Introduction
Adenoid cystic carcinoma (ACC) of the breast (ACCB)
is a rare subtype of triple-negative breast cancer (TNBC), with an
incidence of ~0.92 per million individuals (1) and accounting for 0.06–0.1% of all
breast cancers (2). Although the
exact risk factors for ACCB remain unclear due to its rarity, the
disease predominantly affects postmenopausal women, with rare cases
reported in men, suggesting that age and hormonal status may play a
role (3,4). While genetic alterations such as
MYB-NFIB fusions have been implicated in ACC at other sites, their
etiological significance in ACCB remains to be determined (5). ACCB shares histological features with
ACC of the salivary glands and other sites (6), but differs from other TNBC subtypes
(7). Typically, ACCB is localized
and indolent, with minimal metastasis to axillary lymph nodes or
distant organs (8,9). The prognosis of ACCB is generally
favorable, with a 5-year overall survival (OS) rate of 98–100% and
a 10-year OS rate of 85–100% (10).
However, there is no standardized treatment for ACCB, and surgery
remains the primary treatment strategy, followed by adjuvant
radiotherapy (11). There is no
consensus regarding the need for adjuvant chemotherapy. Therefore,
understanding the unique biological behavior of ACCB and obtaining
an accurate pathological diagnosis are crucial for proper clinical
management and to avoid overtreatment. The present report describes
a rare case of hepatic metastasis from classical ACCB.
Case report
A 70-year-old female patient was admitted to Zhuzhou
Hospital Affiliated to Xiangya School of Medicine, Central South
University (Zhuzhou, China) in March 2024, with a 1-month history
of right upper abdominal pain.
In February 2016, the patient underwent a lumpectomy
for a palpable mass located in the upper outer quadrant of the
right breast. Intraoperative frozen section analysis revealed
invasive carcinoma, and a modified radical mastectomy was
subsequently performed. Postoperative routine pathology was
performed. The tissue samples were fixed using 10% neutral buffered
formalin at room temperature (20–25°C) for 24 h and then cut at a
thickness of 4 μm. Hematoxylin and eosin staining was performed for
3 min and then the sections were examined using an Olympus BX53
optical microscope. The results indicated a classic cribriform
architecture composed of dual-layered epithelial and myoepithelial
cells, with outer basaloid cells surrounding pseudolumina filled
with eosinophilic basement membrane-like material (Fig. 1A). Among the 12 axillary lymph nodes
dissected, one was found to contain metastasis. The tumor measured
1.2×0.8×1.6 cm. For immunihistochemical analysis, paraffin-embedded
tissues were fixed in 10% neutral buffered formalin at room
temperature for 8 h before sectioning to 4 µm. Ready-to-use primary
antibody (anti-ER, cat. no. 790-4325; anti-PR, cat. no. 790-4296;
anti-HER-2, cat. no. 790-4493; all Roche Diagnostics GmbH;
anti-E-cadherin, cat. no. ZM-0092; anti-p63, cat. no. ZM-0406; all
Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.; anti-CD117, cat.
no. kit-0029; Fuzhou Maixin Biotechnology Development Co., Ltd.)
was incubated with the sections at 37°C for 1 h. DAB staining
solution (Polymer method; Beijing Zhongshan Jinqiao Biotechnology
Co., Ltd.) was then added to the sections at room temperature for
20 min. Hematoxylin counterstaining was applied at room temperature
for 3 min and then the sections were examined using an Olympus BX53
optical microscope. The immunohistochemical results were as
follows: Estrogen receptor (ER), negative (Fig. S1B); progesterone receptor (PR),
negative (Fig. S1C); human
epidermal growth factor receptor 2 (HER-2), score 0 (Fig. S1D); CD117, positive (Fig. 1B); p63, positive (Fig. 1C); and E-cadherin, positive
(Fig. S1E). Based on
histomorphological characteristics and the immunophenotype, the
pathological diagnosis was grade II ACCB (12,13).
