Introduction
Primary Central Nervous System Lymphoma is an
uncommon and highly aggressive form of primitive extranodal
Non-Hodgkin Lymphoma (NHL), accounting for 3% of all brain tumors.
Its incidence has increased in recent decades, accounting for
0.47/100,000 people, with a male-to-female ratio of 1.2:1.7, with
the highest rates observed in patients over 60 (1). The main known risk factor for PCNSL is
immunodeficiency, whether primary or acquired, due to HIV infection
or organ transplants (2–4). The most frequent histological subtype
of PCNSL is Diffuse Large B-cell lymphoma, which comprises 95% of
cases (5). PCNSL has been reported
to occur in brain parenchyma, spinal cord, meninges, and ocular
tissues, although the most common site is supratentorial (5). Distinctive topographic features
include the involvement of the midline structures, such as the
corpus callosum and fornix, and locations adjacent to cerebrospinal
fluid spaces, in contact with the ependymal surface (5). Infratentorial locations in the
brainstem and cerebellum or involvement of cranial nerves are
uncommon (5). This site variability
may explain the pleomorphic symptoms, with headache and seizures
being the most common (6). Cerebral
stereotaxic biopsy is considered the gold standard for diagnosis,
although it should be performed without prior corticosteroid
treatment, which can promote lymphocellular necrosis and compromise
accurate diagnosis (5–7). The usual morphological features
include large necrotic areas with evidence of neoplastic
lymphocytes invading the surrounding parenchyma. This proliferation
consists of pleomorphic and atypical B lymphoid cells with large
round nuclei, prominent nucleoli, and many mitoses (8). Additionally, mixed reactive
inflammatory cells, including T lymphocytes, are found among
B-cells (9). The angiocentric
growth pattern, with vessels surrounded by neoplastic lymphocytes,
can be a significant morphological feature (8,10).
Using Hans' algorithm, two main immunohistochemical subtypes can be
identified: Germinal and Non-Germinal Centre B-cell (11). Specifically, the GCB subtype
exhibits immunoreactivity for CD10 and BCL-6, while Non-GCB can be
further subdivided into Activated B-Cell (ABC) with
immunoreactivity for MUM1 and an unclassified subtype (11). The GCB subtype has a reportedly
better clinical outcome compared to Non-GCB (11). The neuroradiological key points for
diagnosing PCNSL include evidence of solid, sometimes multiple,
supratentorial masses with low Apparent Diffusion Coefficient (ADC)
on diffusion-weighted Magnetic Resonance Imaging (MRI) sequences
and homogeneous enhancement after Gadolinium-diethylenetriamine
pentaacetic acid (DTPA) administration. In immunocompromised
patients, the MRI pattern is less specific, often revealing
intralesional hemorrhage or necrosis with ring-like enhancement
after Gadolinium administration (12). This retrospective multidisciplinary
study aims to analyze a cohort of 23 patients affected by
PCNSL-DLBCL, comparing their clinical, pathological, and
neuroradiological features and evaluating the potential effects of
these variables on OS.
Materials and methods
Patients and tumor specimens
Clinical and pathological data from 23 patients
diagnosed with PCNSL-DLBCL between January 2012 and November 2024
were obtained through neurosurgical procedures and collected from
the Department of Human Pathology of Adult and Developmental Age,
Section of Pathological Anatomy, University of Messina, A.O.U.
Gaetano Martino (Messina, Italy). The analysis was performed
according to Good Clinical Practice guidelines and the Declaration
of Helsinki (1975, revised in 2013). Before the surgical
procedures, all patients provided written, anonymized, and informed
consent. Pathology reports and medical records were thoroughly
reviewed. Patients' initials and other personal identifiers were
removed from all images. The Institutional Review Board of the
University Hospital of Messina (Messina, Italy) approved this study
vide protocol no. 47/19 of May 2, 2019. Data collected included age
at diagnosis, gender, and site of disease, classified as
supratentorial or infratentorial. MRI images of the aforementioned
patients were collected from the Department of Biomedical, Dental,
Morphological, and Functional Imaging Sciences, Section of
Neuroradiology, University of Messina, A.O.U. Gaetano Martino
(Messina, Italy), including T1 and T2 weighted images, Late
Gadolinium Enhancement (LGE) imaging, and Diffusion-weighted
Imaging (DWI), considering the ADC as a surrogate for neoplastic
cellularity and the mean Ki-67 value of enhancement as an
expression of blood-brain barrier (BBB) damage. After surgical
procedures, all patients underwent radio-chemotherapy with four
cycles of methotrexate, cytarabine, rituximab, and prednisolone;
additionally, complementary whole-brain radiotherapy (30–36 Gy) was
performed.
Immunohistochemical analysis
All tumor specimens were fixed in 10%
neutral-buffered-formalin at room temperature for 24 h and
paraffin-embedded. Five-micron-thick sections were obtained from
corresponding tissue blocks for hematoxylin and eosin (H&E)
routine staining. Parallel sections from the same tissue blocks
were cut for immunohistochemical procedures; slides were
deparaffinized in xylene, dehydrated, and treated with 3% hydrogen
peroxide for 10 min to block endogenous peroxidase; they were then
washed in deionized water thrice (10 min each time). Sections were
incubated with normal sheep serum to prevent nonspecific adherence
of serum proteins for 30 min at room temperature. Thereafter,
sections were again washed with deionized water and incubated for
30 min at 37°C with the working dilution suggested by the ROCHE
manufacturer for each commercially obtained monoclonal antibody.
The following monoclonal antibodies against CD20 (L26), PAX5
(SP34), CD79a (SP18), GFAP (EP672Y), BCL-2 (124), Ki-67 (MIB 1),
CD10 (SP67), BCL-6 (GI191E/A8), and MUM1 (EP190) were used.
Subsequently, sections were washed with phosphate-buffered saline
solution (PBS) at pH 7.2–7.4 and incubated with corresponding
secondary antibodies (1:300; Abcam, code no. ab7064) for 20 min at
room temperature, incubated with horseradish peroxidase-labeled
secondary antibody for 30 min and developed with diaminobenzidine
tetrahydrochloride, counterstained with hematoxylin using the ULTRA
Staining system (Ventana Medical Systems). Negative controls were
obtained by omitting the specific antisera and substituting PBS for
the primary antibody. The immunohistochemical staining for each
sample was independently scored by two pathologists, who were
blinded to patient outcomes and clinical findings, using a Zeiss
Axioskop microscope (Carl Zeiss Microscopy GmbH) at 40× objective
magnification. Staining for CD10, BCL-6, and MUM-1 was considered
positive when >30% of cells were positively stained; staining
for BCL-2 was considered positive when >50% of cells were
positively stained. Ki-67 expression was evaluated as high when the
percentage of positive neoplastic cells was >50%, counting at
least 1,000 cells.
