mouret-Reynier MA, Pignata S,
Cropet C, González-Martín A, Bogner G, Fujiwara K, Vergote I,
Colombo N, Nøttrup TJ, et al: Efficacy of maintenance olaparib plus
bevacizumab according to clinical risk in patients with newly
diagnosed, advanced ovarian cancer in the phase III
PAOLA-1/ENGOT-ov25 trial. Gynecol Oncol. 164:254–264. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
175
|
Ray-Coquard I, Pautier P, Pignata S, Pérol
D, González-Martín A, Sevelda P, Fujiwara K, Vergote IB, Colombo N,
Maenpaa J, et al: LBA2_PRPhase III PAOLA-1/ENGOT-ov25 trial:
Olaparib plus bevacizumab (bev) as maintenance therapy in patients
(pts) with newly diagnosed, advanced ovarian cancer (OC) treated
with platinum-based chemotherapy (PCh) plus bev. Ann Oncol.
30:2019. View Article : Google Scholar
|
|
176
|
Hardesty MM, Krivak TC, Wright GS,
Hamilton E, Fleming EL, Belotte J, Keeton EK, Wang P, Gupta D,
Clements A, et al: OVARIO phase II trial of combination niraparib
plus bevacizumab maintenance therapy in advanced ovarian cancer
following first-line platinum-based chemotherapy with bevacizumab.
Gynecol Oncol. 166:219–229. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
177
|
Liu JF, Gaillard S, Hendrickson AE, Yeku
O, Diver E, Jackson CG, Arend R, Ratner E, Samnotra V, Gupta D, et
al: Niraparib, dostarlimab, and bevacizumab as combination therapy
in pretreated, advanced platinum-resistant ovarian cancer: Findings
from cohort A of the OPAL phase II trial. JCO Precis Oncol.
8:e23006932024. View Article : Google Scholar : PubMed/NCBI
|
|
178
|
Bukowska B, Gajek A and Marczak A: Two
drugs are better than one. A short history of combined therapy of
ovarian cancer. Contemp Oncol (Pozn). 5:350–353. 2015.PubMed/NCBI
|
|
179
|
Niculescu AG and Grumezescu AM: Novel
tumor-targeting nanoparticles for cancer treatment-a review. Int J
Mol Sci. 23:52532022. View Article : Google Scholar : PubMed/NCBI
|
|
180
|
Jani RK and Krupa G: Active targeting of
nanoparticles: An innovative technology for drug delivery in cancer
therapeutics. J Drug Deliv Ther. 9:408–415. 2019. View Article : Google Scholar
|
|
181
|
Wakaskar RR: Passive and active targeting
in tumor microenvironment. Int J Drug Dev Res. 9:22017.
|
|
182
|
Yallapu MM, Jaggi M and Chauhan SC: Scope
of nanotechnology in ovarian cancer therapeutics. J Ovarian Res.
3:192010. View Article : Google Scholar : PubMed/NCBI
|
|
183
|
Liu H, Zhu X, Wei Y, Song C and Wang Y:
Recent advances in targeted gene silencing and cancer therapy by
nanoparticle-based delivery systems. Biomed Pharmacother.
157:1140652023. View Article : Google Scholar : PubMed/NCBI
|
|
184
|
Blanco E, Shen H and Ferrari M: Principles
of nanoparticle design for overcoming biological barriers to drug
delivery. Nat Biotechnol. 33:941–951. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
185
|
Guo X, Guo N, Zhao J and Cai Y: Active
targeting co-delivery system based on hollow mesoporous silica
nanoparticles for antitumor therapy in ovarian cancer stem-like
cells. Oncol Rep. 38:1442–1450. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
186
|
Madej M, Kurowska N and Strzalka-Mrozik B:
Polymeric nanoparticles-tools in a drug delivery system in selected
cancer therapies. Appl Sci. 12:94792022. View Article : Google Scholar
|
|
187
|
Hascicek C and Gun O: Nano drug delivery
systems for ovarian cancer therapy. Integr Cancer Sci Ther. 4:1–4.
2017.PubMed/NCBI
|
|
188
|
Saripilli R and Sharma DK:
Nanotechnology-based drug delivery system for the diagnosis and
treatment of ovarian cancer. Discov Oncol. 16:4222025. View Article : Google Scholar : PubMed/NCBI
|
|
189
|
Barani M, Bilal M, Sabir F, Rahdar A and
Kyzas GZ: Nanotechnology in ovarian cancer: Diagnosis and
treatment. Life Sci. 266:1189142021. View Article : Google Scholar : PubMed/NCBI
|
|
190
|
Engelberth SA, Hempel N and Bergkvist M:
Development of nanoscale approaches for ovarian cancer therapeutics
and diagnostics. Crit Rev Oncog. 19:281–315. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
191
|
Zhao J, Tan W, Zheng J, Su Y and Cui M:
Aptamer nanomaterials for ovarian cancer target theranostics. Front
Bioeng Biotechnol. 10:8844052022. View Article : Google Scholar : PubMed/NCBI
|
|
192
|
Di Lorenzo G, Ricci G, Severini GM, Romano
F and Biffi S: Imaging and therapy of ovarian cancer: clinical
application of nanoparticles and future perspectives. Theranostics.
