Introduction
Renal cell carcinoma (RCC) is an aggressive
neoplastic disease of the renal parenchyma that accounts for 2–3%
of all diagnosed cancer types (1,2). Among
these subtypes, clear-cell RCC (ccRCC) is the most frequent and is
characterized by intrinsic chemoresistance (3,4).
Overall, ccRCC is diagnosed late in up to 50% of patients, with a
median survival of 6–10 months (5,6).
In cancer, lymphocytes develop a hypofunctional
state known as immune exhaustion, in which cells exhibit increased
expression of immune checkpoints or exhaustion receptors.
Immunotherapy reverses this state of exhaustion by blocking the
interaction between checkpoints and their ligands, leading to the
activation of antitumor immunity (7,8). The
approved therapies for advanced RCC include tyrosine kinase
inhibitors (targeted therapy) and immune checkpoint inhibitors
(ICIs). Currently, there are five ICIs approved for the treatment
of advanced RCC: i) Nivolumab and pembrolizumab, which target
programmed cell death protein-1 (PD-1) expressed on lymphocytes;
ii) ipilimumab, which targets cytotoxic T lymphocyte-associated
protein-4 (CTLA-4) also expressed on lymphocytes; and iii)
atezolizumab or avelumab, which target the programmed death
ligand-1 (PD-L1) on cancer and stromal cells (9–12). In
cancer, the upregulation of ligands such as PD-L1 on stroma and
tumor cells have immunosuppressive effects on lymphocytes via
ligation of the inhibitory receptors CTLA-4 and PD-1. The use of
ICIs block these inhibitory signals, which restores the cytotoxic
function of infiltrating lymphocytes (7). However, the lack of consensus on
predictive tools for guiding the use of ICIs prevents their
widespread applicability, which is a notable issue considering the
cost-effectiveness and side effects associated with these therapies
(13,14).
Since localized disease can be successfully treated
with surgery, there is a lack of studies analyzing the expression
of markers such as PD-1, CTLA-4 and PD-L1 during the early stages
of the disease. To address this knowledge gap, the present study
aimed to evaluate the expression levels of ICI targets [PD-1,
PD-L1, CTLA-4, lymphocyte activation gene 3 (LAG-3) and T-cell
immunoglobulin and mucin domain containing-3 (TIM-3)] in blood and
tumors obtained from a small cohort of patients diagnosed with
localized ccRCC. The expression levels of these markers across
three tissues were compared: Blood and tumor tissue from patients
with ccRCC, and blood from a group of healthy controls.
Furthermore, the expression levels of the markers associated with
the nuclear grade in tumors and changes in blood after a short-term
follow-up of 12 months were evaluated.
Materials and methods
Patient recruitment
The present study included 20 patients diagnosed
with ccRCC who underwent elective localized nephrectomy (median
age, 62 years; range, 44–71 years) and 10 control subjects (median
age, 62 years; range, 55–72 years) who were considered healthy
based on medical history and did not have any prior diagnosis of
cancer, chronic inflammatory diseases, autoimmune disorders or
current infections. Patients and controls were recruited from the
Service of Urology at the University Hospital ‘Dr José Eleuterio
González’ (Nuevo León, Mexico) between January 2022 and January
2024 (Table I). Patients were
excluded if they were diagnosed with metastatic disease or RCC
variants other than ccRCC. The present study was approved by the
Institutional Ethics Committee (approval no. IN23-00002) and all
participants provided written informed consent before enrollment.
Peripheral venous blood samples (4 ml) were obtained on the day
before the scheduled nephrectomy (n=20) and after 12 months of
clinical follow-up from a subgroup of patients who consented to
subsequent blood analysis (n=10). The clinical follow-up consisted
of a complete clinical evaluation by a urologist, including
radiologic studies to evaluate RCC relapse. Healthy controls were
sampled only at the beginning of the study (n=10). Furthermore,
after completing histopathological analysis, 2 g of tumor tissue
was sampled from the macroscopically defined tumor area in each
nephrectomized kidney. The histopathological analysis consisted of
RCC subtype diagnosis, pathological tumor staging (pTNM) according
to the American Joint Committee on Cancer guidelines (15), evaluation of tumor grade according
to the International Society of Urological Pathology (ISUP) grading
system (16), presence of tumor
necrosis and identification of sarcomatoid or rhabdoid
differentiation.
 | Table I.Clinical characteristics of the
participants. |
Table I.
Clinical characteristics of the
participants.
| Variable | Control (n=10) | Patients
(n=20) | P-value |
|---|
| Sex, n (%) |
|
| 0.139a |
|
Male | 4 (40) | 14 (70) |
|
|
Female | 6 (60) | 6 (30) |
|
| Age, years ±
SD | 60.8±6.0 | 59.4±8.0 | 0.610b |
| Tumor size, cm ±
SD | - | 8.2±3.9 | - |
| Necrosis <50%, n
(%) | - | 18 (90) | - |
| Nuclear grade, n
(%) |
|
|
|
| G2 | - | 6 (30) | - |
| G3 | - | 9 (45) | - |
| G4 | - | 5 (25) | - |
| T stage, n (%) |
|
|
|
| T1 | - | 6 (30) | - |
| T2 | - | 2 (10) | - |
| T3 | - | 10 (50) | - |
| T4 | - | 2 (10) | - |
Immunohistochemistry
All studied samples underwent a comprehensive
assessment for multifocality, necrosis and sarcomatoid features.
Representative sections of formalin-fixed paraffin-embedded tissues
were used for further analyses. Briefly, the tissues were fixed
with 10% neutral-buffered formalin for 24 h at room temperature.
After fixation, tissues were embedded in paraffin and sliced to
obtain 4-µm-thick sections. Subsequently, the tissues were
rehydrated through a series of alcohol solutions, from absolute to
70% ethanol, finishing with distilled water. Since standard
formalin-fixed, paraffin-embedded sections do not require
permeabilization, this step was not performed. Immunostaining was
performed using the OptiView DAB IHC Detection Kit (cat. no.
760-700; Roche Tissue Diagnostics; Roche Diagnostics, Ltd.) and the
BenchMark ULTRA stainer module (cat. no. 05342716001; Roche Tissue
Diagnostics; Roche Diagnostics, Ltd.). The OptiView detection kit
includes reagents for peroxidase inhibition, biotin-free
hapten-coupled secondary antibody and horseradish peroxidase
multimer coupled tertiary antibodies. Chromogenic reaction was
performed with the 3,3′-diaminobenzidine tetrahydrochloride (DAB)
chromogen. Antigen retrieval was performed using Discovery CC1
solution (cat. no. 950-500; Roche Diagnostics, Ltd.) for 64 min at
100°C. The primary antibodies used were mouse monoclonal
anti-CTLA-4 (cat. no. BSB 2880; clone BSB-88; Bio SB, Inc.), rabbit
monoclonal anti-PD-L1 (cat. no. 790-4905; clone SP263; Roche Tissue
Diagnostics; Roche Diagnostics, Ltd.) and rabbit monoclonal
anti-CD3 (cat. no. 790-4341; clone 2GV6; Roche Tissue Diagnostics;
Roche Diagnostics, Ltd.) at a dilution of 1:50 for 32 min at 37°C.
