Introduction
Lung cancer is recognized as one of the most
thrombogenic cancer types, wherein venous thromboembolism (VTE)
occurs in up to 14% of patients (1). The risk of VTE is particularly high in
patients with advanced stages of lung cancer, such as those with
adenocarcinoma, which has been associated with a higher incidence
of thrombotic events compared with other histological types of lung
cancer, such as squamous cell carcinoma or small cell lung cancer
(1). The mechanisms underlying the
increased risk of thrombogenesis include immobilization, the
presence of central venous catheters and the direct activation of
the coagulation system by cancer cells, which release procoagulant
factors that stimulate platelet activation and coagulation
pathways. Following vascular endothelial disruption, factor XII
initiates the intrinsic coagulation pathway through contact
activation with subendothelial collagen, thereby mediating
hemostasis in deep tissue injuries. Concurrently, tissue factor
(TF) exposure upon vascular rupture triggers the extrinsic pathway,
enabling rapid thrombin generation via TF-factor VIIa complex
formation. These two pathways synergistically regulate hemostasis
and thrombosis through distinct yet complementary mechanism
(2,3). Chemotherapy, a common treatment for
lung cancer, further exacerbates the risk of thrombosis.
Chemotherapy agents, such as cisplatin, carboplatin, gemcitabine
and paclitaxel, have been demonstrated to elevate procoagulant
activity in endothelial cells and other blood components such as
fibrinogen, antithrombin III and tissue factor pathway inhibitor.
The increase in procoagulant activity is primarily mediated through
mechanisms that involve TF expression and disulfide bond formation,
which contributes to a hypercoagulable state in patients with lung
cancer (4). Furthermore, the
presence of central venous catheters, which are often used for
chemotherapy administration, further increases the risk of
thrombosis. A previous study indicated that patients with lung
cancer who had peripherally inserted central catheters experienced
a high incidence of upper extremity venous thrombosis, particularly
when treated with certain chemotherapeutic agents such as etoposide
(5). The use of antiangiogenic
agents, including bevacizumab, sorafenib, apatinib and
amilorotinib, and immunotherapy has also been linked to an
increased risk of thromboembolic events (2,3). In a
previous study, which included 605 patients diagnosed with
non-small cell lung cancer (NSCLC), the presence of EGFR mutations
was associated with varying risks of VTE. Specifically, patients
with EGFR wild-type status had a higher risk of developing VTE
compared with patients with EGFR mutations. The probability of
developing VTE was 8.3% in patients with EGFR mutations after 1
year, compared with 13.2% in patients without EGFR mutations. After
2 years, the probabilities of developing VTE differed significantly
(9.7 and 15.5%, respectively; P=0.047) (6). Furthermore, another study highlighted
that the mutation of EGFR was associated with a decreased risk of
VTE. In a cohort of 310 patients with lung adenocarcinoma, patients
with EGFR mutations had a hazard ratio (HR) of 0.46 for VTE, which
suggested a protective effect against thrombosis compared with
patients with wild-type EGFR with lung adenocarcinoma (7). This finding indicated that the
presence of the EGFR-L858R mutation may not only be a driver of
tumorigenesis but could also serve a role in modulating the risk of
thrombotic events. Splenic infarction, a rare but clinically
significant thromboembolic event, arises from the occlusion of the
splenic artery or the splenic artery branches by emboli or thrombi.
With an incidence of 0.016% in hospital admissions, VTE
predominantly results from cardiogenic embolism (62.5%), most
notably atrial fibrillation (1).
Secondary etiologies include autoimmune disorders (12.5%),
infections (12.5%) and hematologic malignancies (6%) (8). Clinically, sudden-onset left upper
quadrant abdominal pain (84% of cases) constitutes the classic
presentation, although asymptomatic cases (≤16%) and atypical
manifestations such as fever and nausea may occur (9). Diagnostic delays are common due to
nonspecific symptoms. The prognosis remains favorable with timely
intervention (10,11). The median hospitalization duration
is 6.5 days, and anticoagulation therapy is initiated in most cases
to mitigate thromboembolic risks and promote recovery (12).
The clinical implications of multiple-site
thrombosis in patients with lung cancer are notable (13,14).
Patients with VTE often experience longer hospital stays, higher
rates of inpatient mortality and increased healthcare costs
compared with patients without VTE. Furthermore, the presence of
thrombosis can complicate cancer treatment, as anticoagulation
therapy may increase the risk of bleeding, particularly in patients
undergoing chemotherapy or surgery (15). The present case illustrated the
complex thrombotic evolution in EGFR-driven NSCLC, which emphasizes
the need for precision anticoagulation strategies.
Case report
Case summary
A 37-year-old man was admitted to the First People's
Hospital of Suining (Suining, China) in December 2024, due to ‘pain
and discomfort in the left upper abdomen for >3 days’.
Furthermore, the patient reported persistent chest tightness
accompanied by myocardial discomfort over a 2-week duration.
Physical examination revealed a soft abdomen, fullness in the left
intercostal space, pronounced tenderness in the left upper quadrant
and palpable enlargement of the spleen, accompanied by significant
rebound tenderness in the spleen area. In November 2024, the
patient developed chest pain, exertional dyspnea and fatigue. An
enhanced CT of the abdomen indicated a filling defect and
low-density shadow in the pancreatic body segment of the splenic
artery, with portions of the splenic parenchyma demonstrating no
enhancement, which raised suspicion for splenic artery embolism and
splenic infarction. Upon admission, empiric antimicrobial
prophylaxis with intravenous cefuroxime (4.5 g/day; treatment
duration, 1 week) was initiated to mitigate infectious
complications of splenic infarction. Simultaneous therapeutic
anticoagulation with subcutaneous enoxaparin sodium (8,000 IU every
12 h; treatment duration, 3 weeks) targeted thromboembolic
pathophysiology. The patient was discharged from hospital in
December 2024 and followed up until May 2025, and the splenic
infarction in the patient had not progressed and had gradually
healed spontaneously.