8:4279–4294. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
193
|
Search for: Ovarian Cancer, Other terms:
Nanoparticles, Completed studies | Card Results |
ClinicalTrials.gov [Internet]. [cited 2024 Jun 29]. Available
from:, . https://clinicaltrials.gov/search?cond=Ovarian%20Cancer&term=Nanoparticles&aggFilters=status:com&page=2
|
|
194
|
Gavas S, Quazi S and Karpiński TM:
Nanoparticles for cancer therapy: Current progress and challenges.
Nanoscale Res Lett. 16:1732021. View Article : Google Scholar : PubMed/NCBI
|
|
195
|
Kumah EA, Fopa RD, Harati S, Boadu P,
Zohoori FV and Pak T: Human and environmental impacts of
nanoparticles: A scoping review of the current literature. BMC
Public Health. 23:10592023. View Article : Google Scholar : PubMed/NCBI
|
|
196
|
Pandey RP, Vidic J, Mukherjee R and Chang
CM: Experimental methods for the biological evaluation of
nanoparticle-based drug delivery risks. Pharmaceutics. 15:6122023.
View Article : Google Scholar : PubMed/NCBI
|
|
197
|
Zhang J, Ding H, Zhang F, Xu Y, Liang W
and Huang L: New trends in diagnosing and treating ovarian cancer
using nanotechnology. Front Bioeng Biotechnol. 11:11609852023.
View Article : Google Scholar : PubMed/NCBI
|
|
198
|
Wilson MK, Pujade-Lauraine E, Aoki D,
Mirza MR, Lorusso D, Oza AM, du Bois A, Vergote I, Reuss A, Bacon
M, et al: Fifth ovarian cancer consensus conference of the
gynecologic cancer intergroup: Recurrent disease. Ann Oncol.
28:727–732. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
199
|
Daly MB, Pal T, Berry MP, Buys SS, Dickson
P, Domchek SM, Elkhanany A, Friedman S, Goggins M, Hutton ML, et
al: Genetic/Familial high-risk assessment: Breast, ovarian, and
pancreatic, Version 2.2021, NCCN clinical practice guidelines in
oncology. J Natl Compr Canc Netw. 19:77–102. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
200
|
Konstantinopoulos PA, Norquist B,
Lacchetti C, Armstrong D, Grisham RN, Goodfellow PJ, Kohn EC,
Levine DA, Liu JF, Lu KH, et al: Germline and somatic tumor testing
in epithelial ovarian cancer: ASCO guideline. J Clin Oncol.
38:1222–1245. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
201
|
Zelli V, Compagnoni C, Cannita K, Capelli
R, Capalbo C, Di Vito Nolfi M, Alesse E, Zazzeroni F and Tessitore
A: Applications of next generation sequencing to the analysis of
familial breast/ovarian cancer. High Throughput. 9:12020.
View Article : Google Scholar : PubMed/NCBI
|
|
202
|
Richards S, Aziz N, Bale S, Bick D, Das S,
Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, et al:
Standards and guidelines for the interpretation of sequence
variants: A joint consensus recommendation of the American College
of medical genetics and genomics and the association for molecular
pathology. Genet Med. 17:405–424. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
203
|
Das SK, Menezes ME, Bhatia S, Wang X,
Emdad L, Sarkar D and Fisher PB: Gene therapies for cancer:
Strategies, challenges and successes. J Cell Physiol. 230:259–271.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
204
|
Áyen Á, Martínez YJ, Marchal JA and
Boulaiz H: Recent progress in gene therapy for ovarian cancer. Int
J Mol Sci. 19:19302018. View Article : Google Scholar : PubMed/NCBI
|
|
205
|
Li S, Jiang K, Li J, Hao X, Chu W, Luo C,
Zhu Y, Xie R and Chen B: Estrogen enhances the proliferation and
migration of ovarian cancer cells by activating transient receptor
potential channel C3. J Ovarian Res. 13:202020. View Article : Google Scholar : PubMed/NCBI
|
|
206
|
Cunat S, Hoffmann P and Pujol P: Estrogens
and epithelial ovarian cancer. Gynecol Oncol. 94:25–32. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
207
|
Kozieł MJ and Piastowska-Ciesielska AW:
Estrogens, estrogen receptors and tumor microenvironment in ovarian
cancer. Int J Mol Sci. 24:146732023. View Article : Google Scholar : PubMed/NCBI
|
|
208
|
Zhang M, Xu H, Zhang Y, Li Z, Meng W, Xia
J, Le W, Meng K and Guo Y: Research progress of estrogen receptor
in ovarian cancer. Clin Exp Obstet Gynecol. 50:1992023. View Article : Google Scholar
|
|
209
|
Jeon SY, Hwang KA and Choi KC: Effect of
steroid hormones, estrogen and progesterone, on epithelial
mesenchymal transition in ovarian cancer development. J Steroid
Biochem Mol Biol. 158:1–8. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
210
|
Park SH, Cheung LWT, Wong AST and Leung
PCK: Estrogen regulates snail and slug in the down-regulation of
e-cadherin and induces metastatic potential of ovarian cancer cells
through estrogen receptor alpha. Mol Endocrinol. 22:2085–2098.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
211
|
Smyth JF, Gourley C, Walker G, MacKean MJ,
Stevenson A, Williams ARW, Nafussi AA, Rye T, Rye R, Stewart M, et
al: Antiestrogen therapy is active in selected ovarian cancer
cases: the use of letrozole in estrogen receptor-positive patients.
