Introduction
Gastrointestinal stromal tumors (GISTs) are
mesenchymal tumors that commonly occur in the gastrointestinal
tract. They originate from the precursor cells of Cajal mesenchymal
cells in the muscle plexus. GISTs are rare tumors, with an
incidence of approximately eight cases per million per year
(1). They occur throughout the
gastrointestinal tract from the lower esophagus to the anus. The
most common site is the stomach (60%), followed by the small
intestine (35%) and rectum, esophagus, omentum, and mesentery (less
than 5% each) (2).
The incidence of small intestinal GISTs is
increasing, which may reflect advances in radiologic and endoscopic
techniques and an increase in the frequency of imaging (3,4). Small
intestinal GISTs have a higher recurrence rate compared to gastric
GISTs (5–7), and the 5-year disease-free survival
rate is 44–89% (3,5,8).
Miettinen et al proposed a risk classification system based
on the differences in recurrence rates for each tumor location
(2). However, owing to the rarity
of GISTs, data on GISTs of the small intestine and other intestinal
regions are lacking (2,7). The aim of this study was to identify
the factors involved in the recurrence of small intestinal GISTs
using data obtained from patients treated at our institution.
Patients and methods
Patients
A retrospective review was conducted of adult
patients who underwent surgery for small intestinal tumors at Chiba
University Hospital between January 2002 and December 2021. Among
these, patients who were histologically diagnosed with primary
small intestinal GIST, had undergone preoperative imaging to assess
for distant metastasis, and received curative resection were
included in this study. Patients who underwent palliative surgery,
had concurrent active malignancies, or lacked sufficient
clinicopathological data were excluded. Based on these criteria, 26
patients with 27 lesions were included in the present study. Data
on demographic details, clinical history, preoperative evaluation
including blood tests and imaging studies, treatment details,
histopathological examination results, clinical course, and
survival follow-up were extracted from the patients' medical
records.
Ethical approval
This study was approved by the Ethics Committee of
Chiba University Graduate School of Medicine (Chiba, Japan;
approval no: 3043). Consent for this study was provided on an
opt-out basis by 26 patients.
Statistical analysis
The Fisher's exact test was used for categorical
variables, and the Wilcoxon test was used for continuous variables,
as appropriate. The cut-off values for continuous variables were
set using the receiver operating characteristic (ROC) curve, as the
values vary depending on the report and there are no clear standard
values. The outcome was defined as recurrence. Univariate
predictors of recurrence were determined using a nominal logistic
regression analysis. Correlations between the factors were
estimated using the pairwise method. Statistical analyses were
performed using JMP Pro version 18.1.1 (JMP Statistical Discovery
LLC., Cary, NC, USA), and statistical significance was set at
P<0.05.
Results
Characteristics of patients
The patients' characteristics are listed in Table I. The median patient age was 59
years (15 male and 11 female). The localization of the tumor was as
follows: 10 lesions in the duodenum, 11 lesions in the jejunum, and
five lesions in the ileum, with one lesion in which the tumor was
large and the localization was unclear. Sixteen patients had
clinical symptoms at the time of their first visit, and the main
symptoms were gastrointestinal bleeding, abdominal pain, and
abdominal distension. Positron emission tomography-computed
tomography (PET-CT) data were available for 21 lesions, with a
median maximum standardized uptake value (SUVmax) of 4.16. Fourteen
lesions were classified as moderate or high risk according to
Miettinen's recurrence risk classification, including nine lesions
in the jejunum, four lesions in the ileum, and one large lesion
that spanned the jejunum and ileum. One patient was treated with
imatinib preoperatively, and 12 received postoperative adjuvant
chemotherapy with imatinib. Recurrence occurred in nine patients,
six of whom were administered adjuvant chemotherapy. The sites of
recurrence were the liver, lymph nodes, and peritoneum. Hepatic
resection was performed in four patients with solitary liver
metastasis. The median observation period for all patients was 72
(11–208) months.
 | Table I.Clinicopathological features of all
patients. |
Table I.
