Introduction
The behavioral activities and internal physiology of
organisms exhibit distinct circadian rhythms, which are caused by
the 24-h circadian cycle resulting from Earth's rotation (1,2). The
periodic changes in the external environment of organisms are
transmitted into the body, which has independently evolved a
circadian rhythm system to adapt to these changes (3,4).
Further investigation has shown that the circadian rhythm system
couples the external environment and internal physiology through
complex mechanisms rather than a passive response to changes in the
external environment. This complex interplay not only endows
organisms with greater adaptability and survival advantages in
their external environment but also ensures the precision of the
continuous operation of the body (5).
Transitioning from the theoretical to the practical
implications of this understanding, circadian rhythms are closely
related to the health of the human body (6,7). Based
on substantial evidence from studies (8–11) on
human cancer and animal experiments, the International Agency for
Research on Cancer has classified night shift work as a probable
human carcinogen in Group 2A (12).
Epidemiological and experimental studies have shown that the
circadian clock influences several physiological pathways and that
their disruption can lead to various health issues, including
cancer (13,14). To further emphasize the relevance of
this issue in daily lives, the wide range of connections between
these findings and the living habits of modern society need to be
considered. The disruption of circadian rhythms in modern life is
not limited to night-shift work; jet lag, exposure to nighttime
light, electronic devices and mistimed eating can disrupt the
circadian rhythm (15). This
phenomenon is common in modern life and highlights the need to
understand the mechanisms of action behind disorders involving the
circadian rhythm. However, the mechanism by which circadian
rhythm-related disorders affect cancer is not clear. The
correlation between these activities and cancer, along with the
underlying intrinsic mechanism, need to be determined.
The present review synthesizes emerging evidence
from in vitro mechanistic studies and clinical observational
data to propose molecular pathways linking circadian disruption to
cancer progression, especially the Period (PER) gene family The PER
proteins constitute the circadian output arm of the clock and
function as repressors of heterodimeric clock circadian regulator
(CLOCK)-basic helix-loop-helix ARNT like 1 (BMAL1). Their
expression profiles and roles in cancer have been investigated.
While certain interactions [e.g., period circadian regulator 2
(PER2)-epithelial-mesenchymal transition (EMT) and snail family
transcriptional repressor 2 (SNAI2)-enhancer of zeste 2 polycomb
repressive complex 2 subunit (EZH2)] require further validation
through biochemical assays or in vivo genetic perturbations;
inclusion of these interactions aimed to highlight critical
knowledge gaps and prioritize candidate mechanisms for future
research, particularly in circadian-metabolic-epigenetic
crosstalk.
Circadian dysfunction and cancer
Circadian clock
The circadian clock system is a complex network that
integrates periodic external environmental factors with
intracellular molecular operations. This sophisticated system
functions through the expression and regulation of the circadian
clock genes. Circadian genes are governed by interconnected
oscillators and feedback loops, which function systemically at the
cellular level (16). The central
clock located in the suprachiasmatic nucleus (SCN) of the
hypothalamus is initially concentrated at the systemic level and
helps orchestrate whole-body circadian rhythms. This central clock
not only generates the circadian clock but also constantly renders
the system of circadian rhythm changes synchronized with the
environment (16). This
synchronization involves signal transmission from the external
environment to tissues and cells via the autonomic nervous system
and endocrine system, thereby aligning cellular oscillators with
the circadian rhythm and regulating various physiological factors
in the body. Although light signals are the primary external
signals, the system also exhibits responsive adaptations to other
types of stimuli, including dietary intake, ambient temperature
changes and physical exercise; these stimuli fine-tune the internal
human clock (17–20). Within the SCN of the hypothalamus,
the heart of the circadian rhythm system, these additional signals
generally cannot override the dominant influence of light-dark
signals (16,21). This highlights the close connection
of the circadian system with the light-dark cycle, extending the
scope to a broader physiological context in which the effects of
the circadian system occur in various tissues and organ systems in
the body. Studies using circadian rhythm reporting methods have
shown that tissues of most peripheral organs can independently
express circadian rhythms, even in the absence of central
regulation (16,22–24).
This independent functionality allows these peripheral tissues to
provide feedback to the central clock, completing a complex loop of
regulation and synchronization.
By combining these details, a comprehensive
understanding of the multifaceted nature of the circadian rhythm
system may be gained, from its genetic basis to its systemic and
cellular operations. By conducting further studies at the cellular
level, widely distributed autonomous oscillators consisting of
interconnected feedback loops were identified, each of which serves
a key role in sustaining the circadian rhythm (Fig. 1). In the primary feedback loop
(22), CLOCK and BMAL1 or neuronal
PAS domain protein 2 (NPAS2) proteins translocate from the
cytoplasm to the nucleus, where they bind to E-boxes, promoting the
transcription of PER and cryptochrome circadian regulator (CRY)
genes, which are key components of the circadian system. In the
evening, PER and CRY protein heterodimers relocate to the nucleus,
where they interact with the CLOCK and BMAL1 proteins, inhibiting
their transcription (5,6). This inhibition effectively suppresses
the transcription of their own genes, thereby creating a
self-regulatory loop (5,6).
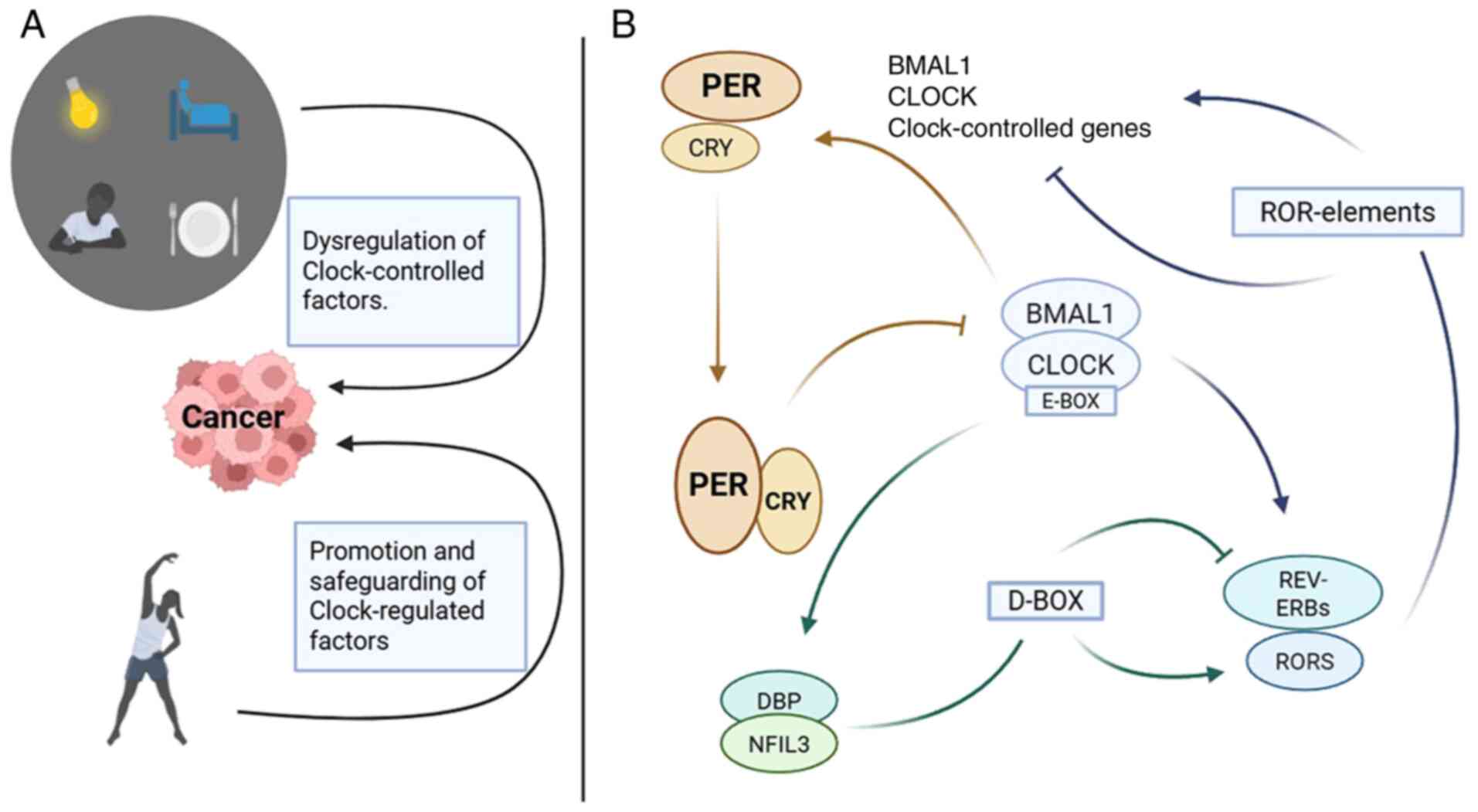 | Figure 1.Circadian feedback loop and its
correlation with cancer. (A) Nighttime light, waking up at night
and eating late at night can increase the susceptibility to cancer.
Morning and evening activity can reduce cancer susceptibility. (B)
Circadian transcriptional and translational feedback loop machinery
in mammals. PER, period circadian regulator; CRY, circadian
regulator; BMAL1, basic helix-loop-helix ARNT like 1; CLOCK,
heterodimeric clock circadian regulator; RORs, RAR related orphan
receptor; REV-ERB, nuclear receptor subfamily 1 group D member 1;
NFIL3, nuclear factor, interleukin 3 regulated; DBP, D-box binding
PAR BZIP transcription factor. |
In the second feedback loop within the nucleus
(22), BMAL1/CLOCK heterodimers
activate the transcription of nuclear receptor subfamily 1 group D
member 1 (REV-ERB) and RAR related orphan receptor (ROR) genes.
RORα and REV-ERBα constitute a supplementary loop, which acts
through RORE elements present in the BMAL1 promoter to activate or
inactivate BMAL1 transcription, respectively. Finally, the third
feedback loop involving proline and acidic amino acid-rich basic
leucine zipper (PAR-bZIP) proteins, including DBP, TEF and HLF,
which interact with the repressor nuclear factor, interleukin 3
regulated at sites containing D-boxes, is driven by the
aforementioned REV-ERB/ROR cycle (5,22).
Epidemiological studies on circadian
rhythms
Following the classification of night shift work as
a probable carcinogen (Group 2A) by the International Agency for
Research on Cancer (12), recent
studies (25–27) have confirmed the close association
between long-term night shift work and the incidence of cancer.
This notable development in the field of occupational health has
led to more focused investigations, with studies on breast,
colorectal, skin, ovarian and prostate cancer showing that
long-term night shift work increases susceptibility to cancer
(25,28–31).
These findings highlight the strong effect of altered circadian
rhythms on health, with multiple factors contributing to deviation
from normal circadian rhythms in individuals engaged in long-term
night shift occupations (Fig. 1),
including fragmented sleep patterns, disordered eating habits,
exposure to nocturnal light and activity, tobacco use, alcohol
consumption and even exposure to carcinogens (25,28–31).
When the focus is shifted to environmental factors, the light-dark
environment serves a key role in modulating the circadian clock. In
this context, several studies have shown a significant association
between exposure to artificial light at night (LAN) and an increase
in the risk of cancer. This correlation was found across various
types of cancer, including prostate, breast, colorectal, thyroid
and pancreatic cancer (28,32–36).
The interaction of light with the central clock in the SCN of the
hypothalamus and subsequent effects on the peripheral clock leading
to cancer illustrate a direct association between environmental
factors and physiological responses. A further study of
epidemiological evidence indicates that light disruption can lead
to circadian rhythm-related disorders in n organs and tissues,
thereby increasing susceptibility to cancer (37). This finding highlights the
importance of studying the relationship between exposure to light
at inappropriate times and the risk of cancer.
Studies on nighttime sleep duration have shown no
correlation with risk of cancer (prostate and breast cancer), but
behaviors such as frequently waking up at night in the previous
year are significantly associated with a greater risk of prostate
and breast cancer (38–40), which may be caused by sleep problems
due to circadian rhythm disturbances resulting from preexisting
diseases. Sleep duration at night shows regional differences.