The secondary reaction was performed using the OptiView DAB
Detection Kit for an additional 12 min at 37°C. All staining
procedures were performed according to the manufacturer's protocol
to ensure consistency and reliability. Clinical analyses of all
samples were performed by three clinical pathologists using a
brightfield optical microscope (Olympus CX31; Olympus
Corporation).
The DP200 slide scanner (cat. no. 08303916001; Roche
Tissue Diagnostics; Roche Diagnostics, Ltd.) was used for
whole-slide scanning. Subsequently, a set of 8–10 randomly selected
images (magnification, ×40) were further analyzed using the QuPath
software for Windows (version 0.5.1) (17). Digital analyses were performed by a
trained non-pathologist (Fig. S1).
The percentage of positive cells was calculated using the following
formula: Percentage of positive cells=[number of positive
cells/(number of positive cells + number of negative cells)]
×100.
Flow cytometry
Cytometric analyses of blood samples were performed
on the day of nephrectomy (n=7-12) and after a 12-month clinical
follow-up (n=10). Some of the blood samples were lost during the
procedure due to a technical error, and only samples deemed
acceptable after quality control were included, defined as properly
anticoagulated blood and a viability >50% based on trypan blue
exclusion (n=7-12). For tissue analysis (n=18), the tumor was
mechanically and enzymatically digested in DMEM supplemented with
5% FBS (cat. no. F2442; Sigma-Aldrich; Merck KGaA). The enzyme
cocktail contained collagenase (cat. no. C9891; MilliporeSigma),
hyaluronidase (cat. no. H3506; MilliporeSigma), DNase (cat. no.
10104159001; Roche Diagnostics, Ltd.) and trypsin (cat. no.
15090-046; Gibco; Thermo Fisher Scientific, Inc.) with EGTA (cat.
no. E3889; MilliporeSigma). Furthermore, the tissues were incubated
with the protease dispase (cat. no. 17105041; Gibco; Thermo Fisher
Scientific, Inc.) and BD FACS™ Lysing Solution (cat. no. 349202; BD
Biosciences) for 10 min at room temperature. After eliminating the
aggregates with a 70 µm cell strainer (cat. no. 352350; Falcon;
Corning Life Sciences), 1×106 cells were stained.
Complete blood samples (50 µl) were stained, followed by
erythrocyte lysis for 20 min. The antibody cocktail for lymphocytes
(CD3+, CD4+ and CD8+ lymphocytes)
contained FITC mouse anti-human CD8 (20 µl/sample; cat. no. 555366;
clone RPA-T8), PE mouse anti-human CD279 (PD-1; 20 µl/sample; cat.
no 557946; clone MIH4), BV605 mouse anti-human CD4 (5 µl/sample;
cat. no. 562658; clone RPA-T4), APC-Cy™7 mouse anti-human CD3 (5
µl/sample; cat. no. 557832; clone SK7), Alexa Fluor® 647
mouse anti-human CD366 (TIM-3; 5 µl/sample; cat. no. 565558; clone
7D3), PE-CF594 mouse anti-human CD223 (LAG-3; 5 µl/sample; cat. no.
565718; clone T47-530) and BV421 mouse anti-human CD152 (CTLA-4; 5
µl/sample; cat. no. 562743; clone BNI3). The antibody cocktail for
myeloid cells (granulocytes and monocytes) contained Alexa
Fluor® 488 mouse anti-human CD14 (5 µl/sample; cat. no.
557700; clone M5E2), BV421 mouse anti-human CD274 (PD-L1; 5
µl/sample; cat. no. 563738; clone MIH1), PE mouse anti-human CD45
(5 µl/sample; cat. no. 561866; clone HI30) and 7-AAD viability
solution (5 µl/sample; cat. no. 559925). All antibodies were
acquired from BD Biosciences.
For each sample, 1×105 events were
acquired using an LSRFortessa flow cytometer (Becton, Dickinson and
Company) and analyzed with DIVA version 8.0 for Windows (BD
Biosciences). The percentages of CD4+ and
CD8+ cells were quantified relative to CD3+
cells. Similarly, the percentages of lymphocytes
(CD14−SSClo), monocytes
(CD14+SSClo) and granulocytes
(CD14−SSChi) were quantified relative to
CD45+ cells (Fig. S2).
PD-1, LAG-3, TIM-3, CTLA-4 and PD-L1 fluorescence was analyzed as
the mean fluorescence intensity (Fig.
S3).
Statistical analysis
After confirming the parametric distribution of the
data, an unpaired Student's t-test or one-way ANOVA followed by
Bonferroni's multiple comparisons test was used for two- and
three-group comparisons, respectively. Fisher's exact test was used
to compare the sex proportions between patients and healthy
controls and between tumor grade categories (Grade 2 vs. Grade
>2). Statistical analyses were performed using GraphPad Prism
(version 5.0; Dotmatics) for Windows and Microsoft Excel. P<0.05
was considered to indicate a statistically significant
difference.
Results
Clinical description of the
participants
Both groups of participants had similar ages and
comorbidities, such as high blood pressure, type 2 diabetes and
obesity. The mean age ± SD of the participants was 59.4±8 years in
the patient group and 60.8±6 years in the healthy control group. A
total of 10 patients were classified as T3 on the pTNM staging
system and 14 patients had an ISUP nuclear grade ≥3. The average
tumor size was 8.2±3.9 cm (Table
I).