Clinicopathological features of all
patients.
| Variables | Groups | Value |
|---|
| Age, years | Median (min-max) | 59 (42–81) |
| Sex, n | Male/female | 15/11 |
| Localization, n | D/J/I/J-I | 10/11/5/1 |
| Clinical symptom,
n | Yes/no | 16/10 |
| Tumor size, mm | Median (min-max) | 47 (10–300) |
| SUVmax | Median (min-max) | 4.16 (0–24) |
| WBC, /µl | Median (min-max) | 5,800
(2,200–11,600) |
| Hb, g/dl | Median (min-max) | 12.3 (9.4–15.7) |
| TP, g/dl | Median (min-max) | 7.1 (4.6–8.1) |
| Alb, g/dl | Median (min-max) | 4.3 (2.2–4.9) |
| CRP, mg/dl | Median (min-max) | 0.1 (0.0–3.7) |
| NLR | Median (min-max) | 2.3 (1.1–8.8) |
| PLR | Median (min-max) | 212.5
(74.4–589.6) |
| OPNI | Median (min-max) | 51.2 (30.5–58.6) |
| CEA, ng/ml | Median (min-max) | 1.5 (0.4–5.7) |
| CA19-9, U/ml | Median (min-max) | 8.3 (0.1–95.8) |
| Operative procedure,
n | PpPD/PR/lap-PR | 2/23/1 |
| Mitotic count,
/50HPF | Median (min-max) | 4 (0–62) |
| Ki-67 index, % | Median (min-max) | 5.0 (1.0–30.8) |
| Risk classification
(Miettinen), n |
None/low/moderate/high/NA | 4/8/4/10/1 |
| Adjuvant
chemotherapy, n | Yes/no | 12/14 |
| Recurrence, n | Yes/no | 9/17 |
| Recurrent
organsa, n | Liver/lymph
node/peritoneal dissemination | 6/2/2 |
| Observation period,
months | Median (min-max) | 72 (11–208) |
Comparison of patients with recurrence
and no recurrence of tumors
Patient backgrounds, tumor factors, and laboratory
values of recurrence and no-recurrence cases are listed in Table II. No consistent trends were
observed in terms of age, sex, or the presence of clinical symptoms
(P=0.571, 0.217, and 0.399, respectively). The tumor locations were
significantly different between the recurrence group and the
no-recurrence group (non-duodenal tumors: 100 (9/9) vs. 44% (8/18);
P=0.009). Regarding tumor factors, the recurrence group had larger
tumor sizes (70 vs. 40 mm; P=0.010), higher SUVmax (8.8 vs. 3.3;
P=0.032), and higher Ki-67 index (12.8 vs. 4.2; P=0.019) than those
in the no-recurrence group. In addition, the recurrence group had
lower hemoglobin level (11.4 vs. 13.2 g/dl; P=0.046), higher
neutrophil-to-lymphocyte ratio (NLR; 4.4 vs. 1.9; P=0.040), and
higher platelet-to-lymphocyte ratio (PLR) (269.8 vs. 161.8;
P=0.036) than those in the no-recurrence group. No differences were
observed in the levels of carcinoembryonic antigen (CEA) and
CA19-9, commonly used tumor markers in gastrointestinal cancer,
between the two groups (P=0.979 and 0.872, respectively). The
median observation period for the recurrence group was 120
(11–208) months, and the period to
recurrence was 66 (4–176) months.
The median observation period for the no-recurrence group was 64
(41–101) months.
 | Table II.Patient characteristics with or
without recurrence. |
Table II.