Studies have shown that in Asia, when sleep time is too short
(<7 h of total sleep among men and overweight individuals),
there is a significant correlation with cancer. However, studies
from Spain and the UK found no correlation between the duration of
sleep and the risk of cancer (38,39).
Another recent case-control study reported a significant
correlation between shortened sleep duration and the risk of breast
cancer (41,42). These regional differences occurred
due to variations in susceptibility or recall bias, highlighting
the need for further investigation into whether the effect of
circadian rhythm disturbances differs among populations.
Population-scale genomic studies have identified inherited variants
that disproportionately increase cancer risk in specific ethnic
groups. For example, BRCA1/2 pathogenic variants are more prevalent
in certain Indian subpopulations and are correlated with higher
hereditary breast/ovarian cancer risk (43). Similarly, cystic fibrosis
transmembrane conductance regulator mutations in Chinese patients
with pancreatic cancer highlight population-specific driver genes
(44). These findings emphasize the
role of germline genetic variability in shaping cancer
susceptibility.
The relationship between dietary patterns and cancer
risk also warrants attention, with studies on disordered eating
showing that late-night eating increases the risk of prostate,
breast and colorectal cancer (45–47).
This finding may be confounded by mistimed light exposure, as
late-night eating implies longer exposure to nighttime light.
Additionally, extending nighttime fasting reduces the risk of
breast cancer recurrence (48),
further supporting the association between circadian rhythm
disturbances and the occurrence, development and recurrence of
cancer. Not only circadian rhythm disruption but also a poor diet,
including the consumption of fast food, canned goods and excessive
sugar, experienced during stressful night shifts are associated
with cancer development in night shift workers (49,50).
An optimal nutritional regimen should systematically integrate key
biological parameters, including chronological age, metabolic
status and neurocognitive expenditure. This adaptive framework must
concurrently consider occupational energy expenditure patterns,
circadian rhythm variations and psychosocial stressors.
Consequently, effective dietary prescriptions require meticulous
evaluation of individual anthropometric measurements, habitual
activity profiles and biochemical markers to ensure congruence with
organismal homeostasis and sustainable health outcomes (49). These epidemiological findings
highlight the harmful effects of disrupting circadian rhythms, with
most disorders leading to an increase in cancer susceptibility
focused on the diurnal cycle transition phase of the circadian
rhythm, suggesting that this may be a particularly vulnerable
period in the circadian rhythm system and a key phase for circadian
rhythm-related disturbances to affect the health of individuals.
This means that the process of transitioning from one daily
activity pattern to another makes organisms more susceptible to
disturbances from abnormal behavior, which in turn can disrupt
their circadian rhythms. Such disruptions can break down the
internal time-regulation systems of organisms, leading to various
negative consequences (51).
Therefore, proper synchronization of the circadian rhythm is key
for maintaining health and preventing diseases.
Circadian rhythm disruption in cancer
animal models
Animal models can be used to validate
epidemiological results and are essential for further understanding
the association between circadian rhythm disruption and cancer. By
disrupting the circadian rhythm of mice through a chronic jet lag
protocol, it was found that mice presented higher tumor growth
rates, greater tumor numbers and more metastatic sites compared to
the control group, leading to more palpable masses at early stages
and larger terminal tumor volumes (52–54).
As nocturnal animals, mice exposed to LAN exhibit suppressed
locomotor activity via negative masking (for example, a 75%
reduction under direct illumination) and disrupted circadian
rhythms, manifesting as delayed activity onset and aberrant
phase-shifting responses (55–57).
LAN also induces anxiety-like behaviors (such as reduced open-field
exploration) and transient spatial memory impairment (58–60).
These findings emphasize the necessity of stringent lighting
control in experimental settings, particularly for studies
investigating circadian or metabolic mechanisms, to avoid
confounding effects of unintended light exposure.
Disrupting the circadian rhythm of mice through
exposure to artificial LAN resulted in greater tumor numbers, more
palpable masses at early stages, larger terminal tumor volumes and
greater weight gain and spleen enlargement (61,62).
When pancreatic cancer cells lacking the BMAL1 gene were implanted
into mice, mice presented faster tumor growth rates, lower survival
rates and drug resistance in tumors (63). These findings confirmed the strong
influence of circadian rhythm disorders on the occurrence,
development and treatment of cancer. Walker et al (64) improved the understanding of the
effect of circadian rhythm disorders on animal behavior through
their research on disrupting the circadian rhythm with
time-restricted feeding. It was reported that such disruptions
cause variations in the activity cycle and vital signs of
tumor-bearing mice, including daily activity patterns, body
temperature rhythms and weight gain (53).
To investigate the spread of cancer, researchers
induced circadian rhythm disruption in mice by implementing a
chronic jet lag protocol. Analysis of blood-related pathways in
tumor-bearing mice demonstrated an increase in the number of cancer
cells in the bloodstream and almost a doubling of disseminated
cancer cells in the bone marrow (53). This significant increase indicated a
greater ability of the cancer to spread via the bloodstream under
circadian rhythm disruption. Lawther et al (65) found that disruption of the circadian
clock through a chronic jet lag protocol exacerbates cancer-induced
inflammation and amplifies disparities in inflammatory signaling
between the body and the brain. The authors also highlighted that
cancer-induced inflammation is organ-specific, further complicating
the interplay between cancer and the circadian clock. Hadadi et
al (53) found that disrupting
the circadian rhythm of mice through a chronic jet lag protocol can
promote EMT and increase the efficiency of mammosphere formation in
cancer cells. These disorders, induced by the chronic jet lag
protocol, contribute to the tumorigenic potential and stemness of
tumor cells, affecting their growth, transport and metastasis while
significantly increasing the tumor burden. In mice with circadian
rhythm disruption, researchers have reported a decrease in the
expression of PER2, CRY2 and REV-ERB, along with a loss of
rhythmicity in the BMAL1, CRY1 and REV-ERB genes (52). Analysis of white blood cells
demonstrated that the clock genes CRY1, CRY2, PER2 and REV-ERBβ
lost their normal rhythmicity, whereas REV-ERBα, PER3 and DBP
maintained significant rhythmicity (66). This disruption in gene expression
and loss of rhythmicity under conditions of circadian rhythm
disruption leads to asynchrony among clock genes. This desynchrony
is closely associated with the occurrence, development and
treatment of cancer (67). To
summarize, the use of animal models has provided invaluable
insights into the complex interplay between circadian rhythm
disruption and cancer, demonstrating intricate details of the
molecular, physiological and behavioral changes associated with
this disruption.
Changes in the immune system due to
circadian rhythm disruption
Circadian rhythm disruption strongly affects tumor
immunity by regulating the cytokine-chemokine network. Hadadi et
al (53) reported that
circadian rhythm disruption induces a switch in protein production
in the tumor immune microenvironment and that this switch is driven
primarily by changes in the CXCL5-CXCR2 axis. It also decreases
antitumor immunity by modulating the chemokine/chemokine receptor
signaling pathway, either through downregulating anti-tumor immune
molecules or upregulating immunosuppressive molecules. Further
investigations by Bishehsari et al (47) provided insights into the
consequences of circadian rhythm disruption on tumor development
and demonstrated that circadian rhythm disruption may promote the
occurrence and development of tumors. This promotion was associated
with a high permeation state, a decrease in the overall and
relative abundance of CD3+ and T cells infiltrating
polyps, a decrease in polyp-related density of Tregs and an
increase in FOXP3+ Treg/RORgt+ Th17 ratio.
These changes in immune cells under circadian rhythm disruption
highlight the importance of the circadian rhythm in immune
responses. The balance between proinflammatory and
anti-inflammatory macrophages in the spleen and tumor is disrupted,
shifting toward a more immunotolerant spectrum. The changes in
immune cells facilitate immune escape and tumor progression,
indicate that circadian disruption affects not only the local
immune microenvironment but also peripheral immunity (68). Zeng et al (69) elucidated how circadian rhythm
disorders speed up the aging and functional decline of natural
killer (NK) cells in the bone marrow and spleen of mice, marked by
an increase in the proportion of senescent
CD27−CD11b+ cells, a reduction in functional
CD27+CD11b+ cells, alterations in receptor
expression and compromised immune functions, including a decrease
in IFN-γ secretion and CD107a activity, leading to a decrease in NK
cell numbers in the spleen and lungs, weakening of immune
surveillance capabilities and a decrease in response to IL-15 due
to lower expression of CD122. In the context of glioma, the effect
of cancer on immunity varies under conditions of circadian rhythm
disruption. In lower-grade glioma, the recruitment of immune cells
increases, but their tumor-killing effect weakens; however, in
glioblastoma multiforme, immune cell recruitment is inhibited
(70). This variation suggests that
circadian disruption can have different effects on the immune
response according to the stage and type of cancer. These studies
collectively showed that circadian disruption can significantly
alter the body's ability to fight cancer by influencing
cytokine-chemokine networks, altering immune cell populations and
changing immune surveillance functions.
Genetic studies linking genes to
circadian rhythms in mouse models
Genetic studies in mouse models demonstrate critical
links between circadian genes and physiological functions. BMAL1
deletion disrupts circadian clock activity, altering respiratory
cycle timing with time-of-day and sex-specific variations,
underscoring the importance of circadian genetics in respiratory
regulation (71). Strain-specific
genetic differences in clock genes between C57BL/6 and BALB/c mice
drive distinct cellular and behavioral adaptations to circadian
disruption, highlighting genetic contributions to circadian
resilience (72). Targeted
disruption of vasoactive intestinal peptide (VIP) neurons in ViptTA
knock-in mice impairs circadian rhythms, demonstrating the
essential role of VIP signaling in maintaining central clock
synchrony and behavioral rhythms (73). Similarly, deleting the Dicer gene in
the SCN shortens circadian periods and reduces locomotor rhythm
precision, proving microRNAs (miRNAs; miRs) are vital for robust
SCN-driven oscillations (74). Loss
of all mature miRNAs in the SCN shortened the circadian period
length by ~37 min at the tissue level and by ~45 min at the
locomotor activity level. Additionally, high-fat diets in female
ICR mice (a genetically diverse outbred strain of albino mice
originally developed by the Institute of Cancer Research) induce
obesity, disrupt diurnal feeding patterns and dysregulate
corticosterone rhythms, linking diet-induced metabolic dysfunction
to circadian and endocrine disruptions through genetic or circadian
pathway interactions (75).
Together, these findings emphasize how genetic variations, neural
circuits and external factors collectively shape circadian
physiology and disease susceptibility (Table I).
 | Table I.Genetic studies linking genes to
circadian rhythms in mouse models. |
Table I.
Genetic studies linking genes to
circadian rhythms in mouse models.
| Gene | Mouse model | Circadian
phenotype | (Refs.) |
|---|
| BMAL1 | BMAL1 knockout | BMAL1 knockout
males exhibit altered respiratory phase durations, while
ventilation remains unaffected. | (71) |
| CLOCK | CLOCK mutant | Altered respiratory
cycle timing and sex-specific metabolic phenotypes observed. | (71) |
| NPAS2 | C57BL/6 and
BALB/c | BALB/c mice adapt
more rapidly to light-dark cycle shifts than C57BL/6 mice and
exhibit significantly reduced Npas2 expression | (72) |
|
| C57BL/6 vs.
BALB/c | Strain-specific
differences in circadian period, entrainment range, and stress
resilience linked to genetic variations. | (72) |
| VIP neurons | VIP-deficient
transgenic | VIP neuronal
ablation disrupts SCN network synchronization; exogenous gene
expression tools developed for studying VIP neuron roles. | (73) |
| Dicer | SCN-specific Dicer
knockout | Loss of Dicer in
the SCN reduces circadian rhythm precision and promotes ultradian
rhythms under constant light. | (74) |
|
| High-fat
diet-induced obesity | Attenuated
corticosterone circadian rhythms observed in obesity models. | (75) |
Potential of circadian clock genes as
cancer biomarkers
High expression levels of the clock genes PER2 and
BMAL1 are associated with good prognosis in colorectal cancer and
gastric cancer, highlighting the key role of these genes in cancer
progression and their lower expression is significantly associated
with distant metastasis, emphasizing the importance of these genes
in cancer metastasis potential (76,77).
