Introduction
In 1982, Nusse and Varmus identified a novel gene
named, integration site 1 (Int-1), whilst investigating oncogenes
utilizing the mouse mammary tumor virus. Int-1 was later confirmed
to be a homolog of the Drosophila Wingless gene. To minimize
the nomenclature conclusion, a consensus was reached in 1991 to
designate genes of the Int-1/Wingless family uniformly as ‘Wnt’
(1).
The Wnt signaling pathway can be subdivided into two
major classes: Canonical and non-canonical signaling pathways. In
the canonical Wnt pathway, β-catenin serves a central regulatory
role by binding T cell-specific transcription factor (TCF)/lymphoid
enhancer-binding factor 1 to regulate the expression of downstream
genes, which controls cell fate, proliferation and differentiation
(2). The role of the canonical Wnt
signaling pathway in tumorigenesis has been extensively reported.
Mutations in β-catenin or loss of function of upstream regulatory
factors, such as adenomatous polyposis coli, Axin and glycogen
synthase kinase 3β, all lead to increased nuclear accumulation of
β-catenin, activating downstream oncogenes, such as c-myc, cyclin
D1 and vascular endothelial growth factor, and promoting tumor cell
proliferation, survival and invasion (3). By contrast, non-canonical Wnt pathways
are independent of β-catenin nuclear translocation and primarily
include the Wnt/Ca2+ pathway and the Wnt/planar cell
polarity (PCP) pathway. Growing research has highlighted the
significance of the Wnt/Ca2+ pathway in tumor biology,
revealing that its effects can differ substantially among several
tumor types. The present review aims to summarize this research
regarding Wnt/Ca2+ signaling, to systematically discuss
its multiple roles and diverse mechanisms in several tumors, and to
provide new insights into the diagnosis and therapeutic strategies
of these malignancies.
Overview of the Wnt/Ca2+
signaling pathway
Core components of the
Wnt/Ca2+ pathway
A vast body of research has revealed the core
components of the Wnt/Ca2+ pathway, including
extracellular ligands, transmembrane receptors and intracellular
signaling molecules (4,5). Specifically, extracellular signaling
is primarily mediated by the following: Wnt proteins; the
transmembrane receptors, R-spondin-2-leucine rich repeat containing
G protein-coupled receptor 5 complex and frizzled (FZD)-2 receptor;
and the intracellular signaling molecules, heterotrimeric guanine
nucleotide-binding protein (G protein), disheveled (Dvl),
phospholipase C (PLC), phosphatidylinositol 4,5-bisphosphate
(PIP2), protein kinase C (PKC), calmodulin (CaM)-dependent protein
kinase II (CaMKII) and calcineurin (CaN). Although these molecules
are independent of the nuclear translocation of β-catenin, they
serve an integral and pivotal role in the Wnt/Ca2+
pathway (6).
Extracellular ligands of the Wnt
signaling pathway
Extracellular ligands of the Wnt signaling pathway
consist of a highly conserved family of secreted glycoproteins
known as Wnt proteins. Currently, 19 Wnt family members have been
identified in mammals. Wnt ligands of the Wnt1 class, including
Wnt2, Wnt3, Wnt3a and Wnt8a, predominantly activate the canonical
Wnt/β-catenin pathway, whereas ligands of the Wnt5a class,
comprising Wnt4, Wnt5a, Wnt5b, Wnt6, Wnt7a and Wnt11, primarily
trigger the non-canonical Wnt pathways. Specifically, the
Wnt/Ca2+ signaling pathway is mainly activated by Wnt5a
and Wnt11 (7).
After being synthesized in the endoplasmic reticulum
(ER), Wnt proteins undergo glycosylation and palmitoylation
modifications prior to their transport to the extracellular space
where they can interact with receptors, subsequently activating
downstream signaling pathways (2).
Palmitoylation, which is crucial for Wnt protein secretion, is
catalyzed by porcupine O-acyltransferase in the ER, attaching the
palmitoleic acid acyl group to the Wnt protein (8). Subsequently, the palmitoylated Wnt
binds to the transmembrane protein Wntless/Evi in the Golgi
apparatus, which encapsulates Wnt within vesicles and facilitates
its secretion into the extracellular space for interaction with
receptors on target cells (9).
Although Wnt signaling primarily occurs between adjacent cells, the
precise mechanism by which extracellular Wnt is delivered to target
cells remains unclear. Previous studies have demonstrated that the
protein core of glypican Dlp can form a groove-like structure that
binds to and shields the palmitoylated moiety of Wnt, increasing
its water solubility and facilitating its extracellular diffusion
and receptor binding, thereby enabling efficient Wnt signal
transmission (10). However, the
intricate mechanisms underlying this process are still not fully
understood and require further exploration.
Transmembrane receptors for Wnt
FZD proteins constitute seven-pass transmembrane
receptors resembling classic G-protein-coupled receptors, which
function as membrane receptors for Wnt secreted glycoproteins
(11). The fundamental structure of
FZD proteins includes an extracellular N-terminal region, seven
transmembrane helixes and an intracellular C-terminal tail
(12). As a unique feature of this
protein subfamily, the N-terminal region contains a conserved
cysteine-rich structural domain (CRD), which is tethered to the
first transmembrane helix via a flexible linker. The CRD possesses
a binding site for the Wnt protein, recognizing both the
palmitoleate moiety at the N-terminus and the hydrophobic amino
acid motifs at the C-terminus of Wnt. The C-terminal intracellular
domain, situated within the plasma membrane (PM), exhibits
variability in length and lacks high conservation among diverse FZD
receptors. It is characterized by a fully conserved KTXXXW motif,
which engages with the post-synaptic density protein/discs
large/zona occludens-1 domain of Dvl proteins, thereby facilitating
intracellular signal transduction (13). There are 19 Wnt genes and 12 FZD
receptors in vertebrates. These FZD receptors exhibit differential
affinity for Wnt ligands and, in combination with different
co-receptors, can selectively activate their respective unique Wnt
signaling pathways (14).
Intracellular components and
downstream molecules of Wnt
Upon binding of the Wnt protein to the FZD receptor,
the intracellular Dvl protein and G protein become activated,
subsequently inducing the activation of PLC. However, the
mechanisms of Dvl and G proteins in Wnt signaling are complex. In
the canonical Wnt/β-catenin signaling pathway, Dvl functions as a
linchpin. It stabilizes β-catenin to prevent degradation and
facilitates its nuclear translocation, enabling the regulation of
downstream target genes transcription (15). By contrast, G proteins do not appear
to participate in the canonical Wnt/β-catenin signaling (16). In non-canonical Wnt pathways, such
as the Wnt/PCP pathway, Dvl employs a distinct mechanism. It
interacts with the disheveled-associated activator of morphogenesis
1 and T-cell lymphoma invasion and metastasis 1 proteins to
activate Ras homolog family member A (RhoA) and Rac family small
GTPase 1 (Rac1), respectively. Both RhoA and Rac1, belonging to the
Rho family of small GTPases, are crucial for regulating actin
polymerization and cytoskeletal reorganization (17). Moreover, another mechanism of G
protein activation in non-canonical Wnt signaling involves Daple
(CCDC88C), a key protein that functions both as a Dvl-binding
protein and as a guanine nucleotide exchange factor (GEF) for G
proteins. Upon ligand stimulation, Daple dissociates from Dvl and
binds to FZDs, forming a Frizzled-Daple-Gαi ternary complex. This
complex activates the Gαi subunit, which inhibits cyclic AMP levels
and releases the Gβγ heterodimer, enhancing Rac1 and PI3K-Akt
signaling (18,19). However, this mechanism has yet to be
validated in the Wnt/Ca2+ signaling pathway. Gong et
al (20) reported that
Sec14-like lipid-binding protein 3 (Sec14L3), a
phosphatidylinositol transfer protein, serves as a critical
mediator that bridges FZD receptors and Dvl within the
Wnt/Ca2+ pathway. As a key component acting as a GTPase
in this signaling cascade, Sec14L3 normally exists in an inactive
GDP-bound state (Sec14L3-GDP), which allows it to form complexes
with FZD receptors and Dvl. Upon stimulation by non-canonical Wnt
signaling, Sec14L3 undergoes a conformational change, transitioning
to its active GTP-bound form (Sec14L3-GTP). Subsequently, the
activated Sec14L3-GTP binds to PLC on the PM, facilitating the
activation of PLC and subsequent Wnt/Ca2+ signaling. In
this context, it is hypothesized that Dvl acts as a scaffolding
protein, recruiting an unknown Sec14L3-GEF to promote the formation
of Sec14L3-GTP (20). Despite this,
the exact molecular mechanisms involving Dvl and G proteins in
activating the Wnt/Ca2+ pathway still require further
investigation (20,21). Once PLC is activated, it rapidly
elicits a transient elevation of intracellular concentrations of
the second messengers diacylglycerol (DAG) and inositol
1,4,5-trisphosphate (IP3), which subsequently activate downstream
effector molecules, such as PKC, CaMKII and CaN (22).
PLC
PLC is a pivotal phospholipase family localized on
the cytoplasmic membrane. It encompasses 13 distinct members, each
possessing conserved EF-hand domains, PH domains and C2 domains,
which together form the structural basis for the function of PLC
(23). Upon activation, PLC
mediates the hydrolysis of PIP2 into IP3 and DAG (24). DAG, as a hydrophobic molecule,
associates with the conserved region 1 (C1) domain of PKC, thereby
facilitating its recruitment and activation at the PM (25). IP3 binds to inositol
1,4,5-trisphosphate receptors (InsP3Rs) on the ER membrane,
inducing a conformational change in the InsP3R complex that opens
Ca2+ channels and releases Ca2+ into the
cytoplasm (26). Ca2+
depletion in the ER triggers the dissociation of Ca2+
from the EF-hand motif of the ER calcium sensor stromal interaction
molecule 1 (STIM1). This process leads to a conformational change
in STIM1, switching it from a tight, inactive form to an extended,
active form. Activated STIM1 then interacts with and activates
Orai1 at the ER-PM junction, converting it from a dimer to a
tetramer, to form a cytoplasmic calcium release-activated calcium
(CRAC) channel (27). The mechanism
by which CRAC channels facilitate the influx of extracellular
Ca2+ is termed store-operated calcium entry (28).