Patient characteristics with or
without recurrence.
| Variables | Group | Recurrence
(n=9/9)a | No-recurrence
(n=17/18)a | P-value |
|---|
| Age, years | Median (min-max) | 59 (43–75) | 59 (42–81) | 0.571b |
| Sex, n | Male/female | 7/2 | 8/9 | 0.217c |
| Localization, n | D/J, I, J-I | 0/6, 2, 1 | 10/5, 3, 0 | 0.009c |
| Clinical symptom,
n | Yes/no | 7/2 | 9/8 | 0.399c |
| Tumor size, mm | Median (min-max) | 70 (42–300) | 40 (10–115) | 0.010b |
| SUVmax | Median (min-max) | 8.8 (3.3–18.7) | 3.3 (0.0–24.0) | 0.032b |
| Mitotic count,
/50HPF | Median (min-max) | 9 (3–23) | 3 (0–62) | 0.073b |
| Ki-67 index, % | Median
(min-max) | 12.8
(2.0–30.0) | 4.2 (1.0–30.8) | 0.019b |
| WBC, /µl | Median
(min-max) | 6,400 (3,700–9,600) | 5,600 (2,200–11,600) | 0.571b |
| Hb, g/dl | Median
(min-max) | 11.4
(9.6–14.9) | 13.2
(9.4–15.7) | 0.046b |
| TP, g/dl | Median
(min-max) | 6.7 (5.8–7.7) | 7.2 (4.6–8.1) | 0.137b |
| Alb, g/dl | Median
(min-max) | 4.0 (3.0–4.8) | 4.3 (2.2–4.9) | 0.449b |
| OPNI | Median
(min-max) | 46.1
(34.7–58.6) | 52.0
(30.5–58.3) | 0.131b |
| CRP, mg/dl | Median
(min-max) | 0.1 (0.0–0.7) | 0.1 (0.0–3.7) | 0.735b |
| NLR | Median
(min-max) | 4.4 (1.4–8.8) | 1.9 (1.1–8.5) | 0.040b |
| PLR | Median
(min-max) | 269.8
(115.9–589.6) | 161.8
(74.4–429.3) | 0.036b |
| CEA, ng/ml | Median
(min-max) | 1.5 (0.7–3.7) | 1.5 (0.4–5.7) | 0.979b |
| CA19-9, U/ml | Median
(min-max) | 8.6 (0.1–24.7) | 8.0 (0.1–95.8) | 0.872b |
| Adjuvant
chemotherapy, n | Yes/no | 5/4 | 6 / 11 | 0.411c |
| Observation period,
months | Median
(min-max) | 120 (11–208) | 64 (41–101) | 0.004b |
| Period to
recurrence, months | Median
(min-max) | 66 (4–176) | - | - |
Factors associated with
recurrence
To investigate the association between recurrence
and clinicopathological characteristics in patients with small
intestinal GISTs, a univariate nominal logistic analysis was
performed. The results showed that jejunal lesions [odds ratio
(OR)=7.8; 95% confidence interval (CI): 1.13–67.4; P=0.023], tumor
size ≥60 mm (OR=11.38; 95% CI: 1.9–101.71; P=0.007), SUVmax ≥8.4
(OR=32.5; 95% CI: 3.25–851.4; P=0.002), mitotic counts ≥4/50 HPF
(OR=9.43; 95% CI: 1.25–199.01; P=0.028), Ki-67 index ≥10% (OR=24.5;
95% CI: 3.13–537.24; P=0.001), NLR ≥3.7 (OR=16.33; 95% CI:
2.57–159.05; P=0.002), PLR ≥227 (OR=11.78; 95% CI: 1.9–101.71;
P=0.007), and Onodera's prognostic nutritional index (OPNI) ≤51
(OR=6.42; 95% CI: 1.13–53.39; P=0.035) were significantly
associated with postoperative recurrence (Table III). Because the number of events
was small, a multivariate analysis was not performed. The
sensitivity and specificity of each factor are listed in Table IV. The mitotic counts and Ki-67
indices had high sensitivity, whereas the SUVmax and NLR values had
high specificity.
 | Table III.Univariate analysis of
recurrence. |
Table III.