Clin Cancer Res. 13:3617–3622. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
212
|
Gershenson DM, Cobb LP and Sun CC:
Endocrine therapy in the management of low-grade serous
ovarian/peritoneal carcinoma: Mounting evidence for therelative
efficacy of tamoxifen and aromatase inhibitors. Gynecol Oncol.
159:601–603. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
213
|
George A, McLachlan J, Tunariu N, Pepa CD,
Migali C, Gore M, Kaye S and Banerjee S: The role of hormonal
therapy in patients with relapsed high-grade ovarian carcinoma: A
retrospective series of tamoxifen and letrozole. BMC Cancer.
17:4562017. View Article : Google Scholar : PubMed/NCBI
|
|
214
|
Colombo N, Sessa C, du Bois A, Ledermann
J, McCluggage WG, McNeish I, Morice P, Pignata S, Ray-Coquard I,
Vergote I, et al: ESMO-ESGO consensus conference recommendations on
ovarian cancer: Pathology and molecular biology, early and advanced
stages, borderline tumours and recurrent disease. Ann Oncol.
30:672–705. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
215
|
National Comprehensive Cancer Network
(NCCN), . Guidelines Detail. NCCN; Plymouth Meeting, PA: 2025,
https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1455May
3–2025
|
|
216
|
Lopresti ML, Bandera CA and Miner TJ: New
approaches to improving survival after neoadjuvant chemotherapy:
The role of intraperitoneal therapy and heated intraperitoneal
chemotherapy in ovarian cancer. Am Soc Clin Oncol Educ Book.
39:19–23. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
217
|
Murphy M, Martin G, Mahmoudjafari Z,
Bivona C, Grauer D and Henry D: Intraperitoneal paclitaxel and
cisplatin compared with dose-dense paclitaxel and carboplatin for
patients with stage III ovarian cancer. J Oncol Pharm Pract.
26:1566–1574. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
218
|
Cascales-Campos P, López-López V, Gil J,
Arévalo-Pérez J, Nieto A, Barceló F, Gil E and Parrilla P:
Hyperthermic intraperitoneal chemotherapy with paclitaxel or
cisplatin in patients with stage III-C/IV ovarian cancer. Is there
any difference? Surg Oncol. 25:164–170. 2016.PubMed/NCBI
|
|
219
|
Sjoquist KM, Espinoza D, Mileshkin L,
Ananda S, Shannon C, Yip S, Goh J, Bowtell D, Harrison M and
Friedlander ML: REZOLVE (ANZGOG-1101): A phase 2 trial of
intraperitoneal bevacizumab to treat symptomatic ascites in
patients with chemotherapy-resistant, epithelial ovarian cancer.
Gynecol Oncol. 161:374–381. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
220
|
Virdi S and Jadavji NM: The impact of
maternal folates on brain development and function after birth.
Metabolites. 12:8762022. View Article : Google Scholar : PubMed/NCBI
|
|
221
|
Gonzalez T, Muminovic M, Nano O and
Vulfovich M: Folate receptor alpha-a novel approach to cancer
therapy. Int J Mol Sci. 25:10462024. View Article : Google Scholar : PubMed/NCBI
|
|
222
|
Elnakat H and Ratnam M: Distribution,
functionality and gene regulation of folate receptor isoforms:
Implications in targeted therapy. Adv Drug Deliv Rev. 56:1067–1084.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
223
|
Mai J, Wu L, Yang L, Sun T, Liu X, Yin R,
Jiang Y, Li J and Li Q: Therapeutic strategies targeting folate
receptor α for ovarian cancer. Front Immunol. 14:12545322023.