Past medical history
The patient was admitted to the First People's
Hospital of Suining in October 2024 due to pleural effusion. Lung
adenocarcinoma cells were identified in the exfoliated cells from
the pleural effusion, which led to a diagnosis of a malignant tumor
in the upper lobe of the left lung, classified as adenocarcinoma
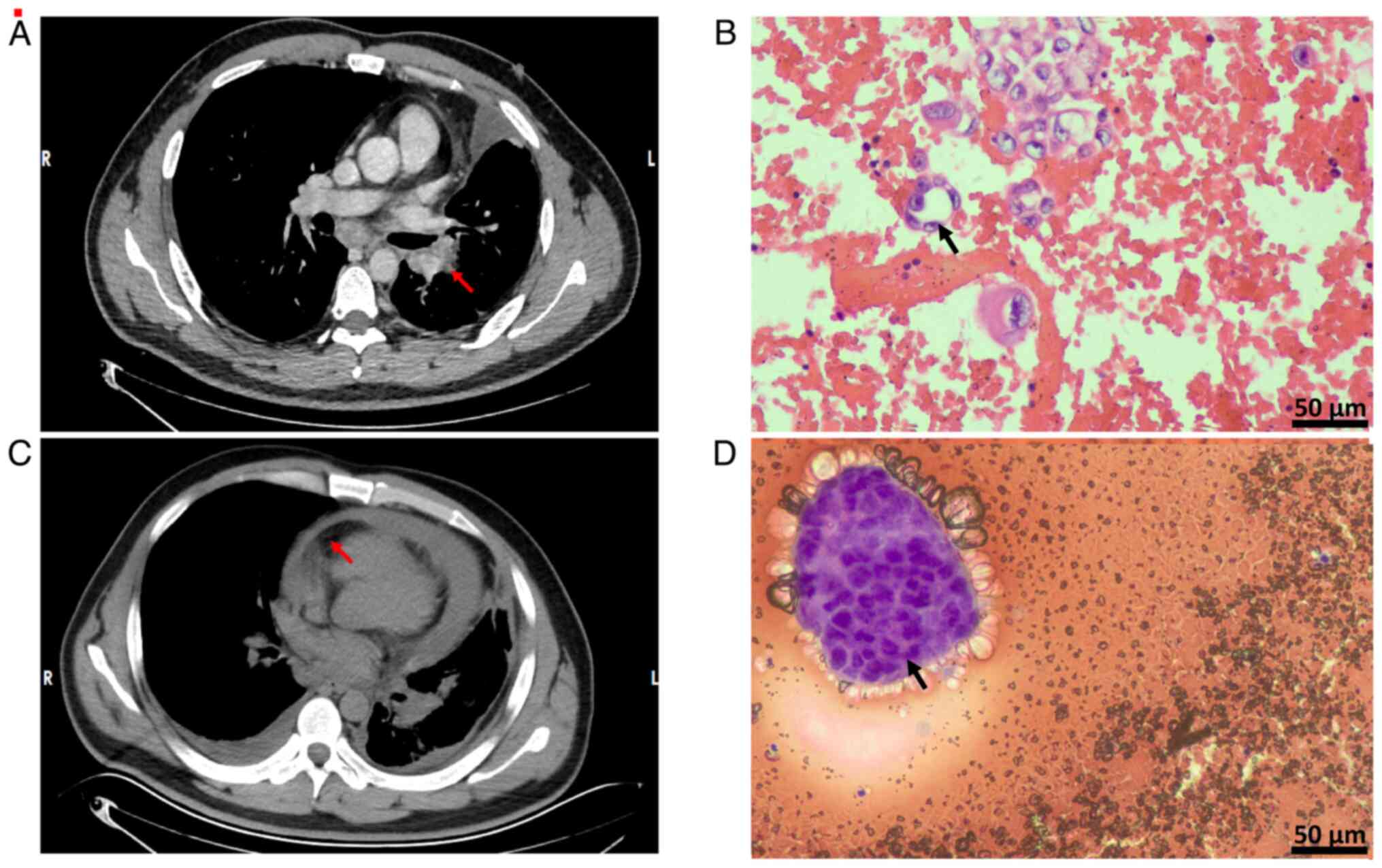
T3N2M1a at IVA stage (16)
(Fig. 1A and B). On admission,
according to the Khorana scoring scale (17), the VTE score of the patient was
classified as low-risk. Additionally, malignant pericardial
effusion (MPE) was diagnosed at the First People's Hospital of
Suining in October 2024 (Fig. 1C and
D), which prompted the performance of pericardiocentesis and
catheter drainage. A week before presentation in December 2024,
color ultrasound examinations of the lower extremity veins and the
internal jugular vein demonstrated thrombosis in the popliteal
vein, posterior tibial vein, peroneal vein and right internal
jugular vein. Following discharge, the patient was prescribed
rivaroxaban at a dose of 10 mg twice daily for anticoagulation for
3 months, with dose and frequency adjusted based on lower extremity
venous ultrasound review.
Genetic findings
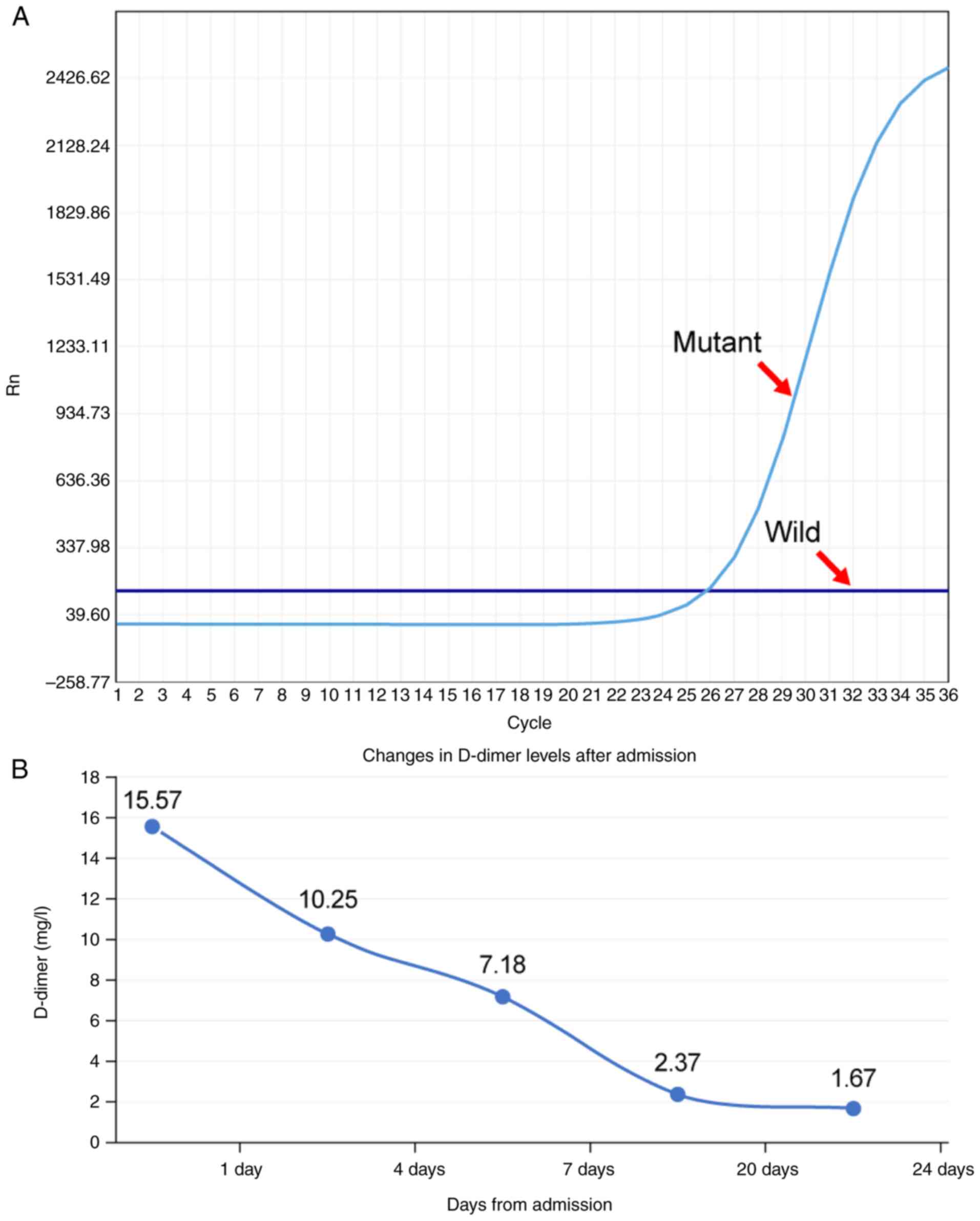
The amplification refractory mutation
system-polymerase chain reaction analysis (experiments were carried
out by the Pathology Laboratory of Suining Central Hospital,
Suining, China; data were obtained from medical records) confirmed
a heterozygous activating mutation at exon 21 of the EGFR gene
(c.2573T>G, p.Leu858Arg), which corresponded to the canonical
L858R substitution (Fig. 2A). The
target EGFR gene primer sequences were as follows: Forward,
5′-GCCAGGAACGTACTGGTGAA-3′and reverse,
5′-CCTTCTGCATGGTATTCTTTCTCTTC-3′.