Univariate analysis of
recurrence.
| Variables | Groups | OR | 95% CI | P-value |
|---|
| Age, year | ≤48 | 2.14 | 0.22–21.12 | 0.491 |
|
| >48 | Ref. |
|
|
| Sex | Male | 3.94 | 0.70–31.99 | 0.123 |
|
| Female | Ref. |
|
|
| Localization | Jejunum | 7.80 | 1.13–67.40 | 0.023 |
|
| Others | Ref. |
|
|
| Tumor size, mm | ≥60 | 11.38 | 1.90–101.71 | 0.007 |
|
| <60 | Ref. |
|
|
| SUVmax | ≥8.4 | 32.50 | 3.25–851.40 | 0.002 |
|
| <8.4 | Ref. |
|
|
| Mitotic count,
/50HPF | ≥4 | 9.43 | 1.25–199.01 | 0.028 |
|
| <4 | Ref. |
|
|
| Ki-67 index, % | ≥10 | 24.5 | 3.13–537.24 | 0.001 |
|
| <10 | Ref. |
|
|
| WBC, /µl | ≥6,200 | 3.67 | 0.70–22.95 | 0.125 |
|
| <6,200 | Ref. |
|
|
| Hb, g/dl | ≤11.4 | 6.00 | 0.90–54.26 | 0.065 |
|
| >11.4 | Ref. |
|
|
| NLR | ≥3.7 | 16.33 | 2.57–159.05 | 0.002 |
|
| <3.7 | Ref. |
|
|
| PLR | ≥227 | 11.78 | 1.90–101.71 | 0.007 |
|
| <227 | Ref. |
|
|
| OPNI | ≤51 | 6.42 | 1.13–53.39 | 0.035 |
|
| >51 | Ref. |
|
|
| CEA, ng/ml | ≥1.8 | 0.71 | 0.12–3.76 | 0.694 |
|
| <1.8 | Ref. |
|
|
| CA19-9, U/ml | ≥2.9 | 0.5 | 0.018–13.75 | 0.642 |
|
| <2.9 | Ref. |
|
|
 | Table IV.Prediction accuracy of each
factor. |
Table IV.
Prediction accuracy of each
factor.
| Variables | Sensitivity, % | Specificity, % | AUC |
|---|
| Jejunal lesion | 75.0 | 70.6 | - |
| Tumor size ≥60
mm | 77.8 | 76.5 | 0.82 |