View Article : Google Scholar : PubMed/NCBI
|
|
224
|
Kurosaki A, Hasegawa K, Kato T, Abe K,
Hanaoka T, Miyara A, O'Shannessy DJ, Somers EB, Yasuda M, Sekino T
and Fujiwara K: Serum folate receptor alpha as a biomarker for
ovarian cancer: Implications for diagnosis, prognosis and
predicting its local tumor expression. Int J Cancer. 138:1994–2002.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
225
|
Moore KN, Martin LP, O'Malley DM,
Matulonis UA, Konner JA, Vergote I, Ponte JF and Birrer MJ: A
review of mirvetuximab soravtansine in the treatment of
platinum-resistant ovarian cancer. Future Oncol. 14:123–136. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
226
|
Li X, Zhou S, Abrahams CL, Krimm S, Smith
J, Bajjuri K, Stephenson HT, Henningsen R, Hanson J, Heibeck TH, et
al: Discovery of STRO-002, a novel homogeneous ADC targeting folate
receptor alpha, for the treatment of ovarian and endometrial
cancers. Mol Cancer Ther. 22:155–167. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
227
|
Matulonis UA, Oaknin A, Pignata S, Denys
H, Colombo N, Van Gorp T, Konner JA, Romeo M, Harter P, Murphy CG,
et al: Mirvetuximab soravtansine (MIRV) in patients with
platinum-resistant ovarian cancer with high folate receptor alpha
(FRα) expression: Characterization of antitumor activity in the
SORAYA study. J Clin Oncol. 40 (16_suppl):S55122022. View Article : Google Scholar
|
|
228
|
Jelovac D and Armstrong DK: Role of
farletuzumab in epithelial ovarian carcinoma. Curr Pharm Des.
18:3812–3815. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
229
|
Fayoud AM, Darwish MY, Nada EA, Helal AA,
Mohamed NS, Elrashedy AA and Abd-ElGawad M: Efficacy and safety of
farletuzumab in ovarian cancer: A systematic review and single-arm
meta-analysis. Cureus. 16:e735032024.PubMed/NCBI
|
|
230
|
Abrahams C, Krimm S, Li X, Zhou S, Hanson
J, Masikat MR, Bajjuri K, Heibeck T, Kothari D, Yu A, et al:
Abstract NT-090: Preclinical activity and safety of STRO-002, a
novel adc targeting folate receptor alpha for ovarian and
endometrial cancer. Clin Cancer Res. 25 (22_Suppl):NT–090. 2019.
View Article : Google Scholar
|
|
231
|
Naumann RW, Braiteh FS, Martin LP,
Hamilton EP, Diaz JP, Diab S, Schilder RJ, Moroney JW, Uyar D,
O'Malley DM, et al: Phase 1 dose-escalation study of STRO-002, an
antifolate receptor alpha (FRα) antibody drug conjugate (ADC), in
patients with advanced, progressive platinum-resistant/refractory
epithelial ovarian cancer (EOC). J Clin Oncol. 39
(15_suppl):S55502021. View Article : Google Scholar
|
|
232
|
Ashburn TT and Thor KB: Drug
repositioning: identifying and developing new uses for existing
drugs. Nat Rev Drug Discov. 3:673–683. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
233
|
Kim JH, Park WH, Suh DH, Kim K, No JH and
Kim YB: Calcitriol combined with platinum-based chemotherapy
suppresses growth and expression of vascular endothelial growth
factor of SKOV-3 ovarian cancer cells. Anticancer Res.
41:2945–2952. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
234
|
Srivastava AK, Rizvi A, Cui T, Han C,
Banerjee A, Naseem I, Zheng Y, Wani AA and Wang QE: Depleting
ovarian cancer stem cells with calcitriol. Oncotarget.
9:14481–14491. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
235
|
Kuittinen T, Rovio P, Luukkaala T, Laurila
M, Grénman S, Kallioniemi A and Mäenpää J: Paclitaxel, carboplatin
and 1,25-D3 inhibit proliferation of ovarian cancer cells in vitro.
Anticancer Res. 40:3129–313. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
236
|
Wang L, Zhou S and Guo B: Vitamin D
suppresses ovarian cancer growth and invasion by targeting long
non-coding RNA CCAT2. Int J Mol Sci. 21:23342020. View Article : Google Scholar : PubMed/NCBI
|
|
237
|
Dovnik A and Dovnik NF: Vitamin D and
ovarian cancer: Systematic review of the literature with a focus on
molecular mechanisms. Cells. 9:3352020. View Article : Google Scholar : PubMed/NCBI
|
|
238
|
Duffy MJ, Murray A, Synnott NC, O'Donovan
N and Crown J: Vitamin D analogues: Potential use in cancer
treatment. Crit Rev Oncol Hematol. 112:190–197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
239
|
Piatek K, Schepelmann M and Kallay E: The
effect of vitamin D and its analogs in ovarian cancer. Nutrients.