Immunophenotype classification
Two main subtypes of PCNSL-DLBCL were identified
through the combined expression of CD10, BCL-6, and MUM-1. All
CD10+ tumor phenotypes were classified as the GCB subgroup. Samples
with CD10-BCL-6+ MUM-1+ and CD10-BCL-6-MUM-1+ profiles were
considered Non-GCB subtypes.
MRI protocol
All MRI examinations were performed in the clinical
routine using a 1.5 Tesla MRI scanner (Ingenia Philips Healthcare,
Best, The Netherlands). The MRI protocol included: axial DWI
single-shot spin-echo (SE) echo planar sequence (repetition
time (TR)/echo time (TE): 3000/85 ms, flip angle (FA): 90°, SL:
71.6 mm, slice thickness (ST): 5 mm, field of view (FOV): 230 mm).
Diffusion-sensitizing gradients were applied sequentially in the x,
y, and z directions with b factors of 0 and 1,000 s/mm2.
ADCs were automatically calculated by the MR scanner and displayed
as corresponding ADC maps: axial SE T1-weighted sequence
(TR: 633 ms, TE: 15 ms, FA: 69°, SL: 71 mm, ST: 5.50 mm); axial
fast SE (FSE) T2-weighted sequence (TR: 4848 ms, TE: 100 ms,
FA: 90°, SL: 71.60 mm, ST: 5 mm); 3D fluid-attenuated inversion
recovery (FLAIR-3D) sequence (TR: 5200 ms, TE: 305 ms, FA: 90°,
SL: 21.44 mm); postcontrast 3D T1-weighted gradient-echo (GE)
sequence (TR/TE: 25/4.58 ms, FA: 30°, 1-mm section thickness,
and 230 mm FOV). A standard dose (0.1 mmol/kg body weight) of
gadoteric acid (Gd-DOTA, Dotarem; Laboratoire Guerbet,
Aulnay-sous-Bois, France) was injected intravenously.
Image analysis
All images were available in digital format. Image
analysis was performed on a Philips Portal Viewer. T2 and ADC maps
were co-registered with the post-contrast T1-weighted 3D-gradient
echo sequence. Image analysis was conducted by two independent
neuroradiologists, who drew a circular Region-of-Interest (ROI)
within the tumor, avoiding regions suspected of intralesional
hemorrhage or necrosis. Three sets of data were collected,
including ADC, T2 signal, and enhancement evaluation. A
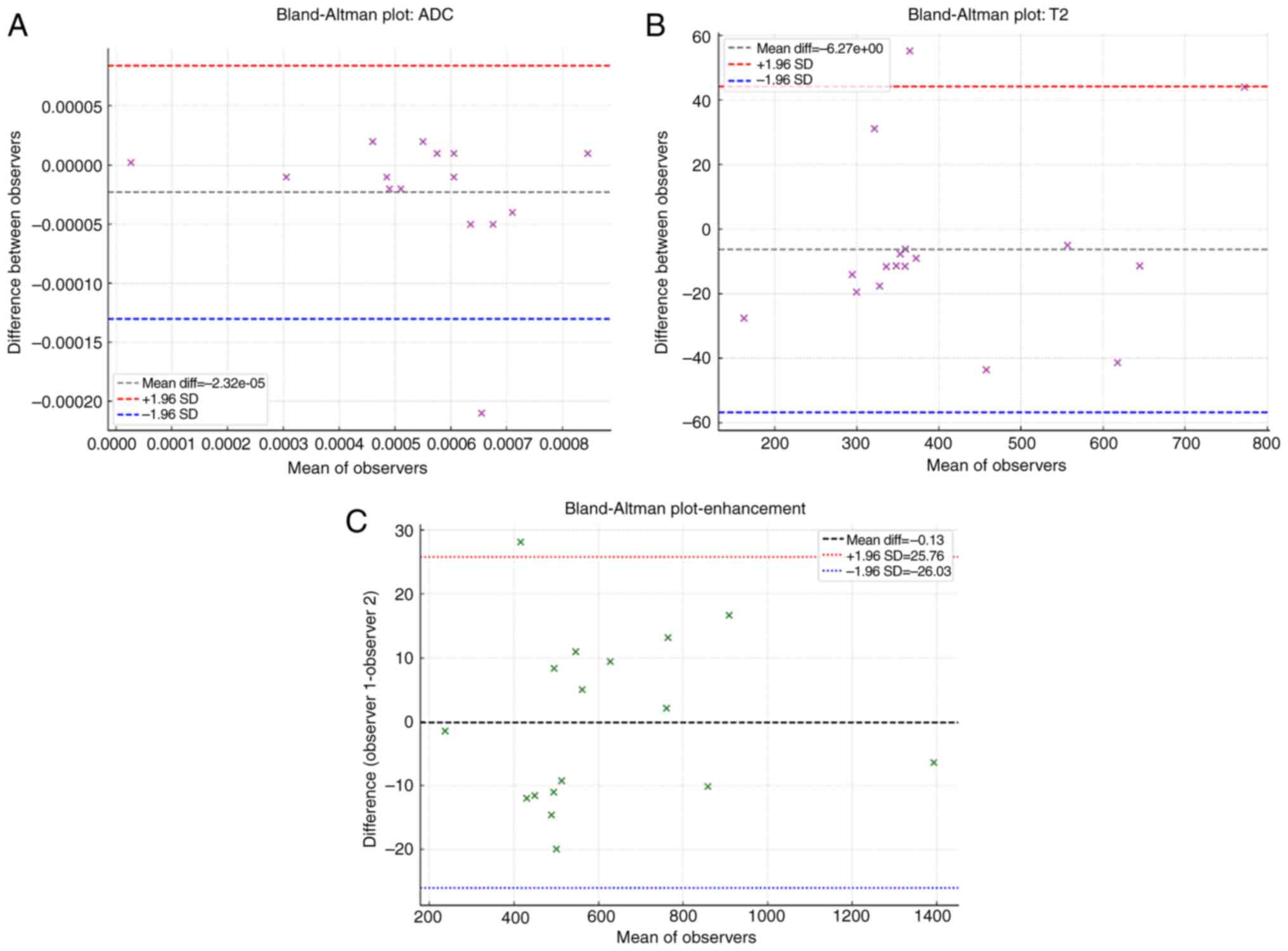
Bland-Altman plot was used to assess agreement between the two
observers (Fig. 1). Good
inter-observer agreement was demonstrated for all MRI parameters:
ADC (Fig. 1A), T2 signal (Fig. 1B), and enhancement (Fig. 1C).