PKC
PKC, a family of phospholipid-dependent
serine/threonine kinases, is subdivided into three subfamilies
based on their structural and activation characteristics:
Conventional or classic PKC isozymes (cPKCs; α, βI, βII and γ),
novel or non-classic PKC isozymes (nPKCs; δ, ε, η and θ), and
atypical PKC isozymes (aPKCs; ζ, ι and λ) (29). The members of the PKC family share a
conserved structure, consisting of an N-terminal regulatory region
and a C-terminal catalytic region, which are connected by the C1-C4
segment and the V0-V5 variable region. Among the three subfamilies,
only cPKC has a unique Ca2+-binding C2 structural
domain, whereas nPKC and aPKC are Ca2+-insensitive
(30). Activation of cPKC
necessitates both Ca2+ and DAG. Initially, the binding
of Ca2+ to the C2 domain induces a conformational change
in cPKC, thereby exposing the PIP2 binding site, which is typically
concealed in its autoinhibited state. This newly exposed site then
interacts with PIP2 on the PM, facilitating the recruitment of cPKC
to the PM. Subsequently, DAG, which is embedded in the membrane,
binds to the C1 domain of cPKC, further inducing a conformational
change that displaces the pseudosubstrate and ultimately leads to
the activation of cPKC (31). The
PKC signaling pathway serves a pivotal role in regulating gene
expression, cell proliferation, differentiation, migration,
survival and apoptosis. Additionally, there is evidence suggesting
its crucial involvement in carcinogenesis (32).
CaMKII
CaMKII is a family of serine/threonine kinases
activated by Ca2+ and CaM. Under commonly physiological
conditions, each CaMKII holoenzyme typically consists of 12
subunits, with each subunit containing an N-terminal catalytic
domain, a central regulatory domain and a C-terminal association
domain (33). In its resting state,
the catalytic domain of CaMKII is inhibited by an autoinhibitory
sequence within the regulatory domain. Upon an increase in
intracellular Ca2+ levels, Ca2+ binds to CaM,
forming a Ca2+/CaM complex. Subsequently, this complex
binds to the CaMKII regulatory domain, inducing a conformational
change that disrupts the interaction between the autoinhibitory
region and the catalytic domain, thereby relieving the
autoinhibition of the kinase. However, this activation is
reversible: When Ca2+ levels fall, CaM dissociates from
CaMKII and kinase activity is re-inhibited. Additionally, the
interaction between the Ca2+/CaM complex and the
regulatory domain exposes the phosphorylation site of Thr287
(Thr286 in CaMKIIα), facilitating autophosphorylation. If
intracellular Ca2+ concentrations remain elevated,
phosphorylation at Thr287 enhances the binding affinity of CaM for
CaMKII, preventing the re-association of the catalytic domain with
the autoinhibitory region and sustaining CaMKII activation
(34,35). CaMKII serves a pivotal role not only
in learning and memory, but also in regulating cancer progression
(36). Within the non-canonical
Wnt/Ca2+/CaMKII pathway, CaMKII activates the
transforming growth factor-β-activated kinase 1 (TAK1)-Nemo-like
kinase (NLK)-MAPK cascade, resulting in the phosphorylation of TCF,
which prevents the binding of the β-catenin-TCF complex to DNA.
This, in turn, antagonizes the canonical Wnt/β-catenin signaling
pathway and inhibits cancer progression (37).
CaN
CaN, a unique calcium and CaM-dependent
serine/threonine phosphatase, serves a crucial role in cellular
signal transduction by converting calcium signals into specific
cellular responses (38). CaN is a
heterodimeric enzyme composed of CaNA and CaNB. CaNA contains a
catalytic domain and three regulatory domains: A CaNB-binding
domain, a CaM-binding domain and an autoinhibitory domain. CaNB
contains four EF-hand motifs for Ca2+ binding (39). In its resting state, the catalytic
center of CaN is inhibited by the autoinhibitory domain, rendering
the enzyme inactive. Upon signal stimulation, increased cellular
cytosolic Ca2+ binds to the EF-hand motifs of CaNB,
causing the dissociation of the CaM-binding domain from the B
subunit regulatory domain. Subsequently, CaM binds to the
CaM-binding domain of CaNA, causing displacement of the
autoinhibitory domain from the catalytic center and effectively
activating CaN. Notably, even in the absence of CaM, the binding of
Ca2+ alone to the EF-hand motifs of CaNB can partially
activate CaN (38). The activation
of CaN results in the dephosphorylation of several substrates,
among which the most extensively studied is nuclear factor of
activated T cells (NFAT). Following dephosphorylation, NFAT
translocates to the nucleus and activates transcription programs,
exerting notable influences on T cell activation and cancer
progression. These effects include promoting cancer cell
proliferation, metastasis, angiogenesis, inflammatory responses and
cancer stem cell (CSC) activity (40,41).
Activation of the Wnt/Ca2+
pathway
The Wnt/Ca2+ pathway, as a crucial branch
of the Wnt signaling pathway, belongs to the category of
non-canonical Wnt signaling pathways. Its activation primarily
depends on the ligands Wnt5a and Wnt11 (7). When these ligands bind to the FZD
receptor on the cell membrane, a series of intracellular molecular
cascade reactions are triggered (19). Initially, the G protein and the FZD
adapter protein Dvl are activated, leading to the activation of
PLC, which further cleaves PIP2 to generate IP3 and DAG (24). Subsequently, IP3 binds to InsP3Rs on
the ER membrane, triggering the release of Ca2+ from the
ER (26). In response to the
decrease in ER Ca2+ levels, STIM1 on the ER membrane
activates the orai protein in the PM, which tetramerizes to form
the CRAC channel, facilitating the entry of extracellular
Ca2+ into the cytoplasm (27). The elevated cytoplasmic
Ca2+ levels primarily regulate three pathways: i)
Together with DAG, Ca2+ activates PKC, which
subsequently stimulates cell division cycle 42, inducing actin
polymerization and promoting cell polarization and migration
(32). This pathway also interacts
with the Wnt/PCP pathway (42); ii)
Ca2+ activates CaMKII, stimulating TAK1, which then
activates NLK to phosphorylate TCF, inhibiting the β-catenin/TCF
complex and blocking gene transcription (37); and iii) Ca2+ activates
CaN, resulting in the phosphorylation of NFAT and the promotion of
downstream gene transcription (40,41)
(Fig. 1).
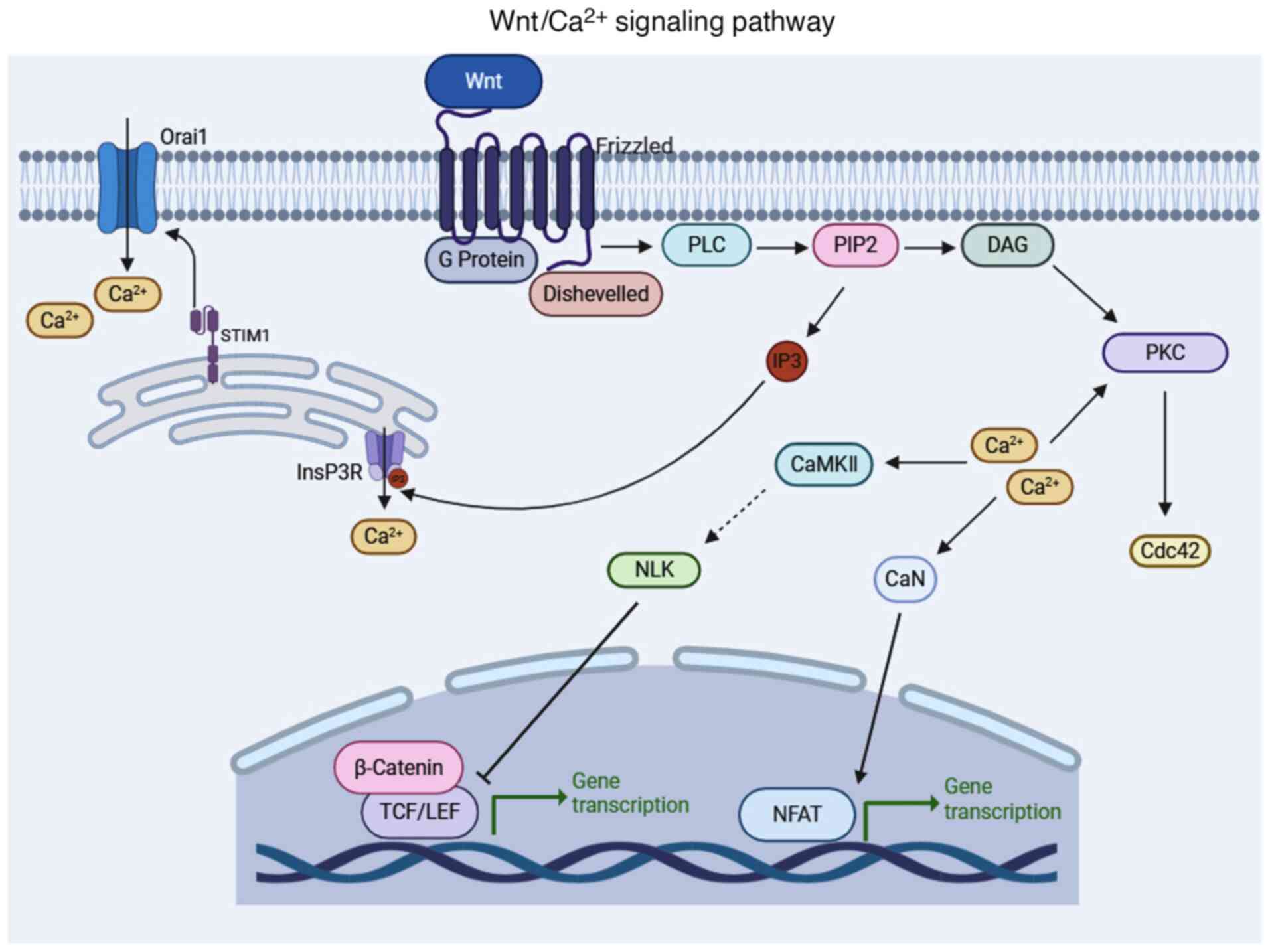 | Figure 1.Schematic diagram of the
Wnt/Ca2+ signaling pathway, created in BioRender. Jing,
L. (2025) https://BioRender.com/t20q988. The
Wnt/Ca2+ pathway is primarily activated by Wnt5a and
Wnt11 ligands, which bind to the FZD receptor on the cell membrane.