14:38672022. View Article : Google Scholar : PubMed/NCBI
|
|
240
|
Rizvi A and Naseem I: Causing DNA damage
and stopping DNA repair-Vitamin D supplementation with
Poly(ADP-ribose) polymerase 1 (PARP1) inhibitors may cause
selective cell death of cancer cells: A novel therapeutic paradigm
utilizing elevated copper levels within the tumour. Med Hypotheses.
144:1102782020. View Article : Google Scholar : PubMed/NCBI
|
|
241
|
Ghaseminejad-Raeini A, Ghaderi A, Sharafi
A, Nematollahi-Sani B, Moossavi M, Derakhshani A and Sarab GA:
Immunomodulatory actions of vitamin D in various immune-related
disorders: A comprehensive review. Front Immunol. 14:9504652023.
View Article : Google Scholar : PubMed/NCBI
|
|
242
|
Munteanu C, Mârza SM and Papuc I: The
immunomodulatory effects of vitamins in cancer. Front Immunol.
15:14643292024. View Article : Google Scholar : PubMed/NCBI
|
|
243
|
Glassman D, Bateman NW, Lee S, Zhao L, Yao
J, Tan Y, Ivan C, Rangel KM, Zhang J, Conrads KA, et al: Molecular
correlates of venous thromboembolism (VTE) in ovarian cancer.
Cancers (Basel). 14:14962022. View Article : Google Scholar : PubMed/NCBI
|
|
244
|
Skorda A, Bay ML, Hautaniemi S, Lahtinen A
and Kallunki T: Kinase inhibitors in the treatment of ovarian
cancer: Current state and future promises. Cancers (Basel).
14:62572022. View Article : Google Scholar : PubMed/NCBI
|
|
245
|
Kasthuri RS, Taubman MB and Mackman N:
Role of tissue factor in cancer. J Clin Oncol. 27:4834–4838. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
246
|
Versteeg HH, Spek CA, Peppelenbosch MP and
Richel DJ: Tissue factor and cancer metastasis: The role of
intracellular and extracellular signaling pathways. Mol Med.
10:6–11. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
247
|
Ruf W, Yokota N and Schaffner F: Tissue
factor in cancer progression and angiogenesis. Thromb Res.
125:S36–S38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
248
|
Coleman RL, Lorusso D, Gennigens C,
González-Martín A, Randall L, Cibula D, Lund B, Woelber L, Pignata
S, Forget F, et al: Efficacy and safety of tisotumab vedotin in
previously treated recurrent or metastatic cervical cancer
(innovaTV 204/GOG-3023/ENGOT-cx6): A multicentre, open-label,
single-arm, phase 2 study. Lancet Oncol. 22:609–619. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
249
|
de Bono JS, Concin N, Hong DS,
Thistlethwaite FC, Machiels JP, Arkenau HT, Plummer R, Jones RH,
Nielsen D, Windfeld K, et al: Tisotumab vedotin in patients with
advanced or metastatic solid tumours (InnovaTV 201): A
first-in-human, multicentre, phase 1–2 trial. Lancet Oncol.