Statistical analysis
The association between the immunohistochemical
profile of tumors, clinicopathological (age, sex, and site of
disease), and neuroradiological features was subjected to
univariate analysis with Fisher's exact test. Multivariate analysis
was performed using the Cox regression model to study the
independent effects of variables on OS, defined as the time from
diagnosis to death. Kaplan-Meier survival curves were generated.
All statistical evaluations were performed using MedCalc version
10.2.0.0 (MedCalc Software). Results were considered statistically
significant when P<0.05.
Results
Histology and immunohistochemical
results
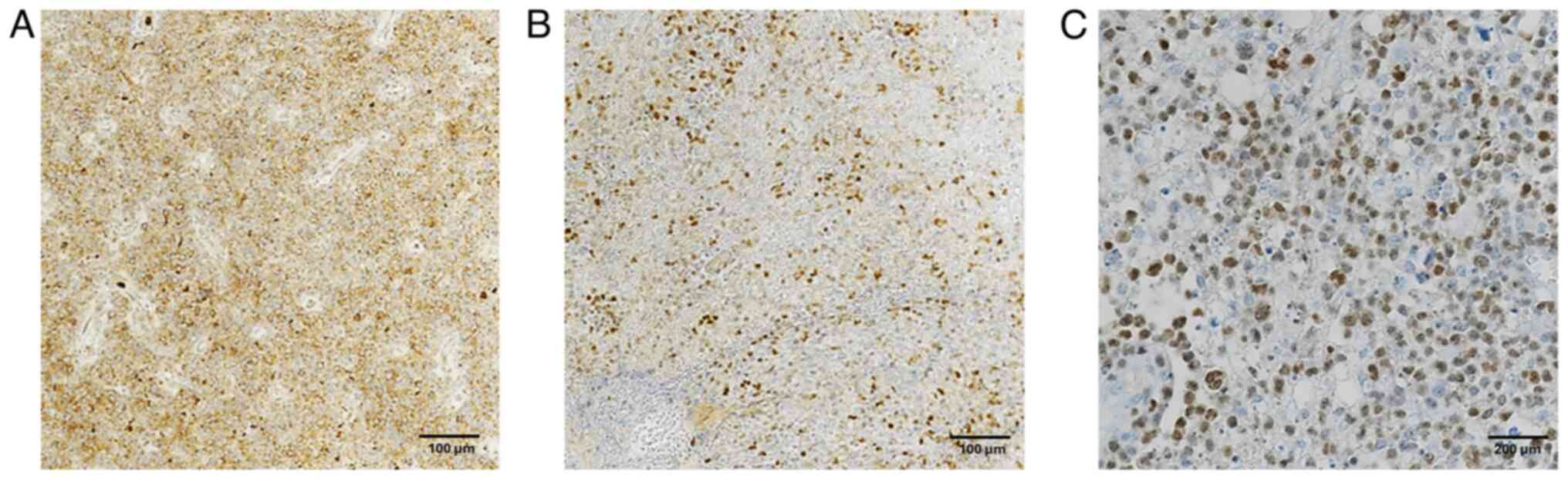
All cases exhibited typical morphological features
of PCNSL-DLBCL in hematoxylin and eosin sections (Fig. 2). B-cell markers (CD20, PAX5, and
CD79a) demonstrated cell membrane staining in all specimens.
Twenty-one out of 23 cases indicated nuclear BCL-2
immunoreactivity. Lymphoid clusters were GFAP negative, with
infiltration of GFAP-positive adjacent nervous tissue. In 17 out of
23 specimens, nuclear immunoreactivity for c-Myc was documented in
>40% of tumor cells. According to Hans' algorithm, 6 samples
(26%) were classified as GC: 4 (67%) were CD10+ BCL-6+ MUM-1+; 2
(33%) were CD10+ BCL-6+ MUM-1-. Seventeen tumors (74%) were
classified as Non-GCB: 16 (94%) were CD10-BCL-6+ MUM-1+; 1 (6%) was
MUM-1+ only (Fig. 3).