This interaction triggers the activation of G proteins and the FZD
adapter protein Dvl, which mediates the activation of PLC. PLC then
cleaves PIP2 into IP3 and DAG. Subsequently, IP3 binds to InsP3R on
the ER membrane, causing the release of Ca2+ from the
ER. In response to the decreased Ca2+ levels in the ER,
STIM1 proteins on the ER membrane activate the Orai protein in the
PM. The Orai protein tetramerizes to form the CRAC channel, which
mediates the influx of extracellular Ca2+ into the
cytoplasm. The increased cytoplasmic Ca2+ primarily
regulates three distinct pathways: i) With DAG, it activates PKC,
further stimulating CDC42, inducing actin polymerization and
promoting cell polarization and migration. This pathway also
cascades through the Wnt/PCP pathway; ii) it activates CaMKII,
stimulating TAK1, which then activates NLK to phosphorylate TCF,
inhibiting the β-catenin/TCF complex and blocking gene
transcription; and iii) it activates CaN, phosphorylating NFAT and
promoting downstream gene transcription. FZD, frizzled; G protein,
heterotrimeric guanine nucleotide-binding protein; Dvl, disheveled;
PLC, phospholipase C; PIP2, phosphatidylinositol 4,5-bisphosphate;
IP3, inositol 1,4,5-trisphosphate; DAG, diacylglycerol; InsP3R,
inositol 1,4,5-trisphosphate receptors; ER, endoplasmic reticulum;
STIM1, stromal interaction molecule 1; PM, plasma membrane; CRAC,
calcium release-activated calcium; CDC42, cell division cycle 42;
Wnt/PCP, Wnt/planar cell polarity; CaMKII, calmodulin-dependent
protein kinase II; TAK1, transforming growth factor-β-activated
kinase 1; NLK, Nemo-like kinase;NFAT, nuclear factor of activated T
cells; TCF, T Cell Factor; CaN, calcineurin; NFAT, nuclear factor
of activated T cells. |
Impact of the Wnt/Ca2+ signaling
pathway on the development and prognosis of several types of
cancers
Dichotomous roles of
Wnt/Ca2+ signaling in tumor progression
The Wnt/Ca2+ signaling pathway exerts a
marked influence on the proliferation, invasion and migration of
tumor cells by precisely regulating critical cellular processes,
such as cell cycle progression, apoptosis, tumor angiogenesis and
epithelial-mesenchymal transition (EMT) (43). This pathway serves as a notable
driver of tumor malignant transformation. Specifically, in prostate
cancer (PCa) (44), melanoma
(45), colorectal cancer (CRC)
(46), ovarian cancer (OC)
(47) and lung cancer (48), the Wnt/Ca2+ signaling
pathway acts as a tumor-promoting factor, accelerating tumor
progression. Conversely, in other types of cancer, such as acute
leukemia (AL) (49–51), thyroid cancer (TC) (52) and hepatocellular carcinoma (HCC)
(53), the pathway exerts tumor
suppressor gene functions by inhibiting malignant behaviors,
including tumor cell proliferation, invasion and migration, thereby
contributing to improved cancer prognosis to a certain extent.
Wnt/Ca2+/CaMKII axis
promotes migration and drug resistance in PCa
PCa is a malignancy that specifically impacts
humans, which serves a substantial role in elevating male mortality
rates worldwide (54). A previous
study reported that the mRNA expression levels of Wnt5a were
markedly elevated in PCa specimens compared with those in healthy
controls; therefore, this may have potential as a genetic marker
for PCa diagnosis (55).
Furthermore, elevated expression of Wnt5a and its receptor FZD2
were reported in an enzalutamide-resistant PCa cell line,
specifically in C4-2B multi-drug-resistant variant (MDVR) cells.
Blocking the expression of Wnt5a and FZD2 not only restored the
sensitivity of C4-2B MDVR cells to enzalutamide, but also
downregulated the expression of genes involved in regulating tumor
cell survival and proliferation (56). Further research reported that Wnt5a
promoted the migration of PCa cells via the
Wnt/Ca2+/CaMKII pathway. In comparison with benign
prostate tissue, malignant prostate tissue exhibited abnormally
high levels of Wnt5a protein expression, which activated the
Wnt/Ca2+/CaMKII pathway. The activated CaMKII then
facilitated the reorganization of the cytoskeleton in PCa cells
through a series of complex signal transduction mechanisms,
ultimately enhancing their migratory capacity (44).
Wnt/Ca2+ pathway activates
CaMKII and PKC, enhancing the invasion, migration and
anti-apoptosis characteristics of melanoma
Melanoma is a malignant tumor originating from
melanocytes, which ranks third in incidence among skin malignancies
(57). Immunohistochemical analysis
has demonstrated that the majority of metastatic melanomas exhibit
intense Wnt5a staining compared with benign tumors (58). Further investigation has revealed
that, by activating the non-canonical Wnt/Ca2+ signaling
pathway, Wnt5a facilitates the phosphorylation of the downstream
molecule CaMKII. It not only augments the angiogenic capacity of
melanoma, but also promotes the formation of vasculogenic mimicry
(VM), which in turn accelerates the processes of tumor cell
proliferation, invasion and migration (59). Additionally, activation of the
Wnt5a/FZD-5 pathway promotes Ca2+-dependent
phosphorylation of PKC in melanoma cells, mediating the invasion
and migration of melanoma cells by downregulating metastasis
suppressor genes (KiSS-1 metastasis suppressor and cadherin 1) and
upregulating metastasis-associated molecules (CD44 and SNAIL)
(60). A previous study reported
that Wnt5a-induced PKC signaling promoted the phosphorylation of
the endogenous substrate myristoylated alanine-rich C-kinase
substrate, which is essential for melanoma cell invasion (45). Furthermore, non-canonical
Wnt/Ca2+ signaling serves a crucial role in inhibiting
apoptosis in melanoma cells. TNF-related apoptosis-inducing ligand
(TRAIL), a member of the TNF superfamily, can induce apoptosis in
melanoma cells. However, Wnt5a-mediated Wnt/Ca2+
signaling protects melanoma cells from apoptosis by activating
CaMKII to upregulate the expression of cellular Fas-associated
death domain-like interleukin-1β converting enzyme inhibitory
protein (c-FLIP), thereby inhibiting TRAIL-mediated cleavage of
caspase-8 and caspase-10. By contrast, the CaMKII inhibitor KN-93
can reduce the expression of c-FLIP and restore the sensitivity of
resistant cells to TRAIL-induced apoptosis (61).
Wnt/Ca2+ pathway activates
NFAT and CaMKII, accelerating the proliferation and migration of
CRC cells
CRC ranks as the third most prevalent cancer
worldwide and is the second leading cause of global cancer-related
mortality (62). In a tumor sphere
formation assay, Wnt3a and Wnt5a ligands were reported to promote
the formation of colon CSC spheroids and cell proliferation by
activating PLC and NFAT. Specific inhibition of PLC or NFAT can
notably impair sphere formation capacity, suggesting that
Wnt/Ca2+ signaling is important for maintaining the
self-renewal of colon CSCs (63).
The Wnt/Ca2+ signaling pathway also promotes CRC cell
migration. Research has demonstrated that both Wnt3a and Wnt5a
ligands are capable of activating the Wnt/Ca2+ signaling
pathway, which subsequently induces PLC-dependent colon cancer cell
migration (64). Furthermore,
tumor-associated macrophages (TAMs), particularly M2-polarized
macrophages, highly express Wnt5a. In co-culture systems of
macrophages and CRC cells, Wnt5a has been reported to promote the
expression of C-C chemokine ligand 2 in TAMs through the
Wnt5a/CaMKII/ERK pathway, thereby enhancing the proliferation and
migration of CRC cells (46). In
addition, the expression levels of Wnt11 are markedly elevated in
CRC, and are closely associated with tumor aggressiveness and
patient prognosis (65). Notably,
Wnt11 may enhance the invasiveness of CRC by promoting the
morphological transformation of intestinal epithelial cells and the
EMT process by enhancing the activation of PKC and CaMKII (66).
Wnt/Ca2+ pathway activates
NFAT and PKCα, promoting EMT, angiogenesis and invasion in OC
OC has the third highest incidence among malignant
tumors of the female reproductive system and epithelial OC (EOC)
constitutes the most prevalent subtype (67). The Wnt5a/Ca2+ signaling
pathway serves a pivotal role in OC invasion. A large-scale study
involving 623 patients revealed that the protein expression levels
of Wnt5a in patients with EOC were markedly higher than in patients
with benign and borderline ovarian tumors, as well as in healthy
control subjects. The upregulation of Wnt5a suppresses the
canonical Wnt pathway in EOCs, induces non-canonical Wnt signaling,
augments the expression of NFAT, JNK and PKCα, diminishes cell
adhesion, and accelerates the EMT (68). Research by Abedini et al
(69) further supported the
aforementioned findings. In mouse ovarian surface epithelium (OSE)
cells, Wnt5a activated CaMKII via Wnt/Ca2+ signaling,
upregulating vimentin and CD44 to facilitate EMT, and transforming
OSE cells into a mesenchymal cell morphology. The expression of
Wnt5a varies across different OC cell lines, with lower expression
observed in the well-differentiated OC cell line SKOV3 relative to
the OVCAR3 cell line. It has been reported that overexpression of
Wnt5a in SKOV3 cells activates PKCα, upregulates vimentin and
suppresses the expression of E-cadherin, thereby enhancing the EMT
and vasculogenic capacity of OC cells in vitro. Notably, the
application of a PKCα inhibitor can reverse these effects (47). In addition, the Wnt5a/PKC/CAMP
responsive element binding protein 1 axis of the non-canonical Wnt
signaling pathway serves an important role in the self-renewal and
dedifferentiation of ovarian CSCs (70).
Wnt/Ca2+ pathway activates
PKC, promoting the proliferation and invasion of lung cancer
cells
Lung cancer has the second highest incidence and
first highest mortality rate among all types of cancer (71). Research has reported that the
expression levels of Wnt5a in non-small cell lung cancer (NSCLC)
tissues are markedly higher than those in corresponding adjacent
normal tissues, and that Wnt5a is associated with a poor prognosis
in NSCLC (72). Wnt5a can activate
the Wnt/Ca2+/PKC signaling pathway, substantially
enhancing the proliferation, migration, invasion and colony
formation of lung cancer cells, whilst suppressing apoptosis.
Notably, the PKC inhibitor GF109203X has been reported to notably
decrease the proportion of aldehyde dehydrogenase-positive lung
CSCs in the cisplatin-resistant A549/DPP cell line, and to reverse
the suppressive effect of Wnt5a on cell apoptosis (48). Previous investigations have reported
that the expression levels of activating transcription factor-2
(ATF-2) in NSCLC were also markedly higher than in normal bronchial
epithelial cells. High expression of ATF-2 can augment the activity
of the Wnt/Ca2+ signaling pathway, boosting the
proliferation and invasion of NSCLC cells. However, knockdown of
ATF-2 in A549 cells can inhibit the activity of the
Wnt/Ca2+ signaling pathway, and substantially reduce the
proliferation and invasion of NSCLC cells (73).