20:383–393. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
250
|
Arter ZL, Desmond D, Berenberg JL, Killeen
JL, Bunch K and Merritt MA: Epithelial ovarian cancer survival by
race and ethnicity in an equal-access healthcare population. Br J
Cancer. 130:108–113. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
251
|
Wagar MK, Mojdehbakhsh RP, Godecker A,
Rice LW and Barroilhet L: Racial and ethnic enrollment disparities
in clinical trials of poly(ADP-ribose) polymerase inhibitors for
gynecologic cancers. Gynecol Oncol. 165:49–52. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
252
|
Fabbro M, Moore KN, Dørum A, Tinker AV,
Mahner S, Bover I, Banerjee S, Tognon G, Goffin F, Shapira-Frommer
R, et al: Efficacy and safety of niraparib as maintenance treatment
in older patients (≥70 years) with recurrent ovarian cancer:
Results from the ENGOT-OV16/NOVA trial. Gynecol Oncol. 152:560–567.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
253
|
Selle F, Colombo N, Korach J, Mendiola C,
Cardona A, Ghazi Y and Oza AM: Safety and efficacy of extended
bevacizumab therapy in elderly (≥70 Years) versus younger patients
treated for newly diagnosed ovarian cancer in the international
ROSiA study. Int J Gynecol Cancer. 28:729–737. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
254
|
Freyer G, Tew WP and Moore KN: Treatment
and trials: Ovarian cancer in older women. Am Soc Clin Oncol Educ
Book. pp227–235. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
255
|
Coleman RL, Fleming GF, Brady MF, Swisher
EM, Steffensen KD, Friedlander M, Okamoto A, Moore KN, Efrat
Ben-Baruch N, Werner TL, et al: Veliparib with first-line
chemotherapy and as maintenance therapy in ovarian cancer. N Engl J
Med. 381:2403–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
256
|
González-Martín A, Pothuri B, Vergote I,
DePont Christensen R, Graybill W, Mirza MR, McCormick C, Lorusso D,
Hoskins P, Freyer G, et al: Niraparib in patients with newly
diagnosed advanced ovarian cancer. N Engl J Med. 381:2391–2402.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
257
|
Swisher EM, Lin KK, Oza AM, Scott CL,
Giordano H, Sun J, Konecny GE, Coleman RL, Tinker AV, O'Malley DM,
et al: Rucaparib in relapsed, platinum-sensitive high-grade ovarian
carcinoma (ARIEL2 Part 1): An international, multicentre,
open-label, phase 2 trial. Lancet Oncol. 18:75–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
258
|
Sha D, Jin Z, Budczies J, Kluck K,
Stenzinger A and Sinicrope FA: Tumor mutational burden as a
predictive biomarker in solid tumors. Cancer Discov. 10:1808–1825.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
259
|
Fan S, Gao X, Qin Q, Li H, Yuan Z and Zhao
S: Association between tumor mutation burden and immune
infiltration in ovarian cancer. Int Immunopharmacol. 89:1071262020.
View Article : Google Scholar : PubMed/NCBI
|
|
260
|
Matulonis UA, Shapira-Frommer R, Santin
AD, Lisyanskaya AS, Pignata S, Vergote I, Raspagliesi F, Sonke GS,
Birrer M, Provencher DM, et al: Antitumor activity and safety of
pembrolizumab in patients with advanced recurrent ovarian cancer:
Results from the phase II KEYNOTE-100 study. Ann Oncol.
30:1080–1087. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
261
|
Lee M, Samstein RM, Valero C, Chan TA and
Morris LGT: Tumor mutational burden as a predictive biomarker for
checkpoint inhibitor immunotherapy. Hum Vaccines Immunother.
16:112–115. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
262
|
Klempner SJ, Fabrizio D, Bane S, Reinhart
M, Peoples T, Ali SM, Sokol ES, Frampton G, Schrock AB, Anhorn R
and Reddy P: Tumor mutational burden as a predictive biomarker for
response to immune checkpoint inhibitors: A review of current
evidence. Oncologist. 25:e147–e159. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
263
|
Lee CY, Cheng WF, Lin PH, Chen YL, Huang
SH, Lei KH, Chang KY, Ko MY and Chi P: An activity-based functional
test for identifying homologous recombination deficiencies across
cancer types in real time. Cell Rep Med. 4:1012472023. View Article : Google Scholar : PubMed/NCBI
|
|
264
|
Korsholm LM, Kjeldsen M, Perino L, Mariani
L, Nyvang GB, Kristensen E, Bagger FO, Mirza MR and Rossing M:
Combining homologous recombination-deficient testing and functional
RAD51 analysis enhances the prediction of Poly(ADP-Ribose)
polymerase inhibitor sensitivity. JCO Precis Oncol. 8:e23004832024.
View Article : Google Scholar : PubMed/NCBI
|
|
265
|
Pettitt SJ, Krastev DB, Brandsma I, Dréan
A, Song F, Aleksandrov R, Harrell MI, Menon M, Brough R, Campbell
J, et al: Genome-wide and high-density CRISPR-Cas9 screens identify
point mutations in PARP1 causing PARP inhibitor resistance. Nat
Commun. 9:18492018. View Article : Google Scholar : PubMed/NCBI
|
|
266
|
Zhang H, Liu T, Zhang Z, Payne SH, Zhang
B, McDermott JE, Zhou JY, Petyuk VA, Chen L, Ray D, et al:
Integrated proteogenomic characterization of human high-grade
serous ovarian cancer. Cell. 166:755–765. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
267
|
Tannock IF and Hickman JA: Limits to
personalized cancer medicine. N Engl J Med. 375:1289–1294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
268
|
Wan JCM, Massie C, Garcia-Corbacho J,
Mouliere F, Brenton JD, Caldas C, Pacey S, Baird R and Rosenfeld N:
Liquid biopsies come of age: Towards implementation of circulating
tumour DNA. Nat Rev. 17:223–238. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
269
|
Mateo J, Lord CJ, Serra V, Tutt A, Balmaña
J, Castroviejo-Bermejo M, Cruz C, Oaknin A, Kaye SB and de Bono JS:
A decade of clinical development of PARP inhibitors in perspective.