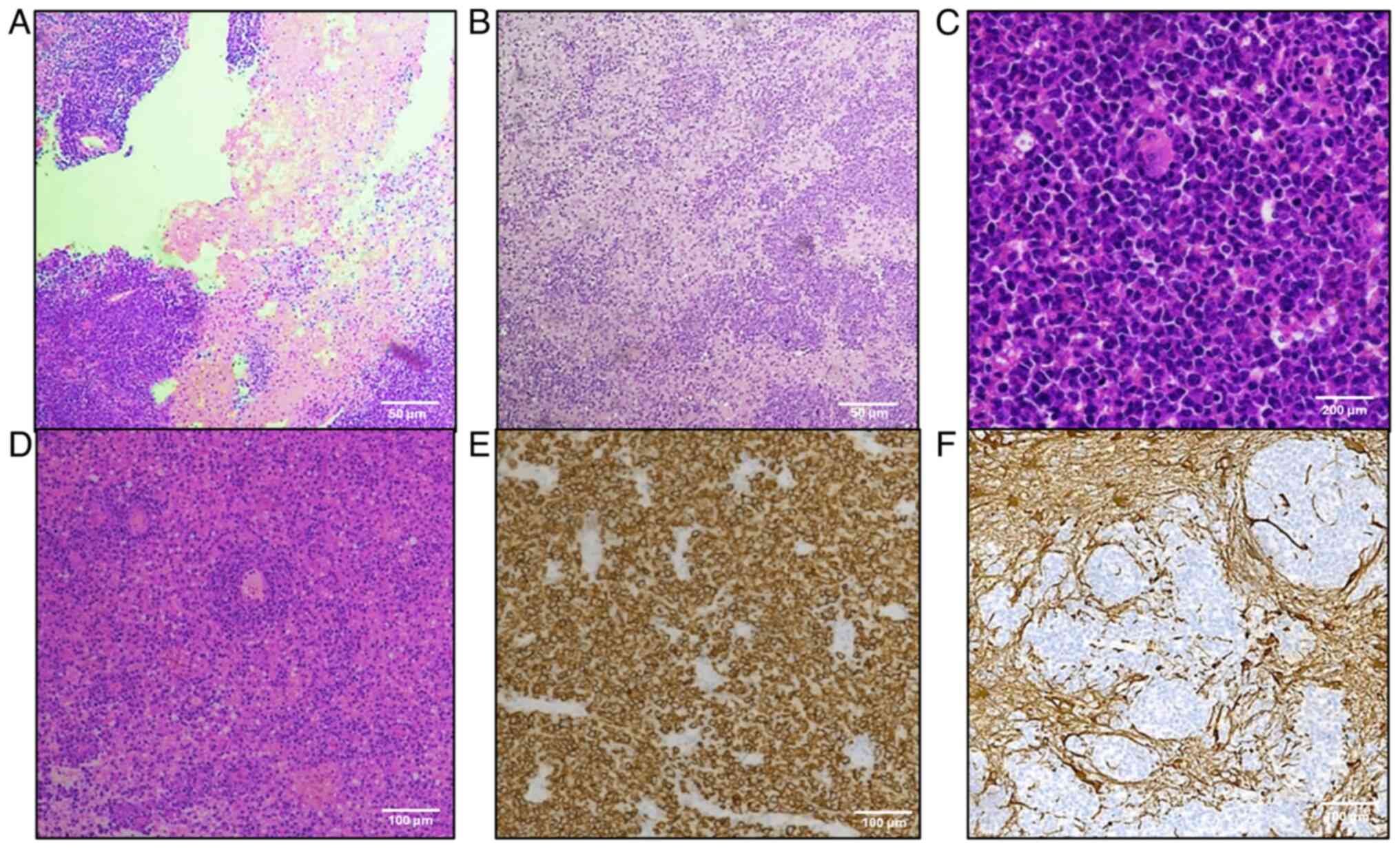 | Figure 2.Primitive diffuse large B-cell
lymphoma: Histologic and immunohistochemical features. (A) Large
areas of geographical necrosis (hematoxylin and eosin staining;
magnification, ×100; scale bar, 50 µm). (B) Clusters of tumor cells
invading brain parenchyma (hematoxylin and eosin staining;
magnification, ×100; scale bar, 50 µm). (C) Atypical B cells with
big, round, vesicular nuclei and numerous mitoses (hematoxylin and
eosin staining; magnification, ×400; scale bar, 200 µm). (D)
Angiocentric pattern of growth (hematoxylin and eosin staining;
magnification, ×100; scale bar, 100 µm). (E) Diffuse cell membrane
CD20-positivity (Mayer's hemalum nuclear counterstain;
magnification, ×200; scale bar, 100 µm). (F) GFAP− tumor
cells invading GFAP+ brain parenchyma (Mayer's hemalum
nuclear counterstain; magnification, ×200; scale bar, 100 µm).
GFAP, glial fibrillary acidic protein. |
Clinicopathological and
neuroradiological features
Of the total of 23 patients, 20 (87%) were ≥60, and
3 (13%) were <60, with a median age of 66 and a mean age of
65.7; 8 patients (35%) were male and 15 (65%) female.
Supratentorial disease was documented in 18 cases (78%), and
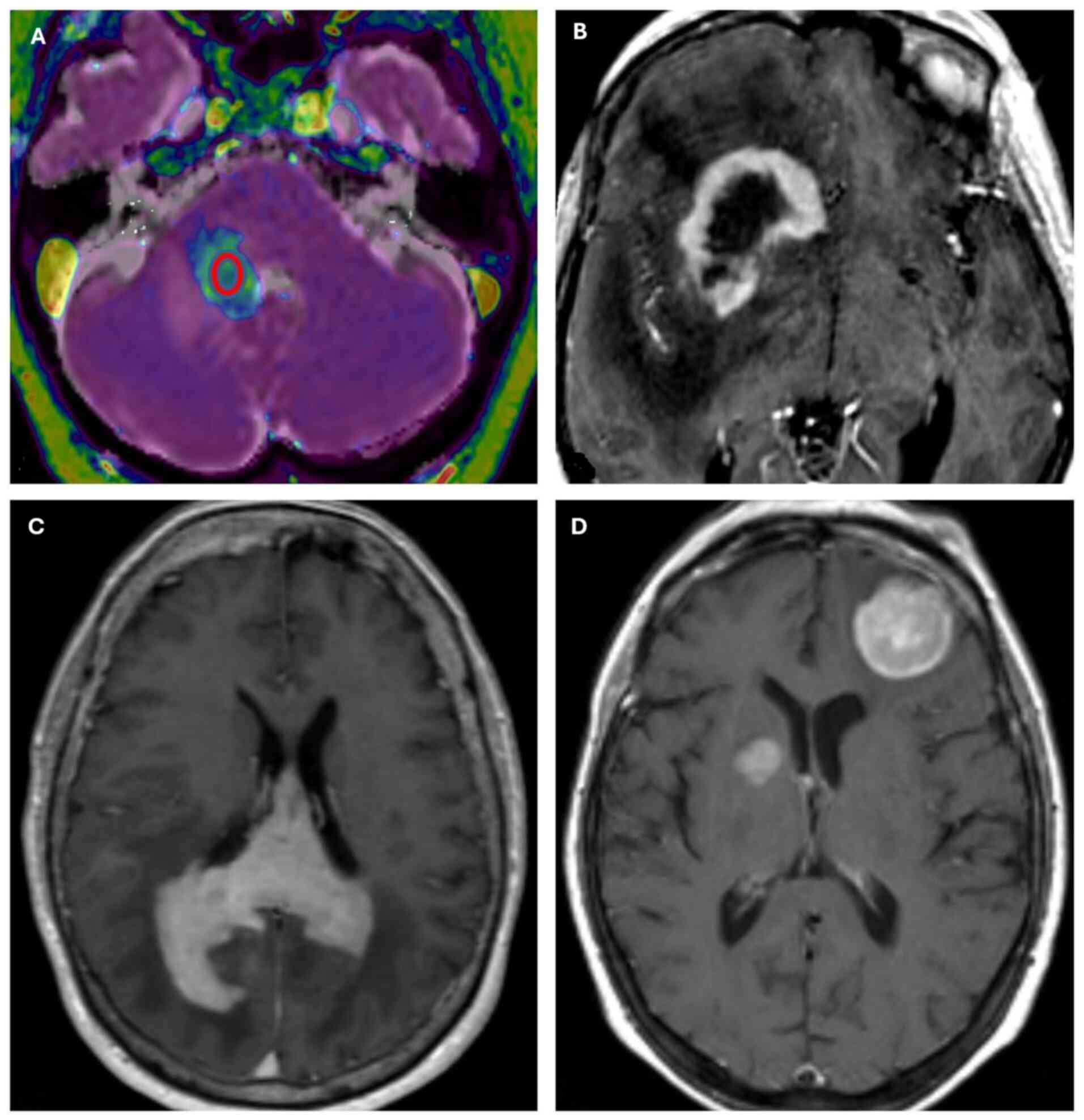
infratentorial disease in 5 cases (22%) (Table I). Regarding neuroradiological
features, MRI images showed homogeneous enhancement in 21 out of 23
cases and ring enhancement in 2 out of 23 patients, which can be
considered an expression of central tumor necrosis. Fourteen out of
18 supratentorial cases revealed invasion of the corpus callosum
and other median line structures, while infratentorial cases did
not exhibit this feature. Multifocal disease was indicated in 14
out of 23 cases and unifocal disease in 9 out of 23 cases (Fig. 4). The mean ADC value was
0.53×10−3 mm2/s, with a standard deviation of
0.18×10−3 mm2/s. The mean enhancement value
was 613.75, with a standard deviation of 264.66. Two subgroups were
identified based on age: those ≥60 included 4 GCB cases (20%) and
16 Non-GCB cases (80%), with a mean Ki-67 value >80%; the second
subgroup included 2 GCB cases and 1 Non-GCB case, with a mean Ki-67
value of 57%. The mean Ki-67 value was 82.5% in male patients and