Immunohistochemistry reveals increased
infiltration of the tumor tissue by CD3+
lymphocytes
Serial sections from RCC tissue were stained for
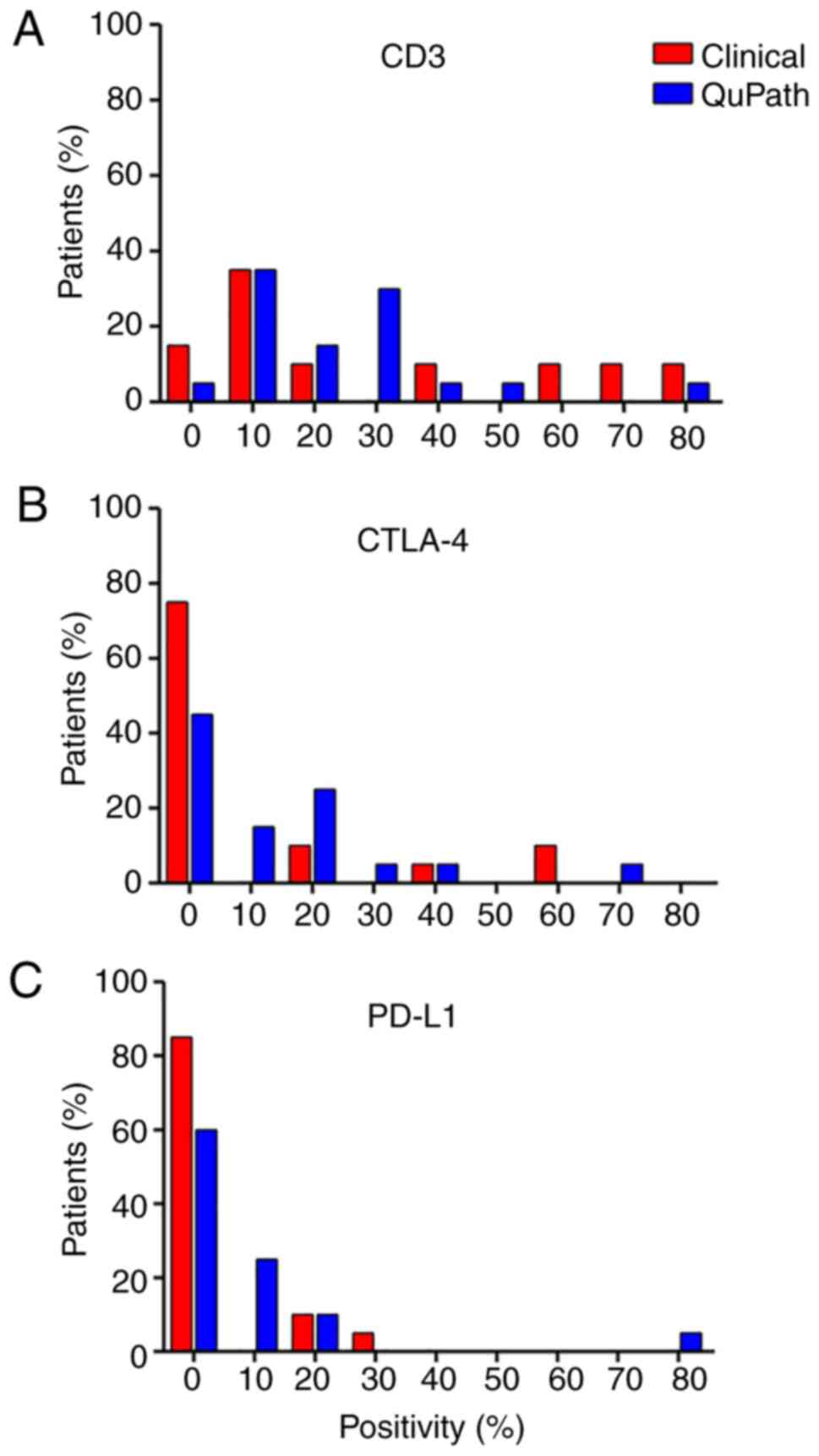
CD3, CTLA-4 and PD-L1 for immunohistochemical analysis. Positivity
was evaluated by both clinical pathologists and digital analysis of
micrographs using QuPath software (see Materials and methods;
Fig. S1). Of all the tumor
samples, >80% were positive for CD3 (Fig. 1A). By contrast, CTLA-4 (Fig. 1B) and PD-L1 (Fig. 1C) were positive in only 50 and 25%
of the samples, respectively. These findings demonstrate that ccRCC
tissue is highly infiltrated with lymphocytes and has low
expression of the exhaustion markers CTLA-4 and PD-L1.
CD8+ lymphocytes and
monocytes dominates the tissue cellularity among infiltrating
leukocytes
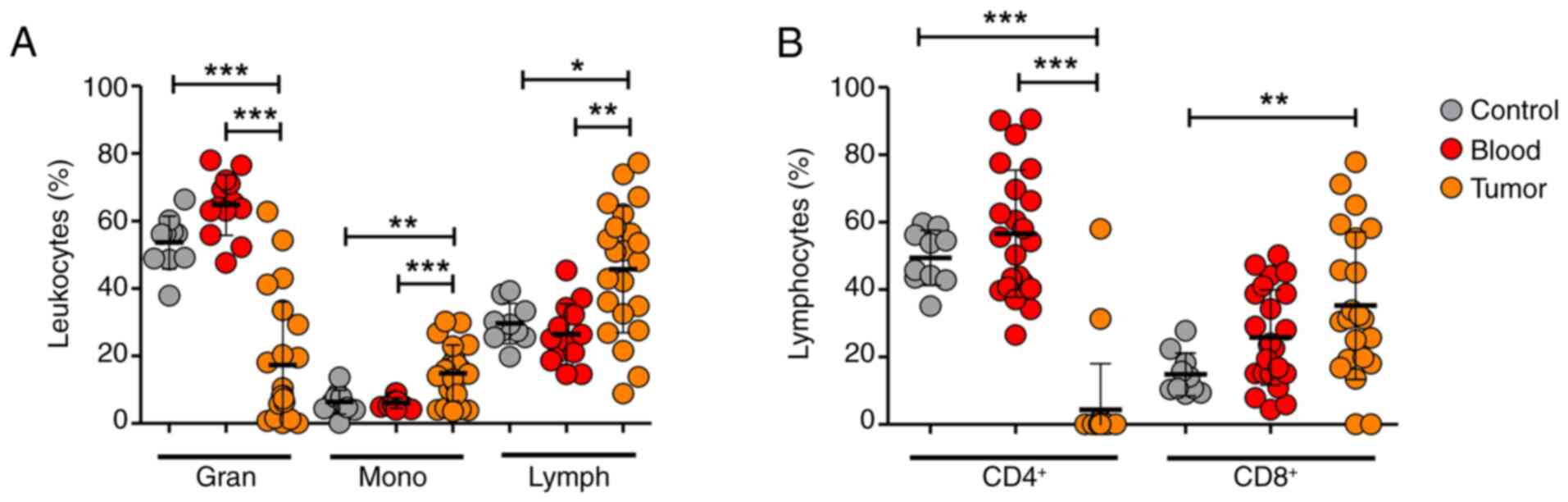
In addition to immunohistochemistry, flow cytometric
analysis of tumor tissue and peripheral blood was performed to
evaluate the changes in leukocyte dynamics (Figs. 2 and S2). The tumor tissue exhibited decreased
infiltration of granulocytes and increased infiltration of
monocytes and lymphocytes compared with the leukocyte percentages
in the blood (Figs. 2A and S2A, C and E). Among the lymphocyte
subpopulations, CD8+ lymphocytes were the dominant
subpopulation in the tumor tissue; notably, the percentage
CD4+ lymphocytes was markedly lower (Figs. 2B and S2B, D and F). There was a trend for
increased percentages of granulocytes and CD4+ and
CD8+ lymphocytes in blood from patients compared with
blood from healthy controls; however, the differences were not
statistically significant.
Tumor-infiltrating leukocytes
demonstrate decreased expression levels of the markers LAG-3,
TIM-3, CTLA-4 and PD-L1
After assessing leukocyte dynamics, the expression
levels of several receptors associated with lymphocyte exhaustion
were evaluated, a principal mechanism involved in antitumor
immunity. Using flow cytometry, the expression levels of PD-1,
LAG-3, TIM-3 and CTLA-4 in lymphocytes, and PD-L1 receptors in
granulocytes and monocytes were evaluated (Figs. 3 and S3). There was no change in PD-1
expression (Figs. 3A-C and S3A) but decreased expression levels of
LAG-3 (Figs. 3D-F and S3B), TIM-3 (Figs. 3H-I and S3C) and CTLA-4 (Figs. 3J-L and S3D) among tumor-infiltrating lymphocytes
were observed compared with blood samples. In addition, decreased
PD-L1 expression levels were reported in granulocytes (Fig. 3M) and monocytes (Figs. 3N and S3E) that infiltrated the tumor tissue
compared with the blood. Only two markers indicated expression
differences between the patient and healthy control blood samples.
The patients had increased TIM-3 levels (Fig. 3G) and decreased PD-L1 levels
(Fig. 3N) in lymphocytes and
monocytes, respectively. Collectively, the present study results
demonstrate decreased expression of several exhaustion-associated
markers within tumor-infiltrating lymphocytes, with the notable
exception of PD-1.
