Introduction
Multiple myeloma (MM) is an incurable malignant
plasma cell disorder characterized by the infiltration of clonal
plasma cells in the bone marrow compartment. It is the second most
common hematologic malignancy with an estimated incidence of
5/100.000 (1). The introduction of
novel agents has significantly prolonged the survival of patients
with MM and enhanced their quality of life (2).
Despite significant progress in the therapeutic
landscape of MM, there remains a proportion of patients, considered
as ‘high risk’ patients, who do not exhibit favorable outcomes
(3). Identifying patients with
‘high risk’ MM is crucial for clinicians since these patients may
benefit from an alternative, more intense therapeutic approach. In
everyday practice, patient stratification is based on the ISS and
R2-ISS staging systems, on cytogenetic abnormalities and
common laboratory findings. Combining these existing approaches
with molecular approaches may offer an improved understanding of
high-risk disease and optimize the recognition of such
patients.
Bone morphogenetic proteins (BMPs) represent a group
of pleiotropic proteins, members of the TGF-β superfamily, that are
now considered multifunctional cytokines. Extensive research in the
previous decades has revealed that BMPs are typically
differentiation factors that are involved in bone metabolism,
tissue development and carcinogenesis (4).
To date, ≥20 members of the BMP family have been
identified (5). Their activity is
mediated upon binding as homodimers or heterodimers to their
respective serine/threonine kinase receptors and ultimately their
signal is intracellularly transduced via SMAD proteins. The diverse
effects that are observed among different BMPs imply a strict
regulation of their activity. It is hypothesized that this strict
regulation is achieved through ligand's availability, through the
concurrent activation or inactivation of different BMP molecules,
through up- or down-regulation of BMP receptors and through the
presence of inhibitory or stimulatory molecules (5).
In MM, BMP signaling is not as well characterized
and its association with the pathogenesis of MM is still poorly
understood. The non-tumor-specific mechanism of action of BMPs is
probably the reason for the limited interest and data of this
pathway in MM, resulting in the lack of knowledge regarding their
prognostic significance. Previous studies attempting to assess
endogenous expression of BMPs in myeloma cells reported elevated
expression of BMP4 and BMP6 in patients with MM compared to healthy
donors (HD) (6,7). Similarly, serum analysis found
increased protein expression levels of BMP9 and BMP2 in patients
with MM compared to controls (8,9).
Prompted by the known tumor-inductive effects of
BMP2 and BMP6 in several cancer types (10–13),
the expression of BMP2 and BMP6 in a cohort of MM patients was
assessed and the clinical impact of their expression was
determined.
Patients and methods
Study design and patients
This was a retrospective study aiming to assess the
clinical significance of BMP2 and BMP6 in patients with newly
diagnosed multiple myeloma. Study endpoints were to evaluate the
expression levels of BMP2 and BMP6 in NDMM and to determine whether
BMP2 or BMP6 could serve as prognostic biomarkers for patients with
NDMM. The study enrolled NDMM patients that were diagnosed and
treated in the Department of Hematology of University Hospital of
Larissa, Greece. Diagnosis of MM was based on the International
Myeloma Working Group consensus criteria (14). The enrollment period was from March
2019 to December 2021. Nine healthy volunteers (male 5, median age
60, range 51–75) were additionally enrolled, at the same period of
time. All participants provided written informed consent, prior to
recruitment, to the use of their biological samples and clinical
data in research and the study was reviewed and approved by the
Institutional Review Board of the University Hospital of Larissa
(approval code 21906) and adhered to the tenets of The Declaration
of Helsinki. Clinical and laboratory data (collected prospectively)
were retrieved from the electronic database and biological samples
were retrieved from the department's biobank. All samples were
acquired at the time of diagnosis and prior to treatment
initiation.
Sampling, RNA extraction, cDNA
synthesis and quantitative PCR (qPCR)
Bone marrow aspirates were collected in EDTA tubes
and immediately processed (within 2 h post sampling). Bone marrow
mononuclear cells (BMMNCs) were isolated using density gradient
separation with a Ficoll Paque Plus kit (MilliporeSigma) (Fig. S1). Bone marrow was diluted 1:1 with
DPBS (Biowest) and was filtered through a 100 µm pore to remove
cell clumps, clots and bone fragments. 4 ml of diluted cell
suspension was carefully layered over 4 ml of Ficoll Paque Plus and
an immediate centrifugation step (400 × g at 20°C for 30 min
without brake) followed. The upper plasma layer was aspirated and
the interphase was drawn for washing steps and further analyses.