Laboratory test results
Laboratory test results of routine blood tests on
admission are shown in Table I.
 | Table I.Laboratory test findings on admission
of the patient. |
Table I.
Laboratory test findings on admission
of the patient.
| Blood
parameters | Value | Normal range |
|---|
| WBC,
×109/l | 4.79 | 4.0–10.0 |
| Neutrophil, % | 63.8 | 50-70 |
| Lymphocyte, % | 19.7 | 20-40 |
| Monocyte, % | 12.9 | 3-8 |
| Eosinophil, % | 3.5 | 1-4 |
| Basophil, % | 0.1 | 0.5–1 |
| RBC,
×1012/l | 4.36 | 3.5–5.5 |
| HGB, g/l | 134 | 120-160 |
| PLT,
×109/l | 168 | 100-300 |
| TG, mmol/l | 1.85 | 0.56–1.7 |
| TC, mmol/l | 5.24 | <5.2 |
| Na, mmol/l | 138.2 | 135-145 |
| K, mmol/l | 4.36 | 3.5–5.0 |
| CL, mmol/l | 103.4 | 96-108 |
| TP, g/l | 73.6 | 60-80 |
| ALB, g/l | 42.1 | 35-55 |
| AKP, U/l | 134 | 45-125 |
| AST, U/l | 24 | 10-40 |
| ALT, U/l | 37 | 10-40 |
| CK-MB, ng/ml | 2.2 | <5 |
| BNP, pg/ml | 82 | <100 |
| TNT-HS, ng/ml | 0.007 | <0.1 |
| D-dimer, mg/l | 15.57 | <0.5 |
| Fibrinogen,
g/l | 6.01 | 2-4 |
| PT, sec | 17.9 | 11-14 |
| APTT, sec | 47.4 | 25-37 |
| FDP, µg/ml | 74.73 | <5 |
| R, min | 3.5 | 5-10 |
| K, min | 2.2 | 1-3 |
| MA, mm | 44.5 | 50-70 |
| CI | −0.7 | −3-+3 |
| EPL, % | 0 | <15 |
| Solidification
angle, ° | 63.6 | 53-72 |
| pCO2,
mmHg | 34 | 35-45 |
| pO2,
mmHg | 99 | 80-100 |
| PROGRP, pg/ml | 29.7 | <63 |
| NSE, ng/ml | 15.9 | <16.3 |
| CYFRA21-1,
ng/ml | 12.1 | <3.3 |
| mSCC, ng/ml | 1.14 | <5 |
| CEA, ng/ml | 1.23 | <1.5 |
| PT-INR | 1.1 | 0.8–1.2 |
| PA, g/l | 3.2 | 1.5–2.5 |
Changes in D-dimer levels
Numerous studies have established that D-dimer
levels serve as an independent risk predictor for VTE in various
cancer types such as kidney, breast, rectal and ovarian cancer
(18–20). In the present study, the D-dimer
levels of the patient were dynamically monitored by detecting the
D-dimer levels in the serum. When the patient presented with
multiple thrombosis sites, the D-dimer levels (15.57 mg/l) were
increased compared with the normal range (<0.5 mg/l) (Fig. 2B). Following anticoagulation
treatment, the D-dimer levels gradually returned to normal levels
(<0.5 mg/l).
Imaging findings
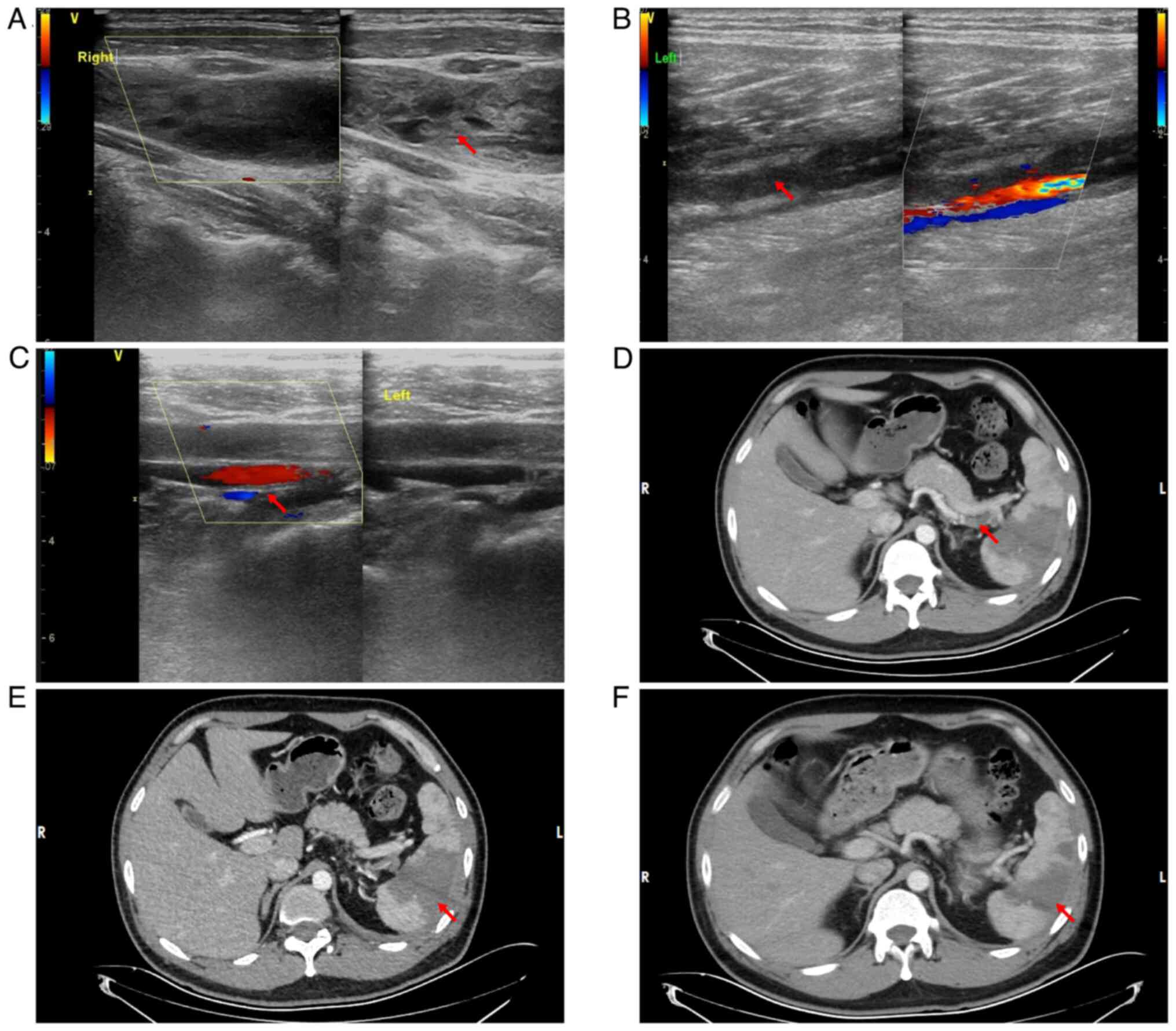
Complementary vascular ultrasonography (December
2023) demonstrated extensive thromboembolic disease: Acute
thrombosis included the distal left superficial femoral vein,
popliteal vein, right internal jugular vein and bilateral posterior
tibial/peroneal veins, with venostasis evident in the remaining
lower extremity vasculature. Cervical venous evaluation identified
aneurysmal dilatation of the right internal jugular vein containing
intraluminal thrombus (Fig. 3A-C).