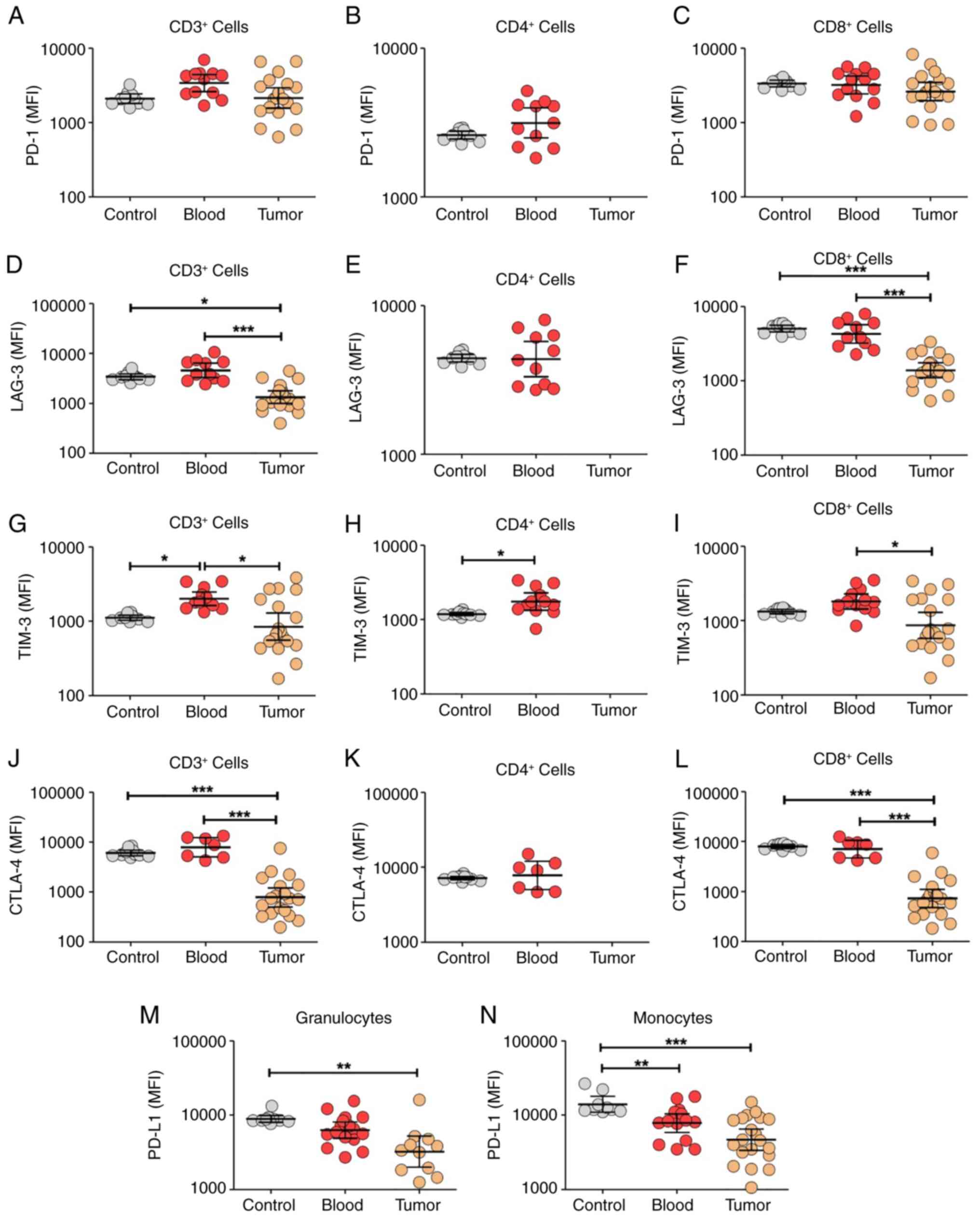 | Figure 3.Compared with circulating leukocytes,
tumor-infiltrating leukocytes exhibit decreased expression of the
exhaustion receptors LAG-3, TIM-3, CTLA-4 and PD-L1 based on flow
cytometry. All receptors were evaluated in blood leukocytes from
healthy controls (control), blood leukocytes from patients (blood)
and tumor-infiltrating leukocytes (tumor). PD-1 was evaluated in
(A) CD3+, (B) CD4+ and (C) CD8+
lymphocytes. LAG-3 was evaluated in (D) CD3+, (E)
CD4+ and (F) CD8+ lymphocytes. TIM-3 was
evaluated in (G) CD3+, (H) CD4+ and (I)
CD8+ lymphocytes. CTLA-4 was evaluated in (J)
CD3+, (K) CD4+ and (L) CD8+
lymphocytes. PD-L1 was evaluated in (M) granulocytes and (N)
monocytes. The tumor-infiltrating leukocytes exhibited decreased
expression levels of LAG-3, TIM-3, CTLA-4 and PD-L1 compared with
blood leukocytes, but no changes were observed in PD-1 expression.
Data are expressed as MFI ± SD. Control group, n=10; blood group,
n=7-12; tumor group, n=18. Data were analyzed using one-way ANOVA
with Bonferroni's post hoc test (except panels B, E, H and K, which
were analyzed using an unpaired Student's t-test). *P<0.05;
**P<0.01; ***P<0.001. PD-L1, programmed death-ligand 1; PD-1,
programmed cell death protein-1; LAG-3, lymphocyte activation
gene-3; TIM-3, T-cell immunoglobulin and mucin domain-containing
protein-3; CTLA-4, cytotoxic T lymphocyte-associated protein-4;
MFI, mean fluorescence intensity. |
Patient stratification according to
nuclear grade indicates no differences in the expression of
exhaustion markers
To assess the clinical significance of quantifying
the exhaustion-associated markers, the differential expression of
these markers was evaluated according to the tumor nuclear grade
(Table II). No overall differences
in marker expression associated with nuclear grade were reported,
except for CD3 positivity based on immunohistochemistry.
Furthermore, the temporal changes in blood expression levels of the
tested markers were compared 12 months after therapeutic
nephrectomy (Table III). The only
change observed was an increase in CTLA-4 expression levels in
blood lymphocytes at 12 months compared with basal levels.
Collectively, ccRCC tissue exhibited increased proportions of
infiltrated CD3+ lymphocytes and monocytes compared with
the proportions in the blood (Figs.
1 and 2). Infiltrated
leukocytes showed decreased expression levels of the exhaustion
markers LAG-3, TIM-3, CTLA-4 and PD-L1, but not PD-1 (Fig. 3). Furthermore, the variables did not
change after stratifying the patients according to nuclear grade
and between baseline and clinical follow-up (Tables II and III).
 | Table II.Analysis of leukocyte markers
according to tumor grade G2-G4. |
Table II.