Wnt/Ca2+ pathway activates
CaMKII and PKC, regulating the differentiation and inhibiting
abnormal proliferation of AL cells
AL is the most common hematopoietic stem cell
malignancy in children and has the highest incidence rate among
pediatric oncological disorders (74). A study encompassing 86 pediatric
specimens of acute lymphoblastic leukemia (ALL) reported that the
Wnt5a promoter was highly methylated in both B cell ALL and T cell
ALL (T-ALL) patient samples, with a decrease in mRNA expression
levels observed in both. Notably, this decrease was more pronounced
in patients with T-ALL (75). In
B-cell tumors, Wnt5a inhibits the expression of cyclin D1 by
activating the Wnt/Ca2+ pathway, thereby negatively
regulating B-cell proliferation (49). Regarding acute myeloid leukemia
(AML), a previous study reported that among 11 AML cell lines and
252 samples from patients with AML, seven cell lines and 43%
(107/252) of patient samples exhibited high methylation of the
Wnt5a promoter. Compared with unmethylated patients,
Wnt5a-methylated patients exhibited downregulated Wnt5a expression
and upregulated cyclin D1 transcription levels (50). The Wnt/Ca2+ signaling
pathway is pivotal for the differentiation of AML cells and
subpopulations induced by 6-benzylthioinosine (6-BT). Following
treatment with 6-BT, HL-60 cells have been reported to exhibit
decreased β-catenin levels and elevated Ca2+
concentrations, as well as increased phosphorylation levels of
CaMKII and PKC. By contrast, pretreatment with the PKC inhibitor
bisindolylmaleimide suppresses the Wnt/Ca2+ signaling
and impedes cell differentiation (51). Foxy-5, a novel and innovative
Wnt5a-mimicking compound, effectively inhibits the abnormally
activated β-catenin, PI3K/AKT and MAPK/ERK signaling pathways in
AML, thereby inducing leukemia cell differentiation and markedly
inhibiting their aberrant proliferation, cell survival and
self-renewal potential (76).
Wnt/Ca2+ pathway inhibits
TC cell proliferation via CaMKII activation and β-catenin
phosphorylation
TC is a prevalent malignancy of the endocrine system
and there has been a substantial rise in its incidence globally
over the past few decades (77).
Studies have reported that Wnt5a exhibits either low or no
expression in normal thyroid tissue and undifferentiated TC, but
demonstrates high expression in differentiated TC (78,79).
Further research has reported that when human follicular TC cells
(FTC-133) are stimulated with recombinant Wnt5a, or when Wnt5a is
overexpressed in FTC-133 cells, the intracellular Ca2+
levels rapidly elevate, subsequently activating CaMKII downstream
of the Wnt/Ca2+ signaling pathway. Activated CaMKII
facilitates the phosphorylated degradation of β-catenin, leading to
the downregulation of nuclear c-myc expression, thereby inhibiting
the proliferative effect of canonical Wnt signaling on FTC-133
cells. This effect can be blocked by the CaM inhibitor W-7 or the
CaMKII inhibitor KN-93 (52).
However, previous studies have also reported that the expression
levels of receptor tyrosine kinase-like orphan receptor 2 (ROR2)
and Wnt5a in papillary thyroid carcinoma (PTC) tissues are markedly
higher than those in adjacent normal tissues, and their expression
levels are closely associated with tumor staging and lymph node
metastasis (79). Long-stranded
non-coding RNA FAM230B is highly expressed in PTC tissues and
functions as a microRNA (miR) sponge, protecting miR-378a-3p from
degrading Wnt5a mRNA through a competitive mechanism, thereby
upregulating Wnt5a expression and enhancing the migration and
invasion of PTC cells (80).
However, there remains a lack of definitive evidence regarding
whether the oncogenic role of Wnt5a in TC is mediated through the
Wnt5a/Ca2+ signaling pathway.
Wnt/Ca2+ pathway activates
PKC and induces β-catenin phosphorylation, inhibiting the
proliferation of HCC cells
Liver cancer is a prevalent global health problem,
with an escalating incidence and substantial mortality rates. It
has risen to become the sixth most common cancer and the third
leading cause of cancer-related deaths worldwide. Moreover, HCC
comprises ~90% of all liver cancer cases (71). Studies have reported that, in
comparison with normal tissues adjacent to human HCC, the mRNA and
protein expression levels of Wnt11 are markedly downregulated in
HCC tissues (53,81). Further in vitro experiments
have reported that Wnt11 effectively inhibits canonical Wnt
signaling by facilitating β-catenin phosphorylation through
activation of the Wnt/Ca2+/PKC signaling pathway,
thereby substantially suppressing HCC cell proliferation. Notably,
this inhibitory effect can be reversed by the PKC inhibitor
bisindolylmaleimide I (53).
Additionally, it has been reported that Wnt5a expression levels are
notably lower in HCC tissues compared with those in adjacent
non-cancerous tissues, and are closely associated with disease
progression and a poor prognosis (82,83).
In HCC cell lines, Wnt5a overexpression not only enhances β-catenin
phosphorylation, but also elevates E-cadherin levels, effectively
inhibiting the proliferation and migration of HCC cells (84,85).
Although the inhibitory effect of Wnt5a on canonical Wnt signaling
in HCC cells has been previously reported (86), the specific mechanisms remain
unclear.
Challenges and future trends of
Wnt/Ca2+ signaling in cancer therapy
The Wnt/Ca2+ signaling pathway serves a
pivotal role in tumorigenesis, metastasis and therapeutic
resistance by regulating cellular migration, invasion and
remodeling of the tumor microenvironment (TME) (43). Epigenetic modifications,
particularly the dynamic regulation of DNA methylation (87–90)
and histone modifications (91–93),
markedly impact the activity of Wnt/Ca2+ signaling,
offering novel therapeutic strategies centered on epigenetic
regulation for cancer treatment.
At the DNA methylation level, aberrant methylation
is a key driver of cancer progression. Notably, hypermethylation of
the SFRP promoter, which encodes a Wnt antagonist, leads to gene
silencing and subsequently disinhibition of Wnt ligand activity,
promoting aberrant pathway activation in CRC, breast cancer (BC)
and other malignancies (87).
Conversely, hypermethylation of Wnt ligand genes (such as WNT5A)
suppresses transcription, resulting in reduced Wnt/Ca2+
signaling pathway activity (88,89).
Additionally, methylation-induced silencing of calcium channel
subunit genes (such as the calcium channel α2-Δ3 subunit) disrupts
intracellular Ca2+ signaling, impairing downstream
Wnt/Ca2+-dependent effects, including CaN-mediated
apoptosis, fostering oncogenesis or neurodevelopmental defects
(90). Notable progress has been
made in developing targeted drugs against DNA methyltransferases.
Hypomethylating agents such as azacitidine and decitabine have
demonstrated substantial clinical importance in treating high-risk
myelodysplastic syndromes, chronic myelomonocytic leukemia and AML
(94).
Complementing the DNA methylation-based strategy,
histone modification regulation emerges as another critical
mechanism in reshaping Wnt/Ca2+ signaling and tumor
phenotypes. Histone deacetylase (HDAC) inhibitors enhance histone
acetylation, upregulating Wnt ligand expression (such as Wnt4,
Wnt5a and Wnt11) and strengthening Wnt/Ca2+ signaling,
promoting cellular migration and differentiation (91). For example, the HDAC6 inhibitor
BML-281 increases Wnt5a expression and activates downstream
Wnt/Ca2+ signaling (92). Furthermore, HDAC inhibitors (HDACis)
restore the expression of the secretory pathway
Ca2+-ATPase secretory pathway calcium ATPase 2 (SPCA2)
in triple-negative BC (TNBC), elevating intracellular
Ca2+ levels and inducing a less aggressive tumor
phenotype via non-canonical Wnt/Ca2+ signaling (93). Currently, five HDACis (vorinostat,
romidepsin, belinostat, panobinostat and tucidinostat) are approved
for treating T-cell lymphomas and multiple myeloma by the US Food
and Drug Administration. Ongoing clinical trials continue to
explore their potential in AML, B-cell lymphomas and several solid
tumors (95,96).
Post-translational modification emerges as another
critical dimension in modulating this pathway. Among these,
palmitoylation is a key post-translational modification for Wnt
protein secretion, ensuring correct Wnt ligand localization.
Porcupine inhibitors (such as WNT974, ETC-159, RXC004 and CGX1321)
block WNT ligand O-palmitoylation, suppressing both canonical and
non-canonical Wnt signaling. These agents have shown preclinical
promise in CRC, pancreatic cancer and HCC, with several entering
Phase I trials (97,98).
Beyond epigenetic therapies, researchers have
developed ligands and receptor-targeted agents against the
Wnt/Ca2+ signaling pathway. For example, two Wnt5a
mimetic peptides, Foxy-5, a WNT5A agonist and Box-5, a WNT5A
antagonist, modulate Wnt5a-dependent signaling to reduce tumor
metastasis. Foxy-5 specifically binds to FZD-5 receptors in BC,
rapidly activating membrane calcium signaling without affecting
canonical β-catenin or JNK pathways (99). Preclinical and clinical studies have
demonstrated its potent anti-metastatic activity, reducing tumor
metastasis by 70–80% in WNT5A-deficient BC models (100). Moreover, Box-5 inhibits
Wnt5a-induced Ca2+/PKC signaling, exhibiting unique
anti-invasive properties in melanoma (101).
In addition to peptide-based agents, monoclonal
antibodies targeting Wnt receptors have shown promise. Vantictumab
(OMP-18R5), a fully humanized monoclonal antibody, targets multiple
FZD receptors, blocking Wnt ligand binding and inhibiting canonical
and non-canonical pathways. Preclinical investigations have
revealed its antiproliferative efficacy across diverse human tumor
models, with improved therapeutic outcomes observed when combined
with chemotherapeutic regimens (102–104).