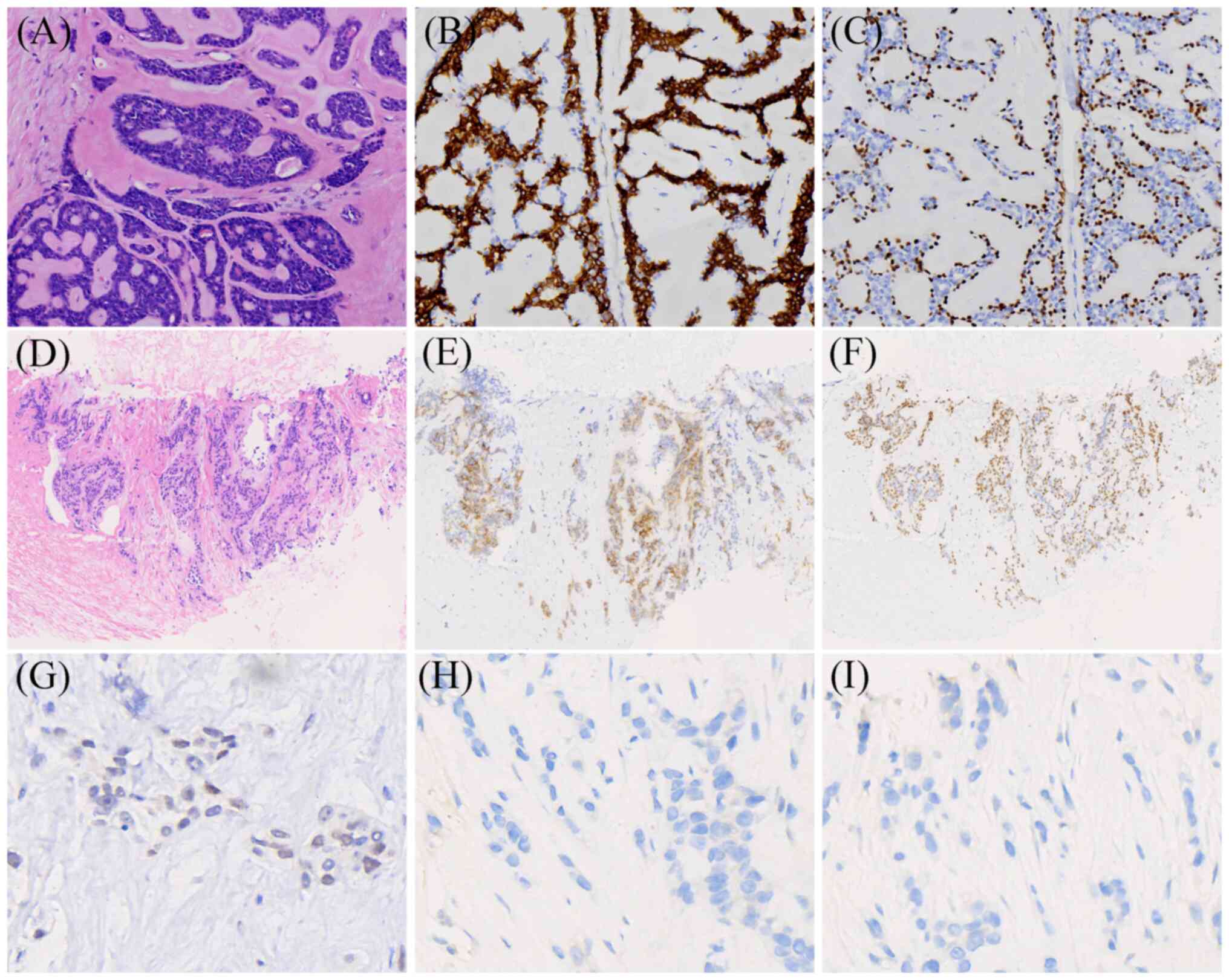 | Figure 1.Histopathological features of the
breast lesion and liver metastasis. (A) Hematoxylin and eosin
staining shows classic cribriform architecture composed of
dual-layered epithelial and myoepithelial cells, with outer
basaloid cells surrounding pseudolumina filled with eosinophilic
basement membrane-like material (original magnification, ×200). (B)
CD117-positive (magnification, ×200). (C) p63-positive
(magnification, ×200). (D) Hematoxylin and eosin staining reveals a
small number of cord-like epithelial cell nests with relatively
uniform size, arranged in glandular and nested patterns (original
magnification, ×100). (E) CD117-positive (magnification, ×100). (F)
p63-positive (magnification, ×100). (G) Progesterone
receptor-weakly positive (~1%; magnification, ×400). (H) Estrogen
receptor-negative (magnification, ×400). (I) Human epidermal growth
factor receptor 2-negative (magnification, ×400). |
Postoperatively, the patient received chemotherapy
consisting of three cycles of fluorouracil (500 mg/m2),
epirubicin (100 mg/m2), and cyclophosphamide (500
mg/m2), followed sequentially by three cycles of
docetaxel (100 mg/m2). All drugs were administered via
intravenous infusion, with each cycle lasting 21 days.