The expression of PER1 and PER2 is related to the differentiation
state of the tumor and their expression decreases as the degree of
tumor differentiation decreases. This relationship suggests a role
for these genes in maintaining tumor differentiation, with
implications for prognosis and treatment strategies (78,79).
Researchers have investigated the significance of various circadian
rhythm genes in cancer prognosis. High expression of CRY1 is an
independent factor for metachronous metastasis, suggesting that it
may serve as a predictive biomarker for future metastatic events
(80). Similarly, high levels of
REV-ERBβ are also an independent prognostic factor for local cancer
recurrence (81). These findings
highlighted the multifaceted roles these genes serve in regulating
cancer progression and recurrence (82). Further emphasizing the prognostic
significance of these genes, high expression of PER2 and BMAL1 was
associated with improved overall and disease-free survival, whereas
high CRY1 expression and low BMAL1 expression were associated with
lower five-year overall and disease-free survival (76). This association provides valuable
insights into the use of these genes as biomarkers for assessing
patient prognosis and guiding treatment decisions. The state of
clock genes and their oscillatory rhythms may be assessed to
evaluate the status and effectiveness of cancer treatment. This
assessment offers a promising avenue for personalized cancer
therapy, where understanding the expression patterns of the
circadian clock genes may guide treatment planning and prognosis
estimation in the future.
Period and cancer
In analyzing polygenic, multifactorial diseases such
as cancer, evidence was integrated across scales, from molecular to
organismal, to reflect the current state of the field. This
approach acknowledges the necessity of combining direct biochemical
evidence (such as protein interaction assays) with indirect
clinical correlations (such as survival analysis of circadian gene
signatures) to construct testable mechanistic hypotheses (83). The PER gene family, consisting of
PER1, PER2 and PER3, forms a core component of the circadian
circuit (69). The protein
functions as a dimer together with the clock gene CRY and PER can
still complete at least one full cycle even in CRY1/2 knockout
cells (84). These findings suggest
that PER may independently inhibit the oscillation of the circadian
rhythm, whereas CRY contributes to the continuation of these
oscillations.
The intricate relationship between the expression of
PER genes and the oscillations of circadian rhythms is closely
intertwined with the onset, progression and treatment responses of
various types of cancer (Table
II). This connection is pivotal when assessing the tumor state
and treatment efficacy, with the level of PER expression and
changes in oscillatory rhythms serving as key indicators. Yin et
al (85) reported significant
alterations in the mesor (rhythm-adjusted mean), amplitude and
acrophase (peak phase of the rhythm) dimensions of PER1 in oral
squamous cell carcinoma. In high-grade breast cancer, the PER2
protein can be detected, but oscillation is absent, which contrasts
with low-grade breast cancer, where oscillation still occurs
(86,87). This pattern is also found in the
transformation of oral cheek mucosal cancer, where there is a
marked decrease in the median and amplitude of PER2 mRNA expression
as the cancer progresses (88). The
role of PER2 extends to non-small cell lung cancer, with its
expression associated with the degree of differentiation and
tumor-node-metastasis stage (89).
Additionally, the significance of PER3 is indicated by its
correlation with the stage of breast and colorectal cancer
(90,91). Several studies have shown that
higher levels of PER protein are associated with a significant
increase in overall survival rates (77–79,92).
Yang et al (93) reported a
significant relationship between the restoration of PER3 and the
likelihood of cancer recurrence, positioning PER genes as
prognostic markers in tumor diagnostics and management. Genetic
variations in PER are closely related to cancer susceptibility.
Rajendran et al (94)
reported a significant association between gastric cancer
susceptibility and gene polymorphisms, including PER1 rs3027178 and
PER2 rs934945. In soft tissue sarcoma; Benna et al (95) identified associations with PER1
rs3027178, PER2 rs934945 and rs7602358 upstream of PER2. Moreover,
associations between the PER3 gene rs228729 and lung cancer and
between rs2640908 and prostate cancer were reported by Couto et
al and Hinoura et al (96,97).
Dagmura et al (98) reported
that the PER2 variable number tandem repeat polymorphism is related
not only to the occurrence of cancer but also to the perineural
invasion of cancer. The PER gene interacts with various
cancer-related genes and pathways to influence the development of
cancer (Fig. 2).
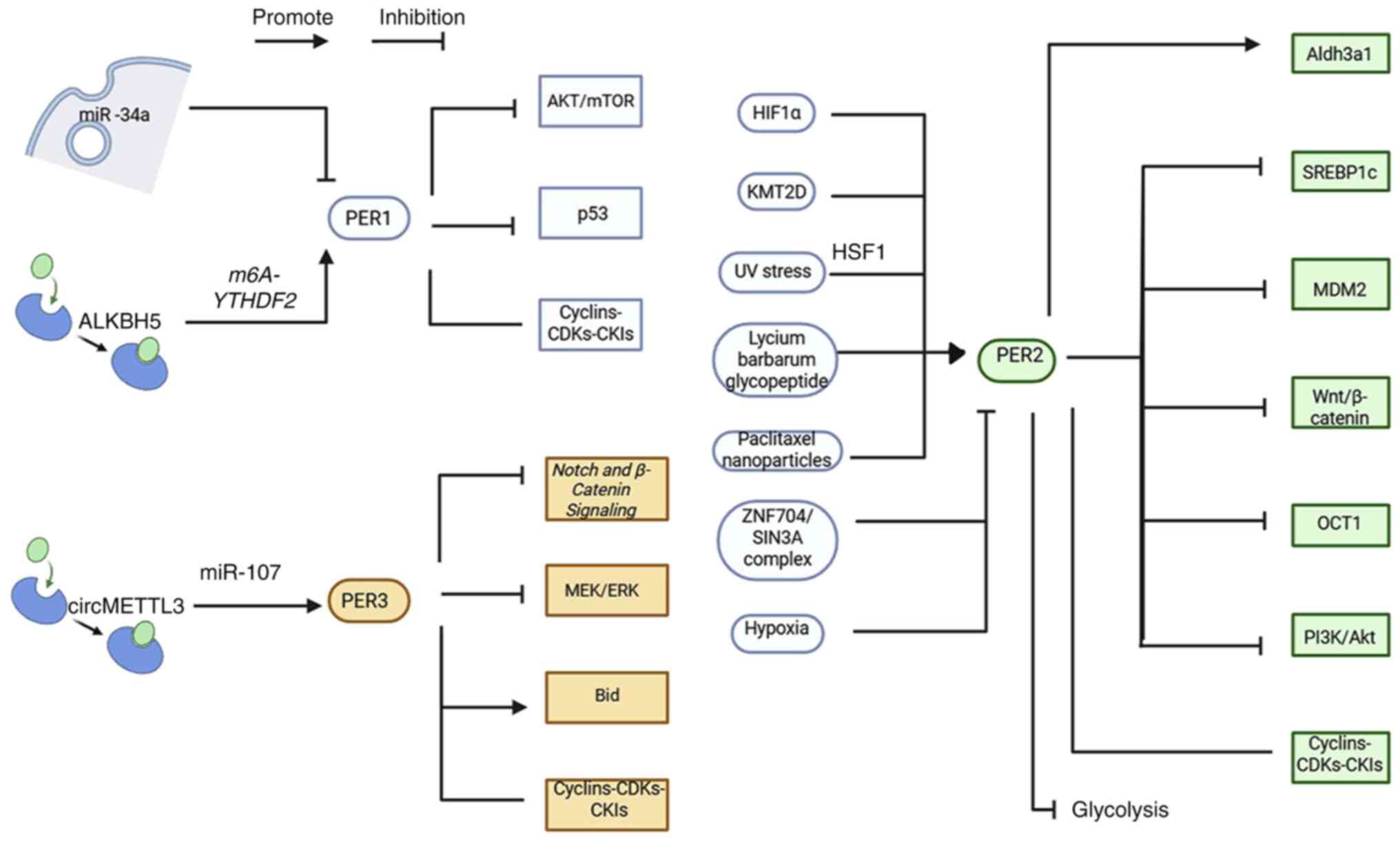 | Figure 2.Mechanisms by which PER inhibits
tumors. The PER gene interacts with various cancer-related genes
and pathways to influence the development of cancer. miR, microRNA;
PER, period circadian regulator; p53, protein 53; ALKBH5, AlkB
homolog 5; m6A, N6-methyladenosine; YTHDF2, YT521-B homology domain
family 2; CDKs, cyclin-dependent kinases; CKIs, CDK inhibitors;
HIF1α, hypoxia-inducible factor 1α; Aldh3a1, aldehyde dehydrogenase
3 family, A member 1; KMT2D, lysine-specific methyltransferase 2D;
SREBP1c, sterol regulatory element-binding protein 1c; UV,
ultraviolet; MDM2, MDM2 proto-oncogene; ZNF704, zinc finger protein
704; SIN3A, SIN3 transcription regulator family member A; OCT1, POU
class 2 homeobox 1; Bid, BH3-interacting domain death agonist. |
 | Table II.Period gene family and cancer. |
Table II.
Period gene family and cancer.