Despite the aforementioned advances, challenges
remain in optimizing Wnt/Ca2+-targeted therapies,
including toxicities and tumor heterogeneity. Whilst epigenetic
drugs are generally less toxic than traditional chemotherapeutics,
they may still cause myelosuppression, gastrointestinal
disturbances and hepatorenal toxicity. Additionally, their efficacy
in solid tumors often lags behind that in hematological
malignancies, with variable patient responses. Combining epigenetic
drugs with other antitumor agents offers a promising strategy to
enhance efficacy and reduce toxicity, yet optimal dosing and
regimen design require further refinement (94,96).
Moreover, Porcupine inhibitors (such as WNT974 and ETC-159) and
pan-FZD antibodies (such as Vantictumab) have shown dose-limiting
toxicities related to loss of bone mass, likely due to the
essential role of Wnt signaling in osteoblast differentiation and
regulation of bone homeostasis (105). Therefore, by integrating the
expression profiles of Wnt ligands and receptors in tumor cells and
the TME, it may be possible to develop inhibitors targeting
specific Wnt ligands and receptors and minimize off-target effects
on bone and other normal tissues, thus preserving skeletal
integrity and avoiding unnecessary side effects.
Discussion
In recent years, integrating interdisciplinary
research methods and the widespread application of innovative
technologies have markedly advanced tumor diagnosis and treatment
(106,107). Tumorigenesis and tumor progression
are governed by intricate biological processes involving numerous
signaling pathways, among which the Wnt signaling pathway serves as
a core regulatory factor and has therefore attracted notable
scientific interest. Until now, several studies have performed
in-depth explorations of the role of the canonical Wnt/β-catenin
signaling pathway in tumorigenesis. This pathway exerts crucial
regulatory effects on multiple biological processes of tumor cells,
including proliferation, differentiation and apoptosis, as well as
invasion and metastasis (108–111). However, the specific role of the
Wnt/Ca2+ signaling pathway, as an important
non-canonical branch of the Wnt signaling pathway, in tumorigenesis
and tumor development has not been fully explored. Therefore, the
present review systematically introduces the research progress of
the Wnt/Ca2+ signaling pathway and explores its
biological effects and potential mechanisms in several tumors
(Table I and Fig. 1). Furthermore, it provides a
comprehensive overview of existing therapeutic strategies targeting
this pathway in cancer and discusses associated challenges.
 | Table I.Wnt/Ca2+ signaling pathway
and cancer. |
Table I.
Wnt/Ca2+ signaling pathway
and cancer.
| Cancer type |
Function/mechanisms | (Refs.) |
|---|
| PCa | Wnt5a is highly
expressed in metastatic PCa; Wnt5a/Ca2+ activates
CaMKII, regulating the cytoskeleton and promoting migration of PCa
cells. | (44,55,56) |
| Melanoma | Wnt5a is highly
expressed in metastatic melanoma; Wnt5a/Ca2+ signaling
activates CaMKII and PKC to inhibit the canonical Wnt signaling,
enhancing melanoma angiogenesis and vasculogenic mimicry formation,
and promoting melanoma cell migration and invasion;
Wnt5a/Ca2+ signaling activates CaMKII and inhibits
TNF-related apoptosis-inducing ligand-induced apoptosis. | (45,58–61) |
| CRC | Wnt/Ca2+
signaling activates PLC and nuclear factor of activated T cells,
promoting the proliferation of colon cancer stem cells, and
accelerating the proliferation and migration of CRC cells; the
Wnt5a/CaMKII/ERK pathway enhances C-C chemokine ligand 2 expression
in tumor-associated macrophages, promoting CRC cell proliferation
and migration; Wnt11/Ca2+ signaling activates PKC and
CaMKII, stimulating proliferation of intestinal epithelial cells,
reducing E-cadherin contacts and promoting CRC metastasis. | (46,63–66) |
| OC | Wnt5a is highly
expressed in epithelial OC; Wnt5a/Ca2+ signaling
activates PKCα and promotes the progression of
epithelial-mesenchymal transition and VM in OC cells;
Wnt5a/PKC/CREB1 signaling promotes the proliferation of ovarian
cancer stem cells. | (47,68–70) |
| Lung cancer | Wnt5a is highly
expressed in non-small cell lung cancer; Wnt5a/Ca2+
signaling activates PKCα and CaMKII, and promotes the proliferation
and invasion of non-small cell lung cancer cells. | (48,72,73) |
| AL | Wnt5a has a low
expression in AL; Wnt5a/Ca2+ signaling downregulates
cyclin D1, inhibiting B-cell proliferation; 6-benzylthioinosine
upregulates Wnt/Ca2+ signaling to activate CaMKII and
PKC, promoting differentiation of AML cells; Foxy-5 blocks
abnormally activated β-catenin, PI3K/AKT, MAPK/ERK and other
signaling pathways in AML to inhibit its progression. | (49–51,75,76) |
| TC | Wnt5a has a low
expression in undifferentiated TC; Wnt5a/Ca2+ signaling
activates CaMKII, promoting β-catenin degradation, downregulating
c-myc and inhibiting the proliferation of FTC-133 cells. | (52,78–80) |
| HCC | Wnt11 has a low
expression in HCC tissues; Wnt11/Ca2+ activates PKC,
downregulates canonical Wnt signaling and inhibits HCC cell
proliferation; Wnt5a has a low expression in HCC tissues; Wnt5a
inhibits canonical Wnt signaling and reduces the proliferation and
migration of HCC cell lines. | (53,81–86) |
The Wnt/Ca2+ signaling pathway
demonstrates remarkable functional heterogeneity in tumors, acting
either as a tumor promoter or suppressor. Such context-dependent
effects may be attributed to differences in the ligand selection of
the signaling pathway, receptor combination and the TME. The tumor
suppressive mechanism of the Wnt/Ca2+ signaling pathway
may involve several reasons. First, the Wnt/Ca2+
signaling pathway antagonizes the canonical Wnt/β-catenin pathway.
Specifically, the Wnt/Ca2+ pathway inhibits the
transcriptional activity of β-catenin by activating the CaMKII and
TAK-NLK pathway, thereby limiting tumor proliferation and invasion
driven by the canonical Wnt/β-catenin pathway (112). For example, in TNBC, HDACi
triggers an increase in resting Ca2+ levels by
upregulating the SPCA2 gene. High levels of Ca2+
activate CAMKII, a downstream component of non-canonical
Wnt/Ca2+ signaling, leading to phosphorylation of
β-catenin and inhibition of its nuclear translocation. This
ultimately leads to the conversion of cancer cells to a less
aggressive ‘epithelial’ state (93). Secondly, the Wnt/Ca2+
signaling pathway can induce cell apoptosis. High concentrations of
intracellular Ca2+, induced by Wnt5a and Wnt11 binding
with FZD-2, activate calcium-dependent phosphatases, such as CaN,
dephosphorylates the pro-apoptotic protein Bad, promotes the
release of cytochrome C, and further induces apoptosis (113). Simultaneously, Ca2+
activates calpain, a CaM-degrading enzyme near the ER, which acts
on caspase-12, activating it and releasing it into the cytoplasm,
thus inducing apoptosis (114). By
contrast, the Wnt/Ca2+ signaling pathway may also
promote tumor development, mainly by enhancing the migration and
invasion of tumor cells. In ameloblastoma, it has been reported
that Wnt5a drives mitochondrial energy production and increases the
number of mitochondria, but reduces their size. Moreover, it also
increases Ca2+ levels, directly leading to altered
mitochondrial dynamics, interactions between the cytoskeleton and
mitochondria, and promotion of cell migration via the nuclear
factor of activated T cells 2 (NFAT2)-coronin 1A-F-actin axis
(115).
In several solid tumors such as PCa, CRC, OC and
lung cancer, the Wnt/Ca2+ signaling pathway markedly
promotes tumor metastasis through processes such as EMT and VM.
This is consistent with the tumor-promoting role of the canonical
Wnt/β-catenin pathway in these tumors, and the two pathways work
synergistically to promote tumor progression (111,116). This synergistic effect is also
reflected in their joint regulation of the TME, particularly by
influencing the polarization state of TAMs, thereby promoting the
occurrence and development of malignant tumors (117). However, in tumors such as AL, TC
and HCC, the Wnt/Ca2+ signaling pathway exhibits
distinct biological effects. In these tumors, this pathway is often
silenced due to epigenetic modifications of its ligands. Upon
activation, it can exert tumor-suppressive effects by inhibiting
the Wnt/β-catenin signaling pathway (78,82,118).
Nevertheless, this inhibitory effect does not always lead to tumor
suppression. For example, in melanoma, Wnt/Ca2+
signaling promotes the transition of tumor cells from a
proliferative state to a highly invasive phenotype by inhibiting
Wnt/β-catenin signaling, ultimately enhancing their metastatic
ability (119). In general, the
tendency of the Wnt/Ca2+ pathway to act as a tumor
suppressor or a tumor promoter in different contexts is influenced
by multiple factors, including tumor type-specific characteristics,
TME factors, the dynamic balance between signaling pathways and
epigenetic regulatory mechanisms. The complex interactions among
these factors determine the functional output of this pathway in
different tumors, providing important clues for explaining its
spatiotemporal heterogeneity.
Despite considerable advances in elucidating the
mechanisms of the Wnt/Ca2+ signaling pathway, several
challenges remain to comprehensively understand its molecular
intricacies. Notably, the precise timing of the interaction between
Dvl and G proteins within the signaling cascade, along with its
kinetic characteristics, requires further investigation to identify
additional potential therapeutic targets for tumors. The
therapeutic landscape of Wnt/Ca2+ pathway-targeted
treatments is further complicated by the challenges of achieving
sustained therapeutic doses of single-agent epigenetic therapies.
Emerging evidence suggests that delivering epigenetic therapies at
low doses or short intervals may act as an adjuvant strategy to
augment the effects of other oncology treatments. This method shows
promise in enhancing the efficacy of existing oncological
treatments, though optimal dosing and treatment regimens require
continued refinement and systematic optimization (94,96).
Profound tumor heterogeneity adds another layer of complexity,
manifested through the variable expression levels of Wnt ligands,
FZD receptors and their co-receptors (120,121). The signaling outcomes critically
depend on the predominant co-receptor context (122). In scenarios where ROR2 serves as
the primary co-receptor, Wnt5a facilitates the degradation of
β-catenin via FZD2 or FZD5 signaling, thereby inhibiting cancer
progression. Conversely, when the low-density lipoprotein receptor
related protein 6 (LRP6) co-receptor prevails, Wnt5a forms a
complex with FZD4/LRP6, leading to the stabilization of β-catenin
and fostering cancer metastasis (123,124). Consequently, by integrating the
expression profiles of Wnt ligands and receptors in tumor cells and
the TME, it is feasible to identify the Wnt signaling subtypes that
dominate during tumorigenesis. This approach enables precise
selection of drugs targeting specific Wnt ligands, receptors or
co-receptors, thus improving the therapeutic efficacy. Furthermore,
synergistic or antagonistic effects between canonical and
non-canonical Wnt pathways can be harnessed to develop targeted
therapies focusing on shared upstream regulators or downstream
effectors, thereby minimizing unnecessary side effects.