Additionally, the patient received 25 sessions of local
radiotherapy.
In 2019, the patient underwent a thyroid fine-needle
aspiration biopsy, which was classified as Bethesda category VI
(14). Total thyroidectomy was
performed at Zhuzhou Hospital Affiliated to Xiangya School of
Medicine, Central South University, during which enlarged lymph
nodes were observed anterior to the cervical trachea (maximum
diameter, ~1.5 cm), and in the left cervical levels II, III, IV and
V (maximum diameter, ~2.0 cm). After communication with the family
of the patient, a total thyroidectomy and left cervical lymph node
dissection were performed. Postoperative pathology, performed as
aforementioned, indicated papillary thyroid carcinoma near the left
isthmus, measuring 0.8×0.5×0.2 cm, with no evidence of metastasis
in the dissected lymph nodes (Fig.
S1A). The patient did not receive postoperative chemotherapy or
radiotherapy.
The patient underwent routine chest imaging
follow-up postoperatively, with the most recent CT scan in October
2020 showing no obvious abnormalities. However, no abdominal
imaging had been performed until March 2024, when the patient
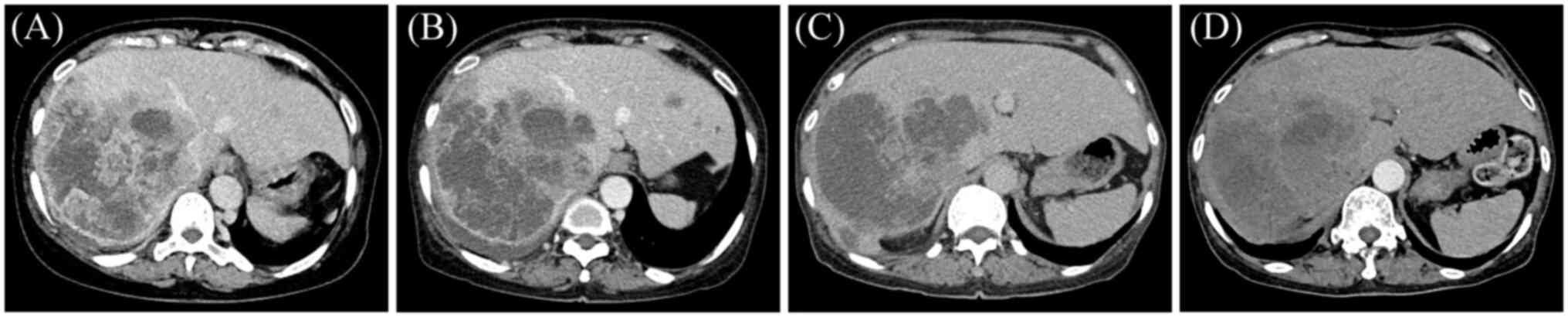
experienced abdominal pain, and abdominal CT revealed multiple
low-density lesions in the liver, with certain lesions
demonstrating indistinct margins and central non-enhancing necrotic
areas (Fig. 2A). These findings
were suggestive of metastatic malignancy. A liver biopsy was
performed, revealing a small number of cord-like epithelial nests
in fibrous tissue, composed of uniform-sized cells with glandular
and nested growth patterns, which was suggestive of malignancy
(Fig. 1D). For immunohistochemical
analysis, paraffin-embedded tissues were fixed in 10% neutral
buffered formalin at room temperature for 8 h before sectioning to
4 µm. The sections were permeabilized with 0.1% Triton X-100 at
room temperature for 10 min and blocked with 5% bovine serum
albumin in PBS at room temperature for 30 min. Ready-to-use primary
antibody (Beijing Zhongshan Jinqiao Biotechnology Co., Ltd.) was
incubated with the sections at 37°C for 1 h. DAB chromogen solution
(Polymer-based method; Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.) with HRP conjugate was applied to the sections at room
temperature for 20 min. Hematoxylin counterstaining was performed
at room temperature for 3 min, and the sections were then examined
using an Olympus BX53 optical microscope. Immunohistochemical
analysis revealed the following results: CD117, partially positive
(Fig. 1E); p63, positive (Fig. 1F); PR, ~1%, weakly positive
(Fig. 1G); ER, negative (Fig. 1H); HER-2, score 0 to negative
(Fig. 1I); E-cadherin, positive
(Fig. S1I); hepatocyte, negative
(Fig. S1F); cytokeratin 19 (CK19),
negative (Fig. S1G);
thyroglobulin, negative (Fig.
S1H); and thyroid transcription factor-1 (TTF-1), negative
(Fig. S1J). Based on the clinical
history, morphology and immunophenotype, the clinicopathological
diagnosis was hepatic metastasis of ACCB.