| SUVmax ≥8.4 | 71.4 | 92.3 | 0.82 |
| Mitotic count
≥4/50HPF | 85.7 | 64.7 | 0.73 |
| Ki-67 index
≥10% | 87.5 | 76.5 | 0.81 |
| NLR ≥3.7 | 77.8 | 82.4 | 0.75 |
| PLR ≥227 | 77.8 | 76.5 | 0.76 |
| OPNI ≤51 | 77.8 | 64.7 | 0.69 |
Correlations among factors associated
with recurrence
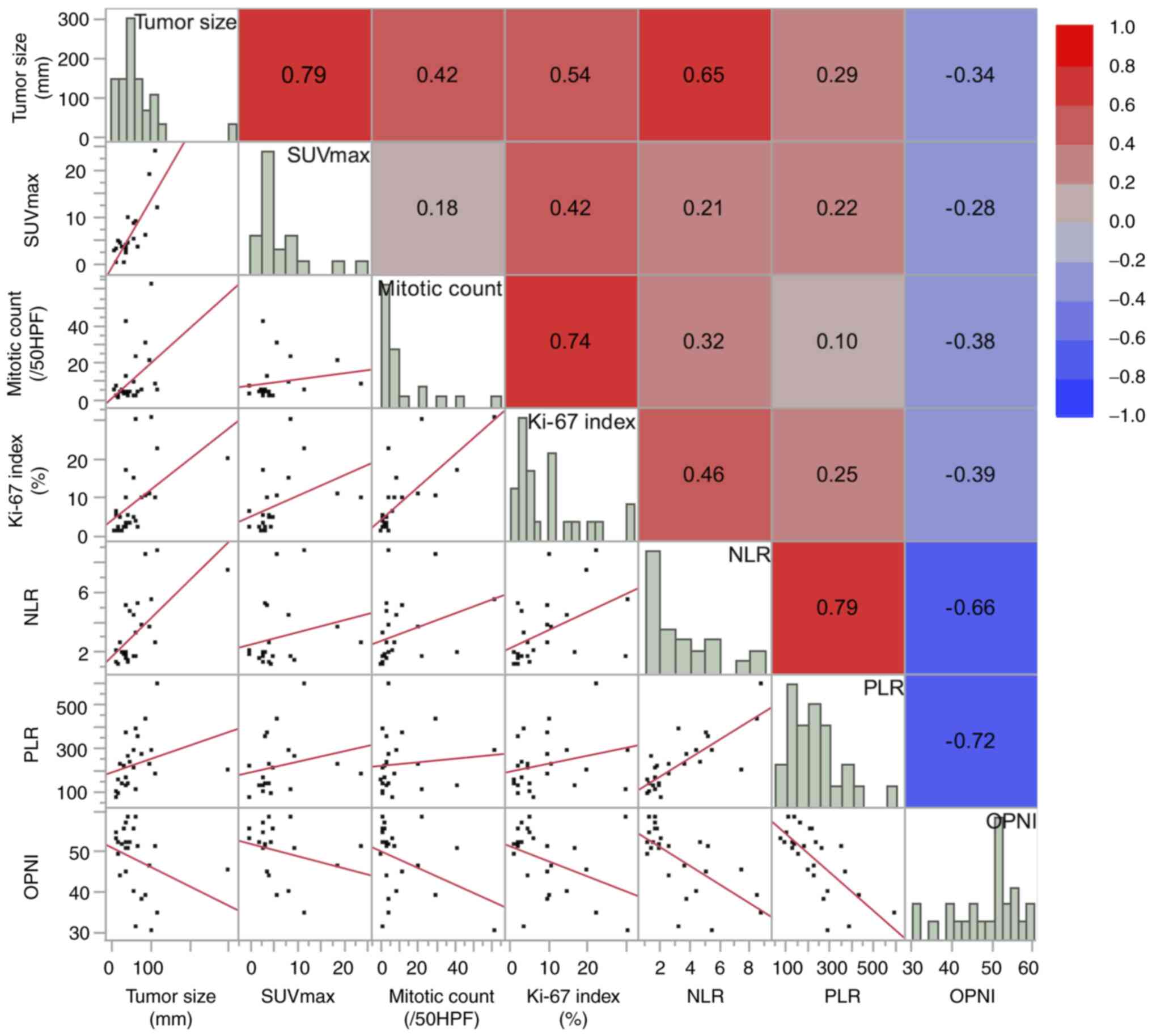
Fig. 1 shows the
correlations between continuous variables. Scatter plots for each
pair of variables are displayed in the lower left of the matrix,
while the corresponding correlation coefficients are shown in the
upper right and presented as a heat map. Among the tumor factors
(tumor size, SUVmax, mitotic counts, Ki-67 index), strong
correlations were observed between tumor size and SUVmax (R=0.79;
P<0.0001) and between mitotic counts and Ki-67 index values
(R=0.74; P<0.0001). Among the patient factors (NLR, PLR, OPNI),
NLR and PLR showed a strong correlation (R=0.79; P<0.0001), and
both were negatively correlated with OPNI (R=−0.66 and −0.72;
P<0.001 and P<0.0001, respectively). Regarding the
relationship between tumor factors and patient factors, NLR showed
a moderate correlation with tumor size (R=0.65; P=<0.001) and
Ki-67 index (R=0.46; P=0.021), whereas PLR and OPNI exhibited only
weak correlations with tumor factors (R ranging from 0.10 to 0.29
for PLR, and −0.38 to −0.28 for OPNI).