71% in female patients.
 | Table I.Clinical and pathological
characteristics of patients with primitive diffuse large B-cell
lymphoma. |
Table I.
Clinical and pathological
characteristics of patients with primitive diffuse large B-cell
lymphoma.
| Characteristics | All patients
(n=23) | GCB | Non-GCB |
|---|
| Mean age, years
(range) | 65.7 (44–77) | 65.2 (57–75) | 65.9 (44–77) |
| Median age,
years | 66 | 65 | 66 |
| Age, n (%) |
|
|
|
| ≥60
years | 20 (87) | 4 (20) | 16 (80) |
| <60
years | 3 (13) | 2 (67) | 1 (33) |
| Sex, n (%) |
|
|
|
| Male | 8 (35) | 2 (25) | 6 (75) |
|
Female | 15 (65) | 4 (27) | 11 (73) |
| Site of disease, n
(%) |
|
|
|
|
Supratentorial | 18 (78) | 3 (17) | 15 (83) |
|
Infratentorial | 5 (22) | 3 (60) | 2 (40) |
| Number of lesions,
n (%) |
|
|
|
| 1 | 9 (39) | 1 (11) | 8 (89) |
| ≥2 | 14 (61) | 6 (43) | 8 (57) |
Statistical analysis
Univariate analysis revealed significant statistical
associations between the following variables: site of disease and
number of lesions (P=0.04), site of disease and immunohistochemical
(IHC) subtype (P=0.03), gender and ADC (P=0.02), and IHC subtype
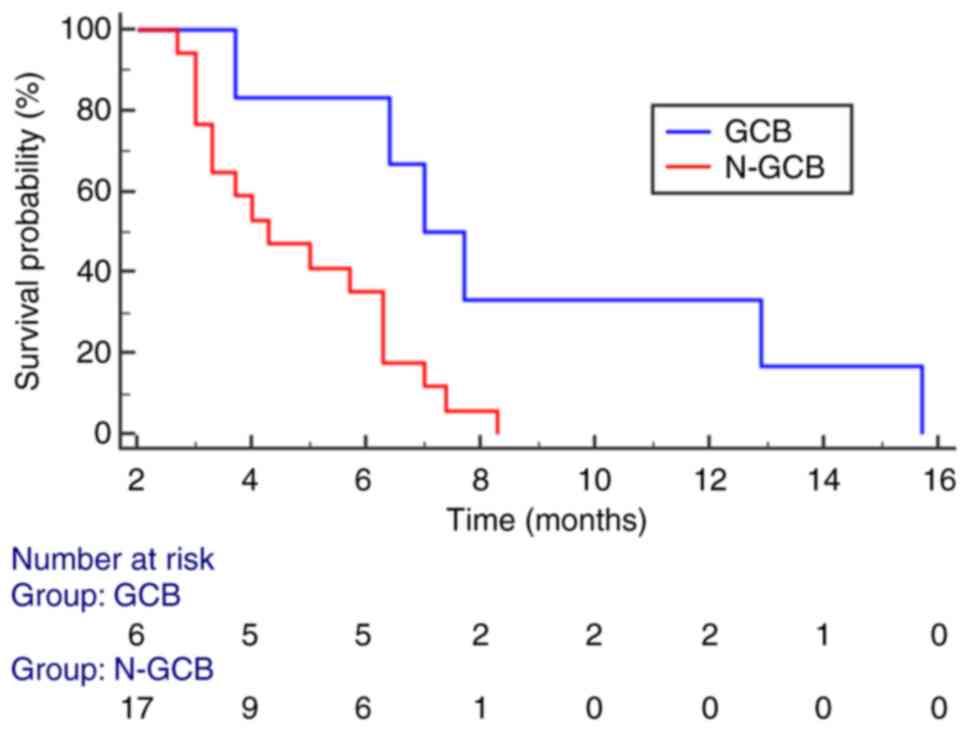
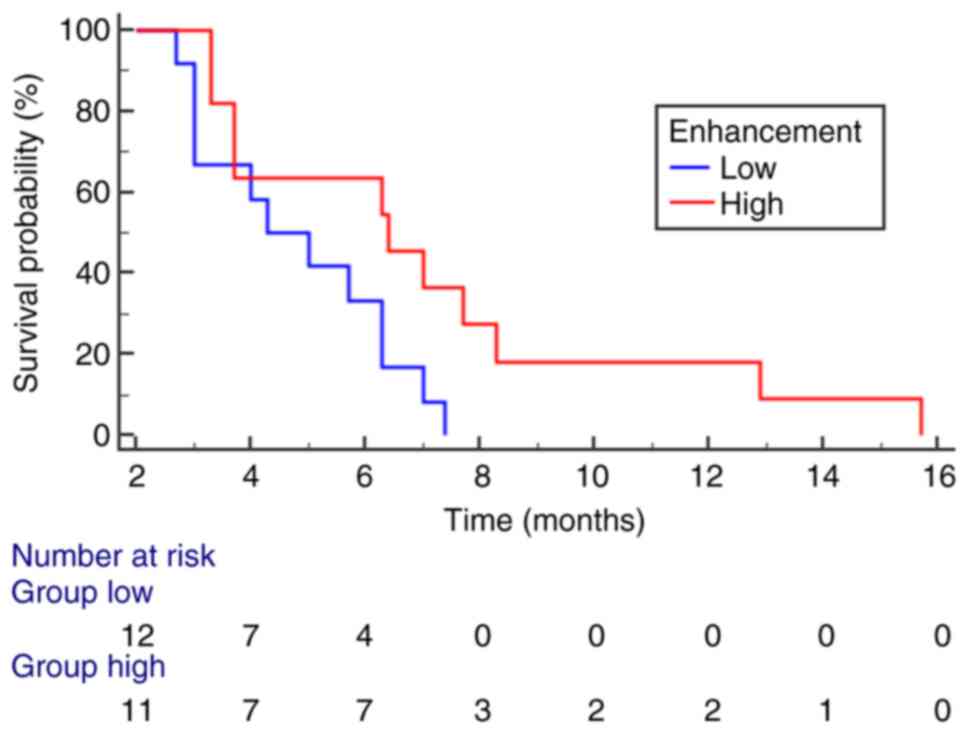
and mean value of enhancement (P=0.008) (Table II, Table III, Table IV, Table V). In multivariate analysis, IHC
subtype (P=0.0158) (Fig. 5) and
mean value of enhancement (P=0.0391) (Fig. 6) emerged as independent variables;
comparing survival curves for GCB vs. Non-GCB and low vs. high
enhancement revealed significant differences in OS. Additionally, a
trend toward statistical significance was observed for c-Myc
(P=0.0648).
 | Table II.Univariate analysis concerning site
and number of lesions in patients with primitive diffuse large
B-cell lymphoma. |
Table II.
Univariate analysis concerning site
and number of lesions in patients with primitive diffuse large
B-cell lymphoma.
|
|
| Number of
lesions |
|
|---|
|
|
|
|
|
|---|
| Site of
disease | No. | Multifocal, n
(n=14) | Unifocal, n
(n=9) | P-value |
|---|
| Supratentorial | 18 | 13 | 5 | 0.04 |
| Infratentorial | 5 | 1 | 4 |
|
 | Table III.Univariate analysis concerning site
and histological subtype in patients with primitive diffuse large
B-cell lymphoma. |
Table III.