After a multidisciplinary discussion, an advanced
breast cancer salvage treatment plan was developed. The patient
began adjuvant chemotherapy in March 2024, consisting of
intravenous albumin-bound paclitaxel (150 mg/m2) and
oral capecitabine (1,000 mg/m2), administered in 21-day
cycles for a total of six cycles. Following two cycles of
chemotherapy, the patient experienced a reduction in right upper
abdominal pain, with noticeable improvement compared with before
treatment, although the symptom did not completely resolve. The
patient returned regularly for subsequent cycles of chemotherapy,
completing a total of six cycles by July 2024. Abdominal CT
performed after the second (Fig.
2B) and fifth (Fig. 2C)
chemotherapy cycles indicated partial remission. Moreover,
follow-up CT in October 2024 (Fig.
2D), indicated slight shrinkage of the liver metastases. The
general condition of the patient remained stable, and the quality
of life of the patient was satisfactory, with ongoing follow-up.
Follow-up included abdominal CT scans every 3 months to monitor
hepatic metastasis and detect any new lesions. Mammography and
chest CT were performed every 3 months to assess for locoregional
recurrence, metastasis and pulmonary involvement. Liver function
tests, complete blood counts and tumor marker analysis (such as
analysis of carcinoembryonic antigen and CA15-3) were conducted
every 3 months. Physical examinations and symptom assessments were
performed to evaluate the patient's general condition, pain,
fatigue or any new symptoms. Quality of life assessments included
patient-reported outcomes regarding physical function,
psychological state and social participation.
Discussion
ACC is most commonly found in the salivary glands
and is rare in the breast (1). The
reported rates of lymph node metastasis for ACCB are <2%, and
the distant metastasis rate is 2.2% (6). The most common site for distant
metastasis is the lung (6).
According to the 2020 World Health Organization classification,
ACCB is categorized into classic, solid and high-grade
transformation types. Among these, the classic type is the most
common, with the most favorable biological behavior and a low rate
of metastasis. The solid and high-grade transformation types are
more prone to local recurrence and distant metastasis. Pathological
grading of ACCB is based on the cellular architecture and the
proportion of solid components: Tumors composed almost entirely of
cribriform and/or tubular structures with no solid areas are
classified as grade I; those primarily cribriform and/or tubular
with ≤30% solid components are classified as grade II; and those
with >30% solid components, notable cytological atypia and
increased mitotic activity are classified as grade III. The higher
the proportion of solid components, the worse the prognosis
(12,13). The present case report describes a
rare instance of distant metastasis in classical grade II ACCB. The
patient had undergone radical surgery, followed by adjuvant
chemotherapy and radiotherapy, but later developed hepatic
metastasis.