Discussion
In the present study, we reviewed the outcomes of
patients with small intestinal GISTs who underwent curative
resection at our institution. Of the 26 patients included in this
study, recurrence occurred in nine patients (34.6%), which is
comparable to the rate observed in previous studies (5). Although some GISTs metastasize within
1–2 years or less than a year of development of the initial tumor,
in some cases, metastasis may occur after a long delay, such as 42
years after the initial tumor development (7). The median period to recurrence in this
study was more than five years, indicating the need for long-term
follow-up. In addition, approximately half of the patients with
recurrent tumors in our study received adjuvant chemotherapy with
imatinib, which may be related to the long time to recurrence.
Adjuvant chemotherapy is recommended to be administered for three
years after surgery for patients with high-risk GISTs after radical
resection; however, the duration of adjuvant chemotherapy
administration remains uncertain. Ongoing randomized clinical
trials are evaluating the efficacy of administering adjuvant
therapy for more than three years (1).
Tumor location, tumor size, and mitotic counts are
important prognostic factors, and risk classifications that include
these factors, such as the Miettinen or modified Fletcher
(mFletcher) classification, are commonly used. These factors were
also found to be associated with recurrence in this study. Ki-67, a
nuclear marker of actively proliferating cells, is an indicator of
cell proliferation and mitotic counts (9); a strong correlation was observed
between the Ki-67 index and mitotic counts. Furthermore, the Ki-67
index was more sensitive and specific for recurrence than the
mitotic counts. Because the Ki-67 index may show less variability
between observers than that shown by mitotic counts (10), use of the Ki-67 index may be a
potential alternative to the use of mitotic counts for analysis of
recurrence.
The SUVmax in GISTs has been reported to be
significantly high in the high-risk groups categorized according to
the Fletcher and mFletcher classifications (11–13).
In this study, the SUVmax showed a high specificity for recurrence
(92.3%), suggesting that SUVmax may be used to accurately identify
patients with a high risk of recurrence. In addition, SUVmax
enables preoperative evaluation, whereas tumor resection is
necessary for accurate evaluation of the mitotic counts and Ki-67
index. This may be useful for determining the indications and
efficacy of preoperative adjuvant chemotherapy. Furthermore, it
provides information on the necessity of postoperative adjuvant
chemotherapy prior to surgery, which is helpful in determining the
treatment schedule and informing patients.
The prognosis of GIST is associated not only with
tumor characteristics, but also with host immune and inflammatory
responses; however, the underlying mechanism has not been
elucidated (14–17). This study showed that the patient
factors NLR, PLR, and OPNI were significantly associated with
recurrence. This suggests that the immune and nutritional status of
patients are involved in tumor progression. These factors could be
modified by drugs that affect white blood cell and platelet counts,
such as steroids, granulocyte colony-stimulating factor
preparations, and thrombopoietin receptor agonists, or by
nutritional interventions. If immune and nutritional status
influence tumor progression, these factors could become novel
therapeutic targets. Furthermore, NLR was moderately correlated
with tumor factors, such as tumor size and Ki-67 index, suggesting
that tumor progression itself may also alter the host's immune
status. These findings may provide a basis for future studies
investigating whether immune status and nutritional status play a
role in the management of GIST.
Most GISTs, approximately 85–90%, contain genetic
mutations in KIT or platelet derived growth factor receptor
(PDGFRA) (7). The vast majority of
KIT mutations are found in exon 11 (66–71%), exon 9 (13–15%), exon
13 (1–3%), and exon 17 (1–3%) (18,19).
GISTs with KIT exon 9 mutations have a poorer prognosis than tumors
with exon 11 mutations and are resistant to imatinib (19). Furthermore, KIT exon 9 mutations are
almost specific to intestinal GIST, and the poor prognosis of small
intestinal GISTs have been suggested to be caused by the high
frequency of exon 9 mutations (2,19).