Contrast-enhanced abdominal CT demonstrated a filling defect and
hypodense intraluminal shadow within the splenic artery distal to
the pancreatic body segment (Fig.
3D). Concomitant absence of parenchymal enhancement in a
portion of the spleen confirmed the diagnosis of splenic artery
embolism with resultant splenic infarction (Fig. 3E).
VTE score
The patient received anticoagulant treatment prior
to admission. According to the ‘Guidelines for the Quality
Evaluation and Management of VTE Prevention and Treatment in
Hospitals (2022 Edition)’ (17),
the patient did not require a VTE score assessment.
Pathological examination
Cytopathological analysis of left pleural
effusion
Malignant tumor cells were identified via left
bloody pleural effusion analysis.
H&E staining and
immunohistochemistry
Histological analysis was performed on tissue
sections fixed in 10% neutral buffered formalin at room temperature
for 24 h. The tissue was processed, embedded in paraffin and
sectioned at a thickness of 5 µm. Sections were stained with
hematoxylin and eosin for 5 min at room temperature, following
standard protocols. The slides were examined using a light
microscope (Leica DM3000; Leica Microsystems GmbH) at a
magnification of ×400. After H&E staining, the following
results were observed: Tumor cells were found in the pleural fluid,
with an enlarged nucleoplasm ratio, deviated nuclei, pronounced
nucleoli and sparse vacuolated cytoplasm (Fig. 1B). Immunohistochemistry (data were
obtained from medical records and are not shown) showed the
following: Transcriptional intermediary factor 1 (+); NapsinA (+);
P40 (−); cytokeratin 5/6 (−); cytokeratin (+); calretinin
mesothelium (+); desmin mesothelium (+); Wilms' tumor protein 1
(−); and Ki67-positive rate, ~10%.
Pericardial effusion analysis
Adenocarcinoma was identified via pericardial
effusion analysis.
H&E staining and
immunohistochemistry
Histological analysis was performed on tissue
sections fixed in 10% neutral buffered formalin at room temperature
for 24 h. The tissue was processed, embedded in paraffin and
sectioned at a thickness of 5 µm. Sections were stained with
hematoxylin and eosin for 5 min at room temperature, following
standard protocols. The slides were examined using a light
microscope (Leica DM3000; Leica Microsystems GmbH) at a
magnification of ×400. After H&E staining, the following
results were observed: Tumor cells were found in the pericardial
effusion cell block, the tumor cells were arranged in an
adenoidal/papillary pattern with deeply stained, enlarged nuclei
with prominent nucleoli and cytoplasm containing mucus vacuoles
(Fig. 1D). Immunohistochemistry
(data were obtained from medical records and are not shown)
revealed the following: Cytokeratin 7 (+); thyroid transcription
factor 1 (weak +); NapsinA (+); p40 (−); and Wilms' tumor protein
(−).
Treatment method
Following the diagnosis of a malignant tumor in the
upper lobe of the left lung (adenocarcinoma; T3N2M1a and IVA
stage), the patient received regular chemotherapy at the First
People's Hospital of Suining. The chemotherapy regimen consisted of
carboplatin (0.7 g) and pemetrexed (1.0 g), which were administered
intravenously (21 days for one cycle, and 6 cycles of continuous
chemotherapy). Concurrently, based on the genetic test results
(Fig. 2A) of the patient, targeted
therapy was initiated with the third-generation tyrosine kinase
inhibitor (TKI) osimertinib (80 mg; oral once a day; long-term
maintenance therapy). Subsequently, after the patient was diagnosed
with thrombosis affecting the popliteal vein, posterior tibial
vein, peroneal vein and right intravenous vein, the patient was
prescribed rivaroxaban (10 mg twice daily; duration, 3 weeks) for
anticoagulation. Upon diagnosis of splenic artery thrombosis with
infarction, a dual therapeutic strategy was implemented: i)
Anti-infective prophylaxis, cefuroxime (4.5 g/dose every 8 h;
duration, 1 week), was empirically administered intravenously,
which targeted the encapsulated organisms [the encapsulated
microorganisms in splenic abscesses after splenic infarction are
predominantly gram-positive (staphylococci and streptococci) and
anaerobic (anaplastic bacilli and clostridia), with gram-negative
(salmonella and Escherichia coli) and fungal
(Candida) bacteria being the next most common (21)] associated with post-infarction
splenic abscess formation; and ii) weight-adjusted therapeutic
enoxaparin (8,000 IU subcutaneously every 12 h; duration, 3 months)
was initiated for anticoagulation to prevent thrombus propagation,
following International Society on Thrombosis and Hemostasis
guidelines for splanchnic vein thrombosis (22). After discharge, the patient
continued with rivaroxaban (15 mg twice daily) to maintain
anticoagulation, and based on the condition of the patient,
permanent maintenance anticoagulation was chosen.
Post-treatment results
The left lower abdominal pain in the patient was
markedly reduced, the splenic infarction did not progress further
and there was no secondary splenic abscess.
Abdominal CT
Contrast-enhanced abdominal imaging demonstrated
persistent non-enhancement of splenic parenchyma consistent with
established infarction. Comparative volumetric analysis indicated
interval reduction (23.4%) in the infarcted volume compared with
the baseline (December 2024; Fig. 3D
and E), which suggested partial splenic reperfusion (Fig. 3F).
Ultrasound of neck veins
Color Doppler ultrasonography identified a
persistent non-occlusive thrombus within the right internal jugular
vein (data not shown), unchanged from prior examinations (Fig. 3A).