ACCB has a complex pathological structure.
Fine-needle aspiration biopsy is associated with a high risk of
missed or incorrect diagnosis. Around half of ACCB cases are
misdiagnosed as other breast diseases (15). Clinical and imaging findings are
often insufficient for definitive diagnosis, and histopathological
examination combined with immunohistochemistry remains the gold
standard for diagnosis. ACCB is mainly composed of epithelial,
myoepithelial and basaloid cells. Co-expression of CD117 (c-kit) in
luminal epithelial cells and p63 in myoepithelial/basaloid cells is
recognized as the immunophenotypic hallmark of ACCB (1,16). In
a study by Mastropasqua et al (17), CD117 and p63 were positive in 95 and
85% of ACCB cases, respectively, but not in common cribriform or
tubular breast carcinomas. In the present case, the breast lesion
exhibited typical cribriform architecture composed of dual-layered
epithelial and myoepithelial cells, with outer basaloid cells
surrounding pseudolumina filled with eosinophilic basement
membrane-like material. The immunophenotype was triple-negative,
with positive CD117 and p63 staining, supporting the diagnosis of
ACCB. Moreover, a liver mass was detected 8 years after breast
surgery. Liver biopsy revealed small cord-like epithelial nests
with uniform cells exhibiting glandular and nested growth. Negative
hepatocyte and CK19 staining ruled out primary liver cancer, and
negative thyroglobulin and TTF-1 staining excluded thyroid cancer.
Based on the clinical history, morphology and immunophenotype, the
diagnosis was hepatic metastasis from ACCB.
ACCB is a unique form of TNBC. Surgery combined with
adjuvant chemotherapy is typically recommended for most patients
with TNBC; however, this approach may not be suitable for ACCB.
Moreover, to date, there is no consensus on the treatment of ACCB
(7). A previous study suggested
that breast-conserving surgery is the preferred treatment, with
adjuvant radiotherapy recommended after the surgery (18). Macias et al (6) performed a systematic review of 4,370
ACCB cases, and surgery was the primary treatment in 3,984
patients, underscoring the importance of surgical management.
Surgical options for ACCB include both breast-conserving surgery
and radical surgery (19). In a
retrospective analysis of 583 patients with early-stage ACCB, Huang
et al (20) reported that
breast-conserving surgery resulted in improved 10-year OS and
disease-specific survival rates compared with modified radical
mastectomy. Furthermore, a recent study reported that
breast-conserving surgery combined with radiotherapy is the main
treatment modality for ACCB (21).
Sun et al (11) also
reported that adjuvant radiotherapy could improve the 5-year
cancer-specific survival rate of patients with ACCB by 4.3%.
The role of adjuvant chemotherapy remains unclear,
and its benefit in terms of survival has not been well established
(7). Certain clinicians treat ACCB
as TNBC, predominantly using chemotherapy (6). Yang et al (22) reported that patients with ACCB did
not benefit from adjuvant chemotherapy. Moreover, Li et al
(7) reported that adjuvant
chemotherapy did not improve the survival of patients with ACCB,
even in subgroups at high risk for recurrence and metastasis. Due
to the absence of hormone receptor (HR) and HER-2 expression, ACCB
is insensitive to endocrine and targeted therapies (23). Certain studies have reported
HR-positive ACCB; however, the clinical features and prognosis of
this subtype are still unclear compared with those of HR-negative
ACCB (1,6). Wenig and Birhiray (24) described cases of ACCB with
isocitrate dehydrogenase [NADP(+)] 2 and fibroblast growth factor
receptor 2 mutations, which responded to targeted therapies with
enasidenib and erdafitinib, suggesting the importance of genetic
testing for rare malignancies. Additionally, Massé et al
(25) identified NOTCH as a
potential therapeutic target in 30% of solid-type ACCB cases.