Cells were then washed with DPBS (Biowest), lysed with red blood
cell lysis buffer (Cell Signaling Technology, Inc.) if required,
aliquoted and stored as dry pellets at −80°C until further
analysis. Total RNA was retrieved from BMMNCs using an E.Z.N.A.
Total RNA kit I (Omega-Biotek Inc.) according to manufacturer's
instructions. Briefly, 5×106 pelleted cells were lysed
using TRK lysis buffer and homogenized with a syringe. The lysate
was then filtered through columns to remove debris and other
contaminants. Washing steps were applied along with a middle
DNA-digestion step and high-yield RNA was eventually eluted in 30
µl nuclease-free water. RNA concentration was quantified at 260 nm
on a Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific,
Inc.). Prior to cDNA synthesis, purity and integrity of eluted RNA
samples was verified by 1% agarose gel electrophoresis and by
A260/280 (median ratio 2.08, range 1.8–2.14)-A260/230 (median ratio
1.93, range 1.4–2.2) absorbance ratios. As expected, agarose gel
electrophoresis of RNA displayed the presence of 2 ribosomal bands
(28S and 18S) with no smearing or any DNA band and the absorbance
ratios were within accepted bounds (15–19).
Total RNA was subsequently reverse transcribed into cDNA in a 20 µl
reaction volume using a QuantiTect Reverse Transcription kit
(Qiagen, GmbH). Approximately, 100 µg RNA template was incubated at
42°C with dNTPs, RNAse inhibitor, Quantiscript's Reverse
Transcriptase, RT Buffer, RT Primer Mix along with RNase-free
water. An initial incubation of 3 min at 95°C was applied to
activate Quantiscript Reverse Transcriptase. cDNA was diluted and
then deposited at −20°C. qPCR was carried out in a 36 well Rotor
Gene Q (Qiagen) with CYBR green 1 as fluorescent dye. Β-2
microglobulin (B2M) was used as housekeeping gene. Intra-spanning
primers with amplicon lengths of around 100 bp (acquired from
Qiagen GmbH, GeneGlobe ID: SBH0312240 for B2M, GeneGlobe ID:
SBH0638609 for BMP2 and GeneGlobe ID: SBH0513495 for BMP6) were
used to ensure that traces of genomic DNA would not affect the PCR.
Template cDNA was mixed with QuantiNova Probe PCR Master Mix and
RNase-free water with volumes according to the manufacturer's
protocol. The cycling conditions included an initial incubation
step at 95°C for 2 min followed by 40 cycles of 95°C for 5 sec and
60°C for 10 sec. Specificity of each reaction was confirmed by
resolving amplification products in a 2% agarose gel, stained with
ethidium bromide (Fig. S2).
Appropriate sample handling was verified by performing PCR
reactions in duplicates. Cq values are presented as means. When for
a specific sample the disagreement between the Cq measurements was
greater than 1, the qPCR was performed for a third time and the
final Cq value was calculated as the mean of the 3 measurements.
Gene expression levels were analyzed using the Livak
2−ΔΔCq method (20),
after validating stable expression of B2M gene between patients and
healthy individuals (median raw Cq value of patients 16.3, range
13.2–24.2 and median raw Cq value of healthy donors 18.5, range
14.4–19.8, P-value 0.1).
Statistical analysis
Statistical analysis was performed with the GraphPad
Prism version 10 (Dotmatics). Results were considered significant
when P-values were <0.05. The normality of distribution was
assessed using a Shapiro-Wilk test. For non-parametric data, a Mann
Whitney U test was used to compare differences between two groups.
For parametric data, an unpaired Student's t-test was applied. The
Fisher's exact test was used to identify differences between the
proportions of categories in two independent groups. Univariate and
multivariate analyses were carried out by Cox regression analysis.