| A, PER1 |
|---|
|
|---|
| Types of
cancer | Pathways of
action | Results of
action | (Refs.) |
|---|
| Oral | PER1 altered the
AKT/mTOR signaling pathway. | PER1 is inhibited
in oral squamous cell carcinoma and overexpression of PER1
suppresses tumor growth. | (85) |
|
| PER1 regulates cell
cycle-related genes. |
| (99) |
|
| PER1 regulates the
AKT/mTOR signaling pathway. |
| (101) |
| Lung | PER1 and P53
negatively regulate each other's expression and activity. | PER1 reduces the
sensitivity of lung cancer cells to drug-induced apoptosis. | (102) |
|
Cholangio-carcinoma | PER1 is regulated
by miRNA-34a. | MiRNA-34a promotes
the progression of cholangiocarcinoma by inhibiting the expression
of PER1. | (104) |
| Pancreatic | ALKBH5 regulates
PER1 post-transcriptional activation by demethy-lating m6A. | The absence of
ALKBH5 leads to reduced activation of PER1, promoting the
progression of pancreatic cancer. | (105) |
| Gastric | In HER2-positive
gastric cancer resistant to trastuzumab, glycolysis is regulated by
the BMAL1-CLOCK-PER1-HK2 axis. | Silencing PER1
enhances the therapeutic effect of trastuzumab in the treatment of
gastric cancer. | (129) |
|
| B, PER2 |
|
| Types of
cancer | Pathways of
action | Results of
action | (Refs.) |
|
| Buccal mucosa
carcinoma | The relative
expression of PER2 mRNA in different stages of carcinogenesis was
analyzed. | PER2 mRNA
expression decreased with tumor progression. | (88) |
| Glioma | Lycium barbarum
polysaccharide may reduce the expression of SREBP1c by upregulating
the expression of PER2. | In glioma, PER2
expression is down-regulated, and overexpression of PER2 inhibits
cancer. | (106) |
|
| PER2 increases TP53
expression, DNA damage repair and apoptosis. |
| (107) |
|
| PER2 acts on
Wnt/β-catenin signaling pathway. |
| (108) |
| Oral | PER2 regulated the
mRNA expression of Ki-67, MDM2, c-Myc, Bcl-2, MMP2, VEGF, p53, Bax
and TIMP-2. | Oral squamous cell
carcinoma cells suppress the expression of PER2 and PER2 inhibits
cancer progression. | (109) |
|
| PER2 regulates the
P53/P14ARF, PIK3CA/AKT, and caspase-8 pathways. |
| (110) |
|
| PER2 upregulates
TP53 and inhibits EMT. |
| (111) |
|
| PER2 regulates the
cyclin/CDK/CKI cell cycle network |
| (112) |
| Breast | Per2 deficiency
maintains mammary epithelial cells in a pre-differentiated
stage. | A defect in the
PER2 gene leads to underdevelopment of glands in mice. | (113) |
|
| Hypoxia promotes
the pathway of EMT by degrading PER2. | PER2 inhibits the
growth and metastasis of breast cancer. | (114) |
|
| The ZNF704/SIN3A
complex inhibits PER2. |
| (115) |
| Ovarian | PER2 gene affects
the PI3K/AKT signaling pathway. | PER2 inhibits
ovarian tumor growth, metastasis and drug resistance. | (116) |
|
| PER2 promoted
NM23-H1 expression by inhibiting MTA-1 expression. |
| (117) |
| Cervical | PER2 affects the
PI3K/AKT pathway. | PER2 overexpression
inhibits tumor progression and cisplatin resistance. | (118) |
| Thyroid | PER2 can induce
AP-1 activity by activating the JNK/MAPK signaling pathway. | Reduced PER2
expression promotes cancer cell proliferation. | (119) |
| Lung | KMT2D deletion
reduces PER2 expression, thereby regulating multiple glycolysis
genes. | PER2 inhibits
glycolysis and blocks tumorigenesis. | (120) |
| Kidney | HIF1α increases the
amplitude of PER2 oscillations. | PER2 inhibits
proliferation of renal cell carcinoma. | (122) |
|
| C, PER3 |
|
| Types of
cancer | Pathways of
action | Results of
action | (Refs.) |
|
| Breast | PER3 affects the
MEK/ERK signaling pathway. | PER3 inhibits
breast cancer. | (90) |
| Colorectal | PER3 affects Notch
and β-catenin signaling pathways. | The expression of
PER3 is decreased in colorectal cancer. Overexpression of PER3 can
inhibit the proliferation, invasion and drug resistance of
colorectal cancer cells. | (91) |
|
| PER3 is targeted by
miRNA-103. |
| (126) |
|
|
circMETTL3/miRNA-107 regulates colorectal
cancer through PER3. |
| (127) |
PER1 and cancer
Oral cancer
Yin et al (85) found that PER1 exhibits abnormal
changes in three key dimensions: the mesor, amplitude and acrophase
dimensions. This finding is important for understanding the role of
genes in cancer. These changes in PER1 lead to a cascading effect
in the clock gene network. The downregulation of PER1 triggers the
upregulation of genes such as PER3, RORA and REV-ERBα while
simultaneously leading to the downregulation of PER2, CRY1, CRY2
and NPAS2 (99). This pattern of
alteration of gene expression indicates a fundamental difference
between cancerous and normal cells, significantly affecting tumor
growth, proliferation, metastasis and treatment responses. By
further investigating the effects of PER1, direct knockout studies
have demonstrated notable outcomes. The removal of PER1 decreases
apoptosis and increases cell proliferation, ultimately promoting
tumor formation (99). The effect
of PER1 extends to the cell cycle, a key aspect disrupted in
cancer. Silencing the PER1 gene in oral squamous cell carcinoma
significantly affects the cyclin-CDK-CKI cell cycle molecular
regulatory network, particularly affecting the G1/S
checkpoint (100). This disruption
is evident in the altered levels of several cell cycle-related
genes. The results of qRT-PCR and Western blotting analyses have
shown an increase in the levels of cyclin D1, cyclin E, cyclin B1,
CDK1 and Wee1, coupled with a decrease in the levels of p53, Cyclin
A2, p16, p21 and cdc25 (100).
Yang et al (101)
contributed to the understanding of the role of PER1 in oral
squamous cell carcinoma. The authors found that overexpressing PER1
promotes autophagy and apoptosis while inhibiting cell
proliferation and the AKT/mTOR pathway and further demonstrated
that PER1, through its effects on AKT activators and autophagy
inhibitors, can inhibit autophagy-mediated apoptosis and promote
cell proliferation in an AKT/mTOR pathway-dependent manner
(101).
Lung cancer
A study by Bellet et al (102) on lung cancer provided significant
insights into the intricate relationship between the circadian
clock gene PER1 and the tumor suppressor factor P53. The authors
reported that PER1 can affect the stability and transcriptional
activity of P53. PER1 reduces the stability and transcriptional
activity of P53. This finding is important because P53 suppresses
tumor development by inducing cell cycle arrest, apoptosis,
cellular senescence and DNA repair (103). A reduction in P53 activity due to
PER1 can decrease the efficacy of these tumor-suppressing actions,
thereby affecting the sensitivity of cancer cells to treatment.
Conversely, P53 can inhibit the binding of CLOCK/BMAL1 to the PER1
promoter. CLOCK and BMAL1 are core components of the molecular
circadian clock and their binding to the PER1 promoter is essential
for regulating PER1 expression. The inhibition of this binding by
P53 suggests a feedback mechanism in which P53 can regulate the
expression of PER1, possibly to maintain cellular homeostasis and
prevent abnormal growth of cells (88).
Cholangiocarcinoma and pancreatic cancer
In the context of cholangiocarcinoma, investigation
on the effect of PER1 on the cyclin-CDK-CKI cell cycle molecular
network has been conducted. Overexpressing the PER1 gene results in
a considerable reduction in G2/M phase blockade and an
increase in apoptosis in cholangiocarcinoma cells, which highlights
the role of PER1 in regulating critical phases of the cell cycle
and promoting cancer cell death (104). In pancreatic cancer, the role of
PER1 has been investigated in the context of the ATM pathway.
Overexpressing PER1 affects the cell cycle molecular network
through this pathway and this overexpression helps restore
molecules associated with the ATM-dependent pathway, including
p-ATM, p-CHK2, pCDC25C, p-P53, P21 and p-CDK1 (105). Reactivating the
ATM-CHK2-P53/CDC25C signaling pathway results in the inhibition of
cell growth, highlighting that PER1 serves a key role in hindering
the progression of pancreatic cancer.
PER2 and cancer
Glioma
In a study involving the Lycium barbarum
glycopeptide, researchers reported that its extract, known as LbGP,
can upregulate the expression of PER2 through the PKA-CREB pathway
(106). An increase in PER2
expression subsequently inhibits the PI3K/AKT/mTOR pathway, which
is key for regulating various cellular processes, including cell
proliferation (106). Due to this
inhibition, sterol regulatory element binding transcription factor
1 (SREBP1c), a key regulator of lipid synthesis, is negatively
affected and the downregulation of SREBP1c reduces lipid synthesis
and cell proliferation in glioblastoma, indicating that SREBP1c may
be a potential therapeutic target (106). Regarding the tumor suppressor gene
P53, which serves a role in cell cycle arrest, apoptosis and aging,
Zhanfeng et al (107)
reported that PER2 regulates apoptosis in glioma cells through the
ATM-P53 pathway. PER2 promotes the expression of ATM and P53 while
inhibiting the expression of c-myc and MDM2 proto-oncogene (MDM2)
and this activity of PER2 is significant during DNA damage and
p53-mediated apoptosis in glioma cells (107). Ma et al (108) reported that PER2 can downregulate
the Wnt/β-catenin signaling pathway, which regulates the stemness
of glioma stem cells. PER2 overexpression results in cell cycle
arrest at the G0/G1 phase, reducing the
stemness, self-renewal ability and migration of glioma stem cells.
These findings suggest that PER2 affects the behavior and
characteristics of glioma stem cells, influencing their potential
for proliferation and invasion.
Oral cancer
The function of the PER2 gene is closely related to
its effect on P53 (109).
Following this line of investigation, researchers found that
attenuating PER2 expression leads to the upregulation of MDM2 mRNA
(109). This is notable because
P14ARF mitigates the ubiquitination and subsequent degradation of
P53 via MDM2, ultimately increasing the concentration of functional
P53 in cells, a key factor in tumor suppression (110). Xiong et al (110) reported that PER2 affects the
PI3K/AKT pathway, a key regulator of tumor cell proliferation,
invasion and metastasis. The lack of PER2 suppresses PTEN
expression, weakening the negative control of the PI3K/AKT pathway,
thereby activating the pathway. PER2 may also affect the PI3K/AKT
pathway through its interaction with the P110 subunit encoded by
PIK3CA (110). Guo et al
(111) reported that PER2 not only
increases the expression of the TP53 gene but also impedes EMT. The
authors reported that the downregulated expression and subcellular
localization of PER2 in oral squamous cell carcinoma tissues and
cells leads to the activation of EMT transcription genes such as
TWIST1/2, ZEB1/2 and MMP1, thereby enhancing the invasiveness of
the tumor (111). Additionally,
the cell cycle network is disrupted in oral cancer. This disruption
is characterized by an increase in mRNA levels of cyclins (A2, B1
and D1) and CDKs (CDK4 andCDK6), whereas the mRNA levels of P53,
P16 and P21 decrease and cancer cells exhibit a significant
reduction in G1/G0 phase cells, increase in
cell proliferation and decrease in apoptosis (112). Finally, knocking down PER2
increases the expression of tumor-related genes such as Ki-67,
MDM2, c-Myc, Bcl-2, MMP2 and VEGF, decreases the expression of p53,
Bax and TIMP metallopeptidase inhibitor 2, increases cancer cell
proliferation, migration and invasion and reduces the number of
apoptotic and G1/G0 phase cells (109).
Breast cancer
McQueen et al (113) provided insights into the role of
PER2 in cancer biology, particularly concerning EMT. The
researchers found that PER2 serves a key role in suppressing the
expression of the EMT-related transcription factor SNAI2. To
further assess the significance of PER2 deficiency, the authors
reported that in PER2−/− mice, mammary epithelial cells
could not fully mature and remained in a precursor state of luminal
and myoepithelial mammary cell types (113). This hindrance in cell maturation
due to the lack of PER2 is an important finding, as it suggests a
mechanism by which PER2 influences cancer progression. Alterations
in EMT-related pathways due to PER2 suppression enhance cancer cell
stemness, along with their migration and invasion ability. This
enhancement is a key factor in the aggressiveness and metastatic
potential of cancer cells (113).
Hwang-Verslues et al (114)
demonstrated that PER2 acts as a corepressor and interacts with the
POU class 2 homeobox 1 (OCT1) gene, which encodes a transcription
factor protein that serves a key role in cell differentiation and
maturation. PER2 then recruits a complex containing EZH2, SUZ12
polycomb repressive complex 2 subunit and histone deacetylase 2
(HDAC2), which may convert the usually active OCT1 sites into
repressive sites through HDAC2-mediated histone deacetylation,
thereby inhibiting EMT. Hwang-Verslues et al (114) also reported a link between EMT
activation and hypoxic conditions, in which the PER2 protein is
degraded, disrupting the connection between the OCT1 site and the
repressor complex and this disruption leads to the activation of
EMT gene expression. Finally, the zinc finger protein 704
(ZNF704)/SIN3 transcription regulator family member A complex,
suppresses PER2 expression and this suppression extends the
circadian clock cycle and reduces its amplitude (115). This intricate network of
interactions and regulatory mechanisms involving PER2 highlights
its potential as a target for therapeutic interventions in cancer
treatment.
Ovarian and cervical cancer
Wang et al (116) demonstrated a strong link between
the decreased protein level of PER2 in various types of cancer and
the methylation of CpG islands, suggesting an epigenetic regulatory
mechanism. The PI3K/AKT signaling pathway is a downstream signal
transduction pathway involving various factors. The PI3K/AKT
signaling pathway participates in antiapoptotic activity and
promotes cell proliferation, migration and carcinogenesis (101). Wang et al (117) reported that overexpression of PER2
inhibits the activation of AKT and further induces the expression
of nm23-H1, subsequently suppressing the expression of
metastasis-associated protein 1, which may be related to the
metastatic potential of cancer cells, thereby inhibiting the
proliferation, angiogenesis, invasion and metastasis of tumor
cells. These findings were also found in ovarian cancer research,
where PER2 was shown to regulate critical elements of the cell
cycle network, including cyclin E and c-myc; the absence of PER2
was found to disrupt this network, impair DNA damage repair, reduce
the efficiency of the cell response to DNA damage through cycle
checkpoints and ultimately lead to the deterioration of the health
of cells (116). Additionally,
Wang et al (116,118) studied treatment resistance in
cervical and ovarian cancer and found that overexpression of PER2
affects the PI3K/AKT pathway, a key factor influencing the
resistance of these types of cancer to chemotherapy.