PDGFRA mutations are found in 30% of KIT wild-type GISTs and are
associated with resistance to imatinib (7). Genetic mutation information was not
obtained in this study, and the association between the recurrence
risk factors identified in this study and genetic mutations is
uncertain. Further investigation of the association between
clinical factors and genetic factors is needed.
This study had several limitations. First, as this
was a retrospective study, it was prone to selection and
information biases. Second, the sample size included in the study
was relatively small. The small number of recurrence events (n=9),
which was insufficient to allow for a reliable multivariate
analysis. Therefore, the independence of each factor could not be
evaluated. Third, the observation period for the group with
non-recurrent tumors was short, and cases of recurrence may not
have been accurately identified. Fourth, the lack of information on
genetic mutations prevented analysis of their association with the
identified risk factors. Fifth, as this was an observational study,
and the potential for intervention of the identified risk factors
could not be evaluated. Sixth, since this was a
single-institutional study, the generalizability of our findings
may be limited. However, larger prospective studies are required to
validate these findings.
In conclusion, in this study, we investigated the
factors associated with recurrence in patients with small
intestinal GISTs who underwent curative resection. Our findings
suggest that tumor location, tumor size, SUVmax, mitotic counts,
Ki-67 index, and inflammatory/nutritional marker levels, such as
NLR, PLR, and OPNI levels, are significantly associated with
recurrence. In addition, NLR was correlated with tumor size and
Ki-67 index. The identification of novel risk factors in addition
to known risk factors may enhance current risk classification and
allow for more accurate risk stratification of small intestinal
GISTs. It may also provide information on the indications and
management of perioperative adjuvant chemotherapy. Furthermore,
elucidating the fundamental mechanisms underlying the association
between inflammation/nutritional indicators and tumor behavior may
clarify the oncological characteristics of GIST and contribute to
the establishment of novel treatment strategies.
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
YN, YM, GO, KH, MM and HM contributed to the
conceptualization of this study. YM, GO, KH, MM and HM conducted
project administration. YN, YM, TT, TM, YK, RO, KO and AH performed
data collection and analysis. GO and KH confirm the authenticity of
all the raw data. YN wrote the manuscript with support from KO and
AH. All authors provided critical feedback and read and approved
the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Chiba University Graduate School of Medicine (Chiba, Japan;
approval no. 3043). Consent for this study was provided on an
opt-out basis by 26 patients.
Patient consent for publication
Patient consent for the publication of their
clinical data was obtained on an opt-out basis.
Competing interests
The authors declare that they have no competing
interests.
Authors' information
Yuri Nishioka, ORCID: https://orcid.org/0009-0003-1479-5123; Yasunori
Matsumoto, ORCID: https://orcid.org/0000-0002-6239-6691.
References
|
1
|
Casali PG, Blay JY, Abecassis N, Bajpai J,
Bauer S, Biagini R, Bielack S, Bonvalot S, Boukovinas I, Bovee
JVMG, et al: Gastrointestinal stromal tumours:
ESMO-EURACAN-GENTURIS Clinical Practice Guid elines for diagnosis,
treatment and follow-up. Ann Oncol. 33:20–33. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors: Review on morphology, molecular pathology,
prognosis, and differential diagnosis. Arch Pathol Lab Med.
130:1466–1478. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Peng F and Liu Y: Gastrointestinal stromal
tumors of the small intestine: Progress in diagnosis and treatment
research. Cancer Manag Res. 12:3877–3889. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Giuliano K, Nagarajan N, Canner J,
Najafian A, Wolfgang C, Schneider E, Meyer C, Lennon AM, Johnston
FM and Ahuja N: Gastric and small intestine gastrointestinal
stromal tumors: Do outcomes differ? J Surg Oncol. 115:351–357.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopez Gordo S, Bettonica C, Miró M,
Estremiana F, Aranda H and Farran L: Gastric and small intestine
gist: Results of 156 cases in 20 years. J Gastrointest Cancer.