Univariate analysis concerning site
and histological subtype in patients with primitive diffuse large
B-cell lymphoma.
|
|
| Subtype |
|
|---|
|
|
|
|
|
|---|
| Site of
disease | No. | GCB, n (n=6) | Non-GCB, n
(n=17) | P-value |
|---|
| Supratentorial | 18 | 3 | 15 | 0.03 |
| Infratentorial | 5 | 3 | 2 |
|
 | Table IV.Univariate analysis concerning sex
and ADC in patients with primitive diffuse large B-cell
lymphoma. |
Table IV.
Univariate analysis concerning sex
and ADC in patients with primitive diffuse large B-cell
lymphoma.
|
|
| ADC |
|
|---|
|
|
|
|
|
|---|
| Sex | No. |
>0.53×10−3
mm2/sec, n (n=12) |
<0.53×10−3
mm2/sec, n (n=11) | P-value |
|---|
| Male | 8 | 7 | 1 | 0.02 |
| Female | 15 | 5 | 10 |
|
 | Table V.Univariate analysis concerning
histological subtype and MRI enhancement in patients with primitive
diffuse large B-cell lymphoma. |
Table V.
Univariate analysis concerning
histological subtype and MRI enhancement in patients with primitive
diffuse large B-cell lymphoma.
|
|
| Mean value of
enhancement |
|
|---|
|
|
|
|
|
|---|
| IHC subtype | No. | >613.75, n
(n=8) | <613.75, n
(n=15) | P-value |
|---|
| GCB | 6 | 5 | 1 | 0.008 |
| Non-GCB | 17 | 3 | 14 |
|
Discussion
PCNSL is reportedly an uncommon extranodal lymphoma
with a poor prognosis (1,2). In the present study, we analyzed a
cohort of PCNSL-DLBCL patients, documenting a higher incidence in
those aged ≥60, consistent with existing literature (5). We also confirmed the highest
occurrence of the disease in supratentorial sites, as found
elsewhere too (13,14). Interestingly, our cohort showed a
higher number of affected female patients than males (13,15).
Notably, concerning the growth fraction in PCNSL-DLBCL, the mean
Ki-67 value appeared slightly reduced in the female subgroup
(Ki-67=71%) compared to male patients (Ki-67=82.5%). However, a
higher mean value (>80%) was observed in patients aged ≥60
compared to those <60 (Ki-67 mean value=57%), highlighting
higher proliferation rates in older patients (≥60), supporting the
potential prognostic significance of age, as indicated by the IELSG
main prognostic clinical score (16–18).
Additionally, high Ki-67 expression may predict poor prognosis in
PCNSL, as Ki-67 >90% has been associated with shorter OS
(15).