Thyroid cancer
Lee et al (119) studied thyroid cancer and found a
significant disruption in the aging-related PER2 gene, which is
characterized by a linear pattern in circadian oscillation and a
substantial reduction in protein levels. They also found that when
the circadian clock gene PER2 is knocked down, it triggers an
increase in AP-1 activity, and this activation occurs through the
JNK signaling pathway, leading to an increase in cell proliferation
(119).
Lung cancer
Alam et al (120) reported the key role of
lysine-specific methyltransferase 2D (KMT2D), an epigenetic
modifier, in the context of lung cancer; KMT2D is one of the most
frequently inactivated modifiers in this type of cancer and
therefore, its connection to PER2 is particularly noteworthy
(105). The authors reported that
the absence of KMT2D leads to a reduction in PER2 expression, which
results in the upregulation of various glycolytic genes, including
ENO1, PGK1, PGAM1, LDHA, GAPDH and CDK1 (120). The upregulation of these genes
strongly promotes tumorigenesis, as indicated by their
findings.
Kidney cancer
The role of hypoxia-inducible factor (HIF) in renal
cell carcinoma is a significant area of interest in cancer research
and HIF1α serves a key role in the cellular response to hypoxia by
regulating various genes involved in key processes such as
angiogenesis, metabolism and cell survival (121). HIF1α increases the amplitude of
PER2 oscillation by directly binding to the HIF-binding site on the
PER2 promoter, thereby altering PER2 activity (122). This increase in transcriptional
activity inhibits tumor proliferation, suggesting that targeting
the HIF1α-PER2 pathway may be a viable strategy for slowing or
inhibiting the progression of renal cell carcinoma.
Hepatocellular carcinoma (HCC)
HCC has a significant global health impact and is
the third leading cause of cancer-related death worldwide. HCC
pathogenesis involves complex interactions between viral infections
(such as hepatitis B/C viruses) and dietary/lifestyle factors
(123). Several studies have
highlighted the role of circadian rhythm disruption and dietary
dysregulation in the development of HCC, particularly among
populations with irregular lifestyles, such as night-shift workers
(124,125). The hepatic physiology follows a
daily rhythm, driven by clock genes that control the expression of
several proteins involved in distinct metabolic pathways.
Alteration of the liver clock results in metabolic disorders, such
as non-alcoholic fatty liver diseases (NAFLD) and impaired glucose
metabolism, which can trigger the activation of oncogenic pathways,
inducing spontaneous HCC.
Downregulation of PER2 in HCC tissues is correlated
with suppressed p53 expression and overexpression of c-Myc,
potentially promoting tumor progression (98,99).
Environmental disruption of circadian rhythms (such as jet lag)
accelerates hepatocarcinogenesis in mice, accompanied by altered
PER2 rhythmicity in liver and tumor tissues (98). PER2 loss of function may exacerbate
genomic instability and inflammation, which are key drivers of HCC
initiation (99). While these
findings highlight the tumor-suppressive role of PER2 in HCC, the
mechanism by which PER-mediated circadian disturbances directly
contribute to HCC remains undetermined.
PER3 and cancer
Colorectal cancer
Hong et al (108) and Zhang et al (91) provided significant insights into the
role of PER3 in colorectal cancer, highlighting its potential as a
target for therapeutic intervention. Hong et al (108) studied colorectal cancer and found
that PER3 overexpression significantly reduces cancer cell
proliferation and invasion while enhancing their apoptosis, largely
through the effect of PER3 on the mitochondrial apoptosis pathway
and the cell cycle network. A key discovery of the study involves
the Bcl-2 family, which is key to the mitochondrial pathway. The
authors noted an increase in Bid and a decrease in Bcl2 expression,
highlighting the role of PER3 in promoting apoptotic pathways in
cancer cells. It was also found that miR-103 targets PER3 by
binding to its 3′ UTR, influencing apoptosis-related genes in the
p53 pathway and altering the expression of cell cycle molecules
such as cyclin B1 and CDC2 (126).
Zhang et al (127) reported
that PER3 is regulated by RUNX3, which activates the transcription
of circMETTL3 by binding to the METTL3 promoter and this
transcription then directly targets PER3 with miR-107 to regulate
cancer cell proliferation and invasion. Additionally, PER3 has a
strong effect on the stemness of colorectal cancer stem cells and
suppressing the Notch or β-catenin signaling pathways may decrease
chemotherapy resistance and self-renewal in these cells (91). PER3 overexpression decreases the
levels of stemness markers such as Notch1, Jagged1, β-catenin,
c-Myc and LGR5 in colorectal cancer stem cells, which is correlated
with the inhibition of the Notch and β-catenin pathways; these
pathways are vital for maintaining stemness and chemotherapy
resistance in these cells (91).
Breast cancer
Liu et al (90) provided valuable insights into the
role of PER3 in breast cancer, particularly concerning the MEK/ERK
signaling pathway, which serves a key role in cell proliferation,
invasion and metastasis. Liu et al (90) demonstrated that silencing PER3 in
breast cancer cells significantly inhibits the MEK/ERK signaling
pathway, as shown by a decrease in the levels of p-MEK and p-ERK1/2
proteins. This pathway serves a vital role in regulating cell
growth and survival, indicating that PER3 silencing substantially
affects the ability of cancer cells to proliferate, invade and
metastasize. The authors also demonstrated the potential of
modulating the effects of PER3 on the MEK/ERK pathway via specific
inhibitors and activators, such as PD98059, an inhibitor of MEK and
TPA, an activator, in cells with altered PER3 expression (90). This approach suggests that
manipulating PER3 expression, along with the targeted use of
MEK/ERK pathway inhibitors or activators, can offer an effective
strategy to control the progression of breast cancer.
Role of the PER family in cancer
PER serves an important role in cancer development
and significantly affects the behavior of cancer cells. Alterations
in PER gene expression affect cell proliferation, apoptosis and
metastasis across various types of cancer cells. In oral cancer,
breast cancer, ovarian cancer, cervical cancer, thyroid and
glioblastoma cells, silencing PER2 promotes cancer cell
proliferation, reduces apoptosis and increases in vivo
metastasis, invasion and tumorigenesis (106,108,109,111,114,117–119). In contrast to the suppressive
effect on p53 observed with PER1 overexpression in lung cancer
(100,102), PER2 overexpression leads to an
increase in p53 expression (111).
Moreover, PER2 overexpression in glioblastoma inhibits lipid
synthesis (106) and induces
morphological changes in cervical cancer cells (118), thereby suppressing cancer cell
proliferation. In breast and colorectal cancer cells, altering
silenced PER3 expression increases proliferation, invasion and
metastatic capabilities and decreases apoptosis (90,126,127). Additionally, overexpressing PER1
increases autophagy in cancer cells (101) and reciprocal negative regulation
between PER1 and p53 gene expression and activity (100,102). PER2 also affects cell autophagy,
potentially related to its interference with the PI3K/PKB pathway
(104). Overexpressing PER1
inhibits the cell cycle network, EMT and angiogenesis capabilities
in cholangiocarcinoma (104). The
PER2 gene also affects the cell cycle cyclin/CDK/CKI network,
angiogenesis, EMT and glycolysis, which are closely associated with
its anticancer effects (108,109,111,112,114,120,128).
The occurrence and development of tumors strongly
influence the circadian rhythm and expression of PER genes. In
lung, gastric, oral and pancreatic cancer, a weakened circadian
rhythm and reduced gene expression of PER1 were found in patients,
with a decrease in PER1 expression as the tumor progresses through
various stages (79,85,89,105).
Low expression of PER1 in patients with pancreatic cancer is
associated with shorter survival (105). Patients with higher levels of PER
expression (1–3) in patients with lung cancer have higher
survival rates compared with those with lower levels of PER
expression (89). Similarly,
weakened circadian rhythms and reduced gene expression of PER2
occur in lung, breast, oral and ovarian cancer, cholangiocarcinoma
and glioma stem cells, with PER2 expression decreasing as the tumor
progresses in stage (85,89,90,104,108,114,117). In high-grade ovarian cancer and
most renal cancer cells, neither the circadian rhythm nor the
protein level of PER2 can be detected (117,122). Additionally, a weakened circadian
rhythm and a decrease in the expression of the PER3 gene were found
in lung, breast and colorectal cancer and leukemia, with a decrease
in the expression of the PER3 gene as the tumor progresses
(89,90,93,114,126,127). Furthermore, recovery of PER3
expression was observed in both patients with AML and those with
ALL who achieved remission but not in patients who relapsed after
treatment (93).
Treatment implications
Overall, the experimental evidence suggests that
PER potentially inhibits cancer progression. However, Bellet et
al (102) introduced a nuanced
perspective, reporting that in mice with xenografted lung cancer
cells, PER1 can reduce the sensitivity of cancer cells to
drug-induced apoptosis. This counterintuitive finding indicates
that while PER1 activation might decrease the effectiveness of
certain cancer therapies, it may benefit normal cells by mitigating
the cytotoxic effects of these drugs. In the context of gastric
cancer, Wang et al (129)
investigated the therapeutic potential of PER1, reporting that PER1
can reverse the drug resistance of cancer cells, specifically
regulating HK2-dependent trastuzumab resistance through interaction
with PPARG and that this specific form of drug resistance can be
reversed by silencing PER1 or using metformin to degrade the PER1
protein and inhibit glycolysis. The association between PER2 and
cancer drug resistance represents a significant area of research.
Wang et al (101,103) reported that overexpressing PER2
can modulate drug resistance factors through the PI3K/AKT pathway.
This modulation decreases the level of multidrug-resistant
proteins, which are major hurdles in the treatment of various types
of cancer, including cervical and ovarian cancer (116,118). By inhibiting drug resistance, PER2
overexpression enhances the effectiveness of chemotherapy, offering
a promising avenue for improving cancer treatment outcomes
(118,130). Katumune et al (131) demonstrated that PER2 can recruit
HDACs to the promoter region of aldehyde dehydrogenase 3 family
member A1 (ALDH3A1), reducing the acetylation level of H3K9 and
acting as an inhibitor of ALDH3A1 expression. However, mutated PER2
could not recruit HDACs, leading to an increase in ALDH3A1
expression and ultimately causing cells to develop resistance to
chemotherapeutic drugs. In the context of lung cancer, paclitaxel
nanoparticles exhibited the most effective antitumor activity and
reduced liver damage 15 h after light onset through the
upregulation of PER2 expression to induce apoptosis in vivo
and in vitro, suggesting a chronotherapy strategy to
optimize drug timing, potentially improving efficacy and reducing
side effects in clinical settings (132). Additionally, the relevance of PER2
in the context of X-ray treatment in glioma cell studies further
highlights its role in various cancer treatments (107). Notably, PER3 enhances the
inhibitory effect of 5-fluorouracil (5-FU) on tumor stem cells,
which is a significant development given that 5-FU is a widely used
chemotherapeutic drug (91). The
enhancement of the effectiveness of 5-FU by PER3 is significant for
targeting tumor stem cells, which serve an important role in
chemotherapy resistance and cancer recurrence, suggesting that PER3
targeting may be a viable strategy to improve treatment outcomes
and address the challenges posed by tumor stem cells in cancer
therapy. The correlation between PER3 and posttreatment side
effects, identified via polygenic risk score analysis, is a
significant aspect of its role in cancer therapy (133). The promising discovery that
altering treatment timing according to different PER3 genotypes can
decrease side effects highlights the potential of personalized
medicine, where treatment is customized based on individual genetic
profiles to optimize efficacy and reduce adverse effects.