Lower extremity vasculature
Color Doppler ultrasonography identified residual
thrombosis in the left popliteal vein and segmental branches of the
posterior tibial/peroneal veins (data not shown), indicative of
chronic thromboembolic remodeling.
Follow-up results
Following discharge, the patient remained
asymptomatic with complete resolution of abdominal pain. Serial
monitoring demonstrated progressive regression of multi-territory
thromboembolic events, with no clinical or radiographic evidence of
new thrombus formation. The last follow-up was in May 2025, and the
follow-up was conducted every 2 weeks in the form of outpatient
follow-up, and changes in the condition of the patient were
clarified by reviewing the CT of the chest and ultrasound of the
veins of the lower limbs and neck at 1-month intervals.
Discussion
Patients with lung cancer are particularly
susceptible to multi-site thrombosis due to a combination of
factors that create a hypercoagulable state (23–25).
Multi-site thrombosis is characterized by an increased tendency for
blood clot formation, which can result in thromboembolic events in
both the venous and arterial systems. A primary reason for this
heightened risk is the presence of procoagulant factors associated
with malignancy (26,27). Cancer cells, including those from
lung cancer, can express various procoagulant substances such as
TF, cancer procoagulant and heparanase. These factors serve a key
role in the activation of the coagulation cascade, which leads to
increased thrombin generation and subsequent clot formation
(3,28). Additionally, tumor cells can secrete
inflammatory cytokines, such as tumor necrosis factor-α and
interleukin-1β, which further stimulate the coagulation process and
promote thrombosis. The presence of a tumor can disrupt normal
blood flow, which leads to stasis, while chemotherapy and other
cancer treatments may cause damage to the vascular endothelium and
thereby increase the risk of clot formation. The hypercoagulable
state observed in patients with cancer is also influenced by the
inflammatory response associated with malignancy. Inflammation can
lead to alterations in the balance between procoagulant and
anticoagulant factors, which shifts the equilibrium towards
increased clotting (29–31). This phenomenon is particularly
relevant in lung cancer, where the inflammatory environment created
by the tumor can heighten the risk of thromboembolic events
(3,32). Additionally, elderly patients with
lung cancer may have comorbid conditions, such as chronic
obstructive pulmonary disease or cardiovascular disease, which can
further elevate the risk of thrombosis (32,33).
The interplay of these factors leads to a markedly increased
incidence of VTE among patients with lung cancer, where previous
studies (24,27) have shown that patients with lung
cancer are four to seven times more likely to develop VTE than the
general population (34). In
summary, the elevated risk of multi-site thrombosis in patients
with lung cancer is multifactorial and arises from the direct
effects of tumor biology, the influence of cancer treatments and
the presence of comorbid conditions. According to the Khorana
scoring scale (17), which assesses
the risk of VTE in patients with cancer, the current patient was
classified as low risk on admission. The current case highlighted
limitations of the Khorana risk assessment model in the prediction
of VTE among patients with cancer. Despite being classified as low
risk using this validated scoring system, the patient developed
multi-site thrombosis, which highlighted the insufficient
sensitivity of the model in certain clinical scenarios. This
finding emphasizes the urgent need for the development of a novel
VTE risk stratification tool that incorporates dynamic variables,
including tumor histology (such as the association between lung
cancer and hypercoagulability), treatment heterogeneity and
biomarkers that reflect individual thrombotic potential. Further
research is warranted to enhance predictive accuracy for high-risk
populations such as patients with lung cancer, who exhibit high
thrombotic event rates. Vigilant thromboprophylaxis monitoring is
advised for patients with cancer, including patients stratified as
low-risk, due to the life-threatening consequences of VTE.
The L858R mutation results in the continuous
activation of EGFR, which in turn activates several downstream
signaling pathways. These include the Ras/Raf/MEK/ERK and
PI3K/AKT/mTOR pathways (35,36).
The Ras pathway is particularly important as it regulates cell
proliferation, survival and migration, which are key processes in
cancer progression and metastasis (35,36).
The activation of these pathways can lead to increased expression
of pro-coagulant factors, which contribute to a hypercoagulable
state.
Previous studies have suggested that the interaction
between EGFR signaling and coagulation pathways can influence
cancer progression. For example, the activation of EGFR can lead to
the upregulation of ligands that promote TF expression and thereby
enhance the pro-coagulant state in tumors (37,38).
The crosstalk is particularly relevant in the context of KRAS
mutations, where both mutations (KRAS and EGFR) can synergistically
promote tumor growth and thrombosis (39). The presence of the EGFR-L858R
mutation in patients with NSCLC may be associated with an increased
risk of thromboembolic events. This association is particularly
important for clinicians to consider when managing patients with
the EGFR-L858R mutation, as the hypercoagulable state can
complicate treatment and increase the risk of adverse outcomes.
However, a previous study, which included 310 patients diagnosed
with lung adenocarcinoma, reported that patients with EGFR
mutations had a HR of 0.46 (95% CI, 0.23–0.94) for VTE, which
suggests a protective effect against thrombosis compared with
patients with wild-type EGFR. Additionally, treatment with TKIs
further reduced the risk of VTE, with an HR of 0.42 (95% CI,
0.29–0.79) when compared with other treatment strategies that did
not include TKIs. This finding suggested that the presence of the
EGFR mutation was associated with a lower incidence of thrombosis
and the therapeutic approach using TKIs may enhance this protective
effect (7). The current patient was
diagnosed with lung adenocarcinoma in October 2024, following the
identification of lung adenocarcinoma cells in pleural effusion via
immunohistochemistry. The patient exhibited pleural and lymph node
metastases, which indicated advanced lung cancer. Subsequent
genetic testing demonstrated the presence of the EGFR-L858R
mutation, which prompted the initiation of targeted therapy with
the TKI osimertinib. However, in October 2024, chest CT indicated
tumor progression and the emergence of pericardial metastasis,
which led to the conclusion that single-agent targeted therapy had
failed. Consequently, the treatment plan was modified to include a
combination of chemotherapy (carboplatin and pemetrexed) and
targeted therapy (osimertinib). At this juncture, a venous color
Doppler ultrasound of the lower limbs had already detected
thrombosis, and rivaroxaban (10 mg twice daily) was prescribed for
anticoagulation. The anticoagulation strategy proved ineffective,
as novel thrombosis was identified when the patient was admitted to
the First People's Hospital of Suining in November 2024. The role
of the EGFR-L858R mutation in thrombosis among patients with lung
cancer remains unclear. A previous study has suggested that the
EGFR-L858R mutation may reduce the incidence of thrombotic events,
while other studies have indicated an increased incidence
associated with the EGFR-L858R mutation (40). Based on the observations in the
current patient, we hypothesized that the EGFR-L858R mutation may
exert a protective effect in thrombosis among patients with lung
cancer. Notably, the patient did not experience thrombosis prior to
the initiation of treatment with TKI inhibitors; however, following
the commencement of TKI therapy, venous thrombosis developed in the
lower extremities, despite treatment with anticoagulants. This
phenomenon may arise from two potential factors: Firstly, the
condition of the patient progressed during treatment, which led to
pericardial metastasis and notable pericardial effusion. This
prolonged state likely impaired systemic circulation, which
resulted in slowed blood flow and increased coagulability.