In the present case, the patient underwent radical
surgery rather than breast-conserving surgery, followed by
chemotherapy and radiotherapy. The chemotherapy regimen, which was
three cycles of fluorouracil, epirubicin and cyclophosphamide,
followed by three cycles of docetaxel, was consistent with
recommended protocols for TNBC (26). Despite the generally good prognosis
of ACCB, the rare occurrence of distant metastasis in the current
case highlights the potential overtreatment associated with radical
surgery and adjuvant chemotherapy in ACCB. Currently, treatment
strategies for ACCB vary widely in clinical practice due to the
lack of specific guidelines. Therefore, further studies with larger
sample sizes and longer follow-up are required to elucidate the
optimal treatment approach for ACCB.
In summary, ACCB is a rare TNBC subtype with
distinct clinical and pathological characteristics. The prognosis
of ACCB is generally favorable, and breast-conserving surgery
combined with radiotherapy may be the ideal treatment option. The
efficacy of chemotherapy requires further evaluation. Therefore,
differentiating ACCB from other TNBC subtypes is crucial to avoid
unnecessary treatments, such as radical surgery and overtreatment
with chemotherapy.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the Youth Project of Hunan
Natural Science Foundation (grant no. 2024JJ7654).
Availability of data and materials
The data generated in the present study may be
requested from the corresponding author.
Authors' contributions
XH, YO, LT and WZ collected the clinical, imaging
and pathological data of the patient, and wrote the manuscript. MT
and TW conceived and designed the study, and revised the
manuscript. MT and TW confirm the authenticity of all the raw data.
All authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Written informed consent was obtained from the
patient for publication of the images and data.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang W, Fang Y, Zhang Z and Wang J:
Management of adenoid cystic carcinoma of the breast: A
single-institution study. Front Oncol. 11:6210122021. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Liao HY, Zhang WW, Sun JY, Li FY, He ZY
and Wu SG: The clinicopathological features and survival outcomes
of different histological subtypes in triple-negative breast
cancer. J Cancer. 9:2962018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Meng M, Xie LJ and Qiu X: A case of
adenoid cystic carcinoma of the breast. Asian J Surg. 47:2415–2416.
2024. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li JX, Zhang XM, Xiao YX, Tang ZM, Huang T
and Ming J: Male adenoid cystic carcinoma of the breast. J Med
Cases. 12:5032021. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
West RB, Kong C, Clarke N, Gilks T,
Lipsick JS, Cao H, Kwok S, Montgomery KD, Varma S and Le QT: MYB
expression and translocation in adenoid cystic carcinomas and other
salivary gland tumors with clinicopathologic correlation. Am J Surg
Pathol. 35:92–99. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Macias J, Canedo FS, Lu SE, Chen C, Heleno
CT, Bridgeman M, Goncalves PH and George MA: Treatment patterns of
adenoid cystic carcinoma of the breast: A systematic review. J Clin
Oncol. 42 (16 Suppl):e131472024. View Article : Google Scholar
|
|
7
|
Li L, Zhang D and Ma F: Adenoid cystic
carcinoma of the breast may be exempt from adjuvant chemotherapy. J
Clin Med. 11:44772022. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghabach B, Anderson WF, Curtis RE, Huycke
MM, Lavigne JA and Dores GM: Adenoid cystic carcinoma of the breast
in the United States (1977 to 2006): A population-based cohort
study. Breast Cancer Res. 12:1–9. 2010. View Article : Google Scholar
|
|
9
|
Gillie B, Kmeid M, Asarian A and Xiao P:
Adenoid cystic carcinoma of the breast with distant metastasis to
the liver and spleen: A case report. J Surg Case Rep.