Pearson correlation was performed to assess the linear relationship
between two continuous variables and Spearman correlation was used
for categorical data. Kaplan-Meier (KM) curves were applied to plot
survival and progression-free survival. The log-rank test was used
to compare the distribution of time to event between 2 groups.
Results
Patient characteristics
A total of 18 newly diagnosed patients with MM were
included in the present study along with 9 additional healthy
volunteers who served as controls (male 5, median age 60 years,
range 51–75 years). Patient baseline characteristics are shown in
Table I. Of the patients with MM,
the median age was 62 years, 50% were male and most had IgG MM
(50%). The median percentage of bone marrow infiltration was 55%
(range 10–90%) and the median beta-2 microglobulin levels were 4.9
mg/l (range 2.3–14.1). A total of 33% of patients presented with
elevated LDH levels. High risk cytogenetics were observed in 5
patients (27%). Regarding the ISS staging, 16% were ISS-1, 38% were
ISS-2 and 44% were ISS-3. The majority of patients presented with
bone disease (72%). The median follow-up period was 50 months
(range 4–67). Patients were primarily treated either with VCD or
VRD as induction therapy and response was evaluated after five
cycles of upfront therapy.
 | Table I.Patient characteristics. |
Table I.
Patient characteristics.
| Characteristic | Value |
|---|
| Median age, years
(range) | 62 (44–86) |
| Sex, n (%) |
|
| Male | 9 (50%) |
|
Female | 9 (50%) |
| MM type, n (%) |
|
| IgG | 9 (50.0%) |
| IgA | 6 (33.3%) |
| IgM | 1 (5.6%) |
| Light
chain only | 2 (11.1%) |
| Median bone marrow
infiltration, % (range) | 55 (10–90) |
| ISS, n (%) |
|
| 1 | 3 (16.7%) |
| 2 | 7 (38.9%) |
| 3 | 8 (44.4%) |
| R2-ISS, n
(%) |