Additionally, PER3 inhibits the resistance and self-renewal of
colorectal cancer stem cells, which is associated with the
inhibition of the Notch and β-catenin signaling pathways,
highlighting its therapeutic potential (91). These pathways are essential for
maintaining cancer cell stemness and drug resistance and the role
of PER3 in inhibiting these pathways indicates that it may hold the
key to addressing major challenges in cancer treatment. A clinical
trial by Yang et al (93)
demonstrated the ability of PER3 to serve as a biomarker for
treatment effectiveness in leukemia, with the restoration of PER3
expression in acute myeloid and acute lymphoblastic leukemia
patients who experienced remission but not in those who relapsed,
indicating that PER3 levels might reflect the response of patients
to treatment. These findings highlight the complex and
contradictory relationship between PER and cancer drug resistance,
which may be attributed to the extensive and complex interactions
of the downstream targets of the PER gene in different genetic
backgrounds.
Conclusions
The current landscape of circadian-cancer research
is characterized by fragmented yet convergent evidence. While
definitive causal links remain scarce for numerous pathways (such
as circadian control of epigenetic reprogramming), the present
review underscored the urgency of bridging in vitro findings
with in vivo validation tools to unravel context-dependent
mechanisms. The circadian clock is extensively present in the human
body, strongly influencing internal functions and helping the body
adapt to external environmental changes, serving a key role in
regulating life activities. In modern society, numerous individuals
experience circadian rhythm disorders for various reasons, such as
shift work, chronic jet lag, high fat intake and abnormal sleep
patterns. Circadian rhythm disruption is an independent risk factor
for cancer and the disruption of circadian rhythm genes is closely
related to the occurrence, development and treatment of cancer. The
members of the PER family, as core components of the circadian
clock cycle, serve vital roles in maintaining these rhythms.
Dysregulation of PER genes is closely associated with the
occurrence, development and treatment of various types of cancer,
as PER dysregulation affects key regulatory pathways related to
cancer, including those governing the cell cycle, apoptosis, DNA
repair, metabolism and stem cell maintenance. These pathways are
important for tumor development and progression, making the PER
family a key focus in cancer research. The understanding of the
mechanisms by which PER genes regulate cancer is currently
evolving. Further in-depth research is needed to decipher these
complex interactions. This includes understanding the
tissue-specific expression of PER genes, as the role and rhythm of
PER expression can vary significantly between different
tissues.
Exploring the potential of lifestyle changes to
restore normal circadian rhythms and consequently, PER expression
is another promising area of research. Such interventions may
reduce cancer incidence and improve patient outcomes. Additionally,
aligning the timing of therapeutic drugs with PER expression and
biological rhythms offers an effective approach for reducing side
effects and enhancing treatment efficacy. Continued research on the
interactions between PER genes and other rhythmically secreted
hormones is also necessary, as this may provide further insights
into the systemic nature of circadian regulation and its effect on
cancer. In conclusion, research on the circadian clock and clock
genes such as PER is not just an academic pursuit but has
real-world implications for cancer prevention, treatment and the
quality of life of patients. Future research in this field holds
the potential to revolutionize the current understanding of cancer
development and progression and open new avenues for more effective
cancer management.
Acknowledgements
Not applicable.
Funding
The present study was funded by the Science and Technology
Program of Binzhou Medical College (grant no. BY2017KJ01).
Availability of data and materials
Not applicable.
Authors' contributions
PG designed the study. WX, XF and PG obtained data
and drafted the manuscript. QG, YW and HZ helped design the studies
and prepare the manuscript. YC drew the figures and tables. The
manuscript was critically reviewed by YH, PG and YC. Data
authentication is not applicable. All authors have read and
approved the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wright KP Jr, McHill AW, Birks BR, Griffin
BR, Rusterholz T and Chinoy ED: Entrainment of the human circadian
clock to the natural light-dark cycle. Curr Biol. 23:1554–1558.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Malik S, Stokes J III, Manne U, Singh R
and Mishra MK: Understanding the significance of biological clock
and its impact on cancer incidence. Cancer Lett. 527:80–94. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pariollaud M and Lamia KA: Cancer in the
fourth dimension: What is the impact of circadian disruption?
Cancer Discov. 10:1455–1464. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sulli G, Lam MTY and Panda S: Interplay
between circadian clock and cancer: New frontiers for cancer
treatment. Trends Cancer. 5:475–494. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Takahashi JS: Transcriptional architecture
of the mammalian circadian clock. Nat Rev Genet. 18:164–179. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ruan W, Yuan X and Eltzschig HK: Circadian
rhythm as a therapeutic target. Nat Rev Drug Discov. 20:287–307.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vasey C, McBride J and Penta K: Circadian
rhythm dysregulation and restoration: The role of melatonin.
Nutrients. 13:34802021. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Behrens T, Rabstein S, Wichert K, Erbel R,
Eisele L, Arendt M, Dragano N, Brüning T and Jöckel KH: Shift work
and the incidence of prostate cancer: A 10-year follow-up of a
German population-based cohort study. Scand J Work Environ Health.
43:560–568. 2017.PubMed/NCBI
|
|
9
|
Schernhammer ES, Laden F, Speizer FE,
Willett WC, Hunter DJ, Kawachi I and Colditz GA: Rotating night
shifts and risk of breast cancer in women participating in the
nurses' health study. J Natl Cancer Inst. 93:1563–1568. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schernhammer ES, Laden F, Speizer FE,
Willett WC, Hunter DJ, Kawachi I, Fuchs CS and Colditz GA:
Night-shift work and risk of colorectal cancer in the nurses'
health study. J Natl Cancer Inst. 95:825–828. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Travis RC, Balkwill A, Fensom GK, Appleby
PN, Reeves GK, Wang XS, Roddam AW, Gathani T, Peto R, Green J, et
al: Night shift work and breast cancer incidence: Three prospective
studies and meta-analysis of published studies. J Natl Cancer Inst.
108:djw1692016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
IARC Monographs Vol 124 Group, :
Carcinogenicity of night shift work. Lancet Oncol. 20:1058–1059.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Rijo-Ferreira F and Takahashi JS: Genomics
of circadian rhythms in health and disease. Genome Med. 11:822019.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shafi AA and Knudsen KE: Cancer and the
circadian clock. Cancer Res. 79:3806–3814. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Walker WH II, Walton JC, DeVries AC and
Nelson RJ: Circadian rhythm disruption and mental health. Transl
Psychiatry. 10:282020. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mohawk JA, Green CB and Takahashi JS:
Central and peripheral circadian clocks in mammals. Ann Rev
Neurosci. 35:445–462. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gabriel BM and Zierath JR: Circadian
rhythms and exercise-re-setting the clock in metabolic disease. Nat
Rev Endocrinol. 15:197–206. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Haupt S, Eckstein ML, Wolf A, Zimmer RT,
Wachsmuth NB and Moser O: Eat, train, sleep-retreat? Hormonal
interactions of intermittent fasting, exercise and circadian
rhythm. Biomolecules. 11:5162021. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Panagiotou M, Michel S, Meijer JH and
Deboer T: The aging brain: Sleep, the circadian clock and exercise.
Biochem Pharmacol. 191:1145632021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Buhr ED, Yoo SH and Takahashi JS:
Temperature as a universal resetting cue for mammalian circadian
oscillators. Science. 330:379–385. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Schroeder AM and Colwell CS: How to fix a
broken clock. Trends Pharmacol Sci. 34:605–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Patke A, Young MW and Axelrod S: Molecular
mechanisms and physiological importance of circadian rhythms. Nat
Rev Mol Cell Biol. 21:67–84. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Scheiermann C, Gibbs J, Ince L and Loudon
A: Clocking in to immunity. Nat Rev Immunol. 18:423–437. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Nagoshi E, Saini C, Bauer C, Laroche T,
Naef F and Schibler U: Circadian gene expression in individual
fibroblasts: Cell-autonomous and self-sustained oscillators pass
time to daughter cells. Cell. 119:693–705. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barber LE, VoPham T, White LF, Roy HK,
Palmer JR and Bertrand KA: Circadian disruption and colorectal
cancer incidence in black women. Cancer Epidemiol Biomarkers Prev.
32:927–935. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Papantoniou K, Konrad P, Haghayegh S,
Strohmaier S, Eliassen AH and Schernhammer E: Rotating night shift
work, sleep, and thyroid cancer risk in the nurses' health study 2.
Cancers (Basel). 15:56732023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang G, Yang Y, Lv K, Wu Y, Song T and
Yuan Q: Night shift work and prostate cancer: A large cohort study
from UK Biobank and Mendelian randomisation study. BMJ Open.
14:e0844012024. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
National Toxicology Program, . NTP Cancer
Hazard Assessment Report on Night Shift Work and Light at Night.
Research Triangle Park (NC), National Toxicology Program, .
2021.
|
|
29
|
Papantoniou K, Devore EE, Massa J,
Strohmaier S, Vetter C, Yang L, Shi Y, Giovannucci E, Speizer F and
Schernhammer ES: Rotating night shift work and colorectal cancer
risk in the nurses' health studies. Int J Cancer. 143:2709–2717.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yousef E, Mitwally N, Noufal N and Tahir
MR: Shift work and risk of skin cancer: A systematic review and
meta-analysis. Sci Rep. 10:20122020. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Carter BD, Diver WR, Hildebrand JS, Patel
AV and Gapstur SM: Circadian disruption and fatal ovarian cancer.
Am J Prev Med. 46 (3 Suppl 1):S34–S41. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Garcia-Saenz A, Sánchez de Miguel A,
Espinosa A, Valentin A, Aragonés N, Llorca J, Amiano P, Martín
Sánchez V, Guevara M, Capelo R, et al: Evaluating the Association
between artificial light-at-night exposure and breast and prostate
cancer risk in spain (MCC-Spain study). Environ Health Perspect.
126:0470112018. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Garcia-Saenz A, de Miguel AS, Espinosa A,
Costas L, Aragonés N, Tonne C, Moreno V, Pérez-Gómez B, Valentin A,
Pollán M, et al: Association between outdoor Light-at-night
exposure and colorectal cancer in spain. Epidemiology. 31:718–727.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang D, Jones RR, James P, Kitahara CM
and Xiao Q: Associations between artificial light at night and risk
for thyroid cancer: A large US cohort study. Cancer. 127:1448–1458.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao Q, Jones RR, James P and
Stolzenberg-Solomon RZ: Light at night and risk of pancreatic
cancer in the NIH-AARP diet and health study. Cancer Res.
81:1616–1622. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sirhan-Atalla M, Gabinet NM and Portnov
BA: Disaggregating the effects of daytime and nighttime light
exposures on obesity, overweight, prostate and breast cancer
morbidity worldwide. Chronobiol Int. 40:483–514. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Jones RR: Exposure to artificial light at
night and risk of cancer: Where do we go from here? Br J Cancer.
124:1467–1468. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Turner MC, Gracia-Lavedan E, Papantoniou
K, Aragonés N, Castaño-Vinyals G, Dierssen-Sotos T, Amiano P,
Ardanaz E, Marcos-Delgado A, Molina-Barceló A, et al: Sleep and
breast and prostate cancer risk in the MCC-Spain study. Sci Rep.
12:218072022. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wong ATY, Heath AK, Tong TYN, Reeves GK,
Floud S, Beral V and Travis RC: Sleep duration and breast cancer
incidence: Results from the Million Women Study and meta-analysis
of published prospective studies. Sleep. 44:zsaa1662021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Chen Y, Tan F, Wei L, Li X, Lyu Z, Feng X,
Wen Y, Guo L, He J, Dai M and Li N: Sleep duration and the risk of
cancer: A systematic review and meta-analysis including
dose-response relationship. BMC Cancer. 18:11492018. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Li X, Hu Y and Aslanbeigi F: Genetic and
epigenetic alterations in night shift nurses with breast cancer: A
narrative review. Cancer Cell International. 25:202025. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Ning D, Fang Y and Zhang W: Association of
habitual sleep duration and its trajectory with the risk of cancer
according to sex and body mass index in a population-based cohort.