2020:rjaa4832020. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Thomas DN, Asarian A and Xiao P: Adenoid
cystic carcinoma of the breast. J Surg Case Rep.
rjy3552019.PubMed/NCBI
|
|
11
|
Sun JY, Wu SG, Chen SY, Li FY, Lin HX,
Chen YX and He ZY: Adjuvant radiation therapy and survival for
adenoid cystic carcinoma of the breast. Breast. 31:214–218. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kashiwagi S, Asano Y, Ishihara S, Morisaki
T, Takashima T, Tanaka S, Amano R, Ohsawa M, Hirakawa K and Ohira
M: Adenoid cystic carcinoma of the breast: A case report. Case Rep
Oncol. 12:698–703. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bhutani N, Kajal P and Singla S: Adenoid
cystic carcinoma of the breast: Experience at a tertiary care
centre of Northern India. Int J Surg Case Rep. 51:204–209. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Cibas ES and Ali SZ: The Bethesda system
for reporting thyroid cytopathology. Thyroid. 19:1159–1165. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sumpio BE, Jennings TA and Merino MJ:
Adenoid cystic carcinoma of the breast. Data from the connecticut
tumor registry and a review of the literature. Ann Surg.
205:295–301. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burusapat C, Buarabporn N, Wongchansom K,
Chanapai P, Parinyanut P and Supaporn S: Mammary adenoid cystic
carcinoma presenting with Ductal carcinoma in situ and axillary
lymph node metastasis. J Surg Case Rep. 2020:rjz3622020. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mastropasqua MG, Maiorano E, Pruneri G,
Orvieto E, Mazzarol G, Vento AR and Viale G: Immunoreactivity for
c-kit and p63 as an adjunct in the diagnosis of adenoid cystic
carcinoma of the breast. Mod Pathol. 18:1277–1282. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Khanfir K, Kallel A, Villette S, Belkacémi
Y, Vautravers C, Nguyen T, Miller R, Li YX, Taghian AG, Boersma L,
et al: Management of adenoid cystic carcinoma of the breast: A rare
cancer network study. Int J Radiat Oncol. 82:2118–2124. 2012.
View Article : Google Scholar
|
|
19
|
Millar BAM, Kerba M, Youngson B, Lockwood
GA and Liu FF: The potential role of breast conservation surgery
and adjuvant breast radiation for adenoid cystic carcinoma of the
breast. Breast Cancer Res Treat. 87:225–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang T, Fang Q, Niu L, Wang L and Sun X:
Optimal surgical procedure for treating early-stage adenoid cystic
carcinoma of the breast. Sci Rep. 13:102222023. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee SM, Jang BS, Park W, Kim YB, Song JH,
Kim JH, Kim TH, Kim IA, Lee JH, Ahn SJ, et al: Locoregional
recurrence in adenoid cystic carcinoma of the breast: A
retrospective, multicenter study (KROG 22-14). Cancer Res Treat.
57:150–158. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang L, Wang C, Liu M and Wang S:
Evaluation of adjuvant treatments for adenoid cystic carcinoma of
the breast: A population-based, propensity score matched cohort
study from the SEER database. Diagnostics (Basel). 12:17602022.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Gomez-Seoane A, Davis A and Oyasiji T:
Treatment of adenoid cystic carcinoma of the breast: Is
postoperative radiation getting its due credit? Breast. 59:358–66.
2021. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wenig E and Birhiray RE: Novel treatment
options in advanced adenoid cystic carcinoma of the breast. J Clin
Oncol. 39 (15 Suppl):e130712021. View Article : Google Scholar
|
|
25
|
Massé J, Truntzer C, Boidot R, Khalifa E,
Pérot G, Velasco V, Mayeur L, Billerey-Larmonier C, Blanchard L,
Charitansky H, et al: Solid-type adenoid cystic carcinoma of the
breast, a distinct molecular entity enriched in NOTCH and CREBBP
mutations. Mod Pathol. 33:1041–55. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yu KD, Ye FG, He M, Fan L, Ma D, Mo M, Wu
J, Liu GY, Di GH, Zeng XH, et al: Effect of adjuvant paclitaxel and
carboplatin on survival in women with triple-negative breast
cancer: A phase 3 randomized clinical trial. JAMA Oncol.
6:1390–1396. 2020. View Article : Google Scholar : PubMed/NCBI
|