Analysis of leukocyte markers
according to tumor grade G2-G4.
| Variable | G2 (n=6) | G3 (n=9) | G4 (n=5) |
P-valuea |
|---|
| Sex, n (%) |
|
|
| 0.354 |
|
Male | 5 (83) | 5 (55) | 3 (60) |
|
|
Female | 1 (17) | 4 (45) | 2 (40) |
|
| Age, years ±
SD | 61.5±8.6 | 59.7±8.9 | 60.2±7.7 | 0.919 |
| Tumor size, cm ±
SD | 7.2±3.2 | 7.8±5.1 | 8.6±2.4 | 0.851 |
| Necrosis, % ±
SD | 16.7±17.2 | 15.5±19.8 | 22.5±14.7 | 0.744 |
| Positive tissue on
immunohistochemistry, % ± SD |
|
|
|
|
|
CD3 | 13.4±7.6 | 19.8±14.6 | 39.6±22.0 | 0.030 |
| CD3
(clinical) | 14.3±22.6 | 25.6±30.7 | 54.0±24.1 | 0.071 |
|
CTLA-4 | 14.9±25.7 | 13.1±14.7 | 9.4±10.0 | 0.876 |
| CTLA-4
(clinical) | 17.0±26.3 | 8.6±19.9 | 4.2±8.8 | 0.566 |
|
PD-L1 | 16.6±31.0 | 6.8±5.8 | 0.6±0.5 | 0.322 |
| PD-L1
(clinical) | 0.5±0.5 | 5.8±11.2 | 4.8±8.5 | 0.514 |
| Flow cytometry of
blood samples, MFI ± SD |
|
|
|
|
| PD-L1
granulocytes | 6,975±5,007 | 6,165±2,272 | 8,873±4,598 | 0.669 |
| PD-L1
monocytes | 5,486±3,618 | 9,543±5,709 | 9,414±2,200 | 0.356 |
| PD-1
CD3 | 475±158 | 925±577 | 2,357±2,451 | 0.134 |
| LAG-3
CD3 | 662±238 | 1,000±530 | 3,093±3,319 | 0.122 |
| TIM-3
CD3 | 552±288 | 775±336 | 1,309±840 | 0.130 |
| CTLA-4
CD3 | 692±226 | 1,166±724 | 4,285±5,624 | 0.181 |
| Flow cytometry of
tumor samples, MFI ± SD |
|
|
|
|
| PD-L1
granulocytes | 6,797±6,332 | 6,031±7,207 | 2,207±1,010 | 0.588 |
| PD-L1
monocytes | 3,032±1,059 | 7,944±3,871 | 29451±48,419 | 0.162 |
| PD-1
CD3 | 2,473±792 | 2,248±1,399 | 3,128±2,616 | 0.729 |
| LAG-3
CD3 | 1,301±294 | 1,488±930 | 1,452±768 | 0.927 |
| TIM-3
CD3 | 502±258 | 731±501 | 1,139±1,015 | 0.395 |
| CTLA-4
CD3 | 444±227 | 801±876 | 1,052±816 | 0.524 |
 | Table III.Changes in leukocyte markers at 12
months follow-up using flow cytometry. |
Table III.
Changes in leukocyte markers at 12
months follow-up using flow cytometry.
| Variable | Basal (n=20) | 12 months
(n=10) |
P-valuea |
|---|
| Granulocytes, % ±
SD | 34.2±32.9 | 45.4±21.4 | 0.320 |
| Monocytes, % ±
SD | 12.2±11.0 | 7.4±2.3 | 0.166 |
| Lymphocytes, % ±
SD | 48.3±25.7 | 42.0±21.4 | 0.497 |
| CD4+, %
± SD | 56.3±18.1 | 52.8±15.1 | 0.604 |
| CD8+, %
± SD | 24.6±13.8 | 18.1±9.2 | 0.197 |
| PD-L1, MFI ±
SD |
|
|
|
|
Monocytes | 6,135±3,916 | 6,807±4,716 | 0.674 |
|
Granulocytes | 15,867±27,880 | 4,951±3,556 | 0.232 |
| PD-1, MFI ± SD |
|
|
|
|
CD3+ | 2,320±1,821 | 3,160±1,967 | 0.260 |
|
CD4+ | 1,973±1,534 | 2,993±1,768 | 0.118 |
|
CD8+ | 2,570±1,499 | 3,041±1,779 | 0.457 |
| LAG-3, MFI ±
SD |
|
|
|
|
CD3+ | 3,043±2,802 | 1,771±1,208 | 0.185 |
|
CD4+ | 2,840±2,321 | 1,826±1,192 | 0.209 |
|
CD8+ | 2,805±2,272 | 1,464±968 | 0.088 |
| TIM-3, MFI ±
SD |
|
|
|
|
CD3+ | 1,358±805 | 1,263±817 | 0.766 |
|
CD4+ | 1,301±772 | 1,210±746 | 0.762 |
|
CD8+ | 1,317±744 | 1,193±786 | 0.680 |
| CTLA-4, MFI ±
SD |
|
|
|
|
CD3+ | 3,672±4,437 | 8,416±6,330 | 0.026 |
|
CD4+ | 3,902±4,297 | 8,539±6,102 | 0.024 |
|
CD8+ | 3,012±4,058 | 6,743±5,130 | 0.041 |
Discussion
RCC primarily affects patients aged 60 to 70 years
old (2). Among its variants, up to
80% of the diagnoses are associated with ccRCC, a severe disease
characterized by intrinsic aggressiveness, chemoresistance and poor
prognosis (3,6). In the early stages, ccRCC may be cured
through nephrectomy; however, advanced disease often requires
systemic and combined therapies such as immunotherapy and systemic
targeted therapy (5). These
treatments present challenges, particularly due to the lack of
validated tools for selecting patients based on their likelihood of
clinical response and the risk of adverse events (13,14).
The role of infiltrating leukocytes in prognosis and response to
treatment is not completely understood and depends on
characteristics such as the cell phenotype, tumor infiltration and
the cell exhaustion status (7). For
example, the increased infiltration of CD8+ and
CD68+ cells and decreased infiltration of
CD4+ cells predict a positive response to ICI treatment
(18). However, in general,
tumor-infiltrating lymphocytes are commonly associated with poor
prognosis (19). Particularly in
RCC, highly infiltrated tumors (also called hot tumors) are
associated with poor prognosis (20). Among exhaustion markers, the
increased expression of PD-L1 is found in ~6% of patients with RCC
and is commonly associated with poor outcomes (21). The relationship between systemic
inflammation and RCC prognosis has also been investigated,
revealing a negative association between these variables (22). For these reasons, the expression
levels of exhaustion receptors should be interpreted with caution
because, while increased levels of PD-L1 are commonly associated
with poor overall prognosis, the increased availability of these
ligands could be predictive of the success of ICI treatments
(23). There is a lack of
information regarding the temporal expression of molecular
immunotherapy targets among tumors and tumor stages, including
kidney tumors. To address this issue, the present study aimed to
characterize the expression of five common immunotherapy targets,
PD-1, PD-L1, LAG-3, TIM-3 and CTLA-4, in tissue and blood samples
obtained from a cohort of 20 patients with localized ccRCC, an
early stage of the disease. According to the literature, the 5-year
cancer-specific survival rates for stage I, II, III and IV patients
are 97.4, 89.9, 77.9 and 26.7%, respectively, and nephrectomy
improves the condition-specific survival, mostly in advanced tumors
(stages III and IV) (24).