Acknowledgements
Not applicable.
Funding
The present work was supported by grants from the National
Natural Science Foundation of China (grant nos. 82071738, 81671541
and 81701545), Jiangsu Natural Science Foundation (grant no.
BK20231236) and the Medical Leadership Program of Jiangsu College
of Nursing (grant no. 2021L001).
Availability of data and materials
Not applicable.
Authors' contributions
LJ and SX designed the review framework, drafted
the manuscript, and created and refined the figures and tables. HW
and QS reviewed and revised the manuscript, and provided financial
support for its publication. All authors read and approved the
final manuscript. Data authentication is not applicable.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Nusse R and Varmus H: Three decades of
Wnts: A personal perspective on how a scientific field developed.
EMBO J. 31:2670–2684. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liu J, Xiao Q, Xiao J, Niu C, Li Y, Zhang
X, Zhou Z, Shu G and Yin G: Wnt/β-catenin signalling: function,
biological mechanisms, and therapeutic opportunities. Signal
Transduct Target Ther. 7:32022. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yu F, Yu C, Li F, Zuo Y, Wang Y, Yao L, Wu
C, Wang C and Ye L: Wnt/β-catenin signaling in cancers and targeted
therapies. Signal Transduct Target Ther. 6:3072021. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chien AJ, Conrad WH and Moon RT: A Wnt
survival guide: From flies to human disease. J Invest Dermatol.
129:1614–1627. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Akoumianakis I, Polkinghorne M and
Antoniades C: Non-canonical WNT signalling in cardiovascular
disease: Mechanisms and therapeutic implications. Nat Rev Cardiol.
19:783–797. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lojk J and Marc J: Roles of non-canonical
Wnt signalling pathways in bone biology. Int J Mol Sci.
22:108402021. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chae WJ and Bothwell ALM: Canonical and
non-canonical Wnt signaling in immune cells. Trends Immunol.
39:830–847. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mehta S, Hingole S and Chaudhary V: The
emerging mechanisms of Wnt secretion and signaling in development.
Front Cell Dev Biol. 9:7147462021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wolf L and Boutros M: The role of
Evi/Wntless in exporting Wnt proteins. Development.
150:dev2013522023. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
McGough IJ, Vecchia L, Bishop B,
Malinauskas T, Beckett K, Joshi D, O'Reilly N, Siebold C, Jones EY
and Vincent JP: Glypicans shield the Wnt lipid moiety to enable
signalling at a distance. Nature. 585:85–90. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Liu HY, Sun XJ, Xiu SY, Zhang XY, Wang ZQ,
Gu YL, Yi CX, Liu JY, Dai YS, Yuan X, et al: Frizzled receptors
(FZDs) in Wnt signaling: Potential therapeutic targets for human
cancers. Acta Pharmacol Sin. 45:1556–1570. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Huang HC and Klein PS: The Frizzled
family: Receptors for multiple signal transduction pathways. Genome
Biol. 5:2342004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng S and Sheng R: The emerging
understanding of Frizzled receptors. FEBS Lett. 598:1939–1954.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Verkaar F and Zaman GJR: A model for
signaling specificity of Wnt/Frizzled combinations through
co-receptor recruitment. FEBS Lett. 584:3850–3854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang L, Zhu R, Wen Z, Fan HJS,
Norwood-Jackson T, Jathan D and Lee HJ: Structural and functional
insights into dishevelled-Mediated Wnt signaling. Cells.
13:18702024. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Bowin CF, Inoue A and Schulte G:
WNT-3A-induced β-catenin signaling does not require signaling
through heterotrimeric G proteins. J Biol Chem. 294:11677–11684.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Boligala GP, Yang MV, van Wunnik JC and
Pruitt K: Nuclear dishevelled: An enigmatic role in governing cell
fate and Wnt signaling. Biochim Biophys Acta Mol Cell Res.
1869:1193052022. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Aznar N, Midde KK, Dunkel Y, Lopez-Sanchez
I, Pavlova Y, Marivin A, Barbazán J, Murray F, Nitsche U, Janssen
KP, et al: Daple is a novel non-receptor GEF required for trimeric
G protein activation in Wnt signaling. Elife. 4:e070912015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Aznar N, Ear J, Dunkel Y, Sun N,
Satterfield K, He F, Kalogriopoulos N, Lopez-Sanchez I, Ghassemian
M, Sahoo D, et al: Convergence of Wnt, growth factor and trimeric G
protein signals on Daple. Sci Signal. 11:eaao42202018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gong B, Shen W, Xiao W, Meng Y, Meng A and
Jia S: The Sec14-like phosphatidylinositol transfer proteins
Sec14l3/SEC14L2 act as GTPase proteins to mediate
Wnt/Ca2+ signaling. Elife. 6:e263622017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sheldahl LC, Slusarski DC, Pandur P,
Miller JR, Kühl M and Moon RT: Dishevelled activates
Ca2+ flux, PKC, and CamKII in vertebrate embryos. J Cell
Biol. 161:769–777. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qin K, Yu M, Fan J, Wang H, Zhao P, Zhao
G, Zeng W, Chen C, Wang Y, Wang A, et al: Canonical and
noncanonical Wnt signaling: Multilayered mediators, signaling
mechanisms and major signaling crosstalk. Genes Dis. 11:103–134.
2023. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bill CA and Vines CM: Phospholipase C. Adv
Exp Med Biol. 1131:215–242. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Kanemaru K and Nakamura Y: Activation
mechanisms and diverse functions of mammalian phospholipase C.
Biomolecules. 13:9152023. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Katti SS, Krieger IV, Ann J, Lee J,
Sacchettini JC and Igumenova TI: Structural anatomy of protein
kinase C C1 domain interactions with diacylglycerol and other
agonists. Nat Commun. 13:26952022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu L and Chen J: Type 3 IP3 receptor: Its
structure, functions, and related disease implications. Channels
(Austin). 17:22674162023. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Derler I, Jardin I and Romanin C:
Molecular mechanisms of STIM/Orai communication. Am J Physiol Cell
Physiol. 310:C643–C662. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kodakandla G, Akimzhanov AM and Boehning
D: Regulatory mechanisms controlling store-operated calcium entry.
Front Physiol. 14:13302592023. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Aquino A, Bianchi N, Terrazzan A and
Franzese O: Protein kinase C at the crossroad of mutations, cancer,
targeted therapy and immune response. Biology (Basel).
12:10472023.PubMed/NCBI
|
|
30
|
Kawano T, Inokuchi J, Eto M, Murata M and
Kang JH: Protein kinase C (PKC) isozymes as diagnostic and
prognostic biomarkers and therapeutic targets for cancer. Cancers
(Basel). 14:54252022. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Newton AC: Protein kinase C: Perfectly
balanced. Crit Rev Biochem Mol Biol. 53:208–230. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kazanietz MG and Cooke M: Protein kinase C
signaling ‘in’ and ‘to’ the nucleus: Master kinases in
transcriptional regulation. J Biol Chem. 300:1056922024. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang X, Connelly J, Levitan ES, Sun D and
Wang JQ: Calcium/calmodulin-dependent protein kinase II in
cerebrovascular diseases. Transl Stroke Res. 12:513–529. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Erickson JR: Mechanisms of CaMKII
activation in the heart. Front Pharmacol. 5:592014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Brown CN and Bayer KU: Studying CaMKII:
Tools and standards. Cell Rep. 43:1139822024. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wang Y, Zhao R and Zhe H: The emerging
role of CaMKII in cancer. Oncotarget. 6:11725–11734. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishitani T, Kishida S, Hyodo-Miura J, Ueno
N, Yasuda J, Waterman M, Shibuya H, Moon RT, Ninomiya-Tsuji J and
Matsumoto K: The TAK1-NLK mitogen-activated protein kinase cascade
functions in the Wnt-5a/Ca(2+) pathway to antagonize
Wnt/beta-catenin signaling. Mol Cell Biol. 23:131–139. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Creamer TP: Calcineurin. Cell Commun
Signal. 18:1372020. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen L, Song M and Yao C: Calcineurin in
development and disease. Genes Dis. 9:915–927. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Hogan PG: Calcium-NFAT transcriptional
signalling in T cell activation and T cell exhaustion. Cell
Calcium. 63:66–69. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin Y, Song Y, Zhang Y, Shi M, Hou A and
Han S: NFAT signaling dysregulation in cancer: Emerging roles in
cancer stem cells. Biomed Pharmacother. 165:1151672023. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Luna-Ulloa LB, Hernández-Maqueda JG,
Castañeda-Patlán MC and Robles-Flores M: Protein kinase C in Wnt
signaling: Implications in cancer initiation and progression. IUBMB
Life. 63:915–921. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Bueno MLP, Saad STO and Roversi FM: WNT5A
in tumor development and progression: A comprehensive review.
Biomed Pharmacother. 155:1135992022. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Wang Q, Symes AJ, Kane CA, Freeman A,
Nariculam J, Munson P, Thrasivoulou C, Masters JRW and Ahmed A: A
novel role for Wnt/Ca2+ signaling in actin cytoskeleton
remodeling and cell motility in prostate cancer. PLoS One.
5:e104562010. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Mohapatra P, Yadav V, Toftdahl M and
Andersson T: WNT5A-induced activation of the protein kinase C
substrate MARCKS is required for melanoma cell invasion. Cancers
(Basel). 12:3462020. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Liu Q, Song J, Pan Y, Shi D, Yang C, Wang
S and Xiong B: Wnt5a/CaMKII/ERK/CCL2 axis is required for
tumor-associated macrophages to promote colorectal cancer
progression. Int J Biol Sci. 16:1023–1034. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Qi H, Sun B, Zhao X, Du J, Gu Q, Liu Y,
Cheng R and Dong X: Wnt5a promotes vasculogenic mimicry and
epithelial-mesenchymal transition via protein kinase Cα in
epithelial ovarian cancer. Oncol Rep. 32:771–779. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Yang J, Zhang K, Wu J, Shi J, Xue J, Li J,
Chen J, Zhu Y, Wei J, He J and Liu X: Wnt5a increases properties of
lung cancer stem cells and resistance to cisplatin through
activation of Wnt5a/PKC signaling pathway. Stem Cells Int.