Secondly, the treatment approach for the patient was modified
following the development of pericardial effusion. The patient was
transitioned to a regimen that included chemotherapy (carboplatin
and pemetrexed) combined with targeted therapy (osimertinib).
Previous studies have indicated that platinum-based antitumor
agents such as cisplatin, carboplatin, oxaliplatin and lopatin may
elevate the risk of VTE in patients with lung cancer, particularly
with regard to catheter-related thrombosis (41,42).
In summary, the antithrombotic effect of the EGFR-L858R mutation
appears to be counteracted by TKI-induced endothelial dysfunction
and pharmacokinetic disturbances. Based on the observations from
the present case, it is necessary to revise current guidelines
(17) to include the following: i)
EGFR mutation status in VTE risk models; ii) TKI-specific
anticoagulation algorithms; and iii) protocolized cytokine
monitoring during metastasis.
MPE markedly impacts hemodynamics and coagulation
properties in patients with lung cancer. The presence of MPE can
lead to alterations in blood flow dynamics due to the accumulation
of fluid in the pericardial space, which can compress the heart and
impede its ability to pump effectively (43–45).
This condition can result in cardiac tamponade, where the pressure
from the fluid prevents the heart chambers from filling properly,
which leads to reduced cardiac output and compromised blood flow to
vital organs (46). In terms of
coagulation properties, patients with lung cancer and MPE often
exhibit a hypercoagulable state. A hypercoagulable state is
characterized by changes in plasma fibrin clot properties, which
can include increased clot density and altered permeability
(47–49). A single study has reported that
patients with lung cancer undergoing chemotherapy exhibited higher
levels of D-dimer, a marker of fibrin degradation, which indicated
increased fibrinolytic activity (50). Additionally, chemotherapy has been
associated with changes in clot structure, such as increased clot
permeability and prolonged clot lysis time, which can contribute to
thromboembolic complications (51).
The presence of MPE can also influence the activity of TF, which is
a key initiator of the coagulation cascade. In patients with lung
cancer, the activity of microparticle-associated TF can be altered,
which leads to an imbalance between coagulation activation and
fibrinolytic potential. This imbalance may predispose patients to
VTE, which is notably more common in patients with lung cancer
compared with the general population (52). The hypercoagulable state is further
exacerbated by the presence of tumor-derived factors that promote
clot formation and inhibit fibrinolysis, which contributes to the
overall risk of thrombotic events in patients with lung cancer
(53). In November 2024, the
patient developed chest pain, exertional dyspnea and fatigue.
Pericardiocentesis and catheter drainage were performed following
detection of a notable amount of pericardial effusion via chest CT.
Lung adenocarcinoma cells were identified in the pericardial
effusion. The pericardial metastasis of the patient resulted in a
substantial accumulation of pericardial effusion. This process was
gradual and continuous, which markedly impacted the systemic
circulation of the patient, and exacerbated the hypercoagulable
state commonly associated with lung cancer. Consequently, the
process led to thrombosis in multiple locations, particularly
arterial thrombosis, including splenic artery thrombosis. Although
pericardiocentesis and catheter drainage were performed to
alleviate the cardiac tamponade in the patient, the effects on the
circulatory state are unlikely to improve in the short term.
In some cases, patients with lung cancer undergoing
chemotherapy may experience acute complications such as splenic
infarction due to embolic events, which can be precipitated by the
underlying malignancy and the treatment (54). Furthermore, the anatomical and
physiological characteristics of the spleen, along with the
vascular supply, can make it susceptible to thrombotic events. The
spleen is typically well-protected from non-hematological
metastasis and splenic artery thrombosis is a rare occurrence
(21,55). However, when splenic artery
thrombosis does occur, it is often part of a broader pattern of
vascular complications associated with malignancies, including lung
cancer (56). In summary, lung
cancer can cause splenic artery thrombosis primarily through the
induction of a hypercoagulable state, either due to the malignancy
itself or as a consequence of chemotherapy. This can lead to
thrombus formation in the splenic artery, which results in
complications such as splenic infarction. The splenic artery, which
is a singular vessel, is particularly susceptible to
thromboembolism, which can result in splenic infarction (10). The current patient experienced
splenic artery thromboembolism and subsequent splenic infarction, a
clinical occurrence that is extremely rare. Notably, splenic artery
thromboembolism and splenic infarction occurred despite
anticoagulation therapy. To the best of our knowledge, there is a
lack of pertinent research reports on this phenomenon, which
necessitates further research on the underlying causes. The causes
of this phenomenon were analyzed and summarized as follows: i)
Hypercoagulable state: Lung cancer may influence the coagulation
status of patients through various mechanisms, which leads to a
persistent hypercoagulable state; ii) cardiac embolism resulting
from pericardial effusion: The patient had a notable history of
MPE, which severely compromised blood circulation and contributed
to thrombosis formation at multiple sites, including the splenic
artery; and iii) treatment modalities: TKIs and platinum-based
chemotherapy agents can elevate the risk of thrombosis in patients
with lung cancer. Additionally, the use of deep vein
catheterization for chemotherapy may lead to catheter-related
thrombosis, as evidenced by the current patient undergoing internal
jugular vein catheterization for chemotherapy. Subsequent cervical
venous color ultrasound revealed thrombosis in the internal jugular
vein.
Venous thrombosis was identified in the lower
extremity and jugular veins in December 2024 via color ultrasound.
In response, anticoagulation with rivaroxaban at a dosage of 10 mg
twice a day was initiated; however, new thrombotic sites continued
to emerge. Following an adjustment of the anticoagulant dosage to
15 mg rivaroxaban twice a day, marked dissolution and disappearance
of the thrombus were observed, with no further occurrences of
thrombosis. This observation led to the conclusion that the initial
anticoagulant dosage was insufficient. Additionally, as the
anticoagulant dosage was modified, the D-dimer levels of the
patient gradually decreased to normal levels, which indicated a
positive association between D-dimer levels and thrombosis, as well
as a potential predictive value regarding anticoagulant efficacy.
Nonetheless, further research is necessary to substantiate the
findings of the present case report.
It is extremely rare for patients with lung
adenocarcinoma and EGFR-L858R mutations to develop multiple
thromboses that lead to splenic infarction. This phenomenon may be
associated with hypercoagulability, cardiac tamponade and genetic
mutations inherent to patients with lung cancer, exacerbated by
factors such as chemotherapy, targeted therapy and insufficient
anticoagulation dosing. The present case highlights the complex
interplay between oncogenic drivers and hemostatic regulation. The
authors propose three clinical practice modifications: i) Mandatory
thrombophilia screening for patients with EGFR-mutant NSCLC
initiating TKIs; ii) implementation of quantitative anti-factor Xa
monitoring for patients with direct oral anticoagulants-treated
cancer; and iii) development of EGFR mutation-specific VTE risk
assessment tools. Further research is warranted to explore the role
of PI3K inhibitors in mitigating TKI-associated thrombosis while
preserving antitumor efficacy.