Cancer. 129:3582–3594. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Vatsyayan A, Mathur P, Bhoyar RC, Imran M,
Senthivel V, Divakar MK, Mishra A, Jolly B, Sivasubbu S and Scaria
V: Understanding the genetic epidemiology of hereditary breast
cancer in India using whole genome data from 1029 healthy
individuals. Cancer Causes Control. Mar 1–2025.(Epub ahead of
print). View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Yin X, Shen H, Wang H, Wang Q, Zhang S,
Zhang C, Jia Q, Guo S, Xu X, Zhang W, et al: Pathogenic germline
variants in Chinese pancreatic adenocarcinoma patients. Nat Commun.
16:22142025. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Srour B, Plancoulaine S, Andreeva VA,
Fassier P, Julia C, Galan P, Hercberg S, Deschasaux M,
Latino-Martel P and Touvier M: Circadian nutritional behaviours and
cancer risk: New insights from the NutriNet-santé prospective
cohort study: Disclaimers. Int J Cancer. 143:2369–2379. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Kogevinas M, Espinosa A, Castelló A,
Gómez-Acebo I, Guevara M, Martin V, Amiano P, Alguacil J, Peiro R,
Moreno V, et al: Effect of mistimed eating patterns on breast and
prostate cancer risk (MCC-Spain Study). Int J Cancer.
143:2380–2389. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Bishehsari F, Engen PA, Voigt RM, Swanson
G, Shaikh M, Wilber S, Naqib A, Green SJ, Shetuni B, Forsyth CB, et
al: Abnormal eating patterns cause circadian disruption and promote
alcohol-associated colon carcinogenesis. Cell Mol Gastroenterol
Hepatol. 9:219–237. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Marinac CR, Nelson SH, Breen CI, Hartman
SJ, Natarajan L, Pierce JP, Flatt SW, Sears DD and Patterson RE:
Prolonged nightly fasting and breast cancer prognosis. JAMA Oncol.
2:1049–1055. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Verbanac D, Maleš Ž and Barišić K:
Nutrition-facts and myths. Acta Pharm. 69:497–510. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Munt AE, Partridge SR and Allman-Farinelli
M: The barriers and enablers of healthy eating among young adults:
A missing piece of the obesity puzzle: A scoping review. Obes Revi.
18:1–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Wang Y, Guo H and He F: Circadian
disruption: From mouse models to molecular mechanisms and cancer
therapeutic targets. Cancer Metastasis Rev. 42:297–322. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Pariollaud M, Ibrahim LH, Irizarry E,
Mello RM, Chan AB, Altman BJ, Shaw RJ, Bollong MJ, Wiseman RL and
Lamia KA: Circadian disruption enhances HSF1 signaling and
tumorigenesis in Kras-driven lung cancer. Sci Adv. 8:eabo11232022.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hadadi E, Taylor W, Li XM, Aslan Y,
Villote M, Rivière J, Duvallet G, Auriau C, Dulong S,
Raymond-Letron I, et al: Chronic circadian disruption modulates
breast cancer stemness and immune microenvironment to drive
metastasis in mice. Nat Commun. 11:31932020. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Koritala BSC, Porter KI, Sarkar S and
Gaddameedhi S: Circadian disruption and cisplatin chronotherapy for
mammary carcinoma. Toxicol Appl Pharmacol. 436:1158632022.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Milićević N, Bergen AA and
Felder-Schmittbuhl MP: Per1 mutation enhances masking responses in
mice. Chronobiol Int. 39:1533–1538. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Oosthuizen T, Pillay N and Oosthuizen MK:
A mouse in the spotlight: Response capacity to artificial light at
night in a rodent pest species, the southern multimammate mouse
(Mastomys coucha). J Environ Manage. 372:1233732024. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Viljoen A and Oosthuizen MK: Dim light at
night affects the locomotor activity of nocturnal African pygmy
mice (Mus minutoides) in an intensity-dependent manner. Proc
Biol Sci. 290:202305262023.PubMed/NCBI
|
|
58
|
Datta S, Samanta D, Tiwary B, Chaudhuri AG
and Chakrabarti N: Sex and estrous cycle dependent changes in
locomotor activity, anxiety and memory performance in aged mice
after exposure of light at night. Behav Brain Res. 365:198–209.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Schoonderwoerd RA, de Torres Gutiérrez P,
Blommers R, van Beurden AW, Coenen TCJJ, Klett NJ, Michel SH and
Meijer JH: Inhibitory responses to retinohypothalamic tract
stimulation in the circadian clock of the diurnal rodent Rhabdomys
pumilio. FASEB J. 36:e224152022. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Tir S, Foster RG and Peirson SN:
Evaluation of the digital ventilated Cage® system for
circadian phenotyping. Sci Rep. 15:36742025. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Walker WH II, Kvadas RM, May LE, Liu JA,
Bumgarner JR, Walton JC, DeVries AC, Dauchy RT, Blask DE and Nelson
RJ: Artificial light at night reduces anxiety-like behavior in
female mice with exacerbated mammary tumor growth. Cancers (Basel).
13:48602021. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Agbaria S, Haim A, Fares F and Zubidat AE:
Epigenetic modification in 4T1 mouse breast cancer model by
artificial light at night and melatonin-the role of
DNA-methyltransferase. Chronobiol Int. 36:629–643. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Schwartz PB, Nukaya M, Berres ME,
Rubinstein CD, Wu G, Hogenesch JB, Bradfield CA and
Ronnekleiv-Kelly SM: The circadian clock is disrupted in pancreatic
cancer. PLoS Genet. 19:e10107702023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Walker WH II, Kaper AL, Meléndez-Fernández
OH, Bumgarner JR, Liu JA, Walton JC, DeVries AC and Nelson RJ:
Time-restricted feeding alters the efficiency of mammary tumor
growth. Chronobiol Int. 39:535–546. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lawther AJ, Phillips AJK, Chung NC, Chang
A, Ziegler AI, Debs S, Sloan EK and Walker AK: Disrupting circadian
rhythms promotes cancer-induced inflammation in mice. Brain Behav
Immun Health. 21:1004282022. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Koritala BSC, Porter KI, Arshad OA, Gajula
RP, Mitchell HD, Arman T, Manjanatha MG, Teeguarden J, Van Dongen
HPA, McDermott JE and Gaddameedhi S: Night shift schedule causes
circadian dysregulation of DNA repair genes and elevated DNA damage
in humans. J Pineal Res. 70:e127262021. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Mishra A, Lin H, Singla R, Le N, Oraebosi
M, Liu D and Cao R: Circadian desynchrony in early life leads to
enduring autistic-like behavioral changes in adulthood. Commun
Biol. 7:14852024. View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Aiello I, Mul Fedele ML, Román F, Marpegan
L, Caldart C, Chiesa JJ, Golombek DA, Finkielstein CV and Paladino
N: Circadian disruption promotes tumor-immune microenvironment
remodeling favoring tumor cell proliferation. Sci Adv.
6:eaaz45302020. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Zeng X, Liang C and Yao J: Chronic
shift-lag promotes NK cell ageing and impairs immunosurveillance in
mice by decreasing the expression of CD122. J Cell Mol Med.
24:14583–14595. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Tian R, Li Y and Shu M: Circadian
regulation patterns with distinct immune landscapes in gliomas aid
in the development of a risk model to predict prognosis and
therapeutic response. Front Immunol. 12:7974502021. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Jones AA and Arble DM: Loss of endogenous
circadian clock function in mice alters respiratory cycle timing in
a time of day- and sex-specific manner. Respir Physiol Neurobiol.
331:1043372025. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Ma C, Li H, Li W, Yang G and Chen L:
Adaptive differences in cellular and behavioral responses to
circadian disruption between C57BL/6 and BALB/c strains. Int J Mol
Sci. 25:104042024. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Peng Y, Tsuno Y, Matsui A, Hiraoka Y,
Tanaka K, Horike SI, Daikoku T and Mieda M: Cell Type-specific
genetic manipulation and impaired circadian rhythms in vip tTA
Knock-In mice. Front Physiol. 13:8956332022. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Du NH, Kompotis K, Sato M, Pedron E,
Androvic S and Brown S: Behavioural phenotypes of Dicer knockout in
the mouse SCN. Eur J Neurosci. 60:6634–6651. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Teeple K, Rajput P, Gonzalez M,
Han-Hallett Y, Fernández-Juricic E and Casey T: High fat diet
induces obesity, alters eating pattern and disrupts corticosterone
circadian rhythms in female ICR mice. PLoS One. 18:e02792092023.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Aroca-Siendones MI, Moreno-SanJuan S,
Puentes-Pardo JD, Verbeni M, Arnedo J, Escudero-Feliu J,
García-Costela M, García-Robles A, Carazo Á and León J: Core
circadian clock proteins as biomarkers of progression in colorectal
cancer. Biomedicines. 9:9672021. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Qiu MJ, Liu LP, Jin S, Fang XF, He XX,
Xiong ZF and Yang SL: Research on circadian clock genes in common
abdominal malignant tumors. Chronobiol Int. 36:906–918. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Deng F, Yang K and Zheng G: Period family
of clock genes as novel predictors of survival in human cancer: A
systematic review and Meta-analysis. Dis Markers. 2020:64862382020.
View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Zhao H, Zeng ZL, Yang J, Jin Y, Qiu MZ, Hu
XY, Han J, Liu KY, Liao JW, Xu RH and Zou QF: Prognostic relevance
of Period1 (Per1) and Period2 (Per2) expression in human gastric
cancer. Int J Clin Exp Pathol. 7:619–630. 2014.PubMed/NCBI
|
|
80
|
Shafi AA, McNair CM, McCann JJ, Alshalalfa
M, Shostak A, Severson TM, Zhu Y, Bergman A, Gordon N, Mandigo AC,
et al: The circadian cryptochrome, CRY1, is a pro-tumorigenic
factor that rhythmically modulates DNA repair. Nat Commun.
12:4012021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Sulli G, Rommel A, Wang X, Kolar MJ, Puca
F, Saghatelian A, Plikus MV, Verma IM and Panda S: Pharmacological
activation of REV-ERBs is lethal in cancer and oncogene-induced
senescence. Nature. 553:351–355. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Liang Y, Wang S, Huang X, Chai R, Tang Q,
Yang R, Huang X, Wang X and Zheng K: Dysregulation of circadian
clock genes as significant clinic factor in the tumorigenesis of
hepatocellular carcinoma. Comput Math Methods Med.
2021:82388332021. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Heng J and Heng HH: Genome chaos: Creating
new genomic information essential for cancer macroevolution. Semin
Cancer Biol. 81:160–175. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Park J, Lee K, Kim H, Shin H and Lee C:
Endogenous circadian reporters reveal functional differences of
PERIOD paralogs and the significance of PERIOD:CK1 stable
interaction. Proc Natl Acad Sci USA. 120:e22122551202023.
View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Yin S, Zhang Z, Tang H and Yang K: The
biological clock gene PER1 affects the development of oral squamous
cell carcinoma by altering the circadian rhythms of cell
proliferation and apoptosis. Chronobiol Int. 39:1206–1219. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Lellupitiyage Don SS, Lin HH, Furtado JJ,
Qraitem M, Taylor SR and Farkas ME: Circadian oscillations persist
in low malignancy breast cancer cells. Cell Cycle. 18:2447–2453.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Lin HH, Qraitem M, Lian Y, Taylor SR and
Farkas ME: Analyses of BMAL1 and PER2 oscillations in a model of
breast cancer progression reveal changes with malignancy. Integr
Cancer Ther. 18:15347354198364942019. View Article : Google Scholar : PubMed/NCBI
|
|
88
|
Ye H, Yang K, Tan X, Zhao D, Lü X and Wang
Q: Circadian variation of clock gene Per2 and cancer-related
clock-controlled genes in buccal mucosa carcinoma of golden hamster
at different cancer stages. Hua Xi Kou Qiang Yi Xue Za Zhi.
33:513–518. 2015.(In Chinese). PubMed/NCBI
|
|
89
|
Liu B, Xu K, Jiang Y and Li X: Aberrant
expression of Per1, Per2 and Per3 and their prognostic relevance in
non-small cell lung cancer. Int J Clin Exp Pathol. 7:7863–7871.