|
| 1 | 3 (16.7%) |
| 2 | 3 (16.7%) |
| 3 | 10 (55.5%) |
| 4 | 2 (11.1%) |
| Cytogenetic risk, n
(%) |
|
|
Standard | 13 (72.2%) |
| High | 5 (27.8%) |
| Lytic bone lesions, n
(%) |
|
| None | 5 (27.8%) |
| 1-3 | 5 (27.8%) |
|
>3 | 8 (44.4%) |
| Skeletal-related
events at diagnosis, n (%) |
|
|
Fractures | 7 (38.9%) |
| Bone
related RT or surgery | 6 (33.3%) |
| Plasmacytoma's at
diagnosis, n (%) | 6 (33.3%) |
| Frontline treatment,
n (%) |
|
| VCD | 7 (38.9%) |
| VRD | 7 (38.9%) |
| RD | 2 (11.1%) |
|
VRD-Autologous | 2 (11.1%) |
Expression of BMP2 and BMP6
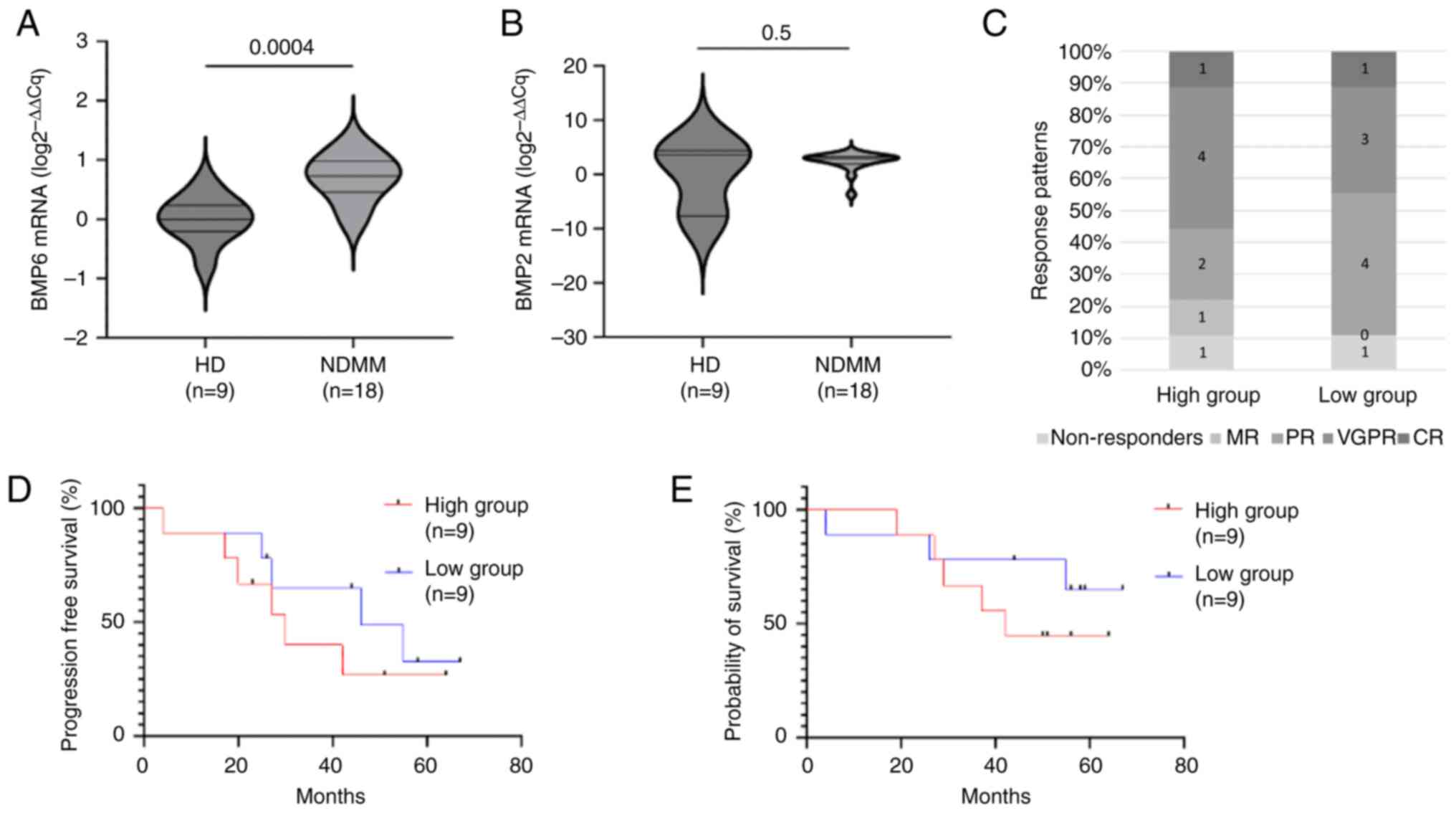
To assess whether BMP2 and BMP6 are differentially
expressed in multiple myeloma, the expression levels of BMP2 and
BMP6 genes between NDMM patients and healthy controls were
assessed. BMP6 mRNA abundance was 5-fold higher in the NDMM group
compared to the HD group (unpaired Student's t-test, P=0.0004)
(Fig. 1A). In contrast, BMP2
expression was not significantly different between the two groups
(Mann Whitney U test, P=0.5) (Fig.
1B). Based on these results, BMP6 was further studied regarding
the clinical impact of its expression. Patients were categorized
into two groups based on the expression levels of BMP6. The median
ΔCq value of the NDMM group was used as the threshold to divide the
patients into high and low BMP6 expression groups, each with 9
patients. In the high expression group, the median ΔCq value was
10.1 (range 9.2–11) while in the low expression group the median
ΔCq value was 11.8 (range 11.2–13.7). The characteristics of each
group are summarized in Table
II.
 | Table II.Characteristics of each patient
group, stratified according to BMP6 expression. |
Table II.
Characteristics of each patient
group, stratified according to BMP6 expression.