2016:16908962016. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Liang H, Chen Q, Coles AH, Anderson SJ,
Pihan G, Bradley A, Gerstein R, Jurecic R and Jones SN: Wnt5a
inhibits B cell proliferation and functions as a tumor suppressor
in hematopoietic tissue. Cancer Cell. 4:349–360. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Martín V, Valencia A, Agirre X, Cervera J,
Jose-Eneriz ES, Vilas-Zornoza A, Rodriguez-Otero P, Sanz MA,
Herrera C, Torres A, et al: Epigenetic regulation of the
non-canonical Wnt pathway in acute myeloid leukemia. Cancer Sci.
101:425–432. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zang S, Liu N, Wang H, Wald DN, Shao N,
Zhang J, Ma D, Ji C and Tse W: Wnt signaling is involved in
6-benzylthioinosine-induced AML cell differentiation. BMC Cancer.
14:8862014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Kremenevskaja N, von Wasielewski R, Rao
AS, Schöfl C, Andersson T and Brabant G: Wnt-5a has tumor
suppressor activity in thyroid carcinoma. Oncogene. 24:2144–2154.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Toyama T, Lee HC, Koga H, Wands JR and Kim
M: Noncanonical Wnt11 inhibits hepatocellular carcinoma cell
proliferation and migration. Mol Cancer Res. 8:254–265. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Sekhoacha M, Riet K, Motloung P, Gumenku
L, Adegoke A and Mashele S: Prostate cancer review: Genetics,
diagnosis, treatment options, and alternative approaches.
Molecules. 27:57302022. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ebrahimi S, Rezaei Fakhrnezhad F,
Jahangiri S, Borjkhani M, Behboodi R and Monfaredan A: The IGSF1,
Wnt5a, FGF14, and ITPR1 gene expression and prognosis hallmark of
prostate cancer. Rep Biochem Mol Biol. 11:44–53. 2022.PubMed/NCBI
|
|
56
|
Ning S, Liu C, Lou W, Yang JC, Lombard AP,
D'Abronzo LS, Batra N, Yu AM, Leslie AR, Sharifi M, et al:
Bioengineered BERA-Wnt5a siRNA targeting Wnt5a/FZD2 signaling
suppresses advanced prostate cancer tumor growth and enhances
enzalutamide treatment. Mol Cancer Ther. 21:1594–1607. 2022.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Garbe C, Amaral T, Peris K, Hauschild A,
Arenberger P, Basset-Seguin N, Bastholt L, Bataille V, Del Marmol
V, Dréno B, et al: European consensus-based interdisciplinary
guideline for melanoma. Part 1: Diagnostics: Update 2022. Eur J
Cancer. 170:236–255. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Da Forno PD, Pringle JH, Hutchinson P,
Osborn J, Huang Q, Potter L, Hancox RA, Fletcher A and Saldanha GS:
WNT5A expression increases during melanoma progression and
correlates with outcome. Clin Cancer Res. 14:5825–5832. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Geng B, Zhu Y, Yuan Y, Bai J, Dou Z, Sui A
and Luo W: Artesunate suppresses choroidal melanoma vasculogenic
mimicry formation and angiogenesis via the Wnt/CaMKII signaling
axis. Front Oncol. 11:7146462021. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Weeraratna AT, Jiang Y, Hostetter G,
Rosenblatt K, Duray P, Bittner M and Trent JM: Wnt5a signaling
directly affects cell motility and invasion of metastatic melanoma.
Cancer Cell. 1:279–288. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Xiao C, Fengyang B, Song J, Schulman H, Li
L and Hao C: Inhibition of CaMKII-mediated c-FLIP expression
sensitizes malignant melanoma cells to TRAIL-induced apoptosis. Exp
Cell Res. 304:244–255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Baidoun F, Elshiwy K, Elkeraie Y, Merjaneh
Z, Khoudari G, Sarmini MT, Gad M, Al-Husseini M and Saad A:
Colorectal cancer epidemiology: Recent trends and impact on
outcomes. Curr Drug Targets. 22:998–1009. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Sarabia-Sánchez MA, Moreno-Londoño AP,
Castañeda-Patlán MC, Alvarado-Ortiz E, Martínez-Morales JC and
Robles-Flores M: Non-canonical Wnt/Ca2+ signaling is
essential to promote self-renewal and proliferation in colon cancer
stem cells. Front Oncol. 13:11217872023. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Flores-Hernández E, Velázquez DM,
Castañeda-Patlán MC, Fuentes-García G, Fonseca-Camarillo G,
Yamamoto-Furusho JK, Romero-Avila MT, García-Sáinz JA and
Robles-Flores M: Canonical and non-canonical Wnt signaling are
simultaneously activated by Wnts in colon cancer cells. Cell
Signal. 72:1096362020. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Gorroño-Etxebarria I, Aguirre U, Sanchez
S, González N, Escobar A, Zabalza I, Quintana JM, Vivanco MD,
Waxman J and Kypta RM: Wnt-11 as a potential prognostic biomarker
and therapeutic target in colorectal cancer. Cancers (Basel).
11:9082019. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Ouko L, Ziegler TR, Gu LH, Eisenberg LM
and Yang VW: Wnt11 signaling promotes proliferation,
transformation, and migration of IEC6 intestinal epithelial cells.
J Biol Chem. 279:26707–26715. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Arnaoutoglou C, Dampala K, Anthoulakis C,
Papanikolaou EG, Tentas I, Dragoutsos G, Machairiotis N,
Zarogoulidis P, Ioannidis A, Matthaios D, et al: Epithelial ovarian
cancer: A five year review. Medicina (Kaunas). 59:11832023.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Ford CE, Punnia-Moorthy G, Henry CE,
Llamosas E, Nixdorf S, Olivier J, Caduff R, Ward RL and
Heinzelmann-Schwarz V: The non-canonical Wnt ligand, Wnt5a, is
upregulated and associated with epithelial to mesenchymal
transition in epithelial ovarian cancer. Gynecol Oncol.
134:338–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
69
|
Abedini A, Sayed C, Carter LE, Boerboom D
and Vanderhyden BC: Non-canonical WNT5a regulates
epithelial-to-mesenchymal transition in the mouse ovarian surface
epithelium. Sci Rep. 10:96952020. View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Fang Y, Xiao X, Wang J, Dasari S, Pepin D,
Nephew KP, Zamarin D and Mitra AK: Cancer associated fibroblasts
serve as an ovarian cancer stem cell niche through noncanonical
Wnt5a signaling. NPJ Precis Oncol. 8:72024. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Sung H, Ferlay J, Siegel RL, Laversanne M,
Soerjomataram I, Jemal A and Bray F: Global cancer statistics 2020:
GLOBOCAN estimates of incidence and mortality worldwide for 36
cancers in 185 countries. CA Cancer J Clin. 71:209–249. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Lu C, Wang X, Zhu H, Feng J, Ni S and
Huang J: Over-expression of ROR2 and Wnt5a cooperatively correlates
with unfavorable prognosis in patients with non-small cell lung
cancer. Oncotarget. 6:24912. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Zhang L, Zeng S, Yu Z, Zhang G, Xiong Z,
Xie F and You Z: Overexpression of activating transcription
factor-2 (ATF-2) activates Wnt/Ca2+ Signaling pathways
and promotes proliferation and invasion in non-small-cell lung
cancer. Dis Markers. 2022:57720892022.PubMed/NCBI
|
|
74
|
Masetti R, Muratore E, Leardini D, Zama D,
Turroni S, Brigidi P, Esposito S and Pession A: Gut microbiome in
pediatric acute leukemia: From predisposition to cure. Blood Adv.
5:4619–4629. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Hatırnaz Ng Ö, Fırtına S, Can İ, Karakaş
Z, Ağaoğlu L, Doğru Ö, Celkan T, Akçay A, Yıldırmak Y, Timur Ç, et
al: A possible role for WNT5A hypermethylation in pediatric acute
lymphoblastic leukemia. Turk J Haematol. 32:127–135. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Bueno MLP, Saad STO and Roversi FM: The
antitumor effects of WNT5A against hematological malignancies. J
Cell Commun Signal. 17:1487–1499. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Torre LA, Siegel RL, Ward EM and Jemal A:
Global cancer incidence and mortality rates and trends-an update.
Cancer Epidemiol Biomarkers Prev. 25:16–27. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Sastre-Perona A and Santisteban P: Role of
the wnt pathway in thyroid cancer. Front Endocrinol (Lausanne).
3:312012. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Chen L, Zhao L, Ding M, Yang M, Yang W,
Cui G and Shan B: Higher expression level of tyrosine kinase-like
orphan receptor 2 and Wnt member 5a in papillary thyroid carcinoma
is associated with poor prognosis. Oncol Lett. 14:5966–5972.
2017.PubMed/NCBI
|
|
80
|
Zhou Q, Feng J, Yin S, Ma S, Wang J and Yi
H: LncRNA FAM230B promotes the metastasis of papillary thyroid
cancer by sponging the miR-378a-3p/WNT5A axis. Biochem Biophys Res
Commun. 546:83–89. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Zhu Y, He Y and Gan R: Wnt signaling in
hepatocellular carcinoma: Biological mechanisms and therapeutic
opportunities. Cells. 13:19902024. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Wang L, Yao M, Fang M, Zheng WJ, Dong ZZ,
Pan LH, Zhang HJ and Yao DF: Expression of hepatic Wnt5a and its
clinicopathological features in patients with hepatocellular
carcinoma. Hepatobiliary Pancreat Dis Int. 17:227–232. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Wakizaka K, Kamiyama T, Kakisaka T, Orimo
T, Nagatsu A, Aiyama T, Shichi S and Taketomi A: Expression of
Wnt5a and ROR2, components of the noncanonical Wnt-signaling
pathway, is associated with tumor differentiation in hepatocellular
carcinoma. Ann Surg Oncol. 31:262–271. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Wang T, Liu X and Wang J: Up-regulation of
Wnt5a inhibits proliferation and migration of hepatocellular
carcinoma cells. J Can Res Ther. 15:904–908. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
85
|
Wakizaka K, Kamiyama T, Wakayama K, Orimo
T, Shimada S, Nagatsu A, Kamachi H, Yokoo H, Fukai M, Kobayashi N,
et al: Role of Wnt5a in suppressing invasiveness of hepatocellular
carcinoma via epithelial-mesenchymal transition. Oncol Lett.