2014.PubMed/NCBI
|
|
90
|
Liu Y, Wu Z, Li Y, Zhang J, Gao Y, Yuan G
and Han M: PER3 plays anticancer roles in the oncogenesis and
progression of breast cancer via regulating MEK/ERK signaling
pathway. J Chin Med Assoc. 85:1051–1060. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Zhang F, Sun H, Zhang S, Yang X, Zhang G
and Su T: Overexpression of PER3 inhibits Self-renewal capability
and chemoresistance of colorectal cancer Stem-Like cells via
inhibition of notch and β-Catenin signaling. Oncol Res. 25:709–719.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Hasakova K, Reis R, Vician M, Zeman M and
Herichova I: Expression of miR-34a-5p is up-regulated in human
colorectal cancer and correlates with survival and clock gene PER2
expression. PLoS One. 14:e02243962019. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Yang MY, Lin PM, Hsiao HH, Hsu JF, Lin HY,
Hsu CM, Chen IY, Su SW, Liu YC and Lin SF: Up-regulation of PER3
expression is correlated with better clinical outcome in acute
leukemia. Anticancer Res. 35:6615–6622. 2015.PubMed/NCBI
|
|
94
|
Rajendran S, Benna C, Marchet A, Nitti D
and Mocellin S: Germline polymorphisms of circadian genes and
gastric cancer predisposition. Cancer Commun (Lond). 40:234–238.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Benna C, Rajendran S, Spiro G, Tropea S,
Del Fiore P, Rossi CR and Mocellin S: Associations of clock genes
polymorphisms with soft tissue sarcoma susceptibility and
prognosis. J Transl Med. 16:3382018. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Hinoura T, Mukai S, Kamoto T and Kuroda Y:
PER3 polymorphisms and their association with prostate cancer risk
in Japanese men. J Prev Med Hyg. 62:E489–E495. 2021.PubMed/NCBI
|
|
97
|
Couto P, Miranda D, Vieira R, Vilhena A,
De Marco L and Bastos-Rodrigues L: Association between CLOCK, PER3
and CCRN4L with non-small cell lung cancer in Brazilian patients.
Mol Med Rep. 10:435–440. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Dagmura H, Yiğit S, Nursal AF, Duman E and
Gumusay O: Possible association of PER2/PER3 variable number tandem
repeat polymorphism variants with susceptibility and clinical
characteristics in pancreatic cancer. Genet Test Mol Biomarkers.
25:124–130. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Zhao Q, Zheng G, Yang K, Ao YR, Su XL, Li
Y and Lv XQ: The clock gene PER1 plays an important role in
regulating the clock gene network in human oral squamous cell
carcinoma cells. Oncotarget. 7:70290–70302. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Xiaojuan F, Kai Y, Hanxue L, Qin Z and Dan
C: Effects and mechanism of the circadian clock gene Per1 on the
proliferation, apoptosis, cycle, and tumorigenicity in vivo of
human oral squamous cell carcinoma. Hua Xi Kou Qiang Yi Xue Za Zhi.
34:255–261, (In Chinese). PubMed/NCBI
|
|
101
|
Yang G, Yang Y, Tang H and Yang K: Loss of
the clock gene Per1 promotes oral squamous cell carcinoma
progression via the AKT/mTOR pathway. Cancer Sci. 111:1542–1554.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Bellet MM, Stincardini C, Costantini C,
Gargaro M, Pieroni S, Castelli M, Piobbico D, Sassone-Corsi P,
Della-Fazia MA, Romani L and Servillo G: The circadian protein PER1
modulates the cellular response to anticancer treatments. Int J Mol
Sci. 22:29742021. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Kastenhuber ER and Lowe SW: Putting p53 in
Context. Cell. 170:1062–1078. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Han Y, Meng F, Venter J, Wu N, Wan Y,
Standeford H, Francis H, Meininger C, Greene J Jr, Trzeciakowski
JP, et al: miR-34a-dependent overexpression of Per1 decreases
cholangiocarcinoma growth. J Hepatol. 64:1295–1304. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Guo X, Li K, Jiang W, Hu Y, Xiao W, Huang
Y, Feng Y, Pan Q and Wan R: RNA demethylase ALKBH5 prevents
pancreatic cancer progression by posttranscriptional activation of
PER1 in an m6A-YTHDF2-dependent manner. Mol Cancer. 19:912020.
View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Yao J, Hui JW, Chen YJ, Luo DY, Yan JS,
Zhang YF, Lan YX, Yan XR, Wang ZH, Fan H and Xia HC: Lycium
barbarum glycopeptide targets PER2 to inhibit lipogenesis in
glioblastoma by downregulating SREBP1c. Cancer Gene Ther.
30:1084–1093. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
107
|
Zhanfeng N, Chengquan W, Hechun X, Jun W,
Lijian Z, Dede M, Wenbin L and Lei Y: Period2 downregulation
inhibits glioma cell apoptosis by activating the MDM2-TP53 pathway.
Oncotarget. 7:27350–27362. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Ma D, Hou L, Xia H, Li H, Fan H, Jia X and
Niu Z: PER2 inhibits proliferation and stemness of glioma stem
cells via the Wnt/β-catenin signaling pathway. Oncol Rep.
44:533–542. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Su X, Chen D, Yang K, Zhao Q, Zhao D, Lv X
and Ao Y: The circadian clock gene PER2 plays an important role in
tumor suppression through regulating tumor-associated genes in
human oral squamous cell carcinoma. Oncol Rep. 38:472–480. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Xiong H, Yang Y, Yang K, Zhao D, Tang H
and Ran X: Loss of the clock gene PER2 is associated with cancer
development and altered expression of important Tumor-related genes
in oral cancer. Int J Oncol. 52:279–287. 2018.PubMed/NCBI
|
|
111
|
Guo F, Tang Q, Chen G, Sun J, Zhu J, Jia Y
and Zhang W: Aberrant expression and subcellular localization of
PER2 promote the progression of oral squamous cell carcinoma.
Biomed Res Int. 2020:85874582020. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Wang Q, Ao Y, Yang K, Tang H and Chen D:
Circadian clock gene Per2 plays an important role in cell
proliferation, apoptosis and cell cycle progression in human oral
squamous cell carcinoma. Oncol Rep. 35:3387–3394. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
McQueen CM, Schmitt EE, Sarkar TR, Elswood
J, Metz RP, Earnest D, Rijnkels M and Porter WW: PER2 regulation of
mammary gland development. Development. 145:dev1579662018.
View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Hwang-Verslues WW, Chang PH, Jeng YM, Kuo
WH, Chiang PH, Chang YC, Hsieh TH, Su FY, Lin LC, Abbondante S, et
al: Loss of corepressor PER2 under hypoxia up-regulates
OCT1-mediated EMT gene expression and enhances tumor malignancy.
Proc Natl Acad Sci USA. 110:12331–12336. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Yang C, Wu J, Liu X, Wang Y, Liu B, Chen
X, Wu X, Yan D, Han L, Liu S, et al: Circadian rhythm is disrupted
by ZNF704 in breast carcinogenesis. Cancer Res. 80:4114–4128. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Wang Z, Wang H, Guo H, Li F, Wu W, Zhang S
and Wang T: The circadian rhythm and core gene Period2 regulate the
chemotherapy effect and multidrug resistance of ovarian cancer
through the PI3K signaling pathway. Biosci Rep. 40:BSR202026832020.
View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Wang Z, Li L and Wang Y: Effects of Per2
overexpression on growth inhibition and metastasis, and on MTA1,
nm23-H1 and the autophagy-associated PI3K/PKB signaling pathway in
nude mice xenograft models of ovarian cancer. Mol Med Rep.
13:4561–4568. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Wang Z, Li F, He S, Zhao L and Wang F:
Period circadian regulator 2 suppresses drug resistance to
cisplatin by PI3K/AKT pathway and improves chronochemotherapeutic
efficacy in cervical cancer. Gene. 809:1460032022. View Article : Google Scholar : PubMed/NCBI
|
|
119
|
Lee J, Sul HJ, Choi H, Oh DH and Shong M:
Loss of thyroid gland circadian PER2 rhythmicity in aged mice and
its potential association with thyroid cancer development. Cell
Death Dis. 13:8982022. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Alam H, Tang M, Maitituoheti M, Dhar SS,
Kumar M, Han CY, Ambati CR, Amin SB, Gu B, Chen TY, et al: KMT2D
deficiency impairs Super-enhancers to confer a glycolytic
vulnerability in lung cancer. Cancer Cell. 37:599–617.e7. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Li Z, You Q and Zhang X: Small-molecule
modulators of the Hypoxia-inducible factor pathway: Development and
therapeutic applications. J Med Chem. 62:5725–5749. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Okabe T, Kumagai M, Nakajima Y, Shirotake
S, Kodaira K, Oyama M, Ueno M and Ikeda M: The impact of HIF1α on
the Per2 circadian rhythm in renal cancer cell lines. PLoS One.
9:e1096932014. View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Yuan H, Xu R, Li S, Zheng M, Tong Q, Xiang
M and Zhang Y: The malignant transformation of viral hepatitis to
hepatocellular carcinoma: Mechanisms and interventions. MedComm.
6:e701212025. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Mteyrek A, Filipski E, Guettier C, Okyar A
and Lévi F: Clock gene Per2 as a controller of liver
carcinogenesis. Oncotarget. 7:85832–85847. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
125
|
Minciuna I, van Kleef LA, Stefanescu H and
Procopet B: Is fasting good when one is at risk of liver cancer?
Cancers (Basel). 14:50842022. View Article : Google Scholar : PubMed/NCBI
|
|
126
|
Hong Z, Feng Z, Sai Z and Tao S: PER3, a
novel target of miR-103, plays a suppressive role in colorectal
cancer in vitro. BMB Rep. 47:500–555. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
127
|
Zhang F, Su T and Xiao M: RUNX3-regulated
circRNA METTL3 inhibits colorectal cancer proliferation and
metastasis via miR-107/PER3 axis. Cell Death Dis. 13:5502022.
View Article : Google Scholar : PubMed/NCBI
|
|
128
|
Kawamura G, Hattori M, Takamatsu K,
Tsukada T, Ninomiya Y, Benjamin I, Sassone-Corsi P, Ozawa T and
Tamaru T: Cooperative interaction among BMAL1, HSF1, and p53
protects mammalian cells from UV stress. Commun Biol. 1:2042018.
View Article : Google Scholar : PubMed/NCBI
|
|
129
|
Wang J, Huang Q, Hu X, Zhang S, Jiang Y,
Yao G, Hu K, Xu X, Liang B, Wu Q, et al: Disrupting Circadian
rhythm via the PER1-HK2 axis reverses trastuzumab resistance in
gastric cancer. Cancer Res. 82:1503–1517. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
130
|
Wang Z, Wang Z, Li F, Wei M, Zhang S and
Wang T: Circadian clock protein PERIOD2 suppresses the PI3K/Akt
pathway and promotes cisplatin sensitivity in ovarian cancer.
Cancer Manag Res. 12:11897–11908. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
131
|
Katamune C, Koyanagi S, Hashikawa KI,
Kusunose N, Akamine T, Matsunaga N and Ohdo S: Mutation of the gene
encoding the circadian clock component PERIOD2 in oncogenic cells
confers chemoresistance by up-regulating the Aldh3a1 gene. J Biol
Chem. 294:547–558. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
132
|
Zhao X, Fan J, Wu P, Wei C, Chen Q, Ming
Z, Yan J and Yang L: Chronic chemotherapy with paclitaxel
nanoparticles induced apoptosis in lung cancer in vitro and in
vivo. Int J Nanomedicine. 14:1299–1309. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
133
|
Webb AJ, Harper E, Rattay T,
Aguado-Barrera ME, Azria D, Bourgier C, Brengues M, Briers E,
Bultijnck R, Chang-Claude J, et al: Treatment time and circadian
genotype interact to influence radiotherapy side-effects. A
prospective European validation study using the REQUITE cohort.
EBioMedicine. 84:1042692022. View Article : Google Scholar : PubMed/NCBI
|
