| Characteristic | High BMP6 Group
(n=9) | Low BMP6 Group
(n=9) |
|---|
| Median age, years
(min-max) | 59 (44–84) | 63 (52–86) |
| Sex (M/F) | 5/4 | 4/5 |
| ISS stage |
|
|
| ISS
stage 1 | 2 | 1 |
| ISS
stage 2 | 3 | 4 |
| ISS
stage 3 | 4 | 4 |
| R2-ISS
stage |
|
|
|
R2-ISS stage 1 | 2 | 1 |
|
R2-ISS stage 2 | 2 | 1 |
|
R2-ISS stage 3 | 4 | 6 |
|
R2-ISS stage 4 | 1 | 1 |
| Median bone marrow
infiltration, % (min-max) | 60 (15–80) | 50 (10–90) |
| Presence of
high-risk cytogenetics | 3 | 2 |
| Frontline
treatment |
|
|
|
VCD | 4 | 3 |
|
VRD | 2 | 5 |
| RD | 1 | 1 |
|
VRD-Autologous | 2 | 0 |
Predictive value of BMP6
The overall response rate (≥PR) was 77% (n=7/9) for
the high expression group and 88% (n=8/9) for the low expression
group, whereas the ≥ VGPR rate was 55% (n=5/9) and 44% (n=4/9),
respectively. Response patterns are shown in Fig. 1C. The median progression free
survival (calculated using the Kaplan Meier curves) was 30 months
for the high expression group compared to 46 months for the low
expression group (log-rank HR=1.6, 95% CI: 0.4–5.3, P=0.3)
(Fig. 1D). The median estimated
overall survival was 42 months for the high expression group and
was not reached for the low expression group (log-rank HR=1.9, 95%
CI: 0.4–7.8, P=0.4) (Fig. 1E). In
the univariate analysis, age (P=0.06, HR=1.06, 95% CI: 0.99–1.13),
LDH levels (P=0.2, HR=1.006, 95% CI: 0.99–1.01), percentage of bone
marrow infiltration (P=0.3, HR=3.9, 95% CI: 0.1–80),
b2-microglobulin levels (P=0.5, HR=1.07, 95% CI: 0.8–1.3) and ΔCq
values of BMP6 (P=0.1, HR=0.6, 95% CI: 0.3–1.1) were factors
affecting survival. Whereas, for most factors statistical
significance was not reached, they are described as affecting
survival based on the hazard ratio values. In the multivariate
analysis, age (P=0.05, HR=1.07, 95% CI: 0.99–1.16) was the only
factor affecting survival. Pearson and Spearman correlation
analyses (Fig. S3) did not show
any correlation between BMP6 ΔCq values and age (r=0.02, P=0.9),
LDH levels (r=−0.08, P=0.7), b2-microglobulin levels (r-0.2,
P=0.4), percentage of bone marrow infiltration (r=−0.1, P=0.4) and
ISS stage (rs=0.1, P=0.6) thus limiting the confounding
factors for the subgroup analysis.
BMP6 and bone disease
Next, whether BMP6 expression affected patient's
bone status at diagnosis was assessed, given that the BMP family of
proteins is a crucial signaling cascade for bone homeostasis and
that BMP6 specifically is an osteogenic mediator (21,22).
The presence of more than 3 spinal osteolytic lesions was used as
the cut-off point to divide the cohort into patients with highly
active bone disease and patients with less active bone disease. A
total of 33% (n=3/9) of patients from the high BMP6 expression
group exhibited highly active bone disease compared to 55% (n=5/9)
of patients from the low BMP6 expression group (Fisher's exact
test, P=0.6). Similarly, the rate of skeletal-related-events (based
on the presence of at least one fracture, the need for bone-related
radiotherapy and the need for bone-related surgery) was 22% (n=2/9)
for the high BMP6 expression group and 55% (n=5/9) for the low BMP6
expression group (Fisher's exact test, P=0.3).
Discussion
MM is traditionally considered a highly heterogenous
disease with distinct subtypes (23,24).
Each subtype is characterized by unique genetic and molecular
abnormalities. This diversity is the primary reason for the diverse
outcomes observed in patients. Stratifying patients, based on their
risk, is a promising approach to guide treatment decision and
treatment intensity. Despite significant progress in stratification
systems, there is still a great need for further optimization.
Combining existing staging parameters with molecular approaches may
offer a better understanding of ‘high risk’ disease and guide
therapeutic protocols.
In the present study, whether bone morphogenetic
protein 2 and 6 could serve as biomarkers and whether they could be
used to predict clinical outcomes of patients with MM was assessed.
The study enrolled 18 newly diagnosed patients and 9 age- and
sex-matched healthy donors. The expression levels of BMP2 and BMP6
genes between patients and donors were first assessed. ELISA
detection was not performed in the present study due to lack of
stored samples. While the expression of BMP6 was significantly
different between the 2 groups, this was not the case for BMP2.