The immunohistochemistry results of the present
study aligned with those of previous reports that characterize
ccRCC as a highly immunogenic tumor (25). The tumor tissue indicated increased
expression levels of CD3, but moderate-to-absent expression of
CTLA-4 and PD-L1. In the majority of cancer types, elevated CD3
expression is generally associated with a favorable prognosis and
response to systemic immunotherapy (26–29);
however, previous studies suggest the opposite for ccRCC, where
high infiltration of lymphocytes and increased expression of
exhaustion markers have been associated with poor overall prognosis
and unresponsiveness to immunotherapy (25,30,31).
The present study assessed the immunohistochemistry data using two
approaches: Clinical analysis by pathologists and digital analysis
using the QuPath software. From an objective point of view, digital
analysis would overcome the possibility of subjective or biased
analysis, although it is more complex and usually restricted to
research settings.
Following immunohistochemistry, the present study
adopted flow cytometry to evaluate the expression of biomarkers in
the blood and tissue samples. The integration of
immunohistochemistry and flow cytometry followed the rationale that
combined methods would enable a broader and more accurate
assessment of immune exhaustion compared with each method alone.
Flow cytometry was selected as a complementary technique to
immunohistochemistry due to its capacity for high-dimensional,
single-cell analysis, which allows for the precise quantification
of immune subsets and co-expression of exhaustion markers. In the
flow cytometry analysis, the present study evaluated the expression
of a set of biomarkers commonly associated with lymphocyte
exhaustion and targeted by therapeutic monoclonal antibodies in
clinical oncology. The results demonstrated that the patients had a
distinct pattern of infiltrating leukocytes, predominantly
cytotoxic lymphocytes and monocytes. Evaluation of the tumor tissue
indicated the absence of CD4+ lymphocytes and a relative
increase in CD3+CD4−CD8− cells
(data not shown). A decrease in the number of infiltrating
CD4+ cells has been associated with poor prognosis in
patients with sarcoma and cervical carcinoma (32,33).
The relative increase in
CD3+CD4−CD8− cells and the
abundance of lipids within the tumor core suggest the presence of
natural killer T lymphocytes because such cells have intrinsic
capabilities to recognize lipidic antigens (34,35).
Furthermore, ccRCC has been associated with the presence of
circulating double-positive lymphocytes
(CD4+CD8+) and dysfunctional lymphocytes in
ex vivo assays (36–38). Other previous studies have also
proposed the use of the neutrophil-to-lymphocyte ratio (39) and C-reactive protein serum levels
(40,41) as potential cost-effective biomarkers
for the prediction of immunotherapy response in advanced ccRCC.
After assessing lymphocyte kinetics, the present
study investigated the expression of exhaustion-associated markers
using flow cytometry. Among circulating leukocytes, patients had
increased TIM-3 expression levels in blood lymphocytes and
decreased PD-L1 expression levels in blood monocytes compared with
that of the control group. In tumors, unchanged expression levels
of PD-1 and decreased expression levels of LAG-3, TIM-3, CTLA-4 and
PD-L1 were reported compared with those in the blood samples.
Similarly, PD-L1 expression levels decreased among infiltrating
monocytes and granulocytes. These results suggest that mild
leukocyte dysfunction is present in the localized stage of ccRCC,
but there is uncertainty about the implications of these findings
in the later response to ICIs in advanced disease, mostly because
the immune exhaustion phenomenon is highly dynamic. It is important
to highlight that immune exhaustion is a complex mechanism
involving multiple receptors and cell phenotypes (42–44).
For example, exhausted CD8+ cells promote tumor
progression, but exhausted T regulatory (Treg) lymphocytes inhibit
tumor progression (45). Treg
lymphocytes mediate immunosuppression in T cells and dendritic
cells by producing the regulatory cytokines interleukin-10 and
transforming growth factor-β (45).
Therefore, detailed and comprehensive studies addressing multiple
markers are warranted to predict immunotherapy responses. Increased
expression levels of TIM-3 in lymphocytes and decreased expression
levels of PD-L1 in monocytes have been associated with worse
outcomes in patients diagnosed with RCC (46,47).
To assess the clinical significance of biomarker
expression in patients with RCC, patients were stratified based on
nuclear grade, due to the well-established inverse association
between nuclear grade and clinical outcomes (31,48).
The present study results did not indicate changes in tissue
leukocyte dynamics and biomarker expression compared with nuclear
grade, except for an increased percentage of CD3 positivity in
grade 4 tumors (Table II). In the
same context, when evaluating the changes in blood biomarker
expression after 12 months of clinical follow-up, the present study
demonstrated that most markers were stable over time, with the
notable exception of CTLA-4 (Table
III). These results suggest that the expression of exhaustion
biomarkers in localized disease was not associated with the nuclear
grade staging and did not demonstrate variation in the short-term
follow-up of 12-months.
The overall results of the present study suggest
that localized ccRCC shows decreased expression levels of the
exhaustion receptors LAG-3, TIM-3, CTLA-4 and PD-L1, implying a
lack of biological support for an immune exhaustion state in this
stage of the disease. In the future, it will be necessary to
analyze an expanded set of markers (OX40, 4-1BB, TOX and
interleukin-10) and cell phenotypes (T regulatory lymphocytes,
myeloid-derived suppressor cells and tumor-associated macrophages)
in localized and metastatic disease to shed light on the
progressive expression of such biomarkers in RCC.
The present study had several limitations, including
the small number of participants, which followed the exclusion of
samples with insufficient cell counts to ensure reliable results
and the short-term follow-up of patients, which complicates the
clinical significance of the findings. In addition, tissue samples
were compared with blood samples because obtaining healthy renal
tissue is challenging due to ethical and practical reasons.
However, the findings remain notable due to the clinical nature of
the present study, the combined use of immunohistochemistry and
flow cytometry, simultaneous analysis of tumor and blood samples
and homogeneous inclusion of patients with localized disease. In
the future, a prospective study is warranted to assess an extended
panel of biomarkers as prognostic and predictive tools to guide the
use of immunotherapy in patients with RCC.
ccRCC is highly infiltrated by lymphocytes and
patients demonstrate decreased expression levels of the exhaustion
biomarkers LAG-3, TIM-3, CTLA-4 and PD-L1 within infiltrating tumor
leukocytes compared with circulating leukocytes. These biomarkers
remain stable in the short term and are not associated with nuclear
grade staging, which suggest they have limited use as a prognostic
tool in localized ccRCC.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was supported by the National Science and
Technology Council (CONACYT), México (grant no. 301133).
Availability of data and materials
The data generated in the present study may be found
on Figshare at the following URL: https://figshare.com/articles/dataset/Flow_cytometry_data_for_tissue_leukocytes_in_a_cohort_of_20_patients_with_clear_cells_renal_cell_carcinoma/26888827/1.
Authors' contributions
RGGa, MMT, AGG and MCSC conceptualized and designed
the present study. RGGa, MMT and NLL developed and devised the
methodology. MMT and RGGu supervised the present study. RGGa, AGG,
VMOJ and MAOM were involved in the clinical evaluation and
follow-up of patients. MMT and RGGa acquired and analyzed the data
for flow cytometry. JPFG, RGGa, RGGu and JHEJ were involved in the
immunohistochemistry analysis. RGGa, MAOM and MMT wrote the first
draft. MMT, MCSC, VMOJ and NLL revised and corrected the
manuscript. RGGa and MMT confirm the authenticity of all the raw
data. All authors read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
The present study was approved by the institutional
ethics committee (approval no. IN23-00002). All patients provided
written informed consent before enrollment in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Padala SA and Barsouk A, Thandra KC,
Saginala K, Mohammed A, Vakiti A, Rawla P and Barsouk A:
Epidemiology of renal cell carcinoma. World J Oncol. 11:79–87.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rose TL and Kim WY: Renal cell carcinoma:
A review. JAMA. 332:1001–1010. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Di S, Gong M, Lv J, Yang Q, Sun Y, Tian Y,
Qian C, Chen W, Zhou W, Dong K, et al: Glycolysis-related biomarker
TCIRG1 participates in the regulation of renal cell carcinoma
progression and tumor immune microenvironment by affecting aerobic
glycolysis and AKT/mTOR signaling pathway. Cancer Cell Int.