The present case underscored the paradoxical
thrombotic risk in patients with EGFR-mutant NSCLC undergoing
targeted therapy, highlighted the insufficiency of the Khorana
score in molecularly defined cohorts, and established visceral
thrombosis screening as a critical component of care in EGFR-mutant
patients with pericardial effusion. It mandates revision of VTE
risk stratification frameworks to incorporate oncogenic driver
status, treatment-specific factors and dynamic biomarkers, while
advocating for intensified anticoagulation protocols in this
high-risk subgroup.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Suining Health Science
and Technology Project (grant no. 24CJDFB38).
Availability of data and materials
The data generated in the present study are included
in the figures and/or tables of this article.
Authors' contributions
YM, MW, HZ and XW conributed to the conception and
design of the study. QW and ZW performed the acquisition, analysis
and interpretation of data. QW and ZW confirm the authenticity of
all the raw data. All authors have read and approved the final
version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written consent was obtained from the patient to
publish the present case report findings and process medical
records.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ruiz-Artacho P, Lecumberri R,
Trujillo-Santos J, Font C, Lopez-Nunez JJ, Peris ML, Pedroche CD,
Lobo JL, Jimenez LL, Reyes RL, et al: Cancer histology and natural
history of patients with lung cancer and venous thromboembolism.
Cancers (Basel). 14:41272022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Hojbjerg JA, Bentsen KK, Vinholt PJ,
Hansen O, Jeppesen SS and Hvas AM: Increased in vivo thrombin
generation in patients with localized non-small cell lung cancer
unfit for surgery. Clin Appl Thromb Hemost.
29:107602962311528972023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lobo FT, Alves R, Judas T and Delerue MF:
Marantic endocarditis and paraneoplastic pulmonary embolism. BMJ
Case Rep. 2017:bcr20172202172017. View Article : Google Scholar
|
|
4
|
Lysov Z, Swystun LL, Kuruvilla S, Arnold A
and Liaw PC: Lung cancer chemotherapy agents increase procoagulant
activity via protein disulfide isomerase-dependent tissue factor
decryption. Blood Coagul Fibrinolysis. 26:36–45. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xu B, Zhang J, Tang S, Hou J and Ma M:
Comparison of two types of catheters through femoral vein
catheterization in patients with lung cancer undergoing
chemotherapy: A retrospective study. J Vasc Access. 19:651–657.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dou F, Li H, Zhu M, Liang L and Zhang Y,
Yi J and Zhang Y: Association between oncogenic status and risk of
venous thromboembolism in patients with non-small cell lung cancer.
Respir Res. 19:882018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Davidsson E, Murgia N, Ortiz-Villalon C,
Wiklundh E, Skold M, Kolbeck KG and Ferrara G: Mutational status
predicts the risk of thromboembolic events in lung adenocarcinoma.
Multidiscip Respir Med. 12:162017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Schattner A, Adi M, Kitroser E and
Klepfish A: Acute splenic infarction at an academic general
hospital over 10 years. Medicine (Baltimore). 94:e13632015.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abdallah A, Kaur V, Mahmoud F and Motwani
P: Image diagnosis: Splenic infarction associated with oral
contraceptive pills in a healthy young woman. Perm J. 21:16–071.
2017. View Article : Google Scholar
|
|
10
|
He YA, Wang DX, Lin LJ and Zheng CQ:
Report of a case of splenic infarction and review of the
literature. J Gastroenterol Hepatol. 29:239–240. 2020.
|
|
11
|
Yin X, He T, Hu H, Sun J and Xu Y: Report
of a case of splenic arteriovenous embolism resulting in necrosis
of the stomach, spleen and pancreatic body tail. Chin J Mod
Operative Surg. 28:434–435. 2024.(In Chinese).
|
|
12
|
Brett AS, Azizzadeh N, Miller EM, Collins
RJ, Seegars MB and Marcus MA: Assessment of clinical conditions
associated with splenic infarction in adult patients. JAMA Intern
Med. 180:1125–1128. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fang Q, Tang M, Chang X, He M, Liu Y and
Yin S: Construction and assessment of a Nomogram model for lung
cancer patients with concurrent deep vein thrombosis. Chinese
Journal of New Clinical Medicine. 17:1019–1025. 2024.(In
Chinese).
|
|
14
|
Square F, Fu XG and Li YB: Deep vein
thrombosis in the lower limbs of elderly lung cancer patients and
its influencing factors. J Pract Cancer. 39:612–614. 2024.
|
|
15
|
Di W, Xu H, Ling C and Xue T: Early
identification of lung cancer patients with venous thromboembolism:
Development and validation of a risk prediction model. Thromb J.
21:952023. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang X and Zhi X: Introduction to the 8th
edition of the TNM Classification of the Internatinoal Association
for the Study of Lung Cancer (IASLC). Chin J Tharac Surg. 3:70–76.
2016.(In Chinese).
|
|
17
|
Writing Expert Group National Expert
Committee on Capacity Building Project for Prevention and Treatment
of Pulmonary Embolism and Deep Vein Thrombosis: Quality Evaluation
and Management Guidelines for Prevention and Treatment of Venous
Thromboembolism in Hospitals (2022 Edition). Natl Med J China.
102:3338–3348. 2022.(In Chinese).
|
|
18
|
Li J, Yan S, Zhang X, Xiang M, Zhang C, Gu
L, Wei X, You C, Chen S, Zeng D and Jiang J: Circulating D-dimers
increase the risk of mortality and venous thromboembolism in
patients with lung cancer: A systematic analysis combined with
external validation. Front Med (Lausanne). 9:8539412022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sanfilippo KM, Fiala MA, Feinberg D,
Tathireddy H, Girard T, Vij R, Di Paola J and Gage BF: D-dimer
predicts venous thromboembolism in multiple myeloma: A nested
case-control study. Res Pract Thromb Haemost. 7:1022352023.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yamanaka Y, Sawai Y and Nomura S:
Platelet-derived microparticles are an important biomarker in
patients with cancer-associated thrombosis. Int J Gen Med.
12:491–497. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lin C, Xu XQ, He SD and Hong T: Clinical
analysis of 21 cases of splenic infarction. J Chin Acad Med Sci.
36:321–323. 2014.
|
|
22
|
Levy JH, Shaw JR, Castellucci LA, Connors
JM, Douketis J, Lindhoff-Last E, Rocca B, Samama CM, Siegal D and
Weitz JI: Reversal of direct oral anticoagulants: Guidance from the
SSC of the ISTH. J Thromb Haemost. 22:2889–2899. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cao L, Duan Z, Zhang T, Yuan R and Wang G:
Progress of Study on Malignant Tumors and Thrombogenesis. Progress
in Modern Biomedicine. 16:2187–2190. 2016.(In Chinese).
|
|
24
|
Wei R: Risk factors and treatment analysis
of combined venous thromboembolism in lung cancer chemotherapy
patients. J Clin Med. 4:15085–15086. 2017.(In Chinese).
|
|
25
|
Khorana AA, Mackman N, Falanga A, Pabinger
I, Noble S, Ageno W, Moik F and Lee AYY: Cancer-associated venous
thromboembolism. Nat Rev Dis Primers. 8:112022. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Cheng Y, Cai X and Liu JW: Malignant
tumours and thrombosis. J Clin Oncol. 15:376–379. 2010.
|
|
27
|
Wang D, Xiong W and Han F: Advances in the
pathogenesis of venous thromboembolism associated with lung cancer.