Thus, only BMP6 was used for subsequent analyses.
The present study used B2M as housekeeping gene. In
general, B2M is an acceptable housekeeping gene which is considered
to have a stable and ubiquitous expression across species and cells
(25) but usually other genes such
as ACTB and GAPDH are more preferable for normalization. To verify
the stable expression of B2M as housekeeping gene, the raw Cq
values of B2M between patients (median Cq value 16.3, range
13.2–24.2) and healthy donors (median Cq value 18.5, range
14.4–19.8) were compared and no significant difference between the
2 groups was found (P-value=0.1). The interquartile range of B2M's
raw Cq values was 2.4 for healthy donors and 6.2 for patients
indicating that the Cq deviation was mostly affected from the
outliers. Verification of results with other housekeeping genes was
not performed due to lack of residual samples for some of the
patients. Literature review indicated that the observed expression
pattern of B2M of this study is in alliance with previous studies
(25–28). Thus, subsequent analysis was
performed.
The patient cohort was then divided into two
subgroups based on the expression values of BMP6. The median ΔCq
served as the cut-off point. The first subgroup included patients
with elevated expression measures of BMP6 and the second subgroup
consisted of patients with lower expression measures. Both groups
consisted of patients with similar characteristics. Comparative
analysis between the two subgroups did not show any significant
difference for response rates, progression free survival and
overall survival.
Bone morphogenetic proteins represent a large family
of proteins with pleiotropic actions both under basal and abnormal
conditions. Previous studies have delineated the significant role
of BMPs in cancer. Paradoxically, BMPs can act both as tumor
promoters and tumor suppressors (29). It is reasonable to assume that this
duality reflects their tumor-specific mechanism of action but
others have suggested that their role is ultimately determined by
their receptors, by inhibitory or stimulatory molecules and by the
concurrent activation and/or inactivation of other BMPs.
Up till now, the role of BMP signaling in MM
pathogenesis remains unclear with contradictory findings from
previous studies. In the present study, the expression of BMP2 and
BMP6 in a cohort of NDMM patients was assessed and the correlation
between their expression and clinical outcomes was determined.
BMP2, particularly, is of great interest since it is implied to
promote lung cancer adenocarcinoma migratory ability via BMP2
receptor activation and subsequent SMAD 1/5/8 phosphorylation,
independently of KRAS pathway activation (10). Although, both BMP2 and BMP6 were
expressed by patient samples, only the expression of BMP6 was
significantly upregulated in patients compared to healthy donors.
This is inconsistent with Maes et al (9) who reported significantly different
BMP2 serum protein levels between patients with MM and controls. It
is hypothesized that the non-linear relationship between mRNA and
protein expression could be a possible explanation for the
discrepancy between the present and past studies.
Consistent with what is known, the expression levels
of BMP6 were significantly elevated in MM samples, although its
expression failed to stratify patients. The relatively small ΔCq
range among patients could be a reason for that failure implying
that a different lab approach, could offer superior results. The
absolute quantification of BMP6 mRNA levels or the serum
measurement of BMP6 protein could be alternative options.
Mechanistically, increased BMP6 expression is considered favorable
as it inhibits MM growth and development. Seckinger et al
(7) reported that the treatment of
human myeloma cell lines with exogenous BMP6 inhibited
proliferation of cultured cells. Similarly, it was recently
demonstrated that the inhibitory effect of BMP6 was mediated
through the ALK-2 receptor (30).
The anti-myeloma effect of BMP6 has also been observed in in-vitro
models where it was observed to inhibit tubule formation, thus
highlighting a potential role of BMP6 in angiogenesis and MM in
general (7). In contrast to MM,
BMP6 is considered as a tumor inductive molecule in breast cancer.
It was reported that BMP6 inhibited MDA-MB-23 cell line apoptosis
via p38 and survivin activation (12).