23:1862023. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ma G, Wang Z, Liu J, Fu S, Zhang L, Zheng
D, Shang P and Yue Z: Quantitative proteomic analysis reveals
sophisticated metabolic alteration and identifies FMNL1 as a
prognostic marker in clear cell renal cell carcinoma. J Cancer.
12:6563–6575. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Scosyrev E, Messing EM, Sylvester R and
Van Poppel H: exploratory subgroup analyses of renal function and
overall survival in European organization for research and
treatment of cancer randomized trial of nephron-sparing surgery
versus radical nephrectomy. Eur Urol Focus. 3:599–605. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bahadoram S, Davoodi M, Hassanzadeh S,
Bahadoram M, Barahman M and Mafakher L: Renal cell carcinoma: An
overview of the epidemiology, diagnosis, and treatment. G Ital
Nefrol. 39:2022–vol3. 2022.PubMed/NCBI
|
|
7
|
González-Garza R, Gutiérrez-González A,
Salinas-Carmona M and Mejía-Torres M: Biomarkers for evaluating the
clinical response to immune checkpoint inhibitors in renal cell
carcinoma (Review). Oncol Rep. 52:1642024. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jiang X, Liu G, Li Y and Pan Y: Immune
checkpoint: The novel target for antitumor therapy. Genes Dis.
8:25–37. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Motzer RJ, Alyasova A, Ye D, Karpenko A,
Li H, Alekseev B, Xie L, Kurteva G, Kowalyszyn R, Karyakin O, et
al: Phase II trial of second-line everolimus in patients with
metastatic renal cell carcinoma (RECORD-4). Ann Oncol. 27:441–448.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Motzer RJ, Tannir NM, McDermott DF,
Frontera OA, Melichar B, Choueiri TK, Plimack ER, Barthélémy P,
Porta C, George S, et al: Nivolumab plus ipilimumab versus
sunitinib in advanced renal-cell carcinoma. N Engl J Med.
378:1277–1290. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Motzer RJ, Penkov K, Haanen J, Rini B,
Albiges L, Campbell MT, Venugopal B, Kollmannsberger C, Negrier S,
Uemura M, et al: Avelumab plus axitinib versus sunitinib for
advanced renal-cell carcinoma. N Engl J Med. 380:1103–1115. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Rini BI, Powles T, Atkins MB, Escudier B,
McDermott DF, Suarez C, Bracarda S, Stadler WM, Donskov F, Lee JL,
et al: Atezolizumab plus bevacizumab versus sunitinib in patients
with previously untreated metastatic renal cell carcinoma
(IMmotion151): A multicentre, open-label, phase 3, randomised
controlled trial. Lancet. 393:2404–2415. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang DY, Salem JE, Cohen JV, Chandra S,
Menzer C, Ye F, Zhao S, Das S, Beckermann KE, Ha L, et al: Fatal
toxic effects associated with immune checkpoint inhibitors. JAMA
Oncol. 4:1721–1728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Saliby RM, Saad E, Kashima S, Schoenfeld
DA and Braun DA: Update on biomarkers in renal cell carcinoma.
American Society of Clinical Oncology Educational Book. 44:22024.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Amin MB; American Joint Committee on
Cancer, : American Cancer Society: AJCC cancer staging manual,
Eight edition/editor-in-chief, Mahul B, Amin, MD, FCAP; Stephen B.
Edge, MD, FACS [and 16 others]; Donna M. Gress, RHIT, CTR-Technical
editor; Laura R. Meyer, CAPM-Managing editor. American Joint
Committee on Cancer, Springer; Chicago IL: 2017
|
|
16
|
Organisation mondiale de la santé, Centre
international de recherche sur le cancer, . Urinary and male
genital tumours. 5th ed. International agency for research on
cancer; Lyon: 2022
|
|
17
|
Bankhead P, Loughrey MB, Fernández JA,
Dombrowski Y, McArt DG, Dunne PD, McQuaid S, Gray RT, Murray LJ,
Coleman HG, et al: QuPath: Open source software for digital
pathology image analysis. Sci Rep. 7:168782017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kazama A, Bilim V, Tasaki M, Anraku T,
Kuroki H, Shirono Y, Murata M, Hiruma K and Tomita Y:
Tumor-infiltrating immune cell status predicts successful response
to immune checkpoint inhibitors in renal cell carcinoma. Sci Rep.
12:203862022. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu C, Cui Y, Liu J, Ma L, Xiong Y, Gong Y,
Zhao Y, Zhang X, Chen S, He Q, et al: Noninvasive evaluation of
tumor immune microenvironment in patients with clear cell renal
cell carcinoma using metabolic parameter from preoperative
2-[18F]FDG PET/CT. Eur J Nucl Med Mol Imaging.
48:4054–4066. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ohe C, Yoshida T, Ikeda J, Tsuzuki T,
Ohashi R, Ohsugi H, Atsumi N, Yamaka R, Saito R, Yasukochi Y, et
al: Histologic-based tumor-associated immune cells status in clear
cell renal cell carcinoma correlates with gene signatures related
to cancer immunity and clinical outcomes. Biomedicines. 10:3232022.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Möller K, Fraune C, Blessin NC, Lennartz
M, Kluth M, Hube-Magg C, Lindhorst L, Dahlem R, Fisch M, Eichenauer
T, et al: Tumor cell PD-L1 expression is a strong predictor of
unfavorable prognosis in immune checkpoint therapy-naive clear cell
renal cell cancer. Int Urol Nephrol. 53:2493–2503. 2021. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ozbek E, Besiroglu H, Ozer K, Horsanali MO
and Gorgel SN: Systemic immune inflammation index is a promising
non-invasive marker for the prognosis of the patients with
localized renal cell carcinoma. Int Urol Nephrol. 52:1455–1463.
2020. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kawashima A, Kanazawa T, Kidani Y, Yoshida
T, Hirata M, Nishida K, Nojima S, Yamamoto Y, Kato T, Hatano K, et
al: Tumour grade significantly correlates with total dysfunction of
tumour tissue-infiltrating lymphocytes in renal cell carcinoma. Sci
Rep. 10:62202020. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cheaib JG, Patel HD, Johnson MH, Gorin MA,
Haut ER, Canner JK, Allaf ME and Pierorazio PM: Stage-specific
conditional survival in renal cell carcinoma after nephrectomy.