20:2682020. View Article : Google Scholar : PubMed/NCBI
|
|
86
|
Yang J, Cusimano A, Monga JK, Preziosi ME,
Pullara F, Calero G, Lang R, Yamaguchi TP, Nejak-Bowen KN and Monga
SP: WNT5A inhibits hepatocyte proliferation and concludes β-catenin
signaling in liver regeneration. Am J Pathol. 185:2194–2205. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
87
|
Yu J, Xie Y, Li M, Zhou F, Zhong Z, Liu Y,
Wang F and Qi J: Association between SFRP promoter hypermethylation
and different types of cancer: A systematic review and
meta-analysis. Oncol Lett. 18:3481–3492. 2019.PubMed/NCBI
|
|
88
|
Zhou HR, Fu HY, Wu DS, Zhang YY, Huang SH,
Chen CJ, Yan JG, Huang JL and Shen JZ: Relationship between
epigenetic changes in Wnt antagonists and acute leukemia. Oncol
Rep. 37:2663–2671. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
89
|
Li J, Ying J, Fan Y, Wu L, Ying Y, Chan
ATC, Srivastava G and Tao Q: WNT5A antagonizes WNT/β-catenin
signaling and is frequently silenced by promoter CpG methylation in
esophageal squamous cell carcinoma. Cancer Biol Ther. 10:617–624.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
90
|
Wong AMG, Kong KL, Chen L, Liu M, Wong
AMG, Zhu C, Tsang JWH and Guan XY: Characterization of CACNA2D3 as
a putative tumor suppressor gene in the development and progression
of nasopharyngeal carcinoma. Int J Cancer. 133:2284–2295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
91
|
Choi J, Hwang J, Ramalingam M, Jeong HS
and Jang S: Effects of HDAC inhibitors on neuroblastoma SH-SY5Y
cell differentiation into mature neurons via the Wnt signaling
pathway. BMC Neurosci. 24:282023. View Article : Google Scholar : PubMed/NCBI
|
|
92
|
Choi J, Gang S, Ramalingam M, Hwang J,
Jeong H, Yoo J, Cho HH, Kim BC, Jang G, Jeong HS and Jang S:
BML-281 promotes neuronal differentiation by modulating
Wnt/Ca2+ and Wnt/PCP signaling pathway. Mol Cell
Biochem. 479:2391–2403. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
93
|
Makena MR, Ko M, Dang DK and Rao R:
Epigenetic modulation of SPCA2 reverses epithelial to mesenchymal
transition in breast cancer cells. Cancers (Basel). 13:2592021.
View Article : Google Scholar : PubMed/NCBI
|
|
94
|
Derissen EJB, Beijnen JH and Schellens
JHM: Concise drug review: Azacitidine and decitabine. Oncologist.
18:619–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
95
|
Bondarev AD, Attwood MM, Jonsson J,
Chubarev VN, Tarasov VV and Schiöth HB: Recent developments of HDAC
inhibitors: Emerging indications and novel molecules. Br J Clin
Pharmacol. 87:4577–4597. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
96
|
Shi MQ, Xu Y, Fu X, Pan DS, Lu XP, Xiao Y
and Jiang YZ: Advances in targeting histone deacetylase for
treatment of solid tumors. J Hematol Oncol. 17:372024. View Article : Google Scholar : PubMed/NCBI
|
|
97
|
Flanagan DJ, Woodcock SA, Phillips C,
Eagle C and Sansom OJ: Targeting ligand-dependent wnt pathway
dysregulation in gastrointestinal cancers through porcupine
inhibition. Pharmacol Ther. 238:1081792022. View Article : Google Scholar : PubMed/NCBI
|
|
98
|
Shah K, Panchal S and Patel B: Porcupine
inhibitors: Novel and emerging anti-cancer therapeutics targeting
the Wnt signaling pathway. Pharmacol Res. 167:1055322021.
View Article : Google Scholar : PubMed/NCBI
|
|
99
|
Säfholm A, Leandersson K, Dejmek J,
Nielsen CK, Villoutreix BO and Andersson T: A formylated
hexapeptide ligand mimics the ability of Wnt-5a to impair migration
of human breast epithelial cells. J Biol Chem. 281:2740–2749. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
100
|
Prasad CP, Manchanda M, Mohapatra P and
Andersson T: WNT5A as a therapeutic target in breast cancer. Cancer
Metastasis Rev. 37:767–778. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
101
|
Mohapatra P, Prasad CP and Andersson T:
Combination therapy targeting the elevated interleukin-6 level
reduces invasive migration of BRAF inhibitor-resistant melanoma
cells. Mol Oncol. 13:480–494. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
102
|
Gurney A, Axelrod F, Bond CJ, Cain J,
Chartier C, Donigan L, Fischer M, Chaudhari A, Ji M, Kapoun AM, et
al: Wnt pathway inhibition via the targeting of Frizzled receptors
results in decreased growth and tumorigenicity of human tumors.
Proc Natl Acad Sci USA. 109:11717–11722. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
103
|
Davis SL, Cardin DB, Shahda S, Lenz HJ,
Dotan E, O'Neil BH, Kapoun AM, Stagg RJ, Berlin J, Messersmith WA
and Cohen SJ: A phase 1b dose escalation study of Wnt pathway
inhibitor vantictumab in combination with nab-paclitaxel and
gemcitabine in patients with previously untreated metastatic
pancreatic cancer. Invest New Drugs. 38:821–830. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
104
|
Diamond JR, Becerra C, Richards D, Mita A,
Osborne C, O'Shaughnessy J, Zhang C, Henner R, Kapoun AM, Xu L, et
al: Phase Ib clinical trial of the anti-frizzled antibody
vantictumab (OMP-18R5) plus paclitaxel in patients with locally
advanced or metastatic HER2-negative breast cancer. Breast Cancer
Res Treat. 184:53–62. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
105
|
Vlashi R, Zhang X, Wu M and Chen G: Wnt
signaling: Essential roles in osteoblast differentiation, bone
metabolism and therapeutic implications for bone and skeletal
disorders. Genes Dis. 10:1291–1317. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
106
|
Wang R, Yang S, Wang M, Zhou Y, Li X, Chen
W, Liu W, Huang Y, Wu J, Cao J, et al: A sustainable approach to
universal metabolic cancer diagnosis. Nat Sustain. 7:602–615. 2024.
View Article : Google Scholar
|
|
107
|
Xie F, Tang S, Zhang Y, Zhao Y, Lin Y, Yao
Y, Wang M, Gu Z and Wan J: Designing peptide-based nanoinhibitors
of programmed cell death ligand 1 (PD-L1) for enhanced
chemo-immunotherapy. ACS Nano. 18:1690–1701. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
108
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
109
|
Parsons MJ, Tammela T and Dow LE: WNT as a
driver and dependency in cancer. Cancer Discov. 11:2413–2429. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
110
|
Song P, Gao Z, Bao Y, Chen L, Huang Y, Liu
Y, Dong Q and Wei X: Wnt/β-catenin signaling pathway in
carcinogenesis and cancer therapy. J Hematol Oncol. 17:462024.
View Article : Google Scholar : PubMed/NCBI
|
|
111
|
Xue W, Yang L, Chen C, Ashrafizadeh M,
Tian Y and Sun R: Wnt/β-catenin-driven EMT regulation in human
cancers. Cell Mol Life Sci. 81:792024. View Article : Google Scholar : PubMed/NCBI
|
|
112
|
Shiah SG, Shieh YS and Chang JY: The Role
of Wnt signaling in squamous cell carcinoma. J Dent Res.
95:129–134. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
113
|
Wang HG, Pathan N, Ethell IM, Krajewski S,
Yamaguchi Y, Shibasaki F, McKeon F, Bobo T, Franke TF and Reed JC:
Ca2+-induced apoptosis through calcineurin
dephosphorylation of BAD. Science. 284:339–343. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
114
|
Nakagawa T and Yuan J: Cross-talk between
two cysteine protease families. Activation of caspase-12 by calpain
in apoptosis. J Cell Biol. 150:887–894. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
115
|
Qiao X, Niu X, Shi J, Chen L, Wang X, Liu
J, Zhu L and Zhong M: Wnt5a regulates ameloblastoma cell migration
by modulating mitochondrial and cytoskeletal dynamics. J Cancer.
11:5490–5502. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
116
|
Katoh M and Katoh M: WNT signaling and
cancer stemness. Essays Biochem. 66:319–331. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
117
|
Yuan Y, Wu D, Hou Y, Zhang Y, Tan C, Nie
X, Zhao Z and Hou J: Wnt signaling: Modulating tumor-associated
macrophages and related immunotherapeutic insights. Biochem
Pharmacol. 223:1161542024. View Article : Google Scholar : PubMed/NCBI
|
|
118
|
Deng G, Li ZQ, Zhao C, Yuan Y, Niu CC,
Zhao C, Pan J and Si WK: WNT5A expression is regulated by the
status of its promoter methylation in leukaemia and can inhibit
leukemic cell malignant proliferation. Oncol Rep. 25:367–376.
2011.PubMed/NCBI
|
|
119
|
Gajos-Michniewicz A and Czyz M: WNT
signaling in melanoma. Int J Mol Sci. 21:48522020. View Article : Google Scholar : PubMed/NCBI
|
|
120
|
Sharma A, Mir R and Galande S: Epigenetic
regulation of the Wnt/β-catenin signaling pathway in cancer. Front
Genet. 12:6810532021. View Article : Google Scholar : PubMed/NCBI
|
|
121
|
Tufail M, Jiang CH and Li N: Wnt signaling
in cancer: From biomarkers to targeted therapies and clinical
translation. Mol Cancer. 24:1072025. View Article : Google Scholar : PubMed/NCBI
|
|
122
|
Grumolato L, Liu G, Mong P, Mudbhary R,
Biswas R, Arroyave R, Vijayakumar S, Economides AN and Aaronson SA:
Canonical and noncanonical Wnts use a common mechanism to activate
completely unrelated coreceptors. Genes Dev. 24:2517–2530. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
123
|
Mikels AJ and Nusse R: Purified Wnt5a
protein activates or inhibits beta-catenin-TCF signaling depending
on receptor context. PLoS Biol. 4:e1152006. View Article : Google Scholar : PubMed/NCBI
|
|
124
|
Xue G, Romano E, Massi D and Mandalà M:
Wnt/β-catenin signaling in melanoma: Preclinical rationale and
novel therapeutic insights. Cancer Treat Rev. 49:1–12. 2016.
View Article : Google Scholar : PubMed/NCBI
|