53:451–459. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Joensuu H, Vehtari A, Riihimäki J, Nishida
T, Steigen SE, Brabec P, Plank L, Nilsson B, Cirilli C, Braconi C,
et al: Risk of recurrence of gastrointestinal stromal tumour after
surgery: An analysis of pooled Population-based cohorts. Lancet
Oncol. 13:265–74. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Miettinen M and Lasota J: Gastrointestinal
stromal tumors. Gastroenterol Clin North Am. 42:399–415. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu TJ, Lee LY, Yeh CN, Wu PY, Chao TC,
Hwang TL, Jan YY and Chen MF: Surgical treatment and prognostic
analysis for gastrointestinal stromal tumors (GISTs) of the small
intestine: Before the era of imatinib mesylate. BMC Gastroenterol.
6:292006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhou Y, Hu W, Chen P, Abe M, Shi L, Tan
SY, Li Y and Zong L: Ki67 is a biological marker of malignant risk
of gastrointestinal stromal tumors: A systematic review and
meta-analysis. Medicine (Baltimore). 96:e79112017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong NA, Young R, Malcomson RD, Nayar AG,
Jamieson LA, Save VE, Carey FA, Brewster DH, Han C and Al-Nafussi
A: Prognostic indicators for gastrointestinal stromal tumours: A
clinicopathological and immunohistochemical study of 108 resected
cases of the stomach. Histopathology. 43:118–126. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Narushima K, Shuto K, Okazumi S, Ohira G,
Mori M, Hayano K, Yanagawa N and Matsubara H: Malignant diagnosis
and prognostic analysis of 89 GIST patients using preoperative
FDG-PET. Sci Rep. 13:22662023. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cho MH, Park CK, Park M, Kim WK, Cho A and
Kim H: Clinicopathologic features and molecular characteristics of
glucose metabolism contributing to
¹8F-fluorodeoxyglucose uptake in gastrointestinal
stromal tumors. PLoS One. 10:e01414132015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du W, Cui G, Wang K and Li S: Clinical
significance of 18F-FDG PET/CT imaging in 32 cases of
gastrointestinal stromal tumors. Eur J Med Res. 27:1822022.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng F, Tian Y, Liu S, Zheng G, Liu Z, Xu
G, Guo M, Lian X, Fan D and Zhang H: Combination of PLR, MLR, MWR,
and tumor size could significantly increase the prognostic value
for gastrointestinal stromal tumors. Medicine (Baltimore).
95:e32482016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wang F, Tao T, Yu H, Xu Y, Yang Z, Xia X,
Wang M, Zong L and Guan W: Prognostic value of Onodera's
nutritional index for intermediate- and high-risk gastrointestinal
stromal tumors treated with or without tyrosine kinase inhibitors.
World J Surg Oncol. 19:2272021. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang H, Xu YY, You J, Hu WQ, Wang SF, Chen
P, Yang F, Shi L, Zhao W and Zong L: Onodera's Prognostic
Nutritional Index is a novel and useful prognostic marker for
gastrointestinal stromal tumors. World J Gastrointest Surg.
13:1202–1215. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ren W, Wang H, Xiang T and Liu G:
Prognostic role of preoperative Onodera's prognostic nutritional
index (OPNI) in gastrointestinal stromal tumors: A systematic
review and Meta-analysis. J Gastrointest Cancer. 54:731–738. 2023.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhou S, Abdihamid O, Tan F, Zhou H, Liu H,
Li Z, Xiao S and Li B: KIT mutations and expression: Current
knowledge and new insights for overcoming IM resistance in GIST.
Cell Commun Signal. 22:1532024. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Debiec-Rychter M, Sciot R, Le Cesne A,
Schlemmer M, Hohenberger P, van Oosterom AT, Blay JY, Leyvraz S,
Stul M, Casali PG, et al: KIT mutations and dose selection for
imatinib in patients with advanced gastrointestinal stromal
tumours. Eur J Cancer. 42:1093–103. 2006. View Article : Google Scholar : PubMed/NCBI
|