Ann Oncol. 30:1437–1447. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
270
|
McAlpine JN, Porter H, Köbel M, Nelson BH,
Prentice LM, Kalloger SE, Senz J, Milne K, Ding J, Shah SP, et al:
BRCA1 and BRCA2 mutations correlate with TP53 abnormalities and
presence of immune cell infiltrates in ovarian high-grade serous
carcinoma. Mod Pathol. 25:740–750. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
271
|
Scully R and Livingston DM: In search of
the tumour-suppressor functions of BRCA1 and BRCA2. Nature.
408:429–432. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
272
|
Venkitaraman AR: Cancer suppression by the
chromosome custodians, BRCA1 and BRCA2. Science. 343:1470–1475.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
273
|
Davies AA, Masson JY, McIlwraith MJ,
Stasiak AZ, Stasiak A, Venkitaraman AR and West SC: Role of BRCA2
in control of the RAD51 recombination and DNA repair protein. Mol
Cell. 7:273–282. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
274
|
Genome Atlas Research Network, .
Integrated genomic analyses of ovarian carcinoma. Nature.
474:609–615. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
275
|
Walsh T, Casadei S, Lee MK, Pennil CC,
Nord AS, Thornton AM, Roeb W, Agnew KJ, Stray SM, Wickramanayake A,
et al: Mutations in 12 genes for inherited ovarian, fallopian tube,
and peritoneal carcinoma identified by massively parallel
sequencing. Proc Natl Acad Sci USA. 108:18032–18037. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
276
|
Kandoth C, McLellan MD, Vandin F, Ye K,
Niu B, Lu C, Xie M, Zhang Q, McMichael JF, Wyczalkowski MA, et al:
Mutational landscape and significance across 12 major cancer types.
Nature. 502:333–339. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
277
|
Levine AJ: p53, the cellular gatekeeper
for growth and division. Cell. 88:323–331. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
278
|
Hoadley KA, Yau C, Wolf DM, Cherniack AD,
Tamborero D, Ng S, Leiserson MDM, Niu B, McLellan MD, Uzunangelov
V, et al: Multiplatform analysis of 12 cancer types reveals
molecular classification within and across tissues of origin. Cell.
158:929–944. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
279
|
Haupt Y, Maya R, Kazaz A and Oren M: Mdm2
promotes the rapid degradation of p53. Nature. 387:296–299. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
280
|
Vaz F, Hanenberg H, Schuster B, Barker K,
Wiek C, Erven V, Neveling K, Endt D, Kesterton I, Autore F, et al:
Mutation of the RAD51C gene in a Fanconi anemia-like disorder. Nat
Genet. 42:406–409. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
281
|
Norquist B, Wurz KA, Pennil CC, Garcia R,
Gross J, Sakai W, Karlan BY, Taniguchi T and Swisher EM: Secondary
somatic mutations restoring BRCA1/2 predict chemotherapy resistance
in hereditary ovarian carcinomas. J Clin Oncol. 29:3008–3015. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
282
|
Guan B, Mao TL, Panuganti PK, Kuhn E,
Kurman RJ, Maeda D, Chen E, Jeng YM, Wang TL and Shih IM: Mutation
and loss of expression of ARID1A in uterine low-grade endometrioid
carcinoma. Am J Surg Pathol. 35:625–632. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
283
|
Wiegand KC, Shah SP, Al-Agha OM, Zhao Y,
Tse K, Zeng T, Senz J, McConechy MK, Anglesio MS, Kalloger SE, et
al: ARID1A mutations in endometriosis-associated ovarian
carcinomas. N Engl J Med. 363:1532–1543. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
284
|
Samartzis EP, Gutsche K, Dedes KJ, Fink D,
Stucki M and Imesch P: Loss of ARID1A expression sensitizes cancer
cells to PI3K- and AKT-inhibition. Oncotarget. 5:5295–5303. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
285
|
Li J, Yen C, Liaw D, Podsypanina K, Bose
S, Wang SI, Puc J, Miliaresis C, Rodgers L, McCombie R, et al:
PTEN, a putative protein tyrosine phosphatase gene mutated in human
brain, breast, and prostate cancer. Science. 275:1943–1947. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
286
|
Song MS, Salmena L and Pandolfi PP: The
functions and regulation of the PTEN tumour suppressor. Nat Rev Mol
Cell Biol. 13:283–296. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
287
|
Obata K, Morland SJ, Watson RH, Hitchcock
A, Chenevix-Trench G, Thomas EJ and Campbell IG: Frequent PTEN/MMAC
mutations in endometrioid but not serous or mucinous epithelial
ovarian tumors. Cancer Res. 58:2095–2097. 1998.PubMed/NCBI
|
|
288
|
Bos JL: Ras oncogenes in human cancer: A
review. Cancer Res. 49:4682–4689. 1989.PubMed/NCBI
|
|
289
|
Davies H, Bignell GR, Cox C, Stephens P,
Edkins S, Clegg S, Teague J, Woffendin H, Garnett MJ, Bottomley W,
et al: Mutations of the BRAF gene in human cancer. Nature.