Chinese Journal of Respiratory and Critical Care. 22:135–141.
2023.(In Chinese).
|
|
28
|
Zhang M, Wu S and Hu C: Do lung cancer
patients require routine anticoagulation treatment? A
meta-analysis. J Int Med Res. 48:3000605198969192020. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Syrigos K, Grapsa D, Sangare R,
Evmorfiadis I, Larsen AK, Van Dreden P, Boura P, Charpidou A,
Kotteas E, Sergentanis TN, et al: Prospective assessment of
clinical risk factors and biomarkers of hypercoagulability for the
identification of patients with lung adenocarcinoma at risk for
cancer-associated thrombosis: The observational ROADMAP-CAT study.
Oncologist. 23:1372–1381. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tang L, Liu XQ, Chen J, Bai Y, Li J, Zhang
YD and Liu QX: Reducing the incidence of postoperative venous
thromboembolism in patients with lung cancer. China Health Quality
Management. 28:66–70. 2021.(In Chinese).
|
|
31
|
Hao EE and Jia Q: Correlation between
coagulation function and tumour metastatic recurrence in non-small
cell lung cancer patients. Thromb Haemost. 28:967–969. 2022.
|
|
32
|
Cukic V and Ustamujic A: Lung cancer and
pulmonary thromboembolism. Mater Sociomed. 27:351–353. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takemoto T, Soh J, Ohara S, Fujino T, Koga
T, Nishino M, Hamada A, Chiba M, Shimoji M, Suda K, et al: The
prevalence and risk factors associated with preoperative deep
venous thrombosis in lung cancer surgery. Surg Today. 51:1480–1487.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Meikle CK, Meisler AJ, Bird CM, Jeffries
JA, Azeem N, Garg P, Crawford EL, Kelly CA, Gao TZ, Wuescher LM, et
al: Platelet-T cell aggregates in lung cancer patients:
Implications for thrombosis. PLoS One. 15:e02369662020. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Minnelli C, Cianfruglia L, Laudadio E,
Mobbili G, Galeazzi R and Armeni T: Effect of
epigallocatechin-3-Gallate on EGFR signaling and migration in
non-small cell lung cancer. Int J Mol Sci. 22:118332021. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Saryeddine L, Zibara K, Kassem N, Badran B
and El-Zein N: EGF-induced VEGF exerts a PI3K-dependent positive
feedback on ERK and AKT through VEGFR2 in hematological in vitro
models. PLoS One. 11:e01658762016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hamza MS and Mousa SA: Cancer-Associated
thrombosis: Risk factors, molecular mechanisms, future management.
Clin Appl Thromb Hemost. 26:10760296209542822020. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Akinbo DB and Ajayi OI: Thrombotic
pathogenesis and laboratory diagnosis in cancer patients, an
update. Int J Gen Med. 16:259–272. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xiao M, Pang C, Xiang S, Zhao Y, Wu X, Li
M, Du F, Chen Y, Wang F, Wen Q, et al: Comprehensive
characterization of B7 family members in NSCLC and identification
of its regulatory network. Sci Rep. 13:43112023. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Durica SS: Venous thromboembolism in the
cancer patient. Curr Opin Hematol. 4:306–311. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Chen P, Wan G and Zhu B: Incidence and
risk factors of symptomatic thrombosis related to peripherally
inserted central catheter in patients with lung cancer. J Adv Nurs.
77:1284–1292. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Feng Y, Fu Y, Xiang Q, Xie L, Yu C and Li
J: Plasminogen activator inhibitor-1 gene promoter 4G/5G
polymorphism and risks of peripherally inserted central
catheter-related venous thrombosis in patients with lung cancer: A
prospective cohort study. Support Care Cancer. 29:6431–6439. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Gao S, Sun S and Song J: Recent advances
in the pathogenesis and treatment of malignant pericardial
effusion. China Research Hospitals. 12:62–67. 2025.(In
Chinese).
|
|
44
|
Hu YX, Jiang Y, Yan J and Liu JL:
Treatment and prognosis of lung cancer with malignant pericardial
effusion in emergency setting. Acad J Sec Mil Med Univ. 30:583–585.
2009. View Article : Google Scholar
|
|
45
|
Suwanwongse K and Shabarek N: Atrial
flutter as an initial presentation of malignant pericardial
effusion caused by lung cancer. Cureus. 12:e117122020.PubMed/NCBI
|
|
46
|
Dequanter D, Lothaire P, Berghmans T and
Sculier JP: Severe pericardial effusion in patients with concurrent
malignancy: A retrospective analysis of prognostic factors
influencing survival. Ann Surg Oncol. 15:3268–3271. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sundar R, Soong R, Cho B, Brahmer JR and
Soo RA: Immunotherapy in the treatment of non-small cell lung
cancer. Lung Cancer. 85:101–109. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Vakamudi S, Ho N and Cremer PC:
Pericardial effusions: Causes, diagnosis, and management. Prog
Cardiovasc Dis. 59:380–388. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Qiu J, Xu L, Zeng X, Wu Z, Wang Y, Wang Y,
Yang J, Wu H, Xie Y, Liang F, et al: NUSAP1 promotes the metastasis
of breast cancer cells via the AMPK/PPARgamma signaling pathway.
Ann Transl Med. 9:16892021. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Hu ZG, Hu K, Li WX and Zeng FJ: Prognostic
factors and nomogram for cancer-specific death in non small cell
lung cancer with malignant pericardial effusion. PLoS One.
14:e02170072019. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Goel K, Ateeli H, Ampel NM and L'Heureux
D: Patient with small cell lung carcinoma and suspected right upper
lobe abscess presenting with a purulent pericardial effusion. Am J
Case Rep. 17:523–528. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Chiruvella V, Ullah A, Elhelf I, Patel N
and Karim NA: Would the addition of immunotherapy impact the
prognosis of patients with malignant pericardial effusion? Front
Oncol. 12:8711322022. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Krolczyk G, Zabczyk M, Czyzewicz G, Plens
K, Prior S, Butenas S and Undas A: Altered fibrin clot properties
in advanced lung cancer: Impact of chemotherapy. J Thorac Dis.
10:6863–6872. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Koyama N, Tomoda K, Matsuda M, Fujita Y,
Yamamoto Y, Hontsu S, Tasaki M, Yoshikawa M and Kimura H: Acute
bilateral renal and splenic infarctions occurring during
chemotherapy for lung cancer. Intern Med. 55:3635–3639. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Wang YL and Fan XZ: A case of splenic
infarction initiated by upper abdominal pain. Clinical Focus.
29:209–210. 2014.(In Chinese).
|
|
56
|
Grant-Freemantle MC, Bass GA, Butt WT and
Gillis AE: Splenectomy for isolated splenic metastasis from primary
lung adenocarcinoma. BMJ Case Rep. 13:e2332562020. View Article : Google Scholar : PubMed/NCBI
|