Our data regarding the prevalence of specific
PCNSL-DLBCL phenotypes revealed more Non-GCB cases, with the
majority expressing MUM1. Non-GCB cases represented the majority in
our cohort (17/23), while GCB cases were only 6/23, aligning with
literature data (13,15). Unfortunately, the Non-GCB subtype is
associated with a worse prognosis, as it carries additional
unfavorable prognostic factors, such as age ≥60 and mean Ki-67
value >80%; conversely, the GCB subtype is associated with a
better prognosis, influenced by younger age (<60) and reduced
Ki-67 value (57%). It is well established that Gadolinium-based
contrast agents leak from blood vessels into brain tissue due to
increased vascular permeability caused by tumor-induced breakdown
of the BBB (19). We therefore
hypothesize that the GCB subtype of PCNS-DLBCL may exhibit a
greater tendency to induce alterations in the capillary bed at the
lesion site, resulting in significant alterations in the BBB and
consequently, in a marked degree of contrast enhancement on MRI
examination. However, in the neuroradiological evaluation of PCNSL,
DWI and ADC maps are essential tools, as they provide crucial
information about tumor microstructure. Specifically, DWI sequences
measure the movement of water molecules in biological tissues,
enabling surrogate assessment of tissue characteristics in both
normal and pathological conditions (20). In highly cellular tumors, for
instance, the movement of water molecules is restricted; thus,
hypercellular tumors such as PCNSL typically exhibit low ADC values
(21). Further, PCNSL shows lower
ADC values than tumors such as glioblastoma multiforme (GBM),
metastases, demyelinating lesions, and infections (21). Although GBM may present solid areas
with restricted diffusion, it exhibits variable cellularity and may
show areas of necrosis or cysts, resulting in greater water
diffusion and higher ADC values. Consequently, ADC values in GBM
tend to be less homogeneous and higher than in PCNSL (21). Previous studies have indicated a
cut-off ADC value of 0.69×10−3 mm2/s
(21) that can help distinguish
lymphoma from glioblastoma with high sensitivity and specificity.
In our case series, all patients had brain lesions with low ADC
values (mean ADC value 0.53×10−3 mm2/s, with
a standard deviation of 0.18×10−3 mm2/s),
with no significant difference between GCB and Non-GCB groups.
Further, we were unable to identify a statistically significant
inverse correlation between lesional ADC values and cellular growth
fraction measured by Ki-67, confirming findings reported elsewhere
(22–24). ADC/cellularity correlation varies
across different tumor types: strong in neoplasms such as gliomas,
ovarian or lung cancer, moderate in epithelial neoplasms, and weak
in lymphomas (25). Therefore, the
low ADC values observed in lymphomas may be attributable not solely
to tumor hypercellularity but to other histopathological features,
such as reduced extracellular matrix, stroma/parenchyma ratio,
and/or microvessel density (25).
Univariate statistical analysis revealed a significant association
between the site of PCNSL-DLBCL and the number of lesions (P=0.04),
indicating that supratentorial localization is related to
multifocality and infratentorial disease to unifocal sites.
Additionally, a relationship between this parameter and the IHC
subtype emerged (P=0.03), with the supratentorial disease being
linked to the Non-GCB subtype and infratentorial disease with the
GCB subtype. Other notable correlations were documented,
particularly between the IHC subtype and the degree of contrast
enhancement (P=0.008), with the GCB subtype demonstrating
significantly increased contrast enhancement than the Non-GCB
group. Nevertheless, previous studies have attempted to examine the
prognostic role of key immunohistochemical markers such as CD10,
BCL-6, MUM-1, and BCL-2, along with clinical parameters (age,
gender, and the number of lesions), although discordant results
have emerged, possibly due to limited cohorts, differing methods of
immunohistochemical analysis, and different treatment (15,26).
Multivariate analysis and comparison of Kaplan-Meier survival
curves indicated that IHC subtype and contrast enhancement on MRI
examination were the most significant parameters influencing OS.
Specifically, GCB cases exhibited a better prognosis, with a longer
OS (16 months), while Non-GCB cases had a worse prognosis with a
shorter OS (8 months). Further, cases with higher MRI enhancement
values, corresponding to the GCB subtype, had longer survival than
cases with lower enhancement values, which aligned with the Non-GCB
subtype. Therefore, more adequate treatment adjustments should be
based on imaging too, suggesting different strategies regarding
immune checkpoint inhibitors and chimeric antigen receptor (CAR) T
cells; thus, immediate therapeutic applicability should be
hypothesized for more aggressive Non-GCB cases.
Finally, the association of morphological and
neuroradiological characteristics in PCNSL-DLBCL may provide a
significant prognostic tool to better define patients' OS,
advocating for a multidisciplinary approach in brain lymphoid
neoplasms. This desirable setting may prevent conflicting data
reported in the literature that exclusively refer to morphological
parameters such as immunophenotype and Ki67 value (15,27).
In conclusion, we assert that PCNSL is characterized by specific
clinicopathological and neuroradiological features (older age,
supratentorial site, Non-GCB immunoprofile, and MRI enhancement);
however, the limited sample size of the present cohort may
introduce bias into the investigation, necessitating further
multicentric studies to corroborate our results. Although the small
cohort analyzed and the single-center retrospective nature of the
study may influence statistics, introducing selection bias and
limiting generalizability, particularly for some subgroups (e.g.,
age/sex), the reduced interpretability of data may arise from
non-significant ADC-Ki67 correlation, rendering potential
confounders less prominent (e.g., tumor microenvironment
heterogeneity, cellular density).
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
IR, CP, FG, AI and GT developed the study design and
drafted the manuscript. IR, CP, SCS, VF, MM and AG were involved in
data acquisition and interpretation. AI, FG and GT reviewed the
manuscript. AG, FG and GT confirm the authenticity of all the raw
data. All authors have read and approved the final version of the
manuscript.
Ethical approval and consent to
participate
The analysis was conducted according to the Good
Clinical Practice guidelines and the Declaration of Helsinki (1975,
revised in 2013). Before surgical procedures, all patients provided
written, anonymized and informed consent. Pathology reports and
medical records were thoroughly reviewed. Patient initials and
other personal identifiers were removed from all images. The
Institutional Review Board of the University Hospital of Messina
(Messina, Italy) approved the present study (approval no. N. 47/19;
May 2, 2019).
Patient consent for publication
Written informed consent was obtained from all
patients for the publication of their data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liu Y, Yao Q and Zhang F: Diagnosis,
prognosis and treatment of primary central nervous system lymphoma
in the elderly population (Review). Int J Oncol. 58:371–387. 2021.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shao L, Xu C, Wu H, Jamal M, Pan S, Li S,
Chen F, Yu D, Liu K and Wei Y: Recent progress on primary central
nervous system lymphoma-From bench to bedside. Front Oncol.