417:949–954. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
290
|
Downward J: Targeting RAS signalling
pathways in cancer therapy. Nat Rev Cancer. 3:11–22. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
291
|
Garnett MJ and Marais R: Guilty as
charged: B-RAF is a human oncogene. Cancer Cell. 6:313–319. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
292
|
Singer G, Oldt R, Cohen Y, Wang BG,
Sidransky D, Kurman RJ and Shih IM: Mutations in BRAF and KRAS
characterize the development of low-grade ovarian serous carcinoma.
JNCI. J Natl Cancer Inst. 95:484–486. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
293
|
Kamb A, Gruis NA, Weaver-Feldhaus J, Liu
Q, Harshman K, Tavtigian SV, Stockert E, Day RS III, Johnson BE and
Skolnick MH: A cell cycle regulator potentially involved in genesis
of many tumor types. Science. 264:436–440. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
294
|
Sherr CJ: The INK4a/ARF network in tumour
suppression. Nat Rev Mol Cell Biol. 2:731–737. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
295
|
Kim WY and Sharpless NE: The regulation of
INK4/ARF in cancer and aging. Cell. 127:265–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
296
|
Pomerantz J, Schreiber-Agus N, Liégeois
NJ, Silverman A, Alland L, Chin L, Potes J, Chen K, Orlow I, Lee
HW, et al: The Ink4a tumor suppressor gene product, p19Arf,
interacts with MDM2 and neutralizes MDM2's inhibition of p53. Cell.
92:713–723. 2025. View Article : Google Scholar : PubMed/NCBI
|
|
297
|
Zhao R, Choi BY, Lee MH, Bode AM and Dong
Z: Implications of genetic and epigenetic alterations of CDKN2A
(p16(INK4a)) in cancer. EBioMedicine. 8:30–39. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
298
|
Reed AL, Califano J, Cairns P, Westra WH,
Jones RM, Koch W, Ahrendt S, Eby Y, Sewell D, Nawroz H, et al: High
frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck
squamous cell carcinoma. Cancer Res. 56:3630–3633. 1996.PubMed/NCBI
|
|
299
|
Cawthon RM, Weiss R, Xu GF, Viskochil D,
Culver M, Stevens J, Robertson M, Dunn D, Gesteland R and O'Connell
P: A major segment of the neurofibromatosis type 1 gene: cDNA
sequence, genomic structure, and point mutations. Cell. 62:193–201.
1990. View Article : Google Scholar : PubMed/NCBI
|
|
300
|
Xu G, O'Connell P, Viskochil D, Cawthon R,
Robertson M, Culver M, Dunn D, Stevens J, Gesteland R and White R:
The neurofibromatosis type 1 gene encodes a protein related to GAP.
Cell. 62:599–608. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
301
|
Dasgupta B and Gutmann DH:
Neurofibromatosis 1: Closing the GAP between mice and men. Curr
Opin Genet Dev. 13:20–27. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
302
|
Ballester R, Marchuk D, Boguski M, Saulino
A, Letcher R, Wigler M and Collins F: The NF1 locus encodes a
protein functionally related to mammalian GAP and yeast IRA
proteins. Cell. 63:851–859. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
303
|
Cancer Genome Atlas Research Network, ;
Kandoth C, Schultz N, Cherniack AD, Akbani R, Liu Y, Shen H,
Robertson AG, Pashtan I, Shen R, et al: Integrated genomic
characterization of endometrial carcinoma. Nature. 497:67–73. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
304
|
Ratner N and Miller SJ: A RASopathy gene
commonly mutated in cancer: The neurofibromatosis type 1 tumour
suppressor. Nat Rev Cancer. 15:290–301. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
305
|
Ortiz M, Wabel E, Mitchell K and Horibata
S: Mechanisms of chemotherapy resistance in ovarian cancer. Cancer
Drug Resist. 5:304–316. 2022.PubMed/NCBI
|
|
306
|
National Library of Medicine (NIH), .
ClinicalTrials.gov. https://clinicaltrials.gov/NIH; Bethesda, MD: 2025
March 15–2025
|