In conclusion, based on the results of the present
study on patients with NDMM, neither BMP2 nor BMP6 accomplished to
serve as biomarkers for patients. The results indicate that BMP2
and BMP6 may not be involved in MM's pathogenesis. However, the
present study is limited by the small sample size, which may have
affected the results obtained and conclusions drawn, indicating
that additional large-scale studies are needed for safer
assumptions. Additionally, it is possible that other members of the
BMP family may influence MM growth and development. Apart from the
potential role of BMPs in patient stratification, future studies
should emphasize in the mechanistic exploration of BMP signaling in
MM. Knockdown assays could, for starters, explain whether BMPs role
is ultimately determined by their receptors or by the concurrent
activation and/or inactivation of other BMPs.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
PS performed experiments. PS, GS and EK collected
data. AG and EK analyzed data. AG wrote the initial manuscript. GV
and NG revised the manuscript. NG designed the study. GV
contributed to study conception and design. GV and NG supervised
the study. GV and NG confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All participants provided written informed consent
prior to recruitment, and the study was reviewed and approved by
the Institutional Review Board of the University Hospital of
Larissa (approval code 21906) and adhered to the tenets of The
Declaration of Helsinki.
Patient consent for publication
All participants provided written informed consent
for their information to be published.
Competing interests
The authors declare no competing interests.
References
|
1
|
Ludwig H, Novis Durie S, Meckl A, Hinke A
and Durie B: Multiple Myeloma incidence and mortality around the
globe; Interrelations between health access and quality, economic
resources, and patient empowerment. Oncologist. 25:e1406–e1413.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eisfeld C, Kajüter H, Möller L, Wellmann
I, Shumilov E and Stang A: Time trends in survival and causes of
death in multiple myeloma: A population-based study from Germany.
BMC Cancer. 23:3172023. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rajkumar SV: Multiple myeloma: 2022 update
on diagnosis, risk stratification, and management. Am J Hematol.
97:1086–1107. 2022. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katagiri T and Watabe T: Bone
morphogenetic proteins. Cold Spring Harb Perspect Biol.
8:a0218992016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bragdon B, Moseychuk O, Saldanha S, King
D, Julian J and Nohe A: Bone morphogenetic proteins: A critical
review. Cell Signal. 23:609–620. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Grcević D, Kusec R, Kovacić N, Lukić A,
Lukić IK, Ivcević S, Nemet D, Seiwerth RS, Ostojić SK, Croucher PI
and Marusić A: Bone morphogenetic proteins and receptors are
over-expressed in bone-marrow cells of multiple myeloma patients
and support myeloma cells by inducing ID genes. Leuk Res.
34:742–751. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Seckinger A, Meissner T, Moreaux J,
Goldschmidt H, Fuhler GM, Benner A, Hundemer M, Rème T, Shaughnessy
JD Jr, Barlogie B, et al: Bone morphogenic protein 6: A member of a
novel class of prognostic factors expressed by normal and malignant
plasma cells inhibiting proliferation and angiogenesis. Oncogene.
28:3866–3879. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Olsen OE, Wader KF, Misund K, Våtsveen TK,
Rø TB, Mylin AK, Turesson I, Størdal BF, Moen SH, Standal T, et al:
Bone morphogenetic protein-9 suppresses growth of myeloma cells by
signaling through ALK2 but is inhibited by endoglin. Blood Cancer
J. 4:1962014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maes K, Nemeth E, Roodman GD, Huston A,
Esteve F, Freytes C, Callander N, Katodritou E, Tussing-Humphreys
L, Rivera S, et al: In anemia of multiple myeloma, hepcidin is
induced by increased bone morphogenetic protein 2. Blood.
116:3635–3644. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wu CK, Wei MT, Wu HC, Wu CL, Wu CJ, Liaw H
and Su WP: BMP2 promotes lung adenocarcinoma metastasis through BMP
receptor 2-mediated SMAD1/5 activation. Sci Rep. 12:163102022.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Raida M, Clement JH, Leek RD, Ameri K,
Bicknell R, Niederwieser D and Harris AL: Bone morphogenetic
protein 2 (BMP-2) and induction of tumor angiogenesis. J Cancer Res
Clin Oncol. 131:741–750. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Du J, Yang S, Wang Z, Zhai C, Yuan W, Lei
R, Zhang J and Zhu T: Bone morphogenetic protein 6 inhibit
stress-induced breast cancer cells apoptosis via both Smad and p38
pathways. J Cell Biochem. 103:1584–1597. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Stieglitz D, Lamm S, Braig S, Feuerer L,
Kuphal S, Dietrich P, Arndt S, Echtenacher B, Hellerbrand C, Karrer
S and Bosserhoff AK: BMP6-induced modulation of the tumor
micro-milieu. Oncogene. 38:609–621. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rajkumar SV, Dimopoulos MA, Palumbo A,
Blade J, Merlini G, Mateos MV, Kumar S, Hillengass J, Kastritis E,
Richardson P, et al: International Myeloma Working Group updated
criteria for the diagnosis of multiple myeloma. Lancet Oncol.