Urol Oncol. 38:6.e1–6.e7. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Giraldo NA, Becht E, Vano Y, Petitprez F,
Lacroix L, Validire P, Sanchez-Salas R, Ingels A, Oudard S, Moatti
A, et al: Tumor-Infiltrating and peripheral blood T-cell
immunophenotypes predict early relapse in localized clear cell
renal cell carcinoma. Clin Cancer Res. 23:4416–4428. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jung M, Lee JA, Yoo SY, Bae JM, Kang GH
and Kim JH: Intratumoral spatial heterogeneity of
tumor-infiltrating lymphocytes is a significant factor for
precisely stratifying prognostic immune subgroups of microsatellite
instability-high colorectal carcinomas. Mod Pathol. 35:2011–2022.
2022. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Mei Z, Liu Y, Liu C, Cui A, Liang Z, Wang
G, Peng H, Cui L and Li C: Tumour-infiltrating inflammation and
prognosis in colorectal cancer: Systematic review and
meta-analysis. Br J Cancer. 110:1595–1605. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tewari N, Zaitoun AM, Arora A, Madhusudan
S, Ilyas M and Lobo DN: The presence of tumour-associated
lymphocytes confers a good prognosis in pancreatic ductal
adenocarcinoma: An immunohistochemical study of tissue microarrays.
BMC Cancer. 13:4362013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rathore AS, Kumar S, Konwar R, Makker A,
Negi MPS and Goel MM: CD3+, CD4+ & CD8+ tumour infiltrating
lymphocytes (TILs) are predictors of favourable survival outcome in
infiltrating ductal carcinoma of breast. Indian J Med Res.
140:361–369. 2014.PubMed/NCBI
|
|
30
|
Kahlmeyer A, Stöhr CG, Hartmann A, Goebell
PJ, Wullich B, Wach S, Taubert H and Erlmeier F: Expression of PD-1
and CTLA-4 are negative prognostic markers in renal cell carcinoma.
J Clin Med. 8:7432019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kawakami F, Sircar K, Rodriguez-Canales J,
Fellman BM, Urbauer DL, Tamboli P, Tannir NM, Jonasch E, Wistuba
II, Wood CG and Karam JA: PD-L1 and tumor infiltrating lymphocytes
status in patients with renal cell carcinoma and sarcomatoid
dedifferentiation. Cancer. 123:4823–4831. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bi Q, Liu Y, Yuan T, Wang H, Li B, Jiang
Y, Mo X, Lei Y, Xiao Y, Dong S, et al: Predicted CD4+ T cell
infiltration levels could indicate better overall survival in
sarcoma patients. J Int Med Res. 49:03000605209815392021.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shah W, Yan X, Jing L, Zhou Y, Chen H and
Wang Y: A reversed CD4/CD8 ratio of tumor-infiltrating lymphocytes
and a high percentage of CD4+FOXP3+ regulatory T cells are
significantly associated with clinical outcome in squamous cell
carcinoma of the cervix. Cell Mol Immunol. 8:59–66. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Paul S, Chhatar S, Mishra A and Lal G:
Natural killer T cell activation increases iNOS+CD206- M1
macrophage and controls the growth of solid tumor. J Immunother
Cancer. 7:2082019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li J, Moresco P and Fearon DT:
Intratumoral NKT cell accumulation promotes antitumor immunity in
pancreatic cancer. Proc Natl Acad Sci USA. 121:e24039171212024.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Nishida K, Kawashima A, Kanazawa T, Kidani
Y, Yoshida T, Hirata M, Yamamoto K, Yamamoto Y, Sawada M, Kato R,
et al: Clinical importance of the expression of CD4+CD8+ T cells in
renal cell carcinoma. Int Immunol. 32:347–357. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Menard LC, Fischer P, Kakrecha B, Linsley
PS, Wambre E, Liu MC, Rust BJ, Lee D, Penhallow B, Orduno NM and
Nadler SG: Renal cell carcinoma (RCC) tumors display large
expansion of double positive (DP) CD4+CD8+ T cells with expression
of exhaustion markers. Front Immunol. 9:27282018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zelba H, Bedke J, Hennenlotter J, Mostböck
S, Zettl M, Zichner T, Chandran A, Stenzl A, Rammensee HG and
Gouttefangeas C: PD-1 and LAG-3 dominate checkpoint
receptor-mediated T-cell inhibition in renal cell carcinoma. Cancer
Immunol Res. 7:1891–1899. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Young M, Tapia JC, Szabados B, Jovaisaite
A, Jackson-Spence F, Nally E and Powles T: NLR outperforms low
hemoglobin and high platelet count as predictive and prognostic
biomarker in metastatic renal cell carcinoma treated with immune
checkpoint inhibitors. Clin Genitourin Cancer. 22:1020722024.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ishihara H, Takagi T, Kondo T, Fukuda H,
Tachibana H, Yoshida K, Iizuka J, Okumi M, Ishida H and Tanabe K:
Predictive impact of an early change in serum C-reactive protein
levels in nivolumab therapy for metastatic renal cell carcinoma.
Urol Oncol. 38:526–532. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Noguchi G, Nakaigawa N, Umemoto S,
Kobayashi K, Shibata Y, Tsutsumi S, Yasui M, Ohtake S, Suzuki T,
Osaka K, et al: C-reactive protein at 1 month after treatment of
nivolumab as a predictive marker of efficacy in advanced renal cell
carcinoma. Cancer Chemother Pharmacol. 86:75–85. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wei F, Zhong S, Ma Z, Kong H, Medvec A,
Ahmed R, Freeman GJ, Krogsgaard M and Riley JL: Strength of PD-1
signaling differentially affects T-cell effector functions. Proc
Natl Acad Sci USA. 110:E2480–E2489. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsujikawa T, Kumar S, Borkar RN, Azimi V,
Thibault G, Chang YH, Balter A, Kawashima R, Choe G, Sauer D, et
al: Quantitative multiplex immunohistochemistry reveals
myeloid-inflamed tumor-immune complexity associated with poor
prognosis. Cell Rep. 19:203–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Pichler R, Siska PJ, Tymoszuk P, Martowicz
A, Untergasser G, Mayr R, Weber F, Seeber A, Kocher F, Barth DA, et
al: A chemokine network of T cell exhaustion and metabolic
reprogramming in renal cell carcinoma. Front Immunol.
14:10951952023. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Denize T, Jegede OA, Matar S, El Ahmar N,
West DJ, Walton E, Bagheri AS, Savla V, Laimon YN, Gupta S, et al:
PD-1 Expression on intratumoral regulatory T cells is associated
with lack of benefit from anti-PD-1 therapy in metastatic
clear-cell renal cell carcinoma patients. Clin Cancer Res.
30:803–813. 2024. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Cai C, Xu YF, Wu ZJ, Dong Q, Li MY, Olson
JC, Rabinowitz YM, Wang LH and Sun Y: Tim-3 expression represents
dysfunctional tumor infiltrating T cells in renal cell carcinoma.
World J Urol. 34:561–567. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yeong J, Zhao Z, Lim JCT, Li H, Thike AA,
Koh VCY, Teh BT, Kanesvaran R, Toh CK, Tan PH and Khor LY: PD-L1
expression is an unfavourable prognostic indicator in Asian renal
cell carcinomas. J Clin Pathol. 73:463–469. 2020. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Warren AY and Harrison D: WHO/ISUP
classification, grading and pathological staging of renal cell
carcinoma: Standards and controversies. World J Urol. 36:1913–1926.
2018. View Article : Google Scholar : PubMed/NCBI
|