11:6898432021. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferreri AJM, Calimeri T, Cwynarski K,
Dietrich J, Grommes C, Hoang-Xuan K, Hu LS, Illerhaus G, Nayak L,
Ponzoni M and Batchelor TT: Primary central nervous system
lymphoma. Nat Rev Dis Primers. 9:292023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
D'Angelo CR: Diagnostic, pathologic, and
therapeutic considerations for primary CNS lymphoma. JCO Oncol
Pract. 20:195–202. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Green K, Munakomi S and Hogg JP: Central
nervous system lymphoma. StatPearls [Internet] Treasure Island
(FL): StatPearls Publishing; 2024
|
|
6
|
Schaff LR and Grommes C: Primary central
nervous system lymphoma. Blood. 140:971–979. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
de Koning ME, Hof JJ, Jansen C, Doorduijn
JK, Bromberg JEC and van der Meulen M: Primary central nervous
system lymphoma. J Neurol. 271:2906–2913. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lebrun L, Allard-Demoustiez S and Salmon
I: Pathology and new insights in central nervous system lymphomas.
Curr Opin Oncol. 35:347–356. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jin Q, Jiang H, Han Y, Zhang L, Li C,
Zhang Y, Chai Y, Zeng P, Yue L and Wu C: Tumor microenvironment in
primary central nervous system lymphoma (PCNSL). Cancer Biol Ther.
25:24251312024. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bödör C, Alpár D, Marosvári D, Galik B,
Rajnai H, Bátai B, Nagy Á, Kajtár B, Burján A, Deák B, et al:
Molecular subtypes and genomic profile of primary central nervous
system lymphoma. J Neuropathol Exp Neurol. 79:176–183. 2020.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hans CP: Confirmation of the molecular
classification of diffuse large B-cell lymphoma by
immunohistochemistry using a tissue microarray. Blood. 103:275–282.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Joshi A, Deshpande S and Bayaskar M:
Primary CNS lymphoma in immunocompetent patients: Appearances on
conventional and advanced imaging with review of literature. J
Radiol Case Rep. 16:1–17. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Radotra BD, Parkhi M, Chatterjee D, Yadav
BS, Ballari NR, Prakash G and Gupta SK: Clinicopathological
features of primary central nervous system diffuse large B cell
lymphoma: Experience from a tertiary center in North India. Surg
Neurol Int. 11:4242020. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ribas GA, de Mori LH, Freddi TAL, Oliveira
LDS, de Souza SR and Corrêa DG: Primary central nervous system
lymphoma: Imaging features and differential diagnosis. Neuroradiol
J. 37:705–722. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu J, Wang Y and Liu Y, Liu Z, Cui Q, Ji
N, Sun S, Wang B, Wang Y, Sun X and Liu Y: Immunohistochemical
profile and prognostic significance in primary central nervous
system lymphoma: Analysis of 89 cases. Oncol Lett. 14:5505–5512.
2017.PubMed/NCBI
|
|
16
|
Puligundla CK, Bala S, Karnam AK, Gundeti
S, Paul TR, Uppin MS and Maddali LS: Clinicopathological features
and outcomes in primary central nervous system lymphoma: A 10-year
experience. Indian J Med Paediatric Oncol. 38:478–482. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Du KX, Shen HR, Pan BH, Luthuli S, Wang L,
Liang JH, Li Y, Yin H, Li JY, Wu JZ and Xu W: Prognostic value of
POD18 combined with improved IELSG in primary central nervous
system lymphoma. Clin Transl Oncol. 26:720–731. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yokogami K, Azuma M, Takeshima H and Hirai
T: Lymphomas of the central nervous system. Adv Exp Med Biol.
1405:527–543. 2023. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Provenzale JM, Mukundan S and Dewhirst M:
The role of blood-brain barrier permeability in brain tumor imaging
and therapeutics. AJR Am J Roentgenol. 185:763–767. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
White NS, McDonald CR, Farid N, Kuperman
JM, Kesari S and Dale AM: Improved conspicuity and delineation of
high-grade primary and metastatic brain tumors using ‘restriction
spectrum imaging’: Quantitative comparison with high B-value DWI
and ADC. AJNR Am J Neuroradiol. 34:958–964. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ahn SJ, Shin HJ, Chang JH and Lee SK:
Differentiation between primary cerebral lymphoma and glioblastoma
using the apparent diffusion coefficient: Comparison of three
different ROI methods. PLoS One. 9:e1129482014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen J, Xia J, Zhou YC, Xia LM, Zhu WZ,
Zou ML, Feng DY and Wang CY: Correlation between magnetic resonance
diffusion-weighted imaging and cell density in astrocytoma.
Zhonghua Zhong Liu Za Zhi. 27:309–311. 2005.(In Chinese).
PubMed/NCBI
|
|
23
|
Guo AC, Cummings TJ, Dash RC and
Provenzale JM: Lymphomas and high-grade astrocytomas: Comparison of
water diffusibility and histologic characteristics. Radiology.
224:177–183. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schob S, Meyer J, Gawlitza M,
Frydrychowicz C, Müller W, Preuss M, Bure L, Quäschling U, Hoffmann
KT and Surov A: Diffusion-weighted MRI reflects proliferative
activity in primary CNS lymphoma. PLoS One. 11:e01613862016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Surov A, Meyer HJ and Wienke A:
Correlation between apparent diffusion coefficient (ADC) and
cellularity is different in several tumors: A meta-analysis.
Oncotarget. 8:59492–59499. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Morales-Martinez A, Nichelli L,
Hernandez-Verdin I, Houillier C, Alentorn A and Hoang-Xuan K:
Prognostic factors in primary central nervous system lymphoma. Curr
Opin Oncol. 34:676–684. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Momota H, Narita Y, Maeshima AM, Miyakita
Y, Shinomiya A, Maruyama T, Muragaki Y and Shibui S: Prognostic
value of immunohistochemical profile and response to high-dose
methotrexate therapy in primary CNS lymphoma. J Neurooncol.
98:341–348. 2010. View Article : Google Scholar : PubMed/NCBI
|