15:538–548. 2014. View Article : Google Scholar
|
|
15
|
Fleige S and Pfaffl MW: RNA integrity and
the effect on the real-time qRT-PCR performance. Mol Aspects Med.
27:126–139. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Giraldo-Parra L, Ramirez LG, Navas A and
Gómez MA: Quality parameters for RNA preparations from biopsies of
ulcerated human skin. Wellcome Open Res. 28:2492023. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Okamoto T and Okabe S: Ultraviolet
absorbance at 260 and 280 nm in RNA measurement is dependent on
measurement solution. Int J Mol Med. 5:657–659. 2000.PubMed/NCBI
|
|
18
|
Pinto FL, Thapper A, Sontheim W and
Lindblad P: Analysis of current and alternative phenol based RNA
extraction methodologies for cyanobacteria. BMC Mol Biol. 7:792009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wilfinger WW, Mackey K and Chomczynski P:
Effect of pH and ionic strength on the spectrophotometric
assessment of nucleic acid purity. Biotechniques. 22:478–481. 1997.
View Article : Google Scholar
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ebisawa T, Tada K, Kitajima I, Tojo K,
Sampath TK, Kawabata M, Miyazono K and Imamura T: Characterization
of bone morphogenetic protein-6 signaling pathways in osteoblast
differentiation. J Cell Sci. 112:3519–27. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Xu H, Tong G, Yan T, Dong L, Yang X, Dou
D, Sun Z, Liu T, Zheng X, Yang J, et al: Transcriptomic analysis
provides insights to reveal the bmp6 function related to the
development of intermuscular bones in zebrafish. Front Cell Dev
Biol. 10:8214712022. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhan F, Huang Y, Colla S, Stewart JP,
Hanamura I, Gupta S, Epstein J, Yaccoby S, Sawyer J, Burington B,
et al: The molecular classification of multiple myeloma. Blood.
108:2020–2028. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Fonseca R, Bergsagel PL, Drach J,
Shaughnessy J, Gutierrez N, Stewart AK, Morgan G, Van Ness B, Chesi
M, Minvielle S, et al: International myeloma working group.
International myeloma working group molecular classification of
multiple myeloma: spotlight review. Leukemia. 23:2210–2221. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuzaki Y, Umemoto T, Tanaka Y, Okano T
and Yamato M: β2-Microglobulin is an appropriate reference gene for
RT-PCR-based gene expression analysis of hematopoietic stem cells.
Regen Ther. 23:91–97. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sabbir H: Validation of internal control
genes for quantitative real-time PCR under different experiment
conditions in Multiple Myeloma. J Bio Sci Biotechnol. 7:79–90.
2019.
|
|
27
|
Nazari F, Parham A and Maleki AF: GAPDH,
β-actin and β2-microglobulin, as three common reference genes, are
not reliable for gene expression studies in equine adipose- and
marrow-derived mesenchymal stem cells. J Anim Sci Technol.
57:182015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin J and Redies C: Histological evidence:
Housekeeping genes beta-actin and GAPDH are of limited value for
normalization of gene expression. Dev Genes Evol. 222:369–376.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bach DH, Park HJ and Lee SK: The dual role
of bone morphogenetic proteins in cancer. Mol Ther Oncolytics.
8:1–13. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ro TB, Holt RU, Brenne AT, Hjorth-Hansen
H, Waage A, Hjertner O, Sundan A and Borset M: Bone morphogenetic
protein-5, −6 and −7 inhibit growth and induce apoptosis in human
myeloma cells. Oncogene. 23:3024–3032. 2004. View Article : Google Scholar : PubMed/